Abstract
Nerve growth factor (NGF), a member of the neurotrophin family, was initially described as neuronal survival and growth factor, but successively has emerged as an active mediator in many essential functions in the central nervous system of mammals. NGF is synthesized as a precursor pro‐NGF and is cleaved intracellularly into mature NGF. However, recent evidence demonstrates that pro‐NGF is not a simple inactive precursor, but is also secreted outside the cells and can exert multiple roles. Despite the vast literature present in mammals, studies devoted to NGF in the brain of other vertebrate models are scarce. Zebrafish is a teleost fish widely known for developmental genetic studies and is well established as model for translational neuroscience research. Genomic organization of zebrafish and mouse NGF is highly similar, and zebrafish NGF protein has been reported in mature and two‐precursors forms. To add further knowledge on neurotrophic factors in vertebrate brain models, we decided to determine the NGF mRNA and protein distribution in the adult zebrafish brain and to characterize the phenotype of NGF‐positive cells. NGF mRNA was visualized by in situ hybridization on whole‐mount brains. NGF protein distribution was assessed on microtomic sections by using an antiserum against NGF, able to recognize pro‐NGF in adult zebrafish brain as demonstrated also in previous studies. To characterize NGF‐positive cells, anti‐NGF was employed on microtomic slides of aromatase B transgenic zebrafish (where radial glial cells appeared fluorescent) and by means of double‐immunolabeling against NGF/proliferative cell nuclear antigen (PCNA; proliferation marker) and NGF/microtube‐associated protein2 (MAP2; neuronal marker). NGF mRNA and protein were widely distributed in the brain of adult zebrafish, and their pattern of distribution of positive perikaryal was overlapping, both in males and females, with few slight differences. Specifically, the immunoreactivity to the protein was observed in fibers over the entire encephalon. MAP2 immunoreactivity was present in the majority of NGF‐positive cells, throughout the zebrafish brain. PCNA and aromatase B cells were not positive to NGF, but they were closely intermingled with NGF cells. In conclusion, our study demonstrated that mature neurons in the zebrafish brain express NGF mRNA and store pro‐NGF.
Keywords: brain, encephalon, fish, neurotrophins, zebrafish neurons
Introduction
Nerve growth factor (NGF) is the first identified factor, together with brain‐derived neurotrophic factor (BDNF), neurotrophin (NT) 3 and NT 4/5, belonging to the neurotrophin family. As all members of the family, NGF is synthesized as a precursor form (pro‐NGF) and either secreted outside the cells, as pro‐ and mature NGF, or cleaved intracellularly to mature NGF. Active forms of pro‐ and mature NGF are homodimers. To date, three types of NGF receptors are known: TrkA, p75 and sortilin. TrkA, the high‐affinity receptor, is a member of receptor tyrosine kinases family, which includes receptor TrkB (for BDNF and NT 4/5) and TrkC (for NT 3). p75NTR is a member of the tumor necrosis factor receptor superfamily, and can transduce signals of all neurotrophins. Sortilin is a member of the family of Vps10p‐domain transmembrane receptors, and was earlier characterized as a receptor for neurotensin. While TrkA mediates trophic signaling of the mature form of NGF, p75NTR bifunctionally mediates: (i) a signal to neuronal survival, especially when binding to mature NGF and acting together with TrkA; or (ii) the induction of neuronal death when forming a receptor complex with sortilin and mediating pro‐NGF signal (for review, see Niewiadomska et al., 2011).
Nerve growth factor, initially described as neuronal survival and growth factor, encompasses roles regarding the density of innervation, synthesis of neurotransmitters and neuropeptides and cell body size, axonal sprouting and dendritic arborization (for review, see Minnone et al., 2017).
The brain of fish possesses, beyond structural organization similar to all vertebrates, extremely high adult neurogenesis and astonishing regenerative properties. These peculiarities make the fish brain suitable for discovering regenerative mechanisms probably suppressed in mammals during evolutive adaptation (Panula et al., 2010; Cacialli et al., 2018b). Among teleost fish, zebrafish (Danio rerio) is widely used as model species for developmental genetic studies and functional mechanisms of numerous genes responsible for human diseases (D'Angelo et al., 2016a,b). In addition, zebrafish brain has been largely utilized in numerous studies devoted to adult neurogenesis (Pellegrini et al., 2007; Diotel et al., 2013; Coumailleau et al., 2015; Than‐Trong & Bally‐Cuif, 2015; Anand & Mondal, 2017) and regenerative ability after injury (Cosacak et al., 2015; Alunni & Bally‐Cuif, 2016; Cacialli et al., 2018a,b).
In fish species, homologs of mammalian neurotrophins have been identified, and an additional member, named NT 6/7, probably originated by a duplication of the ray‐finned fish NGF (Dethleffsen et al., 2003). NT 6/7 has been identified also in zebrafish (Nilsson et al., 1998). Genomic organization of zebrafish and mouse NGF are highly similar, and zebrafish NGF protein has been reported in a mature form of 194 amino acid (a.a.; Dethleffsen et al., 2003). In addition, two pro‐NGF isoforms have been described in zebrafish: isoform 1 (NP_001338647.1) and isoform 2 (NP_954680.2), respectively, of 224 and 261 a.a. (Dethleffsen et al., 2003).
The distribution pattern of neurotrophins and their Trk receptors was described in the brain of adult fish (Dalton et al., 2009a,b; D'Angelo et al., 2012, 2014a,b, 2016a,b; Cacialli et al., 2016; Gatta et al., 2016). Furthermore, BDNF appeared to be involved in repairing mechanisms of adult zebrafish after traumatic brain injury (Cacialli et al., 2018a,b; Lucini et al., 2018).
In the present study, we evaluated NGF mRNA and protein distribution in the brain of zebrafish, and identified neurons as the NGF source cells. Thanks to the conserved adult neurogenesis in fish brain and the potential involvement of neurotrophin in the regenerative ability, the identification of NGF neurons in the adult brain of zebrafish could represent a useful tool to evaluate its involvement in the regenerative process after injury or chemical/genetic‐induced degeneration.
Materials and methods
Animals and brain dissection
Animals used in this study were housed in zebrafish facilities (INRA LPGP, BIOSIT, Rennes, France, agreement number: B 35‐238‐6) under standard conditions of photoperiod (14/10) and temperature (28 °C). This project was approved by the local animal care and Westerfield ethics committee (Comité Rennais d'Ethique en matière d'Expérimentation Animale, Rennes, France), under the number EEA B‐35‐040. Zebrafish did not receive medical treatment prior to or during the experience. No deaths occurred in the facilities before killing of the animals used for in situ hybridization (ISH) and immunohistochemistry (IHC) experiments. Fish were suppressed with an overdose of tricaine methanesulfonate (MS‐222).
ISH
Oligonucleotide primers used to amplify and clone cDNA for the production of NGF ISH probes are: forward 5′‐ACATGTACCATGAGGAGCAC‐3′; reverse 5′‐GTCGCTGGTGTGTGGAAAAT‐3′ (708 bp; NM_001351718.1). For preparation of NGF digoxigenin (DIG)‐labeled antisense riboprobe, the vector pCRII‐TOPO containing NGF was linearized by BamHI digestion, and DIG‐labeled riboprobe was prepared using in vitro transcription with T7 RNA polymerase. For sense riboprobe, the vector containing NGF was linearized by Not1, and DIG‐labeled riboprobe was prepared using in vitro transcription with SP6 RNA polymerase.
In situ hybridization was performed on whole‐mount brains as previously described by Adolf et al. (2006) and Diotel et al. (2015). Briefly, six zebrafish brains (males and females) were excised and fixed in paraformaldehyde (PFA, 4%) dissolved in phosphate‐buffered saline (PBS) for 24 h at 4 °C. Then, the brains were dehydrated in a methanol/PBS concentration (25%; 50%; 75%; 100%) and stored at −20 °C. After rehydrating through methanol/PBS gradient series and washing in PBS, the brains were incubated for 40 min in PBS containing proteinase K (10 μg mL−1) at room temperature (RT). After post‐fixation in 4% PFA for 20 min and washes in PBS, brains were then prehybridized for 1 h and incubated overnight at 65 °C in hybridization buffer (pH 6) containing the DIG‐labeled probe. Then, brains were washed in SCC 2 ×/formamide 50% and SSC 0.2 ×, and pre‐incubated with blocking buffer for 3 h and then overnight with anti‐DIG‐AP, Fab fragments (1:5000; Roche, NJ, USA; Cat# 11093274910, RRID:AB_514497) at 4 °C. The next day the brain sections were washed with PBS before staining with NBT/BCIP buffer (pH 9.5).
Whole‐mount stained brains were embedded in agar 2% and photographed with a Digital camera equipped on Zeiss Stemi. Then, the embedded whole‐mount stained brains were transversally sectioned with a razor blade or vibratome, and the sections were mounted on the slide.
The specificity of the ISH labeling was demonstrated by using sense riboprobe, that showed absence of any staining.
IHC
Imunohistochemical procedures were performed following detailed suggestions reported by de Girolamo & Lucini (2011). Six adult zebrafish brains (male and female) were fixed in Bouin's solution for 24 h and processed for paraffin embedding. Transverse microtome sections were mounted on poly‐lysine slides. Sections were deparaffinized in xylene, rehydrated through graded ethanol, treated with 3% H2O2 for 30 min and rinsed in PBS (pH 7.4), followed by antigen retrieval in sodium citrate buffer (pH 6; 80 °C) for 30 min.
Single IHC
After rinsing twice in 0.2% Triton PBS (PBT), non‐specific binding was blocked by treating sections with 1/5 normal goat serum (Vector, Burlingame, CA, USA; cod S‐1000‐20) for 30 min at RT. Then, sections were incubated overnight at RT in a humidified chamber with rabbit antibody against NGF (Santa Cruz Biotechnology, CA, USA; cod sc‐549; it recognizes N‐terminus of the mature chain of NGF of human origin) diluted 1/100 or 1/300, respectively, depending on the secondary antibody specified at the following point (a) or (b). The next day, the sections were washed several times in PBT and alternatively incubated with (a) Alexa Fluor® goat anti‐rabbit 488 (1:200; Invitrogen Molecular Probes, Eugene, OR, USA; REF: A‐11037; RRID:AB_10561549) for 2 h at RT in a dark and humidified chamber; or (b) EnVision‐horseradish anti‐peroxidase (HRP)‐system (Dako, Santa Barbara, CA, USA; cod. K4002). This system is based on a HRP‐labeled polymer conjugated with goat anti‐rabbit IgG.
Sections treated with Alexa Fluor® goat anti‐rabbit 488, after three washes in PBT, were mounted with the medium Vectashield (Vector) containing 4,6‐diamino‐2‐phenylindole (DAPI), to visualize cell nuclei. Sections treated with EnVision‐HRP‐system were immersed in a fresh solution of 10 μg 3,3′‐diaminobenzidine tetrahydrochloride (Sigma‐Aldrich, St Louis, MO, USA; cod. D5905) in 15 mL of a 0.5 mol, Tris buffer, pH 7.6, containing 1.5 mL of 0.03% H2O2. Then, sections were dehydrated and mounted.
For IHC on cyp19a1bGFP transgenic zebrafish (glial cell marker; Pellegrini et al., 2007), NGF antibody was detected with Alexa Fluor® goat anti‐rabbit 594 (1:200; Invitrogen Molecular Probes, Eugene, OR, USA; REF: A‐11037; RRID: AB_10561549).
The specificity of IHC, assessed by substitution of NGF antiserum, secondary antibody fluorescent dye‐conjugated or the EnVision with PBS or normal serum, achieved no specific immunostaining. Moreover, the incubation of NGF antiserum pre‐incubated with its homologous antigen showed no immunoreactivity (Fig. S1), and NGF antiserum pre‐incubated with its heterologous antigens did not modify the normal pattern of immunostaining.
Double‐immunolabeling
Double‐immunocytochemical staining NGF/proliferative cell nuclear antigen (PCNA) and NGF/microtubule‐associated protein2 (MAP2) was performed as follows: dewaxed and rehydrated consecutive sections were rinsed in PBS, and incubated for 48 h at RT. PCNA, antibody at diluted 1:100, was used to detect proliferative cells (Clone PC10; Dako, Glostrup, Denmark; REF: M0879; RRID: AB_2160651). This antibody is a marker of proliferating cells in vertebrate species, including zebrafish (Pellegrini et al., 2007; Marz et al., 2011; Cacialli et al., 2016, 2018a,b). MAP2 antibody, diluted 1:100, was used to detect neurons [sc‐74422 MAP‐2 (A‐8); Santa Cruz Biotechnology, Santa Cruz, CA, USA]. After rinsing in PBS, the sections were incubated for 2 h at RT with a mixture of secondary antibodies directed against rabbit and mouse IgG: (a) Alexa Fluor® goat anti‐mouse 594 (1:200; Invitrogen Molecular Probes, Eugene, OR, USA; REF: A‐11005; RRID: AB_10561507); (b) Alexa Fluor® goat anti‐rabbit 594 (1:200; Invitrogen Molecular Probes, Eugene, OR, USA; REF: A‐11037; RRID: AB_10561549). Tissue sections were washed in PBS‐Triton 0.2%, and slides were mounted with the Vectashield medium containing DAPI for nuclei counterstaining (Vector Laboratories, Burlingame, CA, USA). Controls for double‐immunolabeling were performed by incubating the sections with one of the two primary antisera and with the mismatched secondary antibodies.
Microscopy
The stained sections were photographed using a Nikon Eclipse 90i microscope, an epifluorescence microscope Olympus equipped with a DP71 digital camera and Leica DM6B, SN: 449492.
The digital raw images were optimized for image resolution, contrast, evenness of illumination and background by using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA, USA).
Results
Nerve growth factor mRNA (Fig. 1A) and protein were widely distributed in the brain of adult zebrafish (Table 1). The pattern of distribution of positive perikarya was overlapping, both in males and females, with few slight differences (Table 1). Specifically, the immunoreactivity to the protein was observed in fibers over the entire encephalon. Thus, regions characterized only by the presence of fibers, such as the glomerular layer of olfactory bulbs and deep and white zone of the optic tect, showed positivity to the protein. Based on these general considerations, for the sake of simplicity, the term NGF in place of NGF mRNA and NGF protein was used in the following description of results. The anatomical terminology follows ‘Neuroanatomy of the zebrafish brain’ by Wulliman et al. (1996).
Figure 1.
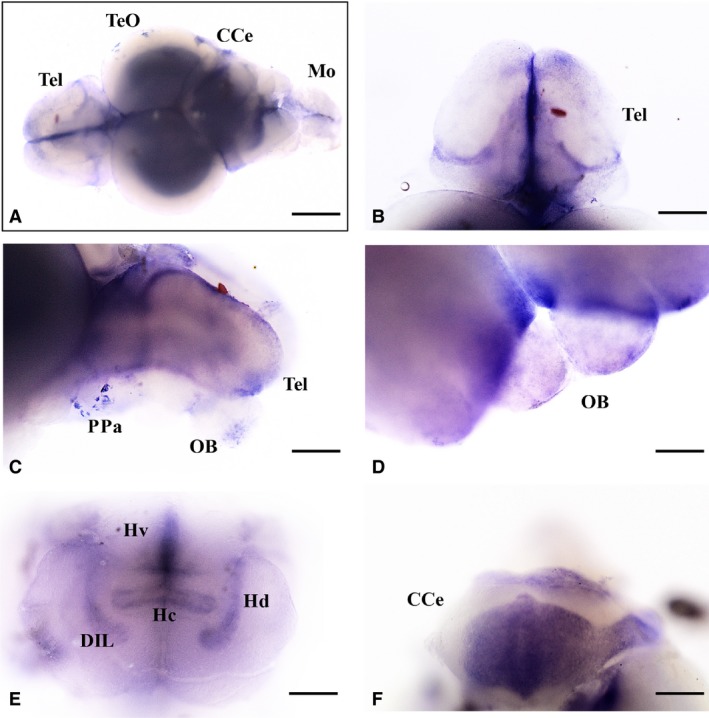
Nerve growth factor (NGF) mRNA in whole‐mount brain of adult zebrafish. NGF mRNA distribution in (A) whole‐mount brain; (B) telencephalon; (C, D) preoptic area and olfactory bulbs; (E) hypothalamus; and (F) cerebellum. CCe, cerebellum; DIL, diffuse nucleus of the inferior lobe; Hv, ventral zone of periventricular hypothalamus; Hc, caudal zone of periventricular hypothalamus; Hd, dorsal zone of periventricular hypothalamus; Mo, medulla oblongata; OB, olfactory bulbs; PPa, preoptic area; Tel, telencephalon; TeO, optic tect; Scale bars: 500 μm (A); 250 μm (B, C, E, F); 150 μm (D).
Table 1.
Distribution of NGF mRNA and protein in the brain of adult zebrafish
| Brain region | NGF mRNA | NGF protein |
|---|---|---|
| Olfactory bulbs | ||
| Glomerular layer | ++ | |
| External cellular layer | + | + |
| Internal cellular layer | + | + |
| Dorsal telencephalic area | ||
| Medial zone of dorsal telencephalic area | + | ++ |
| Dorsal zone of dorsal telencephalic area | + | + |
| Lateral zone of dorsal telencephalic area | + | + |
| Central zone of dorsal telencephalic area | ++ | ++ |
| Posterior zone of dorsal telencephalic area | ++ | + |
| Ventral telencephalic area | ||
| Ventral‐dorsal part | + | ++ |
| Ventral‐central part | + | ++ |
| Preoptic area | ||
| Magnocellular preoptic nucleus | + | + |
| Parvocellular preoptic nucleus, anterior part | ++ | ++ |
| Parvocellular preoptic nucleus, posterior part | + | ++ |
| Epithalamus | ||
| Dorsal habenular nucleus | + | + |
| Ventral habenular nucleus | + | + |
| Dorsal thalamus | ||
| Central posterior thalamic nucleus | + | ++ |
| Ventral thalamus | ||
| Ventromedial thalamic nucleus | + | ++ |
| Ventrolateral thalamic nucleus | + | + |
| Posterior tuberculum | ||
| Posterior tuberal nucleus | ++ | ++ |
| Lateral preglomerular nucleus | + | + |
| Medial preglomerular nucleus | + | ++ |
| Hypothalamus | ||
| Diffuse nucleus of the inferior lobe | + | ++ |
| Ventral zone of periventricular hypothalamus | ++ | ++ |
| Dorsal zone of periventricular hypothalamus | + | + |
| Caudal zone of periventricular hypothalamus | + | + |
| Mammillary body | + | + |
| Synencephalon | ||
| Nucleus of the medial longitudinal fascicle | + | + |
| Optic tectum | ||
| Periventricular gray zone of optic tectum | ++ | ++ |
| Deep white zone | ++ | |
| Central zone | + | + |
| Superficial white gray zone | + | ++ |
| Longitudinal torus | + | + |
| Torus semicircularis | ||
| Central nucleus of semicircular torus | + | + |
| Tegmentum | ||
| Superior reticular formation | + | ++ |
| Cerebellum | ||
| Molecular layer | + | + |
| Purkinje cell layer | ++ | ++ |
| Medulla oblongata | ||
| Inferior reticular formation | + | + |
The scheme was done following qualitative and not quantitative criteria.
NGF, nerve growth factor.
Telencephalon
The olfactory bulbs showed a moderate quantity of NGF (Fig. 1C,D). The cells of both external and internal cellular layers and fibers of the glomerular layer resulted positive.
In the whole telencephalon, more intense NGF positivity was seen in the ventral telencephalon (Fig. 1C,D) and in the posterior zone dorsal telencephalic area (Fig. 1B,C). Positive cells were distributed in the medial (Fig. 3B,B1), dorsal (Fig. 8B,D), lateral (Figs 2A1, 3C,C1, and 10B,D), central (Fig. 11B,D) and posterior parts of the dorsal part of the telencephalic area. In the ventral telencephalic area, small round cells were seen in the dorsal and ventral part.
Figure 2.
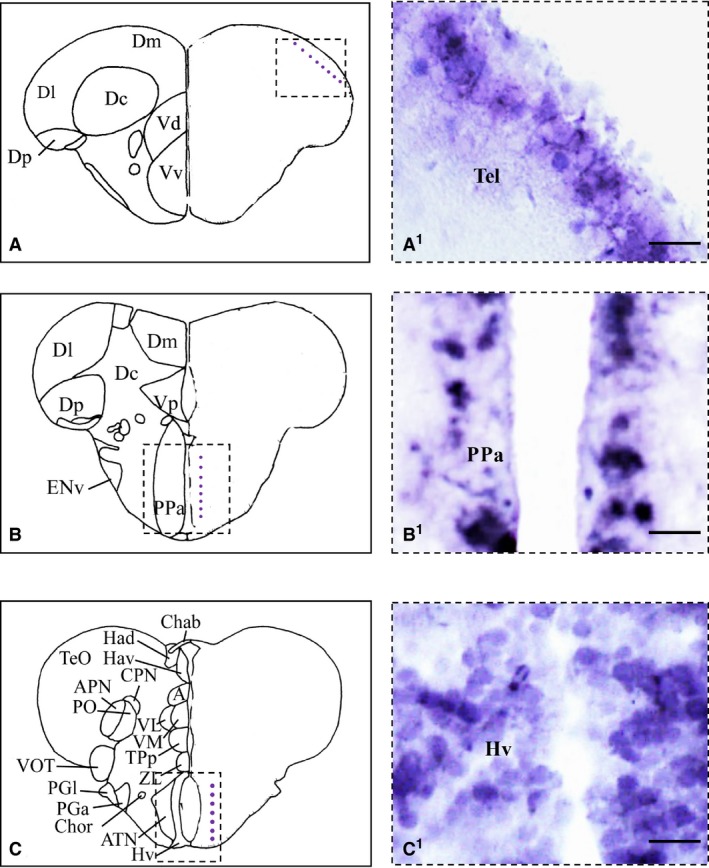
Nerve growth factor (NGF) mRNA in cross‐section of adult zebrafish brain. (A–C) Representative section taken from the zebrafish atlas (Wulliman et al., 1996). NGF‐positive cells are represented by blue dots. (A1–C1) NGF‐positive cells in the dorsal part of the telencephalon (A1), in the anterior parvocellular preoptic nucleus (B1) and along the ventral zone of the periventricular hypothalamus (C1). Hv, ventral zone of periventricular hypothalamus; PPa, anterior parvocellular preoptic nucleus; Tel, telencephalon. Scale bar: 50 μm.
Figure 3.
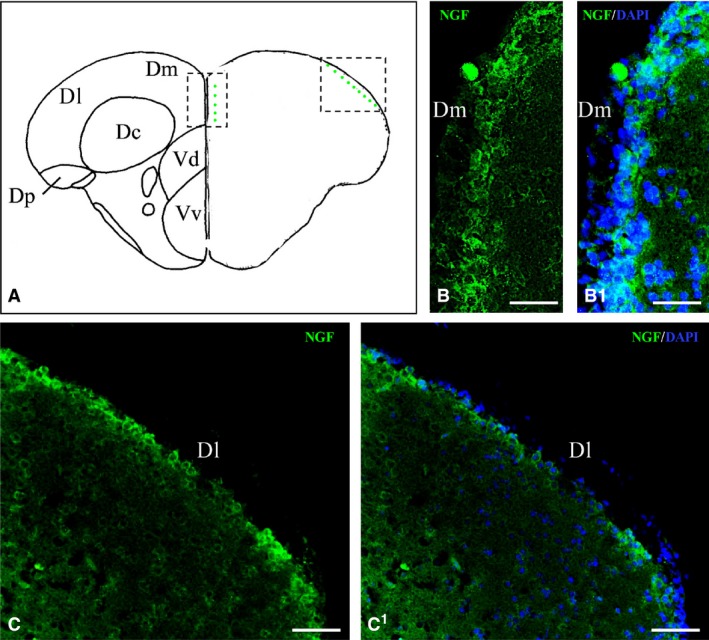
Nerve growth factor (NGF) protein in the telencephalon. (A) Representative section taken from the zebrafish atlas (Wulliman et al., 1996). NGF‐positive cells are represented by green dots. (B, C) Cells positive to NGF in the medial (B) and lateral (C) part of the dorsal telencephalic area. (B1, C1) NGF‐positive cells co‐marked with 4,6‐diamino‐2‐phenylindole (DAPI). Dl, lateral zone of dorsal telencephalic area; Dm, medial zone of dorsal telencephalic area. Scale bars: 100 μm (C, C1); 50 μm (B, B1).
Diencephalon
Numerous intensely stained cells were seen in the anterior parvocellular preoptic nucleus (Figs 1C and 2B1), and in the posterior parvocellular preoptic nucleus (Fig. 9B,D). A weak signal was detected in few cells of the magnocellular preoptic nucleus. In dorsal and ventral habenular nucleus, a few positive cells were detected. A high density of NGF‐positive cells was observed in the ventro‐medial and ventro‐lateral thalamic nuclei (Fig. 9B,D). Few and weak positive cells were seen in the central posterior thalamic nucleus. Numerous positive cells were detected in the posterior tuberal nucleus. In the lateral and medial pre‐glomerular nuclei, positive cells and some fibers were detected.
In the hypothalamus, the ventral zone of the periventricular hypothalamus showed intense positivity in the whole‐mount brain (Fig. 1E), and numerous NGF‐positive cells were seen in histological sections (Figs 1C1 and 4B,C1). In the dorsal (Fig. 1E) and caudal zones of periventricular hypothalamus moderate positivity was seen. Large NGF‐positive cells belonging to the diffuse nucleus of the inferior lobe were seen (Fig. 5A,B1). Few NGF‐positive cells were also present in the mammillary body and in the nucleus of the medial longitudinal fascicle.
Figure 4.
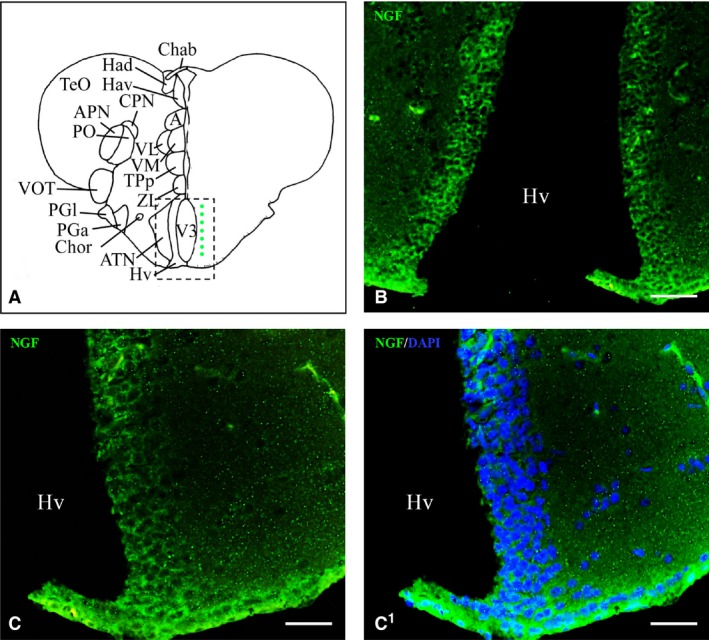
Nerve growth factor (NGF) protein in the hypothalamus. (A) Representative section taken from the zebrafish atlas (Wulliman et al., 1996). NGF‐positive cells are represented by green dots. (B, C) NGF protein (green) along the ventral hypothalamus at low (B) and high (C) magnification. (C1) NGF‐positive cells co‐marked with 4,6‐diamino‐2‐phenylindole (DAPI). Hv, ventral zone of periventricular hypothalamus. Scale bars: 100 μm (B); 50 μm (C, C1).
Figure 5.
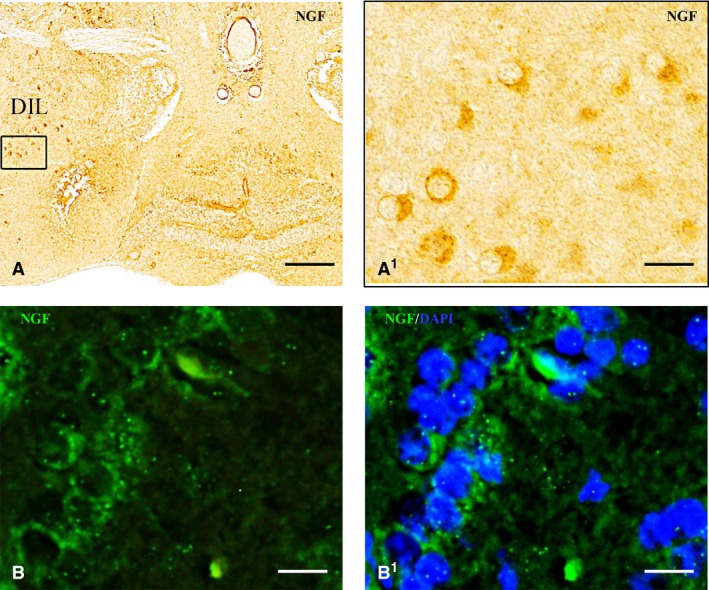
Nerve growth factor (NGF) protein in the hypothalamus. NGF‐positive cells in the diffuse nucleus of inferior lobe (DIL) at low (A) and high (A1, B) magnification. (B1) NGF‐positive cells co‐marked with 4,6‐diamino‐2‐phenylindole (DAPI). Scale bars: 120 μm (A); 30 μm (A1); 20 μm (B, B1).
Mesencephalon
Nerve growth factor‐positive fibers were present in the longitudinal tori and the optic tect, particularly in the deep white zone and superficial white zone. Numerous small NGF‐positive cells appeared scattered in the periventricular gray zone (Fig. 6B1,B2).
Figure 6.
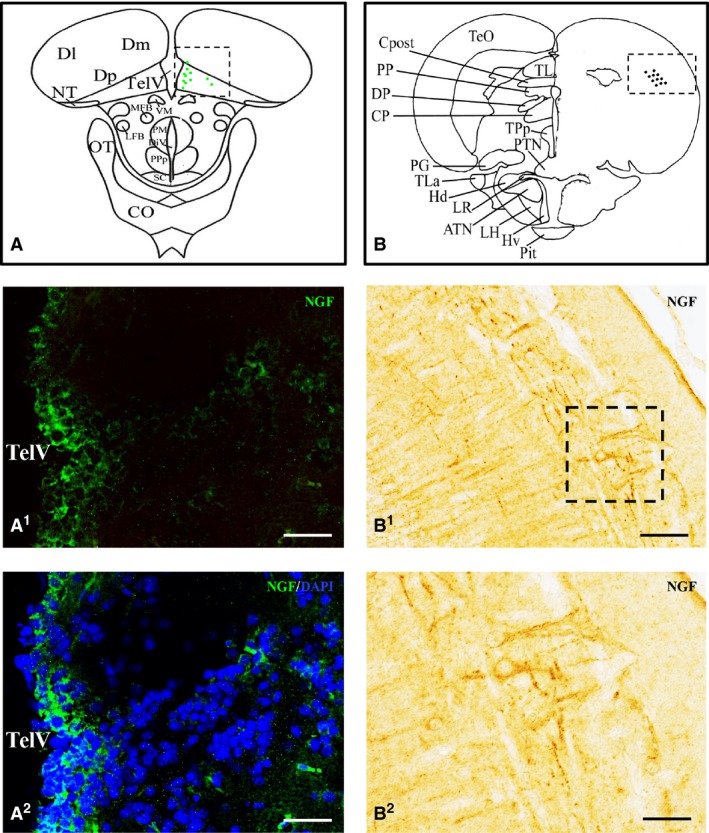
Nerve growth factor (NGF) protein in the forebrain and midbrain. (A, B) Representative sections taken from the zebrafish atlas (Wulliman et al., 1996). NGF‐positive cells are represented by green dots. Cells positive to NGF in the posterior zone of the dorsal telencephalic area (A1) and in the periventricular gray zone of optic tect (B1). (A2, B2) NGF‐positive cells co‐marked with 4,6‐diamino‐2‐phenylindole (DAPI). TelV, telencephalic ventricle. Scale bar: 50 μm.
In the tegmentum, NGF was observed in cells of the central nucleus of semi‐circular torus and superior reticular formation.
Rhombencephalon
The cerebellar body was intensely reactive (Fig. 1F). NGF positivity was observed in numerous large cells of the Purkinje layer and in a few cells of the molecular layer of valvula and body (Fig. 7A–C1). A few weak stained cells were observed in the granular eminence.
Figure 7.
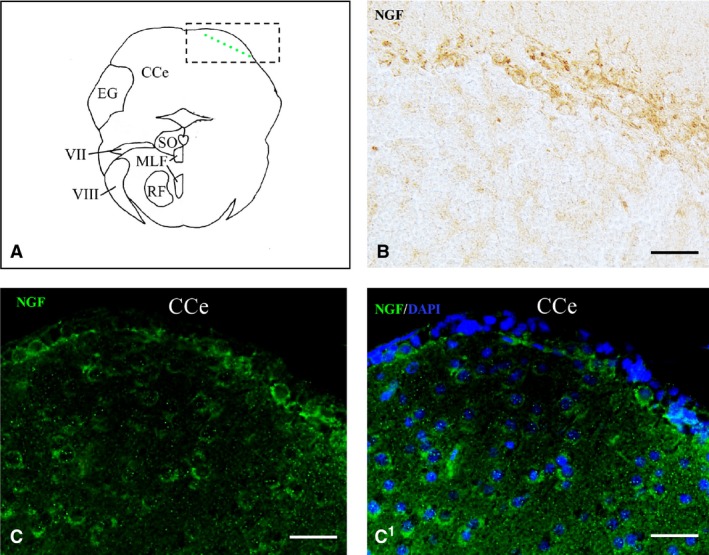
Nerve growth factor (NGF) protein in the cerebellum. (A) Representative section taken from the zebrafish atlas (Wulliman et al., 1996). NGF‐positive cells are represented by green dots. (B, C) NGF‐positive cells in the Purkinje layer of cerebellum. (C1) NGF‐positive cells co‐marked with 4,6‐diamino‐2‐phenylindole (DAPI). CCe, cerebellar body. Scale bar: 50 μm.
In the medulla oblongata, a few large cells containing NGF and belonging to the inferior reticular formation were seen.
Characterization of NGF‐containing cells
In order to identify the nature of NGF‐positive cells in the brain of adult zebrafish, we carried out IHC staining against NGF on slides of aromatase B transgenic fish (cyp19a1b GFP), where radial glial cells appeared green fluorescent. Also, we performed double‐labeling using antibodies against NGF/PCNA (proliferation marker) and NGF/MAP2 (neuronal marker). Aromatase‐positive cells, distributed along ventricles, were closely intermingled with NGF‐positive cells along telencephalic (Fig. 8B–D) and diencephalic (Fig. 9B–D) ventricles. PCNA‐positive cells were positioned along the ventricular lining of the brain, and NGF was detected in the cytoplasm of cells very close to PCNA‐labeled cells (Fig. 10B–D). MAP2 immunoreactivity was present in the majority of NGF‐positive cells, throughout the zebrafish brain (Fig. 11B–D).
Figure 8.
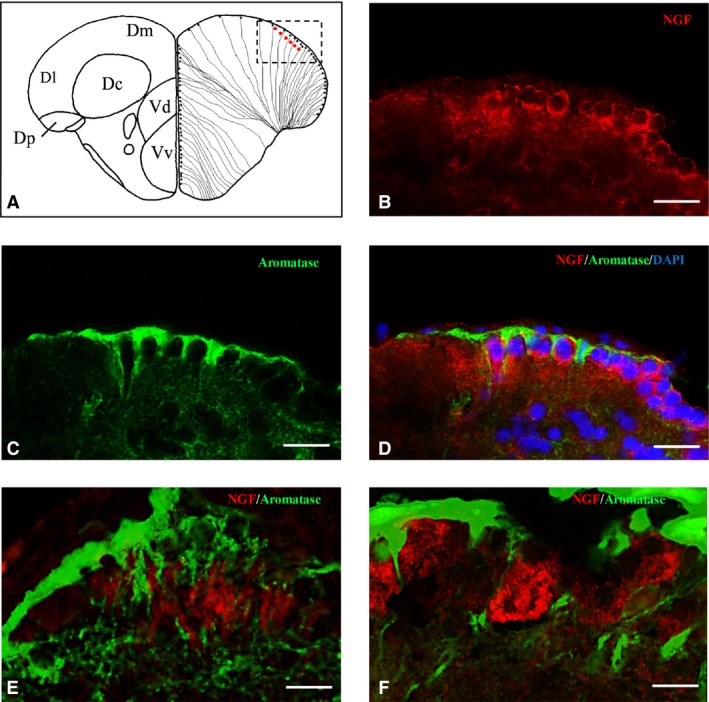
Nerve growth factor (NGF)‐positive cells are close to aromatase B cells along telencephalic ventricle. (A) Representative section of dorsal and ventral telencephalon taken from the zebrafish atlas (Wulliman et al., 1996). NGF‐positive cells are represented by red dots, and aromatase B is represented by black dots with thin lines indicating radial glia cytoplasmic processes. (B–F) Cross‐sections of the dorsal telencephalic area. Double‐staining for (A) NGF (red), (C) aromatase B (green), and merge with (D) 4,6‐diamino‐2‐phenylindole (DAPI). High magnification of NGF and aromatase B cells, closely intermingled (E, F). Scale bars: 40 μm (B–D); 20 μm (E); 10 μm (F).
Figure 9.
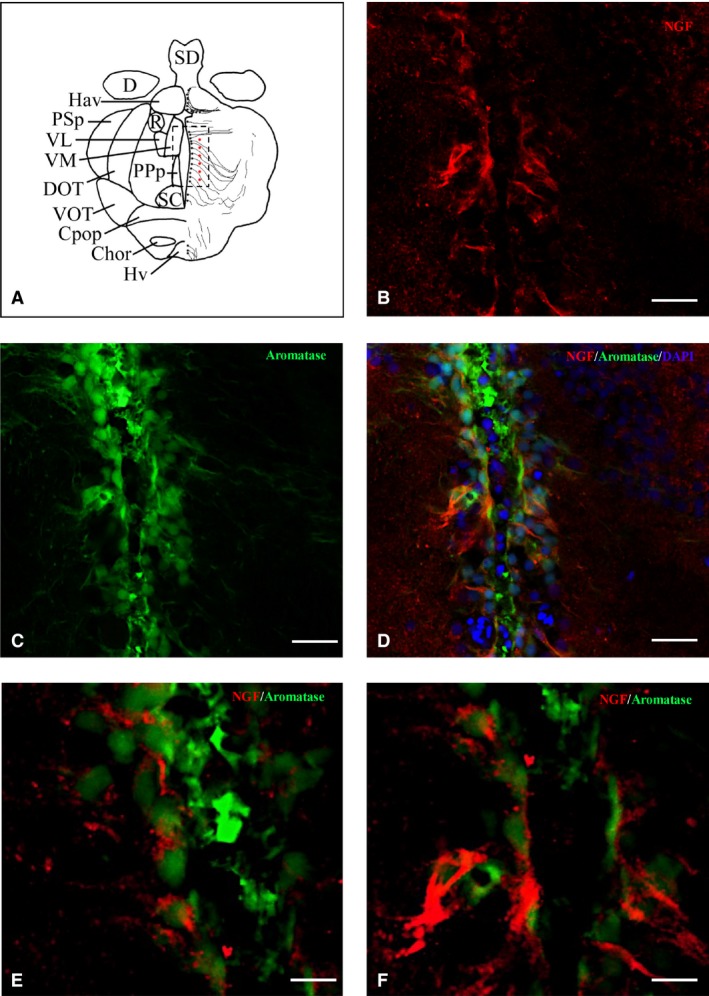
Nerve growth factor (NGF)‐positive cells are close to aromatase B cells along the diencephalic ventricle. (A) Representative section of the diencephalon taken from the zebrafish atlas (Wulliman et al., 1996). NGF‐positive cells are represented by red dots, and aromatase B is represented by black dots with thin lines indicating radial glia cytoplasmic processes. (B–D) Double‐staining for (B) NGF (red), (C) aromatase B (green), and merge with (D) 4,6‐diamino‐2‐phenylindole (DAPI) on cross‐section of the area surrounding diencephalic ventricle. (E, F) High magnifications of (B) and (C) images. Scale bars: 50 μm (B–D); 20 μm (E, F).
Figure 10.
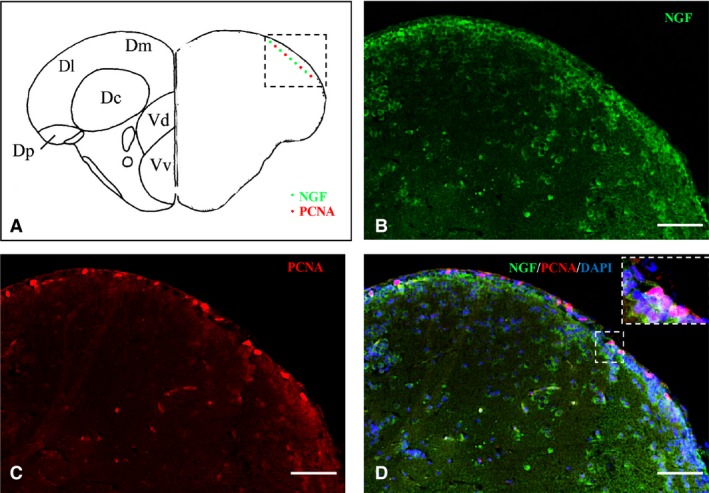
Nerve growth factor (NGF)‐positive cells are intermingled with proliferative cell nuclear antigen (PCNA)‐positive cells. (A) Representative section of dorsal and ventral telencephalon taken from the zebrafish atlas (Wulliman et al., 1996). NGF‐positive cells are represented by green dots and PCNA‐positive cells by red dots. (B–D) Double‐staining for (B) NGF (green), (C) PCNA (red), and merge with (D) 4,6‐diamino‐2‐phenylindole (DAPI) and high‐magnification of a zoom area on cross‐sections through the telencephalon. Scale bar: 50 μm, and particular of the region in (D) 30 μm.
Figure 11.
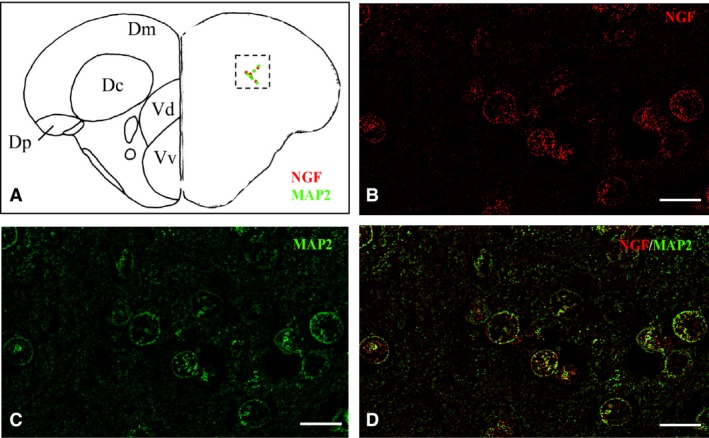
Nerve growth factor (NGF) immunoreactivity is co‐localized with microtube‐associated protein2 (MAP2) in cells of the central zone of the dorsal telencephalic area. Representative section of dorsal and ventral telencephalon taken from the zebrafish atlas (Wulliman et al., 1996). NGF‐positive cells are represented by red dots, and MAP2‐positive cells by green dots. (B–D) Double‐staining for (B) NGF (red), (C) MAP2 (green), and merge (D) on cross‐sections through the telencephalon. Scale bar: 20 μm.
Discussion
This study documents the neuroanatomical distribution of NGF mRNA and protein in zebrafish
The serum raised against NGF was previously characterized (Gatta et al., 2016), where brain homogenates of adult zebrafish showed only a band of 25 kDa, corresponding to the molecular weight of pro‐NGF isoform 1 (NP_001338647.1; Gatta et al., 2016). Accordingly, the presence of pro‐NGF was also reported in the brain of the teleost Nothobranchius furzeri, by employing the same antiserum (D'Angelo et al., 2014a,b). On the other hand, this antiserum detected the mature form of NGF in different organs of fish, such as gut (Lucini et al., 2003) and kidney (Arcamone et al., 2005). Taking into consideration that the employed NGF antiserum is able to detect both pro‐ and mature NGF in fish tissues, our results suggest that that only the pro‐NGF form is present in the brain of zebrafish. Remarkably, in the mammalian brain, precursor and intermediate forms of NGF are expressed (Fahnestock et al., 2001, 2004), and have been demonstrated to actually be the predominant form of NGF in the central nervous system (CNS; Fahnestock et al., 2001), whereas mature NGF appears to be lacking.
The co‐presence of pro‐NGF and the neuronal marker MAP2 immunoreactivity throughout the brain of adult zebrafish demonstrates that pro‐NGF‐containing cells are neurons. This result was further confirmed by the absence of the glial marker aromatase B and proliferative marker PCNA. Consistently with our observations, in the teleost fish N. furzeri, NGF was morphologically detected in neurons widespread throughout all brain regions (D'Angelo et al., 2014a,b). Only some glial cells lining the mesencephalic and rhombencephalic ventricles of N. furzeri seemed to express NGF. In contrast, in goldfish NGF was almost totally localized in radial glia cells lining the ventricles (Benowitz & Shashoua, 1979). Species‐specific characteristics could explain the different results achieved in zebrafish, N. furzeri and goldfish. In agreement with our results, in the rat brain, NGF production was reported in neurons, predominantly localized in GABAergic neurons of the cortex, hippocampus, striatum and basal forebrain (Lauterborn et al., 1993, 1995; Pascual et al., 1998; Bizon et al., 1999; Sofroniew et al., 2001; Biane et al., 2014). NGF was also reported in neuronal populations of adult monkey brain (Hayashi et al., 1993; Zhang et al., 2007). However, oligodendroglial progenitors derived from human embryonic stem cells are a source of NGF (Althaus & Richter‐Landsberg, 2000; Zhang et al., 2006). Remarkably in adult zebrafish, another member of the neurotrophin family, BDNF, was also expressed in neuronal populations of the whole brain (Cacialli et al., 2016) and telencephalon after injury (Cacialli et al., 2018a,b).
Nerve growth factor expression was comparable between zebrafish and N. furzeri (D'Angelo et al., 2014a,b), despite some slight differences in the neuroanatomy, whereas substantial differences in NGF cell localization and distribution were seen between goldfish and zebrafish as previously described. In rat brain, NGF levels were consistent in all regions, with the highest presence in cortical areas (Hoener et al., 1996; Sakamoto et al., 1998). Specifically, the pattern of distribution of both NGF mRNA (Shelton & Reichardt, 1986) and protein (Nishio et al., 1992) was described throughout the rat brain, with the highest intensity in the neocortex, the hippocampal pyramidal layer and striatum (Gall & Isackson, 1989; Rylett & Williams, 1994). Notably, observations in adult zebrafish brain do not support the fact that the forebrain is the NGF prevalent containing region.
In our study, both NGF mRNA and protein were detected in the perikaryon, and only pro‐NGF protein was distributed along neuronal prolongations. Although the cellular co‐presence of NGF mRNA and protein was not investigated, the overlapping pattern of distribution of NGF mRNA and protein throughout the zebrafish brain suggests that NGF expression and translation take place in mature neurons. The presence of NGF protein along neuronal prolongments could be retrogradely transported, according to the classical view on neurotrophins considered as target‐derived retrogradely transported substances, but also anterogradely transported, accordingly to a vast literature. The idea that NGF may be produced locally was suggested two decades ago (Lauterborn et al., 1991; Conner & Varon, 1992), and activity‐dependent release of NGF and its effect on synaptic plasticity was postulated (Blochl & Thoenen, 1995, 1996; Wu et al., 2004). Finally, Guo et al. (2012) demonstrated, by IHC, ELISA and electrophysiological analyses, anterograde delivery of NGF in the hippocamposeptal system of mice.
Pro‐NGF in the CNS is released in the extracellular space (Bruno & Cuello, 2006) and induces activation of the apoptotic machinery with subsequent death of different neuronal populations, mostly after injury and neurodegenerative disorders (for review, see Costa & Willis, 2018).
The present results demonstrate that also in zebrafish brain NGF is synthesized in perykaria; however, future studies are necessary to test whether pro‐NGF is anterogradely transported and released in the extracellular space, at the terminal ending of NGF‐positive fibers.
In conclusion, our study demonstrated that mature neurons of the zebrafish brain express NGF mRNA and store pro‐NGF. Experimental studies reported pro‐NGF as inhibition factor of the proliferation of neural stem cells isolated from postnatal mouse hippocampus, causing cell cycle arrest in the G0/G1 phase (Guo et al., 2013). Thus, it is tempting to speculate that pro‐NGF in the zebrafish brain, where cell proliferation is considerably high and persists along the entire lifespan, could represent a key negative regulator factor of this process.
Author's contribution
PC conceived and planned the experimentation, acquired and analyzed data; CG, AL, AP acquired and analyzed data; LDA, PdG, EP critically revised the manuscript; CL analyzed data and wrote the paper.
Supporting information
Fig. S1 Negative controls performed by nerve growth factor (NGF) antiserum pre‐incubated with its homologous antigen did not show any reactivity.
References
- Adolf B, Chapouton P, Lam CS, et al. (2006) Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol 295, 278–293. [DOI] [PubMed] [Google Scholar]
- Althaus HH, Richter‐Landsberg C (2000) Glial cells as targets and producers of neurotrophins. Int Rev Cytol 197, 203–277. [DOI] [PubMed] [Google Scholar]
- Alunni A, Bally‐Cuif L (2016) A comparative view of regenerative neurogenesis in vertebrates. Development 143, 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand SK, Mondal AC (2017) Cellular and molecular attributes of neural stem cell niches in adult zebrafish brain. Dev Neurobiol 77, 1188–1205. [DOI] [PubMed] [Google Scholar]
- Arcamone N, Lucini C, Borzacchiello G, et al. (2005) Distribution of NGF and NT‐3‐like protein immunoreactivity in the teleost kidney. Microsc Res Tech 66, 17–24. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Shanshoua VE (1979) Immunoreactive sites for nerve growth factor (NGF) in the goldfish brain. Brain Res 172, 561‐565. [DOI] [PubMed] [Google Scholar]
- Biane J, Conner JM, Tuszynski MH (2014) Nerve growth factor is primarily produced by GABAergic neurons of the adult rat cortex. Front Cell Neurosci 8, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Lauterborn JC, Gall CM (1999) Subpopulations of striatal interneurons can be distinguished on the basis of neurotrophic factor expression. J Comp Neurol 408, 283–298. [PubMed] [Google Scholar]
- Blochl A, Thoenen H (1995) Characterization of nerve growth factor (NGF) release from hippocampal‐neurons – evidence for a constitutive and unconventional sodium‐dependent regulated pathway. Eur J Neurosci 7, 1220–1228. [DOI] [PubMed] [Google Scholar]
- Blochl A, Thoenen H (1996) Localization of cellular storage compartments and sites of constitutive and activity‐dependent release of nerve growth factor (NGF) in primary cultures of hippocampal neurons. Mol Cell Neurosci 7, 173–190. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Cuello AC (2006) Activity‐dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci USA 103, 6735–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacialli P, Gueguen MM, Coumailleau P, et al. (2016) BDNF expression in larval and adult zebrafish brain: distribution and cell identification. PLoS ONE 11, e018057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacialli P, D'Angelo L, Kah O, et al. (2018a) Neuronal expression of brain derived neurotrophic factor in the injured telencephalon of adult zebrafish. J Comp Neurol 526, 569–582. [DOI] [PubMed] [Google Scholar]
- Cacialli P, Palladino A, Lucini C (2018b) Role of brain‐derived neurotrophic factor during the regenerative response after traumatic brain injury in adult zebrafish. Neural Regen Res 13, 941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Varon S (1992) Distribution of nerve growth factor‐like immunoreactive neurons in the adult‐rat brain following colchicine treatment. J Comp Neurol 326, 347–362. [DOI] [PubMed] [Google Scholar]
- Cosacak MI, Papadimitriou C, Kizil C (2015) Regeneration, plasticity, and induced molecular programs in adult zebrafish brain. Biomed Res Int 2015, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa CJ, Willis DE (2018) To the end of the line: axonal mRNA transport and local translation in health and neurodegenerative disease. Dev Neurobiol 78, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumailleau P, Pellegrini E, Adrio F, et al. (2015) Aromatase, estrogen receptors and brain development in fish and amphibians. Biochim Biophys Acta Gene Regul Mech 1849, 152–162. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Borich SM, Murphy P, et al. (2009a) Brain‐derived neurotrophic factor mrna expression in the brain of the teleost fish, Anguilla anguilla, the European Eel. Brain Behav Evol 73, 43–58. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Roberts BL, Borich SM (2009b) Brain derived neurotrophic factor and trk B mRNA expression in the brain of a brain stem‐spinal cord regenerating model, the European eel, after spinal cord injury. Neurosci Lett 461, 275–279. [DOI] [PubMed] [Google Scholar]
- D'Angelo L, de Girolamo P, Cellerino A, et al. (2012) Neurotrophin Trk receptors in the brain of a teleost fish, Nothobranchius furzeri. Microsc Res Tech 75, 81–88. [DOI] [PubMed] [Google Scholar]
- D'Angelo L, Castaldo L, Cellerino A, et al. (2014a) Nerve growth factor in the adult brain of a teleostean model for aging research: Nothobranchius furzeri. Ann Anat 196, 183–191. [DOI] [PubMed] [Google Scholar]
- D'Angelo L, De Girolamo P, Lucini C, et al. (2014b) Brain‐derived neurotrophic factor: mRNA expression and protein distribution in the brain of the teleost nothobranchius furzeri. J Comp Neurol 522, 1004–1030. [DOI] [PubMed] [Google Scholar]
- D'Angelo L, Avallone L, Cellerino A, et al. (2016a) Neurotrophin‐4 in the brain of adult Nothobranchius furzeri. Ann Anat 207, 47–54. [DOI] [PubMed] [Google Scholar]
- D'Angelo L, Lossi L, Merighi A, et al. (2016b) Anatomical features for the adequate choice of experimental animal models in biomedicine: I. Fishes. Ann Anat 205, 75–84. [DOI] [PubMed] [Google Scholar]
- Dethleffsen K, Heinrich G, Lauth M, et al. (2003) Insert‐containing neurotrophins in teleost fish and their relationship to nerve growth factor. Mol Cell Neurosci 24, 380–394. [DOI] [PubMed] [Google Scholar]
- Diotel N, Vaillant C, Gabbero C, et al. (2013) Effects of estradiol in adult neurogenesis and brain repair in zebrafish. Horm Behav 63, 193–207. [DOI] [PubMed] [Google Scholar]
- Diotel N, Viales RR, Armant O, et al. (2015) Comprehensive expression map of transcription regulators in the adult zebrafish telencephalon reveals distinct neurogenic niches. J Comp Neurol 523, 1202–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, et al. (2001) The precursor pro‐nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer's disease. Mol Cell Neurosci 18, 210–220. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Yu G, Michalski B, et al. (2004) The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor. J Neurochem 89, 581–592. [DOI] [PubMed] [Google Scholar]
- Gall CM, Isackson PJ (1989) Limbic seizures increase neuronal production of messenger‐RNA for nerve growth‐factor. Science 245, 758–761. [DOI] [PubMed] [Google Scholar]
- Gatta C, Altamura G, Avallone L, et al. (2016) Neurotrophins and their Trk‐receptors in the cerebellum of zebrafish. J Morphol 277, 725–736. [DOI] [PubMed] [Google Scholar]
- de Girolamo P, Lucini C (2011) Neuropeptide localization in nonmammalian vertebrates. Methods Mol Biol 789, 37–56. [DOI] [PubMed] [Google Scholar]
- Guo JJ, Wang JN, Liang CR, et al. (2013) proNGF inhibits proliferation and oligodendrogenesis of postnatal hippocampal neural stem/progenitor cells through p75NTR in vitro. Stem Cell Res 11, 874–887. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamashita A, Shimizu K, et al. (1993) Expression of the gene for nerve growth‐factor (NGF) in the monkey central nervous system. Brain Res 618, 142–148. [DOI] [PubMed] [Google Scholar]
- Hoener MC, Hewitt E, Conner JM, et al. (1996) Nerve growth factor (NGF) content in adult rat brain tissues is several‐fold higher than generally reported and is largely associated with sedimentable fractions. Brain Res 728, 47–56. [PubMed] [Google Scholar]
- Lauterborn JC, Isackson PJ, Gall CM (1991) Nerve growth‐factor messenger RNA‐containing cells are distributed within regions of cholinergic neurons in the rat basal forebrain. J Comp Neurol 306, 439–446. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Tran TMD, Isackson PJ, et al. (1993) Nerve growth‐factor messenger‐rna is expressed by gabaergic neurons in rat hippocampus. NeuroReport 5, 273–276. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Bizon JL, Tran TMD, et al. (1995) NGF messenger‐RNA is expressed by gabaergic but not cholinergic neurons in rat basal forebrain. J Comp Neurol 360, 454–462. [DOI] [PubMed] [Google Scholar]
- Lucini C, Maruccio L, Arcamone N, et al. (2003) Neurotrophin‐like immunoreactivity in the gut of teleost species. Neurosci Lett 345, 33–36. [DOI] [PubMed] [Google Scholar]
- Lucini C, D'Angelo L, Cacialli P, et al. (2018) BDNF, brain, and regeneration: insights from zebrafish. Int J Mol Sci 19, 3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marz M, Schmidt R, Rastegar S, et al. (2011) Regenerative response following stab injury in the adult zebrafish telencephalon. Dev Dyn 240, 2221–2231. [DOI] [PubMed] [Google Scholar]
- Minnone G, De Benedetti F, Bracci‐Laudiero L (2017) NGF and its receptors in the regulation of inflammatory response. Int J Mol Sci 18, 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomska G, Mietelska‐Porowska A, Mazurkiewicz M (2011) The cholinergic system, nerve growth factor and the cytoskeleton. Behav Brain Res 221, 515–526. [DOI] [PubMed] [Google Scholar]
- Nilsson AS, Fainzilber M, Falck P, et al. (1998) Neurotrophin‐7: a novel member of the neurotrophin family from the zebrafish. FEBS Lett 424, 285–290. [DOI] [PubMed] [Google Scholar]
- Nishio T, Akiguchi I, Furukawa S (1992) Detailed distribution of nerve growth‐factor in rat‐brain determined by a highly sensitive enzyme‐immunoassay. Exp Neurol 116, 76–84. [DOI] [PubMed] [Google Scholar]
- Panula P, Chen YC, Priyadarshini M, et al. (2010) The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis 40, 46–57. [DOI] [PubMed] [Google Scholar]
- Pascual M, Rocamora N, Acsady L, et al. (1998) Expression of nerve growth factor and neurotrophin‐3 mRNAs in hippocampal interneurons: morphological characterization, levels of expression, and colocalization of nerve growth factor and neurotrophin‐3. J Comp Neurol 395, 73–90. [PubMed] [Google Scholar]
- Pellegrini E, Mouriec K, Anglade I, et al. (2007) Identification of aromatase‐positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J Comp Neurol 501, 150–167. [DOI] [PubMed] [Google Scholar]
- Rylett RJ, Williams LR (1994) Role of neurotrophins in cholinergic‐neuron function in the adult and aged CNS. Trends Neurosci 17, 486–490. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Kuzuya H, Tamaru M, et al. (1998) Developmental changes in the NGF content in the brain of young, growing, low‐birth‐weight rats. Neurochem Res 23, 115–120. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Reichardt LF (1986) Studies on the expression of the beta‐nerve growth‐factor (NGF) gene in the central nervous system level and regional distribution of NGF messenger RNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc Natl Acad Sci USA 83, 2714–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24, 1217–1281. [DOI] [PubMed] [Google Scholar]
- Than‐Trong E, Bally‐Cuif L (2015) Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia 63, 1406–1428. [DOI] [PubMed] [Google Scholar]
- Wu YJ, Kruttgen A, Moller JC, et al. (2004) Nerve growth factor, brain‐derived neurotrophic factor, and neurotrophin‐3 are sorted to dense‐core vesicles and released via the regulated pathway in primary rat cortical neurons. J Neurosci Res 75, 825–834. [DOI] [PubMed] [Google Scholar]
- Wulliman MF, Rupp B, Reichert H (1996) Neuroanatomy of the Zebrafish Brain. A Topological Atlas. Birkhauser Basel: Birkhauser. [Google Scholar]
- Zhang YH, Vasko MR, Nicol GD (2006) Intracellular sphingosine 1‐phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J Physiol 575, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HT, Li LY, Zou XL, et al. (2007) Immunohistochemical distribution of NGF, BDNF, NT‐3, and NT‐4 in adult rhesus monkey brains. J Histochem Cytochem 55, 1–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Negative controls performed by nerve growth factor (NGF) antiserum pre‐incubated with its homologous antigen did not show any reactivity.


