Abstract
TGF-βs regulate fibroblast responses, by activating Smad2 or Smad3 signaling, or via Smad independent pathways. We have previously demonstrated that myofibroblast-specific Smad3 is critically implicated in repair of the infarcted heart. However, the role of fibroblast Smad2 in myocardial infarction remains unknown. This study investigates the role of myofibroblast specific Smad2 signaling in myocardial infarction, and explores the mechanisms responsible for the distinct effects of Smad2 and Smad3. In a mouse model of non-reperfused myocardial infarction, Smad2 activation in infarct myofibroblasts peaked 7 days after coronary occlusion. In vitro, TGF-β1, -β2 and -β3, but not angiotensin 2 and bone morphogenetic proteins-2, −4 and −7, activated fibroblast Smad2. Myofibroblast-specific Smad2 and Smad3 knockout mice (FS2KO, FS3KO) and corresponding control littermates underwent non-reperfused infarction. In contrast to the increase in rupture rates and adverse remodeling in FS3KO mice, FS2KO animals had mortality comparable to Smad2 fl/fl controls, and exhibited a modest but transient improvement in dysfunction after 7 days of coronary occlusion. At the 28 day timepoint, FS2KO and Smad2 fl/fl mice had comparable adverse remodeling. Although both FS3KO and FS2KO animals had increased myofibroblast density in the infarct, only FS3KO mice exhibited impaired scar organization, associated with perturbed alignment of infarct myofibroblasts. In vitro, Smad3 but not Smad2 knockdown downmodulated fibroblast α2 and α5 integrin expression. Moreover, Smad3 knockdown reduced expression of the GTPase RhoA, whereas Smad2 knockdown markedly increased fibroblast RhoA levels. Smad3-dependent integrin expression may be important for fibroblast activation, whereas RhoA may transduce planar cell polarity pathway signals, essential for fibroblast alignment. Myofibroblast-specific Smad3, but not Smad2 is required for formation of aligned myofibroblast arrays in the infarct. The distinct in vivo effects of myofibroblast Smad2 and Smad3 may involve Smad3-dependent integrin synthesis, and contrasting effects of Smad2 and Smad3 on RhoA expression.
Keywords: TGF-β, Smad, myocardial infarction, fibroblast, planar cell polarity
1. INTRODUCTION
The massive loss of cardiomyocytes following acute myocardial infarction overwhelms the extremely limited regenerative capacity of the adult mammalian heart[1]. Thus, repair of the infarcted myocardium is dependent on formation of a collagen-based scar[2]. Fibroblasts are critical effector cells in cardiac repair, remodeling and fibrosis[3],[4] In addition to their important role in synthesis and metabolism of extracellular matrix proteins, fibroblasts may also exert a wide range of functions, modulating cardiomyocyte survival[5], regulating inflammation[6], and contributing to phagocytic clearance of dead cells[7]. Fibroblasts exhibit remarkable phenotypic plasticity, undergoing dramatic phenotypic changes in the dynamic environment of the infarct[3],[8],[9],[10]. During the inflammatory phase of infarct healing, fibroblasts may produce cytokines, mediating recruitment of leukocytes in the infarcted area[6]. As the infarct is cleared from dead cells and matrix debris, inflammation is suppressed and infarct fibroblasts expand, and undergo myofibroblast conversion, expressing α-smooth muscle actin (α-SMA), and synthesizing large amounts of extracellular matrix proteins[11],[12],[13]. Activated myofibroblasts form well-organized arrays in the infarct border zone[14] and protect the infarcted heart from cardiac rupture[15]. However, excessive fibroblast activation following infarction may accentuate dysfunction and adverse remodeling, precipitating heart failure[16]. Inflammatory cytokines, growth factors and matricellular proteins co-operate to activate fibroblasts in the infarcted heart[17].
Members of the TGF-β superfamily play a crucial role in regulation of fibroblast phenotype and function[18]. TGF-βs promote myofibroblast conversion, stimulate synthesis of extracellular matrix proteins, and exert context-dependent actions on cell proliferation and migration[19]. TGF-βs act by binding and sequentially transphosphorylating type II and type I receptors, transducing signaling through a series of intracellular effectors the Smads, or through stimulation of Smad-independent cascades[20],[19]. Smad2 and Smad3, the receptor-associated Smads activated by TGF-βs, have highly conserved amino acid sequences in their Mad homology (MH)1 and MH2 domains[21]. When Smad2 and Smad3 are activated by TGF-β receptors, they bind to the common Smad, Smad4, and translocate into the nucleus, where they modulate gene transcription. We have recently demonstrated that fibroblast-specific Smad3 signaling plays a critical role in repair and remodeling of the infarcted heart, by mediating formation of organized myofibroblast arrays in the scar, through activation of an integrin-mediated NADPH oxidase (NOX) pathway[15]. Although in vitro studies suggest distinct roles of Smad2 and Smad3 in various cellular responses[21], the in vivo role of Smad2 in regulation of the reparative response following myocardial infarction remains unknown. Accordingly, we hypothesized that activation of Smad2 in infarct fibroblasts may play an important role in repair and remodeling of the infarcted heart.
Our findings demonstrate that, in contrast to the detrimental effects of myofibroblast-specific Smad3 loss, loss of Smad2 in activated cardiac myofibroblasts did not affect scar organization and late remodeling of the infarcted heart, but was associated with a modest and transient improvement in systolic function following infarction, and with delayed dilative remodeling. In vitro, Smad3, but not Smad2 knockdown, downmodulated mRNA expression of α2 and α5 integrins, and reduced expression of the GTPase Ras homolog gene family member A (RhoA) in isolated cardiac fibroblasts. TGF-β-mediated Smad3, but not Smad2 activation, may be important for transduction of integrin-and RhoA-dependent signaling in infarct myofibroblasts, transducing cascades with an important role in scar organization.
2. MATERIALS AND METHODS
Detailed description of the methodology is provided in the online supplement
Generation of mice with Smad2 or Smad3 loss in activated myofibroblasts.
We generated mice with loss of Smad2 in activated infarct fibroblasts (FS2KO) by breeding Smad2fl/fl with a transgenic mouse line in which Cre recombinase is driven by a 3.9-kb mouse Postn promoter[22],[23]. Periostin, which is encoded by Postn, is not expressed in cardiomyocytes, vascular cells, hematopoietic cells or quiescent cardiac fibroblasts[24],[25], but is upregulated in injury site-fibroblasts in infarcted and in pressure-overloaded hearts[25],[26]. In order to generate mice with myofibroblast-specific loss of Smad3 (FS3KO), Smad3fl/fl [27] mice were crossed with Postn-Cre transgenic animals, as previously described[15],[28].
Mouse model of non-reperfused myocardial infarction:
Animal studies were approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine and conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. A mouse model of non-reperfused myocardial infarction was used, as previously described by our group[15]. Female and male mice, 4–6 months of age, were anesthetized using inhaled isoflurane (2%); 55 Smad2fl/fl mice, and 57 FS2KO mice underwent in vivo experiments. For analgesia, buprenorphine (0.05–0.2 mg/kg s.c) was administered at the time of surgery and q12h thereafter for 2 days. Additional doses of analgesics were given if the animals appeared to be experiencing pain (based on criteria such as immobility and failure to eat). At the end of the experiment, euthanasia was performed using 2% inhaled isoflurane followed by cervical dislocation. Early euthanasia was performed with the following criteria, indicating suffering of the animal: weight loss>20%, vocalization, dehiscent wound, hypothermia, clinical signs of heart failure (cyanosis, dyspnea, tachypnea), lack of movement, hunched back, ruffled coat, lack of food or water ingestion.
Echocardiography:
Echocardiographic studies were performed before instrumentation, 7 and 28 days after coronary occlusion using the Vevo 2100 system (VisualSonics. Toronto ON), as previously described [29].
Immunohistochemistry and histology:
For histopathological analysis murine hearts were fixed in zinc-formalin (Z-fix; Anatech, Battle Creek, MI), and embedded in paraffin. Collagen was stained using picrosirius red. Quantitative assessment of myofibroblast density was performed by counting the number of cells/myocardial area.
Isolation and culture of cardiac fibroblasts:
Fibroblasts were isolated from normal mouse (C57/BL6J) hearts as previously described [30],[31].
siRNA knockdown experiments:
For siRNA knockdown experiments, mouse cardiac fibroblasts were either transfected with Smad2 siRNA, Smad3 siRNA or non-silencing control siRNA, using Lipofectamine® 3000 Reagent, as previously described[32]. Briefly, the cells treated with siRNA were suspended in collagen pad or plated in dishes with serum free DMEM/F12 for 72 h. Cell lysates were utilized for qPCR to verify efficacy of mRNA knockdown with siRNA.
Assessment of α-smooth muscle actin (α-SMA) incorporation into the cytoskeleton of cardiac fibroblasts.
Dual fluorescence with phalloidin-AF594 (Invitrogen, A12381) and anti-α-SMA-FITC-labeled antibody (Sigma, F3777) was used to assess decoration of F-actin fibers with α-SMA in fibroblasts cultured in chamber slides in the presence or absence of TGF-β1 (10ng/ml, 72h).
Fibroblast migration assay
A cardiac fibroblast migration assay was performed using a colorimetric transwell system as previously described[31].
RNA extraction, qPCR and qPCR array analysis
Gene expression was assessed using quantitative polymerase chain reaction.
Protein extraction and western blotting:
Protein was extracted from cardiac fibroblasts as previously described [33], and western blotting was performed using established protocols.
Statistics:
For comparisons of two groups unpaired, 2-tailed Student’s t-test using (when appropriate) Welch’s correction for unequal variances was performed. The Mann-Whitney test was used for comparisons between 2 groups that did not show Gaussian distribution. For comparisons of multiple groups, 1-way ANOVA was performed followed by Tukey’s multiple comparison test. The Kruskall-Wallis test, followed by Dunn’s multiple comparison post-test was used when one or more groups did not show Gaussian distribution. Paired t-test was used for comparisons of functional data within the same group. Survival analysis was performed using the Kaplan-Meier method. Mortality was compared using the log rank test.
3. RESULTS:
1. TGF-βs, but not Bone Morphogenetic Proteins (BMPs), or angiotensin II, directly activate Smad2 signaling in mouse cardiac fibroblasts
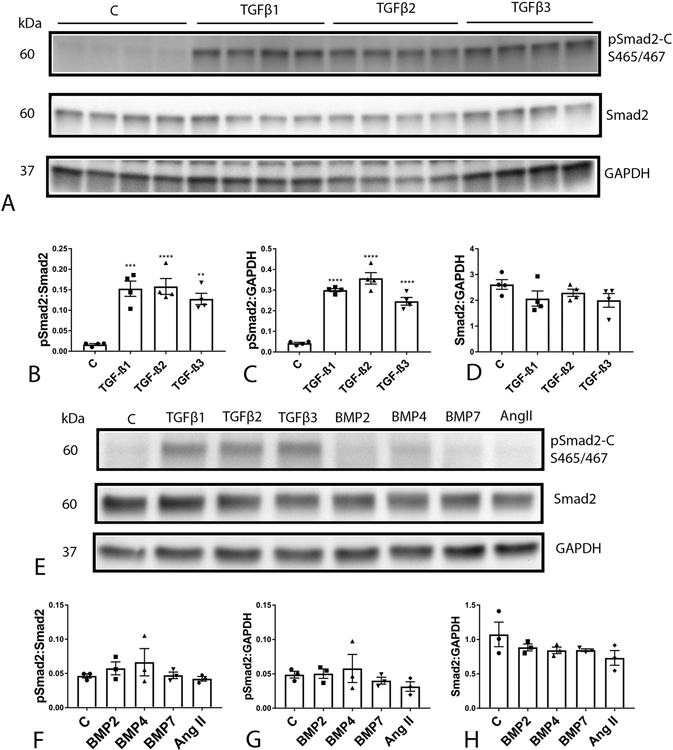
Western blotting experiments showed that isolated mouse cardiac fibroblasts have negligible baseline activation of Smad2 (Fig 1A). TGF-β isoforms (TGF-β1, -β2, or -β3, 10 ng/ml) induced a marked increase in cardiac fibroblast pSmad2 expression levels after 30 min of stimulation (Figure 1A–C). TGF-βs had no effects on total Smad expression by isolated cardiac fibroblasts (Figure 1D).
Figure 1: All three TGF-β isoforms, but not BMPs, or angiotensin II, activate the Smad2 pathway in isolated cardiac fibroblasts.
Representative western blotting experiments demonstrate that TGF-β1 (10ng/ml), -β2 (10ng/ml), and-β3 (10ng/ml) markedly incresase C-terminal Smad2 phosphorylation at the S465/S467 sites in cardiac fibroblasts after 30 min of stimulation (A-D). Quantitative analysis shows that TGF-βs significantly increase the pSmad2:Smad2 ratio (B) and accentuate Smad2 phosphorylation (C) without affecting levels of total Smad2 protein (D). (**P<0.01, ****p<0.0001 vs. control, n=3–4/group-ANOVA followed by Tukey’s multiple comparison test). In contrast, BMP2 (50ng/ml), BMP4 (50ng/ml), BMP7 (50ng/ml) and angiotensin II (50ng/ml) do not directly activate Smad2 (E-H).
In contrast to the effects of TGF-βs, BMP-2, BMP-4 and BMP-7 (50 ng/ml), all known to be induced in the infarcted and remodeling myocardium[34] did not induce Smad2 activation in isolated cardiac fibroblasts after 30 min of stimulation (Figure 1E–H). The critical neurohumoral mediator angiotensin II has also been suggested to activate Smad signaling, directly or indirectly. However, angiotensin II (50ng/ml) did not activate Smad2 in cardiac fibroblasts after 30 min of stimulation (Figure 1E–H).
2. Smad2 is activated in infarct myofibroblasts.
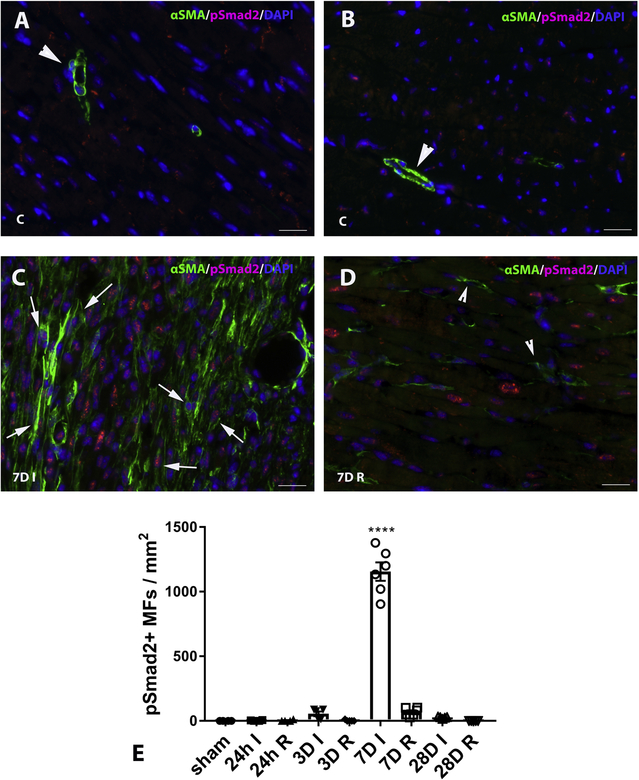
Dual immunofluorescence combining p-Smad2 staining and α-SMA labeling (to identify myofibroblasts as spindle shaped α-SMA+ cells located outside the vascular media), demonstrated that after 7 days of permanent coronary occlusion, abundant infarct myofibroblasts exhibit activation of Smad2 signaling, evidenced by nuclear localization of p-Smad2 (Figure 2A–D). Quantitative analysis showed that the density of p-Smad2+ myofibroblasts in healing infarcts peaked after 7 days of permanent coronary occlusion (Figure 2E). Dual immunofluorescence combining staining for the myofibroblast marker periostin, and p-Smad2 showed that infarct myofibroblasts exhibit Smad2 activation as early as 3 days after coronary occlusion, and confirmed the prominent activation of p-Smad2 in infarct myofibroblasts at the 7-day timepoint (Supplemental figure I). Dual immunofluorescence combing staining for periostin and p-Smad3 demonstrated that the density of myofibroblasts exhibiting Smad3 activation also peaked after 7 days of coronary occlusion (Supplemental figure II).
Figure 2: Smad2 is activated in infarct myofibroblasts.
Dual immunofluorescence combining pSmad2 staining and α-SMA labeling (to label infarct myofibroblasts as spindle shaped α-SMA+ cells located outside the vascular media) was used to identify myofibroblasts exhibiting Smad2 activation. In control (c) hearts (A, B) α -SMA immunoreactivity was localized exclusively in vascular mural cells (arrowheads) and levels of pSmad2 staining were very low. C: After 7 days of permanent coronary occlusion, abundant myofibroblasts in the infarct zone (7DI) exhibited activation of Smad2 signaling, evidenced by nuclear localization of pSmad2 (arrows, C). D: Relatively few myofibroblasts (arrowheads) were noted in the remote remodeling myocardium (7DR) and had negligible pSmad2 expression. E: Quantitative analysis shows that the density of p-Smad2+ myofibroblasts in healing infarcts peaked after 7 days of permanent coronary occlusion. (****p<0.0001 vs. sham, n=4–6/group – Kruskall-Wallis non-parametric ANOVA followed by Dunn’s multiple comparison post-test). Scalebar=20 μm
3. Mice with myofibroblast-specific loss of Smad2 exhibit no baseline defects.
In order to develop mice with cell-specific loss of Smad2 in activated fibroblasts (fibroblast-specific Smad2 knockout mice, FS2KO), we bred periostin-Cre mice with Smad2 fl/fl animals. Dual staining for Smad2 and periostin demonstrated loss of Smad2 immunoreactivity in the majority of periostin+ myofibroblasts infiltrating the healing infarct after 7 days of coronary occlusion (Supplemental figure III). Quantitative analysis showed that FS2KO mice had a marked reduction in the density of Smad2+/periostin+ myofibroblasts in the infarcted myocardium after 7 days of coronary occlusion, when compared with Smad2 fl/fl animals. At the 3-day timepoint, both Smad2 fl/fl and FS2KO mice had a significant number of periostin-negative Smad2-expressing interstitial cells (Supplemental figure III). Thus, the approach using the periostin Cre driver specifically targeted activated myofibroblasts in the healing infarct.
Comparison of echocardiographic parameters between age and gender-matched FS2KO and Smad2 fl/fl mice showed that myofibroblast-specific loss of Smad2 had no effects on baseline cardiac function. Body weight, heart rate, cardiac chamber dimensions, wall thickness, and systolic function were comparable between FS2KO and Smad2 fl/fl animals (Supplemental figure IV).
4. Smad2 loss in activated myofibroblasts does not affect mortality following non- reperfused myocardial infarction.
Mice undergoing non-reperfused infarction protocols exhibit significant mortality during the first week following coronary occlusion, mostly due to cardiac rupture. Gender-matched FS2KO mice and Smad2 fl/fl littermates had comparable mortality following myocardial infarction (Supplemental figure V). Male mice had higher mortality than females for both Smad2 fl/fl and FS2KO groups (Supplemental figure V).
5. Effects of myofibroblast-specific Smad2 loss on cardiac function and adverse remodeling following myocardial infarction.
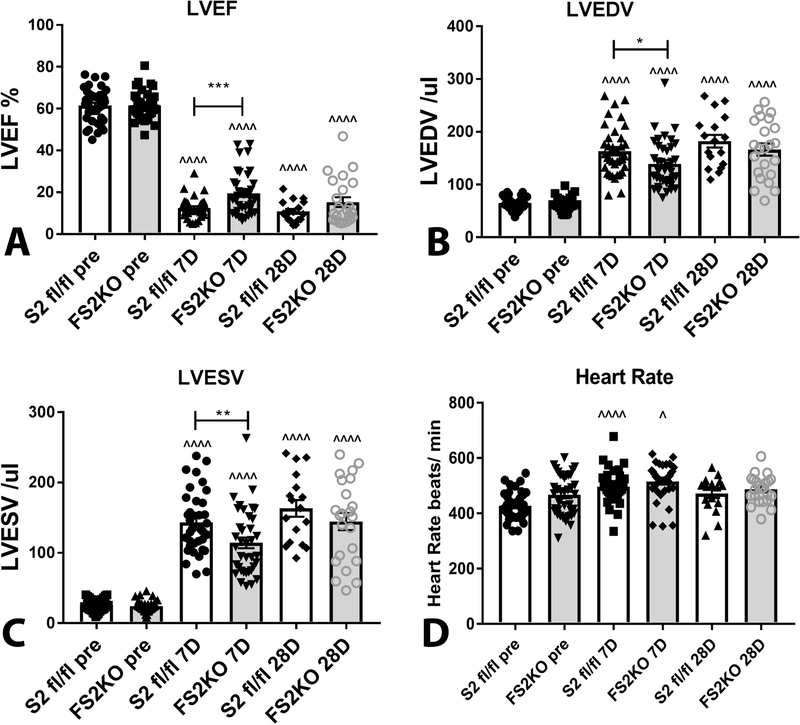
Non-reperfused myocardial infarction was associated with marked depression of systolic function (evidenced by reduction of ejection fraction), and severe dilative remodeling (suggested by increases in LVEDV and LVESV) after 7–28 days of permanent coronary occlusion in both Smad 2 fl/fl and FS2KO groups. FS2KO mice exhibited a modest and transient protection from adverse remodeling and dysfunction after 7 days of coronary occlusion. Ejection fraction was significantly higher in FS2KO mice at the 7 day timepoint (Figure 3A). In contrast, after 28 days of coronary occlusion, FS2KO mice and Smad2 fl/fl animals had no significant differences in ejection fraction (Figure 3). LVEDV (Figure 3B) and LVESV (Figure 3C) were significantly lower in FS2KO mice after 7 days, but not after 28 days of coronary occlusion, suggesting a transient reduction in dilative post-infarction remodeling. Heart rate was comparable between Smad2 fl/fl and FS2KO mice at all timepoints studied (Figure 3D). Analysis of the echocardiographic data for female and male mice is shown in Supplemental figure VI.
Figure 3: Effects of myofibroblast-specific Smad2 loss on cardiac function and adverse remodeling following myocardial infarction.
Non-reperfused myocardial infarction is associated with marked depression of systolic function (evidenced by markedly reduced ejection fraction), and severe dilative remodeling (suggested by increases in LVEDV and LVESV) after 7–28 days of permanent coronary occlusion in both Smad2 fl/fl and FS2KO groups. FS2KO mice exhibited a modest and transient protection from adverse remodeling and dysfunction after 7 days of coronary occlusion. Ejection fraction was significantly higher in FS2KO mice at the 7 day timepoint (A). In contrast, after 28 days of coronary occlusion, FS2KO mice and Smad2 fl/fl animals had no significant differences in ejection fraction (A). LVEDV (B) and LVESV (C) were significantly lower in FS2KO mice after 7 days, but not after 28 days of coronary occlusion, suggesting that myofibroblast-specific Smad2 loss was associated with a transient reduction in dilative post-infarction remodeling. Heart rate (D) was not significantly different between Smad2 fl/fl and FS2KO mice at the same timepoint (*p<0.05, **p<0.01, ***p<0.001 vs. S2fl/fl 7D, ^p<0.05, ^^^^p<0.0001 vs. S2fl/fl pre or FS2KO pre-ANOVA followed by Tukey’s multiple comparison test) (pre-echo: Smad2 fl/fl, n=37; FS2KO, n=38, 7 days: Smad2 fl/fl, n=37; FS2KO, n=37; 28 days: Smad2 fl/fl, n=17; FS2KO, n=23).
6. Myofibroblast-specific Smad2 loss does not affect the morphometric characteristics of the infarcted heart.
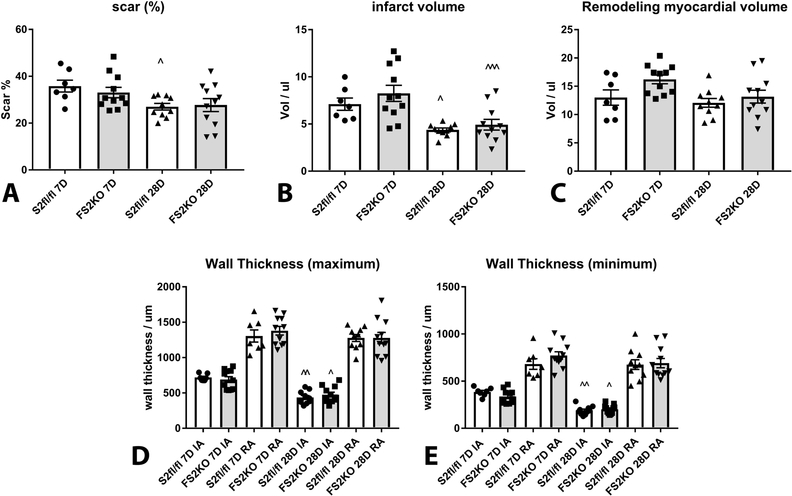
In order to examine whether myofibroblast-specific Smad2 loss affects scar remodeling following myocardial infarction, we performed systematic analysis of morphometric parameters based on reconstruction of the infarcted ventricle from base to apex (Supplemental figure VII). Scar size (Figure 4A), infarct volume (Figure 4B), the volume of the viable remodeling myocardium, (Figure 4C) and the thickness of the infarcted and non-infarcted walls (Figure 4D–E) were comparable between FS2KO and Smad2 fl/fl mice at the 7 and 28-day timepoints. Analysis of the morphometric data for male and female mice is shown in Supplemental figure VIII.
Figure 4: Fibroblast-specific Smad2 loss does not affect the morphometric characteristics of the infarcted heart.
In order to examine whether myofibroblast-specific Smad2 loss affects scar remodeling following myocardial infarction, we performed systematic analysis of morphometric parameters based on reconstruction of the infarcted ventricle from base to apex (strategy shown in Supplemental figure V). Scar size (A), infarct volume (B), the volume of the viable remodeling myocardium (C), and the thickness of the infarcted and non-infarcted walls (D-E) were comparable between FS2KO and Smad2 fl/fl mice after 7–28 days of coronary occlusion. (^p<0.05, ^^p<0.01, ^^^p<0.001 vs. S2fl/fl 7D or FS2KO 7D, n=7–11/group-ANOVA followed by Tukey’s multiple comparison test).
7. FS2KO and Smad2 fl/fl animals exhibit comparable collagen deposition in the infarcted and non-infarcted myocardium following permanent coronary occlusion.
We used sirius red staining to label collagen fibers in the infarcted and remodeling myocardium. FS2KO mice and corresponding Smad2 fl/fl littermates had comparable collagen content in infarcted and remodeling areas at both 7 and 28-day timepoints (Supplemental Figure IX).
8. Myofibroblast-specific loss of Smad3, but not Smad2, perturbs organization of myofibroblast arrays following myocardial infarction
We have previously demonstrated that myofibroblast-specific loss of Smad3 accentuates adverse remodeling following myocardial infarction perturbing formation of organized myofibroblast arrays in the healing infarct[15]. Our current study demonstrates that in contrast to the catastrophic effects of fibroblast-specific Smad3 loss, myofibroblast-specific Smad2 deletion is not associated with impaired repair, but results in a transient improvement of cardiac remodeling following myocardial infarction. In order to explore the basis for the distinct effects of myofibroblast-specific Smad2 and Smad3 signaling in cardiac repair, we compared the effects of Smad2 and Smad3 loss on infarct myofibroblast infiltration.
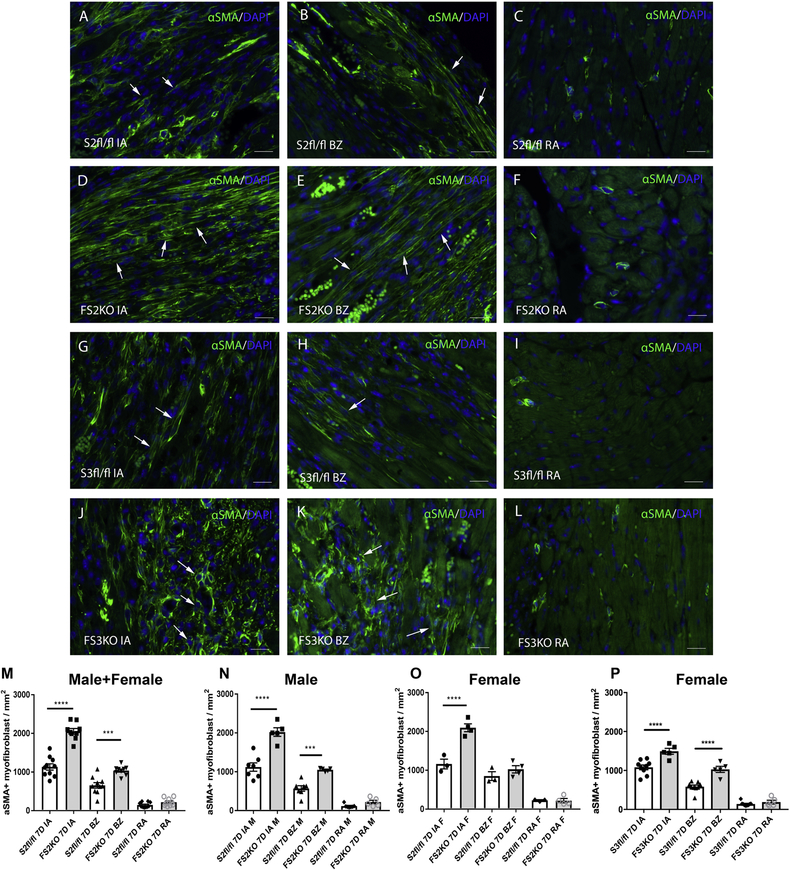
Infarct myofibroblasts were identified in infarcted hearts asα-SMA-immunoreactive cells, located outside the vascular media (Figure 5). Quantitative analysis of myofibroblast density demonstrated that FS2KO mice had increased infiltration of the infarcted area with myofibroblasts, in comparison to Smad2 fl/fl animals (Figure 5A–F, M–O). As we have previously demonstrated, fibroblast-specific Smad3 loss also increased infiltration of the infarct with myofibroblasts (Figure 5G–L, P). Because of the very high mortality of FS3KO mice due to cardiac rupture, analysis of myofibroblast density in Smad3 fl/fl and FS3KO mice in the current study was limited to female animals (Figure 5P). Thus, both Smad2 and Smad3 signaling restrain expansion of the myofibroblast population in the infarcted heart. These observations support the Smad-dependent anti-proliferative effects of TGF-β on fibroblasts, previously reported by our group and by other investigators.
Figure 5: Myofibroblast-specific Smad2 or Smad3 signaling restrain infiltration of the infarcted heart with α-SMA+ myofibroblasts.
Infarct myofibroblasts were identified after 7 days of coronary occlusion as α-SMA-immunoreactive cells (arrows), located outside the vascular media (A-L). Quantitative analysis of myofibroblast density demonstrated that FS2KO mice had increased infiltration of the infarcted area (IA) and of the border zone (BZ) with myofibroblasts, in comparison to Smad2 fl/fl animals (A-F, M). Myofibroblast density in remote areas (RA) was comparable between groups (****p<0.0001, ***p<0.001 vs. S2fl/fl; n=9–10/group). The effects of Smad2 loss were noted in both male (N) and female (O) mice. As we have previously demonstrated, fibroblast-specific Smad3 loss also increased infiltration of the infarct with myofibroblasts (G-L, P) (****p<0.0001, n=5–9/group-ANOVA followed by Tukey’s multiple comparison test). Please note that because of the previously reported high mortality of male FS3KO mice following infarction (due predominantly to rupture), analysis in this study is limited to female animals (P). Scalebar=20μm.
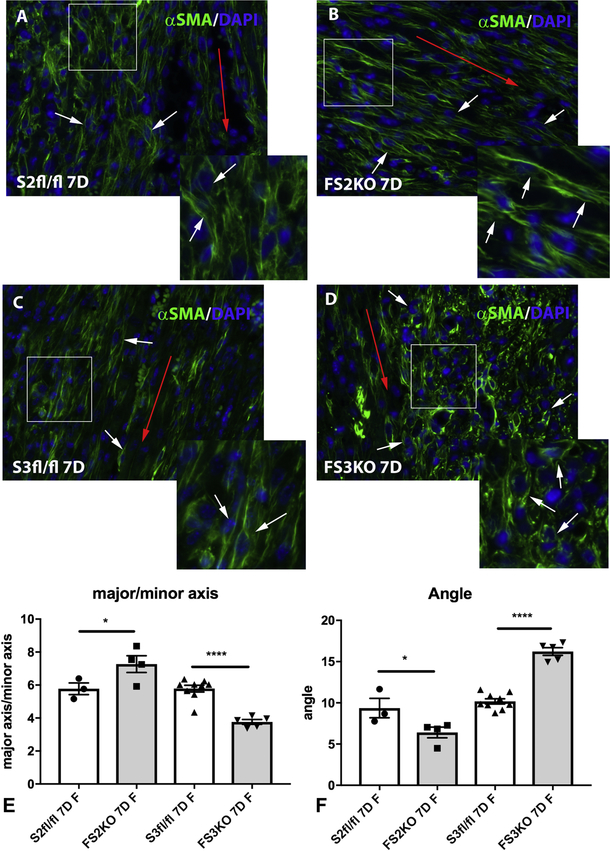
Next, we examined whether Smad2, or Smad3 loss affects the morphologic characteristics and organization of myofibroblast arrays in infarcted hearts. Repair of the infarcted myocardium requires formation of well-organized arrays of polarized myofibroblasts in the infarct zone. Infarct myofibroblasts are aligned along the direction of the left ventricular wall. Consistent with our previously published findings[15], FS3KO mice exhibited infiltration of the infarcted myocardium with abundant malaligned, round-shaped myofibroblasts (Figure 6). In contrast to the perturbed alignment of infarct myofibroblasts in animals with fibroblast-specific loss of Smad3, FS2KO animals exhibited well-organized myofibroblast arrays (Figure 6). Quantitative analysis showed that infarct myofibroblasts in FS3KO mice had a much lower major to minor axis ratio than FS2KO cells and corresponding Smad2 fl/fl and Smad3 fl/fl controls (Figure 6E), suggesting round morphology. Moreover, the average angle of myofibroblasts to the direction of the left ventricle was significantly higher in FS3KO mice than in FS2KO animals, suggesting that alignment of infarct myofibroblasts is dependent on Smad3, and not on Smad2 signaling (Figure 6F). Again, because of the very high mortality of male FS3KO mice following infarction, analysis of myofibroblast alignment was limited to female animals (Figure 6E–F). As previously described by our group, impaired organization of myofibroblast arrays in FS3KO mice was associated with perturbations of the alignment patterns of structural collagen fibers. Sirius red-stained sections visualized under polarized light showed evidence of collagen fiber fragmentation in infarcted FS3KO mice after 7 days of coronary occlusion. In contrast, FS2KO mice had a well-organized network of collagen fibers in the infarcted heart (Supplemental figure X).
Figure 6: Myofibroblast-specific Smad3, but not Smad2 signaling is critical for organization of myofibroblast arrays in infarcted hearts.
After 7 days of coronary occlusion, infarct myofibroblasts in wildtype mice (identified through α-SMA immunofluorescence-white arrows), are typically aligned along the direction of the left ventricular wall (red arrow-A, C). In contrast to the perturbed alignment of infarct myofibroblasts in FS3KO mice (C-D, white arrows), FS2KO animals exhibited well-organized myofibroblast arrays (white arrows, A-B). E: Quantitative analysis showed that infarct myofibroblasts in FS3KO mice had a much lower major to minor axis ratio than Smad3 fl/fl cells, suggesting round morphology. In contrast, FS2KO mice had an increase in the major to minor axis ratio of infarct myofibroblasts, reflecting a more elongated morphology (*p<0.05, ****p<0.0001, n=3–9/group). The average angle of the myofibroblasts (white arrows) to the direction of the left ventricle (red arrow) was assessed as a quantitative indicator of cell alignment. The mean angle was significantly higher in FS3KO mice than in FS2KO animals, (F) (****p<0.0001, n=3–9/group), suggesting that formation of aligned arrays of polarized infarct myofibroblasts is dependent on Smad3, and not on Smad2 signaling. Statistical analysis was performed using ANOVA followed by Tukey’s multiple comparison test. Please note that due to the very high mortality of male FS3KO mice following infarction, comparative analysis in this study was limited to female mice.
9. Effects of Smad2 and Smad3 on expression of myofibroblast-associated genes.
Our in vivo findings suggest an important role for fibroblast Smad3, but not Smad2 in organization of infarct myofibroblast arrays. In order to dissect the mechanism responsible for the contrasting actions of Smad2 and Smad3 signaling, we performed in vitro experiments investigating the effects of Smad2 and Smad3 siRNA knockdown (KD) on fibroblast gene expression in cells cultured in the high tension environment of the culture plate. First, we demonstrated the specificity of Smad2 and Smad3 siRNA KD approaches. Smad2 siRNA KD markedly reduced Smad2 expression by cardiac fibroblasts without affecting Smad3 expression (Supplemental figure XIA–B). Moreover, Smad3 siRNA KD markedly reduced Smad3 expression without affecting Smad2 levels (Supplemental figure XIA–B). Smad2 knockdown did not significantly affect α-SMA and vimentin mRNA expression levels. Cells treated with Smad3 siRNA had trends towards reduced levels of α-SMA and vimentin. Next, we performed dual immunofluorescence experiments for phalloidin and α-SMA to examine whether Smad2 or Smad3 loss affect formation of α -SMA-decorated stress fibers (a feature of myofibroblast conversion) in cells cultured in plates. Smad2 and Smad3 KD did not affect the percentage of α -SMA+ cells at baseline, but significantly reduced the number of αSMA+ cells following stimulation with TGF-β1 (Supplemental figure XII). The findings suggested that in vitro, both Smad2 and Smad3 signaling are implicated in incorporation of α-SMA into stress fibers, a hallmark of myofibroblast conversion. Smad3 may also act by enhancing α -SMA mRNA expression (Supplemental figure XI and [19]).
11. Smad3, but not Smad2 knockdown attenuates α2 and α5 integrin expression in fibroblasts cultured in collagen pads.
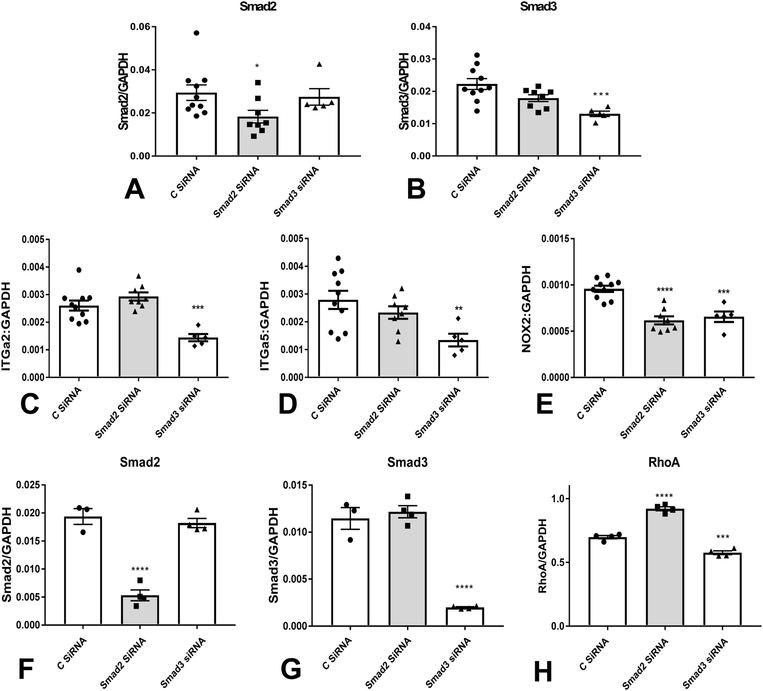
Organization of fibroblast arrays in the healing infarct is dependent, on activation of integrin-mediated interactions between the fibroblasts and the extracellular matrix. We have previously demonstrated that perturbed function of Smad3 KO fibroblasts is due, at least in part, to defective activation of an integrin-dependent, NOX-2 mediated axis[15]. In order to explain the contrasting actions of Smad2 and Smad3 on cardiac fibroblast function, we examined the effects of Smad2 and Smad3 KD (Figure 7A–B) on expression of integrins and NOX-2 in fibroblasts cultured in collagen pads. Smad3, but not Smad2 KD resulted in marked reduction of a2 and a5 integrin mRNA expression (Figure 7C–D). NOX-2 expression on the other hand was significantly reduced in both Smad2 and Smad3 KD cells (Figure 7E). Because integrins are involved in fibroblast migration, we examined whether Smad2 or Smad3 loss affect migration of fibroblasts in a transwell assay. Smad2 and Smad3 siRNA KD had no effects on fibroblast migration in response to serum (1%) and TGF-β1 (20ng/ml) (Supplemental figure XIII).
Figure 7: Smad2 and Smad3 differentially modulate expression of integrins and RhoA in cardiac fibroblasts.
In order to explain the contrasting in vivo actions of myofibroblast Smad2 and Smad3 signaling, we examined the effects of Smad2 and Smad3 siRNA KD on expression of integrins and NOX-2 in fibroblasts cultured in collagen pads. We have previously demonstrated[15] that cardiac fibroblasts harvested from mice with global loss of Smad3 have reduced levels of a2 and a5 integrin and attenuated NOX-2 synthesis. A-B: Effectiveness and specificity of Smad2 (A) and Smad3 (B) siRNA KD in fibroblasts populating collagen pads was demonstrated using qPCR. Smad2 KD reduced Smad2 levels without affecting Smad3 levels, whereas Smad3 KD attenuated Smad3 synthesis without significant effects on Smad2 expression. C-D: Smad3 KD, but not Smad2 KD resulted in marked reduction of α2 and α5 integrin mRNA expression. E: NOX-2 expression on the other hand was significantly reduced in both Smad2 and Smad3 KD cells (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs. control siRNA, n=5–10/group). In order to examine the effects of Smad2 and Smad3 KD (F-G) on expression levels of planar cell polarity (PCP) genes (that may play an important role in formation of aligned myofibroblast arrays in healing infarcts), cardiac fibroblasts cultured in plates were transfected with control siRNA (C siRNA), Smad2 siRNA, or Smad3 siRNA. The effectiveness and specificity of Smad2 and Smad3 KD was demonstrated using qPCR (F, G). A PCR array was performed to assess levels of Wnt/frizzled family genes (full data provided in Supplemental table 1). Smad3 KD markedly suppressed RhoA, a downstream effector of the PCP pathway (H). In contrast, Smad2 KD increased RhoA expression (H). (***p<0.001, ****p<0.0001 vs. C siRNA, n=3–4/group-ANOVA followed by Tukey’s multiple comparison test).
11. Smad2 and Smad3 have opposing effects on RhoA synthesis by cardiac fibroblasts.
The collective alignment of cells across a tissue plane is controlled by the planar cell polarity pathway (PCP)[35]. We hypothesized that perturbed alignment of infarct myofibroblasts in FS3KO, but not in FS2KO mice may reflect Smad3-dependent actions on regulation of PCP genes. Accordingly, we examined the effects of Smad2 and Smad3 KD on expression levels of PCP genes by cardiac fibroblasts (Supplemental figure XIV, Supplemental table I). Smad3 KD was associated with marked suppression of RhoA, a gene critically involved in regulation of the PCP pathway (Figure 7H). In contrast, Smad2 KD increased RhoA expression. Thus, impaired alignment of fibroblasts lacking Smad3 may reflect attenuated RhoA levels. The core PCP pathway involves the Frizzled (Fz) family of multipass transmembrane proteins. Expression of Fzd2 has been previously documented in infarct fibroblasts and has been suggested as a mediator of polarized alignment of myofibroblasts in healing infarcts[14]. Analysis of frizzled gene expression in isolated cardiac fibroblasts did not reveal consistent effects of Smad2 or Smad3 KD that could explain the in vivo observations. Fzd2 and Fzd6 mRNA levels were significantly reduced in both Smad2 and Smad3 KD cells (Supplemental figure XV). Smad2 KD was also associated with reduced Fzd3 expression, whereas Smad3 KD decreased Fzd5 levels. Expression of Fzd1, Fzd4, Fzd7, Fzd8 and Fzd9 (Supplemental figure XV), and of the PCP genes Vangl2, Dvl1 and Dvl2 (Supplemental Table I) was not significantly affected by Smad2 or Smad3 KD. Smad2 and Smad3 also played a role in regulation of Wnt genes. Smad2 KD significantly reduced Wnt5b expression in cardiac fibroblasts; in contrast, Smad3 KD increased Wnt5b mRNA levels. Moreover, Smad2 KD was associated with increased Wnt9a levels, whereas Smad3 KD accentuated Wnt11 expression. In contrast, Wnt2b and Wnt5a were not affected by Smad2 or Smad3 KD. Cardiac fibroblasts also expressed high levels of the secreted frizzle-related proteins Sfrp1 and Sfrp2. However, Sfrp1 and Sfrp2 mRNA expression was not affected by Smad2 or Smad3 KD (Supplemental figure XVI).
4. DISCUSSION
Our study reports for the first time that repair of the infarcted myocardium requires activation of Smad3, but not Smad2 signaling, in activated cardiac myofibroblasts. Myofibroblast-specific loss of Smad3 impairs infarct healing, perturbing alignment of activated fibroblasts and leading to formation of a disorganized scar[15]. In contrast, myofibroblast-specific Smad2 loss does not disrupt scar organization, and is associated with a modest and transient reduction in adverse remodeling. In vitro experiments showed that the detrimental effects of myofibroblast-specific Smad3 loss may involve downmodulation of integrin synthesis, and reduced RhoA expression levels. In contrast, Smad2 loss in cardiac myofibroblasts does not affect integrin expression and is associated with increased RhoA synthesis. The findings highlight the contrasting effects of Smad2 and Smad3 activation in myofibroblast-mediated cardiac repair.
Myofibroblast activation in the infarcted heart.
In the infarcted myocardium, resident fibroblast populations proliferate and undergo myofibroblast conversion, acquiring a matrix-synthetic phenotype[36]. Formation of well-aligned arrays of polarized myofibroblasts plays an important role in repair of the infarcted heart, preserving the structural integrity of the ventricle, and preventing cardiac rupture [37],[38],[15].
On the other hand, excessive or prolonged activation of myofibroblasts following infarction may lead to excessive deposition of extracellular matrix proteins, worsening ventricular compliance, and contributing to the development of heart failure[16],[39]. Regulation of fibroblast phenotype and function in the infarcted heart involves actions of neurohumoral mediators, secreted cytokines and growth factors, and specialized components of the cardiac extracellular matrix. TGF-βs are central effectors of myofibroblast conversion and activation in the infarcted and remodeling myocardium[40],[41]. In the healing infarct, induction of matricellular proteins, oxidative stress and protease activation co-operate to release bioactive TGF-β from its latent stores. Subsequently, the active TGF-β dimer binds to and sequentially transphosphorylates type II and type I TGF-β receptors, transducing Smad-dependent and Smad-independent signaling. Receptor-activated Smads (R-Smads), such as Smad2 and Smad3, are phosphorylated by activated type I receptors, then bind to the common Smad, Smad4 and translocate to the nucleus where they recruit co-activators and co-repressors and regulate transcription[42]. Fibroblasts are major targets of TGF-βs, rapidly activating Smad signaling cascades, and non-Smad pathways upon TGF-β stimulation[19],[20]. In the infarcted myocardium, abundant α-SMA+ myofibroblasts express p-Smad2 in the nucleus (Figure 2), suggesting activation of the Smad2 pathway. All three TGF-β isoforms are induced following myocardial infarction[43], and are capable of activating Smad2 signaling in cardiac fibroblasts (Figure 1).
The distinct roles of Smad2 and Smad3 in myofibroblast-mediated cardiac repair
We have previously demonstrated that mice with myofibroblast-specific Smad3 loss exhibit an increased incidence of cardiac rupture, accentuated dilative remodeling, and worse systolic dysfunction following myocardial infarction[15]. Adverse post-infarction remodeling in the absence of Smad3 is associated with perturbed scar organization, suggesting a critical role for myofibroblast-specific Smad3 signaling in mediating formation of aligned myofibroblast arrays. In contrast to the catastrophic effects of myofibroblast-specific Smad3 loss, myofibroblast-specific Smad2 knockdown did not affect cardiac remodeling after 28 days of coro nary occlusion, but resulted in a modest, but transient improvement in systolic function at the 7 day timepoint (Figure 3). Much like FS3KO animals, FS2KOs had a marked increase in infarct myofibroblast density (Figure 4), possibly reflecting partial loss of the Smad-dependent anti-proliferative actions of TGF-β signaling in cardiac fibroblasts[19]. However, in contrast to the pronounced perturbations in myofibroblast morphology and alignment in mice lacking Smad3, myofibroblast-specific loss of Smad2 did not significantly affect the organization of infarct myofibroblast arrays in the border zone of the infarct. Formation of organized myofibroblast arrays is important to protect the infarcted heart from rupture and may be required to maximize tensile strength of the healing scar, thus preventing dilation and adverse remodeling.
Molecular mechanisms of Smad2 and Smad3 actions in cardiac myofibroblasts
Integrins play a critical role in fibroblast activation[44], serving as a bridge between the cells and the extracellular matrix[45] and transducing signaling cascades that promote myofibroblast conversion[46], and regulate cell motility and polarity[47]. Using an siRNA knockdown approach, we found that Smad3, and not Smad2, mediates α2 and α5 integrin synthesis in cardiac fibroblasts populating collagen pads (Figure 7). Integrin-dependent interactions may play a crucial role in formation of aligned fibroblast arrays in the healing infarct. Thus, perturbed scar organization in mice lacking Smad3 in fibroblasts may reflect low integrin levels, resulting in impaired interaction between the cells and the extracellular matrix. In contrast, preserved integrin expression in fibroblasts lacking Smad2 may be sufficient for formation of a well-organized scar.
The collective alignment of cells in tissues involves activation of the planar cell polarity pathway (PCP). In the healing infarct local alignment of myofibroblasts along the axis of the ventricle may require intercellular communications, mediated through induction of PCP genes, such as Wnt and frizzled family members. In order to test this hypothesis, we examined whether Smad3 loss in cardiac fibroblasts causes perturbations in expression of PCP genes and their downstream effectors. In isolated cardiac fibroblasts, both Smad2 and Smad3 mediated expression of frizzled2 (fzd2) (Supplemental figure XV), a gene highly upregulated in infarct myofibroblasts that may be involved in infarct myofibroblast polarization[14]. Fzd3 expression was dependent on Smad2, whereas fzd5 synthesis was Smad3-dependent and both Smad2 and Smad3 mediated fzd6 expression (Supplemental figure XV). In contrast, fibroblast expression of the frizzled/disheveled pathway genes Fzd1, Fzd4, Fzd7, Fzd8, Fzd9, Vangl2, Dvl1, and Dvl2 was not dependent on Smad2 or Smad3 signaling. Wnt synthesis was also regulated by the Smad cascade. Wnt5b expression was decreased in Smad2 knockdown fibroblasts, but was significantly increased in Smad3 knockdown cells (Supplemental figure XVI). Considering the role of Wnt5b expression in disruption of mesenchymal cell aggregation[48], increased Wnt5b levels in fibroblasts lacking Smad3 may be involved in mediating the perturbed cellular organization of the scar in FS3KO mice. Moreover, Smad2 loss increased Wnt9a levels (D), whereas Smad3 disruption accentuated Wnt11 expression.
The GTPase RhoA is an important downstream mediator of the PCP signaling pathway[49]. Vangl2, a key component of the frizzled/disheveled pathway with a crucial role in regulation of planal cell polarity is part of a multiprotein complex that signals through RhoA[49],[50]. Activation of the RhoA pathway plays a critical role in mediating actin-driven formation of polarized cell protrusions[51]. Our findings demonstrated that Smad3 and Smad2 have contrasting effects on RhoA expression in fibroblasts: Smad3 knockdown reduces RhoA levels, whereas Smad2 inhibition markedly accentuates RhoA synthesis (Figure 7H). Thus, the effects of myofibroblast-specific Smad3 loss on cell polarity may involve attenuation of RhoA-mediated signaling.
What is the basis for the distinct roles of Smad2 and Smad3 in TGF-β-mediated gene transcription?
Considering their similar activation patterns in response to TβRI pohsophorylation, their common interactions with Smad4, and the high degree of homology exhibited by their MH1 and MH2 domains (66% and 96% aminoacid sequence identity, respectively), the divergent actions of Smad2 and Smad3 are somewhat surprising[21]. Several mechanisms may explain the distinct functions of Smad2 and Smad3 in fibroblast-driven cardiac repair. First, activation of Smad2 and Smad3 may involve different subsets of fibroblasts. Recruitment of Smad2 and Smad3 to the activated TβRI is dependent on presentation by regulatory proteins such as Smad anchor for receptor activation (SARA). In vitro experiments in hepatic stellate cells showed that Smad2 is preferentially activated in early cultured cells (quiescent and intermediate cells), while Smad3 is mainly activated in transdifferentiated cells. The more rapid activation of Smad2 in infarct myofibroblasts (Supplemental figure I–II) may reflect preferential recruitment of Smad2 to the activated TGF-β receptor in cells at an early stage of myofibroblast conversion. Differences in SARA expression in various fibroblast subsets may dictate the ability of the receptor to activate Smad2 vs. Smad3[52] in response to TGF-β activation. Second, in mouse embryonic fibroblasts, Smad3 is essentially involved in regulation of immediate-early TGF-β (such as c-fos and Smad7), whereas Smad2 actions are more limited[53, 54]. The promiscuity of Smad3 to engage in multiple mechanisms of nuclear import has been suggested as a potential mechanism for its preferential actions in gene transcription [21]. Third, although both Smad2 and Smad3 interact with a wide range of common transcriptional regulators, some nuclear interacting proteins have been demonstrated to preferentially co-operate with one Smad over the other. For example, the E1A-like inhibitor of differentiation (EID2) exhibits a much stronger interaction with Smad3 than Smad2[55]. Moreover, Smad3 is known to uniquely interact with a wide range of nuclear receptors[21]. Fourth, Smad2 and Smad3 may differentially regulate promoter activity. For example, Smad2 stimulates the goosecoid (gsc) promoter, an early immediate target for TGF-β/activin signaling, in contrast Smad3 has suppressive effects[56]. It should be emphasized that most of the mechanistic evidence suggesting distinct molecular interactions of Smad2 and Smad3 is derived from in vitro experiments. The significance of specific molecular interactions in driving unique Smad2 and Smad3-mediated actions in vivo remains unclear.
Limitations
In vitro experiments showed that both Smad2 and Smad3 play an important role in mediating TGF-β-stimulated incorporation of α-SMA in stress fibers. In contrast to these in vitro findings, in healing infarcts Smad2 and Smad3 loss were associated with increased myofibroblast density. The apparently conflicting in vitro and in vivo data may reflect, at least in part, the use of the periostin Cre driver which specifically targets injury-site myofibroblasts in infarcted and remodeling hearts[15],[23], without affecting fibroblasts prior to myofibroblast conversion. After 3 days of coronary occlusion, we identified numerous Smad2-expressing periostin-negative cells in the infarcted myocardium in both Smad2 fl/fl and in FS2KO animals (Supplemental figure III). Many of these cells may be fibroblasts; in FS2KO infarcts may not exhibit Smad2 loss due to the absence of periostin expression. Thus, use of the periostin Cre driver in myocardial infarction allows dissection of myofibroblast-specific actions without providing information on the mechanisms responsible for fibroblast to myofibroblast conversion.
Conclusions
Although TGF-βs activate both Smad2 and Smad3 in fibroblasts infiltrating the infarcted heart, only myofibroblast-specific Smad3 plays a critical role in cardiac repair, by mediating formation of organized arrays of aligned myofibroblasts. The essential functions of fibroblast Smad3 may reflect induction of integrins in cardiac fibroblasts, leading to activation of a reparative program. Moreover, Smad3-dependent induction of RhoA may be important for regulation of cellular polarity by transducing molecular cascades triggered by the PCP pathway. In contrast to the essential reparative functions of Smad3, Smad2 signaling plays a limited role in activation of a reparative program in fibroblasts (Figure 8). However, myofibroblast-specific Smad2 signaling is transiently implicated in early adverse remodeling of the infarcted myocardium.
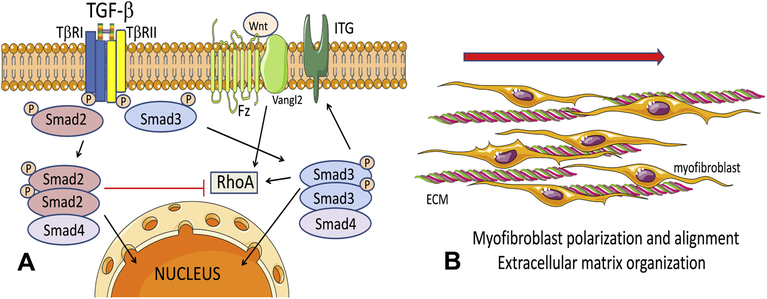
Figure 8: Schematic cartoons illustrate the contrasting roles of Smad2 and Smad3 in activation of infarct myofibroblasts following myocardial infarction.
A: TGF-βs bind and sequentially transphosphorylate type I and type II TGF-β receptors (TβRI, TβRII respectively), leading to downstream phosphorylation of Smad2 and Smad3. Activated Smad2 and Smad3 form complexes with the common Smad, Smad4 and translocate to the nucleus, where they may interact with a wide range of transcriptional activators and repressors and regulate transcription. In activated fibroblasts, Smad3 mediates expression of surface integrins (ITG), promoting interactions with other cells and the extracellular matrix (ECM). Moreover, Smad3 mediates expression of RhoA, a downstream effector of the Wnt/Fz/Vangl2 PCP pathway that may promote fibroblast alignment. In contrast, Smad2 does not critically affect α2 and α5 integrin expression and downmodulates RhoA synthesis. The distinct effects of Smad2 and Smad3 may explain the in vivo phenotypes observed in mice with fibroblast-specific Smad2 and Smad3 loss. Smad3 knockdown, but not Smad2 loss, perturbs myofibroblast polarization and formation of aligned myofibroblast arrays in the infarct, a process critical for organization of the healing scar (B).
Supplementary Material
HIGHLIGHTS.
Smad2 is activated in TGF-β-stimulated fibroblasts, and in infarct myofibroblasts.
Myofibroblast-specific Smad2 loss transiently attenuates post-infarction remodeling.
Smad3, but not Smad2 signaling, mediates formation of organized myofibroblast arrays.
Smad3, but not Smad2 mediates integrin and RhoA expression in cardiac fibroblasts.
Smad3 and Smad2 regulate expression of planar cell polarity genes.
FUNDING SOURCES:
This work was supported by NIH grants R01 HL76246 and R01 HL85440, and by Department of Defense grants PR151134 and PR151029. Dr. Simon J Conway is supported by R01 HL135657. Dr. Shuaibo Huang is supported by China Scholarship Council grant 201603170222. Dr. Bijun Chen is supported by an American Heart Association post-doctoral grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: None declared.
REFERENCES:
- [1].Laflamme MA, Murry CE, Heart regeneration, Nature. 473 (2011) 326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Frangogiannis NG, The extracellular matrix in myocardial injury, repair, and remodeling, J Clin Invest. 127 (2017) 1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shinde AV, Frangogiannis NG, Fibroblasts in myocardial infarction: A role in inflammation and repair, J Mol Cell Cardiol. 70C (2014) 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC, Cardiac Fibrosis: The Fibroblast Awakens, Circ Res. 118 (2016) 1021–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Abrial M, Da Silva CC, Pillot B, Augeul L, Ivanes F, Teixeira G, et al. , Cardiac fibroblasts protect cardiomyocytes against lethal ischemia-reperfusion injury, J Mol Cell Cardiol. 68 (2014) 56–65. [DOI] [PubMed] [Google Scholar]
- [6].Anzai A, Choi JL, He S, Fenn AM, Nairz M, Rattik S, et al. , The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes, J Exp Med. 214 (2017) 3293–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakaya M, Watari K, Tajima M, Nakaya T, Matsuda S, Ohara H, et al. , Cardiac myofibroblast engulfment of dead cells facilitates recovery after myocardial infarction, J Clin Invest. 127 (2017) 383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, et al. , IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium, J Immunol. 191 (2013) 4838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, et al. , Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart, J Clin Invest. 128 (2018) 2127–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lighthouse JK, Small EM, Transcriptional control of cardiac fibroblast plasticity, J Mol Cell Cardiol. 91 (2016) 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Frangogiannis NG, Michael LH, Entman ML, Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb), Cardiovasc Res. 48 (2000) 89–100. [DOI] [PubMed] [Google Scholar]
- [12].Davis J, Molkentin JD, Myofibroblasts: trust your heart and let fate decide, J Mol Cell Cardiol. 70 (2014) 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ivey MJ, Kuwabara JT, Pai JT, Moore RE, Sun Z, Tallquist MD, Resident fibroblast expansion during cardiac growth and remodeling, J Mol Cell Cardiol. 114 (2018) 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blankesteijn WM, Essers-Janssen YP, Verluyten MJ, Daemen MJ, Smits JF, A homologue of Drosophila tissue polarity gene frizzled is expressed in migrating myofibroblasts in the infarcted rat heart, Nat Med. 3 (1997) 541–4. [DOI] [PubMed] [Google Scholar]
- [15].Kong P, Shinde AV, Su Y, Russo I, Chen B, Saxena A, et al. , Opposing Actions of Fibroblast and Cardiomyocyte Smad3 Signaling in the Infarcted Myocardium, Circulation. 137 (2018) 707–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaur H, Takefuji M, Ngai CY, Carvalho J, Bayer J, Wietelmann A, et al. , Targeted Ablation of Periostin-Expressing Activated Fibroblasts Prevents Adverse Cardiac Remodeling in Mice, Circ Res. 118 (2016) 1906–17. [DOI] [PubMed] [Google Scholar]
- [17].Shinde AV, Frangogiannis NG, Mechanisms of Fibroblast Activation in the Remodeling Myocardium, Curr Pathobiol Rep. 5 (2017) 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Biernacka A, Dobaczewski M, Frangogiannis NG, TGF-beta signaling in fibrosis, Growth Factors. 29 (2011) 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. , Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction, Circ Res. 107 (2010) 418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Molkentin JD, Bugg D, Ghearing N, Dorn LE, Kim P, Sargent MA, et al. , Fibroblast-Specific Genetic Manipulation of p38 Mitogen-Activated Protein Kinase In Vivo Reveals Its Central Regulatory Role in Fibrosis, Circulation. 136 (2017) 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brown KA, Pietenpol JA, Moses HL, A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling, J Cell Biochem. 101 (2007) 9–33. [DOI] [PubMed] [Google Scholar]
- [22].Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, et al. , Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer, Dev Biol. 307 (2007) 340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, et al. , Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload, J Clin Invest. 120 (2010) 254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Conway SJ, Molkentin JD, Periostin as a heterofunctional regulator of cardiac development and disease, Curr Genomics. 9 (2008) 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG, Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis, Am J Physiol Heart Circ Physiol. 305 (2013) H1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, et al. , Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling, Circ Res. 101 (2007) 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM, Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo, Mol Cell Biol. 28 (2008) 7001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Russo I, Cavalera M, Huang S, Su Y, Hanna A, Chen B, et al. , Protective Effects of Activated Myofibroblasts in the Pressure-Overloaded Myocardium Are Mediated Through Smad-Dependent Activation of a Matrix-Preserving Program, Circ Res. 124 (2019) 1214–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG, CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells, Am J Pathol. 176 (2010) 2177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, et al. , Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling, Circulation. 116 (2007) 2127–38. [DOI] [PubMed] [Google Scholar]
- [31].Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, et al. , Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction, Circ Res. 105 (2009) 973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shinde AV, Humeres C, Frangogiannis NG, The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling, Biochim Biophys Acta Mol Basis Dis. 1863 (2017) 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, et al. , Smad3 Signaling Promotes Fibrosis While Preserving Cardiac and Aortic Geometry in Obese Diabetic Mice, Circ Heart Fail. 8 (2015) 788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sanders LN, Schoenhard JA, Saleh MA, Mukherjee A, Ryzhov S, McMaster WG Jr., et al. , BMP Antagonist Gremlin 2 Limits Inflammation After Myocardial Infarction, Circ Res. 119 (2016) 434–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Devenport D, The cell biology of planar cell polarity, J Cell Biol. 207 (2014) 171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ, Collagen remodeling after myocardial infarction in the rat heart, Am J Pathol. 147 (1995) 325–38. [PMC free article] [PubMed] [Google Scholar]
- [37].Olsen MB, Hildrestrand GA, Scheffler K, Vinge LE, Alfsnes K, Palibrk V, et al. , NEIL3-Dependent Regulation of Cardiac Fibroblast Proliferation Prevents Myocardial Rupture, Cell Rep. 18 (2017) 82–92. [DOI] [PubMed] [Google Scholar]
- [38].Maruyama S, Nakamura K, Papanicolaou KN, Sano S, Shimizu I, Asaumi Y, et al. , Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from rupture, EMBO Mol Med. 8 (2016) 949–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Micheletti R, Plaisance I, Abraham BJ, Sarre A, Ting CC, Alexanian M, et al. , The long noncoding RNA Wisper controls cardiac fibrosis and remodeling, Sci Transl Med. 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Frangogiannis NG, The role of transforming growth factor (TGF)-beta in the infarcted myocardium, J Thorac Dis. 9 (2017) S52–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, et al. , Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis, J Clin Invest. 127 (2017) 3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moustakas A, Souchelnytskyi S, Heldin CH, Smad regulation in TGF-beta signal transduction, J Cell Sci. 114 (2001) 4359–69. [DOI] [PubMed] [Google Scholar]
- [43].Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, et al. , Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction, Am J Pathol. 164 (2004) 665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu S, Xu SW, Blumbach K, Eastwood M, Denton CP, Eckes B, et al. , Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo, J Cell Sci. 123 (2010) 3674–82. [DOI] [PubMed] [Google Scholar]
- [45].Goldsmith EC, Bradshaw AD, Zile MR, Spinale FG, Myocardial fibroblast-matrix interactions and potential therapeutic targets, J Mol Cell Cardiol. 70 (2014) 92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sarrazy V, Koehler A, Chow ML, Zimina E, Li CX, Kato H, et al. , Integrins alphavbeta5 and alphavbeta3 promote latent TGF-beta1 activation by human cardiac fibroblast contraction, Cardiovasc Res. 102 (2014) 407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gimond C, van Der Flier A, van Delft S, Brakebusch C, Kuikman I, Collard JG, et al. , Induction of cell scattering by expression of beta1 integrins in beta1-deficient epithelial cells requires activation of members of the rho family of GTPases and downregulation of cadherin and catenin function, J Cell Biol. 147 (1999) 1325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bradley EW, Drissi MH, Wnt5b regulates mesenchymal cell aggregation and chondrocyte differentiation through the planar cell polarity pathway, J Cell Physiol. 226 (2011) 1683–93. [DOI] [PubMed] [Google Scholar]
- [49].Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ, Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract, Circ Res. 96 (2005) 292–9. [DOI] [PubMed] [Google Scholar]
- [50].Taylor J, Abramova N, Charlton J, Adler PN, Van Gogh: A new Drosophila tissue polarity gene, Genetics. 150 (1998) 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ulmer B, Hagenlocher C, Schmalholz S, Kurz S, Schweickert A, Kohl A, et al. , Calponin 2 acts as an effector of noncanonical Wnt-mediated cell polarization during neural crest cell migration, Cell Rep. 3 (2013) 615–21. [DOI] [PubMed] [Google Scholar]
- [52].Liu C, Gaca MD, Swenson ES, Vellucci VF, Reiss M, Wells RG, Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent, J Biol Chem. 278 (2003) 11721–8. [DOI] [PubMed] [Google Scholar]
- [53].Yang YC, Piek E, Zavadil J, Liang D, Xie D, Heyer J, et al. , Hierarchical model of gene regulation by transforming growth factor beta, Proc Natl Acad Sci U S A. 100 (2003) 10269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CL, Weinstein M, et al. , Functional characterization of transforming growth factor beta signaling in Smad2-and Smad3-deficient fibroblasts, J Biol Chem. 276 (2001) 19945–53. [DOI] [PubMed] [Google Scholar]
- [55].Lee HJ, Lee JK, Miyake S, Kim SJ, A novel E1A-like inhibitor of differentiation (EID) family member, EID-2, suppresses transforming growth factor (TGF)-beta signaling by blocking TGF-beta-induced formation of Smad3-Smad4 complexes, J Biol Chem. 279 (2004) 2666–72. [DOI] [PubMed] [Google Scholar]
- [56].Labbe E, Silvestri C, Hoodless PA, Wrana JL, Attisano L, Smad2 and Smad3 positively and negatively regulate TGF beta-dependent transcription through the forkhead DNA-binding protein FAST2, Mol Cell. 2 (1998) 109–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.