SUMMARY
Reduced dietary protein intake induces adaptive physiological changes in macronutrient preference, energy expenditure, growth, and glucose homeostasis. We demonstrate that deletion of the FGF21 co-receptor bKlotho (Klb) from the brain produces mice that are unable to mount a physiological response to protein restriction, an effect that is replicated by whole-body deletion of FGF21. Mice forced to consume a low-protein diet exhibit reduced growth, increased energy expenditure, and a resistance to diet-induced obesity, but the loss of FGF21 signaling in the brain completely abrogates that response. When given access to a higher protein alternative, protein-restricted mice exhibit a shift toward protein-containing foods, and central FGF21 signaling is essential for that response. FGF21 is an endocrine signal linking the liver and brain, which regulates adaptive, homeostatic changes in metabolism and feeding behavior during protein restriction.
Graphical Abstract
In Brief
Dietary protein restriction induces changes in macronutrient preference, energy expenditure, growth, and metabolism. Hill et al. show that deletion of the FGF21 co-receptor from the brain produces mice that are unable to mount a physiological response to dietary protein restriction. FGF21 acts in the brain to coordinate homeostatic responses to protein restriction.
INTRODUCTION
The survival of any species is contingent upon the ability to physiologically and behaviorally adapt to a changing nutritional environment. In mammals, this homeostasis is largely mediated by nutritional hormones, which coordinate changes in feeding behavior, energy expenditure, substrate metabolism, and growth in response to fluctuations in nutrient availability. Essential amino acids are required for optimal health and performance, and animals sense and respond to reductions in the consumption of dietary protein (Hill et al., 2018; Morrison and Laeger, 2015). In mice, this metabolic response includes increased energy expenditure, reduced growth, and enhanced insulin sensitivity and glucose homeostasis (Cummings et al., 2018; Fontana et al., 2016; Hill et al., 2017; Laeger et al., 2014a; Maida et al., 2016). Protein restriction also alters feeding behavior to increase protein intake by promoting the overconsumption of a single low-protein diet (protein leverage) or altering food choice, such that higher protein foods are preferred (Chaumontet et al., 2018; Gosby et al., 2014; Huang et al., 2013; Murphy et al., 2018; Simpson and Raubenheimer, 1997; Sørensen et al., 2008).
Although the mechanisms underlying adaptive responses to protein restriction are not well defined, our laboratory and that of others have demonstrated that the metabolic hormone fibroblast growth factor 21 (FGF21) is essential for this metabolic response (Hill et al., 2018, 2017; Laeger et al., 2014a, 2016; Maida et al., 2016). FGF21 is a member of a small subfamily of “endocrine fibroblast growth factors (FGFs),” being produced primarily by the liver and signaling at distant sites via the FGF receptor 1c (FGFR1c) and the co-receptor bKlotho (Klb) (Lee et al., 2018; Markan et al., 2014; Nishimura et al., 2000; Ogawa et al., 2007). Pharmacological FGF21 treatment reduces body weight, increases energy expenditure, improves insulin sensitivity, and improves lipid metabolism in rodents, non-human primates, and humans (Coskun et al., 2008; Gaich et al., 2013; Kharitonenkov and Adams, 2013; Kharitonenkov and DiMarchi, 2017; Potthoff, 2017; Talukdar et al., 2016b), and this effect is argued to be driven by direct effects on both adipose tissue and the brain (BonDurant and Potthoff, 2018). FGF21 has also been linked to macronutrient preference because pharmacological FGF21 treatment reduces the consumption of sweet substances and alcohol (Soberg et al., 2017; Talukdar et al., 2016a; von Holstein-Rathlou et al., 2016).
Because FGF21 has a critical role in the response to protein restriction, we sought to identify the primary site mediating that FGF21-dependent effect, hypothesizing that FGF21 likely acted directly in the CNS to coordinate changes in food intake and energy expenditure. Because protein restriction also produces a macronutrient-specific appetite for protein, we hypothesized that FGF21 mediated that change in macronutrient preference. We demonstrate that FGF21 signaling within the brain is the fundamental mediator of physiological changes in both metabolism and macronutrient preference during protein restriction. In the absence of brain FGF21 signaling, mice are unable to mount a metabolic response to protein restriction, and central FGF21 mediates adaptive changes in macronutrient preference during protein restriction. Collectively, these data not only demonstrate that this liver-to-brain FGF21 signal mechanistically underpins a fundamental aspect of biology, but they also provide a conceptual framework that cohesively defines the physiological relevance of FGF21.
RESULTS
FGF21 Signaling in Brain but not Adipose Tissue Mediates Low-Protein-Induced Metabolic Responses in Lean Mice
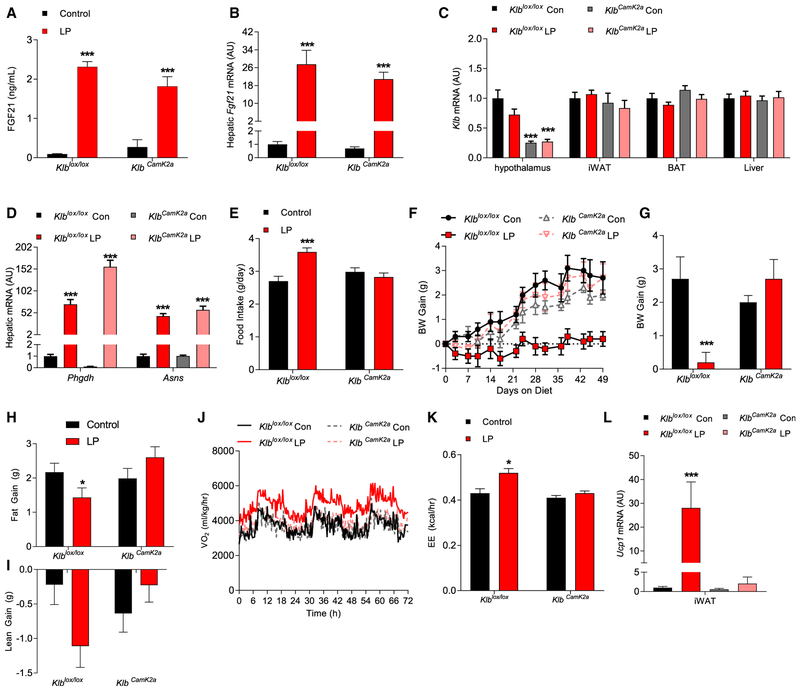
Fgf21-knockout (KO) mice do not exhibit a metabolic response to a low-protein diet (Hill et al., 2017; Laeger et al., 2014a, 2016). To test whether the FGF21-dependent effect is mediated by direct signaling in the brain, we used Camk2a-Cre, which has previously been used to delete Klb from the brain (Bookout et al., 2013; Owen et al., 2013) and which primarily targets neurons within the forebrain (Casanova et al., 2001). KlbCamk2a mice and control littermates (Klblox/lox) were placed ad libitum on isocalorically normal protein (Con) or low-protein (LP) diets for 7 weeks. LP diet increased circulating FGF21 levels (Figure 1A) and hepatic Fgf21 expression (Figure 1B) in both Klblox/lox and KlbCamk2a mice. Klb mRNA expression was selectively reduced within the brain of KlbCamk2a mice (Figure 1C). LP diet also increased liver markers of amino acid restriction (Phgdh and Asns) within both genotypes (Figure 1D).
Figure 1. CNS FGF21 Signaling Mediates Low-Protein-Induced Metabolic Responses in Lean Mice.
(A–L) Brain-specific Klb knockout mice (KlbCamk2a) and control littermates (Klblox/lox) were fed isocaloric control (Con) or low-protein (LP) diet for 7 weeks. (A–I) Circulating FGF21 levels (A), hepatic Fgf21 mRNA levels (B), Klb mRNA levels in various tissues (C), hepatic Phgdh and Asns mRNA levels (D), average daily food intake (E), body weight gain over the experiment (F), final body weight gain (G), final fat gain (H), and final lean gain (I) are shown. (J–L) Oxygen consumption (J) and energy expenditure (K) on days 6–8 of diet exposure and Ucp1 mRNA levels in iWAT and brown adipose tissue (BAT) (L) are depicted.
Data are represented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. 8 mice/group.
Although the LP diet increased food intake in Klblox/lox mice, the effect was lost in KlbCamk2a mice (Figure 1E). Furthermore, the reduction in body-weight gain observed in LP-fed Klblox/lox mice was also lost in KlbCamk2a mice (Figures 1F and 1G). Finally, LP-induced changes in fat and lean gain were not observed in KlbCamk2a mice (Figures 1H and 1I). The observation of increased food intake but reduced body weight in Klblox/lox mice on the LP diet was consistent with increased energy expenditure, and direct measures of the rate of oxygen consumption (VO2) and energy expenditure (EE) confirmed an LP-induced increase only in Klblox/lox mice (Figures 1J and 1K). The LP diet also increased Ucp1 mRNA expression within inguinal white adipose tissue (iWAT) of Klblox/lox, but not KlbCamk2a, mice (Figure 1L). Taken together, these data demonstrate that CNS FGF21 signaling is required for lean mice to metabolically respond to dietary protein restriction.
Because adipose tissue expresses Klb and directly responds to FGF21, adipose-specific Klb KO mice (KlbAdipo) and control littermates (Klblox/lox) were placed on Con or LP diets for 6 weeks ad libitum. The LP diet increased circulating FGF21 and liver Fgf21 mRNA in both Klblox/lox and KlbAdipo mice (Figure S1A and 1B), whereas Klb mRNA was substantially reduced within the adipose tissue of KlbAdipo mice (Figure S1C). Food intake was increased in both LP-fed Klblox/lox and KlbAdipo mice (Figure S1D). The LP fed KlbAdipo and Klblox/lox mice also exhibited similar reductions in body weight, fat, and lean gain (Figures S1E–S1H). Consistent with previously observed effects on EE, the LP diet increased iWAT Ucp1 mRNA expression in both LP groups, and both groups exhibited increased food intake but reduced body-weight gain (Figure S1I). Collectively, these data suggest that FGF21 signaling directly in adipose tissue is not necessary for changes in body weight and food intake in response to protein restriction.
Comparison of the Metabolic Effects of Protein Restriction versus Dietary Restriction in Obese Mice
Dietary protein restriction improves glucose homeostasis in settings of diet induced obesity (Cummings et al., 2018; Fontana et al., 2016; Laeger et al., 2018; Maida et al., 2016, 2017; Solon-Biet et al., 2014) and represents an alternative nutritional strategy to general dietary (calorie) restriction. To directly compare the metabolic effect of protein restriction and dietary (food) restriction in obese mice, wild-type mice were fed either a 60% high-fat diet (HFD) ad libitum (HFCon), a 60% HFD that was low in protein (HFLP), or a diet-restriction group (HFDR), in which mice consumed HFCon but were food restricted to match body weight to the HFLP group. HFLP reduced body weight and fat gain despite ad libitum feeding, and that weight loss was matched by experimentally reducing food intake by 25% in the HFDR group (Figures S2A and S2B; p < 0.05). Thus,Ȉboth energy and protein intake were reduced (Ȉ25%) in HFDR mice, whereas HFLP increased energy intake but reduced protein intake (Figures 2C and 2D; p < 0.05). Fasting insulin levels trended lower in the HFLP, but not the HFDR, group (Figure S3G and S3H), and there was no change in the insulin positive area within pancreatic islets (data not shown). Glucose tolerance at week 4 was improved in both HFLP and HFDR mice (Figures S2E and S2F; p < 0.05). Liver lipogenic genes (Fasn, Scd-1, Srebp-1) were significantly increased by HFDR but unaffected or reduced by HFLP (Figure S2K; p < 0.05). Liver markers of amino acid restriction (Asns and Phgdh) were increased in HFLP, but unchanged in HFDR, mice (Figure S2L; p < 0.05). Consistent with alternative effects on EE, levels of Ucp1 within iWAT were robustly increased by HFLP but unaffected by HFDR (Figure S2M; p < 0.05). Finally, circulating FGF21 levels and liver Fgf21 mRNA expression were robustly increased by HFLP but not HFDR (Figures S2I and S2J; p < 0.01). Taken together, these data demonstrate that protein restriction improves metabolic endpoints in the context of diet-induced obesity but does so by activating pathways that are different from classic dietary (calorie) restriction, most notably the induction of FGF21.
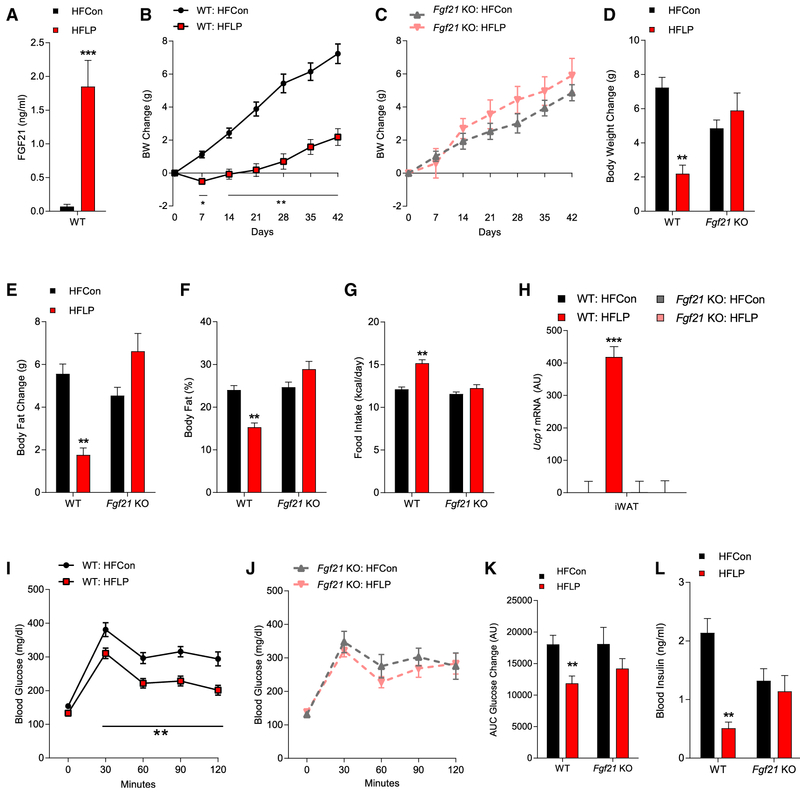
Figure 2. Protein Restriction Requires FGF21 to Protect against HFD-Induced Obesity and Glucose Intolerance.
(A–L) WT and Fgf21-KO mice were fed an HFCon or HFLP diet for 6 weeks. Circulating FGF21 levels (A), body weight (BW) gain in WT mice (B), BW gain in Fgf21KO mice (C), final BW change (D), change in body fat (E), body fat percentage (F), average daily food intake (G), Ucp1 mRNA levels in iWAT (H), glucose tolerance test (GTT) in WT mice (I), and in Fgf21-KO mice (J) at 4 weeks on diet, along with GTT area under the curve (K) and insulin levels (L) are shown.
Data are represented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. 10 mice/group.
Protein Restriction Protects against HFD-Induced Obesity and Glucose Intolerance via FGF21 Action in the Brain
We next tested whether FGF21 is required for the abovedescribed protective effects of protein restriction in the context of diet-induced obesity. Wild-type (WT) and Fgf21-KO mice were placed on HFCon or HFLP for 6 weeks. HFLP increased serum FGF21 levels in LP-fed WT mice, whereas FGF21 levels were undetectable in Fgf21-KO mice (Figure 2A; p < 0.001). WT mice on the HFCon diet exhibited a steady increase in body weight that was reduced by HFLP, with HFLP also reducing fat gain and percentage of body fat (Figures 2B and 2D–2F; p < 0.05). Contrastingly, HFLP had no effect on weight or fat gain in Fgf21-KO mice (Figures 2C and 2D–2F; p < 0.05). The weight-reducing effect of the HFLP diet cannot be explained by changes in food intake because HFLP increased food intake in WT mice but not Fgf21-KO mice (Figure 2G; p < 0.05). Consistent with increased EE, HFLP increased iWAT Ucp1 mRNA expression in WT mice but not Fgf21-KO mice (Figure 2H; p < 0.001). Finally, the HFLP diet improved glucose tolerance (Figures 2I and 2K; p < 0.01) and lowered insulin levels in WT mice (Figure 2L; p < 0.01), and these effects were also lost in Fgf21-KO mice (Figures 2J–2L).
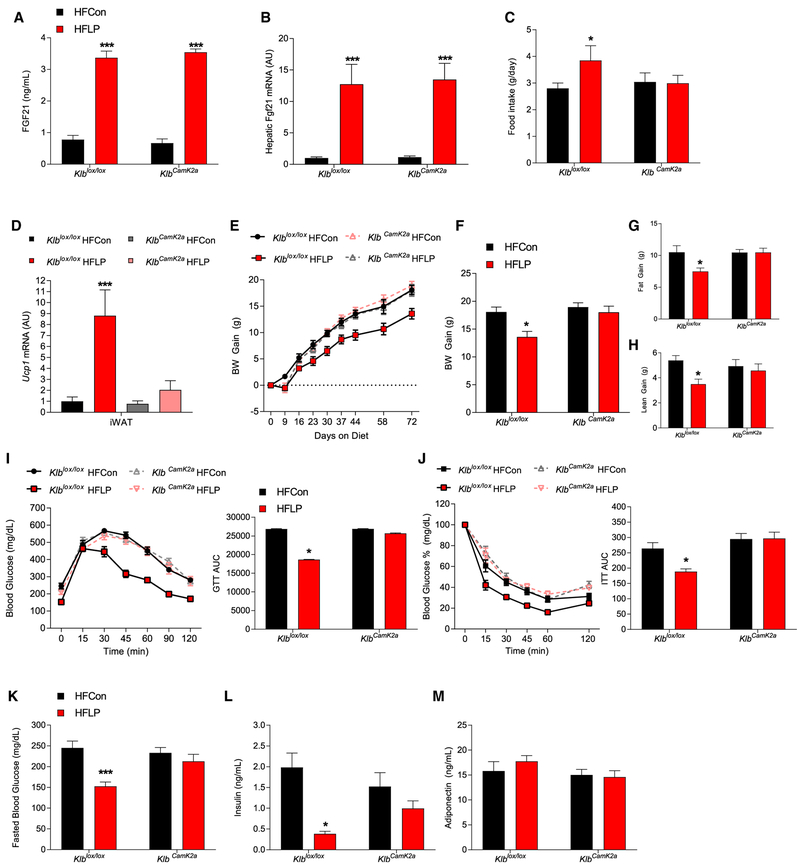
Because dietary protein restriction protects against HFDinduced obesity and glucose intolerance via an FGF21-dependent mechanism, we next tested whether that protective effect also required FGF21 signaling in the brain. Brain-specific Klb KO mice (KlbCamk2a) and control littermates (Klblox/lox) were placed on HFCon or HFLP diets, the same as that above, for 10 weeks. Consistent with previous data, HFLP increased circulating FGF21 levels (Figure 3A; p < 0.001) and hepatic Fgf21 mRNA expression (Figure 3B; p < 0.001) in both Klblox/lox and KlbCamk2a mice. In Klblox/lox mice, HFLP increased food intake (Figure 3C; p < 0.05), increased iWAT Ucp1 mRNA expression (Figure 3D; p < 0.001), reduced body weight and fat and lean gain (Figures 3E–3H; p < 0.05). All of these effects were lost in KlbCamk2a mice. As in WT mice, HFLP improved both glucose tolerance and insulin sensitivity in Klblox/lox mice (Figures 3I and 3J; p < 0.05) and also reduced fasting blood glucose and insulin levels in Klblox/lox mice (Figure 3K; p < 0.001 and 3L; p < 0.001). These effects of HFLP on glucose homeostasis were lost in KlbCamk2a mice. There were no dietary effects on circulating adiponectin levels in either Klblox/lox or KlbCamk2a mice (Figure 3M). Collectively, the data in Figures 2, 3, and S2 use three separate mouse studies and two independent mouse genetic models to demonstrate that dietary protein restriction improves metabolic outcomes in diet-induced obese mice and that these beneficial effects are fully dependent on FGF21 and its signaling via Klb within the brain.
Figure 3. CNS FGF21 Signaling Is Required for Protein Restriction to Protect against Diet-Induced Obesity.
(A–M) Brain-specific Klb knockout mice (KlbCamk2a) and control littermates (Klblox/lox) were fed either a high-fat control (HFCon) or a high-fat low-protein (HFLP) diet for 10 weeks showing circulating FGF21 levels (A), liver Fgf21 mRNA levels (B), average of daily food intake (C), Ucp1 mRNA levels in iWAT and BAT (D), body weight gain over the study (E), final body weight gain (F), fat gain (G), lean gain (H), glucose tolerance test at 6 weeks on diet with area under the curve (AUC) (I), insulin tolerance test (ITT) at 7 weeks on diet with AUC (J), fasted glucose levels at time of GTT (K), insulin levels at week 10 (L), and adiponectin levels at week 10 (M).
Data are represented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. 8 mice/group.
FGF21 Acts in the Brain to Increase Protein Intake
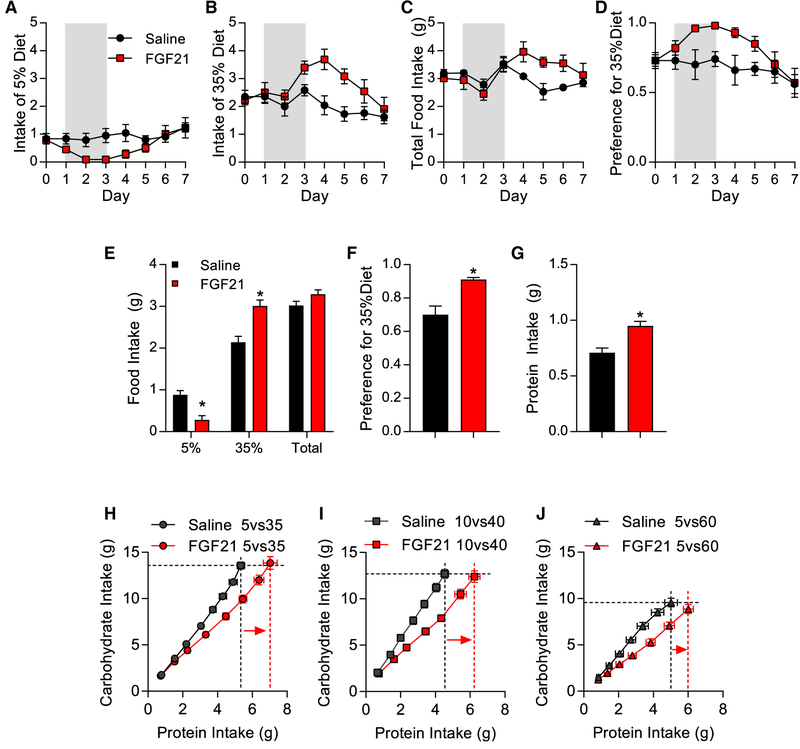
In addition to inducing metabolic adaptations, protein restriction also induces a shift in macronutrient preference toward increased protein intake (Chaumontet et al., 2018; Gosby et al., 2014; Huang et al., 2013; Murphy et al., 2018; Simpson and Raubenheimer, 1997; Sørensen et al., 2008). To test whether FGF21 mediates that shift in macronutrient preference, we first tested the effects of FGF21 on protein selection in mice that were simultaneously offered two isocaloric diets that varied in their protein:carbohydrate ratio (see Table S1 for diet compositions). Mice were given 1 week to adapt to those diets, and then FGF21 (1 mg) was injected into the lateral ventricle for 3 consecutive days, approximately 3 h before lights were turned off. The first diet comparison was mice choosing between 5% and 35% casein diets (Figure 4). Intracerebroventricular (ICV) FGF21 acutely reduced the consumption of the lower (5%) protein diet (Figure 4A; p < 0.05) and produced a slightly delayed and more prolonged increase in consumption of the higher (35%) protein choice (Figure 4B; p < 0.05). Total intake was not consistently affected by FGF21 injection (Figure 4C). Expressing these data as a preference ratio for the 35% diet (intake of 35% divided by total intake) indicates that FGF21 significantly increased preference for 35% by day 2 after the injection (Figure 4D; p < 0.05), with the preference ratio returning to normal by day 6 (3 days after the injection). Averaging consumption across days 1–5 revealed that FGF21 decreased consumption of 5%, increased consumption of 35% and preference for 35%, and increased total protein intake but had no effect on total food intake (Figures 4E–4G; p < 0.05). This FGF21-induced increase in protein intake was replicated in additional dietary combinations, including 10% versus 40% casein diets and 5% versus 60% casein diets (Figure S3). In each case, ICV FGF21 increased preference for the high-protein choice and increased total protein intake but did not increase total food intake.
Figure 4. FGF21 Acts in the Brain to Increase Protein Intake.
(A–G) Wild-type mice selecting between isocaloric diets containing either 5% or 35% casein were given FGF21 injections into the lateral ventricle for three consecutive days. Shaded area denotes days of injection.
(A) Intake of 5% casein diet.
(B) Intake of 35% casein diet.
(C) Total food intake.
(D) Preference for 35% casein diet (35% casein intake divided by total).
(E) Average daily intake of various diets on days 1–5.
(F) Average preference on days 1–5.
(G) Average daily protein intake on days 1–5. 8 mice/group; *p < 0.05.
(H–J) Geometric analysis of FGF21-dependent shifts in cumulative protein and carbohydrate intake over days in mice choosing between multiple dietary combinations.
(H) Geometric analysis of mice choosing between 5% and 35% casein diets.
(I) Geometric analysis of mice choosing between 10% and 40% casein diets.
(J) Geometric analysis of mice choosing between 5% and 60% casein diets.
Horizontal, hashed lines reflect carbohydrate target of the saline group, whereas the vertical, hashed lines reflect protein target of the saline group in black and the FGF21 group in red. Arrows indicate FGF21-induced increase in protein intake. Data are represented as means ± SEM. 7–10 mice/group. *p < 0.05.
We then used the geometric framework (Raubenheimer and Simpson, 1997; Simpson and Raubenheimer, 1997; Sørensen et al., 2008) to directly compare cumulative carbohydrate versus protein intake over time within each individual experiment (Figures 4H–4J). In all three experiments, ICV FGF21 significantly increased cumulative protein intake (p < 0.05), demonstrated by a rightward shift in the FGF21 group (highlighted with a vertical red line). Contrastingly, there was no consistent change along the horizontal carbohydrate axis, although carbohydrate intake was slightly reduced by FGF21 injection in the 5% versus 60% group. These data collectively demonstrate that FGF21 acts in the brain to increase protein intake without significantly altering total food (energy) intake or carbohydrate intake.
Protein-Restricted Mice Exhibit a Specific Appetite for Protein
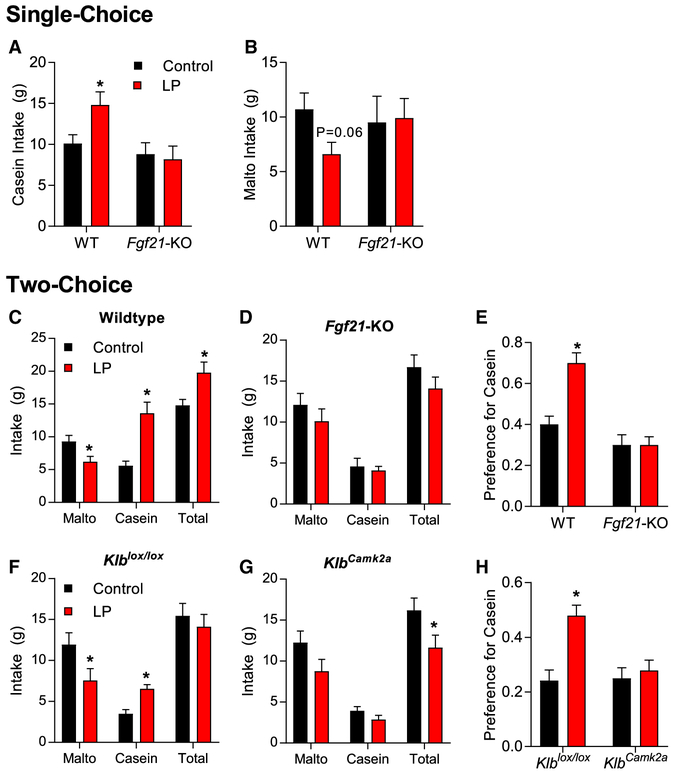
The above data indicate that FGF21 increases protein intake. To further explore protein and carbohydrate consumption, we adopted a protocol previously described by Murphy et al. (2018), in which carbohydrate (maltodextrin) and protein (casein) are offered in liquid form to allow independent consumption and choice between these purified macronutrients. In that work, the protein-restricted rats drank significantly more casein than the control rats, and we, therefore, first sought to reproduce this effect in mice. WT mice were fed either control or LP for 1 week and were then offered 4% casein or 4% maltodextrin solutions for 3 consecutive days. LP mice exhibited a robust and sustained increase in casein intake on all three days of exposure (Figure S4A; p < 0.05), but there was no significant effect on maltodextrin intake (Figure S4B). Casein and maltodextrin were then offered simultaneously in a two-choice paradigm for 3 consecutive days. Although control-fed mice preferred maltodextrin, LP mice exhibited a significant decrease in maltodextrin intake, a significant increase casein intake, and a strong increase in preference for casein (Figures S4C and S4D). These data indicate the protein-restricted mice specifically increased casein, but not maltodextrin, consumption in a single-choice paradigm and shifted away from maltodextrin and toward casein in a twochoice paradigm.
CNS FGF21 Signaling Is Required for Physiological Shifts in Protein Preference
To determine whether FGF21 is also required for LP-induced shifts in macronutrient preference, we repeated the above experiments in additional groups of WT and Fgf21-KO mice. In single-choice, WT LP-fed (WTLP) mice significantly increased casein consumption (Figure 5A; p < 0.05) and tended to reduce maltodextrin intake (Figure 5B; p = 0.06). Fgf21-KO mice on LP exhibited no change in consumption of either macronutrient (Figures 5A and 5B). When both macronutrients were offered simultaneously (two-choice), WTLP mice reduced maltodextrin intake and increased casein intake (Figure 5C; p < 0.05), which translated into a strong and significant shift in casein preference (Figure 5E; p < 0.05). As above, Fgf21-KO mice were completely resistant to these effects of LP diet, exhibiting no change in maltodextrin or casein intake or casein preference in the twochoice setting (Figures 5D and 5E). To test whether CNS FGF21 signaling mediated that effect, we repeated the twochoice paradigm in brain-specific Klb KOs (KlbCamk2a) and their littermate controls (Klblox/lox). The LP diet significantly reduced maltodextrin intake and increased casein intake in Klblox/lox controls (Figure 5F; p < 0.05), resulting in a strong increase in casein preference (Figure 5H; p < 0.05). These effects were completely lost in KlbCamk2a mice (Figures 5G and 5H), replicating the phenotype of Fgf21-KO mice. These data clearly indicate that mice adaptively shift macronutrient preference in response to protein restriction and that FGF21 signaling in the brain mediates the response.
Figure 5. CNS FGF21 Signaling Is Required for Physiological Shifts in Protein Preference.
(A–H) Wild-type and Fgf21-KO mice consumed either control or low-protein (LP) diet for 7 days. Mice were then offered 4% casein or 4% maltodextrin solutions for 3 consecutive days (singlechoice).
(A) Daily consumption of casein in single choice.
(B) Daily consumption of maltodextrin in single choice. Mice were then simultaneously offered casein and maltodextrin solutions for 3 consecutive days (two-choice).
(C) Average maltodextrin, casein, and total liquid intake across the 3 days of two-choice presentation in wild-type mice.
(D) Average maltodextrin, casein, and total liquid intake across the 3 days of two-choice presentation in Fgf21-KO mice.
(E) Preference for casein over that same 3 day period. Mice with brain-specific Klb deletion (KlbCamk2a) or littermate controls were placed on control of LP for 7 days and then simultaneously offered casein or maltodextrin solutions for 3 consecutive days (two-choice).
(F and G) Average maltodextrin, casein, and total liquid intake in Klblox/lox (F) and KlbCamk2a (G) mice across the 3 days of two-choice presentation.
(H) Preference for casein over that same 3 day period.
Data are represented as means ± SEM. *p < 0.05. 10 mice/diet/genotype
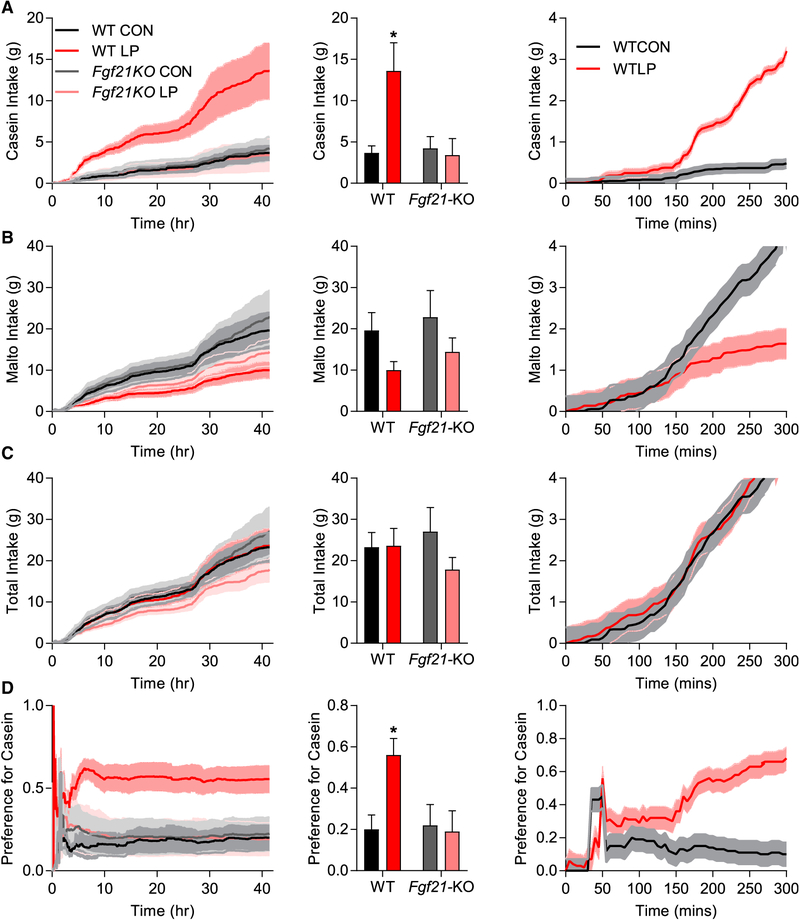
To more closely analyze the timing of the macronutrient preference, we repeated the two-choice paradigm within automated feeding chambers, which allowed continuous assessment of casein and maltodextrin intake over time. WT and Fgf21-KO mice were placed on control and LP diets for 1 week and then provided with maltodextrin and casein solutions for 42 h. Importantly, this design allowed the continuous assessment of macronutrient choice in mice that were naive to the solutions, in contrast to the previous studies in which mice had experience with the solutions before the two-choice study. In this scenario, WTLP mice rapidly and persistently increased casein intake (Figure 6A) and tended to reduce maltodextrin intake (Figure 6B) but did not change total intake (Figure 6C) and, thus, exhibited a strong and persistent shift in casein preference (Figure 6D). These effects were lost in Fgf21-KO mice. We then more narrowly analyzed the first 5 h of liquid consumption in WTCON and WTLP mice. Because the above data were generated in two replicates, which differed slightly (by 45 min) in the time the fluids were offered relative to lights being turned off, we focused on the first replicate (n = 3), in which fluids were presented simultaneously. In WTLP mice, increased casein consumption was visually apparent early during exposure, diverging statistically (p <0.05) at Ȉ150 min after initial presentation. Maltodextrin intake diverged slightly later (180 min), but there was no change in total intake. These data suggest that casein preference manifests relatively rapidly in protein-restricted mice that are naive to the solutions; however, these data are insufficient to distinguish between an innate, unlearned preference verses a preference that is learned through the postingestive consequences.
Figure 6. Rapid Appearance of the FGF21-Dependent Protein Preference.
(A–D) Wild-type and Fgf21-KO mice consumed either control or low protein (LP) diet for 7 days and were then offered 4% casein solution or 4% maltodextrin for 42 h with continuous measurement of fluid intake.
(A) Casein intake.
(B) Maltodextrin intake.
(C) Total intake.
(D) Preference for casein. 5 mice/diet/genotype *p < 0.05.
Dark line indicates the means and shaded area the SEM. Fluid consumption during just the first 300 min of exposure is presented in the final column from a subgroup (3/group) of mice provided solutions at identical times.
CNS FGF21 Signaling Increases Protein Intake in ChowFed Mice
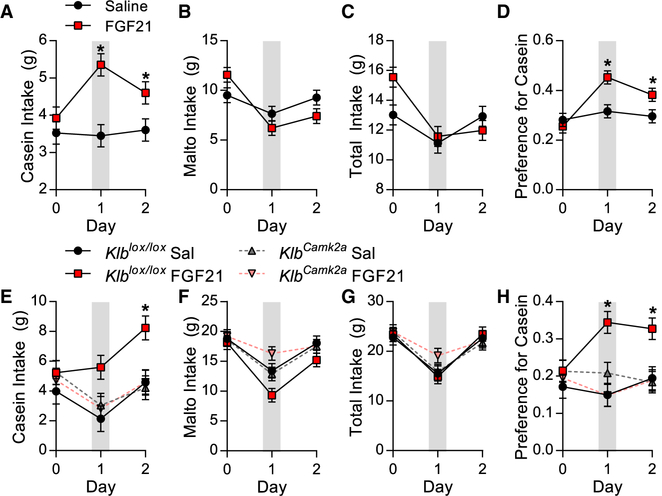
Because CNS FGF21 signaling is necessary for adaptive changes in casein preference, we finally tested whether stimulation of central FGF21 signaling would mimic the effects of protein restriction and cause chow-fed mice to exhibit a protein preference, even though they are not protein restricted. WT mice on chow were offered both casein and maltodextrin (two-choice) for 3 days, with a single ICV FGF21 injection on the second day (day 1). FGF21 significantly increased casein intake (Figure 7A; p < 0.05) without significantly altering maltodextrin (Figure 7B) or total intake (Figure 7C), resulting in a significant increase in preference for casein (Figure 7D; p < 0.05). We then repeated the study in KlbCamk2a and Klblox/lox littermates. As before, ICV FGF21 injection increased casein intake (Figure 7E; p < 0.05) and preference for casein (Figure 7H; p < 0.05) in Klblox/lox mice but had no effect in KlbCamk2a mice. These data demonstrate that FGF21 signaling within the brain shifts macronutrient preference toward protein, and that this effect requires neuronal Klb signaling.
Figure 7. CNS FGF21 Signaling Increases Protein Intake in Chow Fed Mice.
(A–D) Wild-type mice on chow diet were offered casein and maltodextrin solutions (two-choice) for 24h and were then given a single ICV FGF21 injection, and liquid intake was monitored for 2 additional days. Grey shading denotes 24-h period after ICV injection.
(A) Casein intake.
(B) Maltodextrin intake.
(C) Total liquid intake.
(D) Preference for casein. Data are represented as means ± SEM. *p < 0.05; four mice/group.
(E–H) Brain-specific Klb knockouts (KlbCamk2a) and control littermates on chow diet were offered casein and maltodextrin solutions for 24 h and then received a single ICV FGF21 injection as above, and liquid consumption was measured over 2 days.
(E) Casein intake.
(F) Maltodextrin intake.
(G) Total liquid intake.
(H) Preference for casein.
Data are represented as means ± SEM. *p < 0.05. 7–8 mice/group
DISCUSSION
Adapting to reductions in nutrient availability is an essential characteristic of life. Mammals adaptively respond to reductions in nutrients, such as energy, water, or salt, and these adaptive responses are generally mediated by endocrine hormones, which coordinate both metabolic and behavioral adaptations. Protein is also an essential nutrient, and it has long been known that dietary protein restriction triggers changes in food intake, energy expenditure, substrate metabolism, growth, and other metabolic endpoints (Huang et al., 2013; Laeger et al., 2014b; Morrison and Laeger, 2015; Morrison et al., 2012; Rothwell et al., 1982; Solon-Biet et al., 2014, 2015; White et al., 1994, 2000). However, the mechanistic basis for this response has lagged behind other nutrients, despite the intense focus on protein intake and protein supplementation within popular culture and despite the potential power of tapping into this mechanism.
Our laboratory recently demonstrated that the metabolic hormone FGF21 is essential for coordinating the metabolic response to protein restriction in lean mice (Hill et al., 2017; Laeger et al., 2014a, 2016). Here, we define the mechanism through which FGF21 mediates those effects. First, we use tissue-specific deletion of the FGF21 co-receptor Klb to demonstrate that FGF21 signaling within the brain, but not within adipose tissue, is required for the metabolic response to protein restriction. Prior work using either pharmacological FGF21 injection or transgenic overexpression has demonstrated that FGF21 acts in the CNS to reduce body weight, improve glucose homeostasis, and stimulate adipose tissue thermogenesis (Bookout et al., 2013; Douris et al., 2015; Lan et al., 2017; Liang et al., 2014; Owen et al., 2014; Sarruf et al., 2010). Here, we used the physiological model of dietary protein restriction to show that selective deletion of Klb from the CNS fully blocks the effects of protein restriction on food intake, energy expenditure, body weight gain, and iWAT Ucp1 mRNA expression. In their inability to respond to protein restriction, the KlbCamk2a mice, therefore, fully recapitulate the phenotype of whole-body Fgf21-KO mice, and this replication across two independent mouse lines argues against any non-specific effect of either genetic line.
Contrastingly, deletion of Klb from adipose tissue failed to block changes in food intake, growth, or iWAT Ucp1 mRNA expression, suggesting that FGF21 signaling in adipose tissue is not required for metabolic responses to protein restriction. FGF21 was first noted for its ability to promote glucose uptake in adipocytes (Kharitonenkov et al., 2005), and multiple studies have since suggested that FGF21 influences metabolism, at least in part, via direct effects on adipose tissue (Adams et al., 2012; BonDurant et al., 2017; Kharitonenkov et al., 2005). However, recent studies suggested that Klb in adipose tissue is only required for the acute, but not chronic, effects of pharmacological FGF21 treatment (BonDurant et al., 2017). Our data provide strong evidence that the brain is the primary site of FGF21 action in settings of physiological FGF21 induction and, therefore, that this liver-to-brain FGF21 signal is the mechanism through which lean mice sense and respond to protein restriction.
Dietary protein restriction also improves metabolic endpoints in settings of diet-induced obesity, increasing energy expenditure, reducing body weight gain, and improving glucose homeostasis (Cummings et al., 2018; Fontana et al., 2016; Laeger et al., 2018; Maida et al., 2016, 2017; Solon-Biet et al., 2014). In our work initially establishing this high-fat, low-protein model, we compared the effects of protein restriction (HFLP) to an equivalent weight loss induced by typical dietary/caloric restriction (HFDR). Although both interventions produced weight loss and improved glucose homeostasis, there were notable differences in the mechanisms induced. First, HFLP “spontaneously” reduced weight gain and improved glucose tolerance and fasting insulin levels, whereas HFDR required an experimentally imposed 25% food restriction to achieve similar effects. Second, these interventions exerted opposing effects on energy intake and EE because HFLP increased energy intake and EE and browned inguinal white fat (Hill et al., 2017), whereas HFDR reduced energy intake and likely EE (Heilbronn et al., 2006; Leibel et al., 1995). The increased EE in protein-restricted mice is dependent on uncoupling protein 1 (UCP1) (Hill et al., 2017; Wanders et al., 2015) and is thought to be an adaptive, metabolic response, which allows an animal to overconsume nutritionally imbalanced diets, thus increasing intake of the missing nutrient (protein) and disposing of the excess energy via increased EE (Felicetti et al., 2003; Raubenheimer and Simpson, 1997). Third, hepatic expression of genes associated with amino acid biosynthesis and lipogenesis were markedly different between the groups. Finally and importantly, only HFLP produced a significant change in liver Fgf21 mRNA expression and circulating FGF21 protein. These data highlight the differences between protein restriction and dietary restriction, particularly the selective increase of FGF21 (Laeger et al., 2014a; Solon-Biet et al., 2016; Thompson et al., 2014; Zhang et al., 2012).
Brain FGF21 signaling is critical for metabolic effects of protein restriction in lean mice, and protein restriction improves metabolic health in settings of diet-induced obesity. We, therefore, tested whether whole-body FGF21 deletion or brain-specific Klb deletion influenced LP-induced effects in obese mice. Consistent with the above data, HFLP exerted potent metabolic effects in both WT and Klblox/lox mice, including reduced body weight gain, despite increased food intake, increased iWAT Ucp1 mRNA expression, improved glucose tolerance, and lower insulin levels. These effects were completely lost in Fgf21-KO mice and were also lost in mice lacking Klb within the CNS. These data from two independent mouse lines provide convincing evidence that FGF21 signaling within the brain is the mechanism through which protein restriction protects against high-fat diet-induced obesity and glucose intolerance.
To avoid the confounding effects of altered energy density, the LP and HFLP diets were isocaloric to their controls by reciprocally lowering protein and increasing carbohydrate content. Although carbohydrate is known to contribute to FGF21 expression (Dushay et al., 2014; Iroz et al., 2017), we feel that it is unlikely that this increase in carbohydrate substantially contributed to the increase in FGF21 in our diets. First, both LP and HFLP robustly upregulate the expression of liver amino acid biosynthetic genes, which are hallmarks of the protein-restricted state. Second, the HFLP diet robustly increases FGF21 despite being low in carbohydrate, having less carbohydrate than our standard control. Therefore, if high carbohydrate were essential, we would not observe effects with the HFLP diet, yet both LP and HFLP robustly increase FGF21 and induce metabolic effects that are dependent on FGF21 and FGF21 signaling in the brain. Similar observations were made in the research by Laeger et al. (2018), in which LP diet increased FGF21, regardless of whether the diet was balanced by fat or carbohydrate. Thus, our data strongly demonstrate that the diets induce a state of protein restriction, leading to increases in FGF21, which acts in the brain to coordinate the metabolic response to protein restriction.
The work described above examines mice whose only choice is to remain on the low-protein diet, and in that context, FGF21 drives a series of metabolic adaptations as well as an increase in total food intake, consistent with the protein-leverage hypothesis (Sørensen et al., 2008). However, animals will adaptively alter food preferences to compensate for reduced protein intake if other protein sources are available (Chaumontet et al., 2018; Gosby et al., 2014; Huang et al., 2013; Murphy et al., 2018; Simpson and Raubenheimer, 1997, 2012; Sørensen et al., 2008). We tested whether that change in feeding behavior also requires central FGF21 signaling, first testing the hypothesis that elevated FGF21 would increase protein intake in mice choosing between two isocaloric diets differing in their protein:carbohydrate ratio. Using five individual diets in three different dietary combinations, we demonstrated that ICV FGF21 reliably shifts preference among the diets to increase protein intake without altering total food intake. Analyzing these data as the ratio of cumulative carbohydrate versus protein intake (geometric framework; (Raubenheimer and Simpson, 1997) indicates that ICV FGF21 increases intake along the protein axis but not along the carbohydrate axis, indicating that FGF21 acts in the brain to specifically increase protein intake without altering carbohydrate or energy intake. It should be noted that for this and other ICV injections, intake was measured over a 24-h window after once-daily FGF21 injections, and thus, it is possible that results include both short-lived effects of FGF21 and later, compensatory changes. A more detailed temporal assessment of intake would be required resolve such possible outcomes.
Although FGF21 increased protein intake in a mixed macronutrient setting, interpretation of the above data is complicated by that fact that the protein:carbohydrate ratio is altered reciprocally in each diet to maintain equal energy density (isocaloric). This issue is particularly relevant because recent studies have suggested that FGF21 acts to reduce sweet taste, consistent with a reduction in carbohydrate intake (Soberg et al., 2017; Talukdar et al., 2016a; von Holstein-Rathlou et al., 2016), implying that the effects on carbohydrate could contribute to the above results.
However, in those prior studies, FGF21 only reduced sweet taste (sucrose, glucose), not carbohydrate (maltodextrin). The diets used in our studies had equal sucrose content but varied in the non-sweet starch maltodextrin. It is, therefore, unlikely that differences in “sweetness” are driving the observations noted above. Nevertheless, we sought to further extend these data by testing FGF21 in a paradigm in which protein (casein) and carbohydrate (maltodextrin) were independently provided as isocaloric liquid solutions. This design offered three advantages. First, the design separates protein from carbohydrate intake, allowing assessment of nutrient consumption in isolation or when paired/contrasted. Second, prior work using a similar paradigm demonstrated that protein-restricted rats increase casein versus maltodextrin intake (Murphy et al., 2018). Third, the use of the non-sweet carbohydrate maltodextrin avoids confounding effects of sweet taste, although it is acknowledged that mice do exhibit a detectable “taste” for maltodextrin that is independent of sweetness (Spector and Schier, 2018; Zukerman et al., 2009). Using this model, we present compelling evidence that brain FGF21 signaling is required for physiological shifts in macronutrient preference in protein-restricted mice. When the nutrients were provided individually (single-choice), protein-restricted, WT mice increased casein, but not maltodextrin, intake. This observation is important because it suggests that protein restriction does not induce a general state of hyperphagia but, instead, a macronutrient-specific increase in protein intake consistent with the geometric analysis described above. When both nutrients were offered simultaneously (two-choice), protein-restricted WT mice robustly shifted their macronutrient preference toward casein. This shift in preference manifested relatively early (within the first few hours) of exposure, even in mice naive to the solutions. Most important, these effects are completely lost in whole-body Fgf21-KO mice as well as in brain-specific Klb-KO mice, strongly suggesting that CNS FGF21 signaling is necessary for the adaptive changes in protein intake. Finally, we demonstrate that ICV injection of FGF21 recapitulates this casein preference in WT, chow-fed mice but not in mice lacking Klb expression in the brain. This latter observation is highly consistent with a very recently published study demonstrating that FGF21 injection selectively increased protein intake relative to carbohydrate or fat intake and that this effect required central Klb (Larson et al., 2019). Collectively, these data provide compelling evidence that brain FGF21 signaling mediates a physiologically relevant, adaptive change in macronutrient preference. FGF21 is the only known hormone that drives this response.
The above data provide compelling evidence that FGF21 is essential for mice to alter metabolism and food intake during protein restriction, in both lean and obese (diet-induced obesity [DIO]) settings. Considering our previous work (Laeger et al., 2014a, 2016), the essential contribution of FGF21 has now been demonstrated via three completely independent mouse genetic lines that target different components of the FGF21 axis (GCN2-KO, FGF21KO, and KlbCamk2a). This redundancy strongly argues against a potential non-specific effect of any single mouse line. For instance, Klb also mediates the effects FGF15/19, which might contribute to results of the KlbCamk2a model but not the FGF21-KO model. The evidence that FGF21 primarily acts via the brain also begs the question as to the site of FGF21 action. The Camk2a-Cre model has been shown to primarily, but not exclusively, target glutamatergic neurons in the forebrain (Bookout et al., 2013; Casanova et al., 2001; Ding et al., 2012), and thus, it seems very likely that FGF21 is acting within the forebrain to promote the observed changes in metabolism and food intake during protein restriction. However, the precise site of action remains uncertain, and to date, most work has emphasized FGF21 signaling, either within the hypothalamic suprachiasmatic nucleus (SCN) or paraventricular nucleus (PVN) (Bookout et al., 2013; Liang et al., 2014; Matsui et al., 2018; Owen et al., 2013; Santoso et al., 2017; von Holstein-Rathlou et al., 2016). This fact explains why Camk2-Cre reduces but does not abolish Klb expression in the brain. The sites of brain FGF21 action remain an area of active research, and it seems unlikely that a single brain area mediates the broad effects on growth, energy expenditure, sympathetic outflow, glucose homeostasis, and food choice. It is now well accepted that hormones, such as leptin, ghrelin, and GLP-1, act within multiple brain areas (Berthoud et al., 2017; Kanoski et al., 2016; Mason et al., 2014), and defining the brain areas mediating the broad effects of FGF21 in both pharmacological and physiological (protein-restricted) states will require significant effort moving forward.
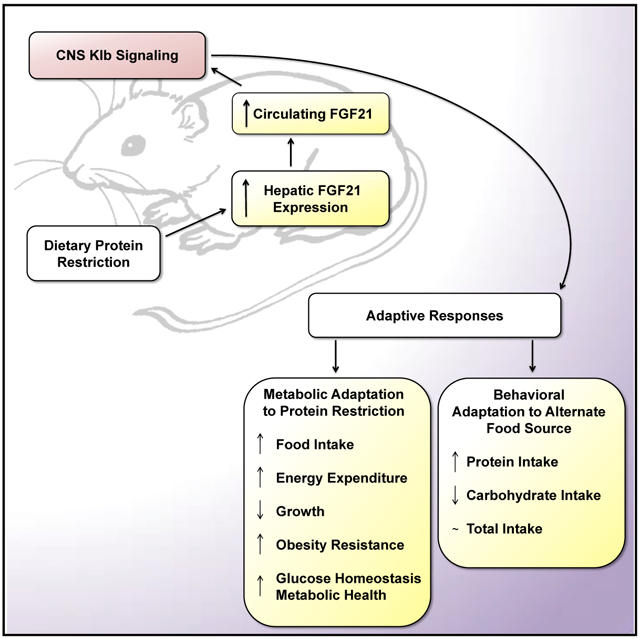
The ability to physiologically and behaviorally adapt to a changing nutritional environment is essential for survival. Central FGF21 signaling provides a clear and compelling mechanism to explain the regulatory control of both metabolism and macronutrient preference in response to physiological protein need. Protein restriction induces a distinct array of responses, including a specific appetite for protein, reduced growth, increased energy expenditure, protection against diet-induced obesity and glucose intolerance, and remodeling of white adipose tissue. Liver FGF21 production is robustly increased in that proteinrestricted state, and FGF21 signaling within the brain is absolutely necessary for mice to mount a physiological response to protein restriction. Notably, the nature of this FGF21-dependent response is dependent on the nutritional environment of the animal. If animals have no choice but to continue consuming the lowprotein diet, central FGF21 signaling mediates metabolic adaptations that include increases in total food intake (protein leverage), increases in energy expenditure, reduced growth, and resistance to diet-induced obesity. If the protein-restricted animal has access to alternative protein sources, FGF21 mediates a preference shift, which increases protein intake without increasing total food intake. In other words, the protein-restricted animal will seek and consume protein if it can but will metabolically adapt if necessary, and each of these homeostatic responses depend on FGF21 signaling within the brain. In summary, this liver-to-brain FGF21 signal mechanistically underpins a fundamental aspect of biology and also provides a conceptual framework that cohesively defines the physiological relevance of FGF21.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Christopher Morrison (Christopher.Morrison@pbrc.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All procedures involving animals were approved by the PBRC Institutional Animal Care and Use Committee and were performed in accordance with the guidelines and regulations of the NIH Office of Laboratory Animal Welfare. Male mice, 8–12 weeks of age, were used in all experiments and were maintained on chow (Lab Diet 5001) unless transitioned to experimental diets (see below). Mouse models were maintained on the C57BL6 background and were either bred in house or purchased from Jackson Labs. Male Fgf21-KO mice on the B6 background were provided by Dr. Steven Kliewer (Potthoff et al., 2009) and bred in the homozygous state with C57BL6 mice (WT; Jackson Labs) used as controls. Klblox/lox were provided by Dr. Steven Kliewer (Bookout et al., 2013; Ding et al., 2012), and tissue specific knockouts generated by crossing female Klblox/lox mice with male Klblox/lox mice also carrying either Adiponectin-Cre (adipose-specific) or Camk2a-Cre (brain-specific) (Casanova et al., 2001). The resulting litters contained Cre positive (knockout) or Cre negative littermate controls, thus maintaining equivalent genetic background. Animals were single housed in 12:12hr light:dark cycle with ad libitum access to food or water unless otherwise noted.
METHOD DETAILS
Experimental Diets
Control versus Low Protein (LP) on both low and high-fat backgrounds For all experiments involving dietary manipulation, single housed mice were transferred from chow to the Control diet for approximately 5 days, at which point a random subgroup of animals were transferred to the respective LP diet, HFCON, or HFLP diet. At the end of the study mice were sacrificed during the mid-light cycle in the fed state (unless otherwise noted) using acute exposure to CO2 followed by rapid decapitation. Trunk blood was also collected at sacrifice, allowed to clot overnight at 4 [notdef]C, centrifuged at 3000×g and serum collected. Tissues were collected and snap frozen in liquid nitrogen for further analysis. Diets were formulated and produced by Research Diets as previously described (Hill et al., 2017; Laeger et al., 2014a) and were designed to be isocaloric by equally varying protein and carbohydrate while keeping fat constant. Control diets contained 20% casein (by weight) as the protein source, while the LP diet contained 5% casein. HFCON and HFLP also contained 20% and 5% casein, respectively, but on a background of 60% fat. All diet compositions are provided in Table S1.
Food choice paradigm
Mixed macronutrient diets Wild-type C57BL6 mice bearing lateral ventricle cannula were simultaneously offered two isocaloric diets that varied in their protein:carbohydrate ratio (Table S1). Mice were offered the choice between 5% versus 35% casein, 10% versus 40% casein, or 5% versus 60% casein. Mice were given one week to adapt to this two-choice paradigm, and then FGF21 (1ug; Eli Lilly and Company, Indianapolis, IN) or saline was injected into the lateral ventricle for 3 consecutive days approximately 3 hours prior to lights off. Food intake was measured daily beginning the day before the injection (Day 0) until after choice normalized.
Casein versus Maltodextrin Solution Intake
Protein versus carbohydrate intake was assessed via a protocol similar to that described by Murphy et al. (2018). First, mice (wildtype or knockout) were randomly assigned to either 20% casein (Control) or 5% casein (LP) diets for at least one week to establish the protein restricted state. Mice were then offered solutions of casein as a protein source or maltodextrin as a carbohydrate source. Solutions contained 4% nutrient and 0.2% saccharin. For single-choice studies, half the mice in each dietary group were offered casein while the other half offered maltodextrin for 3 consecutive days. For two-choice studies, all mice were simultaneously provided both casein and maltodextrin in their cage. Two-choice studies combined with ICV injection were conducted as above, except that mice had one day of access to the solutions before a single ICV injection of FGF21 (1ug), with liquid consumption measured an additional 2 days.
Brain cannulation and ICV injections
Lateral ventricle guide cannula were surgically implanted in mice anesthetized using isofluorane. The superior and dorsal aspect of the head and neck is shaved and the mouse placed into the stereotaxic device. The skin was prepared by successive scrubbing with nolvasan and alcohol. A 1.5-cm midsagittal skin incision was made to expose the skull. Then a small hole was drilled into the skull using a hand-held dremel tool, and a guide cannula (Plastics One) is targeted to specific brain areas or cerebroventricles based on established stereotaxic coordinates. The cannula is permanently affixed to the skull by means of metal bone screws and quickly drying dental acrylic. A removable obdurator seals the guide cannula when not in use. Analgesics were administered following the procedure and animals recovered at least 1 week to prior to study. On experimental days, animals were subjected to intracerebroventricular administration of either 1 ug FGF21 or saline.
Glucose tolerance testing
Sixteen-hour-fasted mice underwent GTT by i.p. injection with 2g glucose per kg of BW. Blood glucose levels were measured at 0, 15, 30, 45, 60, and 120 min with a glucometer (Accu Check; Roche Diabetes Care, Inc. Indianapolis IN) for GTT. The data for GTT are represented as mg/dL and as area under curve (AUC). Fasted glucose levels are reported at the time of GTT.
Insulin sensitivity testing
Three-hour-fasted mice were injected i.p. with 0.75 IU human insulin (Eli Lilly and Company, Indianapolis, IN) per kg of BW. Blood glucose levels were measured at 0, 15, 30, 60 and 120 min with a glucometer (Accu Check; Roche Diabetes Care, Inc. Indianapolis IN). The data for ITT are presented as a percentage of baseline glucose and as area under curve (AUC).
Real-time PCR
RTPCR for assessment of mRNA levels in liver and adipose tissue (white and brown adipose tissue) RNA extraction and real-time PCR was conducted as described previously (Henagan et al., 2016; Laeger et al., 2014b). Total RNA was extracted from liver, iWAT, and BAT using TRIzol reagent following the manufacturer’s protocol (Invitrogen), with the addition of an RNeasy Lipid Tissue Mini Kit (QIAGEN) for the BAT & iWAT. RNA purity and quantity was determined by spectrophotometry using a NanoDrop (Thermo Scientific). cDNA synthesis was performed with iScript (BioRad) and mRNA was quantified on the ABI 7900 platform using the ABI SYBR Green PCR Master Mix in optical 384-well plates (Applied Biosystems). Primer pairs were designed using the IDT RealTime qPCR Primer Design tool with at least one primer spanning an exon-exon boundary. Target gene expression was normalized with cyclophilin as the endogenous Control.
Immunoassay determination of FGF21, Insulin, and Adiponectin
Concentrations of FGF21 in serum were determined in mice with an ELISA according to the procedure recommended by the manufacturer (no. RD291108200R, Mouse and Rat FGF-21 ELISA, BioVendor). The minimal detectable Concentration of FGF21 with this assay was 18.4 pg/ml. For determination of serum FGF21, 50 ml of serum were diluted in 200 ml of dilution buffer before analysis as previously described (Laeger et al., 2014a). Insulin (#EZRMI-13K, Rat/Mouse Insulin ELISA, EMD Millipore Corporation) and adiponectin (#EZMADP-60K/ Mouse Aiponectin EMD Millipore Corporation) was measured with an ELISA according to the procedures per the manufacturer’s recommendation.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data were analyzed using the SAS software package (SAS V9, SAS Institute) using one-way, two-way, or repeated-measures ANOVA using the general linear model procedure. When experiment-wide tests were significant, post hoc comparisons were made using the LSMEANS statement with the PDIFF option, and represent least significant differences tests for pre-planned comparisons. Average daily energy expenditure was analyzed via analysis of covariance (ANCOVA) with body weight as the covariate using the general linear model procedure of SAS. All data are expressed as mean ± SEM, with a probability value of 0.05 considered statistically significant. Groups sizes are described in their respective figure legend.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Human FGF21 | Eli Lilly and Company, Indianapolis, IN | N/A |
| Critical Commercial Assays | ||
| FGF21 ELISA | Biovendor | RD291108200R |
| Insulin ELISA | EMD Millipore | EZRMI-13K |
| Adiponectin ELISA | EMD Millipore | EZMADP-60K |
| Experimental Models: Organisms/Strains | ||
| Mouse:FGF21-KO | Dr. Steven Kliewer | Potthoff et al., 2009 |
| Mouse: Camk2a-Cre | Dr. Steven Kliewer |
Casanova et al., 2001; Bookout et al., 2013 |
| Mouse: Adiponectin-Cre: B6.FVB-Tg(Adipoq-cre)1 Evdr/J | The Jackson Laboratory | Stock No: 028020 |
| Mouse: Klblox/lox (Klbtm1) | Dr. Steven Kliewer | MGI:5446168 Ding et al., 2012 |
| Oligonucleotides | ||
| Fgf21 Forward: CAAATCCTGGGTGTCAAAGC; | Integrated DNA Technologies (IDT) | N/A |
| Fgf21 Reverse: CATGGGCTTCAGACTGGTAC | IDT | N/A |
| Klb Forward: CAGGGATATCTACATCACAGCC | IDT | N/A |
| Klb Reverse: GTAGCCTTTGATTTTGACCTTGTC | IDT | N/A |
| Fas Forward: GGGATCTGGTGAAAGCTGTAG | IDT | N/A |
| Fas Reverse: GTGTTCTCGTTCCAGGATCTG | IDT | N/A |
| Scd-1 Forward: CTGTACGGGATCATACTGGTTC; | IDT | N/A |
| Scd-1 Reverse: CGTGCCTTGTAAGTTCTGTG | IDT | N/A |
| Srebp1 Forward: AGATTGTGGAGCTCAAAGACC | IDT | N/A |
| Srebp1 Reverse: CACl 1CGTAGGGTCAGG1 1C | IDT | N/A |
| Ucp1 Forward: CACCTTCCCGCTGGACAC | IDT | N/A |
| Ucp1 Reverse: CCCTAGGACACCTTTATACCTAATGG | IDT | N/A |
Highlights.
Mice adaptively alter metabolism and food choice during protein restriction
The liver hormone FGF21 is robustly increased by protein restriction
Metabolic responses to protein restriction require FGF21 signaling in the brain
Brain FGF21 also mediates adaptive changes in macronutrient selection
ACKNOWLEDGMENTS
The authors would like to thank the staff of the PBRC Comparative Biology Core and Animal Metabolism and Behavior Core for their skillful assistance and excellent technical support. This work was supported by NIH R01DK105032 and S10OD023703 to C.D.M. T.L. was supported by a research fellowship from the Deutsche Forschungsgemeinschaft (DFG; LA 3042/2–1); C.M.H. was supported by F32DK115137; and S.M.S.-B. was supported by an NHMRC Early Career Fellowship (GNT1110098). H.-R.B. was supported by R01DK047348; H.M. was supported by R01DK092587; and J.J.C. was supported by R21AI138136. This project/work used facilities within the Animal Metabolism & Behavior Core, Genomics Core, and Cell Biology and Bioimaging Core at PBRC that are supported in part by NIH center COBRE (P30GM118430) and NORC (P30DK072476) grants as well as an NIH equipment award S10OD023703.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j. celrep.2019.05.022.
DECLARATION OF INTERESTS
The authors declare no competing interests
REFERENCES
- Adams AC, Yang C, Coskun T, Cheng CC, Gimeno RE, Luo Y, and Kharitonenkov A (2012). The breadth of FGF21’s metabolic actions are governed by FGFR1 in adipose tissue. Mol. Metab 2, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Munzberg H, and Morrison CD (2017). Blaming the Brain for Obesity: Integration of Hedonic and Homeostatic Mechanisms. Gastroenterology 152, 1728–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BonDurant LD, Ameka M, Naber MC, Markan KR, Idiga SO, Acevedo MR, Walsh SA, Ornitz DM, and Potthoff MJ (2017). FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 25, 935–944.e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BonDurant LD, and Potthoff MJ (2018). Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annu. Rev. Nutr 38, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, and Kliewer SA (2013). FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med 19, 1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, Stewart AF, and Schutz G (2001). A CamKIIalpha iCre BAC allows brainspecific gene inactivation. Genesis 31, 37–42. [DOI] [PubMed] [Google Scholar]
- Chaumontet C, Recio I, Fromentin G, Benoit S, Piedcoq J, Darcel N, and Tomé D (2018). The protein status of rats affects the rewarding value of meals due to their protein content. J. Nutr 148, 989–998. [DOI] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, and Kharitonenkov A (2008). Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027. [DOI] [PubMed] [Google Scholar]
- Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, Poudel C, Sherman DS, Yu D, Arriola Apelo SI, et al. (2018). Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol 596, 623–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, and Kliewer SA (2012). bKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 16, 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Stevanovic DM, Fisher FM, Cisu TI, Chee MJ, Nguyen NL, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, et al. (2015). Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology 156, 2470–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, and Maratos-Flier E (2014). Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab 4, 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicetti LA, Robbins CT, and Shipley LA (2003). Dietary protein content alters energy expenditure and composition of the mass gain in grizzly bears (Ursus arctos horribilis). Physiol. Biochem. Zool 76, 256–261. [DOI] [PubMed] [Google Scholar]
- Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, et al. (2016). Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 16, 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, and Moller DE (2013). The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340. [DOI] [PubMed] [Google Scholar]
- Gosby AK, Conigrave AD, Raubenheimer D, and Simpson SJ (2014). Protein leverage and energy intake. Obes. Rev 15, 183–191. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al. ; Pennington CALERIE Team (2006). Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295, 1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henagan TM, Laeger T, Navard AM, Albarado D, Noland RC, Stadler K, Elks CM, Burk D, and Morrison CD (2016). Hepatic autophagy contributes to the metabolic response to dietary protein restriction. Metabolism 65, 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Laeger T, Albarado DC, McDougal DH, Berthoud H-R, Munzberg H, and Morrison CD (2017). Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Sci. Rep 7, 8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Berthoud HR, Munzberg H, and Morrison CD (2018). Homeostatic sensing of dietary protein restriction: a case for FGF21. Front. Neuroendocrinol 51, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Hancock DP, Gosby AK, McMahon AC, Solon SM, Le Couteur DG, Conigrave AD, Raubenheimer D, and Simpson SJ (2013). Effects of dietary protein to carbohydrate balance on energy intake, fat storage, and heat production in mice. Obesity (Silver Spring) 21, 85–92. [DOI] [PubMed] [Google Scholar]
- Iroz A, Montagner A, Benhamed F, Levavasseur F, Polizzi A, Anthony E, Régnier M, Fouché E, Lukowicz C, Cauzac M, et al. (2017). A specific ChREBP and PPARa cross-talk is required for the glucose-mediated FGF21 response. Cell Rep. 21, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Hayes MR, and Skibicka KP (2016). GLP-1 and weight loss: unraveling the diverse neural circuitry. Am. J. Physiol. Regul. Integr. Comp. Physiol 310, R885–R895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, and Adams AC (2013). Inventing new medicines: The FGF21 story. Mol. Metab 3, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, and DiMarchi R (2017). Fibroblast growth factor 21 night watch: advances and uncertainties in the field. J. Intern. Med 281, 233–246. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. (2005). FGF-21 as a novel metabolic regulator. J. Clin. Invest 115, 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, and Morrison CD (2014a). FGF21 is an endocrine signal of protein restriction. J. Clin. Invest 124, 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Reed SD, Henagan TM, Fernandez DH, Taghavi M, Addington A, Munzberg H, Martin RJ, Hutson SM, and Morrison CD (2014b). Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. Am. J. Physiol. Regul. Integr. Comp. Physiol 307, R310–R320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, Gettys TW, Collier JJ, Munzberg H, and Morrison CD (2016). Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 16, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Castaño-Martinez T, Werno MW, Japtok L, Baumeier C, Jonas W, Kleuser B, and Schurmann A (2018). Dietary carbohydrates impair the protective effect of protein restriction against diabetes in NZO mice used as a model of type 2 diabetes. Diabetologia 61, 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan T, Morgan DA, Rahmouni K, Sonoda J, Fu X, Burgess SC, Holland WL, Kliewer SA, and Mangelsdorf DJ (2017). FGF19, FGF21, and an FGFR1/b-klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 26, 709–718.e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson KR, Chaffin AT, Goodson ML, Fang Y, and Ryan KK (2019). Fibroblast growth factor-21 controls dietary protein intake in male mice. Endocrinology 160, 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E, Steyaert J, Lemmon MA, Lax I, and Schlessinger J (2018). Structures of b-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553, 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, and Hirsch J (1995). Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med 332, 621–628. [DOI] [PubMed] [Google Scholar]
- Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, Ding H, Lam KS, and Xu A (2014). FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 63, 4064–4075. [DOI] [PubMed] [Google Scholar]
- Maida A, Zota A, Sjøberg KA, Schumacher J, Sijmonsma TP, Pfenninger A, Christensen MM, Gantert T, Fuhrmeister J, Rothermel U, et al. (2016). A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J. Clin. Invest 126, 3263–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maida A, Chan JSK, Sjøberg KA, Zota A, Schmoll D, Kiens B, Herzig S, and Rose AJ (2017). Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Mol. Metab 6, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, and Potthoff MJ (2014). Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63, 4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BL, Wang Q, and Zigman JM (2014). The central nervous system sites mediating the orexigenic actions of ghrelin. Annu. Rev. Physiol 76, 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S, Sasaki T, Kohno D, Yaku K, Inutsuka A, Yokota-Hashimoto H, Kikuchi O, Suga T, Kobayashi M, Yamanaka A, et al. (2018). Neuronal SIRT1 regulates macronutrient-based diet selection through FGF21 and oxytocin signalling in mice. Nat. Commun 9, 4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, and Laeger T (2015). Protein-dependent regulation of feeding and metabolism. Trends Endocrinol. Metab 26, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, Reed SD, and Henagan TM (2012). Homeostatic regulation of protein intake: in search of a mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol 302, R917–R928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Peters KZ, Denton BS, Lee KA, Chadchankar H, and McCutcheon JE (2018). Restriction of dietary protein leads to conditioned protein preference and elevated palatability of protein-containing food in rats. Physiol. Behav 184, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nakatake Y, Konishi M, and Itoh N (2000). Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 1492, 203–206. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, and Kuro-o M (2007). BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. USA 104, 7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L, Kliewer SA, and Mangelsdorf DJ (2013). FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med 19, 1153–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, and Mangelsdorf DJ (2014). FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 20, 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ (2017). FGF21 and metabolic disease in 2016: a new frontier in FGF21 biology. Nat. Rev. Endocrinol 13, 74–76. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, and Burgess SC (2009). FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA 106, 10853–10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer D, and Simpson SJ (1997). Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev 10, 151–179. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ, and Tyzbir RS (1982). Energy balance and mitochondrial function in liver and brown fat of rats fed “cafeteria” diets of varying protein content. J. Nutr 112, 1663–1672. [DOI] [PubMed] [Google Scholar]
- Santoso P, Nakata M, Shiizaki K, Boyang Z, Parmila K, Otgon-Uul Z, Hashimoto K, Satoh T, Mori M, Kuro-O M, and Yada T (2017). Fibroblast growth factor 21, assisted by elevated glucose, activates paraventricular nucleus NUCB2/Nesfatin-1 neurons to produce satiety under fed states. Sci. Rep 7, 45819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, and Schwartz MW (2010). Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59, 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, and Raubenheimer D (1997). Geometric analysis of macronutrient selection in the rat. Appetite 28, 201–213. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, and Raubenheimer D (2012). The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity (Princeton University Press; ). [Google Scholar]
- Soberg S, Sandholt CH, Jespersen NZ, Toft U, Madsen AL, von Holstein-Rathlou S, Grevengoed TJ, Christensen KB, Bredie WLP, Potthoff MJ, et al. (2017). FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 25, 1045–1053.e1046. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Walters KA, Simanainen UK, McMahon AC, Ruohonen K, Ballard JW, Raubenheimer D, Handelsman DJ, Le Couteur DG, and Simpson SJ (2015). Macronutrient balance, reproductive function, and lifespan in aging mice. Proc. Natl. Acad. Sci. USA 112, 3481–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Cogger VC, Pulpitel T, Heblinski M, Wahl D, McMahon AC, Warren A, Durrant-Whyte J, Walters KA, Krycer JR, et al. (2016). Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 24, 555–565. [DOI] [PubMed] [Google Scholar]
- Sørensen A, Mayntz D, Raubenheimer D, and Simpson SJ (2008). Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring) 16, 566–571. [DOI] [PubMed] [Google Scholar]
- Spector AC, and Schier LA (2018). Behavioral evidence that select carbohydrate stimuli activate T1R-independent receptor mechanisms. Appetite 122, 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, et al. (2016a). FGF21 regulates sweet and alcohol preference. Cell Metab. 23, 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM, et al. (2016b). A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23, 427–440. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Bruss MD, Nag N, Kharitonenkov A, Adams AC, and Hellerstein MK (2014). Fibroblast growth factor 21 is not required for the reductions in circulating insulin-like growth factor-1 or global cell proliferation rates in response to moderate calorie restriction in adult mice. PLoS One 9, e111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, et al. (2016). FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 23, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Burk DH, Cortez CC, Van NT, Stone KP, Baker M, Mendoza T, Mynatt RL, and Gettys TW (2015). UCP1 is an essential mediator of the effects of methionine restriction on energy balance but not insulin sensitivity. FASEB J. 29, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BD, He B, Dean RG, and Martin RJ (1994). Low protein diets increase neuropeptide Y gene expression in the basomedial hypothalamus of rats. J. Nutr 124, 1152–1160. [DOI] [PubMed] [Google Scholar]
- White BD, Porter MH, and Martin RJ (2000). Protein selection, food intake, and body composition in response to the amount of dietary protein. Physiol. Behav 69, 383–389. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, et al. (2012). The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. elife 1, e00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, and Sclafani A (2009). T1R3 taste receptor is critical for sucrose but not Polycose taste. Am. J. Physiol. Regul. Integr. Comp. Physiol 296, R866–R876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.