Abstract
Endothelial cell (EC) mechanochemical transduction is the process by which mechanical stimuli are sensed by ECs and transduced into biochemical signals and ultimately into physiological responses. Identifying the mechanosensor/mechanochemical transducer(s) and describing the mechanism(s) by which they receive and transmit the signals has remained a central focus within the field. The heterotrimeric G protein, Gαq/11, is proposed to be part of a macromolecular complex together with PECAM-1 at EC junctions and may constitute the mechanochemical transducer as it is rapidly activated within seconds of flow onset. The mechanically activated cation channel Piezo1 has recently been implicated due to its involvement in mediating early responses, such as calcium and ATP release. Here, we investigate the role of Piezo1 in rapid shear stress-induced Gαq/11 activation. We show that flow-induced dissociation of Gαq/11 from PECAM-1 in ECs at 15 s is abrogated by BIM-46187, a selective inhibitor of Gαq/11 activation, suggesting that Gαq/11 activation is required for PECAM-1/Gαq/11 dissociation. Although siRNA knockdown of Piezo1 caused a dramatic decrease in PECAM-1/Gαq/11 association in the basal condition, it had no effect on flow-induced dissociation. Interestingly, siRNA knockdown of Piezo1 caused a marked decrease in PECAM-1 expression. Additionally, selective blockade of Piezo1 with ion channel inhibitors had no effect on flow-induced PECAM-1/Gαq/11 dissociations. Lastly, flow onset caused increased association of Gβ1 with Piezo1 as well as with the p101 subunit of phosphoinositide 3-kinase, which were both blocked by the Gβγ inhibitor gallein. Together, our results indicate that flow-induced activation of Piezo1 is not upstream of G protein activation.
Keywords: endothelial cells, Gαq/11, PECAM-1, Piezo1, PLA, shear stress
INTRODUCTION
Vascular endothelial cells (ECs) have the ability to sense external mechanical stimuli, such as fluid shear stress, and transduce them into intracellular biological signals that are critical for both maintaining normal endothelial homeostasis and developing certain vascular pathologies, including atherosclerosis. Identifying the endothelial mechanosensor/mechanochemical transducer(s) and determining how it functions have presented major challenges in fully understanding the mechanism(s) of endothelial mechanochemical transduction. Although several potential mechanosensors/mechanochemical transducers have been described to date, including integrins, receptor tyrosine kinases, adhesion molecules, the endothelial glycocalyx layer, and G protein-coupled receptors (GPCRs), the mechanosensor has yet to be fully elucidated.
The heterotrimeric G proteins Gαq and Gα11 (Gαq/11) have been previously reported to be membrane associated and directly activated within seconds of flow onset, providing one of the earliest flow-induced signaling events in ECs while also suggesting a potential role for Gαq/11 as the mechanochemical transducer (19). In another study, Gαq/11 was reconstituted in phospholipid vesicles in the absence of protein receptors and other components typically found in intact endothelial cells and was capable of being activated by fluid shear stress (18). Additionally, increased bilayer fluidity contributed to increased Gαq/11 activation by flow, whereas decreased fluidity resulted in reduced activation (18). The fact that Gαq/11 was activated in the absence of many of the aforementioned proposed mechanosensors strongly suggests that the membrane on its own is capable of being a mechanosensor.
Gαq/11 has also been shown to be colocalized with PECAM-1 at endothelial cell-cell junctions, to be dependent on heparan sulfates for its association with PECAM-1, and to be rapidly (within 15 s) dissociated from PECAM-1 in response to a step change in shear stress (13, 32). Furthermore, it has been reported that flow-induced activation of Gαq/11 can occur independent of GPCR activation (12). The positioning at the cell-cell junction is an important point since the imposed tension from fluid shear stress is highest at this region (30, 42). Flow-induced propagation of this tension could lead to the rapid increase in membrane fluidity that has been previously observed (6, 20). Together, these studies support a model in which the membrane senses fluid flow and membrane-associated Gαq/11, positioned at the cell-cell junction, acts as the mechanochemical transducer in ECs.
The mechanically activated cation channel Piezo1 has also been implicated as a mechanosensor of fluid shear stress in ECs. Piezo1 has been shown to be expressed by endothelial cells, and both its global and endothelial-specific deletions were determined to be embryonic lethal with severe defects in the developing vasculature (25, 34). Additionally, knockdown of Piezo1 by siRNA or blockade with GsMTx-4, a spider toxin-derived peptide (17), resulted in impaired Ca2+ influx and cell alignment in response to shear stress (25, 34), indicating that Piezo1 is indeed important for transducing flow-induced biological signals. Most recently, it has been reported that Piezo1 is required for ATP release at 5 min, which leads to the activation of the Gαq/11-coupled purinergic P2Y2 receptor, which in turn leads to other downstream flow-induced endothelial responses, including nitric oxide formation and activation of Akt (40, 41). These findings suggest that Piezo1 is an endothelial mechanosensor that is not only upstream of ATP release but also upstream of GPCR-dependent Gαq/11 activation. Based on the ability of Gαq/11 to be rapidly activated by flow in ECs and also in reconstituted phospholipid vesicles in the absence of Piezo1 (18, 19), we hypothesized that flow-induced Gαq/11 activation is not directly downstream of Piezo1 activation. We therefore sought to test whether Piezo1 activation is required for rapid Gαq/11 activation by shear stress in ECs.
In this study, we report that the shear stress-induced dissociation of Gαq/11 from PECAM-1 is a surrogate marker for the activation of Gαq/11 and neither the knockdown of Piezo1 by siRNA nor its inhibition with various ion channel blockers causes a significant decrease in this dissociation. Additionally, shear stress-induced activation of Gαq/11 coincides with an increased association of Gβ1 with Piezo1. Our results indicate that not only is Gαq/11 activation independent of Piezo1 in ECs in response to rapid flow changes, but Piezo1 could potentially be a direct downstream target of Gαq/11 activation.
MATERIALS AND METHODS
Cell culture.
Human coronary artery endothelial cells (HCAECs) from male or female donors were obtained from Lonza (Walkersville, MD) and maintained in complete endothelial growth medium (EGM-2; Lonza) supplemented with 10% heat-inactivated FBS and penicillin-streptomycin. Prior to all experimental procedures, cells were seeded onto glass microscope slides, grown to confluence, and serum-starved overnight in ATP-free endothelial basal medium (EBM-2; Lonza) supplemented with 0.5% BSA. HCAECs within six passages were used for all experiments.
Reagents.
BIM-46187 was purchased from EMD Millipore (Billerica, MA). Gallein, gadolinium (Gd3+) chloride, and Yoda 1 were purchased from Tocris Bioscience (Bristol, UK). Ruthenium Red (RR) was purchased from Cayman Chemical (Ann Arbor, MI). GsMTx-4 was purchased from both Tocris Bioscience and Alomone Laboratories (Jerusalem, Israel). Aluminum chloride (AlCl3) was obtained from Strem Chemicals (Newburyport, MA). Sodium fluoride (NaF) was purchased from Thermo Fisher Scientific (Carlsbad, CA). Rabbit anti-Piezo1 polyclonal antibody (15939-1-AP) was purchased from Proteintech (Rosemont, IL). Goat anti-PECAM-1 polyclonal antibody (sc-1506), anti-β-tubulin-horseradish peroxidase (sc-5274 HRP), and goat anti-Akt (sc-1618) were purchased from Santa Cruz Biotechnology (Dallas, TX). Rabbit anti-phospho-Akt (Ser473) (no. 4060) was obtained from Cell Signaling Technology (Danvers, MA). Rabbit anti-Gαq/11 was custom-made (clone no. 47; Epitomics, Burlingame, CA).
siRNA-mediated knockdown.
Silencing of Piezo1 expression was performed using four different siRNA oligonucleotides, three of which were custom synthesized by Thermo Fisher Scientific based on previously published target sequences (Table 1). The fourth siRNA oligonucleotide, ON-TARGETplus Human PIEZO1 siRNA-SMARTpool (L-020870-03-0005), was purchased from GE Dharmacon (Lafayette, CO). All four sets of siRNAs have been utilized to investigate the role of Piezo1 as a mechanosensor in the literature (25, 33, 34, 40, 43). siRNAs were transfected into HCAECs using Lipofectamine RNAiMax Reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. Cells were reseeded onto glass microscope slides at 24 h after transfection and analyzed at 48 h after transfection.
Table 1.
Oligonucleotide sequences for siRNA-mediated knockdown of human Piezo1
| Oligo Name | Sense (5′→3′) Sequence | Antisense (5′→3′) Sequence | Refs. |
|---|---|---|---|
| Piezo1 1 | GCAAGUUCGUGCGCGGAUUtt | AAUCCGCGCACGAACUUGCtt | (40) |
| Piezo1 2 | GCCUCGUGGUCUACAAGAUtt | AUCUUGUAGACCACGAGGCtt | (25) |
| Piezo1 3 | AGAAGAAGAUCGUCAAGUAtt | UACUUGACGAUCUUCUUCUtt | (25, 40, 43) |
| Piezo1 4 | GCAGCAUGACAGACGACAU | (33, 34) | |
| UGGAGUAUGCCAACGAGAA | |||
| UGGCUGAUGUUGUCGACUU | |||
| GCGCAUCAGUCUACGUUUU |
Shear stress.
Glass microscope slides with HCAECs were mounted on a conventional parallel-plate flow chamber (15) and subjected to a steady fluid shear stress of 14 dyn/cm2 by perfusion with CO2-equilibrated EBM-2 containing 0.5% BSA using a PHD 2000 syringe pump (Harvard Apparatus, Holliston, MA).
In situ proximity ligation assay.
HCAEC monolayers on glass slides were immediately quenched in cold methanol-acetone (−20°C), which is routinely used to quench metabolic activity on a subsecond time scale for quantitative metabolomics (10). After rehydration of cells in ice-cold PBS, the slides were treated according to the manufacturer’s protocol (Olink Biosciences, Uppsala, Sweden). Primary antibodies used were a custom-made rabbit anti-Gαq/11 (clone no. 47; Epitomics, Burlingame, CA), mouse anti-PECAM-1 (BBA7; R&D Systems, Minneapolis, MN), mouse anti-Gβ1 (sc-136307; Santa Cruz Biotechnology), anti-goat phosphoinositide 3-kinase (PI3K) p101 (sc-168955; Santa Cruz Biotechnology), and rabbit anti-Piezo1 (15939-1-AP; Proteintech). Specificity of each primary antibody was confirmed by Western blot analyses (data not shown). The proximity ligation assay (PLA) probes used were Duolink In Situ PLA Probe Anti-Rabbit PLUS (DUO92002), Duolink In Situ PLA Probe Anti-Mouse MINUS (DUO92004), and Duolink In Situ PLA Probe Anti-Goat MINUS (DUO92006), which were purchased from Sigma-Aldrich (Carlsbad, CA). Each probe consists of a secondary antibody that is preconjugated with oligonucleotides that will hybridize to form a closed circle when they are in close proximity (within 40 nm). Polymerase-driven rolling circle amplification (RCA) generates a product to which fluorescence-labeled oligonucleotides hybridize, thereby forming a visible PLA signal. All primary antibody pairs and PLA probes were tested at different concentrations to ensure that the density of PLA signals were in the linear range for detection of effects and did not reach a saturation point (data not shown). Single-sliced images were acquired on a LSM5 PASCAL confocal fluorescence microscope (Carl Zeiss, Germany) equipped with a Plan Apochromatic 63/1.4 numerical aperture oil immersion objective and both the PLA signal (i.e., single dots or pixels), and cell nuclei were quantified using a custom ImageJ image analysis macro. A minimum of ten fields of acquisition were acquired for each of at least three individual experiments.
Western blot analysis.
Proteins were separated on NuPAGE 4–12% Bis-Tris gels (Thermo Fisher Scientific) in MOPS SDS running buffer (Thermo Fisher Scientific) and transferred to PVDF membranes (Immobilon-P; Millipore, Temecula, CA). Membranes were blocked for 1 h with 5% BSA in Tris-buffered saline with 0.1% Tween 20 (TBST) and then incubated with a primary antibody for 2 h or overnight in 5% BSA-TBST at 4°C. After being washed and incubated with HRP-conjugated secondary antibodies for 1 h, the membranes were incubated with chemiluminescence substrate (SuperSignal West Pico or West Femto; Thermo Fisher Scientific). Images were acquired using a C-DiGit Blot Scanner (LI-COR Biosciences, Lincoln, NE), and densitometric analyses performed using ImageJ.
Immunofluorescence staining.
HCAEC monolayers on glass slides were fixed in ice-cold methanol for 5 min followed by ice-cold acetone for 2 min. Cells were then rehydrated in PBS and blocked for 1 h at room temperature with blocking buffer (PBS containing normal goat serum, gelatin, BSA, and Tween 20). Following overnight incubation at 4°C with rabbit anti-Piezo1 antibody (NBP1-78446; Novus Biologicals, Littleton, CO) diluted 1:120 in blocking buffer, cells were incubated for 1 h at room temperature with Alexa Fluor 568-conjugated anti-rabbit secondary antibody (A11011; Thermo Fisher Scientific) and fluorescein-conjugated anti-PECAM-1 antibody (FAB3567F; R&D Systems), both diluted 1:250 in blocking buffer. For Piezo1/Gβ1 colocalization studies, cells were incubated with the same rabbit anti-Piezo1 antibody and mouse anti-Gβ1 antibody (sc-136307; Santa Cruz Biotechnology) diluted 1:20, followed by incubation with Alexa Fluor 488-conjugated anti-rabbit (A21206; Thermo Scientific) and Alexa Fluor 546-conjugated anti-mouse (A10036; Thermo Scientific) secondary antibodies. Cells were mounted with coverslips after adding Vectashield mounting medium with DAPI (H-1200; Vector Laboratories, Burlingame, CA) and imaged by confocal microscopy. Z-stack images were acquired using the Z-sectioning method with the number of slices set to 15 and the interval set to 0.75 μm. XZ-cross-sectional images were generated using Zeiss LSM Image Browser.
Statistical analyses.
All experimental data are expressed as means ± SE from at least three independent experiments. Single comparisons between groups were performed using Student’s t-test, while multiple group comparisons were analyzed using one-way ANOVA with Bonferroni post hoc tests. P values <0.05 were considered statistically significant.
RESULTS
Gαq/11 is activated by shear stress.
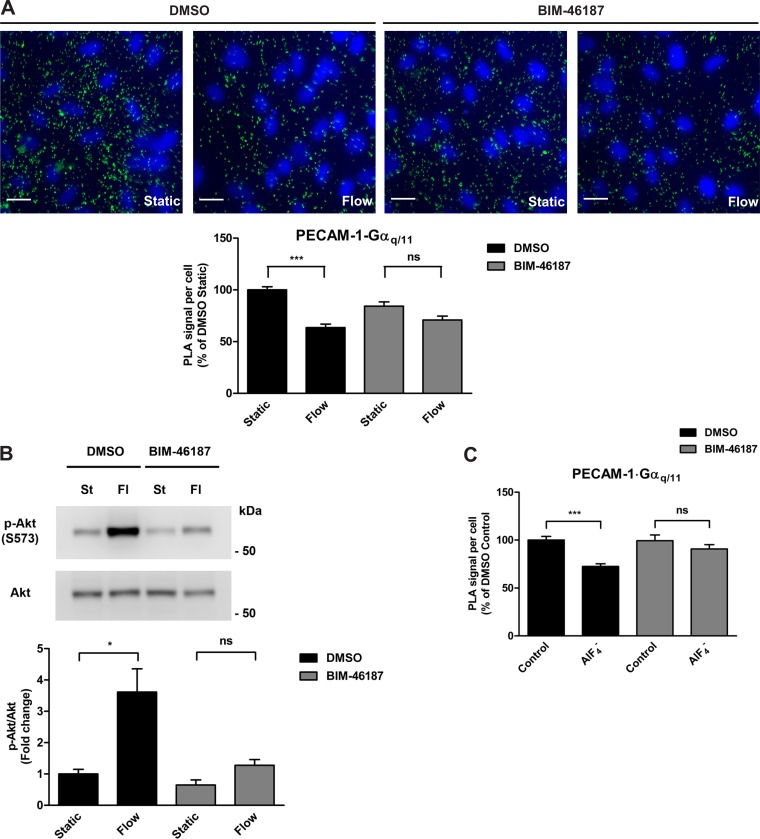
We have previously shown, by coimmunoprecipitation and immunofluorescence colocalization studies in vitro and in vivo, that PECAM-1 and Gαq/11 are two components of a junctional complex in endothelial cells and that their associations are significantly decreased in response to early flow onset (13, 32). Since Gαq/11 is also known to dissociate from ligand-bound GPCRs upon its activation, we hypothesized that the dissociation of Gαq/11 from PECAM-1, which is observed upon shear stress stimulation, occurs when it is activated. To determine whether flow-induced dissociation of Gαq/11 from PECAM-1 is indeed indicative of Gαq/11 activation, we examined their associations in cells that were preincubated with BIM-46187, a Gαq-selective inhibitor (36), before shear stress exposure using in situ proximity ligation assay (PLA). In ECs pretreated with vehicle (DMSO), flow caused a dissociation of Gαq/11 from PECAM-1 at 15 s, as reflected by the 37% decrease in PLA signal compared with static control cells (Fig. 1A). In ECs pretreated with BIM-46187 at 20 μM for 1 h, the level of dissociation compared with its respective static control was only 16%, which represents a decrease in flow-induced dissociation of more than one-half (57%). These findings suggest that G protein activation is necessary for its dissociation from PECAM-1 in acute shear stress conditions. In control experiments, we examined phosphorylation of Akt, a well-known downstream signaling event that is induced by shear stress and mediated by Gαq/11 activation (29, 41). As expected, shear stress caused a robust Akt activation (3.6-fold increase) compared with static control HCAECs (Fig. 1B). Pretreatment of cells with BIM-46187 at 20 μM for 1 h before flow resulted in an attenuated activation of Akt (2-fold increase) when compared specifically to pretreated cells in the absence of flow. In other words, there was a marked decrease in shear stress-induced Akt phosphorylation when Gαq/11 was inhibited. Aluminum fluoride () has been utilized as a stimulatory agent to activate heterotrimeric G proteins. It functions by binding to the inactive GDP-bound Gα subunit and thereby mimicking the activated GTP-bound state. To this end, we next tested the effects of treatment on PECAM-1⋅Gαq/11 associations in cells pretreated with either vehicle or BIM-46187 (Fig. 1C). Treatment of HCAECs with (30 μM AlCl3/10 mM NaF) for 1 min in the presence of vehicle resulted in a 28% decrease in PECAM-1⋅Gαq/11 associations. In the presence of BIM-46187, there was only a 9% decrease in response to stimulation when compared with either DMSO-treated or BIM-46187-treated control cells.
Fig. 1.
Effects of Gαq inhibition on its associations with PECAM-1. A: in situ proximity ligation assay (PLA) was performed on human coronary artery endothelial cells (HCAECs) that were preincubated with vehicle control (DMSO) or BIM-46187 (20 μM, 1 h), a selective inhibitor of Gαq, before stimulation by flow (14 dyn/cm2, 15 s). Specific antibodies against PECAM-1 and Gαq/11 were used to detect associations. Representative images are depicted with the bar graph showing quantification of 4 independent experiments as PLA signal (green dots) per cell (blue nuclei) relative to the DMSO static condition. Error bars indicate SE; ns, not significant. ***P < 0.001. Scale bar, 20 μm. B: HCAECs were preincubated with DMSO or BIM-46187 (20 μM, 1 h) and exposed to flow for 5 min. Immunoblotting was then performed on cell lysates using antibodies against phosphorylated Akt (S473) and total Akt to assess the level of Akt phosphorylation. Representative blots from 3 independent experiments are shown. Bar graph represents the quantification as fold change in the ratio of phosphorylated Akt to total Akt compared with the control static condition, which is set to 1. Error bars indicate SE. *P < 0.05. C: in situ PLA was performed on HCAECs that were preincubated with DMSO or BIM-46187 (20 μM, 1 h) before stimulation with (30 μM AlCl3/10 mM NaF) for 1 min. Specific antibodies against PECAM-1 and Gαq/11 were used to detect associations. Bar graph shows quantification of 4 independent experiments as PLA signal per cell relative to the DMSO static condition, with error bars indicating SE. ***P < 0.001.
siPiezo1 knockdown does not affect Gαq/11 activation induced by shear stress.
It has been recently reported that the mechanosensitive ion channel Piezo1 is required for flow-induced endothelial release of ATP and subsequent activation of Gαq/Gα11-coupled purinergic P2Y2 receptors (40). These findings implicate Piezo1 as a critical participant upstream of Gαq/Gα11-mediated signaling, which directly contradicts the notion that Gαq/11 is the mechanochemical transducer in ECs. To determine whether Piezo1 is required for shear stress-induced rapid activation of Gαq/11, we knocked down the expression of Piezo1 by RNA interference and examined its effect on shear-induced dissociations between PECAM-1 and Gαq/11 by in situ PLA. Our results showed that siRNA targeting of Piezo1 using a previously described siRNA (siPiezo 1 in Table 1) did not inhibit shear-induced PECAM-1⋅Gαq/11 dissociations (Fig. 2A). Specifically, there was a 43% decrease in PLA signal per cell in flow-induced cells in which Piezo1 was knocked down compared with a 42% decrease in flow-induced control cells. Surprisingly, we observed a marked decrease in the detection of PECAM-1⋅Gαq/11 complexes when comparing static conditions (50% decrease). To determine whether this particular siRNA oligonucleotide had nonspecific off target effects, three other siRNA oligonucleotides that specifically target Piezo1 (siPiezo1 2–4 in Table 1) were tested. None of them had a significant inhibitory effect on flow-induced PECAM-1⋅Gαq/11 dissociations (Fig. 2B), and all three siRNA oligos caused a decrease in static associations (Fig. 2C).
Fig. 2.
Effects of siRNA-mediated knockdown of Piezo1 on flow-induced Gαq/11 activation. A: in situ proximity ligation assay (PLA) was performed on human coronary artery endothelial cells (HCAECs) that were exposed to flow 48 h after transfection with either control siRNA (siControl) or siRNA against Piezo1 (Piezo1 1 as described in Table 1). Specific antibodies against PECAM-1 and Gαq/11 were used to detect associations. Representative images are depicted with the bar graph showing quantification of 4 independent experiments as PLA signal (green dots) per cell (blue nuclei) relative to the siControl static condition. Error bars indicate SE. ***P < 0.001. B and C: HCAECs were transfected with either control siRNA or 3 different siRNA oligos against Piezo1: Piezo1 #2, Piezo1 #3, and Piezo1 #4, a predesigned ON-TARGETplus SMARTpool siRNA against siPiezo1. At 48 h after transfection, cells were subjected to flow for 15 s and fixed immediately. In situ PLA was then performed using antibodies against PECAM-1 and Gαq/11. All bar graphs show the quantification of at least 3 independent experiments and are presented as the PLA signal per cell relative to the control condition, which is set arbitrarily to 100%. Error bars indicate SE. B: bar graph represents the relative PLA signal per cell of “Static” versus “Flow”-induced conditions for each siRNA with each “Static” condition set to 100%; n = 12 for siControl; n = 4 for each siPiezo1. *P < 0.05; ***P < 0.001. C: bar graph depicts the relative PLA signal per cell of HCAECs transfected with each Piezo1 siRNA oligo compared with those transfected with siControl under static conditions with siControl set to 100%; n = 4. ***P < 0.001.
Piezo1 is colocalized with PECAM-1 at cell-cell junctions.
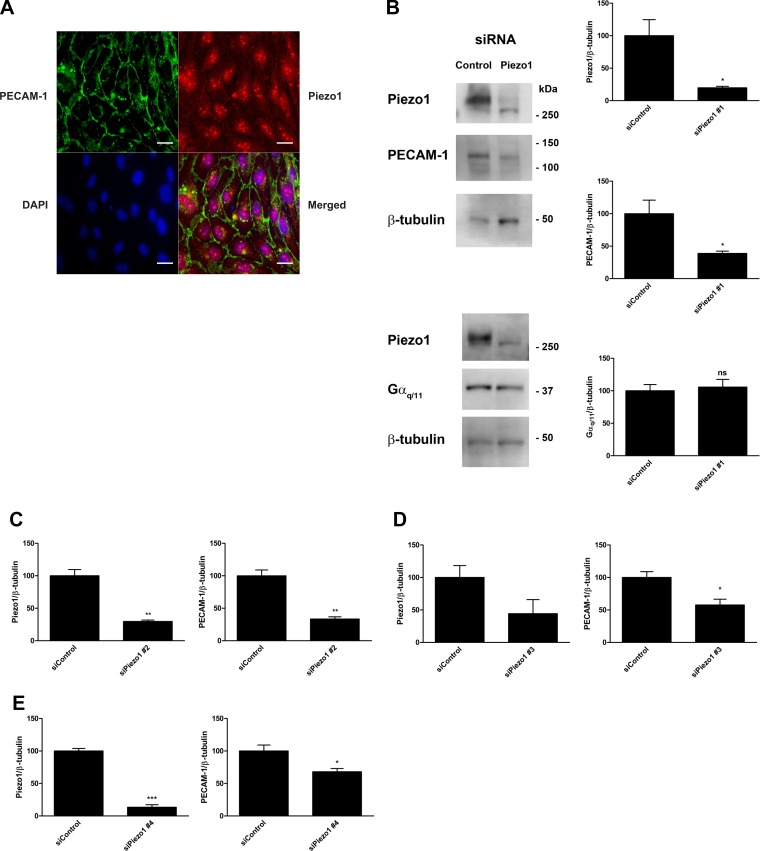
There are a few possible explanations for the decrease in the number of PECAM-1⋅Gαq/11 complexes that we observed under basal conditions. One possibility is that Piezo1 is part of a complex that is required for the association of PECAM-1 and Gαq/11 and its decreased expression due to siRNA knockdown results in the observed decrease in basal complexes. Another possibility is that the protein levels of PECAM-1, Gαq/11, or both are controlled by Piezo1 expression and the decrease in one or both proteins in the pair results in a decrease in their detection as a complex. PECAM-1 and Gαq/11 have previously been shown to be colocalized to the cell-cell junction in endothelial cells by immunofluorescence staining (32). We used this same immunofluorescence staining approach to examine the localization pattern of Piezo1 in HCAECs to determine whether it colocalizes with PECAM-1. Colocalization of Piezo1 with PECAM-1 would indicate that the two proteins are closely associated and therefore could be part of the same complex. Our results showed that Piezo1 (red) has a broad distribution with most of the staining present within the cell (Fig. 3A), which is consistent with data previously reported for Piezo1-GFP transfected into HUVECs as well as for untagged Piezo1 transfected into HEK293T cells and detected using Piezo1 antibody staining (8, 25). Interestingly, we also observed a faint staining at the cell-cell junction that overlaps with PECAM-1 (green), a protein that is known to be abundantly expressed in these areas. The presence of Piezo1 expression between adjacent cells essentially signifies its expression at the plasma membrane, which has been previously reported in non-endothelial cells (8, 38). Based on this finding, it is conceivable that a small subset of Piezo1 is complexed with PECAM-1 at endothelial cell-cell junctions.
Fig. 3.
Piezo1 protein localization and the effects of different Piezo1 siRNA oligonucleotides on Piezo1 and PECAM-1 expression in endothelial cells. A: human coronary artery endothelial cells (HCAECs) were fixed, blocked, and immunostained with an anti-Piezo1 primary antibody followed by an Alexa Fluor 568-conjugated secondary antibody (red). Cells were counterstained with a fluorescein-conjugated anti-PECAM-1 antibody (green) and mounted with medium containing DAPI (blue). A representative image from 3 independent experiments is shown. Scale bar, 20 μm. B: HCAECs were transfected with either control siRNA or siRNA against Piezo1 and lysed after 48 h. Immunoblotting was performed on cell lysates using antibodies against Piezo1, PECAM-1, Gαq/11, and β-tubulin. Representative blots from 3 independent experiments are shown. Bar graphs represent the ratios of Piezo1, PECAM-1, and Gαq/11 expression to β-tubulin expression with control siRNA set to 100%. Error bars indicate SE. *P < 0.05. C–E: HCAECs were transfected with either control siRNA or three different siRNAs against Piezo1: siPiezo1 #2 (C), siPiezo1 #3 (D), and Piezo1 #4 (E). At 48 h after transfection, cells were harvested and lysed. Immunoblotting was performed on cell lysates using antibodies against Piezo1, PECAM-1, and β-tubulin. Bar graphs reflect the quantification of at least 3 independent experiments and are presented as the ratios of Piezo1 and PECAM-1 expression to β-tubulin expression with control siRNA set to 100%. Error bars indicate SE. *P < 0.05; **P < 0.01; ***P < 0.001.
PECAM-1 expression is downregulated in siPiezo1-transfected ECs.
To explore the possibility that siRNA knockdown of Piezo1 may affect the expression levels of either PECAM-1 or Gαq/11 or both, we performed Western blot analyses of lysates from siRNA-transfected HCAECs. Our results showed that transfection with an siRNA against Piezo1 at 20 nM was effective at decreasing Piezo1 protein expression by 80% in HCAECs (Fig. 3B). However, it also unexpectedly caused PECAM-1 protein expression to be markedly decreased by 61%. Gαq/11 protein expression, on the other hand, was essentially unchanged (6% increase). We examined the effects of three other siRNA oligonucleotides against Piezo1 and observed that PECAM-1 protein expression was also decreased, albeit by varying degrees in each case (Fig. 3, C–E).
Gαq/11 activation by flow is not inhibited by gadolinium or ruthenium red.
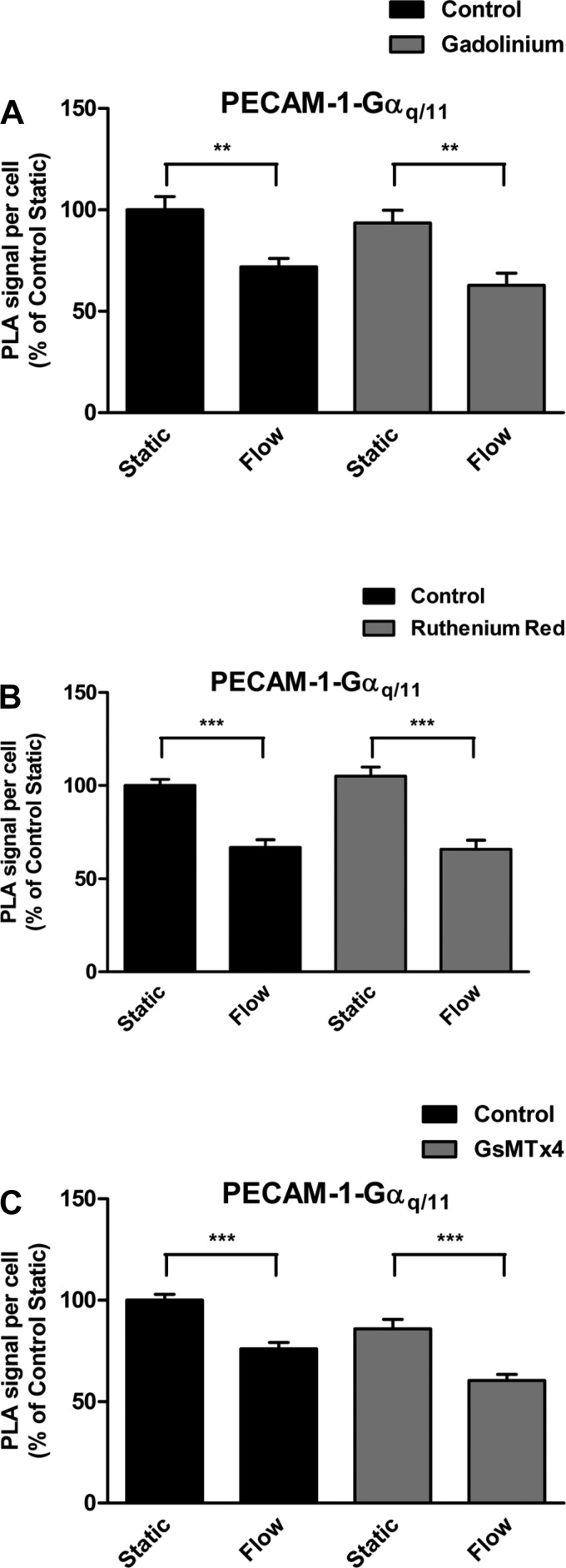
Gadolinium (Gd3+), a potent blocker of stretch-activated calcium channels, has recently been shown to inhibit both Piezo1- and Piezo2-induced mechanically activated (MA) currents (8). To determine whether this particular ion channel blocker has an effect on shear stress-induced Gαq/11 activation, we performed PLA experiments in the presence or absence of Gd3+. In control cells, shear stress caused a decrease (28%) in association of Gαq/11 with PECAM-1 after 15 s (Fig. 4A). In cells that were preincubated with Gd3+ (30 μM) for 30 min before exposure to shear stress, the level of decrease (33%) remained virtually unchanged. Thus there was no apparent effect of Gd3+ on flow-induced activation of Gαq/11 despite an inhibitory effect on Akt phosphorylation induced by the putative selective Piezo1 activator Yoda1 (11).
Fig. 4.
Effects of Piezo1 inhibition on flow-induced Gαq/11 activation. A: human coronary artery endothelial cells (HCAECs) were preincubated with vehicle control (H2O) or the stretch-activated calcium channels inhibitor gadolinium (30 μM, 30 min) before stimulation by flow. In situ proximity ligation assay (PLA) was then performed using antibodies against PECAM-1 and Gαq/11. Bar graph shows quantification of 3 independent experiments as PLA signal per cell relative to the control static condition, with error bars indicating SE. **P < 0.01. B: HCAECs were preincubated with vehicle control (H2O) or the polycationic channel blocker ruthenium red (30 μM, 30 min) before stimulation by flow. Bar graph shows quantification of 4 independent experiments as PLA signal per cell relative to the control static condition, with error bars indicating SE. ***P < 0.001. C: HCAECs were preincubated with vehicle control (H2O) or the Piezo1 channel inhibitor, GsMTx4 (10 μM), for 30 min before exposure to flow. Bar graph shows quantification of 4 independent experiments as PLA signal per cell relative to the control static condition, with error bars indicating SE. ***P < 0.001.
We next tested the effects of another polycationic channel blocker, ruthenium red (RR), which had also been demonstrated to block Piezo-mediated MA currents when applied specifically to the extracellular side of cells (8, 9) and to inhibit Yoda1-induced Akt phosphorylation (11). Our results here showed that Gαq/11 dissociates from PECAM-1 in the absence or presence of RR pretreatment at 30 μM for 30 min before stimulation by shear stress for 15 s (33 vs. 37% decrease in association) (Fig. 4B).
GsMTx4 does not block flow-induced Gαq/11 activation.
Since both gadolinium and ruthenium have low selectivity for Piezo channels, we sought to test the effects of the more specific Piezo1 inhibitor GsMTx4 (1). To determine whether Piezo1 inhibition with GsMTx4 affects shear stress-induced PECAM-1⋅Gαq/11 dissociations, we performed PLA. We examined cells for the presence of complexes with and without shear stress as well as with and without preincubation with GsMTx4 at 10 μM for 30 min. Similar to both Gd3+- and RR-pretreated cells, shear stress-induced dissociation of PECAM-1 and Gαq/11 was essentially unaffected by GsMTx4 (Fig. 4C).
Flow induces association of Gβ1 with Piezo1.
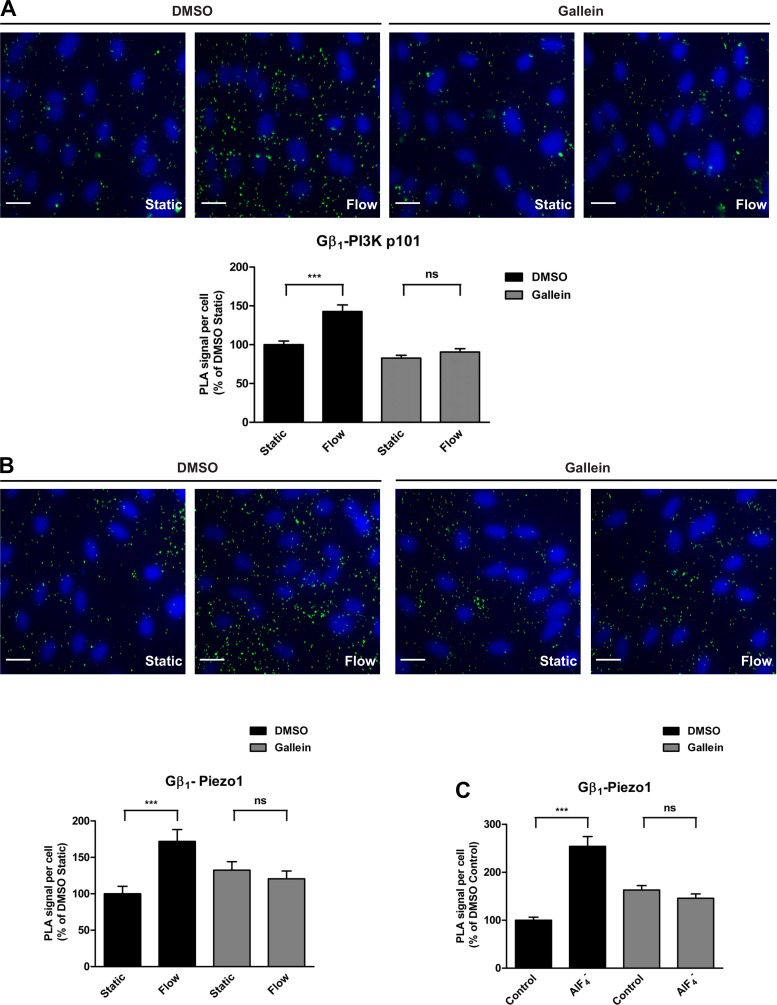
Upon activation, heterotrimeric G proteins dissociate into the GTP-bound α-subunits (Gα) and βγ-dimers (Gβγ). Consequently, Gβγ-subunits are free to interact and activate a host of different effector molecules, including phospholipases and phosphoinositide 3-kinases (PI3Ks). We therefore tested whether activation of G proteins by shear stress causes Gβγ to interact with PI3K (Fig. 5A). In endothelial cells exposed to shear stress for 15 s, we detected an increase in association of the Gβ1 subunit with the p101 subunit of class IB PI3K (43% increase) relative to the static condition. To determine whether increased association of Gβ1 with PI3K p101 in response to flow is due specifically to Gβγ activation, we pretreated ECs with the Gβγ inhibitor gallein at 50 μM for 30 min before shear stress exposure. Although gallein pretreatment alone resulted in a 17% decrease in basal PLA signal, flow-induced association of Gβ1 with p101 was dramatically decreased, with only a minimal increase (9%) in PLA signal compared with its respective static control.
Fig. 5.
Effects of Gβγ inhibition on its associations with downstream effectors. A: human coronary artery endothelial cells (HCAECs) were pretreated with DMSO or the Gβγ-subunit inhibitor gallein (30 μM) for 30 min before being subjected to flow for 15 s. In situ proximity ligation assay (PLA) was performed using antibodies against Gβ1 and the p101 subunit of phosphoinositide 3-kinase (PI3K). Representative images are depicted with the bar graph showing quantification of 3 independent experiments as PLA signal (green dots) per cell (blue nuclei) relative to the DMSO static condition. Error bars indicate SE. ***P < 0.001. B: HCAECs were pretreated with DMSO or gallein (30 μM) for 30 min before 15-s flow. In situ PLA was performed using antibodies against Gβ1 and Piezo1. Representative images are depicted with the bar graph showing quantification of 4 independent experiments as PLA signal (green dots) per cell (blue nuclei) relative to the DMSO static condition. Error bars indicate SE. ***P < 0.001. C: HCAECs were pretreated with DMSO or gallein (30 μM) for 30 min before stimulation with (30 μM AlCl3/10 mM NaF) for 1 min. In situ PLA was performed using antibodies against Gβ1 and Piezo1. Bar graph shows quantification of 3 independent experiments as PLA signal per cell relative to the DMSO static condition, with error bars indicating SE. ***P < 0.001.
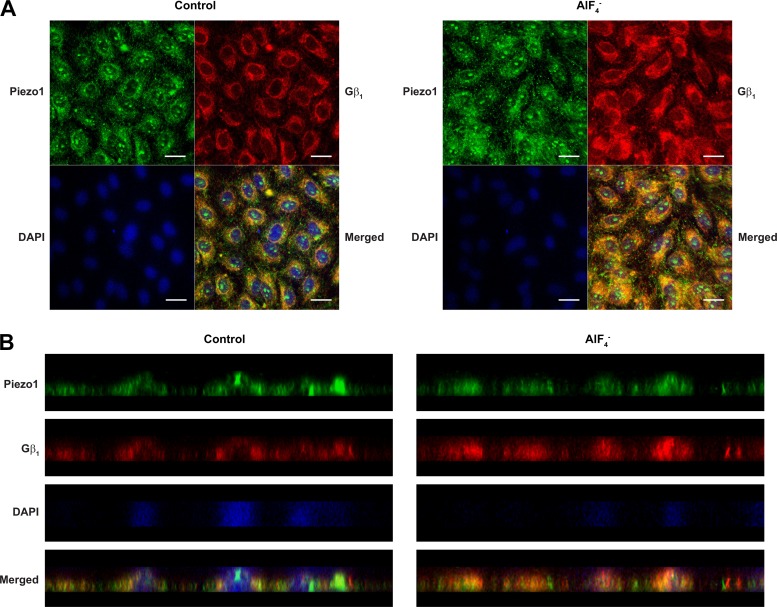
It is also known that Gβγ can directly interact with ion channels and regulate their activities (22, 26). To test whether Gβγ associates with Piezo1 in response to shear stress, we performed PLA using antibodies directed against Piezo1 and Gβ1 (Fig. 5B). Interestingly, in control cells pretreated for 30 min with DMSO before shear stress exposure, we observed a rapid and marked increase (72%) in association between Piezo1 and Gβ1. However, when cells were pretreated with gallein (50 μM, 30 min) to inhibit Gβγ, flow did not cause an increase in Piezo1-Gβ1 association but instead showed a slight decrease (9%). Similarly, treatment of HCAECs with for 1 min to activate G proteins in the absence of flow caused a drastic increase (2.5-fold) in association between Piezo1 and Gβ1 (Fig. 5C). When cells were pretreated with gallein before stimulation, there was a decrease (11%) in association. As an independent approach to verify that Piezo1 and Gβ1 are associated upon activation of G proteins, we performed traditional immunofluorescence staining experiments. Our results showed that treatment of ECs with for 1 min caused an increased colocalization of Gβ1 with Piezo1 when compared with untreated control cells (Fig. 6). The increase was observed at both perinuclear, perhaps in the endoplasmic reticulum, and nuclear areas of the cells.
Fig. 6.
Piezo1 and Gβ1 colocalization and the effects of G-protein activation. Human coronary artery endothelial cells (HCAECs) were left either unstimulated or stimulated with (30 μM AlCl3/10 mM NaF) for 1 min. Cells were then fixed, blocked, and immunostained with rabbit anti-Piezo1 and mouse anti-Gβ1 primary antibodies followed by Alexa Fluor 488-conjugated anti-rabbit (green) and Alexa-Fluor 546-conjugated anti-mouse (red) secondary antibodies. Cells were mounted with medium containing DAPI (blue). A and B: representative confocal Z-section images from 3 independent experiments are shown in A, and the XZ cross-sectional images rendered from the centerline of the same images are depicted in B. Scale bar, 20 μm.
DISCUSSION
The mechanism of heterotrimeric G protein activation upon agonist stimulation of GPCRs is well described and involves the exchange of GDP for GTP on the Gα-subunit. As a result of this exchange, the active GTP-bound Gα-subunit and Gβγ-dimer are released and free to interact with and activate downstream effector proteins and signaling pathways. The mechanism(s) by which shear stress activates G proteins and downstream signaling pathways is still unclear, although it has been recently suggested that the mechanically activated cation channel Piezo1 acts upstream of Gαq/11 activation (40). We have previously described a complex that contains PECAM-1 and Gαq/11, which resides at the endothelial cell junction, as shown by both in vitro and in vivo colocalization, and is responsive to shear stress (13, 32). Specifically, using coimmunoprecipitation, we observed rapid dissociation of Gαq/11 from PECAM-1 in response to flow that is abrogated in the presence of the broad G protein inhibitor GDPβS (13, 32). In this study, we hypothesized that this rapid dissociation is a result of flow-induced activation of Gαq/11. To test our hypothesis, we utilized the small molecule compound, BIM-46187, which has been reported to be a selective inhibitor of Gαq/11 by trapping it in an empty pocket conformation and blocking receptor-catalyzed activation of the G protein (36).
Our results showed that Gαq/11 that is trapped in this inactive conformation in the presence of BIM-46187 is not significantly dissociated from PECAM-1 by flow, suggesting that flow-induced dissociation is G protein activation-dependent. These results are therefore consistent with that of the earlier study, and as such, we identified this dissociation event as a surrogate marker for flow-induced Gαq/11 activation, providing us with a novel and invaluable tool for further examining the mechanism of flow-induced G protein activation and signaling.
The well-established techniques of fluorescence resonance energy transfer (FRET) and bioluminescence resonance energy transfer (BRET) have been previously utilized to examine agonist-induced GPCR/G protein activation by measuring similar (<50%) and often times smaller (<20%) changes in protein dissociations than we reported here using in situ PLA (23, 27). The clear advantage of the PLA approach over both FRET and BRET lies in the ability to measure these changes in protein-protein interactions in the context of their native environment, without the need for overexpression.
The suggestion that Piezo1 mediates ATP release through a Ca2+-mediated mechanism while simultaneously acting upstream of P2Y2/Gαq/11-mediated signaling (40) is somewhat contradictory in light of an earlier finding that siRNA-mediated knockdown of either Gαq/11 or P2Y2 strongly reduced shear stress-induced increases in intracellular Ca2+ concentration ([Ca2+]i) (41). This can only be reconciled if shear stress is able to induce Ca2+ influx in two distinct waves, one mediated through Piezo1 and the other mediated by Gαq/11. In support of this, Scheitlin et al. (35) demonstrated that the initial increase in [Ca2+]i by shear stress in ECs is from intracellular stores, i.e., endoplasmic reticulum and mitochondria, via the G protein/PLC/IP3/IP3R pathway, and thus is unlikely due to Piezo1 activation.
We recently showed that GsMTx4 does not significantly inhibit Yoda1-induced phosphorylation of Akt (11). Although this could suggest that the compound we utilized in our experiments was inactive, it seems highly unlikely considering the fact that we acquired and tested two separate batches from two different sources and used it at a concentration (10 μM) and duration (30 min), which was in line with previously published studies. In light of this, we acknowledge that our results with GsMTx4 cannot stand alone. However, this result is completely consistent with the inability of either Gd3+ or RR, both of which effectively blocked Yoda1-induced phosphorylation of Akt (11), to also block the flow-induced dissociation of Gαq/11 from PECAM-1. Together, our data, which shows that flow-induced dissociation of Gαq/11 from PECAM-1 is essentially unaffected by pharmacological inhibition using three different widely used antagonists as well as by knockdown of Piezo1 using four different siRNA oligos, directly opposes the assertion that Piezo1 is required for or acts upstream of flow-induced activation of Gαq/11.
That siRNA knockdown of Piezo1 results in decreased in vitro expression of PECAM-1, a component of a mechanochemical transducing complex, is another significant finding that should not be glossed over. It is possible that past conclusions made regarding the knockdown of Piezo1 by siRNA may be inaccurate due to the fact that expression of another seemingly unrelated protein may also have been decreased. In support of our finding, it has been previously reported that Piezo1 knockdown also results in decreased endothelial nitric oxide synthase (eNOS) expression (25). Nitric oxide, including that induced by shear, mediates several signaling and gene expression pathways (2, 3, 16, 39). There are also suggestions from the published literature that in vivo PECAM-1 expression may be linked to Piezo1 (21, 25, 34). Specifically, in the vascular plexus of yolk sacs from Piezo1-deficient embryos, PECAM-1 immunostaining appears qualitatively reduced. Obviously, additional quantitative investigations would be necessary to determine whether this is the case.
The fact that four siRNA oligos, each with different targeting sequences against Piezo1, all affected the expression levels of PECAM-1 to some degree suggests that the siRNA oligos are not merely acting nonspecifically in knocking down their intended target protein. It is possible that the expression of the two seemingly unrelated proteins is linked through an unidentified mechanism, perhaps through transcriptional regulation. Interestingly, it has been recently shown that the nuclear localization of the transcriptional coactivator Yes-associated protein (YAP) is reduced in cells in which Piezo1 was knocked down by siRNA (33). Decreased nuclear localization of YAP could conceivably prevent its interaction with transcription factors that are necessary for the expression of certain genes. Although it has not been reported that PECAM-1, or even eNOS, is a specific target of YAP or that its expression is somehow regulated by Piezo1 expression, our findings raise the intriguing possibility that such a mechanism may exist.
Increased association of Gβ1 with both the p101 subunit of PI3K and Piezo1 in response to flow suggests that upon G protein activation (Fig. 7), Gβγ may be targeted to these two downstream effector proteins and may function to activate them. This is further supported by the finding that the Gβγ inhibitor gallein, which has previously been shown to specifically bind with high affinity to Gβγ effector binding sites (4) and to inhibit Gβγ-dependent signaling (24), essentially prevented flow-induced increases in associations between Gβ1 and p101 and between Gβ1 and Piezo1. The finding that association between Gβ1 and Piezo1 is also increased with treatment is consistent with the hypothesis that the flow effects are due to G protein activation. Interestingly, the activity of another Piezo channel, Piezo2, has been previously shown to be modulated by the activation of G proteins and the GPCR bradykinin receptor-β2 in sensory neurons (5, 14).
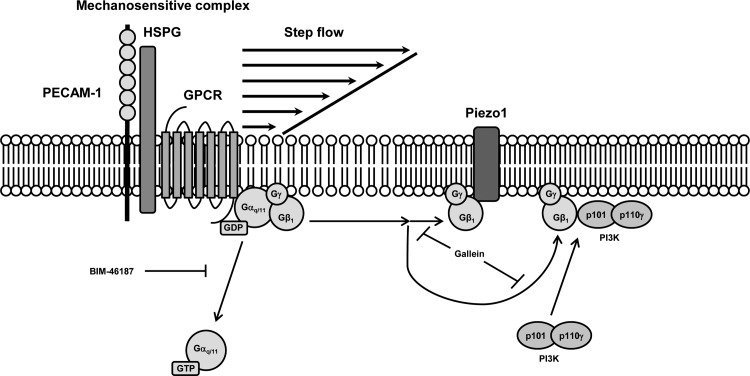
Fig. 7.
Schematic representation of Gαq/11 activation in endothelial cells by step flow. It was previously shown that there is an increase in the fluidity of the lipid bilayer in response to a sudden temporal onset of flow or step change in shear stress (42). Consequently, membrane-associated heterotrimeric Gαq/11 is rapidly dissociated from a mechanosensitive macromolecular complex localized at the endothelial cell-cell junction (12, 13). Pretreatment of ECs with BIM-46187, a selective inhibitor of Gαq activation, blocks this flow-induced dissociation from the complex. Step flow also causes an increased association of βγ-dimer (Gβγ), which is liberated during G protein activation, with its downstream effector, phosphoinositide 3-kinase (PI3K), as well as the mechanosensitive ion channel, Piezo1, which is a membrane-bound protein expressed at both the plasma membrane (8) and endoplasmic reticulum (28). Inhibition of Gβγ signaling with gallein blocks both of these flow-induced associations. GPCR, G protein-coupled receptor; HSPG, heparan sulfate proteoglycan.
Cinar et al. (7) elegantly demonstrated that shear-induced [Ca2+]i influx and ATP release are regulated by Piezo1 in human red blood cells (RBCs). Interestingly, it has also been reported that Gi is critical for mechanically induced ATP release from RBCs (31) and that the activity of G protein β-subunits may mediate this release (37). Our data that shear stress causes an increased association of Gβ1 with Piezo1 in ECs are consistent with both of these observations. However, our data are only suggestive that Gβγ directly interacts with Piezo1 upon flow stimulation of endothelial cells. Further biochemical studies and perhaps electrophysiological studies would be necessary to definitively prove that this is indeed the case and that Gβγ is required for activation of Piezo1 by shear stress.
In summary, we have shown that shear stress induces a G protein activation-dependent dissociation of Gαq/11 from PECAM-1 in endothelial cells that does not require the upstream activation of Piezo1. We have also determined that Piezo1 may possibly be a component of the mechanochemical transduction complex that contains both PECAM-1 and Gαq/11 at the EC junction. Furthermore, knockdown of Piezo1 reduces expression of the mechanochemical transduction complex component PECAM-1. Finally, we present evidence that upon shear-induced activation the β-subunit of G protein may be recruited to Piezo1 and play a role in its activation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute MERIT Award R37 HL040696 (to J. A. Frangos).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.G.d.P. and J.A.F. conceived and designed research; N.G.d.P. performed experiments; N.G.d.P. analyzed data; N.G.d.P. and J.A.F. interpreted results of experiments; N.G.d.P. prepared figures; N.G.d.P. drafted manuscript; N.G.d.P. and J.A.F. edited and revised manuscript; N.G.d.P. and J.A.F. approved final version of manuscript.
REFERENCES
- 1.Bae C, Sachs F, Gottlieb PA. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 50: 6295–6300, 2011. doi: 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao X, Lu C, Frangos JA. Mechanism of temporal gradients in shear-induced ERK1/2 activation and proliferation in endothelial cells. Am J Physiol Heart Circ Physiol 281: H22–H29, 2001. doi: 10.1152/ajpheart.2001.281.1.H22. [DOI] [PubMed] [Google Scholar]
- 3.Bao X, Lu C, Frangos JA. Temporal gradient in shear but not steady shear stress induces PDGF-A and MCP-1 expression in endothelial cells: role of NO, NF kappa B, and egr-1. Arterioscler Thromb Vasc Biol 19: 996–1003, 1999. doi: 10.1161/01.ATV.19.4.996. [DOI] [PubMed] [Google Scholar]
- 4.Bonacci TM, Mathews JL, Yuan C, Lehmann DM, Malik S, Wu D, Font JL, Bidlack JM, Smrcka AV. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science 312: 443–446, 2006. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 5.Borbiro I, Rohacs T. Regulation of piezo channels by cellular signaling pathways. Curr Top Membr 79: 245–261, 2017. doi: 10.1016/bs.ctm.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler PJ, Norwich G, Weinbaum S, Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol 280: C962–C969, 2001. doi: 10.1152/ajpcell.2001.280.4.C962. [DOI] [PubMed] [Google Scholar]
- 7.Cinar E, Zhou S, DeCourcey J, Wang Y, Waugh RE, Wan J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc Natl Acad Sci USA 112: 11783–11788, 2015. doi: 10.1073/pnas.1507309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483: 176–181, 2012. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Koning W, van Dam K. A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Anal Biochem 204: 118–123, 1992. doi: 10.1016/0003-2697(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 11.dela Paz NG, Frangos JA. Yoda1-induced phosphorylation of Akt and ERK1/2 does not require Piezo1 activation. Biochem Biophys Res Commun 497: 220–225, 2018. doi: 10.1016/j.bbrc.2018.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.dela Paz NG, Melchior B, Frangos JA. Shear stress induces Gαq/11 activation independently of G protein-coupled receptor activation in endothelial cells. Am J Physiol Cell Physiol 312: C428–C437, 2017. doi: 10.1152/ajpcell.00148.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.dela Paz NG, Melchior B, Shayo FY, Frangos JA. Heparan sulfates mediate the interaction between platelet endothelial cell adhesion molecule-1 (PECAM-1) and the Gαq/11 subunits of heterotrimeric G proteins. J Biol Chem 289: 7413–7424, 2014. doi: 10.1074/jbc.M113.542514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, Coste B, Patapoutian A. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep 2: 511–517, 2012. doi: 10.1016/j.celrep.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science 227: 1477–1479, 1985. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 16.Fujita M, Imadome K, Endo S, Shoji Y, Yamada S, Imai T. Nitric oxide increases the invasion of pancreatic cancer cells via activation of the PI3K-AKT and RhoA pathways after carbon ion irradiation. FEBS Lett 588: 3240–3250, 2014. doi: 10.1016/j.febslet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb PA, Suchyna TM, Sachs F. Properties and mechanism of the mechanosensitive ion channel inhibitor GsMTx4, a therapeutic peptide derived from tarantula venom. Curr Top Membr 59: 81–109, 2007. doi: 10.1016/S1063-5823(06)59004-0. [DOI] [PubMed] [Google Scholar]
- 18.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA 95: 2515–2519, 1998. doi: 10.1073/pnas.95.5.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gudi SR, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ Res 79: 834–839, 1996. doi: 10.1161/01.RES.79.4.834. [DOI] [PubMed] [Google Scholar]
- 20.Haidekker MA, L’Heureux N, Frangos JA. Fluid shear stress increases membrane fluidity in endothelial cells: a study with DCVJ fluorescence. Am J Physiol Heart Circ Physiol 278: H1401–H1406, 2000. doi: 10.1152/ajpheart.2000.278.4.H1401. [DOI] [PubMed] [Google Scholar]
- 21.Hyman AJ, Tumova S, Beech DJ. Piezo1 channels in vascular development and the sensing of shear stress. Curr Top Membr 79: 37–57, 2017. doi: 10.1016/bs.ctm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380: 255–258, 1996. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 23.Leduc M, Breton B, Galés C, Le Gouill C, Bouvier M, Chemtob S, Heveker N. Functional selectivity of natural and synthetic prostaglandin EP4 receptor ligands. J Pharmacol Exp Ther 331: 297–307, 2009. doi: 10.1124/jpet.109.156398. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol 73: 410–418, 2008. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad RK, Evans PC, Ainscough JF, Beech DJ. Piezo1 integration of vascular architecture with physiological force. Nature 515: 279–282, 2014. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325: 321–326, 1987. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 27.Mastop M, Reinhard NR, Zuconelli CR, Terwey F, Gadella TWJ Jr, van Unen J, Adjobo-Hermans MJW, Goedhart J. A FRET-based biosensor for measuring Gα13 activation in single cells. PLoS One 13: e0193705, 2018. doi: 10.1371/journal.pone.0193705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHugh BJ, Buttery R, Lad Y, Banks S, Haslett C, Sethi T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J Cell Sci 123: 51–61, 2010. doi: 10.1242/jcs.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melchior B, Frangos JA. Distinctive subcellular Akt-1 responses to shear stress in endothelial cells. J Cell Biochem 115: 121–129, 2014. doi: 10.1002/jcb.24639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melchior B, Frangos JA. Shear-induced endothelial cell-cell junction inclination. Am J Physiol Cell Physiol 299: C621–C629, 2010. doi: 10.1152/ajpcell.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol 286: H940–H945, 2004. doi: 10.1152/ajpheart.00677.2003. [DOI] [PubMed] [Google Scholar]
- 32.Otte LA, Bell KS, Loufrani L, Yeh JC, Melchior B, Dao DN, Stevens HY, White CR, Frangos JA. Rapid changes in shear stress induce dissociation of a G alpha(q/11)-platelet endothelial cell adhesion molecule-1 complex. J Physiol 587: 2365–2373, 2009. doi: 10.1113/jphysiol.2009.172643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, Bernardis E, Flanagan LA, Tombola F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA 111: 16148–16153, 2014. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YS, Chien S, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA 111: 10347–10352, 2014. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheitlin CG, Julian JA, Shanmughapriya S, Madesh M, Tsoukias NM, Alevriadou BR. Endothelial mitochondria regulate the intracellular Ca2+ response to fluid shear stress. Am J Physiol Cell Physiol 310: C479–C490, 2016. doi: 10.1152/ajpcell.00171.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz AL, Schrage R, Gaffal E, Charpentier TH, Wiest J, Hiltensperger G, Morschel J, Hennen S, Häußler D, Horn V, Wenzel D, Grundmann M, Büllesbach KM, Schröder R, Brewitz HH, Schmidt J, Gomeza J, Galés C, Fleischmann BK, Tüting T, Imhof D, Tietze D, Gütschow M, Holzgrabe U, Sondek J, Harden TK, Mohr K, Kostenis E. A cell-permeable inhibitor to trap Gαq proteins in the empty pocket conformation. Chem Biol 21: 890–902, 2014. doi: 10.1016/j.chembiol.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprague RS, Bowles EA, Olearczyk JJ, Stephenson AH, Lonigro AJ. The role of G protein beta subunits in the release of ATP from human erythrocytes. J Physiol Pharmacol 53: 667–674, 2002. [PubMed] [Google Scholar]
- 38.Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T, Ueda-Yamaguchi K, Kitamura T, Yoshizaki K, Fukumoto S, Iwamoto T. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep 7: 17696, 2017. doi: 10.1038/s41598-017-18089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tejedo JR, Cahuana GM, Ramírez R, Esbert M, Jiménez J, Sobrino F, Bedoya FJ. nitric oxide triggers the phosphatidylinositol 3-kinase/Akt survival pathway in insulin-producing RINm5F cells by arousing Src to activate insulin receptor substrate-1. Endocrinology 145: 2319–2327, 2004. doi: 10.1210/en.2003-1489. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest 126: 4527–4536, 2016. doi: 10.1172/JCI87343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Iring A, Strilic B, Albarrán Juárez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Müller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S. P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J Clin Invest 125: 3077–3086, 2015. doi: 10.1172/JCI81067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci 362: 1459–1467, 2007. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang T, Chi S, Jiang F, Zhao Q, Xiao B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat Commun 8: 1797, 2017. doi: 10.1038/s41467-017-01712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]