Abstract
Background:
Per- and poly-fluoroalkyl substances (PFAS) are public health concerns because of widespread exposure through contaminated foods/drinking water. Although some determinants of PFAS exposure have been suggested, the role of geographic location and race/ethnicity in PFAS exposure has not been well characterized.
Objectives:
We examined potential determinants of PFAS from the Study of Women’s Health Across the Nation (SWAN).
Methods:
This study includes 1302 women aged 45-56 years from 5 SWAN sites where white women and women from one minority group were recruited (black from Southeast Michigan, Pittsburgh, Boston; Chinese from Oakland; Japanese from Los Angeles). We determined concentrations of 11 PFAS in serum samples collected in 1999-2000 and examined 7 PFAS detected in most women (>97%). Linear regression with backward elimination was used to identify important determinants of PFAS serum concentrations among a set of pre-specified variables (age, body mass index, site, race/ethnicity, education, financial hardship, occupation, born outside the United States (US), parity, menstrual bleeding within the past year, smoking status, alcohol consumption, and consumption of fish, dairy, pizza, salty snack, and French fries).
Results:
Site and race/ethnicity were two major determinants of PFAS. White women had higher concentrations of linear perfluorooctanoic acid (PFOA) compared with the Chinese in Oakland (p<0.0001) and blacks in Pittsburgh (p=0.048). Black women in Southeast Michigan and Boston (vs. white women) had higher concentrations of linear (p<0.001 for Southeast Michigan; p<0.0001 for Boston) and total perfluorooctane sulfonic acid (PFOS) (p<0.001 for both Southeast Michigan and Boston) and 2-(N-methyl-perfluorooctane sulfonamido) acetic acid (p=0.02 for Southeast Michigan; p<0.001 for Boston). Chinese (Oakland) and Japanese (Los Angeles) women had higher concentrations of perfluorononanoic acid (PFNA) compared with white women in each site (p<0.01 for both). Within white women, those in Pittsburgh had relatively higher concentrations of PFAS. Within Chinese and Japanese women, those who were born outside the US had significantly lower concentrations of most PFAS but significantly higher PFNA concentrations. Menstrual bleeding and parity were significantly associated with lower PFAS concentrations. Higher intake of salty snacks including popcorn was significantly associated with higher concentrations of linear PFOA, PFOS and 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid.
Discussion:
Geographic locations and race/ethnicity play an important role in differential exposure to PFAS, with racial/ethnic burdens differing between PFOS, PFOA and PFNA. Menstruation and parity were also determinants of PFAS concentrations possibly as an elimination route.
Keywords: Per- and polyfluoroalkyl substances (PFAS), determinants, midlife women, race/ethnicity, geographic location
INTRODUCTION
Per- and poly-fluoroalkyl substances (PFAS) are a family of synthetic compounds widely used in a variety of industrial applications and consumer products, such as non-stick cookware, carpeting, apparels, upholstery, food packaging, and firefighting forms (OECD 2013). PFAS, especially long-chain PFAS, are very persistent in the environment and in the human body because of strong chemical bonds between carbon and fluorine (Buck et al. 2011). This property has made these chemicals of significant concern as persistent organic compounds (Post et al. 2017). Epidemiologic studies suggest that exposure to PFAS, especially perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), may be associated with elevated cholesterol, thyroid dysfunction, immune suppression, and higher risk of certain types of cancer (DeWitt et al. 2019; Grandjean and Clapp 2015; Kim et al. 2018; Sunderland et al. 2019). These chemicals have recently received enormous attention, especially in Michigan, Pennsylvania, and North Carolina in the United States (U.S.) because of contaminated drinking water (Bagenstose 2018; Barnes 2018; Ellison 2017; Gardner and Ellison 2018; Soechtig and Seifert 2018).
A number of studies have described potential determinants of human exposure to PFAS in the general population. These include age (higher in older adults), sex (male), race/ethnicity (higher in non-Hispanic white and non-Hispanic black vs. Mexican American), socioeconomic status (SES) (lower in the socioeconomically disadvantaged group), fish consumption (higher in the high consumption group), use of Gore-tex goods, and living near PFAS manufacturing or wastewater treatment facilities (Calafat et al. 2007a; Calafat et al. 2007b; Christensen et al. 2017; Fromme et al. 2009; Jain 2014; Lee et al. 2017). Consumption of foods prepared and served using non-greasy contact materials has also been reported as an important source of PFAS exposure (Begley et al. 2008; Berg et al. 2014; Schaider et al. 2017; Tittlemier et al. 2007; Wu et al. 2015). Furthermore, premenopause and parity have been associated with lower PFAS blood concentrations (Berg et al. 2014; Brantsaeter et al. 2013; Knox et al. 2011; Ruark et al. 2017; Taylor et al. 2014; Tsai et al. 2018), suggesting that menstruation and parturition may be a potential elimination pathway of PFAS in reproductive age women (Wong et al. 2014; Zhang and Qin 2014). Although these studies suggest important determinants that can contribute to human body burdens of PFAS in the general population, an important question that remains unanswered is relative importance among those determinants, especially between geographic location and race/ethnicity which are correlated and therefore difficult to disentangle. Such information is of significance as it can guide public health and policy decisions related to mitigating and preventing exposure. In addition, Asian populations have been poorly represented in prior literature on PFAS exposure.
In the present study, we examined potential determinants of PFAS in serum samples from the Study of Women’s Health Across the Nation (SWAN), a multi-site, multi-racial/ethnic cohort of midlife women. This analysis includes four racial/ethnic groups (white, black, Chinese and Japanese). We specifically evaluated the joint influences of geographic location and race/ethnicity on PFAS serum concentrations after accounting for important sociodemographic, behavioral and reproductive factors.
METHODS
Study Population
The SWAN is a multi-site, multi-ethnic, longitudinal study of the natural history of menopause designed to address the effect of the menopausal transition on subsequent health and risk factors for age-related chronic diseases (http://www.swanstudy.org) (Sowers et al. 2000). Between 1996 and 1997, 3302 women were enrolled in the cohort study from 7 study sites where white women and women from one specified minority group were recruited (black from Boston, MA, Pittsburgh, PA, Southeast Michigan, MI, and Chicago, IL; Hispanic from Newark, NJ; Chinese from Oakland, CA; and Japanese from Los Angeles, CA). Eligibility criteria included: age 42 to 52 years; having an intact uterus, having at least 1 menstrual period and not taking hormone medications (e.g., birth control pills, estrogen or progesterone preparations) in the 3 months before the baseline survey; and having self-identified with the site’s designated race/ethnic groups. Data and specimens (serum and urine) have been collected in 16 follow-up visits annually or biannually from 1996/97 through 2016/17. Specimens were collected prior to 11am in the morning. First morning voided urine was collected. Aliquoted specimens were stored in ultra-low freezers at −80°C and without thawing. All specimens were collected and stored in the SWAN Repository (http://swanrepository.com/) using a systematic protocol. Institutional Review Board approval was obtained at each study site, and all participants provided signed informed consent at each study visit.
The SWAN Multi-Pollutant Study funded by the National Institute of Environmental Health Sciences (NIEHS, R01-ES026578 and R01-ES026964, Pi-Park) was initiated in 2016 to examine the associations of multiple environmental pollutants (PFASs, polychlorinated biphenyls, organochlorine pesticides, and polybrominated diphenyl ethers in serum; heavy metals, phenols, phthalates, organophosphate pesticides in urine) individually or as mixtures with metabolic and reproductive health outcomes in midlife women. We used Repository samples available from the third follow-up visit (Visit 3, 1999-2000) for environmental exposure assessment (n=2694). Women from Chicago (n=368) and Newark (n=278) were excluded because urine samples were not collected in these two sites. We excluded 648 women with insufficient serum or urine samples at Visit 3 or insufficient urine samples at Visit 6 (for the assessment of non-persistent phenols and phthalates), yielding the sample size of 1400. Therefore, the SWAN Multi-Pollutant Study includes 4 race groups (white, black, Chinese, and Japanese) and 5 study sites (Boston, Pittsburgh, Southeast Michigan, Los Angeles, Oakland). For the present study, 98 women with missing data in the covariates were additionally excluded, yielding 1302 women eligible for this analysis.
PFAS Assessment
PFAS in serum were analyzed at the Division of Laboratory Sciences at Centers for Disease Control and Prevention’s National Center for Environmental Health. We used the method with a modification described previously (Kato et al. 2011a). Briefly, serum samples were analyzed using online solid-phase extraction coupled to high-performance liquid chromatography-tandem mass spectrometry (SPE-HPLC-MS/MS). A set of 11 PFASs were measured including linear PFOA (n-PFOA), sum of branched PFOA isomers (Sb-PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUA), perfluorododecanoic acid (PFDoA), perfluorohexane sulfonic acid (PFHxS), linear PFOS (n-PFOS), sum of perfluoromethylheptane sulfonic acid isomers (Sm-PFOS), 2-(N-methyl-perfluorooctane sulfonamido) acetic acid (Me-FOSAA), and 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid (Et-FOSAA). Total PFOS was computed as the sum of linear and branched PFOS. Because of low detection in branched PFOA (<20%), we did not include total PFOA in data analysis. The limit of detection (LOD) for all analytes was 0.1 ng/mL. Comprehensive QA/QC procedures were conducted. The coefficients of variation were 5.9-12.1% for the low QC pools; and 5.9-10.6% for the high QC pools.
Covariates
Data on study site, race/ethnicity, educational attainment (high school diploma or less, some college, college, or postgraduate) and financial hardship (“How hard was it to pay for basics: not hard at all, somewhat hard or very hard?”) were collected at the baseline interview. Participants’ reported occupations at Visit 3 were classified as “white collar (e.g., administrative, managerial, professional jobs)”, “blue collar (e.g., manufacture, construction, farming, cleaning and maintenance, transportation jobs)”, “pink collar (e.g., care-oriented jobs, sales, food preparation and serving jobs, secretarial work)”, or “unemployed” based on job titles, job activities and industry. Housewives and students were classified as “unemployed”. Age at Visit 3 was used in analysis. Weight (kg) and height (m) were measured at Visit 3 using standard protocols. Body mass index (BMI) was calculated as kg/m2. At Visit 3 participants reported whether they had any menstrual bleeding (yes/no) since their last visit that occurred approximately 12 months previously). Parity (the sum of the number of livebirths and stillbirths) was self-reported at baseline. Birthplace (“Were you born in the United States?”) was self-reported at Visit 1. Smoking (never, former, current) and alcohol intake (infrequent alcohol use (<once per month), moderate (≥once per month but <2 times per week), and heavy ≥2 times per week)) were self-reported at Visit 3. All participants completed a modified Block food frequency questionnaire (FFQ) (Block et al. 1986) at baseline. Details on the validity of this FFQ with dietary records and 24-hour recalls were published elsewhere (Block et al. 1992; Subar et al. 2001). Chinese, Japanese or Hispanic participants additionally completed ethnic specific food frequency questionnaires. In SWAN, FFQ was administered at baseline (1996-1997). Spearman correlation coefficients for the food items used in our analysis between these two visits ranged from 0.52 to 0.66, suggesting moderate to good correlations. Based on previous studies (Christensen et al. 2017; Jain 2014; Sun et al. 2018; Wu et al. 2015), we selected the intake of fish (fried fish or fish sandwich, tuna, shellfish, other fish, and Japanese-style whole fish or canned fish (Japanese only)), dairy products (milk, cheese, cheese dishes, yogurt and ice cream), Pizza, salty snacks (e.g. potato chips, corn chips, popcorn and crackers), and French fries and fried potatoes. For each food group or item, we calculated the weekly frequency of consumption. Because only a small proportion of women reported no consumption of fish or dairy food, we categorized them into tertiles. The other items were categorized into none, low (less than or equal to the median intake among those who consumed the food) and high intake (above median intake among those who consumed the food).
Statistical Analysis
All analyses were conducted using R (version 3.5.1). Characteristics of the study sample (mean and standard deviation for continuous variables, count and percent for categorical variables) were reported by site. Because PFAS were not normally distributed, we computed geometric means (geometric standard deviations) and percentiles of each PFAS analytes. Pairwise Spearman correlation coefficients were computed.
We computed geometric means (GMs) and 95% confidence intervals (CIs) of each PFAS compound by characteristics. Differences were tested using analysis of variance for nominal variables and tests for linear trends for ordinal variables. To identify important determinants of each PFAS compound, we conducted linear regressions with backward elimination where log-transformed PFAS was regressed on all pre-specified determinants (age, BMI, site, race/ethnicity, education, financial hardship, occupation, birthplace, parity, any menstrual bleeding since last visit, smoking status, alcohol consumption, and frequencies of consumption of fish, dairy, pizza, salty snack, and French fries). Model comparisons (i.e., elimination of predictors) were determined by the Akaike information criterion (AIC) (the step () function in R, a model with smaller AIC was preferred). We computed adjusted ratios of GMs and 95% CIs for the selected variables by back-transforming (i.e., exponentiating) the regression parameters (β coefficients). We also computed partial R2 (the coefficient of partial determination, defined as sum of squares (SS) for each term divided by the SS for each term and SS for error) for each selected variable using rsq. partial () from the package ‘rsq’ that assess the partial effect of each variable by comparing a model with the full set of covariates to a model excluding that particular variable (Zhang 2017). To evaluate geographic differences (site) by race/ethnicity, we computed adjusted least-squared GMs (LSGMs) and 95% CIs using the best model selected, with site and race/ethnicity combined into a 10-level variable (5 sites for white; 3 sites for black; one site for Chinese and Japanese each). This model is equivalent to a model including main effects of site and race/ethnicity along with interaction terms between site and race/ethnicity, allowing us to compare racial/ethnic effects within site or site effects within either white or black women. The LSGMs are the expected GMs of PFAS for women of different racial/ethnic groups at different sites, with all other covariate effects fixed (in our case, age 45-50, overweight, some college education, not hard to pay for basics, not born in the U.S., nulliparous, no menstrual bleeding since last visit, never smokers, infrequent alcohol use, low intake of fish and dairy, and high intake of pizza, salty snacks and French fries).
As a sensitivity analysis, we conducted stratified analyses by race/ethnicity to evaluate whether the associations for the selected determinants are consistent in different racial/ethnic groups.
RESULTS
The mean age of participants included in the current study (Visit 3) was 49.5 (SD=2.6) years (Table 1), which was generally similar by study site. The proportions of each race/ethnic group were 51.0% for white, 20.5% for black, 13.2% for Chinese, and 15.3% for Japanese. In Pittsburgh and Boston, more than 60% of women were white, whereas black women represented 57.4% of participants in Southeast Michigan. Chinese and Japanese women were solely recruited from Oakland and Los Angeles, respectively. Women from Southeast Michigan were more likely to be obese, less educated, had more difficulty to pay for basics, and were current smokers compared with women from other sites.
Table 1.
Characteristics of study participants by study site.
| Characteristics | All (n=1302) |
Oakland, CA (n=300) |
Los Angeles, CA (n=354) |
Southeast Michigan (n=230) |
Pittsburgh, PA (n=217) |
Boston, MA (n=201) |
|---|---|---|---|---|---|---|
| Age, mean ± SD, year | 49.5 ± 2.6 | 49.6 ± 2.7 | 49.7 ± 2.6 | 49.3 ± 2.7 | 49.4 ± 2.5 | 49.7 ± 2.6 |
| BMI, mean ± SD, kg/m2 | 27.9 ± 7.2 | 25.8 ± 6.4 | 25.1 ± 5.3 | 32.7 ± 7.7 | 29.1 ± 6.6 | 29.3 ± 7.9 |
| Race/ethnicity | ||||||
| White | 664 (51.0) | 128 (42.7) | 155 (43.8) | 98 (42.6) | 151 (69.6) | 132 (65.7) |
| Black | 267 (20.5) | 0 (0.0) | 0 (0.0) | 132 (57.4) | 66 (30.4) | 69 (34.3) |
| Chinese | 172 (13.2) | 172 (57.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Japanese | 199 (15.3) | 0 (0.0) | 199 (56.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Education | ||||||
| High school or less | 234 (18.0) | 51 (17.0) | 46 (13.0) | 63 (27.4) | 48 (22.1) | 26 (12.9) |
| Some college | 416 (32.0) | 69 (23.0) | 121 (34.2) | 98 (42.6) | 74 (34.1) | 54 (26.9) |
| College | 319 (24.5) | 90 (30.0) | 104 (29.4) | 32 (13.9) | 42 (19.4) | 51 (25.4) |
| Postgraduate | 333 (25.6) | 90 (30.0) | 83 (23.4) | 37 (16.1) | 53 (24.4) | 70 (34.8) |
| Hardship to pay for basics | ||||||
| Not hard at all | 905 (69.5) | 219 (73.0) | 262 (74.0) | 141 (61.3) | 151 (69.6) | 132 (65.7) |
| Somewhat hard | 316 (24.3) | 69 (23.0) | 79 (22.3) | 59 (25.7) | 54 (24.9) | 55 (27.4) |
| Very hard | 81 (6.2) | 12 (4.0) | 13 (3.7) | 30 (13.0) | 12 (5.5) | 14 (7.0) |
| Occupation | ||||||
| White collar | 600 (46.1) | 158 (52.7) | 157 (44.4) | 75 (32.6) | 95 (43.8) | 115 (57.2) |
| Pink collar | 412 (31.6) | 92 (30.7) | 111 (31.4) | 74 (32.2) | 79 (36.4) | 56 (27.9) |
| Blue collar | 94 (7.2) | 20 (6.7) | 20 (5.6) | 31 (13.5) | 15 (6.9) | 8 (4.0) |
| Unemployed | 196 (15.1) | 30 (10.0) | 66 (18.6) | 50 (21.7) | 28 (12.9) | 22 (10.9) |
| Born in the US | ||||||
| No | 254 (19.5) | 125 (41.7) | 108 (30.5) | 1 (0.4) | 4 (1.8) | 16 (8) |
| Yes | 1048 (80.5) | 175 (58.3) | 246 (69.5) | 229 (99.6) | 213 (98.2) | 185 (92) |
| Parity | ||||||
| Nulliparous | 253 (19.4) | 57 (19.0) | 69 (19.5) | 36 (15.7) | 34 (15.7) | 57 (28.4) |
| 1-2 | 687 (52.8) | 177 (59.0) | 203 (57.3) | 93 (40.4) | 114 (52.5) | 100 (49.8) |
| ≥3 | 362 (27.8) | 66 (22.0) | 82 (23.2) | 101 (43.9) | 69 (31.8) | 44 (21.9) |
| Any menstrual bleeding since last visit | ||||||
| No | 177 (13.6) | 45 (15.0) | 37 (10.5) | 33 (14.3) | 29 (13.4) | 33 (16.4) |
| Yes | 1125 (86.4) | 255 (85.0) | 317 (89.5) | 197 (85.7) | 188 (86.6) | 168 (83.6) |
| Smoking status | ||||||
| Never | 824 (63.3) | 234 (78.0) | 232 (65.5) | 128 (55.7) | 127 (58.5) | 103 (51.2) |
| Former | 349 (26.8) | 59 (19.7) | 90 (25.4) | 60 (26.1) | 68 (31.3) | 72 (35.8) |
| Current | 129 (9.9) | 7 (2.3) | 32 (9.0) | 42 (18.3) | 22 (10.1) | 26 (12.9) |
| Alcohol consumption | ||||||
| <1/month | 684 (52.5) | 190 (63.3) | 175 (49.4) | 142 (61.7) | 109 (50.2) | 68 (33.8) |
| ≥1/month and <2/week | 311 (23.9) | 50 (16.7) | 85 (24.0) | 49 (21.3) | 66 (30.4) | 61 (30.3) |
| ≥2/week | 307 (23.6) | 60 (20.0) | 94 (26.6) | 39 (17.0) | 42 (19.4) | 72 (35.8) |
| Fish consumptiona | ||||||
| Tertile 1 | 441 (33.9) | 102 (34.0) | 103 (29.1) | 80 (34.8) | 80 (36.9) | 76 (37.8) |
| Tertile 2 | 438 (33.6) | 101 (33.7) | 115 (32.5) | 74 (32.2) | 80 (36.9) | 68 (33.8) |
| Tertile 3 | 423 (32.5) | 97 (32.3) | 136 (38.4) | 76 (33.0) | 57 (26.3) | 57 (28.4) |
| Dairy consumptionb | ||||||
| Tertile 1 | 434 (33.3) | 108 (36.0) | 137 (38.7) | 82 (35.7) | 58 (26.7) | 49 (24.4) |
| Tertile 2 | 434 (33.3) | 105 (35.0) | 121 (34.2) | 87 (37.8) | 70 (32.3) | 51 (25.4) |
| Tertile 3 | 434 (33.3) | 87 (29.0) | 96 (27.1) | 61 (26.5) | 89 (41.0) | 101 (50.2) |
| Pizza consumption | ||||||
| Never or <1/month | 283 (21.7) | 86 (28.7) | 81 (22.9) | 52 (22.6) | 30 (13.8) | 34 (16.9) |
| 1/month | 403 (31.0) | 96 (32.0) | 123 (34.7) | 73 (31.7) | 41 (18.9) | 70 (34.8) |
| ≥2/month | 616 (47.3) | 118 (39.3) | 150 (42.4) | 105 (45.7) | 146 (67.3) | 97 (48.3) |
| Salty snacks consumption | ||||||
| Never or <1/month | 230 (17.7) | 80 (26.7) | 66 (18.6) | 35 (15.2) | 24 (11.1) | 25 (12.4) |
| 1-2/month | 401 (30.8) | 105 (35.0) | 119 (33.6) | 66 (28.7) | 62 (28.6) | 49 (24.4) |
| ≥1/week | 671 (51.5) | 115 (38.3) | 169 (47.7) | 129 (56.1) | 131 (60.4) | 127 (63.2) |
| French fries | ||||||
| Never or <1/month | 364 (28.0) | 109 (36.3) | 87 (24.6) | 53 (23.0) | 50 (23.0) | 65 (32.3) |
| 1/month | 278 (21.4) | 63 (21.0) | 76 (21.5) | 44 (19.1) | 39 (18.0) | 56 (27.9) |
| ≥2/month | 660 (50.7) | 128 (42.7) | 191 (54.0) | 133 (57.8) | 128 (59.0) | 80 (39.8) |
All covariates, except age, fish consumption, and menstrual bleeding since last visit, were statistically significantly different by site.
For fish consumption, the tertile breakpoints are <1/week (tertile 1), 1-2/week (tertile 2), >2/week (tertile 3).
For dairy consumption, tertile breakpoints are <1/day (tertile 1), 1-2/day (tertile 2), >2/day (tertile 3).
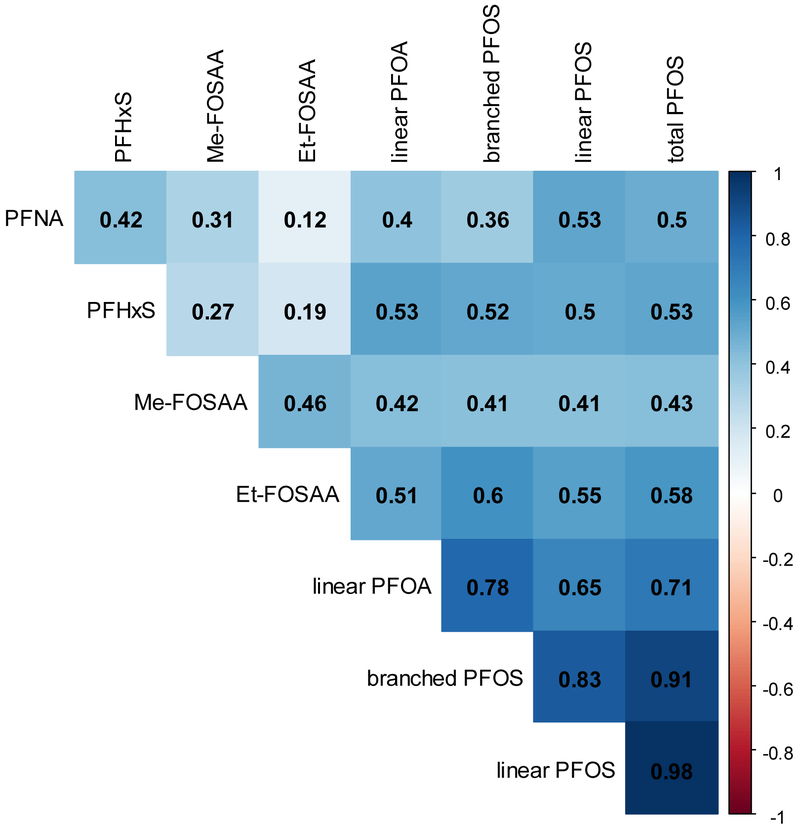
Table 2 shows the distributions of PFAS. Linear PFOA, PFNA, PFHxS, both linear and branched PFOS, and two precursors of PFOS were detected in over 97% of women. Longer chain perfluoroalkyl carboxylic acids (PFDA (40.6%), PFUA (31.3%), PFDoA (3.8%)) and branched PFOA (18.5%) were detected less frequently, and therefore, were not considered in subsequent analyses. Most PFAS were modestly correlated each other (Figure 1). Relatively strong correlations were found between linear PFOS and branched PFOS (Spearman correlation coefficient=0.83), linear PFOA and branched PFOS (0.78), linear PFOA and linear PFOS (0.65), and Et-FOSAA and branched PFOS (0.60). Weak correlations were found between Et-FOSAA and PFNA (0.12) and PFHxS (0.19).
Table 2.
Distributions of PFAS concentrations (ng/mL) in serum collected in 1999-2000 in the Study of Women’s Health Across the Nation.
| Analyte | Detection (%) | GM (GSD) | Percentiles | |||||
|---|---|---|---|---|---|---|---|---|
| LOD=0.1 | 5th | 25th | 50th | 75th | 95th | Maximum | ||
| Perfluoroalkyl carboxylic acids (PFCAs) | ||||||||
| PFOA (total) | 4.18 (1.80) | 1.6 | 2.9 | 4.3 | 6.0 | 10.8 | 56.5 | |
| Linear | 100 | 4.07 (1.79) | 1.5 | 2.9 | 4.1 | 5.8 | 10.3 | 56.5 |
| Branched | 18.5 | 0.11 (2.46) | <LOD | <LOD | <LOD | <LOD | 1 | 5.8 |
| PFNA | 97.2 | 0.55 (1.81) | 0.2 | 0.4 | 0.6 | 0.8 | 1.3 | 4.4 |
| PFDA | 40.6 | 0.13 (2.17) | <LOD | <LOD | <LOD | 0.3 | 0.5 | 2.6 |
| PFUA | 31.3 | 0.12 (2.24) | <LOD | <LOD | <LOD | 0.2 | 0.6 | 3.8 |
| PFDoA | 3.8 | 0.08 (1.38) | <LOD | <LOD | <LOD | <LOD | <LOD | 2.6 |
| Perfluoroalkane sulfonic acids (PFSAs) | ||||||||
| PFHxS | 99.6 | 1.58 (2.22) | 0.5 | 1.0 | 1.5 | 2.3 | 6.9 | 46.5 |
| PFOS (total) | 25.44 (1.79) | 10.4 | 17.6 | 24.9 | 35.8 | 68.5 | 376.0 | |
| Linear | 100 | 17.85 (1.78) | 7.3 | 12.4 | 17.5 | 24.8 | 48.2 | 250.0 |
| Branched | 99.8 | 7.23 (2.00) | 2.6 | 4.7 | 7.4 | 11.2 | 21.8 | 126.0 |
| Perfluoroalkane sulfonamide substances | ||||||||
| Me-FOSAA | 99.6 | 1.45 (2.04) | 0.5 | 0.9 | 1.5 | 2.3 | 4.5 | 13.7 |
| Et-FOSAA | 98.9 | 1.24 (2.53) | 0.3 | 0.7 | 1.2 | 2.2 | 6.0 | 112.5 |
LOD, limit of detection; GM, geometric mean; GSD, geometric standard deviation; PFOA, perfluorooctanoic acid; PFNA, perfluorononanoic acid; PFDA, perfluorodecanoic acid; PFUA, perfluoroundecanoic acid; PFDoA, perfluorododecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFOS, perfluorooctane sulfonic acid; Me-FOSAA, 2-(N-methyl-perfluorooctane sulfonamido) acetic acid; and Et-FOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid.
To compute GMs for the compounds with values below LOD, those values were replaced with LOD/√2.
Figure 1.
A correlation heatmap. Spearman correlation coefficients are shown. More intense color indicates a stronger correlation. PFOA, perfluorooctanoic acid; PFNA, perfluorononanoic acid; PFDA, perfluorodecanoic acid; PFUA, perfluoroundecanoic acid; PFDoA, perfluorododecanoic acid; PFHxS, perfluorohexane sulfonic acid; PFOS, perfluorooctane sulfonic acid; Me-FOSAA, 2-(N-methyl-perfluorooctane sulfonamido) acetic acid; and Et-FOSAA, 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid.
Unadjusted GMs of PFAS by characteristics are presented in Table 3. Overall, study site, race/ethnicity, and born in the U.S. were significantly associated with all PFAS compounds. Oakland had the lowest concentrations consistently in all PFAS, whereas Southeast Michigan had the highest concentrations of linear PFOS and total PFOS, and Pittsburgh had the highest concentrations of linear PFOA, branched PFOS, Me-FOSAA, and Et-FOSAA. Black women had the highest concentrations in both linear and branched PFOS and PFOS precursors, whereas Chinese women had the lowest concentrations in all PFASs except PFNA. White women had the highest concentrations in linear PFOA and Japanese women had the highest concentration in PFNA. Women who were born in the U.S. had higher concentrations of all PFAS except PFNA. Concentrations of all PFAS except linear PFOS and total PFAS significantly increased with parity. Women who had experienced any menstrual bleeding since last visit had lower concentrations of PFAS except PFOS precursors. There were significant increasing trends in PFAS concentrations across BMI (all except PFNA); cigarette smoking status (highest in current smokers and lowest in never smokers in all PFASs except PFNA which was borderline significant); intake of salty snack (all except PFNA and PFHxS); French fries (all except PFNA, PFHxS, and linear PFOS); pizza (all except PFNA, linear PFOS, and total PFOS); alcohol (linear PFOA, PFNA, PFHxS). There were significant decreasing trends in PFAS concentrations across intake of fish (linear PFOA, branched PFOS, total PFOS, and Et-FOSAA). Interestingly, PFNA showed the opposite trend with lower concentrations associated with higher intake of pizza, salty snacks, and French fries, while higher concentrations were associated with higher intake of fish and alcohol.
Table 3.
Unadjusted Geometric means (95% confidence intervals) of PFAS (ng/mL) by characteristics in serum collected in 1999-2000 in the Study of Women’s Health Across the Nation.
| N | PFOA (linear) | PFNA | PFHxS | PFOS (linear) | PFOS (branched) |
PFOS (total) | Me-FOSAA | Et-FOSAA | |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| 45-50 | 832 | 4.13 (3.97, 4.30) |
0.54 (0.52, 0.56) |
1.59 (1.51, 1.68) |
18.22 (17.53, 18.95) |
7.37 (7.03, 7.74) |
25.97 (24.97, 27.02) |
1.52 (1.45, 1.59) |
1.30 (1.22, 1.39) |
| 51-56 | 470 | 3.96 (3.77, 4.17) |
0.57 (0.54, 0.60) |
1.55 (1.44, 1.67) |
17.22 (16.33, 18.16) |
6.97 (6.57, 7.41) |
24.52 (23.26, 25.86) |
1.33 (1.24, 1.43) |
1.14 (1.05, 1.24) |
| P-value | 0.23 | 0.12 | 0.59 | 0.09 | 0.16 | 0.09 | 0.001 | 0.01 | |
| BMI | |||||||||
| Normal or underweight | 553 | 3.68 (3.50, 3.88) |
0.55 (0.52, 0.58) |
1.46 (1.36, 1.56) |
16.21 (15.46, 16.99) |
6.27 (5.92, 6.64) |
22.82 (21.75, 23.93) |
1.31 (1.23, 1.40) |
1.08 (0.99, 1.17) |
| Overweight | 348 | 4.18 (3.96, 4.41) |
0.58 (0.55, 0.61) |
1.58 (1.46, 1.7) |
17.88 (16.84, 18.98) |
7.23 (6.71, 7.79) |
25.49 (23.99, 27.08) |
1.45 (1.35, 1.56) |
1.24 (1.12, 1.37) |
| Obese | 401 | 4.56 (4.32, 4.82) |
0.52 (0.48, 0.55) |
1.77 (1.63, 1.92) |
20.37 (19.25, 21.56) |
8.78 (8.24, 9.35) |
29.51 (27.89, 31.23) |
1.66 (1.56, 1.77) |
1.51 (1.39, 1.64) |
| P-value | <0.0001 | 0.09 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Site | |||||||||
| Oakland, CA | 300 | 2.91 (2.73, 3.11) |
0.49 (0.46, 0.52) |
1.23 (1.13, 1.34) |
15.80 (14.93, 16.73) |
5.64 (5.21, 6.11) |
21.87 (20.63, 23.18) |
1.05 (0.98, 1.14) |
0.90 (0.81, 1.00) |
| Los Angeles, CA | 354 | 4.35 (4.12, 4.59) |
0.54 (0.51, 0.58) |
1.31 (1.22, 1.41) |
16.28 (15.41, 17.21) |
7.48 (7.00, 8.00) |
24.00 (22.68, 25.40) |
1.51 (1.41, 1.62) |
1.39 (1.27, 1.51) |
| Southeast Michigan | 230 | 4.32 (4.00, 4.67) |
0.54 (0.49, 0.59) |
2.07 (1.84, 2.32) |
22.50 (20.71, 24.46) |
8.47 (7.74, 9.27) |
31.31 (28.81, 34.02) |
1.88 (1.73, 2.05) |
1.55 (1.37, 1.74) |
| Pittsburgh, PA | 217 | 5.21 (4.82, 5.64) |
0.59 (0.55, 0.64) |
2.09 (1.87, 2.34) |
20.67 (18.96, 22.53) |
8.55 (7.71, 9.48) |
29.78 (27.34, 32.44) |
1.95 (1.79, 2.12) |
1.63 (1.44, 1.85) |
| Boston, MA | 201 | 4.26 (3.99, 4.55) |
0.62 (0.58, 0.66) |
1.72 (1.56, 1.90) |
16.49 (15.37, 17.70) |
6.84 (6.32, 7.42) |
23.50 (21.87, 25.25) |
1.16 (1.04, 1.30) |
0.95 (0.84, 1.09) |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Race/ethnicity | |||||||||
| White | 664 | 4.62 (4.44, 4.82) |
0.51 (0.49, 0.53) |
1.82 (1.72, 1.94) |
17.30 (16.55, 18.09) |
7.84 (7.44, 8.26) |
25.45 (24.34, 26.62) |
1.44 (1.36, 1.52) |
1.28 (1.19, 1.37) |
| Black | 267 | 4.30 (4.01, 4.61) |
0.59 (0.54, 0.64) |
1.82 (1.64, 2.02) |
23.46 (21.80, 25.25) |
8.53 (7.86, 9.27) |
32.36 (30.06, 34.83) |
1.99 (1.85, 2.15) |
1.62 (1.44, 1.81) |
| Chinese | 172 | 2.26 (2.10, 2.43) |
0.57 (0.52, 0.62) |
1.02 (0.91, 1.13) |
15.34 (14.32, 16.42) |
4.44 (4.05, 4.86) |
20.09 (18.77, 21.50) |
0.92 (0.83, 1.02) |
0.72 (0.64, 0.81) |
| Japanese | 199 | 4.11 (3.84, 4.39) |
0.61 (0.56, 0.65) |
1.18 (1.08, 1.29) |
15.67 (14.64, 16.78) |
6.72 (6.17, 7.31) |
22.57 (21.01, 24.24) |
1.43 (1.31, 1.56) |
1.26 (1.13, 1.41) |
| P-value | <0.0001 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Education | |||||||||
| ≤High school | 234 | 3.66 (3.37, 3.98) |
0.57 (0.52, 0.61) |
1.52 (1.37, 1.69) |
18.86 (17.44, 20.41) |
7.00 (6.38, 7.68) |
26.31 (24.31, 28.48) |
1.50 (1.36, 1.66) |
1.21 (1.06, 1.37) |
| Some college | 416 | 4.31 (4.07, 4.56) |
0.56 (0.53, 0.60) |
1.60 (1.48, 1.73) |
19.06 (17.99, 20.20) |
7.72 (7.22, 8.25) |
27.18 (25.66, 28.79) |
1.62 (1.51, 1.73) |
1.35 (1.24, 1.47) |
| College | 319 | 3.83 (3.59, 4.08) |
0.51 (0.48, 0.55) |
1.41 (1.30, 1.53) |
16.04 (15.08, 17.06) |
6.45 (5.93, 7.00) |
22.88 (21.47, 24.38) |
1.32 (1.22, 1.43) |
1.14 (1.03, 1.26) |
| Postgraduate | 333 | 4.33 (4.10, 4.57) |
0.55 (0.52, 0.58) |
1.77 (1.62, 1.94) |
17.53 (16.57, 18.56) |
7.59 (7.12, 8.10) |
25.33 (23.91, 26.83) |
1.34 (1.25, 1.45) |
1.24 (1.12, 1.37) |
| P-value | 0.04 | 0.27 | 0.11 | 0.007 | 0.88 | 0.04 | 0.001 | 0.55 | |
| Hardship to pay for basics | |||||||||
| Not hard at all | 905 | 4.11 (3.96, 4.27) |
0.55 (0.53, 0.58) |
1.61 (1.53, 1.70) |
17.75 (17.09, 18.43) |
7.28 (6.96, 7.62) |
25.36 (24.41, 26.36) |
1.43 (1.37, 1.50) |
1.24 (1.17, 1.31) |
| Somewhat hard | 316 | 3.94 (3.67, 4.21) |
0.53 (0.49, 0.57) |
1.46 (1.34, 1.59) |
17.43 (16.38, 18.56) |
6.91 (6.39, 7.46) |
24.77 (23.26, 26.38) |
1.44 (1.33, 1.55) |
1.22 (1.10, 1.35) |
| Very hard | 81 | 4.12 (3.63, 4.66) |
0.55 (0.49, 0.63) |
1.66 (1.41, 1.95) |
20.92 (18.48, 23.69) |
7.89 (6.92, 8.99) |
29.19 (25.87, 32.95) |
1.68 (1.41, 2.01) |
1.37 (1.08, 1.73) |
| P-value | 0.49 | 0.42 | 0.34 | 0.14 | 1.00 | 0.27 | 0.15 | 0.61 | |
| Occupation | |||||||||
| White collar | 612 | 4.10 (3.92, 4.29) |
0.54 (0.52, 0.57) |
1.62 (1.52, 1.72) |
17.50 (16.73, 18.30) |
7.12 (6.73, 7.53) |
24.97 (23.86, 26.13) |
1.40 (1.32, 1.48) |
1.25 (1.16, 1.34) |
| Pink collar | 420 | 4.04 (3.83, 4.27) |
0.54 (0.51, 0.57) |
1.53 (1.42, 1.65) |
18.00 (17.03, 19.03) |
7.52 (7.05, 8.03) |
25.86 (24.45, 27.35) |
1.47 (1.37, 1.57) |
1.23 (1.13, 1.35) |
| Blue collar | 100 | 3.90 (3.38, 4.50) |
0.60 (0.52, 0.69) |
1.61 (1.36, 1.92) |
19.94 (17.55, 22.65) |
6.68 (5.76, 7.76) |
27.32 (24.10, 30.97) |
1.54 (1.32, 1.81) |
1.23 (1.00, 1.50) |
| Unemployed | 201 | 4.10 (3.75, 4.48) |
0.57 (0.52, 0.63) |
1.57 (1.40, 1.76) |
17.74 (16.34, 19.26) |
7.19 (6.57, 7.87) |
25.19 (23.20, 27.36) |
1.57 (1.41, 1.75) |
1.24 (1.09, 1.40) |
| P-value | 0.87 | 0.27 | 0.74 | 0.21 | 0.40 | 0.48 | 0.19 | 0.99 | |
| Born in the US | |||||||||
| No | 254 | 2.76 (2.56, 2.97) |
0.65 (0.61, 0.69) |
1.12 (1.03, 1.21) |
14.74 (13.83, 15.72) |
4.74 (4.38, 5.13) |
19.79 (18.57, 21.10) |
1.04 (0.95, 1.14) |
0.78 (0.69, 0.87) |
| Yes | 1048 | 4.47 (4.33, 4.62) |
0.53 (0.51, 0.54) |
1.72 (1.64, 1.8) |
18.70 (18.05, 19.37) |
8.00 (7.69, 8.33) |
27.04 (26.10, 28.01) |
1.57 (1.51, 1.64) |
1.39 (1.32, 1.47) |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Parity | |||||||||
| Nulliparous | 253 | 4.57 (4.26, 4.9) |
0.57 (0.53, 0.62) |
1.87 (1.7, 2.05) |
18.20 (16.99, 19.51) |
7.82 (7.15, 8.55) |
26.37 (24.57, 28.31) |
1.28 (1.17, 1.39) |
1.10 (0.98, 1.23) |
| 1-2 | 687 | 4.03 (3.86, 4.22) |
0.56 (0.53, 0.58) |
1.57 (1.47, 1.66) |
18.03 (17.26, 18.85) |
7.25 (6.88, 7.63) |
25.68 (24.57, 26.84) |
1.43 (1.35, 1.51) |
1.28 (1.19, 1.37) |
| ≥3 | 362 | 3.81 (3.60, 4.04) |
0.51 (0.48, 0.55) |
1.43 (1.32, 1.55) |
17.28 (16.28, 18.33) |
6.81 (6.36, 7.28) |
24.38 (22.97, 25.87) |
1.62 (1.51, 1.74) |
1.28 (1.16, 1.41) |
| P-value | 0.0001 | 0.03 | <0.0001 | 0.31 | 0.02 | 0.12 | <0.0001 | 0.04 | |
| Any menstrual bleeding since last visit | |||||||||
| NO | 177 | 4.57 (4.20, 4.98) |
0.64 (0.59, 0.69) |
1.97 (1.76, 2.21) |
19.51 (17.89, 21.28) |
8.07 (7.32, 8.89) |
27.94 (25.66, 30.42) |
1.49 (1.33, 1.67 |
1.09 (0.95, 1.24) |
| Yes | 1125 | 3.99 (3.86, 4.13) |
0.54 (0.52, 0.55) |
1.53 (1.46, 1.6) |
17.60 (17.02, 18.21) |
7.10 (6.82, 7.40) |
25.07 (24.23, 25.94) |
1.44 (1.38, 1.50) |
1.27 (1.20, 1.34) |
| P-value | 0.004 | 0.0003 | <0.0001 | 0.03 | 0.02 | 0.02 | 0.59 | 0.04 | |
| Smoking status | |||||||||
| Never | 824 | 3.75 (3.60, 3.91) |
0.54 (0.51, 0.56) |
1.47 (1.39, 1.55) |
17.27 (16.62, 17.94) |
6.75 (6.43, 7.10) |
24.41 (23.47, 25.38) |
1.38 (1.32, 1.45) |
1.19 (1.11, 1.26) |
| Former | 349 | 4.64 (4.38, 4.91) |
0.56 (0.53, 0.59) |
1.77 (1.62, 1.93) |
18.61 (17.46, 19.84) |
8.05 (7.53, 8.61) |
26.97 (25.32, 28.73) |
1.49 (1.38, 1.60) |
1.34 (1.22, 1.48) |
| Current | 129 | 4.79 (4.32, 5.31) |
0.60 (0.54, 0.66) |
1.84 (1.60, 2.13) |
19.73 (17.75, 21.94) |
8.29 (7.44, 9.24) |
28.32 (25.52, 31.42) |
1.82 (1.61, 2.07) |
1.33 (1.13, 1.58) |
| P-value | <0.0001 | 0.12 | <0.0001 | 0.01 | <0.0001 | 0.002 | 0.0002 | 0.08 | |
| Alcohol | |||||||||
| < 1/month | 684 | 3.81 (3.64, 3.99) |
0.53 (0.50, 0.55) |
1.47 (1.38, 1.56) |
18.12 (17.34, 18.93) |
7.21 (6.84, 7.59) |
25.69 (24.58, 26.85) |
1.43 (1.36, 1.51) |
1.23 (1.15, 1.32) |
| ≥1/month and < 2/week | 311 | 4.31 (4.04, 4.59) |
0.55 (0.52, 0.59) |
1.63 (1.49, 1.79) |
17.51 (16.42, 18.68) |
7.44 (6.92, 8.00) |
25.22 (23.64, 26.92) |
1.51 (1.39, 1.64) |
1.34 (1.20, 1.49) |
| ≥2/week | 307 | 4.44 (4.18, 4.73) |
0.59 (0.56, 0.63) |
1.80 (1.66, 1.96) |
17.61 (16.52, 18.78) |
7.05 (6.49, 7.66) |
25.10 (23.53, 26.77) |
1.42 (1.32, 1.53) |
1.16 (1.06, 1.28) |
| P-value | <0.0001 | 0.005 | 0.0001 | 0.41 | 0.77 | 0.53 | 0.97 | 0.56 | |
| Fish consumptiona | |||||||||
| Tertile 1 | 441 | 4.37 (4.14, 4.61) |
0.51 (0.48, 0.53) |
1.65 (1.53, 1.78) |
18.50 (17.51, 19.56) |
7.95 (7.43, 8.50) |
26.79 (25.33, 28.34) |
1.51 (1.41, 1.62) |
1.38 (1.27, 1.51) |
| Tertile 2 | 438 | 4.04 (3.83, 4.27) |
0.54 (0.51, 0.56) |
1.55 (1.44, 1.68) |
17.42 (16.53, 18.37) |
7.13 (6.68, 7.60) |
24.90 (23.61, 26.26) |
1.42 (1.34, 1.52) |
1.20 (1.10, 1.30) |
| Tertile 3 | 423 | 3.80 (3.60, 4.03) |
0.61 (0.57, 0.65) |
1.53 (1.42, 1.65) |
17.64 (16.68, 18.64) |
6.64 (6.23, 7.07) |
24.65 (23.32, 26.05) |
1.41 (1.31, 1.51) |
1.15 (1.05, 1.26) |
| P-value | 0.001 | <0.0001 | 0.46 | 0.28 | 0.001 | 0.06 | 0.05 | 0.01 | |
| Dairy consumptionb | |||||||||
| Tertile 1 | 434 | 3.88 (3.65, 4.12) |
0.57 (0.54, 0.61) |
1.37 (1.27, 1.48) |
18.84 (17.76, 19.99) |
7.11 (6.63, 7.63) |
26.36 (24.83, 27.98) |
1.58 (1.47, 1.70) |
1.29 (1.18, 1.41) |
| Tertile 2 | 434 | 4.08 (3.87, 4.30) |
0.54 (0.51, 0.57) |
1.61 (1.50, 1.73) |
17.90 (17.00, 18.84) |
7.36 (6.90, 7.86) |
25.64 (24.33, 27.01) |
1.43 (1.34, 1.53) |
1.25 (1.15, 1.36) |
| Tertile 3 | 434 | 4.26 (4.05, 4.48) |
0.53 (0.50, 0.56) |
1.78 (1.65, 1.93) |
16.87 (16.01, 17.78) |
7.21 (6.79, 7.65) |
24.36 (23.11, 25.68) |
1.34 (1.26, 1.43) |
1.18 (1.08, 1.29) |
| P-value | 0.01 | 0.30 | <0.0001 | 0.003 | 0.66 | 0.04 | 0.005 | 0.44 | |
| Pizza consumption | |||||||||
| Never or <1/month | 283 | 3.64 (3.37, 3.93) |
0.58 (0.54, 0.63) |
1.48 (1.34, 1.64) |
18.02 (16.72, 19.41) |
6.62 (6.05, 7.25) |
25.14 (23.33, 27.08) |
1.31 (1.19, 1.44) |
1.07 (0.95, 1.21) |
| 1/month | 403 | 4.01 (3.79, 4.24) |
0.56 (0.53, 0.60) |
1.55 (1.44, 1.67) |
17.65 (16.68, 18.67) |
7.12 (6.70, 7.58) |
25.06 (23.69, 26.51) |
1.39 (1.29, 1.49) |
1.21 (1.11, 1.33) |
| ≥2/month | 616 | 4.32 (4.14, 4.52) |
0.52 (0.50, 0.55) |
1.64 (1.54, 1.75) |
17.91 (17.15, 18.71) |
7.59 (7.19, 8.02) |
25.83 (24.7, 27.01) |
1.56 (1.48, 1.64) |
1.35 (1.26, 1.44) |
| P-value | <0.0001 | 0.007 | 0.06 | 0.99 | 0.005 | 0.42 | 0.0002 | 0.0005 | |
| Salty snacks consumption | |||||||||
| Never or <1/month | 230 | 3.18 (2.94, 3.44) |
0.60 (0.55, 0.65) |
1.47 (1.33, 1.62) |
15.69 (14.57, 16.89) |
5.51 (5.04, 6.03) |
21.59 (20.08, 23.20) |
1.20 (1.08, 1.34) |
0.82 (0.73, 0.92) |
| 1-2/month | 401 | 3.94 (3.71, 4.18) |
0.55 (0.52, 0.58) |
1.55 (1.43, 1.68) |
17.08 (16.12, 18.09) |
6.70 (6.24, 7.20) |
24.15 (22.78, 25.60) |
1.49 (1.39, 1.60) |
1.13 (1.03, 1.23) |
| ≥1/week | 671 | 4.51 (4.33, 4.70) |
0.53 (0.51, 0.55) |
1.64 (1.54, 1.74) |
19.16 (18.36, 20.01) |
8.29 (7.90, 8.71) |
27.76 (26.59, 28.99) |
1.52 (1.45, 1.60) |
1.51 (1.41, 1.62) |
| P-value | <0.0001 | 0.02 | 0.07 | <0.0001 | <0.0001 | <0.0001 | 0.002 | <0.0001 | |
| French fries consumption | |||||||||
| Never or <1/month | 364 | 3.68 (3.46, 3.92) |
0.56 (0.52, 0.59) |
1.49 (1.37, 1.62) |
16.75 (15.78, 17.78) |
6.47 (6.02, 6.95) |
23.58 (22.22, 25.04) |
1.22 (1.12, 1.31) |
1.04 (0.94, 1.15) |
| 1/month | 278 | 4.12 (3.83, 4.43) |
0.58 (0.55, 0.63) |
1.63 (1.49, 1.78) |
18.41 (17.09, 19.82) |
7.26 (6.70, 7.87) |
26.02 (24.16, 28.01) |
1.37 (1.22/m6, 1.49) |
1.14 (1.02, 1.28) |
| ≥2/month | 660 | 4.28 (4.10, 4.46) |
0.53 (0.50, 0.55) |
1.61 (1.51, 1.71) |
18.25 (17.5, 19.04) |
7.67 (7.27, 8.08) |
26.28 (25.17, 27.43) |
1.63 (1.55, 1.72) |
1.41 (1.32, 1.51) |
| P-value | 0.0005 | 0.04 | 0.27 | 0.08 | 0.0006 | 0.02 | <0.0001 | <0.0001 | |
Differences were tested using analysis of variance for nominal variables and test for linear trends for ordinal variables. Statistically significant p-values (<0.05) were reported in bold.
For fish consumption, the tertile breakpoints are <1/week (tertile 1), 1-2/week (tertile 2), >2/week (tertile 3).
For dairy consumption, tertile breakpoints are <1/day (tertile 1), 1-2/day (tertile 2), >2/day (tertile 3).
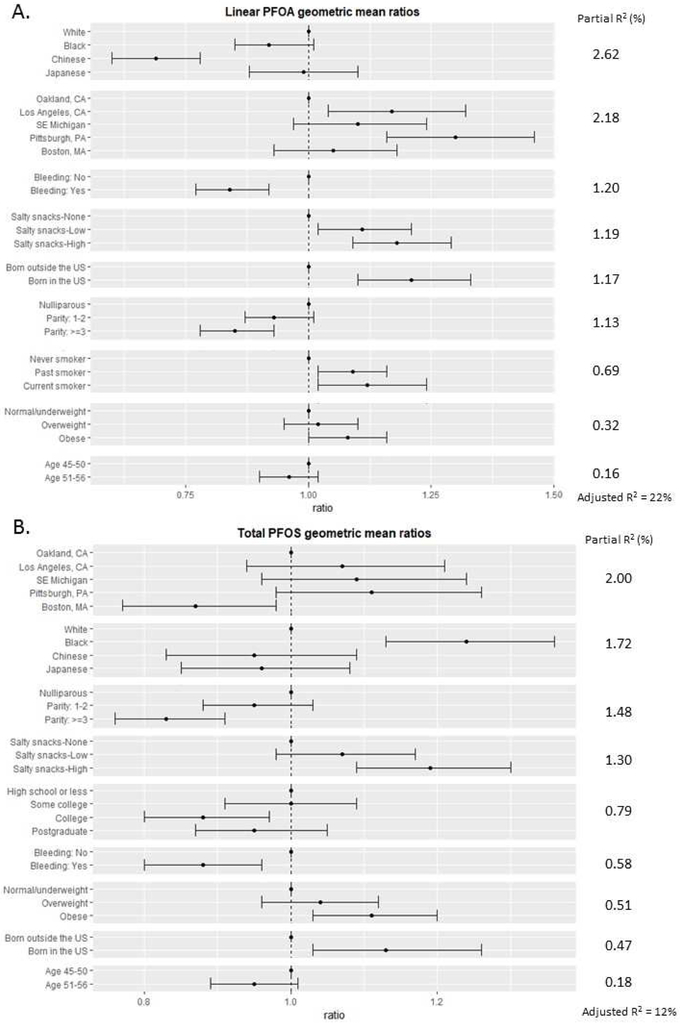
Figure 2 shows adjusted ratios of GMs by order of partial R2 of two major PFAS, linear PFOA and total PFOS, by the characteristics selected by backward elimination. For linear PFOA, race/ethnicity, site, menstrual bleeding, salty snack intake, birthplace, parity, smoking status, obesity status, and age were selected (Figure 2A). Chinese women had significantly lower concentrations of linear PFOA compared with white women who had the highest concentrations (ratio of GM=0.69, 95% CI, 0.60-0.78). Pittsburgh (ratio of GM=1.30, 95% CI, 1.16-1.46) and Los Angeles (1.17, 95% CI, 1.04-1.32) had significantly higher concentrations compared with Oakland which had the lowest concentration. Women who had experienced any menstrual bleeding since their last visit had 16% lower linear PFOA concentrations than women with no menstrual bleeding (ratio of GM=0.84, 95% CI, 0.77-0.92). Salty snack intake (an increasing trend), born in the U.S. (increasing), parity (decreasing), smoking status (increasing), obesity (increasing), and age (decreasing) remained important determinants of linear PFOA concentrations after accounting for other factors. Adjusted R2 with the selected determinants was 22%. For total PFOS, site, race/ethnicity, parity, salty snack intake, education, menstrual bleeding, obesity status, birthplace, and age by order of partial R2 were selected (Figure 2B). Although Oakland had the lowest unadjusted concentration of total PFOS, Boston had even a lower adjusted total PFOS concentration than Oakland after adjustment for other factors including race/ethnicity (ratio of GM=0.87, 95% CI, 0.77-0.98). Black women had a significantly higher concentration compared with white women (1.24, 95% CI, 1.13-1.36). Women with parity 3 or higher had 17% lower total PFOS concentrations than nulliparous women (ratio of GM=0.83, 95% CI, 0.76-0.91). Salty snack intake (increasing), education (decreasing), menstrual bleeding (decreasing), obesity (increasing), born in the U.S. (increasing), and age (decreasing) remain as important determinants of total PFOS concentrations. Adjusted R2 with the selected determinants was 12%. For other compounds, again, site and race were consistently selected as important determinants (Figure A.1). Parity (all other compounds), menstrual bleeding (all except Et-FOSAA), and birthplace (all except PHFxS) were also consistently selected. Adjusted R2’s with the selected determinants were 9.7% for PFNA; 13.4% for PFHxS; 11.1% for linear PFOS; 16.2% for branched PFOS; 17.8% for Me-FOSAA; and 15.3% for Et-FOSAS.
Figure 2.
Adjusted ratios of geometric means for Linear PFOA (A) and total PFOS (B), ordered by partial R2, from linear regression with backward elimination.
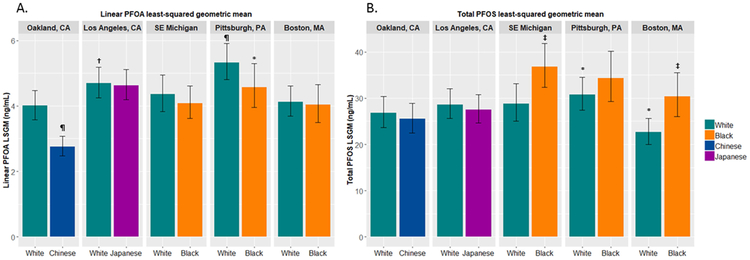
To assess independent roles of geographic location and race/ethnicity, we computed LSGMs of PFAS by study site and race/ethnicity (Figure 3 and Figure A.2). In each site, white women had consistently higher concentrations of linear PFOA than the other minority group, whereas in the sites where black women were recruited (Southeast Michigan, Pittsburgh and Boston), black women had consistently higher concentrations of total PFOS than white women (Figure 3). White women had higher concentrations of linear PFOA compared with the Chinese in Oakland (p<0.0001) and blacks in Pittsburgh (p=0.048). Black women in Southeast Michigan and Boston had higher concentrations of linear (p<0.001 for Southeast Michigan; p<0.0001 for Boston) and total PFOS (p<0.001 for both Southeast Michigan and Boston) and Me-FOSAA (p=0.02 for Southeast Michigan; p<0.001 for Boston) than white women at those sites. Chinese (Oakland) and Japanese (Los Angeles) women had higher concentrations of PFNA compared with white women in each site (p<0.01 for both). Within white women, those in Pittsburgh had relatively higher concentrations of PFAS.
Figure 3.
Least squared geometric means of linear PFOA (A) and total PFOS (B) by site and race/ethnicity, adjusted for covariates in best models selected by backward selection (see Figure 2). Symbols above the bars for White indicate a statistically significant difference comparing with Oakland white women. Symbols above the bars for non-White indicate a statistically significant difference comparing with white women within each site.
*p<0.05, †p<0.01, ‡p<0.001, ¶p<0.0001.
In race/ethnicity-stratified analyses, generally the trends did not differ meaningful from results in models with the total population (Tables A.1-A.3). Within Chinese and Japanese women, those who were born outside the US had lower concentrations of linear PFOA and total PFOS but higher PFNA concentrations.
DISCUSSION
In this study, we evaluated important determinants of PFAS concentrations in serum samples collected between 1999 and 2000 in a multi-site and multi-racial/ethnic cohort of midlife women. We found that geographic location and race/ethnicity are two major determinants observed consistently across different PFAS compounds. Associations with these characteristics were stronger than SES (education attainment or difficulty paying for basics) or behavioral factors (cigarette smoking or consumption of certain foods). Menstruation and parity were also important determinants of most PFAS, supporting the notion that menstrual bleeding and parturition may be PFAS clearance pathways in reproductive age women (Wong et al. 2014; Zhang and Qin 2014). High intake of salty snacks capturing popcorn, potato chips, corn chips, and crackers and obesity status were associated with higher serum concentrations of ‘legacy’ compounds, i.e., linear PFOA, both linear and branched PFOS, and Et-FOASS, a precursor of PFOS and a derivative of N-ethyl perfluorooctane sulfonamidoethanol (EtFOSE) used as a building block for surfactants in paper food packaging (D'Eon J and Mabury 2011).
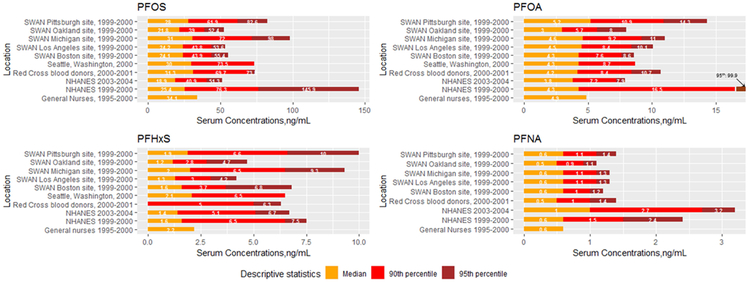
Median concentrations of PFOS and PFHxS in the SWAN Michigan and Pittsburgh sites are higher than those in women of the same age (45-56 years) in the NHANES 1999-2000, similar to those in women living in Seattle (Olsen et al. 2004) and female nurses in the Nurses Health Study (Sun et al. 2018) (Figure 4). Although the medians of PFOA in our study are comparable to that of NHANES 1999-2000, the 95th percentiles of PFOA in our population are much lower than that from NHANES 1999-2000 which may have included participants from areas where PFOA contamination of water supplies was detected, for example, the mid-Ohio River Valley (Frisbee et al. 2009). The higher 90th and 95th percentiles in NHANES 1999-2000 compared to NHANES 2003-2004 may also reflect sampling variability. Serum concentrations of PFOS and PFHxS in SWAN participants from Oakland and Los Angeles, and those of PFOA in women from Oakland seem to be lower, compared with other studies. PFNA concentrations are similar across different studies. It is notable that extreme values (i.e., 95th percentiles and maximums) of PFOS and PFHxS from Michigan and Pittsburgh and those of PFOA from Pittsburgh in this study are higher than those in other comparable studies (Seattle (Olsen et al. 2004); six American Red Cross centers (Olsen et al. 2017)). This suggests that unusually high exposures from point sources, for example, contaminated drinking water, may have happened in these areas.
Figure 4.
Median (orange), 90th percentile (red), and 95th percentile (brown) concentrations among general population women in the United States during similar periods. PFOS, PFOA, PFHxS, and PFNA serum concentrations measured in SWAN between 1999-2000 are compared to NHANES 1999-2000 (n=91) and 2003-2004 (n=137) among women aged 45-56 years and biomonitoring studies (Seattle, Washington (n=120 women aged 65-96 years, Olsen et al. 2004); Red Cross blood donors (n=50 women aged 20-69 years, Olsen et al. 2017); general nurses in Nurse Health Study II (n=555 women aged 40-50 years, data for only median concentrations available, Sun et al. 2018). Note that the median PFHxS was below detection limit among Red Cross blood donors.
This study allowed us to evaluate differential exposure to PFAS between white and black women within the same geographic location. If there were no racial/ethnic differences, we would not have observed differences in PFAS concentrations between white and black women within the same site. It is well documented that racial residential segregation can lead to environmental exposure disparities (Gee and Payne-Sturges 2004; Jesdale et al. 2013; Jones et al. 2014; Morello-Frosch and Lopez 2006; Williams and Collins 2001). Although environmental exposure is frequently higher in racial/ethnic minority groups and disadvantaged neighborhoods, the patterns of PFAS exposure between white and black women depended on the PFAS compounds evaluated. Linear PFOA was higher in white women, whereas PFOS and its precursors were higher in black women. Higher serum PFOA concentrations in white women compared with other racial/ethnic groups were also observed in the NHANES data (Calafat et al. 2007a; Calafat et al. 2007b; Jain 2014). These findings were independent of SES in both our study (education and hardship to pay basics) and the NHANES study (family income). These white/black differences between PFOA and PFOS may indicate different exposure sources and pathways for PFOA and PFOS (Trudel et al. 2008). Race/ethnicity-specific sources of PFAS exposure warrant further investigation.
The observed geographic differences may result from local contamination in drinking water in study areas. A spatial analysis of 2013-2015 national drinking water PFAS concentrations in the U.S. showed that major industrial sites that manufacture or use PFAS, military fire training areas, and wastewater treatment plants are primary contributors to higher PFAS concentrations in public water supplies.(Hu et al. 2016). Their findings are in line with recent reports of industrial sites and military bases as local point sources of PFAS contaminations in Michigan and Pittsburgh, two sites included in our study (Gardner and Ellison 2018; Morrison 2018). In Michigan, a number of plating companies that make chrome part for the auto industry have been discharging excessive levels of PFOS into waterways, which in turn, have contaminated drinking water sources (i.e., ground and surface water), with levels much higher than the EPA guideline of 70 ng/L (ppt) for both PFOS and PFOA (Post et al. 2017). However, limited information on geographic differences in serum PFAS concentrations is available. Studies of adult donor serum samples from 6 American Red Cross blood collection centers reported geographic differences in PFAS concentrations with exposures highest in Charlotte, NC for all PFAS compounds, although information on race/ethnicity and SES were unavailable (Olsen et al. 2003; Olsen et al. 2017). Female nurses living in inland states had higher concentrations of PFHxS but lower concentrations of PFNA and PFDA than those living in coastal regions (Sun et al. 2018), but such large regions are too broad to capture locally contaminated areas.
Asian women, especially Chinese women, had lower concentrations of PFAS (linear PFOA, PFHxS, branched PFOS, Me-FOSAA, and Et-FOSAA, Figure 3 and Figure A2)), compared with white women. Whereas, both Chinese (Oakland) and Japanese (Los Angeles) women had higher concentrations of PFNA compared with white women at the same site. We were limited in our ability to assess whether differences found between Asian and white women were due to race/ethnicity or geographic location because Chinese and Japanese women were each enrolled in only one site. Within Chinese and Japanese, women who were born outside the US had lower concentrations of most PFAS but higher PFNA concentrations (Tables A1-A3). The differences in PFAS serum concentrations between white women and US-born Chinese women in Oakland were not statistically significant (data not shown). As these women immigrated to the US decades before the timing of blood draw, the observed differences in PFAS serum concentrations may be due to different lifestyles being maintained after immigration rather than to lower exposure levels before immigration.
Interestingly, fish intake was inversely associated with serum concentrations of linear PFOA, branched PFOS, and Et-FOSAA, a finding which is in the opposite direction to that reported previously (Christensen et al. 2017; Fromme et al. 2009; Jain 2014). This finding may also be due to the fact that Asian women in this study, especially immigrant Asian women who had lower concentrations of PFAS, were more likely to be in a higher fish intake group. Different PFAS contamination levels by sources (i.e., freshwater vs. sea vs. farmed) and types of fish (Christensen et al. 2017; Hansen et al. 2016; Koponen et al. 2015) may also explain our findings. However, the FFQ used in the present study did not distinguish specific types and sources of fish.
We observed that menstrual bleeding and parity were important determinants of PFAS serum concentrations, consistent with the previous literature (Berg et al. 2014; Brantsaeter et al. 2013; Harada et al. 2005; Monroy et al. 2008; Taylor et al. 2014). The complete substitution of carbon-hydrogen bonds for the strongest carbon-fluorine counterparts lead to increased affinity for proteins, resulting in approximately 90% to 99% of these compounds bound to serum albumin in human blood (Han et al. 2003). Pharmacokinetics modeling has suggested that menstruation could explain 30% of the difference in half-lives of PFOS in men compared to women (Wong et al. 2014). Other possible PFAS elimination pathways that are unique for women include placental transfer, blood loss during delivery, and breastfeeding (Berg et al. 2014; Monroy et al. 2008). Our findings support this notion as both menstrual bleeding and parity were associated with lower concentrations of all PFAS even including PFNA (Figure 2 and Figure A1). In other words, the identified determinants except menstrual bleeding and parity are related to PFAS exposure sources, whereas menstrual bleeding and parity are related to PFAS elimination.
More frequent intake of salty snacks including popcorn was associated with higher concentrations of linear PFOA, PFOS and Et-FOASS. The FFQ used in SWAN (the modified Block FFQ (Block et al. 1986)) was not designed to assess PFAS exposure through food intake, thus it is unclear whether the intake of salty snacks is a direct source or a proxy measure of another source of PFAS. Despite measurement error, our finding suggests that food packaging in snacks may serve as an important source of PFAS exposure given previous reports of high correlations between intake of snacks or popcorn and individual PFAS (Berg et al. 2014; Halldorsson et al. 2008; Ji et al. 2012; Sun et al. 2018; Wu et al. 2015). PFAS are widely used for surface coatings to repel oil and moisture in food contact materials such as microwave popcorn bags, fast food wrappers, baking papers, and paperboard (Begley et al. 2008; Schaider et al. 2017). The migration of PFAS from food packaging and other food contact materials into food therefore represent a potential route of oral exposure to PFAS. Exposure to this source of PFAS seems to be race/ethnicity-dependent in that intake of salty snacks was associated with linear PFOA and total PFOS in white women but not apparent associations were found in Asian women (Tables A.1 and A.2).
In this study, both current and never smokers had higher concentrations of linear PFOA compared with never smokers. A study of pregnant women, the Hokkaido Study on Environment and Children’s Health, found that passive smokers based on blood cotinine levels of 0.22-11.49 ng/mL had higher concentrations of PFOS and PFOA compared with non-smokers (cotinine levels<0.22 ng/mL) (Tsai et al. 2018). In a study using NHANES 2003-2008 data, female smokers had higher concentrations of PFNA compared with non-smokers, but no association was found for PFOA or PFOS (Jain 2014). Other studies have observed negative (Brantsaeter et al. 2013; Fei et al. 2007; Lauritzen et al. 2016) or no associations (Holzer et al. 2008; Lee et al. 2017) between cigarette smoking and PFAS concentrations. As cigarettes do not contain PFASs, cigarette smoking itself may capture PFAS exposure-related behaviors or other factors not reflected by sociodemographic and dietary factors considered in our analysis.
Obese women (vs. women with BMI<25 kg/m2) had significantly higher concentrations of linear PFOA, branched PFOS, total PFOS and Et-FOASS. However, previous literature regarding the association between PFAS and body weight, body size or adiposity is inconsistent (Cardenas et al. 2018; Eriksen et al. 2011; Holzer et al. 2008; Nelson et al. 2010). PFAS as endocrine disruptors have been postulated to interfere with metabolic functions through the activation of nuclear receptors including the peroxisome proliferator-activated receptor α (Takacs and Abbott 2007; Vanden Heuvel et al. 2006). The cross-sectional nature of the current study limits our ability to link causally PFAS exposure and obesity. With longitudinally collected data of adiposity measures and other metabolic phenotypes available in the SWAN cohort, future analyses will be able to assess potential causality.
Several limitations should be noted. First, the timing of blood sampling for PFAS assessment was 1999-2000. As a part of epidemiologic research on PFAS health effects, our exposure was assessed using serum samples collected at the study baseline (1999-2000) rather than at the most recent visit. There has been a decreasing trend in exposure to PFOS and PFOA and an increasing trend in PFNA in the U.S. (Hurley et al. 2018; Jain 2018; Kato et al. 2011b; Olsen et al. 2017). Therefore, racial/ethnic and geographic differences and the other determinants identified in our study may not fully reflect what we would have observed if we used more recent serum samples. However, racial/ethnic and geographic differences in exposure remain an important public health concern and this study allowed us to test racial/ethnic and geographic differences in serum PFAS concentrations simultaneously. We are currently evaluating longitudinal trends in PFAS serum concentrations using repeatedly collected serum samples over 12 years in a small subset (n=75), which will be reported in a separate paper. Second, although this multi-racial/ethnic, multi-site study allowed us to evaluate the role of race/ethnicity and geographic location in PFAS exposure, we were limited to 5 sites and only 3 sites included both white and black women, therefore, we were unable to fully assess the impact of geographic location and race/ethnicity on variability in PFAS exposure in the U.S. A larger scale study with geographic information would provide a clearer picture of the complex relationships between race/ethnicity and geographic location in PFAS exposure. Third, our participants were all females aged 45 and 56 years. In general, males and older individuals have higher concentrations of PFASs (Calafat et al. 2007a; Calafat et al. 2007b; Olsen et al. 2017). Therefore, generalizability of our findings is limited to midlife women. Fourth, although we examined various potential determinants of serum PFAS concentrations, important predictors such as drinking water sources and renal function (a potential PFAS clearance pathway (Harada et al. 2005)) were not included. Finally, our dietary assessment was conducted at baseline, three years before blood draw for PFAS assessment. Temporal changes in diet and dietary exposures could impact the validity and reproducibility of dietary data used in our analysis.
In conclusion, the present study suggests that geographic location and race/ethnicity play an important role in explaining differential exposure to PFAS, but the racial/ethnic determinants differ between PFOS, PFOA and PFNA: such that we observed higher exposure to PFOS in black women, higher exposure to PFOA in white women, and higher exposure to PFNA in Asian women. Menstruation and parity seem to be important determinants of PFAS concentrations likely given their role as an elimination route.
Supplementary Material
Highlights.
We examined the determinants of serum PFAS in midlife women
Geographic locations and race/ethnicity were two major determinants of PFAS
In Oakland and Pittsburgh, white women had higher PFOA than the minority groups
In Southeast Michigan and Boston, black women had higher PFOS than white women
Menstrual bleeding and parity were associated with lower PFAS concentrations
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578, R01-ES026964 and P30-ES017885, and by the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455.
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. The SWAN Repository (U01AG017719). This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor – Siobán Harlow 2013 - Present; Dan McConnell 2011 - 2013; MaryFran Sowers 2000 – 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN. We also thank Drs. Antonia Calafat and Xiaoyun Ye at Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, for their support in PFAS assessment.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagenstose K 2018. Study: High PFAS blood levels in Bucks, Montgomery County residents. The Intelligencer (Doylestown, PA) October 18, 2018. [Google Scholar]
- Barnes G 2018. DHHS Announces Results of Chemours Neighbors’ Blood, Urine Tests. NORTH CAROLINA HEALTH NEWS (North Carolina) October 18, 2018. [Google Scholar]
- Begley TH, Hsu W, Noonan G, Diachenko G. 2008. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25(3):384–390. [DOI] [PubMed] [Google Scholar]
- Berg V, Nost TH, Huber S, Rylander C, Hansen S, Veyhe AS, et al. 2014. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int 69:58–66. [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. 1986. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 124(3):453–469. [DOI] [PubMed] [Google Scholar]
- Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. 1992. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 92(6):686–693. [PubMed] [Google Scholar]
- Brantsaeter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, et al. 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int 54:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. 2007a. Serum concentrations of 11 polyfluoroalkyl compounds in the u.s. population: data from the national health and nutrition examination survey (NHANES). Environ Sci Technol 41(7):2237–2242. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. 2007b. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect 115(11):1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Hauser R, Gold DR, et al. 2018. Association of perfluoroalkyl and polyfluoroalkyl substances with adiposity. JAMA Network Open 1(4):e181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KY, Raymond M, Blackowicz M, Liu Y, Thompson BA, Anderson HA, et al. 2017. Perfluoroalkyl substances and fish consumption. Environ Res 154:145–151. [DOI] [PubMed] [Google Scholar]
- D'Eon JC, Mabury SA. 2011. Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol 45(19):7974–7984. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Blossom SJ, Schaider LA. 2019. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence. J Expo Sci Environ Epidemiol 29(2):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison G 2017. Data shows very high PFAS levels at Wolverine dump. MLive (Grand Rapids, MI) December 21, 2017. [Google Scholar]
- Eriksen KT, Sorensen M, McLaughlin JK, Tjonneland A, Overvad K, Raaschou-Nielsen O. 2011. Determinants of plasma PFOA and PFOS levels among 652 Danish men. Environ Sci Technol 45(19):8137–8143. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. 2007. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect 115(11):1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee SJ, Brooks AP Jr., Maher A, Flensborg P, Arnold S, Fletcher T, et al. 2009. The C8 health project: design, methods, and participants. Environ Health Perspect 117(12):1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. 2009. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int J Hyg Environ Health 212(3):239–270. [DOI] [PubMed] [Google Scholar]
- Gardner P, Ellison G. 2018. Businesses discharging PFAS into Michigan's waterways. MLive (Grand Rapids, MI) November 29, 2018. [Google Scholar]
- Gee GC, Payne-Sturges DC. 2004. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect 112(17):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Clapp R. 2015. Perfluorinated Alkyl Substances: Emerging Insights Into Health Risks. New Solut 25(2):147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. 2008. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environ Sci Technol 42(23):8971–8977. [DOI] [PubMed] [Google Scholar]
- Han X, Snow TA, Kemper RA, Jepson GW. 2003. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem Res Toxicol 16(6):775–781. [DOI] [PubMed] [Google Scholar]
- Hansen S, Vestergren R, Herzke D, Melhus M, Evenset A, Hanssen L, et al. 2016. Exposure to per- and polyfluoroalkyl substances through the consumption of fish from lakes affected by aqueous film-forming foam emissions - A combined epidemiological and exposure modeling approach. The SAMINOR 2 Clinical Study. Environ Int 94:272–282. [DOI] [PubMed] [Google Scholar]
- Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A. 2005. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res 99(2):253–261. [DOI] [PubMed] [Google Scholar]
- Holzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, et al. 2008. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environ Health Perspect 116(5):651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ Sci Technol Lett 3(10):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S, Goldberg D, Wang M, Park JS, Petreas M, Bernstein L, et al. 2018. Time Trends in Per- and Polyfluoroalkyl Substances (PFASs) in California Women: Declining Serum Levels, 2011-2015. Environ Sci Technol 52(1):277–287. [DOI] [PubMed] [Google Scholar]
- Jain RB. 2014. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: data from NHANES 2003-2008. Int J Hyg Environ Health 217(1):52–61. [DOI] [PubMed] [Google Scholar]
- Jain RB. 2018. Time trends over 2003-2014 in the concentrations of selected perfluoroalkyl substances among US adults aged >/=20years: Interpretational issues. Sci Total Environ 645:946–957. [DOI] [PubMed] [Google Scholar]
- Jesdale BM, Morello-Frosch R, Cushing L. 2013. The racial/ethnic distribution of heat risk-related land cover in relation to residential segregation. Environ Health Perspect 121(7):811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Kim S, Kho Y, Paek D, Sakong J, Ha J, et al. 2012. Serum concentrations of major perfluorinated compounds among the general population in Korea: dietary sources and potential impact on thyroid hormones. Environ Int 45:78–85. [DOI] [PubMed] [Google Scholar]
- Jones MR, Diez-Roux AV, Hajat A, Kershaw KN, O'Neill MS, Guallar E, et al. 2014. Race/ethnicity, residential segregation, and exposure to ambient air pollution: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Public Health 104(11):2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM. 2011a. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218(15):2133–2137. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. 2011b. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environ Sci Technol 45(19):8037–8045. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Moon S, Oh BC, Jung D, Ji K, Choi K, et al. 2018. Association between perfluoroalkyl substances exposure and thyroid function in adults: A meta-analysis. PLoS One 13(5):e0197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox SS, Jackson T, Javins B, Frisbee SJ, Shankar A, Ducatman AM. 2011. Implications of early menopause in women exposed to perfluorocarbons. J Clin Endocrinol Metab 96(6):1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen J, Airaksinen R, Hallikainen A, Vuorinen PJ, Mannio J, Kiviranta H. 2015. Perfluoroalkyl acids in various edible Baltic, freshwater, and farmed fish in Finland. Chemosphere 129:186–191. [DOI] [PubMed] [Google Scholar]
- Lauritzen HB, Larose TL, Oien T, Odland JO, van de Bor M, Jacobsen GW, et al. 2016. Factors Associated with Maternal Serum Levels of Perfluoroalkyl Substances and Organochlorines: A Descriptive Study of Parous Women in Norway and Sweden. PLoS One 11(11):e0166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee CK, Suh CH, Kang HS, Hong CP, Choi SN. 2017. Serum concentrations of per- and poly-fluoroalkyl substances and factors associated with exposure in the general adult population in South Korea. Int J Hyg Environ Health 220(6):1046–1054. [DOI] [PubMed] [Google Scholar]
- Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, et al. 2008. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ Res 108(1):56–62. [DOI] [PubMed] [Google Scholar]
- Morello-Frosch R, Lopez R. 2006. The riskscape and the color line: examining the role of segregation in environmental health disparities. Environ Res 102(2): 181–196. [DOI] [PubMed] [Google Scholar]
- Morrison O 2018. Hundreds of thousands of Pennsylvanians have been exposed to dangerous PFAS chemicals, including around Pittsburgh’s airport. PublicSource (Pittsburgh, PA) November 27, 2018. [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 118(2):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. 2013. OECD/UNEP Global PFC Group, Synthesis paper on per- and polyfluorinated chemicals (PFCs). Paris, France. [Google Scholar]
- Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, et al. 2003. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ Health Perspect 111(16):1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Church TR, Larson EB, van Belle G, Lundberg JK, Hansen KJ, et al. 2004. Serum concentrations of perfluorooctanesulfonate and other fluorochemicals in an elderly population from Seattle, Washington. Chemosphere 54(11):1599–1611. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, et al. 2017. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000-2015. Environ Res 157:87–95. [DOI] [PubMed] [Google Scholar]
- Post GB, Gleason JA, Cooper KR. 2017. Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: Contaminants of emerging concern. PLoS Biol 15(12):e2002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruark CD, Song G, Yoon M, Verner MA, Andersen ME, Clewell HJ 3rd, et al. 2017. Quantitative bias analysis for epidemiological associations of perfluoroalkyl substance serum concentrations and early onset of menopause. Environ Int 99:245–254. [DOI] [PubMed] [Google Scholar]
- Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, et al. 2017. Fluorinated Compounds in U.S. Fast Food Packaging. Environ Sci Technol Lett 4(3):105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechtig S, Seifert J. 2018. The Devil We Know. Available: https://thedevilweknow.com/ [accessed March 20 2019].
- Sowers MR, Crawford SL, Sternfeld B, Morganstein D, Gold EB, Greendale GA, et al. 2000. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition In: Menopause: Biology and Pathology (Lobo RA, Kelsey J, Marcus R, eds). San Diego, CA: Academic Press, 175–188. [Google Scholar]
- Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. 2001. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America's Table Study. Am J Epidemiol 154(12):1089–1099. [DOI] [PubMed] [Google Scholar]
- Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. 2018. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environ Health Perspect 126(3):037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs ML, Abbott BD. 2007. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci 95(1):108–117. [DOI] [PubMed] [Google Scholar]
- Taylor KW, Hoffman K, Thayer KA, Daniels JL. 2014. Polyfluoroalkyl chemicals and menopause among women 20-65 years of age (NHANES). Environ Health Perspect 122(2):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao XL, et al. 2007. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J Agric Food Chem 55(8):3203–3210. [DOI] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuhler K. 2008. Estimating consumer exposure to PFOS and PFOA. Risk Anal 28(2):251–269. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Miyashita C, Araki A, Itoh S, Bamai YA, Goudarzi H, et al. 2018. Determinants and Temporal Trends of Perfluoroalkyl Substances in Pregnant Women: The Hokkaido Study on Environment and Children's Health. Int J Environ Res Public Health 15(5):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. 2006. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci 92(2):476–489. [DOI] [PubMed] [Google Scholar]
- Williams DR, Collins C. 2001. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep 116(5):404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT. 2014. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: evidence from population-based pharmacokinetic modeling. Environ Sci Technol 48(15):8807–8814. [DOI] [PubMed] [Google Scholar]
- Wu XM, Bennett DH, Calafat AM, Kato K, Strynar M, Andersen E, et al. 2015. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and adults in California. Environ Res 136:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D 2017. A Coefficient of Determination for Generalized Linear Models. The American Statistician 71(4):310–316. [Google Scholar]
- Zhang T, Qin X. 2014. Assessment of fetal exposure and maternal elimination of perfluoroalkyl substances. Environ Sci Process Impacts 16(8):1878–1881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.