Abstract
Objective:
To determine the long-term outcomes for prostate adenocarcinoma when escalating radiation dose from 70 Gy to 78 Gy.
Methods:
Between 1993 and 1998, 301 patients with biopsy-proven clinical stage T1b – T3 prostate adenocarcinoma, any prostate-specific antigen (PSA) level, and any Gleason score were randomized to 70 Gy in 35 fractions vs. 78 Gy in 39 fractions photon radiation therapy using a four-field box technique without hormone deprivation therapy. The primary outcome was powered to detect a 15% difference in biochemical and/or clinical failure. Secondary outcomes included survival, prostate cancer mortality, biochemical failure, local failure, nodal failure, distant failure, and secondary malignancy rates.
Results:
With a median follow-up of 14.3 years, the cumulative incidence of 15-year biochemical and/or clinical failure was 18.9% vs. 12.0% in the 70-Gy vs. 78-Gy arm, respectively (sHR 0.61, 95% CI 0.38 – 0.98; Fine-Gray P=0.042). The 15-year cumulative incidence of distant metastasis was 3.4% vs. 1.1%, respectively (sHR 0.33, 95% CI 0.13 – 0.82; Fine-Gray P=0.018). The 15-year cumulative incidence of prostate cancer-specific mortality was 6.2% vs. 3.2%, respectively (sHR 0.52, 95% CI 0.27 – 0.98; Fine-Gray P=0.045). There were no differences in overall survival (HR 1.10, 95% CI 0.84–1.45; Log Rank P=0.469) or other-cause survival (sHR 1.33, 95% CI 0.99 – 1.79; Fine-Gray P=0.061). Salvage therapy was more common in the 70-Gy arm at 38.7% vs. 21.9% in the 78-Gy arm (P=0.002). There was a 2.3% secondary solid malignancy rate (1 bladder, 6 rectal) within the radiation treatment field which was not significantly different between treatment arms.
Conclusion:
Dose escalation by 8 Gy (78 Gy versus 70 Gy) provided a sustained improvement in biochemical and clinical failure which translated into lower salvage rates and improved prostate cancer-specific mortality but not overall survival. Long-term follow-up demonstrated a low incidence of potential solid tumor secondary malignancies.
Keywords: Dose escalation, prostate cancer
Introduction
External beam radiation therapy is a curative treatment option for localized prostate cancer, utilized in roughly a quarter of all treated cases in the United States [1]. Based on quantitative biological modeling studies [2, 3] and supported by clinical observations [4], it is well established that high doses of radiation are necessary for adequate prostate cancer cell killing in order to achieve cure. However, until 3-dimensional conformal radiation therapy (3DCRT) and intensity-modulated radiation therapy (IMRT) techniques could be implemented clinically, dose escalation to the prostate was limited due to concern for toxicity to surrounding organs at risk. While several randomized controlled trials have examined dose-escalation in prostate cancer [5-9], none report median follow up beyond ten years. Moreover, these trials had heterogeneous use of concurrent androgen deprivation therapy which is now the standard of care for many patients. Given the frequently indolent nature of the disease and protracted time to failure, extensive follow-up may provide unique and valuable insight. Here we present longterm follow-up of a prospective, trial involving patients with localized prostate adenocarcinoma treated without hormonal therapy, randomized to standard versus dose-escalated radiation therapy.
Methods and Materials
Protocol eligibility and participants
Between 1993 and 1998, patients at our high volume academic institution were enrolled in an Institutional Review Board approved Phase III randomized trial (CRT 93-001). Informed consent was obtained from each participant. Eligibility criteria included clinical stage T1-T3, N0, M0 prostate cancer based on the 1992 American Joint Commission on Cancer staging system; a documented pre-treatment serum prostate-specific antigen (PSA) measurement ≤30 ng/mL; histopathologic confirmation of diagnosis and Gleason score at our institution; and no previous history of pelvic radiation, radical prostatectomy, or androgen therapy. A bone scan or computed tomography (CT) scan was performed for pre-treatment PSA levels >10 ng/mL or >20 ng/mL, respectively. Stratification at protocol entry was done based on pre-treatment PSA level: PSA ≤10, 10–20, and >20 ng/mL. Randomization was done 1:1 using a permuted block randomization allocation scheme performed by the Data Management Section in the Department of Clinical Radiotherapy. Follow-up schedule was a digital rectal exam (DRE) and PSA every 3 months for 2 years, then 6 months for 3 years, and then annually. In later years, patients were followed through clinical visits or yearly letters to determine status.
The Consolidated Standards of Reporting Trials (CONSORT) diagram demonstrates that out of a total of 305 patients, 301 met the eligibility criteria and were enrolled in the study (Appendix Figure 1). The four non-assessable patients include two who withdrew consent before starting radiotherapy, one who chose surveillance, and one who lacked pathologic confirmation review at our institution. Of the 301 assessable patients, 150 patients were randomized to 70 Gy in 35 fractions and 151 patients were randomized to 78 Gy in 39 fractions. There were four protocol violations with two who received adjuvant androgen blockade and two who were randomized to receive 78 Gy but instead received 70 Gy. We report outcomes on an intent-to-treat basis.
Radiation treatment
All patients were prescribed 2 Gy per day to the isocenter and the clinical target volume (CTV) included the prostate and seminal vesicles. Planning was done based on a pre-treatment pelvic CT scan. Pelvic lymph nodes were not included in the target volume. A conventional four-field box design was used for the initial 46 Gy in both treatment arms. The general anterior-posterior field size was 11 × 11 cm while the lateral fields were 11 × 9 cm with a small block over the anterior bladder and posterior half of the rectum. After 46 Gy, the 70-Gy arm had a field reduction to 9 × 9 cm using a four-field design whereas the 78-Gy arm had a six-field 3DCRT design. Margins from the CTV to the block edge were 1.25 to 1.5cm in the anterior-inferior dimension and 0.75 to 1.0 cm in the posterior-superior dimension. 3DCRT included Beam's Eye View shaping with generous margins around the CTV to account for uncertainty. A CT scan was done during the first week of treatment to confirm appropriate CTV coverage.
Endpoints and statistical analysis
The primary endpoint of the study was biochemical and/or clinical disease failure (freedom from failure [FFF]). Secondary endpoints include overall survival (OS), prostate cancer specific-mortality (PCSM), local failure (LF), nodal failure (NF), and distant metastatic failure (DMF). Assuming direct causality from differences in local tumor control, the protocol was designed with 150 patients per treatment arm to detect a 15% difference in FFF [10]. Interim analyses were performed after 180 and 250 patients were enrolled using actuarial freedom from rising PSA as the main endpoint. A planned biopsy two years after completion of radiotherapy was planned to confirm eradication of disease.
Biochemical failure (BF) was defined by the American Society for Therapeutic Radiology and Oncology (ASTRO) Phoenix criteria of PSA nadir + 2 ng/mL [11] on post-hoc analysis given that this is the modern standard of care. BF at the time of the clinical trial was defined as three or more PSA increases on follow-up visit per the 1997 ASTRO criteria [12] which is not commonly used in practice any longer. On post hoc analysis, we stratified patients into risk groups based on the National Comprehensive Cancer Network (NCCN) guidelines which define low risk (Gleason ≤6 and PSA <10 ng/mL and clinical ≤T2a), high risk (Gleason ≥8 or PSA >20 ng/mL or clinical T3), and intermediate risk (any patient not meeting low risk or high risk criteria) groups. Clinical failure was defined as local, nodal, or distant recurrence before PSA failure or the initiation of salvage hormonal therapy. LF was defined as palpable evidence of disease or positive biopsy because of rising serum PSA; NF defined as positive lymph nodes noted on CT scan; DMF based on CT scan or bone scan; PCSM defined as death at time of progressive metastatic disease or death while actively receiving prostate cancer treatment; other-cause mortality defined as all causes of death minus prostate-cancer specific mortality; and distant metastatic-free survival (DMFS) defined as distant metastases or death as an event. Patients who died from other causes, or those without a documented cause of death and last PSA ≤1.0 ng/mL without metastases, or those on prostate cancer treatment (e.g. androgen deprivation) were classified as dying from other causes. Cause of death was determined by death certificate as recorded in the National Death Index (NDI) database or by the institutional tumor registry that regularly contacts patients regarding disease and vital status.
A competing risk analysis was done using the Fine-Gray test for cumulative incidence of clinical and/or biochemical failure, BF, LF, DMF, other-cause mortality, and PCSM [13]. The competing event for all of these analyses is death without failure. Univariate competing risk regression analysis was used to obtain subhazard ratios for clinical and/or biochemical failure, BF, LF, DMF, PCSM and other-cause mortality. Multivariate competing risk regression analysis was also done. Kaplan-Meier analysis was carried out to determine OS and DMFS; comparisons between groups were done using the log-rank test. Univariate Cox regression analysis was used to obtain hazard ratios for OS and DMFS. Differences in prognostic patient characteristics and stratification criteria between treatment groups were assessed using a Chi-squared or Wilcoxon rank-sum test. A P-value of 0.05 or less was considered to be statistically significant. Statistical tests were based on a two-sided significance level. All statistical analyses were performed using Stata/MP 15.1 (College Station, TX).
Results
As of January 2018, the median follow-up was 14.3 years and median time since last contact among non-deceased patients was 1 month (range 0 – 43 months). Out of the 301 patients, 71% (214) were confirmed to be deceased and 7.3% (22) requested to stop contact and/or were lost to follow-up (>48 months since last contact). Examining the lost to follow-up patients more closely, 8 vs. 14 were in the 70-Gy vs. 78-Gy arm, respectively, which was not significantly different (P=0.682). On subgroup analysis, there were no differences in the lost-to-follow-up group in terms of age, ethnicity, pre-treatment PSA, Gleason score, AJCC tumor staging, or NCCN risk classification. The patient and tumor characteristics are outlined in Table 1. The median age at diagnosis was 69 years. There was no difference between treatment arms in age, ethnicity, pre-treatment PSA, Gleason score, AJCC tumor staging, or NCCN risk stratification. The majority of patients were intermediate (45.8%) or high risk (33.6%) per NCCN risk stratification. One patient in the low-dose and high-dose group received unplanned adjuvant hormone therapy after radiation therapy.
Table 1:
Intention to Treat Patient, Disease, and Treatment Characteristics by Treatment Arm
| 70 Gy Arm | 78 Gy Arm | |
|---|---|---|
| Total number of patients | 150 | 151 |
| Median Age at Diagnosis (range), y | 69 (50-79) | 69 (48-81) |
| Race | ||
| White | 124 (82.7%) | 132 (86.1%) |
| Black | 16 (10.7%) | 12 (7.9%) |
| Hispanic | 7 (4.7%) | 4 (2.7%) |
| Asian | 3 (2%) | 3 (2%) |
| Median Pre-Treatment PSA, (range), ng/mL | 7.50 (1.1 – 32.5) | 7.80 (0.6 – 86.1) |
| Pre-T reatment PSA Grouping, ng/mL | ||
| ≤10 | 98 (65.3%) | 98 (64.9%) |
| 10 - 20 | 42 (28%) | 47 (31.1%) |
| >20 | 10 (6.7%) | 6 (4%) |
| Gleason Total | ||
| <6 | 13 (8.7%) | 15 (9.9%) |
| 6 | 57 (38%) | 59 (39.1%) |
| 7 | 54 (36%) | 50 (33.1%) |
| 8 | 19 (12.7%) | 22 (14.6%) |
| 9 | 5 (3.3%) | 5 (3.3%) |
| 10 | 2 (1.3%) | 0 |
| AJCC Tumor Stage | ||
| T1 | 45 (30%) | 43 (28.5%) |
| T2 | 79 (52.7%) | 74 (49%) |
| T3 | 26 (17.3%) | 34 (22.5%) |
| NCCN Risk Group | ||
| Low | 31 (20.7%) | 31 (20.5%) |
| Intermediate | 71 (47.3%) | 67 (44.4%) |
| High | 48 (32%) | 53 (35.1%) |
| Lost to Follow-up | 8 (5.3%) | 14 (9.3%) |
Prostate-specific antigen (PSA); American Joint Commission on Cancer (AJCC); National Comprehensive Care Network (NCCN); Gray (Gy)
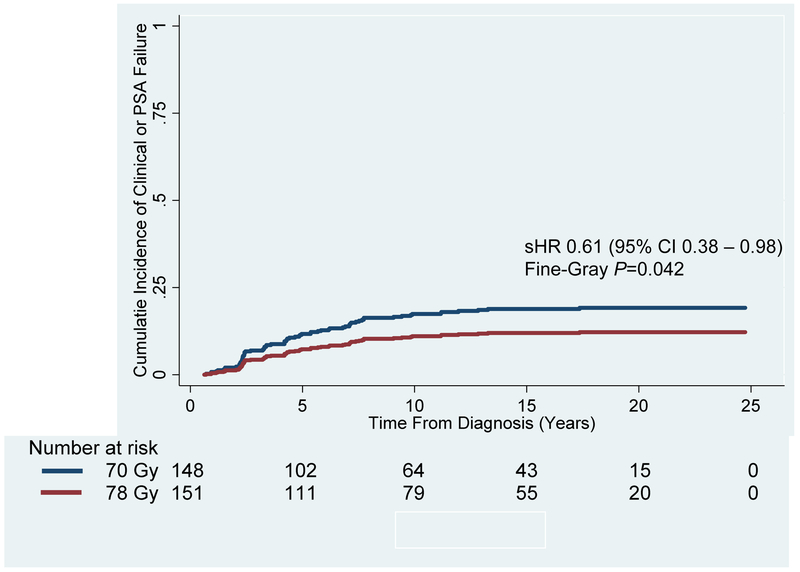
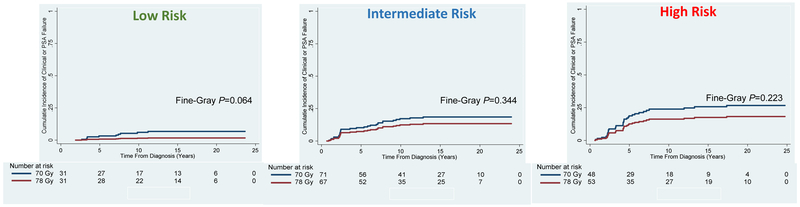
Table 2 summarizes the univariate hazard ratios, P-values, and 15-year survival percentages for the major outcomes in the 70-Gy vs. 78-Gy groups. Figure 1 demonstrates that the primary study endpoint of biochemical and/or clinical failure was significantly better for the 78-Gy arm as compared to the 70-Gy arm (sHR 0.61, 95% CI 0.38 – 0.98; Fine-Gray P=0.042). The 15- and 20-year cumulative incidences were 18.9% and 19.2% for the 70-Gy arm as compared to 12.0% and 12.2% for the 78-Gy arm, respectively. Based on competing risk univariate regression analysis, factors, aside from dose, which were significantly associated with worse biochemical and/or clinical failure included T2 stage (sHR 2.11, 95% CI 1.10 – 4.03; P=0.024) and NCCN low-risk disease (sHR 0.45, 95% CI 0.23 – 0.90; P=0.025). On multivariate analysis, dose (sHR 0.33, 95% CI 0.13 – 0.82; P=0.018), higher Gleason score (sHR 2.14, 95% CI 1.45 – 3.16; P<0.001), T3 stage (sHR 15.34, 95% CI 3.43 – 68.55; P<0.001), pre-treatment PSA (sHR 1.08, 95% CI 1.06 – 1.11; P<0.001), and pre-treatment PSA >10 ng/mL (sHR 4.08, 95% CI 1.76 – 9.49; P=0.001) remained significant (Appendix Table A). Clinical and/or biochemical failure was not significantly different between the 70-Gy and 78-Gy arms (Figure 2) when stratifying by NCCN low risk (Fine-Gray P=0.064), intermediate risk (Fine-Gray P=0.344), and high risk disease (Fine-Gray P=0.223). Appendix Table G, H, and I provide additional information on clinical outcomes when stratifying by treatment arm and NCCN risk grouping.
Table 2:
Cumulative Incidence of Clinical and/or Biochemical Failure, Biochemical Failure, Local Failure, Distant Metastasis Failure, Other-Cause Mortality, Prostate Cancer-Specific Mortality, and Overall Survival Hazard Ratios by Treatment Arm
| Outcome Type | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical and/or Biochemical Failure |
Biochemical Failure | Local Failure | Distant Metastatic Failure |
Other-Cause Mortality | Prostate Cancer- Specific Mortality |
Overall Survival | |||||||||||||||
| 15- Year (%) |
Univariate | 15- Year (%) |
Univariate | 15- Year (%) |
Univariate | 15- Year (%) |
Univariate | 15- Year (%) |
Univariate | 15- Year (%) |
Univariate | 15- Year (%) |
Univariate | ||||||||
| sHR (95 % CI) |
P- value |
sHR (95 % CI) |
P- value |
sHR (95 % CI) |
P- value |
sHR (95% CI) |
P- value |
sHR (95% CI) |
P- value |
sHR (95 % CI) |
P- value |
HR (95 % CI) |
P- value |
||||||||
| 70-Gy Arm | 18.9 | 1 [Ref] | 0.042 | 12.3 | 1 [Ref] | 0.051 | 11.3 | 1 [Ref] | 0.326 | 3.4 | 1 [Ref] | 0.018 | 45.7 | 1 [Ref] | 0.061 | 6.2 | 1 [Ref] | 0.045 | 53.4 | 1 [Ref] | 0.469 |
| 78-Gy arm | 12.0 | 0.61 (0.38-0.98) | 7.1 | 0.56 (0.31-1.00) | 8.4 | 0.72 (0.39-1.37) | 1.1 | 0.33 (0.13-0.82) | 55.6 | 1.33 (0.99-1.79) | 3.2 | 0.52 (0.27-0.98) | 48.9 | 1.10 (0.84-1.45) | |||||||
Subhazard ratio (sHR); Hazard ratio (HR); confidence interval (CI); Gray (Gy); Reference (Ref)
Figure 1: Cumulative Incidence of Biochemical and/or Clinical Failure for the Entire Patient Cohort Treated to 70 Gy vs. 78 Gy.
Biochemical and/or clinical failure at 15 and 20 years was significantly better for patients receiving 78 Gy (Red Line; 12.0% and 12.2%, respectively) compared to 70 Gy (Blue Line; 18.9% and 19.2%, respectively) (Subhazard Ratio [sHR]: 0.61; 95% Confidence Interval [CI]: 0.38 – 0.98; Fine-Gray P=0.042).
Figure 2: Cumulative Incidence of Biochemical and/or Clinical Failure for Low-, Intermediate-, and High-Risk Patients Treated to 70 Gy vs. 78 Gy.
Sub-group analysis demonstrated no difference in the cumulative incidence of biochemical and/or clinical failure in patients with low risk (Fine-Gray P=0.064), intermediate risk (Fine-Gray P=0.344) and high risk (Fine-Gray P=0.223) disease per the National Comprehensive Cancer Network risk stratification criteria.
The cumulative incidence of BF at both 15 and 20 years (Appendix Figure 2) was 12.3% and 12.3% vs. 7.1% and 7.1% in the 70-Gy vs. 78-Gy arm, respectively (sHR 0.56, 95% CI 0.31 – 1.00; Fine-Gray P=0.051). Out of the total of 69 biochemical failures, 29% (20/69) were biopsy-proven. On univariate competing risk regression analysis, dose was the only factor associated with BF; on multivariate analysis, pre-treatment PSA was the only significant factor (Appendix Table B). At 15 years without adjuvant or neoadjuvant androgen deprivation, 6.1% vs. 5.4% of patients had a NF in the 70-Gy vs. 78-Gy arm, respectively.
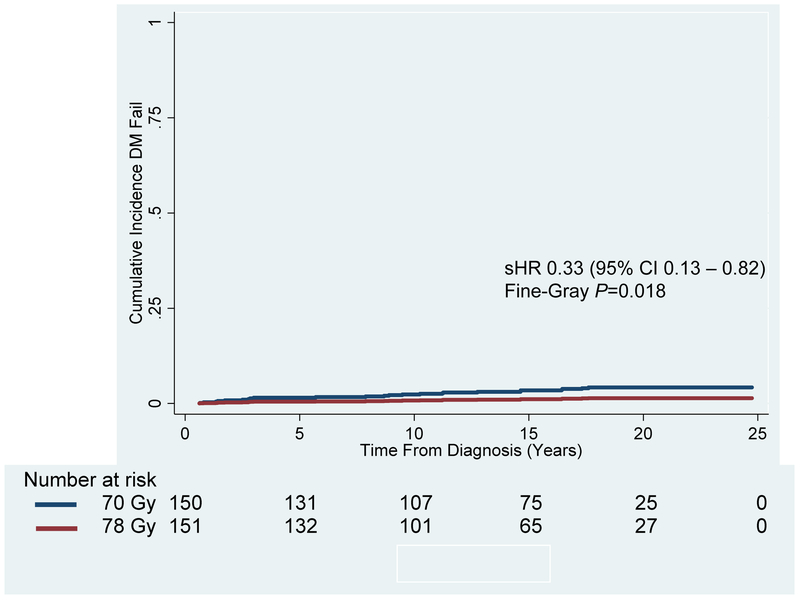
Figure 3 demonstrates that the cumulative incidence of DMF at 15 and 20 years was significantly higher for the 70-Gy arm at 3.4% and 4.2% vs. 1.1% and 1.4% in the 78-Gy arm, respectively (sHR 0.33, 95% CI 0.13-0.82; Fine-Gray P=0.018). Out of the patients who had a distant metastatic failure, 75% (18/24) were high-risk, 17% (4/24) were intermediate-risk, and 8% (2/24) were low risk. On univariate competing risk regression analysis, total Gleason score, dose, T3 stage, NCCN risk grouping, pre-treatment PSA, and pre-treatment PSA >10 ng/mL were factors associated with DMF. On multivariate analysis, total Gleason score and pretreatment PSA >10 ng/mL remained (Appendix Table C). Patients in the NCCN high risk group who received 70 Gy vs. 78 Gy were more likely to experience a DMF at 15-years, 8% vs. 2.6%, respectively (sHR 0.32, 95% CI 0.11 – 0.89; Fine-Gray P=0.03).
Figure 3: Cumulative Incidence of Distant Metastatic Failure for the Entire Patient Cohort Treated to 70 Gy vs. 78 Gy.
Distant metastatic failure at 15 and 20 years was significantly better for patients receiving 78 Gy (Red Line; 1.1% and 1.4%, respectively) compared to 70 Gy (Blue Line; 3.4% and 4.2%, respectively) (Subhazard Ratio [sHR]: 0.33; 95% Confidence Interval [CI] 0.13 – 0.82; Fine-Gray P=0.018).
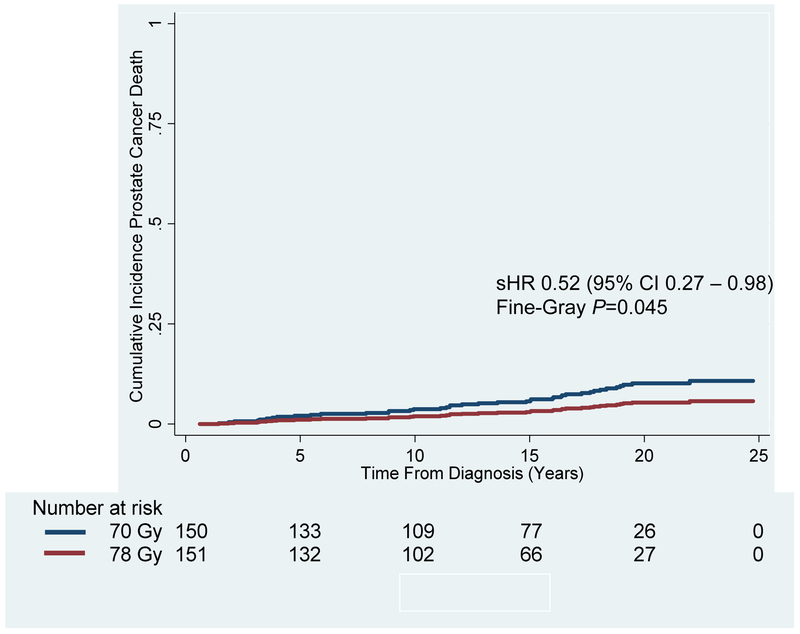
There was no difference in the cumulative incidence of other-cause mortality, also known as non-prostate-cancer death, for patients receiving 70 Gy vs. 78Gy (Appendix Figure 3). The 15- and 20-year cumulative incidences were 45.7% and 64.5% for the 70-Gy arm as compared to 55.6% and 74.8% for the 78-Gy arm, respectively (sHR 1.33, 95% CI 0.99-1.79; Fine-Gray P=0.061). On univariate competing risk regression analysis, age at diagnosis was the only factor associated with other-cause mortality; on multivariate analysis, total Gleason score and age at diagnosis remained (Appendix Table D). As demonstrated in Figure 4, the cumulative incidence of PCSM was significantly higher for patients receiving 70 Gy vs. 78 Gy. The 15- and 20-year cumulative incidences were 6.2% and 10.2% for the 70-Gy arm vs. 3.2% and 5.4%, respectively (sHR 0.52, 95% CI 0.27 – 0.98; Fine-Gray P=0.045). On univariate competing risk regression analysis, factors significantly associated with PCSM were year of diagnosis, total Gleason score, dose, tumor stage, NCCN risk grouping, and pre-treatment PSA. On multivariate analysis, only total Gleason score remained significant (Appendix Table E). Overall survival was not significantly different between the 70-Gy and 78-Gy arm (HR 1.10, 95% CI 0.84-1.45; Log Rank P=0.469). Distant metastasis-free survival (Appendix Figure 4) was not significantly different between the 70-Gy and 78-Gy arm (Log Rank P=0.613).
Figure 4: Cumulative Incidence of Prostate Cancer-Specific Mortality for the Entire Patient Cohort Treated to 70 Gy vs. 78 Gy.
Cumulative incidence of prostate cancer-specific mortality at 15 and 20 years was significantly higher in the patients receiving 70 Gy (Blue Line; 6.2% and 10.2%, respectively) compared to 78 Gy (Red Line; 3.2% and 5.4%, respectively) (Subhazard Ratio [sHR] 0.52, 95% Confidence Interval [CI] 0.27 – 0.98; Fine-Gray P=0.045).
Patients in the 70-Gy arm were significantly more likely (P=0.002) to undergo salvage therapy (38.7% [58/150]) than those in the 78-Gy arm (21.9% [33/151]) such as androgen deprivation, chemotherapy, and/or surgery (Appendix Table F). There was no significant difference in the time to salvage therapy as patients in the 70-Gy group had a median time of 62.9 months (9.0 – 257.5 months) vs. 52.2 months (7.7 – 210.3 months) in the 78-Gy group (P=0.157). Out of 301 patients, 31.2% (94/301) of patients had additional malignancies in their lifetime and 2.3% (7/301) had possible prostate radiation-related, in-field, secondary malignancies: 1 rectal and 6 bladder cancer cases, without a statistically significant difference between treatment arms. The median time to malignancy was 14 years (range 6 – 20 years). Two patients developed a sarcoma in their lifetime and neither was in the radiation treatment field.
Discussion
The long term results of this phase III randomized, single-institution trial found that increasing the radiation dose from 70 to 78 Gy without androgen deprivation resulted in a significant improvement in the primary endpoint of biochemical and/or clinical failure, with significant reductions in the development of distant metastases, the need for subsequent therapy, and death from prostate cancer at a median follow-up of 14 years. Radiation therapy was associated with a 2.3% incidence of in-field secondary malignancy, such as bladder or rectal cancer, for the overall patient cohort.
Dose escalation [5-9] has become widely adopted as the standard of care for external beam radiation therapy for prostate cancer in the United States [14], despite relatively little level one evidence related to survival or survival without prostate cancer to support this approach. Given the indolent nature of localized prostate adenocarcinoma and its propensity for late biochemical or clinical failures, the true benefit, if any, for radiation dose escalation can only be determined after many years of follow-up. With a median follow up of more than 14 years, our study reports one of the longest follow-up intervals available in a group of prospectively treated patients enrolled in a study designed to test the value of dose escalation for prostate cancer. Moreover, the present study did not include the use of neoadjuvant or concurrent androgen deprivation therapy, which offers an unfettered view of the absolute benefit of dose-escalated radiation therapy.
Consistent with previous updates, these results demonstrate improved FFF in those treated with dose escalation [15, 16]. Aside from biochemical failure, dose-escalation also significantly lowered the cumulative incidence of distant metastatic failure at 15 and 20 years in the setting of no hormone deprivation therapy. As a result, the improvement in biochemical and distant relapses translated into lower salvage rates and an improvement in prostate cancer mortality for the dose-escalated group. The discrepancy in higher prostate cancer-specific mortality compared to the cumulative incidence of distant metastatic failure can likely be accounted for by the fact that patients died while on systemic therapy which was initiated for a rising PSA without overt evidence of distant disease on bone scan or CT scan, as was commonly done during this era of less advanced imaging techniques. The decrease in salvage rates with dose escalation are particularly important given the associated morbidity and detrimental effects on quality of life with systemic and, even, local salvage therapy options [17-19]. The advantage of reducing the need for salvage therapy by approximately 15% should be weighed against the small but measurable increase in gastrointestinal and genitourinary toxicity which has been reported by our group in the past [15, 16]. Recent prospective studies demonstrate encouraging results that brachytherapy may be an effective local salvage therapy with decreased toxicity as compared to historical reports and should be further explored in larger patient cohorts [20]. Moreover, reduction in late toxicity has been further enhanced in recent years with the implementation of IMRT, improvements in image-guided radiation therapy, and other modern radiation techniques.
However, despite the significant gains in biochemical and distant control which conferred decreased prostate-cancer mortality, dose-escalation did not translate to an overall survival benefit. This finding may be due, in part, to an elderly patient cohort at the time of enrollment or the relatively low number of prostate cancer-specific deaths compared to other causes. Moreover, the lack of a survival benefit may be due to the successful nature of salvage therapy which can include hormonal therapy, cryotherapy, and surgical intervention first-line. Similar studies [6, 8, 9] have not demonstrated an overall survival benefit for dose-escalation alone, but the addition of neoadjuvant hormone deprivation therapy to lower doses (65 – 70 Gy) of radiation therapy does purport a survival benefit [21]. Similar to salvage therapy, the benefits should be balanced through careful patient selection [22] given the known side effects of androgen deprivation therapy [19] which can be detrimental to quality of life. The present study may provide some guidance to clinicians contemplating the use of external beam radiation alone in patients with relative contraindications to androgen deprivation therapy.
A median follow-up time of 14.3 years allows for the detection of secondary malignancies that may be associated with a history of radiation therapy, as this effect becomes most apparent ≥10 years after completion of treatment [23]. Given a 2.3% incidence of in-field solid secondary malignancies, our clinical findings are well within the range of 1.4% to 4% based on previous studies [24-26]. Of the seven recurrences, four patients (1.3%) died of the secondary malignancy, one from blunt trauma, and two were unknown. As a comparison, in the ProtecT trial, 4.0% (22/545) active monitoring, 4.5% prostatectomy (25/553), and 4.2% radiotherapy (23/545) patients died of a neoplasm other than prostate cancer [27]. We would expect these numbers to dwindle even further with modern techniques [28].
The present study does suffer from some weaknesses. The lack of modern radiation techniques such as IMRT and/or brachytherapy boost is certainly a limitation as this study was conducted during an earlier era using a 4-field box/3D-conformal technique. Similarly, the omission of androgen deprivation in the present study does not reflect the modern standard of care for unfavorable intermediate- and high-risk patients. Therefore, these results are less generalizable to a present-day cohort of patients. Salvage therapy was initiated at the clinician’s discretion as opposed to a standardized threshold. Additionally, the lack of standardized imaging and follow-up long-term may bias the results. Moreover, long-term side effects were not tracked in a prospective manner beyond ten years and, therefore, not reported in this manuscript. While we acknowledge that the patient cohort twenty years after study completion is small due to drop-out and death, this will still likely be the case, however, even with future, more contemporary treatment. Despite this, the reported outcomes should add valuable insight as to the absolute benefit of high dose external beam radiation therapy alone for patients with low- and high-risk prostate cancer and should help to guide future trial design in this patient population.
In conclusion, moderate dose escalation from 70 to 78 Gy demonstrates sustained improvement in clinical and/or biochemical disease control which translated into lower salvage rates and prostate cancer mortality. Long-term follow-up also shows a low incidence of related solid tumor secondary malignancies.
Supplementary Material
Summary:
At a median follow-up of more than fourteen years, this is one of the most mature randomized dose-escalation trials that continues to demonstrate significant improvement in biochemical and/or clinical failure, particularly distant metastases. This translated into a significant improvement in prostate cancer mortality and decreased salvage therapy requirement while also maintaining a low incidence of secondary malignancies.
Acknowledgments
Conflicts of Interest/Funding Acknowledgement: Supported in part by Grants CA 06294 and CA 16672 awarded by the National Cancer Institute, U.S. Department of Health and Human Services, DOD Grant DAMD 17-98-1-8483, and the Prostate Cancer Research Program at M.D. Anderson Cancer Center. Authors A.P., S.J.F., C.T. disclose funding support by Varian Medical Systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: These results were presented at the American Society for Radiation Oncology meeting on October 22, 2018 in San Antonio, TX.
Statistical analysis: Performed by author P.K.A.
References
- 1.Burt LMS, D.C. and Tward JD, Factors influencing prostate cancer patterns of care: An analysis of treatment variation using the SEER database. Adv Radiat Oncol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelius IR and Bentzen SM, Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news? Int J Radiat Oncol Biol Phys, 2013. 85(1): p. 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JZ, Guerrero M, and Li XA, How low is the alpha/beta ratio for prostate cancer? Int J Radiat Oncol Biol Phys, 2003. 55(1): p. 194–203. [DOI] [PubMed] [Google Scholar]
- 4.Pollack A, Smith LG, and von Eschenbach AC, External beam radiotherapy dose response characteristics of 1127 men with prostate cancer treated in the PSA era. Int J Radiat Oncol Biol Phys, 2000. 48(2): p. 507–12. [DOI] [PubMed] [Google Scholar]
- 5.Beckendorf V, et al. , 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys, 2011. 80(4): p. 1056–63. [DOI] [PubMed] [Google Scholar]
- 6.Dearnaley DP, et al. , Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol, 2014. 15(4): p. 464–73. [DOI] [PubMed] [Google Scholar]
- 7.Heemsbergen WD, et al. , Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol, 2014. 110(1): p. 104–9. [DOI] [PubMed] [Google Scholar]
- 8.Michalski JM, et al. , Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zietman AL, et al. , Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95–09. J Clin Oncol, 2010. 28(7): p. 1106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson B and George SL, Sample size requirements and length of study for testing interaction in a 2 x k factorial design when time-to-failure is the outcome [corrected]. Control Clin Trials, 1993. 14(6): p. 511–22. [DOI] [PubMed] [Google Scholar]
- 11.Roach M 3rd, et al. , Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys, 2006. 65(4): p. 965–74. [DOI] [PubMed] [Google Scholar]
- 12.Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys, 1997. 37(5): p. 1035–41. [PubMed] [Google Scholar]
- 13.Fine JP and Gray RJ, A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association, 1999. 94(446): p. 496–509. [Google Scholar]
- 14.Swisher-McClure S, et al. , Increasing use of dose-escalated external beam radiation therapy for men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys, 2014. 89(1): p. 103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuban DA, et al. , Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys, 2008. 70(1): p. 67–74. [DOI] [PubMed] [Google Scholar]
- 16.Pollack A, et al. , Preliminary results of a randomized radiotherapy dose-escalation study comparing 70 Gy with 78 Gy for prostate cancer. J Clin Oncol, 2000. 18(23): p. 3904–11. [DOI] [PubMed] [Google Scholar]
- 17.Caamano AG, et al. , [Quality of life after biochemical recurrence in patients with prostate cancer. How and how long do patients live after biochemical recurrence?]. Arch Esp Urol, 2012. 65(1): p. 193–206. [PubMed] [Google Scholar]
- 18.Sanderson KM, et al. , Salvage radical prostatectomy: quality of life outcomes and long-term oncological control of radiorecurrent prostate cancer. J Urol, 2006. 176(5): p. 2025–31; discussion 2031–2. [DOI] [PubMed] [Google Scholar]
- 19.Crook JM, et al. , Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med, 2012. 367(10): p. 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada Y, et al. , A Phase II study of salvage high-dose-rate brachytherapy for the treatment of locally recurrent prostate cancer after definitive external beam radiotherapy. Brachytherapy, 2014. 13(2): p. 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shelley MD, et al. , A systematic review and meta-analysis of randomised trials of neo-adjuvant hormone therapy for localised and locally advanced prostate carcinoma. Cancer Treat Rev, 2009. 35(1): p. 9–17. [DOI] [PubMed] [Google Scholar]
- 22.Gadia R, et al. , Long-term outcomes of dose-escalated intensity modulated radiation therapy alone without androgen deprivation therapy for patients with intermediate and high-risk prostate cancer. Adv Radiat Oncol, 2016. 1(4): p. 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin T, et al. , Radiation-induced secondary malignancy in prostate cancer: a systematic review and meta-analysis. Urol Int, 2014. 93(3): p. 279–88. [DOI] [PubMed] [Google Scholar]
- 24.Murray L, et al. , Second primary cancers after radiation for prostate cancer: a systematic review of the clinical data and impact of treatment technique. Radiother Oncol, 2014. 110(2): p. 213–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zelefsky MJ, et al. , Secondary cancers after intensity-modulated radiotherapy, brachytherapy and radical prostatectomy for the treatment of prostate cancer: incidence and cause-specific survival outcomes according to the initial treatment intervention. BJU Int, 2012. 110(11): p. 1696–701. [DOI] [PubMed] [Google Scholar]
- 26.Kry SF, et al. , The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys, 2005. 62(4): p. 1195–203. [DOI] [PubMed] [Google Scholar]
- 27.Hamdy FC, et al. , 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med, 2016. 375(15): p. 1415–1424. [DOI] [PubMed] [Google Scholar]
- 28.Journy NM, et al. , Second Primary Cancers After Intensity-Modulated vs 3-Dimensional Conformal Radiation Therapy for Prostate Cancer. JAMA Oncol, 2016. 2(10): p. 1368–1370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.