Abstract
Objectives
The present retrospective cohort study aims to test the hypothesis that elements of swallowing mechanics including hyoid movement, laryngeal elevation, tongue base retraction, pharyngeal shortening, pharyngeal constriction, and head and neck extension can be grouped into functional modules, and that these modules are predictably altered in disease states.
Methods
Modified barium swallow video clips of a thick and a thin liquid swallow from 40 normal patients and 10 dysphagic post‐treatment oropharyngeal head‐and‐neck cancer (HNC) patients were used in this study. Coordinate locations of 12 anatomical landmarks mapping pharyngeal swallowing mechanics were tracked on every frame during the pharyngeal phase of each swallow using a custom‐made MATLAB tool. Morphometric modularity hypothesis testing was performed on these coordinate data to characterize the modular elements of swallowing function in each cohort using MorphoJ software.
Results
The elements of normal swallowing can be grouped into four functional modules including bolus propulsion, pharyngeal shortening, airway protection, and head and neck posture. Modularity in HNC patient showed an intact airway protection module but altered bolus propulsion and pharyngeal shortening modules. To cross‐validate the alteration in modules, a post hoc analysis was performed, which showed significantly increased vallecular (P < .04) and piriform (P < .05) residue but no significant change in aspiration status in the HNC cohort versus controls.
Conclusions
This study suggests that while pharyngeal swallowing mechanics is highly complex, the system is organized into functional modules, and that changes in modularity impacts swallowing performance. This approach to understanding swallowing function may help the patient care team better address swallowing difficulties.
Level of Evidence
2b
Keywords: Deglutition, dysphagia, swallowing mechanics, functional modularity
INTRODUCTION
Swallowing difficulty is a comorbidity of a number of pathologies1, 2, 3, 4 that can dramatically decrease quality of life, leading to malnutrition, dehydration, aspiration pneumonia, mealtime anxiety, and social isolation.5 The pharyngeal phase of swallowing, which involves over 20 muscles suspended by the skull base and mandible to convert a respiratory channel into an alimentary tract and back again in less that 1 second, is complex.6, 7, 8, 9 Although modified barium swallow (MBS) imaging is the standard for visualizing swallowing physiology, the multiple elements of functional anatomy underlying pharyngeal swallowing are difficult to appreciate with this technique.10 Consequently, in the absence of a trained swallowing specialist, MBS imaging is often used to test aspiration status rather than to meaningfully characterize the impaired elements of the swallowing apparatus. The purpose of this study is to determine whether the multiple elements of swallowing mechanics can be simplified into functionally organized modules to provide a clinically useful way to assess swallowing mechanics that can be associated with swallowing performance outcomes such as aspiration or stasis.
Computational analysis of swallowing mechanics (CASM) uses MBS imaging to characterize the multiple interactions of swallowing mechanics.11, 12, 13, 14 This method performs multivariate morphometric analysis of coordinates, mapping various muscle groups that propel a bolus through the upper esophageal sphincter while protecting the airway.15, 16, 17, 18, 19, 20 Since muscle groups change coordinate configuration, shape change analysis can be used to mathematically evaluate and visualize how these various elements of swallowing mechanics interact.11, 12, 13 It remains to be determined whether the elements of swallowing mechanics function independently, or if they function cohesively as modules of a system.
A multivariate morphometric analysis called modularity hypothesis testing, can determine whether elements of a dynamic system are functionally independent or organized into modules.21 Modularity is a characteristic often observed in biological systems that describes the distribution of interaction between the elements that make up the biological system.12 A module is a group of elements that share strong space‐time interactions among themselves, but are relatively independent of the elements within other modules. Using morphometric modularity analysis, the present study aims to test the hypotheses that: elements of swallowing mechanics form distinct modules in normal swallowing (hypothesis 1); functional modules are consistent across age, sex, and bolus types (hypothesis 2); and that these modules are altered as a comorbidity of a dysphagia‐associated disease (hypothesis 3).
MATERIALS AND METHODS
The MBS video clips used in this study were selected from a database of deidentified videos. This database is maintained under ethics approval as non‐human research by the institutional review board where this work was performed. The MBS clips represented the swallowing function of 30 randomly chosen normal patients, 10 randomly chosen post‐treatment oropharyngeal head‐and‐neck cancer (HNC) patients, and 10 additional normal patients that were age‐ and gender‐matched to the HNC group. Randomization was balanced by sex. In this sample, 19 subjects were younger than 60 years old (mean = 53 ± 7.8), and 21 subjects were 60 years old or greater (mean = 71 ± 7.4). Two MBS clips were used from each patient: one 5 mL thin liquid bolus swallow and one 5 mL thick liquid bolus swallow. In total, 80 swallows from 40 control patients and 20 swallows from 10 dysphagic HNC patients were included.
A custom‐built MATLAB tracker tool was used to track the locations of 12 anatomical landmarks through each frame of the swallow (Fig. 1).22 These landmarks have been described previously.23, 24, 25, 26 The landmarks are homologous across individuals and map muscle groups that underlie the various elements of pharyngeal swallowing mechanics including: hyoid movement, laryngeal elevation, hyolaryngeal approximation, pharyngeal shortening, tongue base retraction, pharyngeal constriction, and head and neck posture (Fig. 2).
Figure 1.
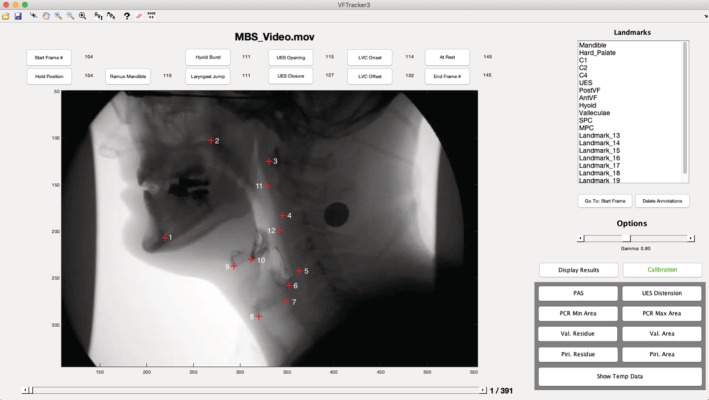
Screenshot of the MATLAB tracker tool used to track the coordinate locations of 12 anatomical landmarks. The landmarks are as follows: 1) mandible, 2) hard palate, 3) C1, 4) C2, 5) C4, 6) upper esophageal sphincter, 7) posterior vocal fold, 8) anterior vocal fold, 9) hyoid bone, 10) pit of the valleculae, 11) superior pharyngeal constrictor, and 12) middle pharyngeal constrictor.
Figure 2.
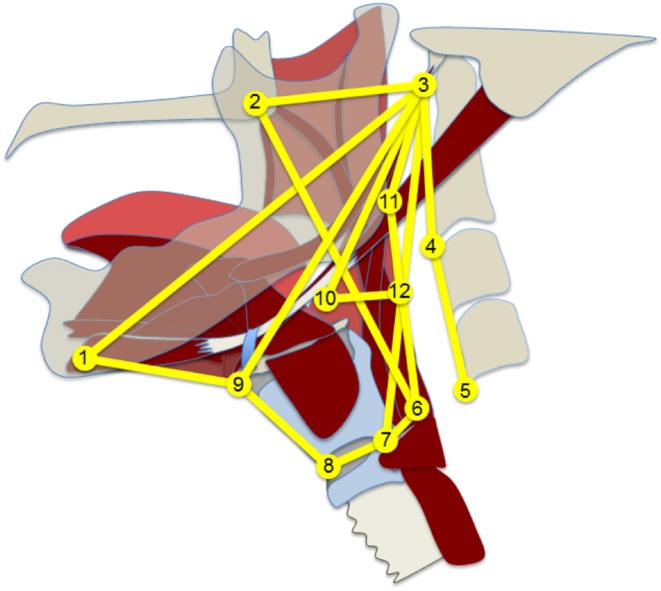
Adjacency graph in yellow represents the functional musculoskeletal organization of the swallowing apparatus. Landmarks approximate muscle attachment sites for muscle groups with lines representing either muscle function or the skeletal levers that suspends the swallowing apparatus. Landmarks are labeled in Figure 1. Line representations are discussed in the “Materials and methods” section.
Two of the authors (PH and YT) upgraded the MATLAB tracker tool to make several improvements. For this study, only coordinate data from the pharyngeal phase of swallowing were included, which was defined as the first frame of hyoid burst to the closure of the upper esophageal sphincter. A compiler function was added to prepare the data captured by the tracker tool for use in MorphoJ, an integrated software package for multivariate morphometric analysis.27 The compiler function generates a unique identifier for each frame from each MBS, concatenates all coordinate data into one large text file, and classifies each frame of data by swallowing stage, type of bolus (5 mL thin or 5 mL thick), and cohort (HNC vs. control). These files are uploaded into MorphoJ for analysis. To perform modularity hypothesis testing, MorphoJ requires three additional user inputs: an adjacency graph, the number of expected functional modules, and hypothesized configuration of the modules.
MorphoJ uses adjacency graphs to characterize the relationships among anatomical landmarks. The adjacency graph must meet two criteria. First, the adjacency graph must be contiguous, and second, the relationships depicted by the adjacency graph must be anatomically meaningful.21 The adjacency graph used for this study is based on prior anatomical work, which described all interactions of the swallowing apparatus that make anatomical sense given that two‐dimensional (2D) videofluoroscopy is a projection of three‐dimensional (3D) morphology (Fig. 2). Landmarks approximate muscle attachment sites for muscle groups with lines representing either muscle function or the skeletal levers that suspends the swallowing apparatus.26 There are multiple lines that represent various muscles or muscle groups including: suprahyoid muscles (landmarks 1‐9‐3), thyrohyoid (landmarks 8‐9), stylopharyngeus (landmarks 7‐3), palatopharyngeus (landmarks 6‐2),25 and pharyngeal constrictor muscles (landmarks 3‐11‐12‐6 with 12‐10 representing the middle pharyngeal constrictor).24 The styloglossus and hyoglossus function together to displace the base of the tongue represented by landmark 10.17, 23 The hyolaryngeal complex, which incorporates the airway and esophagus, is represented by landmarks 6‐7‐8‐9.26 The lines connecting landmarks 1‐3, 2‐3, and 3‐4‐5 represent the mandible, cranial base, and vertebrae, respectively.25
To determine the number of expected functional modules represented in the adjacency graph, an iterative method was used to test every possible number of modules. Each module requires at least two landmarks, thus with 12 landmarks, the number of modules could be 2, 3, 4, 5, or 6. MorphoJ reports the minimum multiset random variable (RV) coefficient used to measure of the strength or degree of modularity.21 The higher the RV coefficient, the less independent the modules are overall. The configuration of landmarks with the lowest multiset RV coefficient (indicating modular independence) with an anatomically meaningful result was selected for this study. This iterative method resulted in four independent modules.
The final required input is an initial configuration of the four modules. This a priori map of hypothesized coordinates belonging to each module is based on previous anatomical work, and is shown in Figure 3a. Landmarks 9, 8, and 7 representing the elements of hyoid and larynx movement were hypothesized to function as a module that relocates and protects the airway. Landmarks 6 and 2 were designated as a hypothesized module representing the function of the long pharyngeal muscles to shorten the pharynx. Landmarks 10, 11, and 12 representing the tongue base and posterior pharyngeal wall were hypothesized to function as a module underlying bolus propulsion. Finally, landmarks 1, 3, 4, and 5 were hypothesized to function as a module underlying head and neck posture.
Figure 3.
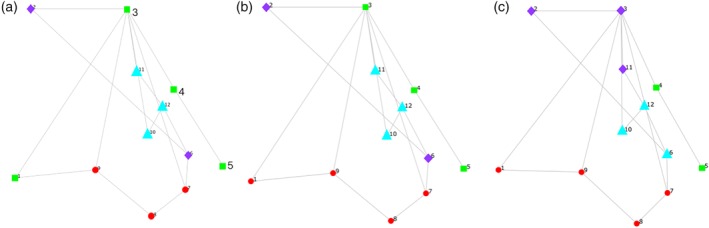
(a) Four hypothesized modules of pharyngeal swallowing in normal patients. (b) Alternative hypothesis representing four modules of pharyngeal swallowing in normal patients. Red circles = airway protection, purple diamonds = pharyngeal shortening, light blue triangles = bolus propulsion, and green squares = head and neck posture. (c) Alteration in modules in head‐and‐neck cancer patients with a disruption to pharyngeal shortening and bolus propulsion modules.
Once the adjacency graph, number of functional modules, and the a priori hypothesis were entered into MorphoJ, the a priori hypothesis was tested using modularity hypothesis testing. MorphoJ performs this test by comparing the a priori hypothesis to all other combinations of modules made possible by the specified adjacency graph and number of modules. The modular configuration with the lowest multiset RV coefficient was considered the best representation of the modularity of the system, and either confirmed the a priori hypothesis or suggested an alternative configuration.
To characterize the modularity in normal swallowing (hypothesis 1), modularity hypothesis testing was performed on the 80 swallows from 40 normal patients. To determine whether sex, age, and bolus type altered modularity (hypothesis 2), the data set was stratified by sex (20 males, 20 females), age (21 subjects >60 years, 19 subjects <60 years), and bolus type (low vs. high viscosity), and each new data set was retested using modularity hypothesis testing. To explore how modules are impacted by a disease state (hypothesis 3), modularity hypothesis testing was applied to the 20 swallows collected from 10 HNC patients and compared with results from age and gender matched controls.
RESULTS
Hypothesis 1: Elements of Swallowing Mechanics Form Distinct Modules in Normal Swallowing
Modularity hypothesis testing of normal swallowing mechanics revealed the configuration shown in Figure 3b. This alternative configuration (RV = 0.311) was statistically equivalent with the a priori hypothesized configuration (RV = 0.314) shown in Figure 3a. Although the alternative configuration was different from the original a priori hypothesis, it still confirmed that airway protection, pharyngeal shortening, and bolus propulsion function as modules. The difference was that landmark 1 (genial tubercle of the mandible) was functionally aligned with airway protection (hyolaryngeal relocation) more so than the extension of the head and neck as seen in the alternative configuration.
Hypothesis 2: Functional Modules of Swallowing Are Consistent across Sex, Age, and Bolus Consistencies
Modularity hypothesis testing normal swallowing mechanics by sex, age, and bolus viscosity showed similar results to hypothesis 1. The best modular configuration among males (RV = 0.35) and low viscosity swallows (RV = 0.33) was found to be the a priori hypothesized configuration (Fig. 3a). However, the best modular configuration among older subjects (RV = 0.30) and younger subjects (RV = 0.35), females (RV = 0.26), and high viscosity bolus swallow (RV = 0.30) was found to be the alternative configuration (Fig. 3b).
Hypothesis 3: Modules of Swallowing Are Altered as a Comorbidity of a Dysphagia Associated Disease
Modularity testing of HNC cohort rejected both the a priori hypothesis of normal swallowing (Fig. 3a, RV = 0.46) and the alternative configuration of normal swallowing (Fig. 3b, RV = 0.45). The altered modularity of the HNC sample resulting in the minimum RV is shown in Figure 3c (RV = 0.41). The age‐ and gender‐matched control group (N = 10 subjects and 20 swallows) matched normal swallowing modularity represented in Figure 3b.
DISCUSSION
Modularity hypothesis testing in the present study suggests that: 1) the elements of swallowing mechanics form distinct functional modules in normal swallowing, 2) these modules are consistent across age, sex, and bolus types, and 3) dysphagia‐associated disease such as HNC alters functional modules of swallowing in this small sample. Functional modules of swallowing mechanics defined by this method include airway protection, bolus propulsion, pharyngeal shortening, and head and neck posture.
The Four Functional Modules of Swallowing Mechanics (Hypothesis 1)
The present study suggests that hyoid movement and laryngeal elevation function as a module to protect the airway.28, 29, 30 This result is supported by previous studies showing that the hyoid and larynx display adaptive behavior in swallowing.31 The importance of hyoid movement has been documented in the dysphagia literature.32 The suprahyoid muscles position the tongue for oral propulsion, and relocate the airway via the thyrohyoid membrane during pharyngeal swallowing.16 The long pharyngeal and thyrohyoid muscles elevate the larynx and ensure laryngeal vestibular closure.15, 16 Additionally, hyolaryngeal displacement aids in stretching open an inhibited upper esophageal sphincter, which is important to the prevention of aspiration.28, 33, 34 Disease or treatment that impacts hyolaryngeal movement, such as radiation to the floor of the mouth, can threaten airway safety.35
This study provides evidence that pharyngeal shortening functions as an independent module.36 The long pharyngeal muscles, including the palatopharyngeus and stylopharyngeus, primarily function to shorten the pharynx.15, 16 Pharyngeal shortening likely has two important functions in pharyngeal swallowing. One is to pull the upper esophageal sphincter around the head of the oncoming bolus. The other function is to help reduce the volume of the pharynx thereby increasing bolus pressure to propel the bolus through the upper esophageal sphincter. Surgeries that impact the long pharyngeal muscles, such as uvulopalatopharyngoplasty, have been associated with dysphagia.37 These muscles can be specifically targeted by swallowing rehabilitation therapy.15, 38
Tongue base retraction, performed by the styloglossus and hyoglossus muscles, and pharyngeal constriction, performed by the pharyngeal constrictor muscles, behave functionally as a module to propel the bolus through the hypopharynx.39 Clinically, it can be observed that when the tongue base does not fully retract, the pharyngeal stripping wave increases. This phenomenon where one element of swallowing mechanics compensates for another suggests modularity. Radiation therapy affecting these structures has been shown to negatively impact pharyngeal clearance of a bolus.40
The vertebra functions as a module to maintain head and neck posture. The a priori hypothesis and the alternative configuration pictured in Figure 3a, 3b were essentially equivalent mathematically. The difference, as noted in the results, was whether the movement of the mandible is associated with the movement of the hyoid and larynx or the vertebrae. The functional similarities between the two configurations suggest that mandibular position is involved in both airway protection and head and neck posture. Slightly flexing the head and neck during swallowing has also been shown to improve airway protection for some subjects.41 It may be that the alternative configuration reflects this adaptation. Both configurations demonstrate the importance of head and neck posture as a functional module, which is underscored by dysphagia associated with cervical neck disorders.42
Consistency across Age, Sex, and Bolus Properties (Hypothesis 2)
Results of hypothesis 2 indicate that the aforementioned four functional modules were consistent across age, sex, and bolus viscosity in this sample of normal patients. The only variation seen was that come cohorts’ modularity matched the a priori hypothesis, and others matched the alternative configuration. As discussed previously, these two configurations (Fig. 3a, 3b) are essentially mathematically equivalent and may represent normal variations in swallowing. Age and bolus properties have been shown to impact various elements of swallowing mechanics.43, 44 However, these changes likely represent adaptions to various swallowing conditions and anatomical changes over the lifespan rather than abnormalities in swallowing function. Modularity may prove to be a more reliable indicator of swallowing dysfunction than isolated alterations in swallowing mechanics.
Disease Alters Modularity (Hypothesis 3)
Modularity analysis of the small sample of HNC patients compared with age‐ and gender‐matched controls suggests that disease alters functional modules. If these modules are important to swallowing function, then a disrupted module should be predictive of impaired swallowing performance. Swallowing performance variables include penetration‐aspiration status and residue as indications of unsafe or inefficient swallowing outcomes, respectively. In this small sample of HNC patients, the bolus propulsion and pharyngeal shortening modules were disrupted whereas the airway protection module was preserved (Fig. 3c). Based on this result, it was predicted that residue would be more prevalent than aspiration in this sample. A post hoc comparison of penetration aspiration scale scores45 and normalized residue ratio scores46 between the HNC cohort with age‐ and gender‐ matched controls showed a significant increase in vallecular residue (P < .04) and piriform residue (P < .05) in the 10 HNC patients with no significant change in penetration‐aspiration status.
Limitations
It should be underscored that the logical association of modular components to functions such as bolus propulsion, while helpful, does not provide a comprehensive paradigm for characterizing pharyngeal swallowing dysfunction. For example, bolus residue can result from a stricture of the upper esophageal sphincter with little to do with impaired modular function. However, understanding the functional anatomy underlying swallowing can provide much greater insight compared with simply reporting the aspiration status of a dysphagic patient. While it is important for swallowing specialists to specifically measure and report swallowing difficulty using tools such as the modified barium swallow impairment profile (MBSImP), a modular explanation of pharyngeal swallowing function is more useful for communicating the significance of those results to colleagues and patients alike.
These findings do not address the question of the chronological sequence of the functional elements of swallowing. Timing data are embedded in this analysis asynchronously as opposed to chronologically since every frame of a 30 fps video is included in the analysis. While variation in the timing of swallowing events occurs in normal swallowing, timing variables not characterized by this method may be critically important in characterizing dysphagia, especially in a stroke population.47, 48, 49, 50 However, it may be that these aberrations in timing also change modularity and should be the subject of future studies.
The results of the present study are from 2D imaging of 3D structures. Repeating this study using coordinates collected from 4D data such as 320 slice helical computed tomography (CT) would further the validity to these findings.51 However, since MBS imaging is primarily how clinicians assess dysphagia, the phenomenon of swallowing physiology needs to be translated for this imaging modality to be clinically useful. Unilateral deficits can be masked by lateral view videofluoroscopy, which presents superimposed 3D structures. Whether CASM is sensitive enough to document the impact of unilateral insults on swallowing mechanics is a subject for future study.
If functional modules are organized at the brainstem level, it is unliklely that age, sex, or bolus type results in fundamental changes to modularity.7 It is also reasonable to predict that a disease state, which impacts effector organs or the neurobiology of swallowing, alters modularity. While the present proof of concept study supports these ideas, more data are needed to document whether age and bolus type impact modularity. How modularity of swallowing function is affected by different disease populations is a subject of future study.
Finally, a limitation of the RV statistic should be noted. This statistic is useful when comparing covariation relative to a single data set, but is not appropriate when comparing covariation between data sets.21 The RV results reported in this study should not lead the reader to think that modularity in normal swallowing is more functionally covariant in normal swallows that in the HNC group, for example. These numbers are primarily useful for determining the minimal covariation to establish which elements belong to a module functionally within a given data set.
As for clinical utility of CASM as a method, the current technology is not easily executed outside of the research environment. If modularity proves to be generalizable, an observational approach to assessment could be developed. Perceptual scoring has been validated in other methods of evaluating swallowing mechanics. Modularity as an explanatory model for swallowing function may facilitate understanding this complicated process in clinical training. Ultimately, artificial intelligence may allow for a clinically accessible technology to emerge.
CONCLUSION
In this pilot study, morphometric modularity hypothesis testing was used to describe four functional modules of swallowing mechanics. Muscles displacing the hyoid and larynx behave as a functional module to protect the airway, whereas extrinsic and intrinsic tongue muscles function with pharyngeal constrictor muscles to drive a bolus through the hypopharynx. The long pharyngeal muscles function as a module to shorten the pharynx to generate pressure and assist in laryngeal elevation. The posture of the head and neck also functions as a module. These functional modules appear to be consistent across sex, age, and bolus type. Alteration of modular function may result in predictably impaired swallowing performance.
Acknowledgments
Imaging data included in this study were acquired through grants from NIH/NIDCD. We acknowledge the grant support provided by Medical Scholars Program, Medical College of Georgia at Augusta University.
Editor's Note: This Manuscript was accepted for publication 30 April, 2019.
This research was conducted in Dr. Pearson's Lab, Department of Cellular Biology and Anatomy, MCG, Augusta University, Augusta, Georgia, U.S.A.
Funding: Imaging data included in this study were acquired through the following grants: NIH/NIDCD K24DC12801, PI: Martin‐Harris; NIH/NIDCD K23 DC005764‐05, PI: Martin‐Harris; NIH/NIDCD R21DC10480‐2. PI: Martin‐Harris.
BIBLIOGRAPHY
- 1. O'Kane L, Groher M. Oropharyngeal dysphagia in patients with chronic obstructive pulmonary disease: a systematic review. Rev CEFAC 2009;11:449–506. [Google Scholar]
- 2. Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol 2006;24:2636–2643. [DOI] [PubMed] [Google Scholar]
- 3. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36:2756–2763. [DOI] [PubMed] [Google Scholar]
- 4. Heffernan C, Jenkinson C, Holmes T, et al. Nutritional management in MND/ALS patients: an evidence based review. Amyotroph Lateral Scler Other Motor Neuron Disord 2004;5:72–83. [DOI] [PubMed] [Google Scholar]
- 5. Ekberg O, Hamdy S, Woisard V, Wuttge–Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia 2002;17:139–146. [DOI] [PubMed] [Google Scholar]
- 6. Shaw SM, Martino R. The normal swallow: muscular and neurophysiological control. Otolaryngol Clin North Am 2013;46:937–956. [DOI] [PubMed] [Google Scholar]
- 7. Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 2001;81:929–969. [DOI] [PubMed] [Google Scholar]
- 8. Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am 2008;19:691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia 2010;25:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin‐Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment‐‐MBSImp: establishing a standard. Dysphagia 2008;23:392–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tran TTA, Martin Harris B, Pearson WG. Improvements resulting from respiratory‐swallow phase training visualized in patient‐specific computational analysis of swallowing mechanics. Comput Methods Biomech Biomed Eng Imaging Vis 2016;6(5):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garand K, Schwertner R, Chen A, Pearson WG. Computational analysis of pharyngeal swallowing mechanics in patients with motor neuron disease: a pilot investigation. Dysphagia 2018;33(2):243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dietsch AM, Rowley CB, Solomon NP, Pearson WG. Swallowing mechanics associated with artificial airways, bolus properties, and penetration–aspiration status in trauma patients. J Speech Lang Hear Res 2017;60:2442–2451. [DOI] [PubMed] [Google Scholar]
- 14. May NH, Pisegna JM, Marchina S, Langmore SE, Kumar S, Pearson WG Jr. Pharyngeal swallowing mechanics secondary to hemispheric stroke. J Stroke Cerebrovasc Dis 2017;26:952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pearson WG, Hindson DF, Langmore SE, Zumwalt AC. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2013;85:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pearson WG, Langmore SE, Yu LB, Zumwalt AC. Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia 2012;27:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gassert RB, Pearson WG. Evaluating muscles underlying tongue base retraction in deglutition using muscular functional magnetic resonance imaging (mfMRI). Magn Reson Imaging 2016;34:204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okuda S, Abe S, Kim H‐J, et al. Morphologic characteristics of palatopharyngeal muscle. Dysphagia 2008;23:258–266. [DOI] [PubMed] [Google Scholar]
- 19. Meng H, Murakami G, Suzuki D, Miyamoto S. Anatomical variations in stylopharyngeus muscle insertions suggest interindividual and left/right differences in pharyngeal clearance function of elderly patients: a cadaveric study. Dysphagia 2008;23:251–257. [DOI] [PubMed] [Google Scholar]
- 20. Gilbert RJ, Napadow VJ, Gaige TA, Wedeen VJ. Anatomical basis of lingual hydrostatic deformation. J Exp Biol 2007;210:4069–4082. [DOI] [PubMed] [Google Scholar]
- 21. Klingenberg CP. Morphometric integration and modularity in configurations of landmarks: tools for evaluating a priori hypotheses. Evol Dev 2009;11:405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Natarajan R, Stavness I, Pearson W. Semi‐automatic tracking of hyolaryngeal coordinates in videofluoroscopic swallowing studies. Comput Methods Biomech Biomed Eng Imaging Vis 2015;5(6):379–389. [Google Scholar]
- 23. Pearson WG Jr, Taylor BK, Blair J, Martin‐Harris B. Computational analysis of swallowing mechanics underlying impaired epiglottic inversion. Laryngoscope 2016;126:1854–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwertner RW, Garand KL, Pearson WG Jr. A novel imaging analysis method for capturing pharyngeal constriction during swallowing. J Imaging Sci 2016;1:1–6. [PMC free article] [PubMed] [Google Scholar]
- 25. Thompson ZT, Obeidin F, Davidoff AA, et al. Coordinate mapping of hyolaryngeal mechanics in swallowing. J Vis Exp 2014;87:e51476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pearson WG, Zumwalt AC. Visualising hyolaryngeal mechanics in swallowing using dynamic MRI. Comput Methods Biomech Biomed Eng Imaging Vis 2014;2:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 2011;11:353–357. [DOI] [PubMed] [Google Scholar]
- 28. Pauloski BR, Rademaker AW, Logemann JA, et al. Relationship between swallow motility disorders on videofluorography and oral intake in patients treated for head and neck cancer with radiotherapy with or without chemotherapy. Head Neck 2006;28:1069–1076. [DOI] [PubMed] [Google Scholar]
- 29. Bingjie L, Tong Z, Xinting S, Jianmin X, Guijun J. Quantitative videofluoroscopic analysis of penetration‐aspiration in post‐stroke patients. Neurol India 2010;58:42–47. [DOI] [PubMed] [Google Scholar]
- 30. Feng X, Todd T, Hu Y, et al. Age‐related changes of hyoid bone position in healthy older adults with aspiration. Laryngoscope 2014;124:E231–E236. [DOI] [PubMed] [Google Scholar]
- 31. Humbert IA, Christopherson H, Lokhande A, German R, Gonzalez‐Fernandez M, Celnik P. Human hyolaryngeal movements show adaptive motor learning during swallowing. Dysphagia 2013;28:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Molfenter SM, Steele CM. Physiological variability in the deglutition literature: hyoid and laryngeal kinematics. Dysphagia 2011;26:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cook IJ, Dodds WJ, Dantas RO, et al. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 1989;257:748–759. [DOI] [PubMed] [Google Scholar]
- 34. Logemann JA, Kahrilas PJ, Cheng J, et al. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol Gastrointest Liver Physiol 1992;262:G338–G344. [DOI] [PubMed] [Google Scholar]
- 35. Kumar R, Madanikia S, Starmer H, et al. Radiation dose to the floor of mouth muscles predicts swallowing complications following chemoradiation in oropharyngeal squamous cell carcinoma. Oral Oncol 2014;50:65–70. [DOI] [PubMed] [Google Scholar]
- 36. Kahrilas PJ. Pharyngeal structure and function. Dysphagia 1993;8:303–307. [DOI] [PubMed] [Google Scholar]
- 37. Jäghagen EL, Berggren D, Dahlqvist Å, Isberg AJ. Prediction and risk of dysphagia after uvulopalatopharyngoplasty and uvulopalatoplasty. Acta Otolaryngol 2004;124:1197–1203. [DOI] [PubMed] [Google Scholar]
- 38. Vasquez‐Miloro K, Pearson W, Langmore S. Effortful pitch glide: a potential new exercise evaluated by dynamic MRI. J Speech Lang Hear Res 2014;57:1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology 1992;103:128–136. [DOI] [PubMed] [Google Scholar]
- 40. Pauloski BR, Logemann JA. Impact of tongue base and posterior pharyngeal wall biomechanics on pharyngeal clearance in irradiated postsurgical oral and oropharyngeal cancer patients. Head Neck 2000;22:120–131. [DOI] [PubMed] [Google Scholar]
- 41. Shanahan TK, Logemann JA, Rademaker AW, Pauloski BR, Kahrilas PJ. Chin‐down posture effect on aspiration in dysphagic patients. Arch Phys Med Rehabil 1993;74:736–739. [DOI] [PubMed] [Google Scholar]
- 42. Papadopoulou S, Exarchakos G, Beris A, Ploumis A. Dysphagia associated with cervical spine and postural disorders. Dysphagia 2013;28:469–480. [DOI] [PubMed] [Google Scholar]
- 43. Leonard R, Kendall K, McKenzie S. Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia 2004;19(2):133–141. [DOI] [PubMed] [Google Scholar]
- 44. Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia 2015;30:2–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration‐aspiration scale. Dysphagia 1996;11:93–98. [DOI] [PubMed] [Google Scholar]
- 46. Pearson W, Molfenter S, Smith Z, Steele C. Image‐based measurement of post‐swallow residue: the normalized residue ratio scale. Dysphagia 2013;28:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Daele DJ, McCulloch TM, Palmer PM, Langmore SE. Timing of glottic closure during swallowing: a combined electromyographic and endoscopic analysis. Ann Otol Rhinol Laryngol 2005;114:478–487. [DOI] [PubMed] [Google Scholar]
- 48. Mendell DA, Logemann JA. Temporal sequence of swallow events during the oropharyngeal swallow. J Speech Lang Hear Res 2007;50:1256–1271. [DOI] [PubMed] [Google Scholar]
- 49. Oommen ER, Kim Y, McCullough G. Stage transition and laryngeal closure in poststroke patients with dysphagia. Dysphagia 2011;26:318–323. [DOI] [PubMed] [Google Scholar]
- 50. Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil 1993;74:1295–1300. [DOI] [PubMed] [Google Scholar]
- 51. Inamoto Y, Fujii N, Saitoh E, et al. Evaluation of swallowing using 320‐detector‐row multislice CT, Part II: kinematic analysis of laryngeal closure during normal swallowing. Dysphagia 2011;26:209–217. [DOI] [PubMed] [Google Scholar]


