Abstract
The mechanisms underlying the functional differences in sympathetic and parasympathetic regulation of the major salivary glands have received little attention. The acute effects of parasympathetic muscarinic (carbachol)-dependent and combined parasympathetic-dependent plus cAMP-dependent pathways on fluid secretion rates, ion composition, and protein content were assessed using a newly developed ex vivo preparation that allows the simultaneous perfusion of the mouse submandibular (SMGs) and sublingual glands (SLGs). Our results confirm that the muscarinic-dependent pathway accounts for the bulk of salivation in SMGs and SLGs, whereas costimulation with a cAMP-increasing agent (forskolin, isoproterenol, or vasoactive intestinal peptide) did not increase the flow rate. Costimulation with carbachol plus the β-adrenergic agonist isoproterenol decreased the concentration of NaCl and produced a substantial increase in the protein and Ca2+ content of SMG but not SLG saliva, consistent with a sparse sympathetic innervation of the SLGs. On the other hand, forskolin, which bypasses receptors to increase intracellular cAMP by directly activating the enzyme adenylate cyclase, enhanced the secretion of protein and Ca2+ by both the SMGs and SLGs. In contrast, isoproterenol and vasoactive intestinal peptide specifically stimulated protein secretion in SMG and SLG salivas, respectively. In summary, cAMP-dependent signaling does not play a major role in the stimulation of fluid secretion in SMGs and SLGs, whereas each cAMP-increasing agonist behaves differently in a gland-specific manner suggesting differential expression of G protein-coupled receptors in the epithelial cells of SMGs and SLGs.
Keywords: epithelium, fluid secretion, ion-transport regulation, protein secretion, salivary gland
INTRODUCTION
Saliva is a complex fluid mainly composed of secretions from the major glands and hundreds of minor salivary glands that drain into the oral cavity where it plays many important roles such as lubrication of oral mucosal surfaces, demineralization and remineralization of teeth, food digestion, and control of the oral microbiome (26). The importance of saliva is clearly inferred from patients suffering from diseases that are associated with salivary gland hypofunction such as Sjögren’s syndrome or the consequence of radiotherapy in head and neck cancers (22).
In humans, the major salivary glands [parotid, submandibular (SMGs), and sublingual glands (SLGs)] secrete between 0.5 and 1 liter of saliva per day. This efficiency is achieved because acinar secretory cells express ion-transporting proteins in their apical and basolateral domains that allow vectorial secretion of solutes and fluid into the luminal space (25).
Salivary gland function is regulated by the parasympathetic and sympathetic branches of the autonomic nervous system. It is known that sympathetic, adrenergic stimulation of parotid glands and SMGs results in the secretion of low amounts of a protein-rich fluid with a very low NaCl content. Conversely, parasympathetic, cholinergic stimulation of parotid glands and SMGs is associated with copious amounts of fluid containing comparatively low amounts of proteins and a relatively higher NaCl concentration (3, 28).
Although the molecular mechanism underlying fluid secretion is well conserved among salivary glands (15), the regulation of ion transport and fluid secretion processes apparently differs between glands. It is known that parotid glands and SMGs receive inputs from sympathetic and parasympathetic fibers, where they modulate ion transport as well as fluid and protein secretion (22, 27). In contrast, mucus-secreting minor glands and SLGs receive extensive inputs from the parasympathetic system, but the innervation by the sympathetic system is sparse (26).
Protein secretion by the rat SLGs is modulated by the parasympathetic activation of cholinergic muscarinic receptors in a PKC-dependent manner (8). The role of the sympathetic system in protein secretion by the SLGs is contradictory. It has been reported that sympathetic adrenergic agonists do not stimulate mucin secretion by isolated rat SLG cells (7), whereas the amount and concentration of protein in the saliva from rat SLGs increased upon intraperitoneal injection of catecholamines compared with cholinergic agonists (1).
On the other hand, little is known about the modulation of protein and fluid secretion of mucus-secreting glands by cAMP-dependent pathways. Vasoactive intestinal peptide (VIP) is a parasympathetic-associated neurotransmitter that elevates intracellular cAMP levels and has been reported to modulate the exocytosis of protein-storing granules in human major salivary gland acinar cells (9). Furthermore, intravenous injections of VIP induced saliva secretion by rat parotid glands and SMGs (11). Of note, increased levels of VIP are observed in the parotid glands and SMGs of sympathectomized rats, suggesting a compensatory mechanism mediated by these cAMP-elevating secretagogues in the absence of β-adrenergic receptor-mediated cAMP cell signaling (10). Nonetheless, the negligible sympathetic innervation of mucus-secreting glands raises the question of which, if any, physiological secretagogues that increase intracellular cAMP levels might be involved in the regulation of fluid and protein secretion in the mucus-secreting sublingual salivary glands.
Taken together, we hypothesize that cAMP-dependent pathways might regulate the activity of mucus-secreting salivary glands. To test this model, we developed an ex vivo assay for simultaneously assessing the role of cAMP-elevating secretagogues in mouse serous fluid-secreting SMGs and mucus-secreting SLGs. Our results show that cAMP-dependent mechanisms differentially regulate serous fluid- and mucus-secreting glands.
MATERIALS AND METHODS
Ex vivo perfused SLGs and SMGs.
Ex vivo SLG and SMG salivas were simultaneously collected by modifying the ex vivo mouse SMG perfusion technique (30). In brief, mice were anesthetized by chloral hydrate injection (400 mg/kg ip), and all branches of the common carotid artery were ligated, except for the artery supplying blood to the SLGs and SMGs. Both glands were then removed and perfused at 37°C via the common artery, which was cannulated with a 31-gauge cannula. SLG and SMG ducts were placed in different calibrated glass capillary tubes (Fig. 1). Salivation was stimulated by perfusion with the muscarinic receptor agonist carbachol (CCh, 0.3 µM) or CCh (0.3 µM) plus cAMP-increasing agents [5 µM isoproterenol (IPR), 100 nM VIP, or 10 µM forskolin]. The perfusion solution was as follows (mM): 4.3 KCl, 120 NaCl, 25 NaHCO3, 1 CaCl2, 1 MgCl2, 5 glucose, and 10 HEPES, pH 7.4 (gassed with 95% O2-5% CO2). Saliva samples were collected and stored at −80°C until analyzed.
Fig. 1.
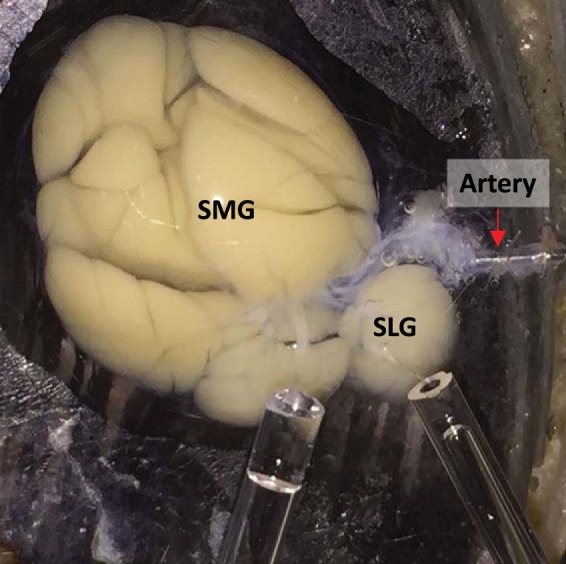
Simultaneous perfusion of mouse submandibular (SMGs) and sublingual glands (SLGs). Image depicting SMGs and SLGs perfused via common carotid artery by a 31-gauge cannula. SMG and SLG main ducts were separated and connected to different calibrated glass capillary tubes for collecting saliva to quantify flow rates and performing ion and protein analysis.
Ion and protein concentrations in SLG- and SMG-secreted fluids.
Na+, K+, and Ca2+ concentrations in saliva were measured by atomic absorption spectrometry using the appropriate lamps (AAnalyst 200 spectrometer; PerkinElmer). The Cl− concentration was analyzed with an Orion Cl− electrode (Thermo Fisher Scientific). To ensure enough sample for measuring the ion composition of saliva secreted in response to cAMP-elevating secretagogues, data shown in Figs. 3 and 4 were obtained from SMGs and SLGs stimulated for 15 min.
Fig. 3.
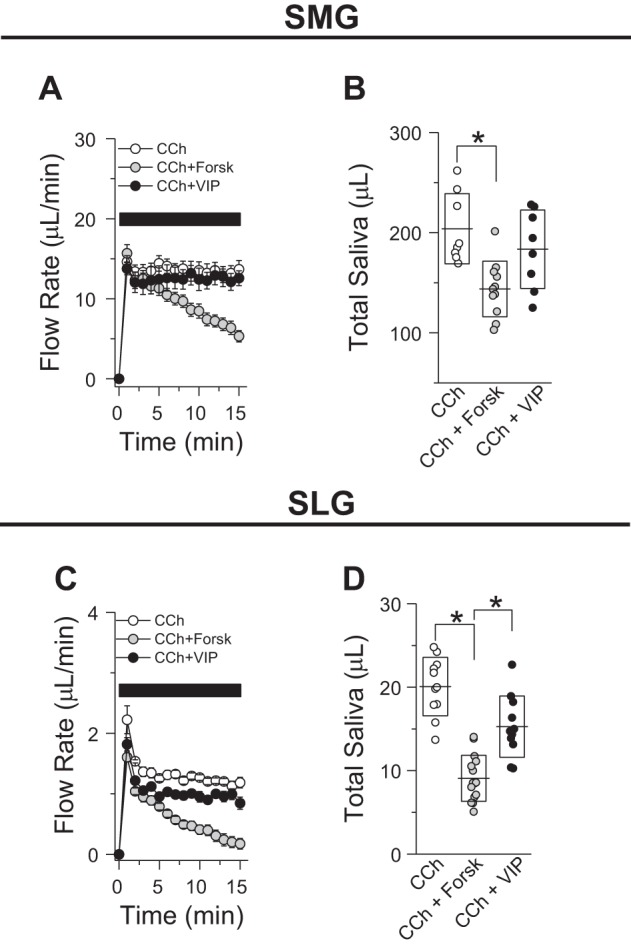
Effect of cAMP-elevating secretagogues on the ex vivo secretions by submandibular (SMGs) and sublingual glands (SLGs). A: flow rates for SMGs (15 min of stimulation) stimulated with 0.3 µM carbachol (CCh; ○, n = 8 glands) or 0.3 µM CCh + 10 µM forskolin (Forsk; gray circles, n = 11 glands) or + 100 nM vasoactive intestinal peptide (VIP; ●, n = 6 glands). B: total amount of saliva secreted by SMGs stimulated with the secretagogues shown in A. C: flow rates for SLGs (15 min of stimulation) stimulated with 0.3 µM CCh (○, n = 11) or 0.3 µM CCh + 10 µM forskolin (gray circles, n = 15) or + 100 nM VIP (●, n = 11). D: total amount of saliva secreted by SLGs stimulated with the secretagogues shown in C. Lines and boxes correspond to means and SDs, respectively, for each experimental condition. *P < 0.05 (ANOVA followed by Bonferroni’s post hoc test).
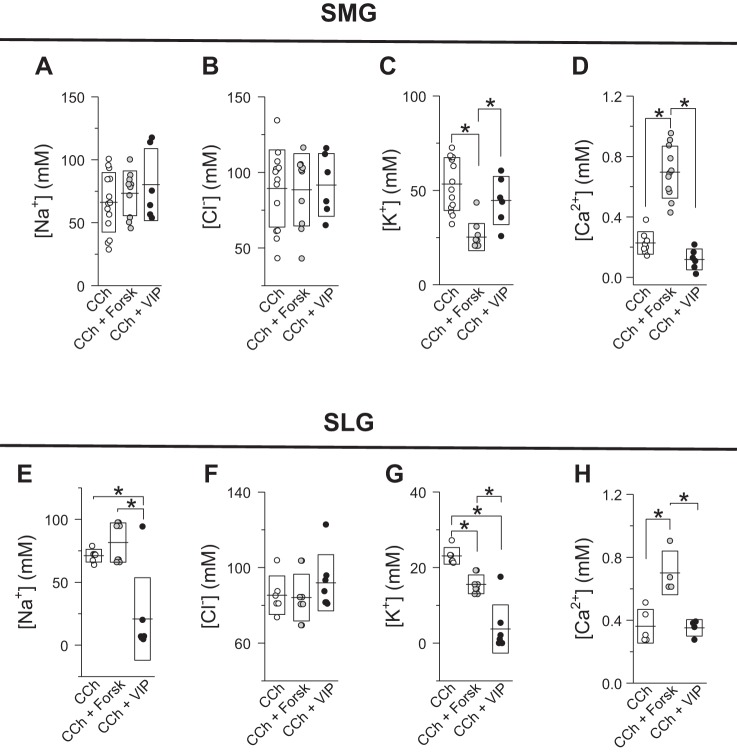
Fig. 4.
Effect of cAMP-elevating secretagogues on the ion composition of submandibular gland (SMG) and sublingual gland (SLG) salivas. Na+ (A), Cl− (B), K+ (C), and Ca2+ (D) concentrations of SMG saliva (from Fig. 3) stimulated with 0.3 µM carbachol (CCh; ○, n = 14) or 0.3 µM CCh + 10 µM forskolin (Forsk; gray circles, n = 10) or + 100 nM vasoactive intestinal peptide (VIP; ●, n = 6). Na+ (E), Cl− (F), K+ (G), and Ca2+ (H) concentrations of SLG saliva (from Fig. 3) stimulated with 0.3 µM CCh (○; Na+, n = 6; Cl−, n = 6; K+, n = 6; Ca2+, n = 5) or 0.3 µM CCh + 10 µM forskolin (gray circles; Na+, n = 8; Cl−, n = 9; K+, n = 8; Ca2+, n = 4) or + 100 nM VIP (●; Na+, n = 7; Cl−, n = 7; K+, n = 7; Ca2+, n = 4). Lines and boxes correspond to means and SDs, respectively, for each experimental condition. *P < 0.05 (ANOVA followed by Bonferroni’s post hoc test).
Protein concentrations were measured according to the manufacturer’s instructions by micro-bicinchoninic assay (Thermo Fisher Pierce, Rockford, IL) using a DU530 spectrophotometer (Beckman) at 562 nm. Bovine serum albumin was used as standard for calibrations. Protein concentrations were calculated from SMGs and SLGs stimulated for 5 min with agonist. This time window was chosen for the protein measurements shown in Figs. 5 and 6 as 75% of amylase (1 of the most abundant proteins in saliva) is secreted within the first 5 min in response to stimulation in rat parotid slices (14).
Fig. 5.
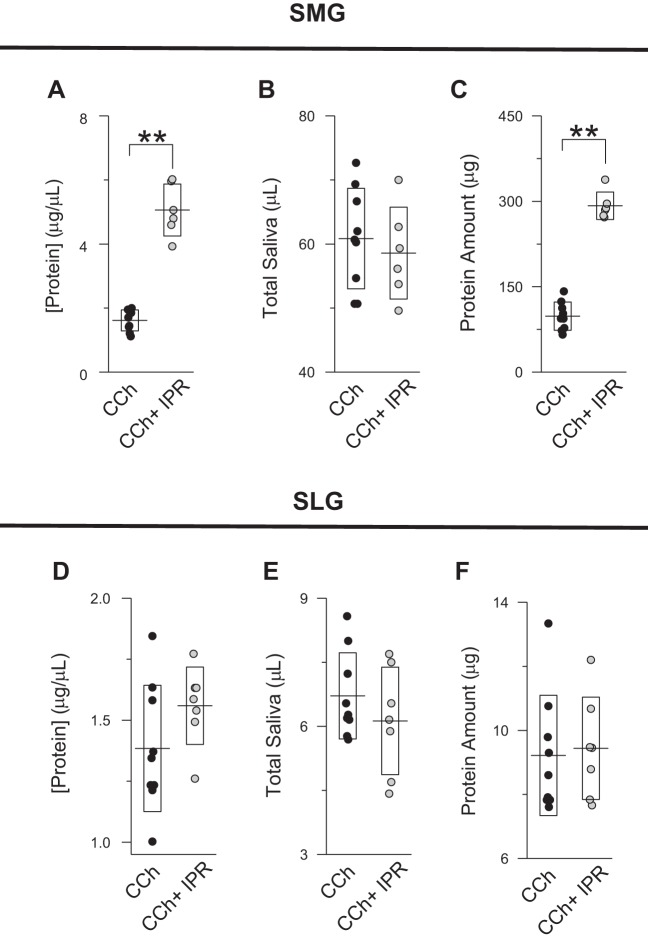
Effect of β-adrenergic agonist on the protein secretion by submandibular (SMGs) and sublingual glands (SLGs). A–C: protein concentration (A), amount of saliva secreted (B), and total amount of protein secreted (C) by SMGs (5 min of stimulation) in response to 0.3 µM carbachol (CCh; ●, n = 9) or 0.3 µM CCh + 5 µM isoproterenol (IPR; gray circles, n = 6). D–F: protein concentration (D), amount of saliva secreted (E), and total amount of protein secreted (F) by SLGs (5 min of stimulation) in response to 0.3 µM CCh (●, n = 9) or 0.3 µM CCh + 5 µM IPR (gray circles, n = 7). Total amount of protein was calculated by multiplying the protein concentration by the secreted saliva volume. Lines and boxes correspond to means and SDs, respectively, of experiments. **P < 0.01 (Student’s t-test).
Fig. 6.
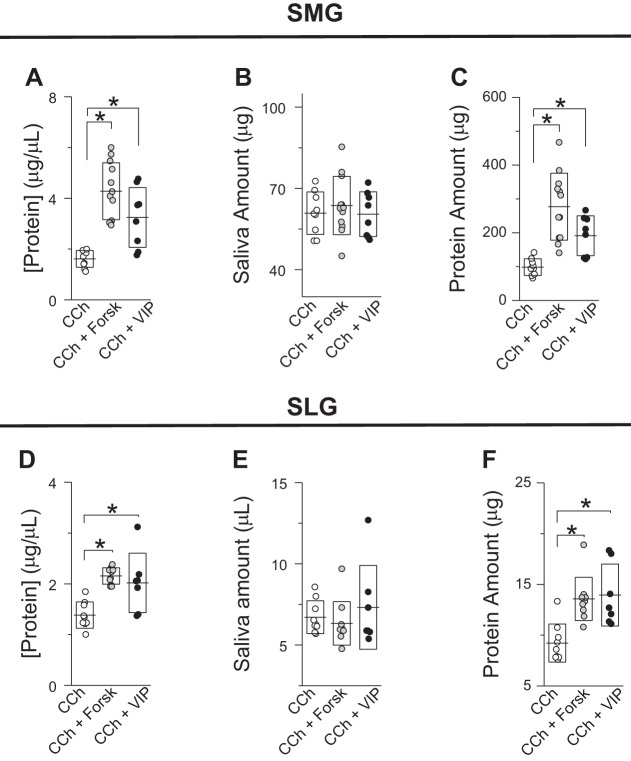
Effect of cAMP-elevating secretagogues on the protein secretion by submandibular (SMGs) and sublingual glands (SLGs). A–C: protein concentration (A), amount of saliva secreted (B), and total amount of protein secreted (C) by SMGs (5 min of stimulation) in response to 0.3 µM carbachol (CCh; ○, n = 9) or 0.3 µM CCh + 10 µM forskolin (Forsk; gray circles, n = 12) or + 100 nM vasoactive intestinal peptide (VIP; ●, n = 8). D–F: protein concentration (D), amount of saliva secreted (E), and total amount of protein secreted (F) by SLGs (5 min of stimulation) in response to 0.3 µM CCh (○, n = 9) or 0.3 µM CCh + 10 µM forskolin (gray circles, n = 10) or + 100 nM VIP (●, n = 7). Lines and boxes correspond to means and SDs, respectively, of experiments. *P < 0.05 (ANOVA followed by Bonferroni’s post hoc test).
Gene expression analysis.
Gene expression analysis of β-adrenergic and VIP receptors and Ca2+-activated K+ channel subunit-α1 (KCa1.1, or Kcnma1) was performed using publicly available RNA-sequencing data of mouse major salivary glands (Sequence Read Archive databases SRX2648311 to SRX2648322; n = 12; 13). The threshold for positive gene expression was 0.1 fragments per kilobase of transcript per million mapped reads (FPKM). Gene-sequencing data from salivary glands from three female and three male mice were used for comparing gene expression levels between SMGs and SLGs.
The Kcnma1 mRNA expression levels for SMGs and SLGs from RNA-sequencing data reported as FPKM values were 4.47 ± 0.55 versus 1.21 ± 0.08 (average ± SE; SMGs vs. SLGs, respectively; n = 6 each gland, P < 0.005, Student’s t-test).
The β-adrenergic receptor expression levels (FPKM) were 5.15 ± 1.16 versus 0.41 ± 0.12 for Adrb1 and 3.61 ± 0.50 versus 1.30 ± 0.41 for Adrb2, SMGs versus SLGs, respectively (n = 6 each gland, P < 0.005, Student’s t-test).
VIP receptor mRNA levels (FPKM) of type 1 VIP receptor (Vipr1) for SMGs versus SLGs were 0.49 ± 0.06 versus 3.31 ± 0.18, respectively (n = 6 each gland, P < 0.005, Student’s t-test). Transcript levels for type 2 VIP receptors encoded by Vipr2 were very low in both SMG and SLG (FPKM values <0.1).
Statistical analysis.
Results are presented as means ± SD. Individual data points are shown in box graphs. Statistical significance was determined using Student’s t-test and one-way ANOVA analysis followed by Bonferroni’s post hoc test. P < 0.05 was considered statistically significant. Origin 8.0 software was used for statistical calculations (OriginLab, Northampton, MA).
Animals and reagents.
Mice (BS/129Svj background) were housed in cages with access to laboratory chow and water ad libitum with a 12-h light-dark cycle. BS/129Svj background mice were generated in house by crossing BS (Charles River Laboratories) and 129Svj (The Jackson Laboratory) mice. Equal numbers of sex- and age-matched (from 6 to 12 wk old) mice were utilized. All animal procedures were approved by the Animal Care and Use Committee of the National Institute of Dental and Craniofacial Research, National Institutes of Health (ASP 13-686). All reagents were from Sigma-Aldrich (St. Louis, MO), unless otherwise indicated.
RESULTS
Saliva secretion by simultaneous ex vivo perfusion of SMGs and SLGs.
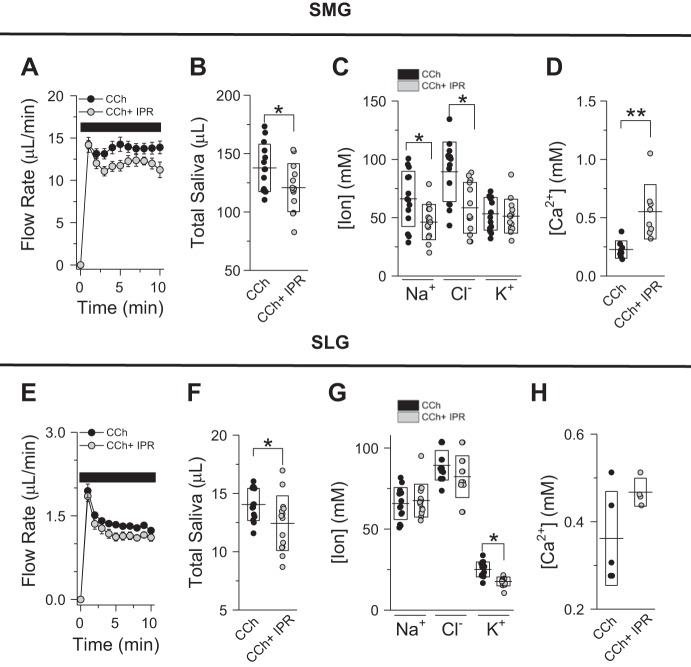
Saliva formation is a two-step process where a NaCl-rich, plasmalike fluid is secreted by acinar cells (stage 1). The ion composition of this primary fluid is subsequently modified (mainly NaCl reabsorption and KHCO3 secretion) as it passes through the ductal epithelium (stage 2; 22, 25). Given that ductal function in salivary glands is regulated by β-adrenergic receptors (5), we evaluated the effect of muscarinic (CCh) plus β-adrenergic IPR receptor stimulation on SMG and SLG flow rates and ion composition. As seen in Fig. 2, a slight but significant reduction in flow rates was observed in SMG (Fig. 2, A and B) and SLG (Fig. 2, E and F) secretion rates when the β-adrenergic agonist IPR (5 µM) was coperfused with the muscarinic agonist CCh (0.3 µM). In terms of duct function, IPR and CCh costimulation induced a substantial NaCl reabsorption in SMG-secreted fluid (Fig. 2C; 38% decrease in [Na+] + [Cl−]) but not in SLG-secreted fluid (Fig. 2G). Moreover, the K+ concentration of SMG saliva did not differ between CCh- and CCh + IPR-stimulated glands (Fig. 2C), whereas the K+ content of SLG saliva was slightly reduced upon β-adrenergic receptor stimulation with IPR (Fig. 2G). Of note, the Ca2+ concentration of SMG saliva increased ~140% but did not significantly change in SLG saliva upon β-adrenergic receptor activation by IPR (Fig. 2, D and H, respectively).
Fig. 2.
Ex vivo flow rates and ion composition of submandibular gland (SMG) and sublingual gland (SLG) saliva upon combined muscarinic acetylcholine and β-adrenergic receptor activation. A: flow rates for SMGs (10 min of stimulation) perfused with 0.3 µM carbachol (CCh; ●, n = 14 glands) or 0.3 µM CCh + 5 µM isoproterenol (IPR; gray circles, n = 13 glands) bicarbonate-containing solutions. B: total amount of saliva secreted by SMGs stimulated with 0.3 µM CCh (●) or 0.3 µM CCh + 5 µM IPR (gray circles). Lines and boxes correspond to means and SDs, respectively, of experiments shown in A. C and D: Na+, K+, and Cl− (C) and Ca2+ (D) concentrations (mM) in SMG saliva stimulated with 0.3 µM CCh (●, n = 14 except for Ca2+ measurements, where n = 8) or 0.3 µM CCh + 5 µM IPR (gray circles, n = 13 except for Ca2+ measurements, where n = 8). E: flow rates for SLGs (10 min of stimulation) perfused with 0.3 µM CCh (●, n = 14 glands) or 0.3 µM CCh + 5 µM IPR (gray circles, n = 14 glands) bicarbonate-containing solutions. F: total amount of saliva secreted by SLGs stimulated with 0.3 µM CCh (●) or 0.3 µM CCh + 5 µM IPR (gray circles). Lines and boxes correspond to means and SDs, respectively, of experiments shown in E. G and H. Na+, K+, and Cl− (G) and Ca2+ (H) concentrations (mM) in SLG saliva stimulated with 0.3 µM CCh (●, n = 14 except for Ca2+ measurements, where n = 5) or 0.3 µM CCh + 5 µM IPR (gray circles, n = 14 except for Ca2+ measurements, where n = 4). *P < 0.05 and **P < 0.01 (Student’s t-test). Lines and boxes correspond to means and SDs, respectively, for each experimental condition.
We then evaluated the effects of other physiological secretagogues that elevate intracellular cAMP levels on SMG and SLG function. To assess whether cAMP secretagogues affect the secretory (acinar) function of SMGs (Fig. 3, A and B) or SLGs (Fig. 3, C and D), the flow rates were measured in response to 0.3 µM CCh or combined stimulation with CCh (0.3 µM) + forskolin or VIP. As seen in Fig. 3, comparison of SMG and SLG secretion rates during 15-min stimulation showed that forskolin, which directly activates adenylate cyclase, reduced CCh-mediated flow rates in both SMGs and SLGs, respectively.
To evaluate whether ductal function in SMGs and SLGs is modulated by cAMP-elevating secretagogues, we then evaluated the ion composition of the SMG- and SLG-secreted fluids collected in response to the experimental conditions shown in Fig. 3. As seen in Fig. 4, the Na+, K+, and Cl− compositions of SMG- and SLG-secreted salivas were differentially affected by different cAMP secretagogues: 1) NaCl content in saliva from SMGs stimulated with by forskolin or VIP was statistically equal to NaCl content from SMG-secreted saliva in response to CCh (Fig. 4, A and B); 2) K+ content in saliva collected from SMGs costimulated with CCh and forskolin was statistically less than that from the other groups tested (Fig. 4C); 3) reduction in Na+ (Fig. 4E) and K+ (Fig. 4G) content in SLG saliva costimulated with VIP was statistically different from that of SLG fluids stimulated with CCh or CCh + forskolin; 4) K+ content in saliva from SLGs stimulated with CCh + forskolin secreted a fluid with lower K+ content; and 5) none of the cAMP-inducing agonists tested in this study modified the Cl− content compared with its control (0.3 µM CCh; Fig. 4, B and F). Regarding the Ca2+ content of saliva, costimulation with CCh + forskolin resulted in higher [Ca2+] in the salivas of both SMGs and SLGs. In contrast, VIP did not induce a significant increase in Ca2+ content in SMG or SLG saliva (Fig. 4, D and H, respectively).
Protein secretion in saliva from perfused SMGs and SLGs.
It has been shown that a higher protein concentration in saliva is associated with sympathetic activity in parotid glands and SMGs. Given that the SLGs receive a sparse innervation of sympathetic fibers, little is known about the regulation of protein secretion in SLGs by agonists that elevate intracellular cAMP levels (7, 26, 27). Taking advantage of the simultaneous SMG and SLG perfusion technique described here, we measured the protein concentration in SMG and SLG salivas obtained by perfusion of both glands with solutions containing different agonists.
We first compared protein concentrations and calculated the total amount of protein secreted from both SMGs and SLGs stimulated for 5 min with CCh and CCh + IPR, respectively. Figure 5, A–C, shows that the protein concentration and the amount of protein secreted by the SMGs were substantially stimulated nearly threefold upon β-adrenergic receptor stimulation. In contrast, no difference was observed in the protein concentration and the amount of protein secreted from SLGs stimulated with CCh compared with CCh+ IPR (Fig. 5, D–F).
We then evaluated the protein concentration and amount of protein secreted by SMGs and SLGs costimulated with CCh and different cAMP-elevating secretagogues. Figure 6, A–C, shows that forskolin and VIP increased the amount and concentration of protein secreted compared with SMGs stimulated with CCh. Similarly, the amount and concentration of protein in SLG saliva increased when SLGs were costimulated with CCh and forskolin or VIP (Fig. 6, D–F).
DISCUSSION
The molecular mechanism underlying fluid secretion in mammalian salivary glands is well conserved (20, 22, 25). Briefly, the basolateral domain of secretory acinar cells expresses Na+-K+-ATPase (21), Na+-K+-2Cl− cotransporter (12, 21), and K+ channels (3, 24, 28, 30), which promote the intracellular accumulation of Cl− above its equilibrium potential. Apical Ca2+-activated Cl− channels allow Cl− efflux across the luminal membrane, which creates a negative transepithelial potential difference that drives Na+ secretion into the luminal space via the paracellular pathway (4, 29, 32). Finally, NaCl accumulation generates an osmotic gradient that energizes the water movement in the secretory direction via transcellular (aquaporin-5-dependent) and paracellular pathways (16, 19). Key proteins involved in fluid secretion have been found in acinar cells from all major salivary glands (15). However, the major salivary glands display distinct secretory properties; that is, the contribution of each gland to whole saliva differs in terms of ion and protein content and the stimuli that trigger secretion.
In this study, we evaluated the dependence of acinar cell fluid secretion on β-adrenergic receptor stimulation in SMGs and SLGs. As seen in Fig. 2, costimulation with CCh + IPR slightly decreased the flow rates of both SMGs and SLGs (<15%). In contrast, the physiological cAMP-elevating agonist VIP had no effect on the flow rates displayed by SMGs and SLGs in response to muscarinic receptor stimulation. Our results contrast with those reported by Larsson and Olgart, in which CCh-induced in vivo salivation by rat parotid glands was potentiated by forskolin and VIP (18). We do not have a clear explanation for such differences, but the in vivo prostimulatory effect of forskolin and VIP might be due to systemic effects that are not present in the isolated perfused-gland preparation.
Sympathetic fibers innervate the ducts of the parotid glands and SMGs, where stimulation enhances NaCl reabsorption, whereas the sympathetic innervation in SLGs is sparse (26). Consistent with these anatomical observations, reabsorption of NaCl was strongly activated upon β-adrenergic receptor stimulation in SMGs but not in SLGs. On the other hand, the NaCl content of SMG saliva produced by CCh costimulation with VIP did not differ from stimulation with only CCh (Fig. 4, A and B). This finding suggests that VIP receptors are not expressed at significant levels in the SMG duct cells responsible for NaCl reabsorption. Alternatively, we cannot rule out that VIP-mediated signals can inhibit ductal function in SMG ducts.
Costimulation of SLGs with CCh + VIP strongly reduced Na+ but not Cl− content in saliva, indicating that VIP receptors are functionally expressed in SLG ducts. This finding revealed that Na+ reabsorption and Cl− reabsorption are independent processes in SLGs. Such a noncoupled NaCl reabsorption mechanism clearly differs from that observed in mouse SMGs (5). Moreover, forskolin did not increase Na+ reabsorption, which clearly differs from the effect mediated by the cAMP-increasing agonists IPR (SMGs) and VIP (SLGs). The cAMP-mediated signals that are receptor mediated or mediated by direct stimulation of adenylate cyclase by forskolin might result in different responses from ion-transporting proteins involved in Na+ reabsorption.
In this study, we found that the K+ content in SMG saliva was higher than that in SLG saliva. According to the present salivary gland secretion model, the final K+ concentration of saliva is mostly determined by active electrodiffusive K+ secretion via the KCa1.1 (Kcnma1) channel (23). Consistent with this model, higher Kcnma1 transcript levels were observed in mouse SMG tissue compared with SLG tissue (13) supporting our finding that the [K+] of saliva is higher in SMGs (see materials and methods for more details).
Given that Na+ reabsorption in SMGs via epithelial Na+ channel (ENaC) is activated by an increase in intracellular cAMP levels (5), we expected a higher K+ content in SMG saliva stimulated with CCh + IPR due to the depolarization caused by ENaC-mediated Na+ influx. However, costimulation with IPR did not increase the K+ content of SMG saliva. We do not have an explanation for this finding, but it is tempting to speculate that K+ secretion might take place in a subset of duct cells that express KCa1.1 but do not express ENaC.
Little is known regarding K+ secretion by SLGs, but it is assumed that K+ secretion occurs in duct cells. Given that K+ content in saliva is inversely proportional to the flow rate, higher K+ content was expected when SLGs were costimulated with CCh + IPR (which reduces flow rate). In contrast, K+ content in SLG saliva was significantly reduced when SLGs were stimulated with CCh + IPR compared with that obtained from SLGs stimulated with only CCh. The same effect was also observed when SLGs were stimulated with CCh + VIP. Furthermore, K+ content in both SMG and SLG salivas was significantly reduced when costimulated with forskolin. Our results differ from those reported by Larsson et al., in which they found that VIP and forskolin potentiated the CCh-mediated K+ efflux in rat parotid fragments (17). The differences between the studies could be due to 1) different experimental approaches (K+ efflux measurements vs. K+ content in saliva; in this case, a higher K+ efflux caused by the activation of a basolateral K+ channel mediated by VIP and forskolin cannot be ruled out) or 2) different species (rat vs. mouse) or glands (parotid vs. submandibular-sublingual).
Together, the data presented in this study show that VIP and IPR decrease K+ secretion in the mouse SLG. Additionally, the reduction in K+ content in SMGs and SLGs in response to costimulation with forskolin strongly suggests that an undescribed cAMP-dependent signaling pathway might directly inhibit K+ secretion in both glands.
Alternatively, given that K+ secretion and secretion are coupled in salivary glands, we could assume a lower content in SLG saliva produced in response to CCh + IPR or CCh + VIP. Interestingly, there is compelling evidence linking the structure of mucins with ions, where lower secretion is associated with more viscous mucus in the airways (6). Together, a lower KHCO3 secretion would result in lower flow rates due to the higher viscosity of saliva.
Na+ (and K+) content in SLG saliva was reduced when costimulated with CCh + VIP. Given that the Cl− was unaffected when costimulated with CCh + VIP and the assumption that [Na+] + [K+] = [Cl−] + [], it is tempting to speculate that such costimulation might reduce the content of in SLG saliva. Further experiments are required to address this important topic.
Regarding calcium content, we found that the [Ca2+] of saliva was dramatically increased when β-adrenergic receptors were costimulated with muscarinic receptors in SMGs, but as expected, the β-adrenergic receptor agonist IPR had no effect in SLGs. Calcium concentration in SMG saliva increased by 140% when stimulated with CCh + IPR. On the other hand, SMG flow rate was decreased by 12.4% when stimulated with CCh + IPR. Therefore, the increase in calcium content in saliva collected in response to CCh + IPR cannot be explained by the lower flow rates observed during CCh + IPR treatment.
Little is known regarding the mechanism underlying Ca2+ transport in salivary glands, but it has been suggested that the final Ca2+ content in saliva is the result of two competing mechanisms, Ca2+ secretion by acinar cells and Ca2+ reabsorption by duct cells (2). On the basis of our findings, the higher Ca2+ content measured in SMG saliva stimulated with CCh + IPR could be due to 1) enhanced Ca2+ secretion by the acinar epithelium, 2) decreased Ca2+ reabsorption by the duct epithelium, or 3) activation of both Ca2+ secretion and inhibition of Ca2+ reabsorption. Given that the Ca2+ content increased in SMG saliva when glands were costimulated with CCh + IPR, it was not surprising that a robust increase in Ca2+ content was observed in SMG and SLG salivas when the glands were costimulated with CCh + forskolin. Together, our results show that the mechanism(s) underlying Ca2+ transport by SMGs and SLGs strongly depend on the intracellular levels of cAMP. However, VIP did not increase Ca2+ content in SMG and SLG salivas, suggesting that other physiological secretagogues might be required to sufficiently elevate cAMP to affect Ca2+ transport by SMGs and SLGs.
The salivary mucins, which are mainly synthesized by the seromucous acinar cells of SMGs and mucous cells of SLGs, form an adherent layer that coats the oral cavity, where it plays a key role in modulating the oral microbiome, hydration and lubrication of oral structures, and tooth remineralization and demineralization (31). In addition to the mucins, the oral mucus also consists of other proteins and lipids secreted by mucus-secreting glands. Taking advantage of the ex vivo perfused SMG-SLG preparation, which allows evaluation of the direct effects of secretagogues on SMG and SLG function, we found that the activation of β-adrenergic receptors stimulates protein secretion by SMGs but not SLGs (Fig. 5). In contrast, VIP induced protein secretion in both SMGs and SLGs. In summary, IPR selectively activated protein secretion by SMGs, whereas VIP induced protein secretion in both glands. Similar results to those displayed by VIP were obtained when glands were costimulated with forskolin, which bypasses receptors to directly activate cAMP production. Together, protein secretion by SMGs and SLGs is activated by increasing intracellular cAMP levels, which suggests a similar molecular secretion mechanism in both glands albeit through different receptors. On the basis of the activation profile of protein secretion by mouse salivary glands, the secretory acinar cells responsible for the bulk of protein secretion express robust levels of β-adrenergic receptors in SMGs. In fact, detailed analysis of transcript levels revealed that the expression is higher for genes encoding β1- and β2-adrenergic receptors (Adrb1 and Adrb2) in SMGs compared with SLGs (see materials and methods; 13).
VIP induced protein secretion in both SMG and SLG salivas, suggesting that VIP receptors are expressed in secretory cells from SMGs and SLGs. Considering that VIP also regulates duct function in SLGs (stimulates Na+ reabsorption; see Fig. 4E), transcript levels of Vipr1 (which encodes for a type 1 VIP receptor) were higher in SLGs compared with SMGs. On the other hand, transcript levels for type 2 VIP receptors encoded by Vipr2 were very low in both SLGs and SMGs (FPKM values <0.1; see materials and methods).
In summary, in the present study we describe the development of a modification of the mouse SMG-perfused preparation where both SMGs and SLGs are simultaneously perfused. Using this experimental approach, we found that cAMP-mobilizing agents modulate diverse aspects of salivary gland function such as flow rate, as well as the ion and protein compositions of saliva.
GRANTS
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Dental and Craniofacial Research (NIDCR) Grants 1-ZIA-DE000738 (J. E. Melvin) and 1-ZIG-DE000740 (NIDCR Veterinary Resources Core), and Proyecto Fondecyt 1171135 (M. A. Catalan) of the Fondo Nacional de Ciencia y Tecnología (Chile).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.E.M. and M.A.C. conceived and designed research; Y.K. performed experiments; Y.K., J.E.M., and M.A.C. analyzed data; Y.K., J.E.M., and M.A.C. interpreted results of experiments; Y.K. and M.A.C. prepared figures; J.E.M. and M.A.C. drafted manuscript; Y.K., J.E.M., and M.A.C. edited and revised manuscript; Y.K., J.E.M., and M.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Yasna Jaramillo for excellent technical assistance and Xin Gao for analyzing and providing RNA-sequencing data.
REFERENCES
- 1.Abe K, Yokota Y, Dawes C. Effects of parasympathomimetic and sympathomimetic drugs on the secretion and composition of rat sublingual saliva. J Dent Res 61: 52–56, 1982. doi: 10.1177/00220345820610011201. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay BC, Swaim WD, Sarkar A, Liu X, Ambudkar IS. Extracellular Ca2+ sensing in salivary ductal cells. J Biol Chem 287: 30305–30316, 2012. doi: 10.1074/jbc.M112.394122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begenisich T, Nakamoto T, Ovitt CE, Nehrke K, Brugnara C, Alper SL, Melvin JE. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem 279: 47681–47687, 2004. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 4.Catalán MA, Kondo Y, Peña-Munzenmayer G, Jaramillo Y, Liu F, Choi S, Crandall E, Borok Z, Flodby P, Shull GE, Melvin JE. A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc Natl Acad Sci USA 112: 2263–2268, 2015. doi: 10.1073/pnas.1415739112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalán MA, Nakamoto T, Gonzalez-Begne M, Camden JM, Wall SM, Clarke LL, Melvin JE. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J Physiol 588: 713–724, 2010. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol 299: L542–L549, 2010. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culp DJ, Graham LA, Latchney LR, Hand AR. Rat sublingual gland as a model to study glandular mucous cell secretion. Am J Physiol Cell Physiol 260: C1233–C1244, 1991. doi: 10.1152/ajpcell.1991.260.6.C1233. [DOI] [PubMed] [Google Scholar]
- 8.Culp DJ, Zhang Z, Evans RL. Role of calcium and PKC in salivary mucous cell exocrine secretion. J Dent Res 90: 1469–1476, 2011. doi: 10.1177/0022034511422817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Fiacco M, Quartu M, Ekström J, Melis T, Boi M, Isola M, Loy F, Serra MP. Effect of the neuropeptides vasoactive intestinal peptide, peptide histidine methionine and substance P on human major salivary gland secretion. Oral Dis 21: 216–223, 2015. doi: 10.1111/odi.12249. [DOI] [PubMed] [Google Scholar]
- 10.Ekström J, Ekman R. Sympathectomy-induced increases in calcitonin gene-related peptide (CGRP)-, substance P- and vasoactive intestinal peptide (VIP)-levels in parotid and submandibular glands of the rat. Arch Oral Biol 50: 909–917, 2005. doi: 10.1016/j.archoralbio.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Ekström J, Månsson B, Tobin G. Vasoactive intestinal peptide evoked secretion of fluid and protein from rat salivary glands and the development of supersensitivity. Acta Physiol Scand 119: 169–175, 1983. doi: 10.1111/j.1748-1716.1983.tb07322.x. [DOI] [PubMed] [Google Scholar]
- 12.Evans RL, Bell SM, Schultheis PJ, Shull GE, Melvin JE. Targeted disruption of the Nhe1 gene prevents muscarinic agonist-induced up-regulation of Na+/H+ exchange in mouse parotid acinar cells. J Biol Chem 274: 29025–29030, 1999. doi: 10.1074/jbc.274.41.29025. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Oei MS, Ovitt CE, Sincan M, Melvin JE. Transcriptional profiling reveals gland-specific differential expression in the three major salivary glands of the adult mouse. Physiol Genomics 50: 263–271, 2018. doi: 10.1152/physiolgenomics.00124.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa Y, Iida H, Skowronski MT, Ishida H. Activation of endogenous nitric oxide synthase coupled with methacholine-induced exocytosis in rat parotid acinar cells. J Pharmacol Exp Ther 301: 355–363, 2002. doi: 10.1124/jpet.301.1.355. [DOI] [PubMed] [Google Scholar]
- 15.Kondo Y, Nakamoto T, Jaramillo Y, Choi S, Catalan MA, Melvin JE. Functional differences in the acinar cells of the murine major salivary glands. J Dent Res 94: 715–721, 2015. doi: 10.1177/0022034515570943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem 276: 23413–23420, 2001. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 17.Larsson O, Detsch T, Fredholm BB. VIP and forskolin enhance carbachol-induced K+ efflux from rat salivary gland fragments by a Ca2+-sensitive mechanism. Am J Physiol Cell Physiol 259: C904–C910, 1990. doi: 10.1152/ajpcell.1990.259.6.C904. [DOI] [PubMed] [Google Scholar]
- 18.Larsson O, Olgart L. The enhancement of carbachol-induced salivary secretion by VIP and CGRP in rat parotid gland is mimicked by forskolin. Acta Physiol Scand 137: 231–236, 1989. doi: 10.1111/j.1748-1716.1989.tb08743.x. [DOI] [PubMed] [Google Scholar]
- 19.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem 274: 20071–20074, 1999. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 20.Martinez JR. Ion transport and water movement. J Dent Res 66, Suppl: 638–647, 1987. doi: 10.1177/00220345870660S106. [DOI] [PubMed] [Google Scholar]
- 21.Martinez JR, Cassity N. Effect of transport inhibitors on secretion by perfused rat submandibular gland. Am J Physiol Gastrointest Liver Physiol 245: G711–G716, 1983. doi: 10.1152/ajpgi.1983.245.5.G711. [DOI] [PubMed] [Google Scholar]
- 22.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469, 2005. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 23.Nakamoto T, Romanenko VG, Takahashi A, Begenisich T, Melvin JE. Apical maxi-K (KCa1.1) channels mediate K+ secretion by the mouse submandibular exocrine gland. Am J Physiol Cell Physiol 294: C810–C819, 2008. doi: 10.1152/ajpcell.00511.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamoto T, Srivastava A, Romanenko VG, Ovitt CE, Perez-Cornejo P, Arreola J, Begenisich T, Melvin JE. Functional and molecular characterization of the fluid secretion mechanism in human parotid acinar cells. Am J Physiol Regul Integr Comp Physiol 292: R2380–R2390, 2007. doi: 10.1152/ajpregu.00591.2006. [DOI] [PubMed] [Google Scholar]
- 25.Nauntofte B. Regulation of electrolyte and fluid secretion in salivary acinar cells. Am J Physiol Gastrointest Liver Physiol 263: G823–G837, 1992. doi: 10.1152/ajpgi.1992.263.6.G823. [DOI] [PubMed] [Google Scholar]
- 26.Proctor GB. The physiology of salivary secretion. Periodontol 2000 70: 11–25, 2016. doi: 10.1111/prd.12116. [DOI] [PubMed] [Google Scholar]
- 27.Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci 133: 3–18, 2007. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Romanenko V, Nakamoto T, Srivastava A, Melvin JE, Begenisich T. Molecular identification and physiological roles of parotid acinar cell maxi-K channels. J Biol Chem 281: 27964–27972, 2006. doi: 10.1074/jbc.M603871200. [DOI] [PubMed] [Google Scholar]
- 29.Romanenko VG, Catalán MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE. Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells. J Biol Chem 285: 12990–13001, 2010. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romanenko VG, Nakamoto T, Srivastava A, Begenisich T, Melvin JE. Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol 581: 801–817, 2007. doi: 10.1113/jphysiol.2006.127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabak LA. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol 57: 547–564, 1995. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 32.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]