Abstract
Purpose
We describe a unique case of CDH3-related hypotrichosis with juvenile macular dystrophy (HJMD) and DNAH5-related primary ciliary dyskinesia (PCD) with progressive vision loss in a young Indian female without positive family history. Both mutations in this patient have not been previously described in the literature.
Observations
An 11-year-old girl of Indian descent from a consanguineous family presented to our clinic with poor central visual acuity, recurrent sinopulmonary infections, hypotrichosis, and gradual hearing loss. Fundus examination was significant for atrophic retinal pigmented epithelial (RPE) changes involving both the macula and periphery of both eyes with central foveal hypoautofluorescence. Optical coherence tomography (OCT) demonstrated RPE loss and significant disruption of the ellipsoid layer in both eyes. Full-field electrophysiology tests on initial presentation demonstrated low cone amplitude reduced to <70% of normal range without prolongation. OCT angiography of the RPE and choriocapillaris demonstrated possible flow voids in the central macular region of both eyes. Genetic testing showed that the proband was homozygous for variants CDH3 c.1660A > C; p. Thr554Pro and DNAH5 c.6688-1G>T.
Conclusion
and Importance: We report two novel variants in the CDH3 and DNAH5 genes that are important for future mutational analysis of both HJMD and PCD respectively. A relationship between the cadherin protein dysfunction in CDH3 mutations and the ciliopathy of DNAH5 mutations has not been established. HJMD is known to cause a longitudinal deterioration of cone and rod mediated function, therefore recognizing the symptoms, visual impairment, physical examination, and photographic and electrophysiological findings is crucial in counseling the patient, the family, and fellow clinicians.
Keywords: Hypotrichosis with juvenile macular dystrophy, Primary ciliary dyskinesia, Ciliopathy, Retinal pigmented epithelium, Photoreceptors
1. Introduction
Hypotrichosis with juvenile macular dystrophy (HJMD; OMIM 601553) is a rare condition that is characterized by impaired hair growth and retinal degeneration due to a mutation in the CDH3 gene (OMIM 114021). This gene encodes for an integral protein, P-cadherin, responsible for cell-cell adhesion and is highly expressed in the development of hair follicle matrix as well as the retinal pigmented epithelium (RPE). CDH3 mutations also cause ectodermal dysplasia, ectrodactyly and macular dystrophy (EEM; OMIM 225280), which leads to degenerative RPE changes as well as limb defects. Though there is a wide range of phenotypic expressions associated with mutant CDH3, no association with symptoms of ciliary dyskinesia or sinopulmonary dysfunction has been reported.
In this case report, we present a young patient with vision loss associated with HJMD as well as primary ciliary dyskinesia (PCD) associated with a mutation in the DNAH5 gene (MIM 603335) resulting in recurrent sinopulmonary infections and conductive hearing loss. This patient was identified to be homozygous for both CDH3 and DNAH5 mutations. To the best of our knowledge, this is the first case of retinal degeneration in a patient diagnosed with both HJMD and PCD described in the literature.
1.1. Case report
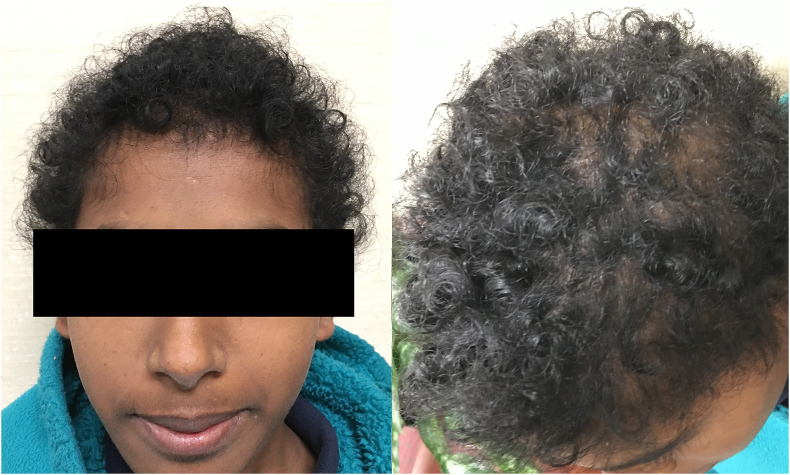
An 11-year-old Indian female was referred to the ophthalmology clinic for evaluation of gradually worsening vision. She was born full-term via normal spontaneous vaginal delivery. There were no complications with pregnancy or developmental delays. Her medical history was unremarkable until age 9, when she developed recurrent, severe episodes of bronchiectasis, chronic pansinusitis, and hypotrichosis of the scalp (Fig. 1) along with gradual vision loss.
Fig. 1.
External photographs of the patient with hypotrichosis as illustrated by the thin and short hair with patchy areas of loss.
Her immediate and extended family history was otherwise unremarkable except for parental consanguinity (first cousins, both of Indian origin). Her older sister was reportedly healthy. Her prior medical work-up included two negative sweat tests, positive skin tests for multiple environmental allergies, and negative immune work-up (B and T cell, immunoglobulin, and vaccine abnormalities). She was also diagnosed with conductive hearing loss of 30 dB in both right and left ears (range 250–8000 Hz) and demonstrated a low level of fraction of exhaled nitric oxide at 88.9 parts per billion on spirometry (predicted range 200–1000). The patient's home medications included albuterol and fluticasone inhalers.
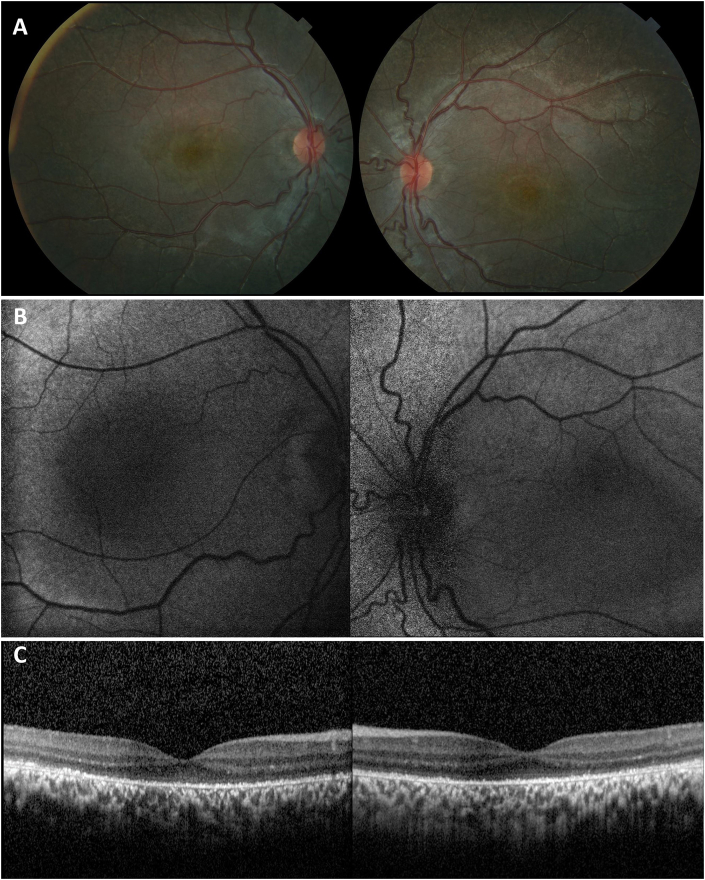
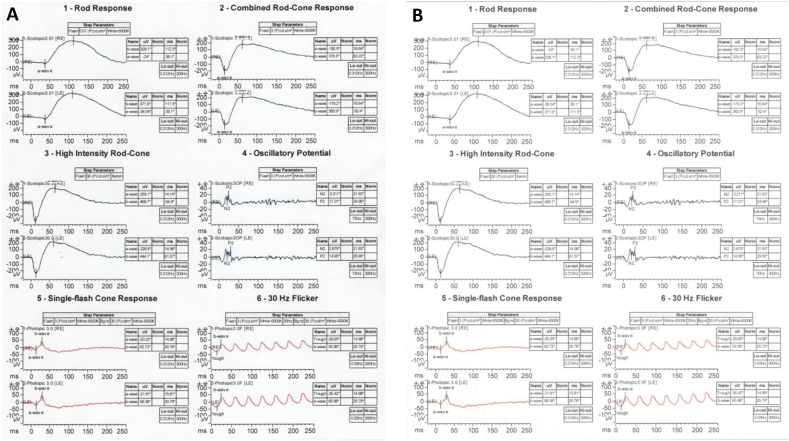
On examination, the patient presented with visual acuities of 20/100 OD and 20/150 OS with normal intraocular pressures. Anterior segment slit lamp examination was unremarkable in both eyes. Fundus examination was significant for atrophic RPE changes involving both macula and periphery of both eyes (Fig. 2A). Fundus autofluorescence was consistent with central foveal hypoautofluorescence surrounded by faint hyperautofluorescence, more significant in the right eye than the left (Fig. 2B). OCT demonstrated RPE loss and significant disruption of the ellipsoid layer in both eyes (Fig. 2C). Full-field electrophysiology tests on initial presentation demonstrated low cone amplitude reduced to <70% of normal range without prolongation (Fig. 3A).
Fig. 2.
Fundus photography, fundus autofluorescence, and optical coherence tomography (OCT) of the right and left eyes. A) Color photographs disclose diffuse atrophy of the retinal pigmented epithelium (RPE) affecting the posterior pole. B) Fundus autofluorescence with symmetric macular hypoautofluorescence corresponding to areas of RPE atrophy. C) OCT demonstrating symmetric disruption of the ellipsoid layer and atrophy of the RPE. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Electrophysiology testing. A) Standard rod, cone, and combined ERG demonstrating reduced cone amplitude to <70% of normal range without prolongation. B) Stable ERG findings four years later as compared to the patient's baseline study.
To reveal a genetic etiology, a chromosomal SNP-based microarray and whole exome sequencing were performed in CLIA-certified diagnostic genetic laboratories. Chromosome microarray revealed no pathogenic copy number variants but showed multiple segments of homozygosity, confirming parental consanguinity. Whole exome sequencing showed that the proband was homozygous for two variants in CDH3 and DNAH5. The CDH3 variant (c.1660A > C, p. Thr554Pro) is a missense change that has not been reported in patients with EEM or HJMD. It is not present in over 120,000 individuals on the Broad Institute Genome Aggregation Database.1 While in silico prediction tools give inconsistent results for its impact on protein, a change from threonine to proline is likely to impact the secondary protein structure. The variant is located at chr16:68721504 (Hg19). The SNP array shows a homozygous run at 16q21q24.3(62,750,519–90,163,275), suggesting that the variant maps to an autozygous segment in the proband's genome. Thus, given the proband's clinical presentation, we concluded that the p. Thr554Pro variant was causative of her retinal and dermal phenotypes. The DNAH5 variant (c.6688-1G>T) destroys a canonical splice site and interpreted as pathogenic to cause primary ciliary dyskinesia (PCD). This variant (chr5:13820608; Hg19) too maps to an autozygous genomic region at 5p15.32p13.3 (5,615,381–29,031,363). No other pathogenic or likely pathogenic variant was found in whole exome sequencing. While recommended, parents were not tested for the identified variants.
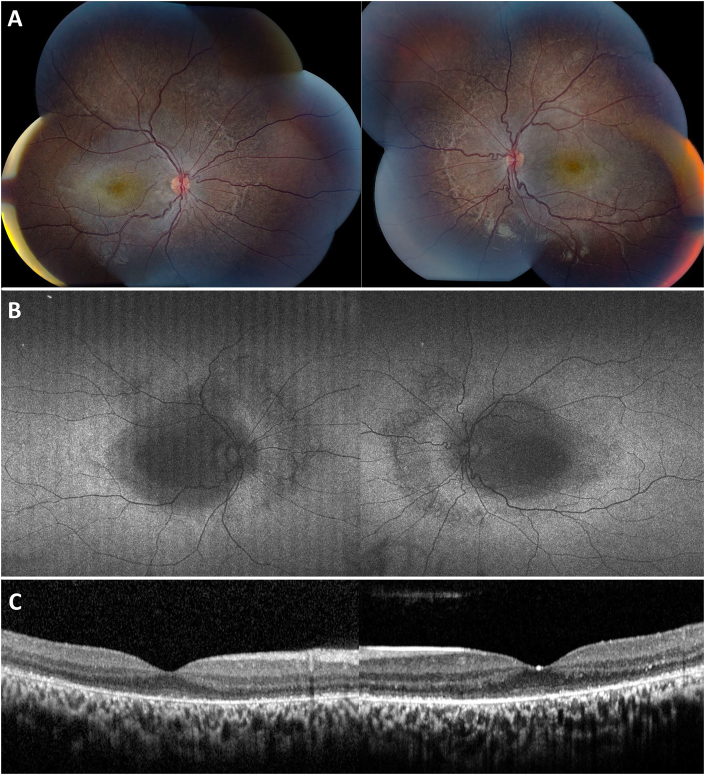
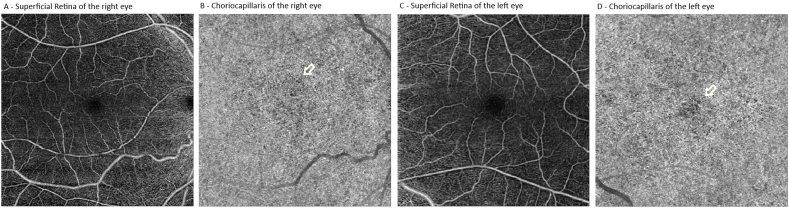
Four years after initial presentation, the patient returned for follow-up with visual acuities of 20/80 OD and 20/200 OS. Imaging was repeated demonstrating slightly worse atrophic RPE changes in both eyes on fundus photos (Fig. 4A). A repeat fundus autofluorescence demonstrated slightly increased macular hypoautofluorescence with involvement of the nasal retinal in both eyes (Fig. 3B). Repeat OCT showed mild progression of ellipsoid zone loss of both eyes (Fig. 4C). An ERG was also repeated showing a similar decrease in cone-response stable compared to prior (Fig. 3B). OCT angiography was obtained that demonstrated possible flow voids of the choriocapillaris in the central macula of both eyes (Fig. 5).
Fig. 4.
Four-year follow-up fundus photography, fundus autofluorescence, and optical coherence tomography (OCT) of the right and left eyes. A) Color photographs demonstrating stable, diffuse atrophy of the retinal pigmented epithelium (RPE) affecting the posterior pole. B) Widefield fundus autofluorescence with symmetrically increased macular hypoautofluorescence and additional visualization of nasal hypoautofluorescence corresponding to extramacular RPE changes. C) OCT findings of increased disruption of the ellipsoid layer and atrophy of the RPE in both eyes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Optical coherence tomography angiography (OCTA) of the right and left eyes. Normal appearing superficial slabs (A and C) of the right and left eye. Choriocapillaris in both right and left eyes with focal, patchy flow voids in the central macula (B and D, yellow arrow indicating area of flow voids).
2. Discussion
Mutations in the CDH3 gene cause EEM or HJMD.2, 3, 4 Prior investigations have established that there is no genotype-phenotype correlation for either EEM or HJMD; the same mutation has been found to cause both diseases in varying phenotypic severity.3, 4, 5, 6 Approximately 50 cases of HJMD have been reported in the literature worldwide. Since the first discovery of the role of CDH3 in HJMD in 2001,2 31 variants have been identified (HGMD Professional Database; accessed on 2/8/2019).3 Our patient's variant is novel.
In our patient, though there was no family history, the constellation of her symptoms (hypotrichosis of the scalp, macular dystrophy, and lack of limb deformities) were phenotypically consistent with HJMD and have been described before.7 Additionally, degenerative changes in retinal and RPE architecture were similar to most reported cases. Like the case series by Hull et al. fundus autofluorescence demonstrated classic symmetric RPE atrophy of the posterior pole with a surrounding ring of hyperautofluorescence along with disruption of ellipsoid layer and RPE loss on OCT in both eyes (Fig. 4).8 This is the first report of HJMD to illustrate OCT angiography findings. Although no normative database of OCT angiography exists for pediatric patients, there appears to be patchy flow voids of the choriocapillaris in both eyes (Fig. 5B and D). Our ERG findings corroborated prior assertions by Leibu et al. that HJMD resembles more of a cone-rod dystrophy on electrophysiology.6
Our patient was also found to have an unrelated DNAH5 mutation that has not been previously described, associated with a clinical presentation consistent with PCD, a genetic disorder affecting 1 in 16,000 individuals.9 While PCD is a heterogenous condition, involvement of the DNAH5 gene is the most common causative mutation, comprising 15–49% of known PCD mutations.10, 11, 12 This patient demonstrated chronic ciliary dysfunction resulting in recurrent sinopulmonary symptoms, bronchiectasis, gradual hearing loss, and low levels of exhaled nitric oxide on spirometry that were consistent with PCD.9 The ciliary dysfunction in PCD is a result of defective outer dynein arms (ODA), resulting in malfunction of ciliary and flagellar beating.13 Fifty percent of PCD patients are diagnosed with Kartagener syndrome, classified as ciliary dyskinesia with the triad of sinusitis, bronchiectasis, and situs inversus.9,14,15
A growing body of evidence continues to explore the role of ciliary dysfunction in photoreceptors and the RPE.9,15, 16, 17, 18, 19 Some studies have discovered that disrupting key genes in ciliary function in mouse models results in severe RPE maturation defects, leading to diseases such as Joubert syndrome.18 Others have found that mutations in the retinitis pigmentosa 1 (RP1) gene result in faulty connecting cilium of photoreceptors, ultimately causing photoreceptor axoneme instability and cell death.17,20 Several recent clinical reports have also begun to link ciliary dysfunction to retinal degeneration. One such case found PCD and RP in two young males with mutations in the X-linked RPGR gene.9 Another report describes early onset RPE atrophy in a patient with Kartagener syndrome.15 In a study of patients with Usher syndrome type 2, photoreceptor axonemes demonstrated an abnormal number of microtubules affecting ciliary function.21 Other research on individuals with RP established that abnormal microtubule organization was present in motile cilia of airway cells and sperm flagella.22,23
In the present case, the role of ciliary dysfunction in the degeneration of the retina and RPE is unclear as no functional interaction between the DNAH5 and CDH3 genes has ever been established and the concurrent mutations have never been reported in a single individual. The chance of having two very rare and unrelated autosomal recessive diseases is increased in children of consanguineous parents. In our case, both mutations map to autozygous genomic segments on different chromosomes, suggesting that there was a common ancestor(s) who was carrier for the two variants. It is possible that the ciliary dyskinesia played a role in our patient's early presentation or more advanced vision loss as compared to other HJMD cases. Interestingly, in a case reported in 2017, two separate heterozygous mutations of ABCA4 and CDH3 were implicated in causing a rare presentation of HJMD.24 Our patient is different since both mutations were homozygous and known causes of unique diseases.2,12 However, the relationship between DNAH5 and CDH3 cannot be ignored in our patient's pathogenic maculopathy, and further investigation is required.
3. Conclusion
Several findings of this case report are unique. This is the first instance of two previously undescribed mutations: CDH3 c.1660A > C and DNAH5 c.6688-1G>T.8,25 Additionally, this the first report of OCT angiography in a patient with HJMD. Our patient's vision loss in conjunction with progressive sinopulmonary disease led to the diagnosis of two separate diseases, one with known retinal degeneration and the other without any prior reported association with vision loss. The relationship between the cadherin protein dysfunction in CDH3 mutations and the ciliopathy of DNAH5 mutations causing progressive retinal and RPE atrophy has not been studied before and should be investigated in the future. Additionally, this specific patient had no family history of HJMD or PCD and presented at a relatively early age of 11 years old with below average visual acuity impairment compared to other reported cases.6 HJMD is known to cause a longitudinal deterioration of cone and rod mediated function,5,6,8 therefore recognizing the symptoms, visual impairment, physical examination, and photographic and electrophysiological findings is crucial in counseling the patient, the family, and fellow clinicians.
Patient consent
The patient's legal guardian consented to the publication of this case report in orally and writing.
Funding
The authors did not receive any funding or grant support.
Conflicts of interest
None of the authors have conflicts of interest.
Authorship
All authors contributed significantly to the creation of this manuscript, each fulfilled criteria as established by the ICMJE.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.100486.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lek M., Karczewski K.J., Minikel E.V. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sprecher E., Bergman R., Richard G. Hypotrichosis with juvenile macular dystrophy is caused by a mutation in CDH3, encoding P-cadherin. Nat Genet. 2001;29:134–136. doi: 10.1038/ng716. [DOI] [PubMed] [Google Scholar]
- 3.Karti O., Abali S., Ayhan Z. CDH3 gene related hypotrichosis and juvenile macular dystrophy - a case with a novel mutation. Am J Ophthalmol Case Rep. 2017;7:129–133. doi: 10.1016/j.ajoc.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basel-Vanagaite L., Pasmanik-Chor M., Lurie R., Yeheskel A., Kjaer K.W. CDH3-Related syndromes: report on a new mutation and overview of the genotype-phenotype correlations. Mol Syndromol. 2010;1:223–230. doi: 10.1159/000327156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason J.O., 3rd, Patel S.A. A case of hypotrichosis with juvenile macular dystrophy. Retin Cases Brief Rep. 2015;9:164–167. doi: 10.1097/ICB.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 6.Leibu R., Jermans A., Hatim G., Miller B., Sprecher E., Perlman I. Hypotrichosis with juvenile macular dystrophy: clinical and electrophysiological assessment of visual function. Ophthalmology. 2006;113:841–847 e3. doi: 10.1016/j.ophtha.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 7.Basora E., Miranda C., Morel D., Tekin M., Colin A. Primary ciliary dyskinesia, hypotrichosis and macular dystrophy: coincidence or a novel syndrome? Am J Respir Crit Care Med. 2016;193 [Google Scholar]
- 8.Hull S., Arno G., Robson A.G. Characterization of CDH3-related congenital hypotrichosis with juvenile macular dystrophy. JAMA Ophthalmol. 2016;134:992–1000. doi: 10.1001/jamaophthalmol.2016.2089. [DOI] [PubMed] [Google Scholar]
- 9.Moore A., Escudier E., Roger G. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet. 2006;43:326–333. doi: 10.1136/jmg.2005.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escudier E., Duquesnoy P., Papon J.F., Amselem S. Ciliary defects and genetics of primary ciliary dyskinesia. Paediatr Respir Rev. 2009;10:51–54. doi: 10.1016/j.prrv.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Horani A., Brody S.L., Ferkol T.W. Picking up speed: advances in the genetics of primary ciliary dyskinesia. Pediatr Res. 2014;75:158–164. doi: 10.1038/pr.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Failly M., Bartoloni L., Letourneau A. Mutations in DNAH5 account for only 15% of a non-preselected cohort of patients with primary ciliary dyskinesia. J Med Genet. 2009;46:281–286. doi: 10.1136/jmg.2008.061176. [DOI] [PubMed] [Google Scholar]
- 13.Witman G.B. Axonemal dyneins. Curr Opin Cell Biol. 1992;4:74–79. doi: 10.1016/0955-0674(92)90061-g. [DOI] [PubMed] [Google Scholar]
- 14.Knowles M.R., Zariwala M., Leigh M. Primary ciliary dyskinesia. Clin Chest Med. 2016;37:449–461. doi: 10.1016/j.ccm.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia M.D., Ventura C.V., Dias J.R., Chang T.C.P., Berrocal A.M. Retinal pigment epithelium changes in Kartagener syndrome. Am J Ophthalmol Case Rep. 2018;10:119–121. doi: 10.1016/j.ajoc.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenekoop R.K., Loyer M., Hand C.K. Novel RPGR mutations with distinct retinitis pigmentosa phenotypes in French-Canadian families. Am J Ophthalmol. 2003;136:678–687. doi: 10.1016/s0002-9394(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 17.Yildiz O., Khanna H. Ciliary signaling cascades in photoreceptors. Vis Res. 2012;75:112–116. doi: 10.1016/j.visres.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May-Simera H.L., Wan Q., Jha B.S. Primary cilium-mediated retinal pigment epithelium maturation is disrupted in ciliopathy patient cells. Cell Rep. 2018;22:189–205. doi: 10.1016/j.celrep.2017.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun D.A., Hildebrandt F. Ciliopathies. Cold Spring Harb Perspect Biol. 2017;9 doi: 10.1101/cshperspect.a028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q., Zuo J., Pierce E.A. The retinitis pigmentosa 1 protein is a photoreceptor microtubule-associated protein. J Neurosci. 2004;24:6427–6436. doi: 10.1523/JNEUROSCI.1335-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrong S.D., Chaitin M.H., Fliesler S.J., Possin D.E., Jacobson S.G., Milam A.H. Ultrastructure of connecting cilia in different forms of retinitis pigmentosa. Arch Ophthalmol. 1992;110:706–710. doi: 10.1001/archopht.1992.01080170128040. [DOI] [PubMed] [Google Scholar]
- 22.Fox B., Bull T.B., Arden G.B. Variations in the ultrastructure of human nasal cilia including abnormalities found in retinitis pigmentosa. J Clin Pathol. 1980;33:327–335. doi: 10.1136/jcp.33.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arden G.B., Fox B. Increased incidence of abnormal nasal cilia in patients with retinitis pigmentosa. Nature. 1979;279:534–536. doi: 10.1038/279534a0. [DOI] [PubMed] [Google Scholar]
- 24.Blanco-Kelly F., Rodrigues-Jacy da Silva L., Sanchez-Navarro I. New CDH3 mutation in the first Spanish case of hypotrichosis with juvenile macular dystrophy, a case report. BMC Med Genet. 2017;18:1. doi: 10.1186/s12881-016-0364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornef N., Olbrich H., Horvath J. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med. 2006;174:120–126. doi: 10.1164/rccm.200601-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.