Abstract
Mitochondrial dysfunction is considered as a critical mechanism in the pathogenesis of Parkinson’s disease (PD). Increasing evidence supports the notion of mitochondria-associated membranes (MAMs) in mitochondrial dysfunction; yet little is known about the role of MAMs-related proteins in the pathogenesis of PD. Herein we exposed the nematode Caenorhabditis elegans to 0.5–10.0 μM rotenone (RO) or 0.2–1.6mM paraquat (PQ) for 3 days. Our results showed that both RO and PQ induced similar Parkinsonism including motor deficits and dopaminergic degeneration. RO/PQ caused mitochondrial damages characterized by the increase of vacuole areas and autophagy vesicles, but the decrease of mitochondrial cristae. RO/PQ-impacted mitochondrial function was also demonstrated by the decrease of ATP level and mitochondrial membrane potential. Additionally, the attachment or surrounding of endoplasmic reticulum to the damaged mitochondria indicates ultrastructural alterations in MAMs. Using fluorescently labeled transgenic nematodes, we further found that the expression of tomm-7 and genes of Complex I, II and III was reduced, whereas the expression of pink-1 was increased in the exposed animals. To determine MAMs in toxicity toward PD, we investigated the mutants of hop-1 and pink-1, encoding presenilin and PTEN-induced putative kinase 1 (PINK1) in mitochondria-associated membranes, respectively. Results demonstrated that the mutation of both hop-1 and pink-1 reduced the vulnerability of lethal, behavioral, and mitochondrial toxicity induced by RO/PQ. These findings suggest that presenilin and PINK1 play important roles in the RO/PQ-induced neurotoxicity through the mechanisms involved in mitochondria-associated membranes.
Keywords: Parkinson’s disease, Mitochondrial toxicity, Mitochondria-associated membranes, hop-1, pink-1, Caenorhabditis elegans
1. Introduction
Parkinson’s disease (PD) is the common neurodegenerative disease characterized by the loss of dopaminergic neurons. Mutations in several genes have been proved to be linked to familial PD and genetics is expected to explain 5–10% of PD cases; however, about 90% of PD cases are sporadic (Pickrell and Youle, 2015; Yu et al., 2018). Until now, the etiology of sporadic PD remains poorly understood. The prevailing hypothesis is that the sporadic PD is most likely associated with environmental toxicants including pesticides and heavy metals (Bellou et al., 2016). Epidemiological studies have proved that pesticide exposure is associated with an increased risk of developing PD (Goldman, 2014). It is also supported by evidence from cell-based and animal studies (Li and Le, 2013; Bellou et al., 2016; Ko and Bezard, 2017). Rotenone and paraquat are the common pesticide or herbicide widely used. Previous studies have demonstrated that both rotenone and paraquat can kill dopaminergic neurons in vitro (Sala et al., 2016). Chronic administrations of paraquat or rotenone could cause key features of PD, including motor deficits and loss of dopaminergic neurons (Tanner et al., 2011). Although rotenone and paraquat were utilized for the PD model, the toxic mechanisms of neurodegeneration remain to be determined (Spivey, 2011).
Mitochondrial dysfunction has been proposed as one of the major contributors to the pathogenesis of PD (Schon and Przedborski, 2011; Esteves et al., 2014; Surmeier et al., 2017). Dysfunction of mitochondrial respiratory chain components was found in the brain, skeletal muscle, and platelets of sporadic PD patients (van der Merwe et al., 2014). PD-related genes including parkin, PINK1, DJ-1, and LRRK2 are involved in mitochondrial regulation, and mutations of these genes are related to mitochondrial dysfunction (Thomas et al., 2011; Smith et al., 2015). Recent studies show that communication between mitochondria and the endoplasmic reticulum (ER) plays key roles in the regulation of various pathophysiological processes (Giorgi et al., 2015; Filadi et al., 2016). Mitochondria-associated membranes (MAMs) are specific regions of contact between mitochondria and the endoplasmic reticulum, which attract increasing attention about their function and physiological consequences (Rodríguez-Arribas et al., 2017). To date, there is a little known about the role of MAMs-related proteins in the toxicity associated with PD (Hattori et al., 2017).
Several proteins located in MAMs, including those encoded by PD-related genes and some ER proteins such as presenilin, are involved in mitochondrial regulation (Gelmetti et al., 2017). As one of the PD-related proteins, PTEN-induced putative kinase 1 (PINK1) is a mitochondrial serine/threonine-protein kinase and takes part in the protection of stress-induced mitochondrial dysfunction (Lazarou et al., 2013). PINK1 interacts with the proautophagic protein BECN1/Beclin1 that is relocalized at MAMs during the process of starvation-induced autophagy (Gelmetti et al., 2017). PINK1 causes the parkin protein to bind into depolarized mitochondria, in order to induce autophagy of those mitochondria (Narendra et al., 2010; Lazarou et al., 2013). On the other hand, presenilin is involved in the control of mitochondrial functions (Filadi et al., 2017), and enriched in MAMs. Presenilin possess a domain involved in different pathways related to ER and mitochondrial functions (van Vliet et al., 2014; Filadi et al., 2017). However, little is known about the potential roles of MAMs-related proteins, including both PINK1 and presenilin, in the mitochondrial toxicity associated with sporadic PD (Checler et al., 2017).
The nematode Caenorhabditis elegans is widely used as an important animal model with the properties of a short life cycle, transparent body, easy handling, well-defined anatomy and well-described genetic and molecular backgrounds (Xu et al., 2017). With only 302 neurons in its nervous system and the lineage and morphology of every neuron described comprehensively, C. elegans also acts as an excellent model organism for neurotoxicology study. C. elegans lacks a functional blood brain barrier; therefore, chemical neurotoxins can quickly diffuse into the nervous system. The nematode C. elegans shares wide homologous genome with mammals. For example, pink-1 and hop-1 in nematodes are the homologous genes of PINK1 and presenilin in mammals, respectively (Li and Greenwald, 1997; Sämann et al., 2009). Therefore, C. elegans has been used as an outstanding model for neurodegenerative diseases such as PD (Caito and Aschner, 2016).
Herein we exposed C. elegans to either rotenone or paraquat to induce behavioral and pathological characteristics associated with Parkinsonism. The morphological changes of mitochondria, endoplasmic reticulum and MAMs were examined by transmission electron microscopy. Using fluorescently-labeled transgenic nematodes, we also investigated the mitochondrial toxicity for its linkage with MAMs-related proteins. We next tested the vulnerability of lethal, behavioral and mitochondrial toxicity induced by rotenone/paraquat in the mutant of hop-1 or pink-1. Our results indicate that the loss of PINK1 and presenilin functions impairs the toxicity of rotenone and paraquat, identifying the importance of both proteins in neurotoxicity. These experiments provide detailed in vivo evidence for MAMs mechanisms of environment-related Parkinsonism.
2. Materials and methods
2.1. Chemicals
Rotenone (RO) was purchased from Aladdin Industrial Corporation (Shanghai, China). Paraquat (Methyl viologen dichloride hydrate, PQ) was purchased from Sigma Aldrich Chemicals Co. (St. Louis, MO, USA). Other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals used in this study were of analytical grade.
2.2. C. elegans strains
All strains of C. elegans were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA) and maintained following standard protocols as previously described (Sulston and Brenner, 1974). Transgenic strains of C. elegans used in this study include the following: BZ555 [dat-1p::GFP] (Jadiya et al., 2011); PD4251 [(pSAK2) myo-3p::GFP::LacZ::NLS + (pSAK4) myo-3p::mitochondrial GFP + dpy-20(+)] (Cao et al., 2007); CB7272 [[(pSAK2) myo-3p::GFP::LacZ::NLS + (pSAK4) myo-3p::mitochondrial GFP + dpy-20(+)] I. mIs12 [myo-2p::GFP + pes-10p::GFP + F22B7.9p::GFP] II. frIs7 [nlp-29p::GFP + col-12p::DsRed] IV. uIs69 [pCFJ90(myo-2p::mCherry) + unc-119p::sid-1] V] (Thompson et al., 2013); DLM14 [eft-3p::CERUL-EAN-VENUS::tomm-7 + unc-119(+)] (Chapin et al., 2015); LA62 [hop-1mt] (Lakowski et al., 2003); BR4006 [pink-1p::pink-1::GFP + myo-2p::mCherry + herring sperm DNA] (Sämann et al., 2009) and RB2547 [pink-1 (ok3538)].
2.3. Nematode maintenance and exposure
Nematodes were maintained on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 at 20 °C (Sulston and Brenner, 1974). Adult nematodes were collected into tubes, and then dissolved a bleach-containing buffer (0.45 mM NaOH, 2% HClO) to release eggs. The eggs were collected and hatched on the plates with food, then synchronized for experiments (Xu et al., 2016).
Rotenone was initially dissolved in dimethyl sulfoxide (DMSO) and diluted with K-medium (32 mM KCl, 51 mM NaCl). PQ solutions were prepared in K-medium. The control group was K-medium. According to results of lethality assays, sublethal concentrations of RO (0.5, 1.0, 2.0, 4.0, 8.0, 10.0 μM) or PQ (0.2, 0.4, 0.6, 0.8, 1.2, 1.6 mM) were used for further research. Nematodes were exposed in the 24-well plates. Each well contained 30–50 age-synchronized nematodes and 45 μL E. coli OP50 solutions for food. Each experimental group included four parallels. L2 stage nematodes were exposed for 3 days.
2.4. Lethality assays
Synchronized wild type or transgenic nematodes were exposed to RO or PQ for 6 days in 24-well plates. To prevent eggs from hatching, 3.3 μL of fluoro-29-deoxyuridine (150 mM) was added to each well. The numbers of dead nematodes were recorded every day. Dead nematodes were identified as unresponsive to a gentle prod of the body with a needle probe. Each experiment was run in quadruplicate. The median lethal concentrations (LC50) of RO and PQ were determined by linear regression analysis with Graphpad Prism (Xu et al., 2017).
2.5. Movement behavior
Body bending was utilized to evaluate locomotion behaviors of nematodes. After the exposure, nematodes were transferred to K-medium. After one minute of recovery, body bending was counted in 20s. Additionally, C. elegans was transferred to surface of fresh NGM after exposure experiments. Following 10 min of adaptation, videos of nematodes were recorded for 1 min using the Motic microscope and Images Advanced 3.2 software (Motic Electric Group, Xiamen, China). Locomotion videos were further analyzed by Wormlab software (MBF Bioscience, Hong Kong, China). The crawling tracks were calculated using the midpoint of the body of nematodes in terms of references (Donnelly et al., 2013). Crawling speeds were recorded in real-time using a bin width of 0.125 s.
2.6. Dopaminergic degeneration analysis
In the BZ555 strain of C. elegans, the dopamine transporter marker dat-1::gfp was expressed in neurons; therefore, bright green fluorescence proteins (GFP) was observable in dopamine neurons (Xu et al., 2017; Jadiya et al., 2011). After exposure, worms were transferred to agar-padded slides. After anesthesia with 100 mM sodium azide, worms were sealed with coverslips. Images of immobilized worms were captured to assay dopamine neurons by a fluorescence microscope (LEICA DM400 B, Leica Microsystems Company, Shanghai, China). Fluorescence intensity of GFP was quantified through Image-Pro Plus software. The intensities of fluorescent puncta for dopaminergic neurons were examined in at least 30 nematodes in each group (Li et al., 2016).
2.7. Fluorescentfy-labeled mitochondria observation
In the strain of PD4251 nematode, GFP is expressed with myo-3p::gfp in all cells of body wall and vulval muscles (Cao et al., 2007). After exposure, worms were transferred to agar-padded slides. Imaging of immobilized nematodes was performed by a fluorescence microscope. The intensities of fluorescent punctum for mitochondria were examined in each group (>30 nematodes).
2.8. Adenosine triphosphate (ATP) measurement
ATP content was measured as previously described (Wang et al., 2007; Zuryn et al., 2010). In brief, 100 age-matched animals in each group were collected in S-Basal buffer and frozen at −80 °C. Frozen worms were immersed in hot water for 15 min, cooled and centrifuged to pellet insoluble debris. The supernatant was moved to a fresh tube and diluted fivefold before measurement. All three replicates were assayed using an ATP assay kit HS II (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. Luminescence was detected with a fluorescence spectrophotometer (Hitachi F-4500). Using an enhanced BCA protein assay kit (Beyotime Biotechnology, Shanghai, China), the protein value was measured at 562 nm with Multiscan MK3 (Bio-Rad, Finland). ATP levels were normalized to total protein content.
2.9. Measurement of mitochondrial membrane potential
According to previously described (Wang et al., 2008; Yee et al., 2014), nematodes were collected and frozen at −80 °C. Frozen worms were immersed in hot water, cooled and centrifuged to pellet insoluble debris. According to the manufacturer’s instructions, three replicates were assayed using a mitochondrial membrane potential assay kit with JC-1 (Beyotime Biotechnology, Shanghai, China). Suspension was incubated with JC-1 for 30 min at 37 °C, rinsed twice with PBS, and then detected with a fluorescence spectrophotometer (Hitachi F-4500). Mitochondrial membrane potential was determined through the ratio of red/green fluorescence intensity.
2.10. Assay for TOM7 and inner membrane complex in mitochondria
Mitochondrial import receptor subunit TOM7 homolog is encoded by the gene of tomm-7. In the strain of DLM14 nematodes, eft-3p::CERULEAN-VENUS::tomm-7 is expressed as fusion proteins, so the intensity of GFP can indicate the expression of TOM7, a key outer membrane protein in mitochondria (Chapin et al., 2015). In another strain of CB7272, GFP is expressed along with Complex I (ccIs4251), Complex II (mIs12) and Complex III (dpy-17) in body wall and pharyngeal muscle, while red fluorescence proteins (RFP) is marked as the fusion with Complex IV (frIs7) and Complex V (uIs69) in epidermis and pharyngeal muscle. Therefore, inner membrane complex I, II, III, IV, IV can be simultaneously assayed basing on the intensity of GFP or RFP. Fluorescence images of nematodes after exposure were performed by a fluorescence microscope. The intensities of fluorescent punctum associated with mitochondrial membrane related proteins were analyzed.
2.11. Assay for the expression level of pink-1
In the transgenic strain of BR4006, pink-1::GFP is expressed in all cells of nematodes. After exposure, the expression level of pink-1 can be assayed by fluorescence intensity of GFP by using a fluorescence microscope. Fluorescence intensity of GFP was quantified through Image-Pro Plus software. Therefore, the expression level of pink-1 was examined in each group (> 30 nematodes).
2.12. Transmission electron microscopy (TEM)
The synchronized worms of wild type, LA62 [hop-1(mt)] and RB2547 [pink-1(ok3538)] were respectively exposed to 2.0 μM RO, 0.8 mM PQ, or the vehicle K-medium (as the control) for 3 days. After exposure, approximately 150 survival nematodes were randomly selected for ultrastructural observation in each group with three independent experiments. In brief, these nematodes were fixed in 2.5% glutaraldehyde in 0.2 M phosphate buffered saline. Nematodes were dehydrated in a graded series of acetone (30–100%) and embedded in epoxy resin in term of a standard procedure (Prasad and Verma, 2013). Thin tissue sections were collected on copper grids, stained with uranyl acetate and lead citrate, and observed by FEI T12 transmission electron microscopy (Thermo Fisher Scientific Co., USA). In a total of nine groups, MAM ultrastructure including mitochondria and endoplasmic reticulum were observed and compared among different exposure groups of the same strain. The vacuolar area and cristae length were measured manually in each mitochondrion. The percentages of vacuolar area in mitochondria were calculated, and compared among different groups. The density of mitochondrial cristae was calculated as the ratio of a total of cristae length to mitochondrial area in each mitochondria. Besides, we counted the percentage of mitochondria with mitophagy vesicles in total mitochondria. At least 30 mitochondria were measured in each exposed group.
2.13. Statistical analysis
All data were expressed as mean ± Standard Error of the mean (SEM). Histograms were generated using SigmaPlot (Systat Software Inc.). Differences between groups were determined in term of results of a one-way analysis of variance (ANOVA) using GraphPad Prism (GraphPad Software Inc.). Probability levels (p-value) of < 0.05 were considered statistically significant.
3. Results
3.1. Motor deficits and dopaminergic loss induced by RO and PQ exposure
After exposure to RO or PQ, survival rates of nematodes were decreased in dose- and time-dependent manners (Fig. S1 in Supplementary Material). Then sublethal concentrations of RO (0.5–10.0 μM) or PQ (0.2–1.6mM) were used to further investigate their neurotoxicity. We first examined the changes in motor behaviors of worms after exposure. Crawling tracks of representative nematodes from the control and exposed groups were presented in a same X/Y axis system (Fig. 1A, C). Compared to the control, the crawling tracks of the exposed worms became irregular and shorten, especially after exposure to RO doses at 2.0 μM and higher (Fig. 1A) or PQ doses at 0.4 mM and higher (Fig. 1C). The mean speed, body bends and wavelength of the crawling movement were further analyzed by WormLab software. As shown (Fig. 1B), 0.5 μM and higher doses of RO caused significant reductions in mean speed and body bends (p < .05, t-test compared to the control). In addition, a significant decrease in wavelength appeared in the RO-exposed groups, which indicated deficits of coordination and balance ability of nematodes. Similarly, 0.4 mM and higher concentrations of PQ induced significant reduction in mean speed, body bends and wavelength of crawling in a dose-dependent manner (Fig. 1D). These results showed that chronic exposure to RO or PQ caused similar motor deficits in C. elegans.
Fig. 1.
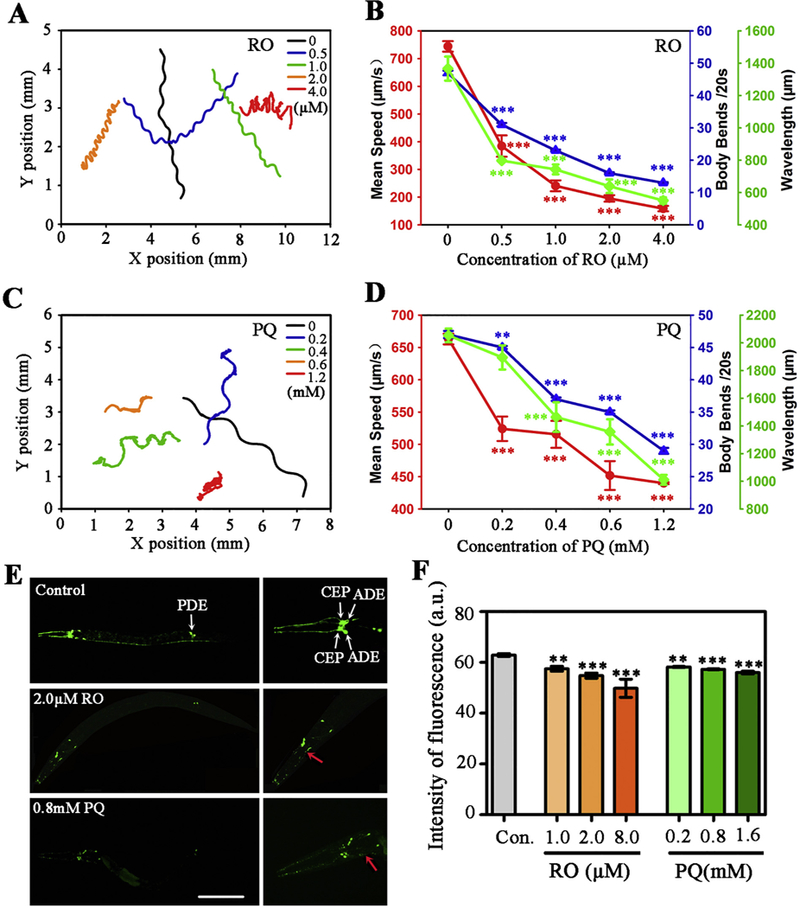
Effects of RO and PQ exposure on locomotor behavior and dopaminergic neurons in C. elegans. (A-F) After exposure to RO (A-B) and PQ (C-D), 60s crawling track line (A, C), mean crawling speed (red), body bends (blue) and wavelength (green) (B, D) of nematodes. (E) In the control nematodes, dopaminergic neurons are visualized by the translational expression of GFP. White arrows show dopaminergic neurons including CEP, ADE and PDE. Fluorescent images show changes of dopaminergic neurons after exposure to 2.0 μM RO and 0.8 mM PQ. Red arrows show the loss of soma or neurite in dopaminergic neurons in the amplified head region. (F) Dopaminergic neurons were quantized by fluorescent intensity (n = 25). Data are means ± SEM of four independent experiments. L2 stage nematodes were exposed for 3 days. **p < .01, ***p < .001, when compared to the control. Bar =200 μM in E. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The dopamine system is important in the modulation of locomotion behavior. There are four bilaterally symmetric pairs of dopaminergic neurons in C. elegans. These neurons include two pairs of cephalic neurons (CEP) and one pair of anterior deirid neurons (ADE) in the head, one pair of posterior deirid neurons (PDE) in the tail. In transgenic strain BZ555 nematodes, morphological patterns of dopaminergic neurons were fluorescently labeled by the expression of the dopamine transporter marker dat-1::GFP (Fig. 1E, control). Results showed exposure to RO or PQ caused obvious decrease in the size of fluorescent puncta in dopaminergic neurons, especially in CEP and ADE (Fig. 1E). Quantized results showed a significant decrease in the fluorescence intensity of dopaminergic neurons; and the mean of reduction values of fluorescence was 5.6–18.0% and 5.3–8.8% for RO and PQ exposure, respectively (Fig. 1F).
3.2. Damages of MAM induced by RO and PQ
In the transgenic nematode PD4251, mitochondria were fluorescently labeled with the expression of myo-3p::mitochondrial GFP in body wall muscles and vulval muscle (Fig. 2A, control). We then used this transgenic line with GFP targeted to mitochondria and under the control of the myo-3 promoter. Images showed that both the density of mitochondria and fluorescence intensity were gradually reduced in nematodes respond to increasing exposure concentrations of RO or PQ (Fig. 2A). Quantized results showed that 1.0–8.0 μM RO caused significant decreases of mitochondrial fluorescence with the mean of 21.5–40.6% reduction. Meanwhile, 0.8 and 1.6 mM PQ resulted in 7.2% and 11.8% decreases of mitochondrial fluorescence, respectively (Fig. 2B).
Fig. 2.
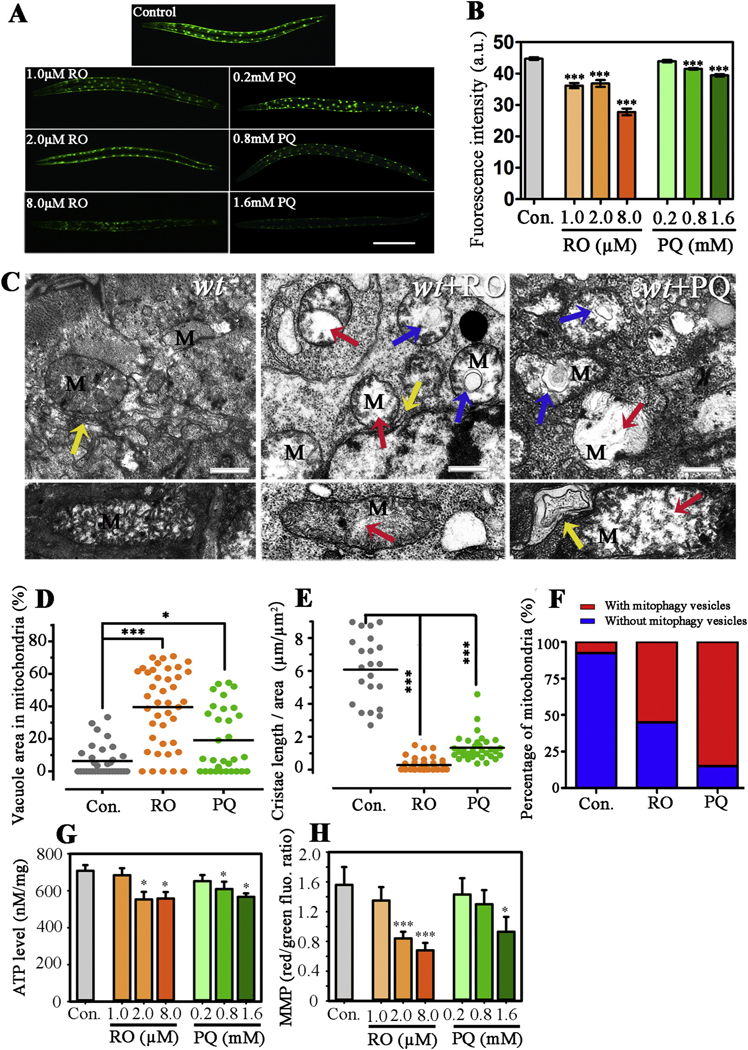
Effects of RO and PQ exposure on mitochondria in C. elegans. (A) Fluorescent images show changes of mitochondria in PD4251 nematodes after respectively exposed to RO and PQ for 3d. (B) Fluorescence intensity of GFP shows the changes of mitochondria. (C) Ultrastructure images show mitochondria (M) and endoplasmic reticulum in untreated and the RO/PQ exposed nematodes. Top and bottom rows show crosscutting and longitudinal images of representative mitochondria, respectively. Red arrows indicate vacuole areas. Blue arrows show the occurrence of autophagy vesicles in mitochondria. Yellow arrows indicate endoplasmic reticulum (ER). (D) The percentages of vacuole area in mitochondrial were analyzed in different groups. (E) Quantitative analysis of cristae membrane length with respect to total mitochondrial area. (F) The percentage of mitochondria with mitophagy vesicles in total mitochondria were analyzed in different groups. (G) The ATP level indicates ATP content in the total proteins. (H) The red/green fluorescence ratio of JC-1 shows the mitochondrial membrane potential (MMP) in different groups. Data are expressed as means ± SEM of four separate experiments (n ≥ 30). *p < .05, ***p < .001, when compared to the control. Bar = 200 μm in A. Bar =600 nm in C. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To further characterize the damages of MAM, we used TEM to examine the ultrastructure of mitochondria and endoplasmic reticulum (ER). Control groups showed a normal pattern of mitochondrial cristae and membrane structure with some elongated and round shaped mitochondria (Fig. 2C, wt). In contrast, RO exposure resulted in the appearance of prominent vacuoles, thickened mitochondrial membranes and reduced mitochondrial cristae (Fig. 2C, wt + RO). PQ exposure also caused development of abnormal mitochondria structures, including prominent vacuoles, irregular shapes, diffuse outer membranes and irregular arrangement of mitochondrial cristae (Fig. 2C, wt + PQ). In addition, the formations of double RE membrane and autophagic vesicles were found to be encapsulated in mitochondria in both RO and PQ exposed nematodes. We observed that some ER included pinched-off segments, and attached to the outer membrane of mitochondria, which showed ER stress in RO/PQ exposed nematodes (Fig. 2C, wt + RO and wt + PQ). In addition, the appearance of vacuolar and autophagic vesicles with the decrease of mitochondrial cristae often represents serious impairments in mitochondria. By quantifying the proportion of vacuole area in mitochondria, we found significant increases in vacuole area, with the mean of 35.0% and 13.0% rise after exposure to 2.0 μM RO and 0.8 mM PQ, respectively (Fig. 2D). By quantifying the cristae length with respect to total mitochondrial area, we found significant decreases in cristae, with the mean of 95.3% and 78.1% reduction after exposure to 2.0 μM RO and 0.8 mM PQ, respectively (Fig. 2E). By analyzing the percentage of mitochondria with autophagy vesicles in total mitochondria, we found obvious increases in percentages of autophagy mitochondria, with 47.5% and 77.5% rise after exposure to 2.0 μM RO and 0.8 mM PQ, respectively (Fig. 2F). In addition, ATP level and mitochondrial membrane potential were measured to evaluate changes of mitochondrial function. Results showed a significant decrease in ATP level, with the mean of 21.9% and 21.2% reduction after exposure to 2.0 and 8.0 μM RO. Similarly, 0.8 and 1.6 mM PQ caused a significant decrease in ATP level with the mean of 13.9% and 19.9% reduction (Fig. 2G). The JC-1 red/green fluorescence ratio was used as a parameter to estimate changes in mitochondrial membrane potential. We found significant reduction of mitochondrial membrane potential after exposure to 2.0–8.0 μM RO and 1.6 mM PQ. However, there was non-significant change between the control and low concentration RO or PQ groups (Fig. 2H).
3.3. Effects of RO and PQ on MAM proteins
In mitochondria, the translocase of outer membrane (TOM) is thought to be responsible for the recognition and translocation of mitochondrial proteins. As a key unit of the TOM complex, TOM-7 is involved in assembly and stability of TOM (Curran et al., 2004). In transgenic strain DLM14 nematodes, TOM-7 was fluorescently labeled by GFP (Fig. 3 A). We found obvious decreases of fluorescent puncta in RO- or PQ-exposed worms. Quantized results showed that higher than 1.0 μM RO or higher than 0. 2 mM PQ induced significant decreases in fluorescence intensity in comparison with the control (Fig. 3 B). The decreased intensity indicated the decreased expression level of TOM-7 induced by RO or PQ.
Fig. 3.
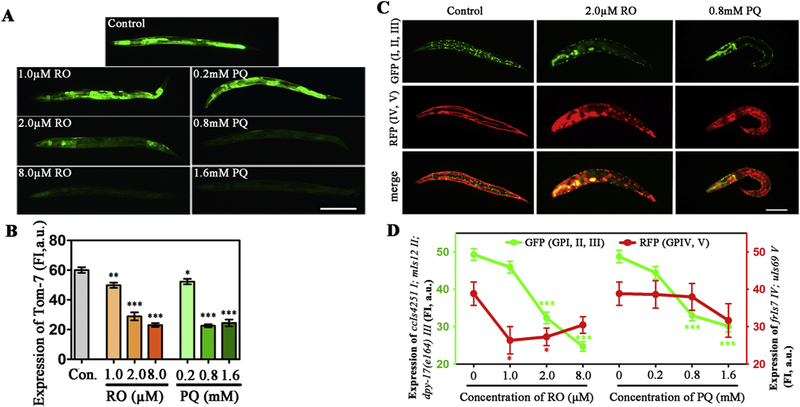
Effects of RO and PQ exposure on mitochondrial membrane proteins in transgenic DLM14 and CB7272 C. elegans. (A) Fluorescent images show the expression of tomm-7 in the control and RO/PQ exposed worms. (B) Fluorescence intensity show changes of TOM-7 in outer membrane of mitochondria after exposure. (C) Green, red and merge fluorescent images show expressions of mitochondrial inter membrane proteins in the control or the RO/PQ exposed nematodes, respectively. In strain CB7272, GFP was co-expressed with mitochondrial inter membrane proteins in body wall muscle nuclei (ccls4251I), pharyngeal muscle (mls12 II), Dumpy (dpy-17 III); and RFP co-expressed in epidermis (frIs7 IV), pharyngeal muscle (uIs69 V). (D) Fluorescence intensity show changes of mitochondrial Complex I, II, III (GFP), and IV, V (RFP) in the nematodes after exposed to RO or PQ. Data are expressed as means ± SEM of four separate experiments (n ≥ 30). Bar = 200 μm.*p < .05, **p < .01, ***p < .001, compared to the control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the mitochondria, the functions of electron transport and redox reactions largely depend on protein complexes of the mitochondrial inner membrane, mostly including Complex I, II, III, IV, and V (Jonckheere et al., 2011). In this study, we used the transgenic strain CB7272 to double-fluorescently label mitochondrial complexes. In the non-exposed nematodes, green fluorescence proteins were expressed along with Complex I (ccIs4251), Complex II (mIs12) and Complex III (dpy-17) in body wall and pharyngeal muscle, while red fluorescence proteins were marked as the co-expression with Complex IV (frIs7) and Complex V (uIs69) in epidermis and pharyngeal muscle (Fig. 3C, control). We found different changes between GFP and RFP expression after exposure to RO or PQ. According to images of 2.0 μM RO or 0.8 mM PQ group, there was obvious reduction of GFP, whereas changes in RFP were not obvious in comparison with the control (Fig. 3C). Data analysis showed that both RO and PQ caused the reduction of GFP in Complex I (ccIs4251), Complex II (mIs12) and Complex III (dpy-17) in a concentration-dependent manner (Fig. 3C, GFP). Although RO induced the slight decrease of RFP in Complex IV (frIs7) and Complex V (uIs69), the dose-effect relationships between RFP intensity and RO/PQ concentration were not obvious (Fig. 3D, RFP). Particularly, no significant changes of expression in frIs7 and uIs69 were found in the PQ-exposed groups. These results indicated that RO/PQ exposure affected mitochondrial inner membrane, and led to the dysfunction of Complex I, Complex II and Complex III, rather than of Complex IV and Complex V.
3.4. Mutation of hop-1 affected vulnerability of RO/PQ-induced toxicity
In strain LA62 nematodes, the mutation of hop-1 (one of presenilin genes) caused the derepression of its transcription (Lakowski et al., 2003). We further investigated the toxic effects of RO or PQ, based on the comparison between wild type and the hop-1 mutant. After exposure to RO/PQ, the survival percentages of both wild type and mutational nematodes were decreased in dose-dependent manners (Fig. 4A, B). Based on comparison, we found that the survival curve of hop-1(mt) worms were above the survival curve of the wild type. The median lethal concentrations (LC50) of RO for hop-1(mt) nematodes is 5.9 μM, which is higher than LC50 (3.2 μM) for wild type. Similarly, LC50 of PQ for hop-1(mt) is higher than that for wild type (1.1 mM vs 0.9 mM). We did not find obvious differences of apoptosis induced by RO/PQ between wild type and hop-1(mt) nematodes (Fig. S2 in Supplementary Material). Compared with wild type, hop-1 mutant worms showed motor deficits including changes of mean speed, body bends and wavelength (Fig. 4C). Furthermore, changed rates of motor behavior were compared between hop-1 (mt) and wild type nematodes, after similarly exposed to a series concentration of RO or PQ. We found that hop-1(mt) nematodes presented relatively slight changes in mean speed, body bends or wavelength of crawling behavior (Fig. 4D–F). It showed that mutation of hop-1 caused the reduction in susceptibility of RO/PQ-induced behavior toxicity.
Fig. 4.
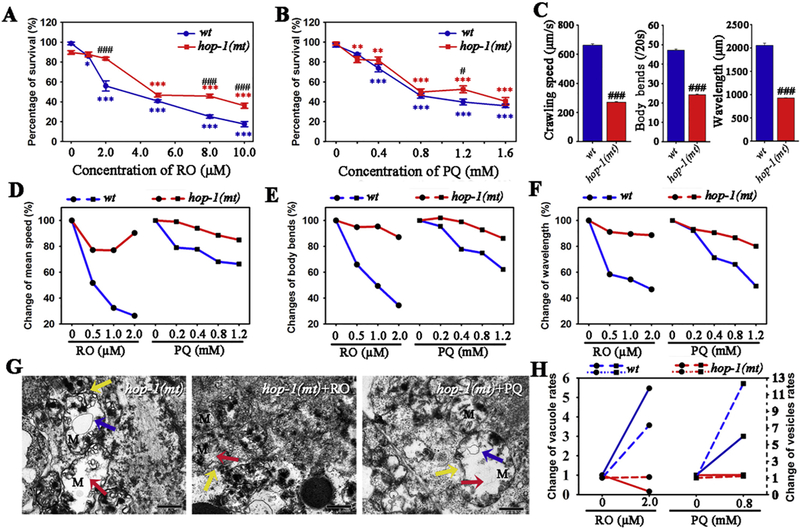
Effects of mutation of hop-1 on the toxicity of RO and PQ in C. elegans. (A-B) Survival percentages of wild type and hop-1(mt) nematodes after exposed to RO (A) and PQ (B). (C) Mutation of hop-1 inhibited movement capability of worms including mean speed, body bends and wavelength. (D-F) Mutation of hop-1 suppresses the vulnerability of RO/PQ-induced behavior toxicity, which is reflected by the comparison of change rates in mean speed (D), body bends (E) and wavelength (F) between wild type and hop-1(mt) worms. (G) Ultrastructure alterations in mitochondria and endoplasmic reticulum of hop-1(mt) nematodes and after exposure to 2.0 μM RO or 0.8 mM PQ. Red and blue arrows indicate vacuole areas and autophagy vesicles in mitochondria, respectively. Yellow arrows show pinched-off segments in endoplasmic reticulum (ER). (H) Changes of vesicle rates (solid lines) and changes of autophagy vesicles rates (dashed lines) in mitochondria respond to RO or PQ, compared between in wild type and the hop-1(mt). L2 stage nematodes were exposed for 3 days. Data are means ± SEM (A-C) or percentage variation (D-H).. *p < .05, **p < .01, ***p < .001, when compared to the control. #p < .05, ###p < .001, when compared between hop-1(mt) and wild type. Bar = 600 nm in G. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To link hop-1(mt)-associated toxicity to MAMs, we investigated the RO/PQ-mediated ultrastructure alterations in mitochondria and endoplasmic reticulum. Untreated hop-1(mt) nematode presented mitochondrial damages, which included irregular shapes of mitochondria along with reduction in the number of crista, and appearance of mitophagy vesicles (Fig. 4G, hop-1(mt)). RO/PQ treatment caused abnormality patterns including mitochondrial vacuoles and autophagy, which were similar to the control hop-1(mt) nematodes (Fig. 4G, hop-1(mt) + RO and hop-1(mt) + PQ). In addition, slight ER stress and establishing contact between the ER membranes and the mitochondrial outer membranes were also found in control or RO/PQ exposure nematodes. However, there were no marked differences between control and exposure groups (Fig. 4G). Moreover, we found that the change of vacuole areas and vesicle rates in hop-1(mt) nematodes were obviously lower than that in wild type, respond to the same exposure to RO or PQ (Fig. 4H). Collectively, we conclude that the mutation of hop-1 remarkably suppress susceptibility of mitochondrial toxicity of RO or PQ.
3.5. RO and PQ exposure increased the expression of pink-1
The gene of pink-1 is an evolutionarily conserved gene that is involved in MAMs to protect cells from stress-induced mitochondrial dysfunction (Gelmetti et al., 2017). PINK1 were observed in the cytoplasm with similar localization as mitochondria (Fig. S3 in Supplementary material). We further investigated changes in the expression of pink-1 after exposure to RO or PQ. Using transgenic strain BR4006 with the expression of fusion protein pink-1::GFP, we found obviously elevated expression in nematodes exposed to 1.0–8.0 μM RO or 0.2–1.6 mM PQ (Fig. 5A). Statistical results demonstrated that both RO and PQ induced a significant increase of pink-1 expression (p < .05, Fig. 5B). In combining data presented below, our results suggest that RO or PQ executes its toxicity via increased PINK1 for increased vulnerability (see data in Fig. 6).
Fig. 5.
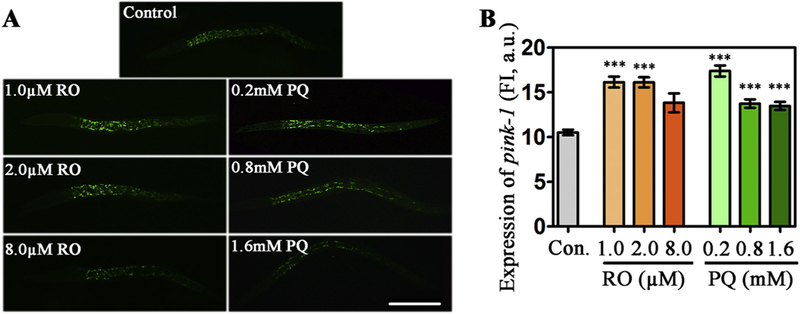
Effects of RO and PQ exposure on the expression of pink-1 in transgenic BR4006 C. elegans. (A) Fluorescent images show pink-1 expression in the control and the RO- or PQ- exposed nematodes. (B) Fluorescence intensity of GFP show changes of pink-1 expression after exposure to RO or PQ. Data are means ± SEM of four separate experiments. ***p < .001, when compared to the control. Bar = 200 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
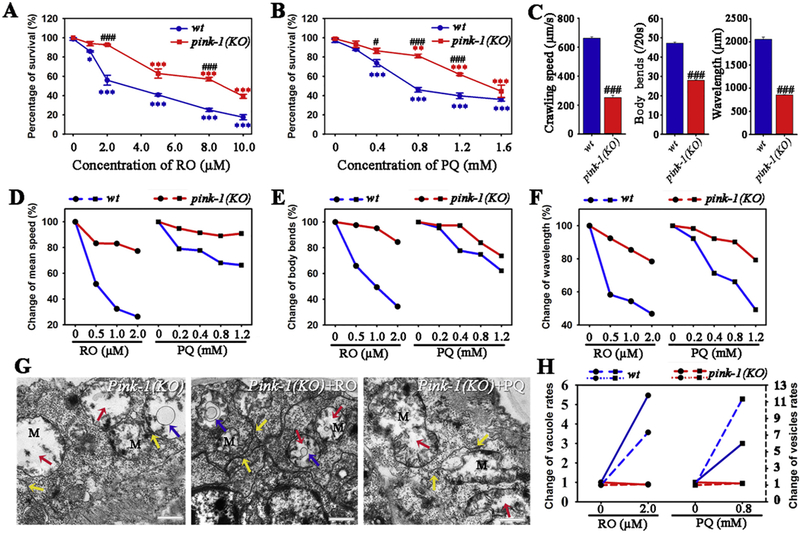
Effects of mutation of pink-1(ok3538) on the toxicity of RO and PQ in C. elegans. (A-B) Survival percentages of wild type and pink-1(ok3538) mutant nematodes after exposed to RO (A) and PQ (B). (C) Movement capability including mean speed, body bends and wavelength of wild type or pink-1(KO) worms. (D-F) Mutation of pink-1 suppresses vulnerability of behavior toxicity, which is reflected by comparison in the RO/PQ-induced change rates of mean speed (D), body bends (E) and wavelength (F) between wild type and the pink-1(KO). (G) Ultrastructure of mitochondria and endoplasmic reticulum in untreated or RO/PQ-exposed pink-1(KO) worms. Red and blue arrows indicate vacuole areas and autophagy vesicles in mitochondria, respectively. Yellow arrows show endoplasmic reticulum adjacent with mitochondria. (H) Changes in vesicle rates (solid lines) and changes of autophagy vesicles rates (dashed lines) of mitochondria in wild type or pink-1(KO) worms after same exposure to RO and PQ. L2 stage nematodes were exposed for 3days. Data are means ± SEM and percentage variation. *p < .05, ***p < .001, when compared to the control. #p < .05, ###p < .001, when compared pink-1(KO) with wild type. Bar =600 nm in G. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. Mutation of pink-1 attenuated vulnerability of RO/PQ- induced toxicity
We further examined the RO/PQ- induced toxicity in the nematode strain of knockout in pink-1 (RB2547, pink-1(KO)). After RO or PQ exposure, the survival percentages were compared between pink-1(KO) and wild type nematodes. Results showed that 2.0–10.0 μM RO caused significantly higher survival rates in pink-1(KO) nematodes than in wild type (p < .05, t-test; Fig. 6A), while 0.4–1.2 mM PQ also cause lower lethality in the pink-1(KO) (p < .05, Fig. 6B). Statistical results demonstrated that LC50s of RO and PQ were 8.2 μM and 1.5 mM for the pink-1(KO), both of which were significantly higher than LC50s for wild type. Additionally, defects of motor behaviors were proved to appear in pink-1(KO) nematodes (Fig. 6B). Respond to RO or PQ exposure, pink-1(KO) nematodes showed more slight behavior toxicity than wild types, which included change percentages of mean speed, body bends and wavelength in crawling behaviors (Fig. 6D–F). These data indicate that mutation of pink-1(ok3538) result in a decreased sensitivity in nematodes respond to RO or PQ exposure.
We further investigated ultrastructure changes of mitochondria-associated membrane in pink-1(KO) worms respond to RO/PQ exposure. Compared to wild type, mitochondria in the pink-1(KO) mutant showed obviously distinguished morphology, including formation of prominent vacuoles and reduction of cristae. Endoplasmic reticulum presented pinched-off segments, and were adjacent with damaged mitochondria. Some mitochondria were fully surrounded by ER (Fig. 6G). After pink-1(KO) nematodes were exposed to 2.0 μM RO, a few autophagy vesicles appeared in mitochondria (Fig. 6G, pink-1(KO) + RO). The pink-1(KO) nematodes also presented autophagy vesicles along with the reduction of mitochondrial crista after exposure to PQ (Fig. 6G, pink-1(KO) + PQ). In addition, RO/PQ exposure triggered the ER stress with unfolded structure, which was found beside the mitochondria. After analysis, we found that change rates of vacuole areas and autophagy vesicles in mitochondria in pink-1(KO) nematodes were obviously lower than that in wild type, in response to similar exposure to RO or PQ (Fig. 6H). There are not obvious differences of apoptosis induced by RO/PQ between wild type and pink-1(KO) nematodes (Fig. S2 in Supplementary Material). Collectively, our data suggest that PINK1 may serve as a node for mediating RO or PQ toxicity for PD pathogenesis.
4. Discussion
In this study, we provide in vivo evidence that MAM is one of the crucial mechanisms in the neurotoxicity induced by rotenone or paraquat. Ultrastructural alterations of MAMs including mitochondrial autophagy and endoplasmic reticulum stress have been revealed in the exposed animals. We have found that several proteins in MAMs, including TOM-7, PINK1, or proteins of Complex I, II and III are involved in the process of toxicity. Furthermore, we reveal that the mutation of both hop-1 and pink-1 reduces the vulnerability of lethal, behavioral and mitochondrial toxicity in nematodes in response to rotenone and paraquat.
4.1. Mitochondrial autophagy and ER stress are key characteristics of the RO/PQ- induced toxicity
Mitochondrial dysfunction has been proposed as one of the mechanisms for dopaminergic degeneration associated with PD (Haddad and Nakamura, 2015). In this study, the fluorescence intensity of mitochondria was concentration-dependently reduced after exposure to RO or PQ. It indicates the obvious dysfunction of mitochondria in environment-related Parkinsonism, which is consistent with previous in vitro studies (Tanner et al., 2011). Furthermore, our results revealed the occurrence of autophagy vesicles in nematodes respond to the exposure of RO and PQ. Mitophagy vesicles appeared in company with the increase of vacuole area, but the decrease of mitochondrial cristae. Additionally, we revealed that exposure to RO and PQ could result in reduction of both ATP level and mitochondrial membrane potential. It indicates an obvious dysfunction of mitochondria, which is consistent with some in vitro studies (Chiu et al., 2017; Delic et al., 2017). These results indicate that mitochondrial autophagy is a key characteristic of the toxicity of Parkinsonism induced by RO/PQ. Mitophagy is the selective degradation of mitochondria by autophagy, which often occurs in defective mitochondria following damage or stress (Lemasters, 2005). Mitophagy is thought to be important to keep cells healthy and to prevent accumulation of dysfunctional mitochondria (Youle and Narendra, 2011), and mitochondrial dysfunction is proposed to be one mechanism in the pathogenesis of Parkinson’s disease. A lot of in vitro experiments showed environmental toxin could lead to mitochondrial swelling and depolarization, autophagic alterations, and even cell death (Tanner et al., 2011). Our study provides in vivo evidence to support mitochondrial dysfunction in the toxicity of environmentally relative PD. Some significant events include mitochondrial dysfunction, energy deficiency and subsequent apoptosis may be involved in the toxic action and cell death.
Mitochondria are surrounded by two distinct membranes: the outer and the inner membrane. A number of proteins in outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM) were proposed to be involved in the mitophagy (Youle and Narendra, 2011). Our results revealed that environmental toxicants, i.e. RO and PQ, caused the reduced expression of Tom-7, one of the highly conserved units of TOM complex (translocase of the mitochondrial outer membrane complex). TOM complex serves as the mitochondrial entry gate for nuclear-encoded protein precursors (Ellenrieder et al., 2015). Tom7 was suggested to be an important regulator by promoting the dissociation of the subunits in TOM complex (Walther and Rapaport, 2009). Our results indicate that the function of Tom7 is inhibited in the toxicity of RO/PQ. Additionally, mitochondrial complex is one type of main proteins in the inner membrane, and involved in ATP production by the oxidative phosphorylation (Chiu et al., 2017; Delic et al., 2017). Our results demonstrate that Complex I, II and III are concentration-dependently inhibited by these two pesticides; however, Complex IV or V is less affected. It is consistent with previous in vitro studies (Javed et al., 2016; Korge et al., 2017; Sousa et al., 2018). In addition, we found that damaged mitochondria were attached or surrounded within endoplasmic reticulum, indicating the occurrence of ER stress under toxin exposure condition. According to previous studies, contact sites between ER and mitochondria, i.e. mitochondria- associated membranes (MAMs) are involved in various biological functions, including lipid metabolism, Ca2+ signaling, inflammation and apoptosis (Pinton, 2018). For the first time, our in vivo data demonstrated that Parkinsonism was linkage with both the ultrastructural alteration of MAMs and the expression of MAMs related proteins. It indicates that the function of mitochondria-associated membranes is closely related to the toxicity of environmentally relative PD.
4.2. Presenilin is involved in the RO/PQ- induced toxicity
MAMs maintain mitochondrial biogenesis (Gelmetti et al., 2017). Within MAMs, Presenilins encoded by hop-1 gene in C. elegans are important proteins (Gelmetti et al., 2017). From our results, the mutation of hop-1 resulted in the appearance of autophagy vesicles and reduction of cristae in mitochondria, and also induced the defects of motor behaviors in nematodes. It indicates that presenilin is closely involved in the mitochondrial toxicity and mitophagy. According to previous studies, mutations of presenilin genes appeared not only in patients of Alzheimer’s disease but also in other patients such as dementia and Parkinson’s disease (Wüst et al., 2016). Another study demonstrated the independent role of presenilin in calcium homeostasis and mitochondrial functions (Sarasija and Norman, 2015). All of these results suggest that presenilin takes part in the toxicity associated with PD.
Furthermore, this study demonstrates the relatively higher survival percentages in the hop-1 mutant than in the wild type nematode in response to the same exposure of RO/PQ. We also found less changed rates of motor behaviors in hop-1 mutants after exposure to concentration-dependent RO/PQ. Moreover, our results revealed that the hop-1 mutants presented relatively slighter mitochondria toxicity than wild type after RO/PQ exposure. These results indicate that mutation of hop-1 reduces the vulnerability of nematodes in response to exposure to RO/PQ. As important proteins in endoplasmic reticulum, presenilins are deemed to take part in ER-mitochondria contact membranes. A recent study also showed that these mitochondria-associated membranes were involved in lipid metabolism, calcium homeostasis, and mitochondrial autophagy (Rodríguez-Arribas et al., 2017). For the first time, this study provides in vivo evidence for this theory. It suggests that presenilin plays important roles in the mechanism of the PD related toxicity.
4.3. Roles of PINK1 in the RO/PQ- induced toxicity
Our results reveal that RO and PQ cause the elevated expressions of pink-1, the homologous gene of PINK1. Previous in vitro studies demonstrated that PINK1 activity induced autophagy of the depolarized mitochondria (Lazarou et al., 2013), which are in keeping with the present study. Additionally, we found that the mutation of pink-1(ok3538) caused defects of motor behaviors and ultrastructure alterations of mitochondria including formation of prominent vacuoles and reduction of cristae. Increasing studies propose that parkin, PINK1 and other MAMs related proteins are involved in mitochondrial dysfunction or mitophagy (Haddad and Nakamura, 2015). Our results bring in vivo evidence for the hypothesis of MAMs mechanism in mitophagy and subsequent toxicity.
In this study, we also found that pink-1(ok3538) nematodes prsented lower lethality, slighter behavioral and mitochondrial toxicity than wild type after the same exposure to RO or PQ. It indicates that the mutation of pink-1 reduces the vulnerability of nematodes in response to toxic stimulus. It implies that the gene of pink-1 plays crucial roles in the RO/PQ-induced neurotoxicity. Other studies also showed that PINK1- dependent ubiquitylation was involved in the quality control of mitochondria, and dysfunction of PINK1 likely resulted in the accumulation of low-quality mitochondria, thereby triggering early-onset familial Parkinson’s disease (Narendra et al., 2010). Additionally, selective localization of PINK1 to low-quality mitochondria facilitated the recruitment of cytosolic Parkin to mitochondria (Akabane et al., 2016). Combining with these, it suggests that PINK1 plays pivotal roles in the neurotoxicity associated with Parkinson’s disease. The study fully compared survival, behavioral and mitochondrial toxicity of in vivo nematodes between mutant and wild type. We found that the mutation of both pink-1 and hop-1 caused the similar reduction in the toxic vulnerability. According to literatures, both pink-1 and hop-1 encode proteins in mitochondria-associated membranes. Therefore, this study provides novel evidence that MAMs proteins, such as PINK1 and presenilin, take part in toxic actions linkage with Parkinsonism. Wong and Holzbaur (2014) found that optineurin was actively recruited to parkin-labeled ubiquitinated mitochondria, and was stabilized by its ubiquitin binding domain. Additionally, multiple proteins in the ubiquitin–proteasome system and MAMs, such as DJ-1 and LRRK2, are related to sporadic forms of Parkinson’s disease (Wolozin et al., 2008; Murdoch et al., 2016; Hattori et al., 2017). However, detailed MAMs mechanisms of toxicity involved in Parkinsonism need further investigations.
In conclusion, our in vivo study revealed the analogousness in mitochondrial mechanisms of parkinsonian toxicity induced by two pesticides: rotenone and paraquat. Mitochondrial autophagy and ER stress are key characteristics with significant alterations in mitochondria-associated membranes, such as damaged mitochondria and surrounded endoplasmic reticulum. Several proteins in mitochondria-associated membranes are related to mitochondrial dysfunction. RO/PQ caused the reduced expression of tomm-7 and genes of Complex I, II and III, but the increased expression of pink-1. Moreover, we found the mutation of both hop-1 and pink-1 reduced the vulnerability of the RO/PQ-induced toxicity. These data suggest that presenilin and PINK1 play important roles in the PD-related neurotoxicity through the mechanism involved in mitochondria-associated membranes.
Supplementary Material
Acknowledgements
This study was supported by grants from the Natural Science Foundation of Shanghai (No. 16ZR1409300) and the National Key Research and Development of China (No. 2016YFC1402204). The laboratory of Z.W was supported by U.S. National Institutes of Health (R01ES25761, U01ES026721 opportunity fund, and R21ES028351) and Johns Hopkins Catalyst Award. Special thanks to Caenorhabditis Genetics Center for contributing transgenic strains.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.expneurol.2018.07.018.
References
- Akabane S, Matsuzaki K, Yamashita S, Arai K, Okatsu K, Kanki T, Matsuda N, Oka T, 2016. Constitutive Activation of PINK1 Protein Leads to Proteasome-mediated and Non-apoptotic Cell Death Independently of Mitochondrial Autophagy. J. Biol. Chem. 291, 16162–16174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP, 2016. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism Relat. Disord. 23, 1–9. [DOI] [PubMed] [Google Scholar]
- Caito SW, Aschner M, 2016. NAD+ supplementation attenuates methylmercury dopaminergic and mitochondrial toxicity in Caenorhabditis elegans. Toxicol. Sci. 151, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Wu Y, Curry K, Wu Z, Christen Y, Luo Y, 2007. Ginkgo biloba extract EGb 761 and Wisconsin Ginseng delay sarcopenia in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 62, 1337–1345. [DOI] [PubMed] [Google Scholar]
- Chapin HC, Okada M, Merz AJ, Miller DL, 2015. Tissue-specific autophagy responses to aging and stress in C. elegans. Aging (Albany NY) 7, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checler F, Goiran T, Alves Da Costa C, 2017. Presenilins at the crossroad of a functional interplay between PARK2/PARKIN and PINK1 to control mitophagy: Implication for neurodegenerative diseases. Autophagy 15, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CC, Yeh TH, Lu CS, Huang YC, Cheng YC, Huang YZ, Weng YH, Liu YC, Lai SC, Chen YL, Chen YJ, Chen CL, Chen HY, Lin YW, Wang HL, 2017. PARK14 PLA2G6 mutants are defective in preventing rotenone-induced mitochondrial dysfunction, ROS generation and activation of mitochondrial apoptotic pathway. Oncotarget 8, 79046–79060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SP, Leverich EP, Koehler CM, Larsen PL, 2004. Defective mitochondrial protein translocation precludes normal Caenorhabditis elegans development. J. Biol. Chem. 279, 54655–54662. [DOI] [PubMed] [Google Scholar]
- Delic V, Griffin JWD, Zivkovic S, Zhang Y, Phan TA, Gong H, Chaput D, Reynes C, Dinh VB, Cruz J, Cvitkovic E, Placides D, Frederic E, Mirzaei H, Stevens SM, Jinwal U, Lee DC, Bradshaw PC, 2017. Individual Amino Acid Supplementation Can Improve Energy Metabolism and Decrease ROS Production in Neuronal Cells Overexpressing Alpha-Synuclein. NeuroMolecular Med. 19, 322–344. [DOI] [PubMed] [Google Scholar]
- Donnelly JL, Clark CM, Leifer AM, Pirri JK, Haburcak M, Francis MM, Samuel AD, Alkema MJ, 2013. Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 11, e1001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenrieder L, Mårtensson CU, Becker T, 2015. Biogenesis of mitochondrial outer membrane proteins, problems and diseases. Biol. Chem. 396, 1199–1213. [DOI] [PubMed] [Google Scholar]
- Esteves AR, Swerdlow RH, Cardoso SM, 2014. LRRK2, a puzzling protein: Insights into Parkinson’s disease pathogenesis. Exp. Neurol. 261, 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P, 2016. Presenilin 2 Modulates Endoplasmic Reticulum-Mitochondria Coupling by Tuning the Antagonistic Effect of Mitofusin 2. Cell Rep. 15, 2226–2238. [DOI] [PubMed] [Google Scholar]
- Filadi R, Theurey P, Pizzo P, 2017. The endoplasmic reticulummitochondria coupling in health and disease: molecules, functions and significance. Cell Calcium 62, 1–15. [DOI] [PubMed] [Google Scholar]
- Gelmetti V, De Rosa P, Torosantucci L, Marini ES, Romagnoli A, Di Rienzo M, Arena G, Vignone D, Fimia GM, Valente EM, 2017. PINK1 and BECN1 relocalize at mitochondria-associated membranes during mitophagy and promote ER-mitochondria tethering and autophagosome formation. Autophagy 13, 654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Missiroli S, Patergnani S, Duszynski J, Wieckowski MR, Pinton P, 2015. Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid. Redox Signal. 2, 995–1019. [DOI] [PubMed] [Google Scholar]
- Goldman SM, 2014. Environmental toxins and Parkinson’s disease. Annu. Rev. Pharmacol. Toxicol. 54, 141–164. [DOI] [PubMed] [Google Scholar]
- Haddad D, Nakamura K, 2015. Understanding the susceptibility of dopamine neurons to mitochondrial stressors in Parkinson’s disease. FEBS Lett. 589, 3702–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Arano T, Hatano T, Mori A, Imai Y, 2017. Mitochondrial-associated membranes in Parkinson’s disease. Adv. Exp. Med. Biol. 997, 157–169. [DOI] [PubMed] [Google Scholar]
- Jadiya P, Khan A, Sammi SR, Kaur S, Mir SS, Nazir A, 2011. Anti-Parkinsonian effects of Bacopa monnieri: insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson’s disease. Biochem. Biophys. Res. Commun. 413, 605–610. [DOI] [PubMed] [Google Scholar]
- Javed H, Azimullah S, Haque ME, Ojha SK, 2016. Cannabinoid type 2 (CB2) receptors activation protects against oxidative stress and neuroinflammation associated dopaminergic neurodegeneration in rotenone model of Parkinson’s Disease. Front. Neurosci. 10, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonckheere AI, Smeitink JA, Rodenburg RJ, 2011. Mitochondrial ATP synthase: architecture, function and pathology. J. Inherit. Metab. Dis. 35, 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W, Bezard E, 2017. Experimental animal models of Parkinson’s disease: A transition from assessing symptomatology to α-synuclein targeted disease modification. Exp. Neurol. 298, 172–179. [DOI] [PubMed] [Google Scholar]
- Korge P, John SA, Calmettes G, Weiss JN, 2017. Reactive oxygen species production induced by pore opening in cardiac mitochondria: The role of complex II. J. Biol. Chem. 292, 9896–9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski B, Eimer S, Göbel C, Böttcher A, Wagler B, Baumeister R, 2003. Two suppressors of sel-12 encode C2H2 zinc-finger proteins that regulate presenilin transcription in Caenorhabditis elegans. Development 130, 2117–2128. [DOI] [PubMed] [Google Scholar]
- Lazarou M, Narendra DP, Jin SM, Tekle E, Banerjee S, Youle RJ, 2013. PINK1 drives Parkin self-association and HECT-like E3 activity upstream of mitochondrial binding. J. Cell Biol. 200, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ, 2005. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 8, 3–5. [DOI] [PubMed] [Google Scholar]
- Li X, Greenwald I, 1997. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc. Natl. Acad. Sci. U. S. A. 94, 12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Le W, 2013. Modeling neurodegenerative diseases in Caenorhabditis elegans. Exp. Neurol. 250, 94–103. [DOI] [PubMed] [Google Scholar]
- Li J, Li D, Yang Y, Xu T, Li P, He D, 2016. Acrylamide induces locomotor defects and degeneration of dopamine neurons in Caenorhabditis elegans. J. Appl. Toxicol. 36, 60–67. [DOI] [PubMed] [Google Scholar]
- Murdoch JD, Rostosky CM, Gowrisankaran S, Arora AS, Soukup SF, Vidal R, Capece V, Freytag S, Fischer A, Verstreken P, Bonn S, Raimundo N, Milosevic I, 2016. Endophilin-A deficiency induces the foxo3a-fbxo32 network in the brain and causes dysregulation of autophagy and the ubiquitin-proteasome system. Cell Rep. 17, 1071–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ, 2010. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ, 2015. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, 2018. Mitochondria-associated membranes (MAMs) and pathologies. Cell Death Dis. 9, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad SB, Verma AK, 2013. Cantharidin-Mediated Ultrastructural and Biochemical Changes in Mitochondria Lead to Apoptosis and Necrosis in Murine Dalton’s Lymphoma. Microsc. Microanal. 19, 1377–1394. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Arribas M, Yakhine-Diop SMS, Pedro JMB, Gómez-Suaga P, Gómez- Sánchez R, Martínez-Chacón G, Fuentes JM, González-Polo RA, Niso-Santano M, 2017. Mitochondria-associated membranes (MAMs): Overview and its role in Parkinson’s Disease. Mol. Neurobiol. 54, 6287–6303. [DOI] [PubMed] [Google Scholar]
- Sala G, Marinig D, Riva C, Arosio A, Stefanoni G, Brighina L, Formenti M, Alberghina L, Colangelo AM, Ferrarese C, 2016. Rotenone down-regulates HSPA8/hsc70 chaperone protein in vitro: A new possible toxic mechanism contributing to Parkinson’s disease. Neurotoxicology 54, 161–169. [DOI] [PubMed] [Google Scholar]
- Sämann J, Hegermann J, von Gromoff E, Eimer S, Baumeister R, Schmidt E, 2009. Caenorhabditits elegans LRK-1 and PINK-1 act antagonistically in stress response and neurite outgrowth. J. Biol. Chem. 284, 16482–16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasija S, Norman KR, 2015. A γ-Secretase Independent Role for Presenilin in Calcium Homeostasis Impacts Mitochondrial Function and Morphology in Caenorhabditis elegans. Genetics 201, 1453–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon EA, Przedborski S, 2011. Mitochondria: the next (neurode) generation. Neuron 70, 1033–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Jansson J, Rocha EM, Osborn T, Hallett PJ, Isacson O, 2015. Fibroblast biomarkers of sporadic Parkinson’s disease and LRRK2 kinase inhibition. Mol. Neurobiol. 53, 5161–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa JS, D’Imprima E, Vonck J, 2018. Mitochondrial Respiratory Chain Complexes. Subcell Biochem. 87, 167–227. [DOI] [PubMed] [Google Scholar]
- Spivey A, 2011. Rotenone and paraquat linked to Parkinson’s disease: human exposure study supports years of animal studies. Environ. Health Perspect. 119, A259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Brenner S, 1974. The DNA of Caenorhabditis elegans. Genetics 77, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Halliday GM, Simuni T, 2017. Calcium, mitochondrial dysfunction and slowing the progression of Parkinson’s disease. Exp. Neurol. 298, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW, 2011. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 119, 866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR, 2011. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum. Mol. Genet. 20, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O, Edgley M, Strasbourger P, Flibotte S, Ewing B, Adair R, Au V, Chaudhry I, Fernando L, Hutter H, Kieffer A, Lau J, Lee N, Miller A, Raymant G, Shen B, Shendure J, Taylor J, Turner EH, Hillier LW, Moerman DG, Waterston RH, 2013. The Million Mutation Project: a new approach to genetics in Caenorhabditis elegans. Genome Res. 23, 1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe C, Loos B, Swart C, Kinnear C, Henning F, van der Merwe L, Pillay K, Muller N, Zaharie D, Engelbrecht L, Carr J, Bardien S, 2014. Mitochondrial impairment observed in fibroblasts from South African Parkinson’s disease patients with parkin mutations. Biochem. Biophys. Res. Commun. 447, 334–340. [DOI] [PubMed] [Google Scholar]
- van Vliet AR, Verfaillie T, Agostinis P, 2014. New functions of mitochondria associated membranes in cellular signaling. Biochim. Biophys. Acta 1843, 2253–2262. [DOI] [PubMed] [Google Scholar]
- Walther DM, Rapaport D, 2009. Biogenesis of mitochondrial outer membrane proteins. Biochim. Biophys. Acta 1793, 42–51. [DOI] [PubMed] [Google Scholar]
- Wang YM, Pu P, Le WD, 2007. ATP depletion is the major cause of MPP+ induced dopamine neuronal death and worm lethality in alpha-synuclein transgenic C. elegans. Neurosci. Bull. 23, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tang X, Li Y, Leu C, Guo L, Zheng X, Zhu D, 2008. 20-Hydroxyeicosatetraenoic acid inhibits the apoptotic responses in pulmonary artery smooth muscle cells. Eur. J. Pharmacol. 588, 9–17. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Saha S, Guillily M, Ferree A, Riley M, 2008. Investigating convergent actions of genes linked to familial Parkinson’s disease. Neurodegener. Dis. 5, 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Holzbaur EL, 2014. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc. Natl. Acad. Sci. U. S. A. 111, E4439–E4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst R, Maurer B, Hauser K, Woitalla D, Sharma M, Krüger R, 2016. Mutation analyses and association studies to assess the role of the presenilin-associated rhomboid-like gene in Parkinson’s disease. Neurobiol. Aging 39, 217 (e13–5). [DOI] [PubMed] [Google Scholar]
- Xu T, Li P, Wu S, Li D, Wu J, Raley-Susman KM, He D, 2016. Chronic exposure to perfluorooctane sulfonate reduces lifespan of Caenorhabditis elegans through insulin/IGF-1 signaling. Bull. Environ. Contam. Toxicol. 97, 119–123. [DOI] [PubMed] [Google Scholar]
- Xu T, Li P, Wu S, Lei L, He D, 2017. Tris (2-chloroethyl) phosphate (TCEP) and tris (2-chloropropyl) phosphate (TCPP) induce locomotor deficits and dopaminergic degeneration in Caenorhabditis elegans. Toxicol. Res. 6, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Yang W, Hekimi S, 2014. The intrinsic apoptosis pathway mediates the prolongevity response to mitochondrial ROS in C. elegans. Cell 157, 897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP, 2011. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Huang Q, Du X, Xu S, Li M, Ma S, 2018. Early activation ofEgr-1 promotes neuroinflammation and dopaminergic neurodegeneration in an experimental model of Parkinson’s disease. Exp. Neurol. 302, 145–154. [DOI] [PubMed] [Google Scholar]
- Zuryn S, Kuang J, Tuck A, Ebert PR, 2010. Mitochondrial dysfunction in Caenorhabditis elegans causes metabolic restructuring, but this is not linked to longevity. Mech. Ageing Dev. 131 (554–161). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


