Abstract
Discovered in 2007, anaplastic lymphoma kinase (ALK) gene rearrangements positive (ALK+) lung cancers compose a small subset of non-small cell lung cancer (NSCLC), with rapidly expanded treatments. There are currently several ALK inhibitors, including crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib which have been licensed by the US Food and Drug Administration or the European Medicines Agency for the treatment of ALK+ NSCLC patients. Along with the multiple therapies, the survival of this subtype of NSCLC has been significantly expanded, even for patients whose disease has spread in the brain. Alectinib (Alecensa), a specific ALK and rearranged during transfection tyrosine kinase inhibitor is approved as first-line therapy for metastatic ALK+ NSCLC patients. It is additionally approved for ALK+ NSCLC previously treated with crizotinib. The main aim of this review is to assemble on the efficacy of alectinib for the treatment of ALK+ NSCLC, to elaborate the activity of the drug in the central nervous system, and to debate on which is the position of this compound in the treatment course of ALK+ lung cancer patients.
Keywords: anaplastic lymphoma kinase, alectinib, lung cancer
Introduction
Precision medicine has made a great impact in the survival of patients with advanced non-small cell lung cancer (NSCLC), particularly those with epidermal growth factor receptor (EGFR) mutations or echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusions (ALK+).1,2 Alterations of the ALK gene were initially described in anaplastic large cell lymphoma (hence the name of the gene).3,4 ALK alterations have a role in the pathogenesis of inflammatory myofibroblastic tumors and neuroblastomas.4,5 In NSCLC, ALK fusions, which join the exons 1–13 of the EML4 gene to exons 20–29 of ALK gene,6 were discovered in 2007.7,8
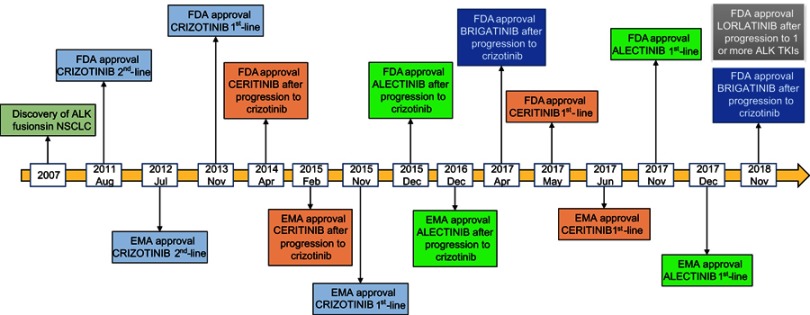
ALK fusions are present in 3–5% of NSCLC and are more common in young patients with lung adenocarcinoma and non-smoking history.9,10 Initially ALK+ patients were treated with chemotherapy until the discovery of crizotinib, an ALK, MET and ROS1 tyrosine kinase inhibitor (TKI), which has demonstrated its superiority compared to standard platinum-based chemotherapy in several clinical trials in ALK+ patients.11–13 As happens with other targeted therapies, resistance to crizotinib soon appears due to ALK-dependent or ALK-independent mechanisms.14–16 Furthermore, the central nervous system (CNS) is a frequent site of metastases in ALK+ NSCLC patients with approximately 26% of them having CNS metastases at the time of the diagnosis.17 The incidence of CNS metastases increases during the course of the disease to as high as 60% for crizotinib-resistant ALK+ patients.17 Still, stage IV ALK+ NSCLC patients have prolonged survival.18 Median survival of 6.8 years can be achieved with the appropriate medical care in stage IV ALK+ NSCLC patients.19 This is due to the development of several second- and third-generation ALK TKIs, like ceritinib,20–21 alectinib,22–24 brigatinib,25 and lorlatinib26,27 which have expanded the treatment options of ALK+ NSCLC (Figure 1). Ensartinib, entrectinib and repotrectinib are under clinical investigation.28–30 It is worth mentioning that ALK+ NSCLC patients have better clinical outcome with pemetrexed chemotherapy, compared to wild-type NSCLC patients or those with other genetic alterations like KRAS mutations.31 The differential outcome to pemetrexed chemotherapy may be attributed to the lower levels of thymidylate synthase in ALK+ compared to wild-type NSCLC patients.31
Figure 1.
ALK inhibitors approved for the treatment of ALK+ NSCLC patients.
Abbreviations: NSCLC, non-small cell lung cancer; FDA, Food and Drug Administration; EMA, European Medicines Agency; TKI, Tyrosine kinase inhibitor; ALK, Anaplastic lymphoma kinase.
Alectinib (marketed as Alecensa) was created at Chugai Kamakura Research Laboratories, which is part of the Hoffmann-La Roche group, as an oral ALK inhibitor. Alectinib is approved for the first-line therapy of ALK+ NSCLC patients as well as for patients pretreated with crizotinib (Figure 1).32 In preclinical studies, alectinib was able to inhibit the growth of EML4-ALK positive tumor cells. It has also shown activity against ALK+ cells with the gatekeeper ALK L1196M mutation, which confers resistance to crizotinib.33,34 However, this activity has not been reconfirmed in the clinical setting.35 Alectinib inhibits ALK autophosphorylation as well as the phosphorylation of signal transducer and activator of transcription 3 (STAT3).33
Alectinib is also a highly selective rearranged during transfection (RET) inhibitor.36 Fusions of the RET gene, such as KIF5B (the kinesin family 5B gene)-RET, CCDC6 (coiled-coil domain containing 6)-RET, and others are driver oncogenes in 1–2% of lung adenocarcinomas.37,38 Alectinib inhibits RET phosphorylation and the tumor growth in xenograft models with RET fusions.36,39 It also has activity against RET gatekeeper mutations, like V804L and V804M.36,39 Currently, alectinib is in clinical trials for RET-rearranged NSCLC. In this review, we will not focus on the activity of alectinib on RET-rearranged tumors, especially because other more specific RET inhibitors are in clinical development.40–43
In this review, we will discuss the clinical status of alectinib in ALK+ NSCLC patients, outline its clinical development, and CNS activity, and comment on the place of alectinib in the management of ALK+ NSCLC patients. Mechanisms of resistance to alectinib will be also revised.
Alectinib in the second-line setting of ALK+ NSCLC patients
The Phase I/II study AF-002JG study was performed in crizotinib-resistant ALK+ NSCLC patients, and established 600 mg of alectinib twice daily as the recommended Phase II dose.44 The study showed promising antitumor activity of alectinib including in patients with brain metastases.44 Indeed, the AF-002JG study confirmed the alectinib CNS penetration. Specifically, a 52% objective response rate (ORR) was observed among 21 patients with baseline brain metastases. In all patients who underwent CNS sampling, measurable concentrations of alectinib were detected.44 In Japan, 300 mg twice daily was tested in ALK+ NSCLC patients who had progressed to previous chemotherapy (Phase I/II AF-001JP study).45 In an updated analysis of the AF-001JP study, median progression-free survival (PFS) was not reached (3-year PFS rate, 62%; 95% CI 45, 75) and the 3-year overall survival (OS) rate was 78%.46
Two Phase II studies evaluated the effect of alectinib in ALK+ NSCLC patients who had progressed on crizotinib (Table 1).47,48 Most of the patients in both studies had received chemotherapy before crizotinib and had brain metastases at inclusion.47,48 ORRs of 49 (95% CI 40, 58) and 48% (95% CI 36, 40) were observed in the global NP28673 and the North American NP28761 studies, respectively.47,48 An updated analysis of the NP28673 with a longer follow-up of 21 months confirmed the ORR of 51% (95% CI 42, 60) with a duration of response (DoR) of 15.2 months (95% CI 11.2, 24,9). Median PFS to alectinib was 8.9 months (95% CI 5.6, 12,8), and median OS was 26 months (95% CI 11.2, not estimable) (Table 1).49 Similarly, an updated analysis of NP28761 with a follow-up of 17 months reported an ORR of 52% (95% CI 40, 65), with a DoR of 15 months. Median PFS and OS were 8 and 22.7 months, respectively.50 A pooled analysis of the NP28673 and NP28761 studies demonstrated an ORR of 51% (95% CI 44, 59), a disease control rate of 79% (95% CI 72, 84), and a median DoR of 14.9 months (95% CI 11.1, 20.4).51 Median PFS was 8.3 months (95% CI 7.0, 11.3) and median OS was 26.0 months (95% CI 21.4, not estimable).51 Based on the results of the NP28673 and NP28761 studies, on 11 December 2015, the US Food and Drug Administration (FDA) granted accelerated approval to alectinib for the treatment of ALK+ NSCLC patients who have progressed on or are intolerant to crizotinib. One year later, on 15 December 2016, the European Medicines Agency (EMA) approved alectinib for the same indication.
Table 1.
Clinical trials of alectinib
| Study | Phase | Design | Population | Results | |||
|---|---|---|---|---|---|---|---|
| PFS (mo) | OS (mo) | ORR (%) | Ref | ||||
| NP28673 | II | Alectinib, single arm | Pretreated with crizotinib | 8.9 | 26 | 51 | 49 |
| NP28761 | II | Alectinib, single arm | Pretreated with crizotinib | 8 | 22.7 | 52 | 50 |
| ALUR | III | Alectinib vs chemotherapy | Pretreated with platinum-based chemotherapy and crizotinib | 7.1 vs 1.6* HR 0.32, P<0.001 | 12.6 vs NE | 38 vs 3 | 22 |
| ALEX | III | Alectinib vs crizotinib | First-line (treatment naïve) | 34.8 vs 10.9, HR 0.43, P<0.001 | – | 83 vs 75 | 24,54 |
| ALEX J | III | Alectinib (300 mg) vs crizotinib | ALK-inhibitor naïve | 25.9 vs 10.2, HR 0.38, P<0.0001 | – | 85 vs 70 | 23,52 |
Note: *Independent review committee.
Abbreviations: PFS, Progression-free survival; OS, Overall survival; ORR, Objective Response Rate; mo, months vs, versus; NE, not estimable.
The Phase III ALUR clinical trial compared alectinib versus chemotherapy in ALK+ metastatic NSCLC patients whose disease had progressed after platinum-based chemotherapy and after crizotinib (Table 1).22 One hundred seven patients were randomized 2:1 to receive alectinb or chemotherapy (pemetrexed or docetaxel). More than three-thirds of the patients had brain metastases at baseline.22 Median PFS was significantly longer with alectinib compared to chemotherapy, as assessed both by the investigators (9.6 months [95% CI 6.9, 12.2; alectinib] and 1.4 months [95% CI 1.3,1.6; chemotherapy], HR 0.15 [95% CI 0.08, 0.29]; P< 0.001) and by an independent review committee (IRC) (7.1 months [95% CI 6.3,10.8, alectinib] and 1.6 months [95% CI 1.3,4.1, chemotherapy], HR 0.32 [95% CI 0.17, 0.59]; P< 0.001) (Table 1).22 The ORR was 38% with alectinib versus 3% with chemotherapy. Median investigator-assessed DoR was 9.3 months (95% CI 6.9, not estimable; alectinib) versus 2.7 months (95% CI not estimable; chemotherapy) (Table 1).22 No significant differences were observed in OS between the two treatment arms of the ALUR study, probably due to the trial design, which was permitting patients in the chemotherapy arm to cross over to alectinib therapy once disease progression was noted.22
Alectinib penetrates through the blood–brain barrier,44 and it is retained within the CNS. The brain protective effect of alectinib has been attributed to the fact that alectinib is not a substrate of the efflux transporters P-glycoprotein (P-gp) and breast cancer resistance protein).32 In the ALUR study, those patients who had brain metastases at baseline had an ORR in CNS of 54% in the alectinib arm, compared to 0% in the chemotherapy arm.22 Alectinib was able to reduce 86% the risk of progression in patients with baseline brain metastases. The 6-month cumulative incidence rate of CNS progression was 11% with alectinib and 48% with chemotherapy. A significantly higher CNS disease control rate of 80% was observed in the alectinib-treated patients compared to 27% for those who received chemotherapy (P<0.001).22 Overall, the ALUR study showed a striking benefit of alectinib versus standard second-line chemotherapy in ALK+ NSCLC patients.
Alectinib in the first-line setting of ALK+ NSCLC patients
The first Phase III trial which compared alectinib (at the dose of 300 mg twice daily) with crizotinib in the first-line setting of ALK+ NSCLC patients took place in Japan (J-ALEX). ALK inhibitor-naïve Japanese patients, who may have received or not previous chemotherapy, were included in the study.23 Overall, median PFS was not estimable (95%CI 20.3, not estimable) at the time of the analysis in the alectinib group compared to 10.2 months (95% CI 8.2, 12.0) in the crizotinib group (HR 0.34 [99.7% CI 0.17, 0.71]; P<0.0001).23 For treatment-naïve patients, median PFS was not estimable (95% CI 17.5, not estimable) with alectinib versus 10.2 months (95% CI 8.3, 13.9) for those receiving crizotinib (HR 0.31 [95% CI 0.17, 0.57]). For patients previously treated with chemotherapy, median PFS was 20.3 months (95% CI 20.3, not estimable) in the alectinib group versus 8.2 months (95% CI 6.4, 15.7 in the crizotinib group (HR 0.40 [95% CI 0.19, 0.87]).23 Patients treated with alectinib obtained an ORR of 85% versus 70% for those receiving crizotinib.23 Updated data from the J-ALEX study showed median PFS of 25.9 months (95% CI 20.3, not estimable) with alectinib and 10.2 months (95% CI 8.3, 12.0) with crizotinib (HR 0.38 [95% CI 0.26, 0.55, P<0.0001)].52 In the J-ALEX study, in 164 patients without brain metastasis at baseline, alectinib prevented CNS progression with a HR of 0.19 (95% CI 0.07, 0.53) compared to crizotinib. The same occurred for the 43 patients with brain metastasis at baseline, who had a lower risk for CNS progression with alectinib compared to crizotinib (HR=0.51, 95% CI 0.16, 1.64).52
The Phase III ALEX trial was conducted in non-Asiatic and Asiatic population.24 A total of 303 ALK+ treatment-naïve NSCLC patients were treated with either 600 mg of alectinib twice daily or 250 mg of crizotinib twice daily. The investigator-assessed median PFS was not reached (95% CI 17.7, not estimable) with alectinib versus 11.1 months (95% CI 9.1, 13.1) with crizotinib (HR 0.47 [95% CI 0.34, 0.65], P<0.001 (Table 1).24 Median PFS as assessed by an IRC was longer with alectinib compared to crizotinb (25.7 months [95% CI 19.9, not estimable) versus 10.4 months [95% CI 7.7, 14.6], HR, 0.50 [95% CI 0.36, 0.70], P<0.001).24 No statistically significant differences occurred in terms of responses with a response rate of 83% (95% CI 76, 88) and 75% (95% CI 68, 82) for alectinib and crizotinib, respectively (P=0.09) (Table 1).24 Finally, in the ALEX study, patients with measurable baseline brain metastases and prior radiotherapy had a CNS ORR of 85.7% with alectinib and 71.4% with crizotinib.53 For those patients who had not received prior radiotherapy, the CNS ORR was 78.6% and 40.0% for alectinib and crizotinib, respectively. Importantly, alectinib significantly delayed CNS progression, independent of the existence or not of baseline brain metastases or the treatment or not with prior radiotherapy.53 Patients with baseline brain metastases had median PFS of 24.7 months (95% CI 9.2, not estimable) with alectinib compared to 7.4 months (95% CI 6.6, 9.6) for crizotinib (HR=0.35, 95% CI 0.22, 0.56).54 With a longer follow-up, median PFS of 34.8 months (95% CI 17.7, not estimable) for alectinib versus 10.9 months (95% CI 9.1, 12.9) for crizotinib (HR 0.43, 95% CI 0.32, 0.58) was reported.54 Patients treated with alectinib had a longer median DoR compared to crizotinib (33.1 [95% CI 31.3, not estimable] versus 11.1 months [95% CI 7.9, 13.0], HR 0.36 [95% CI 0.24; 0.53]).24,54 These updated data consolidate alectinib as the standard-of-care for first-line treatment of ALK+ NSCLC patients. Based on the data of the ALEX study, on 7 November 2017, FDA approved alectinib for the first-line treatment of patients with ALK+ NSCLC. One month later, on 21 December 2017, alectinib was also EMA approved.
Alectinib is a well-tolerated drug. The most frequent of any grade adverse events are gastrointestinal disorders, hepatobiliary disorders, edema, rash, myalgia, anemia and increased body weight.32 This information is derived from a pooled analysis of three alectinib trials (ALEX, NP28673 and NP28761).32 Adverse events of grade more than 3 occurred only in <4% of the patients.32 Liver function disorders are the most common grade ≥3 adverse events, that are transient and resolved, when alectinib is interrupted or when it is given in a lower dose. Grade 1–2 bradycardia has been reported in almost 9% of patients receiving alectinib. Interstitial lung disease (ILD) is uncommon, but grade ≥3 ILD which led to treatment discontinuation, occurred in one patient. Elevated blood creatinine phosphokinase (CPK) and anemia are also common grade ≥3 adverse events. For the above reasons, liver function, heart rate and blood pressure, pulmonary symptoms, CPK blood levels and anemia should be monitored in patients who are treated with alectinib.
Mechanisms of resistance to alectinib
As happens with all targeted therapies, resistance inevitably emerges after treatment with alectinib or other ALK inhibitors. ALK resistant mutations appear to be the main mechanism of resistance to second-generation ALK inhibitors. We have combined data available from three previous publications14,35,55 to summarize the activity of alectinib and other ALK inhibitors against various resistant mutations (Table 2). ALK I1171 mutations are reported to be the second (after ALK G1202R) most common ALK resistance mutations in post-alectinib specimens. Alectinib is also inactive against L1196M, V1180L and T1151Tins mutations (Table 2).
Table 2.
Half-maximal inhibitory concentrations (IC50) of first-, second- and third-generation ALK inhibitors on mutant EML4-ALK (data derived from14,35,55)
| ALK resistantmutations | Crizotinib | Ceritinib | Alectinib | Brigatinib | Lorlatinib |
|---|---|---|---|---|---|
| G1269A | |||||
| E1210K | |||||
| S1206Y | ND | ||||
| S1206F | ND | ||||
| D1203N | |||||
| G1202del | |||||
| G1202R | |||||
| L1196M | |||||
| V1180L | ND | ||||
| F1174C | ND | ||||
| F1174L | ND | ||||
| F1174V | ND | ||||
| C1156Y | |||||
| I1171N | |||||
| I1171S | |||||
| I1171T | |||||
| F1174C | |||||
| L1152R | ND | ND | |||
| L1152P | ND | ||||
| L1198F | |||||
| T1151Tins | ND | ||||
| D1203N+F1174C | |||||
| D1203N+E1210K | ND | ||||
| IC50≤50 nmol/L | |||||
| >50, <200 nmol/L | |||||
| ≥200 nmol/L | |||||
Abbreviation: ND, not determined.
Maintained mitogen-activated protein kinase (MAPK) activation through alternate kinases, including EGFR, KIT, Src, insulin growth factor 1 or the src homology 2 domain-containing phosphatase 2 56 has been also described as a mechanism of resistance to ALK inhibitors.57–60 We have found that KRAS wild-type copy number gain decreased of the dual specificity phosphatase 6 phosphatase reactivates the MAPK pathway in the presence of ALK inhibitors and therefore leads to tumor resistance.61 To this end, a clinical trial with the combination of alectinib with the MEK inhibitor cobimetinib is ongoing in ALK+ NSCLC patients (Table 3).
Table 3.
Ongoing clinical trials with alectinib
| ClinicalTrials.gov Identifier | Phase | Treatment | Objective |
|---|---|---|---|
| NCT03194893 | III, open | Alectinib, crizotinib | To provide continued treatment with alectinib or crizotinib as applicable to patients with ALK- or RET-positive cancer who were previously enrolled in any Roche-sponsored alectinib study and who are deriving continued clinical benefit from alectinib or crizotinib in the parent trial at the time of parent trial closure |
| – | II, open (Japan) | Alectinib | To evaluate the efficacy and safety of alectinib in patients with rare cancer harboring ALK alterations |
| NCT03596866 | III, planned | Alectinib versus brigatinib | To compare the efficacy of brigatinib versus alectinib in participants with ALK+ locally advanced or metastatic NSCLC who have progressed on crizotinib |
| NCT03456076 | III, open | Alectinib versus platinum-based chemotherapy (adjuvant) | To evaluate the efficacy and safety of adjuvant alectinib versus adjuvant platinum-based chemotherapy in patients with completely resected stage IB (tumors equal to or larger than 4 cm) to stage IIIA ALK+ NSCLC |
| NCT03445000 | II, open | Alectinib | To investigate the efficacy of alectinib in patients with advanced stage RET-rearranged NSCLC, treated with at least one platinum-based systemic chemotherapy regimen |
| NCT03202940 | I/II, open | Alectinib plus cobimetinib | To study the combination of alectinib and cobimetinib as a possible treatment for ALK+ NSCLC |
| NCT03779191 | II, planned | Alectinib plus bevacizumab | To assess alectinib plus bevacizumab in untreated and previously treated patients with advanced or metastatic non-squamous ALK+ NSCLC |
| NCT02521051 | I/II, open | Alectinib plus bevacizumab | To evaluate the safety and tolerability of alectinib and bevacizumab in patients with ALK+ NSCLC |
| NCT02091141 (My pathway) | II, open | Alectinib, atezolizumab, vemurafenib/cobimetinib, erlotinib, pertuzumab/trastuzumab, vismodegib | To evaluate trastuzumab/pertuzumab, erlotinib, vemurafenib/cobimetinib, vismodegib, alectinib and atezolizumab in patients who have advanced solid tumors with mutations or gene expression abnormalities predictive of response to one of these agents |
Abbreviations: NSCLC, non-small cell lung cancer; ALK, anapestic lymphoma kinase.
Discussion – sequencing of treatment for ALK+ NSCLC patients
A main point of discussion for ALK+ NSCLC patients is the optimal sequencing of the available agents. More precise diagnosis for identifying the ALK fusion partner is also of great relevance, considering that fusion partners affect the sensitivity to different ALK inhibitors.62 Therefore, in situ hybridization (FISH) or immunohistochemistry are not enough for the accurate diagnosis of ALK+ NSCLC patients. Next-generation sequencing technologies must come to the forefront of clinical diagnostics for the clinicians to know the fusion partner.62 On the other hand, next-generation sequencing platforms allow us to know whether the resistance comes from acquired resistant mutations or from activation of bypass signaling pathways. This is important for selecting next therapeutic options. The activation of bypass signaling pathways may require a tissue re-biopsy as in circulating-free DNA alterations such as small cell transformation or epithelial-mesenchymal transition cannot be easily defined.63 Therefore, liquid biopsies serve for the detection of ALK acquired mutations, but if resistant mutations are not detected, then a tissue biopsy is necessary.63
Alectinib together with crizotinib and ceritinib are approved as first-line therapies for ALK+ NSCLC patients. Brigatinib is expected to gain soon first-line approval. We still do not know whether median PFS with brigatinib will be longer than what has been achieved with alectinib in the first-line setting.25 Lorlatinib is approved for ALK+ NSCLC patients after progression to 1 or 2 prior lines of ALK TKIs. The ongoing clinical trials are comparing the second-generation ALK TKIs with crizotinib in the first-line setting and therefore we speculate that all of them will be positive studies. However, the key question is what the correct second-line therapy after a second-generation ALK inhibitor is? If this is the third-generation inhibitor, lorlatinib, then what comes next? Whether the combination of chemotherapy with immunotherapy is an option, is still debatable? In the subgroup analysis of the IMpower150 study, the combination of carboplatin, paclitaxel, bevacizumab and atezolizumab improved PFS compared to chemotherapy plus bevacizumab alone in ALK+ NSCLC patients.64 Overall, only 34 ALK+ patients were included in the study and therefore the results of this subgroup analysis cannot be conclusive. Furthermore, many doubts have been raised on the design of this study, as well as on the way that the results were presented, especially because they exclude the comparison of carboplatin, paclitaxel, bevacizumab, and atezolizumab versus carboplatin, paclitaxel and atezolizumab.65,66 Therefore, the IMpower150 study did not address the question whether vascular endothelial growth factor blockade enhances the efficacy of immunotherapy.64,65
Crizotinib probably will have no role in the treatment of ALK+ patients in the future. May be at the time that lorlatinib will come in the first-line setting, crizotinib will become useful again as it overcomes some of the lorlatinib-resistant mutations, like the double C1156Y–L1198F.67 Right now, alectinib and ceritinib are the only second-generation ALK inhibitors with CNS activity, approved in the first-line setting. Ceritinib is a very potent ALK inhibitor but with serious toxicity issues, at least when it was given at the initial approved fasting dose of 750 mg daily. Now, 450 mg of ceritinib given with food is the new FDA and EMA approved regimen, and ceritinib becomes again a very relevant compound for the first-line therapy of ALK+ NSCLC patients.68 Brigatinib, which we anticipate that it will be soon approved for the same indication, has shown the longest intracranial PFS of 18 months in the 90–180 mg cohort.69 Therefore, it is possible that soon, neither alectinib nor ceritinib, but brigatinib will be the ALK TKI to be used for the first-line therapy of ALK+ NSCLC patients. Lorlatinib, due to each side effects, is mostly preferred after two second-generation (alectinib and ceritinib, or alectinib and brigatinb) ALK TKIs. It will soon become the second ALK TKI to be used when medical oncologists are familiar with the management of the side effects of lorlatinib.70,71 In the case that an ALK+ NSCLC patient progresses to several ALK TKIs, a chemotherapy regimen, like carboplatin with pemetrexed with or without bevacizumab may control the disease for a certain period and give space for ALK TKIs to regain activity after a “targeted therapy break”.
Conclusion
We searched the Citeline Pharma Intelligence (https://citeline.informa.com/trials/results) for clinical trials with alectinib, that are open or ongoing (Table 3). A Phase III clinical trial plans to compare alectinib versus brigatinib for ALK+ NSCLC patients who have progressed to crizotinib. Alectinib is currently under comparison with adjuvant platinum-based chemotherapy for stage IB-IIIA completely resected ALK+ NSCLC patients. Two studies are planned to test the efficacy of alectinib in combination with bevacizumab in ALK+ NSCLC patients. Considering its efficacy and tolerability, alectinib is the best first-line approach for ALK+ NSCLC patients, especially those with CNS metastasis at the time of the diagnosis. Alectinib is an important treatment option for ALK+ NSCLC patients who have progressed to crizotinib.
Acknowledgments
Work in Dr Rosell´s laboratory is partially supported by a grant from La Caixa Foundation, an Instituto de Salud Carlos III grant (RESPONSE, PIE16/00011), a Marie Skłodowska-Curie Innovative Training Networks European Grant (ELBA No 765492) and a Spanish Association Against Cancer (AECC) grant (PROYE18012ROSE). The Funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet. 2013;382(9893):720–731. doi: 10.1016/S0140-6736(13)61715-8 [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer. Lancet. 2016;387(10026):1354–1356. doi: 10.1016/S0140-6736(15)01125-3 [DOI] [PubMed] [Google Scholar]
- 3.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin‘s lymphoma. Science. 1994;263(5151):1281–1284. [DOI] [PubMed] [Google Scholar]
- 4.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11–23. doi: 10.1038/nrc2291 [DOI] [PubMed] [Google Scholar]
- 5.Webb TR, Slavish J, George RE, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009;9(3):331–356. doi: 10.1586/14737140.9.3.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horn L, Pao W. EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol. 2009;27(26):4232–4235. doi: 10.1200/JCO.2009.23.6661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- 8.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203. doi: 10.1016/j.cell.2007.11.025 [DOI] [PubMed] [Google Scholar]
- 9.Passaro A, Lazzari C, Karachaliou N, et al. Personalized treatment in advanced ALK-positive non-small cell lung cancer: from bench to clinical practice. Onco Targets Ther. 2016;9:6361–6376. doi: 10.2147/OTT.S98347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki T, Rodig SJ, Chirieac LR, Janne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46(10):1773–1780. doi: 10.1016/j.ejca.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. doi: 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 13.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi: 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 14.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118–1133. doi: 10.1158/2159-8290.CD-16-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karachaliou N, Rosell R. Systemic treatment in EGFR-ALK NSCLC patients: second line therapy and beyond. Cancer Biol Med. 2014;11(3):173–181. doi: 10.7497/j.issn.2095-3941.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karachaliou N, Rosell R, Morales-Espinosa D, Viteri S. Systemic treatment in EGFR-ALK NSCLC patients: second line therapy and beyond. Expert Rev Anticancer Ther. 2014;14(7):807–815. doi: 10.1586/14737140.2014.896210 [DOI] [PubMed] [Google Scholar]
- 17.Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10(2):232–236. doi: 10.1097/JTO.0000000000000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non–small cell lung cancer. J Thorac Oncol. 2019;14(4):691–700. doi: 10.1016/j.jtho.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–1197. doi: 10.1056/NEJMoa1311107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917–929. doi: 10.1016/S0140-6736(17)30123-X [DOI] [PubMed] [Google Scholar]
- 21.Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(7):874–886. doi: 10.1016/S1470-2045(17)30339-X [DOI] [PubMed] [Google Scholar]
- 22.Novello S, Mazières J, Oh I-J, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29(6):1409–1416. doi: 10.1093/annonc/mdy121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39. doi: 10.1016/S0140-6736(17)30565-2 [DOI] [PubMed] [Google Scholar]
- 24.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. doi: 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 25.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379(21):2027–2039. doi: 10.1056/NEJMoa1810171 [DOI] [PubMed] [Google Scholar]
- 26.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–1599. doi: 10.1016/S1470-2045(17)30680-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon B, Shaw A, Ou S, et al. OA 05.06 phase 2 study of lorlatinib in patients with advanced ALK+/ROS1+ non-small-cell lung cancer. J Thoracic Oncol. 2017;12(11):S1756. doi: 10.1016/j.jtho.2016.09.002 [DOI] [Google Scholar]
- 28.Horn L, Infante JR, Reckamp KL, et al. Ensartinib (X-396) in ALK-positive non-small cell lung cancer: results from a first-in-human phase I/II, multicenter study. Clin Cancer Res. 2018;24(12):2771–2779. doi: 10.1158/1078-0432.CCR-17-2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent- front mutations. Cancer Discov. 2018;8(10):1227–1236. doi: 10.1158/2159-8290.CD-18-0484 [DOI] [PubMed] [Google Scholar]
- 30.Sullivan I, Planchard D. ALK inhibitors in non-small cell lung cancer: the latest evidence and developments. Ther Adv Med Oncol. 2016;8(1):32–47. doi: 10.1177/1758834015617355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw AT, Varghese AM, Solomon BJ, et al. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann Oncol. 2013;24(1):59–66. doi: 10.1093/annonc/mds242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paik J, Dhillon S. Alectinib: a review in advanced, ALK-positive NSCLC. Drugs. 2018;78(12):1247–1257. doi: 10.1007/s40265-018-0952-0 [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19(5):679–690. doi: 10.1016/j.ccr.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Kodama T, Tsukaguchi T, Yoshida M, Kondoh O, Sakamoto H. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett. 2014;351(2):215–221. doi: 10.1016/j.canlet.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 35.Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov. 2018;8(6):714–729. doi: 10.1158/2159-8290.CD-17-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodama T, Tsukaguchi T, Satoh Y, et al. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther. 2014;13(12):2910–2918. doi: 10.1158/1535-7163.MCT-14-0274 [DOI] [PubMed] [Google Scholar]
- 37.Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18(3):375–377. doi: 10.1038/nm.2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35(13):1403–1410. doi: 10.1200/JCO.2016.70.9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirker R, Filipits M. Alectinib in RET-rearranged non-small cell lung cancer-another progress in precision medicine? Transl Lung Cancer Res. 2015;4(6):797–800. doi: 10.3978/j.issn.2218-6751.2015.03.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drilon AE, Subbiah V, Oxnard GR, et al. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. J Clin Oncol. 2018;36(15_suppl):102. doi: 10.1200/JCO.2018.36.15_suppl.102 [DOI] [Google Scholar]
- 41.Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8(7):836–849. doi: 10.1158/2159-8290.CD-18-0338 [DOI] [PubMed] [Google Scholar]
- 42.BLU-667 targets RET-altered cancers. Cancer Discov. 2018;8(6):OF8. doi: 10.1158/2159-8290.CD-NB2018-050 [DOI] [PubMed] [Google Scholar]
- 43.Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653–1660. doi: 10.1016/S1470-2045(16)30562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–1128. doi: 10.1016/S1470-2045(14)70362-6 [DOI] [PubMed] [Google Scholar]
- 45.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol. 2013;14(7):590–598. doi: 10.1016/S1470-2045(13)70142-6 [DOI] [PubMed] [Google Scholar]
- 46.Tamura T, Kiura K, Seto T, et al. Three-year follow-up of an alectinib phase I/II study in ALK-positive non-small-cell lung cancer: AF-001JP. J Clin Oncol. 2017;35(14):1515–1521. doi: 10.1200/JCO.2016.70.5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34(7):661–668. doi: 10.1200/jco.2016.34.4_suppl.661 [DOI] [PubMed] [Google Scholar]
- 48.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17(2):234–242. doi: 10.1016/S1470-2045(15)00488-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeaiter A, Dingemans A-MC, Balas B, et al. Updated efficacy and safety from the global phase II NP28673 study of alectinib in patients (pts) with previously treated ALK+ non-small-cell lung cancer (NSCLC). Ann of Oncology. 2016;27(suppl_6). doi: 10.1093/annonc/mdw141. [DOI] [Google Scholar]
- 50.Camidge DR, Gadgeel S, Ou S-H, et al. MA07.02 updated efficacy and safety data from the phase 2 NP28761 study of alectinib in ALK-positive non-small-cell lung cancer. J Thoracic Oncol. 2017;12(1):S378. doi: 10.1016/j.jtho.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang JC, Ou SI, De Petris L, et al. Pooled systemic efficacy and safety data from the pivotal phase II studies (NP28673 and NP28761) of alectinib in ALK-positive non-small cell lung cancer. J Thorac Oncol. 2017;12(10):1552–1560. doi: 10.1016/j.jtho.2017.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takiguchi Y, Hida T, Nokihara H, et al. Updated efficacy and safety of the j-alex study comparing alectinib (ALC) with crizotinib (CRZ) in ALK-inhibitor naïve ALK fusion positive non-small cell lung cancer (ALK+ NSCLC). Journal of Clinical Oncology2017; 35(15_suppl): 9064. [Google Scholar]
- 53.Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018;29(11):2214–2222. doi: 10.1093/annonc/mdy405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camidge DR, Peters S, Mok T, et al. Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK+ NSCLC. Journal of Clinical Oncology2018; 36(15_suppl): 9043. [Google Scholar]
- 55.Zhang S, Anjum R, Squillace R, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res. 2016;22(22):5527–5538. doi: 10.1158/1078-0432.CCR-16-0569 [DOI] [PubMed] [Google Scholar]
- 56.Karachaliou N, Cardona AF, Bracht JWP, et al. Integrin-linked kinase (ILK) and src homology 2 domain-containing phosphatase 2 (SHP2): novel targets in EGFR-mutation positive non-small cell lung cancer (NSCLC). EBioMedicine. 2019;39:207–214. doi: 10.1016/j.ebiom.2018.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med. 2012;4(120):120ra117. doi: 10.1126/scitranslmed.3003316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crystal AS, Shaw AT, Sequist LV, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346(6216):1480–1486. doi: 10.1126/science.1254721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lovly CM, McDonald NT, Chen H, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med. 2014;20(9):1027–1034. doi: 10.1038/nm.3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dardaei L, Wang HQ, Singh M, et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med. 2018;24(4):512–517. doi: 10.1038/nm.4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hrustanovic G, Olivas V, Pazarentzos E, et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med. 2015;21(9):1038–1047. doi: 10.1038/nm.3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Childress MA, Himmelberg SM, Chen H, Deng W, Davies MA, Lovly CM. ALK fusion partners impact response to ALK inhibition: differential effects on sensitivity, cellular phenotypes, and biochemical properties. Mol Cancer Res. 2018;16(11):1724–1736. doi: 10.1158/1541-7786.MCR-18-0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ou SI, Lee TK, Young L, et al. Dual occurrence of ALK G1202R solvent front mutation and small cell lung cancer transformation as resistance mechanisms to second generation ALK inhibitors without prior exposure to crizotinib. Pitfall of Solely Relying on Liquid Re-Biopsy? Lung Cancer. 2017;106:110–114. doi: 10.1016/j.lungcan.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 64.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 65.Mountzios G, Calles A, Addeo A. Atezolizumab treatment of nonsquamous NSCLC. N Engl J Med. 2018;379(12):1187. doi: 10.1056/NEJMc1809195 [DOI] [PubMed] [Google Scholar]
- 66.Ashraf N. Atezolizumab treatment of nonsquamous NSCLC. N Engl J Med. 2018;379(12):1187–1188. doi: 10.1056/NEJMc1809195 [DOI] [PubMed] [Google Scholar]
- 67.Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med. 2016;374(1):54–61. doi: 10.1056/NEJMoa1508887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho BC, Obermannová R, Bearz A, et al. OA 05.07 efficacy and updated safety of ceritinib (450 mg or 600 mg) with low-fat meal vs 750 mg fasted in ALK+ metastatic NSCLC. J Thoracic Oncol. 2017;12(11):S1757. doi: 10.1016/j.jtho.2016.09.002 [DOI] [Google Scholar]
- 69.Camidge DR, Kim DW, Tiseo M, et al. Exploratory analysis of brigatinib activity in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer and brain metastases in two clinical trials. J Clin Oncol. 2018;36(26):2693–2701. doi: 10.1200/JCO.2017.77.5841 [DOI] [PubMed] [Google Scholar]
- 70.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654–1667. doi: 10.1016/S1470-2045(18)30649-1 [DOI] [PubMed] [Google Scholar]
- 71.Akamine T, Toyokawa G, Tagawa T, Seto T. Spotlight on lorlatinib and its potential in the treatment of NSCLC: the evidence to date. Onco Targets Ther. 2018;11:5093–5101. doi: 10.2147/OTT.S165511 [DOI] [PMC free article] [PubMed] [Google Scholar]