Abstract
Changes to mammography practice, including revised Breast Imaging Reporting and Data System (BI-RADS) density classification guidelines and implementation of digital breast tomosynthesis (DBT), may impact clinical breast density assessment. We investigated temporal trends in clinical breast density assessment among 2 990 291 digital mammography (DM) screens and 221 063 DBT screens interpreted by 722 radiologists from 144 facilities in the Breast Cancer Surveillance Consortium. After age-standardization, 46.3% (95% CI = 44.1% to 48.6%) of DM screens were assessed as dense (heterogeneously/extremely dense) during the BI-RADS 4th edition era (2005–2013), compared to 46.5% (95% CI = 43.8% to 49.1%) during the 5th edition era (2014–2016) (P = .93 from two-sided generalized score test). Among DBT screens in the BI-RADS 5th edition era, 45.8% (95% CI = 42.0% to 49.7%) were assessed as dense (P = .77 from two-sided generalized score test) compared to 46.5% (95% CI = 43.8% to 49.1%) dense on DM in BI-RADS 5th edition era. Results were similar when examining all four density categories and age subgroups. Clinicians, researchers, and policymakers may reasonably expect stable density distributions across screened populations despite changes to the BI-RADS guidelines and implementation of DBT.
Mammographic breast density is widely recognized as an important predictor of mammography performance and breast cancer risk (1–3). While quantitative measures of breast density exist (4,5), clinical practice relies primarily on the qualitative four-category Breast Imaging Reporting and Data System (BI-RADS) assessment of breast density by radiologists during the interpretation of mammograms (6). It is unknown to what degree changes in the BI-RADS guidelines for density classification in late 2013 (7) or the recent implementation of digital breast tomosynthesis (DBT) have affected clinical breast density assessment.
Since 1993, BI-RADS has provided guidance to radiologists regarding the classification of breast density into four categories: almost entirely fatty, scattered areas of fibroglandular densities, heterogeneously dense, and extremely dense (7). The latter two categories are considered “dense” in mandatory breast density notification laws in most US states (8). The BI-RADS 4th edition, published in 2003, stated that density should be categorized based on the visual assessment of the percentage of fibroglandular tissue within the breast, with the four categories corresponding to less than 25% glandular density; 25%–50% glandular density; 50%–75% glandular density; and greater than 75% glandular density (9). The BI-RADS 5th edition, published in December 2013 (7), omitted this percentage-based system and instead emphasized an assessment of potential for the masking of suspicious lesions behind dense tissue. With this new guidance, women with small areas of focally dense tissue could potentially be categorized in a higher density category than under the 4th edition.
DBT has been widely implemented in US clinical practice since its approval by the US Food and Drug Administration (FDA) in 2011, with half of US facilities having DBT units as of July 1, 2018 (10,11). Previous studies have suggested DBT exams may be less likely to be assessed as having dense breasts compared to digital mammography (DM) exams because DBT images multiple slices through the breast thereby mitigating the effects of masking (12–14), though other studies suggest no difference (15,16). Thus, the impact of DBT dissemination on breast density assessment is also poorly understood.
We evaluated temporal trends and differences by modality in breast density assessment using observational clinical data from the Breast Cancer Surveillance Consortium (BCSC). The BCSC includes six active breast imaging registries: Carolina Mammography Registry, Kaiser Permanente Washington Registry, New Hampshire Mammography Network, Vermont Breast Cancer Surveillance System, San Francisco Mammography Registry, and Metropolitan Chicago Breast Cancer Registry (17,18). Each registry and the BCSC Statistical Coordinating Center received institutional review board approval for either active or passive consenting processes or a waiver of consent. BCSC registries capture imaging modality, exam indication, breast density, and assessment data from participating radiology facilities using standard nomenclature defined by BI-RADS (7). Women complete a standardized questionnaire that collects demographic, risk factor, and medical history information. We estimated each woman’s five-year breast cancer risk using the BCSC version 2.0 risk model (3).
We identified DM and DBT screening mammography exams conducted during 2005–2016 among women ages 40–79 years. Women with breast implants or a history of breast cancer or mastectomy were excluded. We restricted DBT exams to the BI-RADS 5th edition era (2014–2016) because there was insufficient DBT exam volume during prior years. A total of 2 990 291 DM screens and 221 063 DBT screens were identified among 1 116 769 women, interpreted by 722 radiologists at 144 radiology facilities. We used binomial and multinomial logistic regression to estimate the age-standardized and age-stratified distributions of breast density assessments according to calendar year and imaging modality. Models were estimated using generalized estimating equations with a working independence correlation matrix to account for clustering of observations within radiologists, and the robust variance estimates were used to calculate 95% confidence intervals (CIs). P values were determined from a two-sided generalized score test that accounts for correlation of multiple density measures within radiologists. A P value less than .05 was considered statistically significant.
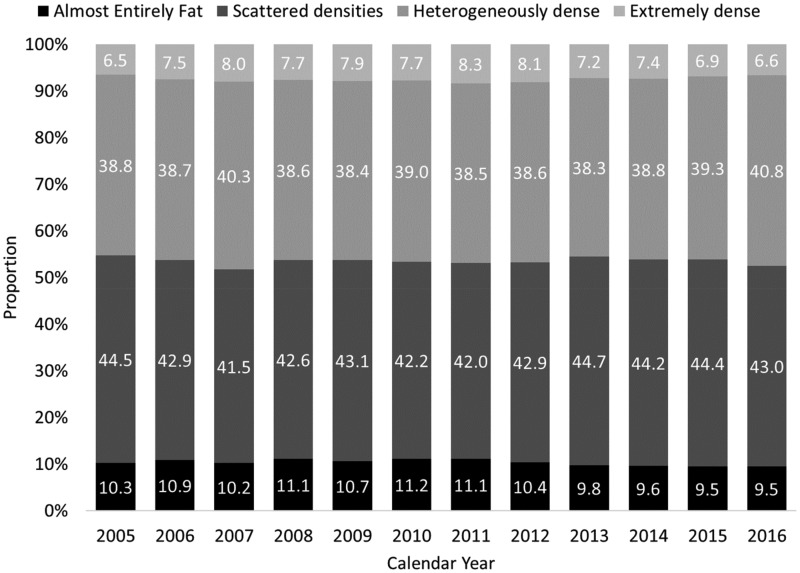
The study population was 69% non-Hispanic white (Supplementary Table 1, available online). The age-standardized distribution of breast density categories was stable across years for DM screens (Figure 1). After age standardization, 46.3% (95% CI = 44.1% to 48.6%) of DM exams had dense breasts during the BI-RADS 4th edition era, compared to 46.5% (95% CI = 43.8% to 49.1%) in the 5th edition era (P = .93). The distributions of the four density categories for DM exams in the two time periods were comparable within subgroups defined by decade of age (Table 1).
Figure 1.
Breast density distribution for 2 990 291 digital screening mammograms among 1 080 427 women in the Breast Cancer Surveillance Consortium by calendar year, 2005–2016.
Table 1.
Distribution of breast density on screening mammograms in the Breast Cancer Surveillance Consortium, 2005–2016
| Age, y | BI-RADS density | Breast density (95% CI), % |
||
|---|---|---|---|---|
| Digital screening mammograms 2005–2013 | Digital screening mammograms 2014–2016 | DBT screening mammograms 2014–2016 | ||
| (N = 2 229 070) | (N = 761 221) | (N = 221 063) | ||
| All ages* | Almost entirely fat | 10.2 (8.6 to 11.7) | 9.1 (7.8 to 10.4) | 8.3 (6.4 to 10.3) |
| Scattered densities | 43.6 (41.8 to 45.4) | 44.6 (42.1 to 47.1) | 46.0 (43.1 to 48.8) | |
| Heterogeneously dense | 39.3 (37.4 to 41.2) | 40.1 (37.6 to 42.5) | 39.7 (36.7 to 42.7) | |
| Extremely dense | 6.9 (6.0 to 7.7) | 6.2 (5.2 to 7.3) | 6.0 (4.7 to 7.4) | |
| 40–49 | Almost entirely fat | 5.4 (4.4 to 6.4) | 4.6 (3.8 to 5.3) | 4.5 (3.3 to 5.6) |
| Scattered densities | 31.0 (29.1 to 32.9) | 32.4 (30.2 to 34.6) | 32.1 (28.8 to 35.3) | |
| Heterogeneously dense | 49.4 (47.4 to 51.4) | 50.5 (48.4 to 52.6) | 50.5 (47.6 to 53.3) | |
| Extremely dense | 14.2 (12.5 to 15.8) | 12.6 (10.9 to 14.3) | 13.0 (10.6 to 15.5) | |
| 50–59 | Almost entirely fat | 9.9 (8.3 to 11.4) | 8.7 (7.4 to 10.0) | 7.9 (5.9 to 9.9) |
| Scattered densities | 42.8 (41.0 to 44.6) | 43.9 (41.4 to 46.3) | 45.5 (42.6 to 48.5) | |
| Heterogeneously dense | 39.5 (37.7 to 41.4) | 40.5 (38.1 to 42.9) | 40.0 (37.1 to 43.0) | |
| Extremely dense | 7.8 (6.8 to 8.7) | 6.9 (5.8 to 8.0) | 6.5 (5.0 to 8.1) | |
| 60–69 | Almost entirely fat | 14.4 (12.4 to 16.3) | 13.1 (11.3 to 14.8) | 12.1 (9.6 to 14.6) |
| Scattered densities | 49.3 (47.4 to 51.2) | 50.2 (47.4 to 53.1) | 52.6 (50.0 to 55.2) | |
| Heterogeneously dense | 32.2 (30.3 to 34.1) | 32.7 (30.0 to 35.5) | 32.0 (29.2 to 34.8) | |
| Extremely dense | 4.1 (3.5 to 4.7) | 4.0 (3.1 to 4.8) | 3.3 (2.5 to 4.1) | |
| 70–79 | Almost entirely fat | 15.2 (13.0 to 17.4) | 14.1 (12.2 to 16.0) | 12.4 (9.6 to 15.2) |
| Scattered densities | 53.2 (51.2 to 55.2) | 54.3 (51.6 to 56.9) | 55.9 (53.5 to 58.3) | |
| Heterogeneously dense | 28.8 (27.0 to 30.6) | 28.9 (26.2 to 31.5) | 28.9 (26.0 to 31.8) | |
| Extremely dense | 2.8 (2.4 to 3.2) | 2.8 (2.2 to 3.4) | 2.8 (2.0 to 3.5) | |
Results for “All ages” are standardized to the age distribution of the total study population. BI-RADS = Breast Imaging Reporting and Data System; CI = confidence interval; DBT = digital breast tomosynthesis.
Among DBT exams conducted during 2014–2016, 45.8% (95% CI = 42.0% to 49.7%) had dense breasts (P = .77 compared to 46.5% [95% CI = 43.8% to 49.1%] dense on DM during 2014–2016). Similar four-category density distributions were observed by modality in each age subgroup (Table 1). In sensitivity analyses that additionally adjusted for BMI and registry, the proportion of exams with dense breasts in each group remained similar (46.4%, 45.6%, and 45.8% for DM 2005–2013, DM 2014–2016, and DBT 2014–2016, respectively).
Our findings demonstrate a stable pattern in clinical breast density assessment in the BCSC despite the changes to density assessment guidance in the BI-RADS 5th edition. These findings stand in contrast to prior reader studies (19–21) and a single institution study (22) that suggested a small increase in dense categories with the BI-RADS 5th edition. The two prior US studies were limited to fellowship-trained breast imagers at a single institution (19,22). The BCSC includes a large, geographically diverse sample of academic and nonacademic facilities and is broadly representative of the United States (17). Our results indicate that clinical density assessment overall did not shift when studied across a large national sample of facilities and radiologists. Our findings parallel prior observations that there was no substantial change in the distribution of breast density categories in the BCSC after the addition of the percentage-based guidance in the BI-RADS 4th edition in 2003 or during the transition from film to digital mammography (7,23). Similarly, our findings suggest that the widespread implementation of DBT in US clinical practice has not substantially affected density assessment.
Our analysis was limited in that we did not evaluate radiologist- or facility-level variation in density assessment. It remains possible and indeed likely that certain radiologists and/or institutions have changed density assessment practice in relation to the new BI-RADS guidance.
In summary, our results suggest that, across screened populations, clinicians and researchers may reasonably expect breast density assessments made since 2014 to be comparable to those recorded previously. Healthcare providers and policymakers should expect the prevalence of dense breasts (24) to remain stable despite changes in the BI-RADS lexicon and the dissemination of DBT.
Funding
This work was supported by the National Cancer Institute (grant P01CA154292). Data collection for this research was additionally supported by grant U54CA163303 from the National Cancer Institute, grant R01 HS018366-01A1 from the Agency for Healthcare Research and Quality, and award PCS-1504–30370 from the Patient-Centered Outcomes Research Institute (PCORI). Ms Bowles’ effort was supported by the National Cancer Institute Research Specialist Award R50CA211115. Dr Lee’s effort was supported by the American Cancer Society (126947-MRSG-1416001CPHPS). The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see: http://www.bcsc-research.org/work/acknowledgement.html.
Notes
Affiliations of authors: Departments of Surgery and Radiology, University of Vermont Cancer Center, University of Vermont, Burlington, VT (BLS); Departments of Medicine and Epidemiology and Biostatistics and General Internal Medicine Section, Department of Veterans Affairs, University of California, San Francisco, CA (KK); Kaiser Permanente Washington Health Research Institute, Kaiser Permanente Washington, Seattle, WA (EJAB), DLM; Department of Epidemiology and Biostatistics, School of Public Health, University of Illinois at Chicago, Chicago, IL (GHR); Department of Radiology, University of Washington School of Medicine, and the Hutchinson Institute for Cancer Outcomes Research, Seattle, WA (CIL); The Dartmouth Institute for Health Policy and Clinical Practice and Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Lebanon, NH (ANAT); Division of Biostatistics, Department of Public Health Sciences, University of California Davis School of Medicine, Davis, CA (DLM).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The statements presented in this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health, or PCORI, its Board of Governors, or Methodology Committee.
Dr Lee reports grant funding from GE Healthcare through his academic institution. Dr Migliroretti reports an honorarium for service on Hologic Scientific Advisory Board. All other authors have no disclosures.
We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. You can learn more about the BCSC at: http://www.bcsc-research.org/.
Supplementary Material
References
- 1. Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;1383:168–175. [DOI] [PubMed] [Google Scholar]
- 2. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;3563:227–236. [DOI] [PubMed] [Google Scholar]
- 3. Tice JA, Miglioretti DL, Li CS, et al. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;3328:3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;16811:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alonzo-Proulx O, Mawdsley GE, Patrie JT, Yaffe MJ, Harvey JA.. Reliability of automated breast density measurements. Radiology. 2015;2752:366–376. [DOI] [PubMed] [Google Scholar]
- 6. Conant EF, Sprague BL, Kontos D.. Beyond BI-RADS density: a call for quantification in the breast imaging clinic. Radiology. 2018;2862:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American College of Radiology. ACR BI-RADS®—mammography. In: D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al., eds. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System 5th ed. Reston, VA: American College of Radiology; 2013:1–175.
- 8. Ray KM, Price ER, Joe BN.. Breast density legislation: mandatory disclosure to patients, alternative screening, billing, reimbursement. AJR Am J Roentgenol. 2015;2042:257–260. [DOI] [PubMed] [Google Scholar]
- 9.American College of Radiology. ACR BI-RADS®—mammography. In: D'Orsi CJ, Bassett LW, Berg WA, Feig SA, et al., eds. ACR Breast Imaging Reporting and Data System, Breast Imaging Atlas 4th Ed. Reston, VA: American College of Radiology; 2003:1–336.
- 10.US Food and Drug Administration. MQSA 2018 Scorecard Statistics. https://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/FacilityScorecard/ucm595007.htm. Accessed August 20, 2018.
- 11. Houssami N, Miglioretti DL.. Digital breast tomosynthesis: a brave new world of mammography screening. JAMA Oncol. 2016;2(6):725–727. [DOI] [PubMed] [Google Scholar]
- 12. Zuckerman SP, Conant EF, Keller BM, et al. Implementation of synthesized two-dimensional mammography in a population-based digital breast tomosynthesis screening program. Radiology. 2016;2813:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aujero MP, Gavenonis SC, Benjamin R, Zhang Z, Holt JS.. Clinical performance of synthesized two-dimensional mammography combined with tomosynthesis in a large screening population. Radiology. 2017;283(1):70–76. [DOI] [PubMed] [Google Scholar]
- 14. Tagliafico AS, Tagliafico G, Cavagnetto F, Calabrese M, Houssami N.. Estimation of percentage breast tissue density: comparison between digital mammography (2D full field digital mammography) and digital breast tomosynthesis according to different BI-RADS categories. Br J Radiol. 2013;861031:20130255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alshafeiy TI, Wadih A, Nicholson BT, et al. Comparison between digital and synthetic 2D mammograms in breast density interpretation. AJR Am J Roentgenol. 2017;2091:W36–W41. [DOI] [PubMed] [Google Scholar]
- 16. Haider I, Morgan M, McGow A, et al. Comparison of breast density between synthesized versus standard digital mammography. J Am Coll Radiol. 2018;15(10):1430–1436. [DOI] [PubMed] [Google Scholar]
- 17. Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the breast cancer surveillance consortium. Radiology. 2017;2831:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast cancer surveillance consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol. 1997;1694:1001–1008. [DOI] [PubMed] [Google Scholar]
- 19. Irshad A, Leddy R, Ackerman S, et al. Effects of changes in BI-RADS density assessment guidelines (fourth versus fifth edition) on breast density assessment: intra- and interreader agreements and density distribution. AJR Am J Roentgenol. 2016;2076:1366–1371. [DOI] [PubMed] [Google Scholar]
- 20. Youk JH, Kim SJ, Son EJ, Gweon HM, Kim JA.. Comparison of visual assessment of breast density in BI-RADS 4th and 5th editions with automated volumetric measurement. AJR Am J Roentgenol. 2017;2093:703–708. [DOI] [PubMed] [Google Scholar]
- 21. Alikhassi A, Esmaili Gourabi H, Baikpour M.. Comparison of inter- and intra-observer variability of breast density assessments using the fourth and fifth editions of breast imaging reporting and data system. Eur J Radiol Open. 2018;5:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Irshad A, Leddy R, Lewis M, et al. Changes in breast density reporting patterns of radiologists after publication of the 5th edition BI-RADS guidelines: a single institution experience. AJR Am J Roentgenol. 2017;2094:943–948. [DOI] [PubMed] [Google Scholar]
- 23. Harvey JA, Gard CC, Miglioretti DL, et al. Reported mammographic density: film-screen versus digital acquisition. Radiology. 2013;2663:752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;10610:dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.