Abstract
Acute lung injury (ALI) is a major cause of morbidity and mortality globally, and is characterized by widespread inflammation in the lungs. Increased production of reactive oxygen species is hypothesized to be associated with ALI. Matrine and lycopene are active products present in traditional Chinese medicine. Matrine is an effective inhibitor of inflammation, whereas lycopene decreases lipid peroxidation. Therefore, it was hypothesized that combinatorial treatment with matrine and lycopene may provide synergistic protection against ALI. In the present study, mice were treated with dexamethasone (DEX; 5 mg/kg), matrine (25 mg/kg), lycopene (100 mg/kg), and matrine (25 mg/kg) + lycopene (100 mg/kg) for 7 days prior to injury induction using lipopolysaccharide (LPS; 5 mg/kg) for 6 h. Lung tissues were collected following the sacrifice of the mice and hematoxylin and eosin staining was used for histological analysis. Malondialdehyde (MDA), glutathione (GSH) and myeloperoxidas (MPO) levels were examined by respective kits. The expressions of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were evaluated by ELISA. The expressions of IκBα and NF-κB p65 were examined by reverse transcription-quantitative polymerase chain reaction, western blotting and immunohistochemistry. The results indicated that the combined treatment exhibited a similar effect to DEX, both of which attenuated lung structural injuries, downregulated the expressions of IL-6, TNF-α, MPO and MDA, and upregulated that of GSH. Furthermore, the combined treatment and DEX inhibited NF-κB p65 activation. The present study revealed that combined treatment with matrine and lycopene exhibited protective effects on an LPS-induced mouse model of ALI, suggesting that they may serve as a potential alternative to glucocorticoid therapy for ALI.
Keywords: matrine, lycopene, acute lung injury, lipopolysaccharides, inflammation
Introduction
Acute lung injury (ALI) is a serious respiratory condition with high morbidity (1) and mortality (~35%) rates (2). It is characterized by damage to alveolar capillary membranes, atelectasis of lung airspaces, and infiltration of neutrophils and protein-rich fluid into the alveolar space (3–5). The mechanisms underlying ALI have not been fully determined. Potential mechanisms include inflammatory reactions (6), apoptosis (7,8) and redox imbalance (9). As a result, the majority of studies have focused on the imbalance of proinflammatory/anti- inflammatory mediators and oxidation/reduction. Neutrophils have important roles in inflammatory processes; neutrophil transepithelial migration is an important pathological feature of ALI (10). Excessive or prolonged activation of neutrophils results in increased permeability of the alveolar/vascular barrier (10). Furthermore, neutrophils release proinflammatory and proapoptotic factors, damaging adjacent cells (10). In addition to neutrophils, free radicals also can damage lung structures (9). Massive production of free radicals leads to lipid peroxidation in lung tissues, promoting the apoptosis of alveolar epithelial cells and vascular endothelial cells (9). During the process, apoptosis induces oxidative stress (7,8). This repeated cycle eventually leads to lung injury (9).
Matrine (MAT; Fig. 1A), a major active component extracted from the traditional Chinese herb Sophora flavescens Ait, is frequently used to treat diseases such as hepatitis, enteritis and atopic dermatitis in China (11). MAT exhibits various biological properties, of which immune modulation and anti-inflammation are the most prominent (12,13). The carotene family is known to suppress oxidative damage by activating antioxidant enzymes and the stimulating the immune system (14). Lycopene (LY; Fig. 1B) is a member of the carotene family (15). As it has been reported that ALI involves superoxide radicals and inflammatory processes (16,17), it was hypothesized that combined treatment with MAT and LY may protect the lungs from ALI. Therefore, in the present study, the effects of MAT, LY and MAT + LY on lipopolysaccharide (LPS)-induced ALI in mice were determined, and the mechanisms were preliminarily investigated. In addition, the efficacy of MAT, LY and MAT + LY were compared with dexamethasone (DEX), to explore the potential use of these compounds as alternatives to glucocorticoid therapy.
Figure 1.
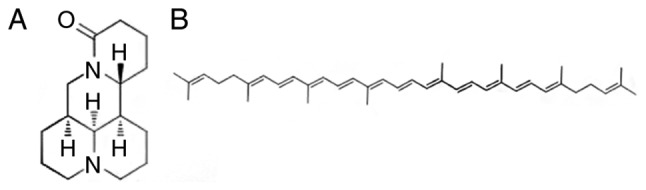
Chemical structures of matrine and lycopene. (A) Matrine is one of the main alkaloid constituents in Sophora flavescens Ait. (B) Lycopene is a carotenoid compound.
Materials and methods
Chemicals
MAT and LY (>98% purity) were obtained from the pharmaceutics laboratory of the Logistics University of Chinese People's Armed Police Force (PAPF; Tianjin, China). DEX was provided by the Affiliated Hospital of Logistics University of Chinese PAPF. LPS (055:B5) was purchased from Sigma-Aldrich (Merck KGaA). Antibodies used during the study included rabbit anti-IκBα (cat. no. CY5026; Abways Technology, Inc.), rabbit anti-phosphorylated (p)-IκBα (cat. no. 2859; Cell Signaling Technology, Inc.), rabbit anti-NF-κB p65 (cat. no. 8242; Cell Signaling Technology, Inc.), rabbit anti-p-NF-κB p65 (cat. no. 3033; Cell Signaling Technology, Inc.), and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat. no. S0001; Affinity Biosciences). ELISA kits for the detection of interleukin-6 (IL-6; cat. no. E-EL-M0044c) and tumor necrosis factor-α (TNF-α; cat. no. E-EL-M0049c) were purchased from Elabscience Biotechnology Co., Ltd. Kits for detecting the activity of malondialdehyde (MDA; cat. no. A003-1), glutathione (GSH; cat. no. A006-2) and myeloperoxidase (MPO; cat. no. A044) were purchased from Nanjing Jiancheng Bio-Engineering Institute Co., Ltd. TRIzol reagent and SuperRT One Step RT-PCR Kit (CW0742) for reverse transcription-polymerase chain reaction (RT-PCR) were purchased from Beijing CoWin Biotech Co., Ltd. Primers were purchased from Integrated DNA Technologies, Inc. BCA Protein Assay kit (cat. no. PC0020) and SDS-PAGE Gel Kit (cat. no. P1200) were purchased from Solarbio Co., Ltd. SPlink Detection kit (cat. no. SP-9001) for immunohistochemistry (IHC) was purchased from OriGene Technologies, Inc. All other chemicals were purchased from Beijing Dingguo Changsheng Biotechnology Co., Ltd.
Animals
Adult male BALB/c mice (18–22 g) were obtained from Vital River Laboratory Animal Technology Co., Ltd. The mice (aged 7 weeks old) were maintained in a 12-h light/dark cycle at 22±2°C with 60±10% humidity, and provided with sufficient food and water ad libitum. All animal experiments were approved by the Ethics Committee of Affiliated Hospital of Logistic University of Chinese People's Armed Police Force (permit no. AF-PJHEC-017-02.0), and were conducted in accordance with the guidelines set by the committee. A total of 36 mice were randomly divided into 6 groups: Control; 6 h LPS; DEX (5 mg/kg) treatment + 6 h LPS; MAT (25 mg/kg) treatment + 6 h LPS; LY (100 mg/kg) treatment + LPS; and MAT (25 mg/kg) + LY (100 mg/kg) treatment + 6 h LPS. DEX was administered via intraperitoneal injection for 7 consecutive days; MAT, LY, and MAT + LY combination treatments were administered orally for 7 consecutive days. The animals in the control group and LPS group received isovolumetric normal saline orally for the same period. At 30 min following the final administrations, the mice in all groups except the control group received an intratracheal instillation of LPS (5 mg/kg), whereas the animals in the control group received an intratracheal instillation of saline. At 6 h after LPS induction, the mice were euthanized via cervical dislocation following anesthetization with diethyl ether. Lung tissues were obtained and stored for further experiments.
Hematoxylin and eosin (H&E) staining
The lower lobe of right lungs were fixed with 4% paraformaldehyde at room temperature (23±2°C) for 1 day, then dehydrated and embedded in paraffin. All samples in paraffin were cut into 5-µm sections. For H&E staining, sections were immersed in hematoxylin for 10 min, followed by eosin for 3 min at room temperature. Following mounting using neutral balsam, the stained slides were observed under a light microscope (Olympus CHK; Olympus Corporation) and histopathology images were captured (C-4040 Zoom; Olympus Corporation). The lung injury score was assessed based on the method reported by Aeffner et al (18): No injury, 0; injury in 25% of the field, 1; injury in 50% of the field, 2; injury in 75% of the field, 3; and injury throughout the field, 4. A total of 10 random microscopic images were acquired for each section, and the fields were scored blindly by two pathologists and averaged. After the lung injury scores were assessed, Q value was used to evaluate the synergistic protective effect (19). Q value was calculated as EA+B/(EA+EB-EA•EB). EA represents the average lung injury score in MAT group, EB represents the average lung injury score in LY group, and EA+B represents the average lung injury score in MAT + LY group. Synergy or antagonism were defined by Q value >1 or <1 respectively, while Q=1 indicated no interaction.
Lung wet-to-dry (W/D) weight ratios
The degree of pulmonary edema can be determined based on lung W/D weight ratios (20). Absorbent paper was used to absorb exudate and blood on the surface of superior lobes of right lungs. Then, the tissues were weighed and recorded as wet weights. The tissues were then dried at 70°C for 72 h to constant weight and recorded as dry weights. Lung W/D weight ratios=wet weight/dry weight.
IHC
The following steps, if not described otherwise, were all performed at room temperature (23±2°C). Lung sections (5 µm) were deparaffinized in xylene (20 min) and rehydrated in a graded alcohol series (absolute ethyl alcohol for 10 min, 90% alcohol for 5 min, 80% alcohol for 5 min, 70% alcohol for 5 min). Antigens were retrieved in 0.01 M citric acid heated by microwave for 20 min. Endogenous peroxidases were inactivated by 3% H2O2 for 20 min. Goat serum was used to block for 1 h at 37°C. Then, slides were incubated with diluted NF-κB p65 antibody (1:500) overnight at 4°C. The following day, the antibody was removed and the sections were washed with PBS prior to incubation with HRP-conjugated secondary antibody for 30 min at 37°C. 3,3′-Diaminobenzidine was used to visualize expression and used to stain the slides for 5 min. Finally, the slides were counterstained with hematoxylin for 30 sec and dehydrated prior to observation under a light microscope. The results were evaluated semi-quantitatively. Five randomly fields were imaged from each section. Images were quantified using two different scores: Intensity score and positive cell ratio score. For the intensity score, negative, weakly positive, positive and strongly positive were graded as 0, 1, 2 and 3, respectively; for the positive cell ratio score, the positive cell area/the total area <1% was scored 0; 1–10% scored 1; >10–50% scored 2; >50–80% scored 3; and, >80% scored 4. The product of the two scores was used as the IHC value for each field.
ELISA for IL-6 and TNF-α
Mouse lung tissues were homogenized and then centrifuged at 12,000 × g at 4°C for 20 min. The supernatant was collected and diluted. The standard ELISA solutions or samples (diluent supernatant) were added to each well, then incubated for 90 min at 37°C. Diluted biotin-labeled antibodies (1:99) were added to the wells, and the plates were covered and incubated for 2 h at 37°C. The plates were then washed with washing buffer three times. HRP conjugate was then added to wells which allows binding with the biotin-labeled antibodies, and plates were incubated for 1 h at 37°C. After washing the plates, the chemiluminescent substrate was detected using colorimetric 3,3′,5,5′-tetramethylbenzidine solution. Finally, the stop solution was added into each well, and optical density of each well was read at 450 nm immediately by a microplate reader (Tecan Sunrise). The absorbance of samples was compared with the standard curve to calculate the concentration.
Western blot analysis
Total lung proteins were prepared from lung homogenates in RIPA buffer and protease/phosphatase inhibitor cocktail (Solarbio Co., Ltd.) and the protein concentration was determined by BCA method. Protein (30 µg/lane) was separated via SDS-PAGE on 10% gels and transferred to PVDF membranes. PVDF membranes were incubated with 5% non-fat milk at 25°C for 1 h with gentle agitation for blocking. Then, the membranes were incubated overnight with the aforementioned antibodies (1:1,000) at 4°C. Following incubation with secondary antibodies (1:3,000) at 25°C for 1 h, the blots were visualized using enhanced chemiluminescence reagent (cat. no. PE0010; Solarbio Co., Ltd.). ImageJ version 2.1.4.7 (National Institutes of Health) was used for densitometric quantification of expression.
Determination of MDA, GSH and MPO
Mouse lung tissues were homogenized, saline was added (tissue weight/g: Saline volume/ml=1:9) and samples were centrifuged at 10,000 × g at 4°C for 10 min. The supernatant was collected and used for subsequent experiments. MDA, GSH and MPO levels were detected using the respective kits, according to the manufacturer's protocols. The absorbances were measured at 532 nm for MDA, 405 nm for GSH, and 460 nm for MPO.
RT-PCR
Total RNA was isolated from lung tissues homogenates using TRIzol reagent (21), and RT-PCR was performed according to manufacturer's protocols. The kit allows reverse transcription and PCR in one step. The protocol includes 1 cycle at 45°C for 30 min followed by 95°C for 2 min; then 40 cycles of 94°C for 30 sec, 60°C for 30 sec, 72°C for 30 sec; and, finally, 1 cycle at 72°C for 5 min. The following primers were used: GAPDH, forward 5′-CCCAGCAAGGACACTGAGCAAG-3′, reverse 5′-GGTCTGGGATGGAAATTGTGAGGG-3′; IL-6, forward 5′-GGATACCACTCCCAACAGACC-3′, reverse 5′-TTCTGCAAGTGCATCATCGT-3′; TNF-α, forward 5′-GGCCTCCCTCTCATCAGTTC-3′, reverse 5′-CTTGGTGGTTTGCTACGACG-3′; and NF-κB p65, forward 5′-TCCGGTTACGTAATGAGTGGT-3′ and reverse 5′-GATCTGGTTCTCTTTCCGAAGTC-3′. PCR products were resolved on 1.5% agarose gels via electrophoresis. Results were visualized using EtBr, and ImageJ version 2.1.4.7 (National Institutes of Health) was used to quantify expression.
Statistical analysis
SPSS version 17.0 (SPSS, Inc.) was used for data analysis. Data are expressed as the mean ± standard deviation. Data were analyzed using one-way analysis of variance (ANOVA) followed by a Bonferroni test for multiple comparisons. For the lung injury scores and IHC values, the Kruskal-Wallis test was used, then all data were transformed logarithmically to make them conform to the normal distribution before using one-way ANOVA to compare specific groups. P<0.05 was considered to indicate a statistically significant difference.
Results
MAT + LY treatment attenuates lung edema induced by LPS
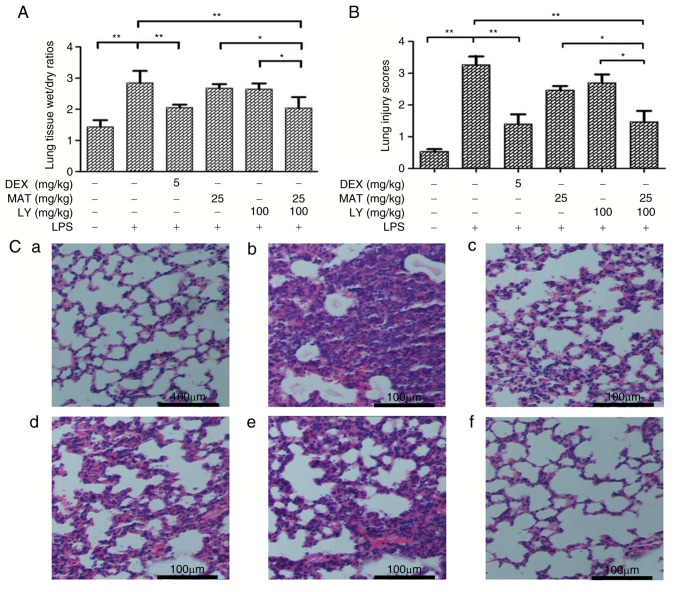
As presented in Fig. 2A, LPS induction significantly increased the W/D ratio of lung tissues compared with the control group. The W/D ratios of tissues from the MAT-treated and LY-treated groups were similar, and were not significantly different to those from the LPS group; however, treatment with DEX or MAT + LY significantly decreased the W/D ratio compared with the LPS group.
Figure 2.
Edema and morphopathological alterations in lung tissues. (A) Superior lobes of right mouse lungs were extracted and weighed. Lung tissue wet/dry ratios=wet weight/dry weight. n=6. (B) Lung injury scores were determined by two pathologists. (C) Hematoxylin and eosin staining of the inferior lobes of right lungs: a, control; b, LPS; c, DEX + LPS; d, MAT + LPS; e, LY + LPS; and f, MAT + LY + LPS. Scale bar, 100 µm. Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01. DEX, dexamethasone; MAT, matrine; LY, lycopene; LPS, lipopolysaccharide.
MAT + LY treatment prevents histopathological changes to the lungs induced by LPS
The synergistic protective effects of MAT + LY were calculated using lung injury scores (Fig. 2B) and the Q value was 1.27, indicating that MAT and LY exhibited synergy. As presented in Fig. 2C, lung tissues from the control group exhibited a normal lung structure, with the alveolar wall mainly composed of single layer of epithelial cells and possessing a complete structure. Conversely, hemorrhage, alveolar wall thickening, alveolar collapse and inflammatory infiltration were observed following LPS administration. The pathological alterations to the lungs were significantly attenuated by MAT + LY or DEX treatment (Fig. 2B and C), but were not significantly reduced in the MAT or LY only-treated groups.
MAT + LY treatment decreases oxidative stress in vivo
MDA is a product of lipid peroxidation and is frequently used as biomarker for determining oxidative stress (22). MDA levels were significantly increased following LPS induction (Fig. 3A); however, treatments with MAT + LY or DEX significantly attenuated LPS-induced MDA increases. GSH is an important antioxidant used as a marker of oxidative stress (23). Administration of LPS induced a reduction in GSH (Fig. 3B). Treatment with MAT or LY alone increased GSH levels compared with the LPS group, although the differences were not statistically significant; however, combined treatment with MAT + LY or DEX significantly increased GSH levels. Furthermore, to determine the effects of MAT + LY on neutrophil accumulation, the levels of MPO were measured. Following intratracheal instillation of LPS, the levels of MPO in lung tissue were significantly elevated (Fig. 3C). MAT or LY treatment alone did not significantly affect MPO levels compared with the LPS group; however, the combination of treatment with MAT + LY significantly attenuated this LPS-induced increase.
Figure 3.
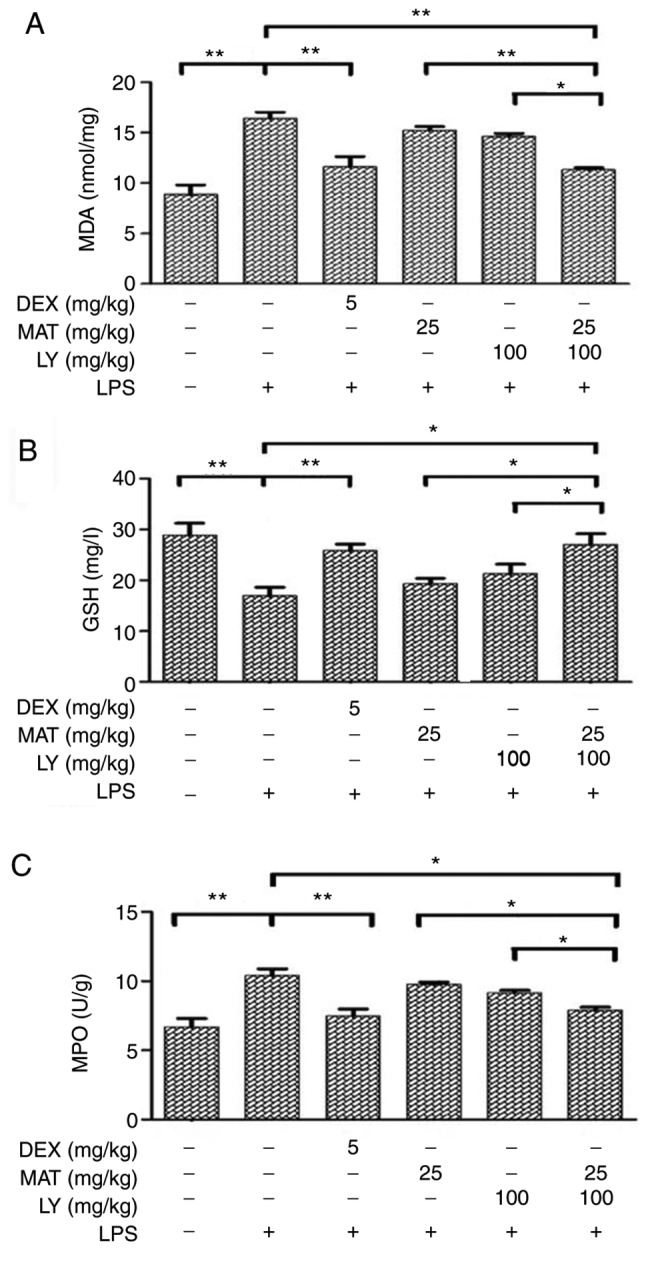
Levels of oxidative stress biomarkers in lung tissues. Lung tissues were collected and homogenized. Levels of (A) MDA, (B) GSH and (C) MPO were determined using respective kits. Data are presented as the mean ± standard deviation. n=6. *P<0.05, **P<0.01. MDA, malondialdehyde; DEX, dexamethasone; MAT, matrine; LY, lycopene; LPS, lipopolysaccharide; GSH, glutathione; MPO, myeloperoxidase.
MAT + LY treatment reduces the inflammatory response in vivo
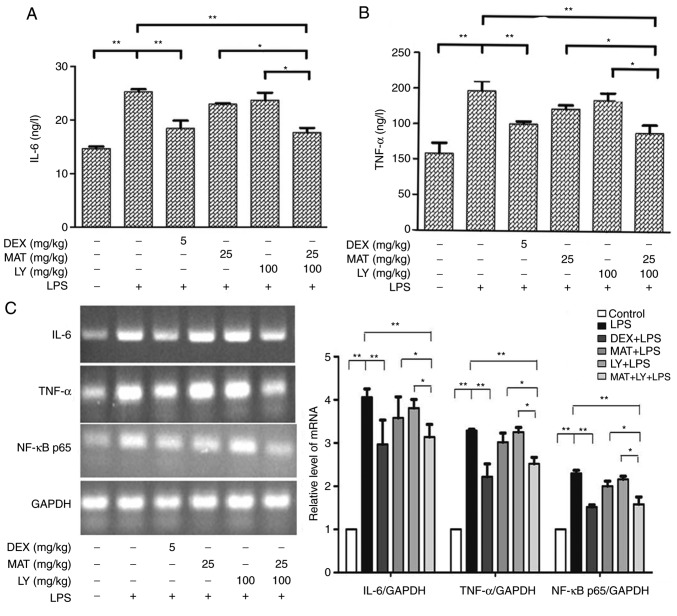
The levels of IL-6 and TNF-α in lung tissues were determined via ELISA. The results indicated that the levels of IL-6 (Fig. 4A) and TNF-α (Fig. 4B) were significantly increased following LPS administration compared with the control group. Treatment with MAT or LY did not significantly affect the LPS-induced increased in IL-6 or TNF-α levels, whereas treatment with MAT + LY or DEX significantly decreased the levels of IL-6 and TNF-α compared with the LPS group. To further validate these results, IL-6 and TNF-α mRNA levels were measured via RT-PCR analysis, with the same trends observed (Fig. 4C).
Figure 4.
Levels of proinflammatory mediators in lung tissues. (A) IL-6 and (B) TNF-α levels in lungs as determined by ELISA. (C) Levels of IL-6, TNF-α and NF-κB p65 mRNA as determined by semi-quantitative reverse transcription-polymerase chain reaction. The expression levels of mRNA were measured by densitometry and normalized to GAPDH mRNA expression levels. Data are presented as the mean ± standard deviation. n=3 for TNF-α. n=5 for IL-6 and NF-κB p65. *P<0.05, **P<0.01. IL-6, interleukin-6; DEX, dexamethasone; MAT, matrine; LY, lycopene; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α.
MAT + LY treatment inhibits the NF-κB signaling pathway
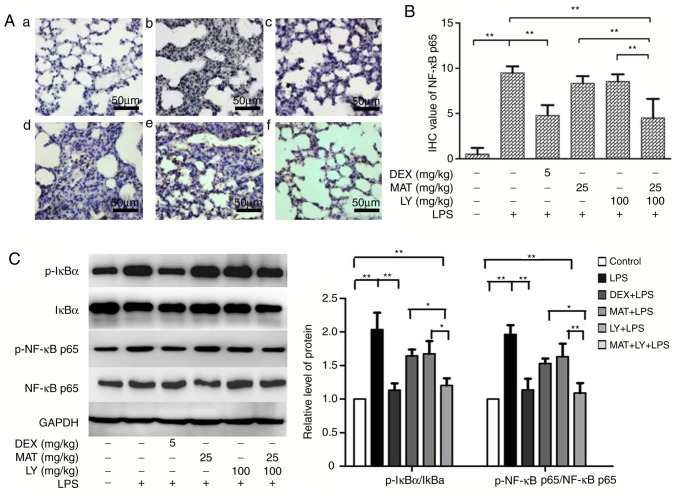
NF-κB signaling pathway is a classical inflammatory signaling pathway. LPS activated the IκB/NF-κB signaling, stimulating the expression of NF-κB p65 compared with the control group (Figs. 4C and 5). Treatment with MAT or LY alone significantly inhibited NF-κB signaling, while the effect of MAT + LY was more pronounced. MAT + LY treatment downregulated the expression of NF-κB p65 at the mRNA and protein levels, and the phosphorylation of NF-κB p65 and IκBα compared with the LPS group, inhibiting the activation of signaling induced by LPS (Fig. 5C).
Figure 5.
Effects of MAT and/or LY on NF-κB signaling. (A) Immunohistochemical staining for NF-κB p65: a, control; b, LPS; c, DEX + LPS; d, MAT + LPS; e, LY + LPS; and f, MAT + LY + LPS. Scale bar, 50 µm. (B) Protein expression of NF-κB p65 as evaluated semi-quantitatively based on immunohistochemistry. (C) Activation levels of IκBα and NF-κB p65 were analyzed via western blotting and densitometry analysis. Data are presented as the mean ± standard deviation. n=6. *P<0.05, **P<0.01. DEX, dexamethasone; MAT, matrine; LY, lycopene; LPS, lipopolysaccharide.
Discussion
ALI is a serious disease characterized by bilateral lung infiltration. Various therapeutic interventions are under development; however, at present, there is no effective cure (24). The mortality rate of ALI is 30–40% (25). Glucocorticoids are potentially useful as anti-inflammatory drugs for the treatment of ALI (26); however, long-term use of glucocorticoids at high doses may lead to immunosuppression, resulting in infections that exacerbate the lung injury, or other complications, including Cushing's syndrome or psychiatric symptoms (27). It was previously reported that treatment with high doses of glucocorticoid for short or long periods of time did not improve the survival of patients with ALI (28). As a result, it is necessary to identify alternative treatments for ALI. Zhang et al (11) reported that intraperitoneal injection of MAT (100 mg/kg) protected against LPS-induced ALI in a mouse model. Türkoğlu et al (15) observed that LY (at 200 times the recommended daily intake in humans) exhibited protective effects in a rat model of oleic acid-induced ALI. The high doses and administration protocols in these studies restricted their clinical usefulness. Therefore, in the present study, these issues were amended; the two compounds were administered orally at a relatively low dosage, and their effects in combination were investigated.
LPS-induced lung injury in rodents is a frequently used ALI model, mimicking a number of the characteristics of ALI in humans (29). In the present study, combined treatment of MAT + LY achieved similar effects to DEX in protecting the lungs in the LPS-induced mouse model. The combination treatment attenuated lung injury and pulmonary edema, potentially via anti-inflammatory and antioxidative mechanisms, exhibiting synergistic effects.
The pathogenesis of ALI has not been fully determined. Inflammatory responses (30) and oxidative stress imbalances (31) have been identified to have important roles during the development and progression of ALI. These two mechanisms can affect the disease progression separately and synchronously. TNF-α is an early endogenous mediator of inflammatory responses produced by monocytes/macrophages (32). Excessive levels of TNF-α will damage lysosomes, leading to injuries in pulmonary vascular endothelial cells and epithelial cells (3). Furthermore, TNF-α induces the secretion of cytokines such as IL-6 (33). ELISA and RT-PCR analyses in the current study revealed that treatment with MAT alone markedly affected TNF-α and IL-6 levels; however, when LY was added, a significant decrease in the levels of the two cytokines was observed compared with the LPS group.
Oxidative stress is increased during inflammation of airways, in turn exacerbating the inflammation (31). This leads to the formation of a positive feedback loop that promotes inflammatory responses, leading to deterioration of the condition (31). The release of reactive oxygen species (ROS), and subsequent induction of oxidative stress, has an important role in this process. ROS mainly attack polyunsaturated fatty acids in plasma membranes, inducing lipid peroxidation and increasing the levels of MDA (34,35); thus, the levels of MDA reflect whether cells are subjected to attack by oxygen free radicals. In addition to interactions with inflammatory factors, ROS induce cell damage via a series of other mechanisms, such as the inactivation of antioxidant defense systems. The system includes various antioxidant enzymes, including superoxide dismutase and GSH peroxidase (31). These enzymes can reduce and eliminate the production of ROS, thereby protecting against damage caused by oxidative stress (36,37). GSH is a member of the antioxidant defense system, and promotes the decomposition of hydrogen peroxide and inhibits the production of free radicals (38). GSH activity indicates the degree of endogenous oxygen free radical scavenging. LY is an antioxidant; however, 100 mg/kg LY did not notably protect mice against ALI. It was observed that the recruitment of neutrophils was decreased when the mice were treated with MAT + LY, as determined by a significant decrease in MPO levels. MAT + LY also decreased the levels of lipid peroxidation in the mouse model of ALI, as determined by decreased MDA levels, and attenuated the reduction in GSH in lung tissue. The increase in GSH level in mice lung tissue may be a result of the upregulation of antioxidant expression or a decrease of ROS production.
The potential mechanism underlying the synergistic protective effect was investigated. NF-κB signaling has been identified as a traditional immune and inflammatory pathway. The NF-κB/Rel family members are dimeric transcription factors. In the absence of stimulating signals, NF-κB dimers are bound to inhibitory IκB proteins. In the canonical pathway, activators, such as proinflammatory cytokines, activate an IκB kinase complex, leading to phosphorylation of IκB (39). Thus, the NF-κB/Rel complexes are freed and further phosphorylated prior to translocation into the nucleus, where they induce gene expression (40). Treatment with MAT + LY significantly suppressed the LPS-induced activation of NF-κB activation compared with the single drug treatment groups. RT-PCR, western blot and IHC analyses indicated that the two agents inhibited NF-κB p65 transcription and translation, in turn decreasing NF-κB signaling. Specifically, MAT + LY treatment reduced the phosphorylation of IκBα, which allows NF-κB p65 to be anchored in the cytoplasm rather than phosphorylate and transfer to the nucleus to regulate proinflammatory gene transcription. The findings suggested that reduced signaling decreased the expression of proinflammatory factors, inhibiting the production of ROS and other oxygen radicals.
In conclusion, the results of the present study demonstrated that MAT or LY treatment alone exhibited limited protection against LPS-induced ALI in mice; however, combined treatment of MAT and LY significantly attenuated the LPS-induced alterations in cytokine expression and oxidative stress, suggesting that there may be a synergistic effect of the two chemicals. One of the mechanisms underlying this potential synergy was the inhibition of the NF-κB signaling pathway. The roles of other signaling pathways requires further investigation. Additionally, it should be noted that the pathology of ALI in clinical settings is more complex that in the mouse model. In the present study, drugs were administered for 7 days prior to LPS induction, an unlikely situation in a clinical setting. Additional groups and time points will be explored in future experiments. According to the present findings, the combined administration of MAT and LY may be a potential alternative to glucocorticoid therapy for the treatment of ALI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Laboratory for Occupational and Environmental Hazards Prevention of Tianjin (grant no. WHKF201705) and the National Natural Science Foundation of China (grant no. 81600051).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
WL and TW were major contributors in writing the manuscript. WL, YR and JW performed the experiments and analyzed the data. TW, BC and BL contributed to the conception and design of the study. FW and LW performed the histological examination. HC and YL designed the present study and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Animal experiments were approved by the Ethics Committee of Affiliated Hospital of Logistic University of Chinese People's Armed Police Force (permit number: AF-PJHEC-017-02.0), and were conducted in accordance with the guidelines set by the committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bersten AD, Edibam C, Hunt T, Moran J; Australian, New Zealand Intensive Care Society Clinical Trials Group Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165:443–448. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 2.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD, NIH NHLBI ARDS Network Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37:1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong WT, Wu YC, Xie XX, Zhou X, Wei MM, Soromou LW, Ci XX, Wang DC. Phillyrin attenuates LPS-induced pulmonary inflammation via suppression of MAPK and NF-κB activation in acute lung injury mice. Fitoterapia. 2013;90:132–139. doi: 10.1016/j.fitote.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Li C, Li J, Xu Y, Guan S, Zhao M. Protective effectsof protocatechuic acid on acute lung injury induced by lipopolysaccharide in mice via p38MAPK and NF-κB signalpathways. Int Immunopharmacol. 2015;26:229–236. doi: 10.1016/j.intimp.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Liang D, Dong L, Ge X, Xu F, Chen W, Dai Y, Li H, Zou P, Yang S, Liang G. Anti-inflammatory effects of novel curcumin analogs in experimental acute lung injury. Respir Res. 2015;16:43. doi: 10.1186/s12931-015-0199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, Zin WA, Rocco PR. Pulmonary and extrapulmonary acute lung injury: Inflammatory and ultrastructural analyses. J Appl Physiol (1985) 2005;98:1777–1783. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 7.Chopra M, Reuben JS, Sharma AC. Acute lung injury: Apoptosis and signaling mechanisms. Exp Biol Med (Maywood) 2009;234:361–371. doi: 10.3181/0811-MR-318. [DOI] [PubMed] [Google Scholar]
- 8.Z'graggen BR, Tornic J, Müller-Edenborn B, Reyes L, Booy C, Beck-Schimmer B. Acute lung injury: Apoptosis in effector and target cells of the upper and lower airway compartment. Clin Exp Immunol. 2010;161:324–331. doi: 10.1111/j.1365-2249.2010.04175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarma JV, Ward PA. Oxidants and redox signaling in acute lung injury. Compr Physiol. 2011;1:1365–1381. doi: 10.1002/cphy.c100068. [DOI] [PubMed] [Google Scholar]
- 10.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: Mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2009;40:519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang B, Liu ZY, Li YY, Luo Y, Liu ML, Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG, Li ZC. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur J Pharm Sci. 2011;44:573–579. doi: 10.1016/j.ejps.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Niu Y, Dong Q, Li R. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-κB signaling. Cell Biol Int. 2017;41:611–621. doi: 10.1002/cbin.10763. [DOI] [PubMed] [Google Scholar]
- 13.Huang WC, Chan CC, Wu SJ, Chen LC, Shen JJ, Kuo ML, Chen MC, Liou CJ. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J Ethnopharmacol. 2014;151:470–477. doi: 10.1016/j.jep.2013.10.065. [DOI] [PubMed] [Google Scholar]
- 14.Prabhala RH, Braune LM, Garewal HS, Watson RR. Influence of beta-carotene on immune functions. Ann N Y Acad Sci. 1993;691:262–263. doi: 10.1111/j.1749-6632.1993.tb26189.x. [DOI] [PubMed] [Google Scholar]
- 15.Türkoğlu S, Muz MH, Ozercan R, Gürsu F, Kırkıl G. Effects of lycopene on the model of oleic acid-induced acute lung injury. Tuberk Toraks. 2012;60:101–107. doi: 10.5578/tt.2468. [DOI] [PubMed] [Google Scholar]
- 16.Allen TC, Kurdowska A. Interleukin 8 and acute lung injury. Arch Pathol Lab Med. 2014;138:266–269. doi: 10.5858/arpa.2013-0182-RA. [DOI] [PubMed] [Google Scholar]
- 17.Mokra D, Kosutova P. Biomarkers in acute lung injury. Respir Physiol Neurobiol. 2015;209:52–58. doi: 10.1016/j.resp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Aeffner F, Bolon B, Davis IC. Mouse models of acute respiratory distress syndrome: A review of analytical approaches, pathologic features, and common measurements. Toxicol Pathol. 2015;43:1074–1092. doi: 10.1177/0192623315598399. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Liu B, Cao B, Wei F, Yu X, Li GF, Chen H, Wei LQ, Wang PL. Synergistic protection of Schizandrin B and Glycyrrhizic acid against bleomycin-induced pulmonary fibrosis by inhibiting TGF-β1/Smad2 pathways and overexpression of NOX4. Int Immunopharmacol. 2017;48:67–75. doi: 10.1016/j.intimp.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L231–L246. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 22.Gaweł S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004;57:453–455. (In Polish) [PubMed] [Google Scholar]
- 23.Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002;8:385–387. doi: 10.1016/S1353-8020(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 24.Del Sorbo L, Goffi A, Ranieri VM. Mechanical ventilation during acute lung injury: Current recommendations and new concepts. Presse Med. 2011;40:e569–e583. doi: 10.1016/j.lpm.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Su ZQ, Mo ZZ, Liao JB, Feng XX, Liang YZ, Zhang X, Liu YH, Chen XY, Chen ZW, Su ZR, Lai XP. Usnic acid protects LPS-induced acute lung injury in mice through attenuating inflammatory responses and oxidative stress. Int Immunopharmacol. 2014;22:371–378. doi: 10.1016/j.intimp.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 26.Thompson BT. Glucocorticoids and acute lung injury. Crit Care Med. 2003;31(Suppl 4):S253–S257. doi: 10.1097/01.CCM.0000057900.19201.55. [DOI] [PubMed] [Google Scholar]
- 27.Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15:457–465. doi: 10.1517/14740338.2016.1140743. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M, National Heart Lung, Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 29.Rittirsch D, Flierl MA, Day DE, Nadeau BA, McGuire SR, Hoesel LM, Ipaktchi K, Zetoune FS, Sarma JV, Leng L, et al. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol. 2008;180:7664–7672. doi: 10.4049/jimmunol.180.11.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward PA. Acute lung injury: How the lung inflammatory response works. Eur Respir J. 2003;(Suppl 44):S22–S23. doi: 10.1183/09031936.03.00000703a. [DOI] [PubMed] [Google Scholar]
- 31.Ward PA. Oxidative stress: Acute and progressive lung injury. Ann N Y Acad Sci. 2010;1203:53–59. doi: 10.1111/j.1749-6632.2010.05552.x. [DOI] [PubMed] [Google Scholar]
- 32.Zelová H, Hošek J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm Res. 2013;62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 33.Ohta K, Naruse T, Ishida Y, Shigeishi H, Nakagawa T, Fukui A, Nishi H, Sasaki K, Ogawa I, Takechi M. TNF-α-induced IL-6 and MMP-9 expression in immortalized ameloblastoma cell line established by hTERT. Oral Dis. 2017;23:199–209. doi: 10.1111/odi.12594. [DOI] [PubMed] [Google Scholar]
- 34.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- 36.Victor VM, Rocha M, De la Fuente M. Immune cells: Free radicals and antioxidants in sepsis. Int Immunopharmacol. 2004;4:327–347. doi: 10.1016/j.intimp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Schettler V, Wieland E, Methe H, Schuff-Werner P, Müller GA. Oxidative stress during dialysis: Effect on free radical scavenging enzyme (FRSE) activities and glutathione (GSH) concentration in granulocytes. Nephrol Dial Transplant. 1998;13:2588–2593. doi: 10.1093/ndt/13.10.2588. [DOI] [PubMed] [Google Scholar]
- 39.Hinz M, Scheidereit C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014;15:46–61. doi: 10.1002/embr.201337983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo K. Signaling CROSS Talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017;9:a022137. doi: 10.1101/cshperspect.a022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.