Abstract
The magnocellular hypothalamic neurons exhibit a substantial degree of structural and functional plasticity over the time of pregnancy, parturition, and lactation. This study has used in situhybridization techniques to examine whether the content of α1, α2, β2, and γ2GABAA receptor subunit mRNAs expressed by these cells fluctuates over this period. A process of regional, followed by cellular and then topographical, analyses within the supraoptic (SON) and posterior paraventricular (PVN) nuclei revealed that an increase in magnocellular α1 subunit mRNA content occurred during the course of pregnancy up to day 19, after which a decline in expression was detected on the day of parturition. Significant fluctuations of this nature were observed only in the oxytocin neuron-enriched regions of the SON and PVN. The expression of α2, β2, and γ2 subunit mRNAs in the SON and PVN and of all subunit mRNAs in the cingulate cortex did not change over this period. During lactation, γ2 subunit mRNA content within the PVN increased significantly on day 14 of lactation as compared with day 7, and topographical analysis suggested that it involved principally magnocellular vasopressin neurons.
These results demonstrate the cell- and subunit-specific regulation of GABAA receptor mRNA expression within the hypothalamic magnocellular system. In particular, they suggest that fluctuations in α1 subunit expression may contribute to the marked variations in electrical activity exhibited by magnocellular oxytocin neurons at the time of parturition. More generally, they provide evidence in support of GABAA receptor plasticity within a physiological context in the adult rat brain.
Keywords: GABAA receptor subunit, in situ hybridization, lactation, oxytocin, paraventricular nucleus, parturition, pregnancy, supraoptic nucleus, vasopressin
The γ aminobutyric acidA(GABAA) receptor is responsible for the majority of fast synaptic inhibition in the mammalian forebrain. Molecular cloning studies now have revealed the existence of a large GABAA receptor gene family encoding six α, four β, three γ, one δ, and two ρ subunits, which are thought to combine as heteropentamers to form pharmacologically distinct receptor isoforms (Macdonald and Olsen, 1994; Sieghart, 1995). Although the exact composition and stoichiometry of native GABAA receptors remains unknown, photolabeling studies and the identification within the brain of different patterns of GABAA receptor ligand binding (Bureau and Olsen, 1993) and receptor subunit expression (Wisden et al., 1992) indicate that a wide variety of GABAA receptor isoforms is likely to be expressed.
The activity of neuronal networks in the forebrain is thought to be critically dependent on GABAergic transmission and substantial interest has focused on the regulatory control of GABAAreceptors. To date, such studies have concentrated on developmental regulation, including changes during aging (MacLennan et al., 1991;Poulter et al., 1993; Fritschy et al., 1994; Gutierrez et al., 1994;Mathews et al., 1994), and examined the response of GABAA receptor expression and function in adults to a variety of allosteric GABAA receptor modulators (Kang and Miller, 1991; Montpied et al., 1991; Primus and Gallager, 1992; Mhatre et al., 1993; Tseng et al., 1994) as well as excitatory and inhibitory amino acids (Kim et al., 1993; Mhatre and Ticku, 1994; Zhu et al., 1995; Fénelon and Herbison, 1996). Further work has examined GABAA receptor expression in pathological states such as epilepsy (Kokaia et al., 1994; Kamphuis et al., 1995), Huntington’s disease (Faull et al., 1993), and chronic stress (Montpied et al., 1993). Although these studies clearly have relevance in understanding the developmental and pathophysiological roles of GABA transmission in the brain, surprisingly little evidence exists for changes in GABAA receptor expression within a physiological context in the adult brain.
The hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei contain the cell bodies of the magnocellular oxytocin neurons, which project to the posterior pituitary gland and play a critical role in enabling parturition and lactation to occur. It is now well established that these cells exhibit marked, reversible changes in their morphology, synaptic connectivity, and electrical activity with each cycle of pregnancy and lactation in the female rat (Hatton, 1990;Theodosis and Poulain, 1993). As such, they provide an excellent example of an adult neuronal population that exhibits a substantial degree of neuronal plasticity within a defined physiological context. We have determined the complement of GABAAreceptor subunits expressed by these hypothalamic magnocellular neurons and shown that, regardless of whether they synthesize oxytocin or vasopressin, they all express mRNA and protein for the α1, α2, β2, and γ2 subunits (Fénelon and Herbison, 1995; Fénelon et al., 1995). Because evidence suggests a critical role for GABA in regulating the activity of adult hypothalamic magnocellular neurons via the GABAA receptor (Moos, 1995; Voisin et al., 1995), we questioned in this study whether these magnocellular neurons may represent a neural population in which fluctuations in GABAA receptor subunit expression may occur within a physiological context in the adult rat brain.
MATERIALS AND METHODS
Animals and tissue preparation. Parturient, pregnant, lactating, and virgin (200–250 gm) Wistar female rats from the Babraham colony were maintained in a controlled environment (lights on from 5:00 A.M. to 7:00 P.M.; 22°C) with food and water freely available. Although gonadal steroids have been shown to influence GABAA receptor subunit expression in other regions of the hypothalamus (Herbison and Fénelon, 1995), ovariectomy has no effect on the subunit mRNA content of magnocellular neurons (V. S. Fénelon and A. E. Herbison, unpublished data). In this study, virgin female rats were ovariectomized under Avertin anesthesia (2% tribromoethanol, 1 ml/100 gm body weight, i.p.) and used as our nonpregnant control group. Six groups of five animals were used; nonpregnant rats, day 10 and day 19 pregnant rats (day 0 of pregnancy equals day after overnight mating), parturient rats 1–2 hr after the delivery of the first pup, and day 7 and day 14 lactating rats (day 1 of lactation equals day of parturition). All rats were killed by cervical dislocation and decapitated; brains were removed quickly and frozen on dry ice. Parturient animals were killed throughout the afternoon, whereas all other rats were killed between 10:00 A.M. and 12:00 A.M.
In situ hybridization histochemistry. Detection of GABAA receptor α1, α2, β2, and γ2 subunit mRNAs was undertaken by usingin situ hybridization procedures reported previously (Fénelon and Herbison, 1995; Fénelon et al., 1995). Briefly, fresh-frozen sections (15 μm thick) were cut in the coronal plane through the hypothalamus (plates 22–26 of Swanson, 1992) and thaw-mounted onto Vectabond-coated slides. Antisense oligonucleotides (42–45 mer) complementary to the coding regions for amino acids 342–356, 340–354, 325–339, and 338–352 of the rat GABAA receptor α1, α2, β2, and γ2 subunits, respectively, were synthesized and 3′ end-labeled with [35S]dATP (1000–1500 Ci/mmol; DuPont NEN, Boston, MA) by using terminal deoxynucleotidyl transferase (50 U, Pharmacia) and resulting in a specific activity of ∼5 × 108 dpm/mg. Fixed sections were dehydrated with alcohol, and 250 μl of hybridization buffer (4× SSC, 50% deionized formamide, 10% dextran sulfate, 1× Denhardt’s solution, 250 μg/ml sheared salmon testis DNA, and 0.3% β mercaptoethanol) containing one of each of the35S-labeled α1, α2, β2, and γ2 subunit probes (28 fmol/ml equivalent to 2 × 109 cpm/ml) was applied to different series of slides containing 6–8 coronal sections.
Hybridization was performed in humidified chambers at 37°C overnight and washed in 1× SSC at room temperature, three times in 1× SSC at 55°C (30 min each), and again in 1× SSC for 1 hr at room temperature. Slides were dipped in Ilford K-5 nuclear track emulsion and exposed for 6–9 weeks in dark, tight boxes. At the appropriate exposure time, as determined by test slides, all slides were photodeveloped with Ilford Phenisol and counterstained lightly with methylene blue. Signal specificity was assessed by use of competition experiments in which radiolabeled probes were hybridized to sections in the presence of an excess (50- to 100-fold) unlabeled probe. Also, specificity was confirmed by reference to previous reports of the distribution of the rat transcripts of the α1, α2, β2, and γ2 subunits of the GABAAreceptor (MacLennan et al., 1991; Zhang et al., 1991; Araki et al., 1992; Wisden et al., 1992).
Data analysis. Using a Joyce–Loebl Magiscan analyzer coupled to a Leica Orthoplan microscope, we undertook an initial quantitative analysis of GABAA receptor subunit mRNA expression by determining silver-grain density (silver grains/μm2) overlying the whole SON in its midportion in the coronal plane (plates 23–24 of Swanson, 1992), the posterior magnocellular division of the PVN (pPVN; plates 25–26), as well as a 200 × 500 μm rectangle overlying layers 2 and 3 of the cingulate cortex. For each animal, four measurements of the SON and pPVN and three measurements of the cortex were taken from two to four sections and averaged to provide mean values for each subunit. Values from each animal were combined to give experimental group means (± SEM). For those subunits and regions in which statistical differences were evident, a subsequent cellular analysis of subunit mRNA expression was undertaken by analyzing 18–25 cells from two to four sections in each region.
Because these results suggested the possibility that subpopulations within the SON and pPVN expressed subunit mRNAs on a differential basis, we reanalyzed these sections on a detailed topographical basis. Despite their potential for oxytocin/vasopressin coexpression, the great majority of magnocellular neurons synthesizes only oxytocin or vasopressin (Jirikowski, 1992) and resides, in general, within different subregions of the PVN and SON (Rhodes et al., 1981; Sawchenko and Swanson, 1982). In this reanalysis, care was taken to identify and analyze, in two to three sections from each animal, a minimum of 40 magnocellular neurons equally from the oxytocin neuron-enriched dorsal half of the SON (Fig. 1A) and anteroventromedial pPVN (Fig. 1B1) and the vasopressin neuron-enriched ventral SON (Fig. 1A) and posterodorsolateral pPVN (Fig. 1B2). In all single-cell silver-grain analyses, the numbers of silver grains overlying cells in the excess unlabeled-probe control sections were determined, and, in experimental sections, only those cells expressing numbers of silver grains greater than five times that of controls were used for analysis. For each rat and each region, an average silver-grain count per cell was determined, and these values were combined to give experimental group means (±SEM). The same procedure was applied to the determination of the median when population shifts in silver-grain numbers per cell were analyzed. For each brain region, nonparametric Mann–Whitney U tests were performed between each temporally contiguous animal group (nonpregnant rats were considered as time point 0 and thus compared with day 10 pregnancy, then day 10 pregnancy versus day 19 pregnancy, and so forth); p ≤ 0.05 was considered statistically significant.
Fig. 1.
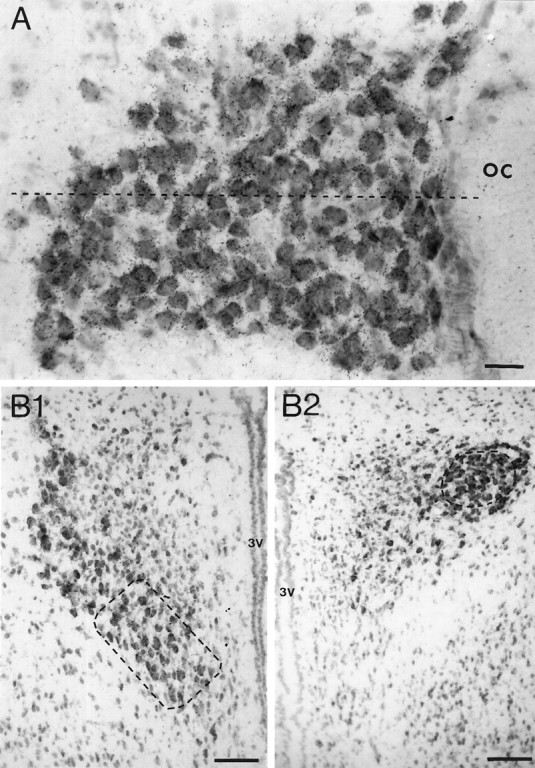
Methylene blue-counterstained sections of the supraoptic (A) and posterior paraventricular (B) nuclei from an ovariectomized rat after hybridization with35S-labeled GABAA receptor α1 subunit oligonucleotide illustrating the different regions in which cellular silver-grain analysis was undertaken. The dashed line in A divides the dorsal from the ventral half of the supraoptic nucleus.B1, The dashed rectangle indicates the region of the anteroventromedial posterior paraventricular nucleus in which magnocellular cells were sampled, whereas the dashedcircle in B2 indicates the position of magnocellular cells sampled from the posterodorsolateral posterior paraventricular nucleus. The anteroposterior level of B1 is rostral to that of B2. oc, Optic chiasm;3V, third ventricle. Scale bars: A, 50 μm;B1, B2, 100 μm.
RESULTS
The distribution of the four GABAA receptor subunit transcripts in the coronal brain sections was the same as that reported in our previous studies with the same oligonucleotide probes (Fénelon and Herbison, 1995; Fénelon et al., 1995). Magnocellular neurons of the SON and PVN displayed hybridization signals for α1, α2, β2, and γ2 subunit mRNAs.
Regional silver-grain density analysis
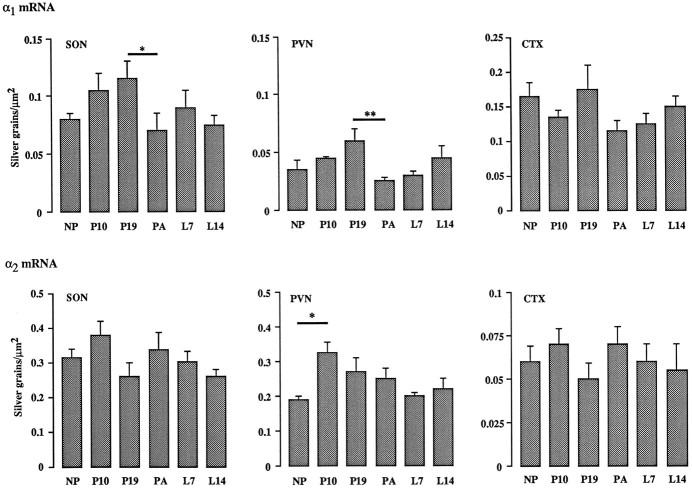
Silver-grain density analysis of the whole SON, pPVN, and layers 2 and 3 of the cingulate cortex detected three significant changes in receptor subunit expression throughout pregnancy, parturition, and lactation (Fig. 2, Table1). In terms of α1 subunit mRNA, a 40–60% decrease in silver-grain density was observed in both the SON (p < 0.05) and pPVN (p < 0.01) on the day of parturition as compared with day 19 of pregnancy (Fig. 2). Before parturition, a nonsignificant increase in α1 subunit mRNA content was observed as pregnancy progressed within both the SON and pPVN (Fig. 2). No significant differences in silver-grain density were detected for the α1 subunit in the cingulate cortex; overall, α1 subunit mRNA content was lower in the pPVN as compared with the SON (Fig. 2). The second significant change was observed with the α2subunit signal in which a significant (p < 0.05) ∼40% increase in silver-grain density was observed in the pPVN alone on day 10 of pregnancy as compared with nonpregnant ovariectomized rats (Fig. 2). Expression of α2 subunit mRNA did not change at any time in the SON or cingulate cortex or at other times in the pPVN (Fig. 2). The final significant alteration in subunit expression was found with the γ2 subunit hybridization signal, in which a significant (p < 0.01) 160% increase in silver-grain density was noted only within the pPVN of day 14 lactating rats as compared with day 7 animals (Table 1). A nonsignificant (p = 0.06) increase in γ2 mRNA content was noted within the SON between day 19 of pregnancy and the day of parturition (Table 1). Apart from the day 14 lactating rats, γ2 subunit mRNA expression was consistently lower in the pPVN as compared with the SON. No significant changes were detected at other time points, and no differences in β2 subunit mRNA expression were found at any time over the course of pregnancy, parturition, and lactation (Table 1). Again, β2 subunit mRNA expression was consistently lower in the pPVN as compared with the SON (Table 1.).
Fig. 2.
Quantitative silver-grain density analysis of α1 (top) and α2 (bottom) GABAA receptor subunit mRNA expression in the supraoptic nucleus (SON), posterior paraventricular nucleus (PVN), and cingulate cortex (CTX) in nonpregnant, ovariectomized (NP) pregnant day 10 (P10) and 19 (P19), parturient (PA), and lactating day 7 (L7) and 14 (L14) rats. Data are expressed as relative numbers of silver grains/μm2. Each bar represents the mean ± SEM of five animals. *p < 0.05; **p < 0.01.
Table 1.
β2 and γ2 subunit mRNA expression in the supraoptic (SON) and posterior paraventricular (PVN) nuclei and cingulate cortex (CTX) over pregnancy, parturition, and lactation
| NP | P10 | P19 | PA | L7 | L14 | |
|---|---|---|---|---|---|---|
| β2 mRNA | ||||||
| SON | 58 ± 4 | 55 ± 2 | 46 ± 3 | 49 ± 3 | 49 ± 9 | 46 ± 3 |
| PVN | 44 ± 5 | 40 ± 3 | 34 ± 2 | 31 ± 3 | 31 ± 5 | 38 ± 3 |
| CTX | 73 ± 1 | 64 ± 5 | 68 ± 5 | 57 ± 4 | 50 ± 3 | 56 ± 3 |
| γ2 mRNA | ||||||
| SON | 44 ± 12 | 33 ± 5 | 19 ± 5 | 38 ± 8 | 34 ± 7 | 47 ± 15 |
| PVN | 19 ± 6 | 26 ± 8 | 14 ± 2 | 16 ± 2 | 19 ± 4 | 46 ± 12* |
| CTX | 21 ± 2 | 20 ± 2 | 17 ± 2 | 16 ± 3 | 16 ± 1 | 20 ± 4 |
Values give mean ± SEM (n = 5) number of silver grains/μm2 (×0.001) in ovariectomized, nonpregnant (NP), day 10 pregnant (P10), day 19 pregnant (P19), parturient (PA), day 7 lactating (L7), and day 14 lactating (L14) rats.
Cellular silver-grain analysis
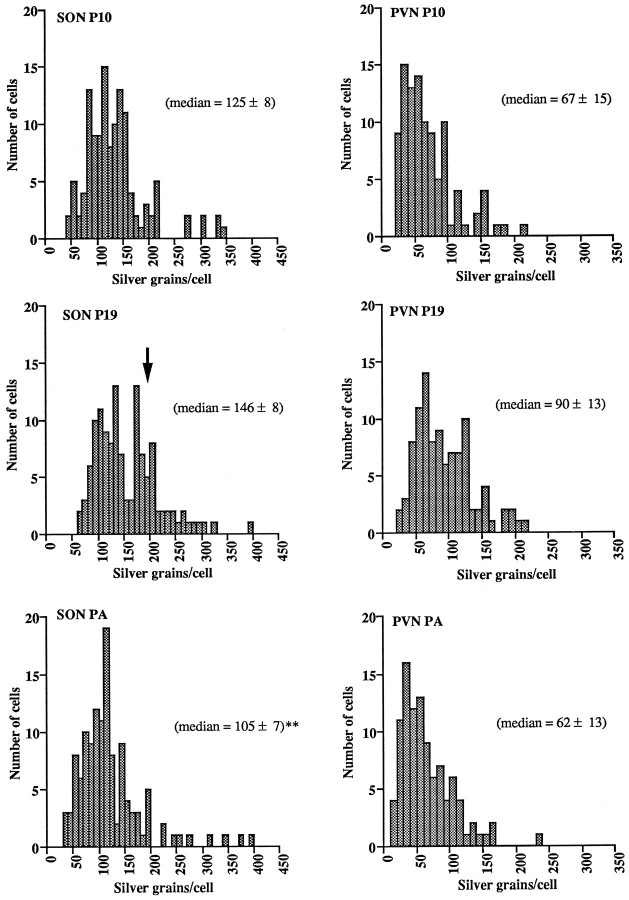
Single-cell silver-grain analysis of α1subunit mRNA hybridizations in the SON and pPVN of pregnant day 10 and day 19 and parturient rats enabled the construction of silver-grain-per-cell distribution histograms (Fig. 3). These histograms indicated a nonsignificant shift toward higher values of silver grains per cell in pregnancy day 19 rats as compared with day 10 pregnant animals in both the SON (medians: day 10 = 125 ± 8 and day 19 = 146 ± 8 silver grains/cell) and pPVN (medians: day 10 = 67 ± 5 and day 19 = 90 ± 13). A return toward lower values in parturient animals as compared with day 19 pregnant rats was found in both the SON (medians: day 19 = 146 ± 8 and parturition = 105 ± 7;p < 0.01) and pPVN (medians: day 19 = 90 ± 13 and parturition = 62 ± 13; Fig. 3). Although the histograms from pregnant day 10 rats were suggestive of an approximately normal distribution of silver grains per cell, those constructed from pregnancy day 19 rats, and in particular the SON, were suggestive of the appearance of a second population of high-expressing cells with a mean of ∼190 silver grains per cell (arrow, Fig. 3). This high-expressing population did not exist in parturient rats (Fig. 3).
Fig. 3.
Frequency distribution histograms of silver grains per magnocellular cell after α1 subunit hybridizations in the supraoptic nucleus (SON, leftcolumn) and paraventricular nucleus (PVN,right column) from pregnant rats on day 10 (P10) and 19 (P19) and parturient rats (PA). Median ± SEM values are given inparentheses for each group (n = 5).Arrow in SON P19 indicates a population of relatively high-expressing cells not detected at other time points. **p < 0.01 as compared with P19.
Reanalysis of the SON and pPVN on a more detailed topographical basis revealed that a significant increase and decrease in α1 subunit mRNA expression over pregnancy was observed only in magnocellular neurons located within the oxytocin neuron-enriched regions of these nuclei. A significant 41–85% increase (p < 0.05) and then 30–40% decrease (p < 0.05) in the number of silver grains per magnocellular cell was found as pregnancy progressed from day 10 to day 19 and then to parturition in only the dorsal SON and anteroventromedial pPVN (Fig. 4A,B). A significant increase (p < 0.05) in the number of silver grains per cell also was found from day 10 to 19 of pregnancy in the ventral SON, but no decreases were detected at the time of parturition in either of the vasopressin neuron-enriched subregions: the ventral SON and posterodorsolateral pPVN (Fig. 4C,D). On day 10 of pregnancy, the number of silver grains per magnocellular cell did not differ in oxytocin- and vasopressin-enriched regions of either the SON or pPVN (Fig. 4).
Fig. 4.
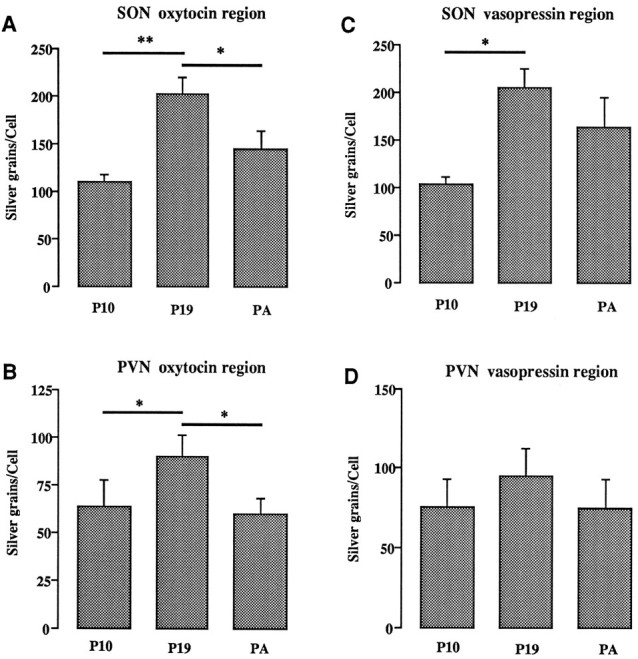
Cellular silver-grain analysis after α1 subunit mRNA hybridization in the dorsal (SON oxytocin region) and ventral (SONvasopressin region) parts of the supraoptic nucleus and in the anteroventromedial (PVN oxytocinregion) and posterodorsolateral (PVN vasopressinregion) parts of the posterior paraventricular nucleus in pregnant day 10 (P10) and 19 (P19) and parturient (PA) rats (n = 5). Bars represent mean number of silver grains per cell ± SEM. *p < 0.05; **p < 0.01.
Histogram analysis of the number of silver grains per cell resulting from the α2 subunit hybridization in the pPVN of nonpregnant and day 10 pregnant rats revealed a normally distributed pattern of silver grains per cell at both times (data not shown) and a nonsignificant shift to the right in the median (medians: 213 ± 5 in nonpregnant animals and 259 ± 14 silver grains/cell in day 10 pregnant rats). The mean number of silver grains per cell also was not significantly different (226 ± 8 in nonpregnant rats and 264 ± 14 silver grains/cell in day 10 pregnant animals; p = 0.095).
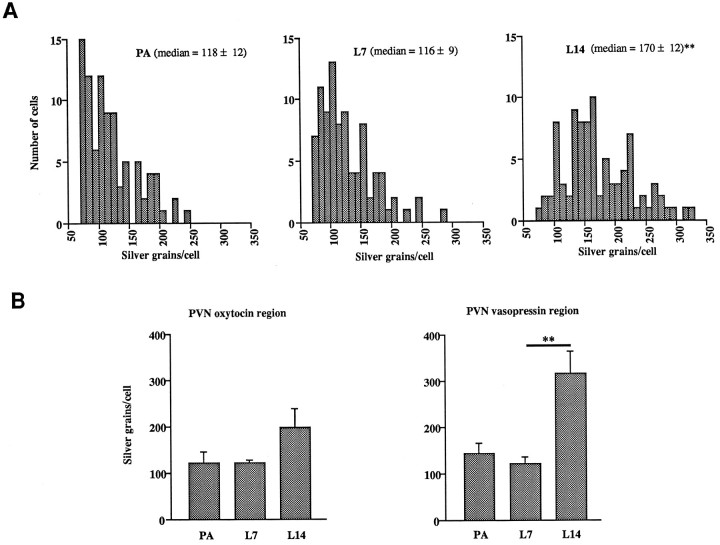
Similar distribution analysis for the γ2subunit showed a significant (p < 0.01) shift to the right in lactating day 14 rats as compared with day 7 lactating rats in only the pPVN (medians: 116 ± 9 on day 7 of lactation and 170 ± 12 silver grains/cell on day 14; Fig.5A). When reanalyzed on a topographical basis, the significant increase in the number of silver grains per cell on day 14 of lactation was found to be restricted to those magnocellular cells within the vasopressin neuron-enriched posterodorsolateral pPVN (p < 0.01; Fig.5B). The number of silver grains per magnocellular cell in the oxytocin- and vasopressin-enriched regions of the pPVN were not different on parturition and day 7 of lactation (Fig.5B).
Fig. 5.
A, Frequency distribution histograms of silver grains per magnocellular cell after γ2subunit hybridizations in the posterior paraventricular nucleus of parturient (PA), day 7 (L7), and day 14 (L14) lactating rats (n = 5). Median ± SEM values are given in parentheses for each group.B, Cellular silver-grain analysis after γ2 subunit mRNA hybridization in the anteroventromedial (PVN oxytocin region) and posterodorsolateral (PVN vasopressin region) parts of the posterior paraventricular nucleus in parturient (PA), day 7 (L7), and day 14 (L14) lactating rats. Bars represent mean number of silver grains per cell ± SEM. **p < 0.01 as compared withL7.
DISCUSSION
These results provide evidence for the cell- and subunit-specific regulation of GABAA receptor mRNA expression within the hypothalamic magnocellular system over the course of pregnancy, parturition, and lactation. Most important, we have shown here with regional silver-grain density analyses that α1 subunit mRNA expression falls between day 19 of pregnancy and the day of parturition in both the SON and pPVN, whereas other subunit mRNAs remain constant. Frequency histogram analysis of cellular silver-grain numbers in these animals indicated the possibility that a specific population of high-expressing α1 subunit mRNA-containing cells existed only on day 19 of pregnancy. A cellular reanalysis of the SON and pPVN on a more detailed topographical basis revealed that a 40–80% increase in α1 subunit mRNA expression between day 10 and 19 of pregnancy, followed by a 30–40% fall at the time of parturition, was found only in the oxytocin neuron-enriched regions of both nuclei. A similar process of analysis revealed a substantial increase in the γ2 subunit mRNA content of putative pPVN vasopressin neurons during late lactation. To the best of our knowledge, these findings represent the first demonstration of plasticity in GABAA receptor subunit mRNA expression within a physiological context in the adult rat brain.
We confirm here our earlier demonstration of α1, α2, β2, and γ2 subunit mRNA expression by hypothalamic magnocellular neurons and note that this correlates well with immunocytochemical evidence for the synthesis of only α1, α2, β2/3, and γ2 subunit proteins by all of these cells (Fénelon and Herbison, 1995;Fénelon et al., 1995). Because the GABAAreceptor subunits expressed by oxytocin and vasopressin neurons do not differ in any qualitative sense, we have taken advantage of the topographical distribution of oxytocin and vasopressin neurons within the SON and pPVN (Rhodes et al., 1981; Sawchenko and Swanson, 1982) to try to differentiate receptor subunit mRNA expression in these two cell types. The partially overlapping topography of these cells within the SON means that we can claim only to be examining oxytocin neuron- and vasopressin neuron-enriched cell populations in this nucleus; indeed, the significant increase in α1 subunit mRNA detected in the ventral SON between day 10 and 19 of pregnancy may reflect this incomplete differentiation between oxytocin and vasopressin neurons. However, our careful attention to analyzing only those cells within the core of the posterodorsolateral and ventral aspect of the anteroventromedial pPVN (Fig. 1) should have ensured that we dealt with almost pure vasopressin and oxytocin neural populations (Sawchenko and Swanson, 1982), respectively, in our subregional pPVN analysis.
GABAA receptor changes within the adult brain
It is clear that the modulation of inhibition in the brain has important implications for normal physiological processes as well as for the generation of pathological states. In principle, changes in GABAA receptor-mediated transmission may result from alterations in either the profile of synaptic GABA concentrations and/or the functioning of the GABAA receptors themselves. It seems likely that most synaptic GABAA receptors in the brain are saturated fully by spontaneous GABA bombardment, with near-maximal numbers of open channels, and as such, it has been suggested that a more effective means of modulating GABAergic transmission in the brain may result from altering the number and/or pharmacodynamics of GABAA receptors (Mody et al., 1994). Although changes in GABAA receptor expression are known to occur in a variety of pathological situations (see introductory remarks), it is not known whether such alterations also occur in the normal adult brain.
One possibility for a physiologically significant regulation of GABAA receptor functioning in the adult brain would be via receptor phosphorylation (Macdonald and Olsen, 1994;Sieghart, 1995). Another mechanism, suggested by the present studies, would involve postsynaptic neurons regulating their own level and type of GABAA receptor expression. In this respect, it is interesting to note recent work in the primate, in which monocular deprivation has been shown to reduce GABAAreceptor subunit mRNA expression in a selective manner within the visual cortex (Huntsman et al., 1994). In that system, reduced mRNA expression is thought to result from a reduction in activity-dependent subunit gene transcription (Huntsman et al., 1994). A similar mechanism seems unlikely within the hypothalamic magnocellular neurons, because an inverse relationship between α1subunit mRNA expression and electrical activity exists (see below). Furthermore, the changes reported here are unlikely to result from autoregulation of GABAA receptor mRNA expression, because increases in endogenous GABA concentrations do not influence α subunit mRNA expression within the SON or PVN, or the γ2 subunit in the PVN (Fénelon and Herbison, 1996).
Although the present study has not evaluated whether the fluctuations in α1 subunit mRNA expression observed here during pregnancy are translated into α1 subunit protein, previous studies have indicated a positive correlation between α1 subunit mRNA expression and α1 subunit protein in vitro (Zheng et al., 1994) and in vivo (Huntsman et al., 1994). It is not unreasonable, therefore, to suggest that the 30–80% variations in mRNA expression observed here may result in changes in functional subunit protein. More difficult, perhaps, is the speculation as to what effects single subunit changes may have on GABAAreceptor functioning in magnocellular oxytocin neurons. All of these cells express the α1, α2, β2, and γ2 subunits of the GABAAreceptor (Fénelon and Herbison, 1995; Fénelon et al., 1995), and current wisdom would suggest that these subunits form pentamers composed of α1, β2, γ2 or α2, β2, γ2, and/or all four subunits (Sieghart, 1995). Hence, in the absence of significant changes in the expression of the other subunits over pregnancy, an increase in α1 subunit abundance before parturition may not change the total number of receptors but, rather, result in a greater proportion of α1 subunit-containing receptors on magnocellular oxytocin neurons. This would alter the overall pharmacodynamics of GABA responses in these cells by moving them toward the benzodiazepine type 1 profile, which shows enhanced benzodiazepine facilitation (Pritchett et al., 1989) and increased allopregnanolone sensitivity (Shingai et al., 1991) as compared with α2 subunit-containing receptor isoforms.
GABAA receptor changes with respect to magnocellular neuron physiology
Approximately 40% of the terminals synapsing on hypothalamic magnocellular neurons contain GABA (Decavel and Van den Pol, 1990; Gies and Theodosis, 1994), and electrophysiological studies indicate that GABA acts via the GABAA receptor to hyperpolarize these cells (Randle and Renaud, 1987). In vivo studies examining the role of GABA in influencing the activity of SON oxytocin neurons in the lactating rat show that occupancy of the GABAA receptor is critical for these neurons to exhibit the intermittent, synchronized bursts of electrical activity necessary for milk ejection (Voisin et al., 1994, 1995; Moos, 1995). Although similar studies have not, as yet, been undertaken in pregnant or parturient animals, it is highly likely that a powerful GABAergic influence also exists on magnocellular oxytocin neurons at this time. On the day of parturition, oxytocin neurons exhibit an increase in basal firing rate, which is followed later by the onset of intermittent, synchronized bursts of electrical activity leading to birth of the pups (Summerlee, 1981; Jiang and Wakerley, 1995). The mechanisms involved in changing the electrical and biosynthetic activity of magnocellular oxytocin neurons over this period remain unclear.
Our present findings of an increase in putative oxytocin neuron α1 subunit mRNA content leading up to day 19 of pregnancy, followed by a fall at the time of parturition, promotes speculation that these magnocellular cells may regulate their GABAA receptor expression to maintain an enhanced level of inhibitory input in late pregnancy. This inhibitory influence then may be relaxed at the time of parturition to enable either the increased basal firing rate and/or the high-frequency discharges required of oxytocin neurons at this time. The neighboring magnocellular vasopressin neurons show relatively little variation in their phasic pattern of electrical activity over this time (Summerlee, 1981), and, accordingly, we have detected no similar significant changes in subunit mRNA profile in putative vasopressin neurons over pregnancy and parturition. As noted above, the changes involving oxytocin neurons may not necessarily involve the appearance of more GABAA receptors within the synapse but, instead, involve more subtle changes in receptor dynamics. The increased sensitivity of α1 subunit-containing receptors to facilitation by progesterone metabolites (Shingai et al., 1991) may be particularly important because progesterone levels are at their highest in late pregnancy, and the GABAAreceptors located on the terminals of magnocellular neurons, at least, have been shown to be sensitive to allopregnanolone (Zhang and Jackson, 1994).
Also, we have found evidence for a substantial increase in γ2 subunit mRNA expression at late pregnancy, and our topographic analysis strongly suggests that this change is restricted to the vasopressin neurons of the pPVN. There is a clear reduction in the responsiveness of oxytocin and vasopressin neurons to osmotic stimuli in lactation (Higuchi et al., 1988; Koehler et al., 1993), and although this may be explained for oxytocin by a reduction in pituitary stores in late lactation, vasopressin content remains unchanged (Koehler et al., 1993). Reduced vasopressin responsiveness could, conceivably, arise from enhanced GABAAreceptor-mediated inhibition at this time.
In conclusion, we present here evidence indicating that GABAA receptor mRNA expression fluctuates under physiological circumstances within the hypothalamic magnocellular system of the adult female rat. Such findings support further the highly plastic nature of this neuronal system and suggest that neuron-specific variations in GABAA receptor expression may be relevant to the regulation of GABAergic transmission within the normal adult brain.
Footnotes
V.S.F. was a Wellcome Trust European Traveling Fellow. A.E.H. is a Lister Institute-Jenner Fellow. We thank Drs. S. Augood and R. J. Bicknell for critical review of this manuscript and Mr. I. King for performing the emulsion autoradiography.
Correspondence should be addressed to Dr Allen E. Herbison at the above address.
Dr. Fénelon’s current address: Centre National de la Recherche Scientifique, Unité de Recherche Associée 1126, Laboratoire de Neurobiologie et Physiologie Comparées, Université de Bordeaux I, Place du Dr Peyneau, 33120 Arcachon, France.
REFERENCES
- 1.Araki T, Sato M, Kiyama H, Manabe Y, Tohyama M. Localization of GABAA receptor γ2-subunit mRNA-containing neurons in the rat central nervous system. Neuroscience. 1992;47:45–61. doi: 10.1016/0306-4522(92)90119-m. [DOI] [PubMed] [Google Scholar]
- 2.Bureau MH, Olsen RW. GABAAreceptor subtypes: ligand-binding heterogeneity demonstrated by photoaffinity labeling and autoradiography. J Neurochem. 1993;61:1479–1491. doi: 10.1111/j.1471-4159.1993.tb13643.x. [DOI] [PubMed] [Google Scholar]
- 3.Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- 4.Faull RLM, Waldvogel HJ, Nicholson LFB, Synek BJL. The distribution of GABAA–benzodiazepine receptors in the basal ganglia in Huntington’s disease and in the quinolinic acid-lesioned rat. Prog Brain Res. 1993;99:105–123. doi: 10.1016/s0079-6123(08)61341-2. [DOI] [PubMed] [Google Scholar]
- 5.Fénelon VS, Herbison AE. Characterisation of GABAA receptor gamma subunit expression by magnocellular neurones in rat hypothalamus. Mol Brain Res. 1995;34:45–56. doi: 10.1016/0169-328x(95)00130-k. [DOI] [PubMed] [Google Scholar]
- 6.Fénelon VS, Herbison AE. In vivo regulation of specific GABAA receptor subunit messenger RNAs by increased GABA concentrations in rat brain. Neuroscience. 1996;71:661–670. doi: 10.1016/0306-4522(95)00492-0. [DOI] [PubMed] [Google Scholar]
- 7.Fénelon VS, Sieghart W, Herbison AE. Cellular localization and differential distribution of GABAA receptor subunit proteins and messenger RNAs within hypothalamic magnocellular neurons. Neuroscience. 1995;64:1129–1143. doi: 10.1016/0306-4522(94)00402-q. [DOI] [PubMed] [Google Scholar]
- 8.Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gies U, Theodosis DT. Synaptic plasticity in the rat supraoptic nucleus during lactation involves GABA innervation and oxytocin neurons: a quantitative immunocytochemical analysis. J Neurosci. 1994;14:2861–2869. doi: 10.1523/JNEUROSCI.14-05-02861.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez A, Khan ZU, Morris SJ, De Blas AL. Age-related decrease of GABAAreceptor subunits and glutamic acid decarboxylase in the rat inferior colliculus. J Neurosci. 1994;14:7469–7477. doi: 10.1523/JNEUROSCI.14-12-07469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatton GI. Emerging concepts of structure–function dynamics in adult brain: the hypothalamo–neurohypophysial system. Prog Neurobiol. 1990;34:437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- 12.Herbison AE, Fénelon VS. Estrogen regulation of GABAA receptor subunit mRNA expression in preoptic area and bed nucleus of the stria terminalis of female rat brain. J Neurosci. 1995;15:2328–2337. doi: 10.1523/JNEUROSCI.15-03-02328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higuchi T, Honda K, Takano S, Negoro H. Reduced oxytocin response to osmotic stimulus and immobilization stress in lactating rats. J Endocrinol. 1988;116:225–230. doi: 10.1677/joe.0.1160225. [DOI] [PubMed] [Google Scholar]
- 14.Huntsman MM, Isackson PJ, Jones EG. Lamina-specific expression and activity-dependent regulation of seven GABAA receptor subunit mRNAs in monkey visual cortex. J Neurosci. 1994;14:2236–2259. doi: 10.1523/JNEUROSCI.14-04-02236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang QB, Wakerley JB. Analysis of bursting responses of oxytocin neurones in the rat in late pregnancy, lactation and after weaning. J Physiol (Lond) 1995;486:237–248. doi: 10.1113/jphysiol.1995.sp020806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jirikowski GF. Oxytocinergic neuronal systems during mating, pregnancy, parturition, and lactation. Ann NY Acad Sci. 1992;652:253–270. doi: 10.1111/j.1749-6632.1992.tb34360.x. [DOI] [PubMed] [Google Scholar]
- 17.Kamphuis W, De Rijk TC, Lopes da Silva FH. Expression of GABAA receptor subunit mRNAs in hippocampal, pyramidal, and granular neurons in the kindling model of epileptogenesis: an in situ hybridization study. Mol Brain Res. 1995;31:33–47. doi: 10.1016/0169-328x(95)00022-k. [DOI] [PubMed] [Google Scholar]
- 18.Kang I, Miller LG. Decreased GABAA receptor subunit mRNA concentrations following chronic lorazepam administration. Br J Pharmacol. 1991;103:1285–1287. doi: 10.1111/j.1476-5381.1991.tb09781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HY, Sapp DW, Olsen RW, Tobin AJ. GABA alters GABAA receptor mRNAs and increases ligand binding. J Neurochem. 1993;62:2334–2337. doi: 10.1111/j.1471-4159.1993.tb07481.x. [DOI] [PubMed] [Google Scholar]
- 20.Koehler EM, McLemore GL, Tang W, Summy-Long JY. Osmoregulation of the magnocellular system during pregnancy and lactation. Am J Physiol. 1993;264:R555–R560. doi: 10.1152/ajpregu.1993.264.3.R555. [DOI] [PubMed] [Google Scholar]
- 21.Kokaia M, Pratt GD, Elmer E, Bengzon J, Fritschy J, Kokaia Z, Lindvall O, Mohler H. Biphasic differential changes of GABAA receptor subunit mRNA levels in dentate gyrus granule cells following recurrent kindling-induced seizures. Mol Brain Res. 1994;23:323–332. doi: 10.1016/0169-328x(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 22.Macdonald RL, Olsen RW. GABAAreceptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 23.MacLennan AJ, Brecha N, Khrestchatisky M, Sternini C, Tillakaratne NJK, Chiang MY, Anderson K, Lai M, Tobin AJ. Independent cellular and ontogenetic expression of mRNAs encoding three α polypeptides of the rat GABAA receptor. Neuroscience. 1991;43:369–380. doi: 10.1016/0306-4522(91)90301-4. [DOI] [PubMed] [Google Scholar]
- 24.Mathews GC, Bolos-Sy AM, Holland KD, Isenberg KE, Covey DF, Ferrendelli JA, Rothman SM. Developmental alteration in GABAA receptor structure and physiological properties in cultured cerebellar granule neurons. Neuron. 1994;13:149–158. doi: 10.1016/0896-6273(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 25.Mhatre MC, Ticku MK. Chronic GABA treatment downregulates the GABAA receptor α2 and α3 subunit mRNAs as well as polypeptide expression in primary cultured cerebral cortical neurons. Mol Brain Res. 1994;24:159–165. doi: 10.1016/0169-328x(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 26.Mhatre MC, Pena G, Sieghart W, Ticku MK. Antibodies specific for GABAA receptor α subunits reveal that chronic alcohol treatment down-regulates α-subunit expression in rat brain regions. J Neurochem. 1993;61:1620–1625. doi: 10.1111/j.1471-4159.1993.tb09795.x. [DOI] [PubMed] [Google Scholar]
- 27.Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 28.Montpied P, Morrow AL, Karanian JW, Ginns EI, Martin BM, Paul SM. Prolonged ethanol inhalation decreases γ-aminobutyric acidA receptor α-subunit mRNAs in the rat cerebral cortex. Mol Pharmacol. 1991;39:157–163. [PubMed] [Google Scholar]
- 29.Montpied P, Weizman A, Weizman R, Kook KA, Morrow AL, Paul SM. Repeated swim-stress reduces GABAA receptor α-subunit mRNAs in the mouse hippocampus. Mol Brain Res. 1993;18:267–272. doi: 10.1016/0169-328x(93)90199-y. [DOI] [PubMed] [Google Scholar]
- 30.Moos FC. GABA-induced facilitation of the periodic bursting activity of oxytocin neurones in suckled rats. J Physiol (Lond) 1995;488:103–114. doi: 10.1113/jphysiol.1995.sp020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulter MO, Barker JL, O’Carroll A, Lolait SJ, Mahan LC. Co-existent expression of GABAA receptor β2, β3, and γ2 subunit messenger RNAs during embryogenesis and early postnatal development of the rat central nervous system. Neuroscience. 1993;53:1019–1033. doi: 10.1016/0306-4522(93)90486-y. [DOI] [PubMed] [Google Scholar]
- 32.Primus RJ, Gallager DW. GABAAreceptor subunit mRNA levels are differentially influenced by chronic FG 7142 and diazepam exposure. Eur J Pharmacol. 1992;226:21–28. doi: 10.1016/0922-4106(92)90078-a. [DOI] [PubMed] [Google Scholar]
- 33.Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 34.Randle JCR, Renaud LP. Actions of γ-aminobutyric acid on rat supraoptic nucleus neurosecretory neurones in vitro . J Physiol (Lond) 1987;387:629–647. doi: 10.1113/jphysiol.1987.sp016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol. 1981;198:45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- 36.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 37.Shingai R, Sutherland ML, Barnard EA. Effects of subunit types of the cloned GABAA receptor on the response to a neurosteroid. Eur J Pharmacol. 1991;206:77–80. doi: 10.1016/0922-4106(91)90149-c. [DOI] [PubMed] [Google Scholar]
- 38.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 39.Summerlee AJS. Extracellular recordings from oxytocin neurones during the expulsive phase of birth in unanaesthetized rats. J Physiol (Lond) 1981;321:1–9. doi: 10.1113/jphysiol.1981.sp013967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swanson LW. Elsevier; Amsterdam: 1992. Brain maps: structure of the rat brain. [Google Scholar]
- 41.Theodosis DT, Poulain DA. Activity-dependent neuronal–glial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience. 1993;57:501–535. doi: 10.1016/0306-4522(93)90002-w. [DOI] [PubMed] [Google Scholar]
- 42.Tseng YT, Wellman SE, Ho IK. In situ hybridization evidence of differential modulation by pentobarbital of GABAA receptor α1- and β3-subunit mRNAs. J Neurochem. 1994;63:301–309. doi: 10.1046/j.1471-4159.1994.63010301.x. [DOI] [PubMed] [Google Scholar]
- 43.Voisin DL, Chapman C, Poulain DA, Herbison AE. Extracellular GABA concentrations in rat supraoptic nucleus during lactation and following haemodynamic changes: an in vivo microdialysis study. Neuroscience. 1994;63:547–558. doi: 10.1016/0306-4522(94)90549-5. [DOI] [PubMed] [Google Scholar]
- 44.Voisin DL, Herbison AE, Poulain DA. Central inhibitory effects of muscimol and bicuculline on the milk ejection reflex in the anaesthetized rat. J Physiol (Lond) 1995;481:211–224. doi: 10.1113/jphysiol.1995.sp020579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J-H, Sato M, Tohyama M. Region-specific expression of the mRNAs encoding β subunits (β1, β2, and β3) of GABAA receptor in the rat brain. J Comp Neurol. 1991;303:637–657. doi: 10.1002/cne.903030409. [DOI] [PubMed] [Google Scholar]
- 47.Zhang SJ, Jackson MB. Neuroactive steroids modulate GABAA receptors in peptidergic nerve terminals. J Neuroendocrinol. 1994;6:533–538. doi: 10.1111/j.1365-2826.1994.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 48.Zheng TM, Zhu WJ, Puia G, Vicini S, Grayson DR, Costa E, Caruncho HJ. Changes in γ-aminobutyrate type A receptor subunit mRNAs, translation product expression, and receptor function during neuronal maturation in vitro . Proc Natl Acad Sci USA. 1994;91:10952–10956. doi: 10.1073/pnas.91.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu WJ, Vicini S, Harris BT, Grayson DR. NMDA-mediated modulation of γ-aminobutyric acid type A receptor function in cerebellar granule neurons. J Neurosci. 1995;15:7692–7701. doi: 10.1523/JNEUROSCI.15-11-07692.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]