Abstract Abstract
We introduce a new genus, Cacaoporus, characterised by chocolate brown to dark brown basidiomata and hymenophore, tubes not separable from the pileus context, white to off-white basal mycelium, reddening when bruised, amygdaliform to ovoid spores and dark brown spore deposit. Phylogenetic analyses of a four-gene dataset (atp6, tef1, rpb2 and cox3) with a wide selection of Boletaceae showed that the new genus is monophyletic and sister to the genera Cupreoboletus and Cyanoboletus in the Pulveroboletus group. Two new species in the genus, C.pallidicarneus and C.tenebrosus are described from northern Thailand. Full descriptions and illustrations of the new genus and species are presented. The phylogeny also confirmed the reciprocal monophyly of Neoboletus and Sutorius, which further support the separation of these two genera.
Keywords: 3 new taxa, atp6, Boletales , cox3, Fungal Diversity, multigene phylogeny, Neoboletus , Pulveroboletus group, Taxonomy
Introduction
In the last decade or so, since molecular techniques and phylogenetic analyses have been used in taxonomy and systematics of the Boletaceae, many new species and genera have been described worldwide (e.g. Halling et al. 2012, 2016; Zeng et al. 2012; Arora and Frank 2014; Gelardi et al. 2014, 2015; Li et al. 2014, Zhao et al. 2014b, Zeng et al. 2014; Wu et al. 2015, 2016; Zhu et al. 2015). In Thailand, although the Boletaceae have been studied for a long time, only a few new Boletaceae species and a new genus have recently been described (Desjardin et al. 2009; Neves et al. 2012; Halling et al. 2014; Raspé et al. 2016; Vadthanarat et al. 2018). At the same time, many new species and genera have been described from southern and south-western China, an area with a climate and forests similar to Thailand (e.g. Li et al. 2011; Wu et al. 2015, 2016; Zhu et al. 2015). Similarly, a high number of new species and possibly new genera are expected to occur in Thailand (Hyde et al. 2018)
During our survey on the diversity of boletes in Thailand, several collections of brown to chocolate to dark brown boletes were obtained. Some collections bearing resemblance to Sutorius Halling, Nuhn & N.A. Fechner species, which typically have brown or reddish to purplish-brown basidiomata with reddish to purplish-brown hymenophore, reddish-brown spore deposit and narrowly ellipsoid to ellipsoid basidiospores (Halling et al. 2012). However, our chocolate brown bolete collections also showed differences, in particular in having a darker hymenophore, as well as in some microscopic characters like spore shape. We therefore performed a family-wide phylogeny, which showed that those brown to chocolate to dark brown boletes belong in a generic lineage, different from Sutorius. Consequently, we introduce the new Boletaceae genus Cacaoporus and describe two new species, C.pallidicarneus and C.tenebrosus, with full descriptions and illustrations.
Materials and method
Specimens collecting
Fresh basidiomata were collected in Chiang Mai Province, northern Thailand during the rainy season in 2013 to 2018. The specimens were photographed in situ, wrapped in aluminium foil and taken to the laboratory. After description of macroscopic characters, the specimens were dried in an electric drier at 45–50 °C. Examined specimens were deposited in the herbaria CMUB, MFLU, BKF and BR (listed in Index Herbariorum; Thiers, continuously updated).
Morphological studies
Macroscopic descriptions were made, based on detailed field notes and photos of fresh basidiomata. Colour codes were taken from Kornerup and Wanscher (1978). Macrochemical reactions (colour reactions) of pileus, pileus context, stipe, stipe context and hymenophore were determined using 10% aqueous potassium hydroxide (KOH) and 28–30% ammonium hydroxide (NH4OH). Microscopic structures were observed from dried specimens, using 5% KOH, NH4OH, Melzer’s reagent or stained with 1% ammoniacal Congo red. A minimum of 50 basidiospores, 20 basidia and 20 cystidia were randomly measured at 1000× with a calibrated ocular micrometer using an Olympus CX51 compound microscope. The notation ‘[m/n/p]’ represents the number of basidiospores “m” measured from “n” basidiomata of “p” collections. Dimensions of microscopic structures are presented in the following format: (a–)b–c–d(–e), in which “c” represents the average, “b” the 5th percentile, “d” the 95th percentile, “a” the minimum and “e” the maximum. Q, the length/width ratio, is presented in the same format. A section of the pileus surface was radially and perpendicularly cut to the surface at a point halfway between the centre and margin of the pileus. Sections of stipitipellis were taken from halfway up the stipe and longitudinally cut, perpendicularly to the surface (Hosen et al. 2013; Li et al. 2011). All microscopic features were drawn by free hand using an Olympus Camera Lucida model U−DA fitted to the microscope cited above. For scanning electron microscopy (SEM), a spore print was mounted on to an SEM stub with double-sided tape. The samples were coated with gold, then examined and photographed with a JEOL JSM–5910 LV SEM.
DNA isolation, PCR amplification and DNA sequencing
Genomic DNA was extracted from fresh tissue preserved in CTAB or about 10–15 mg of dried tissue using a CTAB isolation procedure adapted from Doyle and Doyle (1990). Portions of the genes atp6, tef1, rpb2 and cox3 were amplified by polymerase chain reaction (PCR) and sequenced by Sanger sequencing. The primer pairs ATP6-1M40F/ATP6-2M (Raspé et al. 2016), EF1-983F/EF1-2218R (Rehner and Buckley 2005) and bRPB2-6F/bRPB2-7.1R (Matheny 2005) were used to amplify atp6, tef1 and rpb2, respectively. Part of the mitochondrial gene cox3 was amplified with the newly designed primers COX3M1-F (5’-ATYGGAGCWGTAATGTWYATGC-3’) and COX3M1-R (5’-CCWACTAWTACRTGRATWCCATG-3’), using the following PCR programme: 2 min 30 s at 95 °C; 35 cycles of 25 s at 95 °C, 30 s at 48 °C, 30 s at 72 °C; 3 min at 72 °C. PCR products were purified by adding 1 U of Exonuclease I and 0.5 U FastAP Alkaline Phosphatase (Thermo Scientific, St. Leon-Rot, Germany) and incubated at 37 °C for 1 h, followed by inactivation at 80 °C for 15 min. Standard Sanger sequencing was performed in both directions by Macrogen Europe (The Netherlands) with PCR primers, except for atp6, for which universal primers M13F-pUC(-40) and M13F(-20) were used; for tef1, additional sequencing was performed with two internal primers, EF1-1577F and EF1-1567R (Rehner and Buckley 2005).
Alignment and phylogeny inference
The sequences were assembled in GENEIOUS Pro v. 6.0.6 (Biomatters) and introns were removed prior to alignment based on the amino acid sequence of previously published sequences. All sequences, including sequences from GenBank, were aligned using MAFFT (Katoh and Standley 2013) on the server accessed at http://mafft.cbrc.jp/alignment/server/.
Maximum Likelihood (ML) phylogenetic inference was performed using RAxML (Stamatakis 2006) on the CIPRES web portal (RAxML-HPC2 on XSEDE; Miller et al. 2009). The phylogenetic tree was inferred by a single analysis with three partitions (one for each gene), using the GTRCAT model with 25 categories, two Buchwaldoboletus and nine Chalciporus species from sub-family Chalciporoideae were used as outgroup since Chalciporoideae always appeared as sister to the remainder of the Boletaceae in recent phylogenetic analyses (e.g. Nuhn et al. 2013; Wu et al. 2014, 2016). Statistical support of clades was obtained with 1,000 rapid bootstrap replicates.
For Bayesian Inference (BI), the best-fit model of substitution amongst those implementable in MrBayes was estimated separately for each gene using jModeltest (Darriba et al. 2012) on the CIPRES portal, based on the Bayesian Information Criterion (BIC). The selected models were HKY+I+G for atp6 and rpb2 and GTR+I+G for cox3 and tef1. Partitioned Bayesian analysis was performed with MrBayes 3.2 (Ronquist et al. 2012) on the CIPRES portal. Two runs of five chains were run for 15,000,000 generations and sampled every 500 generations. The chain temperature was decreased to 0.02 to improve convergence. At the end of the run, the average deviation of split frequencies was 0.008147.
Results
Phylogenetic analysis
A total of 325 sequences were newly generated and deposited in GenBank (Table 1). The alignment contained 1,013 sequences from four genes (186 for atp6, 358 for tef1, 326 for rpb2, 143 for cox3) from 362 voucher specimens and was 2946 characters long (TreeBase number 23886).
Table 1.
List of collections used for DNA analyses, with origin, GenBank accession numbers and reference(s).
| Species | Voucher | Origin | atp6 | cox3 | tef1 | rpb2 | Reference(s) |
|---|---|---|---|---|---|---|---|
| Afroboletus aff. multijugus | JD671 | Burundi | MH614651 | MH614794 | MH614700 | MH614747 | This study |
| Afroboletus costatisporus | ADK4644 | Togo | KT823958 | MH614795* | KT824024 | KT823991 | Raspé et al. 2016; *This study |
| Afroboletus luteolus | ADK4844 | Togo | MH614652 | MH614796 | MH614701 | MH614748 | This study |
| Aureoboletus catenarius | HKAS54467 | China | – | – | KT990711 | KT990349 | Wu et al. 2016 |
| Aureoboletus duplicatoporus | HKAS50498 | China | – | – | KF112230 | KF112754 | Wu et al. 2014 |
| Aureoboletus gentilis | ADK4865 | Belgium | KT823961 | MH614797* | KT824027 | KT823994 | Raspé et al. 2016; *This study |
| Aureoboletus mirabilis | HKAS57776 | China | – | – | KF112229 | KF112743 | Wu et al. 2014 |
| Aureoboletus moravicus | VDKO1120 | Belgium | MG212528 | MH614798* | MG212573 | MG212615 | Vadthanarat et al. 2018; *This study |
| Aureoboletus nephrosporus | HKAS67931 | China | – | – | KT990720 | KT990357 | Wu et al. 2016 |
| Aureoboletus projectellus | AFTOL-ID-713 | USA | DQ534604* | – | AY879116 | AY787218 | *Binder and Hibbett 2006; Binder et al., Unpublished |
| Aureoboletus shichianus | HKAS76852 | China | – | – | KF112237 | KF112756 | Wu et al. 2014 |
| Aureoboletus sp. | HKAS56317 | China | – | – | KF112239 | KF112753 | Wu et al. 2014 |
| Aureoboletus sp. | OR0245 | China | MH614653 | MH614799 | MH614702 | MH614749 | This study |
| Aureoboletus sp. | OR0369 | Thailand | MH614654 | MH614800 | MH614703 | MH614750 | This study |
| Aureoboletus thibetanus | HKAS76655 | China | – | – | KF112236 | KF112752 | Wu et al. 2014 |
| Aureoboletus thibetanus | AFTOL-ID-450 | China | DQ534600* | – | DQ029199 | DQ366279 | *Binder and Hibbett 2006; Unpublished |
| Aureoboletus tomentosus | HKAS80485 | China | – | – | KT990715 | KT990353 | Wu et al. 2016 |
| Aureoboletus viscosus | OR0361 | Thailand | MH614655 | MH614801 | MH614704 | MH614751 | This study |
| Aureoboletus zangii | HKAS74766 | China | – | – | KT990726 | KT990363 | Wu et al. 2016 |
| Austroboletus cf. dictyotus | OR0045 | Thailand | KT823966 | MH614802* | KT824032 | KT823999 | Raspé et al. 2016; *This study |
| Austroboletus cf. subvirens | OR0573 | Thailand | MH614656 | MH614803 | MH614705 | MH614752 | This study |
| Austroboletus eburneus | REH9487 | Australia | – | – | JX889708 | – | Halling et al. 2012b |
| Austroboletus olivaceoglutinosus | HKAS57756 | China | – | – | KF112212 | KF112764 | Wu et al. 2014 |
| Austroboletus sp. | HKAS59624 | China | – | – | KF112217 | KF112765 | Wu et al. 2014 |
| Austroboletus sp. | OR0891 | Thailand | MH614657 | MH614804 | MH614706 | MH614753 | This study |
| Baorangia major | OR0209 | Thailand | MG897421 | MK372295* | MG897431 | MG897441 | Phookamsak et al. 2019; *This study |
| Baorangia pseudocalopus | HKAS63607 | China | – | – | KF112167 | KF112677 | Wu et al. 2014 |
| Baorangia pseudocalopus | HKAS75739 | China | – | – | KJ184570 | KM605179 | Wu et al. 2015 |
| Baorangia pseudocalopus | HKAS75081 | China | – | – | KF112168 | KF112678 | Wu et al. 2014 |
| Baorangia rufomaculata | BOTH4144 | USA | MG897415 | MH614805* | MG897425 | MG897435 | Phookamsak et al. 2019; *This study |
| Boletellus ananas | NY815459 | Costa Rica | – | – | KF112308 | KF112760 | Wu et al. 2014 |
| Boletellus ananas | K(M)123769 | Belize | MH614658 | MH614807 | MH614707 | MH614754 | This study |
| Boletellus aff. emodensis | OR0061 | Thailand | KT823970 | MH614806* | KT824036 | KT824003 | Raspé et al. 2016; *This study |
| Boletellus sp. | HKAS59536 | China | – | – | KF112306 | KF112758 | Wu et al. 2014 |
| Boletellus sp. | OR0621 | Thailand | MG212529 | MH614808* | MG212574 | MG212616 | Vadthanarat et al. 2018; *This study |
| Boletus aereus | VDKO1055 | Belgium | MG212530 | MH614809* | MG212575 | MG212617 | Vadthanarat et al. 2018; *This study |
| Boletus albobrunnescens | OR0131 | Thailand | KT823973 | MH614810* | KT824039 | KT824006 | Raspé et al. 2016; *This study |
| Boletus botryoides | HKAS53403 | China | – | – | KT990738 | KT990375 | Wu et al. 2016 |
| Boletus edulis | HMJAU4637 | Russia | – | – | KF112202 | KF112704 | Wu et al. 2014 |
| Boletus edulis | VDKO0869 | Belgium | MG212531 | MH614811* | MG212576 | MG212618 | Vadthanarat et al. 2018; *This study |
| Boletus p.p. sp | JD0693 | Burundi | MH645583 | – | MH645591 | MH645599 | This study |
| Boletus p.p. sp. | OR0832 | Thailand | MH645584 | MH645605 | MH645592 | MH645600 | This study |
| Boletus p.p. sp. | OR1002 | Thailand | MH645585 | MH645606 | MH645593 | MH645601 | This study |
| Boletus pallidus | BOTH4356 | USA | MH614659 | MH614812 | MH614708 | – | This study |
| Boletus pallidus | TDB-1231-Bruns | – | AF002142 | AF002154 | – | – | Kretzer and Bruns 1999 |
| Boletus reticuloceps | HKAS57671 | China | – | – | KF112201 | KF112703 | Wu et al. 2014 |
| Boletus s.s. sp. | OR0446 | China | MG212532 | MH614813* | MG212577 | KF112703 | Vadthanarat et al. 2018; *This study |
| Boletus sp. | HKAS59660 | China | – | – | KF112153 | KF112664 | Wu et al. 2014 |
| Boletus sp. | HKAS63598 | China | – | – | KF112152 | KF112663 | Wu et al. 2014 |
| Boletus violaceofuscus | HKAS62900 | China | – | – | KF112219 | KF112762 | Wu et al. 2014 |
| Borofutus dhakanus | HKAS73789 | Bangladesh | – | – | JQ928576 | JQ928597 | Hosen et al. 2013 |
| Borofutus dhakanus | OR0345 | Thailand | MH614660 | MH614814 | MH614709 | MH614755 | This study |
| Buchwaldoboletus lignicola | HKAS76674 | China | – | – | KF112277 | KF112819 | Wu et al. 2014 |
| Buchwaldoboletus lignicola | VDKO1140 | Belgium | MH614661 | MH614815 | MH614710 | MH614756 | This study |
| Butyriboletus appendiculatus | VDKO0193b | Belgium | MG212537 | MH614816* | MG212582 | MG212624 | Vadthanarat et al. 2018; *This study |
| Butyriboletus cf. roseoflavus | OR0230 | China | KT823974 | MH614819* | KT824040 | KT824007 | Raspé et al. 2016; *This study |
| Butyriboletus frostii | NY815462 | USA | – | – | KF112164 | KF112675 | Wu et al. 2014 |
| Butyriboletus pseudoregius | VDKO0925 | Belgium | MG212538 | MH614817* | MG212583 | MG212625 | Vadthanarat et al. 2018; *This study |
| Butyriboletus pseudospeciosus | HKAS63513 | China | – | – | KT990743 | KT990380 | Wu et al. 2016 |
| Butyriboletus roseoflavus | HKAS54099 | China | – | – | KF739779 | KF739703 | Wu et al. 2014 |
| Butyriboletus roseopurpureus | BOTH4497 | USA | MG897418 | MH614818* | MG897428 | MG897438 | Phookamsak et al., 2019; *This study |
| Butyriboletus sp. | HKAS52661 | China | – | – | KF112169 | KF112676 | Wu et al. 2014 |
| Butyriboletus sp. | HKAS52525 | China | – | – | KF112163 | KF112671 | Wu et al. 2014 |
| Butyriboletus sp. | HKAS57774 | China | – | – | KF112155 | KF112670 | Wu et al. 2014 |
| Butyriboletus sp. | HKAS59814 | China | – | – | KF112199 | KF112699 | Wu et al. 2014 |
| Butyriboletus sp. | HKAS63528 | China | – | – | KF112156 | KF112673 | Wu et al. 2014 |
| Butyriboletus sp. | MHHNU7456 | China | – | – | KT990741 | KT990378 | Wu et al. 2016 |
| Butyriboletus subsplendidus | HKAS50444 | China | – | – | KT990742 | KT990379 | Wu et al. 2016 |
| Butyriboletus yicibus | HKAS55413 | China | – | – | KF112157 | KF112674 | Wu et al. 2014 |
| Cacaoporus pallidicarneus | OR0681 | Thailand | MK372259 | MK372296 | – | MK372283 | This study |
| Cacaoporus pallidicarneus | OR0683 | Thailand | MK372260 | MK372297 | – | MK372284 | This study |
| Cacaoporus pallidicarneus | OR1306 | Thailand | MK372261 | MK372298 | MK372272 | MK372285 | This study |
| Cacaoporus pallidicarneus | SV0221 | Thailand | MK372262 | MK372299 | MK372273 | MK372286 | This study |
| Cacaoporus pallidicarneus | SV0451 | Thailand | MK372263 | MK372300 | MK372274 | MK372287 | This study |
| Cacaoporus sp. | SV0402 | Thailand | MK372270 | – | MK372281 | MK372293 | This study |
| Cacaoporus tenebrosus | OR0654 | Thailand | MK372264 | MK372301 | MK372275 | MK372288 | This study |
| Cacaoporus tenebrosus | OR1435 | Thailand | MK372265 | MK372302 | MK372276 | MK372289 | This study |
| Cacaoporus tenebrosus | SV0223 | Thailand | MK372266 | MK372303 | MK372277 | MK372290 | This study |
| Cacaoporus tenebrosus | SV0224 | Thailand | MK372267 | MK372304 | MK372278 | MK372291 | This study |
| Cacaoporus tenebrosus | SV0422 | Thailand | MK372268 | MK372305 | MK372279 | – | This study |
| Cacaoporus tenebrosus | SV0452 | Thailand | MK372269 | MK372306 | MK372280 | MK372292 | This study |
| Caloboletus aff. calopus | HKAS74739 | China | – | – | KF112166 | KF112667 | Wu et al. 2014 |
| Caloboletus calopus | ADK4087 | Belgium | MG212539 | MH614820 | KJ184566 | KP055030 | Vadthanarat et al. 2018; Zhao et al. 2014a, b; This study |
| Caloboletus inedulis | BOTH3963 | USA | MG897414 | MH614821* | MG897424 | MG897434 | Phookamsak et al. 2019; *This study |
| Caloboletus panniformis | HKAS55444 | China | – | – | KF112165 | KF112666 | Wu et al. 2014 |
| Caloboletus radicans | VDKO1187 | Belgium | MG212540 | MH614822* | MG212584 | MG212626 | Vadthanarat et al. 2018; *This study |
| Caloboletus sp. | HKAS53353 | China | – | – | KF112188 | KF112668 | Wu et al. 2014 |
| Caloboletus sp. | OR0068 | Thailand | MH614662 | MH614823 | MH614711 | MH614757 | This study |
| Caloboletus yunnanensis | HKAS69214 | China | – | – | KJ184568 | KT990396 | Zhao et al. 2014a; Wu et al. 2016 |
| Chalciporus aff. piperatus | OR0586 | Thailand | KT823976 | MH614824* | KT824042 | KT824009 | Raspé et al. 2016; *This study |
| Chalciporus aff. rubinus | OR0139 | China | MH614663 | – | MH614712 | MH614758 | This study |
| Chalciporus africanus | JD517 | Cameroon | KT823963 | MH614825* | KT824029 | KT823996 | Raspé et al. 2016; *This study |
| Chalciporus piperatus | VDKO1063 | Belgium | MH614664 | MH614826 | MH614713 | MH614759 | This study |
| Chalciporus rubinus | AF2835 | Belgium | KT823962 | – | KT824028 | KT823995 | Raspé et al. 2016 |
| Chalciporus sp. | HKAS53400 | China | – | – | KF112279 | KF112821 | Wu et al. 2014 |
| Chalciporus sp. | HKAS74779 | China | – | – | KF112278 | KF112820 | Wu et al. 2014 |
| Chalciporus sp. | OR0363 | Thailand | MH645586 | MH645607 | MH645594 | MH645602 | This study |
| Chalciporus sp. | OR0373 | Thailand | MH645587 | MH645608 | MH645595 | MH645603 | This study |
| Chiua sp. | OR0141 | China | MH614665 | MH614827 | MH614714 | MH614760 | This study |
| Chiua virens | OR0266 | China | MG212541 | MH614828* | MG212585 | MG212627 | Vadthanarat et al. 2018; *This study |
| Chiua viridula | HKAS74928 | China | – | – | KF112273 | KF112794 | Wu et al. 2014 |
| Crocinoboletus cf. laetissimus | OR0576 | Thailand | KT823975 | MH614833* | KT824041 | KT824008 | Raspé et al. 2016; *This study |
| Crocinoboletus rufoaureus | HKAS53424 | China | – | – | KF112206 | KF112710 | Wu et al. 2014 |
| Cupreoboletus poikilochromus | GS10070 | Italy | – | – | KT157072 | KT157068 | Gelardi et al. 2015 |
| Cupreoboletus poikilochromus | GS11008 | Italy | – | – | KT157071 | KT157067 | Gelardi et al. 2015 |
| Cyanoboletus brunneoruber | HKAS80579_1 | China | – | – | KT990763 | KT990401 | Wu et al. 2016 |
| Cyanoboletus brunneoruber | OR0233 | China | MG212542 | MH614834* | MG212586 | MG212628 | Vadthanarat et al. 2018; *This study |
| Cyanoboletus instabilis | HKAS59554 | China | – | – | KF112186 | KF112698 | Wu et al. 2014 |
| Cyanoboletus pulverulentus | RW109 | Belgium | KT823980 | MH614835* | KT824046 | KT824013 | Raspé et al. 2016; *This study |
| Cyanoboletus sinopulverulentus | HKAS59609 | China | – | – | KF112193 | KF112700 | Wu et al. 2014 |
| Cyanoboletus sp. | HKAS52639 | China | – | – | KF112195 | KF112701 | Wu et al. 2014 |
| Cyanoboletus sp. | HKAS76850 | China | – | – | KF112187 | KF112697 | Wu et al. 2014 |
| Cyanoboletus sp. | OR0257 | China | MG212543 | MH614836* | MG212587 | MG212629 | Vadthanarat et al. 2018; *This study |
| Cyanoboletus sp. | HKAS90208_1 | China | – | – | KT990766 | KT990404 | Wu et al. 2016 |
| Cyanoboletus sp. | OR0322 | Thailand | MH614673 | MH614837 | MH614722 | MH614768 | This study |
| Cyanoboletus sp. | OR0491 | China | MH614674 | MH614838 | MH614723 | MH614769 | This study |
| Cyanoboletus sp. | OR0961 | Thailand | MH614675 | MH614839 | MH614724 | MH614770 | This study |
| Fistulinella prunicolor | REH9880 | Australia | MH614676 | MH614840 | MH614725 | MH614771 | This study |
| Gymnogaster boletoides | NY01194009 | Australia | – | – | KT990768 | KT990406 | Wu et al. 2016 |
| Harrya atriceps | REH7403 | Costa Rica | – | – | JX889702 | – | Halling et al. 2012b |
| Harrya chromapes | HKAS50527 | China | – | – | KF112270 | KF112792 | Wu et al. 2014 |
| Harrya moniliformis | HKAS49627 | China | – | – | KT990881 | KT990500 | Wu et al. 2016 |
| Heimioporus cf. mandarinus | OR0661 | Thailand | MG212545 | MH614841* | MG212589 | MG212631 | Vadthanarat et al. 2018; *This study |
| Heimioporus japonicus | OR0114 | Thailand | KT823971 | MH614842* | KT824037 | KT824004 | Raspé et al. 2016; *This study |
| Heimioporus retisporus | HKAS52237 | China | – | – | KF112228 | KF112806 | Wu et al. 2014 |
| Heimioporus sp. | OR0218 | Thailand | MG212546 | – | MG212590 | MG212632 | Vadthanarat et al. 2018 |
| Hemileccinum depilatum | AF2845 | Belgium | MG212547 | MH614843* | MG212591 | MG212633 | Vadthanarat et al. 2018; *This study |
| Hemileccinum impolitum | ADK4078 | Belgium | MG212548 | MH614844* | MG212592 | MG212634 | Vadthanarat et al. 2018; *This study |
| Hemileccinum indecorum | OR0863 | Thailand | MH614677 | MH614845 | MH614726 | MH614772 | This study |
| Hemileccinum rugosum | HKAS84970 | China | – | – | KT990773 | KT990412 | Wu et al. 2016 |
| Hortiboletus amygdalinus | HKAS54166 | China | – | – | KT990777 | KT990416 | Wu et al. 2016 |
| Hortiboletus rubellus | VDKO0403 | Belgium | MH614679 | MH614847 | – | MH614774 | This study |
| Hortiboletus sp. | HKAS50466 | China | – | – | KF112183 | KF112694 | Wu et al. 2014 |
| Hortiboletus sp. | HKAS51239 | China | – | – | KF112184 | KF112695 | Wu et al. 2014 |
| Hortiboletus sp. | HKAS51292 | China | – | – | KF112181 | KF112692 | Wu et al. 2014 |
| Hortiboletus sp. | HKAS76673 | China | – | – | KF112182 | KF112693 | Wu et al. 2014 |
| Hortiboletus subpaludosus | HKAS59608 | China | – | – | KF112185 | KF112696 | Wu et al. 2014 |
| Hourangia cf. pumila | OR0762 | Thailand | MH614680 | MH614848 | MH614728 | MH614775 | This study |
| Hourangia cheoi | HKAS74744 | China | – | – | KF112285 | KF112772 | Wu et al. 2014 |
| Hourangia cheoi | Zhu108 | China | – | – | KP136979 | KP136928 | Zhu et al. 2015 |
| Hourangia nigropunctata | HKAS 57427 | China | – | – | KP136927 | KP136978 | Zhu et al. 2015 |
| Hymenoboletus luteopurpureus | HKAS46334 | China | – | – | KF112271 | KF112795 | Wu et al. 2014 |
| Imleria badia | VDKO0709 | Belgium | KT823983 | MH614849* | KT824049 | KT824016 | Raspé et al. 2016; *This study |
| Imleria obscurebrunnea | OR0263 | China | MH614681 | MH614850 | MH614729 | MH614776 | This study |
| Imleria subalpina | HKAS74712 | China | – | – | KF112189 | KF112706 | Wu et al. 2014 |
| Lanmaoa angustispora | HKAS74759 | China | – | – | KM605155 | KM605178 | Wu et al. 2015 |
| Lanmaoa angustispora | HKAS74765 | China | – | – | KF112159 | KF112680 | Wu et al. 2014 |
| Lanmaoa angustispora | HKAS74752 | China | – | – | KM605154 | KM605177 | Wu et al. 2015 |
| Lanmaoa asiatica | HKAS54094 | China | – | – | KF112161 | KF112682 | Wu et al. 2014 |
| Lanmaoa asiatica | HKAS63516 | China | – | – | KT990780 | KT990419 | Wu et al. 2016 |
| Lanmaoa asiatica | OR0228 | China | MH614682 | MH614851 | MH614730 | MH614777 | This study |
| Lanmaoa carminipes | BOTH4591 | USA | MG897419 | MH614852* | MG897429 | MG897439 | Phookamsak et al. 2019, *This study |
| Lanmaoa flavorubra | NY775777 | Costa Rica | – | – | KF112160 | KF112681 | Wu et al. 2014 |
| Lanmaoa pallidorosea | BOTH4432 | USA | MG897417 | MH614853* | MG897427 | MG897437 | Phookamsak et al. 2019, *This study |
| Lanmaoa sp. | HKAS52518 | China | – | – | KF112162 | KF112683 | Wu et al. 2014 |
| Lanmaoa sp. | OR0130 | Thailand | MH614683 | MH614854 | MH614731 | MH614778 | This study |
| Lanmaoa sp. | OR0370 | Thailand | MH614684 | MH614855 | MH614732 | MH614779 | This study |
| Leccinellum aff. crocipodium | HKAS76658 | China | – | – | KF112252 | KF112728 | Wu et al. 2014 |
| Leccinellum aff. griseum | KPM-NC-0017832 | Japan | KC552164 | – | JN378450* | – | unpublished, *Orihara et al. 2012 |
| Leccinellum corsicum | Buf4507 | USA | – | – | KF030435 | – | Nuhn et al. 2013 |
| Leccinellum cremeum | HKAS90639 | China | – | – | KT990781 | KT990420 | Wu et al. 2016 |
| Leccinellum crocipodium | VDKO1006 | Belgium | KT823988 | MH614856* | KT824054 | KT824021 | Raspé et al. 2016; *This study |
| Leccinellum sp. | KPM-NC-0018041 | Japan | KC552165 | – | KC552094 | – | Orihara et al. 2016 |
| Leccinellum sp. | OR0711 | Thailand | MH614685 | – | MH614733 | MH614780 | This study |
| Leccinum monticola | HKAS76669 | China | – | – | KF112249 | KF112723 | Wu et al. 2014 |
| Leccinum quercinum | HKAS63502 | China | – | – | KF112250 | KF112724 | Wu et al. 2014 |
| Leccinum scabrum | RW105a | Belgium | KT823979 | MH614857* | KT824045 | KT824012 | Raspé et al. 2016; *This study |
| Leccinum scabrum | VDKO0938 | Belgium | MG212549 | MH614858* | MG212593 | MG212635 | Vadthanarat et al. 2018; *This study |
| Leccinum scabrum | KPM-NC-0017840 | Scotland | KC552170 | – | JN378455 | – | Orihara et al. 2016, 2012 |
| Leccinum schistophilum | VDKO1128 | Belgium | KT823989 | MH614859* | KT824055 | KT824022 | Raspé et al. 2016; *This study |
| Leccinum variicolor | VDKO0844 | Belgium | MG212550 | MH614860* | MG212594 | MG212636 | Vadthanarat et al. 2018; *This study |
| Mucilopilus castaneiceps | HKAS75045 | China | – | – | KF112211 | KF112735 | Wu et al. 2014 |
| Neoboletus brunneissimus | HKAS50538 | China | – | – | KM605150 | KM605173 | Wu et al. 2015 |
| Neoboletus brunneissimus | HKAS52660 | China | – | – | KF112143 | KF112650 | Wu et al. 2014 |
| Neoboletus brunneissimus | HKAS57451 | China | – | – | KM605149 | KM605172 | Wu et al. 2015 |
| Neoboletus brunneissimus | OR0249 | China | MG212551 | MH614861* | MG212595 | MG212637 | Vadthanarat et al. 2018; *This study |
| Neoboletus erythropus | VDKO0690 | Belgium | KT823982 | MH614864* | KT824048 | KT824015 | Raspé et al. 2016; *This study |
| Neoboletus ferrugineus | HKAS77718 | China | – | – | KT990789 | KT990431 | Wu et al. 2016 |
| Neoboletus ferrugineus | HKAS77617 | China | – | – | KT990788 | KT990430 | Wu et al. 2016 |
| Neoboletus flavidus | HKAS59443 | China | – | – | KU974136 | KU974144 | Wu et al. 2016 |
| Neoboletus flavidus | HKAS58724 | China | – | – | KU974137 | KU974145 | Wu et al. 2016 |
| Neoboletus hainanensis | HKAS63515 | China | – | – | KT990808 | KT990449 | Wu et al. 2016 |
| Neoboletus hainanensis | HKAS74880 | China | – | – | KT990790 | KT990432 | Wu et al. 2016 |
| Neoboletus hainanensis | HKAS90209 | China | – | – | KT990809 | KT990450 | Wu et al. 2016 |
| Neoboletus hainanensis | HKAS59469 | China | – | – | KF112175 | KF112669 | Wu et al. 2014 |
| Neoboletus junquilleus | AF2922 | France | MG212552 | MH614862* | MG212596 | MG212638 | Vadthanarat et al. 2018; *This study |
| Neoboletus magnificus | HKAS54096 | China | – | – | KF112149 | KF112654 | Wu et al. 2014 |
| Neoboletus magnificus | HKAS74939 | China | – | – | KF112148 | KF112653 | Wu et al. 2014 |
| Neoboletus multipunctatus | HKAS76851 | China | – | – | KF112144 | KF112651 | Wu et al. 2014 |
| Neoboletus multipunctatus | OR0128 | Thailand | MH614686 | MH614863 | MH614734 | MH614781 | This study |
| Neoboletus obscureumbrinus | OR0553 | Thailand | MK372271 | – | MK372282 | MK372294 | This study |
| Neoboletus obscureumbrinus | HKAS63498 | China | – | – | KT990791 | KT990433 | Wu et al. 2016 |
| Neoboletus obscureumbrinus | HKAS77774 | China | – | – | KT990792 | KT990434 | Wu et al. 2016 |
| Neoboletus obscureumbrinus | HKAS89014 | China | – | – | KT990793 | KT990435 | Wu et al. 2016 |
| Neoboletus obscureumbrinus | HKAS89027 | China | – | – | KT990794 | KT990436 | Wu et al. 2016 |
| Neoboletus rubriporus | HKAS57512 | China | – | – | KF112151 | KF112656 | Wu et al. 2014 |
| Neoboletus rubriporus | HKAS83026 | China | – | – | KT990795 | KT990437 | Wu et al. 2016 |
| Neoboletus sanguineoides | HKAS57766 | China | – | – | KT990799 | KT990440 | Wu et al. 2016 |
| Neoboletus sanguineoides | HKAS74733 | China | – | – | KT990800 | KT990441 | Wu et al. 2016 |
| Neoboletus sanguineoides | HKAS55440 | China | – | – | KF112145 | KF112652 | Wu et al. 2014 |
| Neoboletus sanguineus | HKAS80823 | China | – | – | KT990802 | KT990442 | Wu et al. 2016 |
| Neoboletus tomentulosus | HKAS77656 | China | – | – | KT990806 | KT990446 | Wu et al. 2016 |
| Neoboletus tomentulosus | HKAS53369 | China | – | – | KF112154 | KF112659 | Wu et al. 2014 |
| Neoboletus venenatus | HKAS57489 | China | – | – | KF112158 | KF112665 | Wu et al. 2014 |
| Neoboletus venenatus | HKAS63535 | China | – | – | KT990807 | KT990448 | Wu et al. 2016 |
| Neoboletus sp. | HKAS76660 | China | – | – | KF112180 | KF112731 | Wu et al. 2014 |
| Octaviania asahimontana | KPM-NC-17824 | Japan | KC552154 | – | JN378430 | – | Orihara et al. 2016, 2012 |
| Octaviania asterosperma | AQUI3899 | Italy | KC552159 | – | KC552093 | – | Orihara et al. 2016 |
| Octaviania celatifilia | KPM-NC-17776 | Japan | KC552147 | – | JN378416 | – | Orihara et al. 2016, 2012 |
| Octaviania cyanescens | PNW-FUNGI-5603 | USA | KC552160 | – | JN378438 | – | Orihara et al. 2016, 2012 |
| Octaviania decimae | KPM-NC17763 | Japan | KC552145 | – | JN378409 | – | Orihara et al. 2016, 2012 |
| Octaviania tasmanica | MEL2128484 | Australia | KC552157 | – | JN378437 | – | Orihara et al. 2016, 2012 |
| Octaviania tasmanica | MEL2341996 | Australia | KC552156 | – | JN378436 | – | Orihara et al. 2016, 2012 |
| Octaviania zelleri | MES270 | USA | KC552161 | – | JN378440 | – | Orihara et al. 2016, 2012 |
| Parvixerocomus pseudoaokii | OR0155 | China | MG212553 | MH614865 | MG212597 | MG212639 | This study |
| Phylloporus bellus | OR0473 | China | MH580778 | MH614866* | MH580798 | MH580818 | Chuankid et al. 2019; *This study |
| Phylloporus brunneiceps | OR0050 | Thailand | KT823968 | MH614867* | KT824034 | KT824001 | Raspé et al. 2016; *This study |
| Phylloporus castanopsidis | OR0052 | Thailand | KT823969 | MH614868* | KT824035 | KT824002 | Raspé et al. 2016; *This study |
| Phylloporus imbricatus | HKAS68642 | China | – | – | KF112299 | KF112786 | Wu et al. 2014 |
| Phylloporus luxiensis | HKAS75077 | China | – | – | KF112298 | KF112785 | Wu et al. 2014 |
| Phylloporus maculatus | OR0285 | China | MH580780 | – | MH580800 | MH580820 | Chuankid et al. 2019 |
| Phylloporus pelletieri | WU18746 | Austria | MH580781 | MH614869* | MH580801 | MH580821 | Chuankid et al. 2019; *This study |
| Phylloporus pusillus | OR1158 | Thailand | MH580783 | MH614870* | MH580803 | MH580823 | Chuankid et al. 2019; *This study |
| Phylloporus rhodoxanthus | WU17978 | USA | MH580785 | MH614871* | MH580805 | MH580824 | Chuankid et al. 2019; *This study |
| Phylloporus rubeolus | OR0251 | China | MH580786 | MH614872* | MH580806 | MH580825 | Chuankid et al. 2019; *This study |
| Phylloporus rubiginosus | OR0169 | China | MH580788 | MH614873* | MH580808 | MH580827 | Chuankid et al. 2019; *This study |
| Phylloporus sp. | OR0896 | Thailand | MH580790 | MH614874* | MH580810 | MH580829 | Chuankid et al. 2019; *This study |
| Phylloporus subbacillisporus | OR0436 | China | MH580792 | MH614875* | MH580812 | MH580831 | Chuankid et al. 2019; *This study |
| Phylloporus subrubeolus | BC022 | Thailand | MH580793 | MH614876* | MH580813 | MH580832 | Chuankid et al. 2019; *This study |
| Phylloporus yunnanensis | OR0448 | China | MG212554 | MH614877* | MG212598 | MG212640 | Vadthanarat et al. 2018; *This study |
| Porphyrellus castaneus | OR0241 | China | MG212555 | MH614878* | MG212599 | MG212641 | Vadthanarat et al. 2018; *This study |
| Porphyrellus cf. nigropurpureus | ADK3733 | Benin | MH614687 | MH614879 | MH614735 | MH614782 | This study |
| Porphyrellus nigropurpureus | HKAS74938 | China | – | – | KF112246 | KF112763 | Wu et al. 2014 |
| Porphyrellus porphyrosporus | MB97 023 | Germany | DQ534609 | – | GU187734 | GU187800 | Binder and Hibbett 2006; Binder et al. 2010 |
| Porphyrellus sp. | HKAS53366 | China | – | – | KF112241 | KF112716 | Wu et al. 2014 |
| Porphyrellus sp. | JD659 | Burundi | MH614688 | MH614880 | MH614736 | MH614783 | This study |
| Porphyrellus sp. | OR0222 | Thailand | MH614689 | MH614881 | MH614737 | MH614784 | This study |
| Pulveroboletus aff. ravenelii | HKAS50203 | China | – | – | KT990810 | KT990451 | Wu et al. 2016 |
| Pulveroboletus aff. ravenelii | ADK4360 | Togo | KT823957 | MH614882* | KT824023 | KT823990 | Raspé et al. 2016; *This study |
| Pulveroboletus aff. ravenelii | ADK4650 | Togo | KT823959 | MH614883* | KT824025 | KT823992 | Raspé et al. 2016; *This study |
| Pulveroboletus aff. ravenelii | HKAS53351 | China | – | – | KF112261 | KF112712 | Wu et al. 2014 |
| Pulveroboletus brunneopunctatus | HKAS52615 | China | – | – | KT990813 | KT990454 | Wu et al. 2016 |
| Pulveroboletus brunneopunctatus | HKAS55369 | China | – | – | KT990814 | KT990455 | Wu et al. 2016 |
| Pulveroboletus brunneopunctatus | HKAS74926 | China | – | – | KT990815 | KT990456 | Wu et al. 2016 |
| Pulveroboletus fragrans | OR0673 | Thailand | KT823977 | MH614884* | KT824043 | KT824010 | Raspé et al. 2016; *This study |
| Pulveroboletus macrosporus | HKAS57628 | China | – | – | KT990812 | KT990453 | Wu et al. 2016 |
| Pulveroboletus ravenelii | REH2565 | USA | KU665635 | MH614885* | KU665636 | KU665637 | Raspé et al. 2016; *This study |
| Pulveroboletus sp. | HKAS74933 | China | – | – | KF112262 | KF112713 | Wu et al. 2014 |
| Pulveroboletus sp. | HKAS57665 | China | – | – | KF112264 | KF112715 | Wu et al. 2014 |
| Retiboletus aff. nigerrimus | OR0049 | Thailand | KT823967 | MH614886* | KT824033 | KT824000 | Raspé et al. 2016; *This study |
| Retiboletus brunneolus | HKAS52680 | China | – | – | KF112179 | KF112690 | Wu et al. 2014 |
| Retiboletus fuscus | HKAS59460 | China | – | – | JQ928580 | JQ928601 | Hosen et al. 2013 |
| Retiboletus fuscus | OR0231 | China | MG212556 | MH614887* | MG212600 | MG212642 | Vadthanarat et al. 2018; *This study |
| Retiboletus fuscus | HKAS63624 | China | – | – | KT990829 | KT990466 | Wu et al. 2016 |
| Retiboletus fuscus | HKAS74756 | China | – | – | KT990830 | KT990467 | Wu et al. 2016 |
| Retiboletus griseus | MB03 079 | USA | KT823964 | MH614888* | KT824030 | KT823997 | Raspé et al. 2016; *This study |
| Retiboletus griseus | HKAS63590 | China | – | – | KF112178 | KF112691 | Wu et al. 2014 |
| Retiboletus kauffmanii | OR0278 | China | MG212557 | MH614889* | MG212601 | MG212643 | Vadthanarat et al. 2018; *This study |
| Retiboletus nigerrimus | HKAS53418 | China | – | – | KT990824 | KT990462 | Wu et al. 2016 |
| Retiboletus sinensis | HKAS59832 | China | – | – | KT990827 | KT990464 | Wu et al. 2016 |
| Retiboletus zhangfeii | HKAS59699 | China | – | – | JQ928582 | JQ928603 | Hosen et al. 2013 |
| Rhodactina himalayensis | CMU25117 | Thailand | MG212558 | – | MG212602, MG212603 | – | Vadthanarat et al. 2018 |
| Rhodactina rostratispora | SV170 | Thailand | MG212560 | – | MG212605 | MG212645 | Vadthanarat et al. 2018 |
| Rossbeevera cryptocyanea | KPM-NC17843 | Japan | KT581441 | – | KC552072 | – | Orihara et al. 2016 |
| Rossbeevera eucyanea | TNS-F-36986 | Japan | KC552115 | – | KC552068 | – | Orihara et al. 2016 |
| Rossbeevera griseovelutina | TNS-F-36989 | Japan | KC552124 | – | KC552076 | – | Orihara et al. 2016 |
| Rossbeevera pachydermis | KPM-NC23336 | New Zealand | KJ001064 | – | KP222912 | – | Orihara et al. 2016 |
| Rossbeevera vittatispora | OSC61484 | Australia | KC552109 | – | JN378446 | – | Orihara et al. 2016, 2012 |
| Royoungia reticulata | HKAS52253 | China | – | – | KT990786 | KT990427 | Wu et al. 2016 |
| Royoungia rubina | HKAS53379 | China | – | – | KF112274 | KF112796 | Wu et al. 2014 |
| Rubroboletus latisporus | HKAS80358 | China | – | – | KP055020 | KP055029 | Zhao et al. 2014b |
| Rubroboletus legaliae | VDKO0936 | Belgium | KT823985 | MH614890* | KT824051 | KT824018 | Raspé et al. 2016; *This study |
| Rubroboletus rhodosanguineus | BOTH4263 | USA | MG897416 | MH614891* | MG897426 | MG897436 | Phookamsak et al. 2019, *This study |
| Rubroboletus rhodoxanthus | HKAS84879 | Germany | – | – | KT990831 | KT990468 | Wu et al. 2016 |
| Rubroboletus satanas | VDKO0968 | Belgium | KT823986 | MH614892* | KT824052 | KT824019 | Raspé et al. 2016; *This study |
| Rubroboletus sinicus | HKAS68620 | China | – | – | KF112146 | KF112661 | Wu et al. 2014 |
| Rubroboletus sinicus | HKAS56304 | China | – | – | KJ619483 | KP055031 | Zhao et al. 2014a; Zhao et al. 2014b |
| Rubroboletus sp. | HKAS68679 | China | – | – | KF112147 | KF112662 | Wu et al. 2014 |
| Rugiboletus brunneiporus | HKAS68586 | China | – | – | KF112197 | KF112719 | Wu et al. 2014 |
| Rugiboletus brunneiporus | HKAS83009 | China | – | – | KM605146 | KM605169 | Wu et al. 2015 |
| Rugiboletus brunneiporus | HKAS83209 | China | – | – | KM605144 | KM605168 | Wu et al. 2015 |
| Rugiboletus extremiorientalis | HKAS76663 | China | – | – | KM605147 | KM605170 | Wu et al. 2015 |
| Rugiboletus extremiorientalis | OR0406 | Thailand | MG212562 | MH614893* | MG212607 | MG212647 | Vadthanarat et al. 2018; *This study |
| Rugiboletus sp. | HKAS55373 | China | – | – | KF112303 | KF112804 | Wu et al. 2014 |
| Singerocomus inundabilis | TWH9199 | Guyana | MH645588 | MH645609 | MH645596 | LC043089* | *Henkel et al. 2016; This study |
| Singerocomus rubriflavus | TWH9585 | Guyana | MH645589 | MH645610 | MH645597 | – | This study |
| Spongiforma thailandica | DED7873 | Thailand | MG212563 | MH614894** | KF030436* | MG212648 | *Nuhn et al. 2013; Vadthanarat et al. 2018; **This study |
| Strobilomyces atrosquamosus | HKAS55368 | China | – | – | KT990839 | KT990476 | Wu et al. 2016 |
| Strobilomyces echinocephalus | OR0243 | China | MG212564 | – | MG212608 | MG212649 | Vadthanarat et al. 2018 |
| Strobilomyces mirandus | OR0115 | Thailand | KT823972 | MH614896* | KT824038 | KT824005 | Raspé et al. 2016; *This study |
| Strobilomyces strobilaceus | MB03 102 | USA | DQ534607* | – | AY883428 | AY786065 | *Binder and Hibbett 2006, Unpublished |
| Strobilomyces strobilaceus | RW103 | Belgium | KT823978 | MH614895* | KT824044 | KT824011 | Raspé et al. 2016; *This study |
| Strobilomyces verruculosus | HKAS55389 | China | – | – | KF112259 | KF112813 | Wu et al. 2014 |
| Strobilomyces sp. | OR0259 | China | MG212565 | MH614897* | MG212609 | MG212650 | Vadthanarat et al. 2018; *This study |
| Strobilomyces sp. | OR0319 | Thailand | MH614690 | MH614898 | MH614738 | MH614785 | This study |
| Strobilomyces sp. | OR0778 | Thailand | MG212566 | MH614899* | MG212610 | MG212651 | Vadthanarat et al. 2018; *This study |
| Strobilomyces sp. | OR1092 | Thailand | MH614691 | MH614900 | MH614739 | MH614786 | This study |
| Suillellus amygdalinus | 112605ba | USA | – | – | JQ327024 | – | Halling et al. 2012a |
| Suillellus luridus | VDKO0241b | Belgium | KT823981 | MH614901* | KT824047 | KT824014 | Raspé et al. 2016; *This study |
| Suillellus queletii | VDKO1185 | Belgium | MH645590 | MH645611 | MH645598 | MH645604 | This study |
| Suillellus subamygdalinus | HKAS57262 | China | – | – | KF112174 | KF112660 | Wu et al. 2014 |
| Suillellus subamygdalinus | HKAS53641 | China | – | – | KT990841 | KT990478 | Wu et al. 2016 |
| Suillellus subamygdalinus | HKAS74745 | China | – | – | KT990843 | KT990479 | Wu et al. 2016 |
| Sutorius aff. eximius | HKAS52672 | China | – | – | KF112207 | KF112802 | Wu et al. 2014 |
| Sutorius aff. eximius | HKAS56291 | China | – | – | KF112208 | KF112803 | Wu et al. 2014 |
| Sutorius australiensis | REH9441 | Australia | MG212567 | MK386576** | JQ327032* | MG212652 | *Halling et al. 2012a; Vadthanarat et al. 2018; **This study |
| Sutorius eximius | HKAS59657 | China | – | – | KT990887 | KT990505 | Wu et al. 2016 |
| Sutorius eximius | REH9400 | USA | MG212568 | MH614902** | JQ327029* | MG212653 | *Halling et al. 2012a; Vadthanarat et al. 2018; **This study |
| Sutorius eximius | HKAS50420 | China | – | – | KT990750 | KT990387 | Wu et al. 2016 |
| Sutorius sp. | OR0378B | Thailand | MH614692 | MH614903 | MH614740 | MH614787 | This study |
| Sutorius sp. | OR0379 | Thailand | MH614693 | MH614904 | MH614741 | MH614788 | This study |
| Tengioboletus glutinosus | HKAS53425 | China | – | – | KF112204 | KF112800 | Wu et al. 2014 |
| Tengioboletus reticulatus | HKAS53426 | China | – | – | KF112313 | KF112828 | Wu et al. 2014 |
| Tengioboletus sp. | HKAS76661 | China | – | – | KF112205 | KF112801 | Wu et al. 2014 |
| Turmalinea persicina | KPM-NC18001 | Japan | KC552130 | – | KC552082 | – | Orihara et al. 2016 |
| Turmalinea yuwanensis | KPM-NC18011 | Japan | KC552138 | – | KC552089 | – | Orihara et al. 2016 |
| Tylocinum griseolum | HKAS50281 | China | – | – | KF112284 | KF112730 | Wu et al. 2014 |
| Tylopilus alpinus | HKAS55438 | China | – | – | KF112191 | KF112687 | Wu et al. 2014 |
| Tylopilus atripurpureus | HKAS50208 | China | – | – | KF112283 | KF112799 | Wu et al. 2014 |
| Tylopilusballoui s.l. | OR0039 | Thailand | KT823965 | MH614905* | KT824031 | KT823998 | Raspé et al. 2016; *This study |
| Tylopilus brunneirubens | HKAS53388 | China | – | – | KF112192 | KF112688 | Wu et al. 2014 |
| Tylopilus felleus | VDKO0992 | Belgium | KT823987 | MH614906* | KT824053 | KT824020 | Raspé et al. 2016; *This study |
| Tylopilus ferrugineus | BOTH3639 | USA | MH614694 | MH614907 | MH614742 | MH614789 | This study |
| Tylopilus otsuensis | HKAS53401 | China | – | – | KF112224 | KF112797 | Wu et al. 2014 |
| Tylopilus sp. | HKAS74925 | China | – | – | KF112222 | KF112739 | Wu et al. 2014 |
| Tylopilus sp. | HKAS50229 | China | – | – | KF112216 | KF112769 | Wu et al. 2014 |
| Tylopilus sp. | JD598 | Gabon | MH614695 | MH614908 | MH614743 | MH614790 | This study |
| Tylopilus sp. | OR0252 | China | MG212569 | MH614909* | MG212611 | MG212654 | Vadthanarat et al. 2018; *This study |
| Tylopilus sp. | OR0542 | Thailand | MG212570 | MH614910* | MG212612 | MG212655 | Vadthanarat et al. 2018; *This study |
| Tylopilus sp. | OR0583 | Thailand | MH614696 | – | MH614744 | – | This study |
| Tylopilus sp. | OR1009 | Thailand | MH614697 | MH614911 | – | MH614791 | This study |
| Tylopilus vinaceipallidus | HKAS50210 | China | – | – | KF112221 | KF112738 | Wu et al. 2014 |
| Tylopilus vinaceipallidus | OR0137 | China | MG212571 | MH614912* | MG212613 | MG212656 | Vadthanarat et al. 2018; *This study |
| Tylopilus violaceobrunneus | HKAS89443 | China | – | – | KT990886 | KT990504 | Wu et al. 2016 |
| Tylopilus virens | KPM-NC-0018054 | Japan | KC552174 | – | KC552103 | – | Unpublished |
| Veloporphyrellus alpinus | HKAS68301 | China | JX984515 | – | JX984550 | – | Li et al. 2014b |
| Veloporphyrellus conicus | REH8510 | Belize | MH614698 | MH614913 | MH614745 | MH614792 | This study |
| Veloporphyrellus gracilioides | HKAS53590 | China | – | – | KF112210 | KF112734 | Wu et al. 2014 |
| Veloporphyrellus pseudovelatus | HKAS59444 | China | JX984519 | – | JX984553 | – | Li et al. 2014b |
| Veloporphyrellus velatus | HKAS63668 | China | JX984523 | – | JX984554 | – | Li et al. 2014b |
| Xanthoconium affine | NY00815399 | USA | – | – | KT990850 | KT990486 | Wu et al. 2016 |
| Xanthoconium porophyllum | HKAS90217 | China | – | – | KT990851 | KT990487 | Wu et al. 2016 |
| Xanthoconium sinense | HKAS77651 | China | – | – | KT990853 | KT990488 | Wu et al. 2016 |
| Xerocomellus chrysenteron | VDKO0821 | Belgium | KT823984 | MH614914* | KT824050 | KT824017 | Raspé et al. 2016; *This study |
| Xerocomellus cisalpinus | ADK4864 | Belgium | KT823960 | MH614915* | KT824026 | KT823993 | Raspé et al. 2016; *This study |
| Xerocomellus communis | HKAS50467 | China | – | – | KT990858 | KT990494 | Wu et al. 2016 |
| Xerocomellus corneri | HKAS90206 | Philippines | – | – | KT990857 | KT990493 | Wu et al. 2016 |
| Xerocomellus porosporus | VDKO0311 | Belgium | MH614678 | MH614846 | MH614727 | MH614773 | This study |
| Xerocomellus ripariellus | VDKO0404 | Belgium | MH614699 | MH614916 | MH614746 | MH614793 | This study |
| Xerocomellus sp. | HKAS56311 | China | – | – | KF112170 | KF112684 | Wu et al. 2014 |
| Xerocomus aff. macrobbii | HKAS56280 | China | – | – | KF112265 | KF112708 | Wu et al. 2014 |
| Xerocomus fulvipes | HKAS76666 | China | – | – | KF112292 | KF112789 | Wu et al. 2014 |
| Xerocomus magniporus | HKAS58000 | China | – | – | KF112293 | KF112781 | Wu et al. 2014 |
| Xerocomus s.s. sp. | OR0237 | China | MH580796 | – | MH580816 | MH580835 | Chuankid et al. 2019 |
| Xerocomus s.s. sp. | OR0443 | China | MH580797 | MH614917* | MH580817 | MH580836 | Chuankid et al. 2019; *This study |
| Xerocomus sp. | OR0053 | Thailand | MH580795 | MH614918* | MH580815 | MH580834 | Chuankid et al. 2019; *This study |
| Xerocomus subtomentosus | VDKO0987 | Belgium | MG212572 | MH614919* | MG212614 | MG212657 | Vadthanarat et al. 2018; *This study |
| Zangia citrina | HKAS52684 | China | HQ326850 | – | HQ326872 | – | Li et al. 2011 |
| Zangia olivacea | HKAS45445 | China | HQ326854 | – | HQ326873 | – | Li et al. 2011 |
| Zangia olivaceobrunnea | HKAS52272 | China | HQ326857 | – | HQ326876 | – | Li et al. 2011 |
| Zangia roseola | HKAS51137 | China | HQ326858 | – | HQ326877 | – | Li et al. 2011 |
| Zangia roseola | HKAS75046 | China | – | – | KF112269 | KF112791 | Wu et al. 2014 |
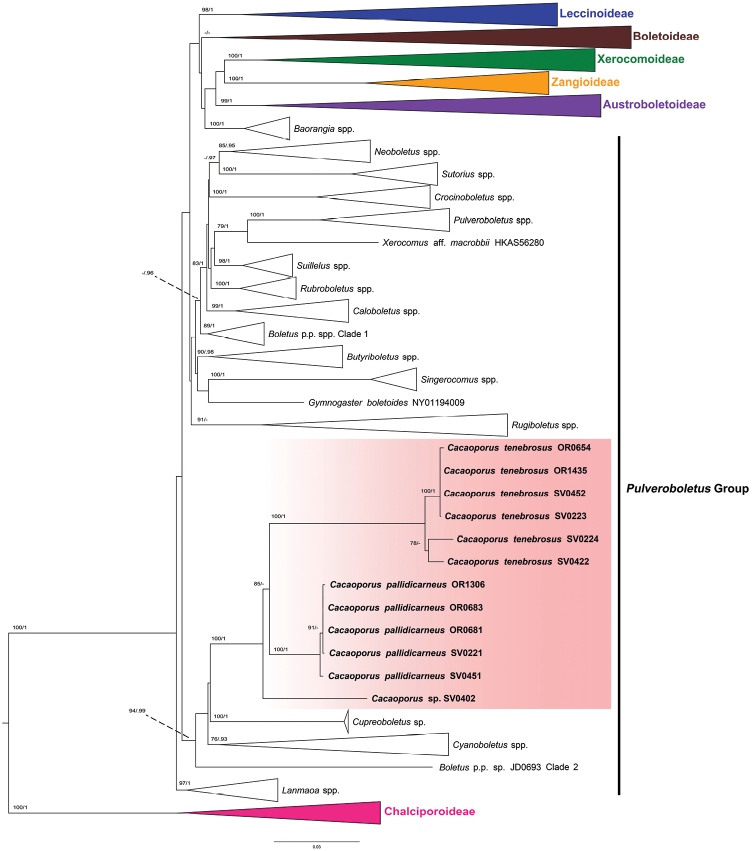
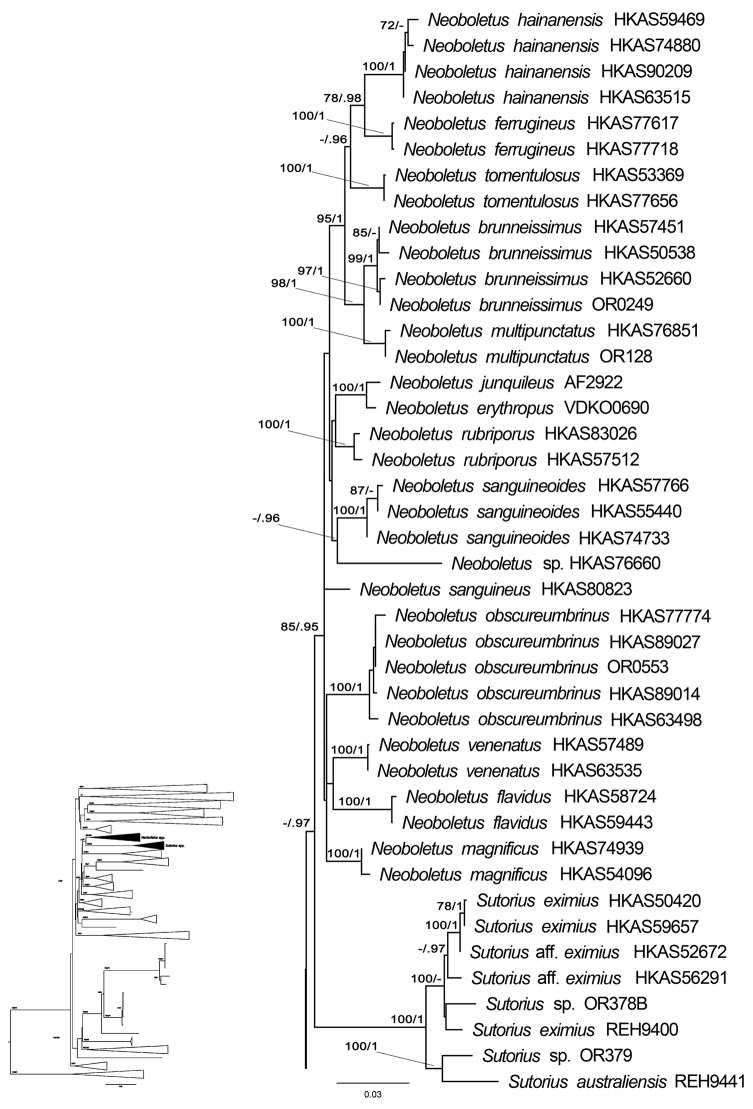
The four-gene analyses retrieved the six subfamilies (Austroboletoideae, Boletoideae, Chalciporoideae, Leccinoideae, Xerocomoideae, Zangioideae) as monophyletic (Fig. 1). The genera belonging to the Pulveroboletus group of Wu et al. (2014, 2016) did not form a monophyletic group. The new genus, Cacaoporus was monophyletic (BS=100% and PP=1) within a clade containing the genera Cupreoboletus Simonini, Gelardi & Vizzini and Cyanoboletus Gelardi, Vizzini & Simonini and one undescribed taxon, Boletus p.p. sp., clade 2 (specimen voucher JD0693) with high support (BS=94% and PP=0.99). The macromorphologically most similar genus, Sutorius, formed another clade (BS=100% and PP=1) sister to Neoboletus Gelardi, Simonini & Vizzini, with 67% BS and 0.97 PP support, in another clade of the Pulveroboletus group.
Figure 1.
Phylogenetic tree inferred from the four-gene dataset (atp6, cox3, rpb2 and tef1), including Cacaoporus species and selected Boletaceae using Maximum Likelihood and Bayesian Inference methods (ML tree is presented). The two Buchwaldoboletus and nine Chalciporus species in subfamily Chalciporoideae were used as outgroup. Most of the taxa not belonging to the Pulveroboletus group were collapsed into subfamilies. All genera clades in Pulveroboletus group that were highly supported were also collapsed. Bootstrap support values (BS ≥ 70%) and posterior probabilities (PP ≥ 0.90) are shown above the supported branches.
Our phylogeny also showed that thirteen Sutorius species including S.brunneissimus (W.F. Chiu) G. Wu & Zhu L. Yang, S.ferrugineus G. Wu, Fang Li & Zhu L. Yang, S.flavidus G. Wu & Zhu L. Yang, S.hainanensis (T.H. Li & M. Zang) G. Wu & Zhu L. Yang, S.junquilleus (Quél.) G. Wu & Zhu L. Yang, S.magnificus (W.F. Chiu) G. Wu & Zhu L. Yang, S.obscureumbrinus (Hongo) G. Wu & Zhu L. Yang, S.rubriporus G. Wu & Zhu L. Yang, S.sanguineoides G. Wu & Zhu L. Yang, S.sanguineus G. Wu & Zhu L. Yang, S.tomentulosus (M. Zang, W.P. Liu & M.R. Hu) G. Wu & Zhu L. Yang and S.venenatus (Nagas.) G. Wu & Zhu L. Yang clustered in the Neoboletus clade with high support (85% BS and 0.95 PP), while the true Sutorius, including the typus generis S.eximius (Peck) Halling, Nuhn & Osmundson, formed a different well-supported clade (BS=100% and PP=1).
Taxonomy
Cacaoporus
Raspé & Vadthanarat gen. nov.
MB829655
Etymology.
Refers to the dark, chocolate brown hymenophore and overall colour of basidiomata.
Diagnosis.
Similar to the genus Sutorius in having brown basidiomata with brown encrustations in the flesh but differs from Sutorius in having the following combination of characters: brown to chocolate brown or greyish-brown to dark brown or blackish-brown basidiomata, without violet tinge, chocolate brown to dark brown hymenophore, tubes not separable from the pileus context, white to off-white basal mycelium which turns reddish-white to pale red when bruised, amygdaliform to ovoid with subacute apex in side view to ovoid basidiospores and dark brown spore deposit.
Description.
Basidiomata stipitate-pileate with poroid hymenophore, small to medium-sized, dull, brown to greyish-brown to dark brown or blackish-brown. Pileus convex when young becoming plano-convex to slightly depressed with age, with deflexed to inflexed margin; surface even to subrugulose, minutely tomentose or slightly cracked at the centre; context soft, yellowish to greyish off-white then slightly greyish-orange to dull orange to greyish-brown when exposed to the air, patchy or marmorated with greyish-brown to dark brown, sometimes with scattered small dark brown to brownish-black encrustations, not or inconsistently reddening when cut. Hymenophore tubulate, adnate, subventricose to ventricose, slightly depressed around the stipe; tubes brown to greyish-brown to dark brown, not separable from the pileus context; pores regularly arranged, mostly roundish at first becoming slightly angular with age, sometimes irregular, elongated around the stipe, dark brown to greyish-brown at first, becoming brown to chocolate brown with age. Stipe central, terete to sometimes slightly compressed, cylindrical to sometimes slightly wider at the base; surface even, minutely tomentose, dull, dark brown to greyish-brown, basal mycelium white to off-white becoming reddish-white to pale red when touched; context solid, yellowish to orange white to yellowish-grey to pale orange to dull orange to reddish-grey, marmorated or virgated with brownish-grey to greyish-brown to dark brown, sometimes scattered with small reddish-brown to brownish-black fine encrustations, unchanged or inconsistently reddening when cut. Spore print dark brown.
Basidiospores amygdaliform to ovoid or ovoid with subacute apex in side view, thin-walled, smooth, slightly reddish to brownish hyaline in water, slightly yellowish to greenish hyaline in KOH or NH4OH, inamyloid. Basidia 4-spored, clavate to narrowly clavate without basal clamp connection. Cheilocystidia fusiform or cylindrical with obtuse apex, sometimes bent or sinuate, thin-walled, often scattered with small brownish-yellow to yellowish-brown crystals on the walls in KOH or NH4OH. Pleurocystidia narrowly fusiform with obtuse apex or cylindrical to narrowly subclavate, sometimes bent or sinuate, thin-walled, densely covered with small reddish-brown to brownish dark encrustations on the walls when observed in H2O, which are discoloured then dissolved in KOH or NH4OH. Pileipellis a trichoderm becoming tangled trichoderm to tomentum, composed of thin-walled hyphae; terminal cells mostly slightly sinuate cylindrical to irregular with rounded apex or clavate to elongated clavate. Stipitipellis a trichoderm to tangled trichoderm or disrupted hymeniderm, composed of loosely to moderately interwoven cylindrical hyphae anastomosing at places. Clamp connections not seen in any tissue.
Typus generis.
Cacaoporus tenebrosus
Distribution.
Currently known from Thailand.
Notes.
Sutorius most closely resembles the new genus. In the field, Cacaoporus is easily distinguished from the Sutorius by the following combination of characters: chocolate brown to dark brown to blackish-brown basidiomata, which are darker than in Sutorius and never purplish-brown like in Sutorius species; chocolate brown to dark brown hymenophore, which is much darker than in Sutorius and never reddish- to purplish-brown like in Sutorius; tubes that are not separable from the pileus context but can be separated in Sutorius; off-white basal mycelium that more or less turns red when bruised, which is never the case in Sutorius.
Cacaoporus pallidicarneus
Vadthanarat, Raspé & Lumyong sp. nov.
MB829657
Figure 2.
Habit of Cacaoporus species. aC.pallidicarneus (SV0221) b–dC.tenebrosus (b - SV0223, c - SV0224, d - SV0422). Scale bars: 1 cm (a–d).
Figure 3.
Close-ups of hymenium/pileus context transition zone in Cacaoporus species, illustrating the non-separability of both tissues aC.pallidicarneus (OR0681) bC.tenebrosus (OR0654) cC.tenebrosus (SV0452). The transition between both tissues is particularly unmarked in C.pallidicarneus (a) Scale bars: 3 mm (a); 5 mm (b–c).
Figure 4.
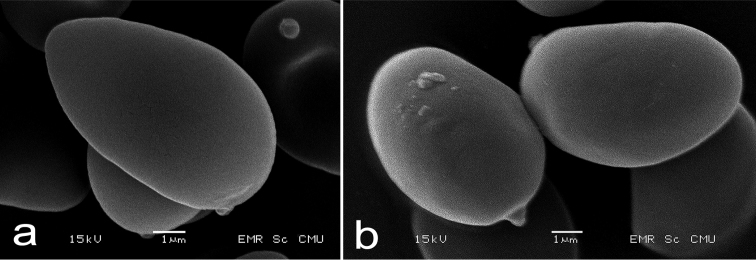
Scanning electron micrographs of Cacaoporus basidiospores aC.pallidicarneus (SV0221) bC.tenebrosus (SV0223). Scale bars: 1 µm (a–b).
Figure 5.
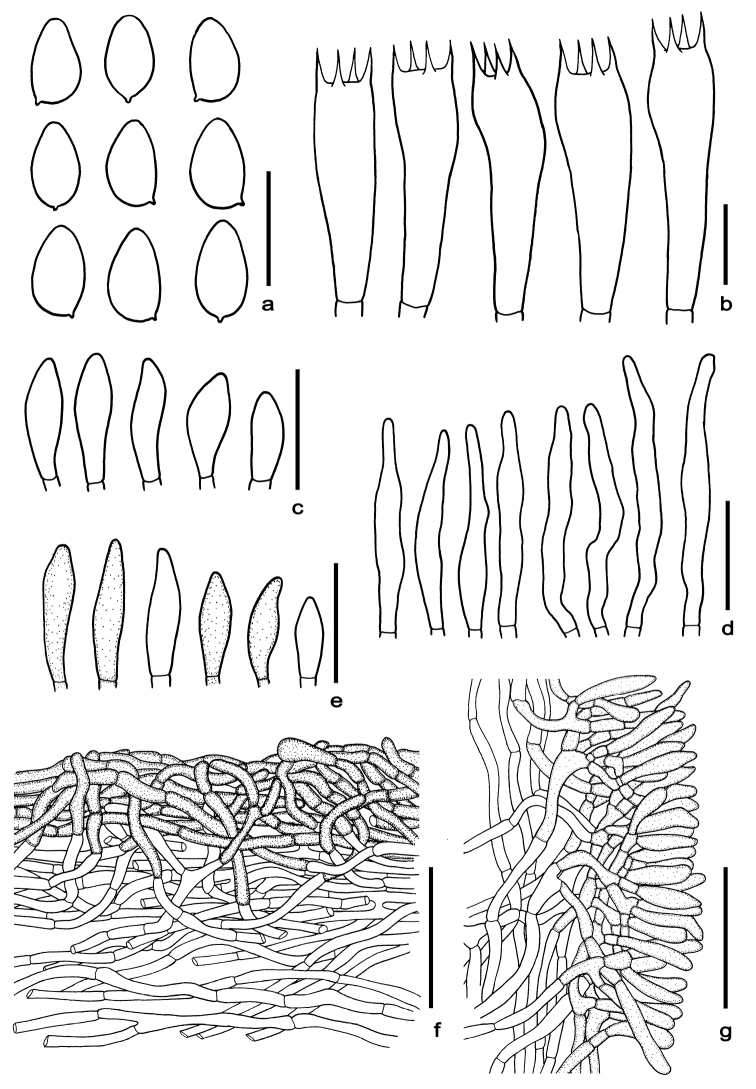
Microscopic features of Cacaoporuspallidicarneusa basidiospores b basidia c cheilocystidia d pleurocystidia e caulocystidia f pileipellis g stipitipellis. Scale bars: 10 µm (a–b); 25 µm (c–e); 50 µm (f–g). All drawings were made from the type (SV0221).
Etymology.
Refers to the context, which is paler than in the other species, especially at the stipe base and in the pileus.
Type.
THAILAND, Chiang Mai Province, Mae On District, 18°52'37"N, 99°18'23"E, elev. 860 m, 15 August 2015, Santhiti Vadthanarat, SV0221 (CMUB!, isotype BR!).
Diagnosis.
Cacaoporuspallidicarneus is characterised by having a paler context than the other species and basidiospores that are amygdaliform or elongated amygdaliform to ovoid in side view, sometimes with subacute apex, shorter basidia and fusiform to narrowly bent fusiform to narrowly fusiform hymenophoral cystidia.
Description.
Basidiomata small to medium-sized. Pileus (1.6)2.4–5.5 cm in diameter, convex when young becoming plano-convex with age; margin deflexed to inflexed, slightly exceeding (1–2 mm), surface even to subrugulose, minutely tomentose, dull, at first brown to greyish-brown to blackish-brown (8F3–4) sometimes paler (8C2) at places, becoming paler to greyish-brown (8E3–5) with age; context 4–9 mm thick half-way to the margin, soft, yellowish to greyish off-white then slightly pale orange to greyish-orange (6A3 to 6B3) when exposed to the air, with patchy or marmorated with greyish-brown (8E3) especially when young, scattered with reddish-brown to brownish-black of fine encrustations at places, slightly reddening when cut. Stipe central, terete or sometimes slightly compressed, cylindrical with slightly wider base, (2.0)2.8–3.7 × 0.4–0.7 cm, surface even, minutely tomentose, dull, greyish-brown to dark brown (8 E/F 3–4 to 8F2), basal mycelium white to off-white becoming pale red (7A3) when bruised; context solid, yellowish to greyish off-white then orange white to pale orange (5A2–3) when exposed to the air, virgate to marmorate with brownish-grey (8F2), less so at the stipe base, at places scattered with brownish-black fine encrustations, unchanged to slowly slightly reddening when cut. Hymenophore tubulate, adnate, subventricose, slightly depressed around the stipe. Tubes (2)4–6 mm long half-way to the margin, brown to greyish-brown (8F3), not separable from the pileus context. Pores 0.4–1.5 mm wide at mid-radius, regularly arranged, mostly roundish to elliptical at first, becoming slightly angular with age, slightly elongated around the stipe, colour distribution even, dark brown to chocolate brown (9F4 to 10F3) at first, becoming chocolate brown to brown (10F4 to 7–8F4–5) with age. Odour rubbery. Taste slightly bitter at first, then mild. Spore print dark brown (8F4/5) in mass.
Macrochemical reactions.KOH, orange brown on cap, yellowish-black on stipe, yellowish-black on the pileus context and stipe context, brownish-black on hymenium; NH4OH, yellowish-brown on cap, yellowish-orange on stipe, orangey yellow to yellowish-orange on the pileus context, stipe context and hymenium.
Basidiospores [437/7/5] (6.5–)6.7–7.7–8.6(–11.5) × (3.8–)4–4.6–5.1(–5.5) µm Q = (1.4–)1.48–1.68–1.9(–2.44). From the type (3 basidiomata, N = 177) (6.8–)7–7.8–8.5(–9.1) × (4–)4.2–4.6–5(–5) µm, Q = (1.49–)1.5–1.69–1.9(–2.21), amygdaliform or elongated amygdaliform sometimes to ovoid with subacute apex in side view, ovoid in front view, thin-walled, smooth, slightly reddish to brownish hyaline in water, slightly yellowish to greenish hyaline in KOH or NH4OH, inamyloid. Basidia 4-spored, (25.3–)25.4–29.7–33.8(–33.8) × (7.3–)7.3–8.4–9.8(–10) µm, clavate without basal clamp connection, slightly yellowish to brownish hyaline in KOH or NH4OH; sterigmata up to 5 µm long. Cheilocystidia (16–)16.3–23.4–32.8(–34) × (5.5–)5.8–7.3–9(–9) µm, frequent, fusiform, thin-walled, yellowish to brownish hyaline to brown in KOH or NH4OH. Pleurocystidia (44–)44.2–54.7–67.6(–68) × (5–)5–6–7(–7) µm, frequent, usually narrowly bent fusiform to narrowly fusiform with obtuse apex, thin-walled, yellowish to brownish hyaline in KOH or NH4OH. Hymenophoral trama subdivergent to divergent, 62–175 µm wide, with 25–100 µm wide, regular to subregular mediostratum, composed of cylindrical, 4–7(11) µm wide hyphae, yellowish to brownish hyaline in KOH or NH4OH. Pileipellis a trichoderm to tangled trichoderm at first, becoming a tomentum to tangled trichoderm with age, 65–110 µm thick, composed of firmly to moderately interwoven thin-walled hyphae; terminal cells 12–55 × 4–6 µm, slightly bent cylindrical with rounded apex, at places clavate to sub-clavate to elongated clavate, 16–34 × 8–10 µm, slightly dark to reddish to brownish dark in water, yellowish to brownish hyaline to yellowish-brown to slightly dark at places in KOH or NH4OH. Pileus context made of moderately interwoven, thin-walled, hyaline hyphae, 6–12 µm wide. Stipitipellis a disrupted hymeniderm, 55–95 µm thick, composed clavate cells, 11–37 × 5–8 µm, yellowish-brown to slightly dark in KOH or NH4OH mixed with caulocystidia. Caulocystidia (17–)17–23.6–31(–31) × (5–)5–6.3–7(–7) µm, frequent, thin-walled, mostly yellowish-brown to slightly dark at places in KOH or NH4OH. Stipe context composed of parallel, 3–7 µm wide hyphae, brownish hyaline to yellowish pale brown in KOH or NH4OH. Clamp connections not seen in any tissue.
Habitat and Distribution.
solitary to gregarious up to 4 basidiomata, on soil in hill evergreen forest dominated by Fagaceae trees, with a few Dipterocarpus spp. and Shorea spp. or in Dipterocarp forest dominated by Dipterocarpus spp. and Shorea spp. with a few Lithocarpus sp., Castanopsis sp. and Quercus sp. Currently known only from Chiang Mai Province, Northern Thailand.
Additional specimens examined.
THAILAND, Chiang Mai Province, Mae Taeng District, 23 km marker (Ban Tapa), 19°08'50"N, 98°46'50"E, elev. 930 m, 2 August 2013, Olivier Raspé & Anan Thawthong, OR0681; Ban Mae Sae, 19°14'70"N, 98°38'70"E, elev. 960 m, 3 August 2013, Olivier Raspé & Anan Thawthong, OR0683; Muang District, Doi Suthep-Pui National Park, 18°48'37"N, 98°53'33"E, elev. 1460 m, 14 July 2016, Olivier Raspé, OR1306; Mae On District, 18°52'35"N, 99°18'16"E, elev. 860 m, 6 June 2018, Santhiti Vadthanarat, SV0451.
Remarks.
We observed some small yellowish to reddish to brownish dark particles or crystals covering the cell walls in pileipellis, stipitipellis and on the hymenium, especially the cystidia and basidia when observed in water. The small particles or crystals were mostly dissolved in KOH.
Cacaoporuspallidicarneus differs from C.tenebrosus by its basidiomata context colour which is paler, especially at the stipe base. A combination of the following characters are also distinctive: spore shape which is amygdaliform or elongated amygdaliform or sometimes ovoid with subacute apex in side view and ovoid in front view, while C.tenebrosus has ovoid spores, shorter basidia and differently shaped hymenophoral cystidia (see note under C.tenebrosus). Cacaoporuspallidicarneus has a stipitipellis which is a disrupted hymeniderm composed of caulocystidia and clavate cells, while the other species has a loose trichoderm or tangled trichoderm. Interestingly, one collection (SV0402) had a slightly paler context than C.tenebrosus but not as pale as C.pallidicarneus. The phylogenetic analyses indicated that this collection might be a species different from C.pallidicarneus and C.tenebrosus. However, the specimen was immature and, therefore, more collections are needed before the species can be formally recognised.
Cacaoporus tenebrosus
Vadthanarat, Raspé & Lumyong sp. nov.
MB829656
Figure 6.
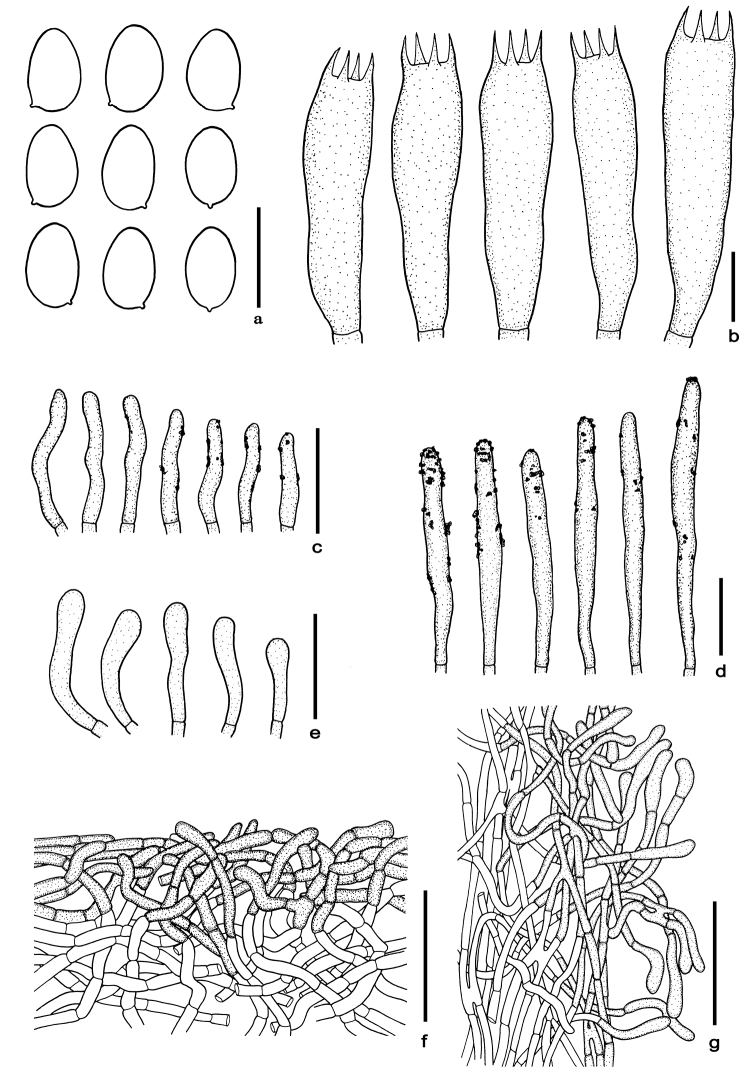
Microscopic features of Cacaoporustenebrosusa basidiospores b basidia c cheilocystidia d pleurocystidia e caulocystidia f pileipellis g stipitipellis. Scale bars: 10 µm (a–b); 25 µm (c–e); 50 µm (f–g). All drawings were made from the type (SV0223).
Etymology.
Refers to the overall darkness of basidiomata, including the context.
Type.
THAILAND, Chiang Mai Province, Mae On District, 18°52'37"N, 99°18'32"E, elev. 940 m, 15 August 2015, Santhiti Vadthanarat, SV0223 (holotype CMUB!, isotype BR!).
Diagnosis.
Cacaoporustenebrosus is characterised by having a darker context than the other species, longer basidia and cylindrical to narrowly subclavate hymenophoral cystidia.
Description.
Basidiomata medium-sized. Pileus (2.3)3.1–5(9) cm in diameter, convex when young becoming plano-convex to slightly depressed with age; margin inflexed to deflexed, slightly exceeding (1–2 mm); surface even to subrugulose, minutely tomentose, slightly cracked at the centre, dull, greyish-brown (10F3) to dark brown to blackish-brown (8F4–5) to the margin; context 5–10 mm thick half-way to the margin, soft, marmorated, greyish-brown to dark brown (10F3–5) with greyish-brown (9B/D3), scattered with reddish-brown to brownish-black, fine encrustations at places, slightly reddening in paler spots when cut. Stipe central, terete, cylindrical to sometimes with slightly wider base, 4.3–7.0 × 0.7–1.4 cm, surface even, minutely tomentose, dull, dark brown to greyish-brown (9F4 to 10F3), basal mycelium white to off-white becoming reddish-white to pale red (7A3–4) when bruised; context solid, greyish-brown to dark brown (9–10F3–5) marmorated with reddish-grey (7/10B2), usually scattered with small reddish-brown to brownish-black fine encrustations, slightly reddening when cut. Hymenophore tubulate, adnate, subventricose to ventricose, slightly depressed around the stipe. Tubes (4)7–13 mm long half-way to the margin, brown to dark brown (8F3 to 9F4), not separable from the pileus context. Pores 0.8–2 mm wide at mid-radius, regularly arranged, mostly roundish at first, becoming slightly angular with age, sometime irregular, elongated around the stipe; colour distribution even, greyish-brown to dark brown (9F4) at first, becoming chocolate brown to brown (10F3 to 7–8F4–5) with age. Odour mild fungoid. Taste slightly bitter at first, then mild. Spore print dark brown (8/9F4) in mass.
Macrochemical reactions.KOH, yellowish then brown to black on cap, stipe, pileus context, stipe context and hymenium; NH4OH, yellowish then orange to brown on cap, stipe, pileus context, stipe context and hymenium.
Basidiospores [290/8/6] (7.4–)7.7–8.4–9.2(–10) × (4.5–)5–5.3–5.7(–6.1) µm Q = (1.25–)1.44–1.57–1.77(–2). From the type (2 basidiomata, N = 134) (7.5–)7.7–8.2–9(–9.9) × (4.9–)5–5.4–5.7(–5.9) µm, Q = (1.41–)1.43–1.54–1.71(–1.9), ovoid, thin-walled, smooth, slightly reddish to brownish hyaline in water, slightly yellowish to greenish hyaline in KOH or NH4OH, inamyloid. Basidia 4-spored, (33.6–)34.3–38.8–45.8(–47) × (7.7–)7.8–9.5–10.8(–10.9) µm, clavate to narrowly clavate without basal clamp connection, yellowish to brownish hyaline to slightly dark in KOH or NH4OH; sterigmata up to 5 µm long. Cheilocystidia (22–)22.1–28.7–37(–37) × (3–)3.1–4.4–5(–5) µm, frequent, cylindrical with obtuse apex, sometimes bent or sinuate, thin-walled, yellowish-brown to dark brown in KOH or NH4OH, often scattered with small brownish-yellow to yellowish-brown crystals on the walls in KOH or NH4OH. Pleurocystidia (62–)62.5–81.5–99(–99) × (7–)7–8–9(–9) µm, frequent, cylindrical to narrowly subclavate, sometimes bent or sinuate, thin-walled, with yellowish-brown to slightly dark content in KOH or NH4OH, densely covered with small reddish-brown to brownish dark encrustations on the walls when observed in H2O, with some scattered small brownish-yellow to yellowish-brown crystals on the walls in KOH or NH4OH. Hymenophoral trama subdivergent to divergent, 80–170 µm wide, with 60–80 µm wide of subregular mediostratum, composed of cylindrical, 4–8(11) µm wide hyphae, slightly yellowish to brownish hyaline in KOH or NH4OH. Pileipellis a tangled trichoderm to tomentum at places, 70–110 µm thick, composed of moderately interwoven thin-walled hyphae; terminal cells 12–48 × 4–7 µm mostly slightly sinuate, cylindrical to irregular with rounded apex, at places clavate to elongated clavate terminal cells 18–33 × 7–9 µm, slightly dark to reddish to brownish dark in water, yellowish-brown to slightly dark in KOH or NH4OH, scattered with small brownish-yellow to yellowish-brown crystals on the walls in KOH or NH4OH. Pileus context made of moderately interwoven, thin-walled, hyaline hyphae, 7–12 µm wide. Stipitipellis a trichoderm to tangled trichoderm, 70–120 µm thick, composed of loosely to moderately interwoven cylindrical hyphae anastomosing at places, brownish dark to dark in KOH or NH4OH. Caulocystidia (17–)17.6–29.4–46.3(–47) × (4–)4.1–5.5–6.9(–7) µm, clavate to cylindrical with obtuse apex, thin-walled, yellowish to brownish dark in KOH or NH4OH. Stipe context composed of parallel, 4–6(12) µm wide hyphae, brownish hyaline to yellowish pale brown in KOH or NH4OH. Clamp connections not seen in any tissue.
Habitat and distribution.
Gregarious (up to 9 basidiomata) to fasciculate or solitary, on soil in hill evergreen forest dominated by Fagaceae trees, with a few Dipterocarpus spp. and Shorea spp. or in Dipterocarp forest dominated by Dipterocarpus spp., Shorea spp. with a few Lithocarpus sp., Castanopsis sp. and Quercus sp. Currently known only from Chiang Mai Province, Northern Thailand.
Additional specimens examined.
THAILAND, Chiang Mai Province, Mae Taeng District, 19°07'15"N, 98°43'55"E, elev. 910 m, 29 July 2013, Olivier Raspé & Benjarong Thongbai, OR0654; ibid. 19°7'29"N, 98°40'59"E, elev. 1010 m, 24 May 2018, Santhiti Vadthanarat, SV0422; Mae On District, 18°52'37"N, 99°18'19"E, elev. 850 m, 15 August 2015, Santhiti Vadthanarat, SV0224; ibid., 18°52'35"N, 99°18'16"E, elev. 860 m, 15 July 2017, Olivier Raspé , OR1435; ibid., 6 June 2018, Santhiti Vadthanarat, SV0452.
Remarks.
There were many small yellowish to reddish to dark brownish particles or crystals on the walls of pileipellis, stipitipellis and hymenium cells, especially on the cystidia and basidia when observed in water. The small particles or crystals are somewhat dissolved and discoloured in KOH.
Microscopically, Cacaoporustenebrosus differs from C.pallidicarneus by having a darker context, longer basidia (33.6–47 µm vs. 25.3–33.8 µm, respectively), longer and larger hymenophoral cystidia, which also differ in shape (cylindrical to narrowly subclavate in C.tenebrosus but fusiform to narrowly fusiform in C.pallidicarneus). Phylogenetically, all Cacaoporus collections with a dark context formed a clade sister to C.pallidicarneus (BS = 85% and PP = 0.88), but some (SV0224 and SV0422) were genetically somewhat distant from the other collections. However, we could not find any difference in morphology. Consequently, we consider them as the same species (C.tenebrosus). Study of more collections is needed to confirm or infirm that they belong to the same species.
Discussion
Morphologically, Cacaoporus is most similar to Sutorius, with which it shares the overall brown colour of basidiomata and encrustations in the flesh. However, the genus Cacaoporus has darker basidiomata, especially the hymenophore and pore surface and is more chocolate brown, not reddish-brown or purplish-brown like Sutorius, tubes that are not separable from the pileus context whereas they are easily separable in Sutorius, white to off-white basal mycelium which becomes reddish when bruised, whereas in Sutorius, the basal mycelium is more or less white and unchanging. Cacaoporus also produces dark brown spore deposits whereas in Sutorius, spore deposits are reddish-brown (Halling et al. 2012). Microscopically, the two genera differ in the shape of basidiospores, which is amygdaliform to ovoid or ovoid with subacute apex in side view in Cacaoporus, whereas Sutorius produces narrowly ellipsoid to ellipsoid or subfusoid to fusoid basidiospores. Phylogenetically, Cacaoporus and Sutorius are not closely related - the two genera belong in two different clades of the Pulveroboletus group.
Some species in Porphyrellus E.-J. Gilbert also have brown to dark brown to umber basidiomata similar to Cacaoporus. However, Porphyrellus differs from the new genus in having white to greyish-white hymenophore when young, becoming greyish-pink to blackish-pink with age, white to pallid context in pileus and stipe variably staining blue and/or reddish when cut and white basal mycelium that does not turn red when bruised (Wolfe 1979; Wu et al. 2016). Some species in Strobilomyces Berk also share some characters with Cacaoporus, including dark brown basidiomata, white to off-white basal mycelium that turns red when bruised and the context turning red when cut. However, Strobilomyces species clearly differ from Cacaoporus, especially in the pileus surface, which is coarsely fibrillose or shows conical to patch-like scales, in the hymenophore, which is whitish-cream or greyish-brown or vinaceous drab and stains reddish then blackish when bruised and also basidiospores, which are subglobose to obtusely ellipsoid with reticulation or longitudinally striate (Gelardi et al. 2012; Antonín et al. 2015; Wu et al. 2016). Moreover, Porphyrellus and Strobilomyces were phylogenetically inferred to belong in subfamily Boletoideae (Wu et al. 2014, 2016; Vadthanarat et al. 2018) distinct from Cacaoporus.
Phylogenetically, Cacaoporus was monophyletic and clustered in a well-supported clade with the genera Cyanoboletus and Cupreoboletus and one undescribed taxon, Boletus p.p. sp. (specimen voucher JD0693), belonging to the Pulveroboletus group of Wu et al. (2014, 2016). Cyanoboletus and Cupreoboletus, however, differ from Cacaoporus in important morphological characters. The former two genera have a yellow hymenophore and yellowish context and tissues instantly discolouring dark blue when injured, and olive-brown spore deposits (Gelardi et al. 2014, 2015; Wu et al. 2016). The undescribed taxon represented by the voucher specimen JD0693, which clustered within the same clade as Cacaoporus, Cyanoboletus and Cupreoboletus, is also morphologically very different from Cacaoporus, in having yellow tubes, reddish pores, pale yellow to off-white context and reddish-brown pileus and stipe.
Our survey on the diversity of Boletes in the north of Thailand has been conducted since 2012 and no Cacaoporus has been found in the forests at elevations lower than 850 m. Cacaoporus was found only between 850 m and 1460 m elevation. However, more collections are needed to confirm that the distribution of the genus is restricted to mid- to high-elevation forests and does not occur in the lower elevation, drier forests. Most collections were made from Fagaceae-dominated, evergreen hill forests. The dominant trees in these forests belong to the Fagaceae, including Lithocarpus, Castanopsis and Quercus, but some Dipterocarpaceae may also occur. At the lower end of its elevation range, however, Cacaoporus was also found in Dipterocarpaceae-dominated forests (in which Fagaceae, especially Quercus spp., also occurs). The Dipterocarpaceae trees include Dipterocarpus, namely D.tuberculatus, D.obtusifolius and Shorea, namely S.obtusa and S.siamensis. The listed trees have previously been reported as ectomycorrhizal hosts of Boletaceae (Moser et al. 2009; Desjardin et al. 2009, 2011; Hosen et al. 2013; Arora and Frank 2014; Halling et al. 2014; Wu et al. 2018) and presumably are also the hosts for Cacaoporus.
Interestingly, our phylogeny indicated that the genera Neoboletus and Sutorius formed two different clades, both with high support (BS = 85% and PP = 0.95 for Neoboletus; BS = 100% and PP = 1 for Sutorius). Recently, Wu et al. (2016) synonymised Neoboletus with Sutorius because, in their phylogeny based on a four-gene dataset (28S+tef1+rpb1+rpb2), Boletusobscureumbrinus, a species morphologically more similar to Neoboletus than to Sutorius, seemed to cluster with Sutorius rather than with the Neoboletus species, although with neither ML nor BI support. Moreover, the Neoboletus clade was not supported either. Later, Chai et al. (2019) treated the two genera as different genetic lineages based on morphology and phylogeny (28S+ITS+tef1+rpb2), in which B.obscureumbrinus clustered with the other Neoboletus species in a well-supported clade. Our phylogenetic analyses, based on a different set of genes (atp6+ tef1+rpb2+cox3), confirm the separation of the two genera Neoboletus and Sutorius. The differences in gene trees obtained could be explained by a long-branch attraction artefact in datasets with different taxon and gene samplings and/or problems in the dataset (e.g. suboptimal alignment). Neoboletusobscureumbrinus is quite atypical amongst Neoboletus species and its phylogenetic affinities within this genus remain unclear (Fig. 7).
Figure 7.
Sub-tree of the phylogram in Fig. 1, showing the well-supported Sutorius and Neoboletus clades and the unsupported sister relationship of Neoboletusobscureumbrinus.
Cacaoporus is the second novel bolete genus described from Thailand, the first one being Spongiforma Desjardin, Manfr. Binder, Roekring & Flegel, described in 2009 (Desjardin et al.). However, fungal diversity in Thailand is high and still poorly known (Hyde et al. 2018), with a large number of species and possibly genera that remain to be described.
Supplementary Material
Acknowledgements
Financial support from the Graduate School, Chiang Mai University, is appreciated. The work was partly supported by a TRF Research Team Association Grant (RTA5880006) to SL and OR. OR is grateful to the Fonds National de la Recherche Scientifique (Belgium) for travel grants. The authors are grateful for the permit number 0907.4/4769 granted by the Department of National Parks, Wildlife and Plant Conservation, Ministry of Natural Resources and Environment for collecting in Doi Suthep-Pui National Park. Beatriz Ortiz-Santana (CFMR), Roy E. Halling (NY), and Terry W. Henkel are gratefully acknowledged for the loan of specimens.
Citation
Vadthanarat S, Lumyong S, Raspé O (2019) Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys 54: 1–29. https://doi.org/10.3897/mycokeys.54.35018
Funding Statement
1. Fonds National de la Recherche Scientifique (Belgium) 2. TRF Research Team Association Grant (RTA5880006) 3. Graduate School, Chiang Mai University
References
- Antonín V, Vizzini A, Ercole E, Leonardi M. (2015) Strobilomycespteroreticulosporus (Boletales), a new species of the S.strobilaceus complex from the Republic of Korea and remarks on the variability of S.confusus. Phytotaxa 219(1): 78–86. 10.11646/phytotaxa.219.1.6 [DOI] [Google Scholar]
- Arora D, Frank JL. (2014) Clarifying the butter Boletes: a new genus, Butyriboletus, is established to accommodate Boletussect.Appendiculati, and six new species are described. Mycologia 106(3): 464–480. 10.3852/13-052 [DOI] [PubMed] [Google Scholar]
- Binder M, Hibbett DS. (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98: 971–981. 10.1080/15572536.2006.11832626 [DOI] [PubMed] [Google Scholar]
- Binder M, Larsson KH, Matheny PB, Hibbett DS. (2010) Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of agaricomycetidae dominated by corticioid forms. Mycologia 102: 865–880. 10.3852/09-288 [DOI] [PubMed] [Google Scholar]
- Chai H, Xue R, Jiang S, Luo SH, Wang Y, Wu LL, Tang LP, Chen Y, Hong D, Liang ZQ, Zeng NK. (2019) New and noteworthy boletes from subtropical and tropical China. MycoKeys 46: 55–96. 10.3897/mycokeys.46.31470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuankid B, Vadthanarat S, Hyde KD, Thongklang N, Zhao R, Lumyong S, Raspé O. (2019) Three new Phylloporus species from tropical China and Thailand. Mycological Progress 18: 603–614. 10.1007/s11557-019-01474-6 [DOI] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed]
- Desjardin DE, Binder M, Roekring S, Flegel T. (2009) Spongiforma, a new genus of gastroid boletes from Thailand. Fungal Diversity 37: 1–8. [Google Scholar]
- Desjardin DE, Peay KG, Bruns TD. (2011) Spongiformasquarepantsii, a new species of gasteroid bolete from Borneo. Mycologia 103(5): 1119–1123. 10.3852/10-433 [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. (1990) Isolation of plant DNA from fresh tissue. Focus, 12: 13–15. 10.2307/2419362 [DOI] [Google Scholar]
- Gelardi M, Vizzini A, Ercole E, Voyron S, Wu G, Liu XZ. (2012) Strobilomycesechinocephalus sp. nov. (Boletales) from southwestern China, and a key to the genus Strobilomyces worldwide. Mycological Progress 12: 575–588. 10.1007/s11557-012-0865-3 [DOI] [Google Scholar]
- Gelardi M, Simonini G, Ercole E, Vizzini A. (2014) Alessioporus and Pulchroboletus gen. nov. (Boletaceae, Boletineae), two novel genera to accommodate Xerocomusichnusanus and X.roseoalbidus from European Mediterranean basin: molecular and morphological evidence. Mycologia 106(6): 1168–1187. 10.3852/14-042 [DOI] [PubMed] [Google Scholar]
- Gelardi M, Simonini G, Ercole E, Davoli P, Vizzini A. (2015) Cupreoboletus (Boletaceae, Boletineae), a new monotypic genus segregated from Boletussect.Luridi to reassign the Mediterranean species B.poikilochromus. Mycologia 107(6): 1254–1269. 10.3852/15-070 [DOI] [PubMed] [Google Scholar]
- Halling RE, Desjardin DE, Fechner N, Arora D, Soytong K, Dentinger BTM. (2014) New porcini (Boletussect.Boletus) from Australia and Thailand. Mycologia 106: 830–834. 10.3852/13-340 [DOI] [PubMed] [Google Scholar]
- Halling RE, Nuhn M, Fechner NA, Osmundson TW, Soytong K, Arora D, Hibbett DS, Binder M. (2012a) Sutorius: a new genus for Boletuseximius. Mycologia 104(4): 951–961. 10.3852/11-376 [DOI] [PubMed] [Google Scholar]
- Halling RE, Nuhn M, Osmundson T, Fechner N, Trappe JM, Soytong K, Arora D, Hibbett DS, Binder M. (2012b) Affinities of the Boletuschromapes group to Royoungia and the description of two new genera, Harrya and Australopilus. Australian Systematic Botany 25: 418–431. 10.1071/SB12028 [DOI] [Google Scholar]
- Henkel TW, Obase K, Husbands D, Uehling JK, Bonito G, Aime MC, Smith ME. (2016) New Boletaceae taxa from Guyana: Binderoboletussegoi gen. and sp. nov., Guyanaporusalbipodus gen. and sp. nov., Singerocomusrubriflavus gen. and sp. nov., and a new combination for Xerocomusinundabilis. Mycologia 108(1): 157–173. 10.3852/15-075 [DOI] [PubMed] [Google Scholar]
- Hosen MI, Feng B, Wu G, Zhu XT, Li YC, Yang ZL. (2013) Borofutus, a new genus of Boletaceae from tropical Asia: phylogeny, morphology and taxonomy. Fungal Diversity 58: 215–226. 10.1007/s13225-012-0211-8 [DOI] [Google Scholar]
- Hyde KD, Norphanphoun C, Chen J, Dissanayake AJ, Doilom M, Hongsanan S, Jayawardena RS, Jeewon R, Perera RH, Thongbai B, Wanasinghe DN, Wisitrassameewong K, Tibpromma S, Stadler M. (2018) Thailand’s amazing diversity: up to 96% of fungi in northern Thailand may be novel. Fungal Diversity 93: 215–239. 10.1007/s13225-018-0415-7 10.1007/s13225-018-0415-7 [DOI] [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. (1978) Methuen Handbook of Colour (3rd edn). Eyre Methuen Ltd, London, 252 pp. [Google Scholar]
- Kretzer AM, Bruns TD. (1999) Use of atp6 in fungal phylogenetics: an example from the Boletales. Molecular Phylogenetics and Evolution 13: 483–492. 10.1006/mpev.1999.0680 [DOI] [PubMed] [Google Scholar]
- Li YC, Feng B, Yang ZL. (2011) Zangia, a new genus of Boletaceae supported by molecular and morphological evidence. Fungal Diversity 49: 125–143. 10.1007/s13225-011-0096-y [DOI] [Google Scholar]
- Li YC, Li F, Zeng NK, Cui YY, Yang ZL. (2014a) A new genus Pseudoaustroboletus (Boletaceae, Boletales) from Asia as inferred from molecular and morphological data. Mycological Progress 13: 1207–1216. 10.1007/s11557-014-1011-1 [DOI] [Google Scholar]
- Li YC, Ortiz-Santana B, Zeng NK, Feng B. (2014b) Molecular phylogeny and taxonomy of the genus Veloporphyrellus. Mycologia 106(2): 291–306. 10.3852/106.2.291 [DOI] [PubMed] [Google Scholar]
- Matheny PB. (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Molecular Phylogenetics and Evolution 35: 1–20. 10.1016/j.ympev.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, Chan L, Hoover P, Warnow T. (2009) The CIPRES portals. CIPRES. http://www.phylo.org/portal2/home.
- Moser AM, Frank JL, D’ Allura JA, Southworth D. (2009) Ectomycorrhizal communities of Quercusgarryana are similar on serpentine and nonserpentine soils. Plant Soil 315: 185–194. 10.1007/s11104-008-9743-9 [DOI] [Google Scholar]
- Neves MA, Binder M, Halling R, Hibbett D, Soytong K. (2012) The phylogeny of selected Phylloporus species inferred from NUC-LSU and ITS sequences, and descriptions of new species from the Old World. Fungal Diversity 55(1): 109–123. 10.1007/s13225-012-0154-0 [DOI] [Google Scholar]
- Nuhn ME, Binder M, Taylor AFS, Halling RE, Hibbett DS. (2013) Phylogenetic overview of the Boletineae. Fungal Biology 117: 479–511. 10.1016/j.funbio.2013.04.008 [DOI] [PubMed] [Google Scholar]
- Orihara T, Lebel T, Ge Z-W, Smith ME, Maekawa N. (2016) Evolutionary history of the sequestrate genus Rossbeevera (Boletaceae) reveals a new genus Turmalinea and highlights the utility of ITS minisatellite-like insertions for molecular identification. Persoonia 37: 173–198. 10.3767/003158516X691212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihara T, Smith ME, Shimomura N, Iwase K, Maekawa N. (2012) Diversity and systematics of the sequestrate genus Octaviania in Japan: two new subgenera and eleven new species. Persoonia 28: 85–112. 10.3767/003158512X650121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phookamsak R, Hyde KD, Jeewon R, Bhat DJ, Jones EBG, Maharachchikumbura SSN et al. (2019) Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Diversity. 10.1007/s13225-019-00421-w [DOI]
- Raspé O, Vadthanarat S, De Kesel A, Degreef J, Hyde KD, Lumyong S. (2016) Pulveroboletusfragrans, a new Boletaceae species from Northern Thailand, with a remarkable aromatic odor. Mycological Progress 15: 38. 10.1007/s11557-016-1179-7 [DOI]
- Rehner SA, Buckley E. (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. 10.3852/mycologia.97.1.84 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, Höhna S, et al. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2006) RAxML-vi-hpc: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Vadthanarat S, Raspé O, Lumyong S. (2018) Phylogenetic affinities of the sequestrate genus Rhodactina (Boletaceae), with a new species, R.rostratispora from Thailand. MycoKeys 29: 63–80. 10.3897/mycokeys.29.22572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CB. (1979) Austroboletus and Tylopilussubg.Porphyrellus, with emphasis on North American taxa. Bibliotheca Mycologica 69: 1–148. [Google Scholar]
- Wu G, Feng B, Xu J, Zhu XT, Li YC, Zeng NK, Hosen MI, Yang ZL. (2014) Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Diversity 69: 93–115. 10.1007/s13225-014-0283-8 [DOI] [Google Scholar]
- Wu G, Lee S ML, Horak E, Yang ZL. (2018) Spongisporatemakensis, a new boletoid genus and species from Singapore. Mycologia 110(5): 919–929. 10.1080/00275514.2018.1496387 [DOI] [PubMed] [Google Scholar]
- Wu G, Li YC, Zhu XT, Zhao K, Han LH, Cui YY, Li F, Xu JP, Yang ZL. (2016) One hundred noteworthy boletes from China. Fungal Diversity 81: 25–188. 10.1007/s13225-016-0375-8 [DOI] [Google Scholar]
- Wu G, Zhao K, Li YC, Zeng NK, Feng B, Halling RE, Yang ZL. (2015) Four new genera of the fungal family Boletaceae. Fungal Diversity 81: 1–24. 10.1007/s13225-015-0322-0 [DOI] [Google Scholar]
- Zeng NK, Cai Q, Yang ZL. (2012) Corneroboletus, a new genus to accommodate the southeastern Asian Boletusindecorus. Mycologia 104(6): 1420–1432. 10.3852/11-326 [DOI] [PubMed] [Google Scholar]
- Zeng NK, Wu G, Li YC, Liang ZQ, Yang ZL. (2014) Crocinoboletus, a new genus of Boletaceae (Boletales) with unusual polyene pigments boletocrocins. Phytotaxa 175: 133–140. 10.11646/phytotaxa.175.3.2 [DOI] [Google Scholar]
- Zhao K, Wu G, Feng B, Yang ZL. (2014a) Molecular phylogeny of Caloboletus (Boletaceae) and a new species in East Asia. Mycological Progress 13: 1127–1136. 10.1007/s11557-014-1001-3 [DOI] [Google Scholar]
- Zhao K, Wu G, Yang ZL. (2014b) A new genus, Rubroboletus, to accommodate Boletussinicus and its allies. Phytotaxa 188: 61–77. 10.11646/phytotaxa.188.2.1 [DOI] [Google Scholar]
- Zhu XT, Wu G, Zhao K, Halling RE, Yang ZL. (2015) Hourangia, a new genus of Boletaceae to accommodate Xerocomuscheoi and its allied species. Mycological Progress 14: 37. 10.1007/s11557-015-1060-0 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.