Abstract
Hypermethylation of transcription factor activating enhancer-binding protein 2e (TFAP2E) has been reported to be associated with chemoresistance to 5-fluorouracil (5-FU) in gastric cancer (GC). In the present study, the molecular mechanism governing this chemoresistance was investigated. Drug-resistant human GC MGC-803/5-FU cells were established and TFAP2E expression and methylation levels were assessed. Autocrine exosomes from GC culture medium were isolated and characterized. MicroRNA (miRNA) microarray analysis was used to determine the miRNA expression profile of GC cell-derived exosomes. Exosomes collected from MGC-803/5-FU cells were co-cultured with control cells, and 5-Aza-2′-deoxycytidine (5Aza) was added into MGC-803/5-FU cells to investigate the relationship between TFAP2E, exosomes and chemosensitivity. In the present study, it was demonstrated that hypermethylation of TFAP2E resulted in its reduced expression and 5-FU chemoresistance in GC cells. miRNAs miR-106a-5p and miR-421 were highly expressed and regulated the chemoresistance induced by TFAP2E methylation. Target gene prediction using miRBase, TargetScan and PicTar revealed that E2F1, MTOR and STAT3 may be TFAP2E target genes in GC. Collectively, our data support an important role of exosomes and exosomal miRNAs in TFAP2E methylation-induced chemoresistance to 5-FU in GC. These results highlight their potential for miRNA-based therapeutics.
Keywords: gastric cancer, chemoresistance, TFAP2E, miRNA
Introduction
Globally, gastric cancer (GC) is the fifth leading cause of cancer and the third leading cause of cancer-related deaths, accounting for 5.7% of cases and 8.2% of deaths (1). Surgery remains the only curative therapy for stomach cancer. Patients with advanced GC are not eligible for surgery and are treated with chemotherapies, such as mitomycin, cisplatin, and 5-fluorouracil (5-FU) (2). However, chemoresistance has contributed to a poor 5-year survival rate in GC patients (3). Chemoresistance is a complex biological problem, and is facilitated by a number of events, including decreased drug uptake, increased drug efflux, changes in the level and structure of intracellular targets, and increased repair of DNA damage (3). The specific mechanism that regulates chemoresistance in GC is not well understood.
Recent studies have reported that transcription factor activating enhancer-binding protein 2e (TFAP2E) methylation may be involved in chemoresistance to 5-FU (4). Activating protein 2 (AP-2) is a family of closely related transcription factors, including AP-2α, AP-2β, AP-2γ, AP-2δ and AP-2ε. TFAP2E is highly homologous to other members of the family in its DNA binding and dimerization domains but less so in its N-terminal domain (5). TFAP2E is located on chromosome 1p34 and has 2 cytosine-phosphate-guanine (CpG) islands, which underscore the potential for regulation of gene expression by CpG methylation. Our previous study revealed that patients with GC also had TFAP2E hypermethylation and were resistant to fluorouracil-based chemotherapy (4). Methylation has a multifaceted link with miRNAs, and exosomes are rich in miRNAs (6). Therefore, the aim of the present study was to determine whether GC cells secreted exosomes and whether exosomal miRNAs were involved in chemoresistance to 5-FU.
Exosomes are cell-derived vesicles that are present in many eukaryotic fluids, including blood, urine, and in conditioned medium of cell cultures (7). Studies have revealed that exosomes can regulate tumor progression and chemosensitivity (8). Exosomes contain a large amount of proteins, a rich variety of mRNAs and miRNAs (9). Previous studies have also shown that miRNA gene promoters are frequent targets of aberrant DNA methylation (6). DNA methylation and miRNAs have been reported to be involved in the chemoresistance of GC (10), glioma (11) and hepatocellular carcinoma (12). Therefore, it was hypothesized that TFAP2E methylation leads to chemoresistance to 5-FU by regulating exosomal miRNAs in GC.
As aforementioned, our previous study indicated a potential role for TFAP2E hypermethylation in chemosensitivity, which may be exploited to develop new treatment strategies for patients with GC (4). In the present study, drug-resistant human GC MGC-803/5-FU cells were established and the mechanism underlying the development of GC chemoresistance to 5-FU was determined. Our analysis of the association between TFAP2E methylation and exosomal miRNAs may increase the development of chemoresistance in GC.
Materials and methods
Antibodies and reagents
Commercially available antibodies and reagents were as follows: Total exosome isolation reagent (cat. no. 4478359) (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), rabbit polyclonal anti-TFAP2E (1:15,000; cat. no. ab56295); rabbit polyclonal anti-CD63 (1:20,000; cat. no. ab68418); rabbit polyclonal anti-CD9 (1:50,000; cat. no. ab223052); rabbit monoclonal anti-CD81 (1:2,000; cat. no. ab109201); Rabbit polyclonal anti-GFP (1:1,000; cat. no. ab6556) (all from Abcam, Cambridge, MA, USA), PKH26 Red Fluorescent Cell Linker Mini kit (cat. no. MINI26) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), Annexin V-FITC apoptosis detection kit (cat. no. AD10), Cell Counting Kit-8 (CCK-8) (cat. no. CK04) (Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
Cell culture and lentivirus transfection
The GC cell line MGC-803 was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Drug-resistant subline, MGC-803/5-FU was successfully established from the parental MGC-803 cells as recently reported (13). MGC-803 was cultured as the control group in some of the experiments. MGC-803/5-FU was maintained in drug-free medium for 1 week prior to subsequent analysis, avoiding toxic effects. Cells used for exosome isolation were cultured in medium with 10% exosome-depleted serum.
TFAP2E was overexpressed via transfection of lenti-TFAP2E (GeneChem, Inc., Shanghai, China). MGC-803/5-FU were transfected with lenti-TFAP2E or lenti-NC at a confluence of 30–50%, >95% of the cells were viable 12 h later. The medium was then changed, the cells were incubated for a further 3 days, and passaged for further experiments. Transfection efficacies were assessed via western blotting.
Cell cytotoxicity and apoptosis assays
Cell cytotoxicity was determined by the CCK-8 according to the manufacturer's instructions. Cells were seeded in 96-well plates with or without the specific treatments, and incubated for 48 h. Then, 10 µl of CCK-8 solution was added into each well. Absorbance values were determined at 450 nm by a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
An equal number of cells was seeded and treated with or without the specific treatments. After 48 h, the cells were collected and resuspended in binding buffer. Apoptotic cells were double-stained by an Annexin V-FITC/PI or an Annexin V-APC/PI Apoptosis Detection kit following the manufacturer's instructions (Dojindo Molecular Technologies, Inc., Shanghai, China). Flow analysis was performed using FACSCalibur flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
TFAP2E methylation analysis
All samples in this research were measured in duplicate. Methylation-sensitive high-resolution melting analysis (MSHRM) was performed with precision melt analysis software to analyze the methylation level of TFAP2E in the cells. Methylation standards (100, 75, 50, 25, 10, 1 and 0%) were established by diluting unmethylated control DNA in a pool of 100% methylated one. Primers were listed as follows: Forward, 5′-GTTTTATTTTAGAAGCGGTTTT-3′ and reverse, 5′-CGAACGCTTACCTACAATCA-3′. The amplicon was located at the second CpG island of the TFAP2E gene in intron 3. The PCR mixture was prepared in a final volume of 20 ml, containing 10 ml LightCycler 480 HRM Master Mix, 2 ml of 25 mmol/l MgCl2, 10 mmol/l of primers, 50 ng DNA template, and ribonuclease-free H2O. The cycling protocol for MS-HRM analysis was initial denaturation at 95°C for 10 min followed by 50 cycles at 94°C for 10 sec, an annealing temperature for 15 sec, and extension at 72°C for 10 sec, followed by an MS-HRM step of 95°C for 1 min, 40°C for 1 min, and continuous acquisition between 65°C and 97°C at 1 min acquisition/0.2°C. The standard curves with known methylation ratios were obtained, and the methylation ratio of each sample via these standard curves was measured.
Exosome isolation and analysis
Exosomes were isolated from the conditioned medium by differential centrifugation. Briefly, the conditioned medium was centrifuged at 300 × g for 10 min and then at 2,000 × g for 30 min at 4°C to remove cells. Subsequently, this supernatant was centrifuged at 10,000 × g for 30 min to remove cell debris, followed by filtration through a 220-nm filter to remove particles with a diameter >200 nm. Then, exosomes were pelleted by ultracentrifugation at 100,000 × g for 70 min (14). MGC-803 in logarithmic phase was transfected with the lentivirus encoding green fluorescent protein (GFP), according to the manufacturer's instructions.
For exosome uptake research, they were labeled with PKH26 Fluorescent Cell Linker kits (Merck KGaA) according to the manufacturer's protocol. Then, exosomes were incubated with GC cells and examined under a confocal microscope.
Microarray analysis
Exosome pellets from 10 ml supernatant of cells were collected and homogenized in TRIzol (Thermo Scientific, Inc.). Total RNA was extracted as aforementioned. The miRNA microarray analysis was performed by Shanghai Biotechnology Corporation (Shanghai, China). The Affymetrix GeneChip miRNA 2.0 Array contains 15,644 probe sets, including 1,105 human mature miRNAs. The raw data were treated using the miRNA QCTool software (version 2.3.0; Petros Eikon, Inc., Orangeville, ON, Canada).
Quantitative real-time PCR
Two differentially expressed miRNAs (hsa-miR-421 and hsa-miR-106a-5p) identified by microarray in exosomes were selected for further validation by quantitative real-time reverse-transcription PCR (qRT-PCR). Total RNA and exosomes were extracted from cells using TRIzol reagent (Shanghai Sangong Pharmaceutical Co., Ltd., Shanghai, China). cDNA synthesis was performed with 1 mg RNA using ReverAid™ First Strand cDNA Synthesis kit (K1622; Fermentas; Thermo Fisher Scientific, Inc.). The oligo-nucleotide primers used are listed in Table I. Equal amounts of each reverse-transcription product (1 mg) were PCR amplified using DreamTaq™ Green PCR Master Mix (2X) (K1081; Fermentas; Thermo Fisher Scientific, Inc.) for 30 cycles consisting of 1 min at 95°C, 30 sec at 55°C, and 1 min 72°C. The amplified cDNA was run on 1% agarose gels and visualized by UV light. Each experiment was performed in duplicate and the results were standardized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Table I.
Primers used for RT-PCR analysis of gene expression.
| Gene symbol | Forward primer | Reverse primer | Accession no. |
|---|---|---|---|
| E2F1 | 5′-GGGGAGAAGTCACGCTATGA-3′ | 5′-CTCAGGGCACAGGAAAACAT-3′ | NM_005225.3 |
| MTOR | 5′-CGCTGTCATCCCTTTATCG-3′ | 5′-ATGCTCAAACACCTCCACC-3 | NM_004958.3 |
| STAT3 | 5′-ACCTGCAGCAATACCATTGAC-3′ | 5′-AAGGTGAGGGACTCAAACTGC-3 | NM_003150.3 |
| TFAP2E | 5′-CAGAGAGAAGTGGGCAGGAG-3′ | 5′-AGGACAGACAGCAACAGGACT-3′ | NM_178548.4 |
| miR-106a-5p | 5′-AAAAGTGCTTACAGTGCAGGTAG-3′ | 5′-GAAAAGTGCTTACAGTGCAGGT-3′ | – |
| miR-421 | 5′-TATGGTTGTTCTGCTCTCTGTGTC-3′ | 5′-CTCACTCACATCAACAGACATTAATT-3′ | – |
E2F1, transcription factor E2F-1; MTOR, mammalian target of rapamycin; STAT3, signal transducer and activator of transcription 3; TFAP2E, transcription factor activating enhancer-binding protein 2e.
Western blot analysis
Total proteins of the isolated exosomes and cells were extracted using a protein extraction kit, according to the manufacturer's protocols. Briefly, the samples were washed with phosphate-buffered saline and lysed in RIPA lysis buffer containing phenylmethylsulfonyl fluoride. The protein concentration was measured using a Bicinchoninic Acid protein assay kit (Sangon Biotech Co., Ltd., Shanghai, China), and 2 mg/ml bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) was used as the standard. Subsequently, 50 µg total protein was loaded in each lane. Proteins were separated by 10% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. The membranes were then blocked with 5% non-fat milk at room temperature for 1 h, and then incubated at 4°C overnight with a primary antibody. After washing, the blots were incubated with Alexa Fluor-conjugated goat-anti-rabbit antibody (1:10,000; cat. no. G-21234; Fermentas; Thermo Fisher Scientific, Inc.) at 37°C for 1 h. Proteins were visualized using an enhanced chemiluminescence kit, and images were analyzed using a Bio-Rad gel imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data were expressed as the mean ± (standard deviation) SD. Wherever appropriate, the data were subjected to unpaired two-tailed Student's t-test. The differences between the experimental groups were evaluated using one-way analysis of variance (ANOVA) followed by a Bonferroni's post hoc test to allow for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
TFAP2E methylation is involved in chemoresistance to 5-FU
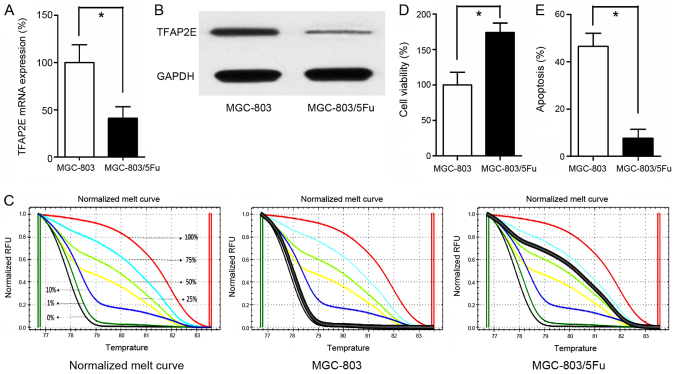
TFAP2E gene expression was significantly lower in the MGC-803/5-FU group than in the MGC-803 group (0.41-fold, P<0.001; Fig. 1A). In addition to reduced gene expression, the TFAP2E protein level was also assessed. As anticipated, the TFAP2E protein level was higher in the MGC-803 group than in the MGC-803/5-FU group (3.42-fold, P<0.001; Fig. 1B). In addition to TFAP2E gene and protein expression level, the methylation status of TFAP2E was detected using MSHRM. First, the normalized melt curve for TFAP2E methylation level was generated. Percentage of methylation (PM) values were then calculated according to the following formula, which also served as a surrogate measure of the methylation level of AP-2E in our study (15):
Figure 1.
TFAP2E methylation status and expression level in MGC-803 and MGC-803/5-FU cells. (A) qRT-PCR detection of TFAP2E mRNA expression in MGC-803 and MGC-803/5-FU cells, GAPDH was assayed as an internal control. (B) Representative western blotting results. (C) MS-HRM analysis for TFAP2E methylation, including standard curves. (D and E) Cell viability and apoptosis were assessed by cell cytotoxicity and apoptosis assays, respectively. All the results were the average from at least 3 independent experiments. Mean ± SD; *P<0.05, two-tailed Student's t-test. TFAP2E, transcription factor activating enhancer-binding protein 2e; 5-FU, 5-fluorouracil; MS-HRM, methylation-sensitive high-resolution melting; SD, standard deviation.
Our data revealed that the drug-resistant MGC-803/5-FU cell line had a higher PM value (1.042±2.552%) than the control group (66.037±11.201%) (P<0.001; Fig. 1C). Additionally, the cells were treated with 10 µg/ml 5-FU for 48 h in 96-well plates. Cell viability and apoptosis assays indicated that treatment with 5-FU significantly decreased the viability and significantly increased apoptosis of the MGC-803 group in comparison to the MGC-803/5-FU group (viability, 100.00±17.944% vs. 173.857±13.558%, P<0.001; apoptosis, 46.543±5.521% vs. 7.729±3.683%, P<0.001, MGC-803 vs. MGC-803/5-FU; Fig. 1D and E).
qRT-PCR analysis of exosomal microRNAs and target gene analysis
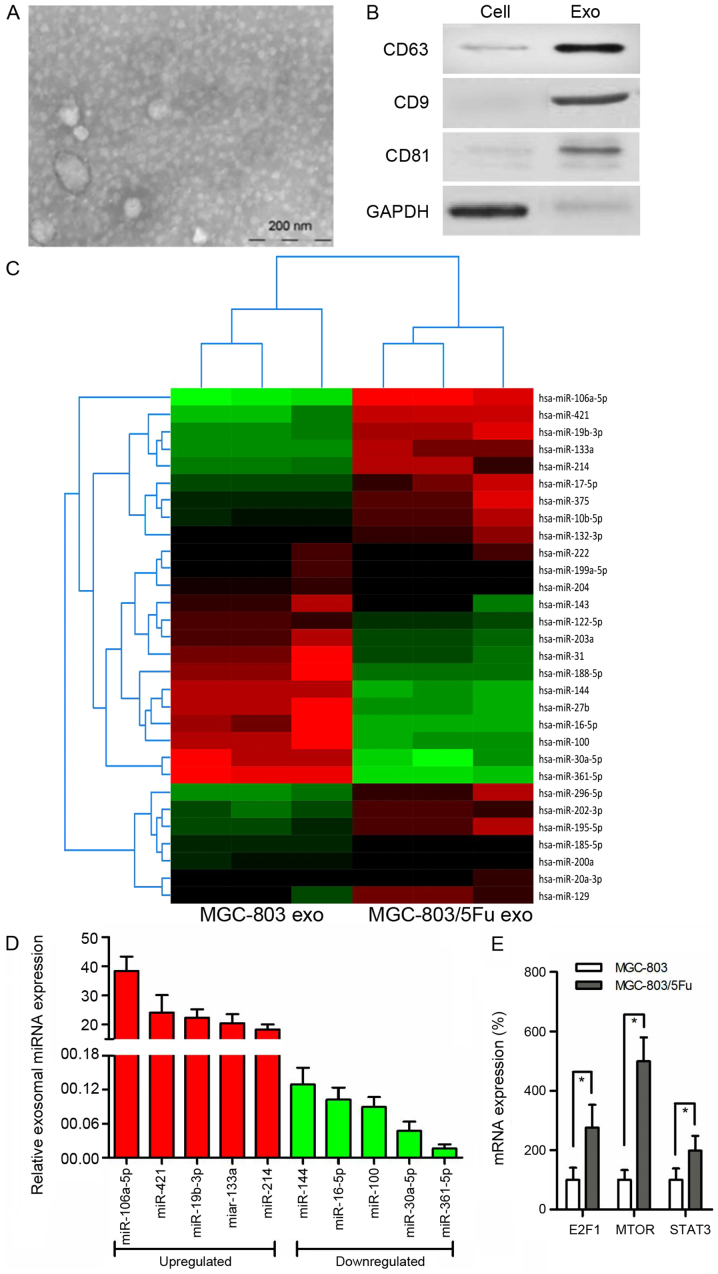
Increasing evidence indicates that tumor-derived exosomes are involved in chemoresistance of GC. We used electron microscopy and western blot analysis to characterize exosomes. The purified exosomes were small round vesicles with diameters ranging from 80 to 120 nm, and expressed the exosomal markers CD9, CD63 and CD81 (Fig. 2A and B). To investigate the functional role of exosomes in chemoresistance in GC cells, miRNA microarray analysis was performed using RNA samples extracted from MGC-803 exosomes and MGC-803/5-FU exosomes (Fig. 2C). Notably, exosomal miR-106a-5p, miR-421, miR-19b-3p, miR-133a, and miR-214 were more highly expressed, whereas miR-144, miR-16-5p, miR-100, miR-30a-5p, and miR-361-5p were more reduced in MGC-803/5-FU exosomes in comparison with MGC-803 exosomes (Fig. 2D). TargetScan 7.1 (version 7.1; http://www.targetscan.org/vert_71/) was used to predict the target genes for these 10 miRNAs. This analysis predicted 2,119 genes as the target mRNAs, and KEGG pathway analysis was performed. According to our data, 90 genes were associated with cancer (P<0.001), and 24 of these genes were associated with chemoresistance. Among these 24 genes, 10 genes [CBLB (16), E2F1 (17), MET (18), SKP2 (19), FGFR3 (20), HIF1A (21), MTOR (22), MAPK1 (23), RHOA (24), and STAT3 (25)] have been revealed to be involved in GC, and 7 of them [CBLB (16), E2F1 (17), HIF1A (21), MTOR (22), MAPK1 (23), RHOA (24), and STAT3 (25)] have been reportedly associated with 5-FU. The relationship between methylation and drug resistance was then explored. Only E2F1 (17), MTOR (22) and STAT3 (26) have been reported to be associated with methylation. E2F1 and STAT3 are targets of miR-106a-5p, while MTOR is a target of miR-421. Therefore, their expression was evaluated. There were higher levels of these 3 genes in the MGC-803/5-FU group in comparison to the MGC-803 group (E2F1, 2.76-fold, P<0.001; MTOR, 4.99-fold, P<0.001; STAT3, 1.98-fold, P= 0.003) (Fig. 2E).
Figure 2.
Exosomes derived from cells and analyzed using microarray. (A) Representative micrograph of scanning electronic microscope of exosomes. Bar indicates 200 nm. (B) Western blotting for the exosome-related proteins. (C) Heat maps revealing the relative expression of miRNAs in exosomes isolated from MGC-803 and MGC-803/5-FU cells. The horizontal axis indicates the sample name, while the vertical axis presents the name of miRNAs. Heat map colors represent relative miRNA expression: Red-high expression; green-low expression. (D) The most significant upregulated and downregulated miRNAs with upregulation and downregulation difference multiples >1.2 fold (all P-value <0.01). (E) Expression of three predicted target mRNAs in MGC-803 and MGC-803/5-FU cells. All the results were the average from at least 3 independent experiments. Mean ± SD; *P<0.05, two-tailed Student's t-test. 5-FU, 5-fluorouracil; SD, standard deviation.
Exosomes mediate target gene expression and chemoresistance to 5-FU
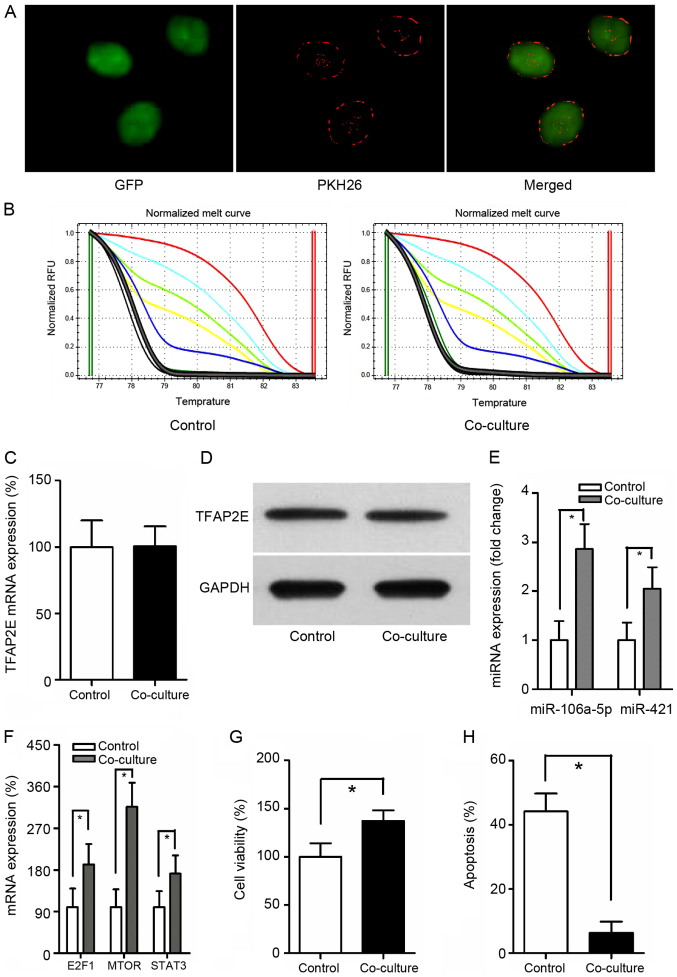
Exosomes from MGC-803/5-FU cells were obtained and co-cultured with MGC-803 cells. It has been reported that exosomes can be transferred from donor cells to recipient cells (27). To better observe the interaction, GFP was employed and exposed to PKH26-labeled exosomes for 24 h. When MGC-803-derived exosomes were labeled with PKH26 (red fluorescent dye), a red staining on the cells could be observed by fluorescence microscopy, suggesting exosome binding and incorporation (Fig. 3A). TFAP2E PM of the MGC-803 (control group) and the co-culture group were assessed. Notably, there was no significant difference between these 2 groups (P=0.549) (Fig. 3B). Similarly, no statistical significance in TFAP2E gene and protein expression levels could be observed in the present study (P=0.953, 0.771, respectively) (Fig. 3C and D), indicating that exosomes may be downstream of and therefore, not directly involved in TFAP2E methylation. The expression of miRNAs was then assessed. The data revealed that the expression of miR-106a-5p and miR-421 were increased with the treatment of MGC-803/5-FU exosomes (miR-106a-5p: P<0.001; miR-421: P=0.001) (Fig. 3E). The expression of the predicted target genes was also assessed. The target genes were higher in the co-culture group than in the control group (E2F1, 1.92-fold, P=0.004; MTOR, 3.16- fold, P<0.001; STAT3, 1.72- fold, P=0.007) (Fig. 3F). Cell viability and apoptosis assays indicated that treatment with 5-FU resulted in a significant decrease of viability and a significant increase of apoptosis in the MGC-803 group than in the co-culture group (viability, 100.00±14.024% vs. 137.286±11.011%, P<0.001; apoptosis, 44.129±5.588% vs. 6.300±3.492%, P<0.001, control vs. co-culture) (Fig. 3G and H).
Figure 3.
TFAP2E, miRNA and mRNA differences in MGC-803 cells co-cultured with exosomes derived from MGC-803/5-FU. (A) Representative confocal microscopy of GFP exposed to PKH26-labeled exosomes. Magnification, ×400. (B) MS-HRM analysis for TFAP2E methylation. (C) qRT-PCR detection of TFAP2E mRNA expression. (D) Western blot analysis of TFAP2E protein level. (E and F) Differences in miRNAs and mRNAs between MGC-803 cells and the co-cultured group. (G and H) Cell viability and apoptosis were assessed by cell cytotoxicity and apoptosis assays, respectively. All the results were the average from at least 3 independent experiments. Mean ± SD; *P<0.05, two-tailed Student's t-test. TFAP2E, transcription factor activating enhancer-binding protein 2e; 5-FU, 5-fluorouracil; GFP, green fluorescent protein; MS-HRM, methylation-sensitive high-resolution melting; SD, standard deviation.
Demethylation reduces chemoresistance by suppressing miRNA expression
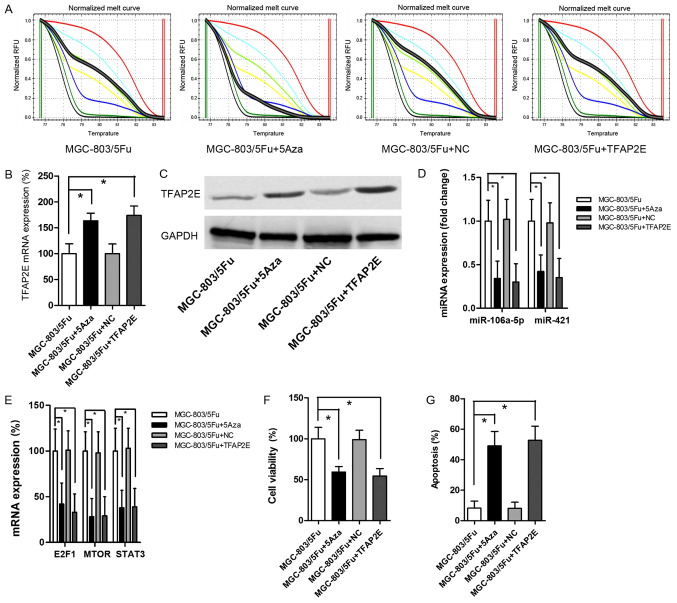
MGC-803/5-FU was used as the control group for this experiment. Cells were treated with 10 µM 5-Aza-2-deoxycytidine (5Aza) to establish a baseline demethylation status as previously reported (28). The data revealed that 5Aza decreased TFAP2E PM significantly (P<0.001) (Fig. 4A). After demethylation, there were significant increases in TFAP2E mRNA expression (1.62-fold) and protein expression (1.80-fold) in MGC-803/5-FU cells (Fig. 4B and C). Additionally, miR-106a-5p and miR-421 were significantly decreased after 5Aza treatment (0.34-, 0.42-fold, respectively; P=0.001, 0.001, respectively; Fig. 4D). The levels of the predicted target gene were also significantly decreased (E2F1, 0.42-fold, P=0.001; MTOR, 0.28-fold, P<0.001; STAT3, 0.38-fold, P<0.001; Fig. 4E). 5-FU chemoresistant tests indicated that 5Aza resulted in a significant decrease in viability and a significant increase in apoptosis (viability, 0.59-fold, P<0.001; apoptosis, 5.99-fold, P<0.001; Fig. 4F and G). MGC-803/5-FU cells were also transfected with a recombinant plasmid to directly upregulate TFAP2E expression. The results revealed a significant increase in TFAP2E expression after transient transfection (Fig. 4B and C). However, there was no obvious difference in TFAP2E PM in comparison to the control group (Fig. 4A). Despite the low methylation rate, high expression of TFAP2E significantly suppressed miR-106a-5p, miR-421, the levels of predicted target genes, and cell viability (Fig. 4D-F). These results recapitulated the effects of 5Aza treatment.
Figure 4.
Effects of transfection and demethylation on TFAP2E, miRNA and mRNA expression and chemoresistance to 5-FU. (A) MS-HRM analysis for TFAP2E methylation after the treatment of 5Aza. (B) qRT-PCR detection of TFAP2E mRNA expression. (C) Western blot analysis of TFAP2E protein level. (D and E) Differences in miRNAs and mRNAs in MGC-803/5-FU cells with or without the treatment of 5Aza or transfection. (F and G) Cell viability and apoptosis were assessed by cell cytotoxicity and apoptosis assays, respectively. All the results were the average from at least 3 independent experiments. Mean ± SD; *P<0.05, two-tailed Student's t-test. TFAP2E, transcription factor activating enhancer-binding protein 2e; 5-FU, 5-fluorouracil; MS-HRM, methylation-sensitive high-resolution melting; 5Aza, 5-Aza-2-deoxycytidine; SD, standard deviation.
Discussion
TFAP2E hypermethylation has been reported to be associated with clinical no-response to chemotherapy in colorectal cancer, and targeting of DKK4 may be an option to overcome TFAP2E-mediated drug resistance (15). It has been reported that 5-FU-resistant gastric cancer (GC) cell lines MKN45 and MKN28 have TFAP2E hypermethylation (29). In the present study, whether TFAP2E methylation affected chemoresistance to 5-FU in MGC-803 cells, was investigated. Our previous study revealed that GC patients with TFAP2E hypermethylation were resistant to fluorouracil-based chemotherapy (4). However, the mechanism has not been fully elucidated. In the present study, a drug-resistant subline MGC-803/5-FU was successfully established, and high TFAP2E PM, low TFAP2E gene and protein expression in this subline were observed, which was consistent with previous research. TFAP2E methylation resulted in reduction of its gene expression. Additionally, the functional role of TFAP2E methylation was investigated in chemoresistance to 5-FU in GC cells.
Studies have demonstrated an increasingly important role for exosomes in GC, as mediators of cell-to-cell crosstalk in the tumor microenvironment (27). Recent studies have indicated that exosomal miRNAs are associated with GC chemoresistance by transferring a variety of RNAs. Zheng et al (30), demonstrated that exosomal transfer of tumor-associated macrophage-derived miR-21 conferred DDP resistance to GC. However, 5-FU is the most frequently used chemotherapy drug in the treatment of GC. Therefore, miRNA microarray analysis was conducted and the target mRNAs were predicted to explore the relationship between miRNAs and chemoresistance to 5-FU.
In our study, 2,119 genes were predicted as target mRNAs. Notably, only 3 genes were previously reported to be associated with chemoresistance to 5-FU and gene methylation in GC. E2F1, a transcription factor, plays a crucial role in the control of cell cycle and tumor suppression and is also a target of the transforming proteins of small DNA tumor viruses. Tahara et al (17), have reported that overexpression of E2F1 promoted the development of MDR in GC, suggesting that E2F1 may represent a viable target for GC therapy. Mammalian target of rapamycin (MTOR) regulates cell growth, cell proliferation, cell motility, cell survival, and transcription. It has been reported that MTOR promoted the development of GC, and its inhibitor interacted with 5-FU in a synergistic manner in scirrhous GC cells by the activation of apoptosis signals (31). Signal transducer and activator of transcription 3 (STAT3) mediates the expression of a variety of genes in response to cell stimuli, and thus plays a key role in many cellular processes such as cell growth and apoptosis. Notably, STAT3 is overactivated in GC stem-like cells (32). Therefore, these 3 mRNAs have been implicated in GC tumorigenesis.
However, the regulatory relationship between TFAP2E and exosomal miRNAs is unclear. Notably, it was revealed that MGC-803 cells co-cultured with exosomes derived from MGC-803/5-FU had increased expression of miR-106a-5p and miR-421. Additionally, predicted target mRNAs were increased. Although co-cultured cells appeared to be chemoresistant to 5-FU, TFAP2E expression and its PM value were not significantly different from the control, indicating that exosomal miRNAs could not meditate methylation of TFAP2E. Therefore, it was hypothesized that TFAP2E methylation may be upstream and regulate the expression of exosomal miRNAs.
In order to assess our hypothesis, the methylation status of MGC-803/5-FU cells was reduced using 5Aza, a commonly used demethylation drug, and subsequently the expression of miR-106a-5p, miR-421 and their predicted target mRNAs was assessed. As anticipated, TFAP2E expression was increased and its PM value was significantly decreased with 5Aza treatment. However, 5Aza significantly suppressed the expression of the aforementioned miRNAs and their predicted target mRNAs, indicating that changes in TFAP2E methylation may result in the decrease of miRNAs. The ultimate result was the increased chemosensitivity of GC cells to 5-FU. These data were also consistent with our previous hypothesis. Additionally, results of a TFAP2E rescue experiment using transfection of a TFAP2E- expressing plasmid supported our hypothesis. Although increased TFAP2E expression did not affect its methylation status, the results indicated that hypermethylation may act as an upstream regulator of its expression.
There were some limitations to the present study. Inhibitors and mimics of miRNAs were not used in our study, and our analysis methods used to predict target genes may have filtered out some important or unreported genes. Additionally, other 5-FU-resistant GC cell lines were not used in the present study and should be analyzed in future research. Future studies are also required to more comprehensively assess the functional role of E2F1, MTOR, and STAT3 in regulating chemoresistance in GC cells.
In summary, our data indicated that chemoresistance to 5-FU in GC cells may be facilitated by TFAP2E hypermethylation-induced release of miRNA-containing exosomes. These results indicated that targeting of miRNAs may be a viable therapeutic strategy for GC patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Suzhou Project (grant no. kjxw2018015).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
XH conceived and designed the study. SJ and ZJ acquired the data. WX analyzed and interpreted the data. LW and LD performed statistical analyses. SJ and WX drafted and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Venerito M, Vasapolli R, Rokkas T, Malfertheiner P. Gastric cancer: Epidemiology, prevention, and therapy. Helicobacter. 2018;23(Suppl 1):e12518. doi: 10.1111/hel.12518. [DOI] [PubMed] [Google Scholar]
- 3.Marin JJ, Al-Abdulla R, Lozano E, Briz O, Bujanda L, Banales JM, Macias RI. Mechanisms of resistance to chemotherapy in gastric cancer. Anticancer Agents Med Chem. 2016;16:318–334. doi: 10.2174/1871520615666150803125121. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Du N, Li J, Zhou J, Tao G, Sun S, He J. Transcription factor AP2epsilon: A potential predictor of chemoresistance in patients with gastric cancer. Technol Cancer Res Treat. 2016;15:285–295. doi: 10.1177/1533034615577028. [DOI] [PubMed] [Google Scholar]
- 5.Tummala R, Romano RA, Fuchs E, Sinha S. Molecular cloning and characterization of AP-2 epsilon, a fifth member of the AP-2 family. Gene. 2003;321:93–102. doi: 10.1016/S0378-1119(03)00840-0. [DOI] [PubMed] [Google Scholar]
- 6.Chhabra R. miRNA and methylation: A multifaceted liaison. Chembiochem. 2015;16:195–203. doi: 10.1002/cbic.201402449. [DOI] [PubMed] [Google Scholar]
- 7.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes. From biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Brinton LT, Sloane HS, Kester M, Kelly KA. Formation and role of exosomes in cancer. Cell Mol Life Sci. 2015;72:659–671. doi: 10.1007/s00018-014-1764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: A common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 10.Toiyama Y, Okugawa Y, Goel A. DNA methylation and microRNA biomarkers for noninvasive detection of gastric and colorectal cancer. Biochem Biophys Res Commun. 2014;455:43–57. doi: 10.1016/j.bbrc.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B, Bian EB, Li J, Li J. New advances of microRNAs in glioma stem cells, with special emphasis on aberrant methylation of microRNAs. J Cell Physiol. 2014;229:1141–1147. doi: 10.1002/jcp.24540. [DOI] [PubMed] [Google Scholar]
- 12.Anwar SL, Lehmann U. DNA methylation, microRNAs, and their crosstalk as potential biomarkers in hepatocellular carcinoma. World J Gastroenterol. 2014;20:7894–7913. doi: 10.3748/wjg.v20.i24.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang H, Zeng L, Wang J, Zhang X, Ruan Q, Wang J, Cui S, Yang D. Reversal of 5-fluorouracil resistance by EGCG is mediate by inactivation of TFAP2A/VEGF signaling pathway and down-regulation of MDR-1 and P-gp expression in gastric cancer. Oncotarget. 2017;8:82842–82853. doi: 10.18632/oncotarget.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015:319–323. doi: 10.1101/pdb.top074476. [DOI] [PubMed] [Google Scholar]
- 15.Giovannetti E, Codacci-Pisanelli G, Peters GJ. TFAP2E-DKK4 and chemoresistance in colorectal cancer. N Engl J Med. 2012;366:966. doi: 10.1056/NEJMc1201170. [DOI] [PubMed] [Google Scholar]
- 16.Feng D, Ma Y, Liu J, Xu L, Zhang Y, Qu J, Liu Y, Qu X. Cbl-b enhances sensitivity to 5-fluorouracil via EGFR- and mitochondria-mediated pathways in gastric cancer cells. Int J Mol Sci. 2013;14:24399–24411. doi: 10.3390/ijms141224399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tahara M, Ochiai A, Fujimoto J, Boku N, Yasui W, Ohtsu A, Tahara E, Yoshida S. Expression of thymidylate synthase, thymidine phosphorylase, dihydropyrimidine dehydrogenase, E2F-1, Bak, Bcl-X, and Bcl-2, and clinical outcomes for gastric cancer patients treated with bolus 5-fluorouracil. Oncol Rep. 2004;11:9–15. [PubMed] [Google Scholar]
- 18.Peng Z, Li Z, Gao J, Lu M, Gong J, Tang ET, Oliner KS, Hei YJ, Zhou H, Shen L. Tumor MET expression and gene amplification in chinese patients with locally advanced or metastatic gastric or gastroesophageal junction cancer. Mol Cancer Ther. 2015;14:2634–2641. doi: 10.1158/1535-7163.MCT-15-0108. [DOI] [PubMed] [Google Scholar]
- 19.Wei Z, Jiang X, Liu F, Qiao H, Zhou B, Zhai B, Zhang L, Zhang X, Han L, Jiang H, et al. Downregulation of Skp2 inhibits the growth and metastasis of gastric cancer cells in vitro and in vivo. Tumour Biol. 2013;34:181–192. doi: 10.1007/s13277-012-0527-8. [DOI] [PubMed] [Google Scholar]
- 20.Piro G, Carbone C, Cataldo I, Di Nicolantonio F, Giacopuzzi S, Aprile G, Simionato F, Boschi F, Zanotto M, Mina MM, et al. An FGFR3 autocrine loop sustains acquired resistance to trastuzumab in gastric cancer patients. Clin Cancer Res. 2016;22:6164–6175. doi: 10.1158/1078-0432.CCR-16-0178. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Zhuang M, Zhong C, Peng J, Wang X, Li J, Chen Z, Huang Y. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1α signaling pathway. Oncol Rep. 2015;33:457–463. doi: 10.3892/or.2014.3550. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Jiang Y, Huang J, Chen H, Liao Y, Yang Z. CISD2 enhances the chemosensitivity of gastric cancer through the enhancement of 5-FU-induced apoptosis and the inhibition of autophagy by AKT/mTOR pathway. Cancer Med. 2017;6:2331–2346. doi: 10.1002/cam4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong HL, Zhou SW, Sun AH, He Y, Li J, Yuan X. MicroRNA197 reverses the drug resistance of fluorouracilinduced SGC7901 cells by targeting mitogenactivated protein kinase 1. Mol Med Rep. 2015;12:5019–5025. doi: 10.3892/mmr.2015.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang WK, Lee I, Park C. Characterization of RhoA-mediated chemoresistance in gastric cancer cells. Cancer Res Treat. 2005;37:251–256. doi: 10.4143/crt.2005.37.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu GY, Tang XJ. Troxerutin (TXN) potentiated 5-Fluorouracil (5-Fu) treatment of human gastric cancer through suppressing STAT3/NF-κB and Bcl-2 signaling pathways. Biomed Pharmacother. 2017;92:95–107. doi: 10.1016/j.biopha.2017.04.059. [DOI] [PubMed] [Google Scholar]
- 26.To KF, Chan MW, Leung WK, Ng EK, Yu J, Bai AH, Lo AW, Chu SH, Tong JH, Lo KW, et al. Constitutional activation of IL-6-mediated JAK/STAT pathway through hypermethylation of SOCS-1 in human gastric cancer cell line. Br J Cancer. 2004;91:1335–1341. doi: 10.1038/sj.bjc.6602133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: Small particle, big player. J Hematol Oncol. 2015;8:33. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seelan RS, Mukhopadhyay P, Pisano MM, Greene RM. Effects of 5-Aza-2′-deoxycytidine (decitabine) on gene expression. Drug Metab Rev. 2018;50:193–207. doi: 10.1080/03602532.2018.1437446. [DOI] [PubMed] [Google Scholar]
- 29.Hong Y, Zhang J, Zhuang M, Li W, Wu P, Li R, Hu N, Bian B, Song Z, Wu F. Efficacy of decitabine-loaded gelatinases-stimuli nanoparticles in overcoming cancer drug resistance is mediated via its enhanced demethylating activity to transcription factor AP-2 epsilon. Oncotarget. 2017;8:114495–114505. doi: 10.18632/oncotarget.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. doi: 10.1186/s13046-017-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuzaki T, Yashiro M, Kaizaki R, Yasuda K, Doi Y, Sawada T, Ohira M, Hirakawa K. Synergistic antiproliferative effect of mTOR inhibitors in combination with 5-fluorouracil in scirrhous gastric cancer. Cancer Sci. 2009;100:2402–2410. doi: 10.1111/j.1349-7006.2009.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajimoradi M, Mohammad Hassan Z, Ebrahimi M, Soleimani M, Bakhshi M, Firouzi J, Samani FS. STAT3 is overactivated in gastric cancer stem-like cells. Cell J. 17:617–628. doi: 10.22074/cellj.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.