Abstract
Interleukin(IL)-17 inhibitors display higher efficacy than both TNFi and IL-12/23i, which increased the goal psoriasis area severity index (PASI) from 75 to PASI 90 or even PASI 100. Ixekizumab, a recombinant, humanized IgG4 monoclonal antibody targeting IL-17A displayed a high efficacy and safety in RCTs, namely UNCOVER-2 and UNCOVER-3. However, few studies examined real-life data for these medications, and those which exist highlight discrepancies in efficacy and safety between RCTs and real-life data, likely due to the heterogeneity of patients treated outside of trials. Thus, we performed a single center large prospective observational study (RLSD) that enrolled 47 psoriatic patients followed for 20 weeks and we compared the obtained data with the UNCOVER studies. At week 20 in RLSD vs UNCOVER-3 both PASI-90 and PASI-100 results were similar, whilst at week 12, the RLSD cohort obtained higher PASI 90 (76% vs 69,3%) and PASI-100 (55% vs 39%) than UNCOVER cohorts. Interestingly we also reported higher injection-site related pain that disappeared after week 12. In conclusion, real-life data together with RCTs contribute to enrich the information background available to dermatologists in daily practice.
Keywords: psoriasis, real-life, ixekizumab, UNCOVER-2, UNCOVER-3, PASI, DLQI, injection-site pain
Introduction
Psoriasis is a systemic inflammatory disease characterized by a growing body of comorbidities; however availability of targeted anti-psoriatic therapy has been largely limited (Al Mutairi,2010, Santus, 2018, Fiore, 2018, Asa’ad, 2018, Jiang, 2015, Yadav, 2018). More recently, clinicians have been able to better treat psoriasis due to the development of targeted therapy allowing for: a) second-step therapy in moderate to severe psoriasis, b) treatment of moderate to severe psoriasis in patients which have contraindication for traditional systemic drugs and/or c) treatment of mild psoriasis not responsive to topical treatments (Ighani, 2018). One of the more recently approved targeted therapies are the new Interleukin (IL)-17 inhibitors(i). Furthermore IL-17i, display higher efficacy than both TNFi and IL-12/23i, which has resulted in increasing the goal psoriasis area severity index (PASI) from 75 to 90[8]. Among IL-17i, we focused on ixekizumab, a high affinity monoclonal antibody which selectively binds and neutralizes IL-17A (Canavan, 2016). However, real-life data regarding ixekizumab efficacy in psoriatic patients are limited to a Spanish retrospective chart review (Deza, 2018). The aim of this study was to evaluate the real life efficacy, with particular attention to PASI 90 and PASI 100, and safety of ixekizumab in an Italian population with moderate-to-severe psoriasis as well as compare our results to two phase-3 randomized controlled trials (RCT), namely UNCOVER-2 and UNCOVER-3 (Griffiths, 2015).
Materials and Methods
Patients with moderate-to-severe plaque psoriasis (Psoriasis Area Severity Index (PASI>10)) were prospectively enrolled in this real-life study (RLSD) for treatment with ixekizumab in a dedicated PSOCARE Center in the Dermatology Unit of San Donato Hospital in Milan, Italy from January to September 2018. The follow up was 20 weeks. The only exclusion criteria were a previous documented history of hypersensitivity reaction to ixekizumab or to any of its excipients or concurrent or even family history of Crohn disease. Tuberculosis(TB) evaluation was performed with Mantoux test, interferon-gamma release assay and chest radiogram. Positive patients were deferred to the department of infectious diseases for TB eradication. Dermatologists explained and showed how to perform the subcutaneous injection in accord with the “Instructions for use” released by the drug company. Each patient could choose the preferred device to perform the subcutaneous injection between auto-injector and prefilled syringe. Patients were treated with 160 mg (two 80 mg injections) during induction at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, and received maintenance dose of 80 mg every 4 weeks, as licensed by the Food and Drug Administration (FDA) (https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125521s004lbl) and European Medicines Agency (EMA) (https://www.ema.europa.eu/medicines/human/EPAR/taltz). Data regarding demographic characteristics (age, gender, weight, body mass index(BMI)), cardiovascular comorbidities (hypertension, diabetes mellitus type I and II, coronary artery disease, stroke, and dyslipidemia), previous anti-psoriatic therapy, pain-numerical rating scale(NRS) at weeks 0, 12 and 20, PASI, PASI 90 and PASI 100 response at weeks 0, 12 and 20, Dermatologic quality of life (DLQI) at weeks 0, 12, 20 and adverse events. These data were further compared to those belonging to two phase-3 RCTs (UNCOVER-2, UNCOVER-3). This study was performed respecting the Declaration of Helsinki and all patients signed an informed consent before starting.
Results
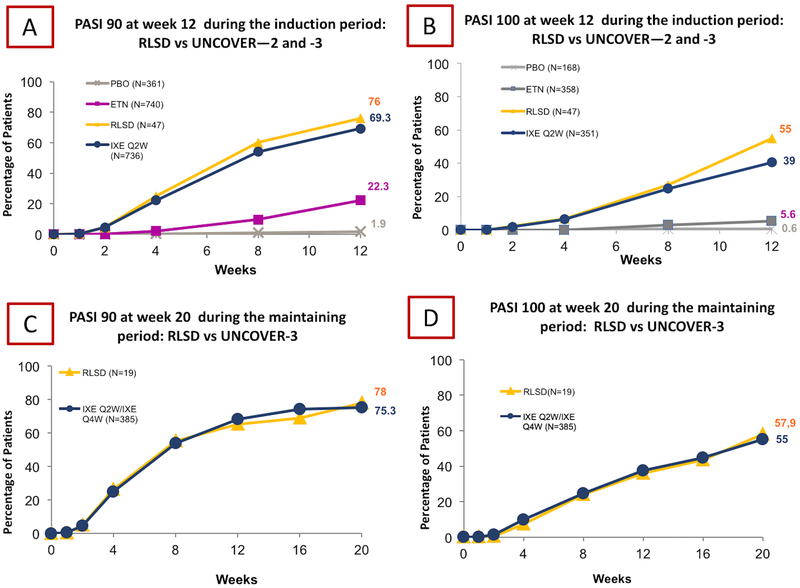
We enrolled 47 patients, 23 males and 24 females respectively, with an average age of 44±13.4 years old and disease duration of 18±15 years, similar to the RCTs (Table I). Family history was positive in 32 patients. At baseline, our cohort displayed an average PASI of 23±5.3, DLQI of 13±7, weight of 81±7kg and BMI of 27±2 kg/m2. The majority of patients (n=45, (95.7%)) received at least one prior topical or systemic anti-psoriatic drug. Topical medication was prescribed to 44 (93.6%) patients, systemic non-biological therapies (acitretin, methotrexate, cyclosporin and fumarate) to 38(80.8%), phototherapy to 9 (19.1%) and biological therapy to 9 (19.1%). All patients that previously had methotrexate used prefilled syringe. Previous medical history included arterial hypertension (n=9, (19.1%)), dyslipidemia (n=8, (17.02%)), diabetes mellitus type 1 (n=1, (2.12%)) diabetes mellitus type 2 (n=1, (2.12%)), and coronary artery disease (n=1, (2.12%))(Table I). Eighteen patients (38.3%) in our cohort would have been excluded under the UNCOVER criteria due to PASI<12 (n=8), overlap with systemic non-biologic therapy (n=6), hepatitis-C infection (n=2), recent squamous cell carcinoma (n=1) and major surgery within the past 8 weeks (n=1). At week 12 at the end of induction phase, PASI 90 was achieved in 76% of patients, compared to 69.3% in the UNCOVER trials (Figure 1A), while PASI 100 was achieved in 55% of our cohort and 39% of the UNCOVER cohorts (Figure 1B). At week 20 during the maintenance phase, PASI 90 was achieved in 75.3% of our cohort and 78% of the UNCOVER-3 cohort (Figure 1C), while PASI 100 was achieved in 57.9% of our cohort and 55% of the UNCOVER-3 cohort(Figure 1D). At the end of induction the average DLQI was 8±1.4 and at week 20 was 5±2, showing a significant difference between baseline-induction and induction-maintenance (p<0.05). There were 55.3% treatment emergent adverse events, similar to the UNCOVER patients on ixekizumab (57.8%); however none were considered serious or resulted in therapy discontinuation. Adverse events (AEs) included injection-site pain in 26 (55.3%) patients, injection-site erythema in 5 (10.6%), nasopharyngitis in 2 (4.2%), upper respiratory tract infection in 2 (4.2%), injection-site reaction in 2(4.2%) and headache in 1 (2.1%) (Table II). Injection-site pain was assessed at the first injection and 26 (55.3%) patients rated that as pain-NRS of 3.2±0.8; however at week 12 only 5 patients complained of injection-site pain it and the average reported pain-NRS was 1.8±0,5, whilst at week 20 only a non-diabetic patient reported a pain-NRS of 3.
Table I.
Demographic and Clinical Characteristics of Patients from Real-life San Donato cohort Vs UNCOVER 2
| RLSD N=47 |
PBO N=168 |
ETN N=358 |
IXE Q2W N=351 |
|
|---|---|---|---|---|
| Age (years), mean (SD) | 44 (13.4) | 45 (12) | 45 (13) | 45 (13) |
| Male, n (%) | 23 (48.9) | 120 (71.4) | 236 (65.9) | 221 (63.0) |
| Weight (kg), mean (SD) | 81 (7) | 92 (22) | 93 (22) | 89 (22) |
| BMI (kg/m2), Mean (SD) | 27 (2) | 30 (6) | 31 (8) | 30 (7) |
| Psoriasis duration (years), mean (SD) | 18 (15) | 18 (13) | 18 (12) | 18 (12) |
| PASI at week 0, mean (SD) | 23 (5.3) | 21 (8) | 19 (7) | 19 (7) |
| DLQI at week 0, mean (SD) | 13 (7) | 13 (7) | 13 (7) | 12 (7) |
| Previous psoriasis therapy | 45 (95.7) | 164 (97.6) | 344 (96.1) | 340 (96.9) |
| • Topical prescription | 44 (93.6) | 142 (84.5) | 287 (80.2) | 289 (82.3) |
| • Topical non-prescribed | 0 (0) | 21 (12.5) | 48 (13.4) | 37 (10.5) |
| Phototherapy | 9 (19.1) | 74 (44.0) | 173 (48.3) | 163 (46.4) |
| Non-biologic systemic | 38 (80.8) | 80 (47.6) | 170 (47.5) | 178 (50.7) |
| Biologic agent | 9 (19.1) | 43 (25.6) | 76 (21.2) | 84 (23.9) |
| • Efalizumab | 0 | 3 (1.8) | 3 (0.8) | 4 (1.1) |
| • Ustekinumab | 1 (2.1) | 9 (5.4) | 25 (7.0) | 32 (9.1) |
| • Infliximab | 3 (6.3) | 5 (3.0) | 16 (4.5) | 16 (4.6) |
| • Etanercept | 0 | 0 | 0 | 0 |
| • Alefacept | 0 | 4 (2.4) | 3 (0.8) | 8 (2.3) |
| • Adalimumab | 5 (10.6) | 9 (5.4) | 27 (7.5) | 35 (10.0) |
| • Golimumab | 0 | 1 (0.6) | 1 (0.3) | 2 (0.6) |
| • Other | 0 | 21 (12.5) | 28 (7.8) | 24 (6.8) |
| CARDIOVASCULAR COMORBIDITIES, n (%) | ||||
| Hypertension | 9 (19.1) | 114 (67.9) | 215 (29.1) | 194 (26.4) |
| Diabetes mellitus, type I | 1 ( 2.12) | 2 (1.2) | 3 (0.4) | 5 (0.7) |
| Diabetes mellitus, type II | 1 ( 2.12) | 33 (19.6) | 61 (8.3) | 55 (7.5) |
| Coronary artery disease | 1 (2.12) | 12 (7.1) | 29 (3.9) | 20 (2.7) |
| Stroke | 0 (0) | 2 (1.2) | 3 (0.4) | 8 (1.1) |
| Dyslipidemia | 8 (17.02) | 41 (24.4) | 85 (11.5) | 91 (12.4) |
Legend:
DLQI: Dermatologic quality of life, ETN: Etanercept; IXE Q2W: Ixekizumab every two weeks cohort; PBO: Placebo, PASI: Psoriasis area severity index, RLSD: Real Life San Donato cohort, SD: standard deviation.
UNCOVER-2 data extracted on the study by Griffiths and collegues [6]
Figure 1.
The figure illustrates the percentage of patients, belonging to RLSD and UNCOVER-2 and -3 pooled, that achieved PASI 90 (A) and PASI 100 (B) after induction at week 12. Furthermore, the percentage of patients, belonging to RLSD and UNCOVER -3, that achieved PASI 90 (C) and PASI 100 (D) during maintenance at week 20 was also assessed.
Table II.
Adverse events of UNCOVER-2 and 3 pooled Vs RLSD
| PBO N=360 |
ETN N=739 |
RLSD N=47 |
IXE Q2W N=734 |
|
|---|---|---|---|---|
| TEAE, n (%) | 160 (44.4) | 399 (54) | 26 (55.3) | 424 (57.8) |
| Death, n (%) | 0 | 0 | 0 | 0 |
| Non fatal serious AEs, n (%) | 7 (1.9) | 14 (1.9) | 0 | 14 (1.9) |
| Any Infection, n (%) | 74 (20.6) | 159 (21.5) | 6 (12.7) | 190 (25.9) |
| Common TEAEs+, n (%) | ||||
| - Nasopharyngitis | 28 (7.8) | 55 (7.4) | 2 (4.2) | 61 (8.3) |
| - Upper respiratory tract infection | 12 (3.3) | 34 (4.6) | 2 (4.2) | 27 (3.7) |
| - Injection-site reaction | 4 (1.1) | 80 (10.8) | 2 (4.2) | 76 (10.4) |
| - Injection-site erythema | 2 (0.6) | 29 (3.9) | 5 (10.6) | 24 (3.3) |
| - Injection-site pain | 5 (1.4) | 9 (1.2) | 26 (55.3) | 21 (2.9) |
| - Pruritus | 5 (1.4) | 8 (1.1) | 0 | 14 (1.9) |
| - Headache | 8 (2.2) | 31 (4.2) | 1 (2.1) | 33 (4.5) |
| - Arthralgia | 8 (2.2) | 17 (2.39 | 0 | 20 (2.7) |
Legend:
ETN: Etanercept; IXE Q2W: Ixekizumab every two weeks cohort; PBO: Placebo, RLSD: Real Life San Donato cohort.
Common in an adverse event with a frequency ≥ 2%
UNCOVER-2 and -3 data extracted on the study by Griffiths and collegues [6]
Discussion
In RLSD data at week 12, PASI 90 was similar to UNCOVERs, while PASI 100 response was better compared to UNCOVERs (Griffiths, 2015), and higher than in Deza’s real-life study (Deza, 2018). Furthermore, patients which did not meet UNCOVERs criteria demonstrated similar PASI 90 and PASI 100 response compared to those who met criteria. The Spanish cohort confirmed these data (Griffiths, 2015). Next, our cohort exhibited lower weight and BMI than the RCTs (Deza, 2018) and Spanish real-life study (Griffiths, 2015), and our enrolled patients had a higher PASI at the baseline. UNCOVER-2(Griffiths, 2015) and Deza et al. enrolled primarily male patients; however in our study gender representation was almost equal. We reported similar occurrence of adverse events compared with UNCOVERs (55.3% vs 57.8%)(Griffiths, 2015); conversely Deza et al. described only 26% of occurrence. Infective events were similar between the two real-life studies (12.7% vs 7%)(Deza, 2018), while UNCOVERs had a double prevalence (25.9%) (Griffiths, 2015). Injection-site related AEs in all studies represented the major category of AEs. Although in RCTs the injection-site reaction represented the most common site-related AEs, in our cohort interestingly injection-site pain was predominant. Furthermore, for the first time we described that this injection-related pain was diminished at the end of induction phase.
In conclusion, our study confirmed the efficacy and safety profile of ixekizumab, previously highlighted in RCTs and to the Spanish study (Deza, 2018). Real life data remarked that PASI 100 during induction with ixekizumab is not uncommon. Interestingly and not previously described is that the injection-site-related pain diminishes after the induction period.
Acknowledgments
Funding
GD and RRZC are supported by the P50 AR 070590 01A1 National Institute Of Arthritis And Musculoskeletal And Skin Diseases, RRZC is supported by the 5 T32 AR 7569–22 National Institute of Health T32 grant.
ABBREVIATIONS
- DLQI
Dermatologic Quality of Life
- EMA
European Medicines Agency
- ETN
Etanercept
- FDA
Food and Drud Administration
- IXE Q2W
80 mg of Ixekizumab Every 2 Weeks
- NRS
Numerical Rating Scale
- RLSD
Real Life San Donato
- PASI
Psoriasis Area and Severity Index
- PBO
Placebo
- RCT
randomized controlled trials
Footnotes
Conflict of interest: No conflict of interests to declare
REFERENCES
- 1.Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy M (2010). Comorbidities associated with psoriasis: an experience from the Middle East. J. Dermatol, 37, 146–155. [DOI] [PubMed] [Google Scholar]
- 2.Asa'ad F, Fiore M, Alfieri A, et al. (2018). Saliva as a Future Field in Psoriasis Research. Biomed. Res. Int, 2018, 7290913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canavan TN, Elmets CA, Cantrell WL, et al. (2016). Anti-IL-17 Medications Used in the Treatment of Plaque Psoriasis and Psoriatic Arthritis: A Comprehensive Review. Am. J. Clin. Dermatol, 17, 33–47. [DOI] [PubMed] [Google Scholar]
- 4.Deza G, Notario J, Lopez-Ferrer A, et al. (2018). Initial results of ixekizumab efficacy and safety in real-world plaque psoriasis patients: a multicentre retrospective study. J Eur Acad Dermatol Venereol. doi: 10.1111/jdv.15288. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Fiore M, Leone S, Maraolo AE, et al. (2018). Liver Illness and Psoriatic Patients. Biomed Res Int, 2018, 3140983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths CE, Reich K, Lebwohl M, et al. (2015). Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet, 386, 541–551. [DOI] [PubMed] [Google Scholar]
- 7.https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125521s004lbl
- 8.https://www.ema.europa.eu/medicines/human/EPAR/taltz
- 9.Ighani A, Partridge ACR, Shear NH, et al. (2018). Comparison of Management Guidelines for Moderate-to-Severe Plaque Psoriasis: A Review of Phototherapy, Systemic Therapies, and Biologic Agents. J Cutan Med Surg, 1203475418814234. doi: 10.1177/1203475418814234. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Jiang S, Hinchliffe TE, Wu T (2015). Biomarkers of An Autoimmune Skin Disease--Psoriasis. Genom. Proteom. Bionf, 13, 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santus P, Rizzi M, Radovanovic D, et al. (2018). Psoriasis and Respiratory Comorbidities: The Added Value of Fraction of Exhaled Nitric Oxide as a New Method to Detect, Evaluate, and Monitor Psoriatic Systemic Involvement and Therapeutic Efficacy. Biomed Res Int, 2018, 3140682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav K, Singh D, Singh MR (2018). Protein biomarker for psoriasis: A systematic review on their role in the pathomechanism, diagnosis, potential targets and treatment of psoriasis. Int J Biol Macromol. , 118(Pt B), 1796–1810. [DOI] [PubMed] [Google Scholar]