Abstract
Background
Genome-wide association studies (GWAS) identify associations of individual single-nucleotide polymorphisms (SNPs) with cancer risk but usually only explain a fraction of the inherited variability. Pathway analysis of genetic variants is a powerful tool to identify networks of susceptibility genes.
Methods
We conducted a large agnostic pathway-based meta-analysis of GWAS data using the summary-based adaptive rank truncated product method to identify gene sets and pathways associated with pancreatic ductal adenocarcinoma (PDAC) in 9040 cases and 12 496 controls. We performed expression quantitative trait loci (eQTL) analysis and functional annotation of the top SNPs in genes contributing to the top associated pathways and gene sets. All statistical tests were two-sided.
Results
We identified 14 pathways and gene sets associated with PDAC at a false discovery rate of less than 0.05. After Bonferroni correction (P ≤ 1.3 × 10−5), the strongest associations were detected in five pathways and gene sets, including maturity-onset diabetes of the young, regulation of beta-cell development, role of epidermal growth factor (EGF) receptor transactivation by G protein–coupled receptors in cardiac hypertrophy pathways, and the Nikolsky breast cancer chr17q11-q21 amplicon and Pujana ATM Pearson correlation coefficient (PCC) network gene sets. We identified and validated rs876493 and three correlating SNPs (PGAP3) and rs3124737 (CASP7) from the Pujana ATM PCC gene set as eQTLs in two normal derived pancreas tissue datasets.
Conclusion
Our agnostic pathway and gene set analysis integrated with functional annotation and eQTL analysis provides insight into genes and pathways that may be biologically relevant for risk of PDAC, including those not previously identified.
Although pancreatic ductal adenocarcinoma (PDAC) only accounts for about 3% of all cancer, it’s the third leading cause of cancer-related death in the United States and its incidence is increasing (1). PDAC is among the most fatal cancers worldwide, and there are few established preventable risk factors beyond cigarette smoking, diabetes, overweight, and obesity (2). There are no effective screening methods for the detection of PDAC; therefore, most people are diagnosed with advanced disease, which contributes to the dismal 5-year survival of 8.2% (1). Understanding the biology underlying the development of PDAC could be useful in developing new treatments or to identify those at high risk for surveillance or targeted intervention
Over the past decade, genome-wide association studies (GWAS) have identified 20 genomic loci associated with PDAC susceptibility in European populations (3–8). Although GWAS have provided valuable insights into the genetic basis of PDAC, the susceptibility loci identified do not fully account for the genetic heritability of this disease because of the relative small effect sizes associated with individual single-nucleotide polymorphisms (SNPs) and the multiple testing correction required for GWAS. Thus, many important susceptibility genes may well remain unidentified. Pathway-based analyses applied to GWAS have the potential to detect associations that may be overlooked by standard single-marker approaches and can be a complementary method to identify groups of genes or biological pathways enriched with disease-associated SNPs (9,10). Pathway analysis, which jointly considers multiple variants in interacting genes and multiple genes in a pathway, may also allow more meaningful biologic interpretation.
We previously conducted a pathway analysis of genes in 23 candidate biological pathways hypothesized a priori to be associated with PDAC (10). PDAC-associated pathways identified included pancreatic development, Helicobacter pylori lacto/neolacto, hedgehog, Th1/Th2 immune response, and apoptosis. This study was limited by the number of participants and pathways/genes examined, suggesting a larger study with a more comprehensive approach may detect associations not previously considered in PDAC. Consequently, in the present study, we included 3795 human canonical pathways and gene sets from the Broad Institute Molecular Signatures Database (MSigDB) using an agnostic data-driven approach to identify genes and pathways associated with PDAC susceptibility within the Pancreatic Cancer Cohort Consortium (PanScan I, II, III) and the Pancreatic Cancer Case Control Consortium (PanC4) GWAS.
Methods
Study Population and Data
Methods for GWAS studies have previously been described (3–6,8). The study sample included 9040 primary pancreatic adenocarcinoma cases (ICD-O-3 code C250-C259) and 12 496 control participants of European genetic ancestry from summary data from previous PanScan and PanC4 GWAS (3–8). All participants gave informed consent and all studies were approved by the institutional review board of each participating institution and the National Cancer Institute.
Pathway and Gene Set–Based Analyses Using GWAS Summary Data
A total of 3795 human-derived gene sets and canonical pathways from The Broad Institute MSigDB v5.0 database (http://software.broadinstitute.org/gsea/msigdb/collections.jsp) were used for the analysis. Canonical pathways (n = 1369) included BioCarta; Hallmark; Kyoto Encyclopedia of Genes and Genomes (KEGG); Pathway Interaction Database (National Cancer Institute and Nature Publishing Group); Reactome; Signaling Gateway (SIG); Signaling Transduction KE, and Sigma Aldrich. Gene sets (n = 2426) included studies with expression signatures (or changes in expression levels) following genetic and chemical perturbations. Gene sets represented genes induced (upregulated) and repressed (downregulated) by the perturbation. The size of gene sets and pathways ranged from 2 to 1668 genes. SNPs were mapped within a genomic region encompassing 20 kb upstream and downstream of each gene.
We conducted a meta-analysis using summary statistics from the four GWAS using an inverse variance fixed-effects model (λ = 1.07). An initial analysis on all of the SNPs resulted in 112 pathways or genes sets associated with PDAC below the Bonferroni statistical significance threshold (P = 1.3 × 10−5, 0.05/3795) (data not shown). Most were driven by one or more previously identified GWAS variants, not a pathway association. Therefore, to identify pathway or gene set associations that were jointly driven by novel SNPs, we excluded previously published GWAS PDAC cancer risk signals at 1q32.1 (NR5A2), 2p13.3 (ETAA1), 3q29 (TP63), 5p15.33 (CLPTM1L-TERT), 7p13 (SUGCT), chr8q24.21 (MYC), 7q32.2 (LINC-PINT), 9q34.2 (ABO), 13q12.2 (PDX1), 13q22.1 (KLF5/KLF12), 16q23.1 (BCAR1), 17q25.1 (LINC00673), and 22q12.1 (ZNRF3) (3–7) and SNPs with meta-analysis at GWAS threshold P value less than 5 × 10−8 (signal at 7p12 [TNS3]) from our analysis plus corresponding genomic regions within roughly 500 kb to eliminate association signals that could be caused by linkage disequilibrium (LD) (11). In total, 207 genes were excluded using these criteria. We excluded SNPs with minor allele frequency less than 1% and applied LD filtering to highly correlated SNP pairs (r2 > .81).
We conducted gene and pathway meta-analyses using the summary-based adaptive rank truncated product (sARTP) method, which combines SNP-level associations across SNPs in a gene or a pathway (11). The signals from up to two of the most associated SNPs in a gene were accumulated. The sARTP method adjusted for the size of genes and pathways (ie, number of SNPs in a gene and number of genes in a pathway) through a resampling procedure to control for false positives. The P values of gene- and pathway-level associations were estimated from the resampled null distribution generated from 100 million resampling steps. A panel of 503 European subjects (population codes: CEU, TSI, FIN, GBR, IBS) in the 1000 Genomes Project (phase 3, v5, 2013/05/024) was used in sARTP to estimate the LDs between SNPs. To eliminate the impact of population stratification, the genomic control inflation factor was adjusted by using to rescale the standard error of the estimated log odds ratio at each SNP. We considered a false discovery rate (FDR) adjusted pathway-level P value less than or equal to .05 to be statistically significant; however we discuss pathways and gene sets below the Bonferroni adjusted α-level (P = 1.3 × 10−5 [.05/3795]). All statistical tests are two-sided.
Functional Annotation and eQTL Analysis
Experimental data from ENCODE (12) custom tracks on the UCSC Genome Browser and Roadmap (13) and information from Ensembl (14), RegulomeDB v1.1 (15), LDlink (16), and HaploReg v4.1 (17) were used to evaluate the regulatory relevance of SNPs (and SNPs in LD) of interest in pancreatic and other tissue types. eQTL was performed to evaluate effects on expression and tissue specificity for the most statistically significant SNPs using publicly available data from the National Institutes of Health Genotype-Tissue Expression (GTEx) v7 (18) in pancreas tissue samples (n = 220). Potential eQTLs from this analysis (P < .05) were then taken forward for further analysis. eQTL analysis of selected SNPs (n = 53 SNPs mapped to 69 genes) (identified by one or more features including SNP P value, RegulomeDB score, and GTEx eQTL result) were validated using data from histologically normal pancreas tissue samples (n = 95) from the Laboratory of Translational Genetics (LTG) as previously described (19). P values for SNP-gene tests were adjusted for multiple comparisons using Bonferroni correction (P = .05/69 = 7.25 × 10−4).
Results
Pathway and Gene Set–Based Analyses
Fourteen pathways and gene sets were associated with PDAC (at FDR < 0.05), of which two gene sets and three pathways remaining statistically significant after the Bonferroni correction (Table 1); including maturity-onset diabetes of the young (MODY) (P = 5.10 × 10−7), regulation of beta-cell development (P = 1.92 × 10−6), Nikolsky breast cancer 17q11-q21 amplicon (P = 2.00 × 10−6), role of EGF receptor transactivation by G protein–coupled receptors (GPCRs) in cardiac hypertrophy (P = 3.79 × 10−6) and Pujana ATM PCC network (P = 1.25 × 10−5).
Table 1.
Pathways and gene sets associated with risk of pancreatic ductal adenocarcinoma (false discovery rate [FDR] < 0.05)*
| Gene set | Pathway description | Pathway source | No. of genes | No. of SNPs | P † | FDR | Genes contributing to the association of pathway and PDAC development |
|---|---|---|---|---|---|---|---|
| Maturity-onset diabetes of the young‡ | MODY is a monogenic form of type 2 diabetes. Mutations of MODY genes lead to dysregulation of genes involved in pancreatic islet development and metabolism. | KEGG | 23 | 1448 | 5.10 × 10−7 | 0.002 | HNF1A, HNF1B, HNF4G, HNF4A, PAX4 |
| Regulation of beta-cell development‡ | Genes involved in the regulation of beta-cell development. | REACTOME | 28 | 2057 | 1.92 × 10−6 | 0.003 | HNF1A, HNF1B, HNF4G, HNF4A |
| Breast cancer chr17q11-q21 amplicon‡ | Genes within amplicon chr17q11-q21 identified in a copy number alterations study of 191 breast tumor samples. | NIKOLSKY | 131 | 3320 | 2.00 × 10−6 | 0.003 | PGAP3, TCAP, ERBB2, PNMT, STARD3, HNF1B, IGFBP4, TNS4, MED24, THRA, FBXL20, STAC2, CSF3, CDK12, PPP1R1B, NEUROD2, PSMD3, RPL19, CRYBA1, CACNB1, CCR7, IKZF3, SGK494, TMEM98, GSDMB, C17orf63, ZPBP2, FOXN1, MIEN1, SEZ6, ERAL1, PIPOX, GRB7, ORMDL3, TIAF1, FLOT2, SPAG5, SLC13A2 |
| Role of EGF receptor transactivation by GPCRs in cardiac hypertrophy‡ | Genes contributing to cardiac hypertrophy through activation of the EGF by GPCRs. | BIOCARTA | 17 | 2133 | 3.79 × 10−6 | 0.004 | EDNRA, AGT |
| ATM PCC Network‡ | Gene network transcripts whose expression positively correlated ATM in normal tissues. | PUJANA | 1350 | 77 404 | 1.25 × 10−5 | 0.01 | SMC2, PNMT, HNF1B, HNF1A, ACTR2, GRP, THRA, HNF4G, TAB1, HEXA, MED1, HIPK3, GHRH, CASP7, MED6, TPP2, KHDRBS2, FCHSD2, OIP5, CSF3, PHOX2B, GRIA1, HNRNPL, HNRNPAB, PMS2P1, PFKFB4, GSR, AOC2, MAPK8, BCL2L11, E2F4, PFDN6, NPHP1, PCF11, LDHB, BTF3, SIKE1, PAX4, NMI, TMEM123, ANGEL1, RPL19, PIBF1, POP4, PSMD3, USP19, GPR3, SP2, CD47, RPL30, KIF20B, BRCA1, NSL1, SNRNP27, BAZ1A, TAF5, ITGBL1, NR2C1, ATXN3, TACR3, TELO2, TRA2B, NAE1, FBL, USP4, CDC123, FAM53B, CR1, PDAP1, CD52, TNFAIP8, F2RL3, ATP5G2, CAMK4, RHOH, PPIP5K2, CHAF1B, XRCC2, KIAA0922, CDH8, GPR171, RAB30, SGPL1, PPM1A, ARID1A, PAICS, DRD1, MR1, SPI1, CYP2C18, TTYH2, ROBO1, POLE, ELF1, IFI44 |
| Developmental biology | Genes involved in developmental biology, including processes of transcriptional regulation and differentiation. | REACTOME | 367 | 37 631 | 1.40 × 10−5 | 0.01 | ERRB2, HNF1A, HNF1B, MED24, HNF4G, MED1, MED6, HNF4A, SRGAP1, NCOR1, EZR, EVL, ROCK1P1, RHOC, HFE2, SMA3E, FES, FURIN, UNC5A, CACNB1, NR2F2, MYL8P, PLXND1, TCF4, MET, KIAA1598, MED21, SDCBP, MED13L, CACNA1I, DPYSL4, ROBO1, HSP90AB1 |
| Response to prostaglandin E2 down | Genes downregulated in CD4+T lymphocytes after stimulation with prostaglandin E2. | CHEMNITZ | 365 | 36 942 | 1.93 × 10−5 | 0.01 | ARL6IP6, PIK3C3, DEGS2, NUS1, ZFP3, WDR48, CNKSR3, PRR15L, BBS4, EZR, LMO7, ANXA11, PKHD1, GORASP1, CD200, FAM60A, OSBPL10, TXNIP, RND2, GALNT11, CHDH, RBMS2, TNFSF13, C19orf46, SENP3, IFT140, CNTN4, MET, FUT3, SLC35E2B, CDKN1A, MITF, PPM1A, CRAMP1L |
| Breast cancer ERBB2 up | Genes upregulated in the erbb2 subtype of breast cancer samples, characterized by higher expression of ERBB2. | SMID | 139 | 9279 | 3.89 × 10−5 | 0.020 | PNMT, PGAP3, ERBB2, STARD3, MED24, CDK12, MED1, GRB7, ELL2, PSMD3, CAMP, LBP, DUSP6, GSDMB, FUT3, SCGB2A2 |
| Breast cancer cluster 8 | Cluster 8: selected ERBB2 amplicon genes clustered together across breast cancer samples. | FARMER | 7 | 159 | 4.40 × 10−5 | 0.02 | PNMT, PGAP3, ERBB2, CDK12, MED1, GRB7, GSDMB |
| Regulation of gene expression in beta cells | Transcriptional network controlling pancreatic development and beta-cell function. | REACTOME | 19 | 1244 | 4.80 × 10−5 | 0.02 | HNF1A, HNF4G, HNF4A |
| Breast cancer basal down | Genes downregulated in basal subtype of breast cancer samples. | SMID | 668 | 60 051 | 6.14 × 10−5 | 0.02 | PGAP3, IGFBP4, PNMT, STARD3, ERBB2, GRP, MED24, CAPN9, CDK12, CXXC4, BBS4, EVL, SLC19A2, KRT8, CANT1, BAI2, IFT140, CAMP, ITGA7, HPGD, SLC48A1, CSF3R, C9orf116, RAPGEF3, ITGBL1, TMEM143, UBA7, C5orf30, RARA, RHOH, MED13L, RAB27B, SCGB2A2, VAV3, SIX1, CNR1 |
| PGR positive meningioma down | Genes downregulated in meningioma samples positive for PGR compared to those without the receptor. | CLAUS | 12 | 1195 | 8.32 × 10−5 | 0.03 | EDNRA, HEG1 |
| Prostate cancer HCP with H3K27ME3 | Genes with high histone H3 trimethylation mark at K27 (H3K27me3) in PC3 cells (prostate cancer) by ChIP-chip assay on a 12K CpG array (high-CpG-density promoters, HCP). | KONDO | 93 | 10 820 | 9.39 × 10−5 | 0.03 | HNF1B, NKX2-3, SAMD11, LINC00273, LIMCH1, KIAA1109, TRIM16, ZNF438, RNASE11 |
| Breast cancer copy no. up | Genes from common regions of gains observed in more than 15% of 148 primary breast cancer tumors. | CHIN | 25 | 1778 | 1.71 × 10−4 | 0.05 | ERBB2, THRA, MED1, CYP24A1 |
Pathway-based meta-analysis used the sARTP method, which combines SNP-level association statistics across SNPs in a gene or a pathway. Each statistically significant contributing gene includes up to two SNPs. The sARTP method adjusts for the size of genes (ie, number of SNPs in a gene) and the size of pathways (ie, number of genes in a pathway) through a resampling procedure. EGF = epidermal growth factor; GPCR = G protein–coupled receptor; HCP = high CpG-density promoters; PGR = progesterone; sARTP = summary-based adaptive rank truncated product; SNP = single-nucleotide polymorphism.
The pathway-level association P values were estimated from the resampled null distribution through up to 100 million resampling steps as described in the Methods section. All statistical tests were two-sided.
Statistically significant pathways/gene sets at Bonferroni-adjusted α-level of P = 1.3 × 10−5 (0.05/3795).
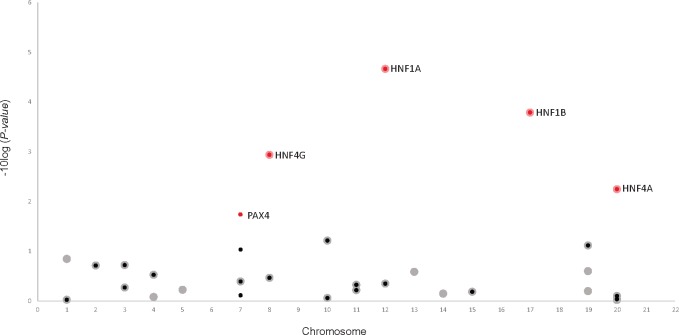
The MODY pathway that contained 1448 SNPs across 23 genes was the most statistically significant pathway. The genes with the strongest association in this pathway and the corresponding top SNP(s) were HNF1A (rs1169296, rs2244608), HNF1B (rs12951345, rs7223387), HNF4A (rs1853150), HNF4G (rs1913641, rs2943547), and PAX4 (rs118117270, rs62483175) (Figure 1, Supplementary Table 1, available online). Four of 28 genes (2057 SNPs) contributing to the regulation of beta-cell development pathway, HNF1A, HNF1B, HNF4A, and HNF4G, had the same corresponding SNPs selected by sARTP as in the MODY pathway (Figure 1, Supplementary Table 1, available online). This suggests an overlap in signals between these two pathways; however, the PAX4 gene was only present in the MODY pathway.
Figure 1.
Genes associated with PDAC in the KEGG maturity-onset diabetes of the young (MODY; small circles, P = 5.10 × 10−7) and Reactome regulation of beta-cell development (large circles, P = 1.92 × 10−6). Red highlighted circles are genes selected by sARTP as contributing the most to each pathway–PDAC association. HNF1A, HNF1B, HNF4G, and HNF4A contributed to both pathways (gene P< .006) and PAX4 (gene P = .02) contributed to the MODY pathway. All statistical tests were two-sided. MODY = maturity-onset diabetes of the young; PDAC = pancreatic ductal adenocarcinoma; sARTP = summary-based adaptive rank truncated product.
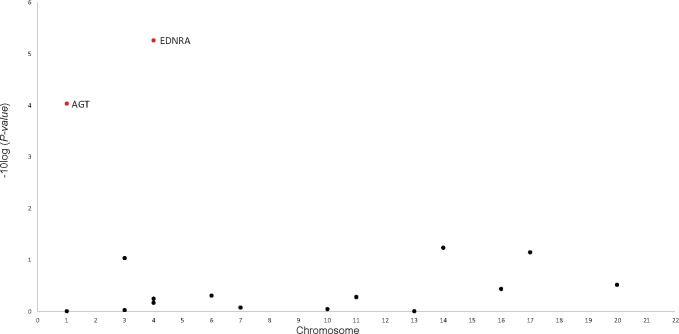
The role of EGF receptor transactivation by GPCRs in the cardiac hypertrophy pathway included 2133 SNPs across 17 genes. EDNRA (rs35232409, rs6537481) and AGT (rs1326889) were the top genes (P = 5.47 × 10−6 and 9.21 × 10−5, respectively) (Figure 2, Supplementary Table 2, available online).
Figure 2.
Genes associated with PDAC in the BioCarta role of EGF receptor transactivation by GPCRs in cardiac hypertrophy (P = 3.79 × 10−6). EDNRA and AGT genes (highlighted in red, P < 9.21 × 10−5) were selected by sARTP as contributing the most to the pathway–PDAC association. All statistical tests were two-sided. PDAC = pancreatic ductal adenocarcinoma; EGF = epidermal growth factor; GPCRs = G protein–coupled receptors; sARTP = summary-based adaptive rank truncated product.
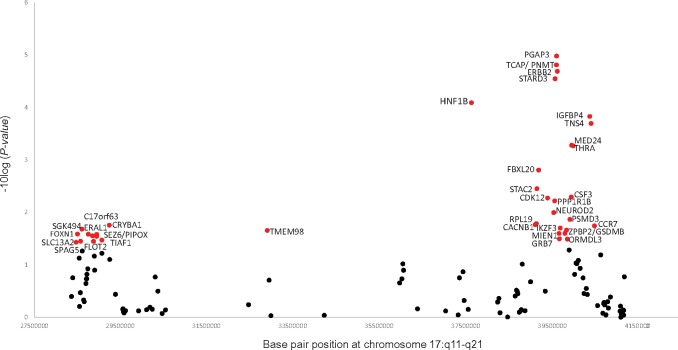
The Nikolsky breast cancer 17q11-q21 amplicon gene set included 131 genes (3320 SNPs) and the association was driven by 36 genes (Figure 3, Supplementary Table 3, available online). The top five genes were PGAP3, PNMT, TCAP, ERBB2, and STARD3 (gene P values < 3.00 × 10−5), all corresponding to two SNPs, rs876493 (P = 1.27 × 10−6) and rs3764351 (P = 1.27 × 10−5) . Additional genes contributing to this pathway included HNF1B (rs12951345, rs7223387), IGFBP4 (rs7225411, rs76592685), TNS4 (rs7225411, rs113557550), MED24/THRA (rs8078692, rs113520394), and FBXL20 (rs62074998, rs12453796) (gene P values < .001). The SNPs selected by the sARTP method in the top 36 genes (gene P < .04) were not all in high LD with each other (r2 range = 0.002–0.6), suggesting multiple signals in this region may be associated with PDAC.
Figure 3.
Genes associated with PDAC in the Nikolsky breast cancer chr17q11-q21 amplicon gene set (P = 2.00 × 10−6). A total of 36 genes (red circles, P < .04) were selected by sARTP as contributing the most to the gene set–PDAC association. All statistical tests were two-sided. PDAC = pancreatic ductal adenocarcinoma; sARTP = summary-based adaptive rank truncated product.
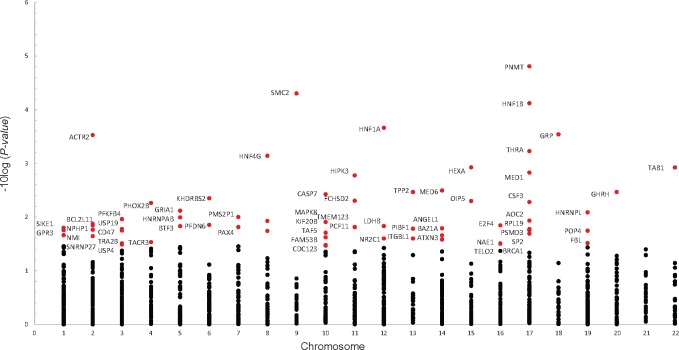
In the Pujana ATM PCC network, 67 of 1350 genes were selected by sARTP as contributing the most to the PDAC association (Figure 4, Supplementary Table 4, available online). The three top genes (gene P < 7.55 × 10−5) were SMC2 (rs7859034), PNMT (rs876493, rs3764351), and HNF1B (rs12951345, rs7223387). Other notable genes (gene P< 0.01) in this gene set included HNF1A (rs1169291, rs1169297), GRP (rs57791062), ACTR2 (rs2160263, rs7579797), THRA (rs8078692, rs113520394), HNF4G (rs1913641, rs2943547), HEXA (rs11636684, rs201611588), TAB1 (rs34825318), MED1 (rs113897737, rs7212868), and CASP7 (rs3124737). The full list of genes and sARTP-selected SNPs that contributed to the Bonferroni statistically significant pathways/gene sets are summarized in Supplementary Table 5 (available online).
Figure 4.
Genes associated with PDAC in the Pujana ATM Pearson correlation coefficient (PCC) network gene set (P = 1.25 × 10−5). A total of 67 genes (red circles, P < .02) were selected by sARTP as contributing the most to the gene set–PDAC association. All statistical tests were two-sided. PDAC = pancreatic ductal adenocarcinoma; sARTP = summary-based adaptive rank truncated product.
Some of our findings were supported by recent consortia efforts in conducting GWAS meta-analysis on PDAC risk (8). By combing the four GWAS used in our study with an additional replication data of selected SNPs (2737 cases; 4752 controls), four new GWAS signals were identified (8). As our analysis was conducted before this, we further excluded these GWAS signal regions at 1p36.33 (NOC2L), 8q21.11 (HNF4G), 17q12 (HNF1B), and 18q21.32 (GRP) (8). Eight of the 14 FDR statistically significant pathways or gene sets included these regions and were attenuated after their exclusion (Supplementary Table 6, available online, P values < .01); however, the Nikolsky breast cancer chr17q11-q21 amplicon gene set remained statistically significant (P = 5.71 × 10−6) after Bonferroni correction. The ATM PCC network, MODY, and regulation of beta-cell development had P values of 3.59 × 10−4, 5.49 × 10−4, and .001, respectively. The other six pathways/gene sets did not include these regions and were unaffected.
eQTL and Functional Annotation
We present the functional annotations and eQTL results from GTEx tissues and replication in an independent eQTL histologically normal pancreas dataset (LTG) (19) for the five Bonferroni statistically significant pathways and gene sets (Table 2 and Supplementary Tables 7 and 8, available online). Top SNP rs876493-A was present in both the Nikolsky breast cancer chr17q11-q21 amplicon and Pujana ATM network gene sets, and was associated with lower PGAP3 expression in normal pancreatic tissue in GTEx (P=3.90 × 10−7, β = −.24) and in the LTG (P=1.16 × 10−5, β = −.43). In addition, we identified and validated three additional SNPs—rs3764351-A, rs4795393-T, and rs12453507-G in LD with rs876493-A—that also act as eQTLs for PGAP3 in both datasets (Table 2). These SNPs were also associated with lower PGAP3 expression in other tissues (Supplementary Table 7, available online). The LD between these four SNPs on chr17 may indicate the same signal is contributing to the associations.
Table 2.
Expression quantitative trait loci (eQTL) for pathway single-nucleotide polymorphisms (SNPs) in normal pancreatic tissue from GTEx and an independent replication set
| Pathway gene | Chr | r 2 with rs876493* | SNP | eQTL gene | GTEx pancreas (n = 220) |
Independent LTG pancreas (n = 95) |
||
|---|---|---|---|---|---|---|---|---|
| P † | Effect size (β)‡ | P † | Effect size (β)‡ | |||||
| ERBB2, PGAP3 | 17 | N/A | rs876493 §,‖ | PGAP3 | 3.90 × 10−7 | −0.24 | 1.16 × 10−5 | −.43 |
| TCAP, STARD3, PNMT | 17 | .636 | rs3764351 §,‖ | PGAP3 | 5.70 × 10−9 | −.30 | 9.70 × 10−5 | −.47 |
| GRB7, MIEN1 | 17 | .3508 | rs4795393§ | PGAP3 | 4.00 × 10−10 | −.30 | 3.83 × 10−5 | −.45 |
| GSDMB, ZPBP2 | 17 | .3046 | rs12453507§ | PGAP3 | 2.70 × 10−7 | −.22 | 2.80 × 10−5 | −.45 |
| CASP7 | 10 | – | rs3124737‖ | CASP7 | 2.50 × 10−8 | .42 | .02 | .28 |
Linkage disequilibrium r2 values are derived from LDLink EUR population data. Chr = chromosome; eQTL = expression quantitative trait loci; FDR = false discovery rate; LTG = Laboratory of Translational Genomics; TSS = transcription start site.
eQTL in pancreas FDR (≤ 0.05) using roughly 1 Mb cis-window around TSS. Statistical test was two-sided.
Beta (β) eQTL directional effect for risk allele.
SNP in the Nikolsky breast cancer chr17 amplicon gene set.
SNP in the Pujana ATM PCC network gene set.
In the Pujana ATM PCC network, we observed that the risk allele rs3124737-G (CASP7) was associated with higher expression of the CASP7 gene in normal pancreatic tissue using GTEx (P = 2.50 × 10−8; β = .48) and LTG (P = 0.02; β = .28) (Table 2). We also observed this eQTL effect in multiple tissues from GTEx including thyroid, subcutaneous adipose, and whole blood (Supplementary Table 7, available online). The two SNPs (rs876493-A [PGAP3] and rs3124737-G [CASP7]) supported by eQTL were not identified or in LD with signals from the recent GWAS meta-analysis and remained statistically significant after exclusion of the new GWAS regions (8).
Discussion
We identified 14 pathways and gene sets associated with PDAC, five of which met the Bonferroni correction for multiple testing. The strongest pathways associated with PDAC included genes involved in susceptibility to MODY, pancreatic beta-cell development, cardiac hypertrophy, breast cancer chr17q11-q21 amplicon, and a network of genes correlated with ATM gene expression.
Our PDAC associations for the MODY and pancreatic beta-cell development pathways add evidence and reinforce previous epidemiologic findings based on candidate genes, pleiotropy, and GWAS approaches (5,7,8,10,20–22). The genes in these pathways are important components for transcriptional networks governing embryonic pancreatic development, differentiation, and pancreatic homeostasis (23,24). MODY accounts for 2% of all diabetes and is caused by genetic mutations that affect islet beta-cell function (25). We found no evidence that the variants linked to the genes in these pathways act as eQTLs in normal adult pancreas tissues. However, functional annotation showed that rs2244608-G (HNF1A) maps to an active transcription start site in normal pancreas and islet cell tissue. PAX4 identified in the MODY pathway has not previously been implicated in PDAC. PAX4 is essential for islet development and adult beta-cell survival and expansion (24). Mutations and germline polymorphisms in PAX4 are associated with type 1 and type 2 diabetes and MODY type 9 (25).
The EDNRA (endothelin receptor type A) and AGT (angiotensinogen) genes were the top genes in the EGF cardiac pathway, a pathway which has not previously been implicated in PDAC susceptibility. This pathway describes cardiac hypertrophy (thickening of the heart muscle) through activation of the EGFR (epidermal growth factor receptor) by GPCRs, which transactivate EGFR in numerous cell types and cancers, resulting in downstream activation of biological processes (26,27). EDNRA is a GPCR for endothelin (ET-1), a potent vasoconstrictor that may play a role in obesity and insulin resistance (28). Overexpression of EDNRA has been associated with many cancers (29–32). Inhibition of EDNRA demonstrated antiangiogenic and antiproliferative activity in pancreatic cancer cell lines (33). AGT encodes an angiotensin precursor, a potent vasoconstrictor involved in blood pressure regulation and a potential cell growth stimulator (34,35). Variant rs1326889 in AGT (SNP P = 4.21 × 10−7) is associated with renal cell carcinoma, with stronger associations observed for overweight/obese or hypertensive participants (34).
The Nikolsky breast cancer chr17q11-q21 amplicon gene set represents genes that exhibit copy number alterations in 191 breast carcinomas (36). The strongest associated SNP rs876493 (P = 1.27 × 10−6) and three additional correlated SNPs (r2 range = .3–.6), rs3764351, rs4795393, and rs12453507, were associated with decreased PGAP3 (post-GPI attachment to proteins 3) expression in both the GTEx and LTG pancreas tissue datasets and all four variants may represent the same signal (Table 2). PGAP3, also known as PERLD1, is an oncogene in breast and gastric cancer, and is frequently coamplified with ERBB2 and CDK12 (37). Cosilencing of STARD3, GRB7, PSMD3, and PGAP3 together with ERBB2 led to an additive inhibition of cell viability and apoptosis in vitro (38). The SNPs rs876493 and rs3764351 were also present in the Pujana ATM PCC network gene set. Our observed PDAC association for this breast cancer–derived gene set may suggest common genetic susceptibility for both cancers.
The Pujana ATM PCC network gene set is based on gene expression integrated with functional genomic and proteomic data from human tissues and cell lines to classify networks associated with ATM (39). Numerous genes across multiple chromosomes contributed to the statistical significance of this gene set with PDAC, including but not limited to the MODY genes HNF1A, HNF1B, HNF4G, and PAX4. The ATM protein is a serine/threonine kinase involved in repair of DNA double-strand breaks (40). Mutations in ATM are responsible for ataxia telangiectasia (40). Germline mutations in ATM are known to be associated with 2%–3% of familial PDAC (41–43) and have recently been found in a case series of sporadic PDAC patients (44). Our analysis identified rs7859034 (SMC2) (SNP P = 3.07 × 10−7) as top signal in this gene set. SMC2 is a central component of the condensin complex required for converting interphase chromatin into mitotic-like condense chromosomes. SMC2 in cooperation with MYCN can transcriptionally regulate DNA damage response genes (45). In this gene set, we additionally identified eQTL and functional annotation evidence for variants in the CASP7 gene (rs3124737, SNP P = 3.29 × 10−5) in pancreas tissue. CASP7 is critical in apoptosis induction, acting as a candidate for susceptibility to insulin-dependent diabetes; inactivating mutations in CASP7have been reported to contribute to the pathogenesis of some human solid cancers (46). This gene set represents a biologic network not previously considered for sporadic PDAC that should be further researched and could have clinical application for classifying those at high risk.
Four of the five Bonferroni statistically significant pathways or gene sets that we observed contained recently published GWAS signal regions (8). When we excluded these regions, the Nikolsky chr17q11-q21 gene set remained Bonferroni statistically significant and the others had P values less than 0.001. Although we do not have a replication study for our analysis, the replication of the four SNPS in these regions in the meta-analysis GWAS study (8) adds to the validity of our pathway findings. Klein et al. (8) also identified four suggestive variants— rs6537481 (EDNRA), rs2417487 (SMC2), rs1182933 (HNF1A), and rs6073450 (HNF4A)—that did not reach genome-wide statistical significance; however, they were in LD with signals (or the same SNP [EDNRA]) that contributed to the biologically relevant pathways and gene sets of multiple smaller association signals observed in our study.
Strengths of our study are its large sample size for PDAC and agnostic pathway and gene set–based statistical approach using GWAS data. Combined with a sophisticated statistical method, sARTP maximized our ability to detect genetic associations that would not be discovered by the single-marker analysis conducted in conventional GWAS. The sARTP method also uses GWAS summary data, which facilitates consortia collaboration in sharing data for large-scale pathway analyses. We excluded reported GWAS SNPs, signals with a P-value threshold less than 5 × 10−8 and regions within roughly 500 kb to identify genetic contributions to PDAC susceptibility beyond the traditional GWAS threshold. The strongest contributing genes and selected SNPs observed within the statistically significant pathways and gene sets in our study may be identified in future GWAS with larger sample sizes.
Limitations of our study include the LD filtering threshold (r2 > .81) used to exclude highly correlated SNPs, meaning potential variants in LD (r2 < .81) could be selected for a gene that represents one signal. However, the sARTP method identifies SNPs and genes contributing the most to the overall pathway associations in a data-driven manner that may help with biologically meaningful result interpretation and has proven to be a powerful and effective strategy to analyze pathways (11). An additional limitation may be the distance (kb) used to map SNPs to genes. Although, there is no agreed exact distance to assign SNPs to their relevant genes (47), we know that some genetic variants can affect RNA expression through cis or trans mechanisms (48). In our study, we mapped SNPs 20 kb upstream and downstream of each gene to identify candidate SNPs that may play a regulatory role in gene expression. We and others have previously used this distance in annotating SNPs/genes in pathway analysis (11), as studies have shown functional variants are located approximately 16–20 kb within transcription start sites (47–49). Ultimately, approaches using causal SNPs based on chromatin interactions, cis- or trans-eQTL functional data for assigning SNPs to genes, will increase the precision of the associations and understanding of the biology beyond the functional annotations we performed using publicly available data and eQTL analyses in two normal pancreas tissue independent datasets.
In conclusion, translating GWAS data into biologically relevant pathways and gene sets expands our knowledge of the potential mechanisms underlying PDAC carcinogenesis, as well as providing evidence for the future development of clinically relevant multigenic predictors for identifying individuals at high risk. Further population, clinical, and laboratory research is needed to confirm our findings. Strategies to accelerate functional biological follow-up may include replication, fine mapping, experimental studies such as whole transcriptomic sequencing, reporter assays, and DNA methylation/epigenetic regulations on gene expression (50) to fully understand the biology and functional nature of the loci contributing to the pathways and gene sets associated with PDAC.
Funding
This work was supported by the Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health. This publication has emanated from research supported in part by a Grant from Science Foundation Ireland under Grant number [15/SIRG/3482](NW) and Health Research Board/Irish Cancer Society (CPFPR-2012–2)(NW). This work was also supported by RO1 CA154823 and federal funds from the National Cancer Institute (NCI), US National Institutes of Health, under contract number HHSN261200800001E. Please see the Supplementary Materials (available online) for a complete list of funding acknowledgments.
Notes
Authors: Naomi Walsh, Han Zhang, Paula L. Hyland, Qi Yang, Evelina Mocci, Mingfeng Zhang, Erica J. Childs, Irene Collins, Zhaoming Wang, Alan A. Arslan, Laura Beane-Freeman, Paige M. Bracci, Federico Canzian, Eric J. Duell, Steven Gallinger, Graham G. Giles, Gary E. Goodman, Phyllis J. Goodman, Charles Kooperberg, Loic LeMarchand, Rachel E. Neale, Sara H. Olson, Ghislaine Scelo, Xiao O. Shu, Stephen K. Van Den Eeden, Kala Visvanathan, Emily White, Wei Zheng, Demetrius Albanes, Gabriella Andreotti, Ana Babic, William R. Bamlet, Sonja I. Berndt, Ayelet Borgida, Marie-Christine Boutron-Ruault, Lauren Brais, Paul Brennan, Bas Bueno-de-Mesquita, Julie Buring, Kari G. Chaffee, Stephen Chanock, Sean Cleary, Michelle Cotterchio, Lenka Foretova, Charles Fuchs, J. Michael M. Gaziano, Edward Giovannucci, Michael Goggins, Thilo Hackert, Christopher Haiman, Patricia Hartge, Manal Hasan, Kathy J. Helzlsouer, Joseph Herman, Ivana Holcatova, Elizabeth A. Holly, Robert Hoover, Rayjean J. Hung, Vladimir Janout, Eric A. Klein, Robert C. Kurtz, Daniel Laheru, I-Min Lee, Lingeng Lu, Núria Malats, Satu Mannisto, Roger L. Milne, Ann L. Oberg, Irene Orlow, Alpa V. Patel, Ulrike Peters, Miquel Porta, Francisco X. Real, Nathaniel Rothman, Howard D. Sesso, Gianluca Severi, Debra Silverman, Oliver Strobel, Malin Sund, Mark D. Thornquist, Geoffrey S. Tobias, Jean Wactawski-Wende, Nick Wareham, Elisabete Weiderpass, Nicolas Wentzensen, William Wheeler, Herbert Yu, Anne Zeleniuch-Jacquotte, Peter Kraft, Donghui Li, Eric J. Jacobs, Gloria M. Petersen, Brian M. Wolpin, Harvey A. Risch, Laufey T. Amundadottir, Kai Yu, Alison P. Klein, Rachael Z. Stolzenberg-Solomon
Affiliations of authors: National Institute for Cellular Biotechnology, Dublin City University, Glasnevin, Dublin, Ireland (NW); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD (HZ, PLH, QY, LBF, DA, GA, SIB, SC, PH, RH, NR, DS, GST, NW, KY, RZSS); Division of Applied Regulatory Science, Office of Translational Science, Center for Drug Evaluation & Research, U.S. Food and Drug Administration, Silver Spring, MD (PLH); Department of Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Baltimore, MD (EM, EJC, DL, APK); Laboratory of Translational Genomics, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD (MZ, IC, ZW, LTA); Division of Epidemiology II, Office of Surveillance and Epidemiology, Center for Drug Evaluation & Research, U.S. Food and Drug Administration, Silver Spring, MD (MZ); Department of Computational Biology, St Jude Children’s Research Hospital, Memphis, Tennessee (ZW); Department of Obstetrics and Gynecology, New York University School of Medicine, New York, NY (AAA); Department of Environmental Medicine, New York University School of Medicine, New York, NY (AAA); Department of Population Health, New York University School of Medicine, New York, NY (AAA, AZJ); Department of Epidemiology and Biostatistics, University of California, San Francisco, CA (PMB, EAH); Genomic Epidemiology Group, German Cancer Research Center (DKFZ), Heidelberg, Germany (FC); Unit of Nutrition and Cancer, Cancer Epidemiology Research Program, Bellvitge Biomedical Research Institute (IDIBELL), Catalan Institute of Oncology (ICO), Barcelona, Spain (EJD); Prosserman Centre for Population Health Research, Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Toronto, ON, Canada (SG, AB, RJH); Cancer Epidemiology and Intelligence Division, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, GS, RLM); Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, The University of Melbourne, Victoria, Australia (GGG, GS, RLM); Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, Victoria, Australia (GGG); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA (GEG, CK, EW, UP, MDT); SWOG Statistical Center, Fred Hutchinson Cancer Research Center, Seattle, WA (PJG); Cancer Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LL); Population Health Department, QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia (REN); Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY (SHO, IO); International Agency for Research on Cancer (IARC), Lyon, France (GS, PB); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (XOS, WZ); Division of Research, Kaiser Permanente Northern California, Oakland, CA (VDE); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD (KV); Department of Epidemiology, University of Washington, Seattle, WA (EW); Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA (AB, LB, EG, BMW); Department of Health Sciences Research, Mayo Clinic College of Medicine, Rochester, MN (WRB, KGC, ALO, GMP); Centre de Recherche en Épidémiologie et Santé des Populations (CESP, Inserm U1018), Facultés de Medicine, Université Paris-Saclay, UPS, UVSQ, Gustave Roussy, Villejuif, France (MCBR, GS); Department for Determinants of Chronic Diseases (DCD), National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (BBM); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (BBM); Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia (BBM); Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA (JB, IML, HDS); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (JB, IML, HDS, PK); Division of Hepatobiliary and Pancreas Surgery, Mayo Clinic, Rochester, MN (SC); Cancer Care Ontario, University of Toronto, Toronto, ON, Canada (MC); Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada (MC); Department of Cancer Epidemiology and Genetics, Masaryk Memorial Cancer Institute, Brno, Czech Republic (LF), Yale Cancer Center, New Haven, CT (CF), Division of Aging, Brigham and Women's Hospital, Boston, MA (JMMG); Boston VA Healthcare System, Boston, MA (JMMG); Department of Pathology, Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins School of Medicine, Baltimore, MD (MG, APK); Department of General Surgery, University Hospital Heidelberg, Heidelberg, Germany (TH, OS); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CH); Department of Epidemiology, University of Texas MD Anderson Cancer Center, Houston, TX (MH); Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Bethesda, MD (KJH); Department of Radiation Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins School of Medicine, Baltimore, MD (JH); Institute of Public Health and Preventive Medicine, Charles University, 2nd Faculty of Medicine, Prague, Czech Republic (IH); Department of Epidemiology and Public Health, Faculty of Medicine, University of Ostrava, Czech Republic (VJ); Faculty of Medicine, University of Olomouc, Olomouc, Czech Republic (VJ); Glickman Urological and Kidney Institute, Cleveland Clinic, Cleveland, OH (EAK); Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY (RCK); Department of Chronic Disease Epidemiology, Yale School of Public Health, New Haven, CT (LL, HAR); Genetic and Molecular Epidemiology Group, Spanish National Cancer Research Center (CNIO), Madrid, Spain (NM); CIBERONC, Madrid, Spain (NM, FXR); Department of Public Health Solutions, National Institute for Health and Welfare, Helsinki, Finland (SM); Epidemiology Research Program, American Cancer Society, Atlanta, GA (AVP, EJJ); CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain (MP); Hospital del Mar Institute of Medical Research (IMIM), Universitat Autònoma de Barcelona, Barcelona, Spain (MP); Epithelial Carcinogenesis Group, Spanish National Cancer Research Centre-CNIO, Madrid, Spain (FXR); Departament de Ciències Experimentals i de la Salut, Universitat Pompeu Fabra, Barcelona, Spain (FXR); Department of Surgical and Perioperative Sciences, Umeå University, Umeå, Sweden (MS); Department of Epidemiology and Environmental Health, University at Buffalo, Buffalo, NY (JWW); MRC Epidemiology Unit, University of Cambridge, Cambridge, UK (NW); Department of Research, Cancer Registry of Norway, Institute of Population-Based Cancer Research, Oslo, Norway (EW); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (EW); Genetic Epidemiology Group, Folkhälsan Research Center and Faculty of Medicine, University of Helsinki, Helsinki, Finland (EW); Department of Community Medicine, Faculty of Health Sciences, University of Tromsø, The Arctic University of Norway, Tromsø, Norway (EW); Information Management Systems, Silver Spring, MD (WW); Cancer Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (HY); Perlmutter Cancer Center, New York University School of Medicine, New York, NY (AZJ); Department of Biostatistics, Harvard School of Public Health, Boston, MA (PK); Department of Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX (DL).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. All authors declare no competing interests. Dr Mingfeng Zhang, the coauthor, is currently an employee of the US Food and Drug Administration; she was involved in this study when she was a postdoctoral fellow at National Cancer Institute. The views expressed in this article are those of the authors and not necessarily those of the Food and Drug Administration.
Supplementary Material
References
- 1. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. JNCI J Natl Cancer Inst. 2017;1099:1827–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stolzenberg-Solomon RZ, Amundadottir LT.. Epidemiology and inherited predisposition for sporadic pancreatic adenocarcinoma. Hematol Oncol Clin North Am. 2015;294:619–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;419:986–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;423:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolpin BM, Rizzato C, Kraft P, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet. 2014;469:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Childs EJ, Mocci E, Campa D, et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat Genet. 2015;478:911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang M, Wang Z, Obazee O, et al. Three new pancreatic cancer susceptibility signals identified on chromosomes 1q32.1, 5p15.33 and 8q24.21. Oncotarget. 2016;741:66328–66343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein AP, Wolpin BM, Risch HA, et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat Commun. 2018;91:556.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wei P, Tang H, Li D.. Insights into pancreatic cancer etiology from pathway analysis of genome-wide association study data. PLoS One. 2012;710:e46887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li D, Duell EJ, Yu K, et al. Pathway analysis of genome-wide association study data highlights pancreatic development genes as susceptibility factors for pancreatic cancer. Carcinogenesis. 2012;337:1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang H, Wheeler W, Hyland PL, et al. A powerful procedure for pathway-based meta-analysis using summary statistics identifies 43 pathways associated with type II diabetes in European populations. PLoS Genet. 2016;126:e1006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;4897414:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kundaje A, Meuleman W, Ernst J, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;5187539:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yates A, Akanni W, Amode MR, et al. Ensembl 2016. Nucleic Acids Res. 2016;44(D1):D710–D716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyle AP, Hong EL, Hariharan M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;229:1790–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Machiela MJ, Chanock SJ.. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;3121:3555–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ward LD, Kellis M.. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016;44(D1):D877–D881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ardlie KG, Deluca DS, Segre AV, et al. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;3486235:648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang M, Lykke-Andersen S, Zhu B, et al. Characterising cis-regulatory variation in the transcriptome of histologically normal and tumour-derived pancreatic tissues. Gut. 2018;67(3):521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pierce BL, Ahsan H.. Genome-wide “pleiotropy scan” identifies HNF1A region as a novel pancreatic cancer susceptibility locus. Cancer Res. 2011;7113:4352–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo Z, Li Y, Wang H, et al. Hepatocyte nuclear factor 1A (HNF1A) as a possible tumor suppressor in pancreatic cancer. PLoS One. 2015;103:e0121082.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoskins JW, Jia J, Flandez M, et al. Transcriptome analysis of pancreatic cancer reveals a tumor suppressor function for HNF1A. Carcinogenesis. 2014;3512:2670–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maestro MA, Cardalda C, Boj SF, Luco RF, Servitja JM, Ferrer J, Distinct roles of HNF1β, HNF1α, and HNF4α in regulating pancreas development, β-cell function and growth In: Scharfmann R, Shield JPH, eds. Development of the Pancreas and Neonatal Diabetes. Vol 12 Basel: KARGER; 2007:33–45. [DOI] [PubMed] [Google Scholar]
- 24. Zhang J, Qin X, Sun Q, et al. Transcriptional control of PAX4-regulated miR-144/451 modulates metastasis by suppressing ADAMs expression. Oncogene. 2015;3425:3283–3295. [DOI] [PubMed] [Google Scholar]
- 25. Timsit J, Saint-Martin C, Dubois-Laforgue D, Bellanné-Chantelot C.. Searching for maturity-onset diabetes of the young (MODY): when and what for? Can J Diabetes. 2016;405:455–461. [DOI] [PubMed] [Google Scholar]
- 26. Ullrich A, Prenzel N, Zwick E, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;4026764:884–888. [DOI] [PubMed] [Google Scholar]
- 27. Fischer OM, Hart S, Gschwind A, Ullrich A.. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31(pt 6):1203–1208. [DOI] [PubMed] [Google Scholar]
- 28. Weil BR, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, Desouza CA.. Enhanced endothelin-1 system activity with overweight and obesity. Am J Physiol Hear Circ Physiol. 2011;3013:H689–H695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laurberg JR, Jensen JB, Schepeler T, Borre M, Ørntoft TF, Dyrskjøt L.. High expression of GEM and EDNRA is associated with metastasis and poor outcome in patients with advanced bladder cancer. BMC Cancer. 2014;14:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wülfing P, Götte M, Sonntag B, et al. Overexpression of endothelin-A-receptor in breast cancer: regulation by estradiol and cobalt-chloride induced hypoxia. Int J Oncol. 2005;264:951–960. [PubMed] [Google Scholar]
- 31. Nie S, Zhou J, Bai F, Jiang B, Chen J, Zhou J.. Role of endothelin A receptor in colon cancer metastasis: in vitro and in vivo evidence. Mol Carcinog. 2014;53(suppl 1):E85–E91. [DOI] [PubMed] [Google Scholar]
- 32. Cook N, Brais R, Qian W, Chan Wah Hak C, Corrie PG.. Endothelin-1 and endothelin B receptor expression in pancreatic adenocarcinoma. J Clin Pathol. 2015;684:309–313. [DOI] [PubMed] [Google Scholar]
- 33. Bhargava S, Stummeyer T, Hotz B, et al. Selective inhibition of endothelin receptor a as an anti-angiogenic and anti-proliferative strategy for human pancreatic cancer. J Gastrointest Surg. 2005;95:703–709. [DOI] [PubMed] [Google Scholar]
- 34. Andreotti G, Boffetta P, Rosenberg PS, et al. Variants in blood pressure genes and the risk of renal cell carcinoma. Carcinogenesis. 2010;314:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wegman-Ostrosky T, Soto-Reyes E, Vidal-Millán S, Sánchez-Corona J.. The renin-angiotensin system meets the hallmarks of cancer. J Renin Angiotensin Aldosterone Syst. 2015;162:227–233. [DOI] [PubMed] [Google Scholar]
- 36. Nikolsky Y, Sviridov E, Yao J, et al. Genome-wide functional synergy between amplified and mutated genes in human breast cancer. Cancer Res. 2008;6822:9532–9540. [DOI] [PubMed] [Google Scholar]
- 37. Singh RR, Patel KP, Routbort MJ, et al. Clinical massively parallel next-generation sequencing analysis of 409 cancer-related genes for mutations and copy number variations in solid tumours. Br J Cancer. 2014;11110:2014–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sahlberg KK, Hongisto V, Edgren H, et al. The HER2 amplicon includes several genes required for the growth and survival of HER2 positive breast cancer cells. Mol Oncol. 2013;73:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pujana MA, Han J-DJ, Starita LM, et al. Network modeling links breast cancer susceptibility and centrosome dysfunction. Nat Genet. 2007;3911:1338–1349. [DOI] [PubMed] [Google Scholar]
- 40. Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;910:759–769. [DOI] [PubMed] [Google Scholar]
- 41. Thompson D, Duedal S, Kirner J, et al. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst. 2005;9711:813–822. [DOI] [PubMed] [Google Scholar]
- 42. Marabelli M, Cheng S-C, Parmigiani G.. Penetrance of ATM gene mutations in breast cancer: a meta-analysis of different measures of risk. Genet Epidemiol. 2016;405:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roberts NJ, Jiao Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012;21:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shindo K, Yu J, Suenaga M, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol. 2017;3530:3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murakami-Tonami Y, Kishida S, Takeuchi I, et al. Inactivation of SMC2 shows a synergistic lethal response in MYCN -amplified neuroblastoma cells. Cell Cycle. 2014;137:1115–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang M-Y, Zhu M-L, He J, et al. Potentially functional polymorphisms in the CASP7 gene contribute to gastric adenocarcinoma susceptibility in an Eastern Chinese population. PLoS One. 2013;89:e74041.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang L, Jia P, Wolfinger RD, Chen X, Zhao Z.. Gene set analysis of genome-wide association studies: methodological issues and perspectives. Genomics. 2011;981:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fagny M, Paulson JN, Kuijjer ML, et al. Exploring regulation in tissues with eQTL networks. Proc Natl Acad Sci USA. 2017;11437:E7841–E7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Veyrieras J-B, Kudaravalli S, Kim SY, et al. High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet. 2008;410:e1000214.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Freedman ML, Monteiro ANA, Gayther SA, et al. Principles for the post-GWAS functional characterization of cancer risk loci. Nat Genet. 2011;436:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.