Abstract
The signaling nucleotide cyclic di-AMP (c-di-AMP) is the only known essential second messenger in bacteria. Recently, c-di-AMP has been identified as being essential for controlling potassium uptake in the model organism Bacillus subtilis and several other bacteria. A B. subtilis strain lacking c-di-AMP is not viable at high potassium concentrations, unless the bacteria acquire suppressor mutations. In this study, we isolated such suppressor mutants and found mutations that reduced the activities of the potassium transporters KtrCD and KimA. Although c-di-AMP–mediated control of KtrCD has previously been demonstrated, it is unknown how c-di-AMP affects KimA activity. Using the DRaCALA screening assay, we tested for any interactions of KimA and other potential target proteins in B. subtilis with c-di-AMP. This assay identified KimA, as well as the K+/H+ antiporter KhtT, the potassium exporter CpaA (YjbQ), the osmoprotectant transporter subunit OpuCA, the primary Mg2+ importer MgtE, and DarB (YkuL), a protein of unknown function, as bona fide c-di-AMP–binding proteins. Further, binding of c-di-AMP to KimA inhibited potassium uptake. Our results indicate that c-di-AMP controls KimA-mediated potassium transport at both kimA gene expression and KimA activity levels. Moreover, the discovery that potassium exporters are c-di-AMP targets indicates that this second messenger controls potassium homeostasis in B. subtilis at a global level by binding to riboswitches and to different classes of transport proteins involved in potassium uptake and export.
Keywords: cyclic diadenosine monophosphate (c-di-AMP), potassium transport, prokaryotic signal-transduction, Bacillus, bacterial genetics, second messenger, Bacillus subtilis, cation homeostasis, cyclic di-AMP, dinucleotide signaling, osmoregulation
Introduction
The essential signaling nucleotide c-di-AMP2 is a recently discovered second messenger that is produced by many bacteria and some archaea (1, 2). The reasons for the essentiality of this dinucleotide have long remained elusive. Recent studies with the Gram-positive bacteria Listeria monocytogenes, Bacillus subtilis, Staphylococcus aureus, and Streptococcus agalactiae revealed that c-di-AMP becomes dispensable, if the bacteria are cultivated on strictly controlled minimal media (3–6). In the Gram-positive model organism B. subtilis, c-di-AMP is dispensable only at low potassium concentrations in minimal medium (4).
Binding assays to search for target proteins of the molecule revealed that c-di-AMP binds several different proteins, with the majority being involved in potassium and compatible solute uptake (see Ref. 7 for review). c-di-AMP binds to the conserved RCK_C (regulator of conductance of K+) domains in the gating components of potassium channels (6, 8–12). Moreover, c-di-AMP binds and inhibits the unrelated Kup potassium transporters in Lactococcus lactis (13). Additionally, c-di-AMP controls the expression of different potassium uptake systems. It binds to the KdpD sensor kinase that controls the expression of the S. aureus and L. monocytogenes Kdp potassium transport systems and to the two copies of the c-di-AMP (formerly ydaO) riboswitch that controls the expression of the high-affinity potassium uptake systems KtrAB and KimA in B. subtilis and other bacteria (4, 14, 15). This makes c-di-AMP the only known second messenger that controls a single biological process by binding both to a protein and to the corresponding mRNA. Similarly, the uptake of osmoprotectants is regulated at levels both of gene expression and protein activity. c-di-AMP binds to the RCK_C domain of the transcription repressor BusR, which controls the expression of the busAB operon for the transport of compatible solutes in lactic acid bacteria (6, 16). Moreover, c-di-AMP binding to the regulatory CBS domain of the ATP-binding subunit directly inhibits osmoprotectant uptake systems in S. aureus and L. monocytogenes (17, 18).
Recently, the concept of sustained sensing has been proposed for second messengers that control a biological process by binding multiple targets (19). The control of potassium and compatible solute transport nicely fits this concept. In addition to these processes, c-di-AMP controls the entry to the citric acid cycle by binding to the pyruvate carboxylase of L. monocytogenes and L. lactis (9, 20). Finally, DarA, a PII-like protein of unknown function binds c-di-AMP in B. subtilis, S. aureus, and L. monocytogenes (8, 9, 21), and CbpB, an unknown protein consisting of two CBS domains, were identified as c-di-AMP–binding protein in L. monocytogenes (9).
In B. subtilis, c-di-AMP is essential for potassium homeostasis (4). However, only very few target molecules of the dinucleotide are known for this model organism. In a first screen for c-di-AMP targets in B. subtilis, only one protein could be identified: the PII-like signal transduction protein DarA showed high and specific affinity for c-di-AMP, although its function has remained enigmatic (21). The other known c-di-AMP target proteins of B. subtilis are the RCK_C domains of the peripheral membrane proteins KtrA and KtrC, which are the regulatory subunits of the KtrAB and KtrCD complexes, respectively (8, 12, 22). For KtrA and KtrC from different organisms, it has been shown that upon binding of c-di-AMP the complexes are inhibited, most likely because of conformational changes within the regulatory subunits (10, 12, 23).
As mentioned above, the control of potassium homeostasis is a key function of c-di-AMP, and this function is crucial for the essentiality of the second messenger. Thus, it is tempting to speculate that c-di-AMP might regulate further potassium transport systems. In addition to the high- and low-affinity potassium uptake systems KtrAB and KtrCD (24), respectively, recently the high-affinity potassium importer KimA was discovered (4, 25). The expression of both high-affinity uptake systems is controlled by a c-di-AMP–responsive riboswitch (4, 15), but it has remained elusive whether KimA is also controlled on the protein level. Much less is known about the potassium export systems of B. subtilis. The KhtSTU complex and the YugO proteins are suggested to be potassium exporters (26, 27). In addition, the YjbQ protein is similar to the S. aureus potassium exporter CpaA, which was also shown to bind c-di-AMP via its RCK_C domain (28).
In general, RCK_C and CBS domains appear to contain conserved c-di-AMP–binding sites (8, 9, 17, 18, 28). B. subtilis encodes five proteins containing an RCK_C domain and 16 proteins with CBS domains (29), many of which are potential potassium and osmolyte transporters, respectively. To get a more comprehensive understanding of c-di-AMP–mediated signaling in this bacterium, we have tested the potential binding of the second messenger to these proteins, as well as to the newly discovered potassium importer KimA. Our results support the idea that c-di-AMP is responsible for the global control of potassium homeostasis, by controlling both its uptake and its export.
Results
Isolation of suppressor mutants that allow growth of a strain lacking c-di-AMP at 20 mm KCl
A B. subtilis strain lacking all three diadenylate cyclases is unable to grow at potassium concentrations of 5 mm or higher. However, the acquisition of suppressor mutations at 5 mm potassium that affect the cation exporter NhaK and result in enhanced potassium secretion prevents the toxic effect of potassium (4). However, those suppressor mutants were not viable at a potassium concentration of 20 mm. To identify the growth-limiting problem, we sought to isolate suppressor mutants that tolerate this potassium concentration. For this purpose, we plated the c-di-AMP–free strain GP2222 (4) on MSSM (modified sodium spizizen minimal) medium containing 20 mm KCl. After 4 days at 37 °C, two colonies could be isolated. Both strains (GP2737 and GP2738) were subjected to whole genome sequencing to identify the responsible mutations. Both strains carried point mutations affecting the potassium transporter KimA (Trp-520 replaced by Gly) and KtrCD's regulatory subunit KtrC (Ala-73 replaced by Val). Moreover, both strains carried a point mutation in FlhA, which is part of the flagellar type III export apparatus. Finally, one of the mutants (GP2737) carried a frameshift mutation in odhA, which results in loss of 2-oxoglutarate dehydrogenase activity as indicated by the reddish color of the colonies (25).
To study the role of the mutations affecting the potassium uptake systems, we first attempted to delete the kimA and ktrC genes in the c-di-AMP–free strain GP2222. Although a control experiment (deletion of the nhaK gene) was successful, the deletion of kimA or ktrC was not possible. This observation suggests that the point mutations did not completely prevent the KimA and KtrC activities. The mutation in KtrC affects a highly conserved residue in the RCK_N domain. Similarly, the Trp-520 residue is conserved in the KimA proteins of B. subtilis, L. monocytogenes, S. aureus, and Mycobacterium tuberculosis. The conservation of the substituted amino acids suggests that the mutant proteins have reduced activity.
Because no detailed information on the activity of KimA is available, we decided to analyze the activity of the mutant protein in a heterologous complementation assay using Escherichia coli LB2003 (30). This strain is deficient in the three major endogenous potassium uptake systems Trk, Kup, and Kdp and is therefore not able to grow at low potassium concentrations without complementation using a gene encoding a potassium transporter. For the IPTG-inducible expression of the WT and mutant KimA variants, we used plasmids pGP2913 and pGP2993, respectively. Accordingly, E. coli LB2003 was transformed with these plasmids or the empty vector control pWH844 (31), and growth was assessed in minimal medium supplemented with increasing KCl concentrations (0.001, 0.01, 0.02, 0.04, 0.06, 0.1, 0.2, 0.5, 10, and 50 mm KCl) (Fig. 1). Although 50 mm KCl was required for growth of the strain carrying the empty vector, expression of WT KimA (Ref. 4 and this study), as well as of the KimA variant W520G, allowed growth at much lower KCl concentrations. The determination of the growth rates at different potassium concentrations allowed fitting according to the Monod equation, an equation describing the growth of cultures, which is based on the Michaelis–Menten equation (32). This revealed the maximum specific growth rate μmax (h−1) and the substrate concentration that supports the half-maximal growth rate KS (mm KCl) of the WT and mutant KimA proteins. The mean KS values of three independent biological replicates are as follows: WT KimA had a KS of 0.029 ± 0.0039 mm for potassium and a μmax of 0.68 ± 0.005 h−1. In contrast, the mutant KimA protein had an apparent KS for potassium of 0.233 ± 0.021 mm and a μmax of 0.84 ± 0.038 h−1. This 8-fold reduction of the apparent affinity for potassium perfectly fits with the conclusion that the suppressor mutant is impaired in potassium uptake without completely preventing it.
Figure 1.
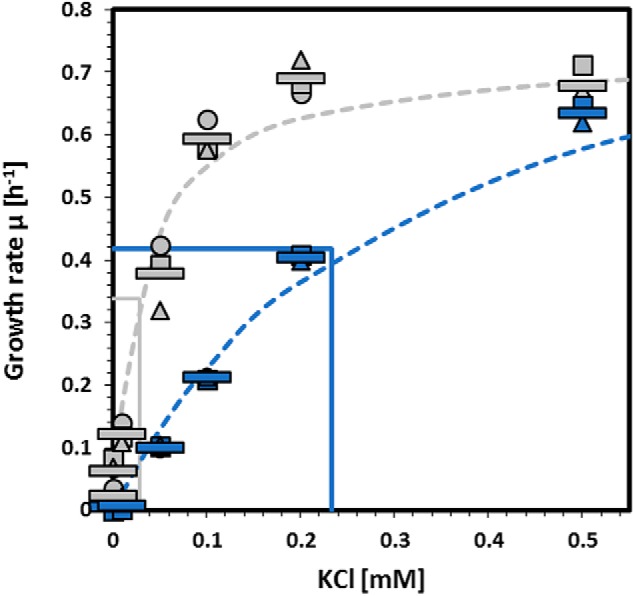
Activity of KimA variant proteins in a heterologous complementation assay. E. coli LB2003 was transformed with pGP2913 (WT KimA, gray) and pGP2993 (KimA-W520G, blue), respectively, and growth at different potassium concentrations was assessed over 24 h. The growth rate μ was determined and plotted against the potassium concentrations. Dashed lines show the ideal progression of μ over the different potassium concentrations according to the Monod equation, and continuous lines show the determined KS. Circles, squares, and triangles represent independent biological replicates. The bars indicate the means of the replicates.
Analysis of a possible interaction between KimA and the PII-like c-di-AMP–binding protein DarA
Often, transporters for molecules that may be toxic upon accumulation are controlled at the levels of expression and of transporter activity to avoid intoxication of the cells. This has been described for the ammonium transporter AmtB, which is only expressed under conditions of ammonium limitation and additionally inhibited by binding of the PII protein GlnK if the ammonium concentrations suddenly increase (33, 34). The expression of the high-affinity potassium transporters KimA and KtrAB is controlled by a c-di-AMP–responsive riboswitch (4, 15). In addition, KtrAB is inhibited upon binding of c-di-AMP, the second messenger that transduces the information on potassium accumulation (4, 8). For the novel potassium transporter KimA, it is not known whether and how the activity of this protein is controlled. Following the concept of sustained sensing, we considered the possibility that the activity of KimA might be controlled by the PII-like c-di-AMP–binding protein DarA (21), because KimA does not contain one of the known c-di-AMP–binding domains. To test this hypothesis, we first analyzed the localization of the DarA protein. The PII protein GlnK is found in the cytoplasm at low ammonium concentrations but is recruited to the membrane via AmtB under conditions of ammonium excess (34). To study whether the localization of DarA depends on potassium availability, we raised antibodies against the protein to facilitate its detection. B. subtilis was cultivated in MSSM minimal medium containing 0.1 or 5 mm of KCl, and the proteins were separated into cytoplasmic and membrane-bound fractions. The fractions were then assayed for the presence of DarA. The cytoplasmic protein CggR and the membrane protein RNase Y served as controls (35, 36). Although the localization of the control proteins was observed as expected, we found DarA to be present exclusively in the cytoplasm (Fig. 2). This result suggests that DarA does not bind to the potassium transporter KimA to control its activity. To confirm this observation, we determined the cellular potassium concentrations in the WT strain B. subtilis 168 and the isogenic darA mutant GP1712 (21) by inductively coupled plasma optical emission spectrometry. Both strains had very similar intracellular potassium concentrations of 5.1 ± 0.4 and 4.8 ± 0.3 μg K+ ml−1 A600−1, respectively. Taken together, these results strongly suggest that the activity of KimA is not controlled by the c-di-AMP–binding protein DarA.
Figure 2.
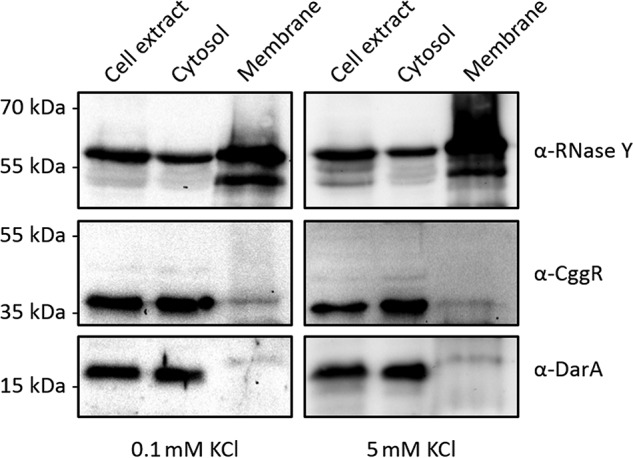
The c-di-AMP–binding protein DarA is a cytoplasmic protein. B. subtilis 168 was cultivated in MSSM minimal medium containing 0.1 or 5 mm KCl. Crude extracts were separated by ultracentrifugation to obtain cytosolic and membrane fractions. The presence of DarA was tested using antibodies recognizing DarA. To check for successful separation, the cytosolic and membrane fraction were tested with the specific antibodies recognizing CggR and Rny, respectively.
Identification of c-di-AMP target proteins
Because our localization studies did not support the idea that the activity of KimA is controlled by DarA, we considered the possibility that c-di-AMP might directly bind and control KimA. Additionally, we aimed at identifying further targets that directly bind c-di-AMP in B. subtilis to detail our understanding of its regulatory function. For this broader approach, we focused on the five proteins containing RCK_C domains and the 16 proteins containing CBS domains encoded in B. subtilis (29) (Table 1). Interestingly, many of these proteins are implicated in ion or compatible solute transport.
Table 1.
Expression plasmids used in the DRaCALA assay
| Plasmid | Protein | Functiona | Domain |
|---|---|---|---|
| pGP2594 | KtrA | Peripheral membrane component K+ transporter | RCK_C |
| pGP2906 | KhtT | K+/H+ antiporter | RCK_C |
| pGP2907 | KtrC | Peripheral membrane component K+ transporter | RCK_C |
| pGP2908 | YrvC | Unknown | RCK_C |
| pGP2908 | YjbQ/CpaA | Unknown | RCK_C |
| pGP2913 | KimA | K+ transporter | b |
| pGP2922 | AcuB | Unknown | CBS |
| pGP2923 | CcpN | Transcriptional repressor | CBS |
| pGP2924 | OpuAA | Glycine betaine ABC transporter (ATP-binding protein) | CBS |
| pGP2927 | MgtE | Primary Mg2+ transporter | CBS |
| pGP2928 | YhdT | Unknown | CBS |
| pGP2929 | YkuL/DarB | Unknown | CBS |
| pGP2930 | YhcV | Unknown, forespore-specific sporulation protein | CBS |
| pGP2931 | YhdP | Potential Mg2+ efflux pump | CBS |
| pGP2932 | YrkA | Unknown | CBS |
| pGP2933 | YtoI | Unknown, similar to transcriptional regulator (GntR family) | CBS |
| pGP2934 | YlbB | Unknown, putative oxidoreductase | CBS |
| pGP2935 | YqhB | General stress protein | CBS |
| pGP2936 | YugS | Unknown | CBS |
| pGP2937 | GuaB | Biosynthesis of GMP | CBS |
| pGP2938 | OpuBA | Choline ABC transporter (ATP-binding protein) | CBS |
| pGP2939 | OpuCA | Glycine betaine/carnitine/choline ABC transporter (ABC-binding protein) | CBS |
a Protein functions have been retrieved from the SubtiWiki database (29).
b For KimA, no conserved c-di-AMP–binding domain has been identified so far.
We used E. coli cells as expression system because c-di-AMP is not synthesized by this bacterium. The 22 selected genes were cloned into the expression vector pWH844 (31). After checking expression of all genes, using strain E. coli BL21 as a host, the lysates of strains carrying the corresponding plasmids were assessed for a possible interaction with c-di-AMP in vitro using the differential radial capillary action of ligand assay (DRaCALA) (37) (see “Experimental procedures”).
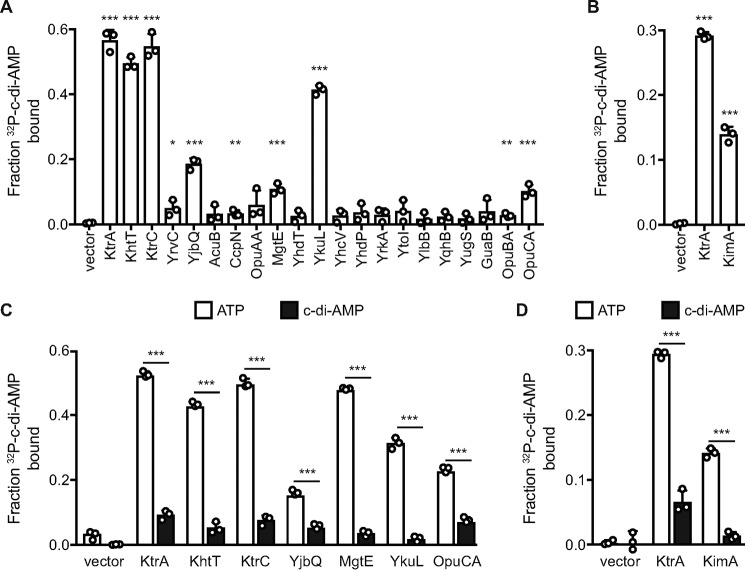
In an initial screen for c-di-AMP binding, eight target proteins could be identified (Fig. 3, A and B). Among these proteins, the known c-di-AMP–binding protein KtrA served as positive control (8, 12). In addition to KtrA, three other proteins containing an RCK_C domain were identified as c-di-AMP–binding proteins. KtrC, the regulatory subunit of the low-affinity potassium transporter KtrCD, the cytoplasmic subunit KhtT of the K+/H+ antiporter KhtSTU and YjbQ, a putative cation exporter, showed strong binding to c-di-AMP. Only YrvC, a protein of unknown function, showed nonsignificant binding. Of the proteins containing a CBS domain, the Mg2+ transporter MgtE, the ATP-binding protein OpuCA of the compatible solute transporter OpuC and YkuL, a protein of unknown function, showed increased binding affinity toward the di-nucleotide (Fig. 3A). Importantly, the novel potassium transporter KimA, which possesses none of the conserved binding motifs, also bound c-di-AMP (Fig. 3B). Strikingly, among the c-di-AMP–binding proteins identified in this screen, YkuL is the only c-di-AMP receptor protein that is not associated to the cell membrane. Binding of OpuCA and YkuL to c-di-AMP is in excellent agreement with previously published results (9, 17, 18). Because YkuL is composed of two reiterated CBS domains and thus seems to have a regulatory function, we refer to this protein as DarB (c-di-AMP receptor protein B, in analogy to DarA) (21). The specificity of c-di-AMP binding to the identified proteins was confirmed by competition experiments with cold ATP or c-di-AMP from overnight grown cultures (Fig. 3, C and D). For all tested proteins, ATP was unable to block c-di-AMP binding, whereas cold c-di-AMP outcompeted the radioactively labeled c-di-AMP. This demonstrates the specificity of c-di-AMP binding and indicates that these proteins are true targets of the second messenger.
Figure 3.
A subset of c-di-AMP–binding proteins overexpressed in E. coli whole cell lysates determined by DRaCALA. A and B, fraction bound of radiolabeled [32P]c-di-AMP is shown for lysates from E. coli induced for the expression of the indicated genes in presence of nonspecific ATP competitor at 100 μm (A) or 200 μm (B). C and D, induced lysates overexpressing the indicated gene are tested for specificity of c-di-AMP binding to [32P]c-di-AMP by competition assays. C, competitors are 100 μm ATP (open bars) or 100 μm ATP and 100 μm c-di-AMP (closed bars). D, competitors are 200 μm ATP (open bars) or 200 μm ATP and 100 μm c-di-AMP (closed bars). Significance of binding was determined using unpaired t test between vector-only control and indicated gene (A and B) or between ATP and c-di-AMP competitors (C and D). p values of <0.05, <0.01, and <0.001 are indicated by *, **, and ***, respectively.
In total, we identified six novel c-di-AMP receptor proteins in B. subtilis. These novel target proteins are involved in potassium uptake and export and also in magnesium and osmolyte uptake. The fact that c-di-AMP does not bind specifically to proteins involved in one homeostatic process suggests that control of potassium homeostasis is not the only function of the essential second messenger (5).
c-di-AMP inhibits potassium transport by KimA
The specific interaction of KimA with c-di-AMP suggested a functional role for c-di-AMP in the control of KimA activity. Therefore, we analyzed the impact of this second messenger nucleotide on the potassium transport activity of KimA by a growth complementation assay. For this purpose, a co-expression system producing the c-di-AMP–synthesizing diadenylate cyclase CdaA and the potassium transporter KimA was established in the potassium transporter-deficient mutant E. coli LB2003 (30). Importantly, E. coli lacks c-di-AMP–synthesizing enzymes and is unable to produce this second messenger (38, 39, 40). The growth phenotype upon co-expression of cdaA from L. monocytogenes, (CdaALmo) and kimA genes reflects the effect of c-di-AMP binding on KimA. The co-expression of KimA with an inactive diadenylate cyclase, CdaALmo*(D171N) (38), served as a negative control. Similarly, the growth phenotype of LB2003 expressing kimA and cdaA alone were analyzed.
Growth curves of LB2003 transformed with the respective plasmids and induced with 0.002% arabinose were recorded at 0.1, 0.2, 0.5, 1, 3, 10, and 30 mm KCl in phosphate-buffered minimal medium for 24 h (Fig. 4). LB2003 harboring pBP370 (CdaALmo) and pBP373 (CdaALmo*) (13) only grew at 30 mm KCl, whereas LB2003 producing KimA (plasmid pB24C3H-KimA) showed growth complementation at all tested potassium concentrations. KimA thus facilitates the uptake of potassium, whereas the expression of cdaA has no general inhibitory effect, which is in agreement with previous observations (4, 13). A very similar growth behavior to LB2003 expressing kimA was observed upon co-production of KimA (pB24C3H-KimA) and the inactive CdaALmo* (pBP373). However, the co-expression KimA (pB24C3H-KimA) with active CdaALmo (pBP370) abolished cell growth below 30 mm KCl. Thus, we can conclude that the binding of c-di-AMP produced by CdaALmo to KimA inhibits its potassium transport activity.
Figure 4.
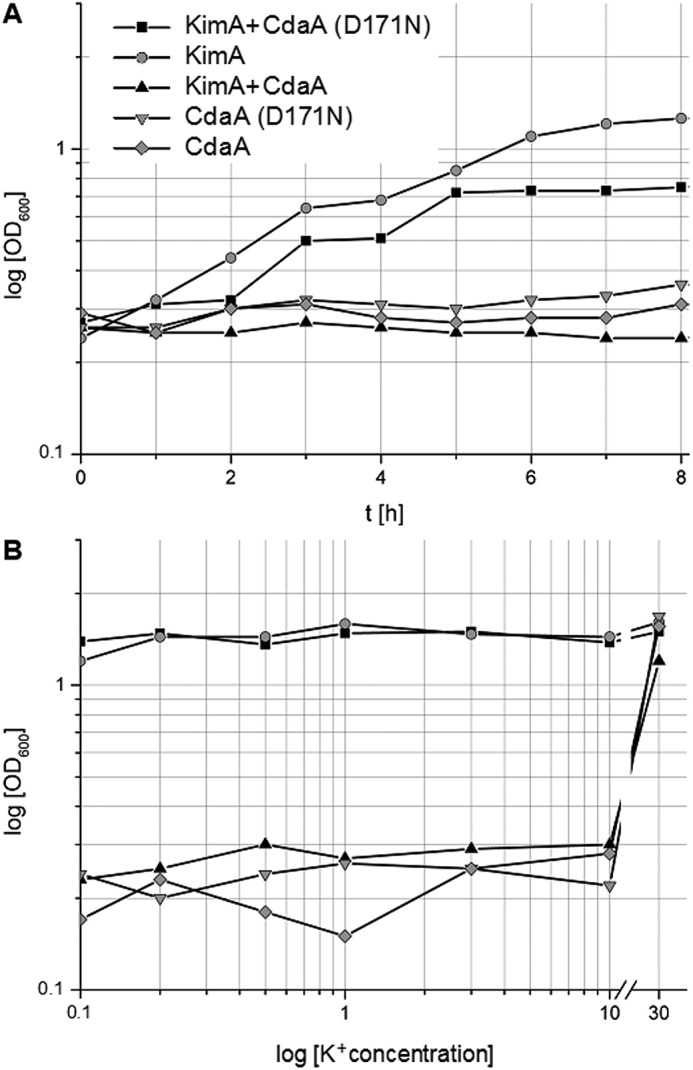
CdaA-dependent inhibition of KimA-mediated growth. A, growth curves of E. coli LB2003 expressing KimA and/or variants of CdaA in K minimal medium containing 3 mm KCl. B, A600 of E. coli LB2003 expressing KimA and/or variants of CdaA grown for 24 h in minimal medium with potassium concentrations ranging from 0.1 to 30 mm.
Discussion
The second messenger c-di-AMP is essential in many bacteria, including the Gram-positive model organism B. subtilis and the closely related pathogenic bacteria L. monocytogenes and S. aureus. Previous pulldown experiments revealed several target proteins for the two pathogenic bacteria (8, 9), but for B. subtilis the unknown c-di-AMP receptor DarA and the potassium channel subunits KtrA and KtrC were the only target proteins that had been identified (12, 21). None of the identified c-di-AMP target proteins was essential for cell viability, and therefore the essential function of the second messenger has long remained elusive (41).
In this study, we tested c-di-AMP binding for the recently discovered potassium transporter KimA, as well as for proteins containing specific domains, either the RCK_C or CBS domain. These protein domains have been shown to bind directly to c-di-AMP (9). Of the 22 tested proteins, we identified six novel c-di-AMP targets, i.e. the potassium transporter KimA, the RCK_C subunit (KhtT) of a potassium/proton antiporter KhtSTU (27), the primary magnesium importer MgtE (42), the glycine-betaine transporter subunit OpuCA (43), and the two proteins of unknown function DarB and YjbQ. Binding of c-di-AMP to DarB, OpuCA, and YjbQ homologs has been reported previously for L. monocytogenes (9), whereas KimA, KhtT, and MgtE have not been previously identified as c-di-AMP targets. The common denominator between KimA, KhtT, YjbQ, MgtE, and OpuCA is that all five proteins are transmembrane proteins. The control of potassium import has been previously linked to the essentiality of c-di-AMP in B. subtilis (4). The here-identified c-di-AMP–binding potassium export systems Kht and YjbQ suggest that c-di-AMP plays a more general role in controlling potassium uptake and release. Moreover, it is tempting to speculate that the control of magnesium and osmolyte homeostasis are additional essential functions of c-di-AMP. Indeed, an implication of c-di-AMP signaling in osmoregulation is a common theme in many bacteria (see Ref. 7 for review). In L. monocytogenes, the essentiality of the second messenger is also not linked to the control of one specific protein but to the general control of the stringent response factor (p)ppGpp (3).
Importantly, this study identified the potassium transporter KimA as an additional target of c-di-AMP. Because potassium is both essential and toxic for the cells (4, 44), the control of its homeostasis is of utmost importance for the bacteria. A strain lacking c-di-AMP can grow at elevated potassium concentrations only if potassium ions can be more efficiently exported (4) or if the uptake of potassium is reduced by mutations affecting conserved residues in the transporters KtrC and KimA (this work). This observation already suggests that the KimA activity is reduced in the presence of c-di-AMP and that a corresponding reduction must be achieved in the absence of c-di-AMP by the acquisition of a mutation. Because the mechanism of KimA control by c-di-AMP had been unknown prior to this study, we hypothesized that the KimA activity might be controlled by the c-di-AMP–binding PII-like protein DarA, in analogy to the control of the ammonium transporter AmtB by the PII protein GlnK (33, 34). However, our experiments did not support this possibility and suggested that KimA might be subject to direct control by c-di-AMP. This idea was confirmed by the detection of binding of c-di-AMP to KimA and by the observation that this interaction inhibits the potassium uptake activity of KimA.
KimA represents a completely novel group of c-di-AMP–binding proteins that does not contain any of the previously identified domains that bind the second messenger. Moreover, KimA is the prototype of the fourth class of proteins that are involved in the control of potassium homeostasis and that are controlled by c-di-AMP in Gram-positive bacteria. So far, the potassium transporters containing an RCK_C domain have been studied in several bacteria, and we have recently identified the KupA and KupB proteins as novel targets of c-di-AMP in L. lactis (13). In all cases, these potassium transporters are inhibited by c-di-AMP. Interestingly, a L. lactis strain that accumulates c-di-AMP was extremely sensitive to salt stress, but the acquisition of a mutation in KupB restored growth. In this case, the variant KupB protein had an increased activity to compensate for the strong inhibition upon c-di-AMP accumulation (16). Thus, accumulation of c-di-AMP had just the opposite effect for the mutational adaptation of the potassium transporter as observed here for lack of c-di-AMP. KimA adds a third protein family to the list of c-di-AMP inhibited potassium transporters.
Interestingly, c-di-AMP does not only bind to proteins involved in potassium uptake but also to a riboswitch that controls the expression of the high-affinity KtrAB and KimA transporters in B. subtilis. Binding of c-di-AMP to the riboswitch prevents expression beyond the riboswitch and does thus inhibit expression of the transporter genes (4, 15). Moreover, c-di-AMP binds to the sensor kinase KdpD, which is required for the expression of the high-affinity potassium transport system KdpFABC in S. aureus. Again, binding of c-di-AMP inhibits the activity of the protein and does thus prevent expression of the transporter (14). In conclusion, c-di-AMP governs potassium uptake by direct binding to and inhibition of potassium uptake systems and by controlling the expression of the high-affinity transporters.
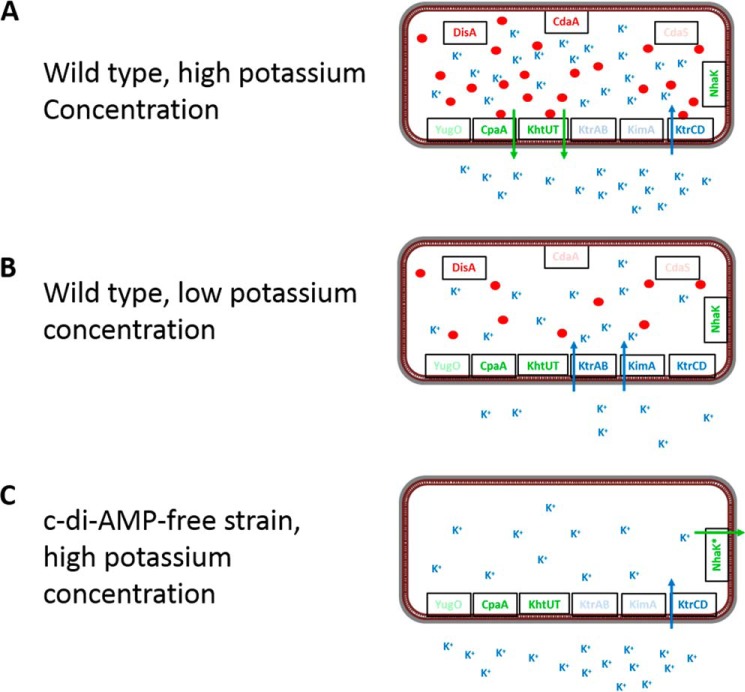
In addition to KimA, we have identified the KhtT and YjbQ proteins as novel targets of c-di-AMP. Although KhtT has been demonstrated to be a subunit of a potassium/proton antiporter in B. subtilis (27), less is known on YjbQ. The corresponding S. aureus protein CpaA (cation/proton antiporter A) has also been shown to bind c-di-AMP (8). This protein is a potassium exporter, and interestingly, its activity is stimulated by c-di-AMP (28). Based on the 52% amino acid identity between the proteins from both organisms and on the common regulation by c-di-AMP, we rename B. subtilis YjbQ to CpaA. It is interesting to mention that in screens for c-di-AMP–free strains that are viable at increased potassium concentrations, we never found mutations that affected the potassium exporters KhtSTU or CpaA. In contrast, variants of the NhaK cation exporter with increased specificity for potassium were selected at 5 mm potassium (4), whereas mutations resulting in reduced potassium uptake were found at 20 mm potassium (this work). These findings support the idea that the potassium exporters are inactive unless they bind and become activated by c-di-AMP. Obviously, mutations that overcome this requirement are rather unlikely.
Taken together, the results from our suppressor screens, the biochemical investigation of S. aureus CpaA, as well as the physiological logics all converge to support the idea that c-di-AMP inhibits potassium uptake at the levels of transporter expression and activity and stimulates potassium export by activating the potassium/proton antiporters (Fig. 5). This extends the idea that the control of potassium homeostasis by controlling potassium uptake is an essential function of c-di-AMP to the export of this cation. The binding of multiple potassium transporters, as well as the dual control of both potassium transporter expression and activity as observed for KtrAB and KimA (Refs. 4, 8, 12, and 15, and this work), makes c-di-AMP–mediated signaling on potassium homeostasis a paradigm for the concept of sustained sensing (19). It is amazing that different classes of (functionally different) proteins and even of a riboswitch have evolved to be controlled by a single second messenger, c-di-AMP. Thus, c-di-AMP is the major effector of potassium uptake in the firmicutes. Further work will be required to understand the roles of the distinct potassium export systems and to unravel the functions of the so far uncharacterized c-di-AMP–binding proteins, DarA and DarB.
Figure 5.
Control of potassium uptake and export in B. subtilis by c-di-AMP. A, in the WT strain, the potassium exporters CpaA and KhtUT, as well as the low-affinity transporter KtrCD, are expressed at high potassium concentrations. Potassium is taken up by KtrCD and triggers the production of c-di-AMP by CdaA and DisA. Upon accumulation of potassium and the second messenger, c-di-AMP binds to KtrC to prevent further potassium uptake. Simultaneous binding of c-di-AMP to CpaA and KhtUT triggers potassium export. Thus, the cell prevents potassium intoxication. B, at low potassium concentrations, all three uptake systems as well as the two exporters are expressed. Potassium is transported by the high-affinity transporters KtrAB and KimA. The low intracellular potassium concentration results only in a very limited synthesis of c-di-AMP that is not sufficient to inhibit or activate potassium uptake or export, respectively. C, a strain lacking c-di-AMP is unable to grow at high potassium concentrations because of the unlimited influx of the ion via KtrCD. The potassium exporters are inactive in the absence of the second messenger. Therefore, growth of this strain in the presence of potassium requires a novel active potassium exporter. Under these conditions, the bacteria acquire mutations affecting the NhaK cation/proton antiporter that result in increased potassium export (4).
Experimental procedures
Strains, media, and growth conditions
E. coli DH5α (45) was used for cloning and for the expression of recombinant proteins. E. coli LB2003 (30) was used to assay potassium transporter activity. All B. subtilis strains used in this study are derivatives of the laboratory strain 168. B. subtilis was grown in LB or in sporulation medium (45, 46). E. coli was cultivated in MSSM medium (4) or in modified M9 medium in which KH2PO4 was replaced by NaH2PO4 and 50 mm KCl was added. The media were supplemented with ampicillin (100 μg/ml), kanamycin (10 and 50 μg/ml for B. subtilis and E. coli, respectively), chloramphenicol (5 μg/ml), tetracyclin (12.5 μg/ml), spectinomycin (150 μg/ml), or erythromycin and lincomycin (2 and 25 μg/ml, respectively) if required.
DNA manipulation and genome sequencing
Transformation of E. coli and plasmid DNA extraction were performed using standard procedures (45). All commercially available plasmids, restriction enzymes, T4 DNA ligase, and DNA polymerases were used as recommended by the manufacturers. Chromosomal DNA of B. subtilis was isolated as described (46). B. subtilis was transformed with plasmid and genomic DNA according to the two-step protocol (46).
To identify the mutations in the suppressor mutant strains GP2737 and GP2738, the genomic DNA was subjected to whole-genome sequencing (47). Briefly, the reads were mapped on the reference genome of B. subtilis 168 (GenBankTM accession number NC_000964) (48). Mapping of the reads was performed using the Geneious software package (Biomatters Ltd.) (49). SNPs were considered as significant when the total coverage depth exceeded 25 reads with a variant frequency of ≥90%. All identified mutations were verified by PCR amplification and Sanger sequencing.
Plasmid constructions
The selected genes were amplified using chromosomal DNA of B. subtilis 168 as the template and appropriate nucleotides that attached BamHI (or BglII) and SalI restriction sites to the fragments and cloned between the BamHI and SalI sites of the expression vector pWH844 (31) (Table 1). The mutant kimA allele encoding KimA (W520G) was amplified using the oligonucleotides JN465 and JN466 (4) and cloned between the BamHI and PstI sites of pWH844. The resulting plasmid was pGP2993. The construct of KimA used in the co-expression of KimA and diadenylate cyclases was cloned by restriction-free cloning into a modified pBAD24 vector that bears a His tag in the C terminus, resulting in the plasmid pB24C3H-KimA.
Identification of c-di-AMP–binding proteins by DRaCALA
Expression of the genes upon induction using 1 mm of IPTG was verified by analyzing the protein patterns of the expression strains by SDS-PAGE. 32P-Labeled c-di-AMP synthesis was performed using purified diadenylate cyclase DisA (8). The analysis of protein–ligand interaction was performed using E. coli whole cell lysates that were grown to an A600 of 0.5–1.0 and induced for 4 h by 1 mm IPTG as described (37, 50) or grown overnight in the presence of 50 μg/ml carbenicillin and 1 mm IPTG. All binding reactions were performed in 1× binding buffer (10 mm Tris, pH 8.0, 100 mm NaCl, 5 mm MgCl2) containing ∼10 pm [32P]c-di-AMP. Protein–ligand mixtures were spotted on nitrocellulose membrane (Amersham Biosciences Hybond-ECL; GE Healthcare) and allowed to dry. The areas and intensities of spots were quantified by exposing phosphorus imaging screens and scanning by FUJI FLA-7000 phosphorus imager. The competition assays were performed with indicated concentrations of unlabeled ATP and c-di-AMP (Axxora).
Preparation of membrane fractions
Cultures of B. subtilis were harvested by centrifugation (4,400 × g, 10 min, 4 °C). The following steps were done as described previously (51). Briefly, the cells were lysed by sonication, the cellular debris was removed, and the fractions of the cell extract were separated by ultracentrifugation. The membrane pellet was washed for three times and finally resuspended in phosphate buffer (50 mm Na2HPO4, 50 mm NaH2PO4, pH 6.8). To assess the quality of the preparations, the fractions were analyzed for the presence of CggR and RNase Y using polyclonal rabbit antibodies raised against these proteins (35, 52).
Western blotting analysis
The DarA protein was purified using E. coli DH5α carrying the expression vector pGP2601 (21) as described previously (21). Purified His6-DarA was used to generate rabbit polyclonal antibodies. For Western blotting analysis, B. subtilis cell extracts were separated on 12.5% SDS-polyacrylamide gels. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad) by electroblotting. The primary antibodies were visualized by using anti-rabbit IgG-AP secondary antibodies (Promega) and the CDP* detection system (Roche Diagnostics) as described previously (53).
Determination of cellular potassium pools
The cellular potassium pools were determined as described previously (25). Briefly, B. subtilis cells were cultivated in MSSM medium supplemented with 0.1 mm KCl. Cells were pelleted, transferred onto ash-free filter discs, and dried. The dried filter disks were cut into small pieces and reduced into a fluid state through pressure and 2 ml of 65% HNO3 for 7 h at 185 °C in 25-ml Teflon beakers (PDS-6 Pressure Digestion System; Loftfield, Göttingen, Germany). After digestion, the fluid content was transferred into an Erlenmeyer flask and diluted with demineralized water to a volume of 50 ml. The total potassium content of the cells in this solution was determined by inductively coupled plasma optical emission spectrometry analysis (Optima 5300 DV; PerkinElmer Life Sciences). Light emission at 766.49 nm that is indicative of the potassium concentration in the sample was recorded.
Determination of specific growth parameters
The growth characteristics of E. coli LB2003 complemented with plasmid-based KimA, KimA-W520G or empty vector were determined as follows. Potassium was used as the growth-limiting factor. The bacteria were inoculated in LB medium containing 50 mm KCl and precultured in MSSM medium, supplemented with thiamin (1 mg/ml) and 50 mm KCl. The cultures were grown until exponential phase and harvested, and the cells were incubated for 1 h in potassium-free medium and washed three times in 1× MSSM buffer. Afterward, the cells were adjusted to A600 1.0 and used to inoculate a 96-well plate (Microtest plate 96-well) containing MSSM medium adjusted to the required potassium concentrations and 50 μm IPTG to induce expression of kimA. Growth was tracked in an Epoch 2 microplate spectrophotometer (BioTek Instruments) at 37 °C with linear shaking at 237 cpm (4 mm) for 20 h, and optical density at 600 nm was measured in 10-min intervals. The exponential growth phase was used to determine the growth rate μ (h−1). The growth rates were then plotted against the potassium concentrations. This allowed fitting to the Michaelis–Menten equation and calculation of vmax (h−1) and the apparent Km (mm KCl) using the solver tool of Excel 2012 (Microsoft). The experiments were repeated with three biological replicates.
Co-expression of KimA and diadenylate cyclases under potassium limitation
E. coli LB2003 was transformed with plasmids pB24C3H-KimA, pBP370, and pBP373 that encode KimA, CdaA, and the inactive mutant of CdaA D171N, alone and in combination. The cells were cultivated in K medium (34 mm Na2HPO4, 17 mm NaH2PO4, 1 mm trisodium-citrate, 7.6 mm (NH4)2SO4) supplemented with 20 μg/ml l-methionine, 6 μm Fe(II)SO4, 1 μg/ml thiamine, 0.4 μm Mg2SO4, 0.2% glycerol, 30 mm potassium (K30, 12 mm K2HPO4, 6 mm KH2PO4,), and the corresponding antibiotics. An overnight culture was harvested and washed two times in K medium (46 mm Na2HPO4, 23 mm NaH2PO4, 1 mm trisodium-citrate, 7.6 mm (NH4)2SO4) supplemented with 10 mm KCl. These samples were then used to inoculate fresh K medium with the same supplements plus 0.002% l-arabinose to induce protein expression and different concentrations of KCl varying from 0.1 to 30 mm. The cultures were incubated at 37 °C with continuous orbital shaking, and A600 measurements were taken every hour.
Author contributions
J. G., R. D., I. H., V. T. L., and J. S. supervision; J. G., I. H., and V. T. L. validation; J. G., L. K., C. H., A. T., A. P., I. T., M. W., and D. H. investigation; L. K., C. H., A. T., A. P., I. T., M. W., and D. H. methodology; A. T., I. H., and V. T. L. visualization; I. H., V. T. L., and J. S. conceptualization; I. H. and J. S. writing-original draft; V. T. L. and J. S. funding acquisition; J. S. project administration.
Acknowledgments
We thank Suzanna Grubek for the help with the cloning experiments. We are grateful to Johannes Gibhardt and Fabian Commichau for helpful discussions.
This work was supported by Deutsche Forschungsgemeinschaft via Priority Program Grant SPP1879 (to J. S.) and by National Institutes of Health Grant AI133670 (to V. T. L.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- c-di-AMP
- cyclic di-AMP
- CBS
- cystathionine β-synthase
- DRaCALA
- differential radial capillary action of ligand assay
- RCK
- regulating conductance of K+
- IPTG
- isopropyl β-d-thiogalactopyranoside.
References
- 1. Witte G., Hartung S., Büttner K., and Hopfner K. P. (2008) Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol. Cell 30, 167–178 10.1016/j.molcel.2008.02.020 [DOI] [PubMed] [Google Scholar]
- 2. Commichau F. M., Heidemann J. L., Ficner R., and Stülke J. (2019) Making and breaking of an essential poison: the cyclases and phosphodiesterases that produce and degrade the essential second messenger cyclic di-AMP in bacteria. J. Bacteriol. 201, e00462–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whiteley A. T., Pollock A. J., and Portnoy D. A. (2015) The PAMP c-di-AMP is essential for Listeria growth in macrophages and rich but not minimal medium due to a toxic increase in (p)ppGpp. Cell Host. Microbe. 17, 788–798 10.1016/j.chom.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gundlach J., Herzberg C., Kaever V., Gunka K., Hoffmann T., Weiss M., Gibhardt J., Thürmer A., Hertel D., Daniel R., Bremer E., Commichau F. M., and Stülke J. (2017) Control of potassium homeostasis is an essential function of the second messenger cyclic di-AMP in Bacillus subtilis. Sci. Signal. 10, eaal3011 10.1126/scisignal.aal3011 [DOI] [PubMed] [Google Scholar]
- 5. Zeden M. S., Schuster C. F., Bowman L., Zhong Q., Williams H. D., and Gründling A. (2018) Cyclic di-adenosine monophosphate (c-di-AMP) is required for osmotic regulation in Staphylococcus aureus but dispensable for viability in anaerobic conditions. J. Biol. Chem. 293, 3180–3200 10.1074/jbc.M117.818716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Devaux L., Sleiman D., Mazzuoli M. V., Gominet M., Lanotte P., Trieu-Cuot P., Kaminski P. A., and Firon A. (2018) Cyclic di-AMP regulation of osmotic homeostasis is essential in group B Streptococcus. PLoS Genet. 14, e1007342 10.1371/journal.pgen.1007342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Commichau F. M., Gibhardt J., Halbedel S., Gundlach J., and Stülke J. (2018) A delicate connection: c-di-AMP affects cell integrity by controlling osmolyte transport. Trends Microbiol. 26, 175–185 10.1016/j.tim.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 8. Corrigan R. M., Campeotto I., Jeganathan T., Roelofs K. G., Lee V. T., and Gründling A. (2013) Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc. Natl. Acad. Sci. U.S.A. 110, 9084–9089 10.1073/pnas.1300595110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sureka K., Choi P. H., Precit M., Delince M., Pensinger D. A., Huynh T. N., Jurado A. R., Goo Y. A., Sadilek M., Iavarone A. T., Sauer J. D., Tong L., and Woodward J. J. (2014) The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell 158, 1389–1401 10.1016/j.cell.2014.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bai Y., Yang J., Zarrella T. M., Zhang Y., Metzger D. W., and Bai G. (2014) Cyclic di-AMP impairs potassium uptake mediated by a cyclic di-AMP binding protein in Streptococcus pneumoniae. J. Bacteriol. 196, 614–623 10.1128/JB.01041-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blötz C., Treffon K., Kaever V., Schwede F., Hammer E., and Stülke J. (2017) Identification of the components involved in cyclic di-AMP signaling in Mycoplasma pneumoniae. Front. Microbiol. 8, 1328 10.3389/fmicb.2017.01328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rocha R., Teixeira-Duarte C. M., Jorge J. M. P., and Morais-Cabral J. H. (2019) Characterization of the molecular properties of KtrC, a second RCK domain that regulates a Ktr channel in Bacillus subtilis. J. Struct. Biol. 205, 34–43 10.1016/j.jsb.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 13. Quintana I. M., Gibhardt J., Turdiev A., Hammer E., Commichau F. M., Lee V. T., Magni C., and Stülke J. (2019) The KupA and KupB proteins of Lactococcus lactis IL1403 are novel c-di-AMP receptor proteins responsible for potassium uptake. J. Bacteriol. 201, e00028–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moscoso J. A., Schramke H., Zhang Y., Tosi T., Dehbi A., Jung K., and Gründling A. (2016) Binding of cyclic di-AMP to the Staphylococcus aureus sensor kinase KdpD occurs via the universal stress protein domain and downregulates the expression of the Kdp potassium transporter. J. Bacteriol. 198, 98–110 10.1128/JB.00480-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nelson J. W., Sudarsan N., Furukawa K., Weinberg Z., Wang J. X., and Breaker R. R. (2013) Riboswitches in eubacteria sense the second messenger cyclic di-AMP. Nat. Chem. Biol. 9, 834–839 10.1038/nchembio.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pham H. T., Nhiep N. T. H., Vu T. N. M., Huynh T. N., Zhu Y., Huynh A. L. D., Chakrabortti A., Marcellin E., Lo R., Howard C. B., Bansal N., Woodward J. J., Liang Z. X., and Turner M. S. (2018) Enhanced uptake of potassium or glycine betaine or export of cyclic di-AMP restores osmoresistance in a high cyclic-di-AMP Lactococcus lactis mutant. PLoS Genet. 14, e1007574 10.1371/journal.pgen.1007574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schuster C. F., Bellows L. E., Tosi T., Campeotto I., Corrigan R. M., Freemont P., and Gründling A. (2016) The second messenger c-di-AMP inhibits the osmolyte uptake system OpuC in Staphylococcus aureus. Sci. Signal. 9, ra81 10.1126/scisignal.aaf7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huynh T. N., Choi P. H., Sureka K., Ledvina H. E., Campillo J., Tong L., and Woodward J. J. (2016) Cyclic di-AMP targets the cystathione β-synthase domain of the osmolyte transporter OpuC. Mol. Microbiol. 102, 233–243 10.1111/mmi.13456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Orr M. W., Galperin M. Y., and Lee V. T. (2016) Sustained sensing as an emerging principle in second messenger signaling systems. Curr. Opin. Microbiol. 34, 119–126 10.1016/j.mib.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi P. H., Vu T. M. N., Pham H. T., Woodward J. J., Turner M. S., and Tong L. (2017) Structural and functional studies of pyruvate carboxylase regulation by cyclic di-AMP in lactic acid bacteria. Proc. Natl. Acad. Sci. U.S.A. 114, E7226–E7235 10.1073/pnas.1704756114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gundlach J., Dickmanns A., Schröder-Tittmann K., Neumann P., Kaesler J., Kampf J., Herzberg C., Hammer E., Schwede F., Kaever V., Tittmann K., Stülke J., and Ficner R. (2015) Identification, characterization and structure analysis of the c-di-AMP binding PII-like signal transduction protein DarA. J. Biol. Chem. 290, 3069–3080 10.1074/jbc.M114.619619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Albright R. A., Ibar J. L., Kim C. U., Gruner S. M., and Morais-Cabral J. H. (2006) The RCK domain of the KtrAB K+ transporter: multiple conformations of an octameric ring. Cell 126, 1147–1159 10.1016/j.cell.2006.08.028 [DOI] [PubMed] [Google Scholar]
- 23. Vieira-Pires R. S., Szollosi A., and Morais-Cabral J. H. (2013) The structure of the KtrAB potassium transporter. Nature 496, 323–328 10.1038/nature12055 [DOI] [PubMed] [Google Scholar]
- 24. Holtmann G., Bakker E. P., Uozumi N., and Bremer E. (2003) KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity. J. Bacteriol. 185, 1289–1298 10.1128/JB.185.4.1289-1298.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gundlach J., Herzberg C., Hertel D., Thürmer A., Daniel R., Link H., and Stülke J. (2017) Adaptation of Bacillus subtilis to life at extreme potassium limitation. mBio 8, e00861–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prindle A., Liu J., Asally M., Ly S., Garcia-Ojalvo J., and Süel G. M. (2015) Ion channels enable electrical communication in bacterial communities. Nature 527, 59–63 10.1038/nature15709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujisawa M., Ito M., and Krulwich T. A. (2007) Three two-component transporters with channel-like properties have monovalent cation/proton antiport activity. Proc. Natl. Acad. Sci. U.S.A. 104, 13289–13294 10.1073/pnas.0703709104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chin K. H., Liang J. M., Yang J. G., Shih M. S., Tu Z. L., Wang Y. C., Sun X. H., Hu N. J., Liang Z. X., Dow J. M., Ryan R. P., and Chou S. H. (2015) Structural insights into the distinct binding mode of cyclic di-AMP with SaCpaA_RCK. Biochemistry 54, 4936–4951 10.1021/acs.biochem.5b00633 [DOI] [PubMed] [Google Scholar]
- 29. Zhu B., and Stülke J. (2018) SubtiWiki in 2018: From genes and proteins to functional network annotation of the model organism Bacillus subtilis. Nucleic Acids Res. 46, D743–D748 10.1093/nar/gkx908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stumpe S., and Bakker E. P. (1997) Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch. Microbiol. 167, 126–136 10.1007/s002030050425 [DOI] [PubMed] [Google Scholar]
- 31. Schirmer F., Ehrt S., and Hillen W. (1997) Expression, inducer spectrum, domain structure, and function of MopR, the regulator of phenol degradation in Acinetobacter calcoaceticus NCIB8250. J. Bacteriol. 179, 1329–1336 10.1128/jb.179.4.1329-1336.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monod J. (1949) The growth of bacterial cultures. Annu. Rev. Microbiol. 3, 371–394 10.1146/annurev.mi.03.100149.002103 [DOI] [Google Scholar]
- 33. Coutts G., Thomas G., Blakey D., and Merrick M. (2002) Membrane sequestration of the signal transduction protein GlnK by the ammonium transporter AmtB. EMBO J. 21, 536–545 10.1093/emboj/21.4.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Detsch C., and Stülke J. (2003) Ammonium utilization in Bacillus subtilis: transport and regulatory functions of NrgA and NrgB. Microbiology 149, 3289–3297 10.1099/mic.0.26512-0 [DOI] [PubMed] [Google Scholar]
- 35. Meinken C., Blencke H.-M., Ludwig H., and Stülke J. (2003) Expression of the glycolytic gapA operon in Bacillus subtilis: differential syntheses of proteins encoded by the operon. Microbiology 149, 751–761 10.1099/mic.0.26078-0 [DOI] [PubMed] [Google Scholar]
- 36. Lehnik-Habrink M., Newman J., Rothe F. M., Solovyova A. S., Rodrigues C., Herzberg C., Commichau F. M., Lewis R. J., and Stülke J. (2011) RNase Y in Bacillus subtilis: a natively disordered protein that is the functional equivalent to RNase E from Escherichia coli. J. Bacteriol. 193, 5431–5441 10.1128/JB.05500-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roelofs K. G., Wang J., Sintim H. O., and Lee V. T. (2011) Differential radial capillary action of ligand assay for high-throughput detection of protein-metabolite interaction in bacteria. Proc. Natl. Acad. Sci. U.S.A. 108, 15528–15533 10.1073/pnas.1018949108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosenberg J., Dickmanns A., Neumann P., Gunka K., Arens J., Kaever V., Stülke J., Ficner R., and Commichau F. M. (2015) Structural and biochemical analysis of the essential diadenylate cyclase CdaA from Listeria monocytogenes. J. Biol. Chem. 290, 6596–6606 10.1074/jbc.M114.630418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corrigan R. M., Abbott J. C., Burhenne H., Kaever V., and Gründling A. (2011) c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 7, e1002217 10.1371/journal.ppat.1002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mehne F. M., Schröder-Tittmann K., Eijlander R. T., Herzberg C., Hewitt L., Kaever V., Lewis R. J., Kuipers O. P., Tittmann K., and Stülke J. (2014) Control of the diadenylate cyclase CdaS in Bacillus subtilis: an autoinhibitory domain limits c-di-AMP production. J. Biol. Chem. 289, 21098–21107 10.1074/jbc.M114.562066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Commichau F. M., Dickmanns A., Gundlach J., Ficner R., and Stülke J. (2015) A jack of all trades: the multiple roles of the unique essential second messenger cyclic di-AMP. Mol. Microbiol. 97, 189–204 10.1111/mmi.13026 [DOI] [PubMed] [Google Scholar]
- 42. Wakeman C. A., Goodson J. R., Zacharia V. M., and Winkler W. C. (2014) Assessment of the requirements for magnesium transporters in Bacillus subtilis. J. Bacteriol. 196, 1206–1214 10.1128/JB.01238-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kappes R. M., Kempf B., Kneip S., Boch J., Gade J., Meier-Wagner J., and Bremer E. (1999) Two evolutionary closely related ABC transporters mediate the uptake of choline for synthesis of theosmoprotectant glycine betaine in Bacillus subtilis. Mol. Microbiol. 32, 203–216 10.1046/j.1365-2958.1999.01354.x [DOI] [PubMed] [Google Scholar]
- 44. Radchenko M. V., Tanaka K., Waditee R., Oshimi S., Matsuzaki Y., Fukuhara M., Kobayashi H., Takabe T., and Nakamura T. (2006) Potassium/proton antiport system of Escherichia coli. J. Biol. Chem. 281, 19822–19829 10.1074/jbc.M600333200 [DOI] [PubMed] [Google Scholar]
- 45. Sambrook J., and Russell D. (2001) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 46. Kunst F., and Rapoport G. (1995) Salt stress is an environmental signal affecting degradative enzyme synthesis in Bacillus subtilis. J. Bacteriol. 177, 2403–2407 10.1128/jb.177.9.2403-2407.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reuss D. R., Altenbuchner J., Mäder U., Rath H., Ischebeck T., Sappa P. K., Thürmer A., Guérin C., Nicolas P., Steil L., Zhu B., Feussner I., Klumpp S., Daniel R., Commichau F. M., et al. (2017) Large-scale reduction of the Bacillus subtilis genome: consequences for the transcriptional network, resource allocation, and metabolism. Genome Res. 27, 289–299 10.1101/gr.215293.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barbe V., Cruveiller S., Kunst F., Lenoble P., Meurice G., Sekowska A., Vallenet D., Wang T., Moszer I., Médigue C., and Danchin A. (2009) From a consortium sequence to a unified sequence: the Bacillus subtilis 168 reference genome a decade later. Microbiology 155, 1758–1775 10.1099/mic.0.027839-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., and Drummond A. (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Orr M. W., and Lee V. T. (2017) Differential radial capillary action of ligand assay (DRaCALA) for high-throughput detection of protein-metabolite interactions in bacteria. Methods Mol. Biol. 1535, 25–41 10.1007/978-1-4939-6673-8_3 [DOI] [PubMed] [Google Scholar]
- 51. Gundlach J., Mehne F. M., Herzberg C., Kampf J., Valerius O., Kaever V., and Stülke J. (2015) An essential poison: synthesis and degradation of cyclic di-AMP in Bacillus subtilis. J. Bacteriol. 197, 3265–3274 10.1128/JB.00564-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zweers J. C., Wiegert T., and van Dijl J. M. (2009) Stress-responsive systems set specific limits to the overproduction of membrane proteins in Bacillus subtilis. Appl. Environ. Microbiol. 75, 7356–7364 10.1128/AEM.01560-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lehnik-Habrink M., Schaffer M., Mäder U., Diethmaier C., Herzberg C., and Stülke J. (2011) RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol. Microbiol. 81, 1459–1473 10.1111/j.1365-2958.2011.07777.x [DOI] [PubMed] [Google Scholar]