Abstract
Objective
To evaluate the safety, efficacy and therapeutic mechanism of BI 655064, an antagonistic anti-CD40 monoclonal antibody, in patients with rheumatoid arthritis (RA) and an inadequate response to methotrexate (MTX-IR).
Methods
In total, 67 patients were randomised to receive weekly subcutaneous doses of 120 mg BI 655064 (n=44) or placebo (n=23) for 12 weeks. The primary endpoint was the proportion of patients who achieved 20% improvement in American College of Rheumatology criteria (ACR20) at week 12. Safety was assessed in patients who received at least one dose of study drug.
Results
At week 12, the primary endpoint was not met, with 68.2% of patients treated with BI 655064 achieving an ACR20 vs 45.5% with placebo (p=0.064); using Bayesian analysis, the posterior probability of seeing a difference greater than 35% was 42.9%. BI 655064 was associated with greater changes in CD40–CD40L pathway-related markers, including reductions in inflammatory and bone resorption markers (interleukin-6, matrix metalloproteinase-3, receptor activator of nuclear factor-κB ligand), concentration of autoantibodies (immunoglobulin [Ig]G rheumatoid factor [RF], IgM RF, IgA RF) and CD95+ activated B-cell subsets. No serious adverse events (AEs) related to BI 655064 treatment or thromboembolic events occurred; reported AEs were mainly of mild intensity.
Conclusion
Although blockade of the CD40–CD40L pathway with BI 655064 in MTX-IR patients with RA resulted in marked changes in clinical and biological parameters, including reductions in activated B-cells, autoantibody production and inflammatory and bone resorption markers, with a favourable safety profile, clinical efficacy was not demonstrated in this small phase IIa study.
Trial registration number
Keywords: rheumatoid arthritis, B-cells, autoantibodies
Key messages.
What is already known about this subject?
Hyperactivation of the CD40–CD40 ligand (CD40L) pathway is associated with disease activity and pathogenesis of autoimmune diseases and is a potential therapeutic target in rheumatoid arthritis (RA).
Former clinical trials of anti-CD40L antibodies were halted because of increased incidence of thromboembolism.
What does this study add?
In this small phase IIa study, treatment with BI 655064, an antagonistic anti-CD40 monoclonal antibody, in inadequate response to methotrexate patients with RA resulted in marked changes in clinical and biological parameters, including reductions in activated B-cells, autoantibody production and inflammatory and bone resorption markers, with a favourable safety profile; however, the primary endpoint (20% improvement in American College of Rheumatology criteria response at week 12) was not met.
How might this impact on clinical practice or future developments?
The CD40–CD40L pathway has been shown to play an important role in the pathogenesis of systemic lupus erythematosus and lupus nephritis (LN).
Given the favourable clinical safety profile of BI 655064 in this study, a clinical trial assessing the efficacy and safety of BI 655064 in LN is ongoing.
Introduction
Hyperactivation of the CD40–CD40 ligand (CD40L) pathway is associated with disease activity and pathogenesis of autoimmune diseases.1–7 CD40L (CD154) is expressed on the T-cell surface following activation. Binding of CD40L to CD40 expressed on B-cell surfaces mediates T-cell-dependent B-cell proliferation, maturation, antibody formation and immunoglobulin isotype switch. Similarly, CD40L binding to CD40 expressed on dendritic cells, monocytes and macrophages, endothelial cells and fibroblasts promotes cell differentiation, proinflammatory cytokine production and upregulated expression of costimulatory ligands. Loss of CD40L expression or function results in X-linked hyperimmunoglobulin (Ig)M syndrome, which is characterised by recurrent infections due to impaired immunoglobulin isotype switching and somatic hypermutations.8
The CD40–CD40L pathway is a potentially attractive therapeutic target in the treatment of rheumatoid arthritis (RA). CD40 expression has been demonstrated on B-cells, dendritic cells and monocytes in synovial fluid and on synovial fibroblasts in joints affected by RA, while enhanced CD40L expression has been found on T-cells in the periphery and synovial tissue in the RA disease setting.3 4 6 9 Anti-CD40 antibody treatment of synovial fibroblasts expressing CD40 from patients with RA resulted in decreased levels of spontaneous tumour necrosis factor (TNF) α production in vitro.6 Furthermore, anti-TNFα treatment in patients with RA resulted in a significant decrease of CD40L expression on T-cells.10 A similar effect was observed with synovial fluid macrophages, whereby CD40 ligation induced the secretion of interleukin (IL)-12, IL-10 and TNFα.11 12 CD40–CD40L pathway inhibition could impact multiple pathological mechanisms in RA and dampen B-cell and T-cell responses and IL-12 production from dendritic cells and macrophages.
BI 655064 is a humanised, antagonistic anti-CD40 monoclonal antibody that selectively binds CD40 and blocks the CD40–CD40L interaction;13 two mutations in the Fc region were introduced to prevent Fc-mediated antibody-dependent or complement-mediated cellular cytotoxicity and platelet activation.13 BI 655064 demonstrated potent and comparable binding properties in both human and cynomolgus monkey B-cells and did not cause platelet activation, aggregation or function.13–15
The objectives of this study were to characterise the efficacy, safety and tolerability of weekly dosing of 120 mg BI 655064 in patients with RA with an inadequate response to methotrexate (MTX-IR) and to elucidate the therapeutic mechanism of anti-CD40 antibody treatment.
Methods
Study design
This 12-week, multicentre, randomised, double-blind, parallel-group, placebo-controlled, phase IIa study with an 8-week follow-up period was conducted at 27 sites in six countries (Czech Republic, Germany, the Netherlands, New Zealand, Poland and Spain) between October 2013 and April 2015. Patients were randomised (via an Interactive Response System) in a ratio of 2:1 to weekly subcutaneous 120 mg BI 655064 or placebo. Randomisation was stratified with respect to region (Eastern Europe vs Western Europe/New Zealand).
The weekly subcutaneous 120 mg dose of BI 655064 was selected based on safety, pharmacokinetic and pharmacodynamic data from the single and multiple dose studies.14 All subcutaneous injections were given by staff at the study site.
Patients
Patients aged 18–70 years were eligible if they had RA diagnosed according to the American College of Rheumatology (ACR) 1987 revised criteria;16 were MTX-IR and had received ≤2 anti-TNFα therapies (patients who were currently using or had previously used a biological disease-modifying antirheumatic drug, such as CTLA4-Ig, anti-IL-6 or anti-CD20 or an oral compound such as Janus kinase inhibitors, were excluded); had active disease, defined as a Disease Activity Score in 28 joints (DAS28) using four variables and C reactive protein (CRP) ≥3.5, ≥6 tender joints (on tender joint count, out of 68) and ≥6 swollen joints (on swollen joint count, out of 66) at screening and confirmed at randomisation; had a serum CRP level ≥0.8 mg/dL or erythrocyte sedimentation rate (ESR) ≥28 mm/hour at screening (added via protocol amendment to improve patient enrolment); and were seropositive for rheumatoid factor (RF) or anticyclic citrullinated protein antibodies (ACPAs). Patients were to continue background treatment with a stable dose (for ≥6 weeks prior to screening) of MTX (≥15 mg/week; for patients who were not able to tolerate this dose due to side effects, a stable weekly dose ≥7.5 mg was permitted); patients were required to have been receiving MTX for ≥3 months prior to screening; changes in MTX dosing were not permitted except in the event of an MTX-related safety issue. Low-dose systemic glucocorticoids (equivalent to ≤10 mg prednisolone/day), non-steroidal anti-inflammatory drugs and analgesics were allowed at the discretion of the investigators; changes in dose were permitted. See online supplementary material for full inclusion/exclusion criteria.
annrheumdis-2018-214729supp001.pdf (373.3KB, pdf)
Assessments
All primary and secondary endpoint assessments were evaluated at week 12. The primary endpoint was the proportion of patients who achieved a 20% improvement in the ACR criteria (ACR20). Secondary endpoints included the proportion of patients who achieved ACR50 or ACR70 response; European League Against Rheumatism (EULAR) response using DAS28-ESR and DAS28-CRP, as defined by EULAR criteria; clinically meaningful improvement in DAS28 (>1.2 decrease from baseline in DAS28-CRP); change in DAS28-CRP from baseline. Further endpoints included change from baseline in Simple Disease Activity Index and Clinical Disease Activity Index.
The safety and general tolerability of BI 655064 were evaluated descriptively by monitoring adverse events (AEs), changes in vital signs, physical examinations, clinical laboratory tests and assessment of tolerability by the investigator. AE intensity was graded according to the Rheumatology Common Toxicity Criteria V.2.0.17
Blood samples for pharmacokinetic, immunogenicity, exploratory clinical biomarkers and pharmacogenomic analysis were collected at prespecified visits (see online supplementary materials). Biomarkers associated with RA disease were assessed, including CD95+ activated B-cell subpopulations, immunoglobulins (IgG RF, IgM RF, IgA RF, IgG ACPA, total IgG, total IgM) and bone remodelling (RANKL, matrix metalloproteinase-3 [MMP3]) and inflammatory (IL-6) biomarkers.
Statistical analysis
Sample size was determined based on a Bayesian approach assuming an ACR20 response rate at week 12 of 65% in the BI 655064 group and 25% in the placebo group.
For binary endpoints, efficacy data were analysed using non-responder imputation; for non-binary endpoints, data were analysed using last observation carried forward. All efficacy endpoints were analysed using the full analysis set (FAS) and (one-sided) p values reported for comparisons of BI 655064 versus placebo. The safety population included all patients who had received at least one dose of study drug.
The primary endpoint was analysed descriptively with a Bayesian approach (details provided in the online supplementary materials); additionally, a logistic regression model was performed, including treatment, geographical region and previous use of anti-TNFα as covariates. Assessments of clinical biomarkers and pharmacogenomic analyses were exploratory. Statistical analyses of secondary endpoints, clinical biomarkers and pharmacogenomic analyses are described in the online supplementary materials.
Results
Patient disposition and baseline characteristics
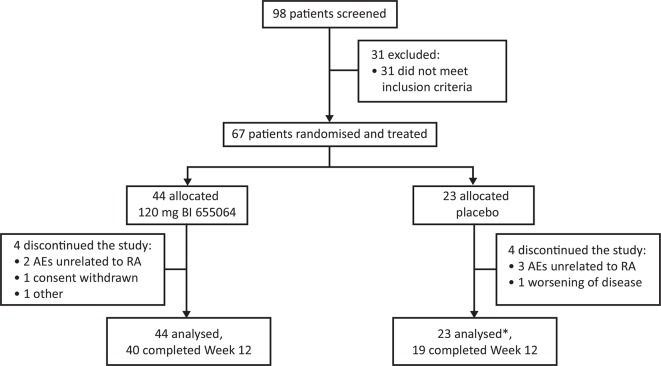
A total of 67 patients were randomised and received treatment: 44 patients were assigned to BI 655064 and 23 to placebo (figure 1). All 67 patients were included in the safety analysis set; 66 patients were included in the FAS population (one patient receiving placebo was excluded from the FAS analysis due to insufficient efficacy data). Overall, eight patients discontinued treatment before week 12; the most common reason for discontinuation was AEs unrelated to RA, in five patients (7.5%). One patient in the placebo group discontinued because of worsening of disease. Demographics at baseline were generally similar across treatment groups (table 1), with the exception of baseline CRP values, which were lower in the BI 655064 group. Most patients were anti-TNFα naive (n=61; 91%).
Figure 1.
Patient disposition. Disposition of the study patients treated with subcutaneous 120 mg BI 655064 or placebo administered once weekly for 12 weeks. Discontinuations due to AEs unrelated to RA were a fatal cerebral haemorrhage and one case of iron deficiency in the BI 655064 group and one case of pleural effusion, one case of elevated ALT and AST and one case of nasopharyngitis in the placebo group.*One patient excluded from full analysis set due to insufficient efficacy data; all 67 patients treated were included in the safety analysis set.AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; RA, rheumatoid arthritis.
Table 1.
Baseline demographic and clinical characteristics
| BI 655064 (n=44) | Placebo (n=23) | |
| Age, years | 53.7±13.3 | 55.1±8.3 |
| Female, n (%) | 37 (84.1) | 18 (78.3) |
| BMI | 26.06±4.57 | 31.09±5.67 |
| Region, n (%) | ||
| Eastern Europe | 28 (63.6) | 15 (65.2) |
| Western Europe | 16 (36.4) | 8 (34.8) |
| Ethnicity, n (%) | ||
| White | 43 (97.7) | 23 (100) |
| Asian | 1 (2.3) | 0 |
| Disease duration, years | 8.3±7.5 | 5.8±4.7 |
| Anti-TNFα naive, n (%) | 38 (86.4) | 23 (100) |
| DAS28-CRP | 5.39±0.85 | 5.53±0.79 |
| DAS28-ESR | 6.21±0.73 | 6.16±0.80 |
| CRP, mg/L | 9.80±12.63 | 23.61±26.46 |
| ESR, mm/hour | 37.45±18.67 | 44.45±23.76 |
| Duration of morning stiffness, mins | 84.1±61.6 | 67.3±53.1 |
| SJC 66 | 14.38±7.41 | 12.86±6.94 |
| TJC 68 | 21.25±11.87 | 19.77±10.84 |
Except where indicated otherwise, values are mean±SD.
BMI, body mass index; CRP, C-reactive protein;DAS28, Disease Activity Score in 28 joints;ESR, erythrocyte sedimentation rate; SD, standard deviation; SJS 66, swollen joint count based on 66 joints; TJC 68, tender joint count based on 68 joints; TNFα, tumour necrosis factor α.
Efficacy
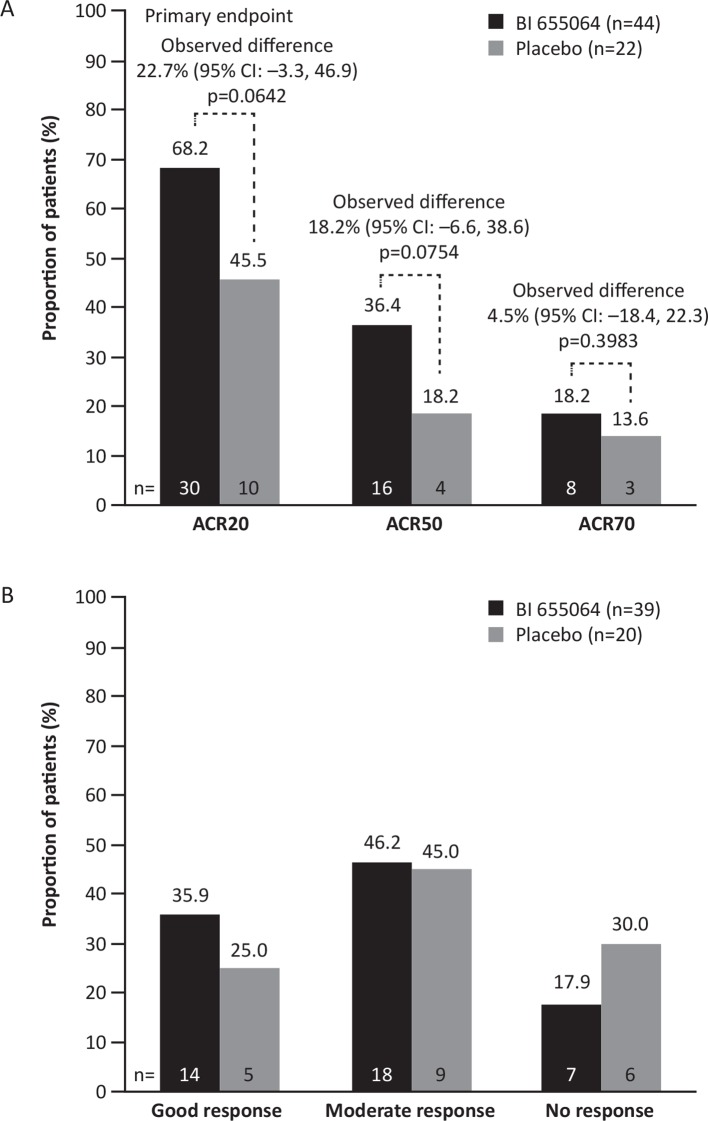
An ACR20 response was seen for 30/44 patients (68.2%) in the BI 655064 group and for 10/22 patients (45.5%) in the placebo group, yielding an observed difference of 22.7% (figure 2A). From the Bayesian analysis using an informative prior (an assumed response rate of 25%) for the placebo group and a non-informative prior for the BI 655064 group, the posterior mean difference between the treatment groups was 33.0%; thus, the primary endpoint was not met, as the observed difference was smaller than the expected difference. When the risk differences were adjusted for treatment, region and anti-TNFα history, the estimate of the adjusted difference was 23.0% (95% CI –3.0% to 46.3%).
Figure 2.
Treatment response at week 12. Responses were defined according to (A) ACR20/50/70 improvement criteria (FAS, non-responder imputation) and (B) EULAR response (DAS28-CRP; FAS, observed). The primary endpoint (ACR20 at week 12) was evaluated with a Bayesian approach. ACR20/50/70, American College of Rheumatology 20/50/70% improvement criteria; DAS28-CRP, Disease Activity score in 28 joints based on C-reactive protein; EULAR, European League Against Rheumatism; FAS, full analysis set.
ACR50 and ACR70 responses were achieved by 36.4% and 18.2% of patients in the BI 655064 group compared with 18.2% and 13.6% of patients in the placebo group, respectively (differences were not statistically significant between BI 655064 and placebo; figure 2A). Of the six patients with previous exposure to anti-TNFα therapy, who were all randomised to the BI 655064 group, four achieved an ACR20 response and two achieved an ACR50 response. EULAR good/moderate responses (DAS28-CRP) were achieved by 82.1% of patients in the BI 655064 group compared with 70.0% in the placebo group (p=0.1894, figure 2B). Mean change in DAS28-CRP at week 12 was –1.61 in the BI 655064 group vs –1.45 in the placebo group (p=0.5463). In patients with CRP values above the median, the difference in DAS28-CRP between BI 655064 and placebo was more pronounced (–1.83 vs –1.38; p=0.2565; online supplementary table S1).
A substantial decrease from baseline to week 12 in median ESR and pain-VAS in the BI 655064 group was observed compared with the placebo group (p=0.0288 and p=0.0456, respectively, online supplementary table S2). BI 655064 led to greater numerical, but not statistically significant, reductions across other ACR core measures compared with the placebo group (online supplementary table S3).
The impact of risk differences for the ACR20 response rate at week 12 based on baseline subcategories was evaluated and indicated a significant difference favouring BI 655064 treatment vs placebo for patients with a time since diagnosis <2.5 years (p=0.0091) (online supplementary figure S1).
Biomarkers
Changes in autoantibodies and B-cell subpopulations
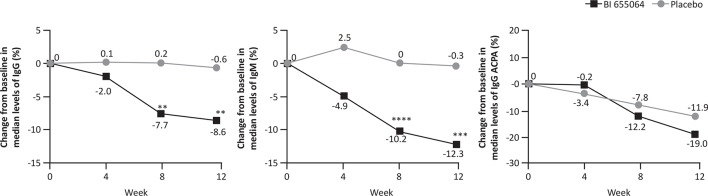
As early as week 4, BI 655064 treatment led to greater, but non-statistically significant, decreases from baseline vs placebo in median levels of total IgG and total IgM (figure 3); at weeks 8 and 12, the reductions were significant between BI 655064 vs placebo for both total IgG (–7.7% vs 0.2%; p=0.0026 and –8.6% vs –0.6%; p=0.0082, respectively) and total IgM (–10.2% vs 0.0%; p<0.0001 and –12.3% vs –0.3%; p=0.0002, respectively). At week 12, a greater reduction in the median levels of disease-specific antibodies was observed in patients receiving BI 655064 vs placebo, with a significant reduction in IgG RF (–22.7% vs 14.3%; p=0.0018) and IgA RF (–13.9% vs 0.0%; p=0.0050); no change was observed in IgM RF (0.0% vs 0.0%; p=0.1743). IgG ACPA was reduced in both treatment groups at week 12 (–19.0% BI 655064 vs –11.9% placebo; p=0.2771).
Figure 3.
Median per cent change from baseline to week 12 in autoantibody levels. Median per cent change from baseline to week 12 in levels of total IgG, IgM and IgG ACPA. FAS, observed. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001. ACPA, anticyclic citrullinated protein antibody; FAS, full analysis set; Ig, immunoglobulin.
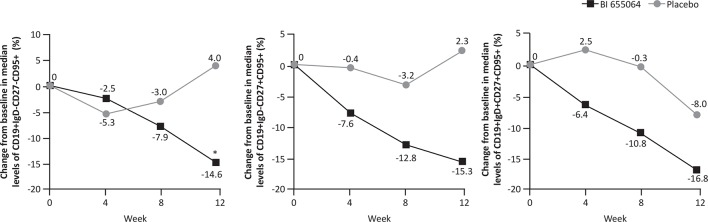
Levels of activated B-cells were modulated by BI 655064 (figure 4). BI 655064 was associated with greater reductions in the median percentage of circulating CD19+IgD−CD27−CD95+ B cells from baseline vs placebo over time and this change was statistically significant at week 12 (–14.6% vs 4.0%; p=0.0097). A reduction in the median percentage of circulating CD19+IgD−CD27+CD95+ B cells and CD19+IgD+CD27+CD95+ B cells from baseline was also observed for BI 655064 vs placebo, but the differences at week 12 did not reach statistical significance (–15.3% vs 2.3%; p=0.0765 and 16.8% vs –8.0%; p=0.0831, respectively).
Figure 4.
Median per cent change from baseline to week 12 in levels of CD95+ activated CD19+ B-cell subsets. Median per cent change from baseline to week 12 in B-cell subsets CD19+IgD−CD27−CD95+, CD19+IgD−CD27+CD95+ and CD19+IgD+CD27+CD95+. FAS, observed. *p≤0.01. FAS, full analysis set.
Inflammatory and bone remodelling biomarkers
The median changes in the levels of select biomarkers associated with inflammation (IL-6) and bone remodelling (MMP3 and RANKL) to week 12 are summarised in online supplementary figure S2 and table S4. BI 655064 was associated with a greater, but not statistically significant, decrease in IL-6 levels vs placebo at week 8 (–28.1% vs –2.75%; p=0.1332) and week 12 (–17.3% vs 18.7%; p=0.1001). Similarly, MMP3 levels were reduced in the BI 655064 group vs placebo at week 8 (–7.5% vs 4.6%; p=0.6176) and week 12 (–7.8% vs 2.3%; p=0.1574) but did not reach statistical significance. Levels of RANKL were significantly decreased following BI 655064 vs placebo at week 8 (–19.6% vs 1.8%; p=0.0045) and week 12 (–29.4% vs 0.0%; p=0.0041). Analysis of the ACR50 responder and non-responder subgroups identified a significant decrease in MMP3 levels versus non-responders receiving BI 655064 at week 12 (–16.4% vs 13.6%; p=0.0005), while a non-statistically significant reduction was observed in IL-6 levels at week 12 in ACR50 responders vs non-responders (–39.0% vs 3.9%; p=0.1215). The per cent change from baseline in MMP3 and IL-6 in ACR20 responders versus non-responders at week 12 was –9.8% vs 3.4% (p=0.5292) and –7.4% vs –35.0% (p=0.2009), respectively. For ACR70 responders, the per cent change from baseline in MMP3 and IL-6 levels was not calculated as the number of patients was considered too small.
Pharmacogenomics
The CD40 rs4810485 G/T polymorphism, which has previously been linked to RA disease,18 was genotyped in all 46 patients who provided informed consent: 21 patients were homozygous for GG, 18 patients were heterozygous for GT and 5 patients were homozygous for TT. T-allele carriers treated with BI 655064 showed a higher, but not statistically significant, ACR20 response (72.7% vs 50.0%; p=0.1558) and significantly higher ACR50 response (54.5% vs 16.7%; p=0.0403) vs placebo at week 12. Similarly, BI 655064 led to a significant decrease in CD19+IgD−CD27+CD95+ (–24.2% vs 4.6%; p=0.0032) and CD19+IgD−CD27−CD95+ (–18.6% vs 7.0%; p=0.0022) B-cell subsets vs placebo in T-allele carriers. No significant reductions were observed in CD19+IgD+CD27+CD95+ B-cell levels following BI 655064 treatment in T-allele carriers (online supplementary table S5).
Safety
There was a higher rate of AEs reported in patients receiving placebo (78.3%) vs BI 655064 (65.9%; table 2). The most frequently reported AEs in both groups were nasopharyngitis and headache.
Table 2.
Safety profile
| BI 655064 (n=44) | Placebo (n=23) | |
| Any AE | 29 (65.9) | 18 (78.3) |
| Other significant AEs* | 1 (2.3) | 3 (13.0) |
| AEs leading to drug discontinuation | 2 (4.5) | 4 (17.4) |
| Serious AEs | 2 (4.5) | 2 (8.7) |
| Death | 1 (2.3)† | 0 |
| Myocardial infarction | 1 (2.3) | 0 |
| Pleural effusion | 0 | 1 (4.3) |
| Anaemia | 0 | 1 (4.3) |
| RCTC AE grade ≥3 | 2 (4.5) | 1 (4.3) |
| Common AEs‡ | ||
| Nasopharyngitis | 6 (13.6) | 5 (21.7) |
| Diarrhoea | 3 (6.8) | 0 |
| Fatigue | 0 | 2 (8.7) |
| Headache | 3 (6.8) | 3 (13.0) |
| Arthralgia | 2 (4.5) | 2 (8.7) |
| Liver disorder | 2 (4.5) | 2 (8.7) |
| Laboratory AEs | ||
| RCTC grade ≥3 | 0 | 0 |
| RCTC grade 2§ | 2 (4.5) | 3 (13.0) |
| RCTC grade 1¶ | 2 (4.5) | 1 (4.3) |
Data are n (%). AEs were coded using MedDRA V.18.0.
*Per ICH E3 Guideline; in the BI 655064 group there was one case of iron deficiency anaemia and in the placebo group there was one case of elevated liver enzymes, one case of worsening RA and one case of nasopharyngitis.
†Patient had pre-existing hypertension and developed cerebral haemorrhage and cardiopulmonary failure.
‡Common AEs are reported by preferred term for ≥5% of patients in any treatment group (ie, for ≥3 patients in the BI 655064 group or ≥2 patients in the placebo group).
§In the BI 655064 group, there was one case of iron deficiency anaemia and one case of hypoglycaemia and in the placebo group there was one case of anaemia, one case of elevated liver enzymes and one case of elevated uric acid.
¶In the BI 655064 group, there was one case of anaemia and one case of elevated liver enzymes and in the placebo group there was one case of hyperglycaemia.
AE, adverse event;ICH, International Conference on Harmonisation;MedDRA, Medical Dictionary for Regulatory Activities;RA, rheumatoid arthritis;RCTC, Rheumatology Common Toxicity Criteria (V.2.0).
Of the 44 patients receiving BI 655064, serious AEs (SAEs) were reported in two patients: one case with pre-existing hypertension who developed a cerebral haemorrhage and cardiopulmonary failure leading to death and one case of myocardial infarction, which occurred 5 weeks after the last dose was administered. One other patient treated with BI 655064 discontinued due to non-treatment-related iron deficiency anaemia. AEs in all other patients (93.1%) receiving BI 655064 were of grade 1 or 2.
Of the 23 patients receiving placebo, SAEs were reported in two patients: one case of pleural effusion requiring hospitalisation and one case of medically significant anaemia. In total, four patients (17.4%) receiving placebo discontinued: one case each of pleural effusion, nasopharyngitis, elevated liver enzymes and worsening RA. There were no clinically relevant changes in safety-related laboratory parameters between groups (table 2). With the exception of decreases in ESR, CRP and RF, there were no relevant changes in the frequencies of patients with values above or below normal limits. Assessments of local tolerability indicated similar signs and symptoms between groups. Baseline and postdose rates of findings were also similar, indicating no substantial change due to administration of study treatment.
There was a three to fourfold accumulation of BI 655064 at the end of treatment compared with that after a single dose. Although BI 655064 trough concentrations were highly variable, steady-state was achieved at weeks 9–12; a correlation between BI 655064 exposure and clinical response could not be clarified due to high pharmacokinetic variability. Overall, five patients (22.7%) receiving placebo and six patients (13.3%) receiving BI 655064 tested positive for anti-drug antibodies at any time (all titres ≤8).
Discussion
In this study, the primary endpoint was not met; BI 655064, a targeted therapy directed against CD40, led to improvements over placebo in ACR responses at week 12, but these were not statistically significant. BI 655064 did, however, show some modulation of select inflammatory and bone resorption biomarkers and autoreactive activated B-cells linked to the pathogenesis of RA,with significant changes in IgG RF (p=0.0018), IgA RF (p=0.0050), RANKL (p=0.0041) and activated CD19+IgD−CD27−CD95+ memory B-cells (p=0.0097) at week 12 compared with placebo. Interestingly, the rs4810485 T-allele, which is linked to RA and systemic lupus erythematosus (SLE) susceptibility, was associated with larger reductions in select memory CD19+B-cell subsets (CD19+IgD−CD27+CD95+; p=0.0032 and CD19+IgD−CD27−CD95+; p=0.0022) post-treatment with BI 655064.19–22 In this study, the GT/TT-allele was associated with greater improvements in ACR50 responses with BI 655064 treatment vs placebo (p=0.0403).
This study had several limitations, but there were three major issues that greatly affected the outcome of this study: the study size, characteristics of the patient population included in the study and the placebo response. Based on historical data, the expected ACR20 placebo response rate was predicted to be 25%; the Bayesian approach used in this study estimated that a sample size of 66 patients would be effective. However, this small sample size, combined with the BI 655064 group having a considerably lower mean CRP than the placebo group, may have influenced the higher than expected placebo response rates. This imbalance may have derived from a change in inclusion criteria based on a protocol amendment, from patients initially requiring a CRP ≥0.8 mg/dL (ULN of assay 0.6 mg/dL) at screening, to requiring a CRP ≥0.8 mg/dL or ESR ≥28 mm/hour. This amendment was made to aid recruitment into the study, however, this inadvertently led to more patients with lower or normal CRP levels being enrolled. Normal CRP values at baseline were observed in 54.5% of patients in the BI 655064 group, which, together with the longer duration of disease, may have reduced the differentiation observed in DAS28-CRP at week 12; this is supported by improved DAS28-CRP differentiation in patients with baseline CRP values above the median. Furthermore, the high ACR20 placebo response rate at week 12 may be a reflection of the patient population, as similar results have been observed in RA trials that enrolled MTX-IR patients.23–25 In the current study, BI 655064 treatment led to a similar ACR20 response rate to that observed previously with TNFα inhibitors. Finally, only investigating a single active dose of BI 655064 (120 mg) meant that it was not possible to determine whether there was a dose effect in this study. A single subcutaneous dose of 120 mg BI 655064 in healthy volunteers resulted in >90% receptor occupancy and >90% inhibition of CD54 upregulation, which was maintained for 1 week.14 Furthermore, multiple subcutaneous dosing of 120 mg BI 655064 once weekly in healthy volunteers resulted in about fourfold increase in exposure compared with a single dose.15 The 120 mg dose was, therefore, anticipated to provide efficacy with a balanced safety profile; however, data obtained after the initiation of this trial suggest that it may take up to 12 weeks to reach steady-state when dosing 120 mg BI 655064 once weekly.15 This finding is supported in this study whereby pharmacokinetic steady-state for BI 655064 was reached within 10–12 weeks26 and that a loading dose is potentially needed in order to achieve steady-state more rapidly. Taking these considerations into account, it is uncertain whether a study with a greater sample size, higher CRP and higher doses or a loading dose of BI 655064 may have resulted in greater efficacy in this patient population.
Former clinical trials of anti-CD40L antibodies were halted because of increased incidence of thromboembolism, initiated by activation and aggregation of platelets, possibly because of the Fc region of anti-CD40L antibodies activating the FcγRIIa (CD32a) platelet receptor;27 anti-CD40L antibodies lacking a functional Fc domain were shown to retain pharmacological activity but were not associated with platelet activation.28–30 In this study, BI 655064 showed a good safety and tolerability profile with no thromboembolic events, demonstrating that targeting CD40 alone in an autoimmune disease such as RA produces a favourable safety profile.
The biomarker results of this small study of BI 655064 in patients with RA have provided insights into key aspects associated with CD40 blockade and may have implications for other autoimmune diseases. The CD40–CD40L pathway has been shown to play an important role in the pathogenesis of SLE and lupus nephritis (LN).31 Patients with LN have been shown to have a high renal expression of CD40 and CD40L and increased expression of CD40L in autoreactive B-cells, resulting in spontaneous autoantibody production.32 Additionally, increased CD40 renal expression correlates with LN activity.32 Consequently, targeting the CD40–CD40L pathway could be an effective approach for the treatment of autoimmune diseases, including RA, SLE and LN. Ongoing clinical trials are assessing the safety and efficacy of BI 655064 in LN; however, further development for RA is not currently planned.
In conclusion, blockade of the CD40–CD40L pathway with BI 655064 in MTX-IR patients with RA was associated with a favourable clinical safety profile, however, the primary endpoint at week 12 was not met.
Acknowledgments
We thank all patients and study investigators who participated in the clinical study described here, and Susanne Svoboda for facilitating the analysis of protein and cellular biomarkers included in this study.
Footnotes
Handling editor: Josef S Smolen
Contributors: SV, UM-L, MR, BR, AGE, RV, PB, DJ, JSF, SJP and JS contributed to study design. SD, RP, AP, HK, ED, BK, RA and CS contributed to data collection. SV, AGE, RV, PB, DJ and JS contributed to data analysis. All authors had full access to the study data, contributed to data analysis, data interpretation, writing and review of the manuscript and approved the final version for publication.
Funding: This study was funded by Boehringer Ingelheim. Professional medical writing support in the development of this manuscript, under the guidance of the authors, was provided by Tina Borg and Leigh Church of OPEN Health Medical Communications (London, UK) and was funded by Boehringer Ingelheim.
Competing interests: SD, RP, AP, HK, ED and BK report no disclosures; UM-L reports being an advisor for Boehringer Ingelheim; RA and CS report having received research/grant support from Boehringer Ingelheim; SV, MR, BR, AGE, RV, PB, DJ, JSF, SJP and JS report being employed by Boehringer Ingelheim.
Patient consent for publication: Not required.
Ethics approval: The study protocol was approved by the institutional review board or ethics committee at each participating centre. The study was conducted according to the Declaration of Helsinki and the International Conference on Harmonisation guidelines. All patients provided written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data and relevant material as needed by them to fulfil their role and obligations as authors under the ICMJE criteria.
Furthermore, clinical study documents (e.g. study report, study protocol, statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the BI Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/transparency_policy.html
Prior to providing access, documents will be examined, and, if necessary, redacted and the data will be de-identified to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants.
Clinical Study Reports and Related Clinical Documents can be requested via this link:https://trials.boehringer-ingelheim.com/trial_results/clinical_submission_documents.html
All such requests will be governed by a Document Sharing Agreement.
Bona fide, qualified scientific and medical researchers may request access to de-identified, analysable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request.
Researchers should use https://clinicalstudydatarequest.com to request access to study data.
References
- 1. Büchner K, Henn V, Gräfe M, et al. CD40 ligand is selectively expressed on CD4+ T cells and platelets: implications for CD40-CD40L signalling in atherosclerosis. J Pathol 2003;201:288–95. 10.1002/path.1425 [DOI] [PubMed] [Google Scholar]
- 2. Desai-Mehta A, Lu L, Ramsey-Goldman R, et al. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest 1996;97:2063–73. 10.1172/JCI118643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berner B, Wolf G, Hummel KM, et al. Increased expression of CD40 ligand (CD154) on CD4+ T cells as a marker of disease activity in rheumatoid arthritis. Ann Rheum Dis 2000;59:190–5. 10.1136/ard.59.3.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacDonald KP, Nishioka Y, Lipsky PE, et al. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest 1997;100:2404–14. 10.1172/JCI119781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kyburz D, Corr M, Brinson DC, et al. Human rheumatoid factor production is dependent on CD40 signaling and autoantigen. J Immunol 1999;163:3116–22. [PubMed] [Google Scholar]
- 6. Liu MF, Chao SC, Wang CR, et al. Expression of CD40 and CD40 ligand among cell populations within rheumatoid synovial compartment. Autoimmunity 2001;34:107–13. 10.3109/08916930109001958 [DOI] [PubMed] [Google Scholar]
- 7. Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest 1996;98:826–37. 10.1172/JCI118855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qamar N, Fuleihan RL. The hyper IgM syndromes. Clin Rev Allergy Immunol 2014;46:120–30. 10.1007/s12016-013-8378-7 [DOI] [PubMed] [Google Scholar]
- 9. Rissoan MC, Van Kooten C, Chomarat P, et al. The functional CD40 antigen of fibroblasts may contribute to the proliferation of rheumatoid synovium. Clin Exp Immunol 1996;106:481–90. 10.1046/j.1365-2249.1996.d01-858.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tung C-H, Lu M-C, Lai N-S, et al. Tumor necrosis factor-α blockade treatment decreased CD154 (CD40-ligand) expression in rheumatoid arthritis. PLoS One 2017;12:e0183726 10.1371/journal.pone.0183726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Möttönen M, Isomäki P, Luukkainen R, et al. Regulation of CD154-induced interleukin-12 production in synovial fluid macrophages. Arthritis Res 2002;4 10.1186/ar589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harigai M, Hara M, Kawamoto M, et al. Amplification of the synovial inflammatory response through activation of mitogen-activated protein kinases and nuclear factor kappaB using ligation of CD40 on CD14+ synovial cells from patients with rheumatoid arthritis. Arthritis Rheum 2004;50:2167–77. 10.1002/art.20340 [DOI] [PubMed] [Google Scholar]
- 13. Ralph K, Nicoletti A, Musvasva E, et al. THU0407 Preclinical Characterization of a Highly Selective and Potent Antagonistic Anti-CD40 mAb. Ann Rheum Dis 2015;74(Suppl 2):344.1–344. 10.1136/annrheumdis-2015-eular.4177 [DOI] [Google Scholar]
- 14. Albach FN, Wagner F, Hüser A, et al. Safety, pharmacokinetics and pharmacodynamics of single rising doses of BI 655064, an antagonistic anti-CD40 antibody in healthy subjects: a potential novel treatment for autoimmune diseases. Eur J Clin Pharmacol 2018;74:161–9. 10.1007/s00228-017-2362-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwabe C, Rosenstock B, Doan T, et al. Safety, Pharmacokinetics, and Pharmacodynamics of Multiple Rising Doses of BI 655064, an Antagonistic Anti-CD40 Antibody, in Healthy Subjects: A Potential Novel Treatment for Autoimmune Diseases. J Clin Pharmacol 2018;58:1566–77. 10.1002/jcph.1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 17. Woodworth T, Furst DE, Alten R, et al. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: the rheumatology common toxicity criteria v.2.0. J Rheumatol 2007;34:1401–14. [PubMed] [Google Scholar]
- 18. Lee YH, Bae S-C, Choi SJ, et al. Associations between the functional CD40 rs4810485 G/T polymorphism and susceptibility to rheumatoid arthritis and systemic lupus erythematosus: a meta-analysis. Lupus 2015;24:1177–83. 10.1177/0961203315583543 [DOI] [PubMed] [Google Scholar]
- 19. Gaffney PM, Ortmann WA, Selby SA, et al. Genome screening in human systemic lupus erythematosus: results from a second Minnesota cohort and combined analyses of 187 sib-pair families. Am J Hum Genet 2000;66:547–56. 10.1086/302767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodríguez-Rodríguez L, Castañeda S, Vázquez-Rodríguez TR, et al. Influence of CD40 rs1883832 polymorphism in susceptibility to and clinical manifestations of biopsy-proven giant cell arteritis. J Rheumatol 2010;37:2076–80. 10.3899/jrheum.100362 [DOI] [PubMed] [Google Scholar]
- 21. Vazgiourakis VM, Zervou MI, Choulaki C, et al. A common SNP in the CD40 region is associated with systemic lupus erythematosus and correlates with altered CD40 expression: implications for the pathogenesis. Ann Rheum Dis 2011;70:2184–90. 10.1136/ard.2010.146530 [DOI] [PubMed] [Google Scholar]
- 22. Raychaudhuri S, Remmers EF, Lee AT, et al. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet 2008;40:1216–23. 10.1038/ng.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huizinga TWJ, Fleischmann RM, Jasson M, et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis 2014;73:1626–34. 10.1136/annrheumdis-2013-204405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol 2016;68:2857–66. 10.1002/art.39808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kivitz AJ, Gutierrez-Ureña SR, Poiley J, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol 2017;69:709–19. 10.1002/art.39955 [DOI] [PubMed] [Google Scholar]
- 26. Daniluk S, Ptaszynski R, Mueller-Ladner U, et al. SAT0147 Safety and Efficacy of BI 655064, An Antagonistic Anti-CD40 Antibody in Rheumatoid Arthritis (RA) Patients: Table 1. Ann Rheum Dis 2016;75(Suppl 2):718.1–718. 10.1136/annrheumdis-2016-eular.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum 2003;48:719–27. 10.1002/art.10856 [DOI] [PubMed] [Google Scholar]
- 28. Shock A, Burkly L, Wakefield I, et al. CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: an in vivo study. Arthritis Res Ther 2015;17 10.1186/s13075-015-0757-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tocoian A, Buchan P, Kirby H, et al. First-in-human trial of the safety, pharmacokinetics and immunogenicity of a PEGylated anti-CD40L antibody fragment (CDP7657) in healthy individuals and patients with systemic lupus erythematosus. Lupus 2015;24:1045–56. 10.1177/0961203315574558 [DOI] [PubMed] [Google Scholar]
- 30. Chamberlain C, Colman PJ, Ranger AM, et al. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann Rheum Dis 2017;76:1837–44. 10.1136/annrheumdis-2017-211388 [DOI] [PubMed] [Google Scholar]
- 31. Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol 2009;21:293–300. 10.1016/j.smim.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yellin MJ, D'Agati V, Parkinson G, et al. Immunohistologic analysis of renal CD40 and CD40L expression in lupus nephritis and other glomerulonephritides. Arthritis Rheum 1997;40:124–34. 10.1002/art.1780400117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2018-214729supp001.pdf (373.3KB, pdf)