Abstract
Regular exercise enhances mitochondrial function by promoting healthy mitochondrial remodeling, but the underlying mechanisms are not thoroughly understood. An emerging hypothesis suggests that, in addition to anabolic events such as mitochondria biogenesis, the selective degradation of dysfunctional mitochondria (i.e., mitophagy) is also a key component of exercise-mediated adaptations in striated muscle which eventually leads to better mitochondrial functions.
Keywords: Mitophagy, Mitochondrial Reticulum, Exercise, Skeletal Muscle, Cardiomyocyte
Summary for Table of Contents
Mitophagy is a key component in exercise-induced adaptations at the mitochondrial reticulum in both skeletal muscle and heart.
Background and Introduction
Exercise is one of the most effective means to prevent and treat many chronic diseases. Many exercise-induced benefits are due to adaptations in striated muscle (e.g., skeletal muscle and heart), which are recruited during exercise. In particular, enhanced mitochondrial function in striated muscles is one of the most important cellular adaptations induced by endurance exercise, where mitochondria remodel to achieve optimal function through a collection of processes referred to as mitochondrial quality control. Historically, anabolic mechanisms that regulate mitochondrial quality, namely mitochondria biogenesis, are the most extensively studied in skeletal muscle and heart, which we have discussed elsewhere (1). More recently, however, there is an increasing appreciation for the roles of catabolic mechanisms of mitochondrial quality control, such as mitophagy. Mitophagy is a multi-step, catabolic process responsible for selective, lysosomal-dependent degradation of damaged and/or dysfunctional mitochondria, which is vital for the maintenance of mitochondrial quality (2). An emerging hypothesis is that mitophagy, a key component of mitochondrial quality control which eventually leads to better mitochondrial function, is critical to exercise-mediated adaptations in striated muscle. It is important to note that other adaptive responses, such as activation of proteases, unfolded protein responses, and antioxidant defense in mitochondria, also contribute to exercise training-induced improvement of mitochondrial quality; however, we will in this review focus on: 1) known mechanisms of mitophagy, 2) how mitophagy is mediated by exercise and its role in exercise training-induced adaptations in both skeletal muscle and heart; and 3) outstanding questions and future research directions with regard to exercise-induced mitophagy and their clinical relevance.
Mechanisms of Mitophagy
The term “mitophagy” was introduced in 1998 by Scott and Klionsky as a proposed example of a regulated, organelle-specific degradation process, though the first documented visualization of mitochondrial fragments in lysosomes via electron microscopy dates back to 1962 by Ashford and Porter (2, 3). Mitophagy was later found to be a tightly regulated multi-step process and a key component of mitochondria quality control (4). In this section, current understanding of the various steps in mitophagy will be reviewed.
i). Initiation of Mitophagy
To date, a few distinct mechanisms where a cascade of events lead to mitophagy initiation have been proposed. One means by which mitophagy is initiated is through the energetic sensor, 5’ AMP-activated protein kinase (AMPK). AMPK is a heterotrimeric kinase consisting of catalytic α and regulatory β and γ subunits. Under energetic stress, cellular AMP and/or ADP accumulates and binds to the γ-subunit of AMPK, resulting in a conformational change. This exposes the activating T172 site on the catalytic α subunit to upstream serine/threonine kinases, liver kinase B1 (LKB1) or CaMKII, resulting in full AMPK activation (5). Once activated, AMPK initiates various processes to restore energetic homeostasis (e.g., mitophagy and β-oxidation). Activated AMPK initiates mitophagy by phosphorylating the most upstream known mitophagy protein, Unc-51-like autophagy activating kinase (ULK1) at S555 (6). Activated ULK1 then initiates the formation of autophagosomes that will engulf targeted mitochondria for mitophagy. Activated Ulk1 may also function to promote the fusion of lysosome with mitochondria-containing autophagosome, which is critical for the completion of the process of mitophagy (6).
Although it is accepted that ULK1 controls the biogenesis of autophagosomes, there is a paucity of evidence for the downstream substrate(s) of ULK1-dependent mitophagy in vivo. Data from in vitro studies suggest that other autophagy-related proteins (ATGs), e.g., ATG2, ATG9, and ATG18, are downstream of ULK1, but the precise mechanisms have not been explored in vivo (7, 8, 9). Also, the activation of FUN14 domain-containing protein 1 (FUNDC1), a mitochondrial outer membrane protein that is required for mitophagy in response to hypoxia, has been shown to be partially dependent on phosphorylation at S17 by ULK1 (10). Taken together, current findings support that energetic stress could initiate mitophagy through a relatively linear AMPK-ULK1 signaling pathway.
Mitophagy can also be initiated by the phosphatase and tensin homolog-induced putative kinase protein 1 (PINK1) system. PINK1 is a serine-threonine kinase that has a mitochondria-targeting sequence in its N-terminus that is normally cleaved at mitochondria, resulting in its degradation by proteasome (11). However, when mitochondria membrane potential is lost cleavage of PINK1 is inhibited, causing accumulation of PINK1 on the outer mitochondrial membrane (OMM) (12). Such accumulation results in recruitment of the primary substrate of PINK1, the E3 ubiquitin ligase PARKIN. PINK1 phosphorylates and activates PARKIN, thus resulting in enhanced ubiquitination of mitochondrial surface proteins (12). This mitophagy initiation mechanism appears to start energetic or oxidative stress from within the mitochondrial reticulum.
Mitophagy can also be initiated by endogenous mitochondrial membrane-bound receptor proteins. For example, BCL2/adenovirus E1B 19kD interacting protein 3 (BNIP3) and its homolog BNIP3L (also known as NIX) are mitophagy receptor proteins that are anchored on OMM through their C-terminal transmembrane domains (13). Their N-termini, facing the cytosol, interact with the autophagosome membrane protein light chain 3 (LC3); therefore, induced expression of BNIP3/NIX could function to initiate mitophagy (13). Importantly, slow-twitch, oxidative muscle and endurance exercise-trained muscles have elevated levels of BNIP3 protein in skeletal muscle, suggesting increased level/capacity of mitophagy (14).
ii). Clearance of Damaged/Dysfunctional Mitochondria
Mitochondria in striated muscle exist as a highly dynamic reticulum, an interconnected architecture that aids in the energetic efficiency of mitochondria (15); topologically, damaged regions must be physically separated from the reticulum in order to be degraded by mitophagy machineries. Mitochondrial fission segregates connected portions of the reticulum and aids mitophagy by isolating areas that have been designated for removal, as discussed above (e.g., PARKIN-mediated ubiquitination) (16). Briefly, dynamin-related protein-1 (DRP-1) is recruited by OMM proteins like fission 1 protein (FIS1), mitochondrial fission factor (MFF), and dynamin-like 120 kDa protein (DLP1) by forming oligomers on the OMM (17). DRP-1 forms a ring or collar that encircles a mitochondrial tubule between the healthy and damaged/dysfunctional regions (18). The collar of DRP-1 then constricts, breaking OMM and completing the first stage in fission (18).
Once separated from the reticulum, the isolated mitochondrion is engulfed by mature autophagosomes promoted by ULK1 activation as discussed above. Autophagosomes are double-membrane structures that engulf recognized “cargo”, such as proteins containing an LC-3 interacting region (LIR) motif, and are formed by elongation of newly formed membranes (“phagophores”) whose lipids can be donated from multiple sources (i.e., ER, mitochondria, plasma membrane). Autophagosome formation is marked by conversion of light chain 3-I (LC3-I) to a lipid-modified form, light chain 3-II (LC3-II, the phosphatidylethanolamine conjugated form of LC3-I) (19). LC-3 serves as a main docking site between the autophagosome and the targeted mitochondria as most OMM-bound mitophagy proteins contain LC-3 interacting region (LIR) motifs. For example, p62/SQSTM1 is an autophagosomal structural protein that is involved in recruitment of damaged mitochondria to LC3 via its LIR domain (20).
After targeting autophagosome to mitochondria, a fusion between autophagosome and lysosome occurs, generating an autolysosome, as indicated by co-localization of mitochondrial proteins with lysosomal-associated membrane protein 1 or 2 (LAMP1 or LAMP2) (6, 21). LAMPs are glycoproteins that are abundant on lysosomal membranes. In particular, LAMP2 is suggested to be required for cytosolic signaling between lysosomes and autophagosomes as Lamp2 knockout mice showed accumulation of autophagic vacuoles containing single mitochondrion in cardiomyocytes (22). Finally, degradation of mitochondria is executed by lysosomal degrative enzymes (21), completing the process of mitophagy. Although muscle-specific Ulk1 knockout mice displayed normal abundance of lysosome in skeletal muscle, exercise-induced mitophagy was impaired along with impaired autophagosome-lysosome fusion, suggesting the importance of this step in mitophagy (6).
Regulation of Mitophagy by Acute Exercise
A potential role of macroautophagy in exercise-induced adaptive responses was first appreciated in a study that reported conversion of LC3-I to LC3-II and decreased p62 content in skeletal muscle following an acute bout of endurance exercise (23). Subsequently, similar findings were reported in mitochondrial fractions from skeletal muscle with enhanced BNIP3 expression, providing more direct evidence of macroautophagy toward clearance of mitochondria (24, 25). Similarly, elevated conversion of LC3-I to LC3-II, coupled with decreased p62 expression and increased DRP1 expression were observed in rodent myocardium following acute endurance exercise, suggesting that exercise-induced mitophagy also occurs in the heart (23, 26). Since these discoveries, the molecular mechanism(s) by which exercise induces mitophagy in adaptations in striated muscle have begun to be elucidated.
Mitophagy activation in response to acute exercise requires virtually simultaneous activation of several processes (e.g., autophagosome biogenesis, mitochondria fission, and autophagosome-lysosome fusion), though some studies have illustrated that the resolution of damaged/dysfunctional mitochondria in mouse and human skeletal muscle occurs during the recovery period post-exercise (3–6 hours), rather than during or early after acute exercise (0–1 hours) (6, 27). A critical node for near-simultaneous activation of these converging processes that make up mitophagy appears to be the energetic sensor AMPK. AMPK has long been studied as a “master regulator” of cellular energetics during acute exercise (5). We have confirmed that immediately following an acute bout of endurance exercise in mice, AMPK is both necessary and sufficient for phosphorylation of ULK1 at S555 in skeletal muscle, which is required for formation of autolysosome in degrading damaged mitochondria post-exercise (6). Activation of AMPK-ULK1 pathway has also been seen in humans as 2-hour high-intensity ergometer cycling increased ULK1 dephosphorylation of the inhibitory site S757 in vastus lateralis muscle (28). As a potential downstream effector of ULK1, FUNDC1 has also been shown to be required in murine skeletal muscle for mitophagy, both at baseline and under exercise-induced energetic stress (29).
A recent study by Chen and colleagues showed that the E3 ubiquitin ligase Parkin, possibly recruited by accumulation of Pink1, is required for exercise-induced mitophagy in murine skeletal muscle (30). Of note, in our hands, exercise-induced mitophagy in mouse skeletal muscle was not accompanied by stabilization of Pink1 on mitochondria in skeletal muscle (31). An important distinction between these findings is the mode of acute exercise employed. Vainshtein et al. and Chen et al. utilized exhaustive treadmill running exercise (25, 30), whereas the treadmill running protocol employed in our laboratory is relatively moderate for a fixed duration of 90 minutes (6). These seemingly conflicting findings highlight the possibility that striated muscle employs multiple/different mechanisms of mitophagy as responses to exercise in a context-dependent manner related to exercise model, duration and/or intensity. Parkin might be phosphorylated by other protein kinases. As we all know, a key link between damaged mitochondrial and autophagy machinery is the presence/activation of certain proteins on mitochondria. Theoretically, this may occur in the absence of the loss of mitochondrial membrane potential, which may explain the variety of mitophagy mechanisms. It remains to be ascertained if local collapses of mitochondrial membrane potential occur prior to the recognition by the autophagy machinery in the context to exercise. Future studies are clearly needed to improve our understanding of different mitophagy pathways.
As mentioned previously, mitochondrial membrane-tethered BNIP3 is involved in initiation of mitophagy potentially by binding with LC3 (13). Elevated Bnip3 levels in skeletal muscle following acute exercise was observed in rodents (24), and such increase is consistent with findings in human skeletal muscle as well (32). However, it remains unclear how BNIP3 contributes to the aforementioned pathways, or whether it acts independently.
The formation of the autophagosome through the AMPK-ULK1 axis appears to be concurrent to mitochondria fission following acute exercise. For example, fragmented mitochondria were found to be separated from the reticulum post-exercise and engulfed in autolysosomes (6). Consistent with the importance of mitochondrial fission in mitophagy process, if fission is blocked, a significant decline of mitophagy would be expected. A recent study showed that Drp1 heterozygote mice had reduced exercise capacity and trainability to exercise (33). However, mitochondria fission may also function to redistribute materials within the mitochondrial reticulum; while required for mitophagy, fission itself may not necessarily lead to mitophagy. We and others have reported phosphorylation of Drp1 is elevated following acute exercise in both skeletal muscle and cardiomyocyte (6, 25, 34). While some studies have connected AMPK activation to mitochondrial fission (35), our findings rejected the hypothesis that exercise-induced activation of AMPK directly phosphorylate Drp1 (6). More recently, proteomic studies have revealed that AMPK directly phosphorylates Mff (36), and Mff phosphorylation is induced by endurance exercise (37). Since Mff is required for recruitment of Drp1 from the cytoplasm to mitochondrial surface to mediate mitochondrial fission (38), it is plausible that exercise-induced AMPK activation promotes mitochondrial fission through phosphorylation of Mff and hence recruitment of Drp1 to aid mitophagy. This scenario remains to be experimentally elucidated.
Lastly, the formation of autolysosomes, where the engulfed mitochondria is degraded, is a critical step in exercise-induced mitophagy. This step appeared to be dependent on Ulk1 (6). Consistent with the notion of the importance of AMPK-Ulk1 axis in exercise-induced mitophagy, mice over expressing a dominant-negative form of AMPK⍺2 subunit showed impaired formation of mitochondria-containing autolysosomes in skeletal muscle in response to acute exercise (6). Furthermore, endurance exercise training has been shown to induce lysosomal biogenesis in skeletal muscle, which may enhance the capacity of mitophagy (39). Using chronic nerve stimulation-induced contractile activity in rodents, Kim and Hood reported increased levels of Lamp1 and Cathepsin D, a lysosomal aspartyl protease, after 3 days of stimulation (39). Since transcription factor EB (TFEB), a transcription factor important for lysosome biogenesis (40), is activated and translocated to the nuclei in murine skeletal muscle by increased contractile activity or exercise (41), and TFEB transcription requires AMPK (42), it is speculated that AMPK is also involved in control of mitophagic response to exercise by influencing lysosomal biogenesis.
Effects of Exercise Training on Mitophagy
Exercise-induced muscle adaptation starts with, but is not simply accumulation of repeated acute responses to exercise. Rather, there could be fundamental differences between acute exercise responses and exercise training-induced adaptations. In this sense, cellular and molecular regulations of mitophagy by long-term exercise training are not as well understood compared to acute exercise responses. One example of the difficulties in interpreting changes related to mitophagy in response to acute exercise vs. exercise training is the differences between changes of mitophagy-related protein levels and mitophagy flux (figure). For example, in resting rodent skeletal muscle, the LC3-II to LC3-I ratio stays relatively unchanged after 4–5 weeks of voluntary wheel running (14, 31, 43, 44). Whereas, in a recent study in human subjects, Brandt and colleagues reported increased LC3-II level in mitochondria fractions of vastus lateralis following 8-week moderate cycling training in young, healthy, and non-athlete men (32). These seemingly conflicting results may not be actually conflicting as LC3-I and LC3-II were measured in whole muscle homogenates in the former studies, providing clues pertinent to macroautophagy, while the later study showed LC3-II in mitochondrial fraction, which is indicative of activation mitophagy. Since BNIP3 and/or BNIP3L are directly involved in mitophagy process, their increased levels following exercise training suggest increased mitophagy flux and/or capacity (14, 31, 43, 44).
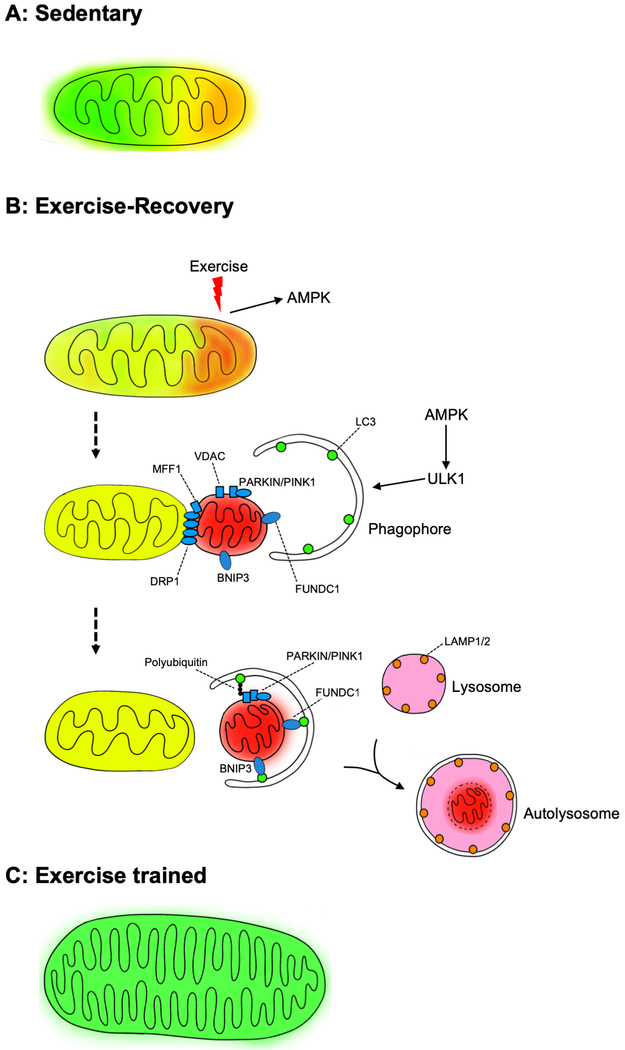
Figure:
Exercise-induced mitophagy. Schematic presentation of exercise-induced mitophagy and its role in mitochondrial quality control by exercise training. A gradient difference in mitochondrial color from green, yellow to red represents healthy, stressed and damaged and/or dysfunctional mitochondria. A) The mitochondrial reticulum in the sedentary muscle exhibits energetic/oxidative stress in some regions; B) Acute exercise induces further mitochondrial stress and initiates the processes of removing damaged/dysfunctional regions of the reticulum through mitochondrial fission and mitophagy; and C) Long-term exercise training results in improved mitochondrial quality.
A logical extrapolation can be made based on the notion that exercise-induced mitophagy is triggered at least in part by the signals within energetically stressed mitochondria during/post exercise. Therefore, since exercise training results in overall improved mitochondrial quality, mitophagy flux may be reduced in the trained state. Indeed, a study by Carter et al. showed decreased mitophagic flux in rat skeletal muscle after 9 days of chronic contractile activity via motor nerve stimulation that mimics exercise training (45). Similarly, mice showed decreased baseline mitophagy in skeletal muscle following 6 weeks of voluntary running (46). These findings are highly consistent with the general principle of exercise training that adaptive responses deem to improve the functionality of various organelles within the challenged organs/tissues to maintain energetic homeostasis to better prepare for the future exercise challenges.
Exercise-induced Mitophagy in Diseases
As mentioned before, exercise training is one of the most effective non-pharmacological approach to remedy many pathological conditions, such as type-II diabetes mellitus and congestive heart failure. Given that long-term endurance exercise training improves mitochondrial quality by effective removal of damaged/dysfunctional mitochondria, it is plausible to hypothesize that mitophagy plays an important role in mediating exercise training-induced benefits against aforementioned pathological conditions. We have previously demonstrated that 6 weeks of voluntary wheel running reversed high-fat diet-induced mitochondrial abnormalities in murine skeletal muscle, in part, via mitophagy (47). Furthermore, we have demonstrated that endurance exercise training-induced improvement of whole-body glucose clearance is dependent on Ulk1 in skeletal muscle (6). These findings raise questions regarding the mechanism by which mitophagy-mediated improvement of mitochondrial quality promotes insulin sensitivity in striated muscle.
Cardiomyocytes are more prone to mitochondrial damage due to higher mitochondrial content, as they rely heavily on β-oxidation of fatty acids as an energy source and are constantly contracting to sustain circulation for the whole body function. In a disease state, such as post-myocardial infarction (MI) in mice, it has been reported that there was an increased conversion of LC3-I to LC3-II along with increased cargo protein p62 (48), which suggests autophagy activation, but insufficient autophagy flux. Notably, 8 weeks of low-intensity swimming resulted in reduced LC3-II along with reduced p62 (48), suggesting exercise training effectively promotes autophagy flux. Consistently, another study showed that mitophagy required for cardioprotection in I/R injuries (49). Recently, Yuan and colleagues have also reported enhancement of Bnip3-mediated mitophagy in the myocardium in rats that underwent an intermittent running protocol as a method of pre-conditioning (50). Likewise, expression levels of Pink1 and Parkin are also suppressed in the MI model by low intensity swimming training (48). Although our own work suggests that Pink1/Parkin is not involved in exercise-induced mitophagy in skeletal muscle (30), their potential function in control of mitophagy in the heart should not be overlooked. In conclusion, although less is known of exercise-induced mitophagy in cardiomyocyte compared to skeletal muscle, its potential role is of great value to be elucidated for better protection of cardiac function in the setting of cardiomyopathies.
Summary and Future Directions in Exercise-induced Mitophagy
In summary, mitophagy is a highly conserved maintenance pathway that is critical to mitochondria quality control in striated muscle, and it plays key protective roles in various disease conditions. Acute exercise induces mitophagy in both skeletal muscle and cardiomyocyte, which appear to be the integral part of the beneficial adaptations induced by long-term exercise training. Distinct pathways and proteins responsible for regulation of mitophagy have been proposed and studied; however, numerous unanswered questions remain outstanding and require further research. Whether there are crosstalks among those identified regulators/pathways of mitophagy is unknown. How damaged or dysfunctional mitochondria are recognized and targeted for degradation with superb specificity remains to be ascertained. Last but not least, given the tight association between mitophagy and cardiometabolic diseases, whether exercise-induced mitophagy as one of the fundamental functional pathways can be targeted for reversing the pathological phenotypes awaits experimental testing.
Key Points.
Mitophagy is induced to counteract exercise-induced stress to mitochondria in skeletal muscle and heart;
Mitophagy is key to ensuring healthy remodeling of mitochondria in skeletal muscle and heart;
5’ AMP-activated protein kinase (AMPK), an energy-sending protein, has been identified to link energetic stress sensing to mitophagy induction;
An emerging direction in this field is to study mechanisms of exercise-induced cardiac mitophagy in cardiomyopathies.
Acknowledgement
This work was supported by NIH (R01-AR050429) to Z.Y., NIH (K99-AG057825) and ADA (1-16-PDF-030) to J.C.D. We thank Elaine Y. Xing for the artistic contributions to the figure. We also thank the members of the Yan lab for critical feedbacks and discussion.
References
- 1.Drake JC, Wilson RJ, Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016;30(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott SV, Klionsky DJ. Delivery of proteins and organelles to the vacuole from the cytoplasm. Curr Opin Cell Biol. 1998;10(4):523–9. [DOI] [PubMed] [Google Scholar]
- 3.Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962;12:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanki T, Klionsky DJ. Mitophagy in yeast occurs through a selective mechanism. J Biol Chem. 2008;283(47):32386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorgensen SB, Rose AJ. How is AMPK activity regulated in skeletal muscles during exercise? Front Biosci. 2008;13:5589–604. [DOI] [PubMed] [Google Scholar]
- 6.Laker RC, Drake JC, Wilson RJ, et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017;8(1):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack HI, Zheng B, Asara JM, Thomas SM. AMPK-dependent phosphorylation of ULK1 regulates ATG9 localization. Autophagy. 2012;8(8):1197–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weerasekara VK, Panek DJ, Broadbent DG, et al. Metabolic-stress-induced rearrangement of the 14–3-3ζ interactome promotes autophagy via a ULK1- and AMPK-regulated 14–3-3ζ interaction with phosphorylated Atg9. Mol Cell Biol. 2014;34(24):4379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou C, Ma K, Gao R, et al. Regulation of mATG9 trafficking by Src- and ULK1-mediated phosphorylation in basal and starvation-induced autophagy. Cell Res. 2017;27(2):184–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu W, Tian W, Hu Z, et al. ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep. 2014;15(5):566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem. 2008;106(1):464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narendra DP, Jin SM, Tanaka A, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1):e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson Å. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem. 2012;287(23):19094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lira VA, Okutsu M, Zhang M, et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013;27(10):4184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glancy B, Hartnell LM, Malide D, et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523(7562):617–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao K, Klionsky DJ. Mitochondrial fission facilitates mitophagy in Saccharomyces cerevisiae. Autophagy. 2013;9(11):1900–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23(15):5409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 2011;18(1):20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinsztein DC, Cuervo AM, Ravikumar B, et al. In search of an “autophagomometer”. Autophagy. 2009;5(5):585–9. [DOI] [PubMed] [Google Scholar]
- 20.Park S, Choi SG, Yoo SM, Son JH, Jung YK. Choline dehydrogenase interacts with SQSTM1/p62 to recruit LC3 and stimulate mitophagy. Autophagy. 2014;10(11):1906–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie. 2008;90(2):313–23. [DOI] [PubMed] [Google Scholar]
- 22.Stypmann J, Janssen PM, Prestle J, et al. LAMP-2 deficient mice show depressed cardiac contractile function without significant changes in calcium handling. Basic Res Cardiol. 2006;101(4):281–91. [DOI] [PubMed] [Google Scholar]
- 23.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481(7382):511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo Verso F, Carnio S, Vainshtein A, Sandri M. Autophagy is not required to sustain exercise and PRKAA1/AMPK activity but is important to prevent mitochondrial damage during physical activity. Autophagy. 2014;10(11):1883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vainshtein A, Tryon LD, Pauly M, Hood DA. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Physiol Cell Physiol. 2015;308(9):C710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Miao W, Ma J, et al. Acute Exercise-Induced Mitochondrial Stress Triggers an Inflammatory Response in the Myocardium via NLRP3 Inflammasome Activation with Mitophagy. Oxid Med Cell Longev. 2016;2016:1987149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwalm C, Deldicque L, Francaux M. Lack of Activation of Mitophagy during Endurance Exercise in Human. Med Sci Sports Exerc. 2017;49(8):1552–61. [DOI] [PubMed] [Google Scholar]
- 28.Schwalm C, Jamart C, Benoit N, et al. Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. FASEB J. 2015;29(8):3515–26. [DOI] [PubMed] [Google Scholar]
- 29.Fu T, Xu Z, Liu L, et al. Mitophagy Directs Muscle-Adipose Crosstalk to Alleviate Dietary Obesity. Cell Rep. 2018;23(5):1357–72. [DOI] [PubMed] [Google Scholar]
- 30.Chen CCW, Erlich AT, Crilly MJ, Hood DA. Parkin is required for exercise-induced mitophagy in muscle: impact of aging. Am J Physiol Endocrinol Metab. 2018;315(3):E404–E15. [DOI] [PubMed] [Google Scholar]
- 31.Drake JC, Laker RC, Wilson RJ, Zhang M, Yan Z. Exercise-induced mitophagy in skeletal muscle occurs in the absence of stabilization of Pink1 on mitochondria. Cell Cycle. 2019;18(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandt N, Gunnarsson TP, Bangsbo J, Pilegaard H. Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Physiol Rep. 2018;6(7):e13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore TM, Zhou Z, Cohn W, et al. The impact of exercise on mitochondrial dynamics and the role of Drp1 in exercise performance and training adaptations in skeletal muscle. Mol Metab. 2019;21:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coronado M, Fajardo G, Nguyen K, et al. Physiological Mitochondrial Fragmentation Is a Normal Cardiac Adaptation to Increased Energy Demand. Circ Res. 2018;122(2):282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Zhang M, Torres G, et al. Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission. Diabetes. 2017;66(1):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ducommun S, Deak M, Sumpton D, et al. Motif affinity and mass spectrometry proteomic approach for the discovery of cellular AMPK targets: identification of mitochondrial fission factor as a new AMPK substrate. Cell Signal. 2015;27(5):978–88. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman NJ, Parker BL, Chaudhuri R, et al. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell Metab. 2015;22(5):922–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koirala S, Guo Q, Kalia R, et al. Interchangeable adaptors regulate mitochondrial dynamin assembly for membrane scission. Proc Natl Acad Sci U S A. 2013;110(15):E1342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y, Hood DA. Regulation of the autophagy system during chronic contractile activity-induced muscle adaptations. Physiol Rep. 2017;5(14). doi: 10.14814/phy2.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci. 2016;129(13):2475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erlich AT, Brownlee DM, Beyfuss K, Hood DA. Exercise induces TFEB expression and activity in skeletal muscle in a PGC-1α-dependent manner. Am J Physiol Cell Physiol. 2018;314(1):C62–C72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young NP, Kamireddy A, Van Nostrand JL, et al. AMPK governs lineage specification through Tfeb-dependent regulation of lysosomes. Genes Dev. 2016;30(5):535–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grumati P, Coletto L, Schiavinato A, et al. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy. 2011;7(12):1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greene NP, Lee DE, Brown JL, et al. Mitochondrial quality control, promoted by PGC-1α, is dysregulated by Western diet-induced obesity and partially restored by moderate physical activity in mice. Physiol Rep. 2015;3(7). doi: 10.14814/phy2.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carter HN, Kim Y, Erlich AT, Zarrin-Khat D, Hood DA. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J Physiol. 2018;596(16):3567–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen CCW, Erlich AT, Hood DA. Role of Parkin and endurance training on mitochondrial turnover in skeletal muscle. Skelet Muscle. 2018;8(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laker RC, Xu P, Ryall KA, et al. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J Biol Chem. 2014;289(17):12005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao D, Sun Y, Tan Y, et al. Short-Duration Swimming Exercise after Myocardial Infarction Attenuates Cardiac Dysfunction and Regulates Mitochondrial Quality Control in Aged Mice. Oxid Med Cell Longev. 2018;2018:4079041. doi: 10.1155/2018/4079041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6(6):e20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan Y, Pan SS, Wan DF, Lu J, Huang Y. H2O2 signaling-triggered PI3K mediates mitochondrial protection to participate in early cardioprotection by exercise preconditioning. Oxid Med Cell Longev. 2018;2018:1916841. doi: 10.1155/2018/1916841. [DOI] [PMC free article] [PubMed] [Google Scholar]