Abstract
Malaria remains a major global health issue, affecting millions and killing hundreds of thousands of people annually. Efforts to break the transmission cycle of the causal Plasmodium parasite, and to cure those that are afflicted, rely upon functional characterization of genes essential to the parasite's growth and development. These studies are often based upon manipulations of the parasite genome to disrupt or modify a gene of interest to understand its importance and function. However, these approaches can be limited by the availability of selectable markers and the time required to generate transgenic parasites. Moreover, there also is a risk of disrupting native gene regulatory elements with the introduction of exogenous sequences. To address these limitations, we have developed CRISPR-RGR, a Streptococcus pyogenes (Sp)Cas9-based gene editing system for Plasmodium that utilizes a ribozyme–guide–ribozyme (RGR) single guide RNA (sgRNA) expression strategy with RNA polymerase II promoters. Using rodent-infectious Plasmodium yoelii, we demonstrate that both gene disruptions and coding sequence insertions are efficiently generated, producing marker-free parasites with homology arms as short as 80–100 bp. Additionally, we find that the common practice of using one sgRNA can produce both unintended plasmid integration and desired locus replacement editing events, whereas the use of two sgRNAs results in only locus replacement editing. Lastly, we show that CRISPR-RGR can be used for CRISPR interference (CRISPRi) by binding catalytically dead SpCas9 (dSpCas9) to the region upstream of a gene of interest, resulting in a position-dependent, but strand-independent reduction in gene expression. This robust and flexible system facilitates efficient genetic characterizations of rodent-infectious Plasmodium species.
Keywords: Plasmodium, CRISPR/Cas, gene regulation, ribozyme (catalytic RNA) (RNA enzyme), parasitology, ALBA, HDR, sgRNA, UIS4
Introduction
Malaria remains one of the world's most daunting public health concerns, with over 200 million infections and nearly half a million fatalities every year (1). Despite gains made to reduce transmission worldwide, there is still a need for a highly effective, licensed vaccine and additional antimalarial drugs to respond to and overcome the emergence and spread of drug resistance. To produce these new therapeutics, further studies of the causal agent of malaria, the Plasmodium parasite, are required. These studies typically rely upon reverse genetic techniques to disrupt or tag genes of interest; however, producing gene modifications in Plasmodium parasites has inherent challenges. First, transfection efficiencies are surprisingly low compared with that of model eukaryotes and human cells despite advances in nucleofection technologies improving these efficiencies a 1000-fold over initial methods (2). Second, selection of transgenic parasites commonly takes months to achieve locus replacement events in Plasmodium falciparum through the use of both positive and negative drug pressure. Moreover, only a single drug-selectable marker (DHFR)2 is commonly available for modifications of the genome of rodent-infectious species of Plasmodium such as Plasmodium yoelii, Plasmodium berghei, and Plasmodium chabaudi (3–5). In response to this, several methodologies have been developed to recycle DHFR expression cassettes (gene in marker out (GIMO), FLP/FRT, and CRE/lox recombination-based methods), but these methods are time-consuming and require multiple interventions (6–8). Finally, exogenous sequences are essentially always left in the parasite genome, and these can include expression cassettes that can influence transcription of neighboring genes, plasmid backbone sequences, and, in the best cases, a single FRT or lox site following recombinase excision (9–11).
The recent development of the clustered, randomly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) gene editing system has been shown to improve the efficiency of genome editing. This has been observed for both P. falciparum, the human-infectious species responsible for the majority of cases and deaths, and P. yoelii, a rodent-infectious species typically favored for rapid genetic manipulation, the ability to interrogate the entire life cycle, and similarities with P. falciparum in mosquito stage development and host–pathogen interactions (12–16). Importantly, CRISPR-based gene editing does not rely on integration of a selection cassette into the genome, and the edited genome can therefore be free of drug resistance/fluorescent markers and scar sequences. CRISPR-based gene editing therefore allows the interrogation of multiple genes either simultaneously or sequentially and, if properly controlled, could be done at multiple points throughout the parasite's life cycle.
CRISPR/SpCas9 gene editing systems function by expressing single guide RNAs (sgRNAs) that recruit the Streptococcus pyogenes Cas9 (SpCas9) endonuclease to a complementary 20-nucleotide sequence of genomic DNA. There, a double-strand break (DSB) is created and typically repaired with either the error-prone nonhomologous end joining or by homology-directed repair (HDR) pathways. Because Plasmodium parasites lack several essential proteins required for nonhomologous end joining, homology-directed repair of double-strand breaks predominates, with infrequent repair also occurring by microhomology-mediated end joining (17–19). Therefore, strategies for CRISPR gene editing in Plasmodium require the introduction of three components: SpCas9, sgRNAs, and an HDR template, which are typically encoded on one or two nuclear plasmids.
CRISPR-based gene editing of P. falciparum has been achieved using dual-plasmid systems, with each plasmid encoding a unique drug resistance marker, and either SpCas9 or the HDR and sgRNAs (12, 13). Efficient expression of sgRNAs was demonstrated with either an engineered T7 RNA polymerase system or the RNA polymerase III–transcribed P. falciparum U6 promoter (12, 13). Upon electroporation, parasites are then pressured with one or both drugs simultaneously to select for parasites containing all of the necessary gene editing elements (12, 13).
CRISPR/Cas9 strategies in P. yoelii, however, are limited by the availability of only one drug-selectable marker (DHFR), so all CRISPR/SpCas9 gene editing elements must be packaged onto a single vector to allow their selection. Previously described single-plasmid systems use the P. yoelii U6 promoter to express sgRNAs; however, the resulting transgenic parasites were found to retain the plasmid sequences and remained resistant to drug pressure (14). Because of this, a second system was constructed that included a negative selectable marker in the plasmid backbone so that parasites that retained a nuclear plasmid or that may have integrated the plasmid could be selected against following gene editing (20). Despite these limitations, the laboratory of J. Yuan (14, 20–23) has used CRISPR to methodically interrogate the ApiAP2 gene family and genes important for ookinete motility and have created transgenic parasites that express a constitutively expressed SpCas9 nuclease or male- and female-enriched fluorescent protein reporters.
Although significant progress has been made to date in the development of CRISPR tools for use in Plasmodium, significant limitations remain. First, most existing systems use RNA polymerase III promoters for sgRNA expression, which is preferred due to the well-defined 5′ and 3′ ends on this class of transcript. However, as RNA polymerase III promoters are strong and constitutively active as required to produce 5S rRNA, tRNAs, and other critical noncoding RNAs, their use in transcribing sgRNAs would not permit stage-specific or readily tunable expression. In systems outside Plasmodium, tools have been generated to produce multiple sgRNAs under the control of a single RNA polymerase II promoter. The ribozyme–guide–ribozyme (RGR) and the microRNA polycistron tools both utilize post-transcriptional modifications to an RNA transcript to generate pristine sgRNAs (24, 25). Second, most studies to date have targeted a single locus with one sgRNA, and those few efforts that have used multiple sgRNAs have used multiple RNA polymerase III–based cassettes to express the sgRNAs. Lastly, the adoption and widespread use of nuclease-dead variants of SpCas9 (dSpCas9) has been used in prokaryotes and eukaryotes for gene regulation by CRISPR activation (CRISPRa) and CRISPR interference (CRISPRi) but has also recently been demonstrated to work in Plasmodium using epigenetic modifiers or the VP65/p65/Rta transactivation domain (26–28). Because Plasmodium parasites lack genes essential for RNA interference (RNAi) (29), genetic tools to regulate transcript abundance (e.g. promoter swap and glmS) or protein abundance (e.g. DD/Shield1, EcDHFR-DD/trimethoprim, and TetR/DOZI) have been developed and utilized (30–33). However, these all require single or multiple modifications to the genome and introduce exogenous sequences into the locus of interest. CRISPRi is therefore a desirable tool as it would allow for regulation of the expression of specific genes without modification of the genome itself.
Here, we show that CRISPR-RGR is able to effectively generate gene deletions, tag insertions, and sequential genome editing and can be used for CRISPRi. Using an RGR method of sgRNA expression, we show that four variants of RNA polymerase II promoters can be used to express multiple sgRNAs simultaneously and that genome editing can be rapidly and efficiently achieved in P. yoelii. Additionally, we demonstrate that the number of sgRNAs used to target a gene influences the outcome of genome repair and that negative selection is not required to produce parasites with only locus replacement events observed. Finally, we demonstrate that CRISPRi is possible in P. yoelii, and we determined the efficacy of 11 individual sgRNAs targeting across the upstream portion of the gene control region of PyALBA4 that also demonstrate positional, but not strand-specific, effects. The contribution of CRISPR-RGR to the growing Plasmodium CRISPR toolbox explains and solves previous issues with current strategies that use one sgRNA, improves how synthetic biology approaches can be used to expedite gene editing, and provides a framework for CRISPRi-based gene regulation.
Results
Single-plasmid, ribozyme-mediated CRISPR/SpCas9 plasmid design for rodent-infectious Plasmodium parasites
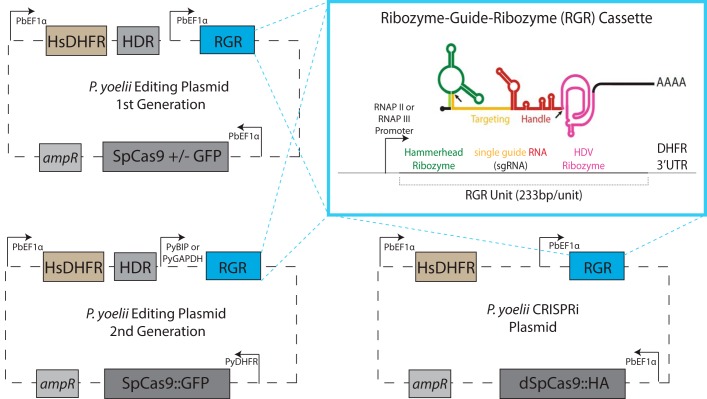
To streamline CRISPR/SpCas9 editing in P. yoelii parasites, we developed a flexible, single-plasmid construct that contains all necessary CRISPR/SpCas9 gene editing elements using a combination of P. yoelii and P. berghei promoters and terminators (Fig. 1, 1st Generation). Expression of SpCas9::GFP, HsDHFR (to provide resistance to antifolate drugs), and sgRNAs was generated by individual iterations of the strong, constitutive pbef1α promoter and the pbdhfr 3′-UTR/terminator. Each of these cassettes is flanked by unique restriction enzyme sites for easy modification and substitutions. In addition to these cassettes, we incorporated an HDR template to enable homology-directed repair of the DSBs that are created by SpCas9.
Figure 1.
Single-plasmid, ribozyme-based CRISPR/SpCas9 constructs for P. yoelii parasites. Each plasmid contains expression cassettes for SpCas9, an HsDHFR drug resistance marker, and sgRNAs via the RGR approach (see inset). For gene editing plasmids, an HDR template is also included. Inset, precise autocatalytic cleavage by both hammerhead and hepatitis delta virus (HDV) ribozymes release the sgRNA without extra bases attached. The location of the ribozyme cleavage is noted by black arrows. RNAP, RNA polymerase.
The major differences between this CRISPR-based editing system for Plasmodium and those previously described lie in the method of expression of the sgRNAs and the preparation of the sgRNA and HDR template sequences. Existing Plasmodium CRISPR systems use RNA polymerase III–driven U6 promoters or T7 RNA polymerase–based systems for sgRNA expression (12, 13). In contrast, we have expressed a transcript encoding an RGR unit that uses a minimal hammerhead ribozyme and a hepatitis delta virus ribozyme to flank the sgRNA on its 5′ and 3′ ends, respectively (Fig. 1, inset). This RGR approach, first described in yeast (24) and since used in Leishmania (34) and zebrafish (35), generates sgRNAs with precisely defined 5′ and 3′ ends and allows for the simultaneous expression of multiple guides under control of a single promoter, including RNA polymerase II promoters. Although RNA polymerase II promoters are far more abundant than RNA polymerase III promoters, they are not typically used for sgRNA expression as their transcripts can initiate from multiple transcriptional start sites and are capped and polyadenylated. The potential impact of these 5′ extensions and modifications upon sgRNA activity are not sufficiently understood to confidently use them for this application. However, the inclusion of autocatalytic, self-cleaving ribozymes within an RNA eliminates these potential problems with RNA polymerase II transcripts, and their beneficial properties of stage-specific and tunable expression levels can be used. Moreover, these individual RGR units can be polymerized into an RGR array on a single transcript and be used to generate multiple sgRNAs. In addition, our sgRNA design contains an extended duplex and a single base change compared with the original sgRNA sequence, which has been shown to have increased editing efficiency (36). Finally, we leverage advances in synthetic biology to create custom DNA fragments that can include the RGR transcript, short HDR templates, or both, which greatly expedites plasmid generation. We anticipate that as the cost of gene synthesis continues to decrease, these approaches can be scaled for use in both forward and reverse genetic screens. A step-by-step tutorial for construct generation is provided in File S1.
Genetic disruption of pyalba4
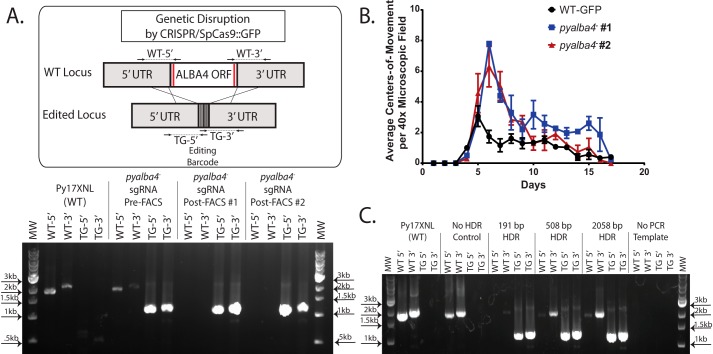
To functionally test this single-plasmid, CRISPR-RGR system, we targeted the gene encoding the PyALBA4 RNA-binding protein, which we have characterized previously (11). Using conventional reverse genetics approaches, we have shown that pyalba4 can be deleted in asexual blood-stage parasites, which results in the production of 2–3-fold more mature male gametocytes that can exflagellate as compared with wildtype (WT) parasites. Additionally, a C-terminal GFP tag can be introduced with no observable effect upon parasite growth or transmission. To delete pyalba4 by CRISPR-RGR, two sgRNA targets were chosen at the 5′ and 3′ ends of its coding sequence by manually scanning for NGG PAM motifs and subsequent computational assessment using the Eukaryotic Pathogen CRISPR guide RNA/DNA design tool (http://grna.ctegd.uga.edu)3 (37) (Fig. 2A, red vertical lines). This tool provides a score for each sgRNA based on the target specificity within the genome as well the GC content and position-specific nucleotide composition for bases that have been shown to affect sgRNA efficiency. Additionally, this tool will flag any sgRNA with long poly(T) tracts (more than four in a row), which can cause early termination of RNA polymerase III transcripts. Because the RGR system utilizes RNA polymerase II promoters (e.g. pbef1α, pygapdh, and pybip), we are not limited by sgRNAs containing poly(T) tracts that are prevalent in Plasmodium genomes (37). For the first trials of our system, we chose to use the pbef1α promoter to drive expression of the RGR. The initial HDR template was designed with homology arms comprising ∼800 bp of sequence homologous to the target gene on either side of the two DSBs with a unique 18-bp DNA barcode between them that could be used for unambiguous, simple genotyping PCR. Notably, this barcode is not necessary for genome editing and could be omitted to produce completely scarless modifications without the introduction of any exogenous sequences.
Figure 2.
A single-plasmid, ribozyme-based CRISPR/SpCas9 system efficiently edits the P. yoelii genome. A, the pyalba4 ORF was deleted by expressing two sgRNAs that target the 5′ and 3′ ends of the coding sequence (red lines in schematic), with transgenic parasites seen within 6 days of constant drug selection. Genotyping PCR showed that the majority of the parasite population had been edited by locus replacement. Enrichment by FACS of parasites expressing SpCas9::GFP resulted in the selection of a completely transgenic (TG) parasite population by genotyping PCR. B, two clones of the CRISPR-generated pyalba4− transgenic parasites produce 2–3-fold more male gametocytes that can be activated into gametes as compared with WT parasites. This activation is measured as centers of movement in a 40× microscopic field. Error bars represent S.E. C, a range of HDR template sizes were tested (191, 508, and 2058 bp). All HDR templates allowed efficient editing of the pyalba4 locus.
Upon transfection of this plasmid into WT P. yoelii (17XNL strain) parasites with constant pyrimethamine selection, mice reached 1% parasitemia in 8 days, which is a similar time frame to that of conventional techniques (3). We observed that a large subset of these parasites showed expression of SpCas9::GFP by live fluorescence microscopy (data not shown), and genotyping PCR analysis showed efficient editing of the pyalba4 locus (Fig. 2A, bottom). Furthermore, by enriching for SpCas9::GFP-positive schizonts via fluorescence-activated cell sorting (FACS), only edited parasites were present by genotyping PCR. Thus, this CRISPR-RGR approach rapidly produced a transgenic parasite population with no observable WT parasites present using as few as two mice.
We further verified that this population of CRISPR-generated pyalba4− parasites had the same phenotype as pyalba4− transgenic parasites generated by conventional reverse genetic approaches. Quantification of the activation of male gametocytes into gametes (measured as centers of movement/exflagellation centers via light microscopy) in the pyalba4− line revealed a similar 2–3-fold increase in the number of activated male gametocytes as compared with WT parasites, which was sustained across the full duration of the infection (Fig. 2B).
Because CRISPR-RGR rapidly and efficiently produced transgenic parasites when providing large homology arms in the HDR template, we tested the effect that lengthening (∼1000 bp each arm) and shortening (∼80–100 bp; ∼250 bp each arm) the homology arms had upon gene editing. We observed that all homology arm lengths allowed for efficient gene editing and that the smallest HDR tested (80–100 bp each arm) had equally efficient editing (as evidenced by the least intense PCR amplicons for WT parasites) and could be selected in the same amount of time as was required for the longer HDR templates (Fig. 2C). Importantly, HDR arms of this length, even with the skewed A–T content of the P. yoelii genome, can be chemically synthesized.
It is notable that over the course of these experiments, we observed that recombination was occurring in Escherichia coli between two instances of the pbef1α promoter and that the RGR portion of the plasmid was being excised. To stabilize the plasmid, we constructed a second generation of editing plasmids with no repeated elements and have not observed spurious recombination events occurring with this new design (Fig. 1, 2nd Generation, and Fig. S1). This second-generation design includes a single iteration of the pbef1α promoter and pyef1α 3′-UTR to control expression of HsDHFR, the pydhfr promoter and pbdhfr 3′-UTR to control expression of SpCas9::GFP, and either the pybip promoter (Fig. S1A) or pygapdh promoter (Fig. S1B) with the pybip 3′-UTR to control transcription of the RGR element. Using the same sgRNA targets and the 191-bp HDR template, we found that both promoters driving RGR expression edited parasites efficiently (Fig. S1). Furthermore, we again verified with FACS and genotyping PCR that parasites expressing SpCas9::GFP only contained the edited pyalba4 locus. Together, these results show that single-plasmid CRISPR-RGR is able to efficiently and robustly create gene deletions in P. yoelii parasites.
Insertion of a GFP reporter by CRISPR-RGR
We next used CRISPR-RGR to append a C-terminal GFP epitope tag to PyALBA4. To accomplish this, we chose a single sgRNA target (1× sgRNA) 18 bp downstream of the stop codon and designed an HDR template with ∼700-bp homology arms flanking an in-frame GFP tag (Fig. 3A). A base change of the NGG PAM sequence (shield mutation) was included in the HDR template so that SpCas9 ceases to bind and cut this locus postediting. These modifications were built into the first-generation CRISPR plasmid with the pbef1α promoter driving sgRNA expression. Transfection of this plasmid, along with constant pyrimethamine selection for 7 days, yielded a largely transgenic parasite population (Fig. 3B, left) with PyALBA4::GFP expression matching that seen in transgenic parasites created by conventional methods (Fig. 3C).
Figure 3.
The number of sgRNAs used influences the outcome of genome repair. A, insertion of a GFP tag at the 3′ end of the pyalba4 coding sequence was accomplished by using either one or two sgRNA targets (red lines in schematic). Each of these approaches requires a shield mutation in the HDR template to destroy the PAM site and prevent SpCas9 cleavage after the repair. B, genotyping PCR shows that the use of one sgRNA resulted in both plasmid integration (PI) into the targeted locus and the desired locus replacement (LR) event. The use of two sgRNAs resulted in only locus replacement and no plasmid integration. C, CRISPR-RGR–modified pyalba4::gfp parasites show an expression pattern of ALBA4 in schizonts similar to that seen with conventionally tagged PyALBA4 parasites. ACP is a marker of the apicoplast morphology and is used for staging of parasites. Scale bar, 5 μm. DAPI, 4′,6-diamidino-2-phenylindole.
In contrast to conventional reverse genetic techniques, CRISPR/SpCas9 gene editing can be accomplished without leaving a drug-selectable marker in the edited locus. This enables multiple, sequential gene edits to be made after curing the delivery plasmid, which is particularly useful with rodent-infectious malaria parasites where only one drug-selectable marker (DHFR) is available. However, previous work has shown that completely curing these plasmids is challenging as resistant parasites can be recovered more than 50 days post-removal of drug pressure (20). To circumvent this issue, methods have been developed to negatively select against parasites that retained the delivery plasmid following genome modification (20). We similarly attempted to cure the plasmid from FACS-selected PyALBA4::GFP parasites produced using one sgRNA and observed that the parasites remained drug-resistant after more than 14 days following removal of drug pressure. An expanded genotyping PCR assay that interrogates for both plasmid integration and locus replacement showed that both editing outcomes had occurred (Fig. 3B).
We reasoned that introduction of two DSBs, and thus two genome repair events, would ensure that only locus replacement events would result. To test this, we selected a second sgRNA target in the pyalba4 3′-UTR downstream of the original sgRNA target and introduced a second shield mutation into the HDR template (Fig. 3A). Transfection of this plasmid, coupled with constant selection with pyrimethamine, produced PyALBA4::GFP-expressing parasites. As before, genotyping PCR showed that a significant fraction of the parasite population had been modified. Notably, this two-sgRNA design yielded only the desired locus replacement events and showed no evidence of plasmid integration (Fig. 3B, right). Furthermore, upon removal of drug pressure, the parasites quickly (<1 week) became sensitive to pyrimethamine once more and were amenable to subsequent transfections.
Sequential modifications with CRISPR-RGR
It is often desirable to make multiple changes in the genome of a single parasite (e.g. tagging multiple proteins or deleting multiple genes). Because CRISPR-RGR genome editing can result in markerless parasites, sequential rounds of editing using the same drug-selectable marker for enrichment of modified parasites are possible. To this end, we deleted pyuis4, which is also dispensable in asexual blood-stage parasites, in both WT and the pyalba4::gfp parasites (Fig. 4, A and B). The pyuis4 CRISPR plasmid was produced using a chemically synthesized DNA fragment that included an HDR template with ∼400 bp of homology on either side of a barcode sequence and an RGR cassette containing two sgRNAs to target the 5′ and 3′ sides of the pyuis4 ORF (Fig. 4, A and B). For expression of the RGR transcript, we subcloned a shorter variant of the pybip promoter that retains full activity. Upon transfection into either WT or pyalba4::gfp parasites, we were able to rapidly create pyuis4− transgenic parasites (Fig. 4, A and B).
Figure 4.
Sequential gene deletions of pyuis4 and pyalba4 in WT and pyalba4::gfp parasites. A, deletion of pyuis4 was accomplished by targeting each end of its coding sequence with as sgRNA (indicated with red vertical lines). The sgRNAs were expressed under the control of a minimal pybip promoter (∼0.5 kb). The HDR template was constructed with ∼400 bp of homology on either side of a barcode sequence. The pyuis4− CRISPR plasmid was transfected into both P. yoelii 17XNL strain (WT) and pyalba4::gfp parasites. B, the gene deletion events were determined using primers that could amplify across both the WT (1.7 kb) and the transgenic (TG; 677 bp) pyuis4 locus. In both technical and biological replicates of WT and pyalba4::gfp parasites, there is a mixed population of WT and TG pyuis4 parasites. This indicates that both naïve parasites and those that have previously been modified can be edited by CRISPR-RGR. NTC, no-template control; MW, molecular weight pPSU ladder. C, to delete the pyalba4 coding sequence in pyalba4::gfp parasites, we used the same sgRNA targets used in the original pyalba4 disruption strategy (red vertical lines). Shield symbols denote the shield mutations that were made during the initial insertion of GFP. D, the genotyping PCR strategy utilized primers that would extend across the pyalba4::gfp locus (knockin (KI); 1.6 kb) or the pyalba4− locus (knockout (KO); 477bp). In the two technical replicates, there is evidence of both knockin and knockout parasites. NTC, no-template control; MW, molecular weight pPSU ladder. E, live fluorescence images of a pyalba4::gfp parasite transfected with the pyalba4− CRISPR plasmid. GFP signal colocalizes with 4′,6-diamidino-2-phenylindole (DAPI), indicating that SpCas9::GFP is present, whereas PyALBA4::GFP is not.
It is also desirable to make sequential edits to the same genomic locus in some circumstances (e.g. introduction of multiple tags or gene deletion/complementation). We therefore targeted the pyalba4::gfp modified locus for gene deletion (Fig. 4, C and D). For this gene deletion, we could use the same sgRNAs as used in our original pyalba4− plasmid, but we modified the HDR template to include sequence homologous to the modified locus (Fig. 4, C and D). As before, the deletion of pyalba4 ORF occurred rapidly in pyalba4::gfp parasites, and we observed a change in the localization of GFP from cytosolic puncta typical of PyALBA4::GFP (Fig. 3C) to a diffuse, nuclear localization consistent with SpCas9::GFP localization due to the presence of multiple nuclear localization signals (Fig. 4E). These results show that sequential editing of the same or different genomic loci is readily accomplished using CRISPR-RGR.
A single-plasmid system for ribozyme-mediated CRISPR interference
CRISPRa and CRISPRi, which target dSpCas9 to locations on the genome to activate or repress transcription, respectively, have emerged as effective methods to study essential genes in prokaryotes, model eukaryotes, and human cells (38–40). CRISPRa and CRISPRi largely rely upon the fusion of transcriptional activation or repression domains to dSpCas9 to produce these effects on transcription. Recently, domains that typically activate transcription in many eukaryotes (e.g. VP64, p65, and Rta) were shown to work similarly in Plasmodium and are important additions to the weakly transactivating sequences from apicomplexan proteins that were used previously (28, 41). Because no trans-repressive domains have been reported to be functional in Plasmodium, we aimed to use CRISPRi with dSpCas9 alone to prevent or dampen association of RNA polymerase II or other critical factors from binding to a specific gene.
For this approach, we constructed a CRISPRi plasmid similar to our first-generation CRISPR plasmid but without an HDR template, which is unnecessary for this application (Fig. 1). This plasmid encodes a nuclease-dead dSpCas9 endonuclease (D10A,H840A) with an appended 1xHA tag, which permits the detection of dCas9:HA-expressing parasites for flow cytometry. To test our CRISPRi plasmid, we utilized the markerless pyalba4::gfp transgenic parasite line, which was enriched by FACS to remove WT parasites and was cloned by limiting dilution. Upon curing the CRISPR-RGR plasmid used to create this parasite line, these parasites regained pyrimethamine sensitivity, thus permitting introduction of another plasmid. We chose this parasite line because the fusion of GFP to the C terminus of PyALBA4 1) has no effect upon parasite growth and transmission, 2) is dispensable to asexual blood-stage parasites, and thus 3) could allow direct testing of CRISPRi upon an endogenous gene and its gene control region (11). This final point is pertinent as exogenous reporter expression cassettes have not accurately represented how well other gene regulation systems can affect the expression of endogenous genes.
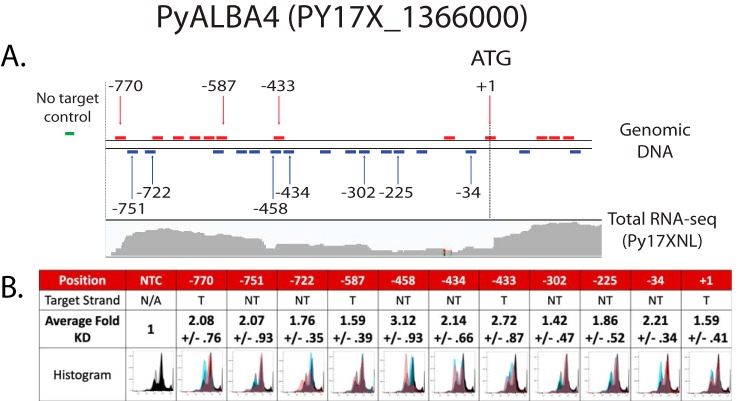
Using RNA-Seq data to estimate the 5′-UTR of pyalba4, we selected 11 individual sgRNA targets between the start of the contiguous RNA-Seq reads (approximately −800) and the ATG (Fig. 5A and Fig. S2). We selected sgRNAs that will target either the template or nontemplate strands of DNA to assess whether strand-specific effects occur in Plasmodium and that target DNA at various distances away from the ATG to assess positional effects (Fig. 5A). The nomenclature used for sgRNAs is based upon the distance of the N of the -NGG PAM sequence (e.g. −770) compared with the A of the ATG translational start codon (+1). As an experimental control, we also included a “no-target” sgRNA control, consisting of conserved sequences found in mCherry, mOrange, and Tomato fluorescence markers (COT control) that are absent in this parasite. These 12 sgRNAs were cloned into independent CRISPRi plasmids, and each was transfected into the drug markerless pyalba4::gfp parasite line.
Figure 5.
CRISPR interference of PyALBA4::GFP expression depends on the sgRNA target location. A, top, a schematic representation of the region upstream of the pyalba4 coding sequence with all possible sgRNAs indicated by colored bars (red bars are sequences found on the template strand; blue bars are on the nontemplate strand). The 11 sgRNAs used for CRISPRi are indicated by arrows, with their numerical names denoting the position of the -N of the -NGG PAM sequence in reference to the ATG. Bottom, RNA-Seq reads of Py17XNL parasites show the contiguous RNA-Seq reads upstream of the pyalba4 coding sequence. B, each sgRNA was tested in biological triplicate, with two technical replicates each, and compared with the no-target control sgRNA. The average -fold knockdown (KD) and S.E. of the mean were calculated and combined across all replicates. Histograms are representative images (all from biological replicate 2), which represent the GFP intensities for two technical replicates of the no-target control sgRNA (black and gray) and the on-target experimental sgRNA (red and blue). NTC, no-target control; T, template; NT, nontemplate.
To assess whether we could impose CRISPRi regulation upon this locus, we used flow cytometry to monitor PyALBA4::GFP protein abundance. Because P. yoelii rapidly evolves de novo resistance to pyrimethamine, effects upon PyALBA4::GFP protein levels were monitored only in dSpCas9::1xHA-positive parasites that are capable of transcriptional control (Fig. 5 and Fig. S3). Parasites were synchronized to the schizont stage to minimize the effect of stage-specific variance in PyALBA4::GFP expression upon these observations. Parasites with background levels (negative or low) of dSpCas9::1xHA did not have a significant change in the median fluorescence intensity (MFI) of PyALBA4::GFP when using any of the on-target sgRNAs when compared with the no-target sgRNA control (Fig. S3 and Table S1). In contrast, parasites expressing dSpCas9::1xHA above background produced a 2–3-fold reduction in PyALBA4::GFP signal when one of six of the 11 on-target sgRNAs was expressed (Fig. 5B; maximum knockdown was 3.12-fold). The two sgRNAs that produced the largest reduction in PyALBA4::GFP expression have targeting sequences that overlap the same sequence by 17 nucleotides, with one (−458) targeting the nontemplate strand and the other (−433) targeting the template strand. This indicates that transcription of pyalba4 is particularly sensitive to the binding of dSpCas9 at this position. Comparison of this region's sequence with known transcriptional effectors of the P. falciparum ApiAP2 family, we found that the core motif of AP2-G (PF3D7_1222600), GTAC, is present in the seed sequence and PAM site of these two sgRNAs, although the functional significance of this remains to be determined (42). We did not observe any significant strand-dependent effects (average of 1.99-fold knockdown for template-strand targets; average of 2.08-fold knockdown for nontemplate-strand targets).
One of the distinct advantages of CRISPR-RGR is that it allows for simultaneous expression of multiple sgRNAs. We reasoned that the expression of multiple sgRNAs that would tile dSpCas9 across the region upstream of pyalba4 coding sequence would increase the knockdown effect as compared with that possible with only one sgRNA. To test this, we generated a 2xRGR-CRISPRi and a 10xRGB-CRISPRi plasmid and transfected them into the pyalba4::gfp parasite line. The 10xRGB (ribozyme–guide–backbone) differs from the RGR system by containing only the hammerhead ribozyme at the 5′ end of each sgRNA as extensions off the 3′ end of sgRNAs are now known to be tolerated (26, 43, 44). Interestingly, we found that the addition of multiple guides had little to no effect on PyALBA4::GFP expression (Fig. S4).
Taken together, these data show that CRISPRi with individual sgRNAs can modestly reduce expression of an endogenous gene in P. yoelii even without the fusion of a transcriptionally repressive domain. However, the addition of multiple sgRNAs does not improve the extent of the gene regulatory effect. These data further underscore the strong need to identify and characterize domains that can repress transcription in Plasmodium to improve the scale of regulation possible by CRISPRi. This system provides an excellent platform on which to build those studies.
Discussion
Here, we present CRISPR-RGR, a ribozyme-based CRISPR system for P. yoelii that allows rapid and efficient gene editing in rodent-infectious parasites. This approach is powerful and provides advantages over current methods. First, the production of multiple sgRNAs from a single transcript greatly reduces the potential size of plasmids required for CRISPR-based gene editing by using a single promoter and terminator for sgRNA expression. Importantly, these sgRNAs can be designed to target a single gene in multiple locations as used here but can be extended to target multiple genes. The utility of using multiple sgRNAs for a single gene is evident from the data presented here: the use of one sgRNA can result in a mixture of gene editing outcomes (plasmid integration and locus replacement), whereas the use of two sgRNAs produced only the desired locus replacement result. We strongly suggest that two or more sgRNAs be used for CRISPR-based gene editing where locus replacement is the desired outcome to eliminate the need for negative selection. Second, CRISPR-RGR can be programmed using synthetically produced DNA fragments that can encode the RGR, HDR, or both elements, which can be inserted in one or two molecular cloning steps. With the anticipated decreases in cost and increases in capacity to synthesize large DNA fragments, this approach will streamline reagent preparation and enable CRISPR screens at scale. Also, because the sequence conservation of these gene control elements between P. yoelii and P. berghei is exceptionally high and that in fact a mixture of elements from both species are used in the plasmids describe here, we anticipate that gene editing using these plasmids will be possible in P. berghei as well. Lastly, the FACS-based selection of transgenic parasites expressing SpCas9::GFP or a protein of interest fused to GFP reduces the number of mice required to produce transgenic parasite lines free from observable WT parasites. Because it is still possible that WT parasites remain in the population at levels lower than the limit of detection of PCR, caution is urged when phenotyping these parasites, and it is our opinion that these parasites should be cloned prior to these studies.
CRISPR-RGR has a range of gene editing efficacies (50–100% editing) and time requirements that are comparable with other CRISPR systems in Plasmodium (12, 13, 20). However, we could rapidly isolate transgenic parasite populations (as per genotyping PCR) by selecting P. yoelii parasites that express SpCas9::GFP by FACS. Coupled with the use of two sgRNAs to produce only locus replacement gene editing events, thus obviating the need for negative selection, CRISPR-RGR can generate the desired transgenic parasites quickly and with a minimum number of mice. Importantly, for subsequent genome modifications to be done, it is essential for these parasites to regain drug sensitivity so that another plasmid can be introduced. CRISPR-RGR achieves this effectively upon curing of the original plasmid, and we show that subsequent modifications are possible in the same or different genomic loci.
Additionally, we demonstrate that CRISPR interference is possible in P. yoelii simply by binding dSpCas9 to the upstream gene control region of an endogenous gene, pyalba4. In contrast to early reports of the “rules” of CRISPRi in prokaryotes, model eukaryotes, and human cells, we did not observe a strand-specific effect on the knockdown efficiency of dSpCas9. However, we did observe a positional effect, with the maximum effect of dSpCas9 binding ∼430–450 bp upstream of the translational start site of pyalba4. This positional effect was further corroborated by observing a similar knockdown effect when using two sgRNAs with overlapping target sequences (17 of 20 nucleotides). An sgRNA (−434) that causes dSpCas9 to be targeted adjacent to the site targeted by −458 and −434 produced a 2.14-fold knockdown as well. Although a 3.12-fold maximum knockdown was observed, it would be advantageous to achieve much higher regulatory control.
Although we anticipated that the simultaneous expression of multiple sgRNAs would improve the extent of transcriptional regulation by CRISPRi, no improvement was observed. This may be due to the effects of the dSpCas9–sgRNA interaction with its target DNA in which the dsDNA is unwound to allow access of the sgRNA, thus overwinding the neighboring bases. Farasat and Salis (45) demonstrate that by increasing the number of targets in a small area of DNA (e.g. 60-bp spacing), an increase in the activation energy would be needed to unwind the overwound, neighboring DNA. This indicates that the spacing of sgRNA targets is an important consideration when attempting to tile dSpCas9 across a genomic locus. This has practical implications not only for CRISPR but also for CRISPRa. For instance, in a preprint, Shrinivas et al. (46) have explored the role that the presence and density of multiple transcriptional factor–binding sites can have in producing transcriptional condensates (liquid–liquid phase-separation droplets) at a promoter. They demonstrate that higher-density binding of specific transcription factors to a promoter correlates with increased transcriptional activation of a luciferase promoter. Taken together, identifying the optimal binding site density for dSpCas9, which occupies ∼30 bp, will be important for its use in gene regulation (47).
Gene regulatory control by CRISPRa/CRISPRi is greatly enhanced by the fusion of transcriptional activation or transcriptional repression domains to dSpCas9. Xiao et al. (28) have recently demonstrated in P. falciparum that fusion of the PfGCN5 histone acetyltransferase or the PfSir2a histone deacetylase to the C terminus of dSpCas9 can up-regulate or down-regulate transcription of targeted genes, respectively, presumably through epigenetic modulation of nucleosomes near the dSpCas9-binding site. However, because epigenetic marks can spread from the initially modified nucleosome, it is important to be mindful of potential effects on neighboring genes when using such a system. To alleviate this concern, they also demonstrated that attaching the VP64-p65-Rta synthetic fusion protein to dSpCas9 can also trans-activate transcription (28). In all instances, they demonstrated that C-terminal fusions to dSpCas9 are viable and functional, which will expedite the search for strong trans-activation/trans-repressive domains that do not rely upon epigenetic modifications.
Based upon these findings, there are several improvements that can be made to improve CRISPR-based gene editing and regulation, some of which can be addressed using CRISPR-RGR. First, the transfection efficiency of all Plasmodium species is woefully low (e.g. 0.01–0.05%) compared with other eukaryotes (often >70%), even with the use of Amaxa nucleofectors (49). However, it is notable that these efficiencies are typically determined by the number of parasites receiving and establishing a plasmid and expressing some marker from it (e.g. GFP) and thus reflect a practical transfection efficiency for most applications. Work from the DeRisi laboratory (50) demonstrated that the transfection of proteins into P. falciparum is much more efficient, with up to 1.7% of parasites receiving a Cas9::RFP protein. Thus, if delivery of SpCas9 bound to sgRNAs and an HDR template can be achieved with Plasmodium, plasmid-based gene editing may become unnecessary. Second, many genes of interest are essential to asexual blood-stage parasites and thus cannot be deleted in this stage using conventional approaches. Instead, recombination-based approaches with stage-specific promoters can enable their excision at later time points, as long as these promoter are not sufficiently leaky or activate prematurely. Although current CRISPR methodologies could use stage-specific promoters to drive SpCas9 expression, the leakiness of a single promoter could present similar problems. Using CRISPR-RGR, two discrete RNA polymerase II promoters can be utilized to express the RGR and SpCas9 components, thus reducing effects of leaky expression by just one of the promoters. Third, the range of efficiencies of sgRNAs is large and hard to predict computationally. Recent work by the Desai laboratory (51) has completed a meta-analysis of a large number of sgRNAs that they have used in P. falciparum and identified parameters that correlate with higher specificities and on-target efficiencies. An alternative approach would be to use multiple sgRNAs for each gene editing attempt, which can be achieved by CRISPR-RGR and appropriately designed HDR templates. Lastly, a similar strategy of using multiple, optimally placed dSpCas9/sgRNA complexes may improve the extent of gene regulation possible. Coupled with effective trans-activation or trans-repression domains or epigenetic regulators, we anticipate that substantial regulatory effects can be achieved using CRISPR-RGR.
Experimental procedures
Ethics
All animal handling followed the Association and Accreditation of Laboratory Animal Care guidelines, and these protocols were approved by the Pennsylvania State University Institutional Animal Care and Use Committee (approval number 42678-01). In addition, all procedures with vertebrate animals were conducted in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health with approved Office for Laboratory Animal Welfare assurance.
Experimental animals and parasite lines
Six- to 8-week-old female Swiss Webster mice were obtained from Envigo and used for all experiments described. P. yoelii 17XNL nonlethal parasites were used for all experiments.
Plasmid construction
A step-by-step guide for creating RGR-based sgRNAs for Plasmodium is provided as File S1. The CRISPR editing and CRISPR interference plasmids (illustrated in Fig. 1 and Fig. S1 with sequences provided in File S2) were constructed from a combination of conventional molecular cloning and gene synthesis (GeneWiz) approaches. S. pyogenes Cas9::GFP and dSpCas9 coding sequences were obtained from Addgene (plasmids 48138 and 42335) and were subcloned into plasmid pSL0444 containing the HsDHFR drug cassette (primer sequences are listed in Table S1). An empty cassette consisting of the pbef1α promoter and pbdhfr 3′-UTR were synthesized (GeneWiz) and cloned into the HsDHFR+Cas9::GFP and HsDHFR+dSpCas9 plasmids and used for expression of synthetic hammerhead RGR transcripts. RGR coding sequences were synthesized (GeneWiz; sgRNA target sequences are provided in Table S3) based on the “optimized” sgRNA structure described previously (36). HDR templates required for gene deletions and gene insertions (mediated by the editing plasmids) were generated by splicing by overlap extension PCR amplification of P. yoelii genomic DNA with oligonucleotides specific to the pyalba4 locus. In addition, exogenous barcode sequences (gene deletions) or a GFPmut2 tag (gene insertion) were included between the two arms of the HDR template. The second-generation editing plasmids were constructed by synthesizing two portions of the first-generation plasmid with different promoters and 3′-UTRs to reduce the possibility of recombination events. A plasmid (pSL1156) containing the pyef1α 3′-UTR, a multiple cloning site, and the pyalba4 191-bp HDR template was used to move these sequences into the first-generation CRISPR editing plasmid (pSL0999) via XbaI and NheI. A DNA fragment including pybip 3′-UTR and pydhfr promoter regions were then cloned into the AgeI and SpeI sites found between the RGR and Cas9 sequences. The penultimate construction (pSL1165) has no repetitive or redundant elements controlling SpCas9, RGR, and HsDHFR expression cassettes, with a multiple cloning site for insertion of unique promoters to drive RGR expression. Candidate promoters for the RGR cassette were generated by PCR-amplifying 1.5 kb of the sequences upstream from (and including) the ATG of pybip (PY17X_0822200) and pygapdh (PY17X_1330200) with NheI and NotI RE sites on the 5′ and 3′ ends, respectively (primer sequences are listed in Table S1). These products were inserted into and sequenced in pCR-Blunt and then subcloned into pSL1165 to create pSL1166 (using the pybip promoter) and pSL1211 (using the pygapdh promoter). The pyuis4-targeting CRISPR plasmid was constructed by replacing the pyalba4 HDR–promoter–RGR sequence of pSL1166 with a synthetic pyuis4 HDR–multiple cloning site–RGR fragment. A minimal (500-bp) pybip promoter was inserted into the multiple cloning site to drive expression of the RGR transcript. These plasmids were transfected into P. yoelii 17XNL strain parasites and were analyzed for their editing efficiency. The empty vector and gene-targeted versions of plasmids described in this work will be available on Addgene.
Generation of transgenic P. yoelii parasites
Transgenic P. yoelii parasites were generated as described previously (3, 52) but with constant pyrimethamine selection post-transfection. The presence of transgenic parasites was confirmed by genotyping PCR using primer with sequences external to the targeting sequences (Table S1). The pPSU molecular weight ladder was used in all genotyping PCR gels (53).
FACS
Synchronized and Accudenz-purified P. yoelii schizonts (3, 52) were sorted on a Beckman Coulter MoFlo Astrios EQ Cell Sorter for SpCas9::GFP or PyALBA4::GFP expression above background (determined using uninfected blood as a negative control). For each replicate, 5,000–10,000 GFP-positive events were sorted and i.v. injected into the tail vein of a naïve mouse. Pyrimethamine pressure was retained when selecting for parasites with a plasmid until parasitemia reached ∼1%. Parasite populations were assessed by genotyping PCR.
Curing the CRISPR:RGR plasmid from pyalba4::gfp parasites
Sorted pyalba4::gfp parasites were injected intravenously into a naïve mouse, and parasites developed for 9–10 days. Once ∼1% parasitemia was reached, these parasites were cloned out by limited dilution to achieve a clonal population of pyalba4::gfp parasites. Naïve mice were infected with these parasites for 4 days and then treated with pyrimethamine-drugged water to test drug sensitivity.
Exflagellation measurements
Male gametocyte activation was determined by counting the number of exflagellation events in 10 40× magnification phase-contrast fields (Leica ICC50 HD) in a confluent monolayer of blood cells obtained from a tail snip. These measurements were taken daily for 18 days postinfection with either WT or transgenic parasite lines. Three biological replicates were performed for each line, each with one mouse.
Live fluorescence and immunofluorescence assays (IFAs)
PyALBA4::GFP-expressing parasites were imaged by both live fluorescence and indirect immunofluorescence assays. Live fluorescence and IFA micrographs were collected on a Zeiss fluorescence/phase-contrast microscope (Zeiss Axioscope A1 with eight-bit AxioCam ICc1 camera) on a 63× objective. IFAs were performed as described previously (48) at room temperature with a 2-h blocking step and 1-h incubations with primary antibodies (mouse anti-GFP (DSHB-GFP-4C9-ds) and rabbit anti-acyl-carrier protein (ACP) (Pocono Rabbit Farm and Laboratory, custom), both diluted 1:1000) and secondary antibodies (anti-mouse and anti-rabbit Alexa Fluor–conjugated AF488 and AF594, respectively (Invitrogen, catalog numbers A-11001 and A-11012), both diluted 1:500). Images from both live fluorescence and IFAs were collected and processed with Zen imaging software (Zeiss).
Flow cytometric assessment of CRISPR interference
Schizonts containing one of the CRISPRi plasmids (1xRGR, 2xRGR, or 10xRGB) were synchronized by overnight in vitro growth and purified by a discontinuous Accudenz density gradient (3, 52) and prepared identically to IFA samples as described above. Samples were stained with rabbit anti-GFPmut2 (Pocono Rabbit Farm and Laboratory, catalog number 32180) bound by anti-rabbit IgG conjugated to AF488 (Invitrogen, catalog number A-11008) and mouse anti-HA (Clone 12CA5) bound by an anti-mouse IgG conjugated to AF405 (Invitrogen, catalog number A-31553). Samples were analyzed on a BD-LSR Fortessa using uninfected red blood cells or with red blood cells infected with Py17XNL WT parasites or PyALBA4::GFP-expressing parasites as negative and positive controls. Gating was established using samples stained with a single primary/secondary antibody for each channel. Data were processed using FlowJo (v10.4.1) to determine the change in MFI of GFP expression in parasites expressing dSpCas9::1xHA. MFIs were averaged within each biological replicate, and -fold change for each experimental sgRNA compared with the no-target control was calculated. The -fold change values were averaged across biological replicates for each sgRNA target.
Author contributions
M. P. W. and S. E. L. conceptualization; M. P. W. and S. E. L. data curation; M. P. W. and S. E. L. formal analysis; M. P. W. and S. E. L. validation; M. P. W. and S. E. L. investigation; M. P. W. and S. E. L. visualization; M. P. W. and S. E. L. writing-original draft; M. P. W. and S. E. L. writing-review and editing; S. E. L. resources; S. E. L. supervision; S. E. L. funding acquisition; S. E. L. methodology; S. E. L. project administration.
Supplementary Material
Acknowledgments
We thank Manuel Llinás and the members of the Lindner and Llinás laboratories as well as Logan Finger, Istvan Albert, Brian Dawson, and Howard Salis for critical discussions and technical assistance for this work. We also thank our animal caretakers and the Penn State Genomics Core Facility and Flow Cytometry Facility, University Park, PA.
This work was supported by National Institutes of Health Grants R01AI123341 and R21AI130692 and Pennsylvania State University start-up funds (to S. E. L.) and by a Huck Institutes of the Life Sciences Graduate Research Innovation award (to M. P. W.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S4, Tables S1–S3, and Files S1 and S2.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party-hosted site.
- DHFR
- dihydrofolate reductase
- Sp
- S. pyogenes
- RGR
- ribozyme–guide–ribozyme
- sgRNA
- single guide RNA
- CRISPRi
- CRISPR interference
- Cas9
- CRISPR-associated protein 9
- dSpCas9
- nuclease-dead variant of SpCas9
- DSB
- double-strand break
- HDR
- homology-directed repair
- CRISPRa
- CRISPR activation
- DD
- destabilization domain
- Ec
- E. coli
- Hs
- Homo sapiens
- Py
- P. yoelii
- PAM
- protospacer adjacent motif
- HA
- hemagglutinin
- MFI
- median fluorescence intensity
- RGB
- ribozyme–guide–backbone
- Pf
- P. falciparum
- IFA
- immunofluorescence assay
- ACP
- acyl-carrier protein
- AF
- Alexa Fluor.
References
- 1. World Health Organization (2018) World Malaria Report 2018, World Health Organization, Geneva [Google Scholar]
- 2. de Koning-Ward T. F., and, Janse C. J., and Waters A. P. (2000) The development of genetic tools for dissecting the biology of malaria parasites. Annu. Rev. Microbiol. 54, 157–185 10.1146/annurev.micro.54.1.157 [DOI] [PubMed] [Google Scholar]
- 3. Jongco A. M., Ting L.-M., Thathy V., Mota M. M., and Kim K. (2006) Improved transfection and new selectable markers for the rodent malaria parasite Plasmodium yoelii. Mol. Biochem. Parasitol. 146, 242–250 10.1016/j.molbiopara.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 4. Janse C. J., Ramesar J., and Waters A. P. (2006) High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 1, 346–356 10.1038/nprot.2006.53 [DOI] [PubMed] [Google Scholar]
- 5. Reece S. E., and Thompson J. (2008) Transformation of the rodent malaria parasite Plasmodium chabaudi and generation of a stable fluorescent line PcGFPCON. Malar. J. 7, 183–183 10.1186/1475-2875-7-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin J-W., Annoura T., Sajid M., Chevalley-Maurel S., Ramesar J., Klop O., Franke-Fayard B. M., Janse C. J., and Khan S. M. (2011) A novel 'gene insertion/marker out' (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PLoS One 6, e29289 10.1371/journal.pone.0029289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lacroix C., Giovannini D., Combe A., Bargieri D. Y., Späth S., Panchal D., Tawk L., Thiberge S., Carvalho T. G., Barale J. C., Bhanot P., and Ménard R. (2011) FLP/FRT-mediated conditional mutagenesis in pre-erythrocytic stages of Plasmodium berghei. Nat. Protoc. 6, 1412–1428 10.1038/nprot.2011.363 [DOI] [PubMed] [Google Scholar]
- 8. Collins C. R., Das S., Wong E. H., Andenmatten N., Stallmach R., Hackett F., Herman J.-P., Müller S., Meissner M., and Blackman M. J. (2013) Robust inducible Cre recombinase activity in the human malaria parasite Plasmodium falciparum enables efficient gene deletion within a single asexual erythrocytic growth cycle. Mol. Microbiol. 88, 687–701 10.1111/mmi.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwach F., Bushell E., Gomes A. R., Anar B., Girling G., Herd C., Rayner J. C., and Billker O. (2015) PlasmoGEM, a database supporting a community resource for large-scale experimental genetics in malaria parasites. Nucleic Acids Res. 43, D1176–D1182 10.1093/nar/gku1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fonager J., Franke-Fayard B. M., Adams J. H., Ramesar J., Klop O., Khan S. M., Janse C. J., and Waters A. P. (2011) Development of the piggyBac transposable system for Plasmodium berghei and its application for random mutagenesis in malaria parasites. BMC Genomics 12, 155 10.1186/1471-2164-12-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muñoz E. E., Hart K. J., Walker M. P., Kennedy M. F., Shipley M. M., and Lindner S. E. (2017) ALBA4 modulates its stage-specific interactions and specific mRNA fates during Plasmodium yoelii growth and transmission. Mol. Microbiol. 106, 266–284 10.1111/mmi.13762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghorbal M., Gorman M., Macpherson C. R., Martins R. M., Scherf A., and Lopez-Rubio J. J. (2014) Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat. Biotechnol. 32, 819–821 10.1038/nbt.2925 [DOI] [PubMed] [Google Scholar]
- 13. Wagner J. C., Platt R. J., Goldfless S. J., Zhang F., and Niles J. C. (2014) Efficient CRISPR-Cas9-mediated genome editing in Plasmodium falciparum. Nat. Methods 11, 915–918 10.1038/nmeth.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang C., Xiao B., Jiang Y., Zhao Y., Li Z., Gao H., Ling Y., Wei J., Li S., Lu M., Su X. Z., Cui H., and Yuan J. (2014) Efficient editing of malaria parasite genome using the CRISPR/Cas9 system. MBio 5, e01414–14 10.1128/mBio.01414-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aly A. S., Vaughan A. M., and Kappe S. H. (2009) Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol. 63, 195–221 10.1146/annurev.micro.091208.073403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butler N. S., Schmidt N. W., Vaughan A. M., Aly A. S., Kappe S. H., and Harty J. T. (2011) Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9, 451–462 10.1016/j.chom.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirkman L. A., Lawrence E. A., and Deitsch K. W. (2014) Malaria parasites utilize both homologous recombination and alternative end joining pathways to maintain genome integrity. Nucleic Acids Res. 42, 370–379 10.1093/nar/gkt881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee A. H., Symington L. S., and Fidock D. A. (2014) DNA repair mechanisms and their biological roles in the malaria parasite Plasmodium falciparum. Microbiol. Mol. Biol. Rev. 78, 469–486 10.1128/MMBR.00059-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singer M., Marshall J., Heiss K., Mair G. R., Grimm D., Mueller A.-K., and Frischknecht F. (2015) Zinc finger nuclease-based double-strand breaks attenuate malaria parasites and reveal rare microhomology-mediated end joining. Genome Biol. 16, 249 10.1186/s13059-015-0811-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang C., Gao H., Yang Z., Jiang Y., Li Z., Wang X., Xiao B., Su X. Z., Cui H., and Yuan J. (2017) CRISPR/Cas9 mediated sequential editing of genes critical for ookinete motility in Plasmodium yoelii. Mol. Biochem. Parasitol. 212, 1–8 10.1016/j.molbiopara.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu C., Li Z., Jiang Y., Cui H., and Yuan J. (2018) Generation of Plasmodium yoelii malaria parasite carrying double fluorescence reporters in gametocytes. Mol. Biochem. Parasitol. 224, 37–43 10.1016/j.molbiopara.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 22. Qian P., Wang X., Yang Z., Li Z., Gao H., Su X.-Z., Cui H, and Yuan J. (2018) A Cas9 transgenic Plasmodium yoelii parasite for efficient gene editing. Mol. Biochem. Parasitol. 222, 21–28 10.1016/j.molbiopara.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang C., Li Z., Cui H., Jiang Y., Yang Z., Wang X., Gao H., Liu C., Zhang S., Su X.-Z., and Yuan J. (2017) Systematic CRISPR-Cas9-mediated modifications of Plasmodium yoelii ApiAP2 genes reveal functional insights into parasite development. MBio 8, e01986–17 10.1128/mBio.01986-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao Y., and Zhao Y. (2014) Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J. Integr. Plant Biol. 56, 343–349 10.1111/jipb.12152 [DOI] [PubMed] [Google Scholar]
- 25. Xie C., Chen Y.-L., Wang D.-F., Wang Y.-L., Zhang T.-P., Li H., Liang F., Zhao Y., and Zhang G.-Y. (2017) sgRNA expression of CRIPSR-Cas9 system based on miRNA polycistrons as a versatile tool to manipulate multiple and tissue-specific genome editing. Sci. Rep. 7, 5795 10.1038/s41598-017-06216-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joung J., Konermann S., Gootenberg J. S., Abudayyeh O. O., Platt R. J., Brigham M. D., Sanjana N. E., and Zhang F. (2017) Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat. Protoc. 12, 828–863 10.1038/nprot.2017.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perez-Pinera P., Kocak D. D., Vockley C. M., Adler A. F., Kabadi A. M., Polstein L. R., Thakore P. I., Glass K. A., Ousterout D. G., Leong K. W., Guilak F., Crawford G. E., Reddy T. E., and Gersbach C. A. (2013) RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods 10, 973–976 10.1038/nmeth.2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao B., Yin S., Hu Y., Sun M., Wei J., Huang Z., Wen Y., Dai X., Chen H., Mu J., Cui L., and Jiang L. (2019) Epigenetic editing by CRISPR/dCas9 in Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 116, 255–260 10.1073/pnas.1813542116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baum J., Papenfuss A. T., Mair G. R., Janse C. J., Vlachou D., Waters A. P., Cowman A. F., Crabb B. S., and de Koning-Ward T. F. (2009) Molecular genetics and comparative genomics reveal RNAi is not functional in malaria parasites. Nucleic Acids Res. 37, 3788–3798 10.1093/nar/gkp239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prommana P., Uthaipibull C., Wongsombat C., Kamchonwongpaisan S., Yuthavong Y., Knuepfer E., Holder A. A., and Shaw P. J. (2013) Inducible knockdown of Plasmodium gene expression using the glmS ribozyme. PLoS One 8, e73783 10.1371/journal.pone.0073783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Russo I., Oksman A., Vaupel B., and Goldberg D. E. (2009) A calpain unique to alveolates is essential in Plasmodium falciparum and its knockdown reveals an involvement in pre-S-phase development. Proc. Natl. Acad. Sci. U.S.A. 106, 1554–1559 10.1073/pnas.0806926106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muralidharan V., Oksman A., Iwamoto M., Wandless T. J., and Goldberg D. E. (2011) Asparagine repeat function in a Plasmodium falciparum protein assessed via a regulatable fluorescent affinity tag. Proc. Natl. Acad. Sci. U.S.A. 108, 4411–4416 10.1073/pnas.1018449108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ganesan S. M., Falla A., Goldfless S. J., Nasamu A. S., and Niles J. C. (2016) Synthetic RNA-protein modules integrated with native translation mechanisms to control gene expression in malaria parasites. Nat. Commun. 7, 10727 10.1038/ncomms10727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang W.-W., and Matlashewski G. (2015) CRISPR-Cas9-mediated genome editing in Leishmania donovani. MBio 6, e00861 10.1128/mBio.00861-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee R. T., Ng A. S., and Ingham P. W. (2016) Ribozyme mediated gRNA generation for in vitro and in vivo CRISPR/Cas9 mutagenesis. PLoS One 11, e0166020 10.1371/journal.pone.0166020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dang Y., Jia G., Choi J., Ma H., Anaya E., Ye C., Shankar P., and Wu H. (2015) Optimizing sgRNA structure to improve CRISPR-Cas9 knockout efficiency. Genome Biol. 16, 280 10.1186/s13059-015-0846-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peng D., and Tarleton R. (2015) EuPaGDT: a web tool tailored to design CRISPR guide RNAs for eukaryotic pathogens. Microb. Genom. 1, e000033 10.1099/mgen.0.000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qi L. S., Larson M. H., Gilbert L. A., Doudna J. A., Weissman J. S., Arkin A. P., and Lim W. A. (2013) Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 10.1016/j.cell.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Larson M. H., Gilbert L. A., Wang X., Lim W. A., Weissman J. S., and Qi L. S. (2013) CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat. Protoc. 8, 2180–2196 10.1038/nprot.2013.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maeder M. L., Linder S. J., Cascio V. M., Fu Y., Ho Q. H., and Joung J. K. (2013) CRISPR RNA–guided activation of endogenous human genes. Nat. Methods 10, 977–979 10.1038/nmeth.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pino P., Sebastian S., Kim E. A., Bush E., Brochet M., Volkmann K., Kozlowski E., Llinás M., Billker O., and Soldati-Favre D. (2012) A tetracycline-repressible transactivator system to study essential genes in malaria parasites. Cell Host Microbe 12, 824–834 10.1016/j.chom.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Campbell T. L., De Silva E. K., Olszewski K. L., Elemento O., and Llinás M. (2010) Identification and genome-wide prediction of DNA binding specificities for the ApiAP2 family of regulators from the malaria parasite. PLoS Pathog. 6, e1001165 10.1371/journal.ppat.1001165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mu W., Zhang Y., Xue X., Liu L., Wei X., and Wang H. (2019) 5′ capped and 3′ polyA-tailed sgRNAs enhance the efficiency of CRISPR-Cas9 system. Protein Cell 10, 223–228 10.1007/s13238-018-0552-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin H., Song C.-Q., Suresh S., Wu Q., Walsh S., Rhym L. H., Mintzer E., Bolukbasi M. F., Zhu L. J., Kauffman K., Mou H., Oberholzer A., Ding J., Kwan S.-Y., Bogorad R. L., et al. (2017) Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat. Biotechnol. 35, 1179–1187 10.1038/nbt.4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Farasat I., and Salis H. M. (2016) A biophysical model of CRISPR/Cas9 activity for rational design of genome editing and gene regulation. PLoS Comput. Biol. 12, e1004724 10.1371/journal.pcbi.1004724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shrinivas K., Sabari B. R., Coffey E. L., Klein I. A., Boija A., Zamudio A. V., Schuijers J., Hannett N. M., Sharp P. A., Young R. A., and Chakraborty A. K. (2018) Enhancer features that drive formation of transcriptional condensates. bioRxiv 10.1101/495606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anders C., Niewoehner O., Duerst A., and Jinek M. (2014) Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature 513, 569–573 10.1038/nature13579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller J. L., Harupa A., Kappe S. H., and Mikolajczak S. A. (2012) Plasmodium yoelii macrophage migration inhibitory factor is necessary for efficient liver-stage development. Infect. Immun. 80, 1399–1407 10.1128/IAI.05861-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Janse C. J., Franke-Fayard B., Mair G. R., Ramesar J., Thiel C., Engelmann S., Matuschewski K., van Gemert G. J., Sauerwein R. W., and Waters A. P. (2006) High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol. Biochem. Parasitol. 145, 60–70 10.1016/j.molbiopara.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 50. Crawford E. D., Quan J., Horst J. A., Ebert D., Wu W., and DeRisi J. L. (2017) Plasmid-free CRISPR/Cas9 genome editing in Plasmodium falciparum confirms mutations conferring resistance to the dihydroisoquinolone clinical candidate SJ733. PLoS One 12, e0178163 10.1371/journal.pone.0178163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ribeiro J. M., Garriga M., Potchen N., Crater A. K., Gupta A., Ito D., and Desai S. A. (2018) Guide RNA selection for CRISPR-Cas9 transfections in Plasmodium falciparum. Int. J. Parasitol. 48, 825–832 10.1016/j.ijpara.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lindner S. E., Mikolajczak S. A., Vaughan A. M., Moon W., Joyce B. R., Sullivan W. J. Jr., and Kappe S. H. (2013) Perturbations of Plasmodium Puf2 expression and RNA-seq of Puf2-deficient sporozoites reveal a critical role in maintaining RNA homeostasis and parasite transmissibility. Cell. Microbiol. 15, 1266–1283 10.1111/cmi.12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Henrici R. C., Pecen T. J., Johnston J. L., and Tan S. (2017) The pPSU plasmids for generating DNA molecular weight markers. Sci. Rep. 7, 2438 10.1038/s41598-017-02693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.