Abstract
BACKGROUND
Clock drawing is a neurocognitive screening tool used in preoperative settings. This study examined hypothesized changes in clock drawing to command and copy test conditions 3 weeks and 3 months after total knee arthroplasty (TKA) with general anesthesia.
METHODS
Participants included 67 surgery and 66 nonsurgery individuals >60 years who completed the digital clock drawing test before TKA (or a pseudosurgery date), and 3 weeks and 3 months postsurgery. Generalized linear mixed models assessed digital clock drawing test latency (ie, total time to completion, seconds between digit placement) and graphomotor output (ie, total number of strokes, clock size). Reliable change analyses examined the percent of participants showing change beyond differences found in nonsurgery peers.
RESULTS
After adjusting for age, education, and baseline cognition, both digital clock drawing test latency measures were significantly different for surgery and nonsurgery groups, where the surgery group performed slower on both command and copy test conditions. Reliable change analyses 3 weeks after surgery found that total time to completion was slower among 25% of command and 21% of copy constructions in the surgery group. At 3 months, 18% of surgery participants were slower than nonsurgery peers. Neither graphomotor measure significantly changed over time.
CONCLUSIONS
Clock drawing construction slowed for nearly one-quarter of patients after TKA surgery, whereas nonsurgery peers showed the expected practice effect, ie, speed increased from baseline to follow-up time points. Future research should investigate the neurobiological basis for these changes after TKA.
Clock drawing is a neuropsychological test used to assess a wide number of cognitive deficits found in neurological disorders.1 The classic version of clock drawing involves a command clock drawing test condition requiring patients to “draw the face of a clock, put in all of the numbers, and set the hands for 10 after 11.” This condition is immediately followed by the copy test condition where patients copy a predrawn clock model. A comparison of command with copy conditions in patients with dementia suggests that complimentary but different neurocognitive constructs underlie performance in each test condition,2,3 and it can be diagnostically helpful to understand impairment related to comprehension, working memory, mental planning, visuoperception, visuospatial integration, and inhibitory functions.4 Cognitive screening tests such as the Montreal Cognitive Assessment (MoCA) protocol5 incorporate portions of the classic clock drawing test to assist with cognitive assessment in rapid medical practices such as the preoperative setting.6
Recently, neuropsychologists and machine learning specialists created a digital clock drawing test (dCDT) to quantify the subtle behaviors of clock drawing.7 The dCDT measures hundreds of variables ranging from latency variables, such as total time to draw the clock, to constructional/graphomotor features, such as the number of strokes used to complete the clock. Machine learning algorithms applied to dCDT variables show improved classification of individuals diagnosed within the early stage of Alzheimer disease, vascular cognitive disorders, and Parkinson disease, and cognitively well controls.7 Some latency variables represent psychomotor processing speed and working memory.8,9 Certain graphomotor variables reflect subtle differences in brain networks for regions of the right hemi-sphere.10 Follow-up validation studies assessing associations between neuropsychological measures, dCDT latency and graphomotor variables are in process with different laboratories (eg, see Dion et al11 and Piers et al12).
The ability to accurately and quickly assess subtle alterations in neurocognition appears advantageous for studying slight neurocognitive changes over time following surgical interventions. As neuropsychological assessment becomes incorporated with perioperative care, digital neuropsychological assessment tools may provide added value for identifying patients at risk for postsurgical complications given the highly nuanced information collected with the assistance of this technology. The field needs longitudinal research investigations assessing how digital measures of behavior change over time with and without surgical interventions.
Toward this end, the current study explored how 2 dCDT latency and graphomotor variables changed over time for research participants undergoing elective total knee arthroplasty (TKA) relative to nonsurgery peers. Participants completed command and copy drawing conditions at baseline (preoperative) and then at 3 weeks and 3 months postoperative. We applied reliable change analyses to examine the percentage of participants per group changing on specific dCDT latency and graphomotor variables.
METHODS
Participants
This is a prospective observational research investigation requiring consent, which was approved through the Institutional Research Board (IRB) of the University of Florida. All participants provided written informed consent, and the authors followed principles from the Declaration of Helsinki. The study is a part of a larger investigation that was registered at clinicaltrials.gov (number: NCT01786577; July 1, 2013; Price) prior to participant enrollment.
Recruitment
Participants were recruited between 2013 and 2016. One surgeon (H.P.) approached eligible individuals scheduled for total knee replacement surgery to consider participation in this voluntary, federally funded research investigation conducted through the University of Florida. Nonsurgery participants were recruited through IRB-approved flyers and brochures distributed through the orthopedic, community and senior centers, and also IRB-approved radio advertisements.
Inclusion Criteria
Each of the groups met the following inclusion criteria: age 60 years or older, English as a primary language, a complaint of osteoarthritis or other comparable joint issues, intact activities of daily living, and baseline neuropsychological testing that was negative for cognitive impairment criteria per Diagnostic and Statistical Manual of Mental Disorders, 5th ed.13
Exclusion Criteria
Exclusion criteria included prior neurologic surgery; a plan to have a surgery within the study timeline; if the patient had a surgery within the study timeline; history of head trauma/neurodegenerative illness; documented learning disability, seizure disorder, or other major neurological illness; substance abuse in the last year; major cardiac disease; chronic medical illness known to induce encephalopathy; administration of a spinal nerve block in this study; inability to tolerate normal dose of hypnotic during anesthesia induction; and unwillingness to complete repeat testing.
Two neuropsychologists reviewed all baseline demographic and cognitive data to confirm if test scores met the expected ranges for nondemented individuals.
Study Design
This prospective study used a yoked procedure to closely match surgery and nonsurgery participants regarding demographic and comorbidity variables. Nonsurgery participants were provided a pseudosurgery date, 1 week after baseline, to closely match the surgery date for their counter participants and maintain longitudinal synchronization between groups.
Procedures
Participants completed a telephone cognitive screening with the telephone interview for cognitive status (TICS)14 and a comprehensive history/systems interview to confirm inclusion/exclusion criteria, followed by an in-person comorbidity rating,15 activities of daily living assessment,16 and neuropsychological testing that included the dCDT. The same examiner completed testing for individual participants. Trained raters blind to group condition scored all behavioral data. Imaging data from a subset of the participants are shown elsewhere.17 Testing was completed at baseline (preoperative TKA/pseudosurgery date) and at 3 weeks and 3 months following the TKA.
Anesthesia and Surgery Protocol
Protocols were standardized, with surgery participants and premedicated with intravenous midazolam (1–4 mg) for anxiety. Participants then received continuous femoral nerve block (CFNB) and single-injection subgluteal sciatic nerve blocks with 20 mL and 30 mL, respectively, of 0.5% ropivacaine as a bolus injection. The CFNB was continued with 0.2% ropivacaine at an infusion rate of 10 mL per hour. No opioids were added. Propofol (1–3 mg/kg intravenously [IV]), fentanyl (1–2 mcg/kg IV), and rocuronium (0.1 mg/kg IV or succinylcholine (1.5 mg/kg IV if intubated) were used for anesthesia induction and intubation. TKA participants were ventilated with an air/oxygen mixture to maintain an end-tidal carbon dioxide at 35 ± 5 mm Hg; FIo2 between 0.5 and 0.7; anesthesia was maintained with inhaled isoflurane and IV fentanyl and rocuronium to maintain between 2 and 4 twitches by using a left frontal 2-channel electroencephalogram with algorithm-derived values (BIS Aspect Medical Systems, Newton, MA). The desirable maintenance BIS range was between 40 and 60; sevoflurane was adjusted to maintain the BIS in this range.
Total knee replacement surgery was done in a standard manner for all TKA participants by the same surgeon. A tourniquet, used for all TKA cases, was set to 250 mm Hg, elevated prior to incision, and deflated just prior to closure. Bony preparation was done by intramedullary instrumentation for the femoral side and extramedullary for the tibial side. The anterior and posterior cruciate ligaments were sacrificed for all TKA participants, and implants were fixed to the bone using bone cement.
Measures
The dCDT
The dCDT comprises the classic command and copy clock drawing test conditions.1–4 In the command condition, participants are instructed to “draw the face of a clock, put in all the numbers, and set the hands to 10 after 11.” In the copy condition, participants were asked to copy a clock model. The same examiner administered the same instructions to each participant at each time point (baseline, 3 weeks, and 3 months). In this study, clock drawing was recorded using a commercially available digitizing pen with a semiautomatic rater-guided software program. The digitizing pen works as an ordinary ballpoint pen while capturing the pen position 80 times/s at ±0.002 inches.7 Semiautomated software classifies each pen stroke (eg, as a clock face, clock hand, digit) with at least 84% accuracy. The spatial resolution of the pen enables the drawing to be enlarged up to 100 times, making it possible to examine phenomena that are fractions of a millimeter in size and not visible on the paper with the naked eye. All strokes are time stamped, allowing an external rater to play and replay a video recording of a patient’s drawings to assist in scoring (eg, the classification of a stroke as a digit, a clock hand) to enhance classification accuracy. The rater completing the first phase of scoring was blind to group condition (TKA, nonsurgery) and had already achieved high (>0.99 intraclass correlation) interrater and intrarater reliability with separate sets of mild, moderate, and severely impaired clocks.
Clock Drawing Outcome Variables
Time and constructional (graphomotor) performance variables examined performance changes in processing speed (latency variables) relative to visuomotor accuracy and planning (graphmotor).
Time/Latency Variables. Total Time to Completion
Total time to completion is the time in seconds to draw all the elements of the clock, from the start of the first stroke to the completion of the final stroke. Total time to draw the face of a clock, put in all the numbers, and set the hands for 10 after 11 requires multiple cognitive domains,1–4 increases with age,12 and positively associates with increased prefrontal oxygen–hemoglobin recruitment.18 The copy condition requires less time and is a metric measuring executive and visuocon-struction measures.1–4,12
Interdigit Latency
Interdigit latency is time from the end of the last stroke of a numeral (numbers 1–12) to the start of the first stroke of the numeral drawn next (this properly assesses multistroke numerals). In a complete clock, there are (at most) 12 numerals, hence 11 latencies, and therefore the average is (sum of latencies)/11. This variable reflects information processing speed, decision-making, and mental planning.9
Graphomotor Variables. Total Strokes
Total strokes are calculated by the total number of pen strokes required to complete the dCDT condition. Number of strokes assesses gross drawing accuracy and stroke number increases with age.12
Clock Face Area
Clock face area is calculated by averaging the horizontal and vertical radii in millimeters (ie, A = pi r[average]^2). Clock face size represents visuospatial planning and execution.3
In addition to scoring clock drawing behavior through digital technology, command and copy clocks were scored using a conventional visual-based, 0 through 3 scoring system as part of the standard administration and scoring of the MoCA,5 a screening tool designed for the rapid assessment of mild cognitive dysfunction and is commonly administered at in-patient units and neurology settings. Within the MoCA, clock drawing is scored from 0 to 3 (3 = best), with 1 point awarded for (1) contour, where the clock face is complete with only minor distortions (eg, the circle is only slightly elongated or there is a small imperfection in closing the circle); (2) numbers, where all numbers are present in the correct clockwise sequence (eg, they must be located within their respective quadrants, and no numbers can be repeated, roman numerals are acceptable, and numbers can be located outside of the clock face); and (3) hands, where both must be set to the correct time with the hour hand distinctively smaller than the minute hand, while both join near the center of the clock face. To reduce rater bias, the software7 provided MoCA clock drawing criteria.
Statistical Analyses
Data were checked for integrity, distributional form, and missing values. Independent sample t tests were used to compare TKA and nonsurgical groups on continuous measures. χ2 tests were used to compare TKA and nonsurgical groups on binary and categorical measures. Spearman correlation testing was used to investigate relationships between general indicators of cognition and variables from the dCDT at baseline. The dCDT variables were log transformed to achieve normality. Generalized linear mixed modeling assessed the initial study aim by examining significant group by time differences for each of the trajectories of dCDT variables. Visual inspection revealed curvilinear trajectories, and thus, quadratic terms (ie, “time by time” and “group by time by time”) were included in generalized linear mixed modeling models. Unstructured variance–covariance structures were specified in the models. All hypothesis testing was 2 sided using a level of significance of 0.05. SAS, version 9.4, software (SAS Institute, Cary, NC) was used for all analyses. R software (R Core Team, Vienna, Austria) was used to generate figures. Reliable change was computed using linear regression described elsewhere.19 By using data from the nonsurgical group, regression models were fit using each 3-week dCDT measure as the dependent variable and the baseline measure as the predictor covarying for group differences found on baseline cognitive screening. Predicted values with corresponding standard errors for individuals in the TKA group were then computed from each regression model. The reliable change score was then transformed into a standardized z-score with the difference of observed and predicted dCDT scores divided by the standard error of the predicted score.19 Finally, the percent of z-scores falling outside of the middle 90% and 95% range of the z-distribution were calculated. The procedure was repeated for 3-month measures.
RESULTS
Supplemental Digital Content, Figure 1, http://links.lww.com/AA/C541, displays the sample size numbers throughout the study for each of the groups. The final participant sample included 133 individuals (67 surgery and 66 nonsurgery) with no statistically significant difference in groups regarding sex, race, or years of education. Table 1 shows baseline descriptors of participants by group. The total TICS score was significantly higher in the nonsurgery group relative to the surgery group by an average of 1 point. Subsequent analyses corrected for TICS scores. The mean (median) number of days between the date of surgery (or date of pseudo-surgery) and the 3-week test date and 3-month test date was 22.3 (21.0) and 79.7 (94.0), respectively, with no significant group differences. Of the final participants meeting inclusion criteria, 3 had evidence of delirium lasting <1 day, and no participant showed evidence of delirium at the time of the postsurgery assessment.
Table 1.
Description of Study Cohort and Results of Bivariate Comparisons Between TKA and Nonsurgical Groups
| Characteristic | All (n= 133), n (%) or Mean ± SD | TKA (n = 67), n (%) or Mean ± SD | No Surgery (n = 66), n (%) or Mean ± SD | P Value |
|---|---|---|---|---|
| Age (y) | 68.5 ± 6.0 | 68.5 ± 6.5 | 68.5 ± 5.6 | .9826 |
| Sex | .4294 | |||
| Female | 61 (46) | 33 (49) | 28 (42) | |
| Male | 72 (54) | 34 (51) | 38 (58) | |
| Race | .2367 | |||
| Caucasian | 121 (91) | 59 (88) | 62 (94) | |
| Non-Caucasian | 12 (9) | 8 (11) | 4 (6) | |
| Charlson comorbidity index | 0.4 ± 0.7 | 0.5 ± 0.8 | 0.3 ± 0.5 | .0758 |
| Years education | 15.8 ± 2.8 | 15.4 ± 2.9 | 16.1 ± 2.6 | .1796 |
| TICS at baseline | 37.8 ± 3.6 | 37.1 ± 3.7 | 38.5 ± 3.3 | .0378 |
Significant differences between surgery and nonsurgery groups are represented in bold.
Abbreviations: SD, standard deviation; TICS, telephone interview for cognitive status; TKA, total knee arthroplasty.
Baseline MoCA and dCDT Clock Metrics Relative to Demographic and General Cognition
Table 2 displays descriptive data for all clock metrics by group type. Table 3 displays Spearman correlations between clock metrics, participant demographics, and TICS. Only dCDT variables of interest showed the expected pattern for clock variable performance, age, and TICS. Increasing age was associated with slower dCDT latency scores and lower (ie, more impaired) TICS scores. Standard MoCA command and copy clock drawing criteria did not significantly correlate with descriptors of interest. For these reasons, only dCDT variables of interest were examined with longitudinal trajectory analyses.
Table 2.
Summary Measures for dCDT Variables Over Time and by TKA and Nonsurgical Groups
| TKA Group |
Nonsurgery Group |
|||||
|---|---|---|---|---|---|---|
| Presurgery, Mean ± SD | 3 wk, Mean ± SD | 3 mo, Mean ± SD | Presurgery, Mean ± SD | 3 wk, Mean ± SD | 3 mo, Mean ± SD | |
| Latency variables | ||||||
| Total time command | 34.4 ± 11.6 | 37.2 ± 12.2 | 35.1 ± 11.1 | 34.1 ± 12.2 | 29.8 ± 7.5 | 30.6 ± 8.2 |
| Total time copy | 28.3 ± 8.6 | 29.8 ± 8.9 | 29.0 ± 8.2 | 29.0 ± 10.0 | 27.0 ± 8.0 | 27.5 ± 8.1 |
| Interdigit latency command | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.8 ± 0.4 | 0.7 ± 0.4 | 0.6 ± 0.2 | 0.7 ± 0.3 |
| Interdigit latency copy | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.2 | 0.6 ± 0.2 |
| Graphomotor variables | ||||||
| Total strokes command | 25.3 ± 5.9 | 25.4 ± 5.2 | 25.5 ± 6.8 | 23.7 ± 2.6 | 23.1 ± 2.3 | 23.9 ± 2.8 |
| Total strokes copy | 24.0 ± 2.3 | 24.3 ± 5.3 | 23.9 ± 2.4 | 23.7 ± 2.6 | 23.6 ± 2.3 | 23.7 ± 2.4 |
| Clock face area command | 3566.7 ± 2072.2 | 3147.1 ± 1925.1 | 3941.8 ± 2446.0 | 4067.7 ± 2350.1 | 4205.7 ± 2211.2 | 4319.5 ± 2197.4 |
| Clock face area copy | 2925.5 ± 1189.7 | 2746.8 ± 1307.7 | 2862.9 ± 1173.7 | 3139.0 ± 1682.4 | 2884.2 ± 1214.4 | 2963.6 ± 1278.4 |
| MoCA clock score | ||||||
| MoCA command | 2.4 ± 0.7 | 2.5 ± 0.6 | 2.6 ± 0.6 | 2.4 ± 0.7 | 2.5 ± 0.6 | 2.6 ± 0.6 |
| MoCA copy | 2.7 ± 0.5 | 2.7 ± 0.5 | 2.7 ± 0.6 | 2.8 ± 0.4 | 2.8 ± 0.4 | 2.7 ± 0.6 |
Abbreviations: dCDT, digital clock drawing test; MoCA, Montreal Cognitive Assessment (0–3 max); SD, standard deviation; TKA, total knee arthroplasty.
Table 3.
Spearman Correlations Between General Cognitive Indicators (Age, Education, and TICS) am dCDT Variables
| Spearman Correlation Coefficient and P Values | |||
|---|---|---|---|
| Age | Education | TICS | |
| Command | |||
| Total time | 0.16 | −0.11 | −0.26 |
| (−0.1% to 32%) | (−28% to 6%) | (−41% to −9%) | |
| .0635 | .1972 | .0034 | |
| Interdigit latency | 0.23 | −0.06 | −0.22 |
| (6% to 39%) | (−23% to 11%) | (−38% to −4%) | |
| .0081 | .4972 | .0145 | |
| Total strokes | 0.12 | −0.01 | −0.07 |
| (−5% to 29%) | (−18% to 16%) | (−24% to 11%) | |
| .1690 | .8917 | .4665 | |
| Clock face area | 0.10 | 0.26 | 0.17 |
| (−7% to 27%) | (9% to 41%) | (−1% to 33%) | |
| .2565 | .0029 | .0637 | |
| MoCA | 0.04 | 0.06 | 0.11 |
| (−13% to 21%) | (−12% to 23%) | (−7% to 28%) | |
| .6483 | .5209 | .2195 | |
| Copy | |||
| Total time | 0.27 | −0.01 | −0.24 |
| (10% to 42%) | (−18% to 16%) | (−40% to | |
| .0018 | .9211 | (−6%) .0070 | |
| Interdigit latency | 0.21 | −0.05 | −0.22 |
| (4% to 37%) | (−22% to 12%) | (−38% to | |
| .0147 | .5562 | (−4%) .0138 | |
| Total strokes | 0.20 | 0.05 | 0.17 |
| (2% to 36%) | (−13% to 22%) | (−1% to 33%) | |
| .0253 | .5861 | .0575 | |
| Clock face area | −0.08 | 0.22 | 0.18 |
| (−25% to 10%) | (5% to 38%) | (0% to 34%) | |
| .3808 | .0106 | .0472 | |
| MoCA | −0.11 | 0.07 | −0.06 |
| (−27% to 7%) | (−11% to 24%) | (−24% to 11%) | |
| .2270 | .4475 | .4717 | |
Significant correlations are represented in bold. Lower and upper bounds of 95% exact CIs are given in brackets.
Abbreviations: CI, confidence interval; dCDT, digital clock drawing test; MoCA, Montreal Cognitive Assessment; TICS, telephone interview for cognitive status.
Trajectory of Change for dCDT Latency Variables
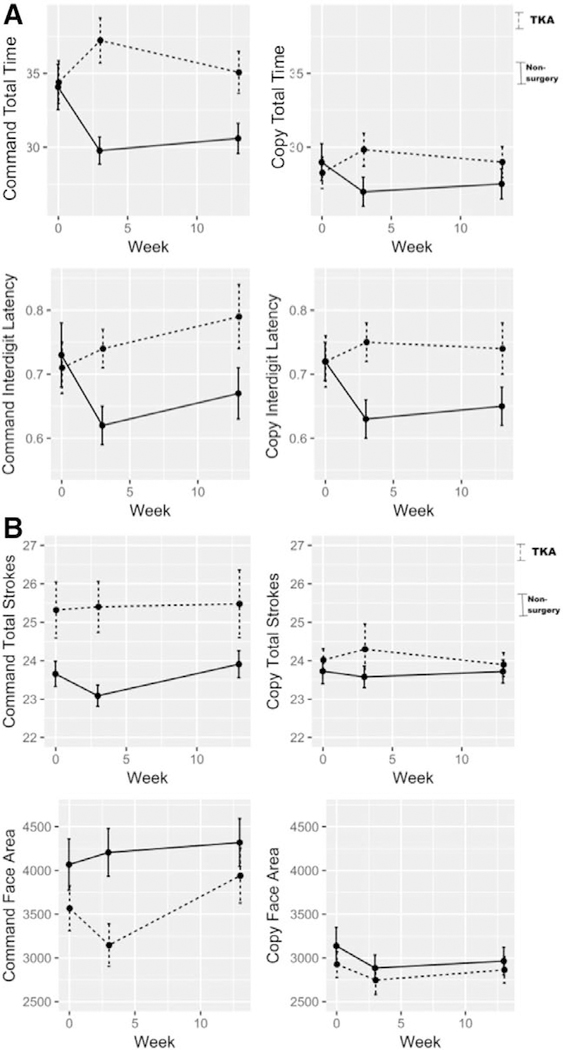
Figure A shows the trajectory of dCDT latency variables by group at each testing time point. Table 4 provides the estimates of curvilinear models. After adjusting for age, education, and TICS, there was a significant group by time by time interaction for dCDT total time to completion and interdigit latency. This was present for only the command condition at 3 weeks and 3 months. At 3 weeks postintervention, TKA patients were 3 seconds slower in total time compared to their baseline. Furthermore, at 3 months, TKA patients were 1 second slower than baseline for total completion time. By contrast, nonsurgery peers were 5 seconds faster in total time at 3 weeks and maintained this improvement after 3 months. The copy condition showed a similar but less dramatic pattern for both groups. For interdigit latency in both command and copy conditions, TKA patients were significantly slower at 3 weeks and 3 months postintervention compared to their nonsurgery peers.
Figure.
Surgery relative to nonsurgery peer differences at each time point (baseline, 3 weeks, and 3 months) for (A) time/latency and (B) graphomotor digital clock drawing test variables of interest. Dashed lines represent the total knee arthroplasty group mean across time; solid lines represent the nonsurgery group mean across time. dCDT indicates digital clock drawing test; TKA, total knee arthroplasty.
Table 4.
Results of Longitudinal Analyses Comparing Trajectories of TKA and Nonsurgery Groups Over Time
| Variable | Group P Value | Time P Value | Group × Time P Value | Time × Time P Value | Group × Time × Time P Value |
|---|---|---|---|---|---|
| Command | |||||
| Total time | .9704 .002 (.06) | .3708 −.007 (.002) | <.0001 .010 (.003) | .5024 .001 (.001) | .0001 −.001 (.001) |
| Interdigit latency | .9902 .001 (.071) | .3399 −.006 (.002) | .0035 .009 (003) | .2614 .001 (.001) | .0075 −.001 (.001) |
| Total strokes | .0284 .061 (.028) | .7332 −.001 (.001) | .1000 .002 (.001) | .6464 .001 (.001) | .0782 −.001 (.001) |
| Clock face area | .4767 −.088 (.124) | .3109 .003 (.005) | .0539 −.014 (.007) | .1429 −.001 (.001) | .0337 .001 (.001) |
| Copy | |||||
| Total time | .5409 −.032 (.052) | .9368 −.004 (.002) | .0051 .007 (.003) | .9267 .001 (.001) | .0067 −.001 (.001) |
| Interdigit latency | .8361 −.013 (.062) | .3051 −.007 (.002) | .0007 .011 (.003) | .4862 .001 (.001) | .0012 −.001 (.001) |
| Total strokes | .2579 .021 (.018) | .9330 −.001 (.001) | .7265 .001 (.001) | .9631 .001 (.001) | .6794 −.001 (.001) |
| Clock face area | .5651 −.042 (.073) | .0371 −.003 (.003) | .8082 −.001 (.004) | .0352 .001 (.001) | .7366 .001 (.001) |
Significant findings are represented in bold. Beneath P values are β estimates and standard errors are in parentheses.
Abbreviation: TKA, total knee arthroplasty.
Trajectory of Change for dCDT Graphomotor Variables
Figure B shows the trajectory of dCDT graphomotor variables by group at each testing time point. Table 4 gives the estimates of curvilinear models. After adjusting for age, education, and TICS, there was a significant group by time by time interaction for dCDT command clock face area supporting different group trajectories over time. For total strokes, there was a significant main effect of group such that the TKA patients produced more strokes than the nonsurgery peers. Neither strokes nor clock face area showed significant differences in the copy condition.
Percent of TKA Participants Showing Reliable Latency Difference Relative to Non-TKA Peers
We completed reliable change analyses on latency variables for clinical utility because the group differences on these variables were pronounced and consistent. Reliable change analyses show that a significant percent of the TKA group demonstrated longer latency, ie, slower total command and copy time to completion (approximately 25% and 21% of the sample, respectively) 3 weeks after surgery relative to nonsurgery peers. This was also seen for interdigit latency for both command and copy, where 18% of surgery participants presented with significant slowness in average pause between drawing clock face numerals relative to the nonsurgery peers. The percentage of participants with significant impairment decreased at 3 months (Table 5).
Table 5.
TKA Group RCI Decline in Latency Performance 3 Weeks and 3 Months Postsurgery Using Moderate and Severe Criteria
| Moderate Criterion |
Severe Criterion |
|||
|---|---|---|---|---|
| Variable | 3 wk | 3 mo | 3 wk | 3 mo |
| Command | ||||
| Total time | 25% (13%−35%) | 21% (10%−31%) | 20% (9%−30%) | 17% (8%−27%) |
| Interdigit latency | 18% (8%−28%) | 11% (3%−19%) | 13% (5%−22%) | 9% (2%−17%) |
| Copy | ||||
| Total time | 21% (11%−34%) | 10% (3%−18%) | 16% (7%—26%) | 7% (2%−17%) |
| Interdigit latency | 18% (8%−28%) | 16% (6%−25%) | 16% (7%—26%) | 10% (3%−18%) |
Moderate criterion: percent of z-scores falling outside of the middle 90% range of the z-distribution.
Severe criterion: percent of z-scores falling outside of the 95% range of the z-distribution.
Lower and upper bounds of 95% exact CIs in parentheses.
Abbreviations: CI, confidence interval; RCI, reliable change index; TKA, total knee arthroplasty.
Consideration for Delirium
We conducted a sensitivity analysis excluding the 3 participants with delirium. Changes in P values from longitudinal analyses were minor (at the hundredths or thousandths decimal place). The sensitivity analysis demonstrated that the findings were robust; digital clock drawing changes over time differed by group (surgery and nonsurgery).
DISCUSSION
This study revealed different clock drawing trajectories for older adults electing TKA relative to nonsurgical peers. On latency measures (total time and interdigit latency) of command and copy clock drawing conditions, TKA participants were slower at 3 weeks following surgery. They required more time to complete their drawings and took longer pauses between placing digits. Their performance after approximately 3 months of recovery approached their original drawing speed. By contrast, nonsurgery peers demonstrated the opposite pattern, speeding up following baseline and then slowing slightly at 3 months. Graphomotor variables were less striking and included only a decrease in clock face area size over time with a trend at 3 weeks post-TKA.
On the dCDT metrics, the TKA group did not show the expected improvement in clock drawing performance with repeat testing. Nearly 25% of TKA participants failed to show a practice effect for the command condition at 3 weeks and 18% at 3 months. A similar percentage of speeded deficits occurred in the clock drawing copy condition. These percentages are consistent with other reported rates of cognitive change following noncardiac procedures.20–23 TKA surgery participants failing to demonstrate the expected practice effect on clock drawing may represent neurocognitive disruption.
Although clock drawing is thought to measure global cognitive function, clock drawing latency variables may be most sensitive to processing speed demands mediated by frontal-subcortical white matter connectivity changes. Previous research shows total clock time to completion associates with increased prefrontal oxygen hemoglobin recruitment.18 A recent Framingham Heart investigation also shows dCDT time to completion correlates with increasing age among neurologically healthy individuals.12 Increasing age and worse processing speed is a well-known pattern24,25 with recent findings demonstrating myelin content contributing to this association.26 Interdigit latency is another dCDT variable to consider for measurement of processing speed and working memory. Interdigit latency is reduced in individuals with multiple sclerosis, a condition well known for white matter disruption and processing speed impairment.9 Clock drawing latency variables, therefore, may provide a heuristically meaningful and noninvasive window into subcortical white matter integrity and changes to these tissues within the frontal cortex. Since the clock drawing test requires numerous cognitive domains for accurate performance (ie, memory, language, visuoconstruction, inhibitory function, etc),1 the latency variables may prove to be the most useful tools to assess subtle cognitive change in nondemented adults following surgery and anesthesia.
By contrast, alterations involving dCDT graphomotor variables may be most reflective of frank gray matter cortical changes from stroke or global inflammation. Lamar et al10 demonstrated that the higher degrees of integration among key cortical gray matter regions explained graphomotor output such as the use of anchoring numerals (the decision to anchor the numbers 12, 3, 6, and 9). Researchers are encouraged to examine latency and graphomotor change relative to preoperative to postoperative brain changes within gray and white matter regions and how these subtle behavioral nuances provide rapid assessment landmarks for neuronal change postoperatively. Graphomotor variables need examination in more cognitively compromised patients before and after surgery and across surgery types with higher risk of perioperative cerebrovascular insults.
The current investigation is not without limitations. The study is limited to nondemented older adults electing TKA. Our findings may not extend well to other surgical patient groups or participant samples. Our study did not consider the number of prior major surgeries, and this should be considered in future investigations. Pain medication dose is also relevant to cognitive outcome studies. We examined TKA and control participant pain mediation dose using a morphine derivative.27 Groups did not differ significantly in pain medication use at baseline or at 3 months, but at 3 weeks, the TKA participants were taking on average one more pain pill than their nonsurgery peers. Unfortunately we could not derive the actual morphine level at 3 weeks due to most patients reporting pain medications “as needed.” Pain medication could have contributed to dCDT latency at 3 weeks and should be considered in future studies. Comorbid risk factors in this study were calculated with the Charlson Comorbidity Index, but we did not quantify severity of cardiovascular risk factors.15 Cardiovascular disease risk factors and cerebrovascular disease contribute to cognitive change and dementia and should be considered in future investigations.7 The current study is also limited by the length of the study interval and the number of follow-up time points; future research should investigate changes over a more extensive time interval with additional time points. Finally, some readers may ask why we did not assess MoCA clock scores for changes after surgery. The limited MoCA score range in our nondemented participant group did not provide any meaningful information relative to demographic information and would not have been appropriate statistically in a longitudinal model. Future studies examining MoCA relative to digital clock metrics in more cognitively compromised patients may provide more meaningful comparisons. Despite study limitations, this study has many strengths including the prospective design with the same surgeon and anesthesia protocol, minimal dropout, and incorporation of digital technology to address the topic of cognitive change after surgery.
We encourage future researchers to conduct similar investigations with time- and graphical-based assessments before and after surgical intervention. Investigators need to examine how surgical/anesthesia procedures coupled with patient comorbidities, surgical history, pain experiences, and surgical outcomes (including incidence of postoperative delirium) alter subtle acute and chronic behavior. We also encourage the integration of neuroimaging methods to explore the underlying neurobiology of subtle clock drawing behaviors before and after surgery with anesthesia.
Supplementary Material
KEY POINTS.
Question: Can a digital version of the classic clock drawing test identify subtle cognitive-behavioral changes in older adults electing total knee replacement surgery?
Findings: Individuals electing total knee replacement surgery significantly slowed relative to nonsurgery peers on clock drawing time to completion and digit placement, but did not show striking graphomotor abnormalities.
Meaning: Clock drawing behaviors can be captured using digital technology and can provide insight into perioperative cognitive changes.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the participants who devoted their valuable time to this investigation. We are very grateful to research coordinators Donna Weber and Kristi Ayers. We thank the Institutional Research Board for their assistance with data monitoring and input. We thank Corey Astrom, ELS, for her editorial expertise and assistance with this manuscript.
Funding: This work was supported by the National Institutes of Health (grant nos. R01 NR014181 to C.C.P.; R01AG055337 to C.C.P. and P.T.; UL1R001427 and P50AG047266) and the National Science Foundation (1404333 to R.D., D.L.P., and C.C.P.).
Footnotes
DISCLOSURES
Name: Loren P. Hizel, MS.
Contribution: This author helped collect the data, draft the first manuscript, and revise the manuscript.
Conflicts of Interest: None.
Name: Eric D. Warner, BS.
Contribution: This author helped collect the data and draft the manuscript.
Conflicts of Interest: None.
Name: Margaret E. Wiggins, BA.
Contribution: This author helped interpret the data and write the manuscript.
Conflicts of Interest: None.
Name: Jared J. Tanner, PhD.
Contribution: This author helped collect the data, interpret the data, and revise the manuscript.
Conflicts of Interest: None.
Name: Hari Parvataneni, MD.
Contribution: This author helped collect the data and revise the manuscript.
Conflicts of Interest: None.
Name: Randall Davis, PhD.
Contribution: This author helped collect the data, revise the manuscript, and secure funding.
Conflicts of Interest: R. Davis owns stock in Digital Cognition Technologies.
Name: Dana L. Penney, PhD.
Contribution: This author helped interpret the data and revise the manuscript.
Conflicts of Interest: D. L. Penney owns stock in Digital Cognition Technologies.
Name: David J. Libon, PhD.
Contribution: This author helped revise the manuscript.
Conflicts of Interest: D. J. Libon owns stock in Digital Cognition Technologies.
Name: Patrick Tighe, MD, MS.
Contribution: This author helped revise the manuscript and secure funding.
Conflicts of Interest: None.
Name: Cynthia W. Garvan, PhD.
Contribution: This author helped analyze the statistical data, write the manuscript, and revise the manuscript.
Conflicts of Interest: None.
Name: Catherine C. Price, PhD.
Contribution: This author helped conceptualization, collect the data, analyze the data, draft all manuscripts, revise the manuscripts, and secure funding.
Conflicts of Interest: None.
This manuscript was handled by: Robert Whittington, MD.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesiaanalgesia.org).
Clinical Trial number and registry URL: NCT01786577; clinicaltrials.gov.
REFERENCES
- 1.Kaplan E The process approach to neuropsychological assessment of psychiatric patients. J Neuropsychiatry Clin Neurosci. 1990;2:72–87. [DOI] [PubMed] [Google Scholar]
- 2.Cosentino S, Jefferson A, Chute D, Kaplan E, Libon D. Clock drawing in dementia: validation of a novel scoring system sensitive to subcortical pathology. Arch Clin Neuropsychol. 2002;17: 720–721. [Google Scholar]
- 3.Libon DJ, Malamut BL, Swenson R, Sands LP, Cloud BS. Further analyses of clock drawings among demented and nondemented older subjects. Arch Clin Neuropsychol. 1996;11:193–205. [PubMed] [Google Scholar]
- 4.Price CC, Cunningham H, Coronado N, et al. Clock drawing in the Montreal Cognitive Assessment: recommendations for dementia assessment. Dement Geriatr Cogn Disord. 2011;31: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 6.Culley DJ, Flaherty D, Reddy S, et al. Preoperative cognitive stratification of older elective surgical patients: a cross-sectional study. Anesth Analg. 2016;123:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souillard-Mandar W, Davis R, Rudin C, et al. Learning classification models of cognitive conditions from subtle behaviors in the digital clock drawing test. Mach Learn. 2016;102:393–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J, Penney DL, Davis R, et al. Digital clock drawing: differentiating “thinking” versus “doing” in younger and older adults with depression. J Int Neuropsychol Soc. 2014;20:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libon DJ, Penney DL, Davis R, et al. Deficits in processing speed and decision making in relapsing-remitting multiple-sclerosis: the digital clock drawing test (dCDT). J Mult Scler. 2014;1:113. [Google Scholar]
- 10.Lamar M, Ajilore O, Leow A, et al. Cognitive and connectome properties detectable through individual differences in graphomotor organization. Neuropsychologia. 2016;85:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dion C, Arias F, Hardcastle C, et al. Cognitive associates of the digital clock drawing test: total clock drawing time, prefirst hand latency, and post-clock face latency. Paper presented at: IARS 2018 Annual Meeting and International Science Symposium; April 29, 2018; Chicago, IL. [Google Scholar]
- 12.Piers RJ, Devlin KN, Ning B, et al. Age and graphomotor decision making assessed with the digital clock drawing test: the Framingham Heart Study. J Alzheimers Dis. 2017;60:1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 14.Cook SE, Marsiske M, McCoy KJ. The use of the modified telephone interview for cognitive status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 16.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 17.Huang H, Tanner J, Parvataneni H, et al. Impact of total knee arthroplasty with general anesthesia on brain networks: cognitive efficiency and ventricular volume predict functional connectivity decline in older adults. J Alzheimers Dis. 2018;62:319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoyama M, Nishioka T, Okumura M, et al. Brain activity during the clock-drawing test: multichannel near-infrared spectroscopy study. Appl Neuropsychol. 2011;18:243–251. [DOI] [PubMed] [Google Scholar]
- 19.Martin R, Sawrie S, Gilliam F, et al. Determining reliable cognitive change after epilepsy surgery: development of reliable change indices and standardized regression-based change norms for the WMS-III and WAIS-III. Epilepsia. 2002;43:1551–1558. [DOI] [PubMed] [Google Scholar]
- 20.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. [DOI] [PubMed] [Google Scholar]
- 21.Monk T, Weldon B, Garvan C, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Surv Anesthesiol. 2008;52:135–136. [DOI] [PubMed] [Google Scholar]
- 22.Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krenk L, Kehlet H, Bsk Hansen T, Solgaard S, Soballe K, Rasmussen LS. Cognitive dysfunction after fast-track hip and knee replacement. Anesth Analg. 2014;118:1034–1040. [DOI] [PubMed] [Google Scholar]
- 24.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. [DOI] [PubMed] [Google Scholar]
- 25.Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54. [DOI] [PubMed] [Google Scholar]
- 26.Chopra S, Shaw M, Shaw T, Sachdev P, Anstey K, Cherbuin N. More highly myelinated white matter tracts are associated with faster processing speed in healthy adults. Neuroimage 2017;171:332–340. [DOI] [PubMed] [Google Scholar]
- 27.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.