Abstract
The present study aimed to ascertain the potential roles and mechanisms of action of micro (mi)RNA-22 in ischemic stroke. The results indicated that miRNA-22 expression was downregulated in ischemic stroke rats model, compared with a control group. The downregulation of miRNA-22 upregulated the expression of inflammatory factors [including tumor necrosis factor-α, interleukin (IL)-1β, IL-6 and IL-18]. It could also induce the expression of macrophage inflammatory protein (MIP-2), prostaglandin E2 (PGE2), cyclooxygenase-2 (COX-2) and inducible NO synthase (iNOS) in the in vitro model. By contrast, the overexpression of miRNA-22 downregulated the expression of inflammatory factors, and suppressed the expression of MIP-2, PGE2, COX-2 and iNOS in the in vitro model. The downregulation of miRNA-22 induced the protein expression of nuclear factor (NF)-κB and phosphorylated-p38 (p-p38) mitogen-activated protein kinase (MAPK) in the in vitro model. By comparison, the overexpression of miRNA-22 suppressed the protein expression of NF-κB and p-p38 in the in vitro model. Typically, LY2228820, the p38 inhibitor (3 nM) would mitigate the pro-inflammatory effects of anti-miRNA-22 in the in vitro model. These results suggested that miRNA-22 can alleviate ischemic stroke-induced inflammation in rats model or vitro model through p38 MAPK/NF-κB pathway suppression.
Keywords: microRNA-22, ischemic stroke, inflammation, nuclear factor-κB, p38 mitogen-activated protein kinase
Introduction
Acute ischemic stroke refers to a brain blood circulation disorder, cerebral blood flow reduction, brain tissue ischemia, anoxic necrosis (1–3). In recent years, the incidence of acute ischemic stroke has shown a steadily increasing trend (2) and it has become a major disease and threat to human health with high incidence, disability rate and fatality rate (3).
Ischemic stroke is the second major factor leading to mortality and disability in patients. Specifically, 20–50% patients will succumb or suffer from despite receiving treatment within one month following a stroke (4). With the development of modern medical science, intensive studies on the mechanism of ischemic stroke have been performed at the cellular and molecular levels (5). In recent years, increasing theoretical and clinical studies have indicated that the genesis and development of the inflammatory reaction involved in ischemic stroke may be used for risk assessment and diagnosis for ischemic stroke at different stages (6).
The inflammatory response is a physiological reaction to repair damaged brain tissue, so as to protect the central nervous system (7,8). Typically, infiltration of a large number of inflammatory factors and cells can be observed within the damaged area (7). However, overexpression of these factors will induce secondary brain injury, and will further participate in the development of cognitive decline. Studies have shown that cognitive decline in stroke patients may be associated with the inflammatory mechanisms (7,8).
There are three types of nitric oxide synthases (NOS) in humans; endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). All can catalyze arginine (Arg) to synthesize NO (9). Under physiological conditions, NO content in the brain tissue is low and mainly catalyzed by nNOS (10,11). NO is involved in in the signal transduction and synaptic plasticity of the nervous system. However, under pathological conditions, including cerebral infarction, cerebral hemorrhage, brain injury and epilepsy (9), brain tissue generates a large amount of NO, which inhibits the activity of neuronal mitochondrial cytochrome oxidase. As a result, the synthesis of ATP is reduced, leading to excessive neuronal depolarization (11).
Previous studies have suggested that the p38 mitogen-activated protein kinase (MAPK) activity is of vital importance to normal immune and inflammatory reactions (12,13). It is involved in in the functional reactions of macrophages and phagocytic neutrophils, including chemotaxis, granule exocytosis, adherence and apoptosis. In addition, it promotes the differentiation and apoptosis of T cells (12). A previous study indicated that p38 MAPK is involved in in the early identification of cells, while blocking the p38 MAPK signaling pathway can inhibit the inflammatory response (13). In addition, a previous study has also suggested that inflammation serves a crucial role in cardiovascular and cerebrovascular diseases, particularly in the pathological and physiological evolution process of cerebral ischemia (14). A previous study also confirmed that p38 MAPK is involved in the inflammatory response of CIBI, which was supported by the observation that p38 MAPK protein expression is upregulated in macrophages at the core area of cerebral ischemia (13).
Micro(mi)RNAs exist in the genome in multiple forms, including single copy, multicopy or gene clusters (15), and a majority of them locate in the intergenic region. miRNA transcription is independent of other genes (15). Of note, miRNA is not translated into protein; instead, it serves multiple regulatory roles during the metabolic processes in vivo. miRNA allows for fine control over the time-sequence expression of genes (16). It serves a key regulatory role in the development and maturation of the central nervous system in addition to multiple neurophysiological processes (16). In addition, it is suggested in a recent study that there are differentially expressed miRNAs in the peripheral plasma in patients with acute ischemic stroke within 24 h of onset (17). These differentially expressed miRNAs may be associated with ischemia-hypoxia stress, blood brain barrier disruption and early reperfusion injury during early ischemic stroke (17). Alternatively, they may serve as novel therapeutic guidelines and targets for the intervention in early ischemic stroke. Jovicic et al (18) demonstrated that the overexpression of miRNA-22 had neuroprotective effects. The present study investigated the possible roles and mechanisms of action of miRNA-22 in ischemic stroke.
Materials and methods
Animal grouping and drug administration
Animal care and the general protocols for animal use were approved by the Institutional Animal Care and Use Committee of Shandong Jining No.1 People's Hospital. Adult male Sprague-Dawley (SD) rats (n=16; 220–240 g, 8–10 week) were purchased form Animal experiment center of Shandong University (Shandong, China) and kept at 22–24°C, 45–55% relative humidity, and in a 12 h light/dark cycle, with free access to water and food. All SD rats were randomly assigned to a sham group (n=6) and a stroke model group (n=10). Prior to experimental surgery, the rats were fasted and then anaesthetized with 35 mg/kg pentobarbital sodium (intraperitoneal). Rats were placed in the supine position and then hair was removed at the neck. The external carotid artery, right common carotid artery, internal carotid artery and the pterygopalatine artery were separated and exposed at the inner edge of sternocleidomastoid. Then, the external carotid and the pterygopalatine arteries were ligated at 1 cm distal and the right common carotid artery was occluded with aneurysm clips. A small incision was made in external carotid artery from ligation at the proximal end and the external carotid artery was pulled at proximal end with the internal carotid artery. A nylon filament silicone resin-coated tip (220 µm × 4 mm) was inserted into the internal carotid artery and the filament was removed after 2 h for the induction of ischemia. The artery was ligated and the wound sutured and sterilized.
Total RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the brain samples or cell samples was extracted using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Then, 1 µg RNA was reverse transcribed using a commercial cDNA synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.) at 42°C for 1 h and 85°C for 5 min. RTqPCR was performed in an ABI Prism 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using SYBR Premix Ex Taq II (Thermo Fisher Scientific, Inc.). qPCR was as follows: 50°C for 10 min, 95°C for 10 min, and 40 cycles of 95°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. The primers sequences were: miR-22 forward, 5′-TGCGCAGTTCTTCAGTGGCAAG-3′ and reverse, 5′-CCAGTGCAGGGTCCGAGGTATT-3′; U6 forward, 5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-AAATATGGAACGCTTCACGA-3′. The relative expression of miRNA-22 was calculated based on the 2−ΔΔCq method (19).
Gene microarray hybridization
Total mRNA was hybridized into the SurePrint G3 Mouse Whole Genome GE 8×60 K Microarray G4852A platform (Agilent Technologies, Inc., Santa Clara, CA, USA). Images were quantified and feature-extracted using Agilent Feature Extraction Software (version A.10.7.3.1; Agilent Technologies, Inc.).
ELISA
All rats were anaesthetized with 35 mg/kg pentobarbital sodium, peripheral blood was collected and then centrifuged at 1,000 g × 10 min at 4°C to obtain serum. Serum was incubated with tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-18, MIP-2, PGE2 (all Elabscience Biotechnology Co. Ltd., Wuhan, China), caspase-3 (C1115, Beyotime Institute of Biotechnology, Nanjing China) and caspase-9 (C1158, Beyotime Institute of Biotechnology) commercial ELISA kits.
Histological examination
The hippocampus was fixed with 4% paraformaldehyde for 24 h at room temperature. Tissue samples were paraffinembedded and sectioned at 5 µm. Tissue samples were stained with hematoxylin and eosin for 15 min at room temperature and examined a Zeiss Axioplan 2 (Carl Zeiss AG, Oberkochen, Germany; magnification, ×50).
Cell culture and transfection
PC12 cells (a rat pheochromocytoma cell line) were acquired from Shanghai cell bank (Chinese academy of sciences) and cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo Fisher Scientific, Inc.) and 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2. miRNA-22, anti-miRNA-22 and negative mimic were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). 100 ng of miRNA-22 (forward 5′-CTCAACTGGTGTCGTGGAGTCGG-3′ and reverse 5′-CAATTCAGTTGAGACAGTTCT-3′), 100 ng of anti-miRNA-22 (5′-ACCTGGCTGAGCCGCAGTAG-3′ and 5′-CCCTCTGCCCCTGGC-3′), and 100 ng of negative mimics (forward 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse 5′-ACGUGACACGUUCGGAGAATT-3′) were transfected into cells with Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Following transfection for 24, 48 and 72 h, the cells were induced into an oxygen glucose deprivation (OGD) model (20), and cultured in neurobasal medium without glucose in a humidified incubator filled with an anoxic gas mixture (5% CO2 and 93.5% N2) at 37°C for 2 h. Following transfection for 24 h, the cells were treated with p38 inhibitor (LY2228820; 3 nM; MedChemExpress LLC, Shanghai, China) for 24 h and were induced for the OGD model.
Luciferase reporter assay
The amplified PCR products of p38 MAPK (5′-TAACAAAATGGCTGAAATGA-3′ and 5′-TGAAAATTCCTTAAAGACGA-3′), the predicted miRNA-22 targeting regions (www.targetscan.org), were inserted into the pMIR-REPORT plasmid (Ambion; Thermo Fisher Scientific, Inc.). Then, 100 ng of p38 MAPK plasmid and anti-miRNA-22 or negative mimics were transfected into PC12 cells using Lipofectamine® 2000 according to the manufacturer's protocol. Cells were the assayed using a luciferase assay kit (Promega Corporation, Madison, WI, USA) at 24 h after transfection according to the manufacturer's protocols. The method of normalization was Renilla luciferase activity.
Western blot analysis
Following treatment with miRNA-22, the rats were anaesthetized with chloral hydrate (400 mg/kg) and brains of all groups were rapidly removed from the skull, washed in ice-cold saline and stored at −80°C. Brain tissue was homogenized using RIPA buffer (Beyotime Institute of Biotechnology) and centrifuged at 10,000 × g for 10 min at 4°C. The protein concentration was measured using the bicinchoninic acid method, and 50 µg protein was electrophoresed with 8–10% Tris-glycine SDS-PAGE. Then, the protein was transcribed onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA) and blocked with 5% skimmed milk in tris-buffered saline containing 0.1% Tween-20 at 37°C for 1 h. The membrane was incubated with anti-COX-2 (cat. no. 12282, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-iNOS (cat. no. 13120, 1:2,000; Cell Signaling Technology, Inc.), anti- NF-κB (cat. no. 8242, 1:1,000; Cell Signaling Technology, Inc.), anti-phosphorylated-(p)-p38 (cat. no. 4511, 1:2,000; Cell Signaling Technology, Inc.) and anti-GADPH (cat. no. 5174, 1:5,000, Cell Signaling Technology, Inc.), which were incubated overnight at 4°C. The membrane was incubated with horseradish peroxidase-conjugated anti-mouse secondary antibody IgG (cat. no. 7074, 1:5,000, Cell Signaling Technology, Inc.) at 37°C for 1 h and then developed by enhanced chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.). Protein expression was calculated using Bio-Rad Image Lab version 3.0 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunofluorescence analysis
Cells were washed with PBS and fixed with 4% paraformaldehyde for 15 min at room temperature. Then they were incubated with 0.25% TrisX100 and 5% BSA in PBS for 1 h at room temperature and then incubated with p-p38 (cat. no. 4511, 1:100; Cell Signaling Technology, Inc.) at 4°C overnight. Cells were stained with donkey anti-rabbit IgG-CFL 555 (sc-362271; 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and stained with DAPI assay (Beyotime Institute of Biotechnology) for 15 min in the dark. Cells were washed with PBST for 15 min and selected at room temperature using a Zeiss Axioplan 2 (Carl Zeiss AG).
Statistical analysis
All data were expressed as the mean ± standard error of mean (Repeats=3). Differences in the parameters were analyzed using one-way analysis of variance followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
miRNA-22 expression in ischemic stroke rats
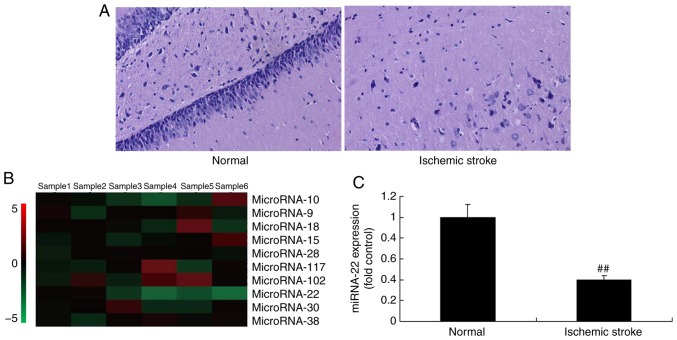
It was identified that the neurological score was increased in stroke rats treated with miRNA-22 acetate compared with the control group (Fig. 1A). Next, the changes of miRNA-22 expression were measured using microarray and qPCR. The results indicated that miRNA-22 expression was downregulated in ischemic stroke rats, compared with the control group (Fig. 1B and C).
Figure 1.
The expression of miRNA-22 in ischemic stroke rats. (A) Hematoxylin and eosin staining of neurocytes. (B) Representative microarray analysis and (C) quantitative polymerase chain reaction analysis of miRNA-22 expression. ##P<0.01 vs. normal rat group. Magnification, ×50. Normal, control normal rat group; Ischemic stroke, ischemic stroke rat group; miRNA, microRNA.
Downregulation of miRNA-22 upregulates the inflammatory factors in vitro
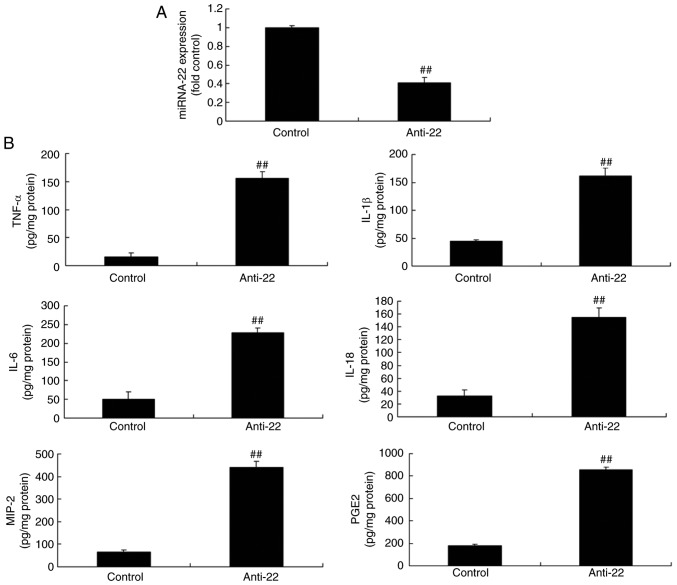
To examine the function of miRNA-22 in the ischemic stroke model in vitro, anti-miRNA-22 mimics were used, which downregulated miRNA-22 expression in the ischemic stroke model compared with the control group (Fig. 2A). In addition, downregulation of miRNA-22 increased the expression of TNF-α, IL-1β, IL-6, IL-18, MIP-2 and PGE2 in the ischemic stroke model in vitro, compared with the control group (Fig. 2B).
Figure 2.
Downregulation of miRNA-22 increases inflammatory factor expression in the in vitro stroke model. (A) miRNA-22 expression and levels of (B) TNF-α, IL-1β, IL-6, IL-18, MIP-2 and PGE2. ##P<0.01 vs. control group. miRNA-22, microRNA-22; Anti-22, downregulation of miRNA-22 group; TNF, tumor necrosis factor; IL, interleukin; MIP, macrophage inflammatory protein; PGE2, prostaglandin E2.
Overexpression of miRNA-22 reduces inflammatory factor expression in vitro
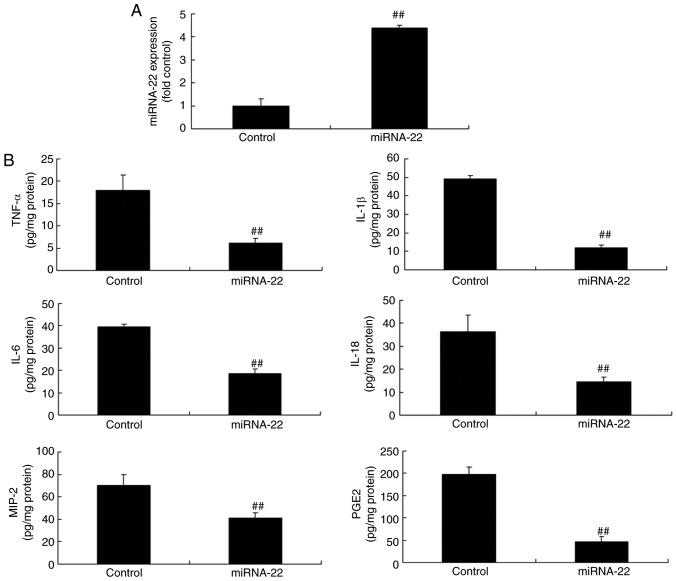
miRNA-22 expression in the in vitro ischemic stroke model was successfully upregulated using miRNA-22 mimics, compared with control negative group (Fig. 3A). The overexpression of miRNA-22 decreased the expression levels of TNF-α, IL-1β, IL-6, IL-18, MIP-2 and PGE2 in the ischemic stroke model in vitro, compared with the control group (Fig. 3B).
Figure 3.
Overexpression of miRNA-22 reduces inflammatory factor expression in the in vitro stroke model. (A) miRNA-22 expression was successfully increased by mimics. (B) Expression of TNF-α, IL-1β, IL-6, IL-18, MIP-2 and PGE2 was determined by ELISA. ##P<0.01 vs. control group. Control, miRNA-22, overexpression of microRNA-22 group; TNF, tumor necrosis factor; IL, interleukin; MIP, macrophage inflammatory protein; PGE2, prostaglandin E2.
miRNA-22 regulates the p38 MAPK/NF-κB pathways in the in vitro ischemic stroke model
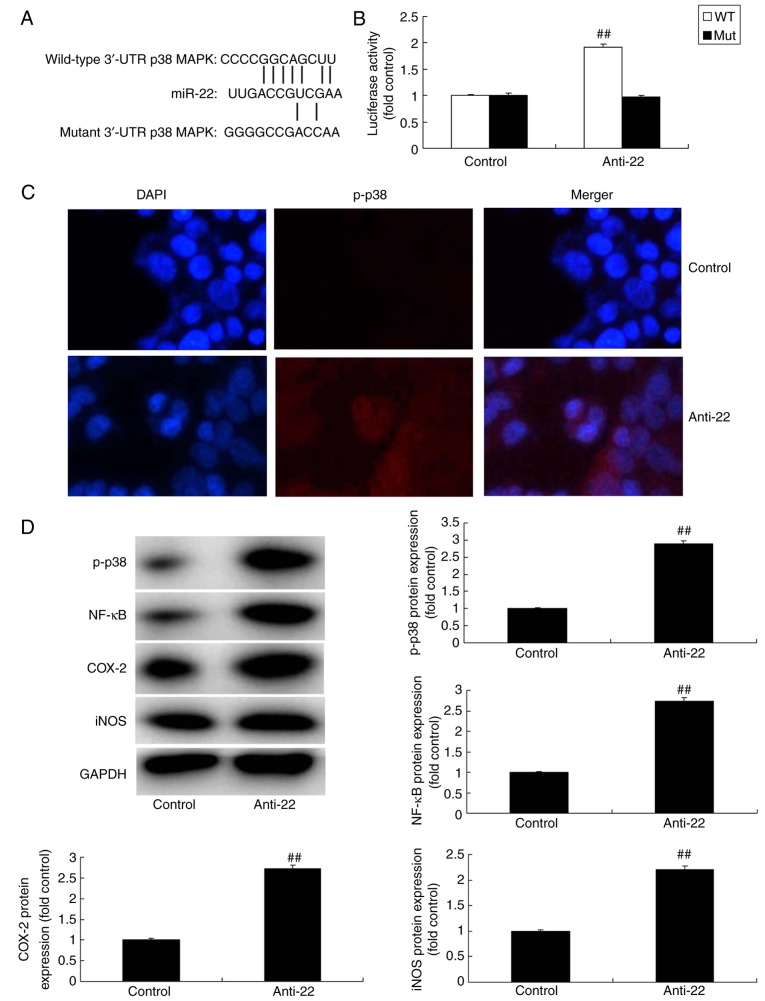
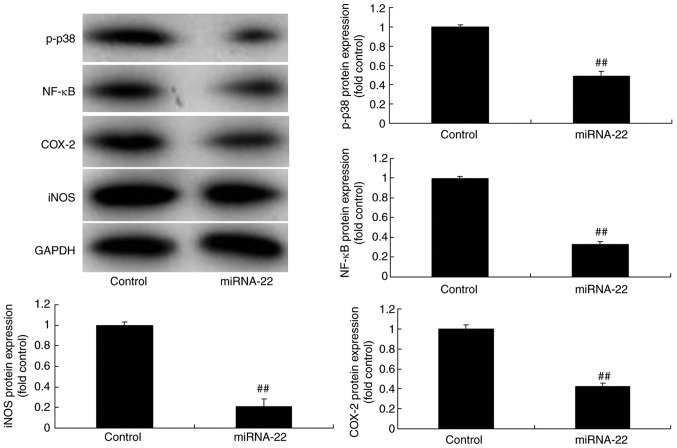
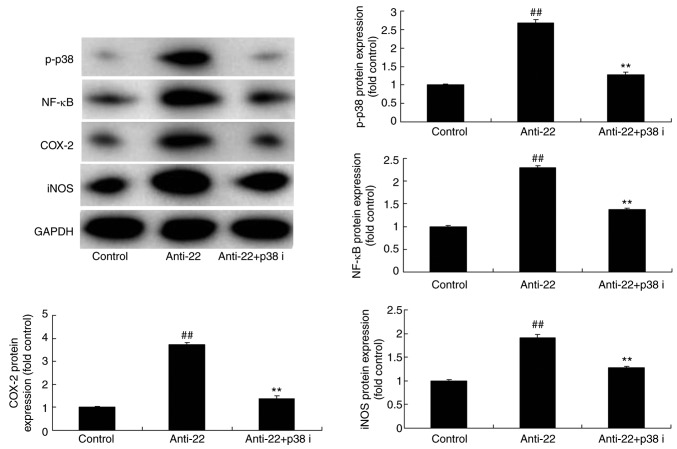
To investigate the mechanism of action of miRNA-22 against stroke, the potential miRNA-22 binding sites in the 3′-untranslated region (3′-UTR) of p38 MAPK were identified (Fig. 4A) and a luciferase reporter assay was performed. Co-transfection of PC12 cells with anti-miR-122 and the wild-type p38/MAPK 3-UTR sequence resulted in an increase in luciferase activity when compared with the mutant 3′-UTR sequence, indicating that miR-122 may target p38 MAPK and regulate its expression (Fig. 4B). Results of immunofluorescence demonstrated that the downregulation of miRNA-22 could induce the protein expression of p-p38 MAPK in the model in vitro, compared with the control group (Fig. 4C). Additionally, the downregulation of miRNA-22 upregulated the protein expression of p-p38, NF-κB, COX-2 and iNOS in the in vitro model, compared with the control group (Fig. 4D). By comparison, overexpression of miRNA-22 suppressed the protein expression of p-p38, NF-κB, COX-2 and iNOS in model in vitro, compared with the control group (Fig. 5).
Figure 4.
Downregulation of miRNA-22 regulates p38 MAPK/NF-κB signaling in the in vitro stroke model. (A) The potential microRNA-22 binding sites in the 3′-untranslated region (3′UTR) of p38 MAPK. (B) Luciferase activity levels. (C) Immunofluorescence for p-p38 protein expression (magnification, ×100). (D) p-p38, NF-κB, COX-2 and iNOS protein expression was examined by western blotting and quantified. ##P<0.01 vs. control negative group. miRNA-22, microRNA-22; Anti-22, downregulation of miRNA-22 group; MAPK, mitogen-activated protein kinase; NF, nuclear factor; p-, phosphorylated; COX, cyclooxygenase; iNOS, inducible NO synthase.
Figure 5.
Overexpression of miRNA-22 regulates p38 MAPK/NF-κB signaling in the in vitro stroke model. p-p38, NF-κB, COX-2 and iNOS protein expression was determined by western blotting and statistically analyzed. ##P<0.01 vs. control negative group. Control, control negative group; miRNA-22, overexpression of microRNA-22 group; MAPK, mitogen-activated protein kinase; NF, nuclear factor; p-, phosphorylated; COX, cyclooxygenase; iNOS, inducible NO synthase.
p38 inhibitor reduces the pro-inflammatory effects of anti-miRNA-22 in the in vitro ischemic stroke model
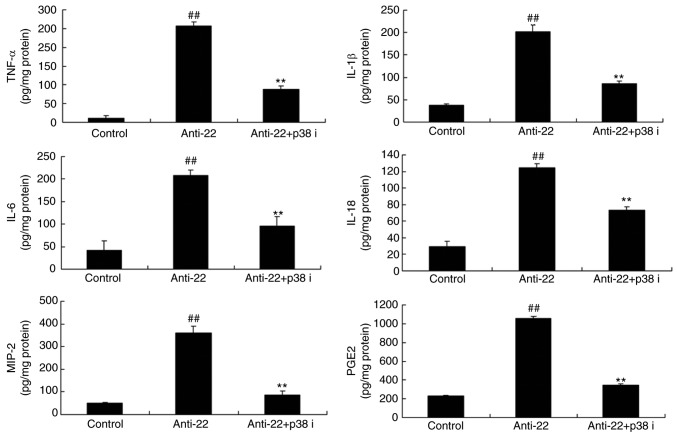
To further investigate the role of p38 in the pro-inflammatory effects of anti-miRNA-22 on stroke, p38 inhibitor (LY2228820; 3 nM) was used in the in vitro ischemic stroke model by anti-miRNA-22. As shown in Fig. 6, p38 inhibitor suppressed the protein expression of p-p38, NF-κB, COX-2 and iNOS in vitro by anti-miRNA-22, compared with the anti-miRNA-22 group. Furthermore, p38 inhibitor also reduced the pro-inflammatory effects of anti-miRNA-22 on the levels of TNF-α, IL-1β, IL-6, IL-18, MIP-2 and PGE2 in model in vitro, compared with the anti-miRNA-22 only group (Fig. 7).
Figure 6.
p38 inhibitor reduces the proinflammatory effects of Anti-22 in model in vitro. p-p38, NF-κB, COX-2 and iNOS protein expression was determined by western blotting and statistically analyzed. ##P<0.01 vs. control negative group, **P<0.01 vs. downregulation of miRNA-22 group. Anti-22, downregulation of miRNA-22 group; Anti-22+p38 i, downregulation of miRNA-22 and p38 inhibitor group; p-, phosphorylated; NF, nuclear factor; COX, cyclooxygenase; iNOS, inducible NO synthase.
Figure 7.
p38 inhibitor reduces the proinflammatory effects of Anti-22 in the in vitro stroke model. TNF-α, IL-1β, IL-6, IL-18, MIP-2 and PGE2 expression was determined by ELISA. ##P<0.01 compared with control group, **P<0.01 compared with downregulation of microRNA-22 group. Anti-22, downregulation of microRNA-22 group; Anti-22+p38 i, downregulation of miRNA-22 and p38 inhibitor group; TNF, tumor necrosis factor; IL, interleukin; MIP, macrophage inflammatory protein; PGE2, prostaglandin E2.
Discussion
Stroke is one of the commonest frequently-occurring diseases at present, with high mortality and disability rate. Fortunately, the survival rate of patients has been improved with the development of medical technology (20). However, the disability rate has also increased accordingly. Cognitive dysfunction often follows a stoke, and a clinical report has suggested it occurs in 50–75% of patients (4). The present study first examined how miRNA-22 expression is downregulated in ischemic stroke rats, compared with a control group. Jovicic et al (18) demonstrated that miRNA-22 overexpression exhibited a neuroprotective effect in a rat and a cell model, this served a basis for the present study.
Following a stroke, inflammatory cells, including CNS astrocytes, microglial cells, peripheral neutrophil and T cells, are activated (21). The activation and infiltration of these inflammatory cells can further induce the release of inflammatory cytokines and chemokines, including IL-1β, TNF-α, MIP-1 and monocyte chemoattractant protein (22,23). A clinical study indicated that inflammatory reaction is closely associated with brain injury and functional recovery in patients with ischemic stroke (24). Consequently, the key links and concrete mechanism of post-stroke inflammatory reaction is essential for the treatment and prognosis of strokes (23). In the current study, it was demonstrated that miRNA-22 reduced TNF-α, IL-1β, IL-6 and IL-18 expression, and inhibited MIP-2 and PGE2 expression in the stroke model. Yang et al (23) suggested that miRNA-22 served an important cardioprotective role, and could partly reduce cell apoptosis and inflammatory damage in myocardial ischemia/reperfusion injury. These results were consistent with those of the present study, which demonstrated that miRNA-22 reduced inflammation in stroke.
COX-2, a rate-limiting enzyme that can transform arachidonic acid to prostaglandin (9), is an important mediator in the stroke-induced inflammation cascade reaction: PGE2, an efficient pro-inflammatory mediator, together with other inflammatory mediators, are generated (25). Subsequently, microglial cells can be activated to form an inflammatory positive feedback loop (26). iNOS is another reactive enzyme in inflammation, the expression of which is associated with neuron damage. It can consistently catalyze a large amount of nitric oxide with potential neurotoxicity (26). These finding suggests that miRNA-22 can suppress the protein expression of COX-2 and iNOS in stroke. Furthermore, Fan et al (27) revealed that miRNA-22 could downregulate the mRNA expression of MMP-3, iNOS and Cox-2 in a rheumatoid arthritis rat model, which is consistent with the results of the present study, which demonstrated that miRNA-22 inhibited COX-2 and iNOS in stroke.
A previous study have suggested that the p38 MAPK pathway can activate NF-κB cell apoptosis. Typically, the p38 MAPK pathway serves an essential role not only in cell apoptosis, but also in a variety of pathological processes, including inflammation, cell stress, cell cycle and growth (28). In addition, the p38 MAPK pathway can be activated by a variety of physical, chemical and inflammatory factors, stress stimulation and gram-positive bacterial cell wall components. Additionally, it has been identified as serving an important role in inflammation and stress reaction (29). On the other hand, the pathway can be activated by cerebral ischemia/reperfusion, as identified in certain studies (13,25). The influence of the central nervous system following activation mainly presents negative regulation, which is achieved through mediating a variety of inflammatory and pathogenic factors. This will cause damage to the plasticity of hippocampal synapses (30). A number of studies have confirmed that the inflammatory factors TNF-α and IL-1β, are associated with the central nervous system (13,30). In the meantime, ischemia exerts essential effects on tissue damage, rehabilitation and immune reaction process (18). These inflammatory factors exhibit toxic effects on neurons and can cause neuron apoptosis and necrosis, promote neuron damage, directly damage the plasticity of hippocampal synapses and lead to long-term potentiation decay (31). Furthermore, the present study demonstrated that miRNA-22 suppressed the protein expression of p-p38 and NF-κB in a stroke model. Liang et al (32) suggested that miRNA-22 impairs the antitumor ability of dendritic cells by targeting p38. The present study only identified the function of p38 in adjusting inflammation, however, the effects of p38 could also regulate cell apoptosis, which requires further study.
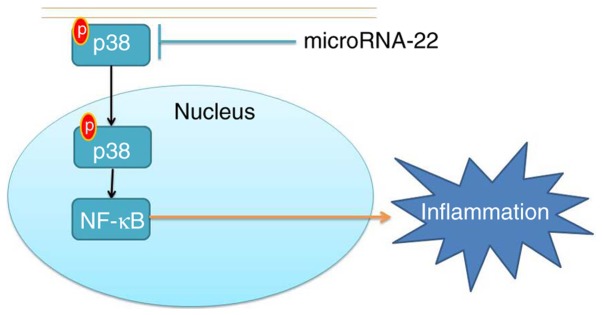
In conclusion, the present study demonstrated that miRNA-22 alleviated ischemic stroke-induced inflammation in rats and thus inhibited the expression of MIP-2, PGE2, COX-2 and iNOS by inhibiting the p38 MAPK/NF-κB pathways (Fig. 8). These results are beneficial for the development of miRNA-22 as a therapy for the prevention and treatment of ischemic stroke, based on its anti-inflammatory effects.
Figure 8.
MicroRNA-22 alleviates inflammation in ischemic stroke via p38 MAPK pathways. MAPK, mitogen-activated protein kinase; p, phosphorylated NF, nuclear factor.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
XH designed the experiments. HD and BC performed the experiments. XH wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Animal care and the general protocols for animal use were approved by the Institutional Animal Care and Use Committee of Shandong Jining No. 1 People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Luo F, Liu J, Yan T, Miao M. Salidroside alleviates cigarette smoke-induced COPD in mice. Biomed Pharmacother. 2017;86:155–161. doi: 10.1016/j.biopha.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Peng R, Zhao W, Liu Q, Guo Y, Zhao S, Xu D. Zhibitai and low-dose atorvastatin reduce blood lipids and inflammation in patients with coronary artery disease. Medicine (Baltimore) 2017;96:e6104. doi: 10.1097/MD.0000000000006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun P, Song SZ, Jiang S, Li X, Yao YL, Wu YL, Lian LH, Nan JX. Salidroside regulates inflammatory response in raw 264.7 macrophages via TLR4/TAK1 and ameliorates inflammation in alcohol binge drinking-induced liver injury. Molecules. 2016;21:E1490. doi: 10.3390/molecules21111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mello EA, Cohen LG, Monteiro Dos Anjos S, Conti J, Andrade KN, Tovar Moll F, Marins T, Fernandes CA, Rodrigues W, Jr, Conforto AB. Increase in short-interval intracortical facilitation of the motor cortex after low-frequency repetitive magnetic stimulation of the unaffected hemisphere in the subacute phase after stroke. Neural Plast. 2015;2015:407320. doi: 10.1155/2015/407320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blesneag AV, Slavoaca DF, Popa L, Stan AD, Jemna N, Isai Moldovan F, Mureșanu F. Low-frequency rTMS in patients with subacute ischemic stroke: Clinical evaluation of short and long-term outcomes and neurophysiological assessment of cortical excitability. J Med Life. 2015;8:378–387. [PMC free article] [PubMed] [Google Scholar]
- 6.Koh GC, Yen SC, Tay A, Cheong A, Ng YS, De Silva DA, Png C, Caves K, Koh K, Kumar Y, et al. Singapore tele-technology aided rehabilitation in stroke (STARS) trial: Protocol of a randomized clinical trial on tele-rehabilitation for stroke patients. BMC Neurol. 2015;15:161. doi: 10.1186/s12883-015-0420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piaggesi A, Sambataro M, Nicoletti C, Goretti C, Lacopi E, Coppelli A. Safety and effectiveness of therapeutic magnetic resonance in diabetic foot ulcers: A prospective randomised controlled trial. J Wound Care. 2016;25:704–711. doi: 10.12968/jowc.2016.25.12.704. [DOI] [PubMed] [Google Scholar]
- 8.Xu ZR, Ran XW, Xian Y, Yan XD, Yuan GY, Mu SM, Shen JF, Zhang BS, Gan WJ, Wang J. Ertapenem versus piperacillin/tazobactam for diabetic foot infections in China: A Phase 3, multicentre, randomized, double-blind, active-controlled, non-inferiority trial. J Antimicrob Chemother. 2016;71:1688–1696. doi: 10.1093/jac/dkw004. [DOI] [PubMed] [Google Scholar]
- 9.You HJ, Han SK, Rhie JW. Randomised controlled clinical trial for autologous fibroblast-hyaluronic acid complex in treating diabetic foot ulcers. J Wound Care. 2014;23:521–522, 524, 526–530. doi: 10.12968/jowc.2014.23.11.521. [DOI] [PubMed] [Google Scholar]
- 10.Amoli MM, Hasani-Ranjbar S, Roohipour N, Sayahpour FA, Amiri P, Zahedi P, Mehrab-Mohseni M, Heshmat R, Larijani B, Tavakkoly-Bazzaz J. VEGF gene polymorphism association with diabetic foot ulcer. Diabetes Res Clin Pract. 2011;93:215–219. doi: 10.1016/j.diabres.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Drela E, Kulwas A, Jundzill W, Góralczyk B, Boinska J, Drewniak W, Gadomska G, Rość D. VEGF-A and PDGF-BB - angiogenic factors and the stage of diabetic foot syndrome advancement. Endokrynol Pol. 2014;65:306–312. doi: 10.5603/EP.2014.0042. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F, Ren Y, Liu P, Ren Y, Wang D. Expression of TGF-beta1 and miRNA-145 in patients with diabetic foot ulcers. Exp Ther Med. 2016;11:2011–2014. doi: 10.3892/etm.2016.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing: Promotive role of miR-21 in fibroblast migration. Int Wound J. 2012;9:355–361. doi: 10.1111/j.1742-481X.2011.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agh F, Mohammadzadeh Honarvar N, Djalali M, Nematipour E, Gholamhoseini S, Zarei M, Ansari S, Javanbakht MH. Omega-3 fatty acid could increase one of myokines in male patients with coronary artery disease: A randomized, double-blind, placebo-controlled trial. Arch Iran Med. 2017;20:28–33. [PubMed] [Google Scholar]
- 15.Liu Q, Du GQ, Zhu ZT, Zhang C, Sun XW, Liu JJ, Li X, Wang YS, Du WJ. Identification of apoptosis-related microRNAs and their target genes in myocardial infarction post-transplantation with skeletal myoblasts. J Transl Med. 2015;13:270. doi: 10.1186/s12967-015-0603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iekushi K, Seeger F, Assmus B, Zeiher AM, Dimmeler S. Regulation of cardiac microRNAs by bone marrow mononuclear cell therapy in myocardial infarction. Circulation. 2012;125:1765–1773. doi: 10.1161/CIRCULATIONAHA.111.079699. [DOI] [PubMed] [Google Scholar]
- 17.Salic K, De Windt LJ. MicroRNAs as biomarkers for myocardial infarction. Curr Atheroscler Rep. 2012;14:193–200. doi: 10.1007/s11883-012-0238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jovicic A, Zaldivar Jolissaint JF, Moser R, Silva Santos Mde F, Luthi-Carter R. MicroRNA-22 (miR-22) overexpression is neuroprotective via general anti-apoptotic effects and may also target specific Huntington's disease-related mechanisms. PLoS One. 2013;8:e54222. doi: 10.1371/journal.pone.0054222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Mou Y, Bernstock JD, Klimanis D, Wang S, Spatz M, Maric D, Johnson K, Klinman DM, Li X, et al. Synthetic oligodeoxynucleotides containing multiple telemeric TTAGGG motifs suppress inflammasome activity in macrophages subjected to oxygen and glucose deprivation and reduce ischemic brain injury in stroke-prone spontaneously hypertensive rats. PLoS One. 2015;10:e0140772. doi: 10.1371/journal.pone.0140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha TS, Hong EJ, Han GD. Diabetic conditions downregulate the expression of CD2AP in podocytes via PI3-K/Akt signalling. Diabetes Metab Res Rev. 2015;31:50–60. doi: 10.1002/dmrr.2562. [DOI] [PubMed] [Google Scholar]
- 22.Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact. 2014;219:101–112. doi: 10.1016/j.cbi.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Chen L, Ding J, et al. Cardioprotective effect of miRNA-22 on hypoxia/reoxygenation induced cardiomyocyte injury in neonatal rats. Gene. 2016;579:17–22. doi: 10.1016/j.gene.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Kim HJ, Kim CW. GLCCI1 is a novel component associated with the PI3K signaling pathway in podocyte foot processes. Exp Mol Med. 2016;48:e233. doi: 10.1038/emm.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: A multicenter randomized controlled trial. Diabetes Care. 2008;31:631–636. doi: 10.2337/dc08-1148. [DOI] [PubMed] [Google Scholar]
- 26.Morley S, Griffiths J, Philips G, Moseley H, O'Grady C, Mellish K, Lankester CL, Faris B, Young RJ, Brown SB, Rhodes LE. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: A new approach to antimicrobial therapy. Br J Dermatol. 2013;168:617–624. doi: 10.1111/bjd.12098. [DOI] [PubMed] [Google Scholar]
- 27.Fan P, He L, Hu N, et al. Effect of 1,25-(OH)2D3 on Proliferation of Fibroblast-Like Synoviocytes and Expressions of Pro-Inflammatory Cytokines through Regulating MicroRNA-22 in a Rat Model of Rheumatoid Arthritis. Cell Physiol Biochem. 2017;42:145–155. doi: 10.1159/000477123. [DOI] [PubMed] [Google Scholar]
- 28.Liang L, Stone RC, Stojadinovic O, Ramirez H, Pastar I, Maione AG, Smith A, Yanez V, Veves A, Kirsner RS, et al. Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair Regen. 2016;24:943–953. doi: 10.1111/wrr.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Sun XJ, Chen J, Hu ZW, Wang L, Gu DM, Wang AP. Increasing the miR-126 expression in the peripheral blood of patients with diabetic foot ulcers treated with maggot debridement therapy. J Diabetes Complications. 2017;31:241–244. doi: 10.1016/j.jdiacomp.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Isner JM, Ropper A, Hirst K. VEGF gene transfer for diabetic neuropathy. Hum Gene Ther. 2001;12:1593–1594. [PubMed] [Google Scholar]
- 31.Laurents DV, Gorman PM, Guo M, Rico M, Chakrabartty A, Bruix M. Alzheimer's Abeta40 studied by NMR at low pH reveals that sodium 4,4-dimethyl-4-silapentane-1-sulfonate (DSS) binds and promotes beta-ball oligomerization. J Biol Chem. 2005;280:3675–3685. doi: 10.1074/jbc.M409507200. [DOI] [PubMed] [Google Scholar]
- 32.Liang X, Liu Y, Mei S, et al. MicroRNA-22 impairs anti-tumor ability of dendritic cells by targeting p38. PLoS One. 2015;10:e0121510. doi: 10.1371/journal.pone.0121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.