Abstract
Despite the immeasurable burden on patients and families, no effective therapies to protect the CNS after an acute injury are available yet. Furthermore, the underlying mechanisms that promote neuronal death and functional deficits after injury remain to be poorly understood. The prevalence, age of onset, pathophysiology, and symptomatology of many CNS insults differ significantly between males and females. In the case of stroke, younger males tend to show a higher risk than younger females, while this trend reverses with age. Accumulating evidence from preclinical studies have shown that sex hormones play a crucial role in providing neuroprotection following ischemic stroke and other acute CNS injuries. Estrogen, in particular, exerts a neuroprotective effect by modulating the immune responses after injury. In addition, there exists a sexual dimorphism in cell death pathways between males and females that are independent of hormones. Meanwhile, recent studies suggest that microRNAs are critically involved in the sex-specific mechanisms of cell death. This review discusses the current knowledge on the contribution of sex and age to outcome after stroke. Implication of the interplay between these two factors on other CNS injuries (spinal cord injury and traumatic brain injury) from the experimental evidence were also discussed.
INTRODUCTION
Acute ischemic stroke, as well as other CNS injuries including traumatic brain injury (TBI) and spinal cord injury (SCI) are major promoters of death and disability worldwide (Benjamin et al., 2018; Corps et al., 2015; Herman et al., 2018). The medical costs of strokes are forecasted to increase from $71.6 billion in 2012 to $184.1 billion by 2030. However, there are no effective treatments to protect the CNS and promote the functional recovery after acute injuries. Although recombinant tissue-type plasminogen activator (r-tPA) is the only FDA-approved thrombolytic agent that shows promise to dissolve blood clots, less than 5% of stroke patients are eligible for tPA therapy (Barber et al., 2001). A major roadblock to developing effective therapies is the lack of understanding of the cellular and molecular mechanisms that promote secondary neuronal damage after an injury.
Numerous successful preclinical animal studies failed to translate into human clinical use for CNS injuries. A major roadblock is the use of only male animals in the majority of the preclinical testing. NIH recently stipulated that all proposed studies should use both male and females. In addition, Stroke Therapy Academic Industry Roundtable (STAIR) formulated guidelines for the animal stroke preclinical studies in order to promote the successful translation of therapies to humans (Fisher et al., 2009). These guidelines emphasize the consideration of age and sex differences along with the use of translational research models representative of the disease state to develop effective treatment. Indeed, results of many studies indicate that sex and age are critical factors in CNS injury pathology (Biswas et al., 2017; Chan et al., 2013; Sealy-Jefferson et al., 2012). In addition, sex and age may also dictate the eligibility of the specific treatment. For example, studies showed that there is a sex difference in rt-PA eligibility as women are less likely to receive rt-PA than men (Boehme et al., 2017; Madsen et al., 2015). Whether this is because of eligibility differences or the exclusion criterion for stroke risk factors remain to be determined. Thus, a better understanding of the effect of these 2 biological variables (age and sex) can improve clinical care and enhance the development of novel therapies.
Sex and age differences in clinical stroke outcome
Approximately 7.2 million Americans aged 20 years or older had a stroke between 2011 and 2014 and the overall stroke prevalence in the U.S during this period was ~2.7% per year (Benjamin et al., 2018). While the prevalence of stroke in the U.S increased with age in both men and women, from 2006 to 2010, stroke prevalence declined in men while remaining stable in women (Centers for Disease and Prevention, 2012). For stroke incidence from 2000 to 2010, overall ischemic stroke rates declined significantly in people aged >60 years but remained largely unchanged over time in those aged 45 to 59 years (Zahuranec et al., 2014). According to the data collected from Greater Cincinnati/Northern Kentucky Stroke Study and National Institutes of Neurological Disorders and Stroke, ~55,000 more women have a stroke than men annually probably due to a larger number of elderly women than men (Kleindorfer et al., 2010). Although women have lower stroke-incidence than men in younger and middle-age groups (before 75 to 80 years of age), this trend reverses in the older age group (>85 years of age) (Hollander et al., 2003; Reeves et al., 2008; Vega et al., 2009). Women, in general, have a higher lifetime risk of stroke than men. The lifetime stroke risk among those 55 to 75 years of age was 1 in 5 for women (~20%) and 1 in 6 for men (~17%) (Seshadri et al., 2006). Although women tend to have less stroke incidence than men, mortality rate appears to be higher in women accounting for 58% of US stroke deaths in 2015 (Xu et al., 2016). Nonetheless, the overall age-adjusted stroke death rate has been decreased from 48% to ~22% for both men and women from 2005 to 2015 and this phenomenon was most salient among people aged 65 to 74 years (Benjamin et al., 2018). The mean age at stroke death was ~79.6 years in 2002 with men having younger mean age than women (Kim and Vemuganti, 2015). As per United States Centers for Disease Control, the 30-day mortality rate between 1995 and 2002 was ~9% for the 65 to 74 years age group (both men and women), while the 74 to 84 years age group showed ~13.1% and the >85 years of age group showed ~23% (Kim and Vemuganti, 2015).
Depression is a major long-term consequence of stroke in humans. Depression occurs in ~33% of individuals after stroke (Poynter B et al., 2009). At 6 months follow-up after stroke in a cohort of 91 patients, females were shown to have much higher depression-like symptoms than males (Nys et al., 2006). A meta-analysis using 56 publications with a total of 75,131 subjects showed that post-stroke depression was observed to be much higher in females than males in 35 studies (Poynter et al., 2009).
Sex and age differences in experimental models of stroke
As observed in clinical stroke cases, women show less incidence of stroke when compared to the age-matched men at a younger age, but that trend reverses at a later age, particularly when women enter the postmenopausal stage. Consistent with the clinical observations, experimental studies confirmed that younger female rodents are more resistant to ischemic brain damage than younger male rodents (Alkayed et al., 1998; Vannucci et al., 2001). In addition, compared to younger animals, ischemic stroke in aged animals showed worsened outcomes including earlier disruption of the blood-brain barrier (BBB), exacerbated neuronal degeneration and reduced functional outcome (DiNapoli et al., 2008; Dinapoli et al., 2006; Rosen et al., 2005). Studies using ovariectomized female animals further elucidated that female sex hormones (estrogen and progesterone) may play a crucial role in providing neuroprotection following ischemia (Alkayed et al., 1998; Suzuki et al., 2007). Estrogen, in particular, is both a sex steroid hormone and a neurosteroid that can also be synthesized in the brain (Arevalo et al., 2015). When administered exogenously, estrogen elicits neuroprotection in both female and male rodents regardless of testosterone availability (Toung et al., 1998). Furthermore, estrogen has been shown to decrease ischemic infarct size in male rats with diabetes which is a comorbid condition for stroke onset (Santizo et al., 2002; Toung et al., 2000).
Although estrogen treatment has been shown to be neuroprotective after stroke in young adult animals, clinical trials demonstrate that estrogens increase the incidence and severity of stroke in aged women. These studies emphasize that the timing of initiation as well as the route of administration of estrogen replacement both impact the risk of stroke in post-menopause women (Carrasquilla et al., 2017; Lokkegaard et al., 2017). In addition, accumulating evidence has reported that estrogen therapy may lead to a pro-thrombotic state and lead to increased stroke risk, suggesting the effects of estrogen may not be limited to the brain but to the coagulation systems as well (Cole et al., 2007; Laliberte et al., 2011). Similar trends have also been observed in the experimental stroke models. When given to aged female rodents, estrogen paradoxically increased infarct volume after ischemic stroke (Carswell et al., 2004; Gordon et al., 2005). Such neurotoxic effects may be associated with decreased availability of IGF-1, a neuroprotectant which decreases with advancing age and is downregulated by estrogen treatment (Selvamani and Sohrabji, 2010). However, these studies were conducted using only females (or ovariectomized females). In addition, it is not clear whether the duration (acute vs. chronic) or the amount of treatment influenced the experimental results although a meta-analysis of stroke/estrogen studies suggested that route and modality of estrogen administration are major factors that affect the outcome of the studies (Strom and Ingberg, 2014). It is worth noting that other sex hormones other than estrogen, but related to ovarian function may also contribute to the stroke outcome as elevated basal follicle stimulating hormone (FSH) in normal cycling women is reported to be associated with unfavorable cholesterol levels and increased cardiovascular risk (Chu et al., 2003). Overall, these findings suggest that estrogen acts in an age-dependent manner in the post-ischemic brain by protecting the younger brain and exacerbating the damage in the aged brain following stroke.
The neuroprotective properties of estrogens are largely mediated by two estrogen receptors: the classical estrogen receptors (ER) and the novel G protein-coupled ER (GPER). The estrogen receptor subtype ER-α mRNA and protein expression were shown to be increased significantly in the cerebral cortex of the female rodent brain following ischemia (Dubal et al., 2006). Loss of ER-α protein expression levels was observed in the cerebral cortex of aged female mice which showed worsened brain damage and neurological deficit compared to young adult mice (Cai et al., 2014). Furthermore, as aged female mice showed no neuroprotection upon estrogen administration, suggesting that the down-regulation of estrogen receptors in the cerebral cortex may contribute to the loss of estrogen efficacy against ischemic injury in aged females (Cai et al., 2014). Deletion of ER-α resulted in larger infarct volume even after administration of a therapeutic dose of estrogen in both male and female mice, suggesting that ER-α is necessary for the estrogen-mediated neuroprotection (Dubal et al., 2006).
In addition to ER-α, recent studies suggested that GPER-mediated second messenger signaling cascades are also neuroprotective. While GPER is reported to be widely distributed throughout the male and female brain, ischemic stroke increases GPER in the brain of male, but not female rodents, suggesting sex-dependent activity on outcome after ischemic stroke (Broughton et al., 2013; Broughton et al., 2014). Although further studies are needed, GPER is a promising molecular target for neuroprotection after stroke by maintaining the integrity of the BBB as well as promoting anti-inflammatory responses (Lu et al., 2016; Zhao et al., 2016).
Estrogen also exerts neuroprotective effects by negatively modulating the immune response after ischemic brain injury. Both in vitro and in vivo studies indicate that estrogen treatment prevents the production and secretion of pro-inflammatory cytokines (Nadkarni and McArthur, 2013; Petrone et al., 2014). When pre-treated with estrogen, male rats showed a significant reduction in the cortical levels of IL-1β, which is a known inflammatory mediator that promotes ischemic cell death (Boutin et al., 2001; Chiappetta et al., 2007). This subsequently leads to suppression of IL-1β-mediated infiltration of neutrophils into the ischemic tissue, resulting in a smaller infarct volume and improved neurological deficits (Galea and Brough, 2013; McColl et al., 2007). Estrogen is also known to regulate levels of TNF, a pro-inflammatory cytokine that acts on receptors found on a variety of cell types including neuronal, glial, and endothelial cells (Koellhoffer and McCullough, 2013). Evidence suggests dual roles of TNF; low levels of TNF can be beneficial to promote the repair after an injury, whereas excessive TNF is neurotoxic (Lambertsen et al., 2012; Smith et al., 2012; Strom et al., 2011). OVX female rats with low to no estrogen levels produce more TNF than normal female rats with physiologic estrogen levels (Rahnama et al., 2002). However, chronic administration of estrogen in mice stimulates the secretion of a variety of pro-inflammatory cytokines, including TNF (Strom et al., 2011). There are several therapeutic agents that target TNF for ischemic stroke therapy. For example, phosphoinositide 3-kinase delta (PI3Kδ) controls intracellular TNF trafficking in macrophages and therefore represents a prospective target to limit neuroinflammation. Deletion of PI3Kδ gene or suppression of PI3Kδ by the inhibitor CAL-101 in mice leads to reduced TNF levels, decreased leukocyte infiltration, reduced infarct size and improved functional outcome (Low et al., 2014).
Another inflammatory molecule that is regulated by estrogen is NF-κB, an inducible transcription factor that mediates inflammatory signaling in a number of cell types including neurons (Matthews and Hay, 1995). Upon activation, NFκB induces pro-inflammatory genes, such as cellular adhesion molecules, cytokines, MMPs, and growth factors (Jin et al., 2013). NF-κB is well known to be activated after ischemic conditions (Jin et al., 2013; Ridder and Schwaninger, 2009) and there is evidence that estrogen can attenuate the activation of NF-κB as well as the delayed cell death mediated by NF-κB during post-ischemic reperfusion (Wen et al., 2004). A study using TNF-α-treated rat aortic smooth muscle cells reported that estrogen binds to intracellular ER-β and promotes synthesis of IκBα, an endogenous inhibitor of NF-κB that reduces the ability of NF-κB to bind DNA (Xing et al., 2012). In addition, estrogen also indirectly suppress NF-κB activation by regulating microRNAs (miRNAs) let-7a and miR-125b that control expression levels of κB-Ras2, an inhibitor of NF-κB (Murphy et al., 2010). Additional studies are needed to elucidate how estrogen interacts with NF-κB signaling in ischemic stroke.
Role of sex chromosomes in the post-stroke outcome
While sex hormones influence the post-stroke outcome, studies investigated the role of X and Y chromosomes independent of hormone levels. The sex chromosome complement is known to be responsible for sexual dimorphism with XX being female and XY being male. Using mouse strains where females lack the second X chromosome, it was demonstrated that neither the number of X chromosomes (XX or XO) nor the parent of origin of the remaining X chromosome influence post-stroke outcome significantly (Turtzo et al., 2011). Furthermore, when the Sry gene was moved from Y chromosome to an autosome that leads to dissociation of sex hormones from sex chromosomes, 4 core genotypes of mice were created (XXM and XYM males and XXF and XYF females) (Manwani et al., 2015). When these mice (gonadally intact) were subjected to focal ischemia at a younger age (~3 months old), XXM and XYM males showed higher infarct volume than XXF and XYF females. This shows that sex hormones than sex chromosome complement mediate the post-stroke sensitivity to stroke (Manwani et al., 2015). However, when focal ischemia was induced in these mice after they aged (18 to 20 months old), XXM and XXF mice showed higher infarct volume, exacerbated microglial activation and increased pro-inflammatory cytokine levels than XYM and XYF cohorts (McCullough et al., 2016). This study shows that ischemic sensitivity will be determined by sex chromosome complement at an older age.
Sexual dimorphism in post-stroke cell death mechanisms
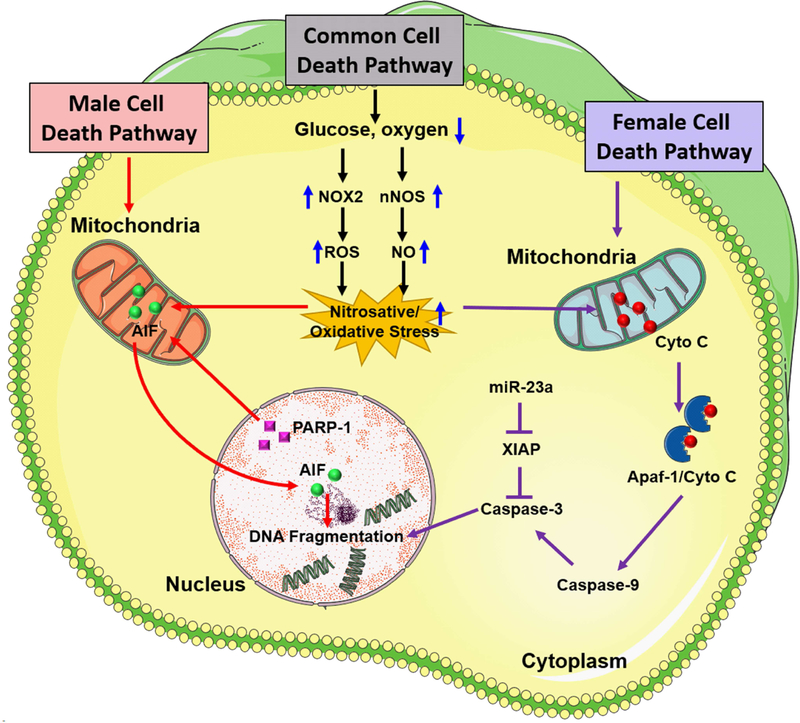
In general terms, estrogen is attributed as the causative factor for the sexual dimorphism in the ischemic injury, but this does not fully account for the various sex-specific outcomes after stroke. Evidence from experimental stroke studies suggests that there is a divergence of cell death pathways between males and females that is independent of sex steroid hormones (Fig. 1). Ischemic cell death in males is predominantly triggered by the activation of a NAD-dependent DNA repair enzyme, poly (ADP-ribose) polymerase (PARP-1) (Lang and McCullough, 2008). Once activated, PARP-1 paradoxically damages DNA by producing PAR polymer which stimulates the release of mitochondrial apoptosis-inducing factor (AIF), resulting in the translocation of AIF to the nucleus (Yu et al., 2006). In the neonatal rodent brain, PARP-1 is activated by hypoxia-ischemia in both sexes, but the neuroprotective effect of the PARP-1 gene deletion was only observed in male pups (Hagberg et al., 2004). Similarly, in adult rodents, genetic deletion or pharmacological inhibition of PARP-1 resulted in neuroprotection in males but exacerbated ischemic brain damage in females (Liu et al., 2011; McCullough et al., 2005). Thus, the male-specific cell death mechanism mediated by PARP-1/AIF pathway may be dependent on androgen availability and androgen receptor signaling (Vagnerova et al., 2010). In contrast, in females, caspase-dependent apoptotic cell death may be the major pathway activated after ischemic injury. Female neurons behaved differentially upon treatment with cytotoxic agents (hydrogen peroxide or peroxynitrite) by demonstrating greater resistance to nitrosative stress than male neurons (Du et al., 2004) and higher levels of cytosolic cytochrome C, an essential component of intrinsic caspase-dependent cell death pathway (Kim and Vemuganti, 2015). Moreover, male and female neurons respond differently to drugs targeting specific proteins and pathways. For example, a study using neonatal rodents showed that females displayed differential temporal expression pattern for caspases after hypoxia-ischemia and were preferentially protected by quinoline-Val-Asp(Ome)-CH2-O-phenoxy (Q-VD-OPh), a pan-caspase inhibitor (Renolleau et al., 2007; Zhu et al., 2006). Additionally, male-derived hippocampal slices exposed to oxygen-glucose deprivation (OGD) were protected with neuronal NOS (nNOS) inhibitors, whereas female slices were not (Li et al., 2005). In adult rodents, females showed an early release of cytochrome C and enhanced caspase activation after ischemic stroke, and caspase inhibition by Q-VD-OPh benefited females, but not males (Liu et al., 2009). Interestingly, the observed sexual dimorphic protective effect of Q-VD-OPh was seen in intact females, ovariectomized (OVX) females, and estrogen-replaced females, suggesting that its neuroprotective effect is independent of ovarian hormone availability (Liu et al., 2009). In summary, these experimental findings bolster the hypothesis that female cell death after an ischemic stroke appears to be mediated primarily by caspase activation, but there might be other factors that need to be understood.
Figure 1:
Sex differences in cell death pathways that contribute to ischemic brain damage. Following focal ischemia, caspase-independent cell death pathway mediated by PARP-1 activation and nuclear translocation of AIF is predominant in males whereas caspase activation predominantly mediates ischemic neuronal death in the female brain. After an ischemic insult, nNOS and NOX2 activation promote high levels of nitric oxide (NO) formation which interacts with superoxide to produce highly reactive peroxynitrite free radicals. Once formed, peroxynitrite free radicals damage protein, lipids as well as DNA, eventually resulting in activation of PARP-1, a DNA repair enzyme that protects the genome under normal physiologic conditions. PARP-1, in turn, induces the release of AIF from mitochondria and its translocation to the nucleus. Overactivation of PARP-1 and nuclear translocation of AIF together promote DNA fragmentation and initiation of caspase-independent cell death pathway in males. In females, ischemia-induced oxidative stress leads to release of mitochondrial cytochrome C into the cytosol which is a hallmark of caspase activation via induction of mitochondrial permeability transition pore (mPTP). In the cytosol, cytochrome C interacts with apoptotic protease activating factor-1 (Apaf-1) to form an Apaf-1/Cyto-C complex in an ATP-dependent manner. This apoptosome complex cleaves procaspase-9 to active caspase-9 which induces activation of effector caspases including caspase-3 which mediates cleavage of most apoptotic substrates including the inhibitor of caspase-activated DNase leading to DNA fragmentation. X-linked inhibitor of apoptosis (XIAP) is a known inhibitor of caspases in females that is regulated by miR-23a.
Sexual dimorphism in microRNA gene regulation in ischemic stroke
In recent years, miRNAs have emerged as potential regulatory molecules in all organisms. They have a broad effect on every aspect of life including embryogenesis, growth and development, metabolism and disease progression (Sharma and Eghbali, 2014). Numerous microRNAs have been identified as key players in post-stroke brain damage and/or recovery. Our lab and others have found that transient focal ischemic stroke can induce extensive temporal changes in rodent cerebral miRNAome and suggested that dysregulation of miRNAs may contribute to the post-stroke outcome (Dharap et al., 2009).
Differential expression of miRNAs in males and females were also observed following ischemic stroke (Lusardi et al., 2014). For example, miR-363 is only found to be elevated in serum of adult female rodents following stroke (Selvamani and Sohrabji, 2017). Interestingly, miR-363 mimic treatment, which suppresses caspase-3 expression and activity, reduced infarct volume and improved sensory motor performance in females but had no effect on stroke outcomes or caspase regulation in adult males (Selvamani and Sohrabji, 2017). Furthermore, microRNAs can have a considerable influence on the regulation of sex steroid hormones as well as X-linked genes, and sexually dimorphic cell death mechanisms may be mediated by differential microRNAs between males and females. An interplay between miR-23a and X-linked inhibitor of apoptosis (XIAP), the primary endogenous inhibitor of caspases was demonstrated recently (Siegel et al., 2011). This study showed that levels of miR-23a which targets XIAP decreased in males and increased in females, whereas XIAP mRNA levels were unaltered in males and decreased in females, following focal ischemia. This indicates that miR-23a might be responsible for post-stroke decreased XIAP in females, but not males indicating a sex-specific response. Furthermore, inhibition XIAP exacerbated ischemic brain damage in females without any effect in males.
Studies also showed that inhibition of miR-181a-5p protects the brain after stroke in male rodents by targeting the stress protein GPR78 (Ouyang et al., 2012). A recent follow-up study showed that miR-181–5p inhibition is also protective in female mice subjected to focal ischemia, but this protection seems to be via modulation of estrogen receptors in astrocytes (Stary et al., 2017). Thus, this study indicates that a miRNA can influence the post-stroke outcome by modulating different downstream pathways in males and females. In female rats, treatment with antagomiR specific for Let-7f which is known to regulate the insulin-like growth factor-1 pathway was observed to be neuroprotective, but the protective effect of anti-Let-7f was abrogated in OVX females and in males (Selvamani et al., 2012). Taken together, these studies suggest that microRNAs can influence sex-specific responses to ischemic disease outcome. Further studies in exploring sex-specific roles of microRNAs are needed for the development of effective therapeutic strategies for sexually dimorphic diseases like ischemic stroke. Interestingly, several miRNAs like miR-98, miR-223, and miR-106 are transcribed from genes located in X-chromosome and their role in sexually dimorphic effects after stroke is yet to be evaluated.
Sex and age differences in the outcome after spinal cord injury
The annual incidence of spinal cord injury (SCI) in the United States is estimated to be 54 cases per million individuals with average age at injury being 42 years (Anonymous, 2016). Typically, men have 3 to 4 times higher incidence rate than women. Interestingly, women tend to experience SCI at a relatively older age compared to men (Devivo, 2012). Leading causes of SCI are vehicle crashes accounting for 38% since 2010, followed by falls (30.5%), acts of violence (primarily gunshot wounds; 13.5%) and sports-related injuries (9%). Approximately 60% of SCI cases are reported at the level of the cervical spine, followed by the thoracic spine (32%) and lumbosacral spine (9%) (Chen et al., 2016). Despite advancement in the modern health care system, the life expectancy of SCI patients remains invariably low compared to age-matched healthy controls. According to a report published by the National Spinal Cord Injury Statistical Center (NSCISC), life expectancy for a 60-year-old individual is approximately 10.3 years after cervical level C5–C8 injury, 8.1 years after C1–C4 injury and 2.2 years if the patient is ventilator dependent (Anonymous, 2016).
Experimental SCI in rodents revealed that females displayed significantly lower secondary tissue loss as well as better functional recovery compared to males (Farooque et al., 2006; Hauben et al., 2002). Female rats presented a greater continuity of myelinated fibers across a lesion at 4 months post-thoracic (T8) SCI than males (Hauben et al., 2002). Likewise, female mice were shown to exhibit a lower degree of macrophage infiltration into the lesion at 2 weeks following thoracic (T10) compression injury (Farooque et al., 2006). It is speculated that these differences might be driven by sex hormone-dependent mechanisms. While testosterone appears to exacerbate tissue damage and inhibits recovery following SCI (Hauben et al., 2002), treatment with exogenous estrogen to bilaterally OVX rats were shown to rescue the nerve cells from apoptosis and promoted functional recovery following SCI (Li et al., 2007). In addition, estrogen treatment to the male rats immediately after the compression injury was shown to protect the spinal cord by stimulating early cytokine release and astroglial responses (Ritz and Hausmann, 2008). Estrogen is also known to exert anti-apoptotic effects by inducing the expression of Bcl-2 via PI3K/Akt-dependent CREB activation pathway and by inhibiting calpain activity after SCI (Sribnick et al., 2006; Yune et al., 2008). Recent evidence sheds light on the neuroprotective role of estrogen in SCI by suppressing the excessive autophagy activation during secondary damage (Lin et al., 2016). However, there are a handful of studies reporting cell death mechanisms after SCI that are independent of gonadal hormones (Fee et al., 2007; Swartz et al., 2007). The disparity in these studies could be attributed to the variation in injury model, duration, dose and complex interactions of sex hormones. Although age differences in functional outcome following SCI are not well-studied, a recent study explored that aging could worsen the functional recovery by exacerbating the oxidative stress, microglial activation, inflammatory mediators and NOX2 expression after a moderate contusion SCI in young and middle-aged rats (von Leden et al., 2017).
Influence of sex and age on the outcome following traumatic brain injury
Traumatic brain injury (TBI) is a leading cause of mortality and long-term disability worldwide. Globally, 69.0 million people suffer a TBI each year. The majority of TBIs are mild (81%) to moderate (11%) in severity (Dewan et al., 2018). Although the severity of TBI is the same in both males and females, sex and age were shown to be a key differentiating factor in determining the outcome. Several earlier findings suggest that younger women have a relatively less post-TBI delayed injury and better functional outcome than older women due to neuroprotective effects of sex hormones in pre-menopausal women (Bayir et al., 2004; Ley et al., 2013). In agreement with these findings, it is also evident that several of the sex hormones decline with age (Niemeier et al., 2013). Although female sex hormones showed a significant protection in experimental animals, results of the major clinical trials that utilized protective effects of female sex hormones have been disappointing (Skolnick et al., 2014; Wright et al., 2014). Despite several studies demonstrated better recovery in women after TBI, women are reported to be more vulnerable to TBI-induced brain damage and mortality than males across the age groups (Biswas et al., 2017). Women show 1.28 times higher post-TBI fatality rates and 1.57 times more likely to suffer from acute/chronic post-traumatic symptoms like severe disability and persistent vegetative state than men (Kraus et al., 2000). Post-TBI neuro-inflammatory response by microglia and astrocytes is reported to be sex-dependent as repeated TBI, in particular, induces a more inflammatory response in women than men (Mychasiuk et al., 2016). Consistent with these findings, the CRASH (Corticosteroid Randomization after Significant Head Injury; a large randomized controlled trial) data collected from TBI patients from different countries suggest that females have worsened short- and long-term outcomes after TBI (Edwards et al., 2005). Interestingly, there is no such observation identified among developing brains. Moreover, studies reported that post-TBI outcomes are better in post-menopausal women compared to age-matched men, while pre-menopausal women have the same outcomes as age-matched men (Davis et al., 2006). Thus, clinical/preclinical studies on sex/age-specific differences in post-TBI vulnerability are still conflicting due to the heterogeneous mode of injury and population.
Experimental studies using TBI animal models primarily focused on males, belying an implicit assumption that sexually dimorphic responses to TBI are relatively unimportant. A recent review on sex-related responses after TBI identified that in 2011, 80% experimental TBI studies used only male animals and the trend of using female animals increased slightly by 7% between 2011 and 2016) (Spani et al., 2018). Meta-analysis of brain cytokines after closed head injury studies found that interleukin-6, tumor necrosis factor α and chemokine ligand 2 are significantly more elevated in female compared to male mice and the anti-inflammatory cytokine interleukin-10 was elevated in males but not in females (Spani et al., 2018). In addition, there is growing evidence that TBI could induce long-lasting adverse events in the vasculature by directly affecting cardiovascular as well as cerebrovascular functions. For example, recent studies concluded that any degree of TBI is an independent acquired risk factor for stroke, and also established a clinical link between TBI and high future stroke risk by approximately 10 fold (Burke et al., 2013; Chen et al., 2011). As female TBI patients are rapidly increasing over the past decade, an in-depth understanding of the intricate interplay between sex and age in post-TBI pathophysiology is critical to find an effective therapy for both sexes.
CONCLUSION
Age and sex have an interactive effect on mortality, motor, cognitive and neuropsychiatric functions following acute CNS insults that include stroke. Many of these effects are mediated by age-related changes in sex hormones, but evidence from experimental studies suggest that chromosomal sex also affects stroke pathophysiology. Studying the mechanisms and consequences of these interactions are critical to the development of safe and effective treatments for patients of both sexes.
Highlights.
In this review, we presented evidence to show that age and sex are major determinants of post-stroke outcome.
We also showed that sex hormones as well as sex chromosomes influences the post-stroke outcome.
We further discussed the significance of microRNAs in promoting ischemic brain damage in a dimorphic manner between males and females.
We discussed the cell death mechanisms that are distinctly different males and females after stroke.
ACKNOWLEDGMENT
This work was supported, in part, by the U.S. Department of Veterans Affairs Merit Review Grant (I01 BX002985) and NIH grants RO1 NS101960, RO1 NS099531, RO1 NS109459. Figures were created using the Servier Medical Art image bank with appropriate modifications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Authors declare no conflict of interest.
REFERENCES
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD, 1998. Gender-linked brain injury in experimental stroke. Stroke 29, 159–165; [DOI] [PubMed] [Google Scholar]
- Anonymous, 2016. Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance. J Spinal Cord Med 39, 493–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo MA, Azcoitia I, Garcia-Segura LM, 2015. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci 16, 17–29. [DOI] [PubMed] [Google Scholar]
- Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM, 2001. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 56, 1015–1020. [DOI] [PubMed] [Google Scholar]
- Bayir H, Marion DW, Puccio AM, Wisniewski SR, Janesko KL, Clark RS, Kochanek PM, 2004. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma 21, 1–8. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on, E., Prevention Statistics, C., Stroke Statistics, S., 2018. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137, e67–e492. [DOI] [PubMed] [Google Scholar]
- Biswas RK, Kabir E, King R, 2017. Effect of sex and age on traumatic brain injury: a geographical comparative study. Arch Public Health 75, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme AK, Carr BG, Kasner SE, Albright KC, Kallan MJ, Elkind MSV, Branas CC, Mullen MT, 2017. Sex Differences in rt-PA Utilization at Hospitals Treating Stroke: The National Inpatient Sample. Front Neurol 8, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ, 2001. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci 21, 5528–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Brait VH, Guida E, Lee S, Arumugam TV, Gardiner-Mann CV, Miller AA, Tang SC, Drummond GR, Sobey CG, 2013. Stroke increases g protein-coupled estrogen receptor expression in the brain of male but not female mice. Neurosignals 21, 229–239. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Brait VH, Kim HA, Lee S, Chu HX, Gardiner-Mann CV, Guida E, Evans MA, Miller AA, Arumugam TV, Drummond GR, Sobey CG, 2014. Sex-dependent effects of G protein-coupled estrogen receptor activity on outcome after ischemic stroke. Stroke 45, 835–841. [DOI] [PubMed] [Google Scholar]
- Burke JF, Stulc JL, Skolarus LE, Sears ED, Zahuranec DB, Morgenstern LB, 2013. Traumatic brain injury may be an independent risk factor for stroke. Neurology 81, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Ma YL, Qin P, Li Y, Zhang LX, Nie H, Peng Z, Dong H, Dong HL, Hou WG, Xiong LZ, 2014. The loss of estrogen efficacy against cerebral ischemia in aged postmenopausal female mice. Neurosci Lett 558, 115–119. [DOI] [PubMed] [Google Scholar]
- Carrasquilla GD, Frumento P, Berglund A, Borgfeldt C, Bottai M, Chiavenna C, Eliasson M, Engstrom G, Hallmans G, Jansson JH, Magnusson PK, Nilsson PM, Pedersen NL, Wolk A, Leander K, 2017. Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies. PLoS Med 14, e1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell HV, Bingham D, Wallace K, Nilsen M, Graham DI, Dominiczak AF, Macrae IM, 2004. Differential effects of 17beta-estradiol upon stroke damage in stroke prone and normotensive rats. J Cereb Blood Flow Metab 24, 298–304. [DOI] [PubMed] [Google Scholar]
- Centers for Disease, C., Prevention, 2012. Prevalence of stroke--United States, 2006–2010. MMWR Morb Mortal Wkly Rep 61, 379–382. [PubMed] [Google Scholar]
- Chan WM, Mohammed Y, Lee I, Pearse DD, 2013. Effect of gender on recovery after spinal cord injury. Transl Stroke Res 4, 447–461. [DOI] [PubMed] [Google Scholar]
- Chen Y, He Y, DeVivo MJ, 2016. Changing Demographics and Injury Profile of New Traumatic Spinal Cord Injuries in the United States, 1972–2014. Arch Phys Med Rehabil 97, 1610–1619. [DOI] [PubMed] [Google Scholar]
- Chen YH, Kang JH, Lin HC, 2011. Patients with traumatic brain injury: population-based study suggests increased risk of stroke. Stroke 42, 2733–2739. [DOI] [PubMed] [Google Scholar]
- Chiappetta O, Gliozzi M, Siviglia E, Amantea D, Morrone LA, Berliocchi L, Bagetta G, Corasaniti MT, 2007. Evidence to implicate early modulation of interleukin-1beta expression in the neuroprotection afforded by 17beta-estradiol in male rats undergone transient middle cerebral artery occlusion. Int Rev Neurobiol 82, 357–372. [DOI] [PubMed] [Google Scholar]
- Chu MC, Rath KM, Huie J, Taylor HS, 2003. Elevated basal FSH in normal cycling women is associated with unfavourable lipid levels and increased cardiovascular risk. Hum Reprod 18, 1570–1573. [DOI] [PubMed] [Google Scholar]
- Cole JA, Norman H, Doherty M, Walker AM, 2007. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 109, 339–346. [DOI] [PubMed] [Google Scholar]
- Corps KN, Roth TL, McGavern DB, 2015. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol 72, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DP, Douglas DJ, Smith W, Sise MJ, Vilke GM, Holbrook TL, Kennedy F, Eastman AB, Velky T, Hoyt DB, 2006. Traumatic brain injury outcomes in pre- and post-menopausal females versus age-matched males. J Neurotrauma 23, 140–148. [DOI] [PubMed] [Google Scholar]
- Devivo MJ, 2012. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50, 365–372. [DOI] [PubMed] [Google Scholar]
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV, Park KB, 2018. Estimating the global incidence of traumatic brain injury. J Neurosurg, 1–18. [DOI] [PubMed]
- Dharap A, Bowen K, Place R, Li LC, Vemuganti R, 2009. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL, 2008. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging 29, 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinapoli VA, Rosen CL, Nagamine T, Crocco T, 2006. Selective MCA occlusion: a precise embolic stroke model. J Neurosci Methods 154, 233–238. [DOI] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS, 2004. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem 279, 38563–38570. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM, 2006. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology 147, 3076–3084. [DOI] [PubMed] [Google Scholar]
- Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, Fernandes J, Gogichaisvili T, Golden N, Hartzenberg B, Husain M, Ulloa MI, Jerbi Z, Khamis H, Komolafe E, Laloe V, Lomas G, Ludwig S, Mazairac G, Munoz Sanchez Mde L, Nasi L, Olldashi F, Plunkett P, Roberts I, Sandercock P, Shakur H, Soler C, Stocker R, Svoboda P, Trenkler S, Venkataramana NK, Wasserberg J, Yates D, Yutthakasemsunt S, collaborators C.t., 2005. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 365, 1957–1959. [DOI] [PubMed] [Google Scholar]
- Farooque M, Suo Z, Arnold PM, Wulser MJ, Chou CT, Vancura RW, Fowler S, Festoff BW, 2006. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord 44, 182–187. [DOI] [PubMed] [Google Scholar]
- Fee DB, Swartz KR, Joy KM, Roberts KN, Scheff NN, Scheff SW, 2007. Effects of progesterone on experimental spinal cord injury. Brain Res 1137, 146–152. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, Group S, 2009. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40, 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea J, Brough D, 2013. The role of inflammation and interleukin-1 in acute cerebrovascular disease. J Inflamm Res 6, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KB, Macrae IM, Carswell HV, 2005. Effects of 17beta-oestradiol on cerebral ischaemic damage and lipid peroxidation. Brain Res 1036, 155–162. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV, 2004. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem 90, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Hauben E, Mizrahi T, Agranov E, Schwartz M, 2002. Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficial autoimmunity? Eur J Neurosci 16, 1731–1740. [DOI] [PubMed] [Google Scholar]
- Herman P, Stein A, Gibbs K, Korsunsky I, Gregersen P, Bloom O, 2018. Persons with Chronic Spinal Cord Injury Have Decreased Natural Killer Cell and Increased Toll-Like Receptor/Inflammatory Gene Expression. J Neurotrauma 35, 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM, 2003. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry 74, 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Liu L, Zhang S, Nanda A, Li G, 2013. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res 6, 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Vemuganti R, 2015. Effect of sex and age interactions on functional outcome after stroke. CNS Neurosci Ther 21, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, Kissela BM, 2010. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 41, 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koellhoffer EC, McCullough LD, 2013. The effects of estrogen in ischemic stroke. Transl Stroke Res 4, 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus JF, Peek-Asa C, McArthur D, 2000. The independent effect of gender on outcomes following traumatic brain injury: a preliminary investigation. Neurosurg Focus 8, e5. [DOI] [PubMed] [Google Scholar]
- Laliberte F, Dea K, Duh MS, Kahler KH, Rolli M, Lefebvre P, 2011. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause 18, 1052–1059. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Biber K, Finsen B, 2012. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 32, 1677–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JT, McCullough LD, 2008. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley EJ, Short SS, Liou DZ, Singer MB, Mirocha J, Melo N, Bukur M, Salim A, 2013. Gender impacts mortality after traumatic brain injury in teenagers. J Trauma Acute Care Surg 75, 682–686. [DOI] [PubMed] [Google Scholar]
- Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD, 2005. Sex differences in cell death. Ann Neurol 58, 317–321. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang S, Xia Y, Wang J, Pan W, Shi Y, Wang M, 2007. Neuroprotective effect of estrogen after chronic spinal cord injury in ovariectomized rats. Neural Regeneration Research 2, 471–474. [Google Scholar]
- Lin CW, Chen B, Huang KL, Dai YS, Teng HL, 2016. Inhibition of Autophagy by Estradiol Promotes Locomotor Recovery after Spinal Cord Injury in Rats. Neurosci Bull 32, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Lang J, Li J, Benashski SE, Siegel M, Xu Y, McCullough LD, 2011. Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke 42, 1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD, 2009. Sex differences in caspase activation after stroke. Stroke 40, 1842–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokkegaard E, Nielsen LH, Keiding N, 2017. Risk of Stroke With Various Types of Menopausal Hormone Therapies: A National Cohort Study. Stroke 48, 2266–2269. [DOI] [PubMed] [Google Scholar]
- Low PC, Manzanero S, Mohannak N, Narayana VK, Nguyen TH, Kvaskoff D, Brennan FH, Ruitenberg MJ, Gelderblom M, Magnus T, Kim HA, Broughton BR, Sobey CG, Vanhaesebroeck B, Stow JL, Arumugam TV, Meunier FA, 2014. PI3Kdelta inhibition reduces TNF secretion and neuroinflammation in a mouse cerebral stroke model. Nat Commun 5, 3450. [DOI] [PubMed] [Google Scholar]
- Lu D, Qu Y, Shi F, Feng D, Tao K, Gao G, He S, Zhao T, 2016. Activation of G protein-coupled estrogen receptor 1 (GPER-1) ameliorates blood-brain barrier permeability after global cerebral ischemia in ovariectomized rats. Biochem Biophys Res Commun 477, 209–214. [DOI] [PubMed] [Google Scholar]
- Lusardi TA, Murphy SJ, Phillips JI, Chen Y, Davis CM, Young JM, Thompson SJ, Saugstad JA, 2014. MicroRNA responses to focal cerebral ischemia in male and female mouse brain. Front Mol Neurosci 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TE, Khoury JC, Alwell KA, Moomaw CJ, Kissela BM, De Los Rios La Rosa F, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, Kleindorfer D, 2015. Analysis of tissue plasminogen activator eligibility by sex in the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 46, 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD, 2015. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab 35, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JR, Hay RT, 1995. Regulation of the DNA binding activity of NF-kappa B. Int J Biochem Cell Biol 27, 865–879. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM, 2007. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci 27, 4403–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F, 2016. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany NY) 8, 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD, 2005. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab 25, 502–512. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Guyre PM, Pioli PA, 2010. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol 184, 5029–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Candy S, Ma I, Esser MJ, 2016. The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J Neurosci Methods 257, 168–178. [DOI] [PubMed] [Google Scholar]
- Nadkarni S, McArthur S, 2013. Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol 13, 576–581. [DOI] [PubMed] [Google Scholar]
- Niemeier JP, Marwitz JH, Walker WC, Davis LC, Bushnik T, Ripley DL, Ketchum JM, 2013. Are there cognitive and neurobehavioural correlates of hormonal neuroprotection for women after TBI? Neuropsychol Rehabil 23, 363–382. [DOI] [PubMed] [Google Scholar]
- Nys GM, van Zandvoort MJ, van der Worp HB, de Haan EH, de Kort PL, Jansen BP, Kappelle LJ, 2006. Early cognitive impairment predicts long-term depressive symptoms and quality of life after stroke. J Neurol Sci 247, 149–156. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, Giffard RG, 2012. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis 45, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone AB, Simpkins JW, Barr TL, 2014. 17beta-estradiol and inflammation: implications for ischemic stroke. Aging Dis 5, 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynter B, Shuman M, Diaz-Granados N, Kapral M, Grace SL, Stewart DE, 2009. Sex differences in the prevalence of post-stroke depression: a systematic review. Psychosomatics 50, 563–569. [DOI] [PubMed] [Google Scholar]
- Rahnama M, Tomaszewski T, Swiatkowski W, 2002. Effect of estrogen replacement therapy on serum cytokines (IL1alpha, IL-6, TNF-alpha) in ovariectomized rats. BULLETIN-VETERINARY INSTITUTE IN PULAWY 46, 273–280. [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L, 2008. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 7, 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C, 2007. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem 100, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Ridder DA, Schwaninger M, 2009. NF-kappaB signaling in cerebral ischemia. Neuroscience 158, 995–1006. [DOI] [PubMed] [Google Scholar]
- Ritz MF, Hausmann ON, 2008. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res 1203, 177–188. [DOI] [PubMed] [Google Scholar]
- Rosen CL, Dinapoli VA, Nagamine T, Crocco T, 2005. Influence of age on stroke outcome following transient focal ischemia. J Neurosurg 103, 687–694. [DOI] [PubMed] [Google Scholar]
- Santizo RA, Xu HL, Ye S, Baughman VL, Pelligrino DA, 2002. Loss of benefit from estrogen replacement therapy in diabetic ovariectomized female rats subjected to transient forebrain ischemia. Brain Res 956, 86–95. [DOI] [PubMed] [Google Scholar]
- Sealy-Jefferson S, Wing JJ, Sanchez BN, Brown DL, Meurer WJ, Smith MA, Morgenstern LB, Lisabeth LD, 2012. Age- and ethnic-specific sex differences in stroke risk. Gend Med 9, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sathyan P, Miranda RC, Sohrabji F, 2012. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One 7, e32662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F, 2010. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci 30, 6852–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F, 2017. Mir363–3p improves ischemic stroke outcomes in female but not male rats. Neurochem Int 107, 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA, 2006. The lifetime risk of stroke: estimates from the Framingham Study. Stroke 37, 345–350. [DOI] [PubMed] [Google Scholar]
- Sharma S, Eghbali M, 2014. Influence of sex differences on microRNA gene regulation in disease. Biol Sex Differ 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Li J, Liu F, Benashski SE, McCullough LD, 2011. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci U S A 108, 11662–11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, Nelson NR, Stocchetti N, Investigators ST, 2014. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med 371, 2467–2476. [DOI] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL, 2012. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 87, 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spani CB, Braun DJ, Van Eldik LJ, 2018. Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Front Neuroendocrinol [DOI] [PMC free article] [PubMed]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL, 2006. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res 84, 1064–1075. [DOI] [PubMed] [Google Scholar]
- Stary CM, Xu L, Li L, Sun X, Ouyang YB, Xiong X, Zhao J, Giffard RG, 2017. Inhibition of miR-181a protects female mice from transient focal cerebral ischemia by targeting astrocyte estrogen receptor-alpha. Mol Cell Neurosci 82, 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom JO, Ingberg E, 2014. Impact of methodology on estrogens’ effects on cerebral ischemia in rats: an updated meta-analysis. BMC Neurosci 15, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E, 2011. Mechanisms of estrogens’ dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia. Int J Mol Sci 12, 1533–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM, 2007. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol 500, 1064–1075. [DOI] [PubMed] [Google Scholar]
- Swartz KR, Fee DB, Joy KM, Roberts KN, Sun S, Scheff NN, Wilson ME, Scheff SW, 2007. Gender differences in spinal cord injury are not estrogen-dependent. J Neurotrauma 24, 473–480. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD, 1998. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke 29, 1666–1670. [DOI] [PubMed] [Google Scholar]
- Toung TK, Hurn PD, Traystman RJ, Sieber FE, 2000. Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke 31, 2701–2706. [DOI] [PubMed] [Google Scholar]
- Turtzo LC, Siegel C, McCullough LD, 2011. X chromosome dosage and the response to cerebral ischemia. J Neurosci 31, 13255–13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnerova K, Liu K, Ardeshiri A, Cheng J, Murphy SJ, Hurn PD, Herson PS, 2010. Poly (ADP-ribose) polymerase-1 initiated neuronal cell death pathway--do androgens matter? Neuroscience 166, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA, 2001. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab 21, 52–60. [DOI] [PubMed] [Google Scholar]
- Vega T, Zurriaga O, Ramos JM, Gil M, Alamo R, Lozano JE, Lopez A, Miralles MT, Vaca P, Alvarez Mdel M, Group of research for the, R.p., 2009. Stroke in Spain: epidemiologic incidence and patterns; a health sentinel network study. J Stroke Cerebrovasc Dis 18, 11–16. [DOI] [PubMed] [Google Scholar]
- von Leden RE, Khayrullina G, Moritz KE, Byrnes KR, 2017. Age exacerbates microglial activation, oxidative stress, inflammatory and NOX2 gene expression, and delays functional recovery in a middle-aged rodent model of spinal cord injury. J Neuroinflammation 14, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW, 2004. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res 1008, 147–154. [DOI] [PubMed] [Google Scholar]
- Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, Goldstein FC, Caveney AF, Howlett-Smith H, Bengelink EM, Manley GT, Merck LH, Janis LS, Barsan WG, Investigators N, 2014. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med 371, 2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Oparil S, Yu H, Gong K, Feng W, Black J, Chen YF, Nozell S, 2012. Estrogen modulates NFkappaB signaling by enhancing IkappaBalpha levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-beta. PLoS One 7, e36890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Murphy SL, Kochanek KD, Arias E, 2016. Mortality in the United States, 2015. NCHS Data Brief, 1–8. [PubMed]
- Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL, 2006. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A 103, 18314–18319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yune TY, Park HG, Lee JY, Oh TH, 2008. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/Akt-dependent CREB activation. J Neurotrauma 25, 1121–1131. [DOI] [PubMed] [Google Scholar]
- Zahuranec DB, Lisabeth LD, Sanchez BN, Smith MA, Brown DL, Garcia NM, Skolarus LE, Meurer WJ, Burke JF, Adelman EE, Morgenstern LB, 2014. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology 82, 2180–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TZ, Ding Q, Hu J, He SM, Shi F, Ma LT, 2016. GPER expressed on microglia mediates the anti-inflammatory effect of estradiol in ischemic stroke. Brain Behav 6, e00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H, 2006. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem 96, 1016–1027. [DOI] [PubMed] [Google Scholar]