Abstract
Previous studies established that leucine stimulates protein synthesis in skeletal muscle to the same extent as a complete mixture of amino acids, and the effect occurs through activation of the mechanistic target of rapamycin in complex 1 (mTORC1). Recent studies using cells in culture showed that the Sestrins bind leucine and are required for leucine-dependent activation of mTORC1. However, the role they play in mediating leucine-dependent activation of the kinase in vivo has been questioned because the dissociation constant of Sestrin2 for leucine is well below circulating and intramuscular levels of the amino acid. The goal of the present study was to compare expression of the Sestrins in skeletal muscle to other tissues and to assess their relative role in mediating activation of mTORC1 by leucine. The results show that the relative expression of the Sestrin proteins varies widely among tissues and that in skeletal muscle Sestrin1 expression is higher than Sestrin3, whereas Sestrin2 expression is markedly lower. Analysis of the dissociation constants of the Sestrins for leucine as assessed by leucine-induced dissociation of the Sestrin·GAP activity toward Rags 2 (GATOR2) complex revealed that Sestrin1 has the highest affinity for leucine and that Sestrin3 has the lowest affinity. In agreement with the dissociation constants calculated using cells in culture, oral leucine administration promotes disassembly of the Sestrin1·GATOR2 complex but not the Sestrin2 or Sestrin3·GATOR2 complex. Overall, the results presented herein are consistent with a model in which leucine-induced activation of mTORC1 in skeletal muscle in vivo occurs primarily through release of Sestrin1 from GATOR2.
Keywords: leucine, mTOR, Sestrin, skeletal muscle
INTRODUCTION
Skeletal muscle comprises ~40% of lean body mass. Consequently, maintaining protein homeostasis in the tissue is critical in health and disease as exemplified by muscle mass being inversely proportional to survival in a number of pathological conditions and critical illnesses, including sepsis, acquired immunodeficiency syndrome, sarcopenia, and cachexia (20). Typically, these conditions are associated with reduced activity that, in combination with decreased appetite and nutrient consumption, results in a repression of protein synthesis and loss of muscle mass. A variety of studies suggest that maintaining protein intake attenuates the loss of muscle mass associated with disuse (reviewed in 19). Moreover, dietary supplementation with essential amino acids further aids in maintaining mass by stimulating muscle protein synthesis (19). Of the essential amino acids, leucine is particularly important because by itself it stimulates protein synthesis in skeletal muscle to the same extent as a complete mixture of amino acids (11). A number of years ago, we showed that leucine stimulates protein synthesis in skeletal muscle by activating a protein kinase referred to as the mechanistic target of rapamycin (mTOR) in complex 1 (mTORC1) (2). However, the mechanism through which leucine mediates activation of mTORC1 remains to be fully elucidated.
Presently, most of the available information regarding activation of mTORC1 by leucine comes from studies using cultures of transformed cells, e.g., HEK293T cells (6, 14, 21). Based on these studies, leucine is proposed to act by binding to a “sensor,” i.e., Sestrin2, that in turn acts to relieve repression of a complex referred to as GAP activity toward Rags 2 (GATOR2), which consists of the proteins meiosis regulator for oocyte development (Mios), WD repeat-domain-containing proteins 24 and 59 (WDR24 and WDR59, respectively), Sec13, and Sec13-like protein (Seh1l) (4). Whether this mechanism explains how leucine mediates activation of mTORC1 in skeletal muscle is unknown. Indeed, whether or not the Sestrins act as leucine “sensors” in vivo has recently been questioned (10). Notably, the dissociation constant (Kd) of Sestrin2 for leucine is reported to be ~20 µM (21), well below the concentration found in fasted rat or human plasma, 129 and 157 µM (2, 5), or muscle, 120 and 167 µM (5, 8), respectively. Thus, if the intracellular concentration of leucine in muscle cells is similar to that found in tissue extracts, Sestrin2 would be fully saturated with leucine under physiological conditions. However, three Sestrin isoforms are expressed in mammalian cells, and, to date, our knowledge of Sestrin function is based primarily on data obtained only for Sestrin2. Moreover, the tissue distribution of the Sestrin isoforms appears to vary widely based on mRNA expression (18), and, thus, the sensitivity of different tissues to leucine-induced mTORC1 activation may vary depending on Sestrin isoform expression.
The purpose of the studies presented herein was to examine the tissue distribution of Sestrin protein expression and to determine which Sestrin(s) might play a role in mediating the activation of mTORC1 by leucine in skeletal muscle. Sestrin isoform expression varied among tissues, with Sestrin1 being more abundant in skeletal muscle than Sestin3, and Sestrin2 expression being markedly lower relative to either Sestrin1 or 3. Thus, based on its low abundance relative to the other two proteins, it seems unlikely that Sestrin2 contributes significantly to leucine “sensing” in skeletal muscle. Based on its ability to promote Sestrin·GATOR2 dissociation, the affinity of the three Sestrins for leucine differed dramatically, with Sestrin1 and Sestrin3 having the highest and lowest affinities, respectively. Importantly, oral administration of leucine to fasted rats promoted dissociation of Sestrin1, but not Sestrin2 or 3, from GATOR2, suggesting that Sestrin1 mediates leucine-induced activation of mTORC1 in skeletal muscle.
MATERIALS AND METHODS
Animal care and experimental design.
The animal facilities and experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine. Adult male Sprague-Dawley rats (∼200–250 g body weight; Charles River Laboratories, Wilmington, MD) were maintained on a 12:12 h light-dark cycle with a standard diet (Rodent Chow 8604, Harlan-Teklad, Indianapolis, IN) and water provided ad libitum for 1 wk. Animal study 1: Rats (n = 6) were food deprived for 18 h and at ~0900 the next day were administered a suspension of l-leucine (54 g/l water; 2.5 ml/100 g body weight; cat. no. L-8000, Sigma Aldrich, St. Louis, MO) by oral gavage. Control rats (n = 6) were administered a similar volume of saline. The amount of leucine given was equivalent to the amount consumed by rats during 24 h of free access to standard laboratory chow (2). Rats were anesthetized 40 min after gavage by isoflurane inhalation (EZ-Anesthesia, Palmer, PA), and 5 min later tissues were rapidly removed in the following order and flash-frozen between aluminum blocks cooled to the temperature of liquid nitrogen: left gastrocnemius, left tibialis anterior, kidney, liver, heart, and brain. Tissues were stored at −80°C until use. Animal study 2: Freely fed rats (n = 6) were deeply anesthetized using isoflurane inhalation. The left gastrocnemius, left tibialis anterior, kidney, liver, heart, and brain were rapidly removed and frozen as described above. Animal study 3: Freely fed rats (n = 14) were deeply anesthetized by isoflurane inhalation. The tibialis anterior muscle in one leg was transfected via in vivo electroporation (100 µl of 2 mg/ml plasmid in 0.9% sterile saline) with a plasmid expressing FLAG-Sestrin1 (pRK5-FLAG-Sestrin1; cat. no. 72594, Addgene, Watertown, MA; a gift from Dr. David Sabatini), and the contralateral leg was transfected with a plasmid expressing FLAG-Sestrin3 (pRK5-FLAG-Sestrin3; cat. no. 72592, Addgene; a gift from Dr. David Sabatini). FLAG-metap2 (pRK5-FLAG-metap2; cat. no. 32004, Addgene; a gift from Dr. David Sabatini) and pmaxGFP (cat. no. VCA-1003, Lonza, Allendale, NJ) served as controls and were transfected into alternate legs in a separate group of animals (n = 6). In a separate experiment, FLAG-Sestrin2 (pRK5-FLAG-Sestrin2; cat. no. RC-201386, Origene, Rockville, MD) was transfected into the left leg of 12 rats. Before use, all plasmids were amplified in XL-1 Blue Supercompetent Cells (cat. no. 200236, Agilent Technologies, Santa Clara, CA) and purified using an EndoFree Plasmid Giga Kit (cat. no. 12391, Qiagen, Germantown, MD). In vivo electroporation was performed as described previously (17). Briefly, the lower hindlimbs of anesthetized rats were shaved with an electric razor to expose the skin, which was then cleaned using 70% ethanol. Plasmid DNA was slowly injected directly into the tibialis anterior through the skin using a 0.3-ml insulin syringe (29-gauge, 1.27-cm needle; Smiths Medical ASD, Dublin, OH). Square-wave electric impulses generated by an electroporator (model 830, BTX, San Diego, CA) were delivered via caliper electrodes (model 384, BTX). The electrodes were applied to the lower hindlimb, and the negative electrode was in contact with the skin directly above the tibialis anterior. Pulse trains were delivered with a 200 V/cm field strength (8 pulses, 20 ms/pulse, 1 Hz, 1-s delay between pulses). On the third day after electroporation, rats were food deprived for 18 h, and the next morning rats were randomly assigned to 2 groups; 1 group received leucine by oral gavage and the other group received saline as described above. The tibialis anterior muscles were rapidly removed 45 min after gavage and immediately homogenized in 7 volumes of ice-cold homogenization buffer containing 40 mM HEPES (pH 7.5), 120 mM NaCl, 1 mM EDTA·2Na, 10 mM NaHP2O7·10H2O, 10 mM β-glycerophosphate, 50 mM NaF, 1 μM microcystin-LE, 1.5 mM sodium vanadate, and 10 µl/ml Protease Inhibitor Cocktail (cat. no. P-8340, Sigma Aldrich). Muscles were homogenized, and 6% CHAPS was added to a final concentration of 0.3%, and samples were kept on ice for 20 min. The samples were then processed as described below under Processing of tissue samples.
Processing of tissue samples.
Tissues from rats in studies 1 and 2 were pulverized under liquid nitrogen and then homogenized in 7 volumes of ice-cold buffer containing 20 mM HEPES (pH 7.4), 2 mM EGTA, 50 mM NaF, 100 mM KCl, 0.2 mM EDTA·Na2, 50 mM β-glycerophosphate, 1 mM DTT, 1 mM benzamidine, 0.5 mM sodium vanadate, and 10 µl/ml Protease Inhibitor Cocktail. The homogenate was centrifuged at 13,000 revolutions/min in an Eppendorf 5415R centrifuge for 10 min at 4°C, and 200 µl of supernatant was added to an equal volume of 2 × SDS sample buffer and boiled for 5 min at 100°C. A Bradford protein assay (cat. no. 5000006, Bio-Rad, Hercules, CA) was performed on an aliquot of the supernatant according to the manufacturer’s protocol, and samples were adjusted to 2.5 µg protein/µl SDS sample buffer.
Cell culture and transfections.
HEK-293T cells were cultured in DMEM containing 4.5 g/l glucose and l-glutamine (cat. no. 11965-092, Gibco, Gaithersburg, MD) supplemented with 100% U.S. Origin Bovine Serum (EquaFETAL, cat. no. EF-0500-A, Atlas Biologicals, Fort Colins, CO) to 10% final concentration and 1% Pen/Strep (cat. no. 15070, Gibco). All cells were maintained at 37°C and 5% CO2. Two days before experimentation, 800,000 cells were plated in 6-cm plates and then transfected using Lipofectamine 2000 (cat. no. 11668-019, Invitrogen, Carlsbad, CA) and 3.7 µg of plasmid expressing FLAG-Sestrin1, FLAG-Sestrin2, FLAG-Sestrin3, FLAG-WDR24 (Flag-WDR24-pRK5; cat. no. 46334, Addgene; a gift from Dr. David Sabatini), or FLAG-metap2. Twenty-four hours after transfection, cells were incubated in medium lacking leucine (DMEM4.5, cat. no. D-22215-L, Atlanta Biologicals, Flowery Branch, GA) and serum for 2 h, and then leucine was returned to the medium for 20 min. Cells were rinsed once with ice-cold PBS and immediately lysed with CHAPS buffer. The cell lysates were centrifuged and supernatants processed as described above under Processing of tissue samples, except that an aliquot of supernatant was reserved for FLAG-immunoprecipitation as described below.
FLAG-immunoprecipitation.
Prior to use, Anti-FLAG-M2 Affinity Gel (cat. no. A-2220, Sigma Aldrich) was washed three times with CHAPS buffer. A 50/50 slurry of the affinity gel was added to clarified supernatants of tibialis anterior homogenates (30 µl; 4 mg protein) or cell lysates (20 µl; 1 mg protein) and incubated with rotation for 2 h at 4°C. The beads then were washed three times with CHAPS buffer. Proteins associated with the beads were denatured by the addition of 70 µl of SDS sample buffer followed by boiling for 5 min, and then subjected to SDS-PAGE and Western blot analysis.
In vitro assessment of leucine-induced dissociation of the Sestrin·GATOR2 complex.
HEK-293T cells that had been transfected with plasmids expressing FLAG-Sestrin1, FLAG-Sestrin2, FLAG-Sestrin3, FLAG-WDR24, or Flag-metap2 were deprived of leucine and serum for 5 h and then lysed as described above. Leucine was added to lysates (1 mg protein) immediately before addition of FLAG affinity gel, and the mixture was incubated and processed as described in the previous paragraph. The Kd for leucine-induced dissociation of the Sestrin·GATOR2 complex was calculated using 3-parameter nonlinear least squares fit [(inhibitor) vs. response] using GraphPad Prism version 7 (GraphPad Software, La Jolla, CA).
Quantitative real-time PCR.
Total RNA was extracted from ~50 mg of tissue using Trizol reagent (cat. no. 15596-018, Invitrogen) according to the manufacturer’s protocol. RNA quality and concentration were determined using a NanoDrop Spectrophotometer (ND-1000, Thermo Fisher Scientific, Waltham, MA). RNA (1 µg) was reverse-transcribed and subjected to quantitative real-time PCR using the following primers: Sestrin1 forward, 5′-GCAGGAAGTGCCTTGGTGAGTG-3′, reverse, 5′-CGAGACACATCTCTCGCTGGTG-3′; Sestrin2 forward, 5′-CTTCATCCCAGTGGAGGAGATCC-3′, reverse, 5′-CCAGAAGCTGCTAAGGTAGTCG-3′; Sestrin3 forward, 5′-ACATTCCGAGCCCAGGACTAC-3′, reverse, 5′-GCGTGGTAGTGTCAACATCC-3′. β-actin primers were purchased from Qiagen (cat. no. QT-00193473). mRNA expression levels were normalized to β-actin mRNA using the 2−ΔΔCt method (12).
Purification of FLAG-tagged proteins from HEK293T cells.
HEK-293T cells (1.2 × 106) were plated in a 10-cm plate, and 48 h later the cells were transfected using Lipofectamine 2000 and 6.1 µg of plasmid expressing FLAG-Sestrin1, FLAG-Sestrin2, FLAG-Sestrin3, FLAG-WDR24, or FLAG-Mios (pRK5-FLAG-Mios; cat. no. 46326, Addgene; a gift from Dr. David Sabatini). Forty-eight hours after transfection, cells were lysed with CHAPS phosphate-free buffer containing 40 mM HEPES (pH 7.5), 120 mM NaCl, 1 mM EDTA·Na2, 0.3% CHAPS, 1.5 mM Na vanadate, and 10 µl/ml Protease Inhibitor Cocktail. Prior to use, Pierce Anti-DYKDDDDK Magnetic Agarose beads (cat. no. A-36799-B, Thermo Scientific, Rockford, IL) were blocked by rotating in 1% bovine serum albumin (cat. no. BP-9703-019, Fisher BioReagents, Fair Lawn, NJ) for 1 h at 4°C, washed thrice in CHAPS phosphate-free buffer, and then suspended in the original volume. Beads (100 µl in lysis buffer) were added to the clarified cell lysate and incubated with rotation at 4°C for 2 h. The beads were then washed 4 times with high salt buffer (40 mM HEPES, pH 7.4, 500 mM NaCl, 2.5 mM MgCl2, 1% Triton X-100) followed by 2 washes with 50 µl 3 × FLAG peptide (150 ng/μl; cat. no. F-4799, Sigma), which were subsequently pooled. The protein concentration of eluate was measured using a NanoOrange Protein Quantitation Kit (cat. no. N-6666, Invitrogen), 2 µg of purified proteins was resolved by electrophoresis on 4%–15% Criterion Tris-HCl Protein Gel (cat. no. 5671084, Bio-Rad), and the gel was stained using SimplyBlue SafeStain (cat. no. LC-6060, Invitrogen). Various concentrations of the purified proteins were subjected to Western blot analysis on the same gel as tissue supernatants.
SDS-PAGE and Western blot analysis.
Samples were resolved by electrophoresis on Criterion Tris-HCl Protein Gels, and proteins in the gel were transferred to a PVDF membrane. The membrane was stained with a Piece Reversible Protein Stain Kit for PVDF Membranes (cat. no. 24585, Thermo Scientific) and then destained following the manufacturer’s instructions. The membrane was then blocked in 5% dry milk in Tris-buffered saline with Tween 20 and incubated overnight at 4°C with primary antibody (7). Antibodies against total 70 kDa ribosomal protein S6 protein kinase (p70S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) were purchased from Bethyl Laboratories, Inc. (Montgomery, TX ; cat. nos. A-300-510-A and A-300-501-A, respectively); antibodies to phospho-S65 4E-BP1 (cat. no. 9451), phospho-T389 p70S6K1 (cat. no. 9205), and Mios (cat. no. 13557) were obtained from Cell Signaling Technology (Danvers, MA); antibodies to WDR24 (cat. no. 20778-1-AP), Sestrin1 (cat. no. 21668-1-AP), and Sestrin2 (cat. no. 10795-1-AP) were from Proteintech Group (Rosemont, IL); antibody to Sestrin3 was from Abgent (cat. no. AP-12471-C, San Diego, CA); antibody to Sec 13 was from R&D Systems (cat. no. MAB-90552-100, Minneapolis, MN); antibody to the FLAG epitope was from Origene (cat. no. TA-50011-100); and antibody to GAPDH was from Santa Cruz Biotechnology (cat. no. sc-32233, Dallas, TX). Following incubation with appropriate secondary antibodies, the antigen-antibody interaction was visualized with Clarity Western ELC Blotting Substrate (cat. no. 1705060, Bio-Rad). Blots were quantified using Image J software (National Institutes of Health, Bethesda, MD).
Green fluorescent protein fluorescence microscopy.
After excision of the tibialis anterior, the middle third of the muscle was excised and fixed in 10% buffered formalin for 24 h. The fixed samples were then suspended in 70% ethanol and submitted to The Pennsylvania State University College of Medicine Molecular and Histopathology Core for paraffin embedding, sectioning, and slide preparation. Fluorescence images were acquired on a Nikon Eclipse E800 microscope using the FITC filter, DXM1200 digital camera, and ACT-1 software (Nikon Instruments, Melville, NY).
Statistical analyses.
Figures and statistical analyses were generated using GraphPad Prism 7.0c (Graphpad Software). Data are presented as means ± SE. Student’s t-test was used for analysis when two groups were compared. Otherwise, one- or two-way analysis of variance was used with Tukey or Sidak correction for multiple comparisons, respectively. Statistical significance was set at P < 0.05.
RESULTS
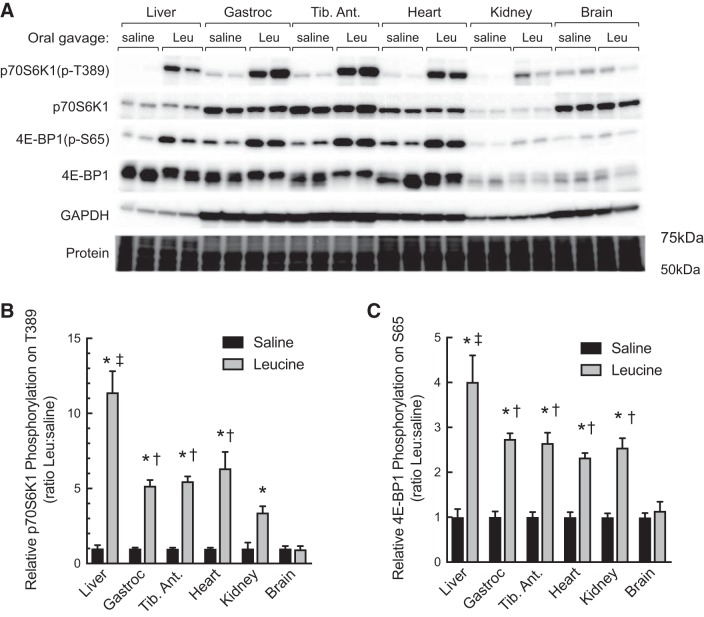
Our previous studies showed that oral administration of leucine to fasted animals promotes activation of mTORC1 in both skeletal muscle (1) and liver (3). However, the sensitivity of mTORC1 to leucine-induced activation in most other tissues in vivo has not been previously assessed. To address this deficit, experiments were conducted wherein rats were fasted overnight and the next morning were administered either a solution containing leucine or saline as an osmotic control. Western blot analyses showed that leucine administration led to activation of mTORC1 in liver, gastrocnemius, tibialis anterior, heart, and kidney, but not brain, as assessed by increased phosphorylation of p70S6K1 on Thr389 and 4E-BP1 on Ser 65 (Fig. 1). Moreover, the magnitude of the leucine-induced activation of mTORC1 was greater in liver than in any of the other tissues examined.
Fig. 1.
Oral administration of leucine to fasted rats differentially activates mTORC1 in liver, gastrocnemius, tibialis anterior, heart, and kidney, but not brain. Sprague-Dawley rats (200–250 g) were fasted overnight and the next morning were administered either saline or leucine (Leu) by oral gavage as described under materials and methods. Tissues were removed 45 min later, and Western blot analysis was performed as described under materials and methods for phosphorylated p70S6K1 [p70S6K1(p-T389)] and 4E-BP1 [4E-BP1(p-S65)], total p70S6K1 and 4E-BP1, and GAPDH (A). PVDF membrane was stained for protein using Pierce Reversible Stain for PVDF and then destained before Western blot analysis. The relative phosphorylation of p70S6K1 (B) and 4E-BP1 (C) is expressed as a fraction of the saline value for the respective tissue. Values represent means ± SE (n = 6). Closed bars, saline; shaded bars, leucine. *P < 0.0001 vs. respective saline condition by two-way ANOVA; ‡P < 0.0001 vs. all other leucine groups by two-way ANOVA; †P < 0.0001 vs. brain, leucine by two-way ANOVA. 4E-BP1, eukaryotic initiation factor 4E-binding protein 1; Gastroc, gastrocnemius; mTORC1, mechanistic target of rapamycin in complex 1; p70S6K1, ribosomal protein S6 protein kinase; Tib. Ant., tibialis anterior.
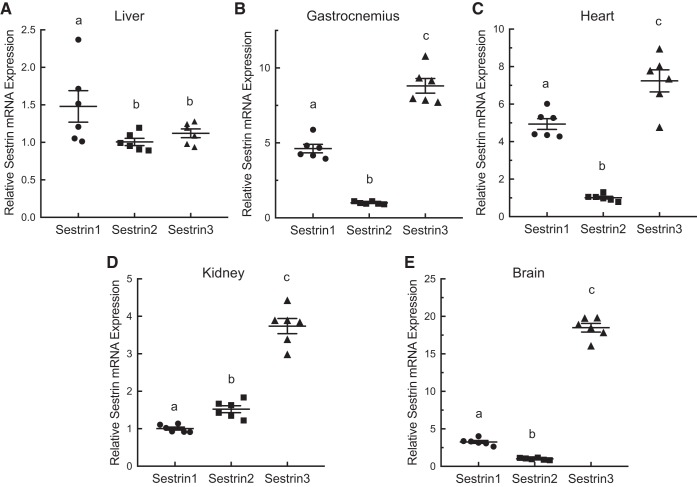
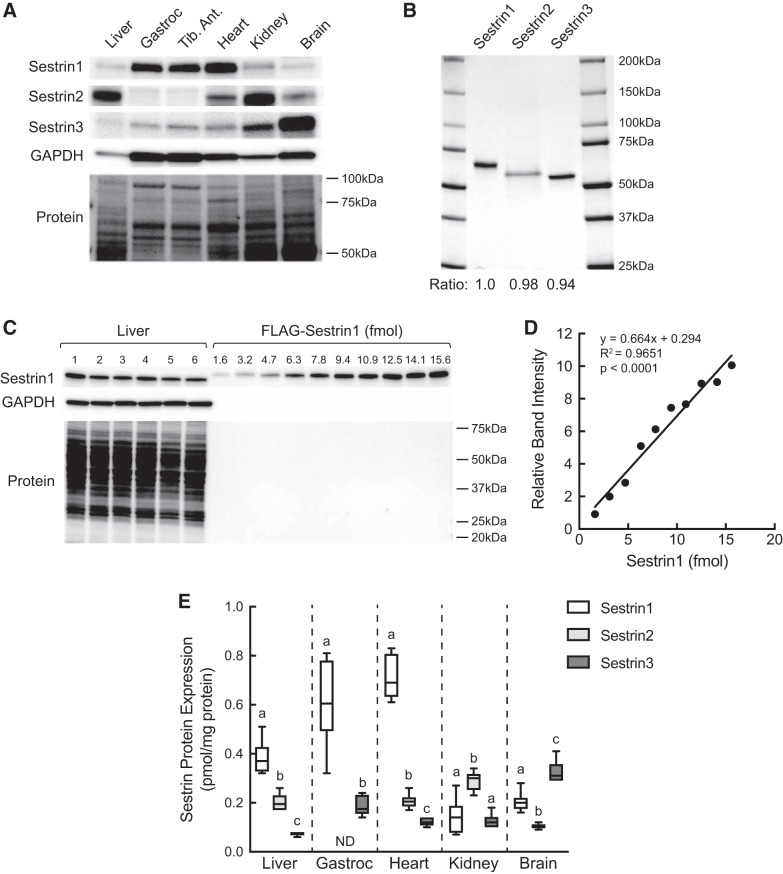
To assess Sestrin gene expression, the mRNAs for Sestrins 1, 2, and 3 were quantified by quantitative real-time PCR analysis of total RNA isolated from liver, gastrocnemius, heart, kidney, and brain. As shown in Fig. 2, Sestrin1 mRNA expression was slightly but significantly higher in liver than either Sestrin2 or 3. However, in the other tissues, Sestrin3 mRNA expression was higher than Sestrin1 or 2. Moreover, in gastrocnemius, heart, and brain, Sestrin2 mRNA expression was markedly lower than Sestrin1. Because mRNA expression is often disproportional to protein expression, Sestrin protein expression was assessed by Western blot analysis. As shown in Fig. 3A, relative Sestrin1 protein expression was higher in gastrocnemius, tibialis anterior, and heart than liver, kidney, or brain. Interestingly, Sestrin2 expression was exceptionally low in gastrocnemius and tibialis anterior, and Sestrin3 expression was low in liver compared with the other tissues. To compare Sestrin1, 2, and 3 protein expression within a tissue, FLAG-tagged variants of the proteins were expressed in HEK293T cells and purified using anti-FLAG beads (Fig. 3B). The purified proteins then were used to generate standard curves to quantitate Sestrin isoform expression (e.g., Fig. 3, C and D). In agreement with the expression pattern of the mRNAs, expression of Sestrin1 protein in liver was higher than either Sestrin2 or 3, whereas in brain, Sestrin3 protein expression was higher than Sestrin1 or 2 (Fig. 3E). In gastrocnemius, heart, and kidney, Sestrin mRNA and protein expression levels correlated less well. In gastrocnemius, Sestrin1 protein expression was significantly greater than Sestrin3, and, notably, Sestrin2 protein was expressed at levels too low to be accurately quantified. Similarly, Sestrin1 was the most abundant Sestrin expressed in heart, but unlike gastrocnemius, heart Sestrin2 was more highly expressed compared with Sestrin3. In contrast, in kidney, Sestrin2 protein expression was significantly greater compared with either Sestrin1 or 3, which were expressed at similar levels.
Fig. 2.
Sestrin1, 2, and 3 mRNA expression in liver (A), gastrocnemius (B), heart (C), kidney (D), and brain (E) of freely fed rats. RNA was extracted from tissues, and Sestrin1, 2, and 3 mRNAs were quantitated by quantitative real-time PCR as described under materials and methods. Values represent means ± SE (n = 6). Values not sharing a letter are significantly different by one-way ANOVA, P < 0.05.
Fig. 3.
Western blot analysis of Sestrin1, 2, and 3 protein expression in liver, gastrocnemius, tibialis anterior, heart, kidney, and brain. A: representative Western blot analysis of Sestrin and GAPDH expression. Membranes were stained with Pierce Reversible Stain for PVDF and then destained before Western blot analysis; a representative membrane is shown. Sestrin1, 2, and 3 antibodies were validated using HEK293T cells lacking all three Sestrin proteins [kindly provided by Dr. David Sabatini (21)]. B: FLAG-tagged Sestrin1, 2, and 3 were individually expressed in and purified from HEK293T cells as described under materials and methods. Purified proteins (2 µg) were resolved by SDS-PAGE, and the gel was stained with SimplyBlue SafeStain. Ratio, band intensity relative to Sestrin1. C: Western blot analysis of Sestrin1 and GAPDH expression in liver (n = 6) and various amounts of purified FLAG-Sestrin1. Membranes were stained for total protein loading before Western blot analysis as described above for A. D: representative standard curve for FLAG-Sestrin1. E: quantification of Western blot analysis of Sestrin1, 2, and 3 expression in various tissues. Values represent means ± SE (n = 6). Method used to quantify Sestrin1 shown in C and D was also used to quantify Sestrins 2 and 3 using the respective purified proteins (B). ND, Sestrin2 expression in gastrocnemius was too low to be accurately quantitated, and therefore is not included on the graph. For each tissue except gastrocnemius, values not sharing the same letter are significantly different by one-way ANOVA, P < 0.001. For gastrocnemius, P < 0.0005 by t-test. Gastroc, gastrocnemius; Tib. Ant., tibialis anterior.
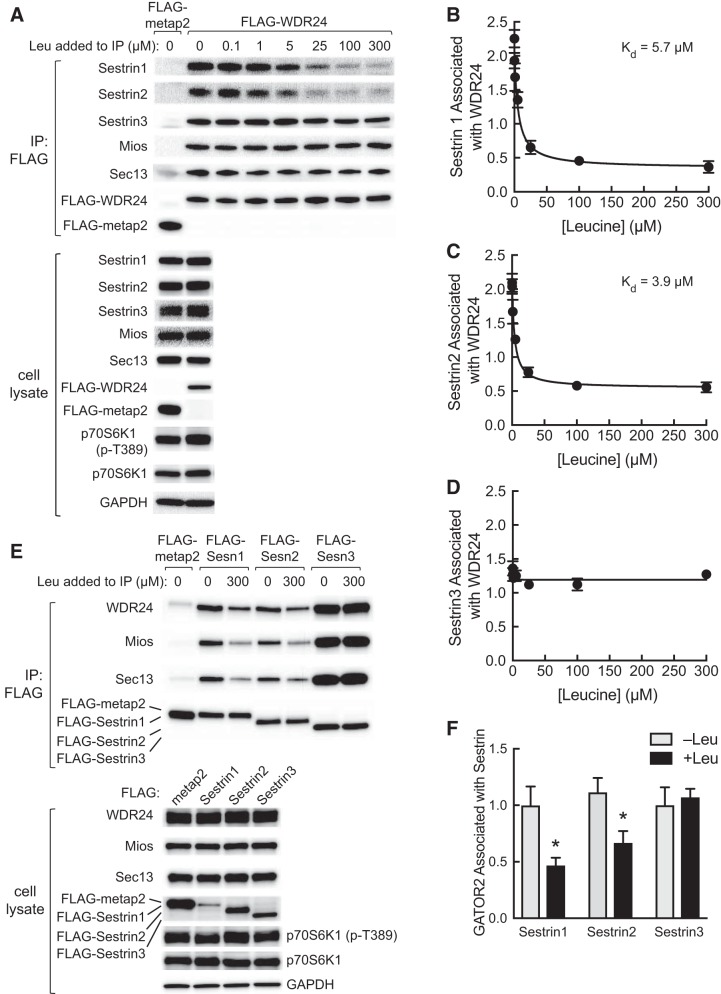
Leucine has been reported to promote Sestrin2 dissociation from GATOR2 when added to GATOR2 immunoprecipitates (21). We took advantage of this finding to obtain a relative assessment of the affinity of Sestrin1, 2, and 3 for leucine. In this assay, a FLAG-tagged variant of the WDR24 subunit of GATOR2 was expressed in and immunoprecipitated from HEK293T cells that had been deprived of leucine. The immunoprecipitates were subsequently incubated without or with leucine at various concentrations. FLAG-tagged metap2 was used as a negative control (21). As shown in Fig. 4A, the association of the Mios and Sec13 subunits of GATOR2 with FLAG-WDR24 was unaffected by leucine addition to the immunoprecipitates. In contrast, the association of Sestrin1 and 2 with FLAG-WDR24 was inversely proportional to leucine concentration. Based on nonlinear curve fit analyses, the Kd of Sestrin1 and Sestrin2 for leucine-induced dissociation of the Sestrin·GATOR2 complex were 5.7 µM and 3.9 µM, respectively (Fig. 4, B and C). In contrast, Sestrin3 binding to FLAG-WDR24 was relatively unaffected by leucine. To confirm the lack of effect of leucine on Sestrin3 association with GATOR2, FLAG-tagged variants of the three Sestrins were expressed in and immunoprecipitated from HEK293T cells, and the immunoprecipitates were incubated in the presence or absence of leucine. In confirmation of the results shown in Fig. 4A, leucine promoted the dissociation of GATOR2 from Sestrin1 and 2 but not Sestrin3 (Fig. 4, E and F). Although the amount of FLAG-tagged Sestrin1, 2, and 3 present in the immunoprecipitates was similar, more of the GATOR2 subunits were found in the FLAG-Sestrin3 immunoprecipitate as compared with the immunoprecipitates of either Sestrin1 or 2. The basis for the difference is unknown and will be examined in future studies.
Fig. 4.
In vitro assessment of Sestrin affinity for leucine. A: FLAG-metap2 or FLAG-WDR24 were expressed in HEK293T cells, and the next day the cells were incubated in medium lacking leucine for 5 h. FLAG-tagged proteins were immunoprecipitated from cell lysates using anti-FLAG beads as described under materials and methods. Beads were incubated for 2 h at 4°C with the concentrations of leucine indicated in the figure and then washed, and then protein bound to the beads was subjected to Western blot analysis. In addition, an aliquot of cell lysate was subjected to Western blot analysis. Representative blots are shown. B–D: Western blots were subjected to densitometric analysis, and a nonlinear curve fit was performed as described under materials and methods. Values represent means ± SE (n = 3). E: FLAG-tagged metap2 or Sestrin1, 2, or 3 was expressed in HEK293T cells, and the next day the cells were incubated in medium lacking leucine for 5 h. FLAG-tagged proteins were immunoprecipitated from cell lysates using anti-FLAG beads as described under materials and methods. Beads were washed and then incubated for 2 h at 4°C with or without 300 µM leucine as indicated in the figure. In addition, an aliquot of cell lysate was subjected to Western blot analysis. Representative blots are shown. F: quantitation of Western blots from FLAG-immunoprecipitates incubated with or without 300 µM leucine. Because WDR24, Mios, and Sec13 are subunits of GATOR2, and leucine-induced changes in their association with the Sestrins were qualitatively similar, values for WDR24, Mios, and Sec13 were combined to provide an estimate for GATOR2 associated with Sestrin. Values represent the means ± SE (n = 3). *P < 0.05 vs. respective −Leu condition. GATOR2, GAP activity toward Rags 2; IP, immunoprecipitation; Kd, dissociation constant; Leu, leucine; Mios, meiosis regulator for oocyte development; p70S6K1, ribosomal protein S6 protein kinase; p70S6K1(p-T389), phosphorylated p70S6K1; Sesn, Sestrin; WDR24, WD repeat-domain-containing protein 24.
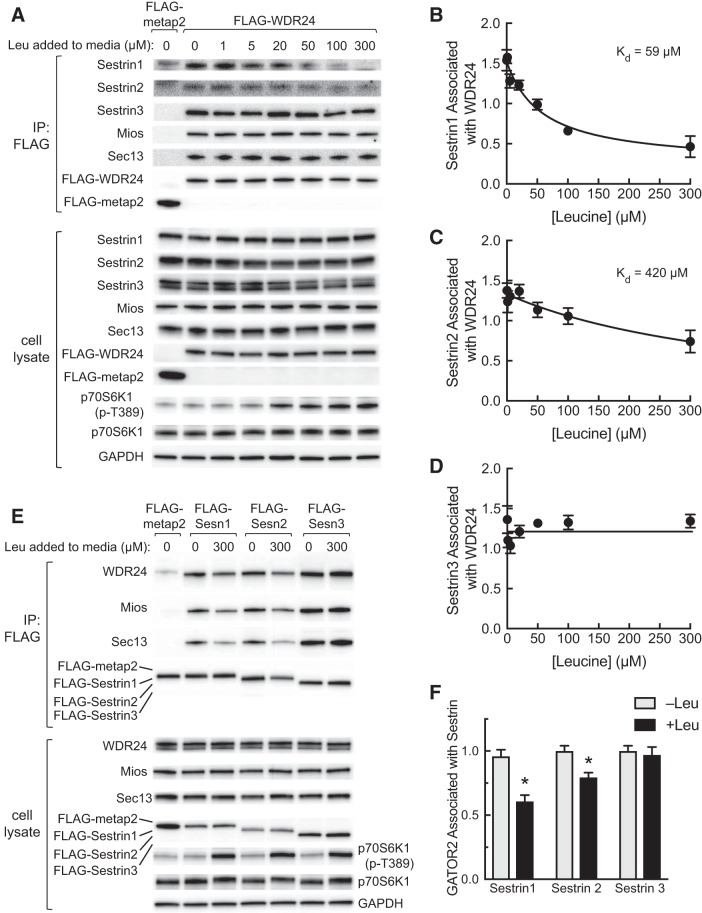
To assess whether the association of the Sestrins with GATOR2 in cells was as sensitive to leucine-induced dissociation as suggested by the in vitro assay shown in Fig. 4, cells were transfected with plasmids expressing either FLAG-WDR24 or FLAG-metap2 and the next day were deprived of leucine. Leucine was restored at various concentrations, and FLAG-tagged WDR24 or metap2 were immunoprecipitated, and proteins present in the immunoprecipitates were assessed by Western blot analysis (Fig. 5A). Similar to the results of the in vitro analyses (Fig. 4), leucine readdition to deprived cells had no effect on Mios, Sec13, or Sestrin3 association with FLAG-WDR24 (Fig. 5, A and D). In contrast, leucine readdition promoted dissociation of both Sestrin1 and 2 from WDR24 with Kd of 59 µM and 420 µM, respectively (Fig. 5, B and C). The ability of leucine readdition to promote Sestrin1 and 2 dissociation from GATOR2 and its failure to induce Sestrin3 dissociation was confirmed by expressing FLAG-tagged Sestrin1, 2, or 3 followed by leucine deprivation and restoration and FLAG-immunoprecipitation (Fig. 5, E and F).
Fig. 5.
Assessment of Sestrin affinity for leucine in cells in culture. A: plasmids expressing FLAG-tagged metap2 or WDR24 were transfected into HEK293T cells, and the next day cells were incubated for 2 h in medium lacking leucine. Leucine was returned to the medium at the concentrations indicated in the figure, and 20 min later cells were harvested. Cell lysates were incubated for 2 h at 4°C with anti-FLAG beads, and proteins remaining associated with the beads were subjected to Western blot analysis. In addition, an aliquot of cell lysate was also subjected to Western blot analysis. Representative blots are shown. B–D: Western blots were subjected to densitometric analysis, and a nonlinear curve fit was performed as described under materials and methods. Values represent means ± SE (n = 3). E: plasmids expressing FLAG-tagged metap2 or Sestrin1, 2, or 3 were transfected into HEK293T cells. The next day, the cells were incubated in medium lacking leucine for 2 h, and then leucine was returned to the medium for some of the cells to a final concentration of 300 µM. Cells were harvested 20 min later, and FLAG-metap2 or FLAG-Sestrin1, 2, or 3 were immunoprecipitated from cell lysates using anti-FLAG beads as described under materials and methods. Proteins remaining bound to the beads were subjected to Western blot analysis. In addition, an aliquot of cell lysate was also subjected to Western blot analysis. Representative blots are shown. F: quantitation of Western blots from FLAG-immunoprecipitates from cells deprived of leucine or deprived of leucine followed by restoration of leucine. Because WDR24, Mios, and Sec13 are subunits of GATOR2, and leucine-induced changes in their association with the Sestrins were qualitatively similar, values for WDR24, Mios, and Sec13 were combined to provide an estimate for GATOR2 associated with Sestrin. Values represent the means ± SE (n = 3). *P < 0.05 vs. respective −Leu condition. GATOR2, GAP activity toward Rags 2; IP, immunoprecipitate; Kd, dissociation constant; Leu, leucine; Mios, meiosis regulator for oocyte development; p70S6K1, ribosomal protein S6 protein kinase; p70S6K1(p-T389), phosphorylated p70S6K1; Sesn, Sestrin; WDR24, WD repeat-domain-containing protein 24.
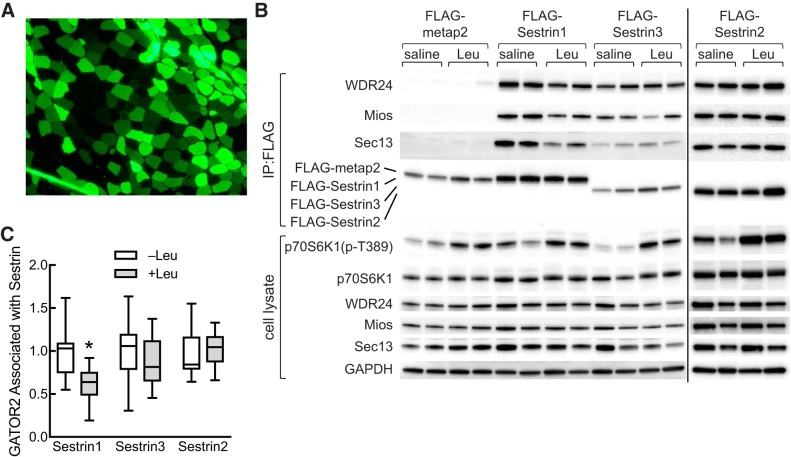
Although the Kd for leucine-induced Sestrin1 dissociation from GATOR2 in cells was closer to the leucine concentration reported in extracts of muscle from fasted animals (5, 8) than the Kd measured in vitro, it was still below the reported in vivo value, suggesting that, in muscle, Sestrin1 might normally be saturated with leucine. To assess whether Sestrin1 is able to “sense” changes in leucine availability in vivo, the two most abundant Sestrin isoforms present in muscle, i.e., Sestrin1 and Sestrin3, were expressed as FLAG-tagged variants in rat tibialis anterior. In this study, FLAG-Sestrin1 was expressed in the tibialis anterior of one leg, and Sestrin3 was expressed in the contralateral leg. In a separate group of control animals, FLAG-metap2 was expressed in one leg and green fluorescent protein (GFP) was expressed in the contralateral leg. The rats were fasted overnight, and the next morning they were administered either saline or a solution containing leucine. Based on GFP expression, ~50%–60% of muscle fibers were transfected (Fig. 6A). As expected, GATOR2 did not associate with FLAG-metap2 as assessed by Western blot analysis for WDR24, Mios, and Sec13 (Fig. 6B). However, all three GATOR2 subunits were present in immunoprecipitates of FLAG-Sestrin1 and 3. Similar to the finding in cells in culture, the amount of GATOR2 associated with Sestrin1, but not Sestrin3, was lower in rats administered leucine compared with saline (Fig. 6C). To assess possible changes in Sestrin2·GATOR2 association in muscle in vivo, in a separate study, FLAG-Sestrin2 was expressed in the tibialis anterior muscle, and the above study was repeated. As shown in Fig. 6, B and C, oral administration of leucine had no detectable effect on Sestrin2·GATOR2 association. Thus, leucine promotes dissociation of the Sestrin1·GATOR2 complex both in cells in culture and skeletal muscle in vivo but has little, if any, effect on the Sestrin2 or Sestrin3·GATOR2 complex.
Fig. 6.
Leucine-induced dissociation of GATOR2 from Sestrin1, but not Sestrin2 or 3, in tibialis anterior muscle. A and B: a plasmid expressing FLAG-tagged Sestrin1 was transfected into the tibialis anterior muscle in one leg of a rat, and a plasmid expressing FLAG-tagged Sestrin3 was transfected into the contralateral muscle as described under materials and methods. In a second set of rats, a plasmid expression FLAG-metap2 was transfected into the tibialis anterior muscle in one leg of a rat, and a plasmid expressing GFP was transfected into the contralateral muscle. In a third set of rats, a plasmid expressing FLAG-Sestrin2 was transfected into the tibialis anterior muscle. Four days later, the rats were fasted for ~18 h and were then gavaged with a suspension containing 54 g/l leucine (2.5 ml/100 g body weight) or an equivalent volume of saline. FLAG-tagged proteins were immunoprecipitated from muscle homogenates using anti-FLAG beads and then subjected to Western blot analysis. Representative blots are shown in B. Muscles transfected with a plasmid expressing GFP were processed for immunofluorescence analysis as described under materials and methods. A representative image is shown in A. C: quantitation of Western blots from FLAG-immunoprecipitates from tibialis anterior muscle. Because WDR24, Mios, and Sec13 are subunits of GATOR2, and leucine-induced changes in their association with the Sestrins were qualitatively similar, values for WDR24, Mios, and Sec13 were combined to provide an estimate for GATOR2 associated with Sestrin. Values represent the means ± SE (n = 6–7 rats/group). *P < 0.05 vs. Leu. GATOR2, GAP activity toward Rags 2; GFP, green fluorescent protein; Leu, leucine; Mios, meiosis regulator for oocyte development; p70S6K1, ribosomal protein S6 protein kinase; p70S6K1(p-T389), phosphorylated p70S6K1; WDR24, WD repeat-domain-containing protein 24.
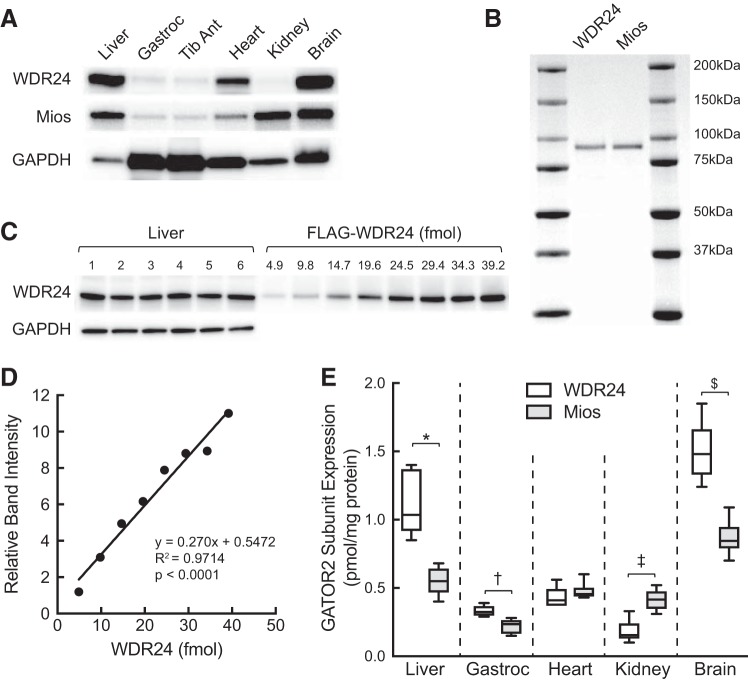
The Sestrins act to inhibit mTORC1 through association with GATOR2 (6, 21). Thus, overexpressing the proteins in skeletal muscle might be expected to attenuate leucine-induced activation of mTORC1. However, as shown in Fig. 6B, oral leucine administration activated mTORC1 regardless of whether Sestrin1 or 3 was expressed. To investigate the basis for this unexpected result, the abundance of two GATOR2 subunits, WDR24 and Mios, was assessed using purified proteins as standards. As shown in Fig. 7, WDR24 abundance in liver and brain was significantly greater compared with Mios, and in gastrocnemius it was slightly but statistically significantly greater. In contrast, in kidney, Mios expression was greater than WDR24, and in heart there was no significant difference in expression. Notably, relative Sestrin1 abundance (Fig. 3) in skeletal muscle was greater than Mios (Fig. 7), suggesting that, at a minimum, Sestrin1 is expressed at approximately three times the level of GATOR2. Moreover, combined, Sestrin1 and 3 are expressed at almost four times the level of GATOR2, based on Mios abundance. Thus, one potential explanation for the apparent lack of effect of ectopic Sestrin expression on leucine-induced mTORC1 activation in muscle is that the amount of endogenous Sestrin is sufficient to sequester all of the GATOR2 complex, and increasing Sestrin expression has no additional repressive effect.
Fig. 7.
Western blot analysis of WDR24 and Mios protein expression in liver, gastrocnemius, tibialis anterior, heart, kidney, and brain of freely fed rats. A: representative Western blot analysis of WDR24, Mios, and GAPDH expression in the same samples analyzed in Fig. 3. B: FLAG-tagged WDR24 and Mios were individually expressed in and purified from HEK293T cells as described under materials and methods. Purified proteins (2 µg) were resolved by SDS-PAGE, and the gel was stained with SimplyBlue SafeStain. Ratio, band intensity relative to WDR24. C: Western blot analysis of WDR24 and GAPDH expression in liver (n = 6) and various amounts of purified FLAG-WDR24. For GAPDH, only the liver samples were analyzed. D: representative standard curve for FLAG-WDR24. E: quantification of Western blot analysis of various tissues. Values represent means ± SE (n = 6). Method used to quantify WDR24 shown in C and D was also used to quantify Mios using the respective purified protein (B). *WDR24 and Mios expression in liver differ significantly, P < 0.005; †WDR24 and Mios expression in gastrocnemius differ significantly, P < 0.005; ‡WDR24 and Mios expression in kidney differ significantly, P < 0.005 kidney; and WDR24 and Mios expression in brain differ significantly, $P < 0.005. Gastroc, gastrocnemius; GATOR2, GAP activity toward Rags 2; Mios, meiosis regulator for oocyte development; Tib Ant, tibialis anterior; WDR24, WD repeat-domain-containing protein 24.
DISCUSSION
In agreement with previous work (21), the results presented herein show that leucine acts to promote dissociation of Sestrin1 and Sestrin2, but not Sestrin3, from GATOR2. This action was observed not only upon readdition of leucine to cells deprived of the amino acid, but also when leucine was added to GATOR2 immunoprecipitates in vitro, suggesting that binding of leucine to Sestrin1 and 2 is sufficient to promote their dissociation from GATOR2. However, the apparent Kd for leucine-induced dissociation of the Sestrin·GATOR2 complex was higher when the amino acid was restored to leucine-deprived cells compared with its addition to immunoprecipitates. Although the basis for the difference is unknown, it is tempting to speculate that in cells, leucine might not only alter Sestrin·GATOR2 interaction by directly binding to Sestrin but also by altering Sestrin phosphorylation. In a recent study (9), we showed that in both HEK293 and HeLa cells, Sestrin2 phosphorylation was enhanced in cells deprived of leucine, and readdition of the amino acid to leucine-deprived cells rapidly (i.e., within 10 min) caused Sestrin2 dephosphorylation. Expression of a phosphorylation-deficient Sestrin2 variant in which T232, S249, and S279 were changed to either Asp or Glu led to constitutive repression of mTORC1 independent of leucine availability. Notably, Sestrin2 phosphorylation was inversely proportional to mTORC1 activity over the physiological range of leucine concentrations. Although leucine-induced changes in Sestrin1 phosphorylation were not assessed in the previous study, we have subsequently identified by mass spec analysis several phosphorylation sites on Sestrin1, including one, S352, that is shared with Sestrin2 (the corresponding residue in Sestrin2 is S279) (S. R. Kimball and L. S. Jefferson, unpublished observation). Whether or not changes in phosphorylation of S352 might modulate Sestrin1 interaction with GATOR2 is unknown but will be investigated in future studies. Unfortunately, we could not assess possible leucine-mediated changes in either Sestrin1 or 2 phosphorylation in rat skeletal muscle. Although the three phosphorylation sites previously identified in human Sestrin2 (9) are conserved in the rat protein, for unknown reasons, unlike the human protein the rat protein does not resolve into multiply phosphorylated forms during SDS-PAGE. Moreover, anti-phospho antibodies for the phosphorylation sites that recognize the endogenous protein are not available, and thus we were unable to address this possibility in the present study.
It is interesting that neither leucine readdition to deprived cells nor leucine addition to GATOR2 immunoprecipitates has any effect on the binding of Sestrin3 to the complex. Based on the model that has been proposed for leucine signaling through Sestrin to mTORC1, i.e., that leucine activates mTORC1 by promoting Sestrin dissociation from GATOR2, the simplest explanation for the finding that leucine has no effect on Sestrin3·GATOR2 interaction is that Sestrin3 does not act as a leucine “sensor.” However, in liver, deletion of all three Sestrins is necessary to engender resistance to fasting-induced repression of mTORC1; i.e., deletion of any two has only a partial effect (13). Similarly, deletion of any pair of Sestrins only partially prevents amino acid-deprivation induced downregulation of mTORC1 in mouse embryo fibroblasts (13) and HEK293T cells (6), whereas deletion of all three proteins has a more marked effect. These findings suggest that all three Sestrins function in a similar manner to regulate mTORC1 activity. A possible explanation for the lack of effect of leucine on Sestrin3·GATOR2 interaction is that although leucine binding to any of the Sestrins induces a conformational change in the Sestrin·GATOR2 complex leading to its inhibition, the altered conformation of the Sestrin1·GATOR2 and Sestrin2·GATOR2 complexes engenders sensitivity to dissociation under the conditions used for immunoprecipitation, whereas the Sestrin3·GATOR2 complexes remains stable.
A recent study (22) assessed the effect of increasing amounts of leucine administered by oral gavage to fasted rats on mTORC1 signaling in liver and skeletal muscle. The highest amount of leucine administered was the same as was used in the present study, and the lowest amount was 12.5% of the maximum. In that study, serum leucine concentrations increased linearly in proportion to the amount of leucine administered. Interestingly, mTORC1, as assessed by changes in phosphorylation of p70S6K1 and 4E-BP1, was maximally activated in muscle at 25% of the highest leucine dose. However, in liver, mTORC1 activity was still increasing at the highest leucine dose. Thus, maximal mTORC1 activation occurs at a significantly lower leucine concentration in muscle compared with liver. Although the basis for the differential response of mTORC1 in liver and muscle to leucine gavage is unknown, it is interesting that the ratio of Sestrin1:Sestrin2 protein expression in muscle is significantly greater than it is in liver. Moreover, in cells in culture, the Kd of leucine-induced Sestrin1 dissociation from GATOR2 is significantly lower than Sestrin2. Based on these findings, we speculate that the greater sensitivity of mTORC1 to leucine-induced activation in skeletal muscle compared with liver is due to relative Sestrin1 expression being markedly greater than Sestrin2 in muscle. In addition, it is tempting to speculate that the liver is able to respond to a wider range of leucine concentrations than muscle because of the relatively equal expression of Sestrins 1 and 2 and their disparate affinities for leucine. The observed difference in Sestrin1-Mios ratio (~3:1 in muscle and ~1:1 in liver) could also contribute to the differential response between liver and muscle to leucine-induced mTORC1 activation.
Overall, the results of the present study extend previous ones to show that Sestrin isoform expression varies dramatically among tissues, with skeletal muscle exhibiting higher Sestrin1 compared with Sestrin 2 or 3 expression. Based on the observation that Sestrin1 exhibits the greatest sensitivity to leucine-induced dissociation from GATOR2, it is tempting to speculate that the higher expression of Sestrin1 in muscle engenders greater sensitivity of mTORC1 to activation at lower leucine concentrations compared with liver. It will therefore be interesting to assess in future studies the effect of lower doses of leucine on mTORC1 activation and Sestrin·GATOR2 interaction in the tissues examined in this study. Moreover, a recent study (23) showed that Sestrin1, but not Sestrin2 or 3, protein expression was increased following an acute bout of resistance exercise. It is well known that resistance exercise enhances the stimulatory effect of amino acids on muscle protein synthesis (15). Whether or not the increase in Sestrin1 expression after exercise enhances muscle sensitivity to leucine-induced mTORC1 activation is unknown but is an interesting topic for future studies. Finally, based on its low abundance relative to the other Sestrins, it seems unlikely that Sestrin2 contributes significantly to leucine-induced activation of mTORC1 in rat gastrocnemius or tibialis anterior muscle. Whether or not Sestrin2 might play a more important role in other skeletal muscles, e.g., human vastus lateralis (23) or pig longissimus dorsi (16), is unknown.
GRANTS
D. Xu was the recipient of financial assistance provided by a grant from the National Natural Science Foundation of China (No. 31602170) and The International Postdoctoral Exchange Fellowship Program 2017 by the Office of China Postdoctoral Council (No.32 Document of OCPC, 2017). This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-15658 and DK-13499.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.R.K conceived and designed research; D.X., K.L.S., H.A.L., and L.K. performed experiments; D.X. and S.R.K. analyzed data; S.R.K. interpreted results of experiments; D.X. and S.R.K. prepared figures; D.X. and S.R.K. drafted manuscript; D.X., K.L.S., H.A.L., L.K., L.S.J., and S.R.K. edited and revised manuscript; D.X., K.L.S., H.A.L., L.K., L.S.J., and S.R.K approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Michael D. Dennis for critical reading of the manuscript before submission.
REFERENCES
- 1.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130: 139–145, 2000. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 2.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130: 2413–2419, 2000. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 3.Anthony TG, Anthony JC, Yoshizawa F, Kimball SR, Jefferson LS. Oral administration of leucine stimulates ribosomal protein mRNA translation but not global rates of protein synthesis in the liver of rats. J Nutr 131: 1171–1176, 2001. doi: 10.1093/jn/131.4.1171. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340: 1100–1106, 2013. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1-13C]leucine. Clin Sci (Lond) 76: 447–454, 1989. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- 6.Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 9: 1–8, 2014. doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grainger DL, Kutzler L, Rannels SL, Kimball SR. Validation of a commercially available anti-REDD1 antibody using RNA interference and REDD1−/− mouse embryonic fibroblasts. F1000 Res 5: 250, 2016. doi: 10.12688/f1000research.7691.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jefferson LS, Li JB, Rannels SR. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem 252: 1476–1483, 1977. [PubMed] [Google Scholar]
- 9.Kimball SR, Gordon BS, Moyer JE, Dennis MD, Jefferson LS. Leucine induced dephosphorylation of Sestrin2 promotes mTORC1 activation. Cell Signal 28: 896–906, 2016. doi: 10.1016/j.cellsig.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Cho US, Karin M. Sestrin regulation of TORC1: Is Sestrin a leucine sensor? Sci Signal 9: re5, 2016. doi: 10.1126/scisignal.aaf2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta 544: 351–359, 1978. doi: 10.1016/0304-4165(78)90103-4. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Peng M, Yin N, Li MO. Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling. Cell 159: 122–133, 2014. doi: 10.1016/j.cell.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 351: 53–58, 2016. doi: 10.1126/science.aad2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokes T, Hector AJ, Morton RW, McGlory C, Phillips SM. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 10: E180, 2018. doi: 10.3390/nu10020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suryawan A, Davis TA. Amino acid- and insulin-induced activation of mTORC1 in neonatal piglet skeletal muscle involves Sestin2-GATOR2, Rag A/C-mTOR, and RHEB-mTOR complex formation. J Nutr 148: 825–833, 2018. doi: 10.1093/jn/nxy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuckow AP, Vary TC, Kimball SR, Jefferson LS. Ectopic expression of eIF2Bepsilon in rat skeletal muscle rescues the sepsis-induced reduction in guanine nucleotide exchange activity and protein synthesis. Am J Physiol Endocrinol Metab 299: E241–E248, 2010. doi: 10.1152/ajpendo.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 19.Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev 71: 195–208, 2013. doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482, 2006. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 21.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351: 43–48, 2016. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshizawa F, Mochizuki S, Sugahara K. Differential dose response of mTOR signaling to oral administration of leucine in skeletal muscle and liver of rats. Biosci Biotechnol Biochem 77: 839–842, 2013. doi: 10.1271/bbb.120737. [DOI] [PubMed] [Google Scholar]
- 23.Zeng N, D’Souza RF, Figueiredo VC, Markworth JF, Roberts LA, Peake JM, Mitchell CJ, Cameron-Smith D. Acute resistance exercise induces Sestrin2 phosphorylation and p62 dephosphorylation in human skeletal muscle. Physiol Rep 5: e13526, 2017. doi: 10.14814/phy2.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]