Abstract
Background:
Falls are a major public health concern in older adults, and the proportion of older adults that has been diagnosed with cancer is growing. Yet, while falls, peripheral neuropathy, and postural instability are more common in aging cancer survivors, it is unclear how these factors interact.
Research Question:
Our objective was to examine how components of sway related to self-reported neuropathy and falls.
Methods:
Postural sway during static stance was recorded with an inertial sensor (APDM Opal), placed on the lumbar spine region in 434 older female cancer survivors (mean age 63) and 49 healthy older female control subjects (mean age 63). Measures of sway were resolved into principal components that were compared between women with and women without self-reported falls in the previous 6 months and between those with and without self-reported symptoms of peripheral neuropathy.
Results:
Cancer survivors had worse sway than healthy control subjects in components related to sway magnitude and mediolateral frequency of sway, but no difference in the component related to resultant / AP sway jerk and frequency. Cancer survivors who reported neuropathy were more likely to have higher resultant / AP sway frequencies and jerk than asymptomatic survivors, while survivors who reported a fall were more likely to have lower frequencies of mediolateral sway than non-fallers. Falls were more strongly associated with mediolateral sway in survivors with more severe neuropathy; whereas falls were more strongly associated with resultant / AP sway frequency in survivors with less severe neuropathy
Significance:
Postural stability, falls, and neuropathy have complex interactions that can vary across components of postural sway. While the frequency of mediolateral sway was associated with falls across our entire cohort, neuropathy influenced the associations between specific characteristics of sway and falls, which may have implications for fall prevention interventions.
Keywords: postural stability, balance, chemotherapy, mediolateral
INTRODUCTION
Preventing falls in older adult populations is an important public health mission that requires understanding the influence of age-related comorbidities. The number of adults age 65 or older in the United States is expected to double by 2050[1], and an increasingly large proportion of these older adults will have a previous diagnosis of cancer. Since 1971, the number of cancer survivors has quadrupled, with 60% of survivors over the age of 65.[2] By 2020, an estimated 11 million older adults will be cancer survivors[2] who have a greater risk of falls[3],[4] and hip fracture [3, 5] compared to older adults with no cancer history.
Since falls increase after cancer diagnoses, the disease and/or treatments must make balance or other known fall risk factors worse or, alternatively, cause new problems. Cancer survivors exhibit poor postural stability[6–9], a strong and independent risk factor for falls in older adults[10]. Additionally, chemotherapy-induced peripheral neuropathy (CIPN)[11] can develop during treatment in up to 90% of cancer patients and can significantly impact mobility and fall risk[12]. However, the relationship between postural instability, neuropathy, and falls is unclear.
Studies using objective measures of postural sway have consistently reported impaired postural stability in cancer survivors[6–9], but clinical interpretations of these deficits are not established. Different sway measures can quantify independent domains of sway (e.g., spatiotemporal measures, frequency-based measures, jerk) and represent unique components of postural control[13, 14]. Direction-dependent deficits may also have clinical significance; increased sway in the mediolateral direction (ML) has been linked to falls in older adults[15] and may be influenced by neuropathy given the reliance on proprioceptive information for control of ML balance[16]. Across clinical populations, peripheral neuropathy has consistently been related to poorer postural sway, increased falls, and decreased quality of life[17, 18]. While both ML sway and neuropathy may worsen over the course of chemotherapy and remain associated with one another up to 3 months after completion of chemotherapy[6], it is unclear if balance, or specific components of balance, are related to falls in cancer survivors and/or the extent to which this relationship is influenced by neuropathy. Additionally, these previous studies used force platforms to quantify sway based on displacement of the center-of-pressure under the feet. While measures derived from center-of-pressure sway and center-of-mass sway estimated by inertial sensors on the pelvis are highly correlated [19], there are no known studies using inertial sensors to quantify body sway in cancer survivors.
The purpose of this study was to investigate the relationship between postural sway, falls, and neuropathy in 434 older female cancer survivors who had received chemotherapy. Using principal component analysis (PCA), we identified three independent components of sway quantified using a wearable inertial sensor located on the lumbar spine. PCA identifies orthogonal projections within a variable space that represent the axes of maximum variance. While others [14] have used this technique as a feature selection tool for postural sway, it is still unclear if variations along these principal components of maximum variance have clinical significance. Here, we examined the relationship of each sway component with self-reported fall history and symptoms of neuropathy, and interpreted the make-up of each component based on a priori knowledge of sway domains. To confirm the interpretations of each principal component, representative variables were examined and step-wise logistic regression models were implemented using individual sway measures. We expected that variation in the principal components of sway would be uniquely associated with falls and neuropathy symptoms, and that principal components would largely align themselves with previously defined domains of sway from other types of cohorts[13, 14]
METHODS
Participants
We analyzed baseline data from 434 female cancer survivors [mean (SD) age = 62.5 (6.4) years] who were enrolled in a randomized clinical trial (ClinTrials.gov NCT01635413)to compare the efficacy of exercise-based fall prevention strategies post-cancer treatment [>3 months, mean (SD) = 61.0 (51.5) months] [20]. All subjects had a diagnosis of cancer that did not have central nervous system (CNS) involvement, were age 50–75 years old, physically inactive, free of metastatic disease and neurologic conditions, ambulatory (assistive device permitted), and medically cleared to exercise. The severity of symptoms associated with CIPN were based on self-reported noticeable dysthesia or parasthesia in the lower extremities using select items in the Functional Assessment of Cancer Therapy Gynecologic Oncology Group Neurotoxicity (FACT/GOG-Ntx). Based upon the presence or absence of symptoms related to neuropathy (numbness, tingling or discomfort in the feet in the past week) women were categorized into one of two groups: symptomatic (CIPN+) or asymptomatic (CIPN-). Individuals were classified as fallers if they reported at least one fall in the previous six months. Forty-nine healthy control participants without a cancer history [mean (SD) age = 63.3 (6.9) years] were recruited from the local community to serve as comparators. All subjects gave written informed consent and the protocol was approved by the Oregon Health & Science University institutional review board.
Procedures
Baseline data collected before subjects were randomly assigned to study arms were used for this analysis. Each subject performed a series of performance tests, including a quiet stance test with eyes open for 30 seconds[19, 21], and completed questionnaires about fall history, cancer history and treatment-related symptoms (i.e., neuropathy). For the purposes of this analysis, only the quiet stance trials were analyzed. During the stance tests, subjects were instructed to stand steady for 30 seconds with their eyes open, arms at their side, and feet spaced apart 10 cm between the heels and 15 cm between halluxes using a template [22]. Subjects were instrumented with an inertial sensor (Opal v1, APDM, Inc. Portland, OR USA) over the lumbar spine that collected tri-axial accelerations and angular velocities at a sampling frequency of 128 Hz.
Analysis
Analysis of the inertial sensor data during quiet standing was performed in Mobility Lab v2 (APDM, Inc. Portland, OR, USA)[19, 23]. The output of this analysis yielded 46 measures of sway (See Supplemental Table A1). Additionally, all sway measures were classified based on the direction of movement: AP-sway, ML-sway, or resultant sway (Euclidean norm of AP and ML sway).
To examine how sway varied within our sample, we performed a principal component analysis (PCA) using the data from our cohort of cancer survivors and healthy controls to reduce our multi-dimensioned sway data into a few, interpretable, orthogonal components. First, all measures of sway were transformed into z-scores with respect to our entire cohort (controls and cancer survivors). Measures of sway that had non-normal distributions were log-transformed prior to the z-score transformation. Next, a PCA was implemented using the z-scores of all measures of sway (using the ‘pca’ function in MATLAB). Principal components (PC) that explained >=10 % of the total variance were retained for interpretation, and the PC coefficients were used to calculate scores for each participant for the respective component.
Interpretation of each sway component was based on: 1) the magnitude of the coefficients for individual sway measures within each component, 2) the correlation between sway components and sway measures, and 3) previously defined domains of sway from other cohorts [13, 14, 19]. Pearson’s correlation coefficients compared the PC scores to each sway measure (See Supplemental Material, Appendices B and C).
Statistical Analysis
Independent t-tests compared PC scores between cancer survivors and healthy controls, and all further analyses were performed in the cohort of cancer survivors only. To test whether variation along each eigenvector was clinically meaningful, comparisons were performed using the PC scores as primary outcomes of interest. Pearson correlation coefficients compared the relationship of each PC score to age, body mass index (BMI), and time since the completion of chemotherapy. Age, BMI and time since treatment completion were examined based on our prior findings linking these to neuropathy severity and mobility impairment[12]. Independent t-tests compared whether the PC scores differed between cancer survivors with (CIPN+) and without (CIPN-) neuropathy, and whether PC scores differed between cancer survivors with a history of falling and those with no falls.
Three logistic regression models were then implemented to test whether, after adjusting for age, BMI, and time since the completion of chemotherapy, 1) PC scores were associated with one or more falls in the previous six months, 2) PC scores were associated with the presence of neuropathy (CIPN+), or 3) PC scores, neuropathy severity, or their interaction were associated with falls in CIPN+ cancer survivors. Comparisons of individual sway measures between cancer survivors and control subjects are presented in the Supplemental Material (Appendix D). To determine the best classifiers of fallers / non-fallers in the cohort of cancer survivors, forward and backward stepwise logistic regression models were implemented using individual sway measures, time since completion of chemotherapy, age, BMI, and CIPN group as independent variables. Constant and linear predictor terms were permitted within the models, and stepwise progression was based on the Bayesian Information Criteria (BIC). All analysis was performed in MATLAB (r2018a, Statistics and Machine Learning Toolbox, The Mathworks, Natick, MA, USA) using a significance level of 0.05.
RESULTS
Sample Characteristics
In general, the cancer cohort was in their early 60’s, overweight to obese based on BMI and had a low prevalence of diabetes (Table 1). The majority (71%) of women were breast cancer survivors and had also had radiation treatment, while the stages of cancer were evenly spread across the sample. Fifty percent of the women with cancer reported that they currently experienced symptoms of sensory loss in their lower extremities and were grouped into CIPN+.
Table 1.
Baseline demographic and clinical characteristics of all participants. Characteristics of the cohort of cancer survivors is presented for the whole cohort, as well as separated by those with (CIPN+) or without (CIPN-) self-reported neuropathy. Data expressed as mean (SD) or % of sample.
| Characteristic | Controls (n=49) | Cancer Cohort (n=434) | CIPN+ (n=216) | CIPN- (n=218) |
|---|---|---|---|---|
| Age (yrs.) | 63.3 (6.9) | 62.5 (6.4) | 63.0 (6.3) | 62.2 (6.3) |
| BMI (kg/m2) | 25.6 (4.0) | 29.3 (6.7) | 30.0 (7.0) | 28.6 (6.2) |
| Time since completion of chemotherapy (months) | 61.0 (51.5) | 55.5 (46.5) | 66.6 (55.6) | |
| Cancer | ||||
| Breast (%) | 71 | 68 | 75 | |
| Colon (%) | 6 | 10 | 3 | |
| Ovarian (%) | 5 | 6 | 4 | |
| LymDhoma (%) | 4 | 3 | 5 | |
| Uterine (%) | 3 | 2 | 4 | |
| Lung (%) | 3 | 3 | 2 | |
| Other (%) | 8 | 8 | 7 | |
| Stageb | ||||
| Stage 0-I (%) | 30 | 25 | 34 | |
| Stage II (%) | 42 | 40 | 45 | |
| Stage III (%) | 28 | 35 | 20 | |
| Received radiation theraDy (%) | 60 | 62 | 59 | |
| Diabetes (%) | 10 | 13 | 8 | |
Independent Postural Sway Components
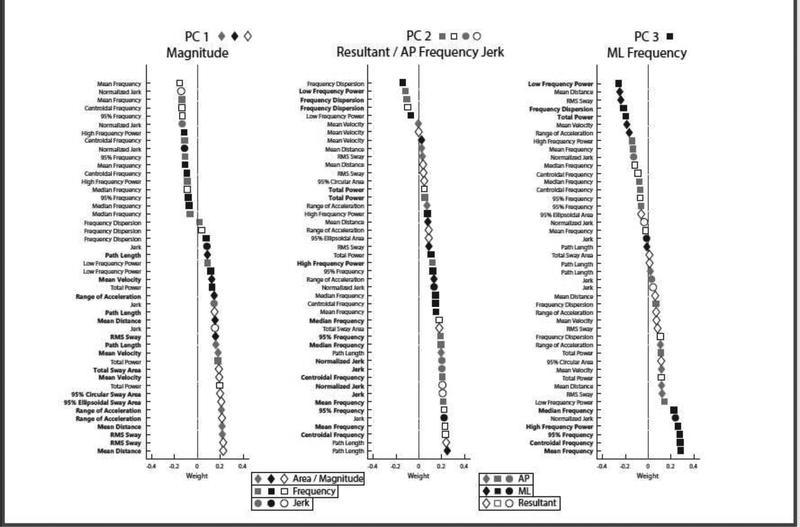
The first three principal components explained over 70% of the total variance across the sway variables (Figure 1). Descriptively, variables related to sway magnitude were dominant in the first PC, with larger sway leading to larger PC1 scores. Resultant and AP frequency and jerk variables were dominant in the second PC, with more jerk and higher frequencies leading to larger PC2 scores. Mediolateral variables, specifically frequency-based ML variables, were dominant in the third PC, with less ML sway and higher ML frequencies leading to larger PC3 scores (Figure 2).
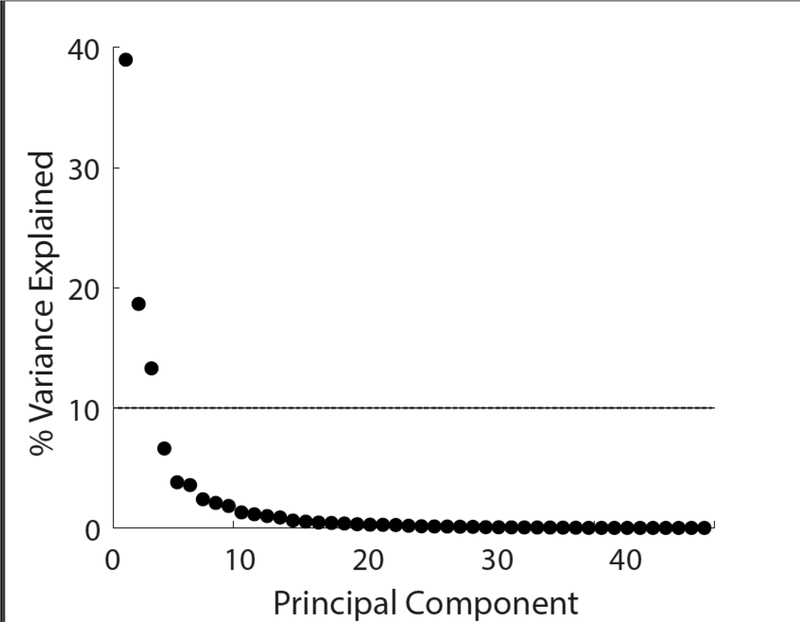
Figure 1.
Scree plot illustrating the percentage of variance explained by the 46 principal components. The first three principle components explained 70.9% of the total variance. The dashed line shows the 10% cut-off used to select components for future analysis.
Figure 2.
Composition of each PC with variables ordered by coefficients. Symbols indicate the domain of sway represented by each variable (magnitude = diamond; frequency = square; jerk = circle), and shades indicate the direction of sway (gray = AP, black = ML, white = resultant). Sway measures that are dominant within the component are bolded, and their icons are shown next to the PC name at the top.
Cancer Survivors Versus Contols
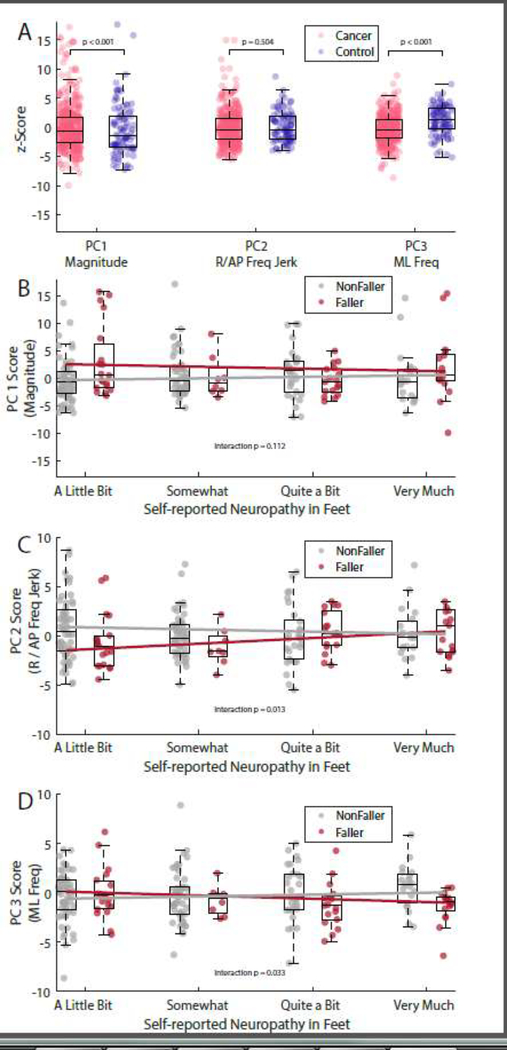
Cancer survivors had significantly higher PC1 scores (p < 0.001, 95% CI of difference = [1.95, 4.39]) compared to healthy control subjects. Cancer survivors also had significantly lower PC3 scores (p < 0.001, 95% CI of difference = [−3.23, −1.84]). No difference in PC2 scores was found between cancer survivors and healthy control subjects (p = 0.504, 95% CI of difference = [−1.16, 0.57]).
Clinical Characteristics
Within the cohort of cancer survivors, age was positively associated with PC1 scores, but not associated with PC2 or PC3 scores (Table 2). Higher BMIs were associated with greater PC1 scores and smaller PC3 scores. Time since the completion of chemotherapy was not associated with any PC score.
Table 2.
Pearson correlation coefficients for correlations of postural sway PC scores with age, BMI, and time since completion of chemotherapy in cancer survivors. Significant correlations (p < 0.05) are indicated in bold.
| Age | BMI | Time Since Chemotherapy (months) | ||||
|---|---|---|---|---|---|---|
| ρ | P-value | ρ | P-value | ρ | P-value | |
| PC 1 (Magnitude) | 0.106 | 0.027 | 0.177 | < 0.001 | −0.012 | 0.805 |
| PC 2 (R/AP Jerk & Frequency) | 0.084 | 0.080 | −0.094 | 0.051 | 0.017 | 0.724 |
| PC 3 (ML Frequency) | 0.074 | 0.124 | −0.162 | < 0.001 | 0.066 | 0.167 |
Neuropathy
In univariate comparisons, CIPN+ women had greater PC2 scores compared to CIPN- women (p = 0.013, 95% CI of difference = [0.15, 1.26]), but no difference in PC1 (p = 0.134, 95% CI of difference = [−0.19, 1.42]) or PC3 (p = 0.450, 95% CI of difference = [−0.62, 0.28]). Results were unchanged when adjusting for covariates in the logistic regression models (Table 3).
Table 3.
Logistic regression models for the presence / absence of neuropathy and falls within cancer subjects using PC scores. Bold values indicate significant effects (p < 0.05).
| Estimate | SE | P-value | |
|---|---|---|---|
| Neuropathy (presence/absence); (n = 434) | |||
| (Intercept) | −2.003 | 1.088 | 0.066 |
| PC1 Magnitude | 0.020 | 0.024 | 0.399 |
| PC2 R / AP Jerk & Frequency | 0.092 | 0.035 | 0.009 |
| PC3 ML Frequency | −0.015 | 0.043 | 0.720 |
| Age | 0.020 | 0.016 | 0.200 |
| BMI | 0.034 | 0.016 | 0.029 |
| Time Since Chemo | −0.005 | 0.002 | 0.014 |
| Falls (>=1 in past 6 months); (n = 434) | |||
| (Intercept) | −2.836 | 1.326 | 0.032 |
| PC1 Magnitude | 0.049 | 0.029 | 0.089 |
| PC2 R / AP Jerk & Frequency | −0.051 | 0.043 | 0.242 |
| PC3 ML Frequency | −0.116 | 0.052 | 0.026 |
| Age | 0.014 | 0.020 | 0.485 |
| BMI | 0.011 | 0.018 | 0.536 |
| CIPN+ | 0.793 | 0.255 | 0.002 |
| Time Since Chemo | −0.004 | 0.003 | 0.158 |
| Falls (>=1 in past 6 months) in CIPN+ subjects; (n = 216) | |||
| (Intercept) | −2.549 | 1.858 | 0.170 |
| PC1 Magnitude | 0.169 | 0.083 | 0.041 |
| PC2 R / AP Jerk & Frequency | −0.346 | 0.143 | 0.016 |
| PC3 ML Frequency | 0.210 | 0.180 | 0.245 |
| Age | −0.006 | 0.028 | 0.830 |
| BMI | 0.022 | 0.024 | 0.363 |
| Neuropathy Severity | 0.278 | 0.165 | 0.092 |
| Time Since Chemo | −0.005 | 0.004 | 0.194 |
| PC1 * Neuropathy Severity | −0.050 | 0.032 | 0.112 |
| PC2 * Neuropathy Severity | 0.129 | 0.052 | 0.013 |
| PC3 * Neuropathy Severity | −0.156 | 0.073 | 0.033 |
Falls
Cancer survivors who reported at least one fall in the previous 6 months had lower ML frequency-dominated PC3 scores (i.e., more ML sway at lower ML frequencies) compared to those who did not report a fall (p = 0.021, 95% CI of difference = [−1.19, −0.10]). Scores for PC1 (p = 0.057, 95% CI of difference = [−0.03, 1.94]) and PC2 (p = 0.525, 95% CI of difference = [−0.90, 0.46]) did not differ between survivors who reported a fall and those who did not. Results remained unchanged when adjusting for covariates in the logistic regression models. Forward and backward stepwise-logistic regression models for individual sway measures agreed on final classifiers of ML Median Frequency (p < 0.001, β = −0.97, SE = 0.31) and CIPN+ status (p < 0.001, β = 0.87, SE = 0.25) to differentiate fallers from non-fallers.
In CIPN+ survivors, self-reported falling was associated with an interaction between neuropathy severity with PC2 and PC3 scores (Table 3). As the severity of CIPN increased, smaller (i.e., more negative) PC3 scores were associated with an increased likelihood of reporting a fall (Figure 3). A significant main effect was found for PC2, indicating that survivors who reported “a little bit” of neuropathy and exhibited smaller PC2 scores were less likely to report a fall. But, as the severity of neuropathy increased, the association between PC2 scores and falls weakened. A significant main effect for PC1 indicated that, after adjusting for the interactions between PC2, PC3, and neuropathy severity, greater PC1 scores were more likely to report a fall. No main effect for PC3 or neuropathy severity was found.
Figure 3.
Box and scatter plots showing the PC scores in cancer survivors and controls (A) and the interactions between neuropathy and postural sway, and falls in CIPN+ cancer survivors (BD). Scattered points indicate individual subjects contained within the boxplot representation of the group. Y-axes values indicate the z-score for each component, relative to the entire sample of cancer survivors and control subjects. For clarity, the scale on the y axes has been adjusted between panels A-D. A.) PC scores for the entire cancer cohort (pink) and healthy controls (blue). B-D.) Individual PC scores in cancer survivors with neuropathy, separated by self-reported faller status (fallers shown in red) and neuropathy severity (x-axis). Lines illustrate the interaction between self-reported neuropathy and postural sway in individuals with (faller, red) or without (nonfaller, gray) one or more reported falls in the previous six months.
DISCUSSION
We investigated how peripheral neuropathy, falls, and age are associated with each component of sway in over 400 female cancer survivors treated with chemotherapy. This is one of the largest studies in any cohort to quantify postural sway and relate sway characteristics to falls and/or signs of peripheral neuropathy. The first three principal components (magnitude, resultant / AP frequency, and ML frequency) explained 39.0, 18.6, and 13.3% of the variance within the sway data, respectively. While each component contained every measure of sway, magnitude components comprised the majority of PC1, whereas resultant and AP frequency and jerk components comprised the majority of PC2, and ML frequency components comprised the majority of PC3. Our results closely match PCA results based on center-of-pressure based sway in much smaller samples young healthy subjects, despite some differences in the measures of sway[14].
Our results show that self-reported falls and neuropathy were both associated with abnormalities in postural sway, but these associations were specific to unique components of sway. PC2, most highly represented by higher frequencies and jerkiness of resultant and AP postural sway was associated with the presence of neuropathy symptoms. In contrast, PC3, mostly represented by the ML direction of sway frequency, was associated with falls. Surprisingly, variation along the first eigenvector (PC1), representing the projection of maximum variance and most highly associated with measures of sway magnitude such as sway area, was not associated with neuropathy or falls across the entire cancer cohort. However, PC1 scores were weakly associated with falls in CIPN+ women. In addition, the association between self-reported falls and sway area depended on the severity of self-reported neuropathy symptoms, and the directionality of this interaction differed by component – ML sway frequency (PC3) was associated with falls if the neuropathy symptoms were severe, whereas AP and resultant frequency and jerk characteristics of sway (PC2) were associated with falls if the neuropathy symptoms were mild.
The present results illustrate that different characteristics of sway have unique relationships to fall risk. Previous studies have identified deficits in postural sway with forceplates in cancer survivors [6–9] but lacked data on falls for clinical interpretations. In those previous studies, cancer survivors exhibited larger magnitudes of sway[7–9], but in the one study that used a control group, frequency-based measures of sway did not differ between cancer patients and controls [8]. Furthermore, ML, but not AP sway was worse in cancer survivors compared to healthy control subjects[8]. Our results confirm other findings of excessive sway magnitude, and especially ML sway, in cancer survivors relative to healthy controls. Additionally, our results suggest that impaired sway in cancer survivors without regard to neuropathy may not be informative for fall risk assessments; variation in sway magnitude measures was not significantly associated with self-reported falls across our entire sample of cancer survivors.
The fact that ML sway was associated with falls across our sample is consistent with other studies in older adults and clinical populations. ML sway is an important predictor of retrospective[10, 24] and prospective[15] falls in older adults, and ML sway is impaired in other populations with neurological injury or disease[25],[26]. Impaired ML sway has also been reported in people with peripheral neuropathy from chronic diabetes[17]. However, neither ML nor spatiotemporal sway was related to the presence of self-reported neuropathy symptoms within our cohort; only the frequency of sway was related to the presence of neuropathy symptoms.
The association between higher PC2 (resultant / AP sway frequency) scores and the presence of neuropathy symptoms may be due to increased sway at the hip joint in people with lower extremity neuropathy. While healthy individuals control sway predominantly at lower frequencies about the ankle joints (i.e., ‘ankle strategy’)[27], individuals with peripheral neuropathy control sway at the hip joints (i.e., ‘hip strategy’) to make use of more accurate proprioceptive information at the hips[28, 29]. As greater hip control is associated with higher sway frequency[30], we speculate that the association between self-reported neuropathy symptoms and high frequency sway was due to increased expression of the ‘hip-strategy’ to compensate for unreliable somatosensory information from the feet and ankles[31]. However, this interpretation of excessive use of a hip strategy by cancer survivors should be confirmed with inertial sensors on both the lower and upper body[32].
Survivors with symptoms of neuropathy (49.3%) showed a different association between sway and falls depending on the severity of their neuropathy. Interestingly, greater sway magnitude was associated with self-reported falls in individuals with neuropathy symptoms, and this association did not change with symptom severity. However, lower frequencies of resultant and AP sway (PC2) were strongly associated with self-reported falls when symptoms were mild, but the association between resultant / AP sway frequency and falls weakened as symptoms became more severe. While this analysis cannot determine the cause of these interactions, sensory reorganization and compensatory adaptation may be a contributing factor. Individuals with impaired somatosensory function from peripheral neuropathy have impaired postural control on firm surfaces if they attempt to depend upon unreliable somatosensory information in the feet and ankles to control sway[33, 34]. However, when somatosensory information is further disrupted in the form of a sway-referenced platform or unstable surface, individuals with peripheral neuropathy down-weight proprioceptive information coming from the feet and ankles, up-weight information from visual and vestibular cues, and control sway more at the hip joints in order to control AP sway[31, 33]. Thus, individuals who can compensate for their mild neuropathy may show normal sway magnitudes with higher resultant and AP sway frequencies that represent an adaptive postural control system capable of using ankle- or hip-strategies, based on the available sensory information[30]. Conversely, individuals with mild neuropathy who exhibit larger sway magnitudes with lower resultant and AP frequencies may continue use an ankle-dominant strategy and impaired somatosensory information from the feet indicative of a rigid postural control system. This interpretation was supported when examining the coefficients of PC2 and individual measures of sway; higher AP and resultant sway frequencies were positively associated with PC2 (Supplemental Material, Table C1), and PC2 was negatively associated with reported falling in those with mild CIPN (Table 3).
Opposite to sway frequency, the association between ML balance and falls increased with increasing neuropathy symptom severity, and may be influenced by the reliance on proprioceptive information for the control of ML sway and biomechanical constraints on ML control. Proprioceptive information is weighted ~37% higher when controlling ML sway compared to AP sway[16] potentially due to a greater number of joints and muscles controlling ML sway[35]. Unlike balance in the AP direction, which can be modeled as an inverted double pendulum with separate torque control at the ankles and hips, ML balance consists of a 4-bar linkage comprised of the right leg, left leg, pelvis, and ground where, due to biomechanical constraints of the feet, torque is controlled almost exclusively by the hip adductors and abductors to shift weight from one leg to the other[36]. The importance of proprioceptive information and the lack of a robust secondary control scheme for the control of ML balance may explain why poor ML sway was more associated with falls as symptom severity increased; unlike AP sway controlled through ankle and hip strategies, there is no robust compensatory strategy for ML sway. Assessing the individual sway measures confirmed this interpretation; higher ML sway frequencies were positively associated with PC3, and the PC3*neuropathy interaction was negatively associated with reporting a fall (i.e., lower ML sway frequency was associated with falls). Accordingly, ML sway may reflect the integrity of the postural control system without being affected by potential compensatory control strategies. The constraints for ML balance control may also explain why ML sway is a consistent predictor of falls and impaired balance across numerous populations[10, 15, 24].
Whereas the large sample size of this cohort enables robust, generalizable conclusions, limitations should also be noted. First, we did not know the types of chemotherapy that women received and different types have varying neurotoxicity. Another limitation was the reliance on retrospective, self-reported falls, rather than prospective falls. Therefore, the ability of sway, specifically ML sway, to prospectively predict falls in cancer survivors needs to be determined. Similarly, neuropathy severity was limited to self-reported symptoms; assessments using objective measures of neuropathy may capture more subtle variation in symptoms than the classifications used here. Co-occurring impairments associated with neuropathy, such as muscle wasting[37, 38] or vestibular ototoxicity[39], may have a significant influence on balance and falls but were not measured in the original trial and thus could not be considered. Here, postural sway was recorded during eyes open quiet standing. It is possible that the effects illustrated here, particularly the effect of neuropathy on specific characteristics of sway, may be magnified in eyes closed quiet standing. Finally, the use of principal component analysis carries several inherent limitations that have been well characterized[40]. Future studies should use these results as a guide for interpreting postural sway measures across different domains. These results can help guide future prospective assessments of neuropathy in relation to balance and falls to determine onset and predictors of fall risk and targets for fall prevention approaches.
Supplementary Material
Highlights.
Postural sway has magnitude, frequency, and mediolateral principal components
Cancer survivors have impaired magnitude and mediolateral sway
Frequency of mediolateral sway was associated with self-reported falls
Relationship between falls, neuropathy, and sway differed by component
ACKNOWLEDGMENTS:
The authors would like to thank Dr. Patty Carlson-Kuhta.
FUNDING:
This work was supported by the National Cancer Institute within the National Institutes of Health [1R01CA163474-01 to K.W.S and HHSN261201600067C to M.E].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
FBH and ME have a significant financial interest in ADPM, a company that may have a commercial interest in the results of this research and technology. This potential conflict has been reviewed and managed by OHSU. No other authors declare a conflict of interest.
References
- [1].Ortman JM, Velkoff VA, Hogan H, An aging nation: the older population in the United States, United States Census Bureau, Economics and Statistics Administration, US Department of Commerce; 2014. [Google Scholar]
- [2].Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH, Cancer survivors: a booming population, Cancer Epidemiol Biomarkers Prev 20(10) (2011) 1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen Z, Maricic M, Aragaki AK, Mouton C, Arendell L, Lopez AM, et al. , Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women’s Health Initiative, Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 20(4) (2009) 527–36. https://www.ncbi.nlm.nih.gov/pubmed/18766294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Winters-Stone KM, Torgrimson B, Horak F, Eisner A, Nail L, Leo MC, et al. , Identifying factors associated with falls in postmenopausal breast cancer survivors: a multi-disciplinary approach, Arch Phys Med Rehabil 92(4) (2011) 646–52. https://www.ncbi.nlm.nih.gov/pubmed/21367394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, Lopez AM, et al. , Fracture risk among breast cancer survivors: results from the Women’s Health Initiative Observational Study, Arch Intern Med 165(5) (2005) 552–8. https://www.ncbi.nlm.nih.gov/pubmed/15767532. [DOI] [PubMed] [Google Scholar]
- [6].Monfort SM, Pan X, Patrick R, Ramaswamy B, Wesolowski R, Naughton MJ, et al. , Gait, balance, and patient-reported outcomes during taxane-based chemotherapy in early-stage breast cancer patients, Breast Cancer Res Treat 164(1) (2017) 69–77. https://www.ncbi.nlm.nih.gov/pubmed/28374323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Monfort SM, Pan X, Patrick R, Singaravelu J, Loprinzi CL, Lustberg MB, et al. , Natural history of postural instability in breast cancer patients treated with taxane-based chemotherapy: A pilot study, Gait Posture 48 (2016) 237–242. https://www.ncbi.nlm.nih.gov/pubmed/27341530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schmitt AC, Repka CP, Heise GD, Challis JH, Smith JD, Comparison of posture and balance in cancer survivors and age-matched controls, Clinical biomechanics (Bristol, Avon) 50 (2017) 1–6. https://www.ncbi.nlm.nih.gov/pubmed/28968535. [DOI] [PubMed] [Google Scholar]
- [9].Wampler MA, Topp KS, Miaskowski C, Byl NN, Rugo HS, Hamel K, Quantitative and clinical description of postural instability in women with breast cancer treated with taxane chemotherapy, Arch Phys Med Rehabil 88(8) (2007) 1002–8. https://www.ncbi.nlm.nih.gov/pubmed/17678662. [DOI] [PubMed] [Google Scholar]
- [10].Lord SR, Rogers MW, Howland A, Fitzpatrick R, Lateral stability, sensorimotor function and falls in older people, J Am Geriatr Soc 47(9) (1999) 1077–81. https://www.ncbi.nlm.nih.gov/pubmed/10484249. [DOI] [PubMed] [Google Scholar]
- [11].Beijers AJ, Mols F, Vreugdenhil G, A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration, Support Care Cancer 22(7) (2014) 1999–2007. https://www.ncbi.nlm.nih.gov/pubmed/24728618. [DOI] [PubMed] [Google Scholar]
- [12].Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, et al. , Falls, Functioning, and Disability Among Women With Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy, J Clin Oncol 35(23) (2017) 2604–2612. https://www.ncbi.nlm.nih.gov/pubmed/28586243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maurer C, Peterka RJ, A new interpretation of spontaneous sway measures based on a simple model of human postural control, J Neurophysiol 93(1) (2005) 189–200. https://www.ncbi.nlm.nih.gov/pubmed/15331614. [DOI] [PubMed] [Google Scholar]
- [14].Rocchi L, Chiari L, Cappello A, Feature selection of stabilometric parameters based on principal component analysis, Med Biol Eng Comput 42(1) (2004) 71–9. https://www.ncbi.nlm.nih.gov/pubmed/14977225. [DOI] [PubMed] [Google Scholar]
- [15].Maki BE, Holliday PJ, Topper AK, A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population, J Gerontol 49(2) (1994) M72–84. https://www.ncbi.nlm.nih.gov/pubmed/8126355. [DOI] [PubMed] [Google Scholar]
- [16].Cenciarini M, Peterka RJ, Stimulus-dependent changes in the vestibular contribution to human postural control, J Neurophysiol 95(5) (2006) 2733–50. https://www.ncbi.nlm.nih.gov/pubmed/16467429. [DOI] [PubMed] [Google Scholar]
- [17].Dickstein R, Shupert CL, Horak FB, Fingertip touch improves postural stability in patients with peripheral neuropathy, Gait Posture 14(3) (2001) 238–47. https://www.ncbi.nlm.nih.gov/pubmed/11600327. [DOI] [PubMed] [Google Scholar]
- [18].Riandini T, Wee HL, Khoo EYH, Tai BC, Wang W, Koh GCH, et al. , Functional status mediates the association between peripheral neuropathy and health-related quality of life in individuals with diabetes, Acta Diabetol 55(2) (2018) 155–164. https://www.ncbi.nlm.nih.gov/pubmed/29185052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mancini M, Salarian A, Carlson-Kuhta P, Zampieri C, King L, Chiari L, et al. , ISway: a sensitive, valid and reliable measure of postural control, Journal of neuroengineering and rehabilitation 9 (2012) 59 https://www.ncbi.nlm.nih.gov/pubmed/22913719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Winters-Stone KM, Li F, Horak F, Luoh SW, Bennett JA, Nail L, et al. , Comparison of tai chi vs. strength training for fall prevention among female cancer survivors: study protocol for the GET FIT trial, BMC Cancer 12 (2012) 577 https://www.ncbi.nlm.nih.gov/pubmed/23217054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Scoppa F, Capra R, Gallamini M, Shiffer R, Clinical stabilometry standardization: basic definitions--acquisition interval--sampling frequency, Gait Posture 37(2) (2013) 290–2. [DOI] [PubMed] [Google Scholar]
- [22].McIlroy WE, Maki BE, Preferred placement of the feet during quiet stance: development of a standardized foot placement for balance testing, Clinical biomechanics (Bristol, Avon) 12(1) (1997) 66–70. https://www.ncbi.nlm.nih.gov/pubmed/11415674. [DOI] [PubMed] [Google Scholar]
- [23].Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB, Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors, Journal of bioengineering & biomedical science Suppl 1 (2011) 007 https://www.ncbi.nlm.nih.gov/pubmed/24955286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Melzer I, Benjuya N, Kaplanski J, Postural stability in the elderly: a comparison between fallers and non-fallers, Age Ageing 33(6) (2004) 602–7. https://www.ncbi.nlm.nih.gov/pubmed/15501837. [DOI] [PubMed] [Google Scholar]
- [25].Mitchell SL, Collins JJ, De Luca CJ, Burrows A, Lipsitz LA, Open-loop and closed-loop postural control mechanisms in Parkinson’s disease: increased mediolateral activity during quiet standing, Neurosci Lett 197(2) (1995) 133–6. https://www.ncbi.nlm.nih.gov/pubmed/8552278. [DOI] [PubMed] [Google Scholar]
- [26].King LA, Mancini M, Fino PC, Chesnutt J, Swanson CW, Markwardt S, et al. , Sensor-Based Balance Measures Outperform Modified Balance Error Scoring System in Identifying Acute Concussion, Ann Biomed Eng 45(9) (2017) 2135–2145. https://www.ncbi.nlm.nih.gov/pubmed/28540448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Horak FB, Nashner LM, Central programming of postural movements: adaptation to altered support-surface configurations, J Neurophysiol 55(6) (1986) 1369–81. https://www.ncbi.nlm.nih.gov/pubmed/3734861. [DOI] [PubMed] [Google Scholar]
- [28].Horak FB, Nashner LM, Diener HC, Postural strategies associated with somatosensory and vestibular loss, Exp Brain Res 82(1) (1990) 167–77. https://www.ncbi.nlm.nih.gov/pubmed/2257901. [DOI] [PubMed] [Google Scholar]
- [29].Simmons RW, Richardson C, Pozos R, Postural stability of diabetic patients with and without cutaneous sensory deficit in the foot, Diabetes Res Clin Pract 36(3) (1997) 153–60. https://www.ncbi.nlm.nih.gov/pubmed/9237781. [DOI] [PubMed] [Google Scholar]
- [30].Creath R, Kiemel T, Horak F, Peterka R, Jeka J, A unified view of quiet and perturbed stance: simultaneous co-existing excitable modes, Neurosci Lett 377(2) (2005) 75–80. https://www.ncbi.nlm.nih.gov/pubmed/15740840. [DOI] [PubMed] [Google Scholar]
- [31].Kuo AD, Speers RA, Peterka RJ, Horak FB, Effect of altered sensory conditions on multivariate descriptors of human postural sway, Exp Brain Res 122(2) (1998) 185–95. https://www.ncbi.nlm.nih.gov/pubmed/9776517. [DOI] [PubMed] [Google Scholar]
- [32].Baston C, Mancini M, Schoneburg B, Horak F, Rocchi L, Postural strategies assessed with inertial sensors in healthy and parkinsonian subjects, Gait Posture 40(1) (2014) 70–5. https://www.ncbi.nlm.nih.gov/pubmed/24656713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Horak FB, Dickstein R, Peterka RJ, Diabetic neuropathy and surface sway-referencing disrupt somatosensory information for postural stability in stance, Somatosens Mot Res 19(4) (2002) 316–26. https://www.ncbi.nlm.nih.gov/pubmed/12590833. [DOI] [PubMed] [Google Scholar]
- [34].Horak FB, Hlavacka F, Somatosensory loss increases vestibulospinal sensitivity, J Neurophysiol 86(2) (2001) 575–85. https://www.ncbi.nlm.nih.gov/pubmed/11495933. [DOI] [PubMed] [Google Scholar]
- [35].Day BL, Steiger MJ, Thompson PD, Marsden CD, Effect of vision and stance width on human body motion when standing: implications for afferent control of lateral sway, J Physiol 469 (1993) 479–99. https://www.ncbi.nlm.nih.gov/pubmed/8271209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Winter DA, Human balance and posture control during standing and walking, Gait Posture 3(4) (1995) 193–214. [Google Scholar]
- [37].Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. , Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy, J Clin Oncol 19(9) (2001) 2381–9. https://www.ncbi.nlm.nih.gov/pubmed/11331316. [DOI] [PubMed] [Google Scholar]
- [38].Nissen MJ, Shapiro A, Swenson KK, Changes in weight and body composition in women receiving chemotherapy for breast cancer, Clin Breast Cancer 11(1) (2011) 52–60. https://www.ncbi.nlm.nih.gov/pubmed/21421523. [DOI] [PubMed] [Google Scholar]
- [39].Sarafraz M, Ahmadi K, Paraclinical evaluation of side-effects of Taxanes on auditory system, Acta Otorhinolaryngol Ital 28(5) (2008) 239–42. https://www.ncbi.nlm.nih.gov/pubmed/19186452. [PMC free article] [PubMed] [Google Scholar]
- [40].Joliffe IT, Morgan BJ, Principal component analysis and exploratory factor analysis, Stat Methods Med Res 1(1) (1992) 69–95. https://www.ncbi.nlm.nih.gov/pubmed/1341653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.