Abstract
Conditioned medium (CM) derived from engineered cells often facilitates the cost-effective culture of a variety of stem cells. Growing emphasis on the importance of rigor and reproducibility in lab-based science requires development of best practices approaches, including quality control procedures for the assessment of CM batches to ensure reliable interpretation and reproducibility. Here, we tested activity level variations of L-WRN CM, which is produced from an L cell line engineered to secrete Wnt3a, R spondin 3, and Noggin into a single CM that is widely used for gastrointestinal stem cell culture. We assessed 14 independent batches of L-WRN CM, produced by 5 laboratories at 3 research institutions, by multiple quantitative assays. We observed highly replicable activity levels among L-WRN CM batches prepared according to a previously published protocol. Quality control assays measuring spheroid growth or mRNA gene marker expression were best able to distinguish the quality L-WRN CM batches, whereas a Wnt reporter assay did not. Thus, we have validated that L-WRN CM activity is highly reproducible over time and between laboratories and have provided guidelines for L-WRN CM quality control testing. These validation procedures and guidelines will benefit experiment replication efforts in stem cell research.
Keywords: Organoid, Spheroid, Media, Lgr5, Intestine
1. Introduction
Organoids derived from pluripotent or adult tissue stem cells can be cultured to model gastrointestinal epithelium. These primary cultures generally contain the mitotic stem and progenitor cells as well as the post-mitotic differentiated cell types normally present in the in vivo tissue. Consequently, organoid culture methods have quickly gained traction in the scientific community for basic and translational research studies of gastrointestinal physiology and pathophysiology (Clevers, 2016; Miyoshi, 2017; Nakamura and Sato, 2018). In order to derive organoids from adult tissue stem cells, the culture conditions must contain components of the stem cell niche (Clevers, 2016). Intestinal organoids form and are maintained when the stem cells are embedded in an extracellular matrix (e.g. Matrigel) and are provided with medium containing 3 necessary protein factors, Wnt3a, R spondin 1, 2, or 3, and Noggin (Sato et al., 2009). These factors can be delivered as recombinant proteins or via conditioned medium (CM) from other cell lines engineered to secrete these proteins individually or in combination (Sato et al., 2009; Heijmans et al., 2013; Miyoshi and Stappenbeck, 2013; Ootani et al., 2009; Wen et al., 2017; Wang et al., 2013). Media composition and the delivery method of stem cell niche factors can alter the cellular composition and behavior of the cultured epithelial cells in downstream assays.
The National Institutes of Health, leading scientitic journal editorial boards, and others in the scientific community are calling for increased transparency, rigor, and reproducibility in experimental science (Collins and Tabak, 2014; Jackson, 2015; McNutt, 2014). This appeal is due in part to reports of cell line cross-contamination and lax validation of key biological reagents leading to erroneous experiment results, wasted time and resources, and diminished public trust in science (Freedman et al., 2015; Baker, 2016). A major action item from these appeals is the incorporation of rigorous procedures to authenticate and validate reagents that could differ over time within a single laboratory or between laboratories (Collins and Tabak, 2014; Jarvis and Williams, 2016). Related to this issue, there is a call for increased transparency in reporting the authentication and validation procedures used by investigators to improve interpretation and replication of scientific findings. The CM used for primary intestinal stem cell culture represents one such key biological reagent that could differ over time or between laboratory groups, and thus it should be subjected to rigorous quality control procedures. Furthermore, the quality control methods and results should be reported to the scientific community.
The Stappenbeck laboratory previously developed a CM cell line, referred to as the L-WRN cell line. L-WRN cells are L cells that secrete Wnt3a, R spondin 3, and Noggin into a single CM (Miyoshi et al., 2012). The L-WRN CM is a specialized biological reagent used to culture epithelial cells from multiple endodermal tissues of a variety of mammals, including mouse and human gastrointestinal epithelial stem cells (Miyoshi and Stappenbeck, 2013; VanDussen et al., 2015; Aly et al., 2013; Powell and Behnke, 2017). Intestinal stem cells embedded in Matrigel and provided with L-WRN CM will form nearly spherical, non-budding three-dimensional structures that have an inner lumen and are comprised of nearly all mitotic stem and progenitor cells (Miyoshi and Stappenbeck, 2013; Miyoshi et al., 2012; VanDussen et al., 2015). These structures have been termed spheroids to distinguish them from the budding structures termed organoids, enteroids, or colonoids, which are comprised of a mixture of proliferating and post-mitotic epithelial cells (Stelzner et al., 2012). Because spheroids are highly enriched for proliferating cells, they expand at a relatively rapid rate to facilitate sufficient production of cells for use in downstream assays, including Transwell culture (VanDussen et al., 2015; Moon et al., 2014). Replacement of the L-WRN CM with a differentiation medium promotes formation of mature epithelial cell lineages, such as enterocytes, colonocytes, goblet cells, or wound-associated epithelial cells (Kaiko et al., 2016; Miyoshi et al., 2017; Patel et al., 2013). Dilution of the L-WRN CM can also promote formation of post-mitotic cells (VanDussen et al., 2015). Because the concentration (i.e. activity) of the L-WRN CM can affect cell state, it is critical to assess batch-to-batch activity levels. In addition, the L-WRN cell line is now being used by laboratories across the world, underscoring the need to investigate L-WRN CM batch-to-batch variation to ensure that experiments using this medium are reproducible within and between laboratories.
We surmised that L-WRN CM activity could be influenced by variables such as passage-to-passage behavioral shifts of the L-WRN cell line, medium storage time at 4 °C, L-WRN CM collection period differences, and slight technical variations in the L-WRN CM production process that potentially occur within or between laboratories. Taking these into account, we undertook an investigation of the variation in L-WRN CM activity levels using 14 batches of L-WRN CM produced by 5 laboratories across 3 research institutions. As a secondary objective, we sought to identify experimental assays that could discrimate the quality L-WRN CM batches in order to provide a framework for L-WRN CM quality control procedures to laboratories utilizing this key biological resource. Overall, this study showed that L-WRN CM activity was highly replicable across laboratory groups when the CM protocol was properly executed. This led us to conclude that L-WRN CM will enable faithful reproduction of spheroid culture experiments over time and between laboratory groups.
2. Materials and methods
2.1. Culture and authentication of L-WRN cells
L-WRN cells (ATCC®; Cat.# CRL-3276™) were cultured in high glucose DMEM (Sigma-Aldrich, Cat.# D5796) supplemented with penicillin (100 units/mL), streptomycin (0.1 mg/mL) (Sigma-Aldrich, Cat.# P4333) and 10% fetal bovine serum (Sigma-Aldrich, Cat.# F2442) (Fig. S1A) (Miyoshi et al., 2012). Mycoplasma infection screening was performed with the LookOut Mycoplasma PCR Detection kit (Sigma-Aldrich, Cat.# MP0035) as recommended by the manufacturer using cultured L-WRN cell supernatant (Dobrovolny and Bess, 2011). For species validation of the L-WRN line, PCR amplification of the mitochondrial cytochrome C oxidase I (COI) gene, which is highly conserved within a species but highly variable between species, was performed using published primer sets (Cooper et al., 2007). Genomic DNA template for species validation was isolated from cultured L-WRN cells using the QIAamp DNA Mini Kit (Qiagen; Cat.# 51304). Genomic DNA was isolated from mouse and human intestinal epithelial spheroids to use as controls. For the genomic DNA isolation, 200 μL of Buffer ATL and 20 μL of Proteinase K was added to cell pellets suspended in ~200 μL of PBS followed by incubation for 3 h at 56 °C. Next, 200 μL of Buffer AL was added and the mixture was incubated for 10 min at 70 °C. Subsequent steps followed the manufacturer’s directions, with 100 μL of Buffer AE used for elution. Sample concentrations were determined using a Cytation5 Multi-mode Reader (BioTek). PCR reactions used 2× JumpStart™ REDTaq® ReadyMix™ Reaction Mix (Sigma, Cat No. P0982), 0.4 μM final concentration of Homo sapiens primers (Hs-F and Hs-R), 0.6 μM final concentration of Mus musculus primers (Mmus-F and Mmus-R), and 10 ng of genomic DNA template. Cycling conditions were as follows: one cycle of 94 °C for 2 min; 35 cycles of 94 °C for 30 s, 60 °C for 30 s and 72 °C for 45 s; one cycle of 72 °C for 5 min; indefinite hold at 4 °C. Products were visualized using a 2% agarose gel stained with ethidium bromide. Expected product lengths were 391 bp for Homo sapiens and 159 bp for Mus musculus.
2.2. Production of L-WRN conditioned medium
The protocols for small-scale and large-scale L-WRN conditioned medium (L-WRN CM) production have been previously described (Miyoshi and Stappenbeck, 2013) (also see Fig. S1B). With the large-scale protocol, a Nunc™ EasyFill™ Cell Factory™ 10 (Fisher Scientific) is used to produce 16 L of 50% L-WRN CM over 8 days, with 8 L being produced during Days 1–4 and another 8 L being produced during Days 5–8. The 50% L-WRN CM is a 50/50 mix of the CM and fresh primary culture media, which is Advanced DMEM/F-12 (Invitrogen; Cat.# 12,634–010) supplemented with 20% fetal bovine serum (Sigma-Alrich; Cat.# F2442), 2 mM L-glutamine, 100 units/mL penicillin and 0.1 mg/ mL streptomycin. All experiments in this study used 50% L-WRN CM, unless indicated otherwise, that was aliquoted and stored at −20 °C or −80 °C for 0–8 months (CM1–13 and technical error (TE) batches were stored for 4–8 months at the time of testing). During L-WRN CM production for the TE batch, the incorrect volume of primary culture medium was added to the cell factory; reagent composition and all other performed procedures were equivalent between the TE and CM1–13 batches.
2.3. Intestinal epithelial spheroid culture
Colonic crypts were isolated from adult mouse or human rectal tissue and grown as three-dimensional spheroids in Matrigel as previously described (Miyoshi and Stappenbeck, 2013; VanDussen et al., 2015). The collection and use of human intestinal tissue for spheroid culture was approved by the Institutional Review Board of Washington University School of Medicine and written informed consent was obtained from the donor prior to inclusion in the study. Spheroids were maintained as enriched for stem and progenitor cells by culturing in L-WRN CM. For mouse spheroid culture, 10 μM Y-27632 (ROCK inhibitor; R&D Systems, Cat.# 1254) was added to the medium provided immediately upon passage. For human spheroid culture, the L-WRN CM was supplemented with 10 μM Y-27632 and 10 μM SB 431542 (TGF-βRI inhibitor; R&D Systems, Cat.# 1614) (VanDussen et al., 2015). To induce colonocyte differentiation, mouse and human spheroids were cultured for 36–48 h in differentiation medium, which is primary culture medium lacking serum and supplemented with 50 ng/mL EGF (Peprotech, Cat.# 315–09B), 10 μM Y-27632, and 10 μM L-161, 982 (EP4 inhibitor; R&D Systems, Cat.# 2514), as previously described (Kaiko et al., 2016; Miyoshi et al., 2017). For general maintenance, spheroids were passaged and embedded into Matrigel on Day 0, medium was changed on Day 2, and spheroids were passaged again on Day 3 or 4. For experiments, a minimum of 3 independent experiments were performed with distinct passages of spheroids (ranging from passage 8–15) for each assay (i.e., n ≥ 3 biological replicates per condition per assay). All of the L-WRN CM test batches and the appropriate controls were assessed concurrently within each experiment.
2.4. Imaging of cultured cells and determination of spheroid area
Live cultures of L-WRN cells and spheroids were imaged with a Pupil Cam camera (Ken-a-Vision) fixed to a phase microscope (Fisher Scientific) equipped with LPL4/0.10 4×, PH10X/0.25, and LWD PH20X/0.40 objective lenses. Adobe Photoshop CS6 was used to convert images to grayscale, adjust brightness and contrast, and crop images. Spheroid area was measured using ImageJ (Schneider et al., 2012), as described previously (VanDussen et al., 2015). The area of 52–70 spheroids was measured and averaged per condition for each of 3 independent experiments.
2.5. CellTiter-Glo assay
Spheroids were seeded into white-walled 96-well plates, with 5 μl droplets of spheroid-Matrigel mixture and 100 μL of L-WRN CM per well. For mouse spheroids, Day 3 spheroids in 1 well of a 12-well plate were used to seed ~12 wells of a 96-well plate. For human spheroids, Day 3 spheroids in 1 well of a 12-well plate were used to seed ~8 wells of a 96-well plate. Medium was changed daily. The CellTiter-Glo® 3D Cell Viability Assay (Promega; Cat.# G9681) was used to assess spheroid growth and viability on Day 3 of culture according to the manufacturer’s directions. Luminescence was detected using a Cytation5 Multi-mode Reader (BioTek). Culture wells containing L-WRN CM and CellTiter-Glo® reagent but without spheroids were used to determine the average background luminescence. For control wells containing spheroids induced to undergo cell death, 50 μg/mL cycloheximide (CHX; Enzo Life Sciences) and 100 ng/mL recombinant carrier-free mouse or human tumor necrosis factor (TNF; Biolegend) were added 15 h prior to addition of the CellTiter-Glo reagent. Assays were performed with quadruplicate technical replicates, which were averaged prior to statistical analysis, for three independent experiments with distinct passages of spheroids.
2.6. Cdc25A-CBR luciferase assay
Analysis of proliferation with the Cdc25A-CBRLuc murine colonic spheroid line has been previously described (Kaiko et al., 2016; Sun et al., 2015). Assays were performed with quadruplicate technical replicates, which were averaged prior to statistical analysis. Data for each of three independent experiments were normalized to the average time 0 h value of all samples within each experiment. As a control, 1 mM of butyrate was added to the L-WRN CM in some wells.
2.7. LDH assay
The Pierce™ LDH Cytotoxicity Assay Kit (Thermofisher Scientific; Cat.# 88953) was used to assess cellular cytotoxicity according to manufacturer’s directions using L-WRN CM alone or the medium supernatant from spheroid cultures. A Cytation5 Multi-mode Reader (BioTek) was used to measure the absorbance at 490 nm and 680 nm of each well. The A680 nm value (background signal from the instrument) was subtracted from the A490 nm value to determine the LDH activity. The LDH positive control (LDH+) was included with the kit. For L-WRN CM without exposure to cultured spheroids, the LDH assay was performed with quadruplicate technical replicates, which were averaged prior to statistical analysis, in three independent experiments with distinct assay reaction mixtures. For L-WRN CM with exposure to cultured spheroids, assays were performed with quadruplicate technical replicates, which were averaged prior to statistical analysis, for three independent experiments with distinct passages of spheroids.
2.8. RNA isolation and qPCR analysis
Total RNA was purified from 1-well of a 12-well plate of cultured spheroids on Day 3 post-seeding using the NucleoSpin RNA II kit (Machery-Nagel, Duren, Germany). Sample concentration was determined using a Cytation5 Multi-mode Reader (BioTek). For quantitative reverse transcription PCR (qPCR), cDNA was synthesized using 1 μg of total RNA and the iScript™ Reverse Transcription Supermix (Bio-Rad: Cat.# 1708841). Quantitative PCR reactions used technical duplicates for each of three biological replicates per experimental condition and contained SYBR Advantage qPCR Premix (Clontech; Cat.# 639676), 200 nM of each primer, and 40 ng cDNA. A BioRad CFX Connect Real-time System Thermocycler was used with the following cycling parameters: 1 cycle of 95 °C for 2 min; 40 cycles of 95 °C for 10 s, 60 °C for 20 s and 72 °C for 30 s; 1 cycle of 72 °C for 5 min; melting curve analysis over a temperature range of 95 °C to 60 °C; indefinite hold at 4 °C. Primers were synthesized at Sigma-Aldrich. All primer pairs were validated to have an efficiency of 100% ± 10% over a range of cDNA concentrations and to generate a single product by agarose gel analysis and Sanger sequencing. Primer sequences are listed in Supplementary Table 1. Relative gene expression levels were determined with the ΔΔCT method and the housekeeper gene glyceraldehyde 3-phosphate dehydrogenase (Gapdh), which was expressed at similar levels in all samples. Technical repeats were averaged prior to determine the average ΔCT value for each sample. The average of these ΔCT values for the Stappenbeck L-WRN CM batches (CM1–7) was used to calculate the ΔΔCT values.
2.9. Wnt reporter assay
The HEK293 TCF/LEF-reporter recombinant cell line (BPS Bioscience; Cat.# 60,501) was cultured according to the vendor’s recommendations. To avoid detachment of the reporter cells from the culture well surface, L-WRN CM or primary culture medium was carefully added at 1:1 vol/vol to the existing HEK293 medium in the culture well for this assay. Viability in the 1:1 medium mixtures was determined using the CellTiter-Glo® 3D Cell Viability Assay, as described above. Recombinant Wnt3a (R&D systems; Cat.# P27467) was used as a positive control. L-WRN CM batches were tested at a final concentration of 5% (i.e., added 10% L-WRN CM at a 1:1 vol/vol to the HEK293 medium) for Wnt activity assays. Luciferase activity was determined using the ONE-Step™ Luciferase Assay System (BPS Bioscience; Cat.# 60690–2) and luminescence was measured with a Cytation5 Multi-mode Reader (BioTek). Culture wells containing L-WRN CM and ONE-Step™ reagent but without cells were used to determine the average back-ground luminescence. Culture wells containing cells, and HEK293:primary culture medium (1:1) were used to determine baseline reporter activity. Experiments included 3 technical replicates, and 3 biological experiments with distinct passages of cells were performed. Technical replicates from each experiment were averaged prior to statistical analysis.
2.10. Statistics
Statistical analysis was performed using GraphPad Prism software (version 7) and parametric tests, as data were determined to be of a normal distribution or were of insufficient sample size to determine normal distribution. A 2-way repeated measures ANOVA was used to assess Cdc25A-CBRLuc data. Comparison of 2 groups (Supplementary Figs. S4, S5, and S6) were analyzed using an unpaired, two-tailed t-test. Comparisons of > 2 groups were analyzed using a 1-way ANOVA followed by a Dunnett’s multiple comparisons post-test with either the 0WK condition (Fig. 2) or average Stappenbeck media values (average results from CM1–7; Figs. 3–7) set as the control condition, unless otherwise stated in the figure legend. P < 0.05 was considered to be significant.
Fig. 2.

L-WRN CM activity is maintained over several weeks of storage following thaw.
(A-E) L-WRN CM was stored at 4C for 0WK, 1WK, 2WK, 3WK or subjected to a second freeze-thaw cycle (2XFT). An aliquot of the 2XFT sample was removed prior to the second freeze-thaw cycle to serve as a direct control (2XFT Cont). Spheroids cultured in differentiation medium with EP4 inhibitor (DM + EP4i), treated with cycloheximide and tumor necrosis factor (CHX + TNF), or treated with butyrate served as negative controls. (A) Schematic of experimental time line for assays in (B) and (C). (B) Graph of CellTiter-Glo data presented as fold change (mean ± s.e.m.) relative to DM + EP4i; n = 3 independent experiments. ****P < 0.0001 by 1-way ANOVA and Dunnett’s post test relative to 0WK. (C) Graphs of mRNA gene expression for indicated genes as determined by qPCR. Data are presented as fold change (mean ± s.e.m.) relative to 0WK; n = 3 independent experiments. (D) Schematic of experimental time line for (E). (E) Graph of Cdc25A-CBRluc data normalized to the average 0 h value of all samples; n = 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by 1-way ANOVA (B, C) or by 2-way repeated measures ANOVA (E) using Dunnett’s post test relative to 0WK (B, C, E).
Fig. 3.
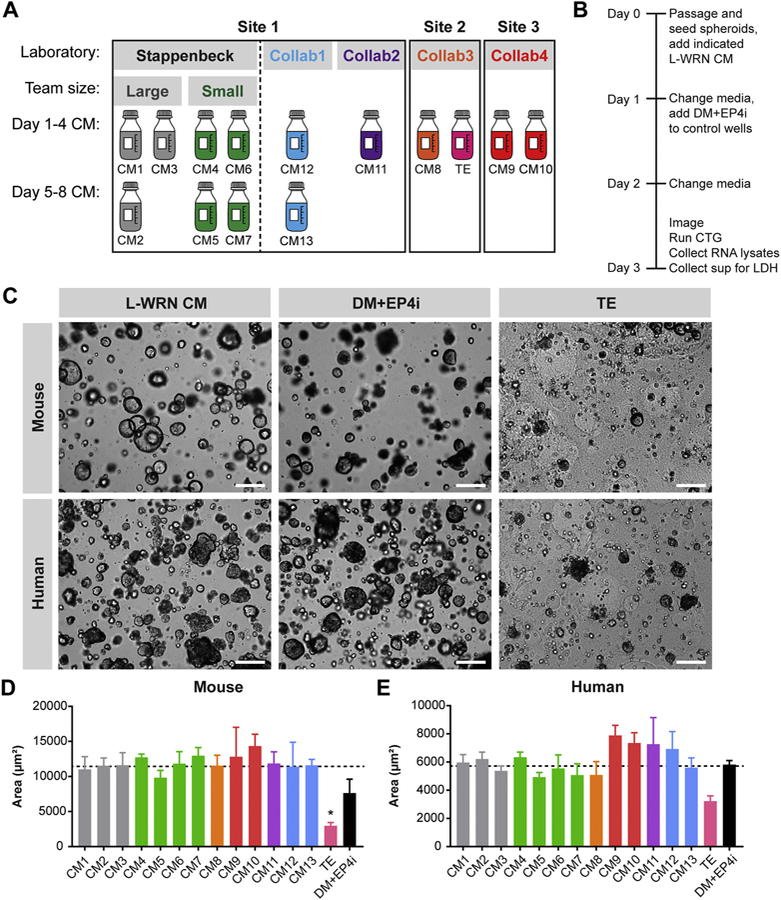
Reproducible spheroid size across multiple L-WRN CM batches.
(A) Schematic of L-WRN CM batches included in the batch-to-batch reproducibility study, including CM1–13 and the technical error (TE) batch. CM batches were collected at one of 3 institutional sites by the Stappenbeck laboratory or a collaborating laboratory (Collab1, Collab2, Collab3, Collab4). The Stappenbeck CM batches were collected by large or small teams. CM batches collected on Days 1–4 and Days 5–8 are in the upper row and lower row, respectively. (B) Schematic of experimental time line for the indicated assays. (C) Representative images from a single experiment of mouse and human colonic spheroids cultured in L-WRN CM, colonocyte differentiation medium (DM + EP4i), or the TE batch. Scale bars, 200 um. (D, E) Graphs of mouse (D) and human (E) spheroid size expressed as the average two-dimensional area (μm2; mean ± s.e.m.) from n = 3 independent experiments. *P < 0.05 by 1-way ANOVA with Dunnett’s post test relative to the average Stappenbeck CM value (represented by dashed line; 11,444 μm2 for mouse spheroids and 5706 μm2 for human spheroids).
Fig. 7.

Reproducible Wnt reporter induction across multiple L-WRN CM batches.
(A) Graph of CellTiter-Glo data from HEK293 Wnt reporter cells cultured in HEK cell media diluted 1:1 with the indicated media, either HEK medium, primary culture medium (1 culture), or L-WRN CM. Data are presented as fold change (mean ± s.e.m.) relative to HEK medium; n = 3 independent experiments. Group comparisons were not significant by 1-way ANOVA and Tukey’s post test. (B) Graph of luciferase activity expressed as fold induction (mean ± s.e.m.) over HEK293 Wnt reporter cells treated with primary culture medium; n = 3 independent experiments. Cells were treated with the indicated final concentrations of a single batch of L-WRN CM or with 1 μg/mL or 0.1 μg/mL of recombinant Wnt3a as a positive control. (C) Graph of luciferase activity expressed as fold induction (mean ± s.e.m.) over HEK293 Wnt reporter cells treated with primary culture medium; n = 3 independent experiments. Cells were treated with a 5% final concentration of the L-WRN CM1–13 or TE batches with 1 μg/mL of recombinant Wnt3a. (B, C) *P < 0.05, ***P < 0.001, ****P < 0.0001 by 1-way ANOVA and Dunnett’s post test relative to 0.1 μg/mL rWnt3a group (B) or the average Stappenbeck CM value (which was 18, represented by dashed line) (C).
2.11. Key resource table
Key resource information is provided in a separate table.
3. Results
3.1. Authentication of the L-WRN cell line
Authenication of the L-WRN cells is a critical initial step in quality control testing of L-WRN CM. To maintain low passage cells and minimize variations that could affect L-WRN CM activity as well as downstream applications with intestinal epithelial cells, we prepare large lots of L-WRN cell line stocks for cryogenic storage (i.e. 64 vials per preparation) (Fig. S1A). We randomly recovered 1 cryogenic vial of L-WRN cells out of this lot and performed authentication procedures for mouse cell lines, consisting of species verification, determination that the cell line is free from mycoplasma, and phase contrast microscopy to confirm the morphology and growth pattern of the cells over multiple days of culture (Freedman et al., 2015; Cooper et al., 2007; Shannon et al., 2016). We verified that the lot of L-WRN cells used in this study was of mouse origin (Fig. 1A) and free of mycoplasma contamination (Fig. 1B) using PCR-based assays. Microscopic assessment of L-WRN cell morphology confirmed that all cells displayed a characteristic fibroblast appearance (Fig. 1C). At 24 h post-thaw, the L-WRN cells were adherent but only a portion exhibited an elongated shape. By 48 h post-thaw, the majority of the L-WRN cells exhibited an elongated shape and were bipolar or multipolar. L-WRN cells should typically be subcultured at 80–90% confluency (e.g. when preparing frozen stocks), but for L-WRN CM production, it is important to culture L-WRN cells until they are post-confluent, with some cells becoming detached from the culture surface, prior to beginning collection of L-WRN CM (Fig. 1C).
Fig. 1.

Authentication of L-WRN cells.
(A, B) Representative images of ethidium bromide-stained agarose gels showing PCR products from species validation assay (A) or mycoplasma detection assay (B). Band sizes for the DNA ladder and expected PCR products are indicated in base pairs (bp). (A) L-WRN cells were purely of mouse origin. As controls, mouse and human spheroid gDNA were used as template either singly or mixed. NT, no template control. (B) Two independent samples from L-WRN cells were determined to be mycoplasma-free. Postive (Pos) and negative (Neg) controls were included in the kit. (D) Representative low- and high-power magnification images of L-WRN cells at the indicated times post-seed demonstrating the fibroblast characteristics of this cell line. Scale bars, 100 μm.
3.2. Sustained activity of L-WRN CM following freeze-thaw
Freeze-thaw cycles and longer storage periods at 4 °C are associated with protein degradation (Cao et al., 2003). Therefore, we tested how varying the length of 4 °C storage periods or one additional freeze-thaw cycle affected L-WRN CM support of stem cell spheroid growth and proliferation. We stored aliquots of a single batch of L-WRN CM for 0, 1, 2, or 3 weeks at 4 °C (0WK, 1WK, 2WK, 3WK, respectively). We also thawed a single L-WRN aliquot and divided it into two portions, one of which served as a control (2XFT Cont) and the other was subjected to a second freeze-thaw cycle (2XFT). Mouse colonic spheroids were cultured in these six media conditions followed by assessment of spheroid growth, stem cell activity, and proliferation (Fig. 2). Spheroids cultured in these media exhibited similar growth as determined by CellTiter-Glo, a luminescent assay that detects ATP present in live cells (Fig. 2A, B). As negative controls for spheroid growth, we treated cells with CHX and recombinant TNF to induce cell death or changed the medium to induce to post-mitotic colonocytes (Kaiko et al., 2016; Miyoshi et al., 2017).
To determine whether these media preparations similarly supported the maintenance of proliferative stem and progenitor cells, we assessed mRNA gene expression of the intestinal stem cell marker Lgr5 (Barker et al., 2007), the proliferative cell marker MKi67 (Gerdes et al., 1983; Sobecki et al., 2016), the Wnt reporter gene Axin2 (Jho et al., 2002; Kim et al., 2007), and the colonocyte differentiation marker Car1 (Parkkila et al., 1994) by qPCR (Fig. 2A, C). The markers associated with proliferative cells (Lgr5, MKi67, and Axin2) were highly expressed in spheroids cultured in L-WRN CM regardless of media storage length or additional freeze-thaw compared to the differentiated colonocyte spheroids. In contrast, Car1 mRNA was only highly expressed in colonocyte spheroids. Only spheroids cultured in 3WK medium (media at 4 °C for 3 weeks) exhibited a statistically significant increase in MKi67 mRNA expression relative to 0WK medium. This pattern is reminiscient of the small increase in the frequency of EdU-positive proliferating cells previously observed in spheroids cultured in 5% L-WRN CM (a 10-fold reduction of the L-WRN CM concentration that optimally supports spheroid growth) (VanDussen et al., 2015). This finding suggests reduced potency of L-WRN media when it is maintained at 4 °C for 3 weeks.
To directly assess spheroid proliferation, we used a mouse colon spheroid line derived from a Cdc25A-click beetle red luciferase cell cycle reporter mouse (Sun et al., 2015). The luminescence of Cdc25A-luciferase spheroids faithfully correlates with cell proliferation (Kaiko et al., 2016; Sun et al., 2015). When spheroids were cultured in L-WRN CM, luminescence increased over a 24-hr period, concomitant with spheroid proliferation and growth (Fig. 2D, E). The addition of the short-chain fatty acid butyrate to L-WRN CM significantly halted spheroid proliferation, similar to our previous report (Kaiko et al., 2016). We did not observe a statistical difference between 0WK and any other L-WRN CM based on storage time or additional freeze-thaw cycle; however, proliferation trended lower in the 3WK vs. 0WK media. Collectively, these experiments demonstrated that L-WRN CM activity was sustained and remained relatively stable when stored at 4 °C for up to several weeks or subjected to one additional freeze-thaw. Because we observed some signs of diminished L-WRN CM activity with the 3WK storage condition, we performed all subsequent experiments using media stored at 4 °C no longer than 2 weeks post-thaw.
3.3. Highly reproducible spheroid growth across multiple L-WRN CM batches
Next, to assess the batch-to-batch variation of L-WRN CM, we gathered 14 batches of L-WRN CM produced according to the same protocol and then stored at −20 °C or −80 °C for 4 to 8 months (Fig. 3A) (Miyoshi and Stappenbeck, 2013). Of these L-WRN CM batches, 7 had been produced in the Stappenbeck laboratory, 3 had been produced by two collaborater laboratories at Washington University, and 4 had been produced by two collaborator laboratories at other institutions. During the production of one of these L-WRN CM batches, a technical error (TE) occurred (see Methods), but we still incorporated this TE batch into our study as a potential negative control. Of the 13 correctly produced batches, 8 were collected on Days 1–4 and 5 were collected on Days 5–8. Of the 7 batches produced by the Stappenbeck laboratory, 3 were produced by large teams consisting of ~15 people and 4 were produced by small teams consisting of ~5 people. Together, these L-WRN CM batches allowed us to test a number of variables for potential effects on CM activity: 1) CM collection on Days 1–4 vs. Days 5–8 of CM production, 2) CM collection across different laboratories; 3) CM collection by large vs. small teams. Mouse or human colonic spheroids were initially passaged and expanded for downstream assays using a single batch of L-WRN CM to ensure equivalency prior to culture in one of the 14 L-WRN CM test batches (Fig. 3B). Spheroids appeared qualitatively similar when cultured in any of CM1–13 (Fig. 3C, Figs. S2, S3). Mouse spheroids cultured in L-WRN CM had a spherical shape, a clear lumen, and thin, smooth edges. In contrast, differentiated spheroids appeared darker due to their smaller lumen and thicker walls. Budding structures, which are typically observed with intestinal organoid culture methods (Sato et al., 2009), were absent in both proliferative and differentiated spheroids. Similar features were observed in the human spheroids, except these were often “clumpy” in appearance, with a less apparent lumen. We measured the average two-dimensional area of the spheroids cultured in each L-WRN CM test batch and confirmed that the spheroid size was similar following culture with CM1–13 within each species (Fig. 3D, E). The human spheroids were smaller than the mouse spheroids, reflective of their lower growth rate (VanDussen et al., 2015; Kaiko et al., 2016; Sun et al., 2015). Following culture with the TE batch, very few spheroids were still apparent and those remaining tended to be significantly smaller than those cultured with CM1–13. Quantitative assessment of spheroid growth using the CellTiter-Glo assay supported these findings, with similar growth observed in spheroids cultured with CM1–13 batches and greatly reduced growth in spheroids cultured with the TE batch (Fig. 4A–B). These results indicate that the TE batch did not support spheroid growth. We did not observe differences in spheroid size or growth based on the CM collection period (Fig. S4A, B) or production laboratory (Fig. S5A, B). However, spheroid growth was slightly, but significantly, elevated in mouse spheroids when cultured in the CM produced by small teams rather than large teams in the Stappenbeck lab (Fig. S6A, B).
Fig. 4.
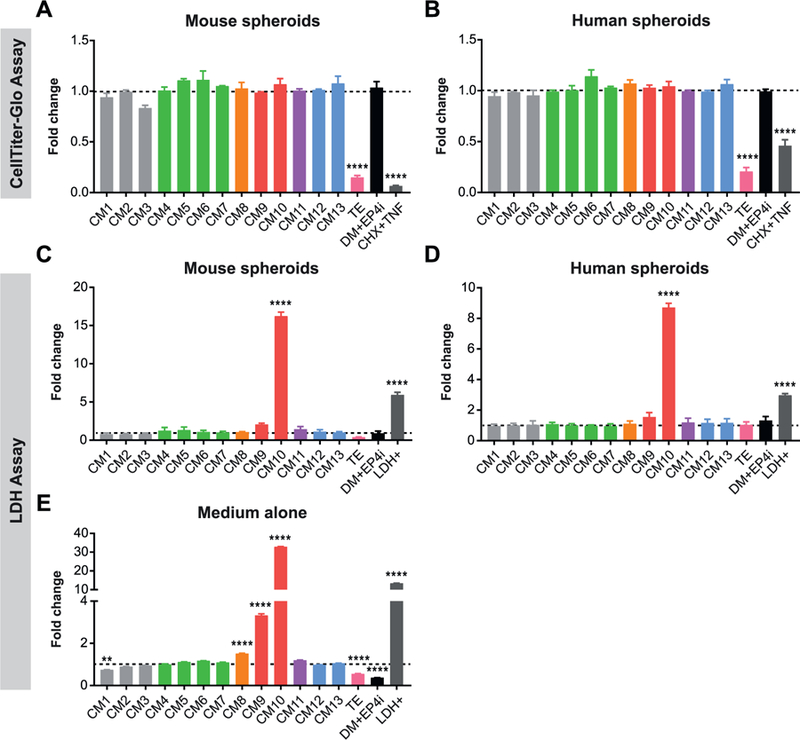
Reproducible spheroid growth across multiple L-WRN CM batches.
(A-D) Mouse (A, C) or human (B, D) spheroids were cultured in CM1–13 or TE batches of L-WRN CM followed by assessment of cell growth with the CellTiter-Glo assay (A, B) or cell death with the LDH assay (C, D). Spheroids cultured in differentiation medium with EP4 inhibitor (DM + EP4i) or treated with cycloheximide and tumor necrosis factor (CHX + TNF) served as negative controls. The positive control for LDH (LDH+) was provided with the assay kit. (A, B) Graphs of CellTiter-Glo data presented as fold change (mean ± s.e.m.) relative to the average Stappenbeck CM value; n = 3 independent experiments. (C, D) Graphs of LDH levels detected in spheroid culture supernatants presented as fold change (mean ± s.e.m.) relative to the average Stappenbeck CM value; n = 3 independent experiments. (E) Graphs of LDH levels detected in L-WRN CM batches presented as fold change (mean ± s.e.m.) relative to the average Stappenbeck CM value; n = 3 technical replicates. **P < 0.01, ****P < 0.0001 by 1-way ANOVA and Dunnett’s post test relative to the average Stappenbeck CM value (represented by dashed line).
Because CellTiter-Glo results were similar between the spheroids cultured in the TE batch and those purposely induced to undergo cell death, we examined whether the TE batch led to spheroid cell death using a colorimetric assay for lactate dehydrogenase (LDH), which is released into media by damaged cells (Fig. 4C–D). LDH levels in spheroid culture supernatants were generally very low. We did not detect increased LDH in the supernatant of spheroids cultured in the TE batch, indicating that cell death or toxicity was likely not the reason for the diminished spheroid growth. However, we found very high levels of LDH in the CM10 spheroid supernatants. Because the CM10-cultured spheroids appeared to be growing normally, we performed the LDH assay using CM alone (i.e., no exposure to spheroids) (Fig. 4E). Elevated LDH levels were observed in the CM10 medium itself. This could be due to insufficient removal of L-WRN cells from the final CM during the collection process, which could result in L-WRN cell damage and LDH release during freeze-thaw of the L-WRN CM.
3.4. Highly reproducible mRNA gene marker expression across multiple L-WRN CM batches
We next used our qPCR gene marker panel to determine whether the L-WRN CM batches similarly supported the maintenance of proliferative stem and progenitor cells. Expression levels of the Lgr5 stem cell and MKi67 proliferative cell markers were similar across the CM1–13 batches, but were very low or undetectable with the TE batch (Fig. 5A, B). Axin2 mRNA expression was similar between spheroids cultured with CM1–13 and the TE medium, with Axin2 mRNA even being significantly higher in the human spheroids cultured in TE vs. CM1–13 (Fig. 5C). This was surprising, as Wnt signaling is the critical pathway supporting intestinal stem cells (Miyoshi, 2017; Kretzschmar and Clevers, 2017); these data indicate that the Wnt-stimulating activity of the TE batch is not diminished, and yet it did not support spheroid growth. The colonocyte marker Car1 was expressed at low levels in spheroids cultured with CM1–13, but highly expressed in spheroids cultured with the TE medium (Fig. 5D). Thus, the TE medium robustly stimulated spheroid differentiation to post-mitotic colonocytes, accounting for its deficiency in supporting spheroid growth. We did not observe any significant differences in the expression of these genes based on batch collection period (Fig. S4C–F), production laboratory (Fig. S5C–F), or production team size, other than significantly higher Car1 mRNA expression in mouse spheroids cultured in medium produced by large teams relative to small teams (Fig. S6C–F).
Fig. 5.
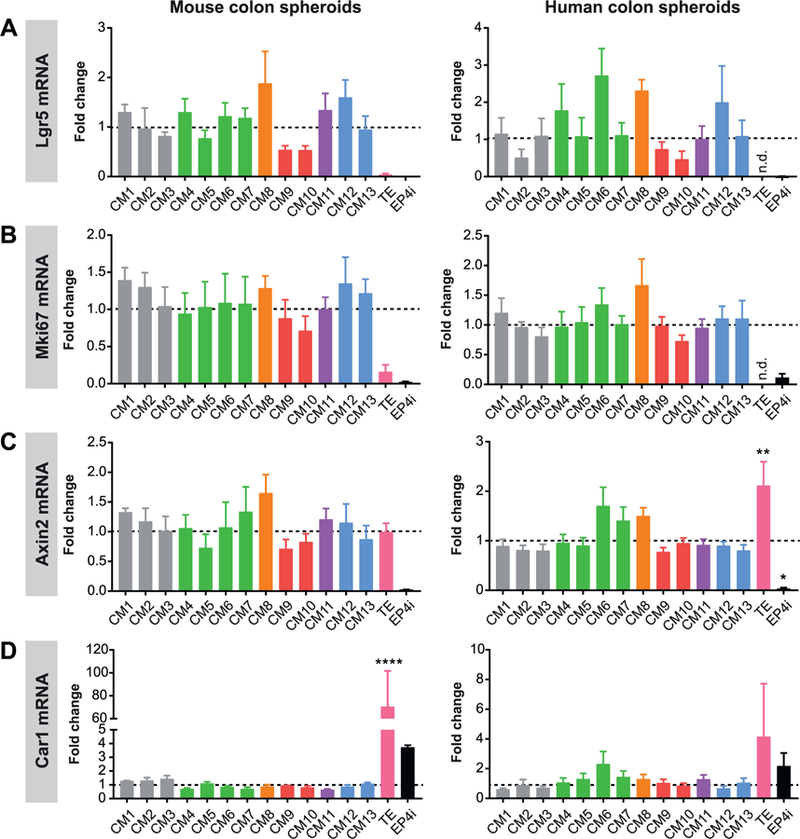
Reproducible spheroid mRNA marker expression across multiple L-WRN CM batches.
(A-D) Mouse or human colonic spheroids were cultured in CM1–13 or TE batches of L-WRN CM followed by qPCR assessment of relative mRNA abundance of Lgr5 (A), MKi67 (B), Axin2 (C), and Car1 (D). Spheroids cultured in differentiation medium with EP4 inhibitor (DM + EP4i) served as a control. Graphs of mRNA expression data are presented as fold change (mean ± s.e.m.) relative to the average Stappenbeck CM value; n = 3 independent experiments. *P < 0.05, **P < 0.01, ****P < 0.0001 by 1-way ANOVA and Dunnett’s post test relative to the average Stappenbeck CM value (represented by dashed line); n.d., not detected.
3.5. Highly reproducible proliferation across multiple L-WRN CM batches
We next directly assessed potential effects of L-WRN CM batch-to-batch variation in spheroid proliferation using Cdc25A-luciferase spheroids (Fig. 6A, B). All of the L-WRN CM batches stimulated proliferation to a similar degree (Fig. 6B). The TE batch did not exhibit statistically diminished proliferation in this assay; however, the curve generated by these data was uniquely high at the 0-hr time point and then diminishing at the 16-hr time point compared to the other CM batches. We did not observe any differences in the capacity of CM1–13 batches to drive spheroid proliferation based on collection period (Fig. S4G), production laboratory (Fig. S5G), or production team size (Fig. S6G).
Fig. 6.
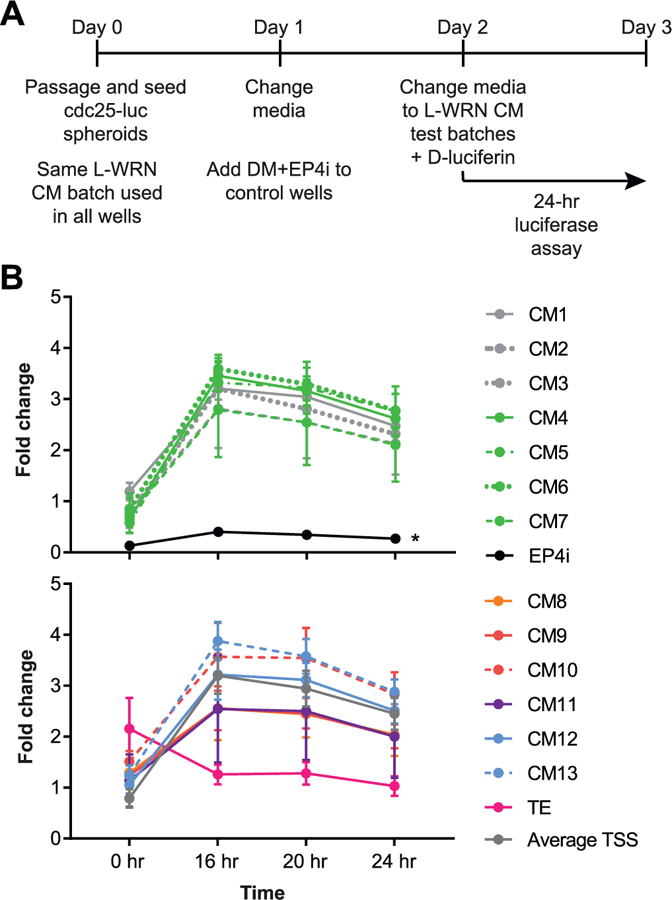
Reproducible spheroid proliferation across multiple L-WRN CM batches.
(A) Schematic of experimental time line for the Cdc25A-CBRluc assay. Mouse Cdc25A-CBRluc spheroids were cultured in CM1–13 or TE batches of L-WRN CM or in differentiation medium with EP4 inhibitor (DM + EP4i) as a negative control for proliferation. (B) Graphs of Cdc25A-CBRluc luminescence of data normalized to the average 0 h value for all samples per experiment and presented as fold change (mean ± s.e.m.) relative to the average Stappenbeck CM value (Average TSS; shown in lower graph); n = 3 independent experiments. The samples in the upper and lower graphs were run in the same experiments but are presented in two graphs for allow for discrimination of individual curves. *P < 0.05 by 2-way repeated measures ANOVA and Dunnett’s post test relative to average Stappenbeck CM.
3.6. L-WRN CM batches similarly activate a Wnt reporter cell line
Wnt is the key signaling pathway supporting renewal of the intestinal stem cell (Miyoshi, 2017; Kretzschmar and Clevers, 2017). The Wnt ligand Wnt3a and the Wnt signaling potentiator R-spondin 3 are key components of L-WRN CM that stimulate high levels of Wnt signaling in the cultured spheroids (Sato et al., 2009; Miyoshi et al., 2012). We hypothesized that testing L-WRN CM activity with HEK293 Wnt (TCF/LEF) luciferase reporter cells would be an easy surrogate for the degree to which L-WRN CM batches would support spheroid growth, such that high Wnt activity should directly correlate with enhanced spheroid growth. The Wnt reporter cells were viable in 1:1 HEK media:L-WRN CM (Fig. 7A). Stimulation of the reporter cells with 5% L-WRN CM yielded a suitable dynamic range for detection of increases and decreases in L-WRN CM Wnt activity (Fig. 7B). Wnt reporter induction was similar between all of the L-WRN CM batches, including the TE batch that did not support spheroid growth (Fig. 7C). These results support the high expression of Axin2 mRNA in spheroids cultured with the TE batch (Fig. 5). Wnt reporter induction was similar between production laboratories (Fig. S5H) and production team sizes (Fig. S6H), but was significantly elevated in medium batches collected on Days 1–4 vs. Days 5–8 (Fig. S4H), despite there being no effect of collection period on spheroid growth or proliferation (Fig. S4A, B, G). Together, these findings indicate that the use of Wnt signaling readouts does not discriminate between L-WRN CM medium batches that efficiently support spheroid growth and those that do not.
4. Discussion
Here, we applied multiple quality control approaches to test the batch-to-batch reproducibility of 14 L-WRN CM batches produced by several independent laboratories and institutions. The CM batches were produced over a several month period by the Stappenbeck laboratory and by collaborating research groups within our institution (i.e. received hands-on training with the Stappenbeck laboratory) and at external institutions (i.e. did not train in person, but had access to the same written protocol, which was clarified via phone and email). Importantly, these independently produced L-WRN CM batches were nearly identical in supporting intestinal spheroid growth and proliferation, with the exception of a single batch where a known technical error had occurred. These data provide strong evidence that experiments utilizing L-WRN CM to culture intestinal epithelial cells will yield reproducible results over time and between laboratory groups when proper quality control testing of the L-WRN CM is performed. This high level of reproducibility is of great benefit to the scientific community, as there are numerous investigators currently using L-WRN CM to culture primary gastrointestinal epithelial cells for studies related to regenerative medicine and tissue engineering, host genetics and physiology, host-microbiome interactions, tumorigenesis, and disease pathogenesis (references provided in Table S2).
A principal challenge in determining the quality of a particular batch of CM (L-WRN or otherwise) using organoid- or spheroid-based assays is that these cells are relatively more difficult to culture than transformed or immortalized cell lines, with results being highly dependent on the technical skill of the individual performing the procedures. Circumventing the requirement for technical expertise in organoid or spheroid culture for CM quality control procedures would be helpful, especially for laboratories new to these techniques. Other groups have reported using transformed cell lines expressing the Wnt-sensitive TOP-Flash/FOP-Flash luciferase reporters or expressing a stable Wnt TCF/LEF reporter for quality control testing of R spondin CM and Wnt3a CM (Gunasekara et al., 2018; Holly and Smith, 2018; Wang et al., 2017). For these reasons, we also explored the use of a Wnt reporter cell line for quality control testing of L-WRN CM. We observed robust Wnt reporter induction in response to L-WRN CM treatment, as expected. However, similar reporter induction was observed in L-WRN CM batches that supported spheroid growth (i.e. CM1–13) and one that did not (i.e. the TE batch). We also observed similar Axin2 mRNA gene expression between these batches, further supporting the equivalency of Wnt-stimulating activity between batches of L-WRN CM of highly variable quality. We conclude that it is insufficient to solely rely on the use of Wnt reporter cells to assess L-WRN CM quality; clearly other medium factors can have dominant effects on spheroid growth. Other laboratory groups have also employed enzyme-linked immunosorbent assays or Western blotting to quantify secreted protein levels of Wnt3a and Noggin in single factor CM (Gunasekara et al., 2018; Holly and Smith, 2018; Wang et al., 2017). We attempted to perform direct protein quantification using L-WRN CM but were unsuccessful, likely due to the serum levels in the medium.
In this study, we found that qualitative and quantitative measures of spheroid growth and proliferation were best able to discriminate between high-quality and low-quality batches of L-WRN CM. Inspection of spheroid visual characteristics and growth by microscopy can be very useful, but requires technical experience and can introduce individual bias. Thus, in addition to careful visual inspection of spheroids, we recommend incorporating two relatively simple quantitative assays into the L-WRN CM quality control procedure. First, we recommend performing an assay for cell growth and viability, such as the CellTiter-Glo assay used in this study. Quantitative spheroid growth should be similar when comparing new batches of L-WRN CM to previous batches known to support spheroid growth. (or higher in the new batch, which has been stored for a lesser amount of time). If a previous batch of high-quality L-WRN CM is not available, perform the assay on sequential days (e.g. spheroids seeded on Day 0 and then assayed on Days 1, 2, and 3 post-seeding) to assess the spheroid growth rate over time (VanDussen et al., 2015). Alternatively, cell cycle analysis (e.g., EdU incorporation followed by flow cytometry) can be used to determine the proportion of cells in S-phase, which should match published results (VanDussen et al., 2015; Kaiko et al., 2016; Sun et al., 2015). Second, we recommend performing qPCR analysis of a small panel of gene markers for tissue-specific stem cells, proliferating cells, and differentiating cells (e.g. Lgr5, MKi67, and Car1 for colonic spheroids). The differentiation medium used as a control is entirely composed of commercially available reagents and therefore should be replicable across laboratory groups. Good quality L-WRN CM will yield spheroids with high levels of stem and proliferation markers and low-to-absent levels of differentiation markers. A summary of the expected results for the quality control assays in this study are provided in Supplementary Table S3. Common problems encountered by investigators using L-WRN CM and the associated trouble-shooting solutions are provided in Supplementary Table S4.
We uncovered some aspects of L-WRN CM batch variation in this study that would be important to consider for particular experiments. Although we did not detect any differences in spheroid growth between L-WRN CM collected on Days 1–4 vs. Days 5–8, we did observe lower Wnt-stimulating activity in Days 5–8 L-WRN CM. Accordingly, studies investigating Wnt signaling should use L-WRN CM from a single collection period. We also observed high levels of LDH in one batch of L-WRN CM that apparently supported normal spheroid growth. If high LDH levels are observed in a L-WRN CM batch, it is possible that other factors associated with cellular damage are also present in that batch. These potentially could affect the results of certain experiments, such as those examining cell death. Together, these results indicate that study-specific quality control procedures for L-WRN CM may need to be performed to ensure reproducible results for particular experiments.
The basic and translational research applications for primary gastrointestinal epithelial cells will continue to rapidly expand as more and more laboratories adopt these culture platforms for their research (Clevers, 2016; Nakamura and Sato, 2018; Huch et al., 2017). Concomittant with this expansion will come many variations on medium composition, culture techniques, and reagent sources, all of which can affect experimental outcomes with cultured primary gastrointestinal epithelium. Thus, it is critical that investigators provide detailed methods that enable critical review and experimental replicability (e.g. see refs (Gunasekara et al., 2018; Holly and Smith, 2018; Wang et al., 2017)). We undertook this study to provide a transparent report of the batch-to-batch variation of L-WRN CM and recommend quality control procedures. We propose that these types of quality control procedures should be adopted as standard operating procedures for any laboratory group using CM (L-WRN or otherwise) and should be reported in experimental methods sections of publications. Implementation of robust quality control measures will reduce resource waste and improve the reproducibility of in vitro culture experiments across the scientific community.
Supplementary Material
Key resources table
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Y-27632 | R&D Systems | Cat #1254 |
| SB 431542 | R&D Systems | Cat #1614 |
| L-161,982 | R&D Systems | Cat #2514 |
| Recombinant EGF | Peprotech | Cat #315–09B |
| Cycloheximide | Enzo Life | Cat #ALX-380–269-G001 |
| Sciences | ||
| Recombinant carrier-free TNF (human) | Biolegend | Cat #570102 |
| Recombinant carrier-free TNF (mouse) | Biolegend | Cat #575202 |
| Recombinant Wnt3a | R&D Systems | Cat.# P27467 |
| Critical Commercial Assays | ||
| LookOut Mycoplasma PCR Detection Kit | Sigma | Cat #MP0035 |
| CellTiter-Glo® 3D Cell Viability Assay | Promega | Cat #G9681 |
| Pierce™ LDH Cytotoxicity Assay Kit | Thermofisher Scientific | Cat #88953 |
| ONE-Step™ Luciferase Assay System | BPS Bioscience | Cat #60690–2 |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| L-WRN cells | ATCC | Cat #CRL-3276; RRID:CVCL_DA06 |
| Cdc25A-CBRLuc murine colonic spheroid line | Sun et al., 2015 | N/A |
| HEK293 TCF/LEF-reporter recombinant cell line | BPS Bioscience | Cat #60501 |
Acknowledgements
The authors thank the laboratories who shared L-WRN CM for assessment in this study and those that contacted the Stappenbeck laboratory for guidance with L-WRN CM production, which helped us compile trouble-shooting recommendations.
Funding
This research report was supported by a grant from the Crohn’s and Colitis Foundation to T. S. Stappenbeck. K. L. VanDussen was supported by a K01 from the NIH (DK109081).
Abbreviations:
- CHX
cycloheximide
- CM
conditioned medium
- COI
cytochrome C oxidase I
- LDH
lactate dehydrogenase
- L-WRN L cell
line engineered to secrete Wnt3a, R spondin 3, and Noggin
- L-WRN CM
conditioned medium from the L-WRN cell line
- TE
technical error
- TNF
tumor necrosis factor
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scr.2019.101430.
Footnotes
Declarations of interest
None.
References
- Aly H, Rohatgi N, Marshall CA, Grossenheider TC, Miyoshi H, Stappenbeck TS, Matkovich SJ, McDaniel ML, 2013. A novel strategy to increase the proliferative potential of adult human beta-cells while maintaining their differentiated phenotype. PLoS ONE 8, e66131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, 2016. Quality time. Nature 529, 456–458. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H, 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Cao E, Chen Y, Cui Z, Foster PR, 2003. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol. Bioeng 82, 684–690. [DOI] [PubMed] [Google Scholar]
- Clevers H, 2016. Modeling development and disease with Organoids. Cell 165, 1586–1597. [DOI] [PubMed] [Google Scholar]
- Collins FS, Tabak LA, 2014. Policy: NIH plans to enhance reproducibility. Nature 505, 612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JK, Sykes G, King S, Cottrill K, Ivanova NV, Hanner R, Ikonomi P, 2007. Species identification in cell culture: a two-pronged molecular approach. In Vitro Cell. Dev. Biol. Anim 43, 344–351. [DOI] [PubMed] [Google Scholar]
- Dobrovolny PL, Bess D, 2011. Optimized PCR-based detection of mycoplasma. J. Vis. Exp 52, 3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LP, Gibson MC, Ethier SP, Soule HR, Neve RM, Reid YA, 2015. Reproducibility: changing the policies and culture of cell line authentication. Nat. Methods 12, 493–497. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H, 1983. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 31, 13–20. [DOI] [PubMed] [Google Scholar]
- Gunasekara DB, DiSalvo M, Wang Y, Nguyen DL, Reed MI, Speer J, Sims CE, Magness ST, Allbritton NL, 2018. Development of arrayed colonic Organoids for screening of Secretagogues associated with enterotoxins. Anal. Chem 90, 1941–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans J, van Lidth de Jeude JF, Koo BK, Rosekrans SL, Wielenga MC, van de Wetering M, Ferrante M, Lee AS, Onderwater JJ, Paton JC, Paton AW, Mommaas AM, Kodach LL, Hardwick JC, Hommes DW, Clevers H, Muncan V, van den Brink GR, 2013. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep 3, 1128–1139. [DOI] [PubMed] [Google Scholar]
- Holly MK, Smith JG, 2018. Adenovirus infection of human enteroids reveals interferon sensitivity and preferential infection of goblet cells. J. Virol 92, e00250–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A, 2017. The hope and the hype of organoid research. Development 144, 938–941. [DOI] [PubMed] [Google Scholar]
- Jackson S, 2015. The importance of being transparent. J. Clin. Invest 125, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Williams M, 2016. Irreproducibility in preclinical biomedical research: perceptions, uncertainties, and knowledge gaps. Trends Pharmacol. Sci 37, 290–302. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F, 2002. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol 22, 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS, 2016. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BM, Mao J, Taketo MM, Shivdasani RA, 2007. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology 133, 529–538. [DOI] [PubMed] [Google Scholar]
- Kretzschmar K, Clevers H, 2017. Wnt/beta-catenin signaling in adult mammalian epithelial stem cells. Dev. Biol 428, 273–282. [DOI] [PubMed] [Google Scholar]
- McNutt M, 2014. Journals unite for reproducibility. Science 346, 679. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, 2017. Wnt-expressing cells in the intestines: guides for tissue remodeling. J. Biochem 161, 19–25. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Stappenbeck TS, 2013. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc 8, 2471–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS, 2012. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science 338, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, Sonnek NM, Lai CW, Stappenbeck TS, 2017. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J 36, 5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, VanDussen KL, Miyoshi H, Stappenbeck TS, 2014. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol 7, 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Sato T, 2018. Advancing intestinal Organoid technology toward regenerative medicine. Cell Mol. Gastroenterol. Hepatol 5, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ, 2009. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med 15, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkila S, Parkkila AK, Juvonen T, Rajaniemi H, 1994. Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut 35, 646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KK, Miyoshi H, Beatty WL, Head RD, Malvin NP, Cadwell K, Guan JL, Saitoh T, Akira S, Seglen PO, Dinauer MC, Virgin HW, Stappenbeck TS, 2013. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J 32, 3130–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell RH, Behnke MS, 2017. WRN conditioned media is sufficient for in vitro propagation of intestinal organoids from large farm and small companion animals. Biol. Open 6, 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H, 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW, 2012. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon M, Capes-Davis A, Eggington E, Georghiou R, Huschtscha LI, Moy E, Power M, Reddel RR, Arthur JW, 2016. Is cell culture a risky business? Risk analysis based on scientist survey data. Int. J. Cancer 138, 664–670. [DOI] [PubMed] [Google Scholar]
- Sobecki M, Mrouj K, Camasses A, Parisis N, Nicolas E, Lleres D, Gerbe F, Prieto S, Krasinska L, David A, Eguren M, Birling MC, Urbach S, Hem S, Dejardin J, Malumbres M, Jay P, Dulic V, Lafontaine D, Feil R, Fisher D, 2016. The cell proliferation antigen Ki-67 organises heterochromatin. Elife 5, e13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzner M, Helmrath M, Dunn JC, Henning SJ, Houchen CW, Kuo C, Lynch J, Li L, Magness ST, Martin MG, Wong MH, Yu J, N.I.H.I.S.C. Consortium, 2012. A nomenclature for intestinal in vitro cultures. Am. J. Physiol. Gastrointest. Liver Physiol 302, G1359–G1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Miyoshi H, Origanti S, Nice TJ, Barger AC, Manieri NA, Fogel LA, French AR, Piwnica-Worms D, Piwnica-Worms H, Virgin HW, Lenschow DJ, Stappenbeck TS, 2015. Type I interferons link viral infection to enhanced epithelial turnover and repair. Cell Host Microbe 17, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS, 2015. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64, 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ahmad AA, Shah PK, Sims CE, Magness ST, Allbritton NL, 2013. Capture and 3D culture of colonic crypts and colonoids in a microarray platform. Lab Chip 13, 4625–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, DiSalvo M, Gunasekara DB, Dutton J, Proctor A, Lebhar MS, Williamson IA, Speer J, Howard RL, Smiddy NM, Bultman SJ, Sims CE, Magness ST, Allbritton NL, 2017. Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell Mol. Gastroenterol. Hepatol 4, 165–182 (e167). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Liao G, Pritchard T, Zhao TT, Connelly JP, Pruett-Miller SM, Blanc V, Davidson NO, Madison BB, 2017. A stable but reversible integrated surrogate reporter for assaying CRISPR/Cas9-stimulated homology-directed repair. J. Biol. Chem 292, 6148–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


