Abstract
Available studies, while limited in number, suggest that e-cigarette vaping induces oxidative stress, with one potential mechanism being the direct formation of reactive oxygen species (ROS) in e-vapor. In the present studies, we measured the formation of hydroxyl radical (•OH), the most destructive ROS, in e-vapor under a range of vaping patterns (i.e., power settings, solvent concentrations, flavorings). Study results show that increased power output and puff volume correspond with the formation of significantly higher amounts of •OH in e-vapor because of elevated coil temperature and oxygen supply. Vegetable glycerin (VG) e-liquids generated higher •OH levels than propylene glycol (PG) e-liquids, as did flavored e-liquids relative to nonflavored e-liquids. E-vapor in combination with ascorbic acid, which is an abundant biological molecule in human epithelial lining fluid, can also induce •OH formation. The dose of radical per puff associated with e-cigarette vaping was 10–1000 times lower than the reported dose generated by cigarette smoking. However, the daily average •OH dose can be comparable to that from cigarette smoking depending on vaping patterns. Overall, e-cigarette users who use VG-based flavored e-cigarettes at higher power output settings may be at increased risk for •OH exposures and related health consequences such as asthma and chronic obstructive pulmonary disease.
Graphical Abstract:
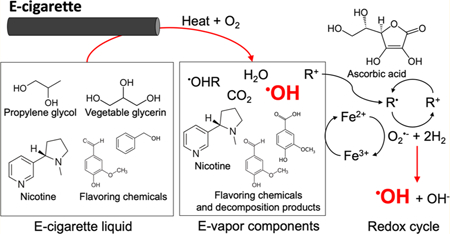
INTRODUCTION
The use of e-cigarettes has been rapidly increasing in the U.S. population.1,2 However, e-cigarettes are not risk-free products. Aerosols emitted from e-cigarettes (i.e., e-vapor) contain potentially harmful chemicals including carbonyls and flavoring chemicals.3,4 In addition, a limited number of available studies have reported the presence of reactive oxygen species (ROS) in e-vapor.4–6 Exposure to ROS in e-vapor has been associated with increased cardiopulmonary disease and decreased pulmonary function.7 Even though previous studies reported that the oxygen radical concentrations found in e-vapor might be much lower than that of other potentially harmful chemical species,5 the radical exposures caused by e-cigarette vaping need to be understood due to high risk potencies of some radical species, especially hydroxyl radical (•OH) in e-vapor.7
Indeed, among the ROS [e.g., superoxide radical (O2 •‑), hydrogen peroxide (H2O2), and •OH], •OH is the most destructive radical and can damage vital biological components in the lung epithelial lining fluid (ELF).7 Moreover, there are no •OH specific antioxidant enzymes such as superoxide dismutase or catalase,8 which can decompose O2•‑ and H2O2, respectively. With respect to the role of ROS in smoking, a conventional cigarette study showed that radicals containing (i.e., Q/QH2 and •OH) tar extract, only accounting for 3% of cigarette tar by mass, induced 70% of the total DNA damage generated by the whole cigarette tar extract.9
However, •OH formed during e-cigarette vaping has not been well studied to date. One study measured e-vapor-induced •OH with the electron spin resonance (ESR) spin trapping method while using limited e-cigarette aerosol generation conditions (i.e., two power output conditions).10 As with other chemicals found in e-cigarette aerosol (e.g., carbonyls), the formation of radicals in e-vapor may, however, be affected by the diverse range of e-cigarette products and their use patterns.6,11–13 Device power settings and vaping topographies may affect radical formation by modifying e-cigarette heating coil temperatures and oxygen supplies.11,13 In addition, various e-liquid compositions, such as the type of base materials (e.g., propylene glycol and vegetable glycerin), nicotine levels, and flavoring agents, may contribute to radical formation through thermal degradation.6,12 Therefore, levels of •OH in e-vapor need to be evaluated under wide ranges of e-cigarette use patterns to appropriately assess e-vaping toxicity.
Also, the formation of •OH induced by e-vapor components under physiologically relevant conditions (i.e., 37 °C, pH 7.4, no light) is unknown. The ELF contains ascorbic acid and iron ions (Fe2+/3+) which may interact with e-vapor components to form •OH through the Fenton-like reaction.14 Previous studies have reported that nicotine and several flavoring chemicals were redox cycled, mediated by transition metal ions.15,16 However, to date, there are no reports on the amounts of •OH induced by e-vapor. As measuring e-vapor-induced •OH formation likely represents a first step to understand longer-term oxidative stress associated with e-cigarette vaping, the current study focused on assessments of the levels of •OH in e-vapor. •OH was assessed in primary e-vapor and following further reactions between e-vapor and biological molecules such as ascorbic acid. •OH levels in e-vapor were analyzed using real-world relevant vaping patterns (i.e., device setting, vaping topography, and e-liquid composition). Next, e-vapor oxidative potential, which measures the capability of e-vapor to induce oxidative stress, was tested with ascorbic acid or Fe3+.
MATERIAL AND METHODS
Preparation of E-Cigarette Device and E-Liquids.
We used a refillable tank type e-cigarette (The Council of Vapor, Walnut, CA, U.S.A.) with adjustable air hole and a replaceable Nichrome heating coil head (dual-bottom coil with 0.8 Ω resistance). Two types of battery boxes, an Apollo Valiant battery (Apolo E-cigarette, Concord, CA, U.S.A.) and a Sigelei-100W battery (Sigelei US, Pomona, CA, U.S.A.), were used to provide heating power ranging from 3 to 100 W.
All e-liquids were freshly prepared in our laboratory using propylene glycol (PG, USP grade, Sigma-Aldrich, MO, U.S.A.), vegetable glycerin (VG, USP grade, J.T. Baker, NJ, U.S.A.), (−)-Nicotine (≥99.0%, Sigma-Aldrich, MO, U.S.A.), and flavoring agents. The eight flavoring agents (strawberry, dragon fruit, menthol, sweet cream, Bavarian, cinnamon, bubble gum, and graham cracker flavors) were obtained from The Perfumer’s Apprentice (Scotts Valley, CA, U.S.A.). These flavors have been listed as the most popular among users in an e-cigarette forum and in vape shops.17 The ingredients of the eight flavors have only partially been released by the manufacturer and appear to consist of natural/artificial flavors in PG, water and/or ethyl alcohol.
Flavoring agents usually contain dozens of flavoring chemicals (e.g., strawberry flavor contains anethole, benzyl acetate, ethyl butyrate, maltol, etc.).18 Flavoring chemicals may redox cycle with transition metal ions, and induce •OH. Nine flavoring chemicals used in this study were selected on the basis of their popularity in the commercially available e-liquids.4 Benzyl alcohol (99%), benzyl acetate (99%), ethyl acetate (99%), trans-anethole (≥98%), transcinnamaldehyde (≥98), 2,3-butanedione (99%, diacetyl), and 2,3-pentanedione (97%, acetylpropionyl) were purchased from Alfa Aesar (Haverhill, MA, U.S.A.). Citral (95%) and vanillin (99%) were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The nine flavoring chemicals represented fruity, citrus, spicy, and creamy/ buttery flavors. Fruity flavoring chemicals consist of hydroxyl and ester functional groups, and other flavoring chemicals usually were aldehydes (Table S1).
E-Vapor Generation Condition.
To obtain real-world vaping patterns, 23 current healthy e-cigarette users (21 men and 2 women, 25 ± 10 years of age, 1.4 ± 0.9 years of e-cigarette use history) were recruited to assess their customary e-vaping patterns using a CReSS Pocket device (Borgwaldt KC Incorporated, North Chesterfield, VA, U.S.A.) with the approval of the IRB at Rutgers University (Pro20140000589). Detailed demographics and observations are shown in Tables S2 and S3.
Combinations of e-cigarette power output, e-liquid composition, and vaping topography were adopted from our previous report19 to generate e-vapors. Detailed experimental conditions are tabulated in Table S4. In brief, the current study used median values from Table S3 as a baseline vaping pattern. Baseline device power output was adjusted to 6.4 W, a level recommended by the e-cigarette forums to be safe.20 The vaping topography was a 90 mL puff volume with a 3.8 s puff duration and comparable to vaping topographies reported in the literature which reported a median puff volume and duration of 91 mL (51–133 mL) and 3.8 s (2.65–4.3 s), respectively.21–26 In addition, observed square shape topographies from the 23 study participants were used instead of the bell-shape topography for the cigarette smoking (Figure S1). Finally, VG-based e-liquid containing 12 mg/mL nicotine, which was the most popular e-liquid base type and nicotine concentration among the 23 study participants, was used throughout the experiment unless otherwise specified.
In order to test the impact of the device power output on •OH formation, the median and the 95th percentile of observed power outputs (Watts) from the 23 study participants (i.e., 6.4 and 31.3 W) were tested. The selected power outputs represent both the safe and the extremely hot ranges of the e-cigarette vaping power chart.20
Air hole size might be an additional, important factor because it determines the air flow through the e-cigarette coil and wick. Three different air hole sizes (i.e., 1, 1.5, and 2 mm), which were the available air hole diameters from the e-cigarette vendor, were used to evaluate the impact of air flow rate on •OH formation.
To test the impacts of vaping topography on •OH formation, combinations of three puff volumes (i.e., 35, 90, and 170 mL) and two puff durations (i.e., 2 and 3.8 s) were used to generate e-vapor. Selected vaping topography conditions represent the cigarette smoking regime (i.e., 35 mL and 2 s puff), the 50th percentile of the observed vaping regime (i.e., 90 mL and 3.8 s puff), and the 95th percentile of the observed puff volume (i.e., 170 mL).
The impact of the e-liquid composition (i.e., different base materials, nicotine levels, and flavoring agents) on the e-vapor •OH concentration was tested because various e-vapor components and their thermal degradation products likely result in different redox potentials and alter •OH formation. In order to test the impact of the base material and nicotine concentrations on •OH formation, nicotine (0, 3, 12, 24, and 36 mg/mL) in VG, PG&VG (v/v = 1:1), or PG-based e-liquid were used to generate e-vapor. The levels of nicotine and base materials were observed from the 23 study participants and e-liquid recipes on the market.17 In addition, e-vapors were generated using freshly prepared e-liquids consisting of the eight flavored e-liquids with low and high levels of flavoring agents (1 and 10% by volume except for the cinnamon flavored e-liquids, which contained 0.1% and 1% of cinnamon flavoring agent). The levels of the flavoring agents were determined on the basis of the 941 914 e-liquid recipes.17
Hydroxyl Radicals in Primary E-Vapor.
For each experimental condition (Table S4), 50 puffs of e-vapor were generated using a LX1 smoking machine (Borgwaldt KC Incorporated, Hamburg, Germany) and collected using a midget impinger with fritted nozzle (Ace Glass Incorporated, NJ, U.S.A.) containing 15 mL of phosphate buffered saline [PBS, pH 7.4, 114 mM sodium chloride (NaCl, ≥ 99.5%, Sigma-Aldrich, MO, U.S.A.), 8 mM sodium phosphate dibasic (Na2HPO4, ≥ 99.0%, Sigma-Aldrich, MO, U.S.A.), 2 mM potassium phosphate monobasic (KH2PO4, ≥ 99.995%, Sigma-Aldrich, MO, U.S.A.)] with 15 mM of disodium terephthalate (TPT, ≥ 99.0%, Alfa Aesar, MA, U.S.A.) as a stable, •OH-specific fluorescence probe.27 Dimethyl sulfoxide (DMSO, ≥ 99.9%, Sigma-Aldrich, MO, U.S.A.) (50 mM) was added immediately after collecting e-vapor using the impinger, and samples were stored at −20 °C until analysis. PBS and other chemicals were prepared using chelex-100 (50–100 mesh, Sigma-Aldrich, MO, U.S.A.) treated deionized water to remove metal ions.
E-Vapor-Induced Hydroxyl Radicals.
E-vapor components may interact with ascorbic acid in ELF to induce •OH. To explore the levels of e-vapor oxidative potential, e-vapor (50 puffs) was collected using an impinger containing 15 mL of PBS (pH 7.4) and then incubated for 2 h with 100 μM of ascorbic acid (Asc, ≥ 99.0%, Sigma-Aldrich, MO, U.S.A.) and 15 mM TPT at 37 °C in the dark. Induced •OH after 2 h of incubation was measured considering background •OH levels at 0 h. The selected conditions included three flavored e-liquids representing fruit, spicy, and creamy/fatty flavors (strawberry, cinnamon, and sweet cream flavor), and 0, 3, 12, 24, 36 mg/mL nicotine in VG. E-vapors were generated under 6.4 W as 90 mL and 3.8 s puffs. The ascorbic acid concentration was determined based on the human respiratory ELF study.28
E-vapor components may also interact with transition metal ions to induce •OH by initiating the Fenton reaction which is a chemical reaction generating •OH from the metal ion and hydrogen peroxide (H2O2). However, transition metals alone (e.g., Fe3+) cannot induce •OH without a reducing agent.8 Therefore, to test •OH inducing capacity, 50 puffs of e-vapor collected using the impinger with 15 mL of PBS (pH 7.4) were incubated for 2 h at 37 °C in the dark after adding 100 μM of Fe3+ (iron[III] nitrate, ≥ 99%, Sigma-Aldrich, MO, U.S.A.) and 15 mM TPT (0 h •OH level was subtracted as background). The tested e-liquids contained strawberry, cinnamon, and sweet cream flavors, and e-liquids containing 0, 3, 12, 24, and 36 mg/mL nicotine in VG. Other vaping parameters were 6.4 W, 90 mL puff volume, and 3.8 s puff duration. In addition, the level of •OH induced by e-vapors containing flavoring chemical and Fe3+ were tested using nine flavoring chemicals selected above, representing fruity (benzyl alcohol, benzyl acetate, and ethyl acetate), sweet (anethole), citrus (citral), spicy (cinnamaldehyde), and creamy/buttery (vanillin, diacetyl, and acetylpropionyl) flavors.
Nicotine was also tested for its ability to generate •OH since it is a redox-active chemical.15 The induced •OH levels were measured using the solutions containing 0.1, 0.3, 0.6, and 0.9 mM of nicotine in PBS (pH 7.4) in the presence of 15 mM of TPT and 100 μM Fe3+ (2 h incubation at 37 °C, avoiding light). These nicotine concentrations correspond to the e-vapor samples containing 3–36 mg/mL nicotine.
Hydroxyl Radical Detection.
Hydroxyl radicals in reaction mixes were measured on the basis of the formation of 2-hydroxyterephthalic acid (2OHTA), the knwon reaction product of •OH and TPT.27 A high-throughput approach was applied using a 96-well fluorescent microplate reader (BioTek Synergy 4 Multidetection Microplate reader) to measure 2OHTA at ex/em = 310/425 nm. A calibration curve was generated using 0.1 to 1.5 μM 2OHTA (2OHTA, 97%, Sigma-Aldrich, MO, U.S.A.) standard samples in phosphate buffer (pH 7.4) containing 15 mM TPT (Figure S2). Limits of detection (LOD) and limits of quantification (LOQ) were the result of adding 3× and 10× the standard deviation, respectively, to the mean blank value. LOD and LOQ of 2OHTA were 4.1 nM and 13.7 nM, respectively.
Quality Assurance and Quality Control.
The amount of TPT in reaction mixes is sufficient to outcompete other •OH scavengers under our experimental conditions.27 The 15 mM of TPT would be sufficient to capture •OH in e-vapor and •OH induced by e-vapor because the levels of •OH in the experimental conditions were much lower than the •OH concentrations induced by transition metal ion and ascorbic acid in our previously published report.27
Lab blanks were obtained for each batch of the experiment. Blank samples were 50 puffs of e-vapor generated under the same experimental conditions and then collected using a midget impinger (Ace Glass Incorporated, NJ, U.S.A.) containing 15 mL of PBS (pH 7.4) with 15 mM of TPT and 50 mM of DMSO. Blank values for each experimental condition were subtracted from the corresponding measured concentrations.
To validate the sampling system, 50 puffs of air that passed through the unpowered e-cigarette were collected using a midget impinger containing 15 mL of PBS (pH 7.4) with 15 mM of TPT. The signals with and without DMSO were 3131 ± 81 RFU (relative florescence unit) and 3025 ± 48 RFU, respectively. The results indicated that the air flow through the e-cigarette did not increase 2OHTA.
Statistical Analyses.
For all the experimental conditions, mean and standard deviations were estimated and presented. Two-tailed Student’s t tests were conducted using R 3.4.3 (R Development Core Team, Vienna, Austria) to compare the means across different e-cigarette vaping conditions.
RESULTS AND DISCUSSION
Impact of Device Settings on •OH Formation.
Figure 1 shows the impact of device settings and e-liquid base material on •OH formation. Higher power output significantly increased •OH generation (p < 0.001). 31.3 W power output formed 2.7, 2.3, and 5.8 times more •OH than 6.4 W conditions for PG, PG&VG, and VG e-liquids, respectively. Furthermore, the 31.3 W power output with a 2 mm air hole size formed 119% and 14.3% more •OH than that with a 1 mm air hole for PG&VG and VG e-liquid, respectively (p < 0.027).
Figure 1.
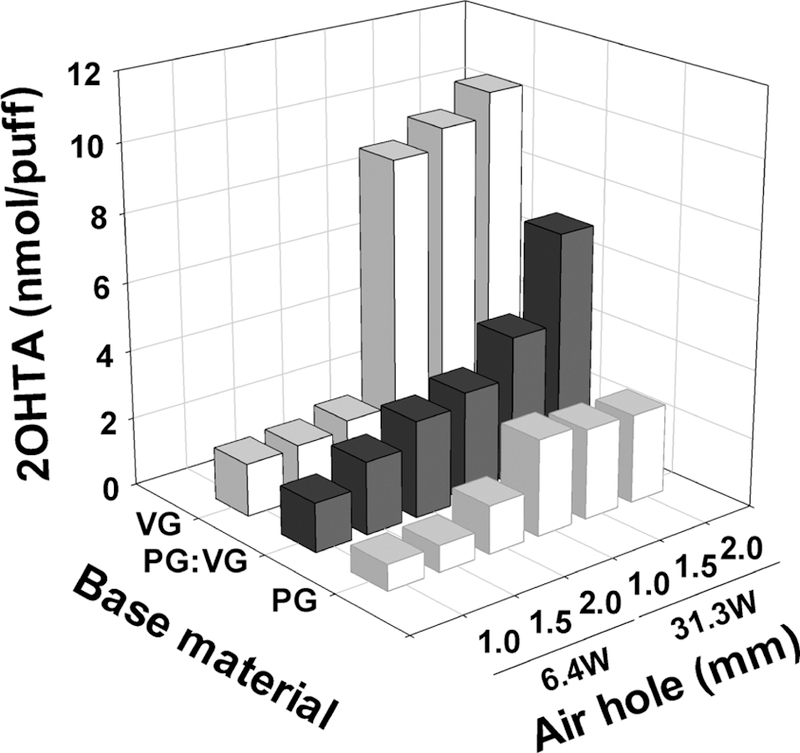
Formed 2OHTA concentrations in e-vapor for different device power settings, air hole sizes, and base materials (n = 5). Twelve mg/mL nicotine was added in all e-liquids. 90 mL puff volume, 3.8 s puff duration, and 24 s puff interval was used for e-vapor generation.
E-cigarette power output alters the amount of e-vapor and its chemical composition. Increased power output (i.e., high coil temperature) was shown to generate higher amount of e-vapor because of the increased e-liquid evaporation rate.29 Larger e-vapor quantity under higher power output settings might increase the amount of OH per puff.
Furthermore, increased device power output and oxygen supply facilitate the partial oxidation of e-liquid given by eq 1 and eq 2.30 The partial oxidation (eq 1) and combustion reaction (eq 2) of VG are thermodynamically favorable at e-cigarette coil temperatures (i.e., 200–300 °C), with higher coil temperatures always favoring these reactions.30 Moreover, higher oxygen supplies due to larger air hole sizes may increase the partial oxidation and combustion reactions. In addition, hydrogen abstracting reaction during the thermal degradation of VG and PG can form hydroxyl group radicals including •OH, •CH2OH, and •C3H7O3 (eq 3).31,32 Reactions between the partial oxidation products (i.e., H2, CO2, H2O, and radicals) and other e-liquid components could lead to the formation of •OH during e-cigarette vaping.8
| (1) |
| (2) |
| (3) |
Impact of E-Liquid Composition on •OH Formation.
Table 1 shows •OH species in e-vapor generated with various e-liquids containing different base materials and different nicotine concentrations. VG-and PG&VG-based e-liquids formed 1.7-and 1.9-fold higher •OH levels than PG based e-liquids (p < 0.015). Similarly, Lerner et al.6 reported that the VG-based e-liquid showed higher ROS level compared with PG-based e-liquid. As of now, there is insufficient knowledge to explain the impact of the base material on •OH formation, but potential impacting factors could be differences in reaction temperatures and oxidation products of PG and VG.31,33 In the presence of O2, PG and VG could initiate the oxidation reaction at 127 °C and 200 °C, respectively, to form the oxidation products derived from the carbon-centered radicals. The higher reaction temperature of VG-based e-liquid might produce more intermediate products (e.g., radicals) because of the incomplete oxidation reaction, while PG-based e-liquid can quickly produce final oxidation products under the same reaction temperature.
Table 1.
Formed 2OHTA in E-Vapor (Mean ± Standard Deviation, nmol/puff, n = 5) from E-Liquids with Different Base Materials and Nicotine Concentrationsa
| nicotine level |
|||||
|---|---|---|---|---|---|
| base material | 0 mg/mL | 3 mg/mL | 12 mg/mL | 24 mg/mL | 36 mg/mL |
| VG | 1.43 ± 0.32 | 1.80 ± 0.30 | 1.71 ± 0.49 | 1.10 ± 0.15 | 1.63 ± 0.62 |
| PG&VG | 1.82 ± 0.79 | 1.78 ± 0.71 | 1.88 ± 0.62 | 0.56 ± 0.94 | 1.48 ± 0.34 |
| PG | 1.08 ± 0.21 | 1.15 ± 0.70 | 0.89 ± 0.53 | 0.82 ± 0.54 | 0.70 ± 0.16 |
6.4 W power output, 1.5 mm air hole, 90 mL puff volume, 3.8 s puff duration, and 24 s puff interval used.
Even though the differences in 2OHTA levels were not statistically significant (p > 0.050), 2OHTA concentrations in e-vapor generated from e-liquids with higher nicotine contents were slightly lower than in samples containing less nicotine up to 24 mg/mL of nicotine in e-liquid (Table 1). VG-and PG:VG-based e-liquid with 36 mg/mL nicotine resulted in higher levels of 2OHTA in e-vapor than e-vapor e-liquids containing 24 mg/mL nicotine, while PG-based e-liquid containing 36 mg/mL nicotine generated lower levels of 2OHTA in e-vapor than e-liquids containing 24 mg/mL of nicotine. Complex interactions between different nicotine concentrations, base materials, and thermal decompositionproducts might be responsible for different •OH levels in e-vapors. In previous research, e-vapor with 24 mg/mL nicotine (PG:VG with tobacco flavor) showed less ROS concentration than e-vapor without nicotine.6 Nicotine is regarded as an antioxidant because of its many oxidation sites that can react with •OH to form electronically neutral radicals.8 Simultaneously, the redox potential of nicotine may facilitate •OH formation.15 The competing effects of nicotine might result in different e-vapor •OH levels.
Figure 2 shows e-vapor •OH levels formed upon use of nonflavored (100% VG) and flavored e-liquids. Flavored e-liquids formed higher levels of •OH in e-vapor than nonflavored e-liquids (p < 0.049). Ranges of the average 2OHTA concentration for low and high flavored e-liquids were 2.29–2.92 nmol/puff and 2.79–3.66 nmol/puff, respectively; while nonflavored e-liquid (100% VG) induced 1.43 ± 0.32 nmol/puff of 2OHTA. Among the flavored e-liquids, fruit (strawberry, dragonfruit) and sweet (bubble gum) flavored eliquids formed slightly more •OH than menthol and creamy/ buttery (Bavarian cream, sweet cream, and graham cracker) flavored e-liquids but the difference was not statistically significant (p > 0.177). Similarly, Bitzer et al.34 reported higher radical formation in sweet, fruit, and citrus-flavored eliquids than in vanillaflavored or nonflavored e-liquids. Flavoring chemicals may decompose to radicals and redox active chemicals through the vaping process,33,34 and interactions between flavoring chemicals and their decomposition products might form more •OH.
Figure 2.
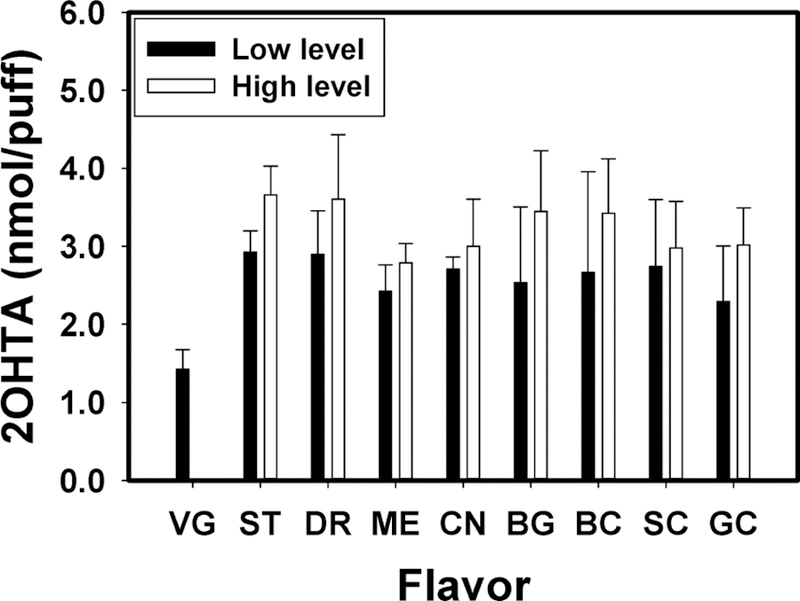
Formed 2OHTA concentrations in e-vapor for the nonflavored e-liquid and the eight flavored e-liquids. Low and high indicates 1% and 10% of flavoring ingredient in VG (by volume) except for the cinnamon flavor (0.1% and 1%) (n = 5, error bars indicate standard deviation). VG: nonflavored e-liquid, ST: strawberry, DR: dragon fruit, ME: menthol, CN: cinnamon, BG: bubble gum, BC: Bavarian cream, SC: sweet cream, and GC: graham cracker flavor. Device setting was 6.4 W and 1.5 mm air hole diameter, and vaping topography was 90 mL puff volume, 3.8 s puff duration, and 24 s puff interval.
Impact of Vaping Topographies on •OH Formation.
The impacts of vaping topography on •OH formation in e-vapor are presented in Table 2. A 90 and 170 mL puff (3.8 s) generated 4.1-and 10.3-fold more •OH than a 35 mL puff (3.8 s), respectively (p < 0.004). •OH levels using a 35 mL puff volume were not significantly different from blank samples (p > 0.101, data not shown). It is therefore likely that toxicological studies adopting a conventional cigarette smoking regime (i.e., 35 mL puff volume and 2 s puff duration) underestimate the oxidative stress affecting real-world e-cigarette users. 2OHTA concentrations for a 170 mL and 3.8 s puff were significantly higher than those from a 170 mL and 2 s puff (p < 0.027). Increased puff volumes are associated with increased air flow rates around the e-cigarette coil which could facilitate e-liquid evaporation.29 Increased oxygen supply was shown to initiate oxidation of VG/PG at a significantly lower temperature compared to anaerobic conditions.31 In addition, high air flow rates rapidly mixed e-vapor with supplied oxygen and could increase the oxidation rate.30
Table 2.
Formed 2OHTA in E-Vapor (Mean ± Standard Deviation, nmol/puff, n = 5) for Different Vaping Topographiesa
| puff volume |
|||
|---|---|---|---|
| puff duration | 35 mL | 90 mL | 170 mL |
| 2 s | 0.66 ± 0.72 | 1.68 ± 0.96 | 2.66 ± 0.73 |
| 3.8 s | 0.34 ± 0.66 | 1.70 ± 0.40 | 3.80 ± 0.59 |
6.4 W power output, 1.5 mm air hole, VG based e-liquid containing 12 mg/mL nicotine, and 24 s puff interval used.
E-Vapor-Induced •OH Formation in Ascorbic Acid Solution.
E-vapors generated with flavored e-liquids reacted with ascorbic acid, which is an abundant molecule in human ELF, and showed higher oxidative potential (i.e., more •OH generation) than e-vapors generated from nonflavored e-liquids under the physiologically relevant incubating condition (37 °C, avoiding light) (Figure 3). Flavored e-vapor samples incubated with 100 μM ascorbic acid generated 1.2 to 1.7-fold higher •OH than nonflavored (100% VG) e-vapor samples (p < 0.016). Among the flavored e-vapor samples, cinnamon and sweet cream flavor induced 39.1% and 35.1% higher •OH than strawberry flavored e-vapor samples (p < 0.034). E-vapor-only samples without ascorbic acid and ascorbic acid-only samples without e-vapor components (PBS samples) did not induce OH.
Figure 3.
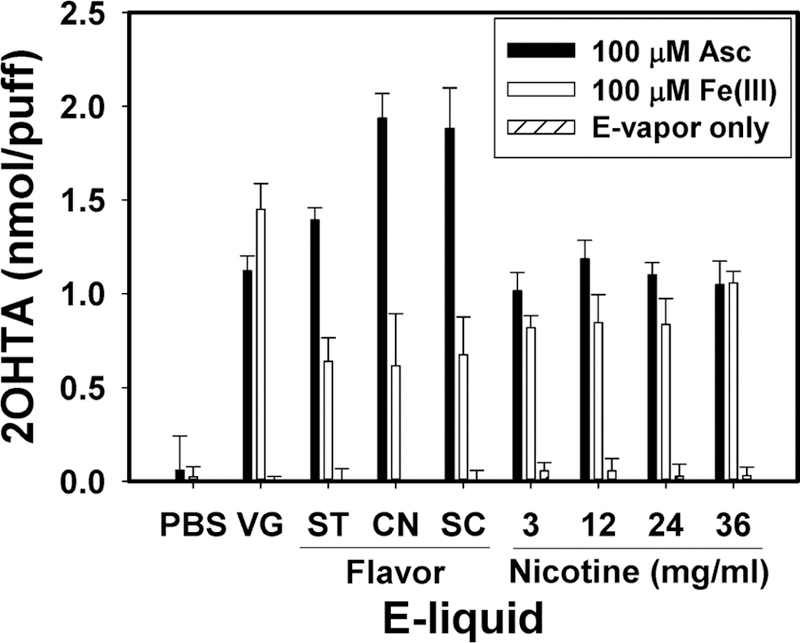
2OHTA concentrations induced by PBS only, 100% VG, flavored (ST: strawberry, CN: cinnamon, SC: sweet cream) and nicotine containing e-vapors (3–36 mg/mL nicotine) with 100 μM ascorbic acid (Asc) or 100 μM Fe3+ after 2 h incubation under 37 °C, avoiding light (n = 5, error bars indicate standard deviation). E-vapor only indicates samples containing e-vapor components without ascorbic acid.
Flavored e-vapors may induce higher •OH levels because of the higher numbers and amount of redox cycling components (i.e., flavoring chemicals or thermal degradation products of flavoring chemicals) than VG-only e-vapor. Ascorbic acid was known to redox cycle transition metal ions (e.g., Fe3+ to Fe2+) to generate •OH through Fenton reaction.14,27 An e-vapor component (e.g., nicotine, flavoring chemical, and other decomposition products) may act as a substitute for metalion in ascorbic acid solution. In our experiments, ascorbic acid (Asc) might reduce e-vapor components (R) through eq 4, and the reduced e-vapor component might increase •OH through the organic Fenton reaction (eq 5).8
| (4) |
| (5) |
Redox potentials of flavoring chemicals are still not well understood because of the large variety of the structures of the flavoring chemicals. A limited number of available studies suggests a reducing capacity of cinnamaldehyde, vanillin, and diacetyl which might be present in cinnamon and sweet cream flavored e-liquids.35,36 Thermal degradation products of vanillin (i.e., vanillic acid) also show a reducing capacity.16 Therefore, flavored e-liquids might form more chemical compounds than nonflavored e-liquid and might redox cycle oxygen and hydrogen to form •OH under the presence of ascorbic acid.
E-Vapor Redox Cycled Fe3+ To Induce •OH.
E-vapor plus Fe3+ induced •OH after 2 h incubation at 37 °C, while neither e-vapor components nor Fe3+ individually induced •OH after 2 h comparing to initial time point (Figure 3). These experiments were performed in the dark. To generate •OH, Fe3+ needs to be reduced to Fe2+ by an e-vapor component (R) (eq 6), and then Fe2+ in the system could generate •OH through the Fenton reaction (eq 7).
| (6) |
| (7) |
Interestingly, VG-only e-vapor with 100 μM Fe3+ induced 1.4–2.4-fold higher •OH than other conditions (p < 0.001), while flavored e-vapors with ascorbic acid induced significantly higher amounts of •OH than VG-only e-vapor. Different 2OHTA formation trends might be affected by the redox potentials of e-vapor components, ascorbic acid (E0 = 0.33 V, pH = 7.0), and Fe3+/2+ (E0 = 0.77 V, pH = 7.0). The redox potential of VG-only e-vapor could be more favorable to the reduction of Fe3+ to Fe2+ than flavored e-liquid samples. In contrast, the redox potential of ascorbic acid might facilitate reducing flavored e-vapor constituents to form more •OH than VG-only e-vapor. Further studies of e-vapor redox potentials are needed to better understand •OH generation mechanisms in e-vapors.
Nicotine solution with Fe3+ could initiate Fenton reaction to form •OH, while nicotine solution without metal ion did not induce •OH (Figure 4). The 0.9 mM nicotine solution with 100 μM Fe3+ induced 2.1-fold more •OH than the 0.1 mM nicotine solution. Figure 3 shows the differential impacts of nicotine and e-vapor on •OH formation with the existence of Fe3+. The levels of •OH in 0.1 and 0.3 mM nicotine solutions were 19.6% and 10.0% lower than that in the e-vapor solutions containing 3 and 12 mg/mL (equivalent to 0.1 and 0.3 mM) nicotine, respectively. However, 0.6 and 0.9 mM nicotine solution induced 40% more •OH than the e-vapor solutions containing 24 and 36 mg/mL (equivalent to 0.6 and 0.9 mM) nicotine (p < 0.019). Nicotine (E0 = 0.84 V, pH = 7.0) might act as either •OH scavengers or competing redox agents with Fe3+ in e-vapor samples.15
Figure 4.
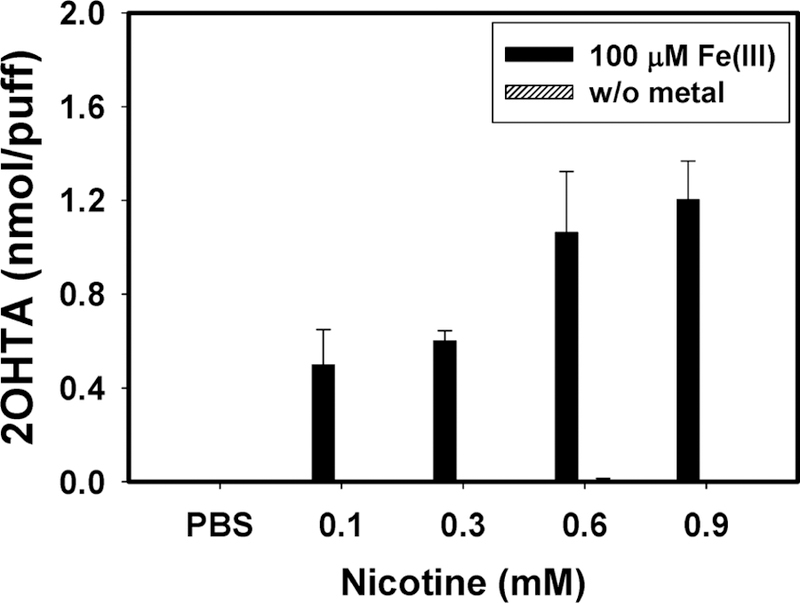
2OHTA concentrations induced by 0.1–0.9 mM nicotine solution in PBS (pH 7.4) with 100 μM of Fe3+ after 2 h incubation under 37 °C, avoiding light (n = 5, error bars indicate standard deviation).
E-vapors generated using the butter flavoring chemicals (i.e., diacetyl and acetylpropionyl) induced higher •OH with Fe3+ than e-vapors containing other flavoring chemicals (Figure 5). At the same molar concentrations, diacetyl-and acetylpropionyl-containing e-vapors with 100 μM Fe3+ formed approximately two times more •OH than the e-vapors containing fruit-like flavoring chemicals (benzyl alcohol, benzyl acetate, and ethyl acetate) (p < 0.001). The results provide evidence that the flavoring chemicals in e-vapor could redox cycle the transition metal ions to form •OH through the Fenton reaction.
Figure 5.
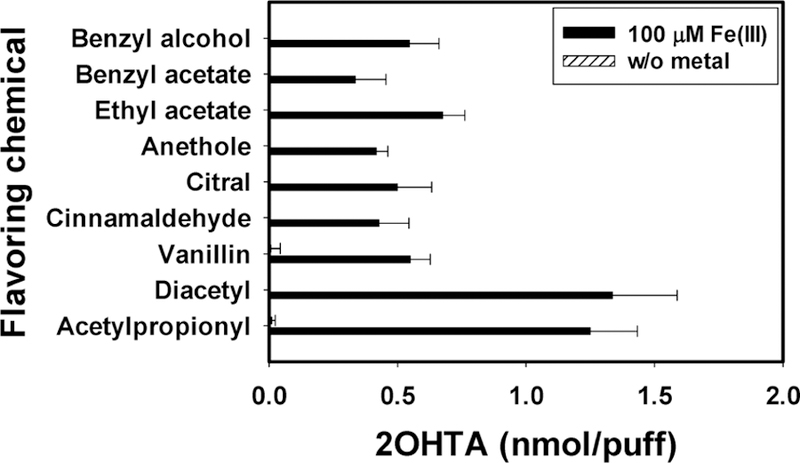
2OHTA generated by e-liquids containing flavoring chemicals with and without 100 μM Fe3+ after 2 h incubation under 37 °C, avoiding light (n = 5, error bars indicate standard deviation). Results were normalized by the molar concentration of flavoring chemicals.
Health Implications.
This study, for the first time, assessed the impact of real-world vaping patterns (e-cigarette device settings, vaping topographies, and e-liquid compositions) on •OH concentrations in e-vapors. •OH is one of the most harmful e-vapor components because of the following: (1) the risk potency of •OH can be much higher than that of other potentially harmful components in e-vapor;9 and (2) the daily dose of free radicals in e-vapor (2 × 1015 radical/day) can exceed the dose caused by ambient particulate matter (2 × 1014 radical/day), assuming vaping frequencies of 200 puffs/ day.5,37
Our study and published results show that e-vapor contains lower levels of radicals per puff than cigarette smoke.5,6 Previously reported free radical levels in tobacco smoke were 10 to 1000 times higher than those observed in e-vapor in our study (0.86–1.09 × 1014 OH radicals/puff) and other earlier studies (0.25–1.03 × 1013 radicals/puff or 0.59–2.94 μM H2O2 equivalents/puff).5,6,8,9
However, it is very likely that daily e-vapor radical exposures in e-cigarette users can reach levels comparable to those of cigarette smokers depending on e-cigarette use patterns. In 2015, on average, daily smokers in the U.S. smoked 14.2 cigarettes per day (range of 5–30 cigarettes/day).38 Assuming a cigarette can last 10–12 puffs, the estimated average •OH exposures, using the published •OH levels in cigarette smoke, range from 5.0 × 1016 to 3.6 × 1019 OH radicals/day (with an average of (9.1 ± 1.3) × 1018 OH radicals/day). In the current study, we found that the daily average radical exposures induced by e-cigarette vaping can be (2.4 ± 2.6) × 1016 OH radicals/day (range of 8.6 × 1014 –1.1 × 1017 OH radicals/ day), assuming puff frequencies from 10 to 1000 puffs/day, a range observed in this study and in published data.21,22 The range of exposures to •OH concentrations from e-cigarette vaping overlaps daily •OH dosages induced by cigarette smoking.
The •OH formation rate induced by e-vapor components through the Fenton reaction is much slower than that induced by cigarette smoke. Both cigarette smoke and e-vapor contain •OH and can induce •OH formation. The Q/QH2 couple in cigarette tar can redox cycle transition metal ions to form •OH.39 In the previous study, the EPR signal intensity of the DMPO–OH spin adduct was increased 4-fold by adding 20 μm Fe3+ in the cigarette tar extract solution (20 mg/mL, pH 9.5).9 We evaluated •OH induced by e-vapor and Fe3+, and the •OH formation rate was 5–10-fold lower than the •OH produced by conventional cigarette smoke. Therefore, it appears that the redox activity of e-vapor components is much lower than the redox activity of conventional cigarette smoke.
Limited numbers of in vivo and in vitro studies have shown oxidative stress caused by e-cigarette vaping. In vivo mouse studies showed that e-vapor extracts in ELF induced lung glutathione depletion and lipid peroxidation,6,40 which might indicate the ROS exposure.7 Limited numbers of in vitro studies have also shown that e-vapor exposure increases DNA damage and apoptotic/necrotic cell death.41–43 However, lacking experimental conditions (e.g., device type, power output, or e-liquid composition) in the previous in vivo and in vitro studies would suggest further needs of e-cigarette oxidative stress research. Specifically, since •OH is the most destructive ROS and it does not have specific antioxidant defense mechanism (e.g., superoxide dismutase and peroxidase),8 the •OH generation mechanism needs to be further studied to be able to reduce adverse health impacts associated with e-cigarette use.
Oxidative stress responses were observed only after longterm e-vapor exposures (24 h or longer).41–43 Up to 3 h of e-vapor exposures using human bronchial epithelial cell line (BEAS-2B) did not induce DNA damage, while earlier research showed that cigarette smoke exposures results in significantly higher DNA damage and cytotoxicity after 3 h.44 Taylor et al.45 incubated human bronchial epithelial cells (NCI-H292) with e-vapor extracts for 6 h, and no cell death, ROS formation, and glutathione consumption was observed. As levels of •OH in e-vapor might not be sufficient to induce acute adverse health impacts, studies are needed to assess the long-term oxidative potential of e-vapors and associated health effects.
Recent developments in e-cigarette devices (i.e., “mod”) may elevate the doses of radicals due to the combinations of recently developed subohm coils (e.g., the Clapton, Twisted, Helix, and Staple coils with the resistance of less than 1 ohm) and battery devices that can provide over 100 W power output. The extremely high-power output settings might cause elevated oxidative stress due to the induction of a large amount of e-vapor radicals. Consequently, the safe range of e-cigarette power output and vaping frequency needs to be assessed, communicated, and regulated to protect public health.
Popular use of flavored e-liquids might increase e-vapor •OH exposures and result in potential health problems. This is a relevant concern as the market share of nonflavored e-cigarettes decreased by 3% between 2012 and 2013, while the market share of fruit and other flavored e-cigarette increased at least by 0.8% and 2.6%, respectively.46 Increased use of the flavored e-liquid may pose potential health problems due to the higher oxidative potential of flavored e-liquids versus nonflavored e-liquids. In addition, e-cigarettes are the most popular flavored tobacco product among high school students.47 Vaping flavored e-cigarettes at early ages should be discouraged because the e-vapor ROS might alter cell proliferation.48
It is worth mentioning that coexisting environmental exposures might further elevate oxidative stress. Airborne particulate matter is a known source of exposure to transition metal ions such as iron and copper. In fact, these two transition metal ions are known to induce •OH in simulated ELF.27 Reactions between redox active components in e-vapor and PM constituents (i.e., transition metal ions) can form •OH through the Fenton reaction. Therefore, the interaction between e-vapor and air pollutants needs to be studied further.
A limitation of the current study is that it had to focus for feasibility reasons on the most popular flavoring agents and chemicals to evaluate the •OH levels only. In fact, there are more than 100 000 e-liquid recipes, which might contain numerous flavoring chemicals.17 All these flavoring chemicals could affect •OH formation in e-vapor and induce •OH with ELF components (e.g., ascorbic acid). In the current study, we had to focus on the most popular flavoring agents and flavoring chemicals after intensive searches for e-liquid recipes. It will be necessary that future research assesses the role of flavoring agents in the formation of •OH radicals on the basis of the redox potentials and chemical structures of the flavoring agents. In addition, there is limited information available on the redox potentials of flavoring chemicals and their thermal degradation products at temperatures of 100–150 °C.16,35,36,49 The redox potential and the thermal oxidation of the flavoring chemicals should be evaluated using comparable heating temperatures of e-cigarettes (i.e., 200–300 °C).
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank Drs. Clifford Weisel and Charles Weschler for valuable discussions. The authors also wish to thank all study participants for their support of the study.
Funding
This study was supported by the Cancer Institute of New Jersey, the New Jersey Health Foundation, and the National Institute of Environmental Health Sciences (P30 ES005022). V.M. and J.D.L. were supported by U01 NS108956 from the National Institute of Neurological Disorders and Stroke and U54 AR055073 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Contributions by O.A.W. were supported in part by K01 CA189301 from the National Cancer Institute and the FDA Center for Tobacco products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
ABBREVIATIONS
- ROS
reactive oxygen species
- ELF
epithelial lining fluid
- PG
propylene glycol
- VG
vegetable glycerin
- PBS
phosphate buffer saline
- TPT
disodium terephthalate
- 2OHTA
2-hydrox-yterephthalic acid
- DMSO
dimethyl sulfoxide
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemres-tox.8b00400.
Characteristics of flavoring chemicals, summary of study participants, E-cigarette vaping patterns observed from the study subjects, detailed experimental conditions, calibration curve for 2OHTA (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, Bunnell RE, Choiniere CJ, King BA, and Cox S (2015) Tobacco use among middle and high school students-United States, 2011–2014. MMWR. Morbidity and mortality weekly report 64, 381–385. [PMC free article] [PubMed] [Google Scholar]
- (2).Schoenborn CA, and Gindi RM (2015) Electronic cigarette use among adults: United States, 2014. NCHS data brief 217, 1–8. [PubMed] [Google Scholar]
- (3).Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, et al. (2014) Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco control 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Tierney PA, Karpinski CD, Brown JE, Luo W, and Pankow JF (2015) Flavour chemicals in electronic cigarette fluids. Tob. Control 25, e10–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Goel R, Durand E, Trushin N, Prokopczyk B, Foulds J, Elias RJ, and Richie JP Jr (2015) Highly reactive free radicals in electronic cigarette aerosols. Chem. Res. Toxicol 28, 1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, and Rahman I (2015) Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10, e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ciencewicki J, Trivedi S, and Kleeberger SR (2008) Oxidants and the pathogenesis of lung diseases. J. Allergy Clin. Immunol 122, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Halliwell B, and Gutteridge JM (1999) Free radicals in biology and medicine, Vol. 3, Oxford University Press, Oxford. [Google Scholar]
- (9).Pryor WA, Stone K, Zang L-Y, and Bermud́ez E (1998) Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem. Res. Toxicol 11, 441–448. [DOI] [PubMed] [Google Scholar]
- (10).Zhao J, Zhang Y, Sisler JD, Shaffer J, Leonard SS, Morris AM, Qian Y, Bello D, and Demokritou P (2018) Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater 344, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Geiss O, Bianchi I, and Barrero-Moreno J (2016) Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 219, 268–277. [DOI] [PubMed] [Google Scholar]
- (12).Khlystov A, and Samburova V (2016) Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ. Sci. Technol 50, 13080–13085. [DOI] [PubMed] [Google Scholar]
- (13).Zhao T, Shu S, Guo Q, and Zhu Y (2016) Effects of design parameters and puff topography on heating coil temperature and mainstream aerosols in electronic cigarettes. Atmos. Environ 134, 61–69. [Google Scholar]
- (14).Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Baeza-Squiban A, Cho A, Duggan S, and Froines J (2008) Evaluating the toxicity of airborne particulate matter and nano-particles by measuring oxidative stress potential -A workshop report and consensus statement. Inhalation Toxicol 20, 75–99. [DOI] [PubMed] [Google Scholar]
- (15).Levent A, Yardim Y, and Senturk Z (2009) Voltammetric behavior of nicotine at pencil graphite electrode and its enhancement determination in the presence of anionic surfactant. Electrochim. Acta 55, 190–195. [Google Scholar]
- (16).Mourtzinos I, Konteles S, Kalogeropoulos N, and Karathanos VT (2009) Thermal oxidation of vanillin affects its antioxidant and antimicrobial properties. Food Chem 114, 791–797. [Google Scholar]
- (17).ELiquidRecipes. (2017) e-Liquid Recipes https://www.nicvape.com/eliquid-recipes.
- (18).Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, and Luch A (2014) Chemical hazards present in liquids and vapors of electronic cigarettes. Arch. Toxicol 88, 1295–1308. [DOI] [PubMed] [Google Scholar]
- (19).Son Y, Wackowski O, Weisel C, Schwander S, Mainelis G, Delnevo C, and Meng Q (2018) Evaluation of e-vapor nicotine and nicotyrine concentrations emitted under various e-liquid compositions, device settings and vaping topographies. Chem. Res. Toxicol 31, 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Misthub. (2013) Tutorial: Variable Voltage and Vaping Power Chart https://www.misthub.com/blogs/vape-tutorials/76788421-tutorial-variable-voltage-and-vaping-power-chart.
- (21).Dautzenberg B, and Bricard D (2015) Real-Time Characterization of E-Cigarettes Use: The 1 Million Puffs Study. J. Addict. Res. Ther 2015, 1000229. [Google Scholar]
- (22).Meng Q, Schwander S, Son Y, Rivas C, Delnevo C, Graber J, Giovenco D, Bruen U, Mathew R, and Robson M (2016) Has the mist been peered through? Revisiting the building blocks of human health risk assessment for electronic cigarette use. Hum. Ecol. Risk Assess 22, 558–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Norton KJ, June KM, and O’Connor RJ (2014) Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob. Induced Dis 12, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Robinson R, Hensel E, Morabito P, and Roundtree K (2015) Electronic cigarette topography in the natural environment. PLoS One 10, e0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, and Eissenberg T (2015) Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob. Res 17, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hua M, and Talbot P (2016) Potential health effects of electronic cigarettes: a systematic review of case reports. Preventive medicine reports 4, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Son Y, Mishin V, Welsh W, Lu S-E, Laskin JD, Kipen H, and Meng Q (2015) A Novel High-Throughput Approach to Measure Hydroxyl Radicals Induced by Airborne Particulate Matter. Int. J. Environ. Res. Public Health 12, 13678–13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).van der Vliet A, O’Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, and Louie S (1999) Determination of lowmolecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol-Lung C 276, L289–L296. [DOI] [PubMed] [Google Scholar]
- (29).Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, and Shihadeh A (2015) Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob. Res 17, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Lin Y-C (2013) Catalytic valorization of glycerol to hydrogen and syngas. Int. J. Hydrogen Energy 38, 2678–2700. [Google Scholar]
- (31).Jensen RP, Strongin RM, and Peyton DH (2017) Solvent chemistry in the electronic cigarette reaction vessel. Sci. Rep 7, 42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hemings EB, Cavallotti C, Cuoci A, Faravelli T, and Ranzi E (2012) A detailed kinetic study of pyrolysis and oxidation of glycerol (propane-1, 2, 3-triol). Combust. Sci. Technol 184, 1164–1178. [Google Scholar]
- (33).Rosado-Reyes CM, and Tsang W (2014) Thermal Stability of Larger Carbonyl Compounds: 2-Methylbutyraldehyde. Int. J. Chem. Kinet 46, 285–293. [Google Scholar]
- (34).Bitzer ZT, Goel R, Reilly SM, Elias RJ, Silakov A, Foulds J, Muscat J, and Richie JP Jr (2018) Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radical Biol. Med 120, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Singh G, Maurya S, deLampasona MP, and Catalan CAN (2007) A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol 45, 1650–1661. [DOI] [PubMed] [Google Scholar]
- (36).Yaylayan V, Haffenden L, Chu F, and Wnorowski A (2005) Oxidative pyrolysis and postpyrolytic derivatization techniques for the total analysis of Maillard model systems: investigation of control parameters of Maillard reaction pathways. Ann. N. Y. Acad. Sci 1043, 41–54. [DOI] [PubMed] [Google Scholar]
- (37).Gehling W, and Dellinger B (2013) Environmentally persistent free radicals and their lifetimes in PM2. 5. Environ. Sci. Technol 47, 8172–8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Jamal A, King BA, Neff LJ, Whitmill J, Babb S, and Graffunder C (2016) Current cigarette smoking among adults-United States, 2005–2015. MMWR. Morbidity and mortality weekly report 65, 1205. [DOI] [PubMed] [Google Scholar]
- (39).Church DF, and Pryor WA (1985) Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect 64, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim J-H, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, et al. (2015) Exposure to Electronic Cigarettes Impairs Pulmonary Anti-Bacterial and Anti-Viral Defenses in a Mouse Model. PLoS One 10, e0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Anderson C, Majeste A, Hanus J, and Wang S (2016) E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol. Sci 154, 332. [DOI] [PubMed] [Google Scholar]
- (42).Rouabhia M, Park HJ, Semlali A, Zakrzewski A, Chmielewski W, and Chakir J (2017) E-Cigarette Vapor Induces an Apoptotic Response in Human Gingival Epithelial Cells Through the Caspase-3 Pathway. J. Cell. Physiol 232, 1539–1547. [DOI] [PubMed] [Google Scholar]
- (43).Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, Tsui T, Aguilera JA, Advani S, Alexander LEC, et al. (2016) Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol 52, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Thorne D, Larard S, Baxter A, Meredith C, and Gaca̧ M (2017) The comparative in vitro assessment of e-cigarette and cigarette smoke aerosols using the γH2AX assay and applied dose measurements. Toxicol. Lett 265, 170–178. [DOI] [PubMed] [Google Scholar]
- (45).Taylor M, Carr T, Oke O, Jaunky T, Breheny D, Lowe F, and Gaca̧ M (2016) E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicol. Mech. Methods 26, 465–476. [DOI] [PubMed] [Google Scholar]
- (46).Giovenco DP, Hammond D, Corey CG, Ambrose BK, and Delnevo CD (2015) E-cigarette market trends in traditional US retail channels, 2012–2013. Nicotine Tob. Res 17, 1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Corey CG, Ambrose BK, Apelberg BJ, and King BA (2015) Flavored tobacco product use among middle and high school students United States, 2014. MMWR Morb Mortal Wkly Rep 64, 1066–1070. [DOI] [PubMed] [Google Scholar]
- (48).Moses E, Wang T, Corbett S, Jackson GR, Drizik E, Perdomo C, Perdomo C, Kleerup E, Brooks D, O’Connor G, et al. (2017) Molecular impact of electronic cigarette aerosol exposure in human bronchial epithelium. Toxicol. Sci 155, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Jeong S-M, Kim S-Y, Kim D-R, Jo S-C, Nam K, Ahn D, and Lee S-C (2004) Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J. Agric. Food Chem 52, 3389–3393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


