Abstract
Mast cells have functional plasticity affected by their tissue microenvironment, which greatly impacts their inflammatory responses. Because lactic acid (LA) is abundant in inflamed tissues and tumors, we investigated how it affects mast cell function. Using IgE-mediated activation as a model system, we found that LA suppressed inflammatory cytokine production and degranulation in mouse peritoneal mast cells, data that were confirmed with human skin mast cells. In mouse peritoneal mast cells, LA-mediated cytokine suppression was dependent on pH- and monocarboxylic transporter-1 expression. Additionally, LA reduced IgE-induced Syk, Btk, and ERK phosphorylation, key signals eliciting inflammation. In vivo, LA injection reduced IgE-mediated hypothermia in mice undergoing passive systemic anaphylaxis. Our data suggest that LA may serve as a feedback inhibitor that limits mast cell-mediated inflammation.
Keywords: allergy, asthma, inflammation, lactate, anaphylaxis
Summary statement:
Lactic acid inhibits IgE-induced mast cell inflammatory responses in vitro and in vivo.
1. INTRODUCTION
Mast cells function as innate sentinels, rapidly responding to bacterial, viral, and parasitic pathogens [1]. However, their detrimental response to allergens is central to allergic disease [2,3]. While mast cells are activated by a variety of stimuli, the best characterized is IgE-antigen crosslinking of the high affinity IgE receptor, FcεRI, which elicits a biphasic response [4]. The early phase is characterized by degranulation, releasing a myriad of pre-formed mediators, including histamine, proteases, proteoglycans, and some cytokines [5]. This is rapidly followed by release of inflammatory lipids, including arachidonic acid metabolites and sphingosine-1-phosphate [6,7]. The late phase consists of de novo cytokine and chemokine synthesis, which can dictate the migration and function of other cell populations [8,9]. These responses are collectively inflammatory. However, anti-inflammatory and pro-angiogenic activities also occur, including synthesis and activation of latent TGFβ1, and angiogenesis induced by VEGF, tryptase, and chymase [10-18].
Mast cell function is altered by tissue microenvironmental cues, including growth factors, cytokines, and chemokines. These stimuli can influence IgE-induced activation. For example, SCF augments FcεRI signals [19], while this pathway is blunted by TGFβ1 [20,21]. Recent interest in immunometabolism prompted us to investigate how the glycolytic by-product LA affects mast cell function. LA concentrations vary widely with metabolic activity and tissue perfusion, and correlate with clinical features. For example, LA or lactate levels are linked to outcomes or severity in cancer [22-25], sepsis [26], pulmonary embolism [27], and asthma [2,28,29]. But more than just a marker of glucose metabolism, LA is functionally important. Lactate or LA suppresses the inflammatory functions of macrophages, dendritic cells, and T cells [30-36].
We recently found that LA suppresses mast cell responses to IL-33 [37]. The current work extends this to IgE-mediated mast cell function. Our results show that LA suppresses FcεRI phosphorylation signals, correlating with reduced degranulation and the production of inflammatory cytokines and chemokines. Furthermore, LA suppressed IgE-mediated anaphylactic shock in vivo, supporting our contention that this metabolic product has immunomodulatory effects that include feedback suppression of IgE-induced inflammation. These data shed new light on how metabolism is tied to allergic inflammation, an area of growing importance.
2. MATERIALS AND METHODS
2.1. Animals
C57BL/6J male and female mice were purchased from The Jackson Laboratory (Bar Harbor, ME), bred in the VCU Animal Care Facility, and used at a minimum of 6 weeks old, with approval from the Virginia Commonwealth University Institutional Animal Care and Use Committee (IACUC).
2.2. Mouse Mast Cell Cultures
Mouse peritoneal mast cells (PMC) were obtained by collecting peritoneal lavage from C57BL/6J mice. Cells were expanded in complete RPMI (cRPMI) 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) containing 10% FBS, 2mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1mM sodium pyruvate, and 1mM HEPES (all from Corning, Corning, NY), cRPMI supplemented with recombinant mouse IL-3 and SCF at 10ng/ml each for 14 days, at which time they were approximately 85% mast cells, based on staining for c-Kit and FcεRI surface expression. Bone marrow derived mast cells (BMMC) were derived by harvesting bone marrow from C57BL/6J mouse femurs, followed by culture in cRPMI supplemented with IL-3-containing supernatant from WEHI-3B cells and SCF-containing supernatant from BHK-MKL cells. The final concentrations of IL-3 and SCF were adjusted to 1.5ng/ml and 15ng/ml, respectively, as measured by ELISA. BMMC were used after 3 weeks of culture, at which point these primary populations were >90% mast cells, based on staining for c-Kit and FcεRI surface expression.
2.3. Human Skin Mast Cell Culture
As approved by the Internal Review Board at the University of South Carolina, surgical skin samples were collected from the Cooperative Human Tissue Network of the National Cancer Institute. Skin mast cells (SkMC) were harvested and cultured from 5 human donors as previously described [38]. Mast cells were used after 6-10 weeks of culture, when purity was nearly 100%, as confirmed with toluidine blue staining.
2.4. Cytokines and Reagents
Recombinant mouse IL-3 and SCF, as well as mouse IL-6, TNF, and MCP-1 (CCL-2) ELISA kits were purchased from BioLegend (San Diego, CA). Mouse MIP-1α(CCL-3) and VEGF ELISA kits were purchased from PeproTech (Rocky Hill, NJ). Mouse IL-13 ELISA kits were purchased from eBioscience (San Diego, CA). L-(+)-lactic acid and Sodium L-lactate were purchased from Sigma-Aldrich (St. Louis, MO). Human TNF and MCP-1 ELISA kits were purchased from BD OptEIA (BD Biosciences; Franklin Lakes, NJ).
2.5. Cell Culture Conditions
For IgE crosslinking, mouse mast cells were incubated with 0.5 μg/ml of mouse IgE clone C38-2 (BD Pharmingen, San Jose, CA) overnight. SkMC were incubated with 1 αg/ml IgE overnight. Cells were washed and resuspended at 2×l06 cells/ml in same media growth factors used for culture. An equal volume of 25mM LA in cRPMI was added to the cell suspension, resulting in a final cell concentration of l×106 cells/ml, 10ng/ml of IL-3 and SCF, and 12.5mM LA. Control conditions received cRPMI in place of LA. After 24 hours of pretreatment in LA media, cells received 50ng/ml of DNP-HSA for 16 hours, after which supernatants were collected. pH was measured for media alone, lactic acid-conditioned media, and lactate-conditioned media using a Beckman Phi 45 pH meter.
2.6. Degranulation Assay
Mast cells were cultured with IgE (0.5 μg/ml) overnight, then treated +/− 12.5 mM LA for 24 hours as described in section 2.5. IL-3 and SCF were removed during the last 4 hours prior to activation. Cells were activated with DNP-HSA (50ng/ml) for 10 minutes at 37°C, then washed twice with PBS and stained for degranulation using PE anti-CD63 and APC anti-CD107a (BioLegend, San Diego, CA), using a BD FACSCelesta (BD Biosciences, San Jose, CA).
2.7. RT-qPCR Assay
TRIzol was purchased from Life Sciences (Grand Island, NY) and used to extract total RNA. Nucleic acid purity was measured using a Nanodrop 1000 UV-Vis Spectrophotometer (Thermo Scientific, Waltham, MA). cDNA was synthesized with the qScript cDNA synthesis kit (Quanta Biosciences, Gaithersburg, MD) following the manufacturer’s protocol. To determine IL-6 and GAPDH mRNA expression, real time quantitative PCR (RT-qPCR) was performed with the CFX96 Touch Real-Time PCR Detection System (Bio-Rad) and PerfeCTa SYBR Green SuperMix (Quanta Biosciences). Primers for IL-6 (forward: 5’-TCCAGTTGCCTTCTTGGGAC-3’, reverse: 5’-GTGTAATTAAGCTCCGACTTG-3’) and GAPDH (GAPDH forward, 5’-GATGACATCAAGAAGGTGGTG-3’, reverse, 5’-GCTGTAGCCAAATTCGTTGTC-3’) were purchased from Eurofins MWG Operon (Huntsville, AL). Amplification conditions were as follows: 95°C (2 min) followed by 40 cycles of 95°C (15 s), 55°C (30 s), and 60°C (1 min). A melting curve analysis was performed between 50°C and 95°C. Results were normalized to GAPDH and the H2O control by using the Relative Livak Method (ΔΔCt).
2.8. Surface Staining with Flow Cytometry
PMC were cultured +/− IgE (0.5 μg/ml) overnight, then treated +/− 12.5 mM LA for 24 hours as in section 2.5. Cells were stained with a PE-anti IgE antibody to determine if LA exposure affected surface staining. Cells cultured without IgE were stained with APC-anti-FcεRIα to determine if LA altered FcεRI surface expression. Samples were analyzed with a FACSCelesta flow cytometer (BD Biosciences, San Jose, CA).
2.9. PhosFlow
PMC were cultured +/− IgE (0.5 μg/ml) overnight, then treated +/− 12.5 mM LA for 24 hours as in section 2.5. IL-3 and SCF were removed during the last 4 hours of culture. Cells were activated with DNP-HSA (50ng/ml) for 10 minutes at 37°C, then fixed with 1.6% paraformaldehyde. For permeabilization, cells were resuspended in PBS and slowly added to ice-cold methanol solution while vortexing. Cells were then washed and stained with APC-labeled antibodies against pERK1/2, pSyk, and pBtk and analyzed by flow cytometry using a FACSCelesta.
2.10. Passive Systemic Anaphylaxis
Age-matched C57BL/6J mice (12 week old mice) were first injected intraperitoneally with anti-DNP IgE (50 μg). 24 hours later, all mice received subcutaneous injection of 1mg/kg ketoprofen (Spectrum Chemical, New Brunswick, NJ), and 30 minutes later received intraperitoneal injection of either 4mg/kg LA, given as a 4% (w/v) solution dissolved in PBS or PBS alone. 24 hours after LA and PBS injections, all mice received intraperitoneal DNP-HSA (100 μg) to induce anaphylaxis. For histamine-induced anaphylaxis, mice were given the same ketoprofen and LA injections, followed by an intraperitoneal injection of histamine (8 mg) 24 hours after LA injection. Core body temperature was measured using a rectal thermometer probe (Physitemp Instruments, Clifton, NJ). Mice were then euthanized via CO2 asphyxiation. Blood was collected by cardiac puncture in EDTA-coated tubes and centrifuged to isolate plasma.
2.12. Statistical Analysis
Data are presented as mean ± SE and analyzed using GraphPad Prism 6 software (GraphPad, La Jolla, CA). Comparisons between two groups were done using unpaired Student’s t test. Comparisons between multiple groups were done using one-way analysis of variance with Tukey’s post-hoc test. All p values <0.05 were deemed significant.
3. RESULTS
3.1. LA suppresses IgE-mediated cytokine production and degranulation by peritoneal mast cells
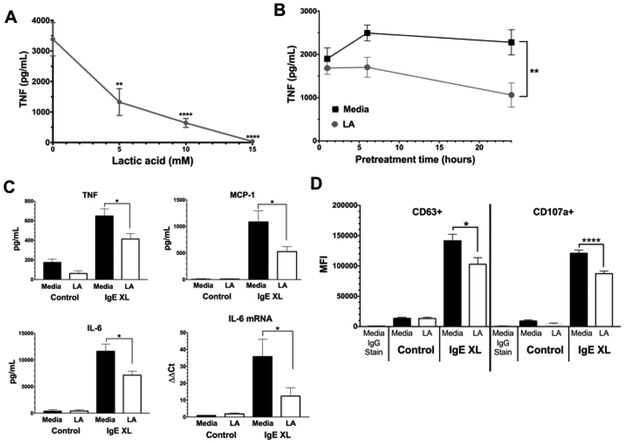
We first examined LA effects using PMC that were expanded in vitro. Cells were cultured +/− IgE overnight, washed, and treated for 24 hours +/− LA at various concentrations, then activated with DNP-HSA for 16 hours. As shown in Figure 1A, LA concentrations ≤5mM greatly reduced TNF secretion. Using 12.5mM LA, we found that a 24-hour culture period consistently yielded suppression (Figure 1B). Because we found no change in cell viability with LA concentrations at or below 20mM (Supplemental Figure 1), 12.5mM LA for 24 hours was used throughout the study. In addition to reducing TNF, LA also suppressed IgE-induced IL-6 and MCP-1 secretion (Figure 1C). The reduction in IL-6 protein was mirrored by changes in mRNA (Figure 1C), suggesting LA reduces cytokine transcription or mRNA stability. In addition to cytokines, LA also inhibited IgE-mediated degranulation. As measured by surface staining for CD63 and CD107a expression [39,40], LA decreased staining intensity (Figure 1D) without altering the percentage of positive cells (Supplemental Figure 2). Collectively, these date show that LA can suppress both the early and late phases of IgE-induced mast cell function.
Figure 1. Kinetics of LA effects on PMC.
(A) PMC were pretreated with the indicated concentrations of lactic acid for 24 hours prior to IgE-Ag crosslinking (IgE XL). Supernatants were analyzed by ELISA 16 hours after activation. Data are from 5 PMC populations analyzed in duplicate. (B) PMC were treated with 12.5mM LA as in (A), with LA given for the indicated times prior to IgE XL. Data are N=6 from 2 independent experiments (C) PMC were pretreated with 12.5 mM lactic acid for 24 hours prior to IgE-Ag crosslinking for 16 hours (ELISA) or 4 hours (RT-qPCR). ELISA results are from 10-12 samples from 2 independent experiments. RT-qPCR data are from 3-4 samples/point. (D) PMC were treated as in (C), with IgE XL for 10 minutes. CD63 and CD107a expression was measured by flow cytometry. Results are representative of three independent experiments using 3 BMMC populations each, and expressed as mean ±SEM. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p0.0001; NS, not significant.
3.2. LA suppresses IgE-mediated cytokine and chemokine production from human skin mast cells
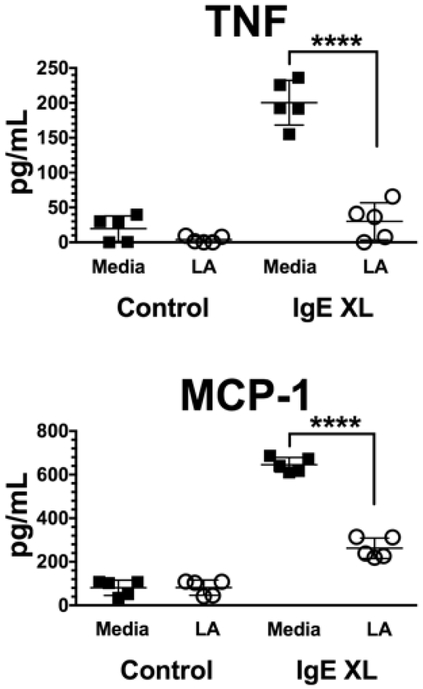
To determine if these results were consistent in human mast cells, we tested SkMC from human donors. Since these cells differentiate in vivo and are expanded ex vivo, they are conceivably one of the most physiologically relevant sources of mast cells. As with PMC, SkMC were sensitized with IgE overnight, washed and treated +/− 12.5 mM LA for 24 hours, then activated with antigen for 16 hours. As shown in Figure 2, LA greatly reduced IgE-mediated TNF and MCP-1 production, an effect consistent among all donors.
Figure 2. LA suppresses IgE-mediated cytokine and chemokine production from human SkMC.
SkMC from 5 donors were cultured with IgE +/− 12.5mM LA and activated as in Figure 1C. Supernatants were collected 16 hours later and analyzed by ELISA. Each icon represents the mean value of triplicate samples from an individual donor. ****, p<0.0001.
3.3. LA does not alter FcεRIα expression or IgE binding
Since acid pH has been used to strip IgE from FcεRI [41], we determined if LA treatment affected IgE receptor occupancy or FcεRI surface expression. PMC were incubated overnight +/− IgE, then with 12.5 mM LA for 24 hours. Cells were stained for surface FcεRIα or surface-bound IgE and analyzed via flow cytometry. LA had no effect on either FcεRI surface expression or surface-bound IgE (Supplemental Figure 3). We conclude that the ability of LA to alter IgE-mediated responses is not due to changes in FcεRI expression or occupancy.
3.5. LA suppresses IgE-mediated cytokine production in a pH- and MCT-1-dependent manner
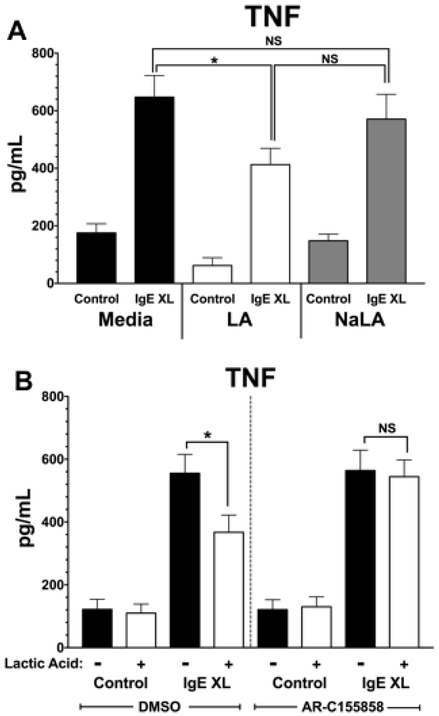
We recently found that LA suppresses IL-33-induced cytokine production in a pH- and MCT-1-dependent manner [37]. We therefore determined if similar mechanisms explain LA effects on IgE signaling. PMC were incubated overnight with IgE, then treated with either media, 12.5 mM LA, or 12.5 mM sodium lactate (NaLA). After treatment for 24 hours, PMC were activated with antigen for 16 hours, and supernatants were analyzed for TNF secretion by ELISA. Figure 3A shows NaLA did not mimic the suppressive effects of LA, indicating that these effects are pH-dependent. To determine if the effect of LA is MCT-1-dependent, PMC were incubated overnight with IgE, then treated with an inhibitor of MCT-1 and MCT-2, AR-C155858. Since we previously found that mast cell do not to express MCT2, any changes with AR-C155858 would be due to MCT-1 blockade [37]. After 1-hour incubation with vehicle (DMSO) or AR-C155858, PMC were treated with media or 12.5 mM LA for 24 hours, then activated for 16 hours with antigen. Figure 3B shows that AR-C155858 treatment prevented the suppressive effects of LA, since IgE-induced TNF was not altered by LA. We concluded that, as with IL-33, LA suppresses IgE-mediated mast cell activation in a pH- and MCT-1-dependent manner.
Figure 3. LA effects are pH- and MCT-1-dependent.
(A) PMC were cultured as in Figure 1C with either 12.5 mM LA or 12.5 mM sodium lactate for 24 hours, then activated by IgE XL for 16 hours. TNF secretion was measured by ELISA. (B) PMC were pretreated with either an MCT-1/2 inhibitor AR-C155858 (100 nM) or DMSO (vehicle) for 1 hour, treated +/− 12.5 mM for 24 hours, then activated via IgE XL for 16 hours. TNF secretion was measured by ELISA. Results are two experiments conducted in triplicate and expressed as mean ±SEM.*, p<0.05; N.S., not significant.
3.6. LA suppresses FcεRI signaling
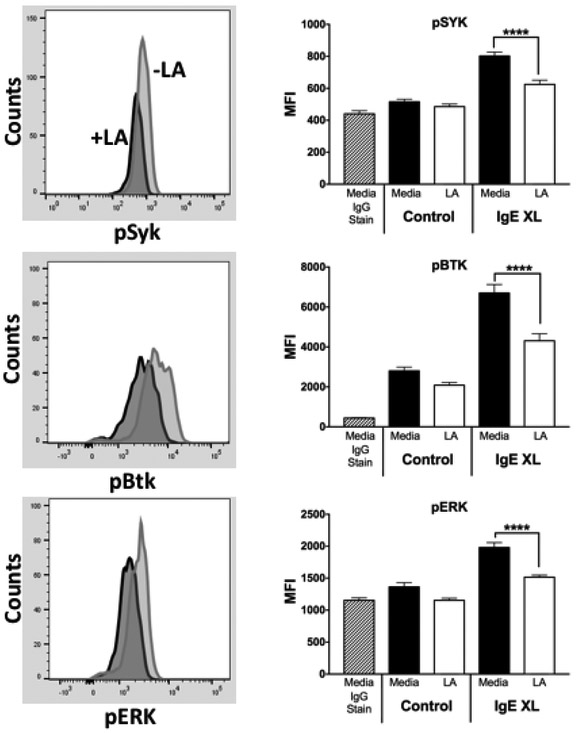
To better understand how LA alters IgE-mediated responses in mast cells, we assessed early events in FcεRI signaling. PMC were incubated overnight with IgE then treated with either media or 12.5 mM LA for 24 hours. PMC were then activated with DNP-HSA for 5 minutes and stained to detected phospho-Syk, phospho-Btk, and phospho-ERK1/2 by flow cytometry analysis. As summarized in Figure 4, LA pre-treatment reduced all three phosphorylation events. These data indicate that LA suppresses the activation of key proteins in the IgE signaling cascade.
Figure 4. LA suppresses FcεRI signaling.
PMC were given IgE overnight and then treated with 12.5 mM LA for 24 hours. During the final 4 hours, IL-3 and SCF were removed. Cells were then given Ag for 10 minutes prior to detection of phospho-proteins as described in Materials and Methods. Left side shows example histograms; right side shows aggregate data. Results are from three experiments conducted in triplicate and expressed as mean ±SEM. ****, p<0.0001.
3.7. LA alters passive systemic anaphylaxis
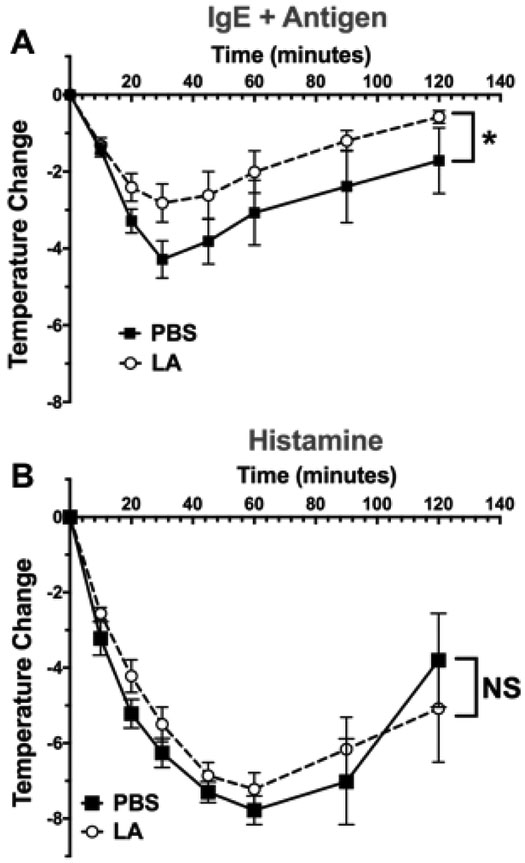
Lastly, we determined if LA alters IgE-mediated mast cell responses in vivo, using the mast cell-mediated PSA model. C57BL/6J mice were injected with IgE, and the next day injected first with ketoprofen to alleviate pain associated with LA, and then either PBS or LA at 4mg/kg [42]. 24 hours after PBS or LA injections, mice were injected with antigen to induce anaphylaxis. As shown in Figure 5A, LA treatment reduced hypothermia, a key indicator of shock. To determine if LA suppressed vascular histamine responses rather than mast cell function, we tested LA effects on histamine-induced shock. We found that LA treatment did not alter histamine-induced hypothermia (Figure 5B), indicating that LA is most likely acting on mast cells rather than the vasculature. These data demonstrate that LA can functionally suppress IgE-mediated mast cell responses in vivo.
Figure 5. LA reduces hypothermia in passive systemic anaphylaxis model.
(A) Age- and gender-matched C57BL/6J mice were intraperitoneally injected with IgE (50 μg). 24 hours later, mice were subcutaneously injected with ketoprofen, and 30 minutes later intraperitoneally injected with LA or PBS. 24 hours later, mice were injected with Ag (100 μg) to induce anaphylaxis. (B) Mice were treated with ketoprofen and LA as in (A), then injected intraperitoneally with 8 mg of histamine. Results shown are from 5 mice per group and expressed as mean ±SEM.*, p<0.05, calculated by Area Under Curve analysis; N.S., not significant.
4. DISCUSSION
LA is abundant in blood and tissues, and likely affects cellular function. LA/lactate levels range from 0.5-2mM in healthy tissue, 18mM in wounds, 25mM in blood during vigorous exercise, and 40mM in tumor sites [43,44]. These elevated concentrations could be due to both reduced tissue perfusion and increased glucose metabolism. Therefore, our in vitro concentrations are physiologically relevant. Adding 12.5mM cRPMI-buffered LA to culture media transiently decreased pH to 6.5, which rebounded to 7.2 over 6 hours (not shown). These conditions mimic sites of inflammation and were previously shown to suppress mast cell and macrophage function by our lab and others [37,45]. There are no published measures of pulmonary lactic acid concentrations in asthmatics, but patients have acidic exhaled breath condensate (EBC) and elevated serum LA/lactate levels [28,46]. Similarly, systemic anaphylaxis is linked to metabolic acidosis [47-49]. It has not been made clear whether LA/lactate has beneficial or pathological effects on asthma or other mast cell-associated diseases.
In this study, LA suppressed IgE-mediated mast cell function. The mechanism of LA effects is partly apparent from these studies. MCT-1 is a H+-dependent transporter. We found that both acidity and MCT-1 expression were required for LA effects. We also noted that LA suppressed early events in the FcεRI signaling cascade, including phosphorylation of the critical kinases Syk and BTK. Syk- or BTK-deficient mast cells have defects in IgE-mediated degranulation and cytokine production, suggesting that antagonizing these kinases could explain the suppressive effects noted in PMC and human SkMC. These and other possibilities warrant further investigation. For example, LA uptake could suppress glucose metabolism, which is critical for IgE-mediated mast cell function [50]. Furthermore, IgE signaling in human LAD2 mast cells stabilizes HIF-1α, which is required for IgE-induced TNF and VEGF secretion [37,51]. Because we and others have previously found that LA induces HIF-1α expression [23,37], this activity should to be at odds with LA suppressing IgE-induced cytokines. This potentially enigmatic role of HIF-1α is a focus of current studies in our laboratory.
Using 5 human donors distinct from our previous work with IL-33-induced function [37], we found that LA effects were consistent in human skin mast cells. While we have noted donor-to-donor variability among human mast cell responses to statins or TGFβ1 [21,39], all 5 donors responded similarly to LA in this study and in our previous work with IL-33. While too small a group to draw broad conclusions, these data suggest that LA effects are less subject to genetic or other variables.
In summary, this study demonstrates that LA, an abundant by-product of glucose metabolism that is increased in inflammatory environments, suppresses IgE-induced cytokine secretion and degranulation, while enhancing angiogenic effects. The suppressive effects were consistent in vitro using mouse or human mast cells, and in vivo using an IgE-induced anaphylaxis model. These data indicate that LA can shape the mast cell response, suppressing inflammation while promoting angiogenesis. Understanding the fundamental biology of LA can reveal important aspects of the inflammatory response, especially as this is now appreciated to rely on increased glucose metabolism.
Supplementary Material
Highlights:
Lactic acid, which is abundant in inflamed tissues and tumor sites, suppresses IgE-induced mast cell inflammatory functions in vitro and in vivo.
Lactic acid effects require acid pH and MCT-1 expression, consistent with proton-dependent transport by MCT-1.
Lactic acid suppressed early IgE receptor-induced phosphorylation events necessary for mast cell function.
Acknowledgements
Supported by grants from the National Institutes of Health 1R01AI59638 and 1R01AI101153 to JJR; and 1R01AI095494 and 1R21AR067996 to CAO.
Abbreviations
- BMMC
Bone marrow derived mast cells
- DNP-HSA
dinitrophenyl-coupled human serum albumin
- LA
Lactic acid
- MCT-1
monocarboxylic transporter-1
- PMC
peritoneal mast cells
- PSA
passive systemic anaphylaxis
- SCF
stem cell factor
- SkMC
human skin mast cells
Footnotes
Conflict-of-interest Disclosures
The authors have no conflicts of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Galli SJ, Borregaard N, Wynn TA, Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils, Nature Immunology. 12 (2011) 1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ishizuka T, Okajima F, Ishiwara M, Iizuka K, Ichimonji I, Kawata T, et al. , Sensitized Mast Cells Migrate Toward the Agen: A Response Regulated by p38 Mitogen-Activated Protein Kinase and Rho-Associated Coiled-Coil-Forming Protein Kinase, The Journal of Immunology. 167 (2001) 2298–2304. doi: 10.4049/jimmunol.167.4.2298. [DOI] [PubMed] [Google Scholar]
- [3].Amin K, The role of mast cells in allergic inflammation, Respiratory Medicine. 106 (2012) 9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- [4].Siraganian RP, de Castro RO, Barbu EA, Zhang J, Mast cell signaling: The role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants, FEBS Letters. 584 (2010) 4933–4940. doi: 10.1016/j.febslet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gilfillan AM, Beaven MA, Regulation of mast cell responses in health and disease, Critical Reviews in Immunology. 31 (2011) 475–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, et al. , Transactivation of Sphingosine-1-Phosphate Receptors by FcεRI Triggering Is Required for Normal Mast Cell Degranulation and Chemotaxis, J Exp Med. 199 (2004) 959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S, Role of ABCC1 in export of sphingosine-1-phosphate from mast cells, Proceedings of the National Academy of Sciences. 103 (2006) 16394–16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Enoksson M, Moller-Westerberg C, Wicher G, Fallon PG, Forsberg-Nilsson K, Lunderius-Andersson C, et al. , Intraperitoneal influx of neutrophils in response to IL-33 is mast cell-dependent, Blood. 121 (2013) 530–536. doi: 10.1182/blood-2012-05-434209. [DOI] [PubMed] [Google Scholar]
- [9].Stone P. Kelly D MD, C.P. MD, D.D.M. MD, IgE, mast cells, basophils, and eosinophils, Journal of Allergy and Clinical Immunology. 125 (2010) S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shaik-Dasthagirisaheb YB, Varvara G, Murmura G, Saggini A, Potalivo G, Caraffa A, et al. , Vascular Endothelial Growth Factor (VEGF), Mast Cells and Inflammation, Int J Immunopathol Pharmacol. 26 (2013) 327–335. doi: 10.1177/039463201302600206. [DOI] [PubMed] [Google Scholar]
- [11].Lee A-J, Ro M, Kim J-H, Leukotriene B 4Receptor 2 Is Critical for the Synthesis of Vascular Endothelial Growth Factor in Allergen-Stimulated Mast Cells, The Journal of Immunology. 197 (2016) 2069–2078. doi: 10.4049/jimmunol.1502565. [DOI] [PubMed] [Google Scholar]
- [12].Ribatti D, Ranieri G, Nico B, Benagiano V, Crivellato E, Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay, Int. J. Dev. Biol 55 (2011) 99–102. doi: 10.1387/ijdb.103138dr. [DOI] [PubMed] [Google Scholar]
- [13].Benitez-Bribiesca L, Wong A, Utrera D, Castellanos E, The Role of Mast Cell Tryptase in Neoangiogenesis of Premalignant and Malignant Lesions of the Uterine Cervix, The Journal of Histochemistry and Cytochemistry. 49 (2001) 1061–1062. [DOI] [PubMed] [Google Scholar]
- [14].Jimenez-Andrade GY, Ibarra-Sanchez A, Gonzalez D, Lamas M, Gonzalez-Espinosa C, Immunoglobulin E induces VEGF production in mast cells and potentiates their pro-tumorigenic actions through a Fyn kinase-dependent mechanism, Journal of Hematology and Oncology. 6 (2013) 1–14. doi: 10.1186/1756-8722-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chumanevich A, Wedman P, Oskeritzian CA, Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor 2 Axis Can Promote Mouse and Human Primary Mast Cell Angiogenic Potential through Upregulation of Vascular Endothelial Growth Factor-A and Matrix Metalloproteinase-2, Mediators of Inflammation. 2016 (2016) 1–8. doi: 10.1155/2016/1503206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, et al. , Mast Cells Can Secrete Vascular Permeability Factor/ Vascular Endothelial Cell Growth Factor and Exhibit Enhanced Release after Immunoglobulin E–dependent Upregulation of Fcε Receptor I Expression, J Exp Med. 188 (1998) 1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blair RJ, Meng H, Marchese MJ, Ren S, Schwartz LB, Tonnesen MG, et al. , Human Mast Cells Stimulate Vascular Tube Formation. Tryptase is a Novel, Potent Angiogenic Factor, The Journal of Clinical Investigation. 99 (1997) 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J, Human Mast Cell Chymase and Leukocyte Elastase Release Latent Transforming Growth Factor-b1 from the Extracellular Matrix of Cultured Human Epithelial and Endothelial Cells, The Journal of Biological Chemistry. 270 (2001) 4689–4696. [DOI] [PubMed] [Google Scholar]
- [19].Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven M, Metcalfe DD, et al. , Btk Plays a Crucial Role in the Amplification of Fc??RI-mediated Mast Cell Activation by Kit, The Journal of Biological Chemistry. 280 (2005) 40261–40270. [DOI] [PubMed] [Google Scholar]
- [20].Gomez G, Ramirez CD, Rivera J, Patel M, Norozian F, Wright HV, et al. , TGF- 1 Inhibits Mast Cell Fc RI Expression, The Journal of Immunology. 174 (2005) 5987–5993. doi: 10.4049/jimmunol.174.10.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fernando J, Faber TW, Pullen NA, Falanga YT, Kolawole EM, Oskeritzian CA, et al. , Genotype-Dependent Effects of TGF- 1 on Mast Cell Function: Targeting the Stat5 Pathway, The Journal of Immunology. 191 (2013) 4505–4513. doi: 10.4049/jimmunol.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, et al. , Targeting the Lactate Transporter MCT1 in Endothelial Cells Inhibits Lactate-Induced HIF-1 Activation and Tumor Angiogenesis, PLoS ONE. 7 (2012) e33418–13. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].De Saedeleer CJ, Copetti T, Porporato PE, Verrax J, Feron O, Sonveaux P, Lactate Activates HIF-1 in Oxidative but Not in Warburg-Phenotype Human Tumor Cells, PLoS ONE. 7 (2012) e46571–12. doi: 10.1371/journal.pone.0046571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O, Lactate Influx through the Endothelial Cell Monocarboxyl ate Transporter MCT1 Supports an NF- B/IL-8 Pathway that Drives Tumor Angiogenesis, Cancer Res. 71 (2011) 2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- [25].Dhup S, Dadhich RK, Porporato PE, Sonveaux P, Multiple Biological Activities of Lactic Acid in Cancer: Influences on Tumor Growth, Angiogenesis and Metastasis, Current Pharmaceutical Design. 18 (2012) 1319–1330. [DOI] [PubMed] [Google Scholar]
- [26].Dahdah A, Gautier G, Attout T, Fiore F, Lebourdais E, Msallam R, et al. , Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis, Journal of Clinical Investigation. 124 (2014) 4577–4589. doi: 10.1172/JCI75212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vanni S, Jimeniz D, Nazaerian P, Morello F, Parisi M, Daghih E, et al. , Short-term clinical outcome of normotensive patients with acute PE and high plasma lactate, Thorax. 70 (2015) 333–338. doi: 10.1136/thoraxjnl-2014-206300. [DOI] [PubMed] [Google Scholar]
- [28].Ostroukhova M, Goplen N, Karim MZ, Michalec L, Guo L, Liang Q, et al. , The role of low-level lactate production in airway inflammation in asthma, American Journal of Physiology-Lung Cellular and Molecular Physiology. 302 (2012) L300–L307. doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Raimondi GA, Gonzalez S, Zaltsman J, Menga G, Adrogué HJ, Acid-base patterns in acute severe asthma, Journal of Asthma. 50 (2013) 1062–1068. doi: 10.3109/02770903.2013.834506. [DOI] [PubMed] [Google Scholar]
- [30].Dietl K, Renner K, Dettmer K, Timischl B, Eberhart K, Dorn C, et al. , Lactic Acid and Acidification Inhibit TNF Secretion and Glycolysis of Human Monocytes, The Journal of Immunology. 184 (2010) 1200–1209. doi: 10.4049/jimmunol.0902584. [DOI] [PubMed] [Google Scholar]
- [31].Errea A, Cayet D, Marchetti P, Tang C, Kluza J, Offermanns S, et al. , Lactate Inhibits the Pro-Inflammatory Response and Metabolic Reprogramming in Murine Macrophages in a GPR81-Independent Manner, PLoS ONE. 11 (2016) e0163694–11. doi: 10.1371/journal.pone.0163694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. , Inhibitory effect of tumor cell–derived lactic acid on human T cells, Blood. 109 (2007) 3812–3819. doi: 10.1182/blood-2006-07. [DOI] [PubMed] [Google Scholar]
- [33].Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W, Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release, Int J Oncol. 39 (2011)453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- [34].Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, et al. , Tumor-derived lactic acid modulates dendritic cell activation and antigen expression, Blood. 107 (2006) 2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- [35].Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E, Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation, Int. J. Cancer 131 (2011)633–640. doi: 10.1002/ijc.26410. [DOI] [PubMed] [Google Scholar]
- [36].Nasi A, Fekete T, Krishnamurthy A, Snowden S, Rajnavolgyi E, Catrina AI, et al. , Dendritic Cell Reprogramming by Endogenously Produced Lactic Acid, The Journal of Immunology. 191 (2013) 3090–3099. doi: 10.4049/jimmunol.1300772. [DOI] [PubMed] [Google Scholar]
- [37].Abebayehu D, Spence AJ, Qayum AA, Taruselli MT, McLeod JJA, Caslin HL, et al. , Lactic Acid Suppresses IL-33-Mediated Mast Cell Inflammatory Responses via Hypoxia-Inducible Factor-1α–Dependent miR-155 Suppression, The Journal of Immunology. 197 (2016) 2909–2917. doi: 10.4049/jimmunol.1600651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kambe N, Kambe M, Kochan JP, Schwartz LB, Human skin–derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes, 97 (2001)2045–2052. [DOI] [PubMed] [Google Scholar]
- [39].Kolawole EM, McLeod JJA, Ndaw V, Abebayehu D, Barnstein BO, Faber T, et al. , Fluvastatin Suppresses Mast Cell and Basophil IgE Responses: Genotype-Dependent Effects, The Journal of Immunology. 196 (2016) 1461–1470. doi: 10.4049/jimmunol.1501932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bahri R, Custovic A, Korosec P, Tsoumani M, Barron M, Wu J, et al. , Mast cell activation test in the diagnosis of allergic disease and anaphylaxis, Journal of Allergy and Clinical Immunology. 142 (2018) 485–496.e16. doi: 10.1016/j.jaci.2018.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Greer AM, Wu N, Putnam AL, Woodruff PG, Wolters P, Kinet J-P, et al. , Serum IgE clearance is facilitated by human FcεRI internalization, Journal of Clinical Investigation. 124 (2014) 1187–1198. doi: 10.1172/JCI68964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Freitas KC, Hillhouse TM, Leitl MD, Negus SS, Effects of Acute and Sustained Pain Manipulations on Performance in a Visual-Signal Detection Task of Attention in Rats, Drug Dev. Res 76 (2015) 194–203. doi: 10.1002/ddr.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wu Y, Dong Y, Atefi M, Liu Y, Elshimali Y, Vadgama JV, Lactate, a Neglected Factor for Diabetes and Cancer Interaction, Mediators of Inflammation. 2016 (2016) 1–12. doi: 10.1155/2016/6456018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Goodwin ML, Harris JE, Hernandez A, Gladden LB, Blood Lactate Measurements and Analysis during Exercise: A Guide for Clinicians, Journal of Diabetes Science and Technology. 1 (2007) 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Colegio OR, Chu N-Q, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. , Functional polarization of tumour-associated macrophages by tumour-derived lactic acid, Nature. 513 (2014) 559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Murata K, Fujimoto K, Kitaguchi Y, Horiuchi T, Kubo K, Honda T, Hydrogen Peroxide Content and pH of Expired Breath Condensate from Patients with Asthma and COPD, COPD: Journal of Chronic Obstructive Pulmonary Disease. 11 (2014) 81–87. doi: 10.3109/15412555.2013.830094. [DOI] [PubMed] [Google Scholar]
- [47].Molls M, Barnauer W, Influence of adrenaline, dibenamine and dopamine on acidosis, hemoconcentration and lethality in protracted anaphylactic shock of guinea pigs, Arch Int Pharmacodyn Ther. 222 (2019) 243–251. [PubMed] [Google Scholar]
- [48].Zielinski J, Koziorowski A, COMBINED RESPIRATORY AND METABOLIC ACIDOSIS CAUSED BY BRONCHOSPASM IN ANAPHYLACTIC SHOCK, Annals of Thoracic Medicine. 21 (2019) 303–310. [DOI] [PubMed] [Google Scholar]
- [49].Post MJ, te Biesebeek JD, Wemer J, van Rooij HH, Porsius AJ, Comparison of the anti anaphylactic effects of milrinone, sulmazole and theophylline in the rat, Int Arch Allergy Appl Immunol. 89 (2019) 6–10. [DOI] [PubMed] [Google Scholar]
- [50].Phong B, Avery L, Menk AV, Delgoffe GM, Kane LP, Cutting Edge: Murine Mast Cells Rapidly Modulate Metabolic Pathways Essential for Distinct Effector Functions, The Journal of Immunology. 198 (2017) 640–644. doi: 10.4049/jimmunol.1601150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sumbayev VV, Yasinska I, Oniku AE, Streatfield CL, Gibbs BF, Involvement of Hypoxia-Inducible Factor-1 in the Inflammatory Responses of Human LAD2 Mast Cells and Basophils, PLoS ONE. 7 (2012) e34259–ll. doi: 10.1371/journal.pone.0034259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.