Abstract
The vestibular system is crucial for movement control during locomotion. As the dimensions of the vestibular system determine the fluid dynamics of the endolymph and, as such, the system's function, we investigate the interaction between vestibular system size, head size and microhabitat use in lizards. We grouped 24 lacertid species in three microhabitat types, we acquired three‐dimensional models of the bony vestibular systems using micro‐computer tomography scanning, and we performed linear and surface measurements. All vestibular measurements scale with a negative allometry with head size, suggesting that smaller heads house disproportionally large ears. As the sensitivity of the vestibular system is positively related to size, a sufficiently large vestibular system in small‐headed animals may meet the sensitivity demands during challenged locomotion. We also found that the microhabitat affects the locomotor dynamics: lizards inhabiting open microhabitats run at higher dimensionless speeds. On the other hand, no statistical relationship exists between dimensionless speed and the vestibular system dimensions. Hence, if the vestibular size would differ between microhabitats, this would be a direct effect (i.e. imposed, for instance, by requirements for manoeuvring, balance control, etc.), rather than depending on the lizards’ intrinsic running speed. However, we found no effect of the microhabitat on the allometric relationship between head and vestibular system size. The finding that microhabitat is not reflected in the vestibular system size (hence sensitivity) of the lacertids in this study is possibly due to spatial constraints of the skull.
Keywords: allometry, bony labyrinth, inner ear, locomotion, manoeuvrability, size constraint
Introduction
Swift displacements are an essential element of the foraging (Perry, 1999), anti‐predatory behaviour (Domenici et al. 2008) and mating behaviour (South & Kenward, 2001) of many animals and, thus, locomotor capacity is widely considered an ecologically relevant performance function (Aerts et al. 2000). Many studies have examined how selection has moulded elements of the locomotor system to match the specific requirements of the habitat (Herrel et al. 2002, 2013; Van Damme & Vanhooydonck, 2002; Biewener, 2003). Performance speed has received a lot of attention in this regard (Huey et al. 1984; Vanhooydonck et al. 2001; Brecko et al. 2008; Herrel et al. 2011), but being able to move in a controlled and stable way, i.e. keeping balance, is equally important for successful locomotion. When (rapidly) manoeuvring through an unknown (hence unpredictable) structurally complex environment, or when perturbed, corrective actions are required (Goyens & Aerts, 2018). This demands a good perception of the structural microhabitat complexity at the relevant scale for the animal, and of the self‐motion (displacement of the body in the environment; Cullen et al. 2011; Cullen, 2012), requiring the integration of vision, vestibular, haptic and proprioceptive sense. As we can assume that animals have adapted to their microhabitat and locomotor mode (e.g. continuous vs. intermitted, fast vs. cautious, etc.; Ritzmann et al. 2004; Toro et al. 2004; Gomes et al. 2009; Herrel et al. 2014), it seems plausible that the interplay between the structural microhabitat complexity and the locomotor mode/behaviour (in this study further together referred to as the microhabitat use s.s.) are reflected in the physiological and morphological structures of motor control (i.e. musculo‐skeletal and neuro‐motoric anatomy).
In vertebrates, the vestibular system is an important sensory organ for movement and balance control. It comprises the upper part of the inner ear, and it consists of the membranous semi‐circular canals (SCs) and the otolith organs (Hill et al. 2016). This balance apparatus senses angular and linear accelerations as well as the spatial orientation of the head, thus being key in perceiving self‐motion (Cullen et al. 2011; Cullen, 2012). At the same time, it is crucial in providing the reference system required to map the proprioceptively modulated movements of the body segments in a body‐bound frame (Angelaki & Cullen, 2008; Carriot et al. 2013; Goyens & Aerts, 2018). This is particularly important for proper anticipatory action when manoeuvring through an unknown complex environment (e.g. when chased by a predator) or for proper corrective actions upon perturbation (e.g. as a result of substrate movements). Its function relies on the hydrodynamics of the endolymph in response to head movements (Angelaki & Cullen, 2008; Carriot et al. 2013; Squire et al. 2013) and it is the absolute dimensions of the vestibular system that determine these fluid dynamics to a large extent (Muller, 1999; Rabbitt et al. 2004; Vogel, 2013). According to basic principles, both the mechanical response time and sensitivity are proportional to the membranous duct's cross‐sectional surface area, whereas, in addition, sensitivity is also proportional to the length of the duct. As such, smaller vestibular systems may face reduced sensitivity because of a too small overall vestibular system size and too narrow ducts (i.e. smaller ducts have a lower endolymph mass, hence only larger head accelerations can result in sensible forces). Larger systems with wide ducts, on the other hand, may lose performance in terms of response time (i.e. more delayed responses). In other words, the size (morphology) of the inner ear affects its performance (for review, see Spoor, 2003). Clearly, the sensitivity and response time of a vestibular system are only meaningful when considered in the context of the animal's natural behaviour in its natural environment, i.e. in the context of the microhabitat use s.s. as it is defined above.
In vertebrates, a generic negative allometric trend of inner ear size with respect to body size (mostly expressed in terms of body mass) exists (Jones & Spells, 1963; Muller, 1990; Spoor, 2003; Spoor et al. 2007; specific exponents varying according to ear dimension used and taxon considered). In this way, overall similarity of canal response is premised to be maintained, as average angular head velocities and accelerations decrease with body mass (i.e. movement frequencies decrease while angular excursions remain identical in larger animals; untested assumptions, Jones & Spells, 1963; Spoor, 2003). However, movement control is likely more challenging for species that must negotiate a priory unknown structurally complex and difficult microhabitat (e.g. climbing slippery, uneven rocky terrains, scurrying narrow, compliant and branching twigs). This is especially true when this needs to be done at high speeds: swiftly manoeuvring will rely on instantaneous, feedback‐controlled anticipatory movements and corrective actions. Accordingly, fast and agile animals that live in challenging microhabitats are expected to have larger vestibular systems for their size than expected on the abovementioned generic negative allometric trend because a larger vestibular size enhances sensitivity (cf. Spoor, 2003: ‘residuals of individual species from the general trend represent specialisation’; species above the trend line are agile/rapid; see fig. 2 in that review).
The vast majority of the species used for the empirical studies revealing this generic negative allometry between ear size and body size have body masses more than 0.5 kg, most even > 1 kg and up to about 6500 kg (Jones & Spells, 1963; Spoor et al. 2007; Davies et al. 2013). Yet, at the other (lower) end of the vertebrate size spectrum (specific body masses below 0.5 kg, down to a few grams only), it seems plausible and worth to question whether the need for sufficient and relevant sensitivity (as expressed by the generic negative allometry) does not trade off with a spatial constraint: the inner ear must fit within the available head space. The inherently smaller head sizes in smaller species should not come at the expense of the performance of the system, unless the animals can ecologically afford to compensate behaviourally (and physiologically) for this by changing their microhabitat use, i.e. by moving about more slowly and cautiously. Therefore, in the absence of such behavioural compensation (e.g. because of predatory pressure, feeding strategy, etc.) and to avoid fitness loss, a negative allometric relationship should in the first place exist between vestibular system size and the head size, rather than with body size. Also, a minimal head size to accommodate an inner ear with ecologically relevant sensitivity seems plausible. Moreover, as for the abovementioned overall generic ear–body size relationship, it can also be expected that the microhabitat use s.s. (structural complexity and locomotor mode) will be reflected in this ear–head size allometry.
In the present study, we aim to assess this possible interaction between head size, vestibular system size and microhabitat use s.s. To do so, we focus on lizard species of the family Lacertidae. All lacertids share a fairly similar body build (Arnold, 1989), can be considered agile/rapid (Spoor, 2003), and occupy a wide variety of habitats (tundra or alpine meadows, Mediterranean climates, tropical forests as well as desert areas; Arnold, 1998; Vanhooydonck & Van Damme, 1999). Moreover, lacertids span a considerable part of the mentioned lower end of the vertebrate size range (i.e. < 0.5 kg; body masses ranging from 0.002 to 0.3 kg; Meiri, 2008), with head sizes (head width) ranging from 5 to 40 mm (own unpublished data on 300 lacertid species). All this makes them ideal to study the premised vestibular scaling with head size and the relationship with the microhabitat use s.s. More specifically, we hypothesize that, within this family and despite the conserved body build, the species with the smallest heads have the relatively largest inner ears in order to respond to the functional demands. This will be reflected in a negative allometry of ear size against head size. Moreover, we predict that there will be an effect of the animals’ microhabitat use s.s. on this allometric relationship in such a way that for animals living in less challenging habitats (open areas), the size of the vestibular system will be smaller and/or will increase more rapidly with head size (i.e. less necessity to boost the sensitivity as much as possible) than for animals inhabiting more complex and demanding environments (densely vegetated areas, areas with vertical elements). Results will also be discussed in the context of the generic ear–body size negative allometry.
Materials and methods
Animals
We obtained a total of 42 preserved specimens representing 24 different lacertid species from the collections of the Functional Morphology Laboratory at the University of Antwerp, from the private collections of Dr A. Herrel (Muséum National d'Histoire Naturelle, Paris) and Dr J. Martín (Museo Nacional de Ciencias Naturales, Madrid, Spain), from the Ellerman Collection of the University of Stellenbosch and the Zoological Museum of Tel Aviv University.
‘Microhabitat use’ and ‘performance’
‘Microhabitat use s.s.’ (see Introduction) is considered as the combined effect of the microhabitat structure [three‐dimensional (3D) complexity] at the lizard‐relevant scale and the locomotor mode, reflecting the lizards’ performance in the unknown (hence unpredictable) complex 3D microhabitats. Based on reports in literature, species were assigned to one of three microhabitat structure groups: (i) open areas; (ii) densely vegetated areas; and (iii) habitats including vertical elements (e.g. rocks, bushes; Martín & Salvador, 1992; Vanhooydonck & Van Damme, 1999; Van Damme & Vanhooydonck, 2002; Arnold & Ovenden, 2004; Tadevosyan, 2007; Bar & Haimovitch, 2011; Bates et al. 2014; Baeckens et al. 2015; Table 1). As such, we consider these microhabitats to be related to different locomotor modes: (i) sprinting on more even surfaces, few manoeuvres; (ii) intermittent sprinting on more structured surfaces, many manoeuvres; (iii) intermittent sprinting on complex surfaces, many manoeuvres, climbing and jumping.
Table 1.
Morphological and performance information for all 24 species
| Species | Head (mm) | Inner ear (mm) | Sprint speed (m s−1) | Microhabitat | |||||
|---|---|---|---|---|---|---|---|---|---|
| Length | Width | Height | Length | Width | Depth | Absolute | Dimensionless | ||
| Acanthodactylus boskianus (1) | 16.43 | 9.71 | 7.97 | 3.38 | 2.44 | 2.84 | 2.98c | 6.38 | oa1 |
| Australolacerta australis (1) | 15.76 | 10.45 | 6.40 | 2.95 | 1.87 | 2.46 | 2.14f | 4.22 | ve5 |
| Dalmatolacerta oxycephala (1) | 15.24 | 9.07 | 6.05 | 3.01 | 2.05 | 2.34 | 2.02a | 4.53 | ve1 |
| Eremias acutirostris (1) | 16.80 | 9.55 | 8.12 | 3.39 | 2.31 | 2.88 | – | – | ve2 |
| Gallotia galloti (1) | 21.46 | 12.87 | 11 | 4.30 | 2.87 | 3.54 | 1.93g | – | dva1 |
| Holaspis guentheri (1) | 10.80 | 7.28 | 3.62 | 2.35 | 1.46 | 1.77 | – | – | ve3 |
| Iberolacerta monticola (1) | 16.74 | 10.91 | 5.99 | 3.47 | 1.95 | 2.54 | 2.76c | 5.28 | ve3 |
| Ichnotropis capensis (1) | 11.45 | 7.59 | 5.5 | 2.41 | 1.59 | 2.07 | 2.48f | 4.91 | oa1 |
| Lacerta agilis (1) | 17.96 | 12.19 | 9.78 | 3.25 | 1.98 | 2.74 | 1.68b | 3.56 | dva3 |
| Latastia longicaudata (1) | 16.03 | 8.67 | 6.81 | 3.25 | 2.14 | 2.84 | 3.34g | 5.74 | oa1 |
| Messalina guttulata (1) | 9.59 | 6.21 | 3.81 | 2.40 | 1.59 | 2.09 | – | – | oa1 |
| Meroles knoxii (1) | 15.82 | 9.96 | 7.85 | 2.62 | 1.65 | 2.31 | 2.36f | 5.17 | oa3 |
| Nucras tesselata (1) | 14.07 | 9.71 | 7.14 | 2.80 | 1.71 | 2.44 | 2.05e | 4.17 | oa7 |
| Ophisops elegans (1) | 9.11 | 5.45 | 3.98 | 2.42 | 1.40 | 2.09 | – | – | oa4 |
| Pedioplanis lineocellata (1) | 13.91 | 8.78 | 6.51 | 2.61 | 1.65 | 2.18 | 2.63e | 5.35 | dva3 |
| Phoenicolacerta laevis (1) | 12.03 | 7.9 | 5.55 | 2.84 | 1.77 | 2.32 | – | – | ve3,4 |
| Podarcis melisellensis (1) | 14.10 | 10.2 | 7.08 | 3.02 | 2.07 | 2.56 | 1.81d | 4.02 | ve3 |
| Podarcis hispanicus (1) | 14.41 | 9.94 | 7.98 | 3.05 | 2.07 | 2.66 | 1.85b | 4.01 | ve3 |
| Podarcis peloponnesiacus (8) | 17.36 | 9.84 | 7.57 | 3.13 | 2.10 | 2.77 | 2.67c | 5.02 | ve6 |
| Podarcis muralis (1) | 12.63 | 6.82 | 5.09 | 2.64 | 1.67 | 2.17 | 2.14a | 4.98 | ve3 |
| Psammodromus algirus (1) | 14.82 | 9.06 | 6.77 | 3.18 | 2.08 | 2.94 | 2.53g | 4.57 | dva3,4 |
| Takydromus sexlineatus (11) | 14.02 | 6.61 | 5.93 | 2.71 | 1.70 | 2.24 | 1.33a | 3.33 | dva3 |
| Tropidosaura gularis (1) | 14.27 | 8.49 | 5.93 | 2.82 | 1.60 | 2.33 | 1.89f | 5.10 | dva5 |
| Zootoca vivipara (1) | 10.61 | 7.21 | 5.22 | 2.46 | 1.47 | 2.13 | 0.87a | 2.14 | dva1,4 |
The number of specimens is indicated in the parentheses. Habitat type is indicated with dva, densely vegetated areas; oa, open areas; and ve, areas including vertical elements.
Sprint speed data acquired from literature: aVanhooydonck et al. (2001); bBauwens et al. (1995); cVerwaijen (2007); dBrecko et al. (2008); eHuey et al. (1984); fUnpublished data (personal observations by Dr Herrel and Dr Vanhooydonck); gVanhooydonck et al. (2007).
Habitat type data acquired from literature: 1Vanhooydonck & Van Damme (1999); 2Tadevosyan (2007); 3Van Damme & Vanhooydonck (2002); 4Baeckens et al. (2015); 5Bates et al. (2014); 6Mayer & Beyerlein (1999); 7Van der Meer et al. (2010). See Materials and methods for the dimensionless speed calculation.
A preliminary test proved that species belonging to open areas move significantly faster than the ones inhabiting densely vegetated areas [P = 0.025, corrected after Tukey hsd test, package ‘agricolae’ (Mendiburu, 2019) in R], and the result remained the same after including phylogeny in the analysis (P = 0.021). For this purpose, we used the maximal sprint speed (on flat terrain) as reported in the literature. As size differences must be considered (perceived microhabitat structure and response may differs for animals of different size), we used the average limb length for each genus (personal unpublished data) to transform maximal sprint speed into dimensionless speed (Hof, 1996):
Micro‐computer tomography scanning
All animals were decapitated, and the heads were placed in a staining solution of 5% phosphomolybdic acid (Sigma‐Aldrich, St Louis, MO, USA) and 70% EtOH for at least 14 days. Next, all the specimens were scanned to obtain a 3D model of the vestibular system.
We obtained high‐resolution shadow images of the inner ears of all 42 specimens, by micro‐computer tomography (μ‐CΤ) scanning. Of the Takydromus sexlineatus specimens, 10 were scanned at the SYREMP beamline of the Elettra synchrotron facility in Bazovizza (Trieste, Italy). The specimens Gallotia galloti and Podarcis muralis were scanned at the Tomolab scanner, which is a conventional µ‐CT scanner at the same facility (by Dr Lucia Mancini). All other specimens were scanned with a SkyScan 1172 high‐resolution μ‐CT scanner [Bruker micro‐CT, Kontich, Belgium, managed by the BiostrμCT Hercules consortium (https://sites.google.com/view/biostruct)] at the Vrije Universiteit Brussel (VUB, Belgium).
We adjusted scanning parameters depending on the specimen's size to optimise image resolution. We used an average voltage of 80 kV and a current of 124 μΑ with an aluminium‐copper filter of 1 mm. The pixel size varied from 4.17 to 13.45 μm, due to the size difference between species. The rotation angle of the scans was 0.40 °, and the exposure time was manually adjusted to 1300 ms.
The projection images were reconstructed in NRecon (Bruker micro‐CT). On the reconstructed slices, the bony labyrinth was clearly visible as a network of connected tunnels at the posterior end of the skull bone. We imported the reconstructed slice images in the specialised 3D image processing software Amira (Amira 5.4.3 VSG systems, Mérignac, France). The voxels belonging to the bony labyrinth were selected by combining automatic grey‐scale thresholding and manual corrections in the three orthogonal views. This resulted in a 3D surface model of the vestibular system of the right inner ear. For the P. muralis individual, we were only able to acquire the left inner ear.
Morphometrics
Head length was measured from the tip of the snout to the posterior extremity of the parietal scale. Head width was measured at its widest point, which is as well the distance across the parietal shields of the animal, and finally head height was the maximal distance measured between the base of the mandible and the parietal surface. We measured all head dimensions with a digital calliper (precision 0.01 mm). We used the 3D computer models to measure the linear dimensions of the inner ear and more specific traits of the bony labyrinth (the most important measurements are shown in Figs 1, 2, 3).
Figure 1.
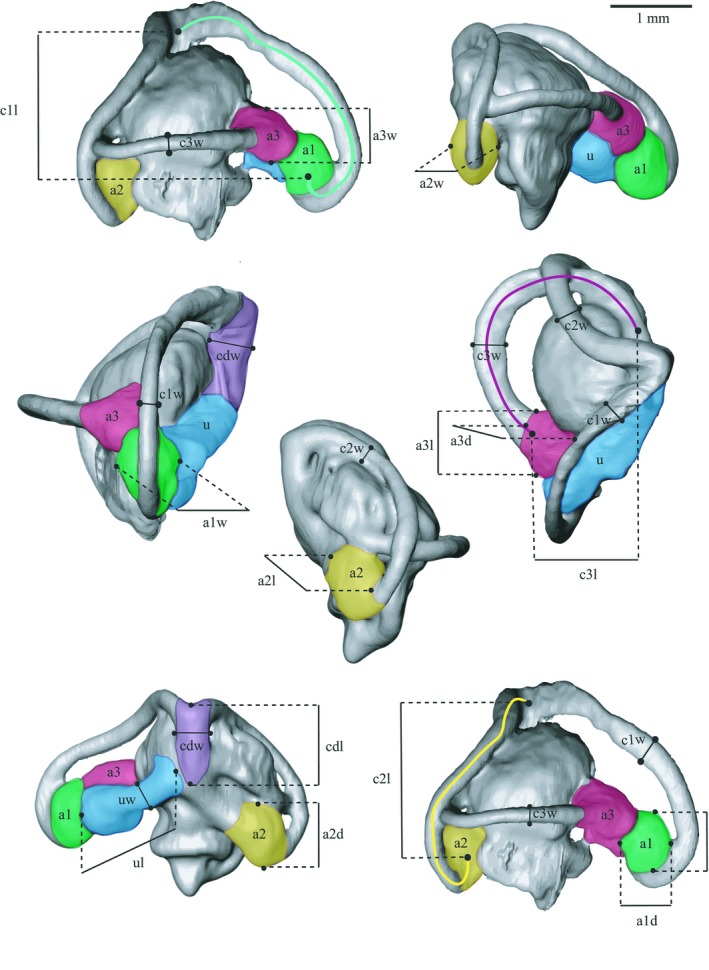
Illustrations of the 3D surface model of the species Iberolacerta monticola and the morphological traits measured. The ampulla width (a1w, a2w, a3w), length (a1l, a2l, a3l) and depth (a1d, a2d, a3d) are indicated, where a1: anterior ampulla (AA); a2: posterior ampulla (PA); and a3: lateral ampulla (LA). The lengths of the semi‐circular canals (SCs) are illustrated with coloured lines: c1l (green line)–ASC length; c2l (yellow line)–PSC length; and c3l (red line)–LSC length. The following traits are illustrated by different colours: green–ASC; yellow–PSC; red–LSC; blue–utricle; purple–common duct. The utricle and common duct length are indicated with ul and cdl, respectively. Finally, the widths of the three SCs are indicated (c1w, c2w, c3w). ASC, anterior semi‐circular canal; LSC, lateral semi‐circular canal; PSC, posterior semi‐circular canal.
Figure 2.
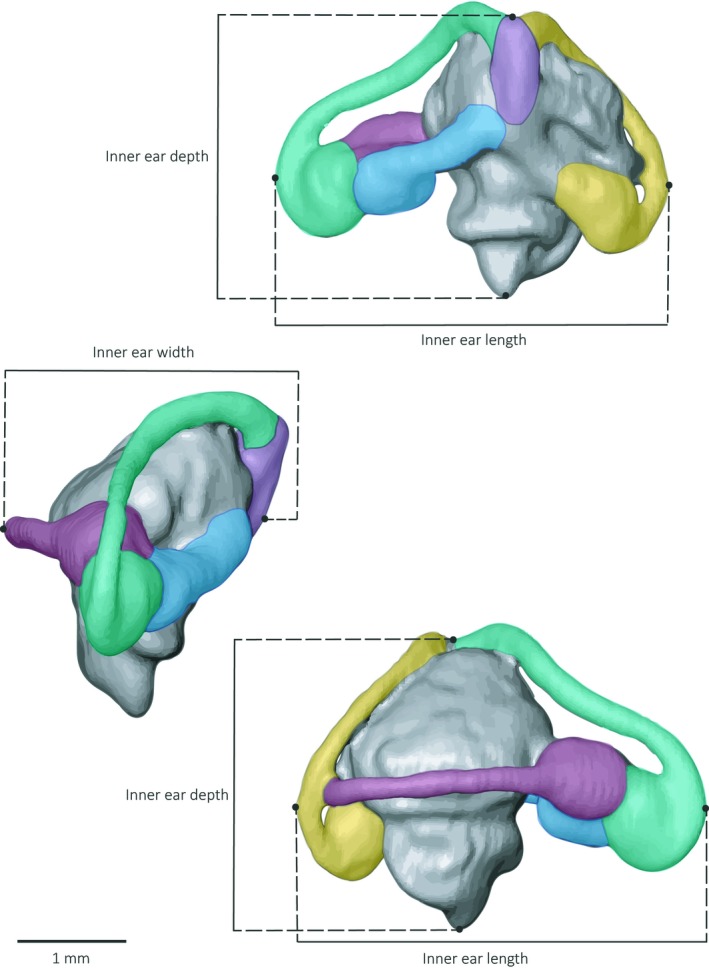
Illustrations of the 3D surface model of the species Iberolacerta monticola and the morphological traits measured. The inner ear length, width and depth measurements are indicated. The semi‐circular canals (SCs) are illustrated with different colours: ASC in green, PSC in yellow, LSC in red. The utricle is shown in blue and the common duct in purple. ASC, anterior semi‐circular canal; LSC, lateral semi‐circular canal; PSC, posterior semi‐circular canal.
Figure 3.
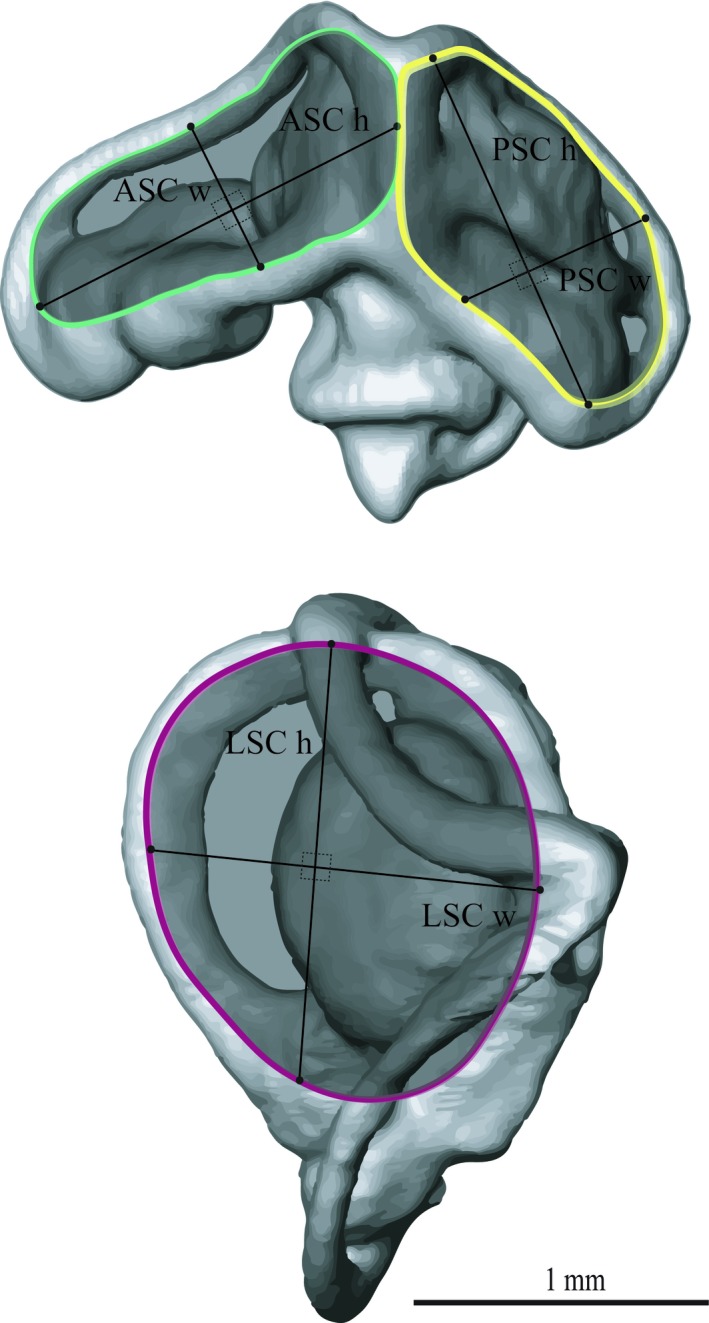
Illustrations of the 3D surface model of the species Iberolacerta monticola and the radius of curvature measurements. For each semi‐circular canal (SC), respectively, the width (ASC w, PSC w, LSC w) and height (ASC h, PSC h, LSC h) measurements of the radius of curvature are illustrated. ASC radius of curvature (ASC R) is indicated in green, PSC radius of curvature (PSC R) is indicated in yellow, and LSC radius of curvature (LSC R) is indicated in red. ASC, anterior semi‐circular canal; LSC, lateral semi‐circular canal; PSC, posterior semi‐circular canal.
We measured the anterior (ASC), posterior (PSC) and lateral (LSC) semi‐circular canal width and height (in lateral and vertical directions, respectively). We used the Amira 3D distance tool at three locations along the canals: (i) close to the ampulla of each SC; (ii) close to the connection with the common duct; and (iii) in the middle of each canal (Fig. 1). Based on these measurements, we calculated the average width and height, as well as the average cross‐sectional area (considering their cross‐sectional shape as an ellipse). The widths and heights of the common duct and the utricle were measured in the middle of the structures (Fig. 1). Further, we measured the height, width and length for the ampullas of the anterior, posterior and lateral SCs and calculated their surface areas considering their surface shape as an ellipsoid (Tables 1 and 2 for all the variables measured). Additionally, we measured the height and width of each canal (perpendicular to each other), and calculated the radius of curvature as R = 0.5 × (height + width)/2 following Spoor et al. (2007) (Fig. 3; Table S1). We used the Meshtools software [ISE‐MeshTools 1.3.3 (Lebrun, 2014)] to place semi‐landmarks at the midline of each canal to create curves that describe their path semi‐automatically. The first and last semi‐landmarks were positioned at the centre of each ampulla, so that their position would be comparable for the whole dataset. The 3D coordinates of these curves were used to calculate the length of the canals.
Table 2.
Morphological information for all 24 species (additional to Table 1)
| Species | Linear measurements (mm) | Cross‐sectional surface areas (mm2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASCl | PSCl | LSCl | Cdl | Ul | ASCa | PSCa | LSCa | Cda | Ua | AAa | PAa | LAa | |
| Acanthodactylus boskianus (1) | 4.19 | 3.82 | 4.29 | 1.08 | 1.57 | 0.05 | 0.06 | 0.06 | 0.14 | 0.12 | 1.67 | 2.04 | 1.83 |
| Australolacerta australis (1) | 3.52 | 3.07 | 3.30 | 1.89 | 1.49 | 0.05 | 0.04 | 0.09 | 0.14 | 0.12 | 1.04 | 1.50 | 1.52 |
| Dalmatolacerta oxycephala (1) | 3.77 | 3.32 | 3.58 | 1.16 | 1.28 | 0.04 | 0.04 | 0.03 | 0.10 | 0.10 | 1.01 | 1.38 | 1.41 |
| Eremias acutirostris (1) | 3.98 | 3.78 | 4.15 | 1.14 | 1.71 | 0.05 | 0.04 | 0.05 | 0.10 | 0.15 | 1.45 | 1.90 | 1.87 |
| Gallotia galloti (1) | 5.58 | 5.02 | 5.77 | 1.37 | 1.94 | 0.07 | 0.05 | 0.06 | 0.17 | 0.15 | 1.97 | 2.59 | 2.23 |
| Holaspis guentheri (1) | 2.66 | 2.80 | 2.52 | 0.97 | 0.96 | 0.03 | 0.03 | 0.04 | 0.08 | 0.10 | 0.84 | 1.11 | 0.90 |
| Iberolacerta monticola (1) | 3.79 | 3.51 | 4.14 | 1.18 | 1.58 | 0.03 | 0.03 | 0.03 | 0.13 | 0.08 | 1.38 | 1.70 | 1.67 |
| Ichnotropis capensis (1) | 2.74 | 2.51 | 2.40 | 1.13 | 1.10 | 0.03 | 0.04 | 0.04 | 0.10 | 0.08 | 0.79 | 0.99 | 1.04 |
| Lacerta agilis (1) | 3.62 | 3.43 | 3.94 | 1.33 | 1.52 | 0.08 | 0.06 | 0.07 | 0.18 | 0.13 | 1.47 | 1.52 | 1.89 |
| Latastia longicaudata (1) | 4.17 | 4.16 | 4.45 | 1.43 | 1.57 | 0.03 | 0.05 | 0.03 | 0.14 | 0.13 | 1.44 | 1.81 | 1.45 |
| Messalina guttulata (1) | 2.87 | 2.85 | 2.67 | 0.96 | 0.99 | 0.04 | 0.04 | 0.04 | 0.10 | 0.05 | 0.57 | 1.05 | 1.10 |
| Meroles knoxii (1) | 3.25 | 2.73 | 2.85 | 1.04 | 1.23 | 0.05 | 0.04 | 0.05 | 0.11 | 0.09 | 0.88 | 1.34 | 1.44 |
| Nucras tesselata (1) | 3.19 | 3.00 | 3.28 | 0.80 | 1.28 | 0.04 | 0.03 | 0.04 | 0.09 | 0.08 | 0.89 | 1.35 | 1.23 |
| Ophisops elegans (1) | 2.67 | 2.52 | 2.63 | 0.94 | 1.09 | 0.03 | 0.03 | 0.04 | 0.09 | 0.05 | 0.83 | 1.07 | 1.08 |
| Pedioplanis lineoocellata (1) | 3.09 | 3.02 | 2.82 | 0.97 | 1.08 | 0.04 | 0.05 | 0.04 | 0.09 | 0.06 | 0.80 | 1.15 | 1.25 |
| Phoenicolacerta laevis (1) | 3.10 | 2.99 | 3.47 | 0.94 | 1.31 | 0.03 | 0.04 | 0.03 | 0.13 | 0.09 | 1.12 | 1.35 | 1.31 |
| Podarcis melisellensis (1) | 3.58 | 3.33 | 3.77 | 1.30 | 1.27 | 0.05 | 0.04 | 0.05 | 0.09 | 0.08 | 1.44 | 1.79 | 1.78 |
| Podarcis hispanicus (1) | 3.46 | 3.49 | 3.73 | 1.52 | 1.33 | 0.06 | 0.04 | 0.05 | 0.15 | 0.13 | 1.15 | 1.56 | 1.59 |
| Podarcis peloponnesiacus (8) | 3.71 | 3.36 | 3.87 | 1.28 | 1.40 | 0.05 | 0.04 | 0.05 | 0.12 | 0.08 | 1.15 | 1.59 | 1.58 |
| Podarcis muralis (1) | 2.98 | 2.68 | 3.11 | 0.85 | 1.16 | 0.05 | 0.03 | 0.04 | 0.09 | 0.07 | 0.57 | 1.18 | 1.14 |
| Psammodromus algirus (1) | 3.85 | 3.56 | 4.06 | 1.30 | 1.44 | 0.05 | 0.04 | 0.04 | 0.14 | 0.11 | 1.09 | 1.45 | 1.41 |
| Takydromus sexlineatus (11) | 3.28 | 2.89 | 3.16 | 0.90 | 1.28 | 0.04 | 0.04 | 0.04 | 0.10 | 0.08 | 0.92 | 1.22 | 1.17 |
| Tropidosaura gularis (1) | 3.36 | 3.07 | 3.28 | 1.13 | 1.35 | 0.04 | 0.03 | 0.02 | 0.11 | 0.10 | 0.86 | 1.13 | 1.24 |
| Zootoca vivipara (1) | 3.05 | 2.88 | 2.82 | 1.01 | 1.10 | 0.03 | 0.03 | 0.04 | 0.09 | 0.07 | 0.70 | 1.07 | 1.27 |
Traits of the vestibular system measured: ASCl, ASC length; Cdl, common duct length; LSCl, LSC length; PSCl, PSC length; Ul, utricle length; cross‐sectional surface areas of ASC (ASCa), PSC (PSCa), LSC (LSCa), anterior ampulla (AAa), posterior ampulla (PAa), lateral ampulla (LAa), utricle (Ua) and common duct (Cda) (Fig. 1).
Statistical analysis
As the species under study cannot be seen as independent data points due to shared‐ancestry, we considered it necessary to implement phylogenetic information into our analyses. To do so, we pruned the Bayesian tree constructed by Baeckens et al. (2015) based on interspecific variation at two nuclear and three mitochondrial gene regions. To test for phylogenetic signal in the traits measured, we calculated Pagel's λ (Pagel, 1999) and Blomberg's K (Bloomberg et al. 2003) for all variables, using the ‘Phylosig’ function in the R‐package ‘Phytools’ (Revell, 2012).
To evaluate the relationship between the size traits of the vestibular system and head size, phylogenetic general least‐squares regressions (‘pgls’) were used. The Log10‐transformed dimensional data of the inner ear were regressed against the Log10‐transformed head width measurements (ordinary least‐squares method). Conforming to existing literature (Georgi et al. 2013; Maddin & Sherratt, 2014), we used head width as a size indicator. We measured the widest point of the skull, which is where the inner ears are positioned and which is, therefore, the potential place of size constraint.
We ran the regression analyses four times, each time using a different model of evolution. Identification of the best evolutionary model was performed using the AIC (Akaike, 1973) and BIC (Findley, 1991) approach. These four models are as follows: the Brownian motion timed model (λ = 1; κ = 1, δ = 1); the Speciational model (κ = 1); the Star model (λ = ~0); and the model in which the best combination of the λ, κ and δ parameters is determined by maximum‐likelihood (ML; R packages ‘nlme’, ‘geiger’, ‘caper’ and ‘phytools’, function: ‘pgls’).
After calculating the regression slopes, we used t‐tests to assess whether the slope values differed significantly from the expected ones [i.e. slope of 1 for linear dimensions; slope of 2 for surfaces (Richard & Wainwright, 1995)]. For the species where we had more than one specimen available, we used the average of the traits measured for the statistical analyses (T. sexlineatus, n = 11; Podarcis peloponnesiacus, n = 8).
To test the effect of microhabitat use on vestibular size variation, phylogenetic analyses of covariance were used using the ‘pgls’ function in the R‐package ‘phytools’. We performed the test twice, once including microhabitat type together with the interaction with head width, and once correcting for head size. In this way, we can assess whether, respectively, the slopes (assessing differences in allometry between microhabitat use groups) and/or the intercepts (referring to overall size as such) differ between microhabitat use. All statistical analyses were conducted in R studio version 1.0.136 (R Core Team, 2012; R Studio, 2012).
Finally, we performed an analysis of covariance (ancova) between the inner ear dimensions measured, the dimensionless sprint speed and the microhabitat types to test whether the intrinsic speed is statistically related to the vestibular system size and if this differs according to the microhabitat type. The relationship between dimensionless speed and vestibular system size has statistical meaning only, as absolute, not normalised, physical variables are perceived by sensors. For all analyses, we assume that the duct morphological parameters are interconnected and are not treated separately.
Results
Morphometrics and microhabitat type
The measured head and inner ear dimensions of the individual animals in this study are reported in Tables 1 and 2. Additionally, Table 1 shows the dimensionless speed based on the maximal sprint speed average for the species as found in the literature. The average head dimensions were 14.4 ± 2.88 mm for head length, 8.94 ± 1.83 mm for head width and 6.57 ± 1.74 mm for head height. The average inner ear dimensions were 2.94 ± 0.45 mm for ear length, 1.88 ± 0.35 mm for ear width and 2.47 ± 0.38 mm for ear depth (Fig. 2 for measurements). Figure 4 illustrates, as an example, the vestibular system anatomy of the species T. sexlineatus focusing on the three SCs of the bony labyrinth. An important part of the skull volume is occupied by the inner ear (both inner ears occupy almost 60% of the total head width for this species).
Figure 4.
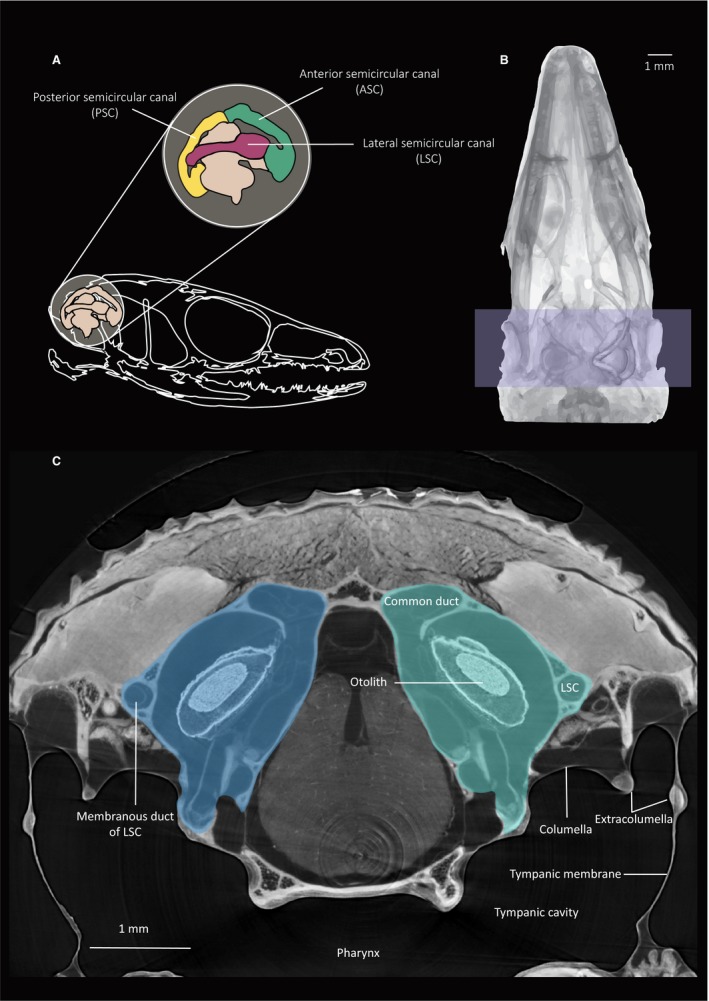
Illustration of the inner ear of the species Takydromus sexlineatus. (A) Graphical illustration of the bony labyrinth. The three semi‐circular bony canals are indicated. (B) D‐V inverse average projection image of the skull with the model of the right inner ear superimposed. Both inner ears are highlighted. (C) Transversal micro‐computed tomography (μ‐CT) slice from the scans made at the Elettra synchrotron facility.
Scaling analysis
Figure 5 illustrates the 3D surface models of the 24 specimens. To visualise size differences, the inner ear size of each species was first scaled to the specific head width. Then, these relative ear sizes were scaled to the relative ear size of G. galloti (the largest in our dataset, see Tables 1 and 2). For all phylogenetic regressions, the ML model explained trait evolution best. Therefore, only the scaling results under this model of evolution were taken into account for further interpretation. The majority of the linear measurements showed a strong, and significant, phylogenetic signal, indicating that closely related species resemble each other more in their inner ear morphology than expected by chance (Table S2). All linear dimensions of the inner ear scaled with a slope significantly lower than a slope of 1, indicating a negative allometry (Fig. 6; Table 3). Furthermore, the cross‐sectional areas of the anterior, posterior and lateral ampullas all scaled with a slope significantly lower than 2, again indicating a negative allometry (Table 4). The same pattern was also followed by the SCs cross‐sectional area (Table 4). The results of the phylogenetic ancova, taking into consideration the interaction between microhabitat type and head size, were not significant (i.e. slopes did not differ significantly), showing no effect of the microhabitat use on the scaling relationships between the measured variables and the head width (all P > 0.42). After correcting for head size, no significant difference was found either; the intercepts of the slopes representing the three microhabitat groups were similar (all P > 0.42). No significant correlation was found between inner ear dimensions, dimensionless speed and microhabitat use (all P > 0.06), indicating that, if a relation between microhabitat structure and ear size would have been present, this does not depend upon intrinsic sprint capacity of the animals.
Figure 5.
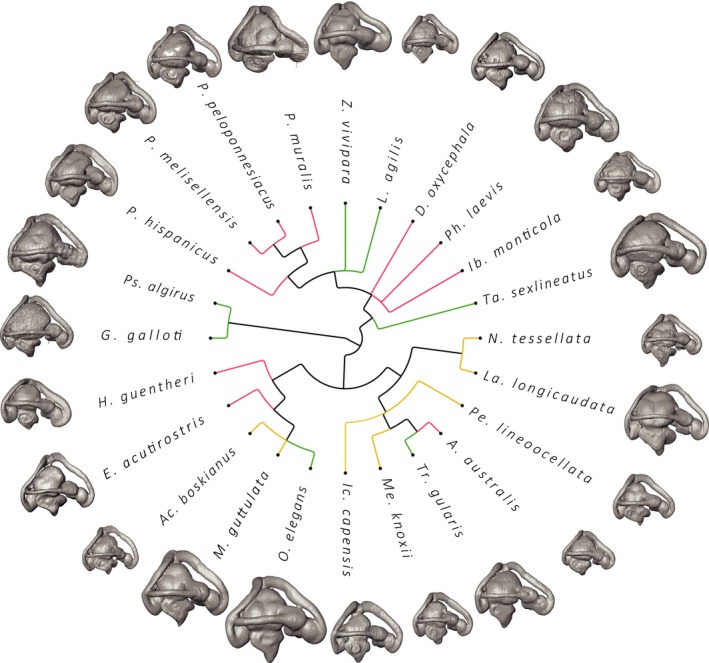
Illustration of the 3D computer models of the inner ears for all lacertid species under study. Relative ear to head size is used and scaling to the Gallotia galloti inner ear. Smaller animals (e.g. Ophisops elegans and Takydromus sexlineatus; Table 1) appear to have relatively the largest inner ears for their head size. Different colours indicate the different habitat types (open areas: yellow; areas with vertical elements: red; densely vegetated areas: green).
Figure 6.
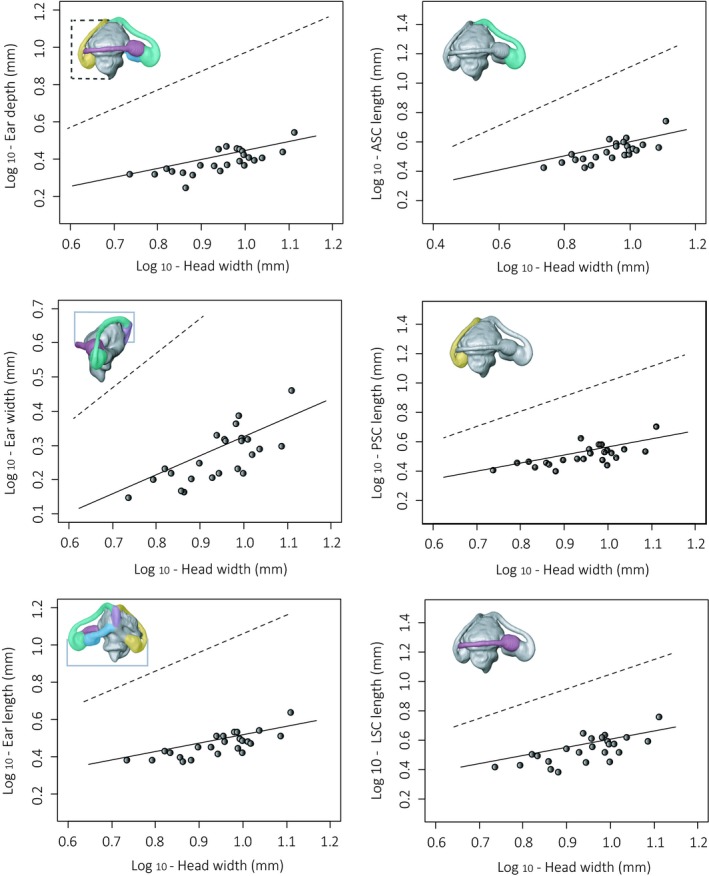
Phylogenetic least‐squares regression (PGLS) examples of the traits measured. The dashed lines indicate the expected slope under isometry. A negative allometry with head size is observed for all the variables. All plots are Log–Log plots.
Table 3.
Phylogenetic scaling results of the ML evolutionary model for linear measurements
| Measurements (mm) | n | Adj. R 2 | Slope | SE | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| Ear width | 24 | 0.72 | 0.56 | 0.07 | 0.41–0.70 | < 0.001 |
| Ear depth | 24 | 0.66 | 0.48 | 0.07 | 0.34–0.61 | < 0.001 |
| Ear length | 24 | 0.68 | 0.45 | 0.07 | 0.33–0.58 | < 0.001 |
| ASC length (c1l) | 24 | 0.69 | 0.48 | 0.07 | 0.35–0.60 | < 0.001 |
| PSC length (c2l) | 24 | 0.67 | 0.55 | 0.08 | 0.40–0.71 | < 0.001 |
| LSC length (c3l) | 24 | 0.65 | 0.55 | 0.08 | 0.39–0.72 | < 0.001 |
| ASC radius of curvature (R) | 24 | 0.45 | 0.37 | 0.08 | 0.20–0.53 | < 0.001 |
| PSC radius of curvature (R) | 24 | 0.66 | 0.54 | 0.07 | 0.38–0.69 | < 0.001 |
| LSC radius of curvature (R) | 24 | 0.47 | 0.47 | 0.08 | 0.32–0.63 | < 0.001 |
| Common duct length (cdl) | 24 | 0.42 | 0.56 | 0.13 | 0.30–0.81 | 0.003 |
| Utricle length (ul) | 24 | 0.57 | 0.65 | 0.12 | 0.42–0.87 | 0.006 |
Coefficient of determination (R 2) and slope of the phylogenetic regression line describing the relationship between the cross‐sectional areas and head length (both Log10‐transformed). The standard error (SE) and 95% confidence interval (CI) are also shown. The P‐value indicates the statistical significance of the difference between the obtained slope with that expected under simple isometry [slope = 1 for linear measurements (Richard & Wainwright, 1995)].
ASC, anterior semi‐circular canal; LSC, lateral semi‐circular canal; PSC, posterior semi‐circular canal.
Table 4.
Phylogenetic scaling results of the ML evolutionary model for surface measurements
| Measurements (mm2) | n | Adj. R 2 | Slope | SE | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| ASC cross‐sectional area | 24 | 0.38 | 0.77 | 0.20 | 0.38–1.17 | < 0.001 |
| PSC cross‐sectional area | 24 | 0.17 | 0.4 | 0.17 | 0.07–0.73 | < 0.001 |
| LSC cross‐sectional area | 24 | 0.43 | 0.71 | 0.17 | 0.38–1.03 | < 0.001 |
| Common duct cross‐sectional area | 24 | 0.45 | 0.78 | 0.18 | 0.43–1.12 | < 0.001 |
| Utricle cross‐sectional area | 24 | 0.49 | 1.01 | 0.21 | 0.60–1.43 | < 0.001 |
| Anterior ampulla cross‐sectional area | 24 | 0.77 | 1.6 | 0.18 | 1.24–1.96 | 0.02 |
| Posterior ampulla cross‐sectional area | 24 | 0.68 | 0.96 | 0.13 | 0.69–1.22 | < 0.001 |
| Lateral ampulla cross‐sectional area | 24 | 0.8 | 0.96 | 0.10 | 0.76–1.16 | < 0.001 |
Coefficient of determination (R 2) and slope of the phylogenetic regression line describing the relationship between the cross‐sectional areas and head length (both Log10‐transformed). The standard error (SE) and 95% confidence interval (CI) are also shown. The P‐value indicates the statistical significance of the difference between the obtained slope with that expected under simple isometry [slope = 2 for surface measurements (Richard & Wainwright, 1995)].
ASC, anterior semi‐circular canal; LSC, lateral semi‐circular canal; PSC, posterior semi‐circular canal.
Discussion
When manoeuvring swiftly through an unknown environment, animals must continuously perform instantaneous feedback‐controlled anticipatory movements to negotiate the microhabitat structure and corrective actions to compensate for (possible) perturbations (Biewener, 2003). It can, therefore, be assumed that movement control is more challenging for animals moving about in complex environments. Basically, there are two ways to deal with this: (i) adjusting the locomotor behaviour by moving more cautiously (i.e. behaving less agile); or (ii) enhancing movement and balance control.
The present results show that, within the tested group of lacertid species, lizards living in the open (less challenging) microhabitat run at a faster dimensionless speed than lizards from the more complex microhabitat types (densely vegetated or habitats where climbing is required). Because the open habitat lends itself to faster running, it is not all too surprising that for two lizards of the same body size, the one living in an open microhabitat is a faster runner. Because we do not find a significant statistical relationship between dimensionless speed and the anatomical measurements, size differences of the inner ear between microhabitats, if present, should relate to other effects of the microhabitat on the locomotion type (e.g. imposed requirement for manoeuvring, balance control) than the intrinsic sprint capacity per se. In this situation, we expected slopes further from isometry (i.e. stronger negative allometry) or larger intercepts in more challenging microhabitat types (overall larger inner ears). As premised on the basis of the hydrodynamic characteristic of the endolymph (see Introduction), we found a negative allometry for the vestibular system, suggesting that species with smaller skulls possess disproportionally larger vestibular systems for their head size. A similar relationship was also found in lizard species of the genus Anolis by Dickson et al. (2017), in this case between head length and the centroid size of the vestibular system. However, contrary to our expectations, the microhabitat that lacertids occupy does not affect the negative allometry or the overall size of the inner ears. Together with the fact that there is no statistical relationship between dimensionless speed on the inner ear dimensions, this finding suggests that microhabitat use (i.e. microhabitat complexity) is not reflected in the inner ear dimensions. So, why is this?
The available data only allow us to speculate about possible reasons. First, this may be due to methodological reasons (e.g. sample size, small number of species per microhabitat group, not specialised enough microhabitat groups). But is it also possible that we have misjudged the demands imposed by habitat use on motor control? If, for these small agile lizards, vestibular sensitivity is not an issue in even the most demanding environment (i.e. hydrodynamics are not constraining and/or vision primarily governs movement and balance control, and/or structural complexity of the three types is after all not too different, but see further), one cannot expect to see a habitat signal in the vestibular morphology (in casu size).
However, the observed negative allometry suggests, in itself, that vestibular function is in fact important in these small species. Because of the negative allometry, small heads have relatively large vestibular systems, which come at the potential cost of a spatial trade off: in small species, the posterior part of the cranium is almost entirely occupied by the inner ears (Fig. 4), limiting the available space for the other vital organs. If the above arguments hold true, it appears that the combined size constraint (Muller, 1999; Lambert et al. 2008) of the inner ear mechanics and the spatial limitations of the small skull size prevent an effect of microhabitat use on inner ear dimensions. Caution has to be taken when comparing with the literature because often different anatomical measurements and different habitat or locomotion groups are compared. Nevertheless, it is interesting to note that also Boistel et al. (2011) show that the vestibular system size does not change in squamates differing in locomotor behaviour and microhabitat, and that Benson et al. (2017) report that the centroid size of the SCs of small birds is constrained by their skull size and not by their flying capacities (using body mass as a body size measure).
On the other hand, this may seem to conflict with Dickson et al. (2017) who did find differences in vestibular system shape (measured using geometric morphometrics) between Anolis ecomorphs. The ecomorphs were defined on the basis of the preferred position in the arboreal environment, which was linked to ‘agility’ by the authors. Hence they are partly comparable to the ‘microhabitat use’ parameter in our study. However, these authors additionally report that SC morphology is correlated with skull and canal size. Contrary to the present lacertid sample, the Anolis ecomorph groups differ in head size, making it possible that the reported link between ecomorph group and vestibular morphology (presumably including size) is indirect, and actually reflects the effect of the head size rather than microhabitat use. Another reason for the discordant findings between Dickson's study and ours might be due to differences in the degree and type of microhabitat specialisation between the two lizard groups. While anole microhabitat specialists from the Greater Antilles differ extremely in their use of structural microhabitat on trees [from high trunks, over low branches of narrow support, to broad, low surfaces (Losos & Ricklefs, 2009)], lacertids occupy not only tree trunks, but also open sandy areas, dense vegetation and steep rock faces (Arnold, 1989). As such, the contrasting set of divergent microhabitats used by anoles and lacertids might affect the evolution of the morphology of the vestibular system on both lizard clades differently.
Maybe, once the spatial constraint of skull size relaxes when body size increases, habitat use can be reflected in vestibular size. Literature suggests that, in larger heads, relationships between vestibular size and locomotor behaviour are present, all or not coupled to the habitat the animals live in. For instance, sloths have relatively small canals for their size as they are less agile and perform slow and sluggish movements (Perier et al. 2016). In addition, it has been observed that modern humans (Homo sapiens) possess ASCs and PSCs that are larger in size than in other hominids and in greater apes (e.g. Australopithecus and Paranthropus; Spoor et al. 1994, 2002), and by contrast they possess LSCs that are significantly smaller (Jeffery & Spoor, 2004). This can probably be linked to the requirement for a higher sensitivity to meet the increased challenge that balance control can present when being bipedal. Similarly, bipedal dinosaurs possessed larger inner ears than quadrupedal ones (Georgi et al. 2013). Obviously, these taxa have very different locomotion styles and occupy very different habitats than the lacertid lizards in this study. Hence, these examples in which habitat use is apparently reflected in vestibular size can only loosely and with much caution be compared with our data.
Most studies scale the vestibular system dimensions with body size, instead of head size. This may well affect the allometry found. For instance, if we would use body size (snout‐to‐vent length; own estimation from unpublished data on 212 lacertids) instead of skull size in our analysis, our conclusions would be different: in lacertids, there is a positive allometry between head and body size [slope = 1.3, P < 0.01 (slope significantly different from a slope of 1)], which together with the negative allometry between inner ear and head size, suggests that the inner ear would scale isometrically with body size [slope = 0.98, P = 0.93 (slope not significantly different from a slope of 1); for these linear regression tests we used species average snout‐to‐vent lengths from unpublished data as mentioned before]. This suggests that the generic negative allometric trend found in vertebrates (ear size vs. body size) disappears at the lower end of the total size range, at least in our sample. Therefore, the size proxy describing the scaling of the vestibular system should always be chosen according to the scientific question, which in our research was to detect the possible size limitations of the small lizard head on their vestibular system's functioning.
In summary, we find no differences in vestibular system allometry between microhabitat groups. The inner ear occupies a large portion of the skull cavity, thereby decreasing space for other organs. This probably constrains the growth of the vestibular system, which may prevent further size adaptation to microhabitat use. Indeed, often no size adaptation to locomotor behaviour is found in animals with small heads, while this is often the case for large‐headed animals where the size constraint is weaker or absent. Considering the complex geometry of the vestibular system, it may be interesting to investigate whether vestibular system shape differs between the microhabitats, as this can happen without taking more space inside the skull (Billet et al. 2015; Pfaff et al. 2015; Grohé et al. 2016; Costeur et al. 2018).
Authors’ contributions
MV‐Κ, PA, JG and RVD conceived and designed the study, participated in the interpretation of the results and helped draft the manuscript; MV‐Κ, SB and JG collected the data; MV‐Κ made the 3D reconstructions, carried out the statistical analysis and drafted the manuscript; RVD and SB participated in the statistical analysis; MV‐Κ designed the illustrations and revised the manuscript. All authors gave final approval for publication.
Supporting information
Table S1. Morphological information for all 24 species.
Table S2. Phylogenetic signal.
Table S3. Comparison of variation among and across species.
Acknowledgements
The present study was funded by FWO project (grant no. G0E0214N). JG and SB are funded by FWO postdoctoral fellowships (JG: grant no. 12R5118N; SB: grant no. 12I8819N). MV‐K is funded as a research assistant by the University of Antwerp. The SkyScan 1172 high‐resolution micro‐CT scanner was funded by the Hercules Foundation (grant no. UABR/11/004). The authors thank the SYREMP beamline of the Elettra synchrotron facility in Bazovizza (Trieste, Italy). The authors thank Dr Renaud Lebrun (ISEM) for the ISE‐MeshTools 1.3.3 software and his advice, and Dr Anthony Herrel and Dr Bieke Vanhooydonck for the sprint speed data of Ichnotropis capensis, Tropidosaura gularis, Meroles knoxii and Australolacerta australis.
References
- Aerts P, Van Damme R, Herrel A, et al. (2000) Lizard locomotion: how morphology meets ecology. Netherlands J Zool 50, 261–277. [Google Scholar]
- Akaike H (1973) Information theory and an extension of the likelihood principle. 2nd Inter‐ Natl. Symp. Inf. Theory Budapest: Akadémia Kiadó. [Google Scholar]
- Angelaki DE, Cullen KE (2008) Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci 31, 125–150. [DOI] [PubMed] [Google Scholar]
- Arnold EN (1989) Towards a phylogeny and biogeography of the Lacertidae: relationships within an Old‐World family of lizards derived from morphology. Bull Br Nat Hist Ser Museum 55, 209–257. [Google Scholar]
- Arnold EN (1998) Structural niche, limb morphology and locomotion in lacertid lizards (Squamata, Lacertidae); a preliminary survey. Bull Nat Hist Museum Zool Ser 64, 63–89. [Google Scholar]
- Arnold N, Ovenden WD (2004) Collins Field guide to the reptiles and amphibians of Britain & Europe. New York: Harper Collins. [Google Scholar]
- Baeckens S, Edwards S, Huyghe K, et al. (2015) Chemical signalling in lizards: an interspecific comparison of femoral pore numbers in Lacertidae. Biol J Linn Soc 114, 44–57. [Google Scholar]
- Bar A, Haimovitch G (2011) A field guide to reptiles and amphibians of Israel. Herzilya: Pazbar Ltd 1989. [Google Scholar]
- Bates MF, Branch WR, Bauer AM, et al. (2014) Atlas and red list of the reptiles of South Africa, Lesotho and Swaziland.
- Bauwens D, Garland T, Castilla AM, et al. (1995) Evolution of sprint speed in lacertid lizards. Evolution 49, 848–863. [DOI] [PubMed] [Google Scholar]
- Benson RBJ, Starmer‐jones E, Close RA, et al. (2017) Comparative analysis of vestibular ecomorphology in birds. J Anat 231, 990–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener AA (2003) Animal Locomotion. Oxford: Oxford University Press. [Google Scholar]
- Billet G, Hautier L, Lebrun R (2015) Morphological diversity of the bony labyrinth (inner ear) in extant xenarthrans and its relation to phylogeny. J Mammal 96, 658–672. [Google Scholar]
- Bloomberg S, Garland T, Ives A (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. [DOI] [PubMed] [Google Scholar]
- Boistel R, Herrel A, Lebrun R, et al. (2011) Shake rattle and roll: the bony labyrinth and aerial descent in squamates. Integr Comp Biol 51, 957–968. [DOI] [PubMed] [Google Scholar]
- Brecko J, Huyghe K, Vanhooydonck B, et al. (2008) Functional and ecological significance of intraspecific variation in body size and shape in the lizard Podarcis melisellensis (Lacertidae). Biol J Linn Soc 94, 251–264. [Google Scholar]
- Carriot J, Brooks JX, Cullen KE (2013) Multimodal integration of self‐motion cues in the vestibular system: active versus passive translations. J Neurosci 33, 19 555–19 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costeur L, Grohé C, Aguirre‐fernández G, et al. (2018) The bony labyrinth of toothed whales reflects both phylogeny and habitat preferences. Sci Rep 8, 7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE (2012) The vestibular system: multimodal integration and encoding of self‐motion for motor control. Trends Neurosci 35, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KE, Brooks JX, Jamali M, et al. (2011) Internal models of self‐motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res 210, 377–388. [DOI] [PubMed] [Google Scholar]
- Davies KTJ, Bates PJJ, Maryanto I, et al. (2013) The evolution of bat vestibular systems in the face of potential antagonistic selection pressures for flight and echolocation. PLoS ONE 8, 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BV, Sherratt E, Losos JB, et al. (2017) Semicircular canals in Anolis lizards: ecomorphological convergence and ecomorph affinities of fossil species. R Soc Open Sci 4, 170 058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici P, Turesson H, Brodersen J, et al. (2008) Predator‐induced morphology enhances escape locomotion in crucian carp. Proc R Soc B Biol Sci 275, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley DF (1991) Counterexamples to parsimony and BIC. Ann Inst Stat Math 43, 505–514. [Google Scholar]
- Georgi JA, Sipla JS, Forster CA (2013) Turning semicircular canal function on its head: dinosaurs and a novel vestibular analysis. PLoS ONE 8, e58517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FR, Rezende EL, Grizante MB, et al. (2009) The evolution of jumping performance in anurans: morphological correlates and ecological implications. J Evol Biol 22, 1088–1097. [DOI] [PubMed] [Google Scholar]
- Goyens J, Aerts P (2018) Head stabilisation in fast running lizards. Zoology 127, 114–120. [DOI] [PubMed] [Google Scholar]
- Grohé C, Tseng ZJ, Lebrun R, et al. (2016) Bony labyrinth shape variation in extant Carnivora: a case study of Musteloidea. J Anat 228, 366–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrel A, Meyers JJ, Vanhooydonck B (2002) Relations between microhabitat use and limb shape in phrynosomatid lizards. Biol J Linn Soc 77, 149–163. [Google Scholar]
- Herrel A, Measey GJ, Vanhooydonck B, et al. (2011) Functional consequences of morphological differentiation between populations of the Cape Dwarf Chameleon (Bradypodion pumilum). Biol J Linn Soc 104, 692–700. [Google Scholar]
- Herrel A, Perrenoud M, Decamps T, et al. (2013) The effect of substrate diameter and incline on locomotion in an arboreal frog. J Exp Biol 216, 3599–3605. [DOI] [PubMed] [Google Scholar]
- Herrel A, Vasilopoulou‐Kampitsi M, Bonneaud C (2014) Jumping performance in the highly aquatic frog, Xenopus tropicalis: sex‐specific relationships between morphology and performance. PeerJ 2, e661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RW, Wyse GA, Anderson M (2016) Animal Physiology, 4th edn Sunderland: Sinauer Associates Inc. [Google Scholar]
- Hof L (1996) Scaling gait data to body size. Gait Posture 4, 222–223. [DOI] [PubMed] [Google Scholar]
- Huey RB, Bennett AF, John‐Alder H, et al. (1984) Locomotor capacity and foraging behawour of kalahari lacertid lizards. Anim Behav 32, 41–50. [Google Scholar]
- Jeffery N, Spoor F (2004) Prenatal growth and development of the modern human labyrinth. J Anat 204, 71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GM, Spells KE (1963) A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc R Soc Lond B Biol Sci 157, 403–419. [DOI] [PubMed] [Google Scholar]
- Labra A, Silva G, Norambuena F, et al. (2013) Acoustic features of the weeping lizard's distress call. Copeia 2016, 206–212. [Google Scholar]
- Lambert FM, Beck JC, Baker R, et al. (2008) Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval Xenopus. J Neurosci 28, 8086–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun R (2014) ISE‐MeshTools, a 3D interactive fossil reconstruction freeware. 12th Annu. Meet. EAVP, Torino.
- Losos JB, Ricklefs RE (2009) Adaptation and diversification on islands. Nature 457, 830–836. [DOI] [PubMed] [Google Scholar]
- Maddin HC, Sherratt E (2014) Influence of fossoriality on inner ear morphology: insights from caecilian amphibians. J Anat 225, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín J, Salvador A (1992) Tail loss consequences on habitat use by the Iberian rock lizard, Lacerta monticola . Oikos 65, 328–333. [Google Scholar]
- Mayer W, Beyerlein P (1999) Ecological niche segregation of seven sympatric lacertid lizards in the Peloponnese highlands. Natl Croat Museum 8, 339–344. [Google Scholar]
- Meiri S (2008) Evolution and ecology of lizard body sizes. Glob Ecol Biogeogr 17, 724–734. [Google Scholar]
- Mendiburu FD (2019) Agricolae: statistical procedures for agricultural research. R Package Version 1.3‐0.
- Muller M (1990) Relationships between semicircular duct radii with some implications for time constants. Netherlands J Zool 40, 173–202. [Google Scholar]
- Muller M (1999) Size limitations in semicircular duct systems. J Theor Biol 198, 405–437. [DOI] [PubMed] [Google Scholar]
- Opazo D, Velásquez N, Veloso A, et al. (2009) Frequency‐modulated vocalizations of Eupsophus queulensis (Anura: Cycloramphidae). J Herpetol 43, 657–664. [Google Scholar]
- Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401, 877–884. [DOI] [PubMed] [Google Scholar]
- Perier A, Lebrun R, Marivaux L (2016) Different level of intraspecific variation of the bony labyrinth morphology in slow‐ versus fast‐moving primates. J Mamm Evol 23, 353–368. [Google Scholar]
- Perry G (1999) The evolution of search modes: ecological versus phylogenetic perspectives. Am Nat 153, 98–109. [DOI] [PubMed] [Google Scholar]
- Pfaff C, Martin T, Ruf I (2015) Bony labyrinth morphometry indicates locomotor adaptations in the squirrel‐related clade (Rodentia, Mammalia). Proc Biol Sci 282, 20 150 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2012) xxxx. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- R Studio (2012) R Studio: integrated development environment for R, Version 0.97.390. Boston, MA: R Studio. [Google Scholar]
- Rabbitt R, Damiano E, Grant JW (2004) Biomechanics of the vestibular semicircular canals and otolith organs In: The Vestibular System. (eds Highstein S, Fay R, Popper A.), pp. 153–201. New York: Springer. [Google Scholar]
- Revell LJ (2012) phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3, 217–223. [Google Scholar]
- Richard BA, Wainwright PC (1995) Scaling the feeding mechanism of largemouth bass (Micropterus Salmoides): kinematics of prey capture. J Exp Biol 198, 419–433. [DOI] [PubMed] [Google Scholar]
- Ritzmann RE, Quinn RD, Fischer MS (2004) Convergent evolution and locomotion through complex terrain by insects, vertebrates and robots. Arthropod Struct Dev 33, 361–379. [DOI] [PubMed] [Google Scholar]
- South AB, Kenward RE (2001) Mate finding, dispersal distances and population growth in invading species: a spatially explicit model. Oikos 95, 53–58. [Google Scholar]
- Spoor F (2003) The semicircular canal system and locomotor behaviour, with special reference to hominin evolution. CFS Cour Forsch Senckenb 243, 93–104. [Google Scholar]
- Spoor F, Wood B, Zonneveld F (1994) Implications of early hominid labyrinthine morphology for evolution of human bipedal locomotion. Nature 369, 645–648. [DOI] [PubMed] [Google Scholar]
- Spoor F, Bajpai S, Hussain ST, et al. (2002) Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature 417, 163–166. [DOI] [PubMed] [Google Scholar]
- Spoor F, Garland T, Krovitz G, et al. (2007) The primate semicircular canal system and locomotion. Proc Natl Acad Sci USA 104, 10 808–10 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L, Berg D, Bloom FE, et al. (2013) Fundamental Neuroscience. Fourth Edi: Academic Press. [Google Scholar]
- Tadevosyan T (2007) The role of vegetation in microhabitat selection of syntopic lizards, Phrynocephalus persicus, Eremias pleskei, and Eremias strauchi from Armenia. Amphib Reptil 28, 444–448. [Google Scholar]
- Toro E, Herrel A, Irschick D (2004) The evolution of jumping performance in Caribbean Anolis lizards: solutions to biomechanical trade‐offs. Am Nat 163, 844–856. [DOI] [PubMed] [Google Scholar]
- Van Damme R, Vanhooydonck B (2002) Speed versus manoeuvrability: association between vertebral number and habitat structure in lacertid lizards. J Zool 258, 327–334. [Google Scholar]
- Van der Meer MH, Whiting MJ, Branch WR (2010) Ecology of Southern African Sandveld Lizards (Lacertidae, Nucras). Copeia 2010, 568–577. [Google Scholar]
- Vanhooydonck B, Van Damme R (1999) Evolutionary relationships between body shape and habitat use in lacertid lizards. Evol Ecol Res 1, 785–805. [Google Scholar]
- Vanhooydonck B, Van Damme R, Aerts P (2001) Speed and stamina trade‐off in lacertid lizards. Evolution 55, 1040–1048. [DOI] [PubMed] [Google Scholar]
- Vanhooydonck B, Herrel A, van Damme R (2007) Interactions between habitat use, behavior, and the trophic niche of lacertid lizards In: Lizard Eclogy. (eds Reilly SM, McBrayer LB, Miles DB.), pp. 427–449. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Verwaijen D (2007) Foraging of (lacertid) Lizards: A Test of the Syndrome Hypothesis. Antwerp: University of Antwerp. [Google Scholar]
- Vogel S (2013) Comparative Biomechanics: Life's Physical World, 2nd edn Princeton: Princeton University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Morphological information for all 24 species.
Table S2. Phylogenetic signal.
Table S3. Comparison of variation among and across species.


