Abstract
Previous work has demonstrated that Lambert–Eaton syndrome (LES) antibodies reduce calcium currents in non-neuronal cells and sensory neurons and reduce the amplitude of extracellularly recorded currents at mouse motor nerve terminals. We compared effects of LES sera on whole-cell currents of cultured nerve and muscle. LES sera more strongly reduced calcium currents in motoneurons than in sensory neurons. Motoneuronal potassium currents were unaffected. The sera minimally affected calcium currents in skeletal and cardiac muscle. In motoneurons, both low voltage-activated (LVA) and high voltage-activated (HVA) components of calcium current were decreased, demonstrating that the sera targeted more than one calcium channel type. The HVA current remaining in LES-treated motoneurons was little affected by micromolar ω-conotoxin MVIIC but was reduced >70% by micromolar nimodipine. This pharmacological profile contrasts with untreated cells and suggests that LES sera primarily spare L-type currents in motoneurons.
Keywords: calcium channels, calcium currents, motoneurons, Lambert–Eaton myasthenic syndrome, neuromuscular transmission, transmitter release
Lambert–Eaton myasthenic syndrome (LES) is an autoimmune disorder characterized by decreased neurotransmitter release at the neuromuscular junction (Elmqvist and Lambert, 1968; Lambert and Elmqvist, 1971). Ultrastructural analysis has revealed that motoneurons from LES patients have fewer active zones, which are less well organized, and contain fewer active zone particles (Fukunaga et al., 1982). Because active zones are the sites of neurotransmitter release and the active zone particles are thought to include the calcium channels necessary for neurotransmitter release (Couteaux and Pecot-Dechavassine, 1970; Heuser et al., 1979), it is widely accepted that LES antibodies target presynaptic calcium channels (Fukunaga et al., 1982). To test this hypothesis, the effects of LES antibodies on radiocalcium fluxes or calcium currents have been examined in a number of systems. The antibodies reduce calcium influx in small-cell lung carcinoma (Roberts et al., 1985), rat anterior pituitary (Login et al., 1987), adrenal chromaffin (Kim and Neher, 1988; Viglione et al., 1992), neuroblastoma (Peers et al., 1990; Grassi et al., 1994), rat thyroid cell line (Kim et al., 1993) and dorsal root ganglion (DRG) cells (García et al., 1996). Recently, it has been shown that LES antibodies decrease mixed sodium and calcium currents at the nerve terminals of mice (Smith et al., 1995). Mice are an appropriate model, because the disease can be transferred passively to them (Fukunaga et al., 1983; Kim, 1986). However, the effects of LES antibodies on isolated calcium currents in motoneurons have not been examined. Here, we describe such experiments on murine motoneurons, which is particularly important because associated proteins, rather than the calcium channels per se, might be the critical antigenic target (Leveque et al., 1992).
Neurons contain a variety of calcium channel types that can be divided into low voltage-activated (LVA) or T-type channels and high voltage-activated (HVA) channels (Nowycky et al., 1985). HVA channels can be subdivided on the basis of molecular, biophysical, and pharmacological properties and include L, N, P, O, Q, and R channels (Nowycky et al., 1985; Fox et al., 1987; Mintz et al., 1992; Regan et al., 1992; Olivera et al., 1994; Randall and Tsien, 1995). Previous experiments have not examined thoroughly the types of calcium channels affected or spared by LES antibodies. Furthermore, the calcium channel makeup of motoneurons is likely to be different from other cell types examined previously. It is especially important to characterize the types of calcium channels affected by LES antibodies in motoneurons, because the specific channel type or types critical for neurotransmitter release seem to vary between synapses (Hirning et al., 1988; Stanley and Goping, 1991; Turner et al., 1993; Wheeler et al., 1994) and the type or types that govern transmitter release at the mammalian neuromuscular junction remain unclear. Based on pharmacological criteria, N, P, and Q channels all have been suggested to be important. A critical role for P-type channels was argued on the basis of the blocking of neuromuscular transmission in mice by a polyamine fraction of funnel-web spider venom (Uchitel et al., 1992). However, divergent results have been reported for the effects of a peptide, ω-AgaIVa, which now is used widely as the spider venom component specific for P-channels at nanomolar concentrations (Mintz et al., 1992). Thus, Hong and Chang (1995) reported that murine neuromuscular transmission was blocked by 10 nmω-AgaIVa but was almost unaffected by 300 μmof the cone shell venom ω-CTx MVIIC. By contrast, Bowersox et al. (1995) found that a complete block required a much higher concentration (∼300 nm) of synthetic ω-AgaIVa (SNX-290) and a much lower concentration (1 μm) of synthetic ω-CTx MVIIC (SNX-230). Thus, these studies make it uncertain whether P or Q channels are involved, because near-micromolar concentrations of ω-AgaIVa and ω-CTx MVIIC block both P and Q channels. Adding further to the controversy, early work demonstrated that the N-channel toxin, ω-CgTx GVIA, did not affect murine neuromuscular transmission (Yoshikami et al., 1989), whereas a more recent report showed significant reduction of nerve-evoked muscle contractions in rats by 3 nm of the toxin (Rossoni et al., 1994).
We report here that LES sera from four patients significantly reduce calcium currents in murine motoneurons. Comparatively, these sera cause a lesser decrease in calcium currents in DRG neurons (previously reported by García et al., 1996) and have little effect on muscle. Within motoneurons, voltage-gated potassium channels are not affected, both LVA and HVA calcium currents are decreased, and the HVA calcium current remaining in LES serum-treated motoneurons is mostly L-type.
MATERIALS AND METHODS
Motoneuron cultures. The procedures used for the preparation of motoneuron cultures were similar to those reported previously (Mynlieff and Beam, 1992a,b). So that they could be identified, neonatal murine motoneurons were labeled retrogradely with a suspension of 2.5 mg/ml 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (diI; Molecular Probes, Eugene, OR), 20% ethanol, and 80% rodent Ringer with 0.1% bovine serum albumin. Each mouse pup was anesthetized with Metofane, and the diI suspension was injected into all four limbs. After being returned to its mother for several hours (9–11) to allow the dye to label motoneuronal cell bodies, the pup was anesthetized and decapitated, and the spinal cord was removed in oxygenated rodent Ringer (in mm): 146 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 11 glucose, pH 7.4. Care was used to dissect away the meninges and any attached DRGs to ensure that the culture was not contaminated with labeled sensory cells. The spinal cord was cut into small pieces (<1 mm3) and placed in 0.5 ml of a 0.1% Type XI trypsin and 0.01% DNase I (both from Sigma, St. Louis, MO) solution in PIPES-buffered saline (in mm): 120 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 25 glucose, and 20 piperazine-N,N′-bis(2-ethanesulfonic acid), pH 7.0. After 15–20 min of incubation at 35°C, the tissue was rinsed with neural basal medium containing B27 supplement (Life Technologies, Grand Island, NY), 100 μg/ml streptomycin, and 60 μg/ml penicillin and triturated with a fire-polished pipette. The cells were plated on 35 mm dishes that had been coated overnight by exposure to poly-l-lysine (4–15 kDa; 1 mg/ml in 0.15 m boric acid, pH 8.4). Serum from either normal humans or one of the LES patients was dialyzed (exclusion of ≥100 kDa) for 24 hr against culture medium at a sample/dialysate ratio of ∼1:100 with one dialysate change at ∼8 hr (García et al., 1996). Dialyzed serum was added to the culture medium at ∼1:20 dilution at the time of cell plating. Cells were recorded from after being maintained overnight in a humidified atmosphere of 95% air/5% CO2 at 37°C.
Ionic currents. The whole-cell patch-clamp configuration (Hamill et al., 1981) was used to record ionic currents at room temperature (20°C) with a Dagan 3900 patch-clamp amplifier (Dagan Corporation, Minneapolis, MN) equipped with a 3911 whole-cell expander. The patch electrodes (3–4 MΩ) were made from soda lime glass and coated with wax to reduce capacitance. Linear components of leak and capacitive currents were removed from test currents by digital subtraction of scaled control currents elicited by 20 mV hyperpolarizations from the holding potential (−80 mV). Currents were filtered electronically at 1 kHz (8 pole Bessel filter) before sampling by the computer. To normalize for differences in total membrane area, current densities were calculated by dividing total current by the linear capacitance of the cell. Data are expressed as mean ± SEM. Least-squares fits were computed with NFIT software (Island Software, Galveston, TX).
To measure calcium currents, recording electrodes contained (in mm):140 Cs-aspartate, 5 MgCl2, 10 Cs2 EGTA, and 10 HEPES, pH 7.4; the extracellular recording medium contained 10 CaCl2, 145 tetraethylammonium-chloride (TEA-Cl), tetrodotoxin (TTX; 0.003 for muscle cells, 0.0005 for motoneurons), and 10 HEPES, pH 7.4. To measure potassium currents, recording electrodes contained (in mm): 140 KCl, 5 MgCl2, 10 K2 EGTA, and 10 HEPES, pH 7.4; the external solution contained 146 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 0.001 TTX, and 10 HEPES, pH 7.4.
Nimodipine was made up as a 10 mm stock solution in ethanol. Stock solutions of 0.5 mm ω-CTx MVIIC were prepared by dissolving the peptide in distilled water with 1 mg/ml bovine serum albumin and stored in aliquots at −20°C. Stock solutions were diluted to final concentrations on the day of use in the external solution used for measuring calcium currents. For population studies, cultures were placed in the solutions containing either ω-CTx MVIIC or nimodipine 1.5 hr before recording. In perfusion experiments, small wells were created that isolated cells in each culture dish. After the currents from a cell under control conditions were recorded, the well was perfused with drug solution (>6× volume exchange).
RESULTS
Serum was obtained from four patients (Patients I, II, III, and IV) diagnosed as having LES. Patient IV was diagnosed also as having myasthenia gravis by virtue of having antibodies characteristic of both conditions (Leys et al., 1989). Spinal cords containing motoneurons labeled by intramuscular injection (Honig and Hume, 1986) of diI were dissociated and incubated overnight with serum from either normal individuals or from one of the four patients. Serum was added to the culture medium at a 1:20 dilution, which results in an immunoglobulin concentration approximately equivalent to that circulating in adult human blood (Isselbacher et al., 1980). Although the majority of recordings (>80%) was from diI-labeled cells, some data were from cells identified as motoneurons on the basis of size and morphology (Smith et al., 1986; Milligan et al., 1994; Mynlieff and Beam, 1994).
Calcium currents obtained from control serum- and LES serum-treated motoneurons
Calcium currents were elicited with 300 msec depolarizing pulses from a holding potential of −80 mV to test potentials from −50 to +50 mV at 10 mV intervals. Figure 1 compares currents from a motoneuron treated with control serum (left) and a motoneuron treated with LES serum (right). In both the control and LES-treated cells, the calcium currents displayed LVA and HVA components. However, at all test potentials the calcium currents in the LES-treated motoneuron were much smaller than those in the control motoneuron. Moreover, the sustained HVA component (+10 mV) represented a larger fraction of the total current in LES-treated cells than in control cells (sustained equaled 55% of total current in control as compared with 66, 74, 65, and 72% in Patients I–IV, respectively). Additionally, the HVA component of current from cells treated with LES serum seemed to have a slower time course of activation, although the small size of the currents made a detailed quantitative analysis difficult.
Fig. 1.
Voltage-activated calcium currents in a motoneuron treated with serum from a control individual (left) and a motoneuron treated with serum from LES Patient II (right). Representative calcium currents were elicited by 300 msec depolarizing pulses to test potentials between −40 and +30 mV at 10 mV intervals. LVA and HVA calcium currents are present in both control and treated cells. Note the prominent transient and sustained components of HVA current in the control motoneuron and that the HVA current in the LES serum-treated cell is predominantly sustained.
Calcium currents were recorded from five groups of motoneurons to quantify the effects of LES serum: a control group that was treated with control serum and four test groups, each of which was treated with serum from one of the four LES patients (Fig. 2). The amplitude of current was divided by cell capacitance and expressed as current density (pA/pF) to normalize for variability in cell size. Current densities recorded from individual cells were averaged for each of the five groups. Currents from the control cells displayed LVA and HVA components similar to those described previously in neonatal murine motoneurons (Mynlieff and Beam, 1992a). In particular, the amplitude of the maximal HVA current (∼5 pA/pF) was almost identical to that found by Mynlieff and Beam. Average current densities were smaller at all test potentials in the LES serum-treated cells, with the largest reductions for Patients II and IV. At both −20 and +20 mV (approximate potentials eliciting peak LVA and HVA currents, respectively), the reduction of current amplitude was statistically significant (p < 0.05) for all four patients.
Fig. 2.
Peak calcium current density plotted as a function of test potential in control (circles) and LES serum-treated (squares) motoneurons. Average current density was smaller at each test potential for cells treated with serum from each of the four LES patients than for cells treated with control serum. The reduction in current was least substantial for cells treated with serum from Patient I (n = 11) and Patient III (n = 8) and greatest for cells treated with serum from Patient II (n = 11) and Patient IV (n = 10).
Calcium conductances from control serum- and LES serum-treated motoneurons
In addition to comparing current densities at specific test potentials, we also fitted the peak current–voltage relationship of individual cells as the sum of current through two populations of channels, each activating in accord with a Boltzmann function (García et al., 1996). These fits yielded a maximal conductance of the LVA and HVA components of current, which were averaged for each group of cells (Fig. 3). LVA and HVA maximal calcium conductances for LES serum-treated motoneurons were decreased, respectively, by 60 and 64% for Patient I, 90 and 91% for Patient II, 80 and 82% for Patient III, and 80 and 73% for Patient IV.
Fig. 3.
To determine whether serum-induced changes in calcium currents could have resulted from effects of LES sera on motoneuron growth, cell size was estimated from measurements of whole-cell capacitance. Averaged capacitances were similar for experimental (26.8 ± 2.9 pF,n = 41) and control (23.2 ± 1.6 pF,n = 17) cells, indicating that the effects of LES antibodies on whole-cell current were not a secondary result of altered cell growth. Additionally, in three cultures in which serum from Patients I or II was used, the percentage of cell survival was determined by counting the number of cells at time of recording relative to the number of cells plated. This ratio was not appreciably different between cells treated with control (47, 57, and 59%) or LES (42, 54, and 62%) serum.
Potassium currents from control serum- and LES serum-treated motoneurons
Because LES sera decreased both the LVA and HVA components of calcium currents, we examined potassium currents to determine whether the serum affected more than one family of voltage-gated ion channels in motoneurons. For these experiments, as well as those on calcium currents in muscle cells (see below), diminishing stocks prevented examination of the effects of sera from all of the patients. As in the case of calcium currents, the potassium currents were measured over a wide range of test potentials (Fig. 4, inset) and normalized to cell capacitance. Figure 4 compares averaged current–voltage relationships for control and serum-treated motoneurons. For strong depolarizations, the average potassium current densities in motoneurons treated with serum from Patients III (open squares) or IV (filledtriangles) were slightly smaller or larger than control, respectively. However, these differences in average current density were not statistically significant for either patient.
Fig. 4.
Normalized, peak potassium current density as a function of test potential in motoneurons treated with control serum (circles; n = 13), serum from Patient III (squares; n = 8), or serum from Patient IV (triangles; n = 8). At high potentials, average potassium currents were slightly smaller than control for motoneurons treated with serum from Patient III and slightly greater for motoneurons treated with serum from Patient IV. However, these changes were not statistically significant. The inset shows a representative family of control potassium currents elicited by test potentials of −30 to 60 mV at 10 mV intervals.
Effects of LES sera on calcium currents recorded from cardiac muscle and skeletal muscle
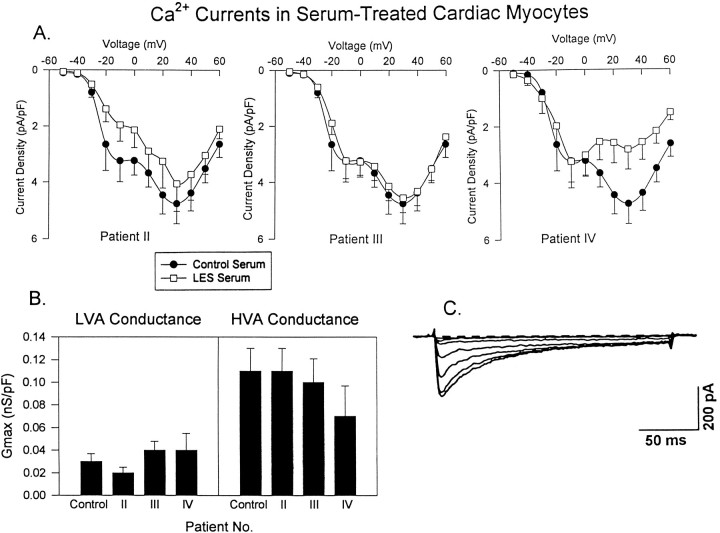
Because LES sera have been reported to reduce calcium currents in a variety of cell types (see introductory remarks) and because neuromuscular weakness is a hallmark of the disease, we investigated the possibility that the sera affect calcium currents in cardiac or skeletal muscle cells via the use of protocols like those for the motoneurons. Averaged calcium current densities in cardiac myocytes treated with serum from Patients II or III were not significantly different from control at either low or high voltages (Fig. 5A). In contrast, serum from Patient IV significantly decreased the HVA calcium current density. The presence or absence of differences in current density were paralleled by differences in the maximal conductances, determined as described above for motoneurons. Thus, the maximal HVA calcium conductance was reduced for cells treated with serum from Patient IV but not for cells treated with serum from Patients II and III (Fig. 5B). Maximal LVA calcium conductance was unaffected by serum from any of the three patients. Although HVA currents were reduced in amplitude by serum from Patient IV, the kinetics were similar to those observed in control cells (Fig. 5C). As for cardiac myocytes, serum from Patient III had little effect on calcium currents in skeletal myotubes, whereas that from Patient IV caused a reduction in the HVA component of current (Fig. 6A). The kinetics and voltage dependence of HVA calcium currents in skeletal myotubes treated with serum from Patient IV were similar to those of control myotubes (Fig.6B) and to HVA currents described previously in skeletal myotubes (Beam and Knudson, 1988).
Fig. 5.
Comparison of calcium currents in cardiac myocytes treated with control or LES sera. A, Normalized, peak current–voltage relationships from control (n = 20) cardiomyocytes and cardiomyocytes treated with serum from Patients II (n = 12), III (n = 14), or IV (n = 14). The LVA current (−20 mV) was not altered significantly by serum from any of the three patients; the only statistically significant (p < 0.05) decrease in HVA current (+20 mV) was for cells treated with serum from Patient IV. B, Average maximal LVA (GmaxL) and HVA (GmaxH) calcium conductances for control and LES serum-treated cardiac myocytes. Differences in conductance were statistically significant only for cells treated with serum from Patient IV. C, Representative calcium currents evoked by test potentials ranging from −20 to +30 mV at 10 mV intervals.
Fig. 6.
Effects of LES serum on calcium currents in skeletal myotubes. A, Normalized, peak current density versus voltage relationship for myotubes treated with control serum (circles; n = 12) or serum (squares) from Patient III (n = 14) or IV (n = 14). Changes in LVA current were not significant. The decrease in HVA current was significant for Patient IV but not for Patient III. For Patient IV, GmaxH(determined as described in Fig. 3) was 0.21 ± 0.06 nS/pF compared with 0.30 ± 0.08 nS/pF in control. B, Representative current traces from a control (left) and Patient IV serum-treated (right) skeletal myotube at test potentials of −30, −20, −10, 20, and 30 mV. Serum from Patient IV had no obvious effect on voltage dependence or kinetics of calcium current.
Pharmacology of residual calcium currents in LES serum-treated motoneurons
Compared with control, calcium currents measured from motoneurons treated with LES serum decayed little during the test pulses, especially at high potentials (Fig. 1), which is reminiscent of L-type calcium current (Bean, 1989; Hess, 1990). Additionally, only serum from Patient IV significantly altered calcium currents in muscle, which are primarily L-type (Bean, 1989; Hess, 1990). These observations suggest that a large portion of the residual calcium current in serum-treated motoneurons may be carried by L-type channels. To further examine the nature of the residual calcium current in LES serum-treated motoneurons, we used pharmacological methods. Nimodipine (10 μm) was selected because it is an L-channel antagonist (Fox et al., 1987; McCarthy and TanPiengco, 1992), and ω-CTx MVIIC (5 μm) was selected because it blocks current via a number of different types of HVA calcium channels (including N, P, and Q) but spares L-type calcium current (Hillyard et al., 1992).
As one approach for examining the pharmacology of the residual current in LES serum-treated motoneurons, the motoneurons were incubated with either nimodipine or ω-CTx MVIIC for at least 1.5 hr before recording. In control serum-treated motoneurons, incubation with either 10 μm nimodipine (Fig.7A) or 5 μm ω-CTx MVIIC (Fig. 7B) did not seem to alter either voltage dependence or kinetics dramatically (compare with Fig. 1). Figure7C illustrates the effects of nimodipine and ω-CTx MVIIC on peak current–voltage relationships in motoneurons treated with control serum (top), serum from Patient II (middle), or serum from Patient III (bottom). The circles plot average densities of the calcium currents from motoneurons not exposed to the calcium channel blockers. In control serum-treated motoneurons, ω-CTx MVIIC (triangles) reduced the maximal HVA current by 70% (n = 7), whereas nimodipine (squares) caused only an 18% decrease (n = 8). By contrast, ω-CTx MVIIC essentially had no effect on the HVA calcium that remained in motoneurons treated with serum from Patients II and III (n = 9 for both), whereas nimodipine caused a large reduction in the residual HVA current; at +10 mV, the reduction was 70% for Patient II (n = 7) and 73% for Patient III (n = 8).
Fig. 7.
Representative calcium currents elicited at test potentials of −30, −20, −10, 10, 20, and 30 mV from motoneurons incubated in control serum and either 10 μmnimodipine (A) or 5 μm ω-CTx MVIIC (B). C, Averaged, peak current density versus test potential in control and test serum-treated motoneurons that had been incubated 1.5 hr in medium with 10 μmnimodipine (squares), 5 μm ω-CTx MVIIC (triangles), or without antagonist (circles; data replotted from Fig. 2). In control serum-treated motoneurons, LVA current was decreased significantly (65%) by nimodipine but not by ω-CTx MVIIC.
In addition to prolonged bath application, acute perfusion of the calcium channel antagonists also was examined in control and Patient II serum-treated motoneurons. HVA current in control serum-treated motoneurons was decreased substantially by perfusion with ω-CTx MVIIC but little affected by nimodipine (Fig. 8A). By contrast, in LES serum-treated motoneurons the HVA current was greatly reduced by perfusion with nimodipine (Fig. 8B,left) but not with ω-CTx MVIIC (Fig. 8B,right, average reduction of −3 ± 5%;n = 4). Even after a 15 min application of ω-CTx MVIIC, the decrease was only 26 and 36% in two experiments, and much of this decrease may have been a consequence of time-dependent rundown. Figure 8C shows the averaged normalized current as a function of time for control serum-treated motoneurons (left) and motoneurons treated with serum from Patient II (right). For control serum-treated motoneurons, maximal HVA current was reduced after 4 min perfusion with ω-CTx MVIIC by 56 ± 7% (n = 3) but <10% by nimodipine (n = 2). In motoneurons treated with serum from Patient II, the HVA current was not affected significantly by perfusion with ω-CTx MVIIC but was reduced >95% by perfusion with nimodipine (n = 2).
Fig. 8.
Effects of acute perfusion of calcium channel blockers on control serum- and Patient II serum-treated motoneurons. Calcium currents were evoked at +10 mV in control serum-treated motoneurons (A) or Patient II serum-treated motoneurons (B) 1 min before and 5 min after acute perfusion with 10 μm nimodipine (left) or 5 μm ω-CTx MVIIC (right).C, Normalized peak calcium current at +10 mV is plotted with respect to time for motoneurons treated with control serum (left) or serum from Patient II (right) and exposed to either nimodipine (squares) or ω-CTx MVIIC (triangles). The circles plot peak current as a function of time in control serum-treated motoneurons not exposed to either antagonist (n = 7).
In summary, it seems that LES serum completely abolishes motoneuronal calcium currents from channels sensitive to 5 μm ω-CTx MVIIC. However, LES serum may have even broader specificity for motoneuronal calcium channels than ω-CTx MVIIC because (1) LES sera also reduce LVA calcium current (Fig. 2), (2) the average reduction in HVA current density is larger for LES sera (Fig. 2) than for ω-CTx MVIIC (Fig. 7C), and (3) the kinetics of calcium current in control serum-treated cells incubated with ω-CTx MVIIC (Fig. 7B) seems to differ from that in LES serum-treated motoneurons (Fig. 1). Despite this rather broad specificity, LES serum seems to spare motoneuronal L-type currents.
DISCUSSION
Since the first descriptions of LES as a neuromuscular disorder (Anderson et al., 1953; Lambert et al., 1956; Eaton and Lambert, 1957), a number of reports have provided evidence that the disease results from the production of autoantibodies that act on the presynaptic terminal (Elmqvist and Lambert, 1968; Lambert and Elmqvist, 1971;Cull-Candy et al., 1980; Kim, 1986). The hypothesis that neuromuscular weakness results from decreased calcium entry at the nerve terminal was first proposed to explain why transmitter release in neuromuscular preparations from LES patients displayed an enhanced sensitivity to changes in calcium concentration (Elmqvist and Lambert, 1968;Cull-Candy et al., 1980). Support for this hypothesis came from structural studies showing the paucity and disruption of active zones and active zone particles (putative calcium channels) at nerve terminals exposed to LES serum (Fukunaga, 1982; Fukuoka et al., 1987a,b; Nagel et al., 1988). Subsequently, numerous studies have established a LES serum-induced decrease in calcium influx in a variety of cells (see introductory remarks). Recently it has been demonstrated that LES antibodies decrease mixed sodium and calcium currents at mouse nerve terminals (Smith et al., 1995). Here, we provide the first study directly quantifying the effects of LES sera on isolated calcium currents in motoneurons. Although the antibodies clearly target more than one type of calcium channel, their effects display specificity, because they have little effect on motoneuronal potassium currents and calcium currents in cardiac and skeletal muscle.
Previous studies have supported the idea that LES effects are restricted to calcium channels. Thus, potassium currents in DRG cells (García et al., 1996) and sodium currents in chromaffin cells (Viglione et al., 1992) are not altered substantially by LES antibodies. The present report demonstrates that this is also true for potassium currents in motoneurons. Potassium currents were of particular interest because recent studies have raised the possibility that active zone particles may contain colocalized calcium and potassium channels (Roberts et al., 1990; Robitaille et al., 1993). If this is true for mammalian motoneurons, LES serum-induced destruction of active zones might result in simultaneous decreases in potassium and calcium currents. This did not occur in the cultured motoneurons used in our studies, although potassium and calcium channel colocalization may depend on the formation of neuromuscular synapses, which does not occur with the system we have used. Moreover, an additional argument against destruction of potassium channels in vivo is that decreased potassium current would tend to prolong the presynaptic depolarization and thus increase calcium influx, which would lessen the pathological consequences of destruction of calcium channels.
In various cells (Blandino and Kim, 1993; Grassi et al., 1994; Johnston et al., 1994; Lennon et al., 1995; García et al., 1996), LES antibodies have been shown to decrease currents or immunoprecipitate binding sites for antagonists associated with a number of calcium channel types, including LVA (T) and HVA (L, N, P, Q, others?). Our results demonstrate that in motoneurons, also, LES sera decrease both LVA and HVA calcium currents. Thus, LES sera do not target exclusively the channels controlling transmitter release, which is thought to be controlled by HVA, not LVA, channels (Hirning et al., 1988; Uchitel et al., 1992; Turner et al., 1993; Rossoni et al., 1994; Wheeler et al., 1994).
Although LES antibodies affect more than one type of calcium channel in motoneurons, the spared current seemed to be predominantly L-type. Thus, the spared current seemed to have slower activation, had a transient phase that was small compared with the sustained phase, and was reduced substantially by a dihydropyridine antagonist. The sensitivity to the dihydropyridine antagonist contrasted with control motoneurons, in which micromolar nimodipine blocked 18% of HVA current. L-type current also represents only 6.6% of HVA calcium current in rat hypoglassal motoneurons (Umemiya and Berger, 1994). Additionally, 5 μm ω-CTx MVIIC, which blocks several types of HVA calcium channels including N, P, and Q (Hillyard et al., 1992; Randall and Tsien, 1995), had little effect on the motoneuronal current spared by LES antibodies, whereas it blocked a large fraction of HVA current (70%) in control motoneurons. In conclusion, LES antibodies nearly eliminated the non-L HVA current in murine motoneurons while sparing significant L-type current. This conclusion is in agreement with a recent report that a large fraction of the extracellularly recorded calcium current in mouse motor nerve terminals exposed to LES antibodies is blocked by dihydropyridines (Smith et al., 1995).
LES sera seem to have a much more profound effect on calcium currents in motoneurons than in other native tissues examined (Fig.9). For example, serum from Patients I, II, and III caused a moderate reduction of both LVA and HVA conductance in DRG neurons (the remaining conductance was 28–46% of control for LVA and 46–57% for HVA). These sera caused a much larger reduction of calcium conductance in motoneurons (remaining conductance, 10–40% of control for LVA and 9–36% for HVA). The difference between DRG neurons and motoneurons was particularly striking for serum from Patient IV (LVA, 81 and 20% in DRGs and motoneurons, respectively; HVA, 91 and 27% in DRGs and motoneurons, respectively). LES sera have little effect on muscle calcium channels (Fig. 9). Of the serum examined, only that from Patient IV had an effect on muscle HVA (L-type) calcium conductance, and this effect was modest. Thus, our whole-cell current measurements are in agreement with an earlier study that found no difference in the waveform of the cardiac action potential in ventricular muscle from mice injected with LES antibodies (Lang et al., 1988). The modest effect of the serum from Patient IV on muscle L-channels raises the possibility that some LES patients produce antibodies that cross-react with muscle. Alternatively, Patient IV was diagnosed as having both LES and myasthenia gravis (García et al., 1996). Thus, the effects of serum from Patient IV on muscle channels may be a consequence of neuromuscular inflammation by anti-AChR antibodies (Rash et al., 1976; Maselli et al., 1991).
Fig. 9.
Summary of effects of LES sera on LVA and HVA calcium conductances in DRG neurons, motoneurons, and cardiac myocytes. The vertical axis plots the average calcium conductance remaining in treated cells as a percentage of control.
The result in this paper that LES sera spare L-type channels in motoneurons seemingly contradicts some earlier reports demonstrating that LES antibodies affect L-type calcium channels in neuroblastoma X glioma (Peers et al., 1990) and bovine adrenal chromaffin cells (Blandino and Kim, 1993). Perhaps motoneurons express a different L-channel isoform than do these other tissues, because L-channels are encoded by at least three genes, and individual genes undergo alternative splicing (Perez-Reyes et al., 1990; Snutch and Reiner, 1992). Furthermore, LES sera from different patients may display differing degrees of cross-reactivity between calcium channel types. Alternatively, the channel categories affected may depend on the type of cell examined, as suggested by the data summarized in Figure 9.
LES antibodies seem to interact with a variety of, but not all, calcium channels. It is possible that the antibodies react with an antigen common to the α1 subunit of all affected channels. Calcium channel α1 subunits are evolutionarily and structurally related, although the non-L channels (classes A, B, and E) have been hypothesized to be closely related phylogenetically and to have diverged from L-type channels (classes C, D, and skeletal) at an early time point (Fujita et al., 1993; Zhang et al., 1993). It is equally plausible that the antibodies target an accessory subunit (α2/δ or β) of α1 or some other channel-associated protein. One suggested candidate is the protein synaptotagmin (Leveque et al., 1992; Takamori et al., 1994), although this idea is controversial (Hajela and Atchison, 1995).
The present work raises another interesting question: why are motoneurons more profoundly affected? Among the possibilities are that, in motoneurons, the critical epitopes are more accessible, the targeted channel(s) comprise a larger fraction of the total, or the rates of calcium channel biosynthesis and degradation are different. Whatever the mechanism, the present results, together with previous work (García et al., 1996), show that LES antibodies preferentially target neurons and more profoundly affect motor than sensory neurons.
Footnotes
This work was supported by National Institutes of Health Grant NS26416 to K.G.B. We thank Dr. Donald Sanders for the serum samples and Robin Morris for help with the tissue culture. This work is from a thesis submitted to the Academic Faculty of Colorado State University in partial fulfillment of the requirements for the degree of Ph.D. to K.D.G.
Correspondence should be addressed to Dr. Beam at the above address.
REFERENCES
- 1.Anderson HJ, Churchill-Davidson HC, Richardson AT. Bronchial neoplasm with myasthenia: prolonged apnea after administration of succinylcholine. Lancet. 1953;2:1291–1293. doi: 10.1016/s0140-6736(53)91358-0. [DOI] [PubMed] [Google Scholar]
- 2.Beam KG, Knudson CM. Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol. 1988;91:781–798. doi: 10.1085/jgp.91.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- 4.Blandino JK, Kim YI. Lambert–Eaton syndrome IgG inhibits dihydropyridine-sensitive, slowly inactivating calcium channels in bovine adrenal chromaffin cells. Ann NY Acad Sci. 1993;681:394–397. doi: 10.1111/j.1749-6632.1993.tb22918.x. [DOI] [PubMed] [Google Scholar]
- 5.Bowersox SS, Miljanich GP, Sugiura Y, Li C, Nadasdi L, Hoffman BB, Ramachandran J, Ko CP. Differential blockade of voltage-sensitive calcium channels at the mouse neuromuscular junction by novel ω-conopeptides and ω-agatoxin IVA. J Pharmacol Exp Ther. 1995;273:248–256. [PubMed] [Google Scholar]
- 6.Couteaux R, Pecot-Dechavassine M. Vesicules synaptiques et poches au niveau des “zones actives” de la jonction neuromusculaire. C R Acad Sci Series D. 1970;271:2346–2349. [PubMed] [Google Scholar]
- 7.Cull-Candy SG, Miledi R, Trautmann A, Uchitel OD. On the release of transmitter at normal, myasthenia gravis, and myasthenic syndrome-affected human end-plates. J Physiol (Lond) 1980;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton LM, Lambert EH. Electromyography and electric stimulation of nerves in diseases of motor unit: observations on myasthenic syndrome associated with malignant tumors. JAMA. 1957;163:1117–1124. doi: 10.1001/jama.1957.02970480021005. [DOI] [PubMed] [Google Scholar]
- 9.Elmqvist D, Lambert EH. Detailed analysis of neuromuscular transmission in a patient with the myasthenic syndrome sometimes associated with bronchogenic carcinoma. Mayo Clin Proc. 1968;43:689–713. [PubMed] [Google Scholar]
- 10.Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol (Lond) 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita Y, Mynlieff M, Dirksen RT, Kim MS, Niidome T, Nakai J, Friedrich T, Iwabe N, Miyata T, Furuichi T, Furutama D, Mikoshiba K, Mori Y, Beam KG. Primary structure and functional expression of the ω-conotoxin-sensitive N -type calcium channel from rabbit brain. Neuron. 1993;10:585–598. doi: 10.1016/0896-6273(93)90162-k. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga H, Engel AG, Osame M, Lambert EH. Paucity and disorganization of presynaptic membrane active zones in the Lambert–Eaton myasthenic syndrome. Muscle Nerve. 1982;5:686–697. [Google Scholar]
- 13.Fukunaga H, Engel AG, Lang B, Newsom-Davis J, Vincent A. Passive transfer of Lambert–Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci USA. 1983;80:7636–7640. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuoka T, Engel AG, Lang B, Newsom-Davis J, Prior C, Wray DW. Lambert–Eaton myasthenic syndrome. I. Early morphological effects of IgG on the presynaptic membrane active zones. Ann Neurol. 1987a;22:193–199. doi: 10.1002/ana.410220203. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka T, Engel AG, Lang B, Newsom-Davis J, Vincent A. Lambert–Eaton myasthenic syndrome. II. Immunoelectron microscopy localization of IgG at the mouse motor end-plate. Ann Neurol. 1987b;22:200–211. doi: 10.1002/ana.410220204. [DOI] [PubMed] [Google Scholar]
- 16.García KD, Mynlieff M, Sanders DB, Walrond JW, Beam KG (1996) Lambert–Eaton sera reduce low-voltage and high-voltage activated calcium currents in murine dorsal root ganglion neurons. Proc Natl Acad Sci USA, in press. [DOI] [PMC free article] [PubMed]
- 17.Grassi C, Magnelli V, Carbelli V, Sher E, Carbone E. Inhibition of low- and high-threshold calcium channels of human neuroblastoma IMR32 cells by Lambert–Eaton myasthenic syndrome (LEMS) IgGs. Neurosci Lett. 1994;181:50–56. doi: 10.1016/0304-3940(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 18.Hajela RK, Atchison WD. The proteins synaptotagmin and syntaxin are not general targets of Lambert–Eaton myasthenic syndrome autoantibody. J Neurochem. 1995;654:1245–1251. doi: 10.1046/j.1471-4159.1995.64031245.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamill OP, Morty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques from high-resolution current recordings from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 20.Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- 21.Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillyard DR, Monje VD, Mintz IM, Bean BP, Nadasdi L, Ramachandran J, Miljanich G, Azimi-Zoonooz A, Mcintosh JM, Cruz LJ, Imerial JS, Olivera BM. A new conus peptide ligand for mammalian presynaptic calcium channels. Neuron. 1992;9:69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- 23.Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ. Dominant role of N-type calcium channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988;239:57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- 24.Hong SJ, Chang CC. Inhibition of acetylcholine release from mouse motor nerve by a P-type calcium channel blocker, ω-agatoxin IVA. J Physiol (Lond) 1995;482:283–290. doi: 10.1113/jphysiol.1995.sp020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honig MG, Hume RI. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studies in long-term cultures. J Cell Biol. 1986;103:171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isselbacher KJ, Adams RD, Braunwald E, Petersdorf RG, Wilson JD. Principles of internal medicine, McGraw-Hill; New York: 1980. Laboratory values of clinical importance. pp. A2–A3. [Google Scholar]
- 27.Johnston I, Lang B, Leys K, Newsom-Davis J. Heterogeneity of calcium channel autoantibodies detected using a small-cell lung cancer line derived from a Lambert–Eaton myasthenic syndrome patient. Neurology. 1994;44:334–338. doi: 10.1212/wnl.44.2.334. [DOI] [PubMed] [Google Scholar]
- 28.Kim YI. Passively transferred Lambert–Eaton syndrome in mice receiving purified IgG. Muscle Nerve. 1986;9:523–530. doi: 10.1002/mus.880090608. [DOI] [PubMed] [Google Scholar]
- 29.Kim YI, Neher E. IgG from patients with Lambert–Eaton syndrome blocks voltage-dependent calcium channels. Science. 1988;239:405–408. doi: 10.1126/science.2447652. [DOI] [PubMed] [Google Scholar]
- 30.Kim YI, Blandino JK, O’Shaughnessy TJ. Inhibitory action of Lambert–Eaton syndrome IgG on calcium currents in a thyroid c-cell line. Ann NY Acad Sci. 1993;681:398–401. doi: 10.1111/j.1749-6632.1993.tb22919.x. [DOI] [PubMed] [Google Scholar]
- 31.Lambert EH, Elmqvist D. Quantal components of end-plate potentials in the myasthenic syndrome. Ann NY Acad Sci. 1971;183:183–199. doi: 10.1111/j.1749-6632.1971.tb30750.x. [DOI] [PubMed] [Google Scholar]
- 32.Lambert EH, Eaton LM, Rooke ED. Defects of neuromuscular conduction associated with malignant neoplasms. Am J Physiol. 1956;187:612–613. [Google Scholar]
- 33.Lang B, Newsom-Davis J, Wray DW. The effect of Lambert–Eaton myasthenic syndrome antibody on slow action potentials in mouse cardiac ventricle. Proc R Soc Lond [Biol] 1988;235:103–110. doi: 10.1098/rspb.1988.0065. [DOI] [PubMed] [Google Scholar]
- 34.Lennon VA, Kryzer TJ, Griesmann MS, O’Suilleabhasin PE, Windebank AJ, Woppmann A, Miljanich GP, Lambert EH. Calcium-channel antibodies in the Lambert–Eaton syndrome and other paraneoplastic disorders. N Engl J Med. 1995;332:1467–1474. doi: 10.1056/NEJM199506013322203. [DOI] [PubMed] [Google Scholar]
- 35.Leveque C, Hoshino T, David P, Shoji-Kasai Y, Leys K, Omori A, Lang B, Far EO, Sato K, Martin-Moutot N, Newsom-Davis J, Takahashi M, Seagar MJ. The synaptic vesicle protein synaptotagmin associates with calcium channels and is a putative Lambert–Eaton myasthenic syndrome antigen. Proc Natl Acad Sci USA. 1992;89:3625–3629. doi: 10.1073/pnas.89.8.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leys K, Lang B, Vincent A, Newsom-Davis J. Calcium channel autoantibodies in Lambert–Eaton myasthenic syndrome. Lancet. 1989;2:1107. doi: 10.1016/s0140-6736(89)91129-x. [DOI] [PubMed] [Google Scholar]
- 37.Login IS, Kim YI, Judd AM, Spangelo BL, MacLeod RM. Immunoglobulins of Lambert–Eaton myasthenic syndrome inhibit rat pituitary hormone release. Ann Neurol. 1987;22:610–614. doi: 10.1002/ana.410220509. [DOI] [PubMed] [Google Scholar]
- 38.Masselli RA, Richman DP, Wollmann RL. Inflammation at the neuromuscular junction in myasthenia gravis. Neurology. 1991;41:1497–1504. doi: 10.1212/wnl.41.9.1497. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy RT, TanPiengco PE. Multiple types of high-threshold calcium channels in rabbit sensory neurons: high-affinity block by nimodipine. J Neurosci. 1992;12:2225–2234. doi: 10.1523/JNEUROSCI.12-06-02225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milligan CE, Oppenheim RW, Schwartz LM. Motoneurons deprived of trophic support in vitro require new gene expression to undergo programmed cell death. J Neurobiol. 1994;25:1005–1016. doi: 10.1002/neu.480250809. [DOI] [PubMed] [Google Scholar]
- 41.Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 42.Mynlieff M, Beam KG. Developmental expression of voltage-dependent calcium currents in identified mouse motoneurons. Dev Biol. 1992a;152:407–410. doi: 10.1016/0012-1606(92)90148-a. [DOI] [PubMed] [Google Scholar]
- 43.Mynlieff M, Beam KG. Characterization of voltage-dependent calcium currents in mouse motoneurons. J Neurophysiol. 1992b;68:85–92. doi: 10.1152/jn.1992.68.1.85. [DOI] [PubMed] [Google Scholar]
- 44.Mynlieff M, Beam KG. Adenosine acting at an A1 receptor decreases N-type calcium current in mouse motoneurons. J Neurosci. 1994;14:3628–3634. doi: 10.1523/JNEUROSCI.14-06-03628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagel A, Engel AG, Newsom-Davis J, Fukuoka T. Lambert–Eaton myasthenic syndrome IgG depletes presynaptic membrane active zone particles by antigenic modulation. Ann Neurol. 1988;24:552–558. doi: 10.1002/ana.410240412. [DOI] [PubMed] [Google Scholar]
- 46.Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 47.Olivera BM, Miljanich GP, Ramachandran J, Adams ME. Calcium channel diversity and neurotransmitter release: the ω-conotoxins and ω-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- 48.Peers C, Lang B, Newsom-Davis J, Wray DW. Selective action of myasthenic syndrome antibodies on calcium channels in a rodent neuroblastoma X glioma cell line. J Physiol (Lond) 1990;421:293–308. doi: 10.1113/jphysiol.1990.sp017945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Reyes E, Wei X, Castellano A, Birnbaumer L. Molecular diversity of L-type calcium channels. J Biol Chem. 1990;265:20430–20436. [PubMed] [Google Scholar]
- 50.Randall A, Tsien RW. Pharmacological dissection of multiple types of calcium channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rash JE, Albuquerque EX, Hudson CS, Mayer RF, Satterfield JR. Studies of human myasthenia gravis: electrophysiological and ultrastructural evidence compatible with antibody attachment to the acetylcholine receptor complex. Proc Natl Acad Sci USA. 1976;73:4584–4588. doi: 10.1073/pnas.73.12.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Regan LJ, Sah DWY, Bean BP. Calcium channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and ω-conotoxin. Neuron. 1992;6:269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- 53.Roberts A, Perera S, Lang B, Vincent A, Newsom-Davis J. Paraneoplastic myasthenic syndrome IgG inhibits45Ca flux in a human small-cell carcinoma line. Nature. 1985;317:737–739. doi: 10.1038/317737a0. [DOI] [PubMed] [Google Scholar]
- 54.Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 56.Rossoni G, Berti G, La Maestra L, Clementi G. ω-Conotoxin GVIA binds to and blocks rat neuromuscular junction. Neurosci Lett. 1994;176:185–188. doi: 10.1016/0304-3940(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 57.Smith DO, Conklin MW, Jensen PJ, Atchison WD. Decreased calcium currents in motor nerve terminals of mice with Lambert–Eaton myasthenic syndrome. J Physiol (Lond) 1995;487:115–123. doi: 10.1113/jphysiol.1995.sp020865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith RG, Vaca K, McManaman J, Appel SH. Selective effects of skeletal muscle extract fractions on motoneuron development in vitro . J Neurosci. 1986;6:439–447. doi: 10.1523/JNEUROSCI.06-02-00439.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snutch TP, Reiner PB. Ca2+channels: diversity of form and function. Curr Opin Neurobiol. 1992;2:247–253. doi: 10.1016/0959-4388(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 60.Stanley EF, Goping G. Characterization of a calcium current in a vertebrate cholinergic presynaptic nerve terminal. J Neurosci. 1991;11:985–993. doi: 10.1523/JNEUROSCI.11-04-00985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takamori M, Hamada T, Komai K, Takahashi M, Yoshida A. Synaptotagmin can cause an immune-mediated model of Lambert–Eaton myasthenic syndrome in rats. Ann Neurol. 1994;35:74–80. doi: 10.1002/ana.410350112. [DOI] [PubMed] [Google Scholar]
- 62.Turner TJ, Adams ME, Dunlop K. Multiple calcium channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci USA. 1993;90:9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uchitel OD, Protti DA, Sanchez VA, Cherksey BD, Sugimori M, LLinas R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci USA. 1992;89:3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Umemiya M, Berger AJ. Properties and function of low- and high-voltage-activated calcium channels in hypoglossal motoneurons. J Neurosci. 1994;14:5652–5660. doi: 10.1523/JNEUROSCI.14-09-05652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Viglione MP, Creutz CE, Kim YI. Lambert–Eaton syndrome: antigen–antibody interaction and calcium current inhibition in chromaffin cells. Muscle Nerve. 1992;15:1325–1333. doi: 10.1002/mus.880151206. [DOI] [PubMed] [Google Scholar]
- 66.Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type calcium channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 67.Yoshikami D, Bagabaldo Z, Olivera BM. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann NY Acad Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal calcium channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]