Abstract
Objective:
It is unclear if a low or high-volume intravenous (IV) fluid resuscitation strategy is better for patients with severe sepsis and septic shock.
Design:
Prospective randomized controlled trial.
Setting:
Two adult acute care hospitals within a single academic system.
Patients:
Patients with severe sepsis and septic shock admitted from the emergency department to the intensive care unit (ICU) from November 2016 to February 2018.
Interventions:
Patients were randomly assigned to a restrictive IV fluid resuscitation strategy (≤ 60ml/kg of IV fluid) or usual care for the first 72 hours of care.
Measurements and Main Results:
We enrolled 109 patients, of whom 55 were assigned to the restrictive resuscitation group and 54 to the usual care group. The restrictive group received significantly less resuscitative IV fluid than the usual care group (47.1 vs. 61.1 ml/kg; p=0.01) over 72 hours. By 30 days there were 12 deaths (21.8%) in the restrictive group and 12 deaths (22.2%) in the usual care group (OR 1.02, 95% CI: 0.41 to 2.53). There were no differences between groups in the rate of new organ failure, hospital or ICU length of stay, or serious adverse events.
Conclusion:
This pilot study demonstrates that a restrictive resuscitation strategy can successfully reduce the amount of IV fluid administered to patients with severe sepsis and septic shock compared to usual care. While limited by the sample size, we observed no increase in mortality, organ failure, or adverse events. These findings further support that a restrictive IV fluid strategy should be explored in a larger multi-center trial.
Keywords: Septic shock, intravenous fluid, resuscitation, restrictive fluid strategy
Introduction
In 2001, the success of early goal directed therapy (EGDT) ushered in the modern era of high-volume intravenous (IV) fluid resuscitation for patients with severe sepsis and septic shock [1]. EGDT administered an average of more than 160 ml/kg of IV fluid to patients during the first 72 hours of care. Over a decade later, the subsequent EGDT validation trials administered a more moderate amount of IV fluid (98–130 ml/kg) to septic patients yet observed reduced mortality rates (Supplementary Table 1) [2–4]. Research indicates that administering IV fluid to increase stroke volume and organ perfusion concurrently damages the vascular integrity [5] leading to organ edema and dysfunction [6–9]. With a growing concern that increased amounts of IV fluid may be harmful, the resuscitative community is re-examining the risks and benefits of high-volume IV fluid resuscitation for patients with sepsis [10–12].
Following an initial resuscitation bolus of IV fluid (30 ml/kg), current evidence does not provide clear guidance on IV fluid resuscitation strategies for severe sepsis and septic shock. The Rivers, ProCESS, ARISE and ProMISe studies [1–4] evaluated IV fluid as a component of a larger sepsis protocol, which limits any inferences solely based on the IV fluid strategies used within these trials. Observational studies using national or trial databases have associated high-volume IV fluid resuscitation with increased mortality [13–17]. However, these studies likely are affected by unmeasured confounders and confounding by indication (i.e., clinicians generally administer more IV fluid to sicker patients). A 2017 randomized trial in Zambia found that higher volume IV fluid administration increased mortality among septic patients, but its generalizability is limited by the high prevalence of HIV/AIDS among study participants and a resource-limited study setting that lacked intensive care units (ICUs) [18].
Given the continued clinical uncertainty surrounding the optimum IV fluid resuscitation strategy in sepsis, we designed the Restrictive Intravenous Fluid Trial in Severe Sepsis and Septic Shock (RIFTS) pilot study. The primary objective of RIFTS was to assess the feasibility and initial efficacy of a restrictive resuscitation strategy that significantly limits the amount of IV fluid administered to septic patients (≤60 ml/kg) over the first 72 hours of emergency department and ICU care.
Materials and Methods
Study Oversight and Setting
We conducted the Restrictive Intravenous Fluid Trial in Severe Sepsis and Septic Shock (RIFTS) in the emergency department and the medical ICUs of two hospitals in the United States. The institutional review board of the Lifespan hospitals approved the study and the protocol was registered at clinicaltrial.gov (NCT03137446). Informed consent was obtained from the patient or their surrogate medical decision maker prior to study enrollment. Patients were then randomly assigned to one of two study groups in a 1:1 ratio by a computer-generated random number sequence in blocks of 20. Stratification was not performed. Group allocation was concealed until after randomization. Study investigators remained blinded to the results until the conclusion of the study.
Patients
We recruited patients admitted from the emergency department to the ICU who were suspected by the treating physicians of having severe sepsis or septic shock, per the Sepsis 2 International Consensus definitions [19]. Since approximately 12% of patients with sepsis do not meet systemic inflammatory response syndrome (SIRS) criteria [20], we allowed patients with <2 SIRS criteria to be enrolled if the treating attending physician believed that the primary cause of their critical illness was due to sepsis. Following 1000 ml of IV fluid, patients were required to have either: refractory hypotension (mean arterial pressure (MAP) <65 mmHg), or a lactic acid ≥ 4 mmol per liter. Patients were not eligible for enrollment if they had a primary admitting diagnosis other than severe sepsis or septic shock, an active fluid wasting condition, a diagnosis where the established treatment is high-volume IV fluid resuscitation, had a requirement for immediate surgery or extracorporeal membrane oxygenation; were <18 years old; pregnant, incarcerated, or had received >60 ml/kg of IV fluid before randomization. (See supplementary appendix for details).
Study Interventions
We randomly assigned participants to a restrictive IV fluid strategy or to usual care for their first 72 hours of treatment, which began when their emergency department triage vital signs were collected. Participants randomized to the restrictive study group were permitted to receive up to 60 ml/kg of resuscitative IV fluids during the 72-hour study period. The usual care group received resuscitative IV fluid without any specified or suggested limits; as per the clinical decisions of the treatment team. Resuscitative IV fluid included all IV crystalloid boluses (normal saline and ringers lactate) and maintenance IV fluid infusions (normal saline, ringers lactate, and sodium bicarbonate). PlasmaLyte and other balanced salt solutions were not available and therefore were not administered as resuscitative fluids. In both intervention groups a target MAP of ≥ 65 mmHg was obtained by IV fluid and vasopressor administration. The type of vasopressors used and the timing of vasopressor initiation were not restricted in either group or specified by protocol. IV fluid administered with medications (vasopressors, antibiotics, electrolyte replacement, and other drugs), termed non-resuscitative IV fluid, was not restricted. Albumin (25 grams/100 ml), enteral nutrition, and blood products were not considered resuscitative fluid and therefore not restricted. Participants in the restrictive group weighing over 100 kg received a maximum of 6000 ml (60 ml/kg for a 100 kg person) of resuscitative fluid over the 72-hour study period. IV fluid received prior to randomization counted towards the participant’s IV fluid total.
The study team monitored participants and communicated IV fluid caps to the treating nurses and physicians verbally and through posted IV fluid logs within participant hospital rooms. The study team reviewed the IV fluid cap with the treatment team at least every 12 hours, at the start of the day or evening ICU physician shift. The study team did not prompt the administration of IV fluids in either study group. Participant crossover from the restrictive IV fluid group to the usual care group was permitted if the attending critical care physician of record believed that the clinical circumstances demanded further IV fluid resuscitation. Study participants were permitted to withdraw from the study at any time.
Outcomes
The primary outcome was 30-day all-cause mortality. Secondary outcomes were: 60-day all-cause mortality; ICU length of stay; hospital length of stay; the development of new organ failure (cardiovascular, pulmonary, and renal); vasopressor free days; vasopressor hours; ventilator free days; mechanical ventilation hours; electrolyte abnormalities, and adverse events (myocardial infarction, acute kidney injury defined as a doubling in the triage creatinine, repeat intubations, disseminated intravascular coagulation, and limb ischemia).
Statistical Analysis
Differences between the two groups at baseline were determined using Fisher’s exact test for categorical variables with only two categories, chi-square tests for categorical variables with more than two categories, t-tests for normally distributed continuous variables and Wilcoxon’s rank-sum test for non-normally distributed continuous variables. Differences in fluid amounts were examined using means/standard deviations and medians/interquartile ranges (IQRs). In a secondary analysis resuscitative fluid was adjusted as a fraction of the patient’s total blood volume; TBV= (70/√(body mass index/22)) [21]. Fisher’s exact test was used to assess the primary outcome of 30-day mortality and the secondary outcomes of 60-day mortality, vasopressor use for shock, new mechanical ventilation, new hemodialysis and adverse events. Wilcoxon’s rank-sum test was used to compare the distribution of ICU and hospital length of stay between the two treatment groups. Differences in 30-day mortality were compared using Kaplan-Meier curves, log-rank testing, and logistic regression. Odds ratios with corresponding 95% confidence intervals (CIs) were estimated. Sensitivity analyses using logistic regression for the odds of mortality controlling for chronic kidney disease and amount of non-resuscitative fluids also were performed. All analyses were performed initially using an intention-to-treat approach and afterwards using a per-protocol approach. An alpha level of 0.05 was used for all analyses.
Results
Participants
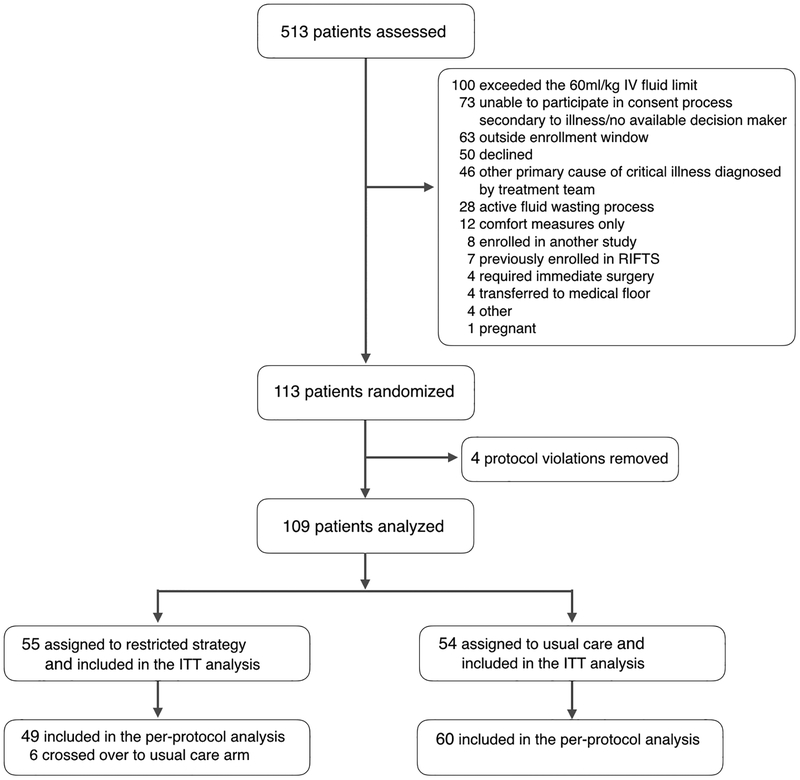
Between November 2016 and February 2018, we enrolled and completed follow up for 113 participants (Figure 1). Three participants (two restrictive and one usual care) who received more than 60 ml/kg of IV fluid before randomization, and one patient in the usual care group who did not meet enrollment criteria, were identified after randomization and subsequently removed from the final analysis. This produced a study cohort of 109 participants: 55 in the restrictive group and 54 in the usual care group. Zero patients were lost to follow-up. Participant characteristics were similar for the two groups, except for a greater proportion of chronic kidney disease in the restrictive group (Table 1 and Supplementary Table 2).
Figure #1.
Enrollment, randomization, and follow up of trial participants. Other reasons for exclusion include: no available translator, intravenous fluids not documented, patient weight not documented, and left against medical advise.
Table #1.
Characteristics of the patients at baseline
| Characteristic | Restrictive fluid group (n=55) |
Usual care group (n=54) |
|---|---|---|
| Age – yr, median (IQR) | 71 (60–82) | 73.5 (54–81) |
| Male sex – n (%) | 24 (43.6) | 26 (48.2) |
| Weight – kg, median (IQR) | 84.3 (75.4–103.5) | 78.3 (69.9–92.3) |
| BMI – kg/m2, median (IQR) | 30.7 (26.8–35.6) | 28.7 (24.3–33.0) |
| Long term care facility resident – n (%) | 10 (18.2) | 9 (16.7)) |
| Physiologic variables | ||
| APACHE II score – meana | 35.4 ±6.9 | 35.7 ±7.4 |
| SOFA score – median (IQR) | 8 (7–11) | 8.5 (7–12) |
| Mean arterial pressure – mmHg, median (IQR) | 51.7 (46.7–55.7) | 53.5 (46.3–58.7) |
| Serum lactate – mmol/liter, median (IQR) | 2.0 (1.4–3.7) | 3.0 (1.5–5.3) |
| Entry criteria – n (%) | ||
| Refractory hypotension | 52 (93.4) | 47 (87.0) |
| Lactic >4mmol/Lb | 2 (3.6) | 7 (13.0) |
| Time from triage to randomization – hours, median (IQR) | 8.8 (4.3–20.8) | 9.1 (5.5–14.5) |
| Chronic medical condition – n (%) | ||
| Diabetes mellitus | 23 (41.8) | 18 (33.3) |
| Chronic kidney diseasec | 20 (36.4) | 6 (11.1) |
| Baseline dialysis | 7 (12.7) | 1 (1.9) |
| Congestive heart failure | 18 (32.7) | 13 (24.1) |
| Chronic respiratory failure | 12 (21.8) | 15 (27.8) |
| Baseline mechanical ventilation | 2 (3.6) | 2 (3.7) |
| Immune modifying medication | 11 (20.0) | 7 (13.0) |
| Chronic liver disease | 5 (9.1) | 8 (14.8) |
| Lymphoma, leukemia, multiple myeloma | 5 (9.1) | 4 (7.4) |
| Metastatic Cancer | 2 (3.6) | 5 (9.3) |
| Organ transplant | 4 (7.3) | 0 (0) |
| Site of infection – n (%) | ||
| Pulmonary | 13 (23.6) | 17 (31.5) |
| Urinary tract | 15 (27.3) | 14 (25.9) |
| Abdominal | 7 (12.7) | 4 (7.4) |
| Skin/soft tissue | 8 (14.6) | 2 (3.7) |
| Endocarditis | 2 (3.6) | 1 (1.9) |
| Indwelling catheter or device | 1 (1.8) | 1 (1.9) |
| Multiple sites | 6 (10.9) | 7 (13.0) |
| Unknown | 3 (5.5) | 8 (14.8) |
± indicates standard deviation
One patient has missing data
Patient baseline characteristics are equally balanced except for chronic kidney disease
IQR= interquartile range
IV Fluid Resuscitation
Adherence to the study protocol was high (94.5%): six participants randomized to the restrictive group were crossed over to the usual care group. The reasons for crossover are presented in Supplementary Table 3. In the primary intention-to-treat analysis, participants in the restrictive fluid group received 14.0 ml/kg (95% CI: 3.5 to 24.5) less resuscitative IV fluid, as compared to usual care participants (47.1 vs. 61.1 ml/kg, p=0.01) over the 72-hour study period (Table 2). The 14.0 ml/kg difference between groups represents an 823 ml volume difference (Supplementary Table 4). Median IV fluid values are presented in Supplementary Table 5. The restrictive group also received a lower fraction of IV fluid per total blood volume and less non-resuscitative IV fluid than the usual care group. In the per-protocol analysis, the difference in resuscitative IV fluid administered between groups was larger: 23.3 ml/kg (41.2 vs. 64.5 ml/kg; p=<0.0001), or 1384 ml (Supplementary Tables 6 and 7). There were no differences between groups in adjunct resuscitative measures administered (albumin, packed red blood cell transfusion, or stress dose steroids) (Table 2).
Table #2.
Intravenous fluid (ml/kg) and resuscitative measures delivered during the 72-hour study period, study outcomes, and adverse events; intention-to-treat analysis
| Restrictive fluid group (n=55) |
Usual care group (n=54) |
P value | |
|---|---|---|---|
| Intervention | |||
| Resuscitative IV fluid: ml/kga | |||
| Prior to randomization | 34.4 ± 13.2 | 36.2 ±14.3 | 0.49 |
| Randomization to 24 hoursb | 7.8 ± 13.3 | 16.6 ±23.2 | 0.02 |
| Hours 24 to 48 | 3.6 ± 11.4 | 6.8 ±11.2 | 0.15 |
| Hours 48 to 72 | 1.3 ± 4.4 | 1.5 ± 5.1 | 0.82 |
| Total | 47.1 ± 22.3 | 61.1 ± 32.0 | 0.01 |
| Non-resuscitative IV fluid and fluid totals | |||
| Non-resuscitative IV fluid, ml/kgc | 23.7 ± 17.5 | 37.6 ± 35.4 | 0.01 |
| Total all forms IV fluid, ml/kg | 70.8 ± 31.8 | 98.8 ± 54.5 | 0.002 |
| Resuscitative IV fluid as a fraction of total blood volumed | 0.79 ± 0.34 | 1.00 ± 0.49 | 0.01 |
| Adjunct Interventions: n (%) | |||
| Albumine | 14 (25.5) | 11 (20.4) | 0.65 |
| Blood transfusion | 10 (18.2) | 8 (14.8) | 0.80 |
| Stress dose steroidsf | 20 (36.0) | 20 (37.0) | >0.99 |
| Outcomes | |||
| Death: no./total (%) | |||
| 30-day mortality: primary outcome | 12/55 (21.8) | 12/54 (22.2) | >0.99 |
| 60-day mortality | 15/55 (27.3) | 15/54 (27.8) | >0.99 |
| New organ failure: n (%) | |||
| Cardiovascular – vasopressors for shock | 47/55 (85.4) | 43/54 (79.6) | 0.46 |
| Respiratory – new mechanical ventilationg | 15/53(28.3) | 17/52 (32.7) | 0.68 |
| Renal – new hemodialysish | 1/48 (2.1) | 2/53 (3.8) | >0.99 |
| Duration of organ support: median (IQR) | |||
| Vasopressor free daysi | 28 (26 to 29) | 28 (7 to 28) | 0.12 |
| Vasopressor hoursi | 17.0 (8.8 to 30.3) | 19.8 (9.8 to 57.8) | 0.33 |
| Ventilator free daysj | 26 (0 to 28) | 19 (1 to 28) | 0.91 |
| Mechanical ventilation hoursj | 16.8 (7.0 to 26.5) | 37.8 (22.0 to 126.5) | 0.02 |
| Use of hospital resources: median (IQR) | |||
| ICU length of stay hours | 53.5 (28.8 to 92.0) | 69.0 (26.3 to 105.8) | 0.61 |
| Hospital length of stay hours | 189.0 (95.3 to 294.5) | 179.3 (97.0 to 280.8) | 0.82 |
| Electrolyte measurements: mean, SDk | |||
| Change in chloride, mmol/L | 4.1 ± 4.3 | 5.5 ± 4.9 | 0.15 |
| Change in bicarbonate, mmol/L | 0.55 ± 3.9 | 1.2 ± 5.0 | 0.45 |
| Serious adverse events: | |||
| Myocardial infarction n (%) | 3 (5.5) | 0 (0) | 0.24 |
| Acute kidney injuryl | 1 (1.8) | 1 (1.9) | >0.99 |
| Required re-intubation | 1 (1.8) | 3 (5.6) | 0.36 |
| Disseminated intravascular coagulation | 0 (0) | 0 (0) | |
| Limb ischemia | 0 (0) | 0 (0) |
± indicates standard deviation
Six participants (5 restrictive and 1 standard) were randomized after 24 hours, none received additional IV fluid during that time
Non-resuscitative fluid includes all IV fluid administered with medications in volumes of ≥100ml
(Total resuscitative IV fluid)/(total blood volume); total blood volume = (70/√(BMI/22))
Only 25% albumin available at study sites
Hydrocortisone 100mg three times daily or 50mg four times daily
Four participants with mechanical ventilation at baseline excluded
Eight participants with dialysis at baseline excluded
Includes the 90 participants who were on vasopressors, out of 30 days
Includes the 32 participants with new mechanical ventilation, out of 30 days
Includes only the participants alive at 72 hours, ± indicates standard deviation
Defined as a doubling of creatinine from the first recorded value during the study period
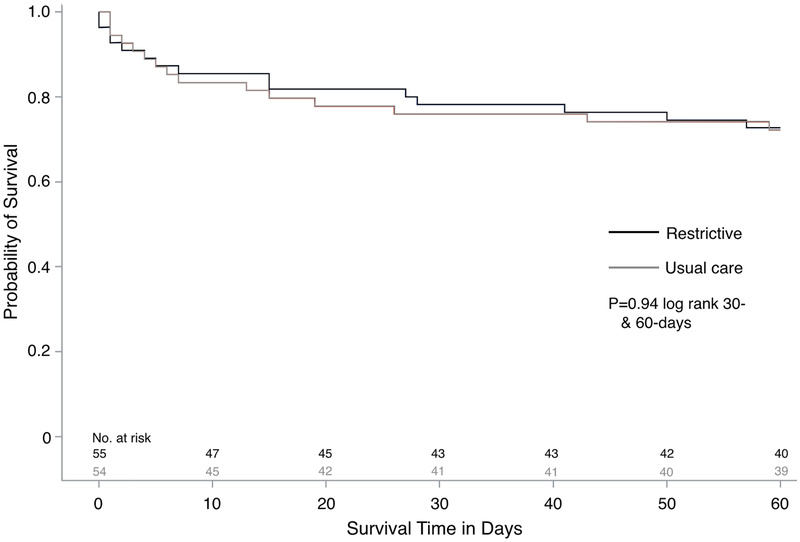
Primary Outcome
By day 30, 12 participants (21.8%) in the restrictive group and 12 participants (22.2%) in the usual care had died: OR=1.02 (95% CI: 0.41 to 2.53), (Figure 2, Table 2). A per-protocol analysis yielded similar results (Supplementary Table 8). Adjusting for the baseline imbalances in chronic kidney disease and the amount of non-resuscitative IV fluid administered yielded no changes in observed 30-day mortality risk between groups (Supplementary Table 9). There were no deaths among the four protocol violations removed prior to analysis.
Figure #2:
Kaplan Meier survival estimate in the intention-to-treat population during the 60-day trial period.
Secondary Outcomes and Adverse Events
There were no differences in 60-day mortality, ICU or hospital lengths of stay, rates of new organ failure, or changes in electrolytes between study groups (Table 2, Supplementary Table 7). Fifteen participants (28.3%) in the restrictive group and 17 participants (31.5%) in the usual care group required new mechanical ventilation (p=0.67). While we did not observe a significant difference between groups in the number of ventilator free days among the 32 participants with respiratory failure, the restrictive group spent 22 fewer hours mechanically ventilated compared to usual care (p=0.02) (Table 2). The number of participants who required vasopressors, the total number of vasopressor hours, and vasopressor doses were similar between the study groups (Table 2 and Supplementary Table 10). There were no differences in serious adverse events between study groups.
Discussion
We conducted a pilot randomized trial to examine the feasibility and initial effects of limiting the amount of resuscitative IV fluid administered over the first 72 hours to patients with severe sepsis and septic shock. The restrictive strategy significantly reduced the amount of IV fluid administered to critically ill septic patients compared to usual care. Although our study was not powered to detect differences in patient centered primary or secondary outcomes, and a larger trial is needed to determine if our findings hold, we observed no increased rates of death, organ dysfunction, or adverse events with a restrictive strategy. Our results suggest that following effective initial resuscitation (30ml/kg), a strategy of fluid minimization, using less IV fluid than previously given, may be appropriate for patients with severe sepsis and septic shock. The recently initiated National Heart, Lung and Blood Institute “Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) trial” will be powered to answer questions of mortality and may shed further light on lung injury outcomes [22]. For CLOVERS to make the maximal clinical impact their design should ensure a larger fluid difference between study arms than we obtained in RIFTS.
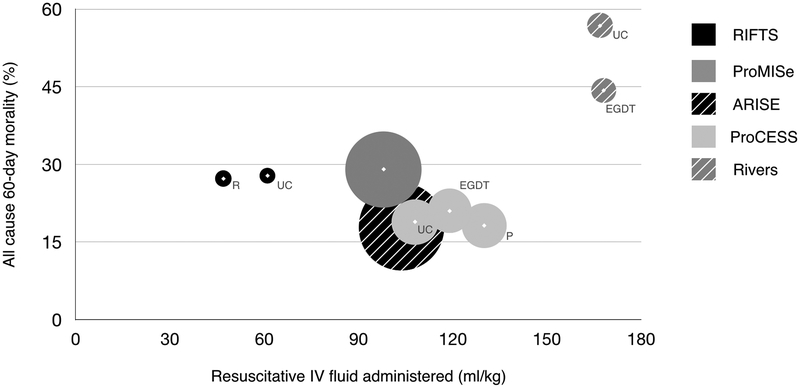
There is growing concern in emergency and critical care medicine that high-volume IV fluid resuscitation is harmful to patients [23]. Clinicians are favoring resuscitation strategies that initiate early vasopressors and utilize lower volumes of IV fluid to achieve blood pressure goals, but there is limited evidence to support this practice [13–18]. In our trial prior to randomization, participants received 34.4 ml/kg (restrictive) and 36.2 ml/kg (usual care) of IV fluid, an amount consistent with the 2016 Surviving Sepsis Campaign guidelines [24], the CMS Sep-1 Core Measure [25], and recent sepsis trials [2–4]. Yet, following randomization, both groups received a small volume of additional resuscitative fluid over the remainder of the trial (12.7 vs. 24.9 ml/kg). In fact, the total amount of resuscitative IV given in either group, 47.1 ml/kg (restrictive) and 61.1 ml/kg (usual care), is two- to three-fold less than what was administered in the Rivers (168 ml/kg), ProCESS (108–130 ml/kg), ProMISe (98 ml/kg), or ARISE (108–109 ml/kg) trials (Supplementary Table 1) and in comparison, both groups could be considered restrictive. An internal review of the medical records of 374 patients admitted to our medical ICUs in the 18 months immediately preceding RIFTS, showed that patients with severe sepsis and septic shock received an average of 75.5 ml/kg of IV fluid over 72 hours. This suggests the Hawthorne effect influenced physician behavior and reduced the amount of IV fluid administered to RIFTS participants. In effect this created two study arms that tested fluid minimization, one more limited than the other, and notably neither group produced high rates of organ dysfunction or serious adverse events. This, coupled with our observed mortality rates that are similar to contemporary sepsis outcomes [2–4], suggests that reducing the amount of IV fluid used to resuscitate septic patients to amount far below what was used in previous research may be safe (Figure #3), and should inform future trial design.
Figure #3:
Amount of resuscitative IV fluid administered in select sepsis trials and 60-day mortality.
The area of the circle is proportional to trial sample size. Resuscitative fluid volume in ml/kg for Rivers, ProMISe and ProCESS are estimated based upon an average 80kg participant. ProMISe and ARISE study groups combined into single circle secondary to significant overlap in IV fluid administered and mortality between study groups. EGTD = early goal directed therapy, P = protocol, R = restricted, UC = usual care.
Our findings align with the 2016 CLASSIC trial that suggests a restrictive IV fluid resuscitation strategy is safe for septic ICU patients [26]. The CLASSIC restrictive study arm received a total of 8057 ml of fluid (4800 ml of resuscitative IV fluid and 3257 ml of non-resuscitative fluid with medication), in the first 72 hours. In contrast, our study’s restrictive group patients received 23% less combined resuscitative and non-resuscitative fluid (6213 ml; 70.8 ml/kg), suggesting an even lower volume of IV fluids might be safe for a restrictive resuscitation strategy. It may be that following the initial bolus of resuscitative IV fluid, critically ill septic patients require little if any further fluid boluses because they receive enough daily IV fluid with medications to sustain organ perfusion. Notably our restrictive group received significantly less non-resuscitative IV fluid compared to the usual care group (23.7 vs 37.6 ml/kg). This difference might be due to chance or could be a downstream effect of a restrictive strategy. If a restrictive strategy limits organ edema and dysfunction it may decrease the patient’s critical illness severity and thereby reduce the amount of medications (i.e. vasopressors, sedatives, antibiotics) needed to treat them.
We also observed that intubated patients who received a restrictive strategy required fewer hours of mechanical ventilation as compared to those patients in the usual care group (16.8 vs. 33.6 hours; p=0.01). Although this finding is hypothesis generating and might be driven by the small number of intubated patients (n=32), it nevertheless suggests that a restrictive resuscitation strategy may limit lung injury. The concept that the liberal use of IV fluids induces lung injury is supported by observations in ARDS research [27] and warrants further investigation in sepsis research.
Our study has limitations. First, the sample size of our pilot trial makes it underpowered to detect superiority or non-inferiority in mortality and secondary outcomes. With our sample size of 109 patients, assuming a baseline mortality rate of 22%, we estimate that we could have detected an absolute morality difference of ≥19% (α=0.05, power 80%), which indicates that very large samples are needed to detect small differences in mortality or assess non-inferiority. Second, the patients and providers were not blinded to the intervention, which may have allowed for the introduction of bias in fluid administration practices. Future trials could consider implementing a step-wedge approach across randomized enrolling departments to mitigate this effect. Third, the relatively small difference in IV fluid between study arms (14ml/kg or 823ml) may not reach the threshold of clinical significance. However, a recent multi-center trial of balanced salt solutions versus normal saline showed a reduced combined outcome of death and renal dysfunction with a 1000ml difference of IV fluid between study arms, suggesting that limiting even moderate amounts of IV fluid may confer clinical significance [28]. Fourth, our study did not incorporate a formalized measurement of participant volume status or fluid responsiveness. Future efforts may find improved outcomes with strategies that include a patient tailored approach to fluid resuscitation. Finally, the large number of patients with altered mental status and those who had received more than 60 ml/kg of IV fluid were excluded from the study. This may have introduced a selection bias that favored a less-sick study cohort; however a mean participant APACHE score of 35 and the observed 30-day mortality rates argue against this.
Conclusion
A restrictive resuscitation strategy that significantly limited the amount of IV fluid administered to patients with severe sepsis and septic shock, did not appear to increase mortality, organ dysfunction, or adverse events. Our data contribute to the current state of clinical equipoise surrounding the use of IV fluids in sepsis, support a larger multicenter trial addressing this topic, and inform future study design.
Supplementary Material
Acknowledgements:
We are grateful to the ICU nurses, research staff, and physicians as well as the participants and their families. Without your collective generosity this trial would not have been possible.
Funding: This work was supported by the Division of Pulmonary and Critical Care and the Department of Emergency Medicine, Alpert Medical School of Brown University.
Copyright form disclosure: Dr. Merchant’s institution received funding from the National Institutes of Health (NIH). Dr. Amass received support for article research from the NIH. Dr. Jay disclosed he is a Covidien scientific advisory board member. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Conflicts of interests: no authors have any conflicts of interest
References
- 1.Rivers E, Nguyen B, Havstad S, et al. : Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377 [DOI] [PubMed] [Google Scholar]
- 2.Peake SL, Delaney A, Bailey M, et al. : Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506 [DOI] [PubMed] [Google Scholar]
- 3.Yealy DM, Kellum JA, Huang DT, et al. : A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mouncey PR, Osborn TM, Power GS, et al. : Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372:1301–1311 [DOI] [PubMed] [Google Scholar]
- 5.Alphonsus CS, Rodseth RN: The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 2014; 69:777–784 [DOI] [PubMed] [Google Scholar]
- 6.D’Orio V, Wahlen C, Rodriguez LM, et al. : Effects of intravascular volume expansion on lung fluid balance in a canine model of septic shock. Crit Care Med 1987; 115:863–868 [DOI] [PubMed] [Google Scholar]
- 7.Brooks HF, Moss RF, Davies NA, et al. : Caecal ligation and puncture induced sepsis in the rat results in increased brain water content and perimicrovessel oedema. Metab Brain Dis 2014; 29:837–843 [DOI] [PubMed] [Google Scholar]
- 8.Glassford NJ, Eastwood GM, Bellomo R: Physiological changes after fluid bolus therapy in sepsis: a systematic review of contemporary data. Crit Care 2014; 18:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marik PE: Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care 2014; 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marik PE: Early management of severe sepsis: concepts and controversies. Chest 2014; 145:1407–1418 [DOI] [PubMed] [Google Scholar]
- 11.Marik P, Bellomo R: A rational approach to fluid therapy in sepsis. Br J of Anaesth 2016; 116:339–349 [DOI] [PubMed] [Google Scholar]
- 12.Byrne L, Van Haren F: Fluid resuscitation in human sepsis: Time to rewrite history? Ann Intensive Care 2017; 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd JH, Forbes J, Nakada TA, et al. : Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39:259–265 [DOI] [PubMed] [Google Scholar]
- 14.Micek ST, McEvoy C, McKenzie M, et al. : Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care 2013; 17:R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelm DJ, Perrin JT, Cartin-Ceba R, et al. : Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 2015; 43:68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marik PE, Linde-Zwirble WT, Bittner EA, et al. : Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 2017; 43:625–632 [DOI] [PubMed] [Google Scholar]
- 17.Sakr Y, Rubatto Birri PN, et al. : Higher Fluid Balance Increases the Risk of Death From Sepsis: Results From a Large International Audit. Crit Care Med 2017; 45:386–394 [DOI] [PubMed] [Google Scholar]
- 18.Andrews B, Semler MW, Muchemwa L, et al. : Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. JAMA 2017; 318:1233–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC, et al. : 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 20.Kaukonen KM, Bailey M, Pilcher D, et al. : Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015; 372:1629–1638 [DOI] [PubMed] [Google Scholar]
- 21.Lemmens HJ, Bernstein DP, Brodsky JB: Estimating blood volume in obese and morbidly obese patients. Obesity Surgery 2006; 16:773–776 [DOI] [PubMed] [Google Scholar]
- 22.Self WH, Semler MW, Bellomo R, et al. : Liberal Versus Restrictive Intravenous Fluid Therapy for Early Septic Shock: Rationale for a Randomized Trial. Ann Emerg Med 2018; 72:457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuter DA, Chappell D, Perel A: The dark sides of fluid administration in the critically ill patient. Intensive Care Med 2018; 44:1138–1140 [DOI] [PubMed] [Google Scholar]
- 24.Rhodes A, Evans LE, Alhazzani W, et al. : Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2016; 43:304–377 [DOI] [PubMed] [Google Scholar]
- 25.NQF-Endorsed Voluntary Consensus Standards for Hospital Care – Sepsis, SEP-1. Version 5.0a. Available at: https://www.nhfca.org/psf/resources/Updates1/SEP-1%20Measure%20Information%20Form%20(MIF).pdf. Accessed on October 1, 2018.
- 26.Hjortrup PB, Haase N, Bundgaard H, et al. : Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med 2016; 42:1695–1705 [DOI] [PubMed] [Google Scholar]
- 27.Wiedemann HP, Wheeler AP, Bernard GR, et al. : Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 28.Semler MW, Self WH, Wanderer JP, et al. : Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med. 2018; 378(9):829–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.