Abstract
Iron–sulfur clusters are essential cofactors of proteins. In eukaryotes, iron–sulfur cluster biogenesis requires a mitochondrial iron–sulfur cluster machinery (ISC) and a cytoplasmic iron–sulfur protein assembly machinery (CIA). Here we used mitochondria and cytoplasm isolated from yeast cells, and [35S]cysteine to detect cytoplasmic Fe–35S cluster assembly on a purified apoprotein substrate. We showed that mitochondria generate an intermediate, called (Fe–S)int, needed for cytoplasmic iron–sulfur cluster assembly. The mitochondrial biosynthesis of (Fe–S)int required ISC components such as Nfs1 cysteine desulfurase, Isu1/2 scaffold, and Ssq1 chaperone. Mitochondria then exported (Fe–S)int via the Atm1 transporter in the inner membrane, and we detected (Fe–S)int in active form. When (Fe–S)int was added to cytoplasm, CIA utilized it for iron–sulfur cluster assembly without any further help from the mitochondria. We found that both iron and sulfur for cytoplasmic iron–sulfur cluster assembly originate from the mitochondria, revealing a surprising and novel mitochondrial role. Mitochondrial (Fe–S)int export was most efficient in the presence of cytoplasm containing an apoprotein substrate, suggesting that mitochondria respond to the cytoplasmic demand for iron–sulfur cluster synthesis. Of note, the (Fe–S)int is distinct from the sulfur intermediate called Sint, which is also made and exported by mitochondria but is instead used for cytoplasmic tRNA thiolation. In summary, our findings establish a direct and vital role of mitochondria in cytoplasmic iron–sulfur cluster assembly in yeast cells.
Keywords: yeast, mitochondria, iron, sulfur, ABC transporter, iron–sulfur protein, cytoplasm, export, metal cofactor, tRNA thiolation
Introduction
Iron–sulfur clusters are vital cofactors of proteins that perform critical functions inside mitochondria (e.g. electron transfer by respiratory complexes or aconitase activity in the TCA cycle) and outside of mitochondria (e.g. ribosome biogenesis, protein synthesis, DNA repair, DNA transcription, iron regulation, and tRNA modifications) (1–5). The biosynthesis of iron–sulfur cluster cofactors is compartmentalized, with a mitochondrial iron–sulfur cluster machinery (termed ISC)3 and a cytoplasmic iron–sulfur protein assembly machinery (termed CIA). In the yeast Saccharomyces cerevisiae, the initial step in mitochondrial iron–sulfur cluster biogenesis is the formation of an iron–sulfur cluster intermediate on a scaffold protein, Isu (Isu1 or Isu2 isoform) (1, 2). The cysteine desulfurase enzyme complex (Nfs1/Isd11/Acp1) provides sulfur from amino acid cysteine for the intermediate (6–11). Frataxin (Yfh1) plays a role in enhancing binding of the substrate cysteine to Nfs1, transfer of the persulfide sulfur from Nfs1 to Isu1/2, and/or iron delivery from an unknown source (1, 12–18). The NAD(P)H-dependent ferredoxin reductase–ferredoxin redox couple supplies reducing equivalents that are needed for formation of the [2Fe–2S] cluster intermediate on Isu1/2 (1, 19–21). A GTPase is also likely to be involved at this stage (22, 23), although the GTPase remains to be identified. In a subsequent step, the iron–sulfur cluster intermediate bound to Isu1/2 is delivered to the monothiol glutaredoxin Grx5 by the ATP-dependent Ssq1 Hsp70 chaperone/Jac1 cochaperone (24, 25). Several other proteins are subsequently involved in modification of the [2Fe–2S] cluster intermediate into a [4Fe–4S] cluster intermediate and/or in targeting the preformed clusters to specific recipients such as aconitase (1, 2). In analogous fashion, an iron–sulfur cluster intermediate for the cytoplasmic iron–sulfur cluster assembly system, the CIA, is first assembled on a scaffold protein complex comprised of Cfd1 and Nbp35 (26–29). Reducing equivalents from the Dre2/Tah18 reductase are required at this stage (30, 31). Subsequently, the newly assembled iron–sulfur cluster is transferred from Cfd1/Nbp35 and inserted into target apoproteins via Nar1 and the CIA targeting complex (Cia1/Cia2/Mms19), which perform chaperone and targeting functions (2, 32–34).
Twenty years ago, studies with whole yeast cells predicted that mitochondria lie upstream of the cytoplasm for cytoplasmic iron–sulfur cluster assembly (35). However, such a mitochondria–cytoplasm interaction has thus far not been mechanistically understood. Mitochondria isolated from yeast cells contain a complete ISC machinery, and isolated mitochondria by themselves are capable of forming iron–sulfur clusters when supplemented with cysteine, iron, and nucleotides (GTP, NADH, and ATP) (19, 22, 23). The situation in the cytoplasm is different. The CIA machinery is apparently incomplete, requiring a contribution from mitochondria to function as an assembly apparatus for cytoplasmic iron–sulfur clusters. This idea was originally hinted at by in vivo experiments in which mitochondrial Nfs1 cysteine desulfurase was depleted from yeast cells using a regulated promoter. These experiments resulted in deficient sulfur use in both mitochondria and cytoplasm (35), suggesting export of a sulfur species, called “X-S” by Lill and co-workers (2). A long-standing hypothesis is that X-S is exported from mitochondria by the ABC transporter Atm1 in the mitochondrial inner membrane to the cytoplasm and is then utilized for cytoplasmic iron–sulfur cluster synthesis by the CIA machinery (2, 35). However, the X-S molecule exported from mitochondria has thus far not been detected or identified. The crystal structure of Atm1 has been determined (36), and yet the export substrate X-S remains elusive (1, 2). Suggestions have been made that X-S contains sulfur as a persulfide or glutathione (GSH) derivative, perhaps as a GSSH (36) or GSSSG (37) compound. A chemically synthesized compound containing GSH-conjugated iron–sulfur cluster has alternatively been proposed (38). However, none of these proposed intermediates has been verified or tested in a biological context (2).
Here we show that mitochondria export an essential intermediate, called (Fe–S)int, to the cytoplasm that is then used for cytoplasmic iron–sulfur cluster assembly. However, the situation is even more complex, because another intermediate, a sulfur-only intermediate called Sint, is made and exported by mitochondria and is required for cytoplasmic tRNA thiolation (39, 40). The wobble uridines (U34) of the cytoplasmic tRNAs specific for lysine, glutamate, and glutamine contain a thio-modification (41). The thiolation of these cytoplasmic tRNAs enhances their interactions with ribosomes, prevents frameshifting, and ensures accurate protein synthesis (42–44). The thio-modification involves a sulfur relay/transfer system mediated by several cytoplasmic proteins including a ubiquitin-related modifier, Urm1 (40, 41). Note that the pathways for biosynthesis of cytoplasmic iron–sulfur clusters and cytoplasmic tRNA thiolation possess many intersecting and overlapping features. The sulfur-only Sint, like the (Fe–S)int, is also made in mitochondria in a Nfs1-dependent manner and exported to the cytoplasm via Atm1 (39, 40). However, these intermediates are distinct; they are not biochemically equivalent, and they are not interchangeable. A key feature that emerges from this study is that iron for (Fe–S)int originates in mitochondria.
Results
Requirement of mitochondria for cytoplasmic iron–sulfur cluster assembly
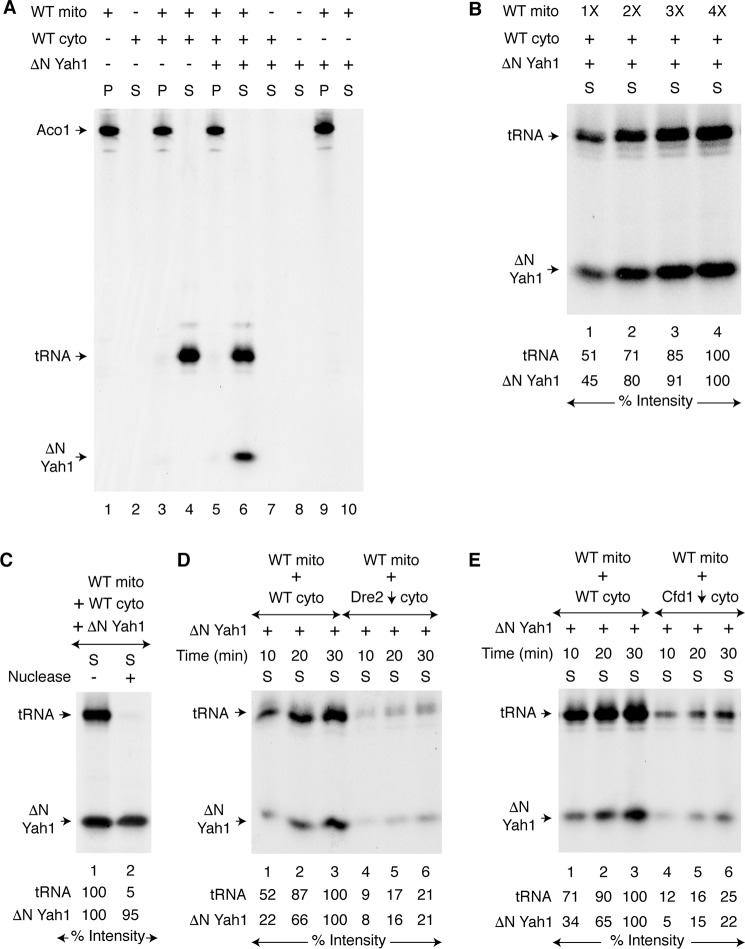
The TCA cycle enzyme aconitase [4Fe-4S] resides in the mitochondrial matrix. We found that mitochondria isolated from wild-type (WT) yeast cells contain a pool of apoaconitase (apo-Aco1) that can serve as a substrate for [4Fe-4S] cluster assembly (19, 22). For example, when isolated WT mitochondria were incubated with [35S]cysteine, nucleotides (GTP, NADH, and ATP), and iron, the endogenous apo-Aco1 became radiolabeled because of insertion of newly formed Fe–35S clusters (Fig. 1A, lane 1, mitochondrial pellet P). In contrast, no radiolabeled signal was detected when isolated WT cytoplasm was incubated with [35S]cysteine under identical conditions (Fig. 1A, lane 2, cytoplasmic supernatant S). WT mitochondria were then added to WT cytoplasm and incubated with [35S]cysteine in a similar manner. The reaction mixture was centrifuged, separating mitochondria from cytoplasm. As expected, radiolabeled aconitase was found in the mitochondrial pellet (Fig. 1A, lane 3). A strong radiolabeled band was now detected in the cytoplasm, and this was due to 35S-thiolation of endogenous tRNAs (39) (Fig. 1A, lane 4). However, no significant radiolabeling of an endogenous cytoplasmic protein was detected (Fig. 1A, lane 4). Perhaps cytoplasmic apoproteins are much less abundant.
Figure 1.
Requirement of mitochondria for cytoplasmic iron–sulfur cluster assembly. A, WT mitochondria (200 μg of proteins) alone, WT cytoplasm (200 μg of proteins) alone, or both were mixed with [35S]cysteine (10 μCi), nucleotides (1 mm GTP, 2 mm NADH, and 4 mm ATP), iron (10 μm ferrous ascorbate), and as indicated, apo-ΔN60 Yah1 protein (1 μg). The samples were incubated at 30 °C for 30 min. After centrifugation, the pellet (P; mitochondria) and supernatant (S; cytoplasm) fractions were analyzed by native PAGE followed by autoradiography. B, WT mitochondria (1× = 100 μg of proteins) were supplemented with WT cytoplasm (200 μg of proteins), apo-ΔN60 Yah1, [35S]cysteine, nucleotides, and iron. After incubation at 30 °C for 30 min, the samples were centrifuged, and the cytoplasm/supernatant (S) fractions were analyzed. C, reaction mixtures containing WT mitochondria, WT cytoplasm, apo-ΔN60 Yah1, [35S]cysteine, nucleotides, and iron were incubated at 30 °C for 30 min. After centrifugation, the cytoplasm/supernatant (S) fraction was treated with S7 micrococcal nuclease (800 units/ml) at 30 °C for 10 min as indicated and analyzed. D, WT mitochondria were added to WT cytoplasm or Dre2-depleted (Dre2↓) cytoplasm. The samples were incubated with apo-ΔN60 Yah1, [35S]cysteine, nucleotides, and iron at 30 °C for 10–30 min as indicated. After centrifugation, the cytoplasm/supernatant (S) fractions were analyzed. E, WT mitochondria were added to WT cytoplasm or Cfd1-depleted (Cfd1↓) cytoplasm, and assays were performed as in D. WT mito, WT mitochondria; WT cyto, WT cytoplasm.
We therefore added an apoprotein indicator to assess cytoplasmic iron–sulfur cluster assembly activity. We used bacterial expressed and purified apoferredoxin (apo-ΔN60 Yah1; Fig. S1A, lane 2) as a substrate for cytoplasmic iron–sulfur cluster assembly. The protein was incubated with cytoplasm alone, with buffer alone, or with mitochondria alone, in the presence of [35S]cysteine, nucleotides, and iron. After centrifugation, the cytoplasm/supernatant fractions were analyzed, but there was no signal (Fig. 1A, lanes 7, 8, and 10, respectively). Only when mitochondria were added to cytoplasm did ΔN60 Yah1 become radiolabeled in the cytoplasm, similar to thiolated tRNAs (Fig. 1A, lane 6). We believe that mitochondria exported an 35S-labeled intermediate that was then utilized by the CIA machinery to generate the radiolabeled ΔN60 Yah1 in the cytoplasm. The ΔN60 Yah1 protein remains in the cytoplasm because of the lack of its mitochondrial targeting sequence, providing compartment-specific readout on iron–sulfur cluster assembly activity (see also Fig. S1, A and B). In a separate experiment, increasing concentrations of WT mitochondria were added to reaction mixtures containing fixed concentrations of WT cytoplasm and apo-ΔN60 Yah1. After incubation with [35S]cysteine, iron, and nucleotides, samples were centrifuged to remove mitochondrial pellets. In the cytoplasm/supernatant fractions, the radioactive signals for both tRNAs and ΔN60 Yah1 were found to be enhanced with increasing concentrations of added mitochondria (Fig. 1B). As expected, nuclease treatment of the cytoplasm following the mixing assay abrogated the 35S-tRNA signal, whereas radiolabeled ΔN60 Yah1 was completely unaffected (Fig. 1C). The radiolabeling of ΔN60 Yah1 was most likely due to insertion of a newly formed [2Fe-235S] cluster into the protein. To validate this notion, the cytoplasm/supernatant fractions obtained after the mixing assay were analyzed by SDS-PAGE followed by autoradiography. This time only the 35S-tRNA signal was detected, and no 35S-labeled ΔN60 Yah1 was visible (Fig. S1C, right panel). The 35S label is covalently attached to tRNAs, and therefore thiolated tRNAs can be analyzed by either native PAGE or SDS-PAGE (Fig. S1C) (39). In contrast, iron–sulfur clusters are noncovalently attached to proteins via cysteine residues in most cases and are destroyed by denaturants such as SDS. Thus, (Fe–35S)–labeled proteins can only be analyzed by native gels (22). Importantly, these assays are not limited to yeast ferredoxin (Yah1); bacterial expressed and purified Chlamydomonas reinhardtii ferredoxin (45) (PetF; Fig. S1A, lane 3) can also be used as an indicator for cytoplasmic [2Fe–2S] cluster assembly but again only in the presence of mitochondria (Fig. S1D, lane 4). Most likely mitochondria generate and export critical sulfur-containing intermediates that are used by the cytoplasm for cytoplasmic tRNA thiolation and iron–sulfur cluster assembly.
We then sought to determine whether ΔN60 Yah1 loading depends on components of the CIA machinery such as Dre2 or Cfd1, known to be required for cytoplasmic iron–sulfur cluster assembly. Dre2-depleted (Dre2↓) cytoplasm was isolated after turning off Dre2 expression in a GAL1-DRE2 promoter swap strain (39) (Table S1). WT mitochondria were added to WT cytoplasm or Dre2↓ cytoplasm and incubated with apo-ΔN60 Yah1, [35S]cysteine, nucleotides, and iron. Compared with WT cytoplasm, radiolabeling of ΔN60 Yah1 occurred only poorly with Dre2↓ cytoplasm (Fig. 1D). Likewise, Cfd1-depleted (Cfd1↓) cytoplasm also failed to efficiently synthesize [2Fe-235S] clusters, and accordingly, 35S labeling of ΔN60 Yah1 occurred only poorly (Fig. 1E). Radiolabeling of cytoplasmic tRNAs was also impaired (Fig. 1, D and E). This could be due to loss of function of cytoplasmic iron–sulfur proteins such as Elp3 (46) and Ncs6 (47), which are involved in tRNA thiolation (39–41). Note that [2Fe–2S] cluster biogenesis of the transcription factor Yap5 in vivo appears to occur independent of Cfd1 or any other CIA components (48). There might exist a canonical pathway for ΔN60 Yah1 [2Fe–2S] cluster assembly that requires at least some of the CIA components such as Dre2 and Cfd1 (Fig. 1, D and E) and a noncanonical CIA-independent pathway for Yap5 [2Fe–2S] cluster biogenesis (48).
Role of the mitochondrial ISC machinery in promoting cytoplasmic iron–sulfur cluster assembly
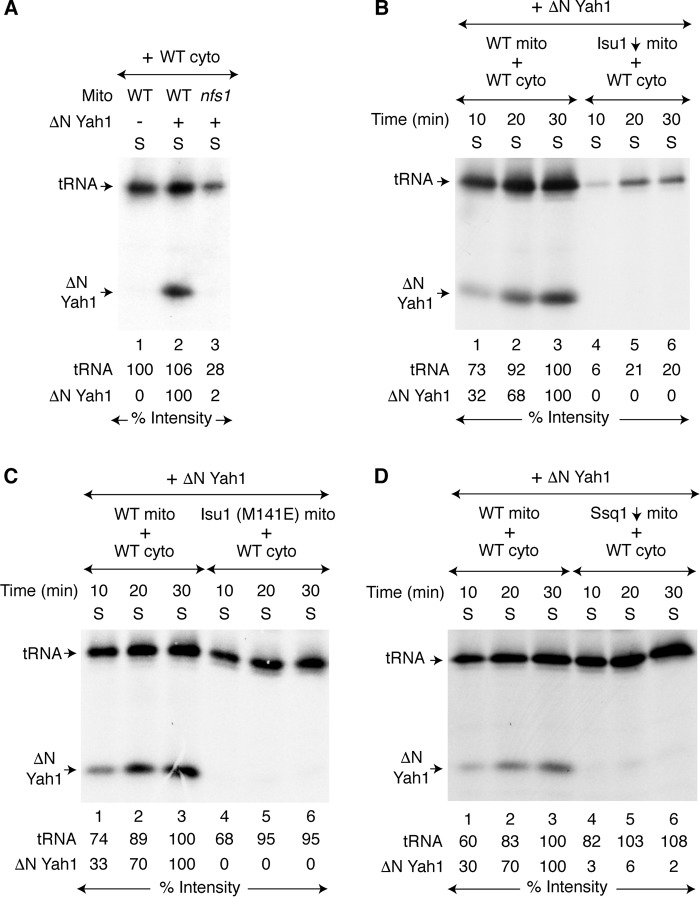
The hypomorphic mutant nfs1-14 carries a missense NFS1 allele (I191S). Mitochondria isolated from this mutant are deficient in cysteine desulfurase activity and are severely compromised in iron–sulfur cluster assembly activity (10, 39). When used in the mixing assay with WT cytoplasm, no radiolabeling of ΔN60 Yah1 was detected, and only a weak 35S-tRNA signal was observed (Fig. 2A, lane 3). Thus, cytoplasm depends on mitochondrial Nfs1 for supply of sulfur-containing species required for both tRNA thiolation and iron–sulfur cluster assembly. What about the other components of the mitochondrial ISC? The central events in mitochondrial iron–sulfur cluster assembly are the formation of cluster intermediates on Isu1/2 scaffolds and the subsequent transfer to recipients by Ssq1 Hsp70 chaperone and other proteins. Isu1 was depleted (Isu1↓) from Δisu2 cells, and mitochondria were isolated (39, 49). These Isu1↓ mitochondria were then incubated with WT cytoplasm and apo-ΔN60 Yah1 in the presence of [35S]cysteine, nucleotides, and iron. No radiolabeled aconitase (Aco1) was detected in mitochondria (Fig. S2A, lanes 4–6). Likewise, no radiolabeled ΔN60 Yah1 was detected in the cytoplasm, and very little cytoplasmic tRNA was radiolabeled (Fig. 2B, lanes 4–6). We also examined mitochondria isolated from a strain expressing a hypomorphic Isu1 (M141E) mutant protein (49) in a similar manner. These mutant mitochondria exhibited weak radiolabeling of Aco1 (Fig. S2B, lanes 4–6), and no ΔN60 Yah1 radiolabeling was detected in the cytoplasm (Fig. 2C, lanes 4–6). Surprisingly, however, these mutant mitochondria were almost as efficient as WT mitochondria in promoting cytoplasmic tRNA thiolation (Fig. 2C, compare lanes 1–3 with lanes 4–6, respectively). Interestingly, the point mutation M141E in the Isu1 sequence lies adjacent to the Ssq1 interaction site motif LPPVK (50), and this location may explain the apparent phenocopy of ssq1 mutants. Ssq1-depleted (Ssq1↓) mitochondria exhibited poor radiolabeling of mitochondrial aconitase (Fig. S2C, lanes 4–6) and practically undetectable radiolabeling of cytoplasmic ΔN60 Yah1 (Fig. 2D, lanes 4–6). Furthermore, Ssq1↓ mitochondria supported strong cytoplasmic tRNA labeling to the WT level (Fig. 2D, compare lanes 1–3 with lanes 4–6, respectively). To summarize, Nfs1, Isu1/2, and Ssq1 are required for generating sulfur-containing intermediate species for both mitochondrial and cytoplasmic iron–sulfur clusters. However, Nfs1 and Isu1/2, but not Ssq1, are required for cytoplasmic tRNA thiolation. Two distinct intermediates are likely generated by WT mitochondria and subsequently exported to the cytoplasm: one for cytoplasmic tRNA thiolation (called Sint) (39) and another for cytoplasmic iron–sulfur cluster assembly (called (Fe–S)int).
Figure 2.
Mitochondrial ISC components involved in cytoplasmic iron–sulfur cluster assembly. A, WT or nfs1-14 mitochondria (200 μg of proteins) were added to WT cytoplasm (200 μg of proteins). Apo-ΔN60 Yah1 protein (1 μg) was added as indicated. The samples were then incubated with [35S]cysteine (10 μCi), nucleotides (1 mm GTP, 2 mm NADH, and 4 mm ATP), and iron (10 μm) at 30 °C for 30 min. After centrifugation, the cytoplasm/supernatant (S) fractions were analyzed. B, WT or Isu1-depleted (Isu1↓, Isu2 absent) mitochondria were added to WT cytoplasm, and reaction mixtures were incubated with apo-ΔN60 Yah1, [35S]cysteine, nucleotides, and iron at 30 °C for 10–30 min as indicated. After centrifugation, the cytoplasm/supernatant (S) fractions were analyzed. C, WT mitochondria or mitochondria with the Isu1 (M141E) mutant protein (WT Isu1 depleted, Isu2 absent, plasmid YCplac22-Isu1 (M141E)) were added to WT cytoplasm, and assays were performed as in B. D, WT mitochondria or Ssq1-depleted (Ssq1↓) mitochondria were added to WT cytoplasm, and assays were performed as in B. WT mito, WT mitochondria; WT cyto, WT cytoplasm.
Mitochondrial production of (Fe–S)int occurs without any help from CIA
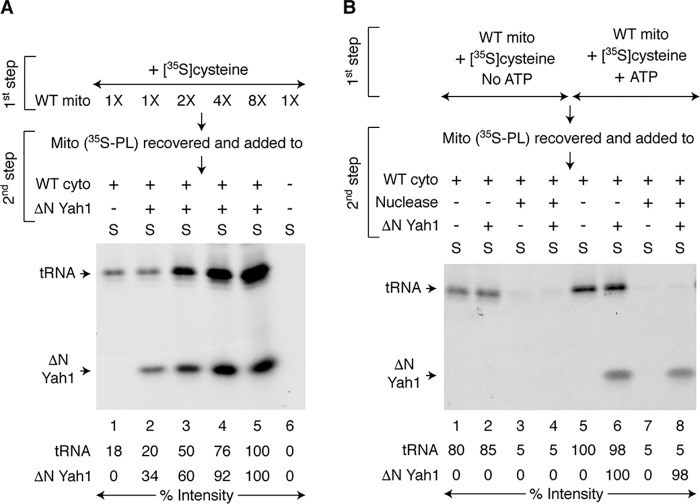
In the assays described above, generation of (Fe–S)int in mitochondria, its export from mitochondria, and its cytoplasmic utilization by CIA were all performed in a single step. We asked whether mitochondria can generate (Fe–S)int by themselves in the absence of cytoplasm. For this purpose, we designed a two-step assay as follows. Increasing concentrations of WT mitochondria were incubated with [35S]cysteine, iron, and nucleotides (Fig. 3A, first step, intermediate generation). These 35S-prelabeled mitochondria (called “Mito (35S-PL)”) were recovered and washed to remove free and excess [35S]cysteine. Iron and nucleotides were added back, and reaction mixtures were incubated without or with fixed amounts of WT cytoplasm and/or apo-ΔN60 Yah1 (Fig. 3A, second step, export and utilization). As in the case for 35S-tRNAs, the 35S-ΔN60 Yah1 signal was also enhanced with increasing concentrations of mitochondria used during the first step (Fig. 3A, lanes 2–5). These results suggest that increasing concentrations of mitochondria led to increased productions of both 35Sint and (Fe–35S)int and that the process occurred even in the absence of cytoplasm during the first step. Accordingly, when cytoplasm and apo-ΔN60 Yah1 were added, increasing amounts of these already formed intermediates were exported from mitochondria and utilized in the cytoplasm during the second step.
Figure 3.
Mitochondrial production of (Fe–S)int and ATP dependence. A, increasing concentrations of WT mitochondria (1× = 100 μg of proteins) were incubated with [35S]cysteine (10 μCi), nucleotides (1 mm GTP, 2 mm NADH, and 4 mm ATP), and iron (10 μm) at 30 °C for 20 min (first step). Mitochondria were recovered and washed. These prelabeled mitochondria (called Mito (35S-PL)) were then incubated with nucleotides and iron at 30 °C for 30 min, in the absence or presence of WT cytoplasm (200 μg of proteins) and/or apo-ΔN60 Yah1 (1 μg) as indicated (second step). After centrifugation, the cytoplasm/supernatant (S) fractions were analyzed. B, WT mitochondria (200 μg of proteins) were supplemented with 1 mm GTP, 2 mm NADH, and 10 μm iron and prelabeled with [35S]cysteine (10 μCi), in the absence (lanes 1–4) or presence (lanes 5–8) of 4 mm ATP as in A (first step). Mitochondria (35S-PL) were recovered, washed, and then mixed with WT cytoplasm (200 μg of proteins). In some cases, S7 micrococcal nuclease (300 units/ml) was added to degrade endogenous tRNAs in the cytoplasm. Reaction mixtures were supplemented with nucleotides (1 mm GTP, 2 mm NADH, and 4 mm ATP), iron (10 μm), and as indicated, apo-ΔN60 Yah1 (1 μg). After incubation at 30 °C for 30 min (second step), the samples were centrifuged, and the cytoplasm/supernatant (S) fractions were analyzed. WT mito, WT mitochondria; WT cyto, WT cytoplasm.
ATP requirement for mitochondrial production of (Fe–S)int
Ssq1 is an ATPase. It is required for cytoplasmic iron–sulfur cluster assembly but not for cytoplasmic tRNA thiolation (Fig. 2D). We therefore sought to determine the role of ATP specifically in generating (Fe–S)int using a two-step assay similar to the one described above. Briefly, WT mitochondria were incubated with [35S]cysteine, iron, GTP, and NADH, in the absence or presence of added ATP (Fig. 3B, first step, intermediate generation). These 35S-prelabeled mitochondria (Mito (35S-PL)) were recovered, washed, and then incubated with WT cytoplasm supplemented with iron and nucleotides including ATP (i.e. GTP, NADH, and ATP), in the absence or presence of added apo-ΔN60 Yah1 (Fig. 3B, second step, intermediate export and utilization). In some cases, a nuclease was included to degrade endogenous tRNAs in the cytoplasm. Radiolabeling of ΔN60 Yah1 was detected only when ATP was added during the first step (Fig. 3B, compare lanes 2 and 4 with lanes 6 and 8, respectively). In contrast, tRNA radiolabeling occurred without any added ATP at this step (Fig. 3B, lanes 1 and 2). Thus, (Fe–S)int generation specifically requires ATP addition, and most likely ATP hydrolysis by the Ssq1 ATPase plays a critical role in the biosynthetic process.
Mitochondrial origin of iron for cytoplasmic iron–sulfur cluster assembly
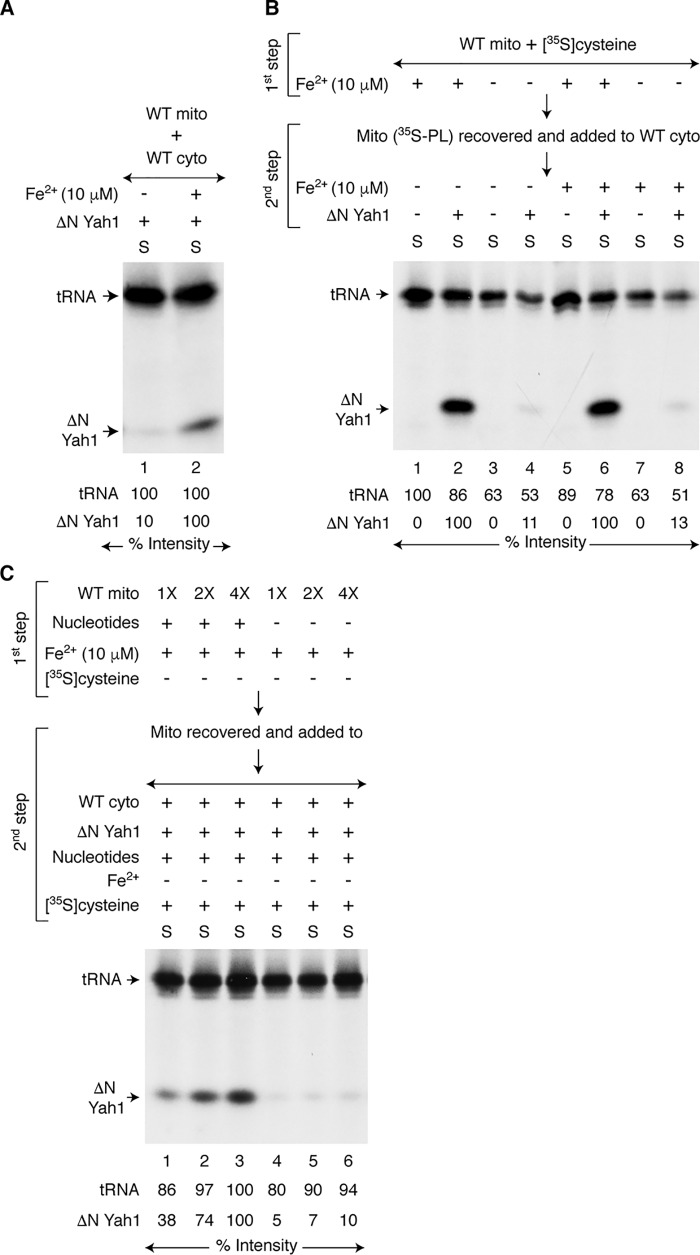
Iron was expected to be needed for cytoplasmic iron–sulfur cluster assembly, because it is a constituent of the cytoplasmic iron–sulfur cluster cofactor itself. To evaluate the iron requirement, WT mitochondria were incubated with WT cytoplasm, apo-ΔN60 Yah1, [35S]cysteine, and nucleotides, in the absence or presence of added iron. The addition of iron greatly stimulated [2Fe-235S] labeling of ΔN60 Yah1 in the cytoplasm but had no effect on 35S labeling of tRNAs (Fig. 4A, lane 2). In the absence of added iron, very little ΔN60 Yah1 was radiolabeled (Fig. 4A, lane 1). This experiment, however, cannot distinguish between mitochondrial versus cytoplasmic origin for the iron, because it is a one-step assay. We therefore performed a two-step assay as follows. WT mitochondria were incubated with [35S]cysteine and nucleotides, in the absence or presence of added iron (Fig. 4B, first step, intermediate generation). Mitochondria were recovered and washed to remove free [35S]cysteine and iron. These prelabeled mitochondria (Mito (35S-PL)) were then incubated with WT cytoplasm and nucleotides. Apo-ΔN60 Yah1 and iron were added as indicated (Fig. 4B, second step, intermediate export and utilization). A strong radiolabeling of ΔN60 Yah1 was detected only when iron was added during the first step (mitochondria present but no cytoplasm) (Fig. 4B, lanes 2 and 6). Furthermore, radiolabeling of ΔN60 Yah1 was not enhanced by the addition of iron during the second step (Fig. 4B, compare lanes 2 and 6 and also lanes 4 and 8). The addition of 5–10 μm iron during the first step was optimal, and higher concentrations had no further stimulatory effects (Fig. S3). These results suggest an important role for mitochondrial iron in the production of (Fe–S)int. Most likely, (Fe–S)int exported from mitochondria contains both iron and sulfur species required for cytoplasmic iron–sulfur cluster assembly. Interestingly, radiolabeling of tRNAs occurred even when iron addition was omitted in both the first and second steps (Fig. 4B, lanes 3 and 4).
Figure 4.
Requirement of mitochondrial iron for cytoplasmic iron–sulfur cluster assembly. A, WT mitochondria (200 μg of proteins) were incubated with WT cytoplasm (200 μg of proteins), apo-ΔN60 Yah1, [35S]cysteine, and nucleotides (1 mm GTP, 2 mm NADH, and 4 mm ATP) at 30 °C for 30 min, in the absence or presence of added iron (Fe2+; 10 μm ferrous ascorbate). After centrifugation, the cytoplasm/supernatant (S) fractions were analyzed. B, WT mitochondria (400 μg of proteins) were prelabeled by incubating with [35S]cysteine and nucleotides at 30 °C for 20 min, in the absence or presence of iron (10 μm) (first step). Mitochondria (35S-PL) were recovered, washed, and then mixed with WT cytoplasm (200 μg of proteins), nucleotides, and as indicated, apo-ΔN60 Yah1. The samples were incubated at 30 °C for 30 min, with or without added iron (10 μm) (second step). After centrifugation, the cytoplasm/supernatant (S) fractions were analyzed. C, increasing concentrations of WT mitochondria (1× = 200 μg of proteins) were incubated with iron (10 μm) at 30 °C for 20 min, in the absence or presence of added nucleotides (first step). Mitochondria were recovered, washed, and mixed with WT cytoplasm (200 μg of proteins), [35S]cysteine, nucleotides, and apo-ΔN60 Yah1. No iron was added at this stage. The samples were incubated at 30 °C for 30 min (second step). After centrifugation, the cytoplasm/supernatant (S) fractions were analyzed. WT mito, WT mitochondria; WT cyto, WT cytoplasm.
To further define iron-dependent production of (Fe–S)int, increasing concentrations of WT mitochondria were incubated with iron but no [35S]cysteine, in the absence or presence of added nucleotides (Fig. 4C, first step). Mitochondria were recovered, washed, and then incubated with WT cytoplasm, apo-ΔN60 Yah1, [35S]cysteine, and nucleotides, but no added iron (Fig. 4C, second step). Efficient radiolabeling of ΔN60 Yah1 was detected only when mitochondria were preloaded with iron in the presence of nucleotides during the first step (Fig. 4C, compare lanes 1–3 with lanes 4–6, respectively). Nucleotides may be required for iron import into mitochondria and/or maintenance of imported iron in bioavailable form. In any case, mitochondria can be preloaded with iron for subsequent production of (Fe–35S)int with the addition of [35S]cysteine. It is not necessary that mitochondria be supplemented with both iron and [35S]cysteine at the same time for efficient generation of (Fe–35S)int.
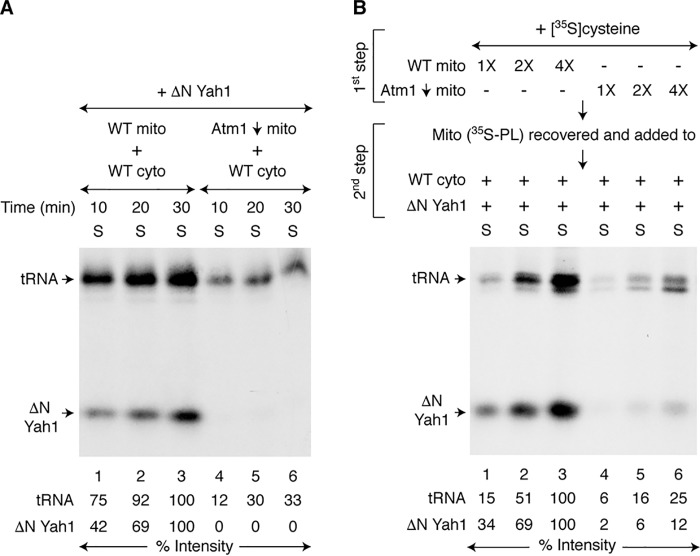
Mitochondrial export of (Fe–S)int involving the Atm1 transporter
The (Fe–S)int species generated within mitochondria must be exported to the cytoplasm for iron–sulfur cluster assembly. Atm1 is an ABC transporter in the mitochondrial inner membrane (51), and we sought to determine whether it is required for (Fe–S)int export. The Atm1 protein was depleted from cells using the GAL1-ATM1 promoter swap, and mitochondria isolated from this strain (Atm1↓) failed to promote any detectable iron–sulfur cluster assembly in WT cytoplasm in a one-step assay (Fig. 5A, lanes 4–6). The cytoplasmic tRNA thiolation was also much less efficient and occurred slowly as previously observed (39). A two-step assay was then performed specifically looking at the export step. Briefly, increasing concentrations of mitochondria (WT or Atm1↓) were prelabeled with [35S]cysteine, nucleotides, and iron (Fig. 5B, first step). Mitochondria (35S-PL) were recovered, washed, and then incubated with WT cytoplasm, apo-ΔN60 Yah1, nucleotides, and iron (Fig. 5B, second step). Compared with WT mitochondria, Atm1↓ mitochondria promoted only very little [2Fe-235S] labeling of ΔN60 Yah1 or 35S labeling of tRNAs (Fig. 5B, lanes 1–3 versus lanes 4–6, respectively). In a parallel two-step assay, a fixed concentration of mitochondria (WT or Atm1↓) was prelabeled with [35S]cysteine during the first step, but the export/utilization reaction was performed for different time periods during the second step. Again, cytoplasmic iron–sulfur cluster assembly or tRNA thiolation promoted by Atm1↓ mitochondria (35S-PL) occurred very poorly (Fig. S4). In the absence of adequate levels of Atm1, mitochondria were unable to efficiently export active (Fe–35S)int or 35Sint.
Figure 5.
Role of Atm1 in mitochondrial export of (Fe–S)int to the cytoplasm. A, WT or Atm1-depleted (Atm1↓) mitochondria (200 μg of proteins) were added to WT cytoplasm (200 μg of proteins), and reaction mixtures were incubated with apo-ΔN60 Yah1, [35S]cysteine, nucleotides (1 mm GTP, 2 mm NADH, and 4 mm ATP), and iron (10 μm) at 30 °C for 10–30 min as indicated. After centrifugation, the cytoplasm/supernatant (S) fractions were analyzed. B, mitochondria (WT or Atm1↓; 1× = 200 μg of proteins) were prelabeled by incubating with [35S]cysteine, nucleotides, and iron at 30 °C for 20 min (first step). Mitochondria (35S-PL) were recovered, washed, and then incubated with WT cytoplasm (200 μg of proteins), nucleotides, iron, and apo-ΔN60 Yah1 at 30 °C for 30 min (second step). The samples were centrifuged, and the cytoplasm/supernatant (S) fractions were analyzed. WT mito, WT mitochondria; WT cyto, WT cytoplasm.
Detection of exported (Fe–S)int in active form
In the assays described above, mitochondrial export of (Fe–S)int and its cytoplasmic utilization occurred at the same time. We wondered whether (Fe–S)int could be exported from mitochondria in the absence of cytoplasm. In a different two-step assay, increasing concentrations of mitochondria were incubated with [35S]cysteine, nucleotides, and iron (Fig. 6A, first step). The samples were centrifuged to remove mitochondria, and the resulting supernatant fractions (called “SupExported”) were added to WT cytoplasm and incubated without or with a fixed concentration of added apo-ΔN60 Yah1 (Fig. 6A, second step). Radiolabeled signals for both ΔN60 Yah1 and tRNAs were enhanced with increasing concentrations of mitochondria used during the first step (Fig. 6A, lanes 2–5). Thus, mitochondria were able to export both (Fe–35S)int and 35Sint outside the organelle even in the absence of cytoplasm, allowing us to directly detect these intermediates. We know that the exported intermediates remained in active form, because when added to cytoplasm, they were utilized for their respective cytoplasmic processes. Importantly, cytoplasmic utilization of the intermediates occurred, even though mitochondria were no longer present. Note that radiolabeling of tRNAs or ΔN60 Yah1 during the second step was not due to free [35S]cysteine that was carried over from the first step, because no radiolabeled band can be detected when [35S]cysteine was directly added to cytoplasm containing apo-ΔN60 Yah1 in the absence of mitochondria (Fig. 1A, lane 7). Furthermore, unlabeled cysteine failed to inhibit radiolabeling of cytoplasmic ΔN60 Yah1 or tRNAs by exported and active intermediates in the SupExported fraction (Fig. S5A). However, as expected, unlabeled cysteine inhibited radiolabeling of both ΔN60 Yah1 or tRNAs in a concentration-dependent manner when added to a one-step assay mixture containing WT mitochondria, WT cytoplasm, apo-ΔN60 Yah1, nucleotides, iron, and [35S]cysteine at the same time (Fig. S5B). Because unlabeled cysteine was present together with [35S]cysteine during production of the intermediates, their 35S labeling was greatly diminished.
Figure 6.
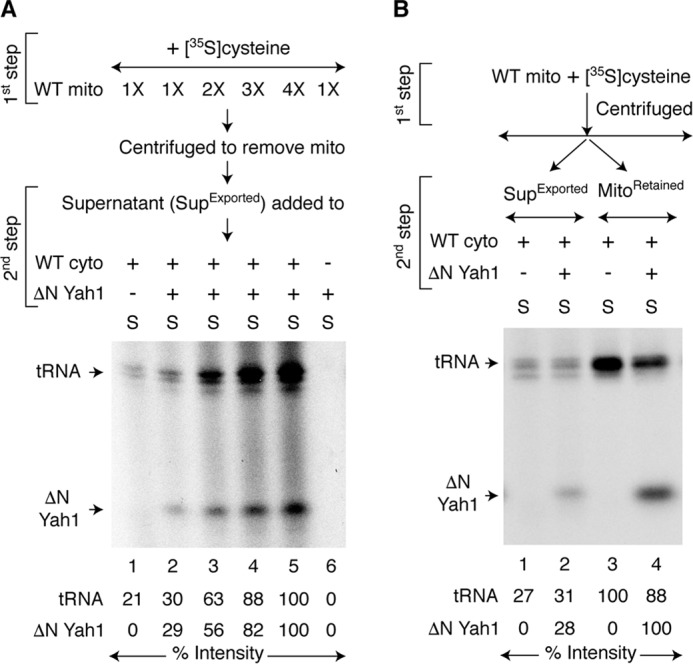
Comparison of (Fe–S)int export from mitochondria in the absence or presence of cytoplasm and apoprotein substrate. A, WT mitochondria (1× = 200 μg of proteins) were incubated with [35S]cysteine, nucleotides (1 mm GTP, 2 mm NADH, and 4 mm ATP), and iron (10 μm) at 30 °C for 30 min (first step). Mitochondria were removed by centrifugation. As indicated, the supernatant fractions (SupExported) were incubated with WT cytoplasm (200 μg of proteins) and/or apo-ΔN60 Yah1 at 30 °C for 30 min (second step) and analyzed. B, WT mitochondria (400 μg of proteins) were incubated with [35S]cysteine, nucleotides, and iron at 30 °C for 30 min (first step). The samples were centrifuged, separating the pellet (MitoRetained) and supernatant (SupExported) fractions. These fractions were then separately mixed with WT cytoplasm (200 μg of proteins) and, as indicated, apo-ΔN60 Yah1 protein. Samples containing MitoRetained fractions were supplemented with nucleotides and iron. After incubation at 30 °C for 30 min (second step), the samples were centrifuged, and the cytoplasm/supernatant (S) fractions were analyzed. WT mito, WT mitochondria; WT cyto, WT cytoplasm.
Mitochondrial export of (Fe–S)int is enhanced in the presence of cytoplasm and apoprotein substrate
Within cells, mitochondria likely export (Fe–S)int directly into the cytoplasm with apoprotein substrates. We therefore compared the mitochondrial export efficiency in the absence or presence of cytoplasm and apoprotein substrate in our in vitro assays as follows. WT mitochondria were incubated with [35S]cysteine, nucleotides, and iron in the absence of cytoplasm or apoprotein substrate (Fig. 6B, first step). After centrifugation, the supernatant fractions containing already exported intermediates (SupExported; Fig. 6B, lanes 1 and 2) were separated from mitochondrial pellets containing residual intermediates (called MitoRetained; Fig. 6B, lanes 3 and 4). These fractions were then individually supplemented with WT cytoplasm, incubated with or without added apo-ΔN60 Yah1 (Fig. 6B, second step), and analyzed. As judged by radiolabeling of ΔN60 Yah1, a relatively small portion of total exportable and active (Fe–35S)int was found to be released in the absence of cytoplasm and apoprotein substrate during the first step in the SupExported fraction. The rest was initially retained within mitochondria but was exported when supplemented with cytoplasm and apoprotein substrate during the second step (Fig. 6B, compare 35S-ΔN60 Yah1 signals in lanes 2 and 4). Thus, (Fe–35S)int export from mitochondria was more efficient in the presence of cytoplasm and ΔN60 Yah1 apoprotein substrate. Interestingly, 35Sint export also occurred more efficiently in the presence of cytoplasm with endogenous tRNA substrates as judged by radiolabeling of tRNAs (Fig. 6B, compare 35S-tRNA signals in lanes 1 and 3, and also lanes 2 and 4).
Mitochondrial sensing of the cytoplasmic need for iron–sulfur cluster assembly
We then wondered whether the presence of apoprotein substrate in the cytoplasm is necessary for efficient mitochondrial export of (Fe–35S)int, reflecting the cytoplasmic need for iron–sulfur cluster assembly. To test this possibility, two sets of reactions were performed with prelabeled WT mitochondria (35S-PL) that contained already formed 35Sint and (Fe–35S)int. In one case (Fig. 7, area to the left of the dotted line), these mitochondria were incubated with WT cytoplasm and apo-ΔN60 Yah1 at the same time. A strong 35S labeling of endogenous tRNAs, as well as added ΔN60 Yah1, was observed, implying that both 35Sint and (Fe–35S)int were efficiently exported from mitochondria (Fig. 7, lanes 1–3). In another case (Fig. 7, area to the right of the dotted line), WT mitochondria (35S-PL) were incubated with WT cytoplasm, but no apo-ΔN60 Yah1 was added at this stage. Again, 35Sint was efficiently exported and was utilized for tRNA thiolation (Fig. 7, lanes 4–6). What about (Fe–35S)int export under these conditions? Surprisingly, 35S labeling of ΔN60 Yah1 was markedly weaker in an additional step in which the apoprotein substrate was added to cytoplasm after removal of mitochondria (Fig. 7, lanes 4–6). The implication is that because the apoprotein substrate ΔN60 Yah1 was not present at the time the mitochondrial export of (Fe–35S)int was taking place, the efficiency of the process was compromised. Interestingly, the 35S labeling of ΔN60 Yah1 was completely unchanged whether the apoprotein was added immediately to exported (Fe–35S)int or after incubation of exported (Fe–35S)int at 30 °C for 10–30 min (Fig. S6). These results further substantiate that the weak radiolabeling of ΔN60 Yah1 (Fig. 7, lanes 4–6) was most likely due to low amounts of (Fe–35S)int that was exported from mitochondria in the absence of apoprotein substrate rather than progressive destabilization or loss of activity of the exported intermediate prior to the addition of apoprotein substrate. Mitochondria may sense the cytoplasmic demand for iron–sulfur cluster biogenesis and export (Fe–S)int as needed.
Figure 7.
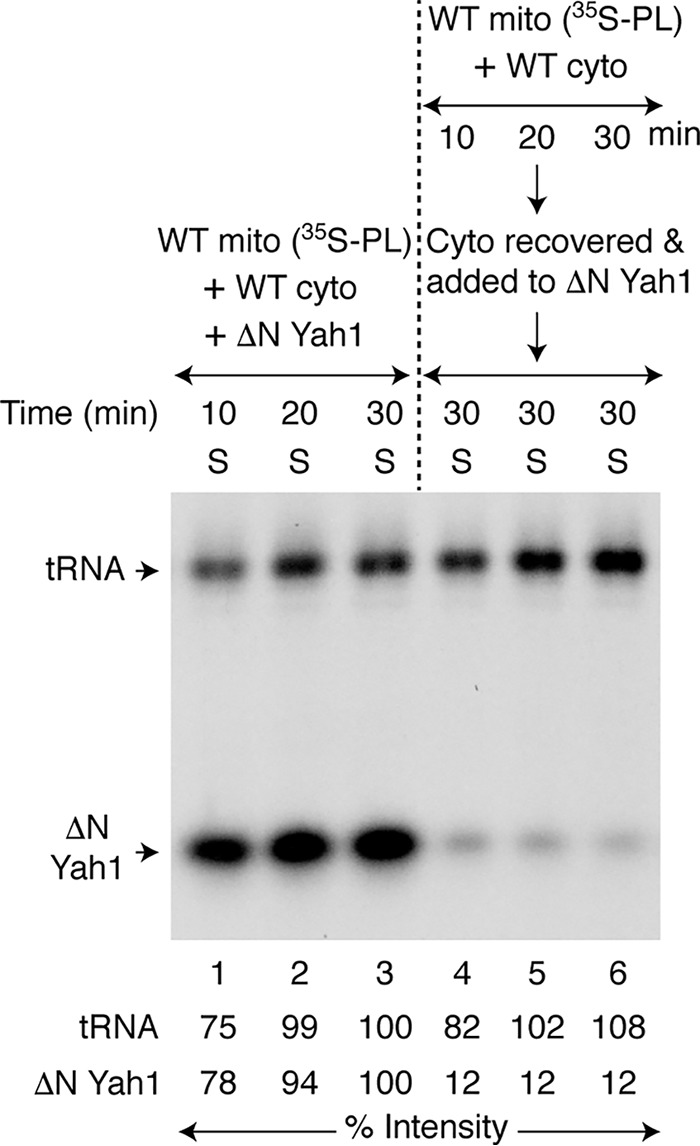
Mitochondrial sensing of the cytoplasmic need for iron–sulfur cluster assembly. WT mitochondria (400 μg of proteins) were prelabeled with [35S]cysteine in the presence of nucleotides (1 mm GTP, 2 mm NADH, and 4 mm ATP) and iron (10 μm) at 30 °C for 20 min. Mitochondria (35S-PL) were recovered and used for two sets of reactions. Area to the left of the dotted line (lanes 1–3): mitochondria (35S-PL) were incubated with WT cytoplasm (200 μg of proteins), apo-ΔN60 Yah1, nucleotides, and iron at 30 °C for 10–30 min. After centrifugation, the cytoplasm/supernatant (S) fractions were analyzed. Area to the right of the dotted line (lanes 4–6): mitochondria (35S-PL) were incubated with WT cytoplasm, nucleotides, and iron at 30 °C for 10–30 min. After removal of mitochondria by centrifugation, the cytoplasm/supernatant (S) fractions were supplemented with apo-ΔN60 Yah1, incubated again at 30 °C for 30 min, and analyzed. WT mito, WT mitochondria; WT cyto, WT cytoplasm.
Discussion
In eukaryotic cells, the biogenesis of metal cofactors often involves compartment-specific functions. Here we have ascertained a direct and critical role of mitochondria in cytoplasmic iron–sulfur cluster assembly. Using mitochondria and cytoplasm isolated from yeast cells, we show that cytoplasm by itself cannot make iron–sulfur clusters without a vital contribution from mitochondria. Specifically, mitochondria synthesize an intermediate, called (Fe–S)int, and export it to the cytoplasm. The exported intermediate then provides both iron and sulfur components for the assembly of cytoplasmic iron–sulfur clusters by the CIA machinery. The biochemical assays described here are unique, allowing sequential dissection of mitochondrial generation of the intermediate, its export to the cytoplasm and finally, its utilization for cytoplasmic iron–sulfur cluster assembly.
The exported (Fe–S)int species detected here is distinct from the previously described Sint species. Recall that mitochondria generate and export Sint for cytoplasmic tRNA thiolation (39, 40). Sint synthesis depends on Nfs1 cysteine desulfurase and Isu1/2 scaffold proteins but not Ssq1 chaperone (Fig. 2). On the other hand, mitochondrial production of (Fe–S)int requires Nfs1, Isu1/2, and Ssq1. Thus, (Fe–S)int synthesis requires additional processing by the mitochondrial ISC machinery. Interestingly, formation of both Sint and (Fe–S)int requires Isu1/2 proteins (Fig. 2B). However, mitochondria containing the Isu1 (M141E) mutant protein (WT Isu1 depleted, Isu2 absent) efficiently generate Sint but completely fail to produce (Fe–S)int. Consequently, these mutant mitochondria promote cytoplasmic tRNA thiolation but not iron–sulfur cluster assembly (Fig. 2C). The location of the substitution in the Isu1 protein, (131SLPPVKLHCSM(E)141) adjacent to the LPPVK chaperone-binding site (50), suggests a scenario according to which the mutation inhibits interaction with the Ssq1 Hsp70 chaperone, thereby interfering with (Fe–S)int production. Indeed, Isu1 (M141E) mitochondria and Ssq1-depleted mitochondria strongly resemble each other in terms of their inability to promote both mitochondrial and cytoplasmic iron–sulfur clusters, whereas both support cytoplasmic tRNA thiolation (Fig. 2, C and D, and Fig. S2, B and C). Furthermore, Sint generation does not require ATP addition or ATP hydrolysis, and it is produced even in the presence of ATPγS (39) because the process occurs independent of Ssq1 (Fig. 2D). In contrast, (Fe–S)int production in mitochondria is strictly dependent on ATP addition (Fig. 3B), and ATP is likely hydrolyzed by the Ssq1 ATPase (Fig. 2D). Additionally, whereas (Fe–S)int production is greatly stimulated by the addition of iron, Sint is generated regardless of added iron (Fig. 4A). Thus, mitochondrial ISC generates two distinct intermediates: Sint for cytoplasmic tRNA thiolation and (Fe–S)int for cytoplasmic iron–sulfur cluster assembly. Whereas the pathway for Sint formation appears to branch off from the general ISC pathway at the Isu1/2 site, the pathway for formation of (Fe–S)int may bifurcate downstream of Ssq1 (Fig. 8). Implied here is that Isu1/2 is a dual function protein. It may serve as a sulfur relay in the transfer of persulfide sulfur from Nfs1 to Sint, which is then exported to the cytoplasm. Isu1/2 also acts as a scaffold for a [2Fe–2S] cluster intermediate, which is transferred by Ssq1 and its cochaperone Jac1 to downstream components such as Grx5, ISA (iron–sulfur assembly) complex, and other proteins for use in de novo iron–sulfur cluster synthesis for aconitase and other mitochondrial proteins (1). During this process, (Fe–S)int is formed and is subsequently exported to the cytoplasm (Fig. 8).
Figure 8.
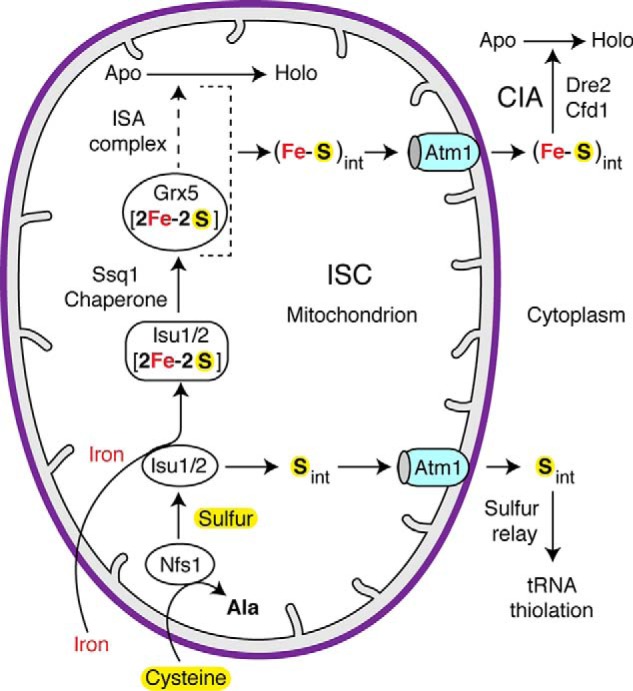
A model for mitochondrial contributions to cytoplasmic tRNA thiolation and iron–sulfur cluster assembly. The mitochondrial ISC machinery synthesizes two different intermediates: one for cytoplasmic tRNA thiolation (Sint) and another for cytoplasmic iron–sulfur cluster assembly ((Fe–S)int). The Nfs1 cysteine desulfurase abstracts sulfur from the amino acid cysteine, forms a persulfide, and donates the persulfide sulfur to Isu1/2. This sulfur then leaves the conventional pathway for mitochondrial iron–sulfur cluster assembly and is used for production of Sint in a process that does not require Isu1/2 downstream components such as Ssq1 of the ISC machinery. Sint is subsequently exported to cytoplasm by the Atm1 transporter and is utilized for tRNA thiolation by a sulfur relay process. In the conventional ISC pathway, Isu1/2 forms a [2Fe–2S] cluster intermediate utilizing the persulfide sulfur from Nfs1 and mitochondrial iron from an unknown source. Ssq1 and its cochaperone Jac1 promote transfer of the Isu1/2-bound [2Fe–2S] cluster to downstream components such as Grx5 and the ISA (iron–sulfur assembly) complex. A [4Fe-4S] cluster is formed, which is inserted into apoproteins such as aconitase. During this process, the pathway for (Fe–S)int production diverges downstream of Ssq1. As in the case for Sint, (Fe–S)int is also exported by Atm1. Once exported, (Fe–S)int is utilized for cytoplasmic iron–sulfur cluster assembly by the CIA machinery involving Dre2 and Cfd1.
A surprising and novel finding is that the iron required for cytoplasmic iron–sulfur cluster assembly originates in mitochondria and is exported as part of (Fe–S)int. Iron in the cytoplasm cannot be directly used for cytoplasmic iron–sulfur cluster biogenesis. For example, mitochondria by themselves are able to generate (Fe–35S)int when they are incubated with [35S]cysteine in the presence of both iron and nucleotides. These mitochondria containing already formed (Fe–35S)int promote strong cytoplasmic [2Fe-235S] cluster assembly with no further requirement for iron addition, and added iron has no effect on the biosynthetic process (Fig. 4B). Interestingly, isolated mitochondria are able to import added iron and store it in a usable form in a nucleotide-dependent manner. When supplemented with [35S]cysteine, mitochondria efficiently utilize the stored iron for (Fe–35S)int production and promote cytoplasmic iron–sulfur cluster assembly (Fig. 4C). Thus, it is the mitochondrial iron that is specifically needed for cytoplasmic iron–sulfur cluster biogenesis. The exported (Fe–S)int intermediate provides both iron and sulfur species required for cytoplasmic iron–sulfur cluster assembly. This notion is further substantiated by the observation that 35Sint (i.e. the intermediate required for tRNA thiolation) cannot be converted to (Fe–35S)int by the cytoplasm for iron–sulfur cluster assembly. For example, Isu1 (M141E) mitochondria (Fig. 2C) or Ssq1↓ mitochondria (Fig. 2D) produce and export 35Sint, which is efficiently utilized by the WT cytoplasm for tRNA thiolation but not for iron–sulfur cluster assembly even in the presence of added iron. Note that our assays allow mixing of mitochondria and cytoplasm from different sources, thereby specifically pinpointing compartmental contributions or defects.
An interesting observation is that (Fe–S)int and Sint are two distinct species in terms of their productions and activities, and yet both are exported by Atm1 (Fig. 5). The ABC transporters in mitochondria may have a broad substrate specificity. For example, the plant Arabidopsis thaliana ATM3 is orthologous to yeast Atm1 (52). In addition to being involved in maturation of extramitochondrial iron–sulfur proteins (53), ATM3 also appears to transport cyclic pyranopterin monophosphate from mitochondria to the cytoplasm for molybdenum cofactor synthesis (54). It is possible that (Fe–S)int and Sint intermediates might have a common component that is recognized by Atm1. For example, both intermediates might contain some form of GSH derivative, and this would be consistent with Atm1's ability to bind GSH (36).
Underlying all these experiments was a technical challenge: the exported intermediates were rapidly and efficiently used in the cytoplasm, making detection extremely difficult. The key to detecting active (Fe–S)int in isolation was to perform production and export of the intermediate in the absence of cytoplasm and apoprotein substrate. After mitochondria were removed by centrifugation, the resulting supernatant/exported material was found to contain (Fe–S)int in active form because it was utilized by cytoplasm for iron–sulfur cluster assembly (Fig. 6A). Thus, once exported, (Fe–S)int by itself was able to promote the cytoplasmic process with no further help from mitochondria. Exported Sint behaved similarly for tRNA thiolation. Although detectable, the export process occurred inefficiently in the absence of cytoplasm and apoprotein substrate. A major portion of (Fe–S)int (or Sint) remained trapped within mitochondria but was exported and utilized with the addition of cytoplasm and apoprotein substrate (Fig. 6B). Presence of an apoprotein substrate in the cytoplasm markedly enhanced mitochondrial export of already formed (Fe–S)int (Fig. 7).
Under physiological conditions within the cell, the mitochondria–cytoplasm interaction is likely to be regulated. We propose that mitochondria can synthesize (Fe–S)int and store it. They then sense the cytoplasmic need for iron–sulfur cluster assembly and export (Fe–S)int via Atm1 accordingly. Once the export process is initiated, CIA component(s), other protein(s), and/or small molecule(s) in the cytoplasm interact with exiting (Fe–S)int. Such an interaction not only renders the export process unidirectional and more efficient but also makes the utilization process more productive.
The mitochondrial ISC, Atm1 exporter, and CIA components are conserved from yeast to humans (3, 55). Importantly, iron–sulfur proteins in the cytoplasm/nucleus are essential for cell viability because they play vital roles in crucial processes of life including protein synthesis, DNA replication, and DNA repair. Malfunction of iron–sulfur enzymes (e.g. DNA polymerases, helicases, primases, and endonucleases) leads to genomic instability, a hallmark of aging and cancer (2, 56, 57). Furthermore, Atm1 is orthologous to the human protein ABCB7 (58), and mutations in ABCB7 cause the human disease XLSA/A (X-linked sideroblastic anemia and ataxia syndrome), in which failure to export the substrate(s) causes iron accumulation in red cell precursors and consequent cell death (59). Defining the roles of human mitochondrial ISC and the ABCB7 exporter in cytoplasmic/nuclear iron–sulfur cluster assembly will certainly be useful for better understanding of these diseases. A similar mitochondrial regulation of cytoplasmic iron–sulfur cluster assembly as described here in yeast might also occur in human cells.
Experimental procedures
Yeast strains, culture conditions, and isolation of mitochondria and cytoplasm
Yeast strains used in this study are described in Table S1. Two new strains were generated for this study. For Ssq1 depletion, a shuffle strain was generated in the YPH501 background, followed by insertion of a GAL1-SSQ1 regulated construct in the vector pEMBLyex4i, which was targeted in its entirety to the ura3–52 locus by digestion with StuI. The covering plasmid, pRS318-SSQ1, was removed by counterselection with cycloheximide, and the sole remaining copy of SSQ1 remained under control of the GAL1 promoter (strain 71–1). For GAL1-ATM1 (strain 124–25), the parental strain BY4741 was subjected to promoter swap at the ATM1 locus, using the method described by Longtine et al. (60).
All cells were grown at 30 °C. Yeast extract–peptone–adenine–dextrose (YPAD) medium and synthetic complete (SC) medium containing raffinose, with or without dextrose or galactose, were made as described (61). Matched WT and mutant S. cerevisiae strains were examined. The nfs1-14 mutant (strain 68-8) was grown in SC, 2% raffinose, 0.5% dextrose medium. Isu1 depletion of GAL1-ISU1/Δisu2 (strain 115-10) and Dre2 depletion of GAL1-DRE2 (strain 105-59) were performed as described (39). For Isu1 depletion of GAL1-ISU1/Δisu2/YCplac22-Isu1 (M141E) (strain 114-74), an overnight culture in YPAD was diluted 200-fold with the same medium, and the cells were grown for 20 h. For Ssq1 depletion of GAL1-SSQ1 (strain 71–1), an overnight culture in SC, 2% raffinose was diluted 40-fold with the same medium and cells were grown for 20 h. For Atm1 depletion of GAL1-ATM1 (strain 124–25), the cells were initially grown in SC, 2% raffinose, 0.5% galactose (100 ml) for 20 h. The cells were harvested, washed, and then grown in SC, 2% raffinose, 0.5% dextrose medium (2 liter) for 20 h. For Cfd1 depletion of GAL1-CFD1 (strain 98–39), the cells were initially grown in SC, 2% raffinose (100 ml) for 24 h. The cells were harvested, washed, and then grown in SC, 2% raffinose, 0.5% dextrose medium (2 liter) for 20 h. Mitochondria and cytoplasm were isolated from various strains as described (22, 23, 39).
Bacterial expression and purification of apoprotein substrates
In S. cerevisiae, ferredoxin (Yah1) is a mitochondrial matrix protein that contains a [2Fe–2S] cluster. The Yah1 precursor protein (pYah1) with a C-terminal His6 tag was expressed in bacteria, purified, and used as a substrate for mitochondrial iron–sulfur cluster assembly (22, 23) (Fig. S1, A and B). To be able to use the ferredoxin specifically as an indicator for cytoplasmic iron–sulfur cluster biogenesis, the N-terminal 60 amino acids including the mitochondrial targeting signal were removed from the Yah1 precursor protein, generating ΔN60 Yah1. Briefly, the PCR product NdeI-ΔN60 Yah1-XhoI was digested with NdeI and XhoI and cloned into the same sites of pET21b (23). This introduces a His6 tag in frame at the C terminus of the protein. BL21 (DE3) cells carrying the plasmid pET21b/ΔN60 Yah1-His6 were grown at 37 °C in Luria–Bertani medium containing 100 μg/ml ampicillin to an A600 of ∼0.8. Protein expression was carried out in the presence of 0.5 mm isopropyl-1-thio-β-d-galactopyranoside for 20 h at 20 °C. Under these conditions, the expressed protein was found mostly in soluble form. The cells were harvested, and washed with buffer A (20 mm Tris/HCl, pH 7.5, 0.25 m NaCl, 1 mm PMSF). The cells were resuspended in buffer A and disrupted using a probe sonicator (Branson Sonifier 450). Cell lysates were centrifuged at 15,000 × g for 30 min at 4 °C, and the supernatant fraction was incubated with nickel–nitrilotriacetic acid–agarose by end-over-end mixing for 3 h at 4 °C. The resin was washed with buffer A containing 5 mm imidazole, and bound proteins were eluted with 20 mm Tris/HCl, pH 7.5, containing 0.4 m imidazole, 0.15 m NaCl, 10% glycerol, and 1 mm PMSF and stored in aliquots at −80 °C. As needed, the purified protein (25 μg) was treated with 0.2 n HCl in a final volume of 125 μl for 1 min on ice and then neutralized with 1 m Tris/HCl, pH 8.0 (75 μl). The apo-ΔN60 Yah1 thus generated was supplemented with 10 mm tris(2-carboxyethyl)phosphine, pH 7.5 and used as a substrate for cytoplasmic iron–sulfur cluster assembly. C. reinhardtii ferredoxin (PetF) (45) was similarly expressed in soluble form in BL21DE3 cells carrying the plasmid pET21b/PetF-His6. The purified protein was converted to the apo form by acid treatment as described above and was used as another indicator protein for cytoplasmic iron–sulfur cluster biogenesis in some experiments (Fig. S1, A and D).
Biochemical assays for cytoplasmic iron–sulfur cluster assembly
A typical one-step reaction mixture (100 μl) contained isolated and intact mitochondria (100–400 μg of proteins), isolated cytoplasm (100–200 μg of proteins), apo-ΔN60 Yah1 (0.5–1 μg), [35S]cysteine (5–10 μCi; 1075 Ci/mmol), nucleotides (1 mm GTP, 2 mm NADH, and 4 mm ATP), iron as ferrous ascorbate (10 μm), KOAc (40 mm), Mg(OAc)2 (10 mm), and tris(2-carboxyethyl)phosphine (1 mm) in HS buffer (20 mm Hepes/KOH, pH 7.5, 0.6 m sorbitol). The samples were incubated at 30 °C for 10–30 min. The reaction mixtures were centrifuged at 15,000 × g for 10 min at 4 °C, and the resulting pellet (P, mitochondria) and supernatant (S, cytoplasm) fractions were processed as follows.
The mitochondrial pellets were washed with ice-cold HS buffer and resuspended in 35 μl of 50 mm Tris/HCl, pH 8.0, containing 1 mm PMSF. Mitochondrial membranes were ruptured as previously described (22, 23, 39). After centrifugation at 15,000 × g for 30 min at 4 °C, the supernatant fractions containing soluble matrix proteins were supplemented with 10 mm DTT and were used for gel analysis. The cytoplasmic supernatant fractions (see above) were subjected to precipitation with 67% ammonium sulfate for 2–3 h on ice. The samples were centrifuged at 15,000 × g for 45 min at 4 °C. The pellets thus obtained were dissolved in 35 μl of 50 mm Tris/HCl, pH 8.0, containing 1 mm PMSF and 10 mm DTT and were used for gel analysis. The samples were analyzed by native PAGE followed by autoradiography (39). Numerous variations of these assays are described in the figure legends.
Radiolabeled protein and tRNA bands were quantified by densitometric analysis of autoradiographs using the National Institutes of Health ImageJ software. The data for both cytoplasmic iron–sulfur cluster assembly and cytoplasmic tRNA thiolation are shown in all of the figures. Thus, the data as presented are internally controlled. The reproducibility of different assays was confirmed with biological replicates using different batches of mitochondria and/or cytoplasm isolated from various yeast strains and using different sets of bacterial expressed and purified apoprotein substrates.
Author contributions
A. K. P. and J. P. data curation; A. K. P., J. P., and D. P. formal analysis; A. K. P., J. P., and D. P. investigation; A. K. P., J. P., A. D., and D. P. methodology; A. D. and D. P. resources; A. D. and D. P. funding acquisition; A. D. writing-review and editing; D. P. conceptualization; D. P. supervision; D. P. validation; D. P. writing-original draft; D. P. project administration.
Supplementary Material
Acknowledgments
We thank M. M. Balamurali, Arnab K. Ghosh, and Anjaneyulu Murari for participation at different stages of this work. We also thank Paul A. Lindahl for critical evaluation of the data.
This work was supported by National Institutes of Health Grant R01 GM107542 (to D. P. and A. D.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Table S1 and Figs. S1–S6.
- Sint
- sulfur intermediate
- (Fe–S)int
- iron–sulfur intermediate
- ΔN60 Yah1
- the N-terminal 60 amino acids including the mitochondrial targeting signal removed from the Yah1 precursor protein
- ISC
- mitochondrial iron–sulfur cluster machinery
- CIA
- cytoplasmic iron–sulfur protein assembly machinery
- PMSF
- phenylmethylsulfonyl fluoride.
References
- 1. Melber A., and Winge D. R. (2018) Steps toward understanding mitochondrial Fe/S cluster biogenesis. Methods Enzymol. 599, 265–292 10.1016/bs.mie.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 2. Lill R., Dutkiewicz R., Freibert S. A., Heidenreich T., Mascarenhas J., Netz D. J., Paul V. D., Pierik A. J., Richter N., Stümpfig M., Srinivasan V., Stehling O., and Mühlenhoff U. (2015) The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron–sulfur proteins. Eur. J. Cell Biol. 94, 280–291 10.1016/j.ejcb.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 3. Rouault T. A. (2015) Mammalian iron-sulphur proteins: novel insights into biogenesis and function. Nat. Rev. Mol. Cell Biol. 16, 45–55 10.1038/nrm3909 [DOI] [PubMed] [Google Scholar]
- 4. Li L., Jia X., Ward D. M., and Kaplan J. (2011) Yap5 protein-regulated transcription of the TYW1 gene protects yeast from high iron toxicity. J. Biol. Chem. 286, 38488–38497 10.1074/jbc.M111.286666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dlouhy A. C., and Outten C. E. (2013) The iron metallome in eukaryotic organisms. Met Ions Life Sci. 12, 241–278 10.1007/978-94-007-5561-1_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li J., Kogan M., Knight S. A., Pain D., and Dancis A. (1999) Yeast mitochondrial protein, Nfs1p, coordinately regulates iron–sulfur cluster proteins, cellular iron uptake, and iron distribution. J. Biol. Chem. 274, 33025–33034 10.1074/jbc.274.46.33025 [DOI] [PubMed] [Google Scholar]
- 7. Boniecki M. T., Freibert S. A., Mühlenhoff U., Lill R., and Cygler M. (2017) Structure and functional dynamics of the mitochondrial Fe/S cluster synthesis complex. Nat. Commun. 8, 1287 10.1038/s41467-017-01497-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cory S. A., Van Vranken J. G., Brignole E. J., Patra S., Winge D. R., Drennan C. L., Rutter J., and Barondeau D. P. (2017) Structure of human Fe–S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl-ACP-ISD11 interactions. Proc. Natl. Acad. Sci. U.S.A. 114, E5325–E5334 10.1073/pnas.1702849114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Vranken J. G., Jeong M. Y., Wei P., Chen Y. C., Gygi S. P., Winge D. R., and Rutter J. (2016) The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis. Elife 5, e17828 10.7554/eLife.17828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pandey A., Yoon H., Lyver E. R., Dancis A., and Pain D. (2012) Identification of a Nfs1p-bound persulfide intermediate in Fe–S cluster synthesis by intact mitochondria. Mitochondrion 12, 539–549 10.1016/j.mito.2012.07.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandey A., Golla R., Yoon H., Dancis A., and Pain D. (2012) Persulfide formation on mitochondrial cysteine desulfurase: enzyme activation by a eukaryote-specific interacting protein and Fe–S cluster synthesis. Biochem. J. 448, 171–187 10.1042/BJ20120951 [DOI] [PubMed] [Google Scholar]
- 12. Pandey A., Gordon D. M., Pain J., Stemmler T. L., Dancis A., and Pain D. (2013) Frataxin directly stimulates mitochondrial cysteine desulfurase by exposing substrate-binding sites, and a mutant Fe–S cluster scaffold protein with frataxin-bypassing ability acts similarly. J. Biol. Chem. 288, 36773–36786 10.1074/jbc.M113.525857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerber J., Mühlenhoff U., and Lill R. (2003) An interaction between frataxin and Isu1/Nfs1 that is crucial for Fe/S cluster synthesis on Isu1. EMBO Rep. 4, 906–911 10.1038/sj.embor.embor918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang T., and Craig E. A. (2008) Binding of yeast frataxin to the scaffold for Fe–S cluster biogenesis, Isu. J. Biol. Chem. 283, 12674–12679 10.1074/jbc.M800399200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bridwell-Rabb J., Fox N. G., Tsai C. L., Winn A. M., and Barondeau D. P. (2014) Human frataxin activates Fe–S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry 53, 4904–4913 10.1021/bi500532e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colin F., Martelli A., Clémancey M., Latour J. M., Gambarelli S., Zeppieri L., Birck C., Page A., Puccio H., and Ollagnier de Choudens S. (2013) Mammalian frataxin controls sulfur production and iron entry during de novo Fe4S4 cluster assembly. J. Am. Chem. Soc. 135, 733–740 10.1021/ja308736e [DOI] [PubMed] [Google Scholar]
- 17. Cook J. D., Kondapalli K. C., Rawat S., Childs W. C., Murugesan Y., Dancis A., and Stemmler T. L. (2010) Molecular details of the yeast frataxin-Isu1 interaction during mitochondrial Fe–S cluster assembly. Biochemistry 49, 8756–8765 10.1021/bi1008613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ranatunga W., Gakh O., Galeano B. K., Smith D.,Y. 4th, Söderberg C. A., Al-Karadaghi S., Thompson J. R., and Isaya G. (2016) Architecture of the yeast mitochondrial iron–sulfur cluster assembly machinery: the sub-complex formed by the iron donor, yFH1 protein, and the scaffold, Isu1 protein. J. Biol. Chem. 291, 10378–10398 10.1074/jbc.M115.712414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pain J., Balamurali M. M., Dancis A., and Pain D. (2010) Mitochondrial NADH kinase, Pos5p, is required for efficient iron–sulfur cluster biogenesis in Saccharomyces cerevisiae. J. Biol. Chem. 285, 39409–39424 10.1074/jbc.M110.178947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Webert H., Freibert S. A., Gallo A., Heidenreich T., Linne U., Amlacher S., Hurt E., Mühlenhoff U., Banci L., and Lill R. (2014) Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nat. Commun. 5, 5013 10.1038/ncomms6013 [DOI] [PubMed] [Google Scholar]
- 21. Outten C. E., and Culotta V. C. (2003) A novel NADH kinase is the mitochondrial source of NADPH in Saccharomyces cerevisiae. EMBO J. 22, 2015–2024 10.1093/emboj/cdg211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amutha B., Gordon D. M., Gu Y., Lyver E. R., Dancis A., and Pain D. (2008) GTP is required for iron–sulfur cluster biogenesis in mitochondria. J. Biol. Chem. 283, 1362–1371 10.1074/jbc.M706808200 [DOI] [PubMed] [Google Scholar]
- 23. Amutha B., Gordon D. M., Dancis A., and Pain D. (2009) Nucleotide-dependent iron–sulfur cluster biogenesis of endogenous and imported apoproteins in isolated intact mitochondria. Methods Enzymol. 456, 247–266 10.1016/S0076-6879(08)04414-5 [DOI] [PubMed] [Google Scholar]
- 24. Schilke B., Williams B., Knieszner H., Pukszta S., D'Silva P., Craig E. A., and Marszalek J. (2006) Evolution of mitochondrial chaperones utilized in Fe–S cluster biogenesis. Curr. Biol. 16, 1660–1665 10.1016/j.cub.2006.06.069 [DOI] [PubMed] [Google Scholar]
- 25. Uzarska M. A., Dutkiewicz R., Freibert S. A., Lill R., and Mühlenhoff U. (2013) The mitochondrial Hsp70 chaperone Ssq1 facilitates Fe/S cluster transfer from Isu1 to Grx5 by complex formation. Mol. Biol. Cell 24, 1830–1841 10.1091/mbc.e12-09-0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hausmann A., Aguilar Netz D. J., Balk J., Pierik A. J., Mühlenhoff U., and Lill R. (2005) The eukaryotic P loop NTPase Nbp35: an essential component of the cytosolic and nuclear iron–sulfur protein assembly machinery. Proc. Natl. Acad. Sci. U.S.A. 102, 3266–3271 10.1073/pnas.0406447102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Netz D. J., Pierik A. J., Stümpfig M., Bill E., Sharma A. K., Pallesen L. J., Walden W. E., and Lill R. (2012) A bridging [4Fe-4S] cluster and nucleotide binding are essential for function of the Cfd1-Nbp35 complex as a scaffold in iron–sulfur protein maturation. J. Biol. Chem. 287, 12365–12378 10.1074/jbc.M111.328914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Netz D. J., Pierik A. J., Stümpfig M., Mühlenhoff U., and Lill R. (2007) The Cfd1-Nbp35 complex acts as a scaffold for iron–sulfur protein assembly in the yeast cytosol. Nat. Chem. Biol. 3, 278–286 10.1038/nchembio872 [DOI] [PubMed] [Google Scholar]
- 29. Roy A., Solodovnikova N., Nicholson T., Antholine W., and Walden W. E. (2003) A novel eukaryotic factor for cytosolic Fe–S cluster assembly. EMBO J. 22, 4826–4835 10.1093/emboj/cdg455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y., Lyver E. R., Nakamaru-Ogiso E., Yoon H., Amutha B., Lee D. W., Bi E., Ohnishi T., Daldal F., Pain D., and Dancis A. (2008) Dre2, a conserved eukaryotic Fe/S cluster protein, functions in cytosolic Fe/S protein biogenesis. Mol. Cell. Biol. 28, 5569–5582 10.1128/MCB.00642-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Netz D. J., Stümpfig M., Doré C., Mühlenhoff U., Pierik A. J., and Lill R. (2010) Tah18 transfers electrons to Dre2 in cytosolic iron–sulfur protein biogenesis. Nat. Chem. Biol. 6, 758–765 10.1038/nchembio.432 [DOI] [PubMed] [Google Scholar]
- 32. Balk J., Pierik A. J., Netz D. J., Mühlenhoff U., and Lill R. (2004) The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron–sulphur proteins. EMBO J. 23, 2105–2115 10.1038/sj.emboj.7600216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gari K., León Ortiz A. M., Borel V., Flynn H., Skehel J. M., and Boulton S. J. (2012) MMS19 links cytoplasmic iron–sulfur cluster assembly to DNA metabolism. Science 337, 243–245 10.1126/science.1219664 [DOI] [PubMed] [Google Scholar]
- 34. Srinivasan V., Netz D. J., Webert H., Mascarenhas J., Pierik A. J., Michel H., and Lill R. (2007) Structure of the yeast WD40 domain protein Cia1, a component acting late in iron–sulfur protein biogenesis. Structure 15, 1246–1257 10.1016/j.str.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 35. Kispal G., Csere P., Prohl C., and Lill R. (1999) The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 18, 3981–3989 10.1093/emboj/18.14.3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Srinivasan V., Pierik A. J., and Lill R. (2014) Crystal structures of nucleotide-free and glutathione-bound mitochondrial ABC transporter Atm1. Science 343, 1137–1140 10.1126/science.1246729 [DOI] [PubMed] [Google Scholar]
- 37. Schaedler T. A., Thornton J. D., Kruse I., Schwarzländer M., Meyer A. J., van Veen H. W., and Balk J. (2014) A conserved mitochondrial ATP-binding cassette transporter exports glutathione polysulfide for cytosolic metal cofactor assembly. J. Biol. Chem. 289, 23264–23274 10.1074/jbc.M114.553438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li J., and Cowan J. A. (2015) Glutathione-coordinated [2Fe–2S] cluster: a viable physiological substrate for mitochondrial ABCB7 transport. Chem. Commun. (Camb.) 51, 2253–2255 10.1039/c4cc09175b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pandey A., Pain J., Dziuba N., Pandey A. K., Dancis A., Lindahl P. A., and Pain D. (2018) Mitochondria export sulfur species required for cytosolic tRNA thiolation. Cell Chem. Biol. 25, 738–748.e3 10.1016/j.chembiol.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Braymer J. J., and Winge D. R. (2018) Sulfur from within: cytosolic tRNA thiouridinylation. Cell Chem. Biol. 25, 645–647 10.1016/j.chembiol.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 41. Nakai Y., Nakai M., and Yano T. (2017) Sulfur modifications of the wobble U34 in tRNAs and their intracellular localization in eukaryotic cells. Biomolecules 7, E17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rezgui V. A., Tyagi K., Ranjan N., Konevega A. L., Mittelstaet J., Rodnina M. V., Peter M., and Pedrioli P. G. (2013) tRNA tKUUU, tQUUG, and tEUUC wobble position modifications fine-tune protein translation by promoting ribosome A-site binding. Proc. Natl. Acad. Sci. U.S.A. 110, 12289–12294 10.1073/pnas.1300781110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nedialkova D. D., and Leidel S. A. (2015) Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell 161, 1606–1618 10.1016/j.cell.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Urbonavicius J., Qian Q., Durand J. M., Hagervall T. G., and Björk G. R. (2001) Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 20, 4863–4873 10.1093/emboj/20.17.4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Winkler M., Hemschemeier A., Jacobs J., Stripp S., and Happe T. (2010) Multiple ferredoxin isoforms in Chlamydomonas reinhardtii: their role under stress conditions and biotechnological implications. Eur. J. Cell Biol. 89, 998–1004 10.1016/j.ejcb.2010.06.018 [DOI] [PubMed] [Google Scholar]
- 46. Paraskevopoulou C., Fairhurst S. A., Lowe D. J., Brick P., and Onesti S. (2006) The elongator subunit Elp3 contains a Fe4S4 cluster and binds S-adenosylmethionine. Mol. Microbiol. 59, 795–806 10.1111/j.1365-2958.2005.04989.x [DOI] [PubMed] [Google Scholar]
- 47. Liu Y., Vinyard D. J., Reesbeck M. E., Suzuki T., Manakongtreecheep K., Holland P. L., Brudvig G. W., and Söll D. (2016) A [3Fe-4S] cluster is required for tRNA thiolation in archaea and eukaryotes. Proc. Natl. Acad. Sci. U.S.A. 113, 12703–12708 10.1073/pnas.1615732113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rietzschel N., Pierik A. J., Bill E., Lill R., and Mühlenhoff U. (2015) The basic leucine zipper stress response regulator Yap5 senses high-iron conditions by coordination of [2Fe–2S] clusters. Mol. Cell. Biol. 35, 370–378 10.1128/MCB.01033-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoon H., Knight S. A., Pandey A., Pain J., Turkarslan S., Pain D., and Dancis A. (2015) Turning Saccharomyces cerevisiae into a frataxin-independent organism. PLoS Genet. 11, e1005135 10.1371/journal.pgen.1005135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dutkiewicz R., Schilke B., Cheng S., Knieszner H., Craig E. A., and Marszalek J. (2004) Sequence-specific interaction between mitochondrial Fe–S scaffold protein Isu and Hsp70 Ssq1 is essential for their in vivo function. J. Biol. Chem. 279, 29167–29174 10.1074/jbc.M402947200 [DOI] [PubMed] [Google Scholar]
- 51. Leighton J., and Schatz G. (1995) An ABC transporter in the mitochondrial inner membrane is required for normal growth of yeast. EMBO J. 14, 188–195 10.1002/j.1460-2075.1995.tb06989.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen S., Sánchez-Fernández R., Lyver E. R., Dancis A., and Rea P. A. (2007) Functional characterization of AtATM1, AtATM2, and AtATM3, a subfamily of Arabidopsis half-molecule ATP-binding cassette transporters implicated in iron homeostasis. J. Biol. Chem. 282, 21561–21571 10.1074/jbc.M702383200 [DOI] [PubMed] [Google Scholar]
- 53. Bernard D. G., Cheng Y., Zhao Y., and Balk J. (2009) An allelic mutant series of ATM3 reveals its key role in the biogenesis of cytosolic iron–sulfur proteins in Arabidopsis. Plant Physiol. 151, 590–602 10.1104/pp.109.143651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Teschner J., Lachmann N., Schulze J., Geisler M., Selbach K., Santamaria-Araujo J., Balk J., Mendel R. R., and Bittner F. (2010) A novel role for Arabidopsis mitochondrial ABC transporter ATM3 in molybdenum cofactor biosynthesis. Plant Cell 22, 468–480 10.1105/tpc.109.068478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lill R. (2009) Function and biogenesis of iron-sulphur proteins. Nature 460, 831–838 10.1038/nature08301 [DOI] [PubMed] [Google Scholar]
- 56. Veatch J. R., McMurray M. A., Nelson Z. W., and Gottschling D. E. (2009) Mitochondrial dysfunction leads to nuclear genome instability via an iron–sulfur cluster defect. Cell 137, 1247–1258 10.1016/j.cell.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vergara S. V., and Thiele D. J. (2009) Ironing out a midlife crisis. Cell 137, 1179–1181 10.1016/j.cell.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 58. Bekri S., Kispal G., Lange H., Fitzsimons E., Tolmie J., Lill R., and Bishop D. F. (2000) Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron–sulfur protein maturation. Blood 96, 3256–3264 [PubMed] [Google Scholar]
- 59. Boultwood J., Pellagatti A., Nikpour M., Pushkaran B., Fidler C., Cattan H., Littlewood T. J., Malcovati L., Della Porta M. G., Jädersten M., Killick S., Giagounidis A., Bowen D., Hellström-Lindberg E., Cazzola M., et al. (2008) The role of the iron transporter ABCB7 in refractory anemia with ring sideroblasts. PLoS One 3, e1970 10.1371/journal.pone.0001970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Longtine M. S., McKenzie A. 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., and Pringle J. R. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 61. Sherman F. (2002) Getting started with yeast. Methods Enzymol. 350, 3–41 10.1016/S0076-6879(02)50954-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.