Abstract
Brain ischemia induced by cardiac arrest or ischemic stroke is a severe form of metabolic stress that substantially disrupts cellular homeostasis, especially protein homeostasis (proteostasis). As proteostasis is fundamental for cellular and organismal health, cells have developed a complex network to restore proteostasis impaired by stress. Many components of this network – including ubiquitination, small ubiquitin-like modifier (SUMO) conjugation, autophagy, and the unfolded protein response (UPR) – are activated in the post-ischemic brain, and play a crucial role in cell survival and recovery of neurologic function. Importantly, recent studies have shown that ischemia-induced activation of these proteostasis-related pathways in the aged brain is impaired, indicating an aging-related decline in the self-healing capacity of the brain. This impaired capacity is a significant factor for consideration in the field of brain ischemia because the vast majority of cardiac arrest and stroke patients are elderly. In this review, we focus on the effects of aging on these critical proteostasis-related pathways in the brain, and discuss their implications in translational brain ischemia research.
Keywords: aging, brain ischemia, cardiac arrest, stroke, protein homeostasis, neuroprotection
Brain ischemia is a leading cause of death as well as long-term disability worldwide (Benjamin et al, 2018; Hayashi et al, 2015; Pandian et al, 2018). Global brain ischemia can be induced by cardiac arrest (CA), while focal brain ischemia is primarily a result of embolic or thrombotic stroke (ischemic stroke). Every year, more than one million people in the US are affected by CA or ischemic stroke, which presents a major burden on families and healthcare systems (Benjamin et al, 2018). This is expected to become even more pronounced, as our senior population continues to increase and age is a key risk factor for both CA and ischemic stroke (Benjamin et al, 2018). However, despite massive basic and translational research investments during the past several decades, clinical treatment options to improve neurologic outcome after brain ischemia remain extremely limited. This disappointing situation has ignited extensive discussions on the potential limitations of current brain ischemia research. One obvious, yet still less appreciated, limitation is that experimental brain ischemia studies are conducted predominantly in young animals, which seems to ignore the fact that the majority of CA and stroke patients are elderly. Therefore, there is a major mismatch between preclinical studies and clinical relevance (Shi et al, 2018; Yang and Paschen, 2017). Although it is conceivable that young and aged brains respond to ischemia similarly in many aspects, there are notable differences in overall cerebrovascular structure and neuronal responses to stress (Kang et al, 2016; Yang and Paschen, 2017). A better understanding of these differences is fundamental to the success of future efforts to develop new effective therapeutics for CA and stroke patients of advanced age.
Notably, the effect of age on disease progression is an area of extensive research in neurodegenerative diseases such as Alzheimer’s, Huntington’s, and Parkinson’s. One hallmark of these diseases is the deposition of abnormal protein aggregates, which causes proteotoxicity in neurons. The formation of these aggregates is largely attributed to the aging-related decline in the capacity of cells to maintain protein homeostasis (proteostasis) (Labbadia and Morimoto, 2015). Such findings have important implications in brain ischemia because 1) brain ischemia is a severe form of metabolic stress that disrupts cellular proteostasis; 2) timely restoration of proteostasis is crucial for cell survival (Labbadia and Morimoto, 2015); and 3) clinical outcome after brain ischemia worsens with increasing age. Therefore, it is imperative to understand how aging affects the brain’s capacity to restore proteostasis after ischemia.
In the ischemic brain, the supply of vital oxygen and nutrients is not sufficient to meet the metabolic demand. This causes depletion of ATP, disruption of Ca2+ homeostasis, and oxidative stress, which inevitably disturbs cellular proteostasis (Schaller and Graf, 2004). For example, excess production of reactive oxygen species (ROS) can have a detrimental effect on the protein degradation process, protein structure, and the cellular/subcellular redox state, resulting in accumulation of damaged and misfolded/unfolded proteins in brain cells, which disrupts proteostasis (Keller et al, 2000; Korovila et al, 2017; Manzanero et al, 2013). Proteostasis is a dynamic equilibrium that involves biogenesis, folding, and degradation of proteins, and is achieved by a complex protein quality control network in the cell (Kaushik and Cuervo, 2015; Labbadia and Morimoto, 2015). Within this network, many components including ubiquitination and small ubiquitin-like modifier (SUMO) conjugation (SUMOylation) pathways, autophagy, and the unfolded protein response (UPR) pathway have been implicated in cell survival in the post-ischemic brain, as discussed below. Importantly, recent studies have shown that activation of these proteostasis-related pathways in the post-ischemic brain is impaired with increasing age (Jiang et al, 2017; Liu et al, 2016; Shen et al, 2018). Our goal in this review is to briefly summarize the current understanding of the effects of aging on these pathways, highlight the findings related to these pathways in brain ischemia, and discuss their implications in translational brain ischemia research. It is important to note that the findings on these pathways in brain ischemia have been obtained predominantly from animal studies.
Ubiquitination
Ubiquitination is a highly conserved post-translational protein modification by which ubiquitin, a 76-amino acid protein, is covalently conjugated to lysine residues of target proteins in an ATP-dependent manner via an enzymatic cascade consisting of E1-activating, E2-conjugating, and E3-ligating enzymes (Hochrainer, 2018). The process of ubiquitination can be reversed by de-ubiquitinating enzymes.
Many ubiquitinated proteins contain polyubiquitin chains. Seven internal lysine (K) residues (K6, K11, K27, K29, K33, K48, and K63) of ubiquitin are available for ubiquitination, thus giving rise to 7 common polyubiquitin linkages, of which the K48 linkage is the most frequently observed (Xu et al, 2009). Importantly, ubiquitinated proteins with K48-linked polyubiquitin chains are translocated to the 26S proteasome, and then degraded by proteolysis. This degradation system is, therefore, called the ubiquitin-proteasome system (UPS). The UPS is a major protein quality control mechanism in the cell that eliminates misfolded, aggregated, or unwanted proteins, and thus, is vital to maintaining cellular proteostasis (Kevei and Hoppe, 2014). Notably, UPS function declines with increasing age (Baraibar and Friguet, 2012; Vilchez et al, 2014). For example, aging-related reduction in proteasome activity is evidenced by decreased expression of proteasome subunits and perturbed 26S proteasome assembly (Huber et al, 2009; Kapetanou et al, 2017; Lee et al, 1999; Vernace et al, 2007). Data also show that cortical expression of Ube3A, an E3 ubiquitin ligase, decreases with age in humans, monkeys, and cats (Williams et al, 2010). In fact, it is believed that the aging-related decline in UPS function contributes to the accumulation of damaged and aggregated proteins in neurons and thereby, accounts for the high incidence of neurodegenerative diseases in the elderly (Labbadia and Morimoto, 2015).
Although only indirect evidence indicates involvement of ubiquitination in human brain ischemia (Fink et al, 2016), it is well established in animal studies that levels of ubiquitinated proteins increase in the brain after both global and focal brain ischemia (Hayashi et al, 1991; Hochrainer et al, 2012; Hu et al, 2001; Hu et al, 2000; Iwabuchi et al, 2014; Sharma et al, 2015). In 2000, Hu et al provided electron and confocal microscopic evidence that during reperfusion after brain ischemia in the rat, ubiquitinated proteins form aggregates that accumulate in dying CA1 neurons (Hu et al, 2000). Post-ischemic accumulated ubiquitinated proteins are largely insoluble in Triton X-100 solution (Hu et al, 2001; Iwabuchi et al, 2014). Later, Hochrainer et al demonstrated that reperfusion, rather than ischemia, leads to an increase in ubiquitinated aggregates in the mouse brain after transient middle cerebral artery occlusion (MCAO; a focal brain ischemia model), and that this increase may be caused by enhanced ubiquitination activity (Hochrainer et al, 2012). They further showed that increased ubiquitination is more pronounced in the cortex containing salvageable penumbra tissue than in the striatum corresponding to the irreversibly damaged ischemic core (Hochrainer et al, 2012). Thus, in contrast to the traditional notion of a detrimental role for ubiquitination in brain ischemia, these data suggest that the formation of ubiquitin aggregates may be beneficial for tissue viability as it results in the labeling and removal of abnormal proteins caused by the ischemia/reperfusion insult. In support of this, our ubiquitin proteomics study found that K48-linked polyubiquitinated proteins are the most increased among ubiquitinated proteins that form aggregates in the post-ischemic mouse brain (Iwabuchi et al, 2014). Interestingly, ischemic preconditioning also increases ubiquitination. In in vitro studies, a short period of oxygen and glucose deprivation (OGD; an in vitro ischemia model), used to provide ischemic preconditioning, leads to ubiquitination of pro-apoptotic and postsynaptic proteins for rapid degradation by the UPS, which may contribute to the ischemic tolerance that preconditioning affords (Meller et al, 2006; Meller et al, 2008).
Not surprisingly, all brain ischemia studies mentioned above have been conducted in young animals. Recently, we reported that after global brain ischemia, activation of ubiquitination is moderately impaired in aged mice (Liu et al, 2016). In an earlier study, Gozal et al provided evidence of increased susceptibility to intermittent hypoxia in aged rats due to a marked decrease in UPS capacity to clear degraded proteins (Gozal et al, 2003). Thus, considering an aging-related decline in degradation activity under physiologic conditions, and in the UPS response under ischemic or hypoxic conditions, therapeutic interventions that target the UPS after an ischemic event in the aged brain need be designed to increase both degradation and ubiquitination activities of the UPS. Importantly, many small molecules that target various components of the ubiquitin system have been identified, due to the broad implications of the UPS in human diseases (Bedford et al, 2011; Boland et al, 2018). Future studies can take advantage of this rich resource, and explore the therapeutic potential of targeting the UPS in brain ischemia (Caldeira et al, 2014).
SUMOylation
Among many other ubiquitin-like molecules that are present in mammals are interferon-stimulated gene 15 (ISG15) and SUMO. Levels of both ISG15 and SUMO conjugated (SUMOylated) proteins are markedly increased after brain ischemia (Nakka et al, 2011; Yang et al, 2008a, b); however, the follow-up studies have primarily focused on SUMOylation. Similar to ubiquitination, SUMOylation is mediated by E1, E2 (Ubc9 as the only conjugating enzyme), and E3 enzymes, and can be reversed by SUMO-specific proteases (SENPs) via a de-SUMOylation process (Bernstock et al, 2018a; Yang et al, 2016). Unlike ubiquitin, there are 3 SUMO isoforms in mammalian cells: SUMO1, 2, and 3. Accumulating evidence points to a prominent role for SUMOylation in cellular proteostasis in both a UPS-dependent and independent manner (Liebelt and Vertegaal, 2016). Several proteomics studies have revealed an extensive interplay between SUMOylation and ubiquitination under stress conditions (Schimmel et al, 2008; Tatham et al, 2011; Yang et al, 2014; Yang et al, 2012). SUMO-targeted ubiquitin ligases that recognize SUMOylated proteins for degradation have been identified (Liebelt and Vertegaal, 2016; Tatham et al, 2008). On the other hand, since many SUMO targets are transcription factors or nuclear proteins involved in regulating gene expression, activation of SUMOylation can modulate many protein levels and thus, influence proteostasis independent of the UPS.
As a protein quality control mechanism, SUMOylation plays a major role in aging-related neurodegenerative diseases (Anderson et al, 2017; Princz and Tavernarakis, 2017). For example, key proteins involved in Alzheimer’s, Parkinson’s, and Huntington’s diseases are also SUMO targets. Data have shown that SUMOylation reduces generation of amyloid beta peptide (Li et al, 2003), increases solubility of α-synuclein (Krumova et al, 2011), and decreases formation of mutant huntingtin protein aggregates (Steffan et al, 2004). It is important to note that negative effects of SUMOylation on protein solubility and aggregation in neurodegenerative diseases have also been reported (Liebelt and Vertegaal, 2016). In brain ischemia, however, most agree that SUMOylation is neuroprotective (Bernstock et al, 2018a; Datwyler et al, 2011; Lee et al, 2009; Lee et al, 2014; Lee et al, 2011; Zhang et al, 2017). For example, overexpression of Ubc9 in mice leads to elevated levels of SUMOylated proteins in the brain, and confers neuroprotection against ischemia-induced brain injury (Lee et al, 2011). Further, when SUMOylation is suppressed by silencing SUMO1–3 expression in the mouse brain, the functional outcome after transient forebrain ischemia is worse (Zhang et al, 2017). Moreover, in vitro and in vivo data support the notion that SUMOylation contributes to the ischemic tolerance induced by preconditioning (Cuomo et al, 2016; Lee et al, 2009; Tong et al, 2015; Yang et al, 2008a). For example, knockdown of SUMO1 dramatically attenuates benefits from OGD preconditioning in cells (Lee et al, 2009); SUMOylation is increased after a short preconditioning period of transient MCAO (Yang et al, 2008a); and Ubc9 is required for isoflurane-induced preconditioning against ischemic neuronal injury (Tong et al, 2015). Notably, a recent case report showed an increase in SUMO1–3 immune reactivity in the ischemic penumbral neurons of a post-stroke human brain, a pattern similar to those observed in animal studies (Bernstock et al, 2017). This finding suggests clinical relevance of the SUMOylation pathway in brain ischemia diseases. In future experimental studies, an important next step is to determine how each of the 3 SUMO isoforms affects outcome after brain ischemia. This requires individual SUMO knockout mouse lines. In the case of SUMO2, however, a mouse line with conditional knockout is needed since global deletion of Sumo2 in mice is lethal (Wang et al, 2014a).
Information about the effect of age on the SUMOylation pathway is limited. A recent study by Ficulle et al showed that global SUMOylated protein levels in the brain peak at 6 months and then progressively decrease (Ficulle et al, 2018). We found that after transient forebrain ischemia and after CA, activation of SUMOylation is less pronounced in aged vs young brains (Liu et al, 2016; Shen et al, 2018). Considering that SUMOylation is neuroprotective, and that evidence supports a prominent role for SUMOylation in proteostasis, these aging-related changes in the SUMO conjugation pathway may contribute to worse outcome in aged vs young animals after an ischemic injury. Therefore, enhancing activation of the SUMOylation pathway would be expected to protect even aged brains against ischemic injury. Currently, however, there are few pharmacologic tools that can specifically target the SUMOylation pathway and thus, translational brain ischemia research in this area has been impeded (Bernstock et al, 2018a; Yang et al, 2016). Large-scale drug screenings are needed to address this deficit. Toward this end, an adaptation of our recently established high-throughput platform screening for SENP inhibitors could be useful (Bernstock et al, 2018b).
Autophagy
In the protein quality control network, autophagy is another essential protein degradation component, which is mediated by lysosomal degradation of protein aggregates and damaged organelles (Kroemer et al, 2010). Although there are 3 types of autophagy – macroautophagy, microautophagy, and chaperone-mediated autophagy – macroautophagy has been the most widely studied in aging and aging-related diseases (Liang and Sigrist, 2018; Nixon, 2013). Therefore, this discussion is focused on macroautophagy (hereafter called autophagy).
The autophagy process is tightly controlled, and engages multiple autophagy-related (ATG) proteins in 5 sequential steps: 1) initiation, 2) nucleation and formation of phagophores, 3) phagophore elongation and sequestration of cytoplasmic cargo to form autophagosomes, 4) fusion with lysosomes to form autolysosomes, and 5) degradation. Autophagy is primarily regulated negatively by the mammalian target of rapamycin (mTOR) pathway, and positively by the AMP-activated protein kinase (AMPK) pathway (Hansen et al, 2018). During the process, the cytosolic form of light chain 3 (LC3) is conjugated to phosphatidylethanolamine (PE) to form PE-conjugated LC3 (LC3-II), which is required for the autophagosomal membranes. Thus, LC3-II is commonly used as a marker for autophagy activity.
Autophagic activity declines with age, as has been observed in several species (Carnio et al, 2014; Chang et al, 2017; Kaushik et al, 2012; Sarkis et al, 1988; Simonsen et al, 2008). In C. elegans, for example, the number of autophagic vesicles increases and the level of lysosomal protease activity decreases with advancing age, indicating impaired autophagic activity (Chang et al, 2017; Sarkis et al, 1988). Further, in Drosophila, an aging-related reduction in expression of several autophagy genes in neural tissues has been observed, and enhancing autophagy in neural tissues extends lifespan and prevents accumulation of protein aggregates (Simonsen et al, 2008). Compared to young mouse brains, levels of the key autophagy-associated component ATG7 and steady-state LC3-II are decreased in aged mouse brains, and the rate of autophagolysosomal fusion is also reduced (Kaushik et al, 2012). Further, when ATG7 is deleted in mouse brain neurons, insoluble protein aggerates progressively accumulate, and these neurons then degenerate (Komatsu et al, 2006). Together, these findings show age-dependent impairment of autophagic function and lysosomal degradation. This impairment is believed to be responsible for the aging-related decline in proteostasis, which is associated with accumulation of misfolded and aggregated proteins, a hallmark of neurodegenerative diseases (Nixon, 2013). Indeed, pharmacologic enhancement of autophagy alleviates neuropathology and neurodegeneration in experimental models of neurodegenerative diseases (Boland et al, 2018; Liu et al, 2018; Spilman et al, 2010).
In contrast to the considerable research on autophagy in chronic neurodegenerative diseases, we have only begun to understand its role in acute brain ischemia. Moreover, these early findings are often inconsistent and sometimes overtly contradictory [reviewed in (Galluzzi et al, 2016; Wang et al, 2018)]. It is well established, however, that autophagy is activated after experimental brain ischemia, including stroke and CA (Carloni et al, 2010; Cui et al, 2016; Gao et al, 2012; Papadakis et al, 2013; Sheng et al, 2010; Zhang et al, 2014; Zhu et al, 2018), and imaging analysis shows that after ischemic stroke, autophagic activity is significantly increased in the neurons of the periischemic area in mouse brain (Tian et al, 2010). To dissect the role of autophagy in brain ischemia, a variety of pharmacologic tools have been used. Although 2 earlier animal studies reported marked reduction in infarct volumes after pre- or post-treatment with the autophagy inhibitor 3-methyladenine (3-MA) or bafilomycin A1 (Puyal et al, 2009; Wen et al, 2008), a more recent study found that pre-treatment with 3-MA worsens stroke outcome (Wang et al, 2014b). Moreover, the autophagy activator rapamycin, which targets mTOR activity, is neuroprotective in neonatal hypoxiaischemia (Carloni et al, 2010), and the beneficial effects of various pre-conditioning treatments are dependent on autophagy activation (Jiang et al, 2014; Jiang et al, 2015; Sheng et al, 2010).
Similar to other experimental brain ischemia studies, almost all studies on autophagy in brain ischemia have been performed on young animals. As discussed above, autophagy plays essential roles in maintaining cellular homeostasis and in the pro-survival response to stress, but its capacity to fulfill these roles declines with advancing age. Therefore, it is reasonable to speculate that the autophagic response to ischemic stress is different in aged vs young brains, which may contribute to the severity of brain damage after an ischemic event. Thus, to harness this important pathway and thereby, reduce injury after brain ischemia, future studies should focus not only on clarifying the role of autophagy in brain ischemia, but also on understanding how autophagy is impaired in the aged brain. Of note, there is a rich resource of pharmacologic tools that can be used to interfere with various steps of the autophagy process (Boland et al, 2018; Galluzzi et al, 2017).
Unfolded protein response
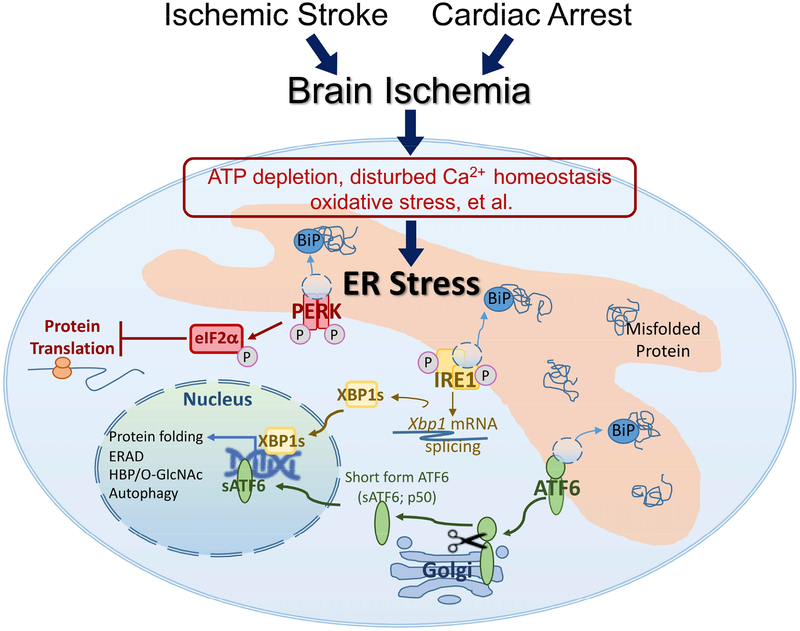
In addition to the ubiquitination and autophagy pathways largely in the cytosol, cells have also developed delicate systems to maintain proteostasis in subcellular organelles, especially in the endoplasmic reticulum (ER) where almost one-third of the total proteins are synthesized and folded (Braakman and Bulleid, 2011). ER function is sensitive to stress conditions, and impaired ER function (ER stress) leads to accumulation of misfolded or unfolded protein in the ER lumen. To restore ER function and reestablish proteostasis impaired by stress, cells activate a battery of adaptive processes, collectively known as the unfolded protein response (UPR; Figure 1). The UPR is driven by 3 ER stress sensors – protein kinase R-like ER kinase (PERK), activating transcription factor-6 (ATF6), and inositol-requiring enzyme-1 (IRE1) (Yang and Paschen, 2016). Upon activation, PERK phosphorylates eukaryotic initiation factor 2α (eIF2α), which inhibits global translation and thus, decreases the ER workload. Activation of the ATF6 and IRE1 branches generates 2 transcriptional factors – short form ATF6 (sATF6) and spliced Xbp1 (XBP1s). Both transcriptional factors can upregulate expression of genes encoding for ER chaperons (eg, GRP78/Bip), folding enzymes (eg, PDI), and ER-associated degradation (ERAD) proteins, and thereby enhance the ER capacity to correctly fold proteins and clear the unfolded/misfolded proteins. Interestingly, XBP1s also upregulates expression of multiple enzyme-genes in the hexosamine biosynthetic pathway (HBP). HBP generates uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), the substrate for O-GlcNAc protein modification (O-GlcNAcylation), a post-translational protein modification. Thus, under ER stress conditions, XBP1s couples UPR to O-GlcNAc modification via HBP. O-GlcNAcylation is involved in protein quality control, and is protective under a variety of stress conditions (Yang and Qian, 2017; Zhu et al, 2015).
Figure 1. The unfolded protein response (UPR).
Brain ischemia causes endoplasmic reticulum (ER) stress that activates UPR. The UPR pathway has 3 response branches controlled by 3 ER stress sensors – protein kinase R-like ER kinase (PERK), activating transcription factor-6 (ATF6), and inositol-requiring enzyme-1 (IRE1). Dissociation of GRP78/BiP (a major ER chaperone) from the ER stress sensors provides a mechanism to activate the UPR branches. Activation of the PERK branch results in phosphorylation of eukaryotic initiation factor 2?? (eIF2α), which inhibits global protein translation. Activation of the ATF6 and IRE1 branches generates 2 transcriptional factors – short form ATF6 (sATF6) and spliced Xbp1 (XBP1s). These transcriptional factors regulate expression of genes that are involved in the processes of protein folding, ER-associated degradation (ERAD), hexosamine biosynthetic pathway (HBP)/O-linked β-N-acetylglucosamine (O-GlcNAc), and autophagy.
UPR activity also declines with age (Martinez et al, 2017). Indeed, expression of key ER resident chaperones and folding enzymes, eg GPR78 and PDI, is decreased in aged vs young rat brains (Paz Gavilan et al, 2006). Under stress conditions, including proteasome inhibition and sleep deprivation, activation of all 3 UPR branches appears to be impaired in the aged brain (Gavilan et al, 2009; Naidoo et al, 2008). Given that the UPR is an essential component of the proteostasis network, it is not surprising that many studies suggest a key role for the aging-related decline in UPR activity in the abnormal accumulation of protein aggregates, which eventually contributes to the pathogenesis of neurodegenerative diseases (Hetz and Saxena, 2017; Martinez et al, 2017). Notably, the IRE1/XBP1s pathway is the most conserved of the UPR branches. Overexpression of XBP1s in neurons can increase lifespan in C. elegans by 30% (Taylor and Dillin, 2013), highlighting not only the importance of proteostasis in aging cells, but also the significance of the IRE1/XBP1s UPR branch in proteostasis.
Many experimental studies have demonstrated that both global and focal brain ischemia disrupt cellular proteostasis, and thus activate the UPR (Yang and Paschen, 2016), which may also be the case in ischemic human brains (Duan et al, 2010). However, an experimental study suggests dysfunction in regulation of the UPR in the post-ischemic brain, as it does not appear to be sufficiently activated to restore ER functions impaired by ischemia/reperfusion (Kumar et al, 2003). Specifically, the IRE1 and PERK branches are clearly activated after brain ischemia, reflected by a marked increase in Xbp1s mRNA levels and phosphorylated eIF2α protein levels. However, there is no convincing evidence that the ATF6 branch is activated in the post-CA or post-ischemic stroke brain (Kumar et al, 2003; Shen et al, 2018; Yu et al, 2017), even though it may be critical to cell recovery from disrupted proteostasis. Indeed, sATF6 can upregulate the expression of many pro-survival genes, especially grp78, which encodes for the most abundant ER chaperon. Recently, we discovered that forced activation of the ATF6 branch in neurons significantly reduces infarct sizes, and improves recovery of neurologic function after ischemic stroke in mice (Yu et al, 2017). Thus, activation of UPR to restore ER function impaired by ischemia is a pro-survival stress response. This is also supported by other studies using pharmacologic tools. For example, BIX is a small molecule that can preferentially induce GRP78 expression, and is neuroprotective in ischemic stroke (Kudo et al, 2008). Treatment with the chemical chaperone sodium 4-phenylbutyrate (4-PBA) attenuates ER stress-mediated cell death, and improves neurologic function in a mouse model of hypoxia-ischemia (Qi et al, 2004). Further, post-ischemic treatment with salubrinal, a specific inhibitor of dephosphorylation of p-eIF2α, results in long-lasting post-ischemic eIF2α phosphorylation and reduced ischemic brain damage (Anuncibay-Soto et al, 2016). Moreover, ischemic preconditioning increases expression of the ER resident chaperon GRP78, which prevents delayed neuronal cell death after subsequent harmful global brain ischemia in the rat (Hayashi et al, 2003).
Recently, we systematically compared activation of the UPR and associated O-GlcNAcylation in young and aged brains after transient forebrain ischemia and CA (Liu et al, 2016; Shen et al, 2018). In these studies, one prominent finding is that activation of O-GlcNAcylation is dysfunctional in the post-ischemic brain of aged mice. Ischemia-activated O-GlcNAcylation in the brain is neuroprotective, and largely dependent on the IRE1/XBP1s UPR branch, as deletion of XBP1 in the forebrain suppresses activation of O-GlcNAcylation, and leads to worse stroke outcome (Jiang et al, 2017). These findings suggest that the IRE1/XBP1s/O-GlcNAc axis plays an important role in protecting against brain damage by facilitating restoration of proteostasis impaired by ischemia. Notably, our comparative studies clearly indicate that activation of the IRE1/XBP1s/O-GlcNAc axis is impaired in the aged brain after ischemia. Thus, pharmacologic interventions that reverse this aging-related impairment would be expected to improve outcome after brain ischemia in aged animals. Indeed, we provided the first evidence that treatment with thiamet-G, which is able to increase O-GlcNAcylation in the aged brain, significantly reduces infarct sizes, and improves neurologic function in aged mice after ischemic stroke (Jiang et al, 2017). These data also indicate a promising new strategy in the ongoing search for novel interventions that are neuroprotective in brain ischemia, namely to focus on pro-survival pathways that are activated in the young, but not the aged, ischemic brain.
Conclusions and perspectives
Stroke and CA are potentially devastating medical conditions that most often strike elderly people, and can cause severe brain damage. Clinical studies show that outcomes after CA and stroke worsen with increasing age (Ay et al, 2005; Knoflach et al, 2012; Terman et al, 2015). Such findings indicate an aging-related decline in the brain’s capacity to recover from an ischemic injury. More specifically, mounting evidence suggests that the capacity of the aged brain to preserve/restore proteostasis after ischemia is compromised (Jiang et al, 2017; Kaushik and Cuervo, 2015; Liu et al, 2016; Shen et al, 2018). Therefore, enhancing this capacity by boosting relevant pathways would be expected to improve outcomes after brain ischemia, even in elderly patients. This strategy is believed to work more efficiently in the acute phase after brain ischemia/reperfusion because cell survival depends upon timely restoration of proteostasis, which has been disrupted by ischemic stress in the brain. Such evidence of proof-of-concept has recently been provided. Activation of O-GlcNAcylation in the post-ischemic brain is severely impaired in the aged vs young brains; however, if O-GlcNAcylation is boosted pharmacologically at 30 minutes after ischemia, stroke outcome is improved in aged mice (Jiang et al, 2017). Thus, pharmacologic interventions to boost proteostasis-related pathways that are impaired in aged animals, may hold great promise to improve outcome in elderly brain ischemia patients.
Although animal models may not be able to faithfully mimic all aspects of human disease, experimental studies that have used these models have provided tremendous insights into the pathophysiology of stroke and CA (Dirnagl and Endres, 2014; Vognsen et al, 2017). Consequently, such animal research is deemed essential for the discovery of new and promising therapeutic targets in human brain ischemia. However, most experimental stroke and CA studies are conducted in young animals, which may critically contribute to the disappointing results in translational brain ischemia research (Yang and Paschen, 2017). Thus, although we acknowledge the obvious challenges in experimental brain ischemia studies on aged animals – including difficulty in performing surgeries, relatively high animal cost, and considerably higher mortality – more brain ischemia studies in aged animals are clearly justified from a translational perspective. First, most CA and stroke patients are elderly. Second, compelling evidence indicates that the stress response of many key cellular processes, especially proteostasis, is impaired in aged brains compared to young brains. Finally, many studies have demonstrated that enhancing the aged brain’s capacity to restore proteostasis, prevents proteotoxicity and exerts remarkably neuroprotective effects in a variety of brain disorders and diseases including brain ischemia.
Highlights:
Proteostasis is essential for cellular and organismal health.
Brain ischemia disrupts cellular proteostasis and activates adaptive pathways.
Activation of proteostasis pathways in the post-ischemic aged brain is impaired.
This impairment needs to be considered in translational brain ischemia research.
Acknowledgement:
We thank Kathy Gage for her excellent editorial contribution.
Funding Sources: This work was supported by the National Institutes of Health [grant numbers NS099590 and NS097554] and the American Heart Association [grant numbers 18CSA34080277 and 16GRNT30270003].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson DB, Zanella CA, Henley JM, Cimarosti H, 2017. Sumoylation: Implications for Neurodegenerative Diseases. Adv Exp Med Biol 963, 261–281. [DOI] [PubMed] [Google Scholar]
- Anuncibay-Soto B, Perez-Rodriguez D, Santos-Galdiano M, Font E, Regueiro-Purrinos M, Fernandez-Lopez A, 2016. Post-ischemic salubrinal treatment results in a neuroprotective role in global cerebral ischemia. J Neurochem 138, 295–306. [DOI] [PubMed] [Google Scholar]
- Ay H, Koroshetz WJ, Vangel M, Benner T, Melinosky C, Zhu M, Menezes N, Lopez CJ, Sorensen AG, 2005. Conversion of ischemic brain tissue into infarction increases with age. Stroke 36, 2632–2636. [DOI] [PubMed] [Google Scholar]
- Baraibar MA, Friguet B, 2012. Changes of the proteasomal system during the aging process. Prog Mol Biol Transl Sci 109, 249–275. [DOI] [PubMed] [Google Scholar]
- Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE, 2011. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov 10, 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on, E., Prevention Statistics, C., Stroke Statistics, S., 2018. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137, e67–e492. [DOI] [PubMed] [Google Scholar]
- Bernstock JD, Yang W, Ye DG, Shen Y, Pluchino S, Lee YJ, Hallenbeck JM, Paschen W, 2018a. SUMOylation in brain ischemia: Patterns, targets, and translational implications. J Cereb Blood Flow Metab 38, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstock JD, Ye D, Smith JA, Lee YJ, Gessler FA, Yasgar A, Kouznetsova J, Jadhav A, Wang Z, Pluchino S, Zheng W, Simeonov A, Hallenbeck JM, Yang W, 2018b. Quantitative high-throughput screening identifies cytoprotective molecules that enhance SUMO conjugation via the inhibition of SUMO-specific protease (SENP)2. FASEB J 32, 1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstock JD, Ye DG, Griffin A, Lee YJ, Lynch J, Latour LL, Friedman GK, Maric D, Hallenbeck JM, 2017. Cerebral Ischemia Increases Small Ubiquitin-Like Modifier Conjugation within Human Penumbral Tissue: Radiological-Pathological Correlation. Front Neurol 8, 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E, Pastores GM, Rubinsztein DC, Nixon RA, Duchen MR, Mallucci GR, Kroemer G, Levine B, Eskelinen E-L, Mochel F, Spedding M, Louis C, Martin OR, Millan MJ, 2018. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov 17, 660–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I, Bulleid NJ, 2011. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem 80, 71–99. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Salazar IL, Curcio M, Canzoniero LM, Duarte CB, 2014. Role of the ubiquitinproteasome system in brain ischemia: friend or foe? Prog Neurobiol 112, 50–69. [DOI] [PubMed] [Google Scholar]
- Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W, 2010. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy 6, 366–377. [DOI] [PubMed] [Google Scholar]
- Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, Reischl M, Canepari M, Loefler S, Kern H, Blaauw B, Friguet B, Bottinelli R, Rudolf R, Sandri M, 2014. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep 8, 1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Kumsta C, Hellman AB, Adams LM, Hansen M, 2017. Spatiotemporal regulation of autophagy during Caenorhabditis elegans aging. Elife 6, e18459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Shang H, Zhang X, Jiang W, Jia X, 2016. Cardiac arrest triggers hippocampal neuronal death through autophagic and apoptotic pathways. Sci Rep 6, 27642. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cuomo O, Pignataro G, Sirabella R, Molinaro P, Anzilotti S, Scorziello A, Sisalli MJ, Di Renzo G, Annunziato L, 2016. Sumoylation of LYS590 of NCX3 f-Loop by SUMO1 Participates in Brain Neuroprotection Induced by Ischemic Preconditioning. Stroke 47, 1085–1093. [DOI] [PubMed] [Google Scholar]
- Datwyler AL, Lattig-Tunnemann G, Yang W, Paschen W, Lee SL, Dirnagl U, Endres M, Harms C, 2011. SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metab 31, 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Endres M, 2014. Found in translation: preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke 45, 1510–1518. [DOI] [PubMed] [Google Scholar]
- Duan SR, Wang JX, Wang J, Xu R, Zhao JK, Wang DS, 2010. Ischemia induces endoplasmic reticulum stress and cell apoptosis in human brain. Neurosci Lett 475, 132–135. [DOI] [PubMed] [Google Scholar]
- Ficulle E, Sufian MDS, Tinelli C, Corbo M, Feligioni M, 2018. Aging-related SUMOylation pattern in the cortex and blood plasma of wild type mice. Neurosci Lett 668, 48–54. [DOI] [PubMed] [Google Scholar]
- Fink EL, Berger RP, Clark RS, Watson RS, Angus DC, Panigrahy A, Richichi R, Callaway CW, Bell MJ, Mondello S, Hayes RL, Kochanek PM, 2016. Exploratory study of serum ubiquitin carboxyl-terminal esterase L1 and glial fibrillary acidic protein for outcome prognostication after pediatric cardiac arrest. Resuscitation 101, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Bravo-San Pedro JM, Blomgren K, Kroemer G, 2016. Autophagy in acute brain injury. Nat Rev Neurosci 17, 467–484. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G, 2017. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov 16, 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Jiang T, Guo J, Liu Y, Cui G, Gu L, Su L, Zhang Y, 2012. Inhibition of autophagy contributes to ischemic postconditioning-induced neuroprotection against focal cerebral ischemia in rats. PLoS One 7, e46092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilan MP, Pintado C, Gavilan E, Jimenez S, Rios RM, Vitorica J, Castano A, Ruano D, 2009. Dysfunction of the unfolded protein response increases neurodegeneration in aged rat hippocampus following proteasome inhibition. Aging Cell 8, 654–665. [DOI] [PubMed] [Google Scholar]
- Gozal D, Row BW, Kheirandish L, Liu R, Guo SZ, Qiang F, Brittian KR, 2003. Increased susceptibility to intermittent hypoxia in aging rats: changes in proteasomal activity, neuronal apoptosis and spatial function. J Neurochem 86, 1545–1552. [DOI] [PubMed] [Google Scholar]
- Hansen M, Rubinsztein DC, Walker DW, 2018. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol 19, 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Shimizu W, Albert CM, 2015. The spectrum of epidemiology underlying sudden cardiac death. Circ Res 116, 1887–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Chan PH, 2003. Induction of GRP78 by ischemic preconditioning reduces endoplasmic reticulum stress and prevents delayed neuronal cell death. J Cereb Blood Flow Metab 23, 949–961. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Takada K, Matsuda M, 1991. Changes in ubiquitin and ubiquitin-protein conjugates in the CA1 neurons after transient sublethal ischemia. Mol Chem Neuropathol 15, 75–82. [DOI] [PubMed] [Google Scholar]
- Hetz C, Saxena S, 2017. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13, 477–491. [DOI] [PubMed] [Google Scholar]
- Hochrainer K, 2018. Protein Modifications with Ubiquitin as Response to Cerebral Ischemia-Reperfusion Injury. Transl Stroke Res 9, 157–173. [DOI] [PubMed] [Google Scholar]
- Hochrainer K, Jackman K, Anrather J, Iadecola C, 2012. Reperfusion rather than ischemia drives the formation of ubiquitin aggregates after middle cerebral artery occlusion. Stroke 43, 2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BR, Janelidze S, Ginsberg MD, Busto R, Perez-Pinzon M, Sick TJ, Siesjo BK, Liu CL, 2001. Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metab 21, 865–875. [DOI] [PubMed] [Google Scholar]
- Hu BR, Martone ME, Jones YZ, Liu CL, 2000. Protein aggregation after transient cerebral ischemia. J Neurosci 20, 3191–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber N, Sakai N, Eismann T, Shin T, Kuboki S, Blanchard J, Schuster R, Edwards MJ, Wong HR, Lentsch AB, 2009. Age-related decrease in proteasome expression contributes to defective nuclear factor-kappaB activation during hepatic ischemia/reperfusion. Hepatology 49, 1718–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi M, Sheng H, Thompson JW, Wang L, Dubois LG, Gooden D, Moseley M, Paschen W, Yang W, 2014. Characterization of the ubiquitin-modified proteome regulated by transient forebrain ischemia. J Cereb Blood Flow Metab 34, 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Yu S, Yu Z, Sheng H, Li Y, Liu S, Warner DS, Paschen W, Yang W, 2017. XBP1 (X-Box-Binding Protein-1)-Dependent O-GlcNAcylation Is Neuroprotective in Ischemic Stroke in Young Mice and Its Impairment in Aged Mice Is Rescued by Thiamet-G. Stroke 48, 1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD, Tan L, 2014. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 171, 3146–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, Wang HF, Shi JQ, Gao L, Qin H, Zhang YD, Tan L, 2015. Ischemic preconditioning provides neuroprotection by induction of AMP-activated protein kinase-dependent autophagy in a rat model of ischemic stroke. Mol Neurobiol 51, 220–229. [DOI] [PubMed] [Google Scholar]
- Kang HM, Sohn I, Jung J, Jeong JW, Park C, 2016. Age-related changes in pial arterial structure and blood flow in mice. Neurobiol Aging 37, 161–170. [DOI] [PubMed] [Google Scholar]
- Kapetanou M, Chondrogianni N, Petrakis S, Koliakos G, Gonos ES, 2017. Proteasome activation enhances stemness and lifespan of human mesenchymal stem cells. Free Radic Biol Med 103, 226–235. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Arias E, Kwon H, Lopez NM, Athonvarangkul D, Sahu S, Schwartz GJ, Pessin JE, Singh R, 2012. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep 13, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM, 2015. Proteostasis and aging. Nat Med 21, 1406–1415. [DOI] [PubMed] [Google Scholar]
- Keller JN, Huang FF, Zhu H, Yu J, Ho YS, Kindy TS, 2000. Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab 20, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Kevei E, Hoppe T, 2014. Ubiquitin sets the timer: impacts on aging and longevity. Nat Struct Mol Biol 21, 290–292. [DOI] [PubMed] [Google Scholar]
- Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C, Seyfang L, Kiechl S, Willeit J, Austrian Stroke Unit Registry, C., 2012. Functional recovery after ischemic stroke--a matter of age: data from the Austrian Stroke Unit Registry. Neurology 78, 279–285. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K, 2006. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884. [DOI] [PubMed] [Google Scholar]
- Korovila I, Hugo M, Castro JP, Weber D, Hohn A, Grune T, Jung T, 2017. Proteostasis, oxidative stress and aging. Redox Biol 13, 550–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B, 2010. Autophagy and the integrated stress response. Mol Cell 40, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumova P, Meulmeester E, Garrido M, Tirard M, Hsiao HH, Bossis G, Urlaub H, Zweckstetter M, Kugler S, Melchior F, Bahr M, Weishaupt JH, 2011. Sumoylation inhibits alpha-synuclein aggregation and toxicity. J Cell Biol 194, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, Tabira T, Imaizumi K, Takeda M, 2008. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ 15, 364–375. [DOI] [PubMed] [Google Scholar]
- Kumar R, Krause GS, Yoshida H, Mori K, DeGracia DJ, 2003. Dysfunction of the unfolded protein response during global brain ischemia and reperfusion. J Cereb Blood Flow Metab 23, 462–471. [DOI] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI, 2015. The biology of proteostasis in aging and disease. Annu Rev Biochem 84, 435–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA, 1999. Gene expression profile of aging and its retardation by caloric restriction. Science 285, 1390–1393. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Castri P, Bembry J, Maric D, Auh S, Hallenbeck JM, 2009. SUMOylation participates in induction of ischemic tolerance. J Neurochem 109, 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Mou Y, Klimanis D, Bernstock JD, Hallenbeck JM, 2014. Global SUMOylation is a molecular mechanism underlying hypothermia-induced ischemic tolerance. Front Cell Neurosci 8, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Mou Y, Maric D, Klimanis D, Auh S, Hallenbeck JM, 2011. Elevated global SUMOylation in Ubc9 transgenic mice protects their brains against focal cerebral ischemic damage. PLoS One 6, e25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang H, Wang S, Quon D, Liu YW, Cordell B, 2003. Positive and negative regulation of APP amyloidogenesis by sumoylation. Proc Natl Acad Sci U S A 100, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Sigrist S, 2018. Autophagy and proteostasis in the control of synapse aging and disease. Curr Opin Neurobiol 48, 113–121. [DOI] [PubMed] [Google Scholar]
- Liebelt F, Vertegaal AC, 2016. Ubiquitin-dependent and independent roles of SUMO in proteostasis. Am J Physiol Cell Physiol 311, C284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu W, Lu Y, Tian H, Duan C, Lu L, Gao G, Wu X, Wang X, Yang H, 2018. Piperlongumine restores the balance of autophagy and apoptosis by increasing BCL2 phosphorylation in rotenone-induced Parkinson disease models. Autophagy 14, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Sheng H, Yu Z, Paschen W, Yang W, 2016. O-linked beta-N-acetylglucosamine modification of proteins is activated in post-ischemic brains of young but not aged mice: Implications for impaired functional recovery from ischemic stress. J Cereb Blood Flow Metab 36, 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanero S, Santro T, Arumugam TV, 2013. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int 62, 712–718. [DOI] [PubMed] [Google Scholar]
- Martinez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, Hetz C, 2017. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell 16, 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller R, Cameron JA, Torrey DJ, Clayton CE, Ordonez AN, Henshall DC, Minami M, Schindler CK, Saugstad JA, Simon RP, 2006. Rapid degradation of Bim by the ubiquitin-proteasome pathway mediates short-term ischemic tolerance in cultured neurons. J Biol Chem 281, 7429–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller R, Thompson SJ, Lusardi TA, Ordonez AN, Ashley MD, Jessick V, Wang W, Torrey DJ, Henshall DC, Gafken PR, Saugstad JA, Xiong ZG, Simon RP, 2008. Ubiquitin proteasome-mediated synaptic reorganization: a novel mechanism underlying rapid ischemic tolerance. J Neurosci 28, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N, Ferber M, Master M, Zhu Y, Pack AI, 2008. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci 28, 6539–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakka VP, Lang BT, Lenschow DJ, Zhang DE, Dempsey RJ, Vemuganti R, 2011. Increased cerebral protein ISGylation after focal ischemia is neuroprotective. J Cereb Blood Flow Metab 31, 2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, 2013. The role of autophagy in neurodegenerative disease. Nat Med 19, 983–997. [DOI] [PubMed] [Google Scholar]
- Pandian JD, Gall SL, Kate MP, Silva GS, Akinyemi RO, Ovbiagele BI, Lavados PM, Gandhi DBC, Thrift AG, 2018. Prevention of stroke: a global perspective. Lancet 392, 1269–1278. [DOI] [PubMed] [Google Scholar]
- Papadakis M, Hadley G, Xilouri M, Hoyte LC, Nagel S, McMenamin MM, Tsaknakis G, Watt SM, Drakesmith CW, Chen R, Wood MJ, Zhao Z, Kessler B, Vekrellis K, Buchan AM, 2013. Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat Med 19, 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz Gavilan M, Vela J, Castano A, Ramos B, del Rio JC, Vitorica J, Ruano D, 2006. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging 27, 973–982. [DOI] [PubMed] [Google Scholar]
- Princz A, Tavernarakis N, 2017. The role of SUMOylation in ageing and senescent decline. Mech Ageing Dev 162, 85–90. [DOI] [PubMed] [Google Scholar]
- Puyal J, Vaslin A, Mottier V, Clarke PG, 2009. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol 66, 378–389. [DOI] [PubMed] [Google Scholar]
- Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y, 2004. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol 66, 899–908. [DOI] [PubMed] [Google Scholar]
- Sarkis GJ, Ashcom JD, Hawdon JM, Jacobson LA, 1988. Decline in protease activities with age in the nematode Caenorhabditis elegans. Mech Ageing Dev 45, 191–201. [DOI] [PubMed] [Google Scholar]
- Schaller B, Graf R, 2004. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab 24, 351–371. [DOI] [PubMed] [Google Scholar]
- Schimmel J, Larsen KM, Matic I, van Hagen M, Cox J, Mann M, Andersen JS, Vertegaal AC, 2008. The ubiquitin-proteasome system is a key component of the SUMO-2/3 cycle. Mol Cell Proteomics 7, 2107–2122. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Patnaik R, Sharma A, Lafuente JV, Miclescu A, Wiklund L, 2015. Cardiac Arrest Alters Regional Ubiquitin Levels in Association with the Blood-Brain Barrier Breakdown and Neuronal Damages in the Porcine Brain. Mol Neurobiol 52, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Shen Y, Yan B, Zhao Q, Wang Z, Wu J, Ren J, Wang W, Yu S, Sheng H, Crowley S, Ding F, Paschen W, Yang W, 2018. Aging is associated with impaired activation of protein homeostasis-related pathways after cardiac arrest in mice. J Am Heart Assoc 7, e009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng R, Zhang LS, Han R, Liu XQ, Gao B, Qin ZH, 2010. Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy 6, 482–494. [DOI] [PubMed] [Google Scholar]
- Shi L, Rocha M, Leak RK, Zhao J, Bhatia TN, Mu H, Wei Z, Yu F, Weiner SL, Ma F, Jovin TG, Chen J, 2018. A new era for stroke therapy: Integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab 38, 2073–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD, 2008. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 4, 176–184. [DOI] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V, 2010. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 5, e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, Pandolfi PP, Thompson LM, Marsh JL, 2004. SUMO modification of Huntingtin and Huntington’s disease pathology. Science 304, 100–104. [DOI] [PubMed] [Google Scholar]
- Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT, 2008. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol 10, 538–546. [DOI] [PubMed] [Google Scholar]
- Tatham MH, Matic I, Mann M, Hay RT, 2011. Comparative proteomic analysis identifies a role for SUMO in protein quality control. Sci Signal 4, rs4. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Dillin A, 2013. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153, 1435–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman SW, Shields TA, Hume B, Silbergleit R, 2015. The influence of age and chronic medical conditions on neurological outcomes in out of hospital cardiac arrest. Resuscitation 89, 169–176. [DOI] [PubMed] [Google Scholar]
- Tian F, Deguchi K, Yamashita T, Ohta Y, Morimoto N, Shang J, Zhang X, Liu N, Ikeda Y, Matsuura T, Abe K, 2010. In vivo imaging of autophagy in a mouse stroke model. Autophagy 6, 1107–1114. [DOI] [PubMed] [Google Scholar]
- Tong L, Wu Z, Ran M, Chen Y, Yang L, Zhang H, Zhang L, Dong H, Xiong L, 2015. The Role of SUMO-Conjugating Enzyme Ubc9 in the Neuroprotection of Isoflurane Preconditioning Against Ischemic Neuronal Injury. Mol Neurobiol 51, 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernace VA, Arnaud L, Schmidt-Glenewinkel T, Figueiredo-Pereira ME, 2007. Aging perturbs 26S proteasome assembly in Drosophila melanogaster. FASEB J 21, 2672–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D, Saez I, Dillin A, 2014. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun 5, 5659. [DOI] [PubMed] [Google Scholar]
- Vognsen M, Fabian-Jessing BK, Secher N, Lofgren B, Dezfulian C, Andersen LW, Granfeldt A, 2017. Contemporary animal models of cardiac arrest: A systematic review. Resuscitation 113, 115–123. [DOI] [PubMed] [Google Scholar]
- Wang L, Wansleeben C, Zhao S, Miao P, Paschen W, Yang W, 2014a. SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development. EMBO Rep 15, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Shao BZ, Deng Z, Chen S, Yue Z, Miao CY, 2018. Autophagy in ischemic stroke. Prog Neurobiol 163–164, 98–117. [DOI] [PubMed] [Google Scholar]
- Wang P, Xu TY, Wei K, Guan YF, Wang X, Xu H, Su DF, Pei G, Miao CY, 2014b. ARRB1/beta-arrestin-1 mediates neuroprotection through coordination of BECN1-dependent autophagy in cerebral ischemia. Autophagy 10, 1535–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH, 2008. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 4, 762–769. [DOI] [PubMed] [Google Scholar]
- Williams K, Irwin DA, Jones DG, Murphy KM, 2010. Dramatic Loss of Ube3A Expression during Aging of the Mammalian Cortex. Front Aging Neurosci 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J, 2009. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Paschen W, 2016. Unfolded protein response in brain ischemia: A timely update. J Cereb Blood Flow Metab 36, 2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Paschen W, 2017. Is age a key factor contributing to the disparity between success of neuroprotective strategies in young animals and limited success in elderly stroke patients? Focus on protein homeostasis. J Cereb Blood Flow Metab 37, 3318–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Sheng H, Thompson JW, Zhao S, Wang L, Miao P, Liu X, Moseley MA, Paschen W, 2014. Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: putative protective proteins/pathways. Stroke 45, 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Sheng H, Wang H, 2016. Targeting the SUMO pathway for neuroprotection in brain ischaemia. Stroke Vasc Neurol 1, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Sheng H, Warner DS, Paschen W, 2008a. Transient focal cerebral ischemia induces a dramatic activation of small ubiquitin-like modifier conjugation. J Cereb Blood Flow Metab 28, 892–896. [DOI] [PubMed] [Google Scholar]
- Yang W, Sheng H, Warner DS, Paschen W, 2008b. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab 28, 269–279. [DOI] [PubMed] [Google Scholar]
- Yang W, Thompson JW, Wang Z, Wang L, Sheng H, Foster MW, Moseley MA, Paschen W, 2012. Analysis of oxygen/glucose-deprivation-induced changes in SUMO3 conjugation using SILAC-based quantitative proteomics. J Proteome Res 11, 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Qian K, 2017. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol 18, 452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Sheng H, Liu S, Zhao S, Glembotski CC, Warner DS, Paschen W, Yang W, 2017. Activation of the ATF6 branch of the unfolded protein response in neurons improves stroke outcome. J Cereb Blood Flow Metab 37, 1069–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu X, Sheng H, Liu S, Li Y, Zhao JQ, Warner DS, Paschen W, Yang W, 2017. Neuron-specific SUMO knockdown suppresses global gene expression response and worsens functional outcome after transient forebrain ischemia in mice. Neuroscience 343, 190–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yuan Y, Jiang L, Zhang J, Gao J, Shen Z, Zheng Y, Deng T, Yan H, Li W, Hou WW, Lu J, Shen Y, Dai H, Hu WW, Zhang Z, Chen Z, 2014. Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: Involvement of PARK2-dependent mitophagy. Autophagy 10, 1801–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Liu K, Huang K, Gu Y, Hu Y, Pan S, Ji Z, 2018. Metformin Improves Neurologic Outcome Via AMP-Activated Protein Kinase-Mediated Autophagy Activation in a Rat Model of Cardiac Arrest and Resuscitation. J Am Heart Assoc 7, e008389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu TW, Cecioni S, Eskandari R, Zandberg WF, Vocadlo DJ, 2015. O-GlcNAc occurs cotranslationally to stabilize nascent polypeptide chains. Nat Chem Biol 11, 319–325. [DOI] [PubMed] [Google Scholar]