Abstract
Background
This is an updated version of the original Cochrane Review published in the Cochrane Library in 2013 (Issue 8) on the risk of ovarian cancer in women using infertility drugs when compared to the general population or to infertile women not treated. The link between fertility drugs and ovarian cancer remains controversial.
Objectives
To evaluate the risk of invasive ovarian cancer and borderline ovarian tumours in women treated with ovarian stimulating drugs for subfertility.
Search methods
The original review included published and unpublished observational studies from 1990 to February 2013. For this update, we extended the searches from February 2013 to November 2018; we evaluated the quality of the included studies and judged the certainty of evidence by using the GRADE approach. We have reported the results in a Summary of findings table to present effect sizes across all outcome types.
Selection criteria
In the original review and in this update, we searched for randomised controlled trials (RCTs) and non‐randomised studies and case series including more than 30 participants.
Data collection and analysis
At least two review authors independently conducted eligibility and 'Risk of bias' assessments and extracted data. We grouped studies based on the fertility drug used for two outcomes: borderline ovarian tumours and invasive ovarian cancer. We conducted no meta‐analyses due to expected methodological and clinical heterogeneity.
Main results
We included 13 case‐control and 24 cohort studies (an additional nine new cohort and two case‐control studies), which included a total of 4,684,724 women.
Two cohort studies reported an increased incidence of invasive ovarian cancer in exposed subfertile women compared with unexposed women. One reported a standardised incidence ratio (SIR) of 1.19 (95% confidence interval (CI) 0.54 to 2.25) based on 17 cancer cases. The other cohort study reported a hazard ratio (HR) of 1.93 (95% CI 1.18 to 3.18), and this risk was increased in women remaining nulligravid after using clomiphene citrate (HR 2.49, 95% CI 1.30 to 4.78) versus multiparous women (HR 1.52, 95% CI 0.67 to 3.42) (very low‐certainty evidence). The slight increase in ovarian cancer risk among women having between one and three cycles of in vitro fertilisation (IVF) was reported, but this was not clinically significant (P = 0.18). There was no increase in risk of invasive ovarian cancer after use of infertility drugs in women with the BRCA mutation according to one cohort and one case‐control study. The certainty of evidence as assessed using GRADE was very low.
For borderline ovarian tumours, one cohort study reported increased risk in exposed women with an SIR of 3.61 (95% CI 1.45 to 7.44), and this risk was greater after treatment with clomiphene citrate (SIR 7.47, 95% CI 1.54 to 21.83) based on 12 cases. In another cohort study, the risk of a borderline ovarian tumour was increased, with an HR of 4.23 (95% CI 1.25 to 14.33), for subfertile women treated with IVF compared with a non‐IVF‐treated group with more than one year of follow‐up. A large cohort reported increased risk of borderline ovarian tumours, with HR of 2.46 (95% CI 1.20 to 5.04), and this was based on 17 cases. A significant increase in serous borderline ovarian tumours was reported in one cohort study after the use of progesterone for more than four cycles (risk ratio (RR) 2.63, 95% CI 1.04 to 6.64). A case‐control study reported increased risk after clomiphene citrate was taken, with an SIR of 2.5 (95% CI 1.3 to 4.5) based on 11 cases, and another reported an increase especially after human menopausal gonadotrophin was taken (odds ratio (OR) 9.38, 95% CI 1.66 to 52.08). Another study estimated an increased risk of borderline ovarian tumour, but this estimation was based on four cases with no control reporting use of fertility drugs. The certainty of evidence as assessed using GRADE was very low.
However, although some studies suggested a slight increase in risks of ovarian cancer and borderline ovarian tumour, none provided moderate‐ or high‐certainty evidence, as summarised in the GRADE tables.
Authors' conclusions
Since the last version of this review, only a few new relevant studies have provided additional findings with supporting evidence to suggest that infertility drugs may increase the risk of ovarian cancer slightly in subfertile women treated with infertility drugs when compared to the general population or to subfertile women not treated. The risk is slightly higher in nulliparous than in multiparous women treated with infertility drugs, and for borderline ovarian tumours. However, few studies have been conducted, the number of cancers is very small, and information on the dose or type of fertility drugs used is insufficient.
Plain language summary
Is there an increased risk of ovarian cancer in women treated with drugs for subfertility?
Background Drugs to stimulate ovulation have been used to treat subfertility since the early 1960s. There is uncertainty about the safety of these drugs and their potential risk of causing cancer. Moreover, it has already been shown that infertility itself increases the risk of ovarian cancer.
The aim of the review We aimed with this updated systematic review to summarise current published research on the risk of ovarian cancer in subfertile women treated with fertility drugs compared to the general population and to subfertile women not treated with fertility drugs.
What are the main findings? Overall, based on 37 studies, which included a total of 4,684,724 women, we did not find enough strong evidence suggesting a potentially higher risk of ovarian cancer in women treated with fertility drugs.
A cumulative analysis of 12 case‐control studies from the USA revealed increased risk of ovarian cancer in women using fertility drugs, and this risk was higher in nulliparous women (women who have not given birth) when compared to multiparous women (women who have given birth to more than one child). One of the 37 included studies reported a two‐fold increase in development of serous borderline ovarian tumour in women after more than four cycles of progesterone; however the number of cases included in this group was very small. One cohort study also suggested an increased risk of borderline ovarian tumour in infertile women treated with clomiphene citrate when compared to infertile women who did not undergo treatment to conceive.
Quality of the evidence Studies showing an increase in the risk of ovarian cancer were of low methodological quality, with short follow‐up periods and with lack of adjustment for important confounding factors; therefore the results are too unreliable. However, compared with older studies, recent studies have tended to report both the dose and the number of cycles of infertility drugs and have included more contemporary drug regimens; this has made the final results more reliable.
What are the conclusions? Infertility has been found to be an important risk factor for ovarian cancer. However, the association between infertility drugs and ovarian cancer needs to be addressed with consideration of other factors such as age, body mass index, parity, genetic factors (i.e. family history for ovarian cancer), and aetiology of the infertility, along with longer follow‐up times.
Summary of findings
Summary of findings for the main comparison. Ovarian stimulating drugs in subfertile women compared to subfertile women not treated or versus general population for subfertile women.
| Ovarian stimulating drugs in subfertile women compared to subfertile women not treated or versus general population for subfertile women | |||
| Patient or population: subfertile women Setting: hospital setting Intervention: ovarian stimulating drugs in subfertile women Comparison: subfertile women not treated or general population | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| Risk of invasive ovarian cancer in subfertile women exposed to ovarian stimulating drug vs unexposed women (summary of cohort studies suggesting increased risk) assessed with hazard ratio (HR), standardised incidence ratio (SIR) | Increased risk in women using clomiphene citrate vs unexposed women was reported: HR 1.93 (95% CI 1.18 to 3.18), nulliparous women HR 2.49 (95% CI 1.30 to 4.78), and multiparous women HR 1.37 (95% CI 0.64 to 2.96). Increased risk of SIR was reported at 1.19 (95% CI 0.54 to 2.25), and this was even higher in women using gonadotrophin treatment SIR 5.89 (95% Ci 1.91 to 13.75). When risk was adjusted for age, parity, and subfertility cause, the HR was 2.14 (95% CI 1.07 to 4.25). For increased risk in exposed women after IVF and adjusted for age and obesity, HR was 3.9 (95% 1.2 to 12.6) | 194,583 (4 observational studies) | ⊕⊝⊝⊝ VERY LOWa‐g |
| Risk of invasive ovarian cancer in subfertile women exposed to ovarian stimulating drugs vs unexposed women (summary of case‐control studies suggesting increased risk) assessed with odds ratio (OR) | An increase in risk of ovarian cancer was described in women taking clomiphene for longer than 12 months with SIR 2.5 (95% CI 1.3 to 4.5); this was based on 11 cases, and it included borderline and invasive ovarian tumours. Increased risk was estimated for any infertility drugs with OR 1.78 (95% CI 0.97 to 3.27), for clomiphene citrate with OR 1.32 (95% CI 0.57 to 3.01), for human menopausal gonadotrophin with OR 3.95 (95% 1.33 to 12.2), and for human menopausal gonadotrophin and clomiphene citrate with OR 1.97 (95% CI 1.03 to 3.77) | 35 cases, 543 controls (2 observational studies) | ⊕⊝⊝⊝ VERY LOWa‐e,h |
| Risk of borderline ovarian tumours in subfertile women exposed to ovarian stimulating drugs vs unexposed women (summary of cohort studies suggesting increased risk) assessed with risk ratio (RR), hazard ratio (HR) | Increased risk of borderline ovarian tumours was reported: SIR 3.61 (95% CI 1.45 to 7.44) for women exposed to any ovarian stimulating drugs, and for women exposed to clomiphene citrate SIR 7.47 (95% CI 1.54 to 21.83). Adjusting for age, parity, and subfertility caused a risk increase HR 4.23 (95% CI 1.25 to 14.33). Women undergoing IVF had an increased rate with HR 2.46 (95% CI 1.20 to 5.04); this result was adjusted for parity, age, calendar year, socioeconomic status, infertility diagnoses including pelvic inflammatory disorders and endometriosis, and surgical procedures such as hysterectomy and tubal ligation. However, the risk was not changed after birth (HR 0.89, 95% CI 0.43 to 1.88) nor after hysterectomy (HR 1.02, 95% CI 0.24 to 4.37) nor after sterilisation (HR 1.48, 95% CI 0.63 to 3.48). Risk of serous borderline tumour was increased in women having more than 4 cycles of progesterone (RR 2.63, 95% CI 1.04 to 6.64). A slight increase in borderline was reported with HR 1.95 (95% CI 1.18 to 3.23). However, stratified analyses on parity showed there was no significant difference in risk between nulliparous women (HR 1.69, 95% CI 0.75 to 3.79) and parous women (HR 2.12, 95% CI 1.11 to 4.04) with P = 0.9 | 1,381,732 (5 observational studies) | ⊕⊝⊝⊝ VERY LOWa,b,d,e,i |
| Risk of borderline ovarian cancer in subfertile women exposed to ovarian stimulating drugs vs unexposed women (summary of case‐control studies suggesting an increase) assessed with hazard ratio (HR), odds ratio (OR) | One study suggested an increase in borderline ovarian tumours in women using human menopausal gonadotrophin (OR 3.95, 95% CI 1.33 to 12.2), and risk did not change much after adjustments for age, parity, BMI, region of birth, education, or family history (OR 3.19, 95% CI 0.86 to 11.82). Another study reported increased risk and based its findings on 4 cases with no control reporting the use of fertility drugs | 28 cases, 29 controls (2 observational studies) | ⊕⊝⊝⊝ VERY LOWb‐f,h |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; OR: odds ratio; RR: risk ratio; SIR: standardised incidence ratio. | |||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
aFollow‐up according to type of cancer is not reported. bAll fertility drugs used, dosages, and cycles are not reported. cCancer cases were obtained from medical records; however no blinding of assessors to exposure status is reported. dIt is not reported how cases were ascertained and if there was any blinding of assessors to exposure status. eIt is unclear if all fertility drugs used were investigated. fFertility drugs used and duration are not reported. gAdjustments were made for region of residence, birth cohort, and concomitant exposure to clomiphene citrate. hCancer cases were obtained from a cancer registry, but assessors were not blinded to exposure status. iCancer registry; no blinding of assessors to exposure status is reported.
Background
Description of the condition
Subfertility has been defined as failure to conceive after frequent unprotected sexual intercourse for one year in the absence of known causes of subfertility (NICE 2013). The prevalence of subfertility in Western societies ranges from 3% to 33% (Boivin 2007; Chandra 1998; Greenhall 1990; Healy 1994; Karmaus 1999; Schmidt 1995). It is reported that in the UK, one in seven heterosexual couples suffer from subfertility (NICE 2013). In less developed countries, prevalence has been reported as 6.9% to 9.3% (Boivin 2007). It is presumed that differences in the prevalence of subfertility among different populations in the industrialised countries are due mainly to differences in the definitions and methods of measurement used.
Description of the intervention
Fertility drugs are used during the follicular phase of the menstrual cycle to increase the serum concentration of gonadotrophins, with the aim of promoting maturation of multiple follicles and consequently multiple ovulations. Commonly used ovulation induction agents include (1) anti‐oestrogens, such as clomiphene citrate; (2) tamoxifen, a selective oestrogen receptor modulator (SERM); (3) human menopausal gonadotrophin (HMG), which contains follicle‐stimulating hormone (FSH) and luteinising hormone; (4) human chorionic gonadotrophin (HCG); (5) gonadotrophin‐releasing hormone agonist (GnRH‐AG); (6) gonadotrophin‐releasing hormone antagonist (GnRH‐A); (7) purified FSH; (8) growth hormone; (9) insulin‐like growth factor (IGF); (10) progesterone; and (11) letrozole, which is a third‐generation aromatase inhibitor (Demir 2016; Duffy 2010; Pabuccu 2016). These hormones are used either alone or in combination depending on the cause of infertility and the protocol used. In addition, other fertility drugs used in most regimens of assisted reproductive technologies, such as in vitro fertilisation (IVF), include progestogens to support the luteal phase of the menstrual cycle (Genc 2011). For isolated anovulatory infertility, letrozole and clomiphene citrate alone or in combination with metformin are currently preferred drugs (Wang 2017).
How the intervention might work
Clomiphene citrate and tamoxifen (a selective oestrogen receptor modulator) are used for women whose failure to ovulate is due to a hypothalamic‐pituitary dysfunction type II (World Health Organization Classification (WHO)) (Rowe 1993). Both drugs are prescribed during the early phase of the menstrual cycle (day two to six) to reduce the negative feedback caused by oestrogen and to result in an increase in GnRH secretion from the hypothalamus, which in turn leads to a rise in FSH and luteinising hormone production. These natural gonadotrophin hormones then stimulate the ovary to ovulate. Gonadotrophins (HMG or HCG or FSH) are used in the treatment of subfertility in women with proven hypopituitarism and in those who have not responded to clomiphene, or in superovulation treatment for assisted contraception, such as IVF. They are given with the aim of amplifying and prolonging the endogenous secretion of FSH and to ensure that at least two or three follicles are developed to maximise pregnancy potential.
Growth hormone, IGF, and GnRH all increase the sensitivity of the ovaries to gonadotrophin stimulation and enhance follicular development (Poretsky 1999); they have been shown to have a role in fertility treatment, in that they can improve the outcome of ovarian stimulation therapy. Co‐treatment with growth hormone combined with HMG and HCG for ovulation induction has been suggested as a way to improve follicle growth, and probably pregnancy rate, in women with hypogonadotrophic hypogonadism (Homburg 1995). This reduces the gonadotrophin dose requirement, reduces the duration of HMG treatment, and improves success rates (Duffy 2010). The IGF system is composed of two ligands (IGF‐1 and IGF‐2), two receptors, and insulin‐like growth factor binding protein (IGFBP). Women treated for subfertility with IGF require a lower gonadotropin stimulation dose and reduced induction time (Genc 2011).
Progesterone is used to prepare the endometrium for pregnancy, and its production is supported by HCG, which usually is produced by the corpus luteum. This happens during the luteal phase of the menstrual cycle. During assisted reproduction, levels of progesterone, HCG, or both are low; therefore the natural process may be insufficient to ensure good production of progesterone. This problem is overcome by the use of progesterone, HCG, or GnRH agonists (Demir 2016; Pabuccu 2016; Van der Linden 2011).
Letrozole is a modern third‐generation aromatase inhibitor (AI). Aromatase is a cytochrome P‐450 haemoprotein responsible for catalysing the conversion of androgens to oestrogens. Letrozole effectively blocks the production of oestrogen without exerting effects on steroidogenic pathways. By reducing oestrogen levels, letrozole increases FSH levels and therefore the number of mature follicles with no adverse endometrial effects because it has a shorter half‐life than clomiphene citrate (Allaway 2017).
Studies have suggested that one long‐term effect of fertility drugs could be the development of borderline ovarian tumours or ovarian cancer. Borderline ovarian tumours possess many of the same morphological features as their malignant counterparts, but they do not destructively invade the ovarian stroma, and women in whom they develop have a significantly more favourable prognosis than those with invasive ovarian cancers. Because the aetiology is largely unknown, it is difficult to explain the possible causal association between infertility, fertility drugs, and other reproductive risk factors and borderline ovarian tumours and invasive ovarian cancers. However, it has largely been established that risk factors for the disease relate mostly to reproductive events, and there is general agreement on the protective effects of pregnancy and oral contraceptive use (Rish 1994; Whittemore 1992a). Several hypotheses have postulated ovulation as a potential biologic promoter of ovarian cancer. Research has shown that epithelial ovarian cancer might be caused by repeated ovulation, which disrupts the ovarian epithelium and leads to malignant transformation of the epithelial cells ‐ the so called 'incessant ovulation' hypothesis. Genetic alterations may develop due to the many micro‐traumata and the high mitotic activity associated with ovulation, eventually causing autonomic growth of malignant cells (Fathalla 1971). According to the 'incessant ovulation' theory, promoting ovulation by ovulation induction medications would increase the frequency of invasive ovarian tumours, whilst any factor that suppresses ovulation, such as pregnancy, oral contraception, lactation, and early menopause, would reduce the risk of cancer.
Fertility medication stimulates multiple oocytes so there is simultaneous maturation and ovulation during a single cycle. This serves to increase the mechanical trauma and the number of epithelial inclusions in the surface epithelium of the ovary (Meirow 1996). It has been estimated that a single cycle of ovulation induction in preparation for IVF can be equivalent to two years of normal menstrual cycles, in terms of the number of follicles produced and the oestrogen concentrations achieved (Attia 2006). However, some epidemiological studies contradict this link (Booth 1989; Brinton 1989; Ron 1987; Rossing 1994; Whittemore 1992a). The risk of ovarian cancer in these studies was increased in women with ovulatory disturbances (either lack of ovulation or reduction in the number of ovulations over one year), while according to the 'incessant ovulation' theory, these women would have been expected to have reduced risk of ovarian cancer. Moreover, Balasch 1993 critically reviewed the literature concerning follicular stimulation and ovarian cancer and concluded that even if an association between ovulation induction and ovarian cancer was found, this would not necessarily indicate an effect of ovarian stimulation. A more likely explanation is that an underlying ovulatory disorder or the absence of pregnancy predisposes the woman to cancer of the ovary (Balasch 1993).
The second hypothesis ‐ the gonadotrophin hypothesis ‐ proposes a model in which persistent stimulation of gonadotrophins increases the risk of malignant changes directly, or by acting in combination with a raised concentration of oestrogen (Rish 1998). This theory is based on the animal studies of Biskind carried out in 1944 (Biskind 1944). Biskind found that rats developed ovarian tumours of stromal origin (no epithelial tumours occurred) when they were manipulated to produce high concentrations of gonadotrophins.
Nevertheless, these data do not prove the existence of a casual relationship between iatrogenically raised serum gonadotrophin concentrations (i.e. prescribed by a healthcare provider and not naturally produced by the body) and the development of granulosa cell tumours, as it is possible that the tumour was present before fertility treatment, or association of cancer with the use of gonadotrophins is confidential (Meirow 1996). The gonadotrophin model is consistent with the known protective effects of each additional pregnancy and the duration of oral contraceptive use (Henderson 1998).
Another hypothesis frequently suggested is that undiagnosed early ovarian cancer causes, in some manner, subfertility. This hypothesis was based upon epidemiological data that showed an increased rate of subfertility among women with ovarian cancer (Harris 1992; Whittemore 1992a).
Why it is important to do this review
In spite of an increase in the number of women requesting fertility treatments, the question of whether ovarian stimulation increases the incidence of invasive ovarian cancer, borderline ovarian tumours, or both, as an independent factor remains unanswered. Some studies suggest that the risk of ovarian tumours is not increased among women with primary infertility who do not undergo fertility treatment (Adami 1994; Asante 2013; Hartge 1989; McGowan 1979; Ness 2002; Rish 1994; Rossing 1994). However, it remains difficult to provide reassurance to subfertile women regarding their risk of developing an ovarian tumour due to exposure to fertility treatment.
Ovarian cancer is a relatively rare outcome; it occurs most often late in life ‐ many years after normal childbearing age or completion of fertility therapy. Furthermore, there is uncertainty over the role of various drugs because limited information is available on their different potential effects. An evaluation of risk factors for ovarian cancer was published in a combined analysis of 12 US case‐control studies of ovarian cancer diagnosed between 1956 and 1986 and conducted by the Collaborative Ovarian Cancer Group (US) (Whittemore 1992b). Only three of the 12 studies examined the association between the use of fertility drugs and invasive ovarian cancer; the others evaluated different reproductive and menstrual risk factors. This study showed a 2.7‐fold increased risk of ovarian cancer in subfertile women who had used fertility drugs as compared to those who had not used these drugs, and a 27‐fold higher risk in subfertile women who had never been pregnant compared to subfertile women who had been treated and conceived. In this study, subfertile women who had not used fertility drugs experienced no increase in risk of ovarian cancer compared with women without a history of subfertility (Whittemore 1992b). This study had limitations, for example, few of the women had used fertility medications, making the confidence interval around the risk estimates wide, and some of the fertility drugs when used (such as conjugated oestrogen and diethylstilbestrol) were outdated (Mahdavi 2006). Moreover, poor information was given about the reasons for subfertility among the women included, and this made it impossible to separate treatment effects from ovulatory abnormalities that themselves may increase the risk of ovarian cancer. Moreover, little or no information was provided on the types of medications used or the duration of treatment, and women with ovarian cancer may have been more likely than control participants to recall their exposure to fertility drugs (recall bias), which could have overestimated the risk of association. Subsequently, a large cohort study also suggested increased risk of invasive and borderline ovarian tumours among women using clomiphene citrate for 12 months or longer (Rossing 1994). This finding was confirmed by other studies (Harris 1992; Ness 2002; Nugent 1998; Parazzini 1998; Shushan 1996).
In contrast, several other epidemiological studies failed to confirm the above findings and showed no association between women exposed to treatment with ovulation‐inducing drugs and untreated infertile women (Brinton 2004; Dor 2002; Doyle 2002; Franceschini 1994; Jensen 2009; Modan 1998; Mosgaard 1997; Mosgaard 1998; Rossing 2004; Venn 1999).
Another important group of women for consideration are those at increased risk of developing ovarian cancer due to an inherited germline mutation (BRCA1 and BRCA2 gene mutations). Recent studies suggest that BRCA mutation carriers may have decreased ovarian reserve compared with women without BRCA mutations, as well as an earlier natural menopause (Finch 2013; Wang 2014). This may impact the fertility and reproductive health of BRCA mutation carriers; therefore two studies have looked at any relationship between fertility drugs and ovarian cancer in these groups of women (Gronwald 2015; Perri 2015).
Several reviews have evaluated the long‐term effects of ovulation‐promoting drugs on cancer risk (Brinton 2005; Brinton 2012; Gadducci 2013; He 2012; Mahdavi 2006). To our knowledge, eight reviews and meta‐analyses have evaluated the literature regarding the relationship between fertility drugs and ovarian cancer (Diergaarde 2014; Gadducci 2013; Kashyap 2003; Li 2013; Siristatidis 2013; Tomao 2014; Zarchi (a) 2013; Zhao 2015). One included seven case‐control and three cohort studies (Kashyap 2004), another included only six cohort studies (Li 2013), one four cohort studies and three case‐control studies (Gadducci 2013), and one nine cohort studies calculating the risk of ovarian cancer in infertile women treated with fertility drugs (Siristatidis 2013); yet another included 10 cohort studies (Tomao 2014). The authors for two of these meta‐analyses reported a significantly elevated risk of ovarian cancer in treated subfertile women when compared to the general population (Kashyap 2003; Li 2013). However, data from cohort studies that compared treated versus untreated subfertile women suggest that treated women may tend to have a lower incidence of ovarian cancer (Kashyap 2004). The last published meta‐analysis reported that fertility treatment is not associated with an elevated risk of ovarian cancer (Siristatidis 2013). This meta‐analysis included only some of the observational studies published on this topic up to 2013. A more recent published review and meta‐analysis included 10 cohort studies that assessed the risk of ovarian cancer; however review authors did not include the most recent large cohort and case‐control studies published on the same topic (Zhao 2015). On the contrary, one older review published data on two large case‐control studies and three cohort studies, and highlighted that these recent studies based on large samples of women utilising infertility drugs have yielded reassuring results (Diergaarde 2014). It is therefore important to conduct an updated systematic review including all available evidence.
Objectives
To evaluate the risk of invasive ovarian cancer and borderline ovarian tumours in women treated with ovarian stimulating drugs for subfertility.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), non‐randomised studies (cohort studies and case‐control studies), and case series including more than 30 participants were eligible for inclusion.
Types of participants
Women aged 18 years and older with at least one ovary were included.
Types of interventions
Interventions or exposures of interest include the following fertility medications: clomiphene citrate; selective oestrogen receptor modulator (SERM); luteinising hormone; follicle‐stimulating hormone (FSH); purified FSH; human chorionic gonadotrophin (HCG); gonadotrophin‐releasing hormone agonist (GnRH‐AG); gonadotrophin‐releasing hormone antagonist (GnRH‐A); growth hormone; progesterone; and letrozole. Comparison groups included subfertile women not treated with any of the above mentioned fertility drugs or women from the general population who did not receive fertility treatment.
Types of outcome measures
Primary outcomes
The primary outcome or case of interest is a new diagnosis of primary borderline ovarian tumour or malignant ovarian tumour of epithelial, germ cell, or stromal origin and confirmed by histological investigations.
Search methods for identification of studies
Electronic searches
In the original review, we carried out a comprehensive search for published and unpublished observational studies from 1990 to February 2013. We restricted our search to start from 1990, as subfertility and especially fertility treatment increased in the UK and the USA after 1988. In addition, in initial scoping searches, we did not find any articles referring to any significant research on this topic area published before 1990. For this update, we extended the searches from February 2013 to November 2018. We used the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 11) (Appendix 1);
MEDLINE via Ovid (November week 2 2018) (Appendix 2);
Embase via Ovid (2018 week 46) (Appendix 3).
Searching other resources
Unpublished and grey literature
We searched for published or ongoing studies using the MetaRegister (http://www.controlled‐trials.com), Physicians Data Query (http://www.nci.nih.gov), http://www.clinicaltrials.gov, and http://www.cancer.gov/clinicaltrials.
We searched conference proceedings and abstracts through ZETOC (http://zetoc.mimas.ac.uk) and WorldCat Dissertations. Moreover, we checked the citation lists of included studies, key textbooks, and previous systematic reviews through handsearching, and we contacted experts in the field to identify further reports of trials. If we identified other relevant articles, we searched them for candidate articles. We handsearched reports of conferences in the following sources: Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologists), International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society), British Journal of Cancer, British Cancer Research Meeting, Annual Meeting of the European Society of Medical Oncology (ESMO), and Annual Meeting of the American Society of Clinical Oncology (ASCO).
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database and removed duplicates (EndNote); two review authors (IR and RB) independently examined the remaining references. We excluded studies that clearly did not meet the inclusion criteria, and we obtained copies of the full text of potentially relevant references. At least two review authors (IR and RB or LS) assessed independently the eligibility of retrieved papers. Disagreements were resolved by discussion between the two review authors and, if necessary, by the third review author. We documented reasons for exclusion and contacted study authors to clarify results when necessary.
Data extraction and management
For included studies, we extracted data on study design, characteristics of women (such as eligibility criteria, age, parity, use of oral contraceptive pill, medical diagnosis of subfertility, age of menarche, and family history of ovarian cancer), interventions (type of treatment, dosage and number of treatment cycles), risk of bias, duration and person‐years of follow‐up, histological type of ovarian cancer, summary effect estimates, factors adjusted for, unadjusted and adjusted summary statistics, and location where the study was conducted.
We extracted the number of participants with ovarian cancer in each treatment or exposure group and the number of participants assessed at endpoint and unadjusted and adjusted summary statistics. We noted the time points at which outcomes were collected and reported. Two review authors (IR and RB) abstracted data independently onto a data abstraction form specially designed for the review, and a third review author (LS) checked the extraction in addition to resolving any differences between review authors.
Assessment of risk of bias in included studies
As we did not find any RCTs, assessment of risk of bias focused exclusively on non‐randomised studies.
We assessed risk of bias in non‐randomised studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions Sections 13.5 and 8.5 (Higgins 2011).
We assessed the likelihood of bias due to selection bias, control of confounding, performance bias, detection bias, and attrition bias. We rated studies eliciting a positive response to the following questions as having low risk of bias.
Selection bias and control of confounding
Demonstration that women did not have ovarian cancer at the start of the study and had at least one ovary (cohort studies)
All eligible cases over a defined period of time or a random sample or consecutive series of those cases (case‐control studies)
Community controls derived from the same population as the cases (case‐control studies)
Control of confounding
We pre‐specified the following factors as potential confounders and noted whether they were balanced at baseline (or at outcome assessment for studies that allocated participants to groups on the basis of outcome) between the two groups, or balanced through matching at the time when participants were allocated to groups, or adjusted through an adjusted analysis. These factors were chosen as they are known risk factors for ovarian cancer (cohort studies/case‐control studies).
Risk factors included age, parity, use of oral contraceptive pill, family history of ovarian cancer, age at menarche, age at menopause, smoking, body mass index (BMI), breastfeeding, use of hormone replacement therapy (HRT), social class, hysterectomy status, and causes of subfertility.
Performance bias
Exposure to fertility drugs was ascertained by medical record review (cohort studies/case‐control studies)
The same method was used to ascertain exposure to fertility drugs for cases and controls (case‐control studies)
Assessors of exposure to fertility drugs were blinded to the presence or absence of ovarian cancer (cohort studies/case‐control studies)
Detection bias
Ovarian cancer was confirmed by histology (cohort studies)
Ovarian cancer was confirmed by histology in the cases and no clinical evidence of cancer was used to define the controls (case‐control studies)
Assessors of cancer status were blinded to exposure status (cohort studies/case‐control studies)
Attrition bias
Women exposed to ovarian stimulating drugs and unexposed women in the control group were followed up for the same length of time (cohort studies/case‐control studies)
At least 80% of women in all groups were included in the final analysis, or the description of those not included was not suggestive of bias (cohort studies/case‐control studies)
Measures of treatment effect
We extracted all summary statistics as reported from each study. These included crude and adjusted odds ratio (OR), risk ratio (RR), and hazard ratio (HR) with their respective 95% confidence intervals (CIs). For studies not reporting relative treatment effects, we report the standardised incidence ratio (SIR) with 95% CI. For studies that reported both relative treatment effects and incidence ratios, we preferentially focused on relative effect estimates in the text but reported incidence ratios for completeness.
Unit of analysis issues
None were expected.
Dealing with missing data
We did not impute missing outcome data for the primary outcome. We did not contact study authors to obtain missing outcome data.
Assessment of heterogeneity
As non‐randomised studies are expected to be more heterogeneous than randomised trials due to methodological diversity and greater susceptibility to bias, we showed variation in study findings by presenting a forest plot with the pooled estimate suppressed.
Assessment of reporting biases
We did not formally assess publication bias, as we did not anticipate conducting a meta‐analysis. We conducted a qualitative assessment of the likely impact of publication bias only.
Data synthesis
Our protocol specified that meta‐analysis would be conducted where appropriate. However, meta‐analysis was not performed due to methodological and clinical heterogeneity between studies, suggesting that any overall statistical summary may be misleading. Instead we grouped studies by type of drug given and presented results as a narrative summary in the text and in tables and as a forest plot without an overall summary statistic. Synthesis of the data focused on describing the consistency of effect of ovarian stimulating drugs in causing ovarian cancer, assessing risk of bias, and investigating factors that may explain differences between the results of studies.
Summary of findings
We will present the overall certainty of evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results (Langendam 2013; Schünemann 2011). We created Table 1 based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used GRADEPro GDT 2014. We will use the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014). We will downgrade the evidence from 'high' certainty by one level for serious (or by two for very serious) concerns for each limitation.
High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
As we did not perform meta‐analyses due to expected heterogeneity, we were unable to conduct quantitative subgroup analyses. Instead, we provide a qualitative description of the differences in results between different types of fertility drugs, by whether control groups included infertile women untreated with ovarian stimulating drugs or women from the general population, by parity, and for different histological types of ovarian cancer.
Sensitivity analysis
Sensitivity analysis was not specified as we did not plan to conduct a meta‐analysis.
Results
Description of studies
Results of the search
A search of all databases resulted in a large number of additional studies (1694) to add to the 5176 included in the original version of the review (Rizzuto 2013). We identified 1648 articles after de‐duplication and selected an additional 18 articles for full‐text review after title and abstract screening. We excluded seven articles that did not meet the inclusion criteria. Therefore, we identified a total of 37 studies from the original and new searches for inclusion. See the PRISMA flow diagram for the study selection process (Figure 1). We did not find any articles that required translation among those that met the eligibility criteria. All included articles had an abstract prepared in the English language. We did not identify any RCTs for inclusion.
1.
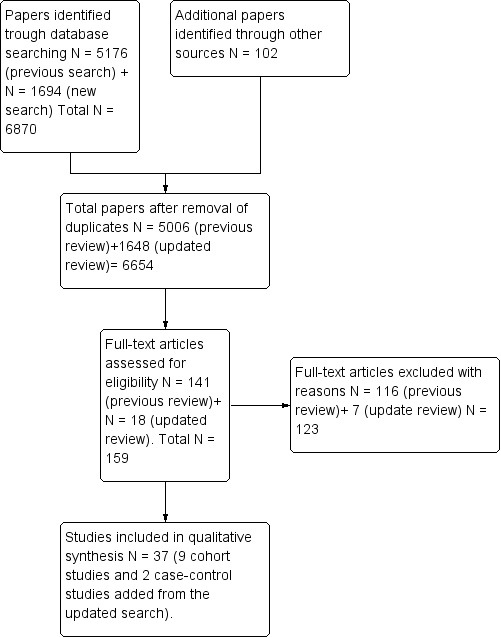
Identification and selection of studies.
Included studies
Cohort studies
We included 24 cohort studies (Bjornholt 2015; Brinton 2013; Calderon‐Margalit 2009; Dor 2002; Dos Santos Silva 2009; Doyle 2002; Kallen 2011; Kessous 2016; Lerner‐Geva 2003; Lerner‐Geva 2012; Luke 2015; Modan 1998; Perri 2015; Potashnik 1999; Reigstad 2015; Reigstad 2017; Sanner 2009; Stewart 2013; Stewart 2013a; Trabert 2013; Van Leeuwen 2011; Venn 1995; Venn 1999; Yli‐Kuha 2012). Ten studies compared the risk of ovarian cancer in subfertile women treated with ovarian stimulating drugs versus the risk in untreated subfertile women attending the same fertility clinics (Brinton 2013; Calderon‐Margalit 2009; Dos Santos Silva 2009; Doyle 2002; Luke 2015; Modan 1998; Sanner 2009; Stewart 2013; Trabert 2013; Van Leeuwen 2011). Two studies reported the risk of invasive ovarian cancer (Gronwald 2015; Perri 2015), and one also reported on borderline ovarian cancer in women with BRCA1 and BRCA2 mutations (Gronwald 2015). Both studies compared treated versus untreated infertile women with the same mutation. One cohort study was reported in two papers. The first looked at the risk of invasive ovarian cancer and borderline ovarian tumours among infertile women who underwent IVF and infertile women who underwent infertility treatment different from IVF, and the other only looked at the increased risk for borderline ovarian cancer associated with IVF (Stewart 2013). Three of these cohort studies also reported the standardised incidence ratio (SIR) for comparison with the general population (Sanner 2009; Van Leeuwen 2011; Venn 1999). Nine studies only compared the risk of ovarian cancer in women treated with ovarian stimulating drugs versus the risk in the general population (Dor 2002; Kessous 2016; Lerner‐Geva 2003; Lerner‐Geva 2012; Modan 1998; Reigstad 2015; Reigstad 2017; Venn 1999; Yli‐Kuha 2012). Three compared the risk in women who gave birth after IVF treatment versus the risk in women who gave birth during the same observation period but with no previous infertility treatment (Kallen 2011; Luke 2015; Reigstad 2015). Two cohort studies reported the risk of ovarian cancer for women who were childless after infertility treatment and for women who were parous after treatment (Stewart 2013). One looked only at the risk of borderline ovarian tumours in infertile women treated with fertility drugs when compared to infertile woman not treated (Bjornholt 2015).
Two cohort studies were conducted in the USA (Luke 2015; Trabert 2013), nine in Israel (Brinton 2013; Calderon‐Margalit 2009; Dor 2002; Kessous 2016; Lerner‐Geva 2003; Lerner‐Geva 2012; Modan 1998; Perri 2015; Potashnik 1999), two in the UK (Dos Santos Silva 2009; Doyle 2002), four in Australia (Stewart 2013; Stewart 2013a; Venn 1999; Venn 1995), two in Sweden (Kallen 2011; Sanner 2009), one in the Netherlands (Van Leeuwen 2011), one in Denmark (Bjornholt 2015), one in Finland (Yli‐Kuha 2012), and two in Norway (Reigstad 2015; Reigstad 2017). All were retrospective, and almost all (30 out of 38) sampled women from fertility clinics. The remainder selected their sample from women enrolled in the Jerusalem Perinatal Study (Calderon‐Margalit 2009), genetics clinics (Perri 2015), a national database called the Assisted Reproductive Technology Clinic Outcomes Reporting System (SART CORS) (Luke 2015), a hospital morbidity database (Stewart 2013;Stewart 2013a), and a hospital database collecting births and admissions (Kessous 2016), and two studies searched data from a database including births (Reigstad 2015; Reigstad 2017). All cohort studies were conducted between 1960 and 2014.
All women in the cohort studies either were premenopausal or had a premature menopause with at least one ovary and were free from ovarian cancer at the start of the study. Almost all studies used HCG, clomiphene citrate, HMG, and GnRH alone or as co‐therapy with each other as ovarian stimulating drugs, and one study analysed the effect of progesterone as well (Bjornholt 2015), but the numbers of cycles and doses used were not reported in 15 studies (Bjornholt 2015; Calderon‐Margalit 2009; Dor 2002; Dos Santos Silva 2009; Doyle 2002; Kallen 2011; Lerner‐Geva 2003; Lerner‐Geva 2012; Modan 1998; Potashnik 1999; Reigstad 2015; Reigstad 2017; Stewart 2013; Venn 1999; Yli‐Kuha 2012). Duration of follow‐up was longer than 10 years in 13 studies (Calderon‐Margalit 2009; Dos Santos Silva 2009; Kessous 2016; Lerner‐Geva 2012; Modan 1998; Perri 2015; Potashnik 1999; Reigstad 2015; Stewart 2013; Stewart 2013a; Trabert 2013; Van Leeuwen 2011; Venn 1999). In four cohort studies, the length of follow‐up was not reported clearly (Bjornholt 2015; Brinton 2013; Dor 2002; Lerner‐Geva 2003), and in another four cohort studies, subfertile women treated were followed up for less than 10 years (Doyle 2002; Kallen 2011; Luke 2015; Yli‐Kuha 2012). One study reported 30 years of follow‐up (Lerner‐Geva 2012).
Case‐control studies
Thirteen case‐control studies were included (Asante 2013; Franceschini 1994; Gronwald 2015; Jensen 2009; Kurta 2012; Mosgaard 1997; Mosgaard 1998; Parazzini 1997; Parazzini 1998; Parazzini 2001; Rossing 1994; Rossing 2004; Shushan 1996), two of which were nested case‐control studies (Jensen 2009; Rossing 1994). Two were conducted in Israel (Shushan 1996; Jensen 2009), four in the USA (Asante 2013; Kurta 2012;Rossing 1994; Rossing 2004), two in Denmark (Mosgaard 1997; Mosgaard 1998), and four in Italy (Franceschini 1994; Parazzini 1997; Parazzini 1998; Parazzini 2001), and one included women from six countries including Sweden, United Kingdom, China, Austria, Italy, and the Netherlands (Gronwald 2015). All studies were conducted between 1994 and 2012. Characteristics of the study samples can be seen in Characteristics of included studies.
Two of 13 case‐control studies involved women from a single hospital (Parazzini 1998; Rossing 1994), and the others were multi‐centre studies. In one study, cases and controls were selected from the Hormones and Ovarian Cancer Prediction (HOPE) study, a national population case‐control study (Kurta 2012). In six case‐control studies, cases were selected from the National Cancer Registry and controls from the same hospital or from the same geographical area (Jensen 2009; Mosgaard 1997; Mosgaard 1998; Rossing 1994; Rossing 2004; Shushan 1996). In five other case‐control studies, cases were selected from hospital clinics (Asante 2013; Franceschini 1994; Parazzini 1997; Parazzini 1998; Parazzini 2001), and in one from genetics clinics (Gronwald 2015). Cases included ages ranging from 18 to 79 years and included invasive ovarian cancer and borderline ovarian tumours. Controls were of a similar age, ranging from 16 to 79 years, and in one study were matched for the same genetic mutation (Gronwald 2015).
In only six case‐control studies was the type of ovarian‐stimulating drug clearly reported (Gronwald 2015; Jensen 2009; Kurta 2012; Mosgaard 1997; Mosgaard 1998; Rossing 1994), consisting of clomiphene citrate, HCG, HMG, and gonadotrophins alone or as co‐therapy, and in seven, specific drugs used were unreported (Asante 2013; Franceschini 1994; Parazzini 1997; Parazzini 1998; Parazzini 2001; Rossing 2004; Shushan 1996). Moreover, the numbers of cycles and doses of drugs used were clearly reported in two studies only (Jensen 2009; Rossing 2004). The duration between exposure and follow‐up was the same for cases and controls in four case‐control studies (Mosgaard 1998; Parazzini 1998; Parazzini 1997; Parazzini 2001). This information was unclear in four studies (Asante 2013; Gronwald 2015; Kurta 2012; Shushan 1996), and it was not the same in three studies (Franceschini 1994; Mosgaard 1997; Rossing 2004).
Excluded studies
In the previous version of this Cochrane Review, we excluded 116 studies after we had read the entire text, most often because they reported on multiple risk factors for invasive ovarian cancer in subfertile women or in the general population. We excluded four studies because they were reviews of case reports (Artini 1997; Balasch 1993; Franco 2000; Lopes 1993), three because they were case series reporting 30 or fewer cases (Dos Santos 2002; Goldberg 1992; Willemsen 1993), and one because the diagnosis of ovarian cancer in these cases was not confirmed by histological reports but was based on ultrasonographic findings (Pozlep 2001). We excluded six articles as they were not primary studies but were pooled (secondary) analyses of case‐control and cohort studies reporting the risk of ovarian cancer in subfertile women using ovarian‐stimulating drugs (Harris 1992; Horn‐Ross 1992; Negri 1991; Ness 2000; Ness 2002; Whittemore 1994), and we excluded one study because the data were published only as an abstract and were not fully informative of the risk of ovarian cancer calculated by the study author (Croughan‐Minihane 2001). In the updated review, we excluded seven articles from the 18 full‐text articles screened. Among the articles excluded, we found four new reviews (Gadducci 2013; Diergaarde 2014; Tomao 2014; Zarchi (a) 2013), as well as one meta‐analysis (Zhao 2015), which did not include all the articles in this review. We also excluded a published editorial on the risk of epithelial ovarian cancer (Mendola 2013). We excluded one of the cohort studies included in the previous review (Brinton 2004), as this was superseded by another, more recent cohort study (Trabert 2013).
Risk of bias in included studies
We found that overall study quality was highly variable between studies, and as all studies were non‐randomised, we judged none of them to be at low risk of bias (Figure 2).
2.
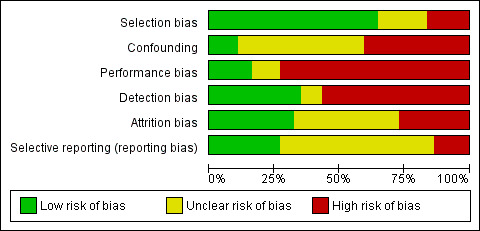
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In all 24 cohort studies, we identified that selection bias was minimised. The sample consisted of all women attending fertility or gynaecological clinics or both, or from national or hospital databases during the defined study period, and they were recruited consecutively. At the study inception, women had no history of ovarian cancer and all had at least one ovary.
In six case‐control studies (Jensen 2009; Mosgaard 1997; Mosgaard 1998; Rossing 1994; Rossing 2004; Shushan 1996), cases were selected from the National Cancer Registry and controls from the same hospital or from the same geographical area as the cases. On the contrary, in the other five case‐control studies (Asante 2013; Franceschini 1994; Parazzini 1997; Parazzini 1998; Parazzini 2001), cases were selected from hospitals and one (Gronwald 2015) from genetic clinics. Age‐matched controls were selected from the general population in the same geographical area from which cases arose in three studies (Mosgaard 1997; Mosgaard 1998; Rossing 2004). In five studies, hospital‐based controls were selected for non‐gynaecological conditions from hospital clinics serving the same areas as those from which cases were selected in four studies (Franceschini 1994; Parazzini 1997; Parazzini 1998; Parazzini 2001), and they were selected from gynaecological clinics in one study (Asante 2013). In four studies, controls were women attending hospital clinics for non‐neoplastic gynaecological conditions (Franceschini 1994; Parazzini 1997; Parazzini 1998; Parazzini 2001), and controls were women from a gynaecological clinic in one study (Asante 2013); in another study, women were part of the control group used in the Women's Contraceptive and Reproductive Experiences (CARE) study of breast cancer, which was another study conducted contemporaneously (Rossing 2004). Two nested case‐control studies randomly selected controls from the entire cohort of women in the study (Jensen 2009; Rossing 1994). One case‐control study obtained cases and controls from a national case‐control study involving several hospitals (Kurta 2012). In another study, cases and controls were recruited from the same genetics clinics and were matched for inherited genetic mutation (Gronwald 2015).
Only two studies matched or adjusted for all or most of the pre‐specified risk factors that we identified as potential confounders, such as age, parity, use of oral contraceptive pill, family history of ovarian cancer, age at menarche, age at menopause, smoking, high BMI, breastfeeding, and use of HRT (Jensen 2009; Mosgaard 1997), and another study adjusted for age, race, duration of use of an oral contraceptive pill (OCP), number of pregnancies, and number of live births (Asante 2013).
Of the 24 cohort studies, five reported the SIR, which was adjusted for age (Dor 2002; Lerner‐Geva 2003; Modan 1998; Potashnik 1999; Venn 1999). One reported calendar time and area of residence (Trabert 2013), two types of infertility (Luke 2015; Sanner 2009), six parity (Bjornholt 2015; Luke 2015; Rossing 1994; Sanner 2009; Stewart 2013; Trabert 2013), eight age (Calderon‐Margalit 2009; Kallen 2011; Luke 2015; Perri 2015; Rossing 2004; Sanner 2009; Trabert 2013; Van Leeuwen 2011), one use of an OCP (Sanner 2009), one smoking and year of delivery after IVF (Kallen 2011), one presence of endometriosis or tubal factor as the reason for subfertility (Van Leeuwen 2011), one marital status and socioeconomic position (Yli‐Kuha 2012), one clinic site, calendar year of first infertility evaluation, and gravidity status at study entry (Trabert 2013), and one age and obesity (Kessous 2016). One study adjusted for age, age at the start of follow‐up, parity, region of residence, and calendar period (Reigstad 2015), and another adjusted for age, calendar year, and socioeconomic status (Stewart 2013). One cohort study reported on invasive ovarian cancer and borderline ovarian tumour combined in the same analysis (Stewart 2013).
Of the 13 case‐control studies, only one did not control for confounding in the analyses and reported a crude estimate (Parazzini 2001). All other studies adjusted for age (Asante 2013; Franceschini 1994; Gronwald 2015; Jensen 2009; Kurta 2012; Mosgaard 1997; Mosgaard 1998; Parazzini 1997; Parazzini 1998; Rossing 1994; Rossing 2004; Shushan 1996). Two adjusted for ethnicity (Kurta 2012; Rossing 2004), and one for region of birth (Shushan 1996). Five studies adjusted for family history of ovarian cancer (Asante 2013; Jensen 2009; Kurta 2012; Mosgaard 1997; Shushan 1996), one for smoking (Mosgaard 1998), eight for parity (Asante 2013; Franceschini 1994; Jensen 2009; Parazzini 1997; Parazzini 1998; Rossing 1994; Rossing 2004; Shushan 1996), one for history of previous cancer (Mosgaard 1997), four for area of residence (Franceschini 1994; Mosgaard 1997; Mosgaard 1998; Rossing 2004), six for education (Franceschini 1994; Jensen 2009; Kurta 2012; Parazzini 1997; Parazzini 1998; Shushan 1996), two for HRT (Mosgaard 1997; Mosgaard 1998), one for intrauterine device (Mosgaard 1997), three for oral contraceptive pill use (Asante 2013; Jensen 2009; Kurta 2012), two for BMI (Mosgaard 1997; Shushan 1996), two for menopausal status (Jensen 2009; Mosgaard 1997), one for age at menopause, history of subfertility, spontaneous miscarriage, and termination of pregnancy (Jensen 2009), three for the number of births (Asante 2013; Kurta 2012; Rossing 2004), and one for race, tubal ligation, age at menarche, duration of breastfeeding, perineal talc use, and family history of ovarian or breast cancer (or both) (Kurta 2012).
Blinding
Recall bias may be a factor in all studies as fertility drug treatment received was obtained by self‐report or retrospective review of case notes and therefore may be incompletely or inaccurately recalled or recorded.
In 15 cohort studies, ascertainment of exposure to fertility drugs was conducted by review of medical records (Bjornholt 2015; Brinton 2013; Dor 2002; Dos Santos Silva 2009; Doyle 2002; Kallen 2011; Lerner‐Geva 2003; Lerner‐Geva 2012; Modan 1998; Potashnik 1999; Sanner 2009; Trabert 2013; Venn 1995; Venn 1999; Yli‐Kuha 2012), and in one cohort study, this was done via a self‐completed questionnaire given to all women in the study (Calderon‐Margalit 2009). In three cohort studies, information was obtained via a self‐completed questionnaire and by review of medical records (Perri 2015; Trabert 2013; Van Leeuwen 2011), and in two from a national database (Luke 2015; Stewart 2013); one cohort study did not specify this information (Kessous 2016). Two cohort studies did not specify how information about infertility and treatment used was ascertained (Reigstad 2015; Reigstad 2017). Blinding of assessors to the presence or absence of ovarian cancer status was not reported in all 24 cohort studies.
In two case‐control studies, exposure to fertility drugs was conducted by review of medical records (Jensen 2009; Rossing 1994). In nine case‐control studies, exposure to fertility drugs was ascertained by a standard questionnaire given to all women in case and control groups, and some information was derived from the medical notes (Asante 2013; Franceschini 1994; Kurta 2012; Mosgaard 1997; Mosgaard 1998; Parazzini 1997; Parazzini 2001; Rossing 2004; Shushan 1996), and in two, the method used was unclear (Gronwald 2015; Parazzini 1998). In five case‐control studies, it is unclear if assessors were blinded to case/control status (Gronwald 2015; Mosgaard 1997; Parazzini 1998; Parazzini 2001; Rossing 2004), and in seven case‐control studies assessors were not blind to the presence or absence of ovarian cancer (Asante 2013; Franceschini 1994; Jensen 2009; Mosgaard 1998; Parazzini 1997; Rossing 1994; Shushan 1996). In all studies, the same method was used to ascertain exposure to fertility drugs for cases and for controls.
Detection bias in relation to ascertainment of outcome was rare across all studies, as all used histology reports to confirm the diagnosis of ovarian cancer, and all control groups had no histological evidence of previous ovarian cancer. However, blinding of investigators to exposure status was not reported.
Incomplete outcome data
Eight studies were at risk of attrition bias because less than 80% of the sample was followed up (Dor 2002; Franceschini 1994; Parazzini 1998; Rossing 2004; Shushan 1996; Stewart 2013; Trabert 2013; Van Leeuwen 2011); in five studies, this information was unclear (Bjornholt 2015; Brinton 2013; Gronwald 2015; Kessous 2016; Rossing 1994). In one cohort study, follow‐up was provided for a mean of 4.87 (± 2.01) years (Luke 2015). In another cohort study, only 60% of the women were followed‐up for longer than 5 years to 10 years (Reigstad 2015).
Selective reporting
In seven cohort studies, the fertility drugs investigated were clearly reported; therefore we judged risk of reporting bias to be low (Bjornholt 2015; Brinton 2013; Luke 2015; Mosgaard 1997; Mosgaard 1998; Trabert 2013; Van Leeuwen 2011). In 10 cohort studies (Calderon‐Margalit 2009; Dos Santos Silva 2009; Kallen 2011; Kessous 2016; Lerner‐Geva 2003; Modan 1998; Perri 2015; Reigstad 2015; Stewart 2013; Yli‐Kuha 2012) it was unclear the fertility drugs investigated and included in the final analysis.
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
See: Table 1
Invasive ovarian cancer
Any fertility drug
Twenty‐one cohort studies ‐ Brinton 2013; Calderon‐Margalit 2009; Dor 2002; Dos Santos Silva 2009; Doyle 2002; Kallen 2011; Kessous 2016; Lerner‐Geva 2003; Luke 2015; Modan 1998; Perri 2015; Potashnik 1999; Reigstad 2015; Reigstad 2017; Sanner 2009; Stewart 2013; Trabert 2013; Van Leeuwen 2011; Venn 1995; Venn 1997; Yli‐Kuha 2012 ‐ and eight case‐control studies ‐ Asante 2013; Franceschini 1994; Kurta 2012; Mosgaard 1997; Parazzini 1997; Parazzini 2001; Rossing 2004; Shushan 1996 ‐ evaluated the incidence of invasive ovarian cancer with use of any fertility drug (Analysis 1.1;Figure 3). Two studies included borderline tumours and invasive ovarian tumours (Rossing 1994; Shushan 1996).
1.1. Analysis.
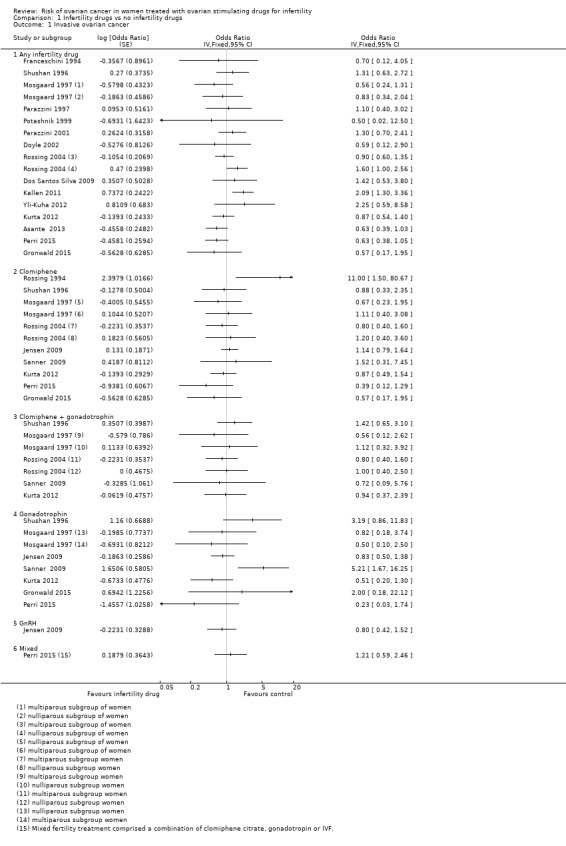
Comparison 1 Infertility drugs vs no infertility drugs, Outcome 1 Invasive ovarian cancer.
3.
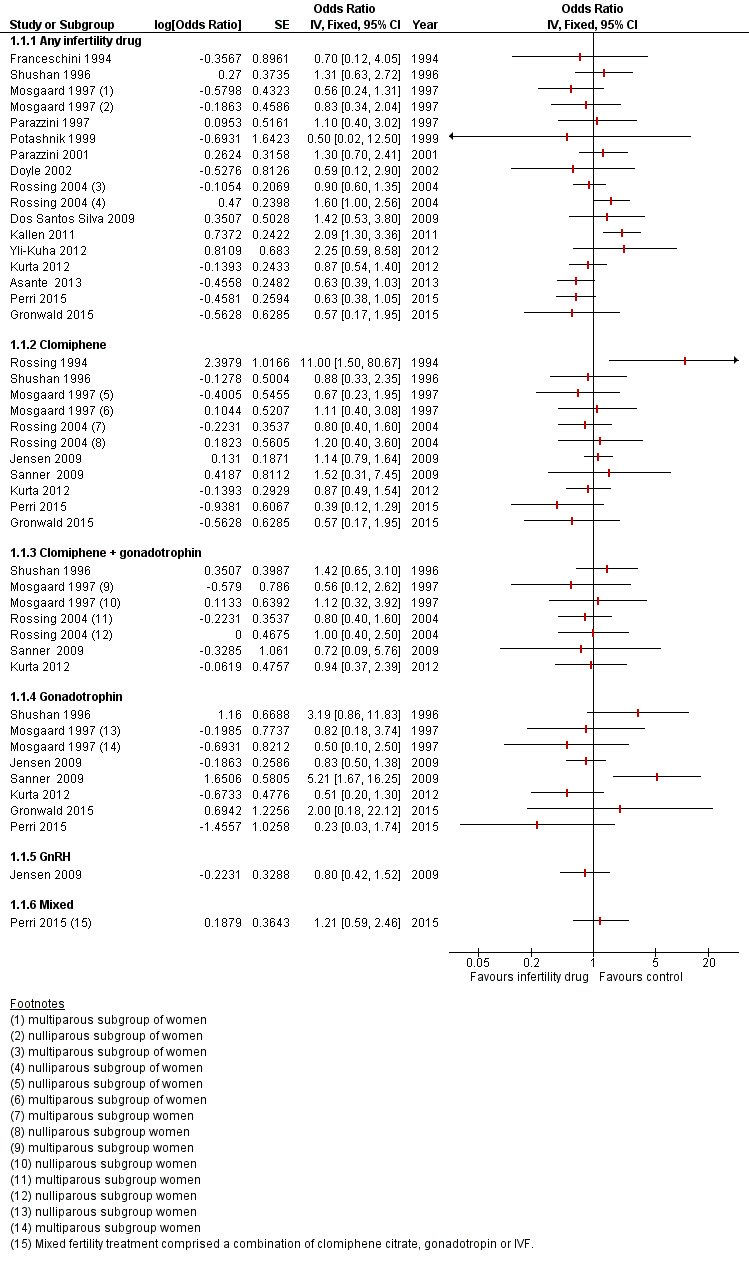
Analyses include only studies reporting risk of ovarian cancer as an odds ratio (OR).
There was no evidence of increased risk with any fertility drug used compared with non‐use in the general population in 15 cohort studies that analysed the risk of ovarian cancer (Brinton 2013; Calderon‐Margalit 2009; Dor 2002; Dos Santos Silva 2009; Doyle 2002; Kallen 2011; Luke 2015; Modan 1998; Perri 2015; Potashnik 1999; Stewart 2013; Trabert 2013;Venn 1995; Venn 1997; Yli‐Kuha 2012).
Five analysed the risk of invasive ovarian cancer according to the number of IVF cycles used (Brinton 2013; Dor 2002; Luke 2015; Van Leeuwen 2011; Venn 1999). One of these studies estimated risk of ovarian cancer with a hazard ratio (HR) of 1.58 (95% confidence interval (CI) 0.75 to 3.29), with higher risk noted among those receiving more than four cycles of IVF with an HR of 1.78 (95% CI 0.76 to 4.13) but with P = 0.18 (Brinton 2013). One cohort study reported no increase in invasive ovarian cancer in subfertile women carriers of a BRCA1 and/or BRCA2 mutation and exposed to fertility drugs. The estimated age‐adjusted odds ratio (OR) was 0.63 (95% CI 0.38 to 1.05) for any type of fertility drug used (Perri 2015). No information about number of cycles was provided.
Six cohort studies suggested increased risk of ovarian cancer (Kessous 2016; Lerner‐Geva 2003; Reigstad 2015; Reigstad 2017; Sanner 2009; Van Leeuwen 2011). One study reported increased risk of ovarian cancer among subfertile women treated with ovarian stimulating drugs when compared to the general population (standardised incidence ratio (SIR) 5.0, 95% CI 1.02 to 14.6) (Lerner‐Geva 2003), which decreased when cancer cases diagnosed within one year of treatment were excluded from the analysis (SIR 1.67, 95% CI 0.02 to 9.27) (Lerner‐Geva 2003). One study showed an increase in invasive ovarian cancer in women given gonadotrophin treatment (SIR 5.89, 95% CI 1.91 to 13.75); four of the five cases reported HCG treatment only (Sanner 2009). One cohort study reported a slight increase in invasive ovarian cancer in women after IVF treatment with any drugs and an HR of 2.14 (95% CI 1.07 to 4.25) (Van Leeuwen 2011). In two cohort studies, ovarian cancer risk was increased more in nulliparous women (HR 2.49, 95% CI 1.30 to 4.78) than in multiparous women (HR 1.37, 95% CI 0.64 to 2.96) (Reigstad 2015; Reigstad 2017). One cohort study suggested an increase in women having IVF when compared to unexposed women and an adjusted HR for age or obesity of 3.9 (95% CI 1.2 to 12.6) (Kessous 2016).
Only two cohort studies clearly reported the different histological types of cancer among included cases (Kallen 2011; Van Leeuwen 2011), as did two case‐control studies (Rossing 1994; Shushan 1996). One cohort study ‐ Perri 2015 ‐ and one case‐control study ‐ Gronwald 2015 ‐ evaluated the risk of ovarian cancer in women carriers of a BRCA1 and/or BRCA2 gene mutation.
One case‐control study suggested a slight increase in the risk of ovarian cancer among women using ovarian stimulating drugs (Shushan 1996). For any type of ovarian stimulating drugs, the OR was 1.78 (95% CI 0.97 to 3.27), and this was based on 24 cases over 200 included cases and 29 controls. The adjusted OR was 1.31 (95% CI 0.63 to 2.74) and was adjusted for age, parity, BMI, region of birth, education, and family history of ovarian cancer.
Eight case‐control studies showed no evidence of increased risk in women who used any fertility drug compared with controls, who were women of a similar age and variably matched for reproductive risk factors (Asante 2013; Franceschini 1994; Gronwald 2015; Jensen 2009; Mosgaard 1997; Parazzini 1997; Parazzini 2001; Rossing 2004). One of those case‐control studies reported no associations among any fertility drugs and numbers of cycles of use, length of follow‐up, or parity (Jensen 2009). Another study suggested no increased risk of ovarian cancer in women using ovarian stimulating drugs even for more than 12 cycles with an adjusted OR of 1.3 (95% CI 0.1 to 13.7) in nulliparous women and an adjusted OR of 0.5 (95% CI 0.1 to 4.2) in multiparous women (Rossing 2004). This was adjusted for age, race, study site, and duration of oral contraceptive use. Another case‐control study reported no increase among parous as well as nulliparous women after treatment with fertility drugs (Mosgaard 1997). In this study, the risk of ovarian cancer among treated infertile versus non‐treated infertile women was given as OR of 0.83 (95% 0.35 to 2.01) for nulliparous and OR of 0.56 (95% CI 0.24 to 1.29) for multiparous women. There was no significant difference in risk even when different treatment regimens were used. Another study reported an OR of 0.84 (95% CI 0.19 to 3.73) adjusted for age and area of residence and an OR of 0.73 (95% 0.16 to 3.30) adjusted for age, area of residence, education, use of oral contraceptives, and number of pregnancies (Franceschini 1994). Another study did not show any increase in risk of ovarian cancer in subfertile women exposed to fertility drugs; this was based on only five cases and 11 controls (Parazzini 1997). The OR was 0.7 (95% CI 0.1 to 7.9) in women using fertility drugs for fewer than six cycles and was 1.0 (95% CI 0.2 to 3.8) in women using fertility drugs for longer than six months. In nulliparous women, the use of any type of fertility drugs was estimated with OR of 0.6 (95% CI 0.1 to 3.5). Another study based the conclusion on 15 cases and 26 controls and reported no increase in risk of ovarian cancer among women using fertility drugs when compared to unexposed women (Parazzini 2001). The OR was 1.3 (95% 0.7 to 2.5), and for women with longer than 25 years from the last use of fertility drugs, the OR was 1.3 (95% CI 0.5 to 3.5). This finding was confirmed even in women with a BRCA inherited mutation by one cohort study (Perri 2015), along with one case‐control study (Gronwald 2015) (Figure 3). In the case‐control study, there was no relationship between the use of any fertility medication or IVF treatment and risk of ovarian cancer in BRCA1 and BRCA2 carrier women with an OR of 0.57 (95% CI 0.17 to 1.95) and an adjusted OR of 0.66 (95% CI 0.18 to 2.33); this was adjusted for age at menarche and was based on four cases (Gronwald 2015). Another case‐control study suggested no increase in risk of ovarian cancer in exposed subfertile women based on 38 cases and 44 controls with an OR of 0.63 (95% CI 0.39 to 1.03) and with an adjusted OR of 0.64 (95% CI 0.37 to 1.11) (Asante 2013). This was adjusted for age, race, duration of oral contraceptive pill use, numbers of pregnancies and live births, and family history of ovarian cancer. The unadjusted OR for subfertile nulliparous women exposed to fertility drugs was 0.57 (95% CI 0.15 to 2.21), and the adjusted OR was 0.59 (95% CI 0.14 to 2.52); this was adjusted for age, race, duration of use of OCP, and family history of ovarian cancer. The estimate was based on seven cases and four controls. The risk of ovarian cancer in multiparous subfertile women had an unadjusted OR of 0.70 (95% CI 0.41 to 1.19) and an adjusted OR of 0.69 (95% CI 0.37 to 1.26); this information was adjusted for age, race, duration of use of OCP, numbers of pregnancies and live births, and family history of ovarian cancer.
Clomiphene
Seven cohort studies ‐(Lerner‐Geva 2012; Perri 2015; Reigstad 2015; Reigstad 2017; Rossing 1994; Sanner 2009; Trabert 2013) and six case‐control studies (Brinton 2013; Jensen 2009; Kurta 2012; Mosgaard 1997; Rossing 2004; Shushan 1996 ) evaluated the incidence of invasive ovarian cancer with clomiphene. Six cohort studies showed no convincing evidence for increased risk of invasive cancer with clomiphene use compared with no use in women with subfertility (Brinton 2013; Calderon‐Margalit 2009; Modan 1998; Perri 2015; Reigstad 2017; Venn 1999) (Analysis 1.1;Figure 3).
One cohort study reported an HR of 0.98 (95% CI 0.14 to 7.11), indicating no evidence of increased risk with clomiphene compared with non‐use in the general population (Calderon‐Margalit 2009), and one reported an HR of 0.75 (95% CI 0.36 to 1.58) with clomiphene (Brinton 2013). One cohort study suggested no increase in risk of invasive ovarian cancer among women carriers of BRCA1 and/or BRCA2 with an adjusted OR of 0.87 (95% CI 0.46 to 1.63) in women taking clomiphene citrate (Perri 2015).
Three cohort studies reported only SIR for exposure to clomiphene and invasive ovarian cancer (Lerner‐Geva 2012; Modan 1998; Venn 1999); these studies provided no evidence of an increase in women who used clomiphene when compared to subfertile untreated women (Modan 1998), or when compared to the general population in two studies (Lerner‐Geva 2012; Venn 1999); one provided 30 years of follow‐up (Lerner‐Geva 2012). Only one case‐control study reported data with SIR estimation (Rossing 1994).
One of the cohort studies that had suggested increased risk of invasive ovarian tumour with gonadotrophins did not show the same degree of increase with the use of clomiphene citrate (Sanner 2009). Trial authors suggested a risk ratio (RR) of 1.12 (95% CI 0.24 to 5.29) in women using clomiphene, which was similar after adjustment for age and reasons of infertility, with an RR of 1.52 (95% CI 0.31 to 7.39) and an RR of 1.57 (95% CI 0.32 to 7.62) when adjusted for pregnancy during follow‐up. One cohort study reported a slightly increased risk of developing ovarian cancer among women treated with clomiphene citrate who remained nulliparous at the end of treatment when compared to parous women at the end of therapy, with P = 0.04 (Reigstad 2017). Clomiphene citrate‐exposed nulliparous women had increased risk of ovarian cancer (HR 2.49, 95% CI 1.30 to 4.78), and risk was not increased in parous women (HR 1.37, 95% CI 0.64 to 2.96; P = 0.04). The magnitude of the HRs appeared to increase with higher doses of clomiphene citrate at the lowest dose (1.76, 95% CI 0.68 to 4.58) versus the highest dose (3.46, 95% CI 1.19 to 10.0), although a test for trend revealed P = 0.269 (Reigstad 2017).
One case‐control study reported an increase in ovarian tumours with an SIR of 2.5 (95% CI 1.3 to 4.5); this was based on 11 cases (six cases of ovarian invasive tumours and five cases of borderline ovarian tumours), and higher risk of developing cancer was noted in patients using clomiphene for longer than 12 months (Rossing 1994). One case‐control study reported a slight increase in risk among women using clomiphene (OR 1.32, 95% CI 0.57 to 3.01); this was based on 11 cases and 18 controls (Shushan 1996). The adjusted OR for the same group of patients was 0.88 (95% CI 0.33 to 2.34), and this was adjusted for age, parity, BMI, region of birth, education, or family history of ovarian cancer. However, trial authors did not report any information on duration of therapy.
Three case‐control studies showed no evidence of increased risk in women who used clomiphene compared with women of a similar age and variably matched for reproductive risk factors (Jensen 2009; Mosgaard 1997; Rossing 2004). One of those reported an adjusted OR of 1.14 (95% CI 0.79 to 1.64) with the use of clomiphene, and this was adjusted for parity and number of additional births (Jensen 2009). In one study, the adjusted OR was 1.3 (95% CI 0.1 to 13.5) in nulliparous women using clomiphene citrate for longer than 12 months and the adjusted OR was 0.7 (95% CI 0.1 to 6.4) in multiparous women (Rossing 2004). This was adjusted for age, race, study site, and duration of oral contraceptive use. Another case‐control study reporting no increase in risk of ovarian cancer after treatment with clomiphene estimated an OR of 0.69 and an adjusted OR of 0.67 (95% CI 0.23 to 1.96) in nulliparous women and an OR of 0.91 and an adjusted OR of 1.11 (95% CI 0.40 to 3.06) in multiparous women (Mosgaard 1997). The adjusted OR was adjusted for age, residence, use of oral contraceptives and intrauterine device, menopausal status, previous cancer, familial cancer, HRT, and BMI. This was based only on nine cases and 11 controls in nulliparous women and on six cases and 16 controls in multiparous women.
Clomiphene plus gonadotrophin
Four cohort studies ‐ Lerner‐Geva 2012; Modan 1998; Sanner 2009; Trabert 2013 ‐ and five case‐control studies ‐ Gronwald 2015; Kurta 2012; Mosgaard 1997; Rossing 2004; Shushan 1996 ‐ evaluated the incidence of invasive ovarian cancer with clomiphene plus gonadotrophin. All four cohort studies showed no convincing evidence for increased risk of invasive cancer with clomiphene plus gonadotrophin use compared with no use in women with subfertility (Lerner‐Geva 2003; Modan 1998; Sanner 2009; Trabert 2013).
Two studies reported only SIR for exposure to clomiphene and HMG and invasive ovarian cancer (Modan 1998; Venn 1999); these studies showed no evidence of an increase in women who used clomiphene plus HMG when compared to infertile women not treated (Modan 1998), or when compared to the general population (Lerner‐Geva 2012; Venn 1999).
Four case‐control studies also showed no evidence of increased risk in women who used clomiphene plus gonadotrophin compared with women of similar age and variably matched for reproductive risk factors (Asante 2013; Franceschini 1994; Mosgaard 1997; Parazzini 1997), and this was confirmed even in women with BRCA mutation in one case‐control study (Gronwald 2015). One of those studies reported an OR of 1.99 and an adjusted OR of 1.12 (95% CI 0.32 to 3.96) in nulliparous women and an OR of 0.24 and an adjusted OR of 0.56 (95% CI 0.12 to 2.70) in multiparous women (Mosgaard 1997). The OR was adjusted for age, residence, use of oral contraceptives, intrauterine device, menopausal status, previous cancer, HRT, and BMI. In nulliparous women, this was based on seven cases and three controls, and in multiparous women on one case and 10 controls.
One case‐control study suggested only a slight increase in risk of ovarian cancer with use of clomiphene and HMG with an OR of 1.92 (95% CI 1.03 to 3.77); this was based on 22 cases and 24 controls (Shushan 1996). The adjusted OR was 1.42 (95% CI 0.65 to 3.12), and this was adjusted for age, parity, BMI, region of birth, education, and family history of ovarian cancer (Analysis 1.1).
Gonadotrophin
Eight cohort studies ‐ Brinton 2013; Lerner‐Geva 2012; Luke B. 2015; Modan 1998; Perri 2015; Sanner 2009; Venn 1999; Trabert 2013 ‐ and five case‐control studies ‐ Jensen 2009; Kurta 2012; Mosgaard 1997; Rossing 1994; Shushan 1996 ‐ evaluated the incidence of invasive ovarian cancer with gonadotrophin use. Only one of the cohort studies showed increased risk of invasive ovarian tumour in women using gonadotrophin (SIR 5.89, 95% CI 1.91 to 13.75), and four of the five cases reported the use of HCG (Sanner 2009).
One cohort study ‐ Brinton 2013 ‐ reported no increase in risk of ovarian cancer in women treated with gonadotrophin (HR 0.93, 95% CI 0.40 to 2.16), including women with a BRCA genetic mutation as reported by two studies: one cohort study with an adjusted OR of 0.59 (95% CI 0.26 to 1.31) (Perri 2015), and one case‐control study (Gronwald 2015). One study divided the women into four groups according to the cumulative dose of FSH used, which ranged from 2000 IU to more than 7000 IU; however trial authors reported P = 0.17 (Luke B. 2015).
One study reported SIR for invasive ovarian cancer with exposure to HMG and showed no evidence of an increase in women who used HMG when compared to the general population (Lerner‐Geva 2012).
Three case‐control studies provided no evidence of increased risk among women who used gonadotrophin compared with women of a similar age and variably matched for reproductive risk factors (Gronwald 2015; Jensen 2009; Mosgaard 1997). One of those studies suggested no increase in the use of gonadotrophins (FSH and HMG) with adjusted OR of 0.83 (95% CI 0.50 to 1.37), and this was adjusted for parity and number of additional births (Jensen 2009). Another case‐control study reporting no increase in risk of ovarian cancer in women using gonadotrophins estimated an OR of 1.06 and an adjusted OR of 0.82 (95% CI 0.18 to 3.71) in nulliparous women and an OR of 0.54 and an adjusted OR of 0.50 (95% CI 0.10 to 2.47) in multiparous women (Mosgaard 1997). The OR was adjusted for age, residence, use of oral contraceptives and intrauterine device, menopausal status, previous cancer, familial cancer, HRT, and BMI. This was based on five cases and four controls in nulliparous women and on two cases and nine controls in multiparous women. Another study reported an OR of 1.35 (95% CI 0.11 to 16.62) and an adjusted OR of 1.10 (95% CI 0.09 to 13.23), and this was adjusted for age at menarche (Gronwald 2015).
One case‐control study reported a slight increase in ovarian cancer risk (OR 3.95, 95% CI 1.33 to 12.2); this was based on 11 cases and six controls (Shushan 1996). The adjusted OR was 3.19 (95% CI 0.86 to 11.82), and this was adjusted for age, parity, BMI, region of birth, education, and family history of ovarian cancer.
Two case‐control studies reported results separately by parity (Mosgaard 1997; Rossing 2004). Although risk estimates for invasive ovarian cancer were slightly lower for parous women compared with nulliparous women, there was no evidence of a real difference between these two groups for any of the drugs investigated (Analysis 1.1;Figure 3).
Progestogens
One cohort study included subfertile women who used progestogens; study authors did not report any increase in their risk of developing ovarian cancer (HR 0.77, 95% CI 0.37 to 1.60) (Brinton 2013).
Borderline cancer
Any fertility drug
Five cohort studies suggested an increase in the risk of borderline tumours (Bjornholt 2015; Reigstad 2017; Sanner 2009;Stewart 2013a ; Van Leeuwen 2011). One cohort study reported only SIR for exposure to any fertility drug use and borderline ovarian tumours. This study suggested a threefold overall increase in risk of borderline ovarian tumours (SIR 3.61, 95% CI1.45 to 7.44) (Sanner 2009). One study reported significantly increased risk of borderline ovarian tumours in IVF‐treated versus subfertile untreated women with more than one year of follow‐up (HR 4.23, 95% CI 1.25 to 14.33); this was adjusted for age, parity, and infertility causes (Van Leeuwen 2011). One cohort study did not show any significant increase in the risk of borderline ovarian cancer and reported an OR of 2.25 (95% CI 0.59 to 8.68) in the exposed group compared to the general population, excluding the first year after IVF (Yli‐Kuha 2012); another study reported data on borderline ovarian tumours only and included the number of cases according to histology type and did not show any increase in risk among women using any fertility drugs versus women treated and reported data (RR 1.00, 95% CI 0.67 to 1.51) (Bjornholt 2015). However, the same study suggested increased risk among women using progesterone for more than four cycles (Bjornholt 2015). One cohort study suggested increased risk of borderline ovarian tumour in infertile women who underwent infertility in unadjusted analysis (HR 2.48, 95% CI 1.22 to 5.04) and in adjusted analysis using as confounding data age, calendar year, socioeconomic status, and causes of infertility (HR 2.46, 95% CI 1.20 to 5.04) (Stewart 2013a). This increased risk did not change in women who conceived (HR 0.89, 95% 0.43 to 1.88) nor after hysterectomy (HR 1.02, 95% CI 0.24 to 4.37) nor after sterilisation (HR 1.48, 95% 0.63 to 3.48); therefore these factors were not protective. The rate was not increased in women suffering from endometriosis (HR 0.31, 95% CI 0.04 to 2.29) (Stewart 2013a). The risk of borderline ovarian tumour was reported as slightly elevated for all women exposed to infertility drugs in one cohort study (HR 1.95, 95% CI 1.18 to 3.23) (Reigstad 2017). However, stratified analyses on parity showed no significant difference in risk between nulliparous women (HR 1.69, 95% CI 0.75 to 3.79) and parous women (HR 2.12, 95% CI 1.11 to 4.04; P = 0.9).
Two case‐control studies reported the incidence of borderline ovarian cancer, and showed increased risk in women who used any fertility drug compared with women of a similar age and variably matched for reproductive risk factors (Parazzini 1998; Shushan 1996). The estimate for one study should be interpreted with caution as only four cases were exposed to fertility drugs compared with none of the controls, generating a wide confidence interval (Parazzini 1998) (Analysis 1.2;Figure 4). In one case‐control study, the OR was 5.03 (95% CI 2.04 to 12.22); this was based on 10 cases and 29 controls (Shushan 1996). The adjusted OR was 3.52 (95% CI 1.23 to 10); this was adjusted for age, parity, BMI, region of birth, education, and family history of ovarian cancer. In one case‐control cohort study, invasive ovarian cancer and borderline tumours cases were included in the same analysis; therefore the real difference in incidence among these two clinical conditions is unclear (Asante 2013).
1.2. Analysis.
Comparison 1 Infertility drugs vs no infertility drugs, Outcome 2 Borderline ovarian cancer.
4.
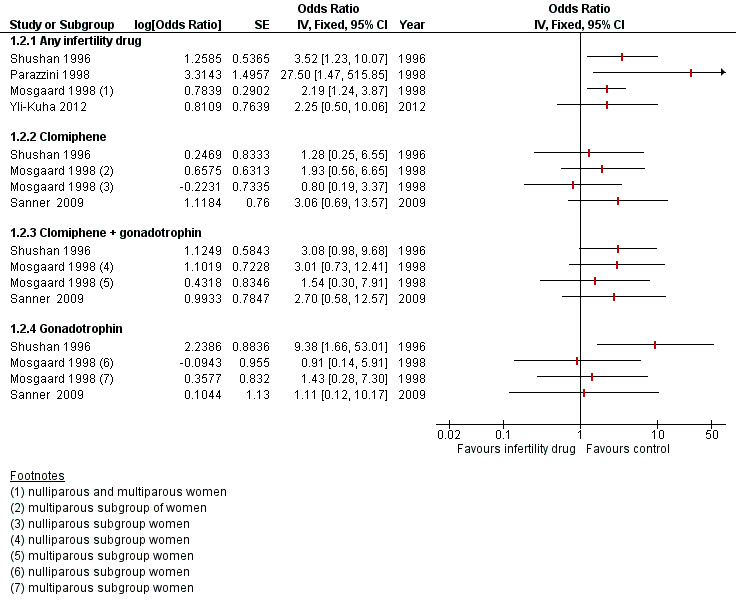
Analyses include only studies reporting risk of ovarian cancer as an odds ratio (OR).
One case‐control study reported no increase in risk of borderline ovarian tumour in subfertile women using fertility drugs (OR 2.27, adjusted OR 2.19, 95% CI 1.24 to 3.85), and this was adjusted for area of residence and for age (Mosgaard 1998). The OR for nulliparous women using fertility drugs was 1.78 and the adjusted OR was 1.70 (95% CI 1.20 to 2.39); this was adjusted for age and residence.
Clomiphene
One cohort study showed no convincing evidence for women with increased risk of borderline tumours with clomiphene compared with women in the cohort unexposed to hormonal fertility treatment (RR 0.96, 95% CI 0.64 to 1.44) (Bjornholt 2015).
Two case‐control studies showed no convincing evidence for increased risk of borderline tumours with clomiphene compared with no use in women of similar age and variably matched for reproductive risk factors (Mosgaard 1998; Shushan 1996). One of the two case‐control studies reported an OR of 1.62 (95% CI 0.25 to 7.87) (Shushan 1996); this was based on two cases and six controls. The adjusted OR was 1.28 (95% CI 0.25 to 6.87), and this was adjusted for age, parity, BMI, region of birth, education, and family history of ovarian cancer (Analysis 1.2;Figure 4).
Clomiphene plus gonadotrophin
One cohort study showed increased risk in women exposed to clomiphene citrate (SIR 7.47, 95% CI 1.54 to 21.83) but provided no convincing evidence for women with increased risk of borderline tumours with clomiphene plus gonadotrophin use compared to women in the cohort unexposed to hormonal fertility treatment (Analysis 1.2;Figure 4) (Sanner 2009).
Two case‐control studies showed no convincing evidence for increased risk of borderline tumours with clomiphene plus gonadotrophin compared with no use in women of similar age and variably matched for reproductive risk factors (Mosgaard 1998; Shushan 1996). One of the two case‐control studies reported an OR of 4.86 (95% CI 1.81 to 12.79) based on 22 cases and 24 controls (Shushan 1996). The adjusted OR was 3.08 (95% CI 0.98 to 9.69); this was adjusted for age, parity, BMI, region of birth, education, and family history of ovarian cancer (Analysis 1.2;Figure 4).
Gonadotrophin
Two cohort studies showed no convincing evidence for increased risk of borderline tumours with gonadotrophin use (RR 1.10, 95% CI 0.66 to 1.81) compared to women in one cohort unexposed to hormonal fertility treatment (Bjornholt 2015), and in the other study, an SIR of 1.88 (95% CI 0.05 to 10.45) (Analysis 1.2;Figure 4) (Sanner 2009).
One case‐control study reported increased risk among users of HMG (OR 14.58, 95% CI 3.82 to 55.91) with an adjusted OR of 9.38 (95% CI 1.66 to 52.08) (Shushan 1996); however these data should be interpreted with caution because only six of the cases ever used HMG, generating wide confidence intervals, which weaken any conclusions. One case‐control study showed no convincing evidence for increased risk of borderline tumours with gonadotrophin compared with no use in women of similar age and variably matched for reproductive risk factors (Analysis 1.2;Figure 4) (Mosgaard 1998).
One case‐control study reported results separately by parity (Mosgaard 1998). Although risk estimates for borderline ovarian tumours were slightly lower for parous women than for nulliparous women, there was no evidence of a real difference between these two groups for any of the drugs investigated.
Progesterone
One study reported data on the use of progesterone as fertility treatment and the risk of borderline ovarian tumours (Bjornholt 2015). Trial findings suggested increased risk of borderline ovarian tumours, especially serous histological type, in women using progesterone (RR 1.82, 95% CI 1.03 to 3.24), and this was especially evident among women using more than four cycles of progesterone during their treatments (RR 2.63, 95% CI 1.04 to 6.64) and when followed for four or more years. However, this increase was not statistically different between nulliparous women with RR of 1.12 (95% CI 0.57 to 2.18) and parous women with RR of 2.09 (1.03 to 4.25) (P = 0.17) (Bjornholt 2015).
GnRH analogues
One cohort study included the use of GnRH analogues and the risk of borderline ovarian tumours was not increased (RR 1.10, 95% CI 0.66 to 1.81) (Bjornholt 2015). The risk of borderline ovarian tumours was not markedly affected by parity status with RR of 0.85 (95% CI 0.44 to 1.62) in nulliparous women and RR of 1.52 (95% CI 0.77 to 3.02) in multiparous women (P = 0.19).
Discussion
Summary of main results
Overall we found no convincing evidence of an increase in the risk of invasive ovarian tumours with fertility drug treatment, and this has been confirmed even in women with a BRCA genetic mutation. Risk of borderline ovarian tumours may be increased in subfertile women treated with in vitro fertilisation (IVF). Studies showing an increase in the risk of ovarian cancer had a high overall risk of bias due to retrospective study design, lack of accounting for potential confounding, and lack of details about fertility drug treatments given; estimates were based on a small number of cases, giving rise to wide confidence intervals. Studies with more robust estimates based on a larger number of cases did not detect differences between exposed and unexposed women.
One study reported higher risk in women with long‐term use of clomiphene citrate (12 or more cycles) (Rossing 1994). This was observed in subfertile women who conceived following treatment, as well as in subfertile women who were refractory to therapy. The same was not shown with the use of human chorionic gonadotrophin (HCG) in the same cohort of women. This study was limited by the small number of tumours, with almost half of them borderline (five out of 11 neoplasms), which gives strong evidence of selection bias. Moreover, the study author included two women with granulosa cell tumour. This histological type of invasive ovarian cancer often presents with abnormalities of fertility and ovulation, which may be the cause of the tumour, rather than the use of ovulation‐stimulating drugs. Another study suggested increased risk of ovarian cancer in women who remain nulliparous after using clomiphene citrate (Trabert 2013), but this conclusion is based on only 13 women and should be viewed with caution. However, this study highlights the importance of stratifying the estimate of the risk of ovarian cancer in women according to gravidity at the end of infertility treatment rather than according to the types of drugs used.
Overall three case‐control studies reported increased risk of developing borderline ovarian cancer (Mosgaard 1998; Parazzini 1998; Shushan 1996). In one of these studies, subfertile women treated with ovarian stimulation drugs were reported to have increased risk of developing borderline ovarian tumours and also invasive ovarian cancer when compared to subfertile women who were not treated (Shushan 1996). Investigators did not provide any information on the causes of subfertility, and 36% of patients had died before contact was established, which could have caused selection bias. Another case‐control study was based on a very small number of patients (only four) who had used fertility medications (Parazzini 1998). In all three studies, the higher proportion of borderline tumours may also suggest that the increased risk is attributed to increased medical surveillance and the younger age of subfertile women. The cohort study reporting an increase in borderline ovarian tumours in subfertile women highlighted that risk was particularly high during the first year after IVF (Van Leeuwen 2011), which may be supported by reported evidence suggesting that ovarian stimulation may induce growth in existing highly differentiated tumours (Brinton 2005).
One cohort study suggested an increase in risk of developing borderline ovarian tumours in women receiving more than four cycles of progesterone (Bjornholt 2015). However, women treated with progesterone had used it as part of an IVF regimen, and this might have meant an overlap of the effect of progesterone and the effect of the IVF procedure used. Study authors could not distinguish if the increased risk of borderline ovarian tumours was due solely to the progesterone or may be due to the IVF treatment regimen. Further limitations of the study were that study authors provided no information on the dosage of fertility drugs and the type of progestin used for fertility treatment. Follow‐up of these women was long (median 11.3 years); however, median age at the end of follow‐up was 42.5 years, which is below the peak age for diagnosis of borderline ovarian tumours in Denmark (52 years), and this may have had an effect on the final results (Bjornholt 2015). A cohort study reported a slight increase in development of borderline ovarian tumour in infertile women who seek infertility treatment when compared to women who do not receive infertility treatment (Stewart 2013a). Study authors argued the assumption that a possible explanation may be a surveillance bias in infertile women who undergo infertility treatment, and this was suggested by other authors in the past to explain this slight increase in the risk of borderline ovarian tumour (Brinton 2005; Ness 2002; Shushan 1996). Women who undergo infertility treatment undergo more investigations, and this provides more opportunities for detection of the condition. One study adjusted the analysis for time from the last infertility admission to the diagnosis of borderline ovarian tumours and the age at diagnosis and concluded that this occurred 9.4 years after the last fertility treatment, and in infertile women who did not have fertility treatment after 7.3 years (Stewart 2013a). In conclusion, researchers did not offer definitive evidence in favour of a casual relationship, and they did not provide any information on dosages and numbers and types of drugs used. However, they did not even support the hypothesis that the observed increase in risk of borderline tumours after fertility treatment might be due to detection bias.
It is interesting to note that only one cohort study reported a slight increase in ovarian cancer in women after one to three cycles of IVF treatment (Brinton 2013), but the risk was similar between four and six cycles, with a hazard ratio (HR) of 3.18 (95% confidence interval (CI) 1.37 to 7.40), and after more than seven cycles (HR 2.12, 95% CI 0.73 to 6.12; P = 0.18).
One case‐control study focused on women with a BRCA genetic mutation and concluded that there is not a significant increase in the risk of ovarian cancer among women with a BRCA mutation (Gronwald 2015). Given the high lifetime risk of ovarian cancer in this population, prophylactic bilateral salpingo‐oophorectomy has been advised at age 35 years for BRCA1 mutation carriers, and at age 40 years for BRCA2 mutation carriers (Manchanda 2018). These data support that it is safe to use fertility drugs before surgery, but results of this study were based on small numbers.
One cohort study had the advantage of including a large cohort sample (Luke 2015); however few ovarian cancer cases were included, and this was due to short‐term follow‐up. The population‐based design was reliable, as it used a national database to identify women who searched for infertility treatment to validate the data.
One cohort study highlighted the importance in these types of studies of distinguishing the risk of ovarian cancer among women who conceived as a result of infertility treatment regardless of the type of treatment from the risk among women who were not successful in conceiving and for whom this may be due to the protective effect of pregnancy (Stewart 2013). The same trial authors suggested a slight increase in risk of ovarian cancer in nulliparous versus multiparous women; however they did not conclude that the difference was significant in view of the small number of cancers included. The short‐term follow‐up made interpretation of data difficult, as ovarian cancer typically occurs in old age. Previous reports have rarely examined separately nulliparous women and parous women. However, the same study team published another report on the risk of borderline ovarian tumour in the same cohort of women and reported that parity, hysterectomy, and sterilisation were not protective and that endometriosis was not associated with an increase in the rate of borderline ovarian tumour (Stewart 2013; Stewart 2013a).
Only one cohort study reported an increase in risk of ovarian cancer when the analysis was adjusted for age and obesity (HR 3.9, 95% CI 1.2 to 12.6) (Kessous 2016); however the confidence interval was very large, suggesting a large spread of values. This was based on a small number of cancer cases when compared to the sample of women analysed, and researchers provided no clear information on types of drugs used, dosages, or numbers of cycles. In addition, the result was not supported in the crude analysis, which showed no increase in risk (HR 0.05, 95% CI not recorded).
Overall completeness and applicability of evidence
Results from the cohort studies are broadly generalised to women who seek fertility treatment, as on the whole, samples consisted of all women who attended fertility clinics at major hospitals within a particular time frame. The most recently published evidence shows more details about types of drugs used, numbers of IVF cycles completed, and types of infertility drugs examined when compared to the oldest published evidence on the same topic. Additionally, as complete case ascertainment was maximised in most of the non‐randomised studies included by the use of cancer registries as the source of ovarian cancer cases, this also optimised identification and selection of cases within a given time frame and area.
In addition, all studies were investigating the effects of fertility drugs that are currently used during fertility treatment.
Quality of the evidence
One strength of our review is that almost all of the included studies reported the outcome taken from reliable sources (i.e. cancer registry).
We identified a few factors in the observational studies included in our review that may have biased our final conclusions. First of all, exposed and non‐exposed (to fertility drugs) groups were not always balanced, and not many studies adjusted their data for important confounding factors.
Subfertile populations have lower pregnancy rates than the general population, as has already been proved, and low parity is an important risk factor for ovarian cancer. Risk estimates for ovarian cancer reported in cohort studies that are based solely on comparison with the general population are likely to be biased towards overestimation. In addition, nulliparity, subfertility, and lack of use of an oral contraceptive pill make subfertile women already at higher risk of ovarian cancer compared to non‐subfertile women. Second, in all studies, exposure was ascertained retrospectively; therefore researchers provided limited information on specific types, dosages, and numbers of cycles of fertility drugs. Despite the inclusion of new studies including information on the number of IVF cycles used, we still are not able to draw conclusions, and this can have an impact on the actual clinical use of fertility drugs, especially because cases of cancer included in the studies are few, and adjusted analysis for confounding factors is not always reported. Third, the length of follow‐up in some studies may be insufficient, as ovarian cancer tends to develop in women of postmenopausal age, and the cancer may not have had time to develop within the time that women were followed up, reducing the reported number of women with ovarian cancer. Ovarian cancer cases recorded in the included studies are few; most likely this is due to the fact that the average for ovarian cancer diagnosis is 68 years and the follow‐up period was not reported or was too short.
Moreover, we were unable to contact researchers to obtain missing data; therefore we rely on the data reported in the published article. In some cases, factors related to study quality were not clearly reported, such as measurement of confounding variables and whether these were balanced at baseline and/or adjusted for in the analysis, numbers of fertility drug cycles, dosages, types of drugs used, and duration of subfertility.
Several cohort studies used the standardised incidence ratio (SIR) to compare cancer risk in subfertile women versus risk in the general population. This statistical parameter is difficult to interpret, as it does not make a comparison between comparable women and does not take into account the influence of factors associated with subfertility that may influence the development of ovarian cancer. The new cohort studies included used HRs or ORs.
Lack of blinding of investigators to case status and exposure status was another potential source of bias, along with potential attrition bias in some studies. It is difficult to gauge the impact of selective reporting bias, as studies were conducted retrospectively and participants may have been excluded from the sample, if exposure could not be ascertained. No studies provided a pre‐specified list of all drugs investigated.
None of the included studies specified histological subtypes of ovarian cancer in the cases found. Future studies should address fertility relationships for cancer histological subtypes, as recognition of the aetiological heterogeneity of ovarian cancer is increasing (Gates 2010; Rish 1996).
Overall, we judge that the certainty or confidence we have in the findings of this review as very low, mainly because of very serious risk of bias and serious inconsistency between study findings. We acknowledge the uncertainty remaining and the potential of future studies to change these conclusions.
Potential biases in the review process
We acknowledge that publication bias may limit our conclusions, and that it is difficult to predict the direction in which bias would operate. On one hand, it is likely that studies with non‐significant associations for particular fertility drugs remain unpublished due to perceived unimportance. On the other hand, however, there is the chance that studies with positive associations remain unpublished, although it is more likely that publication bias would favour publication of positive studies.
We did not put any limits on our search (such as language restrictions), and we sought published and unpublished data.
We are aware that missing information limits our ability to explore the exact relationship between fertility drugs and ovarian cancer. The strength of this review could have been greatly improved if it have been possible to contact all researchers to obtain original data. Obtaining individual participant data for each study would have allowed us to perform a standard adjustment for confounding factors in all studies, if appropriate variables had been measured. Although this could have reduced the likelihood of bias in the included studies, on the other hand it would not have resolved the major problems inherent to the observational studies.
Agreements and disagreements with other studies or reviews
Our findings are in broad agreement with those presented in the most current systematic reviews and meta‐analyses on this topic (Kashyap 2004; Li 2013). These reviews included some of the studies in our review, but not those published since 2001 and not all of the cohort studies included here.
Authors' conclusions
Implications for practice.
It is difficult to give clear advice about the safety of fertility treatment based on our findings, but available evidence does not suggest that there is a clinically significant adverse effect. Current guidance recommends treatment with clomiphene citrate for a maximum of six months (NICE 2013; Nugent 1998). We found no evidence that fertility treatment with clomiphene citrate increases or does not increase the risk of ovarian cancer. Furthermore, we found no conclusive evidence that IVF treatment utilising other fertility drugs confers higher risk of ovarian cancer compared with clomiphene citrate alone.
Implications for research.
Although it seems clear that more epidemiological research is needed, the organisation of this is problematic. Known risk factors for ovarian cancer and the rarity and later onset of incidence necessitate large, long‐term prospective studies with carefully selected cohorts. Although retrospective studies such as case‐control studies are attractive (due to the low incidence of ovarian cancer, fewer participants are required to achieve adequate power), they present methodological challenges such as selection of an adequate control group, retrospective collection of data on drug exposure increasing the likelihood of recall bias, and attrition bias from missing data on exposure and other important risk factors.
Collaboration between fertility services should be encouraged to facilitate data sharing. Data on drug types, dosages, and duration of treatment could be collected prospectively and linked to cancer registries, which collect data on the incidence of ovarian cancer. This would provide estimates of the risk of ovarian cancer with different treatment strategies such as monotherapy versus multi‐therapy or high‐dose versus low‐dose treatment. Information on confounding factors such as parity, oral contraception use, and family history should be collected, along with information on fertility diagnoses, types, dosages, duration of medications, and outcomes of treatment. Ovarian cancer of different histological types and borderline ovarian tumours should be analysed separately to obtain a more reliable difference in incidence between cancer and borderline tumours.
Currently, no 'safe' limits on dose or duration of any of the other drugs used in ovarian stimulation are recommended. One important question for women and practitioners to be determined is whether clomiphene citrate alone is less likely to cause cancer compared with multi‐therapy; also, risks associated with the number of IVF stimulation cycles remain to be clarified.
What's new
| Date | Event | Description |
|---|---|---|
| 24 November 2018 | New search has been performed | We have updated literature searches to November 2018 |
| 24 November 2018 | New citation required but conclusions have not changed | We have analysed and included all new eligible studies There is only a slight increase in the risk of ovarian cancer, and this is supported by weak evidence |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 8, 2013
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | We have updated contact details |
Acknowledgements
We thank Jo Morrison for providing clinical and editorial advice, Jo PLat for running the literature searches, and Gail Quinn, Clare Jess, and Tracey Harrison for contributing to the editorial process.
We also thank the staff of the Ipswich Hospital Library, who provided the articles to us and helped us with the search strategy for some databases.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology, and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 Mesh descriptor: [Ovarian Neoplasms] explode all trees #2 ovar* near/5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*) #3 #1 or #2 #4 Mesh descriptor: [Ovulation Induction] explode all trees #5 Mesh descriptor: [Fertility Agents] explode all trees #6 (fertil* or infertil*) near/5 (agent* or drug*) #7 (stimul* or induc*) near/5 (ovar* or ovul*) #8 MeSH descriptor: [Selective Estrogen Receptor Modulators] explode all trees #9 SERM or (selective next (estrogen or oestrogen) next receptor next modulator*) or clomiphene or chloramiphene or clomid* or clomifen* or tamoxifen #10 Mesh descriptor: [Gonadotropins] explode all trees #11 gonadotropin‐releasing hormone #12 Mesh descriptor: [Gonadotropin‐Releasing‐Hormone] explode all trees #13 gonadotropin* or (luteinizing hormone*) or (follicle stimulating hormone*) or LH or FSH or hMG or hCG or GnRH* #14 Mesh descriptor: [Growth Hormone] explode all trees #15 Mesh descriptor: [Insulin‐Like Growth Factor 1] explode all trees #16 (growth hormone*) or (insulin near/5 (growth factor) or GH or IGF #17 Mesh descriptor: [Reproductive Techniques, Assisted] explode all trees #18 (assist* near/5 reproduct*) or ART or (in vitro near/5 fertili*) or IVF #19 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 #3 and #19
Appendix 2. MEDLINE search strategy
1 exp Ovarian Neoplasms/ 2 (ovar* adj5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)).mp. 3 1 or 2 4 exp Ovulation Induction/ 5 exp Fertility Agents/ 6 ((fertil* or infertil*) adj5 (agent* or drug*)).mp. 7 ((stimul* or induc*) adj5 (ovar* or ovul*)).mp. 8 exp Selective Estrogen Receptor Modulators/ or (selective adj (estrogen or oestrogen) adj receptor adj modulator*).mp. 9 (SERM* or clomiphene or chloramiphene or clomid* or clomifen* or tamoxifen).mp. 10 exp Gonadotropins/ 11 exp Gonadotropin‐Releasing Hormone/ 12 (gonadotropin* or luteinizing hormone* or follicle stimulating hormone* or LH or FSH or hMG or hCG or GnRH*).mp. 13 exp Growth Hormone/ 14 Insulin‐Like Growth Factor I/ 15 (growth hormone* or (insulin adj5 growth factor) or GH or IGF).mp. 16 exp Reproductive Techniques, Assisted/ 17 ((assist* adj5 reproduct*) or ART or (in vitro adj5 fertili*) or IVF).mp. 18 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19 3 and 18 20 randomized controlled trial.pt. 21 controlled clinical trial.pt. 22 randomized.ab. 23 placebo.ab. 24 drug therapy.fs. 25 randomly.ab. 26 trial.ab. 27 groups.ab. 28 exp Cohort Studies/ 29 cohort*.mp. 30 exp Case‐Control Studies/ 31 (case* and control*).mp. 32 (case* and series).mp. 33 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 34 19 and 33 35 exp animals/ not humans.sh. 36 34 not 35
key: [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
Appendix 3. Embase search strategy
1 exp ovary tumor/ 2 (ovar* adj5 (cancer* or neoplas* or carcinoma* or malignan* or tumor* or tumour* or adenocarcinoma*)).mp. 3 1 or 2 4 exp ovulation induction/ 5 exp fertility promoting agent/ 6 (((fertil* or infertil*) adj5 (agent* or drug*)) or ((stimul* or induc*) adj5 (ovar* or ovul*))).mp. 7 selective estrogen receptor modulator/ or (selective adj (estrogen or oestrogen) adj receptor adj modulator*).mp. 8 (SERM* or clomiphene or chloramiphene or clomid* or clomifen* or tamoxifen).mp. 9 exp gonadotropin/ 10 gonadorelin/ 11 (gonadotropin* or luteinizing hormone* or follicle stimulating hormone* or LH or FSH or hMG or hCG or GnRH*).mp. 12 growth hormone/ 13 somatomedin/ 14 (growth hormone* or (insulin adj5 growth factor) or GH or IGF).mp. 15 exp infertility therapy/ 16 ((assist* adj5 reproduct*) or ART or (in vitro adj5 fertili*) or IVF).mp. 17 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 18 3 and 17 19 exp controlled clinical trial/ 20 crossover procedure/ 21 double‐blind procedure/ 22 randomized controlled trial/ 23 single‐blind procedure/ 24 random*.mp. 25 factorial*.mp. 26 (crossover* or cross over* or cross‐over*).mp. 27 placebo*.mp. 28 (double* adj blind*).mp. 29 (singl* adj blind*).mp. 30 assign*.mp. 31 allocat*.mp. 32 volunteer*.mp. 33 exp cohort analysis/ 34 cohort*.mp. 35 retrospective study/ 36 prospective study/ 37 prospective study/ 38 (case* and (control* or series)).mp. 39 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 40 18 and 39 41 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 42 40 not 41
key: [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Data and analyses
Comparison 1. Infertility drugs vs no infertility drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Invasive ovarian cancer | 18 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Any infertility drug | 15 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Clomiphene | 9 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Clomiphene + gonadotrophin | 5 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Gonadotrophin | 7 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 GnRH | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Mixed | 1 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Borderline ovarian cancer | 5 | Odds Ratio (Fixed, 95% CI) | Totals not selected | |
| 2.1 Any infertility drug | 4 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clomiphene | 3 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Clomiphene + gonadotrophin | 3 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Gonadotrophin | 3 | Odds Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asante 2013.
| Methods | Matched 'case‐control study'; Mayo Clinic Ovarian Cancer Study | |
| Participants | Cases and controls lived in 6‐state region that defines the primary service population of the Mayo Clinic (Minnesota, Iowa, Wisconsin, Illinois, North Dakota, and South Dakota). Cases (N = 1028) were women with prevalent and incidental epithelial ovarian cancer and borderline ovarian tumour attending clinics from December 1999 at Mayo Clinic (Rochester, MN) through May 2012. Controls (N = 872) were women with at least 1 ovary intact who had presented to the clinic for other gynaecological medical problems. These women were frequency matched on age (5‐year age categories) and region of residence to cases. Mean age for controls was 60.5 (SD 13.2), and for cases was 61.3 (SD 12.8) | |
| Interventions | Use of 'fertility drugs', drugs, dosage, and number of cycles not reported | |
| Outcomes | Prevalent and incidental epithelial ovarian tumours and borderline ovarian tumours by histological diagnosis (see Table 2) | |
| Notes | Duration of follow‐up and dose and timing of exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Controls selected from women attending Mayo Clinic for general medical examination |
| Confounding | Low risk | Frequency matched on age (5‐year age categories) and region of residence to cases. Adjusted for age, race, duration of OCP use, parity, and number of live births |
| Performance bias | High risk | Information obtained through a self‐report questionnaire about history of infertility and fertility medication use. Clinicians involved in recruitment were not blinded to case‐control status |
| Detection bias | Low risk | Prevalent and incidental epithelial ovarian tumours and borderline ovarian tumours by histological diagnosis; "most" enrolled within a year of diagnosis |
| Attrition bias | High risk | Length of follow‐up from exposure not reported. Original sample included 1157 cases and 1096 controls; women with incomplete information on infertility and infertility drug use were excluded from final analysed sample |
| Selective reporting (reporting bias) | High risk | Fertility drugs used and duration were not reported |
Bjornholt 2015.
| Methods | 'Retrospective case cohort study'. The Danish Infertility Cohort (N = 96) comprises 545 women with fertility problems referred to all Danish fertility clinics in the period from 1963 to 2006. All women were followed for first occurrence of a borderline tumour from the initial date of infertility evaluation until date of migration or death or until 31 December 2006, whichever occurred first | |
| Participants | All women with a borderline ovarian tumour by 31 December 2006 selected from the Danish Infertility Cohort (N = 96) constituted a group of 545. 142 cases and 1328 controls randomly selected from the cohort were stratified by age and year of entry. Median age at first fertility evaluation and hence entry into the cohort was 30.3 years, and median age at the end of follow‐up was 42.5 years. The 142 cases of borderline ovarian tumours consisted 100 with serous, 36 with mucinous, and 6 with other borderline tumours | |
| Interventions | To obtain information on use of fertility drugs, hospital files and medical records of infertility‐associated visits to all Danish fertility clinics were collected and supplemented with information from the Danish IVF Register. Fertility drugs were used by 89/142 (63%) patients with borderline ovarian tumours and by 683/1328 (51%) control patients. The most commonly used fertility drug was HCG ‐ 65 (45%) cases and 488 (37%) controls ‐ followed by clomiphene citrate in 56 (39%) cases and 440 (33%) controls, gonadotrophins in 55 (39%) cases and 256 (19%) controls, GnRH analogues in 40 (28%) cases and 180 (14%) controls, and progesterone in 39 (27%) cases and 153 (12%) controls | |
| Outcomes | Cases were identified by linkage to the Danish Cancer Register and the Danish Register of Pathology via personal identification numbers. Ovarian borderline tumours were identified by histological diagnosis (see Table 2). Histological types of borderline ovarian tumours included were serous, mucinous, and other morphology types. If a woman was recorded as having more than 1 borderline ovarian tumour, only the first recorded incident tumour was used | |
| Notes | The median length of follow‐up was 11.3 years, with a maximum of 49 years. Age at diagnosis of borderline ovarian cancer ranged from 21.7 to 65.1 years, with a median of 40.2 years. The median time between entry into the cohort and diagnosis was 10.7 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All eligible women were selected with no history of ovarian cancer at the beginning of the study; all women had at least 1 ovary |
| Confounding | High risk | Adjusted for parity and stratified by age and year of study entry |
| Performance bias | Low risk | Medical record review, no blinding of assessors to exposure status (low because exposure was recorded by the fertility clinic and preceded development of the outcome) |
| Detection bias | Low risk | Information on the development of cancers was obtained from clinic records and cancer registries. This was not reported if assessors were blind to exposure status |
| Attrition bias | High risk | Unclear how many women were followed up for longer than 10 years. Exposure data were missing for 27% of cases and 9% of controls |
| Selective reporting (reporting bias) | Low risk | Results for all fertility drugs investigated were reported |
Brinton 2013.
| Methods | Retrospective cohort study at the Maccabi Healthcare Services ‐ a large health maintenance organisation in Israel. Record linkage with the Israel Cancer Register (ICR) information on demographic factors (date of birth, district of residence, enumeration area), potential cancer risk factors (parity status at cohort entry, parity status at cohort exit, number of children at exit, weight, height, ever smoked cigarettes, infertility indication), and data on fertility treatments were obtained from women's electronic medical records (EMRs). Socioeconomic status was categorised according to the poverty index of the member's enumeration area as defined by 1995 national census data based on several parameters such as household income, educational qualifications, crowding, material conditions, and car ownership. 704,241 person‐years of follow‐up (mean years of follow‐up 8.1, SD 3.8) | |
| Participants | All women evaluated and/or treated for infertility on or after 25 September 1994 until 22 June 2011 (N = 87,403). At cohort entry, the mean age of women was 31.1 (SD 6.4) | |
| Interventions | IVF defined as hormonal exposure with or without oocyte retrieval, number of IVF cycles, GnRH, clomiphene citrate, and progestogen. 67,608 (77.4%) received fertility treatment; numbers given any infertility treatment were N = 34, any IVF treatment N = 21 (1 to 3 cycles, 10 women; 4 to 6 cycles, 7 women; more than 7 cycles, 4 women), GnRH treatment N = 11, clomiphene N = 20, and progestogen N = 23. Of those included, 55 were nulliparous; of those, N = 19 received any fertility treatment, N = 11 were given IVF treatment, N = 5 received GnRH treatment, N = 9 were given clomiphene treatment, and N = 11 received progestogen treatment. The number of parous women included was 54; of those, N = 15 were given any infertility treatment, N = 10 received any IVF treatment, N = 6 were treated with GnRH, N = 11 were given clomiphene citrate, and N = 12 received progestogen | |
| Outcomes | Invasive ovarian cancer ascertained by histology report recorded with the Israel Cancer Register (ICR) | |
| Notes | Adjusted values for age at entry, BMI, smoking, and parity at exit, were not included in the analysis, as those estimates were not reported according to all the different cancers included by authors of the paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All eligible women were selected; there was no history of ovarian cancer at the beginning of the study and all women had at least 1 ovary |
| Confounding | High risk | Adjusted analysis was not reported; parity and type of infertility treatment were used according to the type of cancer included in the study. Complete information for all potential confounders is not available |
| Performance bias | Low risk | Medical record review; no blinding of assessors to exposure status |
| Detection bias | Low risk | Information on development of cancers was obtained from clinic records and cancer registries. It was not reported if assessors were blind to exposure status |
| Attrition bias | Low risk | Time‐to‐event analysis was performed |
| Selective reporting (reporting bias) | Low risk | Results for all fertility drugs investigated were reported |
Calderon‐Margalit 2009.
| Methods | 'Retrospective cohort'. All women who gave birth in 1974 to 1976 at 3 major obstetrical units in Israel and included in the Jerusalem Perinatal cohort study were linked with the Israel Population Registry and the Israel Cancer Registry | |
| Participants | N = 15,426; mean age 27.5 for exposed and NR for unexposed | |
| Interventions | Fertility treatment, dosage, and number of cycles were not reported. Clomiphene citrate (N = 312), human menopausal gonadotrophins (N = 61), other (N = 54), and unknown (N = 87). Follow‐up by exposure group was not reported | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 3) | |
| Notes | 424,193 person‐years follow‐up (median 29 years) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women in a given area with no history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Analysis adjusted for age at first birth, geographical origin, social class, education, parity, mean body mass index, time to conception, ovulation disorders, and mechanical treatment |
| Performance bias | High risk | Questionnaires; no blinding of assessors to case‐control status reported |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status used |
| Attrition bias | Low risk | HR was estimated and missing data were censored (8%) |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
1. Narrative summary of studies included.
| Cohort studies | |
| Any Infertility drugs | |
|
Brinton 2013 Calderon‐Margalit 2009 Doyle 2002 Dor 2002 Dos Santos Silva 2009 Kallen 2011 Luke 2015 Modan 1998 Perri 2015 Potashnik 1999 Stewart 2013 Trabert 2013 Venn 1995a Venn 1997 Yli‐Kuha 2012 |
No increase in risk of ovarian cancer |
|
Sanner 2009 Van Leeuwen 2011 Reigstad 2015 Kessous 2016 Reigstad 2017 |
Slight increase in risk of ovarian cancer |
| Case‐control studies | |
| Any infertility drugs | |
|
Franceschini 1994 Mosgaard 1997 Parazzini 1997 Parazzini 2001 Rossing 2004 Jensen 2009 Asante 2013 Gronwald 2015 |
No increase in risk of ovarian cancer |
| Shushan 1996 | Slight increase in risk of ovarian cancer |
Dor 2002.
| Methods | 'Retrospective cohort'. All women who underwent IVF from 1981 to 1992 identified from medical records in 2 fertility clinics and Israel, and linked to Israel National Cancer Registry | |
| Participants | Women who received at least 1 treatment cycle: N = 5026. Mean age at first IVF treatment was 34.0 ± 6.4 years, and mean age at end of follow‐up was 37.5 ± 7.1 years | |
| Interventions | Fertility treatment and number of cycles reported but not dosage. Between 1 and 2 cycles, 663 women; between 3 and 5 cycles, 417 women; 6 or more cycles, 174 women. Length of follow‐up by exposure status was not reported, but cancer cases diagnosed within 1 year of IVF treatment were excluded | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 3) | |
| Notes | 18,291 women‐years follow‐up; mean follow‐up 3.6 ± 3.4 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Factors adjusted for place of birth, type of subfertility, and numbers of IVF cycles and pregnancies |
| Performance bias | High risk | Medical record review; no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | High risk | 73% (5026/18,291) of women were followed up (mean follow‐up 3.6 ± 3.4 years). Length of follow‐up by exposure status was not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
Dos Santos Silva 2009.
| Methods | 'Retrospective cohort study'. All women with ovulatory disorders attending 2 IVF clinics from 1963 to 1999 at 2 centres in the UK. Identified from clinic records; linked to National Health Service Central Register in England and Wales | |
| Participants | N = 7355; mean age 28.1 years; N = 3196 (44.5%) received fertility drugs | |
| Interventions | Fertility drugs; no dosage and cycles reported (1976 (62%) used clomiphene and 1198 (38%) used clomiphene and HMG). Length of follow‐up by exposure status was not reported | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 3) | |
| Notes | Mean follow‐up was 21.4 years (89% of participants were followed up for at least 10 years and 14% for at least 30 years) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with ovulatory disorders and at least 1 ovary |
| Confounding | High risk | No adjusted analysis was reported |
| Performance bias | High risk | Medical notes; no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Low risk | 7444/9152 (81.3%) followed up; 7355 analysed with complete data. Length of follow‐up by exposure status was not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
Doyle 2002.
| Methods | 'Retrospective cohort' of women who were UK residents attending 1 fertility clinic and who had received at least 1 cycle of fertility treatment from 1975 to 1989. Identified from clinic records. Linked to National Health Service Central Register in England and Wales | |
| Participants | N = 5556; age 20 years or more at the time of treatment; resident in the UK; alive and cancer‐free from 1990. Exposed group (4188; 75%) received drugs to stimulate ovulation; unexposed group did not receive drugs | |
| Interventions | Fertility treatment; number of cycles was reported but no dosage was mentioned. Fewer than 2 cycles ‐ 20 (0.5%) women, between 2 and 4 cycles ‐ 1246 (30%) women, between 5 and 9 cycles ‐ 1770 (42%) women, 10 or more cycles ‐ 1152 (28%) women. Follow‐up for women who received ovarian stimulation ‐ 32,986 person‐years at risk; for women with no ovarian stimulation 9753 person‐years at risk | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 3) | |
| Notes | Follow‐up from 1990 to 1997; 43,811 person‐years at risk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women attending a single centre with no history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Factors adjusted for age at first clinical visit, years of first clinical visit, parity, time since first treatment, and age at the end of follow‐up |
| Performance bias | High risk | Medical records; no blinding of assessors to exposure status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to case status |
| Attrition bias | High risk | N = 74 women (451 person‐years) excluded as follow‐up was restricted to 1990 onwards rather than the date of first treatment. These women had died, emigrated, or were diagnosed with cancer before 1990. Follow‐up for women who received ovarian stimulation ‐ 32,986 person‐years at risk; for women with no ovarian stimulation 9753 person‐years at risk |
| Selective reporting (reporting bias) | Low risk | All fertility drugs investigated were reported |
Franceschini 1994.
| Methods | 'Case‐control study'. Cases were 195 women with incident epithelial ovarian cancer admitted to major teaching and general hospitals at 4 centres. Women with borderline tumours were excluded. Controls were 1339 women from the same geographical area and admitted to the same network of hospitals as cases for a wide range of acute non‐neoplastic conditions. Women with hormonal or gynaecological diseases or bilateral oophorectomy were excluded. From 1992 to 1993; multi‐centre in Italy | |
| Participants | Age range for cases was 18 to 75 (median 55); age range for controls was 19 to 79 years (median 56) | |
| Interventions | Use of 'fertility drugs', drug, dosage, and number of cycles not reported | |
| Outcomes | Epithelial ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Duration of follow‐up and timing of exposure were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | All women admitted with ovarian cancer |
| Confounding | Unclear risk | Factors adjusted for age, education, parity, medical diagnosis of infertility, and length of attempt to first pregnancy |
| Performance bias | High risk | Self‐reported during an interview; unclear if interviewers were blinded to case‐control status |
| Detection bias | Low risk | Epithelial ovarian cancer by histological diagnosis |
| Attrition bias | Unclear risk | Unclear if exclusions were based on incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all fertility drugs investigated were reported |
Gronwald 2015.
| Methods | Matched case‐control study involving women who are BRCA1 or BRCA2 mutation carriers. Women were identified in genetic clinics at centres in Sweden, United Kingdom, China, Austria, Italy, and the Netherlands | |
| Participants | Cases were women with a diagnosis of invasive epithelial cancer (N = 941). Controls were women with BRCA1 or BRCA2 mutation but with no ovarian cancer selected from women attending genetics clinics (N = 941). Each case was matched to one of the controls according to mutation in the same gene (BRCA1 or BRCA2), year of birth (within 2 years), parity (nulliparous; 1, 2, 3, or 4 or more births), country of residence, date of completion of the baseline questionnaire (within 2 years), oral contraceptive (OC) use (ever/never), and previous diagnosis of breast cancer (yes/no). A control participant was eligible to be matched to a given case if the date of the interview or the date of prophylactic bilateral salpingo‐oophorectomy was after the year of ovarian cancer diagnosis in the case | |
| Interventions | N = 12 women used selective oestrogen receptor modulators (SERMs); these included clomiphene citrate (CC), serophene; N = 22 controls for this group. N = 2 women with gonadotrophin, which included FSH or FSH/LH combination, and N = 1 control for this group. N = 4 women using mixed protocols such as SERM and recombinant FSH/LH, and N = 4 controls. As IVF treatment, with no information on the drugs used, there were N = 4 women and N = 7 controls, and for Intrauterine insemination (IUI), N = 4 women were included as were N = 5 controls Information regarding treatment of infertility was self‐reported via a routinely administered questionnaire |
|
| Outcomes | Epithelial ovarian cancer by histological diagnosis | |
| Notes | Dose, timing, and duration of infertility treatment not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | All women with ovarian cancer and women matched according to their genetic mutation. Cases selected from clinic‐based setting where patients were seeking genetic counselling/advice |
| Confounding | Low risk | Matched on mutation in the same gene (BRCA1 or BRCA2), year of birth (within 2 years), parity (nulliparous, 1, 2, 3, or 4 or more births), country of residence, date of completion of the baseline questionnaire (within 2 years), oral contraceptive (OC) use (ever/never), and previous diagnosis of breast cancer (yes/no) |
| Performance bias | High risk | Self‐reported during an interview; unclear if interviewers were blinded to case‐control status |
| Detection bias | Low risk | Epithelial ovarian cancer by histological diagnosis. Women excluded if cancer other than breast or ovarian was diagnosed, or if information on personal history of breast or ovarian cancer was missing |
| Attrition bias | Unclear risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Results were presented for all fertility drugs investigated, as stated in the methods |
Jensen 2009.
| Methods | 'Nested case‐control study'. Women with subfertility problems and referred to all Danish private fertility clinics or hospitals from 1963 to 1998, and all women with ICD diagnosis of infertility from the national patient registry (a nationwide register of virtually all discharges from Danish hospitals for somatic conditions since 1977). Linked to civil registration database to obtain date of migration or death. Linked to Danish cancer registry and Danish registry of pathology for ovarian cancer diagnosis. Cases included women with ovarian cancer by 30 June 2006. Controls were randomly selected from 4 age strata and 5 strata according to year of entry to cohort | |
| Participants | Cohort comprised N = 54,449 women with primary or secondary infertility; N = 176 cases; 1360 controls. Median age at first evaluation of infertility was 30 years (range 16 to 55), and median age at the end of follow‐up was 47 years (range 18 to 81) | |
| Interventions | Fertility drugs and number of cycles reported for each drug, but dosage not reported. Gonadotrophins 1 to 4 cycles 18/130, 5 to 9 cycles 7/46 women, 10 or more cycles 1/8. Clomiphene citrate 1 to 4 cycles 35/226 women, 5 to 9 cycles 15/117 women, 10 or more cycles 8/74 women. HCG between 1 and 4 cycles 31/232 women, between 5 and 9 cycles 13/121 women, and 10 or more cycles 5/60 women. GnRH between 1 and 4 cycles 14/100 women, between 5 and 9 cycles 1/10 women, and 10 or more cycles 0 women. Duration of follow‐up by fertility drug not reported | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 3) | |
| Notes | 95% of women (54,362) were followed up for a median of 16.0 years (range 0.0 to 42.6 years); 25% were followed for longer than 23 years; 957,454 person‐years of observation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with infertility treated at a private clinic or a public hospital or with a diagnosis of infertility on the national disease registry. No history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Factors adjusted for include parity, number of births, maternal age at birth of first child, and maternal age at birth of last child |
| Performance bias | High risk | Medical records; no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Low risk | 95% of women had similar length of follow‐up |
| Selective reporting (reporting bias) | Low risk | All fertility drugs investigated were reported |
Kallen 2011.
| Methods | Retrospective cohort; Sweden, multi‐centre | |
| Participants | All women who gave birth following IVF treatment from 1982 to 2007, identified from all IVF clinics in Sweden and Swedish Medical Birth Register (24,058). A control group comprised 95,775 women recorded in the Medical Birth Register. Mean age at first delivery after IVF was 40.3 years | |
| Interventions | There was no clear report of the number of IVF cycles, dosage, or type of fertility drugs used | |
| Outcomes | Ovarian cancer by histological diagnosis; Swedish Cancer Registry | |
| Notes | Average follow‐up time was 8.3 years for IVF women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All eligible women selected; no history of ovarian cancer at the beginning of the study, and all women had at least 1 ovary |
| Confounding | Unclear risk | Adjustment was made in the analysis for maternal age and year of birth, smoking, and parity |
| Performance bias | High risk | Medical record review; no blinding of assessors to case‐control status |
| Detection bias | Unclear risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | High risk | 75% (24,058/95,775) of women were followed up (mean follow‐up time 8.3 years) |
| Selective reporting (reporting bias) | Unclear risk | Dosage, number of IVF cycles, and types of drugs used were not reported |
Kessous 2016.
| Methods | Population‐based retrospective cohort study comprising consecutive women who delivered between 1988 and 2013 at the Soroka University Medical Center in Israel. Data were obtained from the computerised perinatal database comprising information recorded after delivery by an obstetrician, and the computerised database of the Soroka University Medical Centre, which includes ICD‐9 codes for all medical diagnoses made during hospitalisations. Mean follow‐up 11.6 years | |
| Participants | N = 106,031 consecutive women who delivered from 1988 to 2013. Women with a known predisposition for malignancy or with cancers before the infertility treatment were excluded from the study | |
| Interventions | N = 101,668 women with no fertility treatment and N = 4363 women with infertility and treated. Among those, N = 1149 underwent IVF treatment and N = 3214 had ovarian stimulation therapy | |
| Outcomes | Invasive ovarian cancer confirmed by histology | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women coming to the hospital to deliver. All women had no history of ovarian cancer at the beginning of the study and at least 1 ovary |
| Confounding | High risk | Adjusted for obesity, maternal age |
| Performance bias | Low risk | Hospital databases; no blinding of assessors to exposure status |
| Detection bias | Low risk | Information on the development of cancer was obtained from hospital database. Not reported whether assessors were blind to exposure status |
| Attrition bias | Unclear risk | It is not reported clearly if women were excluded from the study |
| Selective reporting (reporting bias) | Unclear risk | States "no increased risk of ovarian cancer throughout the study period in women that underwent ovarian stimulation" but no results were presented |
Kurta 2012.
| Methods | Retrospective case‐control study; multi‐centre in USA | |
| Participants | Participants were residents in Western Pennsylvania, Eastern Ohio, and Western New York State participating in the Hormones and Ovarian Cancer Prediction Study (national population‐based study). All cases were histologically confirmed to have primary epithelial ovarian cancers diagnosed between 2003 and 2008. Eligible women were at least 25 years old and were within 9 months of initial diagnosis at the time of recruitment. A total of 155 cases. 290 controls were frequency matched to cases (about 2:1) by 5‐year age group and telephone area code through random digit dialling. Women who had undergone a bilateral oophorectomy were ineligible. Trained interviewers collected questionnaire data that included detailed reproductive, gynaecological, and medical histories, as well as information about lifestyle and family medical history. Mean age for cases and controls was not reported | |
| Interventions | Fertility drugs used were raloxifene, danazol, unknown hormone pills, bromocriptine, progesterone, and metformin. Fertility drug doses were not reported. Most used fertility drugs for less than 12 months (66.7%); mean duration was 11.4 months (range 1 to 134 months). Among the cases, 105/155 (67%) were not exposed to fertility drugs and 50/155 (32%) were exposed. Among the controls, 192/290 (66%) were not exposed to fertility drugs and 98/290 (34%) were exposed to fertility drugs | |
| Outcomes | Invasive epithelial ovarian cancer by histological diagnosis | |
| Notes | Duration of exposure was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Only live cases included as well as women who had a confirmed histological diagnosis and returned a questionnaire about exposure |
| Confounding | Low risk | Matched for age at the time of diagnosis and area of residence. Factors adjusted for age, race, education, tubal ligation, age at menarche, duration of oral contraceptive use, number of live births, duration of breastfeeding, perineal talc use, and family history of breast or ovarian cancer |
| Performance bias | High risk | Self‐reported by questionnaire on exposure status. Unclear if blinding of assessors to case‐control status was used |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Low risk | 71% (902/1270) of total cases eligible returned the questionnaire; 97% (1802/1844) of controls participated in the study |
| Selective reporting (reporting bias) | Low risk | Results for all drugs investigated were reported |
Lerner‐Geva 2003.
| Methods | 'Retrospective cohort'. All infertile women who attended 1 IVF clinic and who received at least 1 treatment cycle in Israel from 1984 to 1992 were identified from the medical records. Linked to the Israel National Cancer Registry | |
| Participants | N = 1082 with 7002 person‐years follow‐up. Mean age at the first IVF treatment was 32.7 ± 4.8 years, and mean age at the end of follow‐up 38.7 ± 5.2 years | |
| Interventions | Fertility drug not reported. 650 women received 1 to 2 cycles of treatment, 323 received 3 to 5 cycles, and 109 received more than 6 cycles | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 3) | |
| Notes | Mean years of follow‐up: 6.5 ± 2.2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | No history of ovarian cancer at the beginning of the study and with at least 1 ovary. Women with diagnosis of cancer within 1 year of IVF treatment were excluded from analyses |
| Confounding | Unclear risk | Factors were adjusted for continent of birth, type of infertility, diagnosis of infertility, number of IVF cycles, and treatment outcome (pregnancy or not) |
| Performance bias | High risk | Medical records; no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 85% (1082/7002) of women were followed up |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether all fertility drugs were investigated and reported |
Lerner‐Geva 2012.
| Methods | Retrospective cohort; Israel 1964 to 1974; only 1 centre | |
| Participants | 2431 subfertile women treated at the Sheba Medical Center compared to the general population | |
| Interventions | Fertility treatment with clomiphene (N = 884), clomiphene and HMG (N = 238), and HMG (N = 159) | |
| Outcomes | Ovarian cancer by histological diagnosis | |
| Notes | Mean age at the end of follow‐up 62.7 ± 8.1 years; 88,181 person‐years follow‐up (over 30 years of follow‐up) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women coming to the infertility centre where the study was started. All women had no history of ovarian cancer at the beginning of the study and at least 1 ovary |
| Confounding | Unclear risk | Adjusted analysis was not reported |
| Performance bias | High risk | Medical notes review; no blinding of assessors to exposure status |
| Detection bias | High risk | Information on the development of cancer was obtained from a cancer registry; not reported whether assessors were blind to exposure status |
| Attrition bias | Low risk | Almost all women (94%) were followed up (2431/2575) throughout the time |
| Selective reporting (reporting bias) | Unclear risk | Type of drug used was reported. There was no information about dosage of drugs used and number of cycles |
Luke 2015.
| Methods | 'Longitudinal cohort study' of women resident in New York, Texas, and Illinois treated with infertility drugs between 2004 and 2009. Data were obtained from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System (SART CORS) database, which contains data from more than 90% of all clinics providing infertility treatment. Follow‐up until December 2010 in New York and December 2012 for Texas and Illinois, with 263,457 person‐years of follow‐up (mean 4.87 ± 2.01) | |
| Participants | 53,872 women treated with ART with no prior ART treatment; mean age at treatment start was 35.3 ± 5.3 years | |
| Interventions | Number of ART cycles, cumulative FSH dose, cumulative clomiphene citrate dose obtained from SART CORS database. Accuracy of data records validated via independent AUDIT procedure | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 3). SART CORS data were linked with cancer registries; 21 ovarian cancer cases. Age at cancer diagnosis was 40.8 ± 5.7 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Low risk | Adjusted analysis for diagnosis of infertility, parity, age at the first cycle, state of origin, and year when the infertility treatment was done |
| Performance bias | Low risk | Large national database; no blinding of assessors to case‐control status |
| Detection bias | Low risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | High risk | Women were followed up for a short time; this meant few cases of cancers were identified despite a large cohort |
| Selective reporting (reporting bias) | Low risk | Drugs used, doses, and number of cycles were reported |
Modan 1998.
| Methods | 'Retrospective cohort' of women with diagnosis of infertility from 1964 to 1974 who had visited the clinic more than once (2 centres in Israel) identified from medical records. Linked to Israel Cancer Registry | |
| Participants | Women with primary or secondary infertility. N = 2496. Mean age at entry was 28.7; mean age at the end of follow‐up was 50.0 | |
| Interventions | Fertility treatment; 908 women with clomiphene citrate, 242 women with clomiphene citrate + HMG, 159 women with HMG. No dosage or number of cycles was reported. Women received at least 1 cycle of fertility drugs. Duration of follow‐up by exposure group not reported | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 3) | |
| Notes | 54,413 person‐years follow‐up; mean follow‐up 21.4 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | High risk | Adjusted analysis not reported |
| Performance bias | High risk | Medical records; no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 96% of women followed (2496/54,413) |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
Mosgaard 1997.
| Methods | 'Prospective case‐control study'. Cases were all women with a first diagnosis of ovarian cancer from 1989 to 1994 selected from the Danish Cancer Registry with histological diagnosis, who returned a completed questionnaire with exposure data (N = 684). A random sample of 3 controls per case was selected from the National Person Register, matched by area of residence, age at time of cancer diagnosis, with at least 1 ovary and completed questionnaire from 1989 to 1994. Multi‐centre in Denmark, but number of centres not reported | |
| Participants | N = 1721 women. Mean age for cases = 47.2 (range 18 to 59). Mean age for controls = 46 (range 19 to 59) | |
| Interventions | Fertility drugs, dosage, and number of cycles not reported. 28/684 (20.7%) cases were exposed to infertility drugs, and 58/1721 (23.8%) controls were exposed to fertility drugs | |
| Outcomes | Invasive epithelial and non‐epithelial ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Duration of exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Only live cases included and women who had a confirmed histological diagnosis and returned a questionnaire about exposure |
| Confounding | Unclear risk | Matched for age at time of diagnosis and area of residence. Factors adjusted for included age, menarche, parity, age at first birth, duration of infertility, other causes of infertility, use of oral contraceptive pill, use of intrauterine devices, menopausal status, age at menopause, use of hormonal replacement therapy, age at sterilisation, history of cancer and family history for cancer, smoking, and body mass index |
| Performance bias | High risk | Self‐reported exposure status; unclear if blinding of assessors to case‐control status was used |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 88% of questionnaires returned for cases, 79.8% for controls. 80.7% of questionnaires for the cases were valid for analysis, as were 97% of questionnaires for the controls |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
Mosgaard 1998.
| Methods | 'Case‐control study'. All Danish women < 60 years of age with histologically confirmed borderline ovarian tumours identified from the Danish Cancer Registry from 1989 to 1994 with histological diagnosis, who returned a completed questionnaire with exposure data (N = 263). Random sample of 3 controls per case were selected from the National Person Register, were matched by area of residence and age at time of cancer diagnosis, and completed a questionnaire. National study in Denmark from 1989 to 1994 | |
| Participants | N = 1721 women with at least 1 ovary. Mean age for cases 43.6 (range 22 to 59) years. Mean age for controls 46 (range 19 to 59) years | |
| Interventions | Fertility drugs, dosage, and number of cycles not reported | |
| Outcomes | Borderline ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Duration of exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | High risk | Live cases only and women who responded to questionnaire on exposure |
| Confounding | Unclear risk | Cases and controls were matched for age at time of diagnosis and area of residence. Factors adjusted for included parity, use of an oral contraceptive pill, menopause, use of hormonal replacement therapy, and smoking |
| Performance bias | High risk | Self‐reported (type of treatment ‐ oral/injection) with some checks with fertility clinics for confirmation. No blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 87.8% of questionnaires were returned, and all were selected for cases to analyse; 79.8% of questionnaires were returned for controls, and all were used for analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
Parazzini 1997.
| Methods | 'Case‐control study'. Cases were women < 75 years of age with diagnosis of invasive ovarian cancer within 1 year of interview and admitted to a major teaching or general hospital in Milan, Italy, from 1983 to 1991. Controls were women admitted to the same hospitals where the cases were identified with acute non‐gynaecological, non‐hormonal, or non‐neoplastic conditions | |
| Participants | N = 971 cases; age 22 to 74 (median 54 years). N = 2758 controls, age 23 to 74 (median 52 years) | |
| Interventions | Fertility drugs and number of cycles reported but not dosage used per cycle. Fewer than or equal to 6 cycles; 1/971 cases and 3/2758 controls. 6 or more cycles; 4/971 cases and 7/2758 controls | |
| Outcomes | Invasive epithelial ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Duration of exposure per number of cycles reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Cases and controls were recruited from the same geographical area |
| Confounding | Unclear risk | Factors adjusted for included age, education, parity, oral contraceptive use, difficulties in conception |
| Performance bias | High risk | Questionnaires; no blinding of assessors to case‐control status used |
| Detection bias | High risk | How cases were ascertained was not reported, and blinding of assessors to exposure status was used |
| Attrition bias | Low risk | Cases and controls assessed for exposure and outcome at the same time when admitted to hospital |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all fertility drugs used were investigated |
Parazzini 1998.
| Methods | 'Case‐control study'. Cases were women with histologically confirmed borderline ovarian tumours admitted to 1 hospital in Milan, Italy. Controls were women admitted to hospitals serving the same catchment area in which cases lived with acute non‐gynaecological, non‐hormonal, non‐neoplastic conditions from 1986 to 1991 | |
| Participants | N = 93 cases, age 23 to 64 years. N = 273 controls, age 24 to 64 years | |
| Interventions | Fertility drugs, dosage, and number of cycles not reported. 4/93 (4.3%) cases and 0/273 controls exposed to fertility drugs | |
| Outcomes | Borderline ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | States that cases in this report were not included in previous articles on relationship between fertility drugs and ovarian cancer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Cases and controls were recruited from the same geographical area |
| Confounding | Unclear risk | Factors adjusted for included age, education, parity, oral contraceptive use, and difficulty in conception |
| Performance bias | High risk | Face‐to‐face interview; blinding unclear |
| Detection bias | High risk | How cases were ascertained was not reported, and assessors were not blinded to exposure status |
| Attrition bias | Unclear risk | Cases and controls assessed for exposure and outcome at the same time when admitted to hospital |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether all fertility drugs were used and investigated |
Parazzini 2001.
| Methods | 'Case‐control study'. Cases were women with incident histologically confirmed ovarian cancer admitted to the major teaching and general hospitals in 4 geographical regions in Italy (women with borderline tumours were excluded) from 1992 to 1999. Controls were women from the same geographical area who were admitted to the same network of hospitals as cases for a wide range of acute non‐neoplastic conditions (women with hormonal or gynaecological diseases or bilateral oophorectomy were excluded) | |
| Participants | N = 1031 cases, median age 56, range 18 to 79 years. N = 2411 controls, median age 57, range 17 to 79 years | |
| Interventions | Fertility drugs, dosage, and number of cycles not reported. 15/1031 (1.5%) cases were exposed to fertility drugs, and 26/2411 (1.1%) controls were exposed to fertility drugs | |
| Outcomes | Epithelial ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Length of exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Cases and controls were recruited from the same geographical area |
| Confounding | Unclear risk | Factors adjusted for included age, education, menopausal status, age at menopause, parity, spontaneous miscarriages, termination of pregnancy, oral contraceptive use, family history for ovarian cancer, and history of infertility |
| Performance bias | High risk | Structured interviewer‐administered questionnaire and checked with medical records. Unclear if blinding of assessors to case‐control status was used |
| Detection bias | High risk | How cases were ascertained has not been specified, and it is unclear if blinding of assessors to exposure status was used |
| Attrition bias | Unclear risk | Cases and controls assessed for exposure and outcome at the same time as admitted to hospital |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all fertility drugs used were investigated |
Perri 2015.
| Methods | 'Historical cohort study' involving a single institution in Israel between 1995 and 2013. All participants had provided written informed consent for genetic testing and data collection and during the first clinical appointment had completed a questionnaire regarding family history as well as reproductive and selective lifestyle factors. Items on the questionnaire included age at menarche, oral contraceptive use (yes/no and duration), fertility treatments (yes/no and type), age at first pregnancy and pregnancy details (live births, abortions), age at first pregnancy and pregnancy details (live births, abortions), duration of breastfeeding (if any, in months), hormone replacement therapy (yes/no and type), and prior gynaecological surgeries (including risk‐reducing salpingo‐oophorectomy). During the follow‐up period until cancer diagnosis, death of other cause, or end of the study, relevant data were collected from women's files and participant parameters were updated. In the ovarian cancer women, only fertility treatments that preceded cancer diagnosis were recorded. Information on cancer diagnosis, type, and age at diagnosis was provided by the Israel National Cancer Registry up to the end of 2013 | |
| Participants | Consecutive Jewish Israeli females with BRCA1 or BRCA2 mutation confirmed following genetic counselling due to family history of BRCA mutation‐associated cancers between 1995 and 2011 (N = 1073 women) | |
| Interventions | Fertility treatments ascertained by self‐report included medications containing clomiphene citrate (CC) N = 82 or gonadotrophin N = 69, in vitro fertilisation (IVF n = 66), and some combination of these treatments (N = 50 women) | |
| Outcomes | Epithelial ovarian cancer by histological diagnosis obtained from the National Cancer Registry up to the end of 2013 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with family risk for genetic mutation who attended a particular clinic. No person had ovarian cancer at the start of the study and with at least 1 ovary |
| Confounding | High risk | Adjusted for age |
| Performance bias | High risk | Medical record review for ovarian cancer women only and self‐reported questionnaire for all women; no blinding of assessors to case‐control status |
| Detection bias | Low risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Low risk | Case ascertainment 90% to 95% complete; missing data on exposure for 97 women |
| Selective reporting (reporting bias) | Unclear risk | Fertility drugs used reported but not dosage and number of cycles |
Potashnik 1999.
| Methods | 'Retrospective cohort'. All women with infertility attending 1 fertility clinic (Soroka University Hospital), in Israel, from 1960 to 1984 identified from medical records. Linked to the National Cancer Registry | |
| Participants | Women with at least 2 recorded visits to the clinic. N = 1197. Mean age at first visit 27.5 ± 5.4 years, mean age at the end of follow‐up 44.8 ± 6.4 years for cohort. Mean age for exposed at first visit 27.5 ± 5.1, mean age at the end of follow‐up for exposed 27.7 ± 5.8 years, mean age at the first visit for unexposed 27.7 ± 5.8, at the end of the follow‐up 44.8 ± 7.1 years for unexposed | |
| Interventions | Infertility treatment, 0 with clomiphene citrate, 531/780 treated with clomiphene citrate + HMG, 6/780 treated with HMG, 780 (65.2%) exposed to fertility drugs. Duration of follow‐up for women exposed to fertility drugs 18.0 ± 4.9 years; non‐exposed 17.6 ± 5.9 years | |
| Outcomes | Ovarian cancer by histological diagnosis recorded on the National Cancer Registry | |
| Notes | 21,407 person‐years follow‐up. Mean follow‐up 17.9 ± 5.3 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with infertility who attended a particular clinic. No person had ovarian cancer at the start of the study and with at least 1 ovary |
| Confounding | Unclear risk | Factors adjusted for included age and ethnic origin |
| Performance bias | High risk | Medical records; no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | Case ascertainment 90% to 95% complete; missing data by exposure group not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all fertility drugs used were investigated |
Reigstad 2015.
| Methods | A population‐based cohort study of women registered in the Medical Birth Registry of Norway as having given birth between 1 January 1984 and 31 December 2010. Study participants were followed from start of first pregnancy until first cancer, death, emigration, or 31 December 2010. Median follow‐up for all women who underwent assisted reproductive techniques was 7.3 years, and for women who did not undergo assisted reproductive technique 16 years. More than 50% were followed > 5 to 10 years | |
| Participants | 806,248 women who had at least 1 pregnancy: 16,525 with ART; 789,723 without ART | |
| Interventions | Assisted reproductive techniques categorised as (1) conventional IVF, (2) IVF with ICSI, (3) a combination of the 2 or treatment with a different assisted reproductive technique such as frozen embryo replacement, gamete donation, or treatment abroad, and (4) unknown or unspecified. N = 11 had IVF; N = 2 ICSI; N = 3 Other | |
| Outcomes | Invasive ovarian cancer confirmed by histology and identified by linkage to the Cancer Registry of Norway: 16 ART; 800 non‐ART | |
| Notes | Among 16 cases were 2 cases followed up for < 1 year; 3 between 1 and 5 years, 6 for more than 5 to 10 years, and 5 for > 10 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with at least 1 ovary and no history of ovarian cancer |
| Confounding | High risk | Adjusted for age, age at start of follow‐up, parity, region of residence, and calendar period |
| Performance bias | Unclear risk | Not clear how information on infertility treatment was ascertained. Any exposure outside the IVF clinics included was unknown. No blinding of assessors to case‐control status reported |
| Detection bias | Low risk | Cancer registry; no blinding of assessors to exposure status was reported |
| Attrition bias | Low risk | Adjusted hazard ratios reported to account for losses to follow‐up |
| Selective reporting (reporting bias) | High risk | All fertility drugs used, dosages, and cycles were not reported |
Reigstad 2017.
| Methods | Registy‐based cohort study assessing risk of ovarian cancer in women treated with infertility drugs compared with women unexposed to infertility drugs. Exposure data obtained from the Norwegian Prescription Database; partus status obtained from the Birth Registry of Norway; and cancer diagnosis from the Cancer Registry of Norway | |
| Participants | Women born in Norway (n = 1,353,724) between 1960 and 1996. 598,983 (44%) women were classified as nulliparous, of whom 14,645 (2.4%) had received fertility treatment; 41,549 (5.5%) of 764,741 parous women received fertility treatment. Of those receiving fertility drugs, 33,431 received treatment with assisted reproductive technique, and 38,927 only with clomiphene citrate. Median age at entry was 27 years for nulliparous women with fertility treatment and 18 years for nulliparous women without fertility treatment. Nulliparous women with cancer were younger at diagnosis (median 40 years and 37 years for those without and with fertility treatment, respectively) compared with parous women (median 43 and 38 years) | |
| Interventions | Drug exposure data from 2004 to 2014 were obtained from the Norwegian Prescription Database (drugs used in ART and clomiphene citrate). Each treatment cycle with clomiphene citrate comprised 50 mg for 5 consecutive days, and dose was categorised as ≤ 3 cycles, 3 to 6 cycles, or > 6 cycles. Duration of follow‐up by exposure group was not reported | |
| Outcomes | Ovarian cancer by histological diagnosis (see Table 3) | |
| Notes | 12,354,392 person‐years of follow‐up; 20,128 women received a diagnosis of cancer; of those, 32 were cases of ovarian cancer. Median follow‐up 11 years and median exposure time for exposed women 5.8 years. Follow‐up started January 2004 for all women born between 1960 and 1985. Women who were born in 1986 or later started follow‐up on turning 18 years because receiving fertility treatment before this age was deemed unlikely. Follow‐up ended upon diagnosis of the first cancer of interest, death, emigration, or 31 December 2014 (the latest update of the Cancer Registry of Norway) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with at least 1 ovary and no history of ovarian cancer |
| Confounding | High risk | Adjusted for region of residence, birth cohort, and concomitant exposure to clomiphene citrate |
| Performance bias | Unclear risk | Information on infertility treatment was ascertained from the Norwegian Prescription Database. Any exposure outside the IVF clinics included was unknown. No blinding of assessors to case‐control status reported |
| Detection bias | Low risk | Cancer registry; no blinding of assessors to exposure status was reported |
| Attrition bias | Unclear risk | Follow‐up according to type of cancer not reported |
| Selective reporting (reporting bias) | High risk | All fertility drugs used, dosages, and cycles were not reported |
Rossing 1994.
| Methods | 'Nested case‐control study'. The cohort (N = 3837) comprised women undergoing fertility treatment at participating clinics in Seattle, USA, from 1974 to 1985. Cases were women with ovarian cancer after enrolment in the study until 1992 identified from a cancer registry. Controls were a random selection of women from the cohort stratified by age at enrolment 3:1 for each case within each strata | |
| Participants | Women who had made at least 2 clinic visits and lived in an area covered by the cancer surveillance system. Mean age of women at enrolment 29.7 years | |
| Interventions | Clomiphene dosage and number of cycles not reported | |
| Outcomes | Ovarian cancer by histological diagnosis recorded in cancer surveillance system (see Table 3) | |
| Notes | 43,438 person‐years of observation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No person had ovarian cancer at the start of the study and with at least 1 ovary |
| Confounding | Unclear risk | Adjusted analysis presented, but factors adjusted for not reported |
| Performance bias | High risk | Medical records; no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Unclear risk | 74.2% of controls were eligible to be interviewed |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all investigated fertility drugs were reported |
Rossing 2004.
| Methods | 'Population‐based case‐control study'. From 1994 to 1998 in 3 regions (Atlanta, Georgia; Detroit, Michigan; Seattle, Washington) in the USA (cancer registry ‐ local US born, with no history of breast cancer (to match controls)). Cases identified from cancer registry. Controls randomly selected from the Women's Contraceptive and Reproductive Experiences (CARE) study of breast cancer (English‐speaking women born in the USA, white/black, in 5 geographic regions), age 35 to 64 at reference date | |
| Participants | N = 378 cases; N = 1637 controls. Age range between 35 and 54 for cases and between 35 and 64 for controls | |
| Interventions | Fertility drugs, dosage, and cycles not reported | |
| Outcomes | Epithelial, non‐epithelial, and borderline ovarian cancer by histological diagnosis (see Table 2) | |
| Notes | Controls were more likely to have black ethnicity: 27.1% vs 13.5%. Length of follow‐up from exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Only women still alive were selected as cases; controls were matched on geographical area and age |
| Confounding | Unclear risk | Factors adjusted for included study site, race, age, marital status, education, cigarette smoking, age at menarche, oral contraceptive use in months |
| Performance bias | High risk | Information obtained through face‐to‐face interview and not from medical records. Interviewers were not blinded to case‐control status |
| Detection bias | High risk | Cancer registry |
| Attrition bias | Unclear risk | Length of follow‐up from exposure not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all fertility drugs used were investigated |
Sanner 2009.
| Methods | 'Retrospective cohort'. Women with infertility or infertility‐associated disorders attending 3 university hospital fertility clinics in Sweden from 1961 to 1975. Linked to Swedish Cancer Register | |
| Participants | N = 2768, median age 27 (16 to 45) exposed. N = 1615 (58%) unexposed who did not receive hormonal treatment. Median age 27 (16 to 45) | |
| Interventions | Fertility treatment: 389 (34%) with clomiphene citrate; 325 (28%) with gonadotrophins; and 439 (38%) with clomiphene citrate + HMG. Median follow‐up time for the cohort was 33 years (range 1 to 47 years). Duration of follow‐up by exposure group was not reported | |
| Outcomes | Primary invasive epithelial or borderline ovarian cancer by histological diagnosis obtained from National Cancer Registry (see Table 3) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with at least 1 ovary and no history of ovarian cancer |
| Confounding | High risk | No adjusted analysis reported |
| Performance bias | High risk | Medical record review at IVF clinics. Any exposure outside IVF clinics included was unknown. No blinding of assessors to case‐control status reported |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status was reported |
| Attrition bias | Unclear risk | 81% of women were followed up |
| Selective reporting (reporting bias) | Low risk | All fertility drugs used were reported |
Shushan 1996.
| Methods | 'Population‐based case‐control study' in Israel. Cases were women with invasive and borderline epithelial ovarian cancer reported to registry from 1990 to 1993. Cases were selected from National Cancer Registry (included only living cases). Controls were randomly selected from the same telephone dialling code (matched for geographical area) | |
| Participants | N = 164 cases with invasive cancer; N = 36 cases with borderline cancer; N = 408 controls | |
| Interventions | Fertility drugs, dosage, and number of cycles not reported | |
| Outcomes | Primary invasive epithelial or borderline ovarian cancer by histological diagnosis from Israel Cancer Registry (see Table 2) | |
| Notes | Length of follow‐up post exposure not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | Only women still alive were selected as cases; controls were matched on geographical area and age |
| Confounding | Unclear risk | Factors adjusted for included age, parity, BMI, region of birth, education, family history, interviewer |
| Performance bias | High risk | Self‐reported during an interview; interviewer not blind to case‐control status |
| Detection bias | High risk | Cancer registry |
| Attrition bias | Unclear risk | 200/287 (70%) living selected cases interviewed. Length of follow‐up post exposure not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear if other fertility drugs investigated but not reported |
Stewart 2013.
| Methods | Population‐based cohort study during the years 1982 to 2002. Data obtained from WA Data Linkage System. Mean duration of follow‐up 16.5 ± 5.9 years (median 16.5 years). Total duration of follow‐up (person‐years): 365,775 | |
| Participants | Women with a diagnosis of infertility or procreative management according to ICD codes in all hospitals in Western Australia (N = 21,639 for borderline ovarian cancer analysis and 21,646 for invasive epithelial cancer) aged 20 to 44 years; mean age 31.2 years | |
| Interventions | IVF treatment obtained from the Reproductive Technology Register (N = 14,095 did not undergo infertility treatment and N = 7544 women underwent infertility treatment) | |
| Outcomes | Borderline ovarian and invasive epithelial cancer diagnosis ascertained by histology and recorded by the Western Australia Cancer Registry | |
| Notes | Results published in 2 articles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with no history of ovarian cancer at the beginning of the study and with at least 1 ovary Retrospective analysis |
| Confounding | High risk | Analysis was adjusted only for parity |
| Performance bias | Low risk | Information based on national databases |
| Detection bias | Low risk | Cancer registry; no blinding of assessors to exposure status used |
| Attrition bias | High risk | Less than 80% of the sample was followed up with a mean of 17 years (366,041 person‐years) |
| Selective reporting (reporting bias) | High risk | Infertility drugs and numbers of cycles were not reported |
Stewart 2013a.
| Methods | Population‐based cohort study during the years 1982 to 2002. Data obtained from WA Data Linkage System. Mean duration of follow‐up 16.5 ± 5.9 years (median 16.5 years). Total duration of follow‐up (person‐years): 365,775 | |
| Participants | Women with a diagnosis of infertility or procreative management according to ICD codes in all hospitals in Western Australia (N = 21,639 for borderline ovarian cancer analysis and 21,646 for invasive epithelial cancer) aged 20 to 44 years; mean age 31.2 years | |
| Interventions | IVF treatment obtained from the Reproductive Technology Register (N = 14,095 did not undergo infertility treatment and N = 7544 women underwent infertility treatment) | |
| Outcomes | Borderline ovarian and invasive epithelial cancer diagnosis ascertained by histology and recorded by the Western Australia Cancer Registry | |
| Notes | Results published in 2 articles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women with no history of ovarian cancer at the beginning of the study and with at least 1 ovary Retrospective analysis |
| Confounding | High risk | Analysis was adjusted only for parity |
| Performance bias | Low risk | Information based on national databases |
| Detection bias | Low risk | Cancer registry; no blinding of assessors to exposure status used |
| Attrition bias | High risk | Less than 80% of the sample was followed up with a mean of 17 years (366,041 person‐years) |
| Selective reporting (reporting bias) | High risk | Infertility drugs and numbers of cycles were not reported |
Trabert 2013.
| Methods | 'Retrospective cohort study' involving women who had sought advice for infertility at 5 large reproductive endocrinology practices in the USA, between 1965 and 1988, identified from clinic records. A short questionnaire was used to ascertain cancer diagnoses and cancer risk factors that may have changed over time (e.g. reproductive and menopausal status). A questionnaire was used from 1998 to 2001, which included information on menstrual and reproductive history, use of exogenous hormones, anthropometric factors, cigarette smoking, alcohol consumption, and screening for breast and ovarian diseases. A shorter questionnaire was added in 2010 with updated information on reproductive behaviour, body size, gynaecological operations, use of menopausal hormones, and mammographic screening history | |
| Participants | All women evaluated for infertility at 5 clinical sites in the USA between 1965 and 1988 with follow‐up through 2010, who were treated with infertility drugs and with at least 1 intact ovary (N = 9825). Mean follow‐up was 17.6 years for ovarian cancer cases n = 85 and 26.2 years for women who did not develop cancer. Mean age at first clinic visit of all women was 30.1 years, and study population was predominantly white. Questionnaires were obtained from 6,582 women (67%) | |
| Interventions | N = 3745 women used CC; of those, 37 (9%) cases developed ovarian cancer (RR 1.34, 95% CI 0.86 to 2.07), N = 13 women used CC and remained nulligravid and among these the RR was 3.63 (95% CI 1.36 to 9.72) vs N = 16 women who did conceive with the use of CC who had RR 0.88 (95% CI 0.47 to 1.63) with less risk to develop cancer N = 952 used gonadotrophins; of these, 8 (8%) with RR 1.00 (95% CI 0.48 to 2.08) had ovarian cancer. N = 7 cases had sequential CC and gonadotrophins (RR 1.20, 95% CI 0.54 to 2.68), and the total number of women who received combined treatment was not reported |
|
| Outcomes | Information about ovarian cancer cases was obtained for only 68 of the 85 ovarian cancer cases (80%) through cancer registry and medical records. Cancers were also identified by linkages to cancer registries in the 14 states in which most women resided. Information on women outside these states was achieved by the outcome documented in the questionnaire, and records were requested by contacting their treating physicians. New deaths were also identified by searching the Social Security Administration Death Master File. Information regarding infertility drugs used such as total cumulative dosage, number of treatment cycles, and age at first use was ascertained from clinic records | |
| Notes | Longer‐term follow‐up of study reported by Brinton 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women in a given area with no history of ovarian cancer at the beginning of the study and with at least 1 ovary. Retrospective analysis |
| Confounding | High risk | Analysis was adjusted for clinic site, calendar year of first infertility evaluation, and gravidity status at study entry (ever pregnant at first visit vs nulligravid at first clinic visit) |
| Performance bias | Unclear risk | Information based on medical records and for women without medical record data; information added from health questionnaire |
| Detection bias | Low risk | Cancer registry; no blinding of assessors to exposure status used |
| Attrition bias | High risk | Analytical cohort 9825 IVF treated, Questionnaires were obtained from 6582 women (67% of the analysis participants); 5349 completed the 1998 to 2001 questionnaire, 4772 the 2010 questionnaire, and 3538 both. Medical verification for cancers was obtained for 68 cases among the 85 cases of ovarian cancer (80%). 3538/9825 (36%) at 12 years' follow‐up and 4772/9825(48%) at 10 years' follow‐up through the use of questionnaires |
| Selective reporting (reporting bias) | Low risk | All fertility drugs and doses used were reported |
Van Leeuwen 2011.
| Methods | Historical cohort (OMEGA), Netherlands; multi‐centre (12 hospitals) | |
| Participants | Subfertile women who received at least 1 IVF cycle with ovarian stimulation (19,861) from 1983 to 1995. The control group comprised subfertile women not treated with IVF (6604) selected from the 4 IVF clinics with a computerised registry of all subfertile women evaluated from 1980 to 1995 before IVF was a routine procedure. Mean age of IVF‐treated women was 47.5 years and for women who did not receive IVF 49.4 years | |
| Interventions | In the IVF group, 32.9% of women had 1 to 2 stimulated IVF cycles, 32.8% had 3 to 4 cycles, and 17.5% received 5 or more cycles. Clomiphene/HMG or FSH/HMG stimulation protocols were used until 1988 to 1989, whereas stimulation with GnRH agonists became more common after 1990 (from 20% in 1986 to about 90% after 1990). From 1984 to 1994, the number of ampoules of gonadotrophins strongly increased | |
| Outcomes | Ovarian cancer including borderline ovarian tumours by histological diagnosis; linkage with national cancer registry | |
| Notes | Median duration of follow‐up was 14.3 years for the exposed and 16.4 years for the non‐exposed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Unclear risk | All women in a given area with no history of ovarian cancer at the beginning of the study and with at least 1 ovary. Women in cohort who did not receive IVF were slightly older and had a slightly longer median duration of follow‐up than women who did receive IVF |
| Confounding | High risk | Analysis was adjusted for age at the end of follow‐up, endometriosis, tubal problems, and parity |
| Performance bias | Unclear risk | Information based on medical records, and for women without medical record data, information was added from health questionnaire |
| Detection bias | Unclear risk | Cancer registry; no blinding of assessors to exposure status used |
| Attrition bias | High risk | Analytical cohort 19,146 IVF treated, 6006 non‐IVF treated. 67.3% responded and consented to future record linkage, 4.3% of responders refused, 28.2% were non‐responders, 0.2% were deceased at initial approach of the IVF group. 40.7% responded and consented to future record linkage, 3.1% responders refused, 55.4% were non‐responders, 0.9% were deceased at initial approach of non‐IVF group |
| Selective reporting (reporting bias) | Unclear risk | 10,343/19,146 (54%) at 10 years' follow‐up; 7621/19,146 (40%) at more than 15 years' follow‐up |
Venn 1995.
| Methods | Retrospective cohort, Ausralia, 1978 to 1992, 1 centre | |
| Participants | Women treated or referred for IVF with known age and time of entry to cohort, N = 10,358, median age 32 (range 18 to 49), median age at the end of the follow‐up 38 (range 21 to 57) in exposed (N = 5564).Median age 31 (range 19 to 51), median age at the end of the follow‐up 38 (range 22 to 59) in unexposed (4794). | |
| Interventions | Infertility treatment, type of treatment CC/HCG/HMG, dosage NR, 2052 women underwent 1 cycle, 1362 2 cycles, 1637 between 3 and 5 cycles and 191 more than 6 cycles. | |
| Outcomes | Ovarian cancer by histological diagnosis from the Victorian Cancer Registry | |
| Notes | Total person‐years contributed was 31,272 for the exposed group and 35,655 for the unexposed group. Median length of follow‐up for exposed 5.2 (range 1 to 15.1), median length follow‐up for unexposed 7.6 (range 1 to 15.5) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No person had ovarian cancer at the start of the study and at least with one ovary |
| Confounding | High risk | No adjusted analysis was reported and groups were not matched or balanced for confounding factors at baseline |
| Performance bias | High risk | Medical records, no blinding of assessors to exposure status reported |
| Detection bias | High risk | Cancer registry, no blinding of assessors to case status |
| Attrition bias | Low risk | 67% for exposed group and 71% for the unexposed. |
| Selective reporting (reporting bias) | Low risk | All the infertility drugs used were reported. |
Venn 1999.
| Methods | 'Retrospective cohort'. Women who registered with at least 1 of 10 participating clinics in Australia before 1994: 30% before 1986, 70% from 1986 to 1996. Linked to cancer registry | |
| Participants | Women who received at least 1 IVF treatment. N = 29,700, median age 31 (range 18 to 50) in exposed, median age 30 (range 18 to 53) in unexposed | |
| Interventions | Fertility treatment used: 1182 (6.9%) with clomiphene citrate, 6543 (38.2%) with clomiphene citrate + HMG, 1464 (8.5%) with HMG, 11,153 (65%) with HMG + GnRH agonist, 1771 (8.6%) with other treatments NR. Dosage NR. 6346 (37.0%) with 1 cycle, 3712 (21.6%) with 2 cycles, 5157 (30.1%) between 3 and 5 cycles, 1933 (11.3%) with more than 6 cycles. 134,240 person‐years follow‐up in exposed, 96,794 person‐years in unexposed. Median follow‐up in exposed 7 (range < 1 to 21) years; in unexposed 10 (< 1 to 22) years | |
| Outcomes | Invasive ovarian cancer by histological diagnosis from the Victoria Cancer Registry (see Table 3) | |
| Notes | 80% of the cohort sample was followed up until 1996 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | No person had ovarian cancer at the start of the study and had at least 1 ovary |
| Confounding | High risk | No adjusted analysis reported, and groups were not matched or balanced for confounding factors at baseline |
| Performance bias | High risk | Medical records; no blinding of assessors to case‐control status |
| Detection bias | High risk | Cancer registry; no blinding of assessors to exposure status |
| Attrition bias | Low risk | 81% exposed and 72% unexposed were followed up |
| Selective reporting (reporting bias) | Low risk | All drugs used were reported |
Yli‐Kuha 2012.
| Methods | Retrospective cohort, Finland 1996 to 1998; single centre | |
| Participants | Subfertile women (N = 9175) who purchased drugs for IVF between 1996 and 1998 and their age and residence‐matched controls were randomly selected from the general population register (N = 9175) | |
| Interventions | Fertility treatment reported, but dosage, number of cycles, and type of drug used not reported | |
| Outcomes | Ovarian cancer by histological diagnosis; Finnish cancer registry | |
| Notes | Mean follow‐up time for exposed subfertile women was 7 years 9 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias | Low risk | All women in a given area with no history of ovarian cancer at the beginning of the study and with at least 1 ovary |
| Confounding | Unclear risk | Cases were age and residence matched with controls and further adjusted for socioeconomic position and marital status |
| Performance bias | High risk | Medical record review; no blinding of assessors to exposure status |
| Detection bias | Unclear risk | Information on development of the cancers was obtained from the medical notes and cancer registry. Not reported if assessors were blind to exposure status |
| Attrition bias | Low risk | All women (9175) were followed up for 7 years and 9 months |
| Selective reporting (reporting bias) | Unclear risk | The fertility drugs used were not reported |
amp: ampoule. ART: assisted reproductive technology. BMI: body mass index. CARE: Women's Contraceptive and Reproductive Experiences study. CC: clomiphene citrate. CI: confidence interval. EMR: electronic medical record. FSH: follicle‐stimulating hormone. GnRH: gonadotropin‐releasing hormone. HCG: human chorionic gonadotrophin. HR: hazard ratio. ICD: International Classification of Diseases. ICSI: intracytoplasmic sperm injection. IVF: in vitro fertilisation. LH: luteinising hormone. NR: not reported. OC: oral contraceptive. OCP: oral contraceptive pill. RR: risk ratio. SART CORS: Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. SD: standard deviation. SERM: selective oestrogen receptor modulator.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adami 1994 | General article about risk factors for ovarian cancer |
| Adelson 1993 | General article |
| Al‐Shawaf 2005 | Review article |
| Albrektsen 1996 | Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Allaway 2017 | General article on infertility and discussion of indications for the use of clomiphene citrate |
| Althuis 2005 | General article on infertility and risk of ovarian cancer |
| Anderson 1996 | Case report |
| Artini 1997 | Case series (fewer than 30 patients) |
| Attia 2006 | General article on infertility and risk of ovarian cancer |
| Ayhan 2004 | General article on infertility |
| Badawy 2009 | General article on infertility and risk of ovarian cancer |
| Balasch 1994 | Case series (fewer than 30 patients) |
| Bandera 2005 | General article on infertility and risk of ovarian cancer |
| Bayar 2006 | Case report |
| Bose 2008 | General article on infertility and risk of ovarian cancer |
| Brekelmans 2003 | Review article |
| Brinton 1996 | General article on infertility and risk of ovarian cancer |
| Brinton 1997 | General article on infertility |
| Brinton 2007 | Review article |
| Brinton 2012 | Review of some observational studies investigating risk of ovarian cancer and use of infertility drugs |
| Bristow 1996 | Review article |
| Bristow 1996 | Review article |
| Burger 2004 | Review article |
| Cetin 2008 | Review article |
| Chene 2009 | General article on infertility and risk of ovarian cancer |
| Clinton 1997 | General article on infertility and risk of ovarian cancer |
| Cohen 1993 | General article on infertility and risk of ovarian cancer |
| Cramer 1998 | Cohort study about other risk factors (no infertility or infertility drugs included) for ovarian cancer |
| Crosbie 2005 | Review article |
| Croughan‐Minihane 2001 | Unpublished data. Abstract not fully informative about risk calculated by study authors |
| Cusido 2007 | Case‐control study evaluating risk of borderline ovarian cancer. Controls were women treated for benign ovarian pathology requiring surgery. Only crude estimates presented, no attempt at controlling for confounding. No details on how ovarian cancer was confirmed |
| Demirol 2006 | Review article on infertility and ovarian cancer |
| Devesa 2010 | Review article on infertility and risk of ovarian cancer |
| Dos Santos 2002 | Case series (fewer than 30 patients) |
| Duckitt 1998 | General article on infertility and risk of ovarian cancer |
| Duska 1996 | Review article |
| Franceschini 1991 | Pooled analysis of 3 European case‐control studies |
| Franco 2000 | Case series (fewer than 30 patients) |
| Gadducci 2004 | Review article |
| Gadducci 2013 | Review of some observational studies investigating risk of ovarian cancer and use of infertility drugs |
| Genc 2011 | General article on infertility and risk of ovarian cancer |
| Glud 1998 | Review article |
| Goldberg 1992 | Case series (fewer than 30 patients) |
| Goodman 2001 | Research article |
| Goshen 1998 | Review article |
| Gwinn 1990 | Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Hankinson 1995 | Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Harris 1992 | Collaborative analysis of 12 US case‐control studies |
| He 2012 | Review of some observational studies investigating risk of ovarian cancer and use of infertility drugs |
| Helzlsouer 1995 | General article on infertility and risk of ovarian cancer |
| Horn‐Ross 1992 | Collaborative analysis of 12 US case‐control studies |
| Jensen 2007 | Cohort study on reproductive factors and risk of breast cancer |
| Jensen 2008 | Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of breast cancer |
| Kashyap 2003 | Review article |
| Kaufman 1995 | Review article |
| Kelly 2003 | General article on infertility and risk of ovarian cancer |
| King 1994 | General article on infertility and ovarian cancer |
| Klip 2000 | General article on infertility |
| Klip 2001 | Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Konishi 1999 | Review article |
| Kristiansson 2007 | General article on infertility and risk of ovarian cancer |
| Kurian 2004 | Review article |
| La Vecchia C 2011 | General article on infertility and risk of ovarian cancer |
| Land 1993 | Review article |
| Lerner‐Geva 2010 | Review article |
| Lerner‐Geva 2004 | Review article |
| Lerner‐Geva 2006 | Cohort study on reproductive factors and risk of breast cancer |
| Li 2013 | Meta‐analysis of only some of the cohort studies published on the risk of ovarian cancer in women treated with ovulation stimulation drugs |
| Lopes 1993 | General article on infertility and ovarian cancer |
| Mandai 2007 | Review article |
| McGuire 2004 | General article on infertility and risk of ovarian cancer |
| McSorley 2009 | General article on infertility and risk of ovarian cancer |
| Mendola 2013 | Commentary on Asante's paper published in 2013 |
| Menon 2009 | Article on sensitivity and specificity of possible ovarian cancer screening |
| Miao 2006 | General article on infertility/article in Chinese (abstract in English) |
| Modugno 2001 | Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Negri 1991 | Pooled analysis of case‐control studies |
| Ness 2000 | Pooled analysis of case‐control studies |
| Ness 2003 | Review article |
| Ness 2011 | General article on risk of ovarian cancer |
| Nieto 2001 | General article on infertility and risk of ovarian cancer |
| Oktay 2010 | Review article on infertility and risk of ovarian cancer |
| Ozcan 2009 | Review article on infertility and risk of ovarian cancer |
| Ozdemir 2005 | Research article |
| Parazzini 2004 | General article on infertility and risk of ovarian cancer |
| Paulson 1996 | Review article |
| Persson 1995 | General article on infertility/article in Swedish (abstract in English) |
| Purdie 1995 | Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Riman 1998 | General article on infertility and ovarian cancer |
| Riman 2002 | Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Rish 1996 | Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Rodriguez 1998 | Reported risk of ovarian cancer only in infertile women who were not treated with ovarian stimulating drugs |
| Ron 1995 | Review article |
| Rosemberg 1994 | Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Rosen 1997 | General article on infertility |
| Rosenblatt 1993 | Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Rossing 1996 | Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of breast cancer |
| Schildkraut 1996 | General article on infertility and risk of ovarian cancer |
| Shoham 1994 | Review article |
| Siristatidis 2013 | A meta‐analysis on risk of ovarian cancer and women treated with ovarian stimulating drugs for infertility |
| Smith 2001 | General article on infertility and ovarian cancer |
| So 2008 | General article on infertility and ovarian cancer |
| Soegaard 2007 | Case‐control study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Spirtas 1993 | General article on infertility and ovarian cancer |
| Stein 1997 | General article on infertility and ovarian cancer |
| Tarlatzis 1995 | Review article |
| Trifonov 2000 | Article in Bulgarian/review article |
| Unkila‐Kallio 1997 | Case series (fewer than 30 patients) |
| Unkila‐Kallio 2000 | Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Venn 2003 | Review article |
| Venn 1997 | Review article |
| Venn 2001 | Cohort study about reproductive factors (no infertility or infertility drugs included) and risk of ovarian cancer |
| Vlahos 2010 | Review article |
| Wakeley 2000 | Review article |
| Wang 2017 | Review on options available to manage infertility due to anovulation |
| Whittemore 1994 | Commentary/letter |
| Willemsen 1993 | Case series (fewer than 30 patients) |
| Zarchi 2013 | Review on infertility as risk factor for all gynaecological cancers |
| Zreik 2008 | Review article |
Differences between protocol and review
We decided to add the following fertility medications to the review: selective oestrogen receptor modulator (SERM); gonadotrophin‐releasing hormone agonist (GnRH‐AG); gonadotrophin‐releasing hormone antagonist (GnRH‐A); and growth hormone.
Contributions of authors
IR and LS selected studies and contributed to writing of the text. IR, RB, and LS extracted data. RB reviewed drafts and suggested revisions. LS performed statistical analysis. All review authors approved the final version.
Sources of support
Internal sources
-
Ipswich Hospital NHS Trust, UK.
The staff at the library helped us in searching and in providing articles
External sources
No sources of support supplied
Declarations of interest
Ivana Rizzuto: none known. Renee Behrens: none known. Lesley Smith: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Asante 2013 {published and unpublished data}
- Asante A, Leonard PH, Weaver AL, Goode EL, Jensen JR, Stewart EA, et al. Fertility drug use and the risk of ovarian tumours in infertile women: a case‐control study. Fertility and Sterility 2013;99(7):2031‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjornholt 2015 {published data only}
- Bjornholt S, Kruger Kjaer S, Svane Nielsen TS, Jensen A. Risk for borderline ovarian tumours after exposure to fertility drugs: results of a population‐based cohort study. Human Reproduction 2015;30(1):222‐31. [DOI] [PubMed] [Google Scholar]
Brinton 2013 {published data only}
- Brinton LA, Trabert B, Shalev V, Lunenfield E, Sella T, Chodick G. In vitro fertilization and risk of breast and gynaecologic cancers: a retrospective cohort study within the Israeli Maccabi Healthcare services. Fertility and Sterility 2013;99(5):1189‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calderon‐Margalit 2009 {published and unpublished data}
- Calderon‐Margalit R, Friedlander Y, Yanetz R, Kleinhaus K, Perrin MC, Manor O, et al. Cancer risk after exposure to treatments for ovulation induction. American Journal of Epidemiology 2008;169(3):365‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dor 2002 {published and unpublished data}
- Dor J, Lerner‐Geva L, Rabinovici J, Chetrit A, Levran D, Lunenfeld B, et al. Cancer incidence in a cohort of infertile women who underwent in vitro fertilization. Fertility and Sterility 2002;77(2):324‐7. [DOI] [PubMed] [Google Scholar]
Dos Santos Silva 2009 {published and unpublished data}
- Dos Santos Silva I, Wark PA, McCormack VA, Mayer D, Overton C, Little V, et al. Ovulation‐stimulation drugs and cancer risks: a long‐term follow‐up of a British cohort. British Journal of Cancer 2009;100:1824‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2002 {published and unpublished data}
- Doyle P, Maconochie N, Beral V, Swerdlow AJ, Tan SL. Cancer incidence following treatment for infertility at a clinic in the UK. Human Reproduction 2002;17(8):2209‐13. [DOI] [PubMed] [Google Scholar]
Franceschini 1994 {published and unpublished data}
- Franceschini S, Vecchia C, Negri E, Guarneri S, Montella M, Conti E, et al. Fertility drugs and risk of epithelial ovarian cancer in Italy. Human Reproduction 1994;9(9):1673‐5. [DOI] [PubMed] [Google Scholar]
Gronwald 2015 {published data only}
- Gronwald J, Glass K, Rosen B, Karlan B, Tung N, The Hereditary Breast Cancer Clinical Study Group, et al. Treatment of infertility does not increase the risk of ovarian cancer among women with a BRCA1 or BRCA 2 mutation. Fertility and Sterility 2015;105(3):781‐5. [DOI] [PubMed] [Google Scholar]
Jensen 2009 {published and unpublished data}
- Jensen A, Sharif H, Frederiksen K, Kjaer SK. Use of fertility drugs and risk of ovarian cancer: Danish population based cohort study. BMJ 2009;338:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kallen 2011 {published and unpublished data}
- Kallen B, Finnstrom O, Lindam A, Nilsson E, Nygren K‐G, Olausson PO. Malignancies among women who gave birth after in vitro fertilization. Human Reproduction 2011;26(1):253‐8. [DOI] [PubMed] [Google Scholar]
Kessous 2016 {published data only}
- Kessous K, Davidson E, Meirovitz M, Sergienko R, Sheiner E. The risk of female malignancies after fertility treatments: a cohort study with 25 year follow‐up. Journal of Cancer Research and Clinical Oncology 2016;142:287‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurta 2012 {published and unpublished data}
- Kurta ML, Moysich KB, Weissfeld JL, Youk AO, Bunker CH, Edwards RP, et al. Use of fertility drugs and risk of ovarian cancer: results from a US‐based case‐control study. Cancer Epidemiology, Biomarkers and Prevention 2012;21(8):1282‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerner‐Geva 2003 {published and unpublished data}
- Lerner‐Geva L, Geva E, Lessing JB, Chetrit A, Modan B, Amit A. The possible association between in vitro fertilization treatments and cancer development. International Journal of Gynecological Cancer 2003;13:23‐7. [DOI] [PubMed] [Google Scholar]
Lerner‐Geva 2012 {published and unpublished data}
- Lerner‐Geva L, Jaron R, Liraz O, Tzvia B, Shlomo M, Bruno L. Are infertility treatments a potential risk factor for cancer development? Perspective of 30 years of follow‐up. Gynecological Endocrinology 2012;28(10):809‐14. [DOI] [PubMed] [Google Scholar]
Luke 2015 {published data only}
- Luke B, Brown MB, Spector LG, Missmer SA, Leach RE, William M, et al. Cancer in women after assisted reproductive technology. Fertility and Sterility 2015;104(5):1218‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Modan 1998 {published and unpublished data}
- Modan B, Ron E, Lerner‐Geva L, Blumstein T, Menczer J, Rabinovici J, et al. Cancer incidence in a cohort of infertile women. American Journal of Epidemiology 1998;147:1038‐42. [DOI] [PubMed] [Google Scholar]
Mosgaard 1997 {published and unpublished data}
- Mosgaard BJ, Lidegaard BJ, Kjaer SK, Schou G, Anderson AN. Infertility, fertility drugs, and invasive ovarian cancer: a case‐control study. Fertility and Sterility 1997;67(6):1005‐12. [DOI] [PubMed] [Google Scholar]
Mosgaard 1998 {published and unpublished data}
- Mosgaard BJ, Lidegaard O, Kjaer SK, Schou G, Anderson AN. Ovarian stimulation and borderline ovarian tumours: a case‐control study. Fertility and Sterility 1998;70(6):1049‐55. [DOI] [PubMed] [Google Scholar]
Parazzini 1997 {published and unpublished data}
- Parazzini F, Negri E, Vecchia C, Moroni S, Franceschini S, Crosignani PG. Treatment for infertility and risk of invasive epithelial ovarian cancer. Human Reproduction 1997;12(10):2159‐61. [DOI] [PubMed] [Google Scholar]
Parazzini 1998 {published and unpublished data}
- Parazzini F, Negri E, Vecchia C, Moroni S, Polatti A, Chiaffarino F, et al. Treatment for fertility and risk of ovarian tumours of borderline malignancy. Human Reproduction 1998;68:226‐8. [DOI] [PubMed] [Google Scholar]
Parazzini 2001 {published and unpublished data}
- Parazzini F, Pelucchi C, Negri E, Franceschini S, Talamini R, Montella M, et al. Use of fertility drugs and risk of ovarian cancer. Human Reproduction 2001;16(7):1372‐5. [DOI] [PubMed] [Google Scholar]
Perri 2015 {published data only}
- Perri T, Lifshitz D, Sadetzki S, Oberman B, Meirow D, Ben‐Baruch G, et al. Fertility treatments and invasive epithelial ovarian cancer risk in Jewish Israeli BRCA 1 or BRCA 2 mutation carriers. Fertility and Sterility 2015;103(5):1305‐12. [DOI] [PubMed] [Google Scholar]
Potashnik 1999 {published and unpublished data}
- Potashnik G, Lerner‐Geva L, Genkin L, Chetrit A, Lunenfeld E, Porath A. Fertility drugs and the risk of breast and ovarian cancers: results of a long‐term follow‐up study. Fertility and Sterility 1999;71(5):853‐9. [DOI] [PubMed] [Google Scholar]
Reigstad 2015 {published data only}
- Reigstad MM, Larsen IK, Myklebust TA, Robsahm TE, Oldereid NB, Omland AK, et al. Cancer risk among parous women following assisted reproductive technology. Human Reproduction 2015;30(8):1952‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reigstad 2017 {published data only}
- Reigstad MM, Storeng R, Myklebust TA, Oldereid NB, Omland AK, Robsahm TE, et al. Cancer risk in women treated with fertility drugs according to parity status ‐ A registry‐based cohort study. Cancer Epidemiology, Biomarkers & Prevention 2017;26(6):953‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossing 1994 {published and unpublished data}
- Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumours in a cohort of infertile women. New England Journal of Medicine 1994;331(12):771‐6. [DOI] [PubMed] [Google Scholar]
Rossing 2004 {published and unpublished data}
- Rossing MA, Tang Mei‐Tzu C, Flagg EW, Weiss LK, Wicklund KG. A case‐control study of ovarian cancer in relation to infertility and the use of ovulation‐inducing drugs. American Journal of Epidemiology 2004;160(11):1070‐8. [DOI] [PubMed] [Google Scholar]
Sanner 2009 {published and unpublished data}
- Sanner K, Conner P, Bergfeldt K, Dickman P, Sundfeldt K, Bergh T, et al. Ovarian epithelial neoplasia after hormonal infertility treatment: long‐term follow‐up of a historical cohort in Sweden. Fertility and Sterility 2009;91:1152‐8. [DOI] [PubMed] [Google Scholar]
Shushan 1996 {published and unpublished data}
- Shushan A, Paltiel O, Isovich J, Elchalal U, Peretz T, Schenker JG. Human menopausal gonadotropin and the risk of epithelial ovarian cancer. Fertility and Sterility 1996;65(1):13‐8. [PubMed] [Google Scholar]
Stewart 2013 {published data only}
- Stewart LM, Holman CDJ, Aboagye‐Sarfo P, Finn JC, Preen DB, Hart R. In vitro fertilization, endometriosis, nulliparity and ovarian cancer risk. Gynecologic Oncology 2013;128:260‐4. [DOI] [PubMed] [Google Scholar]
- Stewart LM, Holman CJ, Finn JC, Preen DB, Hart R. In vitro fertilization is associated with an increased risk of borderline ovarian tumours. Gynecologic Oncology 2013;129:372‐6. [DOI] [PubMed] [Google Scholar]
Stewart 2013a {published data only}
- Stewart L.M, Holman C. D'Arcy J, J. C. Finn, D.B. Preen, R. Hart. In‐vitro fertilization is associated with an increased risk of borderline ovarian tumours. Gynaecol Oncology 2013;129(2):372‐376. [DOI] [PubMed] [Google Scholar]
Trabert 2013 {published data only}
- Trabert B, lamb EJ, Scoccia B, Moghissi KS, Westhoff CL, Niwa A, et al. Ovulation‐inducing drugs and ovarian cancer risk: results from an extended follow‐up of a large US infertility cohort. Fertility and Sterility 2013;100(6):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Leeuwen 2011 {published data only}
- Leeuwen FE, Klip H, Mooij TM, Swaluw AMG, Lambalk CB, Kortman M, et al. Risk of borderline and invasive ovarian tumours after ovarian stimulation for in vitro fertilization in a large Dutch cohort. Human Reproduction 2011;26(12):3456‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venn 1995 {published data only}
- Venn A, Watson L, Lumley J, Giles G, King C, Healy D. Breast and ovarian cancer incidence after infertility and in vitro fertilisation. Lancet 1995;346:995‐1000. [DOI] [PubMed] [Google Scholar]
Venn 1999 {published and unpublished data}
- Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of infertility drugs with in‐vitro fertilisation. Lancet 1999;354:1586‐90. [DOI] [PubMed] [Google Scholar]
Yli‐Kuha 2012 {published and unpublished data}
- Yli‐Kuha A‐N, Gissler M, Klemetti R, Luoto R, Hemminki E. Cancer morbidity in a cohort of 9175 Finnish women treated for infertility. Human Reproduction 2012;27(4):1149‐55. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adami 1994 {published data only}
- Adami H‐O, Hsieh C‐C, Lambe M, Trichopoulos D, Leon D, Persson I, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet 1994;344:1250‐4. [DOI] [PubMed] [Google Scholar]
Adelson 1993 {published data only}
- Adelson MD, Reece MT. Effects of gonadotropin‐releasing hormone analogues on ovarian epithelial tumours. Clinical Obstetrics & Gynaecology 1993;36(3):690‐700. [DOI] [PubMed] [Google Scholar]
Albrektsen 1996 {published data only}
- Albrektsen G, Heuch I, Kvale G. Reproductive factors and incidence of epithelial ovarian cancer: a Norwegian prospective study. Cancer Causes and Control 1996;7(4):421‐7. [DOI] [PubMed] [Google Scholar]
Allaway 2017 {published data only}
- Allaway HCM, Chizen DR, Adams GP, Pierson RA. Effects of a single 20 mg dose of letrozole on ovarian function post dominant follicle selection: an exploratory randomized controlled trial. Journal of Ovarian Research 2017;10(6):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Shawaf 2005 {published data only}
- Al‐Shawaf T, Zosmer A, Dirnfeld M, Grudzinskas G. Safety of drugs used in assisted reproduction techniques. Drug Safety 2005;28(6):513‐28. [DOI] [PubMed] [Google Scholar]
Althuis 2005 {published data only}
- Althuis MD, Moghissi KS, Westhoff CL, Scoccia B, Lamb EJ, Lubin JH, et al. Uterine cancer after use of clomiphene citrate to induce ovulation. American Journal of Epidemiology 2005;161(7):607‐15. [DOI] [PubMed] [Google Scholar]
Anderson 1996 {published data only}
- Anderson SM, Dimitrievich E. Ovulation induction for infertility: is it safe or not?. South Dakota Journal of Medicine 1996;49(11):419‐21. [PubMed] [Google Scholar]
Artini 1997 {published data only}
- Artini PG, Fasciani A, Cela V, Battaglia C, Micheroux AA, D'Ambrogio G, et al. Fertility drugs and ovarian cancer. Gynecological Endocrinology 1997;11(1):59‐68. [DOI] [PubMed] [Google Scholar]
Attia 2006 {published data only}
- Attia A. EBM in action: does ovulation induction increase the risk of ovarian cancer?. Middle East Fertility Society Journal 2006;11(2):135‐9. [Google Scholar]
Ayhan 2004 {published data only}
- Ayhan A, Salman MC, Celik H, Dursun P, Ozyuncu O, Gultekin M. Association between fertility drugs and gynaecologic cancers, breast cancer, and childhood cancers. Acta Obstetricia et Gynecologica Scandinavica 2004;83:1104‐11. [DOI] [PubMed] [Google Scholar]
Badawy 2009 {published data only}
- Badawy A, Abdel Aal I, Abulatta M. Clomiphene or anastrozole for ovulation for ovulation induction in women with polycystic ovary syndrome? A prospective controlled trial. Fertility and Sterility 2009;92:860‐3. [DOI] [PubMed] [Google Scholar]
Balasch 1994 {published and unpublished data}
- Balasch J, Penarrubia J, Marquez M, Mirkin SR, Carmona F, Barri PN, et al. Ovulation side and ovarian cancer. Gynecological Endocrinology Journal 1994;8(1):51‐4. [DOI] [PubMed] [Google Scholar]
Bandera 2005 {published data only}
- Bandera C. Advances in the understanding of risk factors for ovarian cancer. Journal of Reproductive Medicine 2005;50:399‐406. [PubMed] [Google Scholar]
Bayar 2006 {published data only}
- Bayar U, Demirtas E, Usubütün A, Basaran M, Esinler I, Yarali H. Ovarian adenomyoma following gonadotrophin treatment for infertility. Reproductive Biomedicine Online 2006;13(5):676‐9. [DOI] [PubMed] [Google Scholar]
Bose 2008 {published data only}
- Bose CK. Clomiphene may not cause neoplasia in the ovary: an animal study on ovarian morphology, biochemistry and histochemistry. Journal Turkish‐German Gynecological Association 2008;9(3):158‐63. [Google Scholar]
Brekelmans 2003 {published data only}
- Brekelmans CTM. Risk factors and risk reduction of breast and ovarian cancer. Current Opinion in Obstetrics and Gynecology 2003;15:63‐8. [DOI] [PubMed] [Google Scholar]
Brinton 1996 {published data only (unpublished sought but not used)}
- Brinton LA. Hormones and risk of cancers of the breast and ovary. Cancer Causes Control 1996;7(6):569‐71. [DOI] [PubMed] [Google Scholar]
Brinton 1997 {published data only (unpublished sought but not used)}
- Brinton LA, Gridley G, Persson I, Baron J, Berqqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. American Journal of Obstetrics and Gynecology 1997;176:572‐9. [DOI] [PubMed] [Google Scholar]
Brinton 2007 {published data only (unpublished sought but not used)}
- Brinton L. Long‐term effects of ovulation‐stimulating drugs on cancer risk. Reproductive Biomedicine Online 2007;15(1):38‐44. [DOI] [PubMed] [Google Scholar]
Brinton 2012 {published and unpublished data}
- Brinton LA, Sahasrabuddhe VV, Scoccia B. Fertility drugs and the risk of breast and gynaecologic cancers. Seminars in Reproductive Medicine 2012;30:131‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bristow 1996 {published data only}
- Bristow RE, Karlan BY. The risk of ovarian cancer after treatment for infertility. Current Opinion in Obstetrics and Gynaecology 1996;8:32‐7. [PubMed] [Google Scholar]
Bristow 1996 {published and unpublished data}
- Bristow RE, Karlan B. Ovulation induction, infertility, and ovarian cancer risk. Fertility and Sterility 1996;66(4):499‐507. [DOI] [PubMed] [Google Scholar]
Burger 2004 {published and unpublished data}
- Burger CW, Klip H, Leeuwen FE. In vitro fertilization, ovulation induction and the risk of cancer. CME Journal of Gynaecologic Oncology 2004;9:256‐63. [Google Scholar]
Cetin 2008 {published and unpublished data}
- Cetin I, Cozzi V, Antonazzo P. Infertility as a cancer risk factor ‐ A review. Placenta 2008;29:169‐77. [DOI] [PubMed] [Google Scholar]
Chene 2009 {published and unpublished data}
- Chene G, Penault‐Llorca F, Bouedec G, Mishellany F, Dauplat MM, Jaffeux P. Ovarian epithelial dysplasia after ovulation induction: time and dose effects. Human Reproduction 2009;24(1):132‐8. [DOI] [PubMed] [Google Scholar]
Clinton 1997 {published and unpublished data}
- Clinton GM, Hua W. Estrogen action in human ovarian cancer. Critical Reviews in Oncology Hematology 1997;25(1):1‐9. [DOI] [PubMed] [Google Scholar]
Cohen 1993 {published and unpublished data}
- Cohen J. Fertility drugs and ovarian cancer. Fertility and Sterility 1993;60(3):406‐8. [PubMed] [Google Scholar]
Cramer 1998 {published and unpublished data}
- Cramer DW, Harlow BL, Titus‐Ernstoff L, Bohlke K, Welch WR, Greenberg ER. Over‐the‐counter analgesics and risk of ovarian cancer. Lancet 1998;351:104‐7. [DOI] [PubMed] [Google Scholar]
Crosbie 2005 {published data only (unpublished sought but not used)}
- Crosbie E, Menon U. Epithelial ovarian cancer and induction of ovulation. Reviews in Gynaecological Practice 2005;5:131‐8. [Google Scholar]
Croughan‐Minihane 2001 {unpublished data only}
- Croughan‐Minihane M, Camarano L, Feigenbaum S, Adamson NGD, Cadieux M. The risk of ovarian cancer associated with infertility and infertility treatments. Fertility and Sterility 2001;76:S68. [Google Scholar]
Cusido 2007 {published data only (unpublished sought but not used)}
- Cusido M, Fabregas R, Pere Barris S, Escayola C, Nolsac Barr P. Ovulation induction treatment and risk of borderline ovarian tumours. Gynaecological Endocrinology 2007;23(7):373‐6. [DOI] [PubMed] [Google Scholar]
Demirol 2006 {published data only}
- Demirol NA, Guven S, Gurgan T. Ovulation induction agents and cancer. Journal of the Turkish German Gynecological Association 2006;7(2):139‐45. [Google Scholar]
Devesa 2010 {published data only}
- Devesa M, Barri PN, Coroleu B. Assisted reproductive technology and ovarian cancer. Minerva Endocrinologica 2010;35(4):247‐57. [PubMed] [Google Scholar]
Dos Santos 2002 {published and unpublished data}
- Dos Santos Silva J, Maclean AB, Mayer D, Hardiman PJ, Lieberman G, Nieto JJ, et al. Does ovarian stimulation increase the risk of ovarian cancer?. Reproductive Medicine Review 2002;11(1):57‐66. [Google Scholar]
Duckitt 1998 {published data only}
- Duckitt K, Templeton A. Cancer in women with infertility. Current Opinion of Obstetrics and Gynaecology 1998;10(3):199‐203. [DOI] [PubMed] [Google Scholar]
Duska 1996 {published and unpublished data}
- Duska L, Wallach EE. Infertility, ovulation induction, and epithelial ovarian cancer. Postgraduate Obstetrics & Gynaecology 1996;16:1‐6. [Google Scholar]
Franceschini 1991 {published data only (unpublished sought but not used)}
- Franceschini S, Vecchia C, Booth M, Tzonou A, Negri E, Parazzini F. Pooled analysis of 3 European case‐control studies of ovarian cancer: II. Age at menarche and at menopause. International Journal of Cancer 1991;49:57‐60. [DOI] [PubMed] [Google Scholar]
Franco 2000 {published data only}
- Franco C, Coppola S, Prosperi Porta R, Patella A. Ovulation induction and risk of ovarian cancer [Induzione dell'ovulazione e rischio di neoplasie ovariche]. Minerva Ginecologica 2000;52:103‐9. [PubMed] [Google Scholar]
Gadducci 2004 {published and unpublished data}
- Gaducci A, Gargini A, Palla E, Genazzani AR. Reproductive variables, fertility drugs and epithelial ovarian tumour risk. CME Journal of Gynaecologic Oncology 2004;9:245‐52. [Google Scholar]
Gadducci 2013 {published and unpublished data}
- Gadducci A, Guerrieri ME, Genazzani AR. Fertility drug use and risk of ovarian tumors: a debated clinical challenge. Gynaecological Endocrinology 2013;29(1):30‐5. [DOI] [PubMed] [Google Scholar]
Genc 2011 {published and unpublished data}
- Genc G, Yilmaz N, Vygur D, Dogan M, Mollamahmutogli L. The effect of intrafollicular IGF 1 and IGFBP3 on IVF outcome in patients using different gonadotropins: a prospective study. Journal of Assisted Reproduction and Genetics 2011;28:405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glud 1998 {published data only (unpublished sought but not used)}
- Glud E, Kjaer SK, Troisi R, Brinton LA. Fertility drugs and ovarian cancer. Epidemiologic Reviews 1998;20(2):237‐57. [DOI] [PubMed] [Google Scholar]
Goldberg 1992 {published data only}
- Goldberg G, Runowicz CD. Ovarian carcinoma of low malignant potential, infertility, and induction of ovulation ‐ Is there a link?. American Journal of Obstetrics and Gynecology 1992;166:853‐4. [DOI] [PubMed] [Google Scholar]
Goodman 2001 {published data only}
- Goodman MT, McDuffie K, Kolonel LN, Terada K, Donlon TA, Wilkens LR, et al. Case‐control study of ovarian cancer and polymorphisms in genes involved in catecholestrogen formation and metabolism. Cancer Epidemiology Biomarkers and Prevention 2001;10:209‐16. [PubMed] [Google Scholar]
Goshen 1998 {published and unpublished data}
- Goshen R, Wessman A, Shoham Z. Epithelial ovarian cancer, infertility and induction of ovulation: possible pathogenesis and updated concepts. Baillieres Clinical Obstetrics and Gynaecology 1998;12(4):581‐91. [DOI] [PubMed] [Google Scholar]
Gwinn 1990 {published and unpublished data}
- Gwinn ML, Lee NC, Rhodes PH, Layde PM, Rubin GL. Pregnancy, breast feeding, and oral contraceptives and the risk of epithelial ovarian cancer. Journal of Clinical Epidemiology 1990;43(6):559‐68. [DOI] [PubMed] [Google Scholar]
Hankinson 1995 {published data only (unpublished sought but not used)}
- Hankinson S, Colditz G, Hunter DJ, Willett WC, Stampfer MJ, Rosner B. A prospective study of reproductive factors and risk of epithelial ovarian cancer. Cancer 1995;76:284‐90. [DOI] [PubMed] [Google Scholar]
Harris 1992 {published data only (unpublished sought but not used)}
- Harris R, Whittemore AS, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case‐control studies. III. Epithelial tumours of low malignant potential in white women. Collaborative Ovarian Cancer Group. American Journal of Epidemiology 1992;136(10):1204‐11. [DOI] [PubMed] [Google Scholar]
He 2012 {published data only}
- He F, Gan X‐L, Hu L‐N. Ovulation induction and risk of ovarian cancer: a systematic review. Chinese Journal of Evidence‐Based Medicine 2012;12(9):1129‐34. [Google Scholar]
Helzlsouer 1995 {published and unpublished data}
- Helzlsouer KJ, Alberg AJ, Gordon GB, Longcope C, Bush TL, Hoffman SC, et al. Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA 1995;274:1926‐30. [PubMed] [Google Scholar]
Horn‐Ross 1992 {published data only}
- Horn‐Ross P, Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case‐control studies. VI. Nonepithelial cancers among adults. Collaborative Ovarian Cancer Group. Epidemiology 1992;3:490‐5. [DOI] [PubMed] [Google Scholar]
Jensen 2007 {published data only (unpublished sought but not used)}
- Jensen A, Sharif H, Svare E, Frederiksen K, Kjaer SK. Risk of breast cancer after exposure to fertility drugs: results from a large Danish cohort study. Cancer Epidemiology of Biomarkers and Prevention 2007;16(7):1400‐7. [DOI] [PubMed] [Google Scholar]
Jensen 2008 {published data only}
- Jensen A, Sharif H, Olsen JH, Kjaer SK. Risk of breast cancer and gynecologic cancers in a large population of nearly 50,000 infertile Danish women. American Journal of Epidemiology 2008;168(1):49‐57. [DOI] [PubMed] [Google Scholar]
Kashyap 2003 {published data only (unpublished sought but not used)}
- Kashyap S, Davis OK. Ovarian cancer and fertility medications: a critical appraisal. Seminars in Reproductive Medicine 2003;21(1):65‐71. [DOI] [PubMed] [Google Scholar]
Kaufman 1995 {published data only}
- Kaufman S, Spirtas R, Alexander NJ. Do fertility drugs cause ovarian tumours?. Journal of Women's Health 1995;4(3):247‐59. [Google Scholar]
Kelly 2003 {published data only}
- Kelly AM, Buckett WM, William M, Tan Seang L. Effect of repeated assisted reproductive technology on ovarian response. Current Opinion in Obstetrics and Gynecology 2003;15:219‐24. [DOI] [PubMed] [Google Scholar]
King 1994 {published data only (unpublished sought but not used)}
- King TM. Ovarian cancer and fertility drugs. The Cancer Bulletin 1994;46(2):181‐4. [Google Scholar]
Klip 2000 {published data only}
- Klip H, Burger CW, Kenemans P, Leeuwen FE. Cancer risk associated with subfertility and ovulation induction: a review. Cancer Causes and Control 2000;11(4):319‐44. [DOI] [PubMed] [Google Scholar]
Klip 2001 {published and unpublished data}
- Klip H, Burger CW, Kraker J, Leeuwen FE, OMEGA Project Group. Risk of cancer in the offspring of women who underwent ovarian stimulation for IVF. Human Reproduction 2001;16(11):2451‐8. [DOI] [PubMed] [Google Scholar]
Konishi 1999 {published and unpublished data}
- Konishi I, Kuroda H, Mandai M. Review: gonadotropins and development of ovarian cancer. Oncology 1999;57(2):45‐8. [DOI] [PubMed] [Google Scholar]
Kristiansson 2007 {published and unpublished data}
- Kristiansson P, Bjor O, Wramsby H. Tumour incidence in Swedish women who gave birth following IVF treatment. Human Reproduction 2007;22(2):421‐6. [DOI] [PubMed] [Google Scholar]
Kurian 2004 {published data only}
- Kurian AW, Balise RR, McGuire V, Whittemore AS. Histologic types of epithelial ovarian cancer: have they different risk factors?. Gynaecologic Oncology 2005;96:520‐30. [DOI] [PubMed] [Google Scholar]
Land 1993 {published data only}
- Land JA. Ovulation, ovulation induction and ovarian carcinoma. Bailliere's Clinical Obstetrics and Gynaecology 1993;7(2):455‐71. [DOI] [PubMed] [Google Scholar]
La Vecchia C 2011 {published and unpublished data}
- Vecchia C. Infertility, ovulation, induced ovulation and female cancers. European Journal of Cancer Prevention 2011;20(3):147‐9. [DOI] [PubMed] [Google Scholar]
Lerner‐Geva 2004 {published data only}
- Lerner‐Geva L. The possible association between infertility, ovulation induction treatments and the incidence of cancer. CME Journal of Gynecologic Oncology 2004;9:236‐44. [Google Scholar]
Lerner‐Geva 2006 {published and unpublished data}
- Lerner‐Geva L, Keinan‐Boker L, Blumstein T, Boyko V, Olmar L, Mashiach S, et al. Infertility, ovulation induction treatments and the incidence of breast cancer: a historical prospective cohort of Israeli women. Breast Cancer Research and Treatment 2006;100:201‐12. [DOI] [PubMed] [Google Scholar]
Lerner‐Geva 2010 {published and unpublished data}
- Lerner‐Geva L, Rabinovici J, Lunenfeld B. Ovarian stimulation: is there a long‐term risk for ovarian, breast and endometrial cancer?. Women's Health (London England) 2010;6(6):831‐9. [DOI] [PubMed] [Google Scholar]
Li 2013 {published and unpublished data}
- Li LL, Zhou J, Qian XJ, Chen YD. Meta‐analysis on the possible association between in vitro fertilization and cancer risk. International Journal of Gynecological Cancer 2013;23:16‐24. [DOI] [PubMed] [Google Scholar]
Lopes 1993 {published data only}
- Lopes P, Mensier A. Ovarian cancer and assisted reproductive technology. European Journal of Obstetrics and Gynecology and Reproductive Biology 1993;51:171‐3. [DOI] [PubMed] [Google Scholar]
Mandai 2007 {published and unpublished data}
- Mandai M, Konishi I, Kuroda H, Fujii S. LH/hCG action and development of ovarian cancer ‐ A short review on biological and clinical/epidemiological aspects. Molecular and Cellular Endocrinology 2007;269:61‐4. [DOI] [PubMed] [Google Scholar]
McGuire 2004 {published and unpublished data}
- McGuire V, Felberg A, Mills M, Ostrow KL, Dicioccio R, John EM, et al. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and non carriers of BRCA 1 gene mutations. American Journal of Epidemiology 2004;160(7):613‐8. [DOI] [PubMed] [Google Scholar]
McSorley 2009 {published data only}
- McSorley MA, Alberg AJ, Allen DS, Allen NE, Brinton LA, Dorgan JF, et al. Prediagnostic circulating follicle stimulating hormone concentrations and ovarian cancer risk. International Journal Cancer 2009;125(3):674‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendola 2013 {published data only}
- Mendola P. Epithelial ovarian tumors and fertility drugs ‐ Are we asking the right questions?. Fertility and Sterility 2013;99(7):1853‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menon 2009 {published and unpublished data}
- Menon U, Gentry‐Maraj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers results of the prevalence screen of the UK collaborative trial of ovarian cancer screening (UKCTOCS). Lancet Oncology 2009;10(4):327‐34. [DOI] [PubMed] [Google Scholar]
Miao 2006 {published and unpublished data}
- Miao Q, Kong BH. A case‐control study on etiology of epithelial ovarian cancer in Shandong Province. Al Zheng 2006;25(7):871‐5. [PubMed] [Google Scholar]
Modugno 2001 {published and unpublished data}
- Modugno F, Ness RB, Wheeler JE. Reproductive risk factors for epithelial ovarian cancer according to histologic type and invasiveness. American Journal of Epidemiology 2001;11:568‐74. [DOI] [PubMed] [Google Scholar]
Negri 1991 {published data only}
- Negri E, Franceschini S, Tzonou A, Booth M, Vecchia C, Parazzini F, et al. Pooled analysis of 3 European case‐control studies: I. Reproductive factors and risk of epithelial ovarian cancer. International Journal of Cancer 1991;49:50‐6. [DOI] [PubMed] [Google Scholar]
Ness 2000 {published data only}
- Ness RB, Grisso JA, Klapper J, Schlesselman J, Silberzweig S, Vergona R, SHARE Study Group, et al. Risk of ovarian cancer in relation to estrogen and progestin dose and use characteristics of oral contraceptives: steroid hormones and reproductions. American Journal of Epidemiology 2000;152(3):233‐41. [DOI] [PubMed] [Google Scholar]
Ness 2003 {published and unpublished data}
- Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, et al. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case‐control studies. American Journal of Epidemiology 2002;155:217‐24. [DOI] [PubMed] [Google Scholar]
Ness 2011 {published and unpublished data}
- Ness RB, Dodge RC, Edwards RP, Baker JA, Moysich KB. Contraception methods, beyond oral oral contraceptives and tubal ligation, and risk of ovarian cancer. Annals of Epidemiology 2011;21:188‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nieto 2001 {published and unpublished data}
- Nieto JJ, Crow J, Sundaresan M, Constantinovici N, Perrett CW, MacLean AB, et al. Ovarian epithelial dysplasia in relation to ovulation induction and nulliparity. Gynecologic Oncology 2001;82:344‐9. [DOI] [PubMed] [Google Scholar]
Oktay 2010 {published and unpublished data}
- Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA 1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. Journal of Clinical Oncology 2010;28(2):240‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozcan 2009 {published and unpublished data}
- Ozcan Z, Celik H, Gurates B, Ozercan HI, Hanay F, Nalbant M, et al. Effects of ovulation induction agents on ovarian surface epithelial in rats. Reproductive Biomedicine Online 2009;19(3):314‐8. [DOI] [PubMed] [Google Scholar]
Ozdemir 2005 {published and unpublished data}
- Ozdemir I, Ustundag N, Guven A, Duran B, Dermici F. Effect of clomiphene citrate on ovarian, endometrial, and cervical histologies in a rat model. Gynecologic and Obstetric Investigation 2005;60:181‐5. [DOI] [PubMed] [Google Scholar]
Parazzini 2004 {published and unpublished data}
- Parazzini F, Chiaffarino F, Negri E, Surace M, Benzi G, Franceschini S, et al. Risk factors for different histological types of ovarian cancer. International Journal of Gynecologic Cancer 2004;14:431‐6. [DOI] [PubMed] [Google Scholar]
Paulson 1996 {published and unpublished data}
- Paulson RJ. Fertility drugs and ovarian epithelial cancer: is there a link?. Journal of Assisted Reproduction and Genetics 1996;13(10):751‐6. [DOI] [PubMed] [Google Scholar]
Persson 1995 {published data only}
- Persson I, Janson PO. Ovarian neoplasms. Protective or hazardous effects of sterilization, hysterectomy and hormonal infertility therapy?. Lakartidningen 1995;92(5):371‐4. [PubMed] [Google Scholar]
Purdie 1995 {published data only}
- Purdie D, Green A, Bain C, Siskind V, Ward B, Hacker N, et al. Reproductive and other factors and risk of epithelial ovarian cancer: an Australian case‐control study. International Journal of Cancer 1995;62:678‐84. [DOI] [PubMed] [Google Scholar]
Riman 1998 {published and unpublished data}
- Riman T, Persson I, Nilsson S. Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clinical Endocrinology 1998;49:695‐707. [DOI] [PubMed] [Google Scholar]
Riman 2002 {published and unpublished data}
- Riman T, Dickman PW, Nilsson S, Correira N, Nordlinder H, Magnusson CM. Risk factors for invasive epithelial ovarian cancer: results from a Swedish case‐control study. American Journal of Epidemiology 2002;156(4):363‐73. [DOI] [PubMed] [Google Scholar]
Rish 1996 {published and unpublished data}
- Rish HA, Marrett LD, Jain M, Howe G. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case‐control study. American Journal of Epidemiology 1996;144(4):363‐72. [DOI] [PubMed] [Google Scholar]
Rodriguez 1998 {published and unpublished data}
- Rodriguez C, Tatham LM, Calle EE, Thun MJ, Jacobs EJ, Health CW. Infertility and risk of fatal ovarian cancer in a prospective cohort of US women. Cancer Causes and Control 1998;9:645‐51. [DOI] [PubMed] [Google Scholar]
Ron 1995 {published and unpublished data}
- Ron E, Lunenfeld B. A review of infertility and its treatment in the etiology of female reproductive and other cancers. Journal of Women's Health 1995;4(3):261‐72. [Google Scholar]
Rosemberg 1994 {published and unpublished data}
- Rosemberg L, Palmer JR, Zauber AG, Warshauer ME, Lewis JL, Strom BL, et al. A case‐control study of oral contraceptive use and invasive epithelial ovarian cancer. American Journal of Epidemiology 1994;139(7):654‐61. [DOI] [PubMed] [Google Scholar]
Rosen 1997 {published and unpublished data}
- Rosen B, Irvine J, Ritvo P, Shapiro H, Stewart D, Reynolds K, et al. The feasibility of assessing women's perceptions of the risks and benefits of fertility drug therapy in relation to ovarian cancer risk. Fertility and Sterility 1997;68(1):90‐4. [DOI] [PubMed] [Google Scholar]
Rosenblatt 1993 {published and unpublished data}
- Rosenblatt KA, Thomas DB, WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Lactation and the risk of epithelial ovarian cancer. International Journal of Epidemiology 1993;22(2):192‐7. [DOI] [PubMed] [Google Scholar]
Rossing 1996 {published and unpublished data}
- Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Risk of breast cancer in a cohort of infertile women. Gynecologic Oncology 1996;60:3‐7. [DOI] [PubMed] [Google Scholar]
Schildkraut 1996 {published and unpublished data}
- Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstetrics and Gynecology 1996;88:554‐9. [DOI] [PubMed] [Google Scholar]
Shoham 1994 {published data only}
- Shoham Z. Epidemiology, etiology, and fertility drugs in ovarian epithelial carcinoma: where are we today?. Fertility and Sterility 1994;62(3):433‐48. [DOI] [PubMed] [Google Scholar]
Siristatidis 2013 {published data only}
- Siristatidis C, Sergentanis TN, Kanavidis P, Trivella M, Sotiraki M, Mavromatis I, et al. Controlled ovarian hyperstimulation for IVF: impact on ovarian, endometrial and cervical cancer. A systematic review and metananalysis. Human Reproduction Update 2013;19(2):105‐23. [DOI] [PubMed] [Google Scholar]
Smith 2001 {published data only}
- Smith JA. The controversial association of the treatment of infertility with fertility agents and development of ovarian cancer: what should patients be told?. Oncology Spectrums 2001;2(6):428‐31. [Google Scholar]
So 2008 {published and unpublished data}
- So W‐K, Cheng J‐C, Poon S‐L, Leung PCK. Gonadotropin‐releasing hormone and ovarian cancer: a functional and mechanistic overview. FEBS Journal 2008;275:5496‐511. [DOI] [PubMed] [Google Scholar]
Soegaard 2007 {published and unpublished data}
- Soegaard M, Jensen A, Hogdall E, Christensen L, Hogdall C, Blaakaer J, et al. Different risk factor profiles for mucinous and non mucinous ovarian cancer: results from the Danish MALOVA study. Cancer Epidemiology Biomarkers Prevention 2007;16(6):1160‐6. [DOI] [PubMed] [Google Scholar]
Spirtas 1993 {published and unpublished data}
- Spirtas R, Kaufman SC, Alexander NJ. Fertility drugs and ovarian cancer: red alert or red herring?. Fertility and Sterility 1983;59(2):291‐3. [DOI] [PubMed] [Google Scholar]
Stein 1997 {published and unpublished data}
- Stein DE, Santoro N. Infertility, gonadotropins, and ovarian cancer. Controversies in Infertility Management 1997;8(2):289‐303. [Google Scholar]
Tarlatzis 1995 {published and unpublished data}
- Tarlatzis BC, Grimbizis G, Bontis J, Mantalenakis S. Ovarian stimulation and ovarian tumours: a critical reappraisal. Human Reproduction Update 1995;1(3):284‐301. [DOI] [PubMed] [Google Scholar]
Trifonov 2000 {published and unpublished data}
- Trifonov I, Todorova M, Uzunova ZH. Stimulation of ovulation and ovarian cancer. Literature. Akush Ginekol (Sofiia) 2000;40(4):39‐41. [PubMed] [Google Scholar]
Unkila‐Kallio 1997 {published and unpublished data}
- Unkila‐Kallio L, Leminen A, Tiitnen A, Lehtovirta P, Wahlstrom T, Ylikorkala O. Malignant tumours of the ovary or the breast in association with infertility: a report of thirteen cases. Acta Obstetricia et Gynecologica Scandinavica 1997;76:177‐81. [DOI] [PubMed] [Google Scholar]
Unkila‐Kallio 2000 {published and unpublished data}
- Unkila‐Kallio L, Tiitinen A, Wahlstrom T, Lehtovirta P, Leminen A. Reproductive features in women developing ovarian granulosa cell tumour at a fertile age. Human Reproduction 2000;15(3):589‐93. [DOI] [PubMed] [Google Scholar]
Venn 1997 {published and unpublished data}
- Venn AJ, Healy DL. Fertility drugs and cancer. Reproductive Medicine Review 1997;6(3):185‐98. [Google Scholar]
Venn 2001 {published data only (unpublished sought but not used)}
- Venn A, Hemminki E, Watson L, Bruinsma F, Healy D. Mortality in a cohort of IVF patients. Human Reproduction 2001;16(12):2691‐6. [DOI] [PubMed] [Google Scholar]
Venn 2003 {published data only (unpublished sought but not used)}
- Venn A, Healy D, McLachlan R. Cancer risks associated with the diagnosis of infertility. Best Practice & Research Clinical Obstetrics & Gynaecology 2003;17(2):343‐67. [DOI] [PubMed] [Google Scholar]
Vlahos 2010 {published and unpublished data}
- Vlahos N, Economopoulos KP, Creatsas G. Fertility drugs and ovarian cancer risk: a critical review of the literature. Annuals of the New York Academy Sciences 2010;1205:214‐9. [DOI] [PubMed] [Google Scholar]
Wakeley 2000 {published and unpublished data}
- Wakeley KE, Grendys EC. Reproductive technologies and risk of ovarian cancer. Current Opinion of Obstetrics and Gynecology 2000;12:43‐7. [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- Wang R, Kim BV, Wely M, Johnson NP, Costello MF, Zhang H, et al. Treatment strategies for women with WHO group II anovulation: systematic review and network meta‐analysis. BMJ 2017;356:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittemore 1994 {published data only (unpublished sought but not used)}
- Whittemore AS. The risk of ovarian cancer after treatment for infertility. New England Journal of Medicine 1994;331(12):805‐6. [DOI] [PubMed] [Google Scholar]
Willemsen 1993 {published data only (unpublished sought but not used)}
- Willemsen W, Kruitwagen R, Bastiaans B, Hanselaar T, Rolland R. Ovarian stimulation and granulosa‐cell tumour. Lancet 1993;341:986‐8. [DOI] [PubMed] [Google Scholar]
Zarchi 2013 {published data only}
- Zarchi MK, Rouhi M, Abdolahi AH, Hekmatimoghaddam S. The effect of assisted reproductive technologies on gynaecological cancer: report of our experiences and literature review. International Journal of Biomedical Science 2013;9(3):129‐34. [PMC free article] [PubMed] [Google Scholar]
Zreik 2008 {published data only}
- Zreik TG, Ayoub CM, Hannoun A, Karam CJ, Munkarah AR. Fertility drugs and risk of ovarian cancer: dispelling the myth. Current Opinion in Obstetrics and Gynecology 2008;20:313‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Balasch 1993
- Balasch J, Barri PN. Follicular stimulation and ovarian cancer?. Human Reproduction 1993;8:990‐6. [DOI] [PubMed] [Google Scholar]
Biskind 1944
- Biskind MS, Biskind GS. Development of tumours in the rat ovary after transplantation into the spleen. Proceedings of the Society for Experimental Biology and Medicine 1944;55:176‐9. [Google Scholar]
Boivin 2007
- Boivin J, Bunting L, Collins J, Nygren KG. International estimates of infertility prevalence and treatment‐seeking: potential need and demand for infertility medical care. Human Reproduction 2007;22(6):1506‐12. [DOI] [PubMed] [Google Scholar]
Booth 1989
- Booth M, Beral V, Smith P. Risk factors for ovarian cancer: a case control study. British Journal of Cancer 1989;60:592‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brinton 2005
- Brinton L, Moghiss K, Scoccia B, Westhoff C, Lamb E. Ovulation induction and cancer risk. Fertility and Sterility 2005;83(2):261‐74. [DOI] [PubMed] [Google Scholar]
Brinton 1989
- Brinton LA, Melton LJ, Malkasian GD Jr, Bond A, Hoover R. Cancer risk after evaluation for infertility. American Journal of Epidemiology 1989;129:712‐22. [DOI] [PubMed] [Google Scholar]
Brinton 2004
- Brinton LA, Lamb E, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, et al. Ovarian cancer risk after the use of ovulation stimulating drugs. Obstetrics and Gynaecology 2004;103:1194‐203. [DOI] [PubMed] [Google Scholar]
Chandra 1998
- Chandra A, Stephen EH. Impaired fecundity in the United States: 1982‐1995. Family Planning Perspectives 1998;30:34‐42. [PubMed] [Google Scholar]
Demir 2016
- Demir B, Kahyaoglu I, Guvenir A, Yerebasmaz N, Altinbas S, Dilbaz B, et al. Progesterone change in the late follicular phase affects pregnancy rates in both agonist and antagonist protocols in normoresponders: a case‐controlled study in ICSI cycles. Gynecological Endocrinology 2016;32(5):361‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diergaarde 2014
- Diergaarde B, Kurta ML. Use of fertility drugs and risk of ovarian cancer. Current Opinion in Obstetrics & Gynecology 2014;26(3):125‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2010
- Duffy JMN, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A. Growth hormone for in vitro fertilization. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD000099.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fathalla 1971
- Fathalla MF. Incessant ovulation: a factor in ovarian neoplasia?. Lancet 1971;2:163. [DOI] [PubMed] [Google Scholar]
Finch 2013
- Finch A, Valentini A, Greenblatt E, Lynch HT, Ghadirian P, Armel S, et al. Hereditary Breast Cancer Study Group. Frequency of premature menopause in women who carry a BRCA 1 or BRCA2 mutation. Fertility and Sterility 2013;99(6):1724‐8. [DOI] [PubMed] [Google Scholar]
Gates 2010
- Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histological subtype. American Journal of Epidemiology 2010;171:45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenhall 1990
- Greenhall E, Vessey M. The prevalence of subfertility: a review of the current confusion and a report of two new studies. Fertility and Sterility 1990;54:978‐83. [DOI] [PubMed] [Google Scholar]
Hartge 1989
- Hartge P, Schiffman MH, Hoover R, McGowan L, Lesher L, Norris HJ. A case‐control study of epithelial ovarian cancer. American Journal of Obstetrics and Gynecology 1989;161:10‐6. [DOI] [PubMed] [Google Scholar]
Healy 1994
- Healy DL, Trounson AO, Andersen AN. Female infertility: causes and treatment. Lancet 1994;343:1539‐44. [DOI] [PubMed] [Google Scholar]
Henderson 1998
- Henderson BE, Ross R, Bernstein L. Estrogens as a cause of human cancer. The Richards and Hinda Rosenthal Foundation Award Lecture. Cancer Research 1988;48:246‐53. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systemic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Homburg 1995
- Homburg R, Ostergard H. Clinical applications of growth hormone for ovarian stimulation. Human Reproduction Update 1995;1:264‐75. [DOI] [PubMed] [Google Scholar]
Karmaus 1999
- Karmaus W, Juul S. Infertility and subfecundity in population‐based samples from Denmark, Germany, Italy, Poland and Spain. European Journal of Public Health 1999;9:229‐35. [Google Scholar]
Kashyap 2004
- Kashyap S, Moher D, Fung MF, Rosenwaks Z. Assisted reproductive technology and the incidence of ovarian cancer: a meta analysis. Obstetrics and Gynecology 2004;103:785‐94. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;23(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahdavi 2006
- Mahdavi A, Pejovic T, Nezhat F. Induction of ovulation and ovarian cancer: a critical review of the literature. Fertility and Sterility 2006;85(4):819‐26. [DOI] [PubMed] [Google Scholar]
Manchanda 2018
- Manchanda R, Menon U. Setting the threshold for surgical prevention in women at increased risk of ovarian cancer. International Journal of Gynecological Cancer 2018;28(1):34‐42. [DOI] [PubMed] [Google Scholar]
McGowan 1979
- McGowan L, Parent L, Lednar W, Norris HJ. The woman at risk for developing ovarian cancer. Gynecologic Oncology 1979;7:325‐44. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meirow 1996
- Meirow D, Schenker JG. The link between female infertility and cancer: epidemiology and possible aetiologies. Human Reproduction 1996;2(1):63‐75. [DOI] [PubMed] [Google Scholar]
Ness 2002
- Ness RB, Cramer DW, Goodman MT. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case‐control studies. American Journal of Epidemiology 2002;155:217‐24. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Clinical Excellence (NICE). Fertility: assessment and treatment for people with fertility problems (CG156), 2013. NICE (guidence.nice.org.uk/cg156) (accessed February 2013).
Nugent 1998
- Nugent D, Salha O, Balen AH, Rutherford AJ. Ovarian neoplasia and subfertility treatments. British Journal of Obstetrics and Gynaecology 1998;105:584‐91. [DOI] [PubMed] [Google Scholar]
Pabuccu 2016
- Pabuccu EG, Pabuccu R, Evliyaoglu Ozdegirmenci O, Bostanci Durmus A, Keskin M. Combined progesterone (IM+V) versus vaginal progesterone for luteal support in cleavage‐stage embryo transfer cycles of good prognosis patients. Gynecological Endocrinology 2016;32(5):366‐9. [DOI] [PubMed] [Google Scholar]
Poretsky 1999
- Poretsky L, Cataldo NA, Resenwaks Z, Giudice IC. The insulin‐related ovarian regulatory system in health and disease. Endocrine Reviews 1999;20:535‐82. [DOI] [PubMed] [Google Scholar]
Pozlep 2001
- Pozlep B, Meden‐Vrtovec H. Ovulation induction agents and ovarian cancer. Zdravstveni Vestnik 2001;70:525‐9. [Google Scholar]
Rish 1994
- Rish HA, Marrett LD, Hove GR. Parity, contraception, infertility and the risk of epithelial ovarian cancer. American Journal of Epidemiology 1994;140:585‐97. [DOI] [PubMed] [Google Scholar]
Rish 1998
- Rish HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. National Cancer Institute Journal 1998;90:1774‐86. [DOI] [PubMed] [Google Scholar]
Ron 1987
- Ron E, Lunenfeld B, Menczer J, Blumstein T, Katz L. Cancer incidence in a cohort of infertile women. American Journal of Epidemiology 1987;125:780‐90. [DOI] [PubMed] [Google Scholar]
Rowe 1993
- Rowe PJ, Hargreave TB, Mellows HJ, Comhaire FH. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple. Cambridge, UK: Cambridge University Press, 1993. [Google Scholar]
Schmidt 1995
- Schmidt L, Munster K. Infertility, involuntary infecundity, and the seeking of medical advice in industrialized countries 1970‐1992: a review of concepts, measurements and results. Human Reproduction 1995;10:1407‐18. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tomao 2014
- Tomao F, Lo Russo G, Spinelli P, Stati V, Prete AA, Prinzi N, et al. Fertility drugs, reproductive strategies and ovarian cancer risk. Journal of Ovarian Research 2014;7(51):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Linden 2011
- Linden M, Buckingham K, Farqhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database of Systematic Reviews 2015;7(7):1‐10. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2014
- Wang ET, Pisarska MD, Bresee C, Chen YD, Lester J, Afshar Y, et al. BRCA 1 germline mutations may be associated with reduced ovarian reserve. Fertility and Sterility 2014;102(6):1723‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittemore 1992a
- Whittemore AS. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case‐control studies. American Journal of Epidemiology 1992;136(10):1175‐83. [DOI] [PubMed] [Google Scholar]
Whittemore 1992b
- Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case‐control studies. II. Invasive epithelial ovarian cancers in white women. American Journal of Epidemiology 1992;136:1184‐203. [DOI] [PubMed] [Google Scholar]
Zarchi (a) 2013
- Zarchi MK, Rouhi M, Abdolahi AH, Hekmatimoghaddam S. The effect of assisted reproductive technologies on gynecological cancer: report of our experiences and literature review. International Journal of Biomedical Science 2013;9(3):129‐34. [PMC free article] [PubMed] [Google Scholar]
Zhao 2015
- Zhao J, Yanping L, Zhang Q, Wang Y. Does ovarian stimulation for IVF increase gynaecological cancer risk? A systematic review and meta‐analysis. Reproductive Biomedicine Online 2015;31:20‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rizzuto 2010
- Rizzuto I, Larsen‐Disney P, Smith LA. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rizzuto 2013
- Rizzuto I, Behrens RF, Smith LA. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD008215.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


