Abstract
A goal of osteoporosis therapy is to restore lost bone with structurally sound tissue. Mice lacking the transcription factor nuclear matrix protein 4 (Nmp4, Zfp384, Ciz, ZNF384) respond to several classes of osteoporosis drugs with enhanced bone formation compared with wild-type (WT) animals. Nmp4−/− mesenchymal stem/progenitor cells (MSPCs) exhibit an accelerated and enhanced mineralization during osteoblast differentiation. To address the mechanisms underlying this hyperanabolic phenotype, we carried out RNA-sequencing and molecular and cellular analyses of WT and Nmp4−/− MSPCs during osteogenesis to define pathways and mechanisms associated with elevated matrix production. We determined that Nmp4 has a broad impact on the transcriptome during osteogenic differentiation, contributing to the expression of over 5,000 genes. Phenotypic anchoring of transcriptional data was performed for the hypothesis-testing arm through analysis of cell metabolism, protein synthesis and secretion, and bone material properties. Mechanistic studies confirmed that Nmp4−/− MSPCs exhibited an enhanced capacity for glycolytic conversion: a key step in bone anabolism. Nmp4−/− cells showed elevated collagen translation and secretion. The expression of matrix genes that contribute to bone material-level mechanical properties was elevated in Nmp4−/− cells, an observation that was supported by biomechanical testing of bone samples from Nmp4−/− and WT mice. We conclude that loss of Nmp4 increases the magnitude of glycolysis upon the metabolic switch, which fuels the conversion of the osteoblast into a super-secretor of matrix resulting in more bone with improvements in intrinsic quality.
Keywords: bone biomechanics, metabolism, osteoporosis, parathyroid hormone, transcriptome
INTRODUCTION
Osteoporosis is a disease of attenuated bone mass and strength that significantly increases the risk of fragility fractures (92). Teriparatide (PTH) and abaloparatide (PTHrP) are currently the only Food and Drug Administration (FDA)-approved osteoanabolic therapies for this disease (52, 61). These drugs add new bone to the osteoporotic skeleton whereas the primary effect of anticatabolic drugs is a reduction in the pathologically elevated bone resorption (30). The benefits of PTH treatment include an increase in bone mass through a combination of new bone modeling and the sustained bone remodeling with a positive balance as well as improved bone material properties (13, 18, 29, 32, 59). However, the potency of PTH precipitously declines and there is an FDA-mandated 2-yr limit on treatment (18), emphasizing the need for new strategies that improve the efficacy of the drug, such as by combining hormone treatment with an anticatabolic drug or targeting PTH directly to bone (26, 83). Neutralizing intrinsic pathways that temper PTH-induced osteoblast secretion of bone matrix might improve drug efficacy. Indeed, a similar strategy of “inhibiting the inhibitor” (46) has led to the development of the osteoanabolic romosozumab, a monoclonal antibody that neutralizes the action of the osteoinhibitory protein sclerostin, currently under consideration by the FDA for clinical approval (3, 94).
We reported that the transcription factor nuclear matrix protein 4 (Nmp4, Zfp384, Ciz, ZNF384) suppresses the action of osteoanabolics (15, 16, 41, 70, 90, 95), and thus elucidation of the upstream and downstream effectors in the Nmp4 pathway may provide a map of the innate barriers to PTH-induced bone formation. Indeed, as a transacting protein Nmp4 is well positioned to control multiple aspects of bone formation. Genome-wide chromatin immunoprecipitation followed by high-throughput sequencing [chromatin immunoprecipitation sequencing (ChIP-seq)] analysis in MC3T3-E1 cells suggested that Nmp4 has wide-ranging effects on the transcriptome, with over 15,000 Nmp4 binding sites in the osteoblast genome. Of importance, nearly 70% of these sites are within −5 and +2 kb from a transcription start site (TSS) or within introns, both DNA regions that often harbor regulatory regions (16).
Nmp4−/− mice exhibit more bone marrow osteoprogenitors than their wild-type (WT) littermates (16, 41, 95). Expanded cultures of Nmp4−/− mesenchymal stem/progenitor cells (MSPCs) induced with osteogenic medium exhibit elevated mRNA expression of the bone matrix proteins type I collagen (Col1a1), osteocalcin (Bglap2), and osteopontin (Spp1). Additionally, the anabolic process of ribosome biogenesis is elevated in these cells, as is the expression of Gadd34 (PPP1r15a), which helps maintain translation and ultimately contributes to the continued trafficking of secretory protein through the endoplasmic reticulum (ER) despite increased protein loads (16, 20, 114).
To address the cellular pathways by which Nmp4 suppresses osteoblast-mediated bone formation, we performed high-throughput RNA sequencing (RNA-seq) of WT and Nmp4−/− expanded MSPCs during osteogenesis. Network analyses of the RNA-seq output were used for driving hypothesis testing, i.e., select pathways that were significantly altered in the transcriptome were evaluated experimentally. The results phenotypically anchored bioinformatic predictions to changes in metabolic and biochemical properties of the Nmp4−/− osteogenic cells. Based on the RNA-seq data, we hypothesized that Nmp4−/− osteoblasts elaborate a matrix that improves bone material and structural characteristics. Therefore we examined these bone properties from experimental WT and Nmp4−/− mice that had undergone various osteoporosis therapies. These data reveal new aspects of how loss of Nmp4 alters bone matrix secretion as well as the impact of this single gene on bone quality.
MATERIALS AND METHODS
Cell culture.
MSPCs were derived from individual mice as previously described (16, 109). Briefly, long bone marrow was harvested from euthanized mice 6–8 wk of age, and a Ficoll gradient was used to isolate the mononuclear cells. These cells were seeded in Mesencult Media + Mesencult Stimulatory Supplement (StemCell Technologies, Vancouver, Canada) and sustained for 3–4 wk without passage while being fed every 5–7 days by removing 50% of the old media and adding 50% fresh media, so as not to disturb the cells. Upon reaching 80% confluence, the cells were passaged at 1:3 dilution for 2 additional passages before use in experiments or were frozen for stock vials. Cells were used for study between passages 5–10. To assess the mineralization phenotype of each MSPC preparation, cells were seeded in α-MEM supplemented with 100 IU/ml penicillin, 100 µg/ml streptomycin, 25 µg/ml amphotericin, 2 mM l-glutamine (GIBCO-BRL, Life Technologies, Grand Island, NY), and 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO). At 48 h postseeding, the medium was refreshed and further supplemented with ascorbic acid (5 µg/ml; Sigma-Aldrich), dexamethasone (10 nM; Sigma-Aldrich), and 10 mM glycerol 2-phosphate disodium salt hydrate (BGP; Sigma-Aldrich). To visualize the mineralization in culture, cells were stained with alizarin red as previously described (16).
RNA-seq analysis.
To compare transcriptome profiles of nondifferentiating and osteogenic-differentiating WT and Nmp4−/− MSPCs, cells were seeded into 12-well plates at either 10,000 cells/well (25 cells/mm2) or 25,000 cells/well (62 cells/mm2). The cells seeded at the lower density were maintained in Mesencult Media + Mesencult Stimulatory Supplement (nondifferentiating medium) for 3 days postseeding and then harvested for total RNA. Cells plated at the higher density were maintained in α-MEM complete medium throughout the experiment. At 48 h postseeding, the medium was refreshed with the ascorbic acid, dexamethasone, and BGP supplement. These cells were harvested at 7 days postseeding as early osteogenic cells.
Total RNA was harvested using RNeasy (Qiagen, Valencia, CA) and measured for quality using the Agilent 2100 Bioanalyzer and Qubit 2.0 Fluorometer. High RNA integrity is critical for evaluating the transcriptome. The RNA integrity number is an algorithm for assigning integrity values to RNA measurements and assigns an electropherogram a value of 1 to 10, with 10 being the least degraded. All RNA integrity number numbers for our samples ranged between 8.2 and 9.7. A conservative cut-off value in the context of RNA degradation lies between 6.4 and 7.9 (31), well below our values. Four technical replicates were harvested for each time point and genotype. Total RNA samples were submitted to the Beijing Genomics Institute for transcriptome sequencing. In brief, magnetic beads with Oligo (dT) were used to isolate mRNA. The mRNA was fragmented and then constructed into HiSeq 2000 strand-specific libraries. The 2 × 100-nt paired-end reads were generated by Illumina HiSeq 2000. Clean reads filtered from raw sequence reads were returned from Beijing Genomics Institute. Raw reads were filtered into clean reads by employing the following rules: 1) remove reads in which the percentage of bases with quality <10 was >50%; 2) remove reads in which unknown bases were >10%; 3) remove reads with adapters; 4) map the clean reads to Mus musculus reference mm10 using STAR (version 2.4.2a) (23); 5) quantify gene-based expression levels with featureCounts (58); and 6) determine differential expression of genes across different treatments with edgeR (88) (Gene Expression Omnibus accession no. GSE112694).
RNA-seq determines the relative amount of each gene in each RNA sample but does not provide any measure of the total RNA output on a per-cell basis. This can be important when some genes are very highly expressed in one sample but not another (89), which is the case for our Nmp4−/− phenotype. We have previously shown that the Nmp4−/− MSPCs express upwards to twofold more RNA/cell than WT cells (114). Therefore, we used GusB as a scaling factor for the present RNA-seq data since our previous work identified GusB as an appropriate normalizer for microarray data (16).
Pathway enrichment analysis was performed using the Ingenuity Pathway Analysis software (IPA; Ingenuity Systems, Redwood City, CA) to distinguish significant canonical pathways in which the differentially expressed genes identified in the WT and Nmp4−/− samples were enriched. Fisher’s exact test was used to compute a P value that denotes the probability of the differentially expressed genes in the pathway being found together due to random chance. We also applied the Benjamini-Hochberg false discovery rate (q < 0.05) correction to account for multiple comparisons in the IPA.
We define a candidate Nmp4 direct target gene as a gene whose expression is altered with the loss of Nmp4 and also supports Nmp4 occupancy. To identify candidate genes, we performed Venn diagram analysis with the gene lists from the present RNA-seq data set and lists derived from our previous study of the Nmp4 genome-wide occupancy by ChIP-Seq in MC3T3-E1 preosteoblasts (16). This cell line is an established in vitro model for osteoblastogenesis. Genes that were identified as supporting Nmp4 occupancy exhibited ChIP-seq peaks within −5 to +2 kb from a TSS and/or within the range defined by the TSS and the transcription end site and not within the promoter range of the same gene (Supplemental Table S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.7496147; Gene Expression Omnibus accession no. GSE112693 for complete ChIP-Seq data set) (16). Additionally, we further refined this definition by using only genes contained in both the ChIP-seq and RNA-seq lists.
Seahorse assay.
Four independent MSPC preparations were used in the metabolic stress tests. The MSPCs 1957RWT and 1957NKO were derived from male littermates obtained from an Nmp4+/− × Nmp4+/− cross. The 1584LWT and 1515RRKO MSPCs were derived from mice obtained from different litters and different parents. Cells were seeded into an XFe24 well plate and grown for ~24 h in culture. MSPCs were then subjected to mitochondrial stress tests using oligomycin, carbonyl cyanide-4 (trifluoromethoxy)phenylhydrazone, rotenone, and antimycin A per the manufacturer’s instructions (Seahorse Biosciences, Lexington, MA). Glycolysis stress tests were performed using oligomycin and 2-deoxy-d-glucose (Seahorse Biosciences). After each analysis, total cell number was quantified and normalized to O2 consumption rate or extracellular acidification rate (ECAR), respectively. Glycolytic and mitochondrial stress tests were repeated four to five times each (biological replicates). We pooled all data (each well) obtained from the glycolytic or mitochondrial tests as technical replicates for statistical analysis.
Collagen secretion analysis.
All six independent MSPC preparations were used in the collagen secretion assays including 1957RWT, 1584LWT, 2001RLWT, 1957NKO, 1515RRKO, and 1986RKO. Collagen levels were determined using the Sircol assay (Biocolor, Carrickfergus, Northern Ireland) (1, 62). Nondifferentiating WT and Nmp4−/− MSPCs, cells were seeded into 12-well plates at 10,000–20,000 cells/well (25–50 cells/mm2). These cells were maintained in Mesencult Media + Mesencult Stimulatory Supplement (nondifferentiating medium) for 4 days postseeding. To harvest the acid soluble fraction, cultures were washed twice with ice-cold PBS and then scraped into PBS containing 0.5 M acetic acid and digested overnight at 4–8°C. The samples were then snap frozen. Collagen was concentrated from these acid-soluble fractions and then analyzed according to the manufacturer’s instructions. The collagen amount was normalized to cell number or presented as collagen/well versus cell number/well. All experiments were repeated at least twice. All the data shown in the assays are an average of at least four to five different wells per group.
Col1a1 polysome analysis.
Preparations from four independent MSPC preparations, designated 1957RWT, 1957NKO, 1584LWT, and 1515RRKO, were used to measure collagen mRNA in polysomes. Equal amounts of WT and Nmp4−/− MSPCs were cultured into 10-cm culture plates and maintained in Mesencult Media + Mesencult Stimulatory Supplement for 4 days. On day 4, cycloheximide was added to each culture dish for 10 min before harvesting. Cells were rinsed with ice-cold PBS solution containing 50 µg/ml cycloheximide and then lysed with 500 µl of cold lysis buffer containing 10 mM Tris·HCl (pH 7.4), 300 mM KCl, 10 mM MgCl2, 1 mM DTT, and 50 µg/ml cycloheximide, followed by centrifugation at 13,000 rpm for 10 min at 4°C. Cell lysates were then applied to the top of 10–50% sucrose gradients and subjected to ultracentrifugation in a Beckman SW41Ti rotor at 40,000 rpm for 2 h at 4°C. With the use of a piston gradient fractionator, polysome profiles of each sample was recorded at 254 nM by a UV monitor with Data Quest software as described previously (103). TRIzol LS reagent (Life Technologies) was used to purify RNA present in each of the sucrose gradient fractions. To ensure that there was uniform RNA preparation between fractions, an equal amount of firefly luciferase mRNA was added to each fraction. RNA prepared from equal volumes of each fraction was then used as a template for cDNA synthesis utilizing the TaqMan RT kit (Life Technologies). The quantitative PCR analyses of firefly luciferase and Col1a1 transcripts were measured as described previously (2). Equal amounts of firefly luciferase mRNA was measured in each of the fractions. Primer sequences for both transcripts were Col1a1 forward: 5′-ACGTCCTGGTGAAGTTGGTC-3′ and reverse: 5′-CAGGGAAGCCTCTTTCTCCT-3′; and firefly luciferase forward: 5′-CCAGGGATTTCAGTCGATGT-3′ and reverse: 5′-AATCTCACGCAGGCAGTTCT-3′. Experiments were carried out two independent times with similar results.
Mice.
WT and Nmp4−/− mice were generated as previously described and maintained at Indiana University Bioresearch Facility School of Dentistry (90). Briefly, the strategy for preparing the global Nmp4−/− mice involved removing the region of this gene containing coding exons 4–7 via homologous recombination (90). The correctly targeted embryonic stem cell lines from 129SvEv embryonic stem clones were microinjected into C57BL/6J blastocysts, and the chimeric mice were crossbred with the C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) to generate germline transmission. These mice were backcrossed for seven generations on the C57BL/6J background. Their WT littermates were used as the control mice for these experiments. The mice were housed, two to four mice/cage, under a 12:12-h light-dark regimen, and Labdiet Rodent 5001 diet was provided ad libitum. The Indiana University Institutional Animal Care and Use Committee approved all experimental procedures described in the present study.
Therapies.
At 10 wk of age, virgin female mice were randomly sorted into eight treatment groups by weight and genotype. Each mouse received two sequential 100-µl injections/day containing the drugs or vehicle(s) 7 days/wk for 7 wk. Mice in select groups were injected subcutaneously with synthetic hPTH 1–34 acetate salt (Bachem Americas, Torrance, CA) at 30 µg·kg−1·day−1, daily, a dose often used in rodents to evaluate PTH bone anabolic action in vivo (37, 63). The dose of the anticatabolic agent raloxifene (RAL; Sigma-Aldrich) is based on human clinical doses. RAL is normally administered as a 60-mg daily dose; therefore, based on a 60-kg patient, the quantity would be 1 mg·kg−1·day−1. The assumption is 100% absorption; therefore, the full dose was administered as a subcutaneous injection (95). Our euthanasia protocol involves using carbon dioxide inhalation at 20%V/min followed by bilateral pneumothorax or cervical dislocation in compliance with the guidelines of our Animal Care and Use Committee. This is an approved method by the Panel on Euthanasia of the American Veterinary Medical Association.
Microcomputed tomography.
Femurs and L5 vertebra were dissected from the 17-wk-old mice. The femurs were soaked in 0.9% saline, wrapped with gauze, and stored at −20°C. The L5 vertebra were transferred to 10% formalin for 2 days and then stored in 70% ethanol. Left femurs were thawed to room temperature and scanned while being hydrated with a 8.5-μm voxel size using a Skyscan 1172 microcomputed tomography (μCT) system (176 mA, 0.5-mm Aauminum filter). Scans were reconstructed with voxel attenuation coefficients ranging from 0 to 0.11, a beam hardening correction of 40%, and a ring artifact correction of 5. Mineral density was calculated using daily scans of manufacturer-supplied hydroxyapatite phantoms of 0.25 and 0.75 g/cm3. L5 vertebrae were scanned with a 6-μm voxel size using the Skyscan 1172 µCT system (176 mA, 0.5-mm aluminum filter). Scans were reconstructed with voxel attenuation coefficients ranging from 0 to 0.08, a beam hardening correction of 20%, and a ring artifact correction of 10. Three-dimensional reconstructions using Skyscan software provided femur and L5 vertebra trabecular bone volume per total volume (BV/TV; %). Parameters obtained for femoral cortical bone included total cross-sectional area (mm2), marrow area (mm2), cortical thickness (mm), periosteal bone surface (mm), endocortical bone surface (mm), anterior-posterior width (AP; mm), medial-lateral width (ML; mm), AP/ML, moment of inertia about the AP axis (mm4), moment of inertia about the ML axis (mm4), maximum moment of inertia (mm4), minimum moment of inertia (mm4), medial extreme (mm), and tissue mineral density (g/cm3 hydroxyapatite).
Mechanical testing.
Left femurs from each animal were thawed to room temperature and monotonically tested to failure in three-point bending at a displacement rate of 0.025 mm/s using a support span of 9 mm (4). The bones were oriented in the anterior-posterior direction with the anterior side in tension. The moment of inertia about the medial-lateral axis and the extreme fiber in the anterior direction were obtained from the μCT images using a seven slice region centered on the failure site and were utilized to map load-displacement to stress-strain, employing standard beam bending equations. Structural-level mechanical and tissue-level material properties were then obtained from the load-displacement and stress-strain curves.
Statistical analysis.
We used the statistical package JMP version 7.0.1 (SAS Institute, Cary, NC) to evaluate osteoporosis treatment response in our experimental mice. We tested three experimental therapies and a vehicle control using two genotypes of mice, yielding a total of eight treatment groups. Outliers in the data sets were identified using the interquartile range (IQR) method to assess statistical dispersion (68). The remaining data were analyzed with a two-way ANOVA for effects of genotype and treatment followed by a Tukey-Kramer post hoc test for comparison of more than two groups or Student’s t-test post hoc test for comparing WT and Nmp4−/− parameters as two groups. Experimental data were sorted by either treatment or genotype to determine whether either or both influenced the value of the end point parameter and whether genotype affected the response to treatment (genotype × treatment interaction). To assess if the combination treatment provided a synergistic effect over the monotherapies we performed 2-way ANOVA tests using PTH and RAL as the independent variables on both WT and Nmp4−/− data sets. Statistical significance was set at P ≤ 0.05. To evaluate the metabolic profiles of the MSPCs, we used the Statistical Analysis System version 9.4 (SAS Institute) and JMP to perform Student’s t-test in comparing specific metabolic parameters. Finally, ggplot2 was used to create all the heatmaps, volcano plots, and boxplots (107).
RESULTS
Nmp4 regulates a large portion of the osteogenic transcriptome.
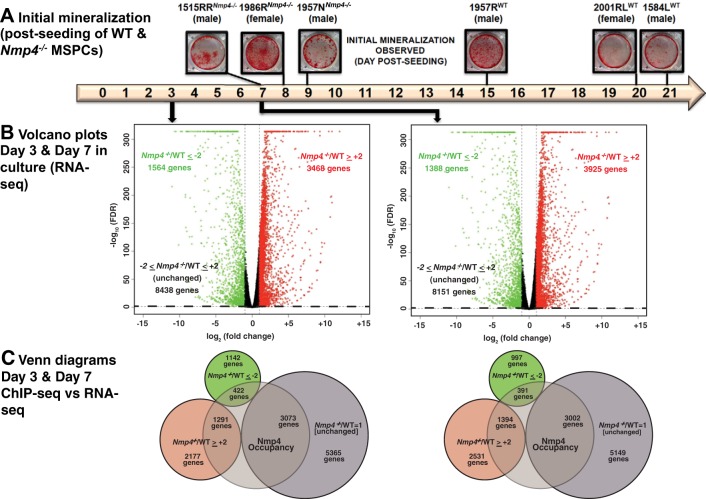
We previously showed that expanded cultures of Nmp4−/− bone marrow MSPCs exhibited a precocious and enhanced mineralization compared with WT cells (16). For the present study, three independently derived WT MPSCs from individual isogenic mice, along with three Nmp4−/− preparations confirmed that the null cells exhibited mineralization typically within 1 wk of exposure to osteogenic medium compared with 2–3 wk for the WT cells (Fig. 1A).
Fig. 1.
Loss of nuclear matrix protein 4 (Nmp4) accelerates and enhances mesenchymal stem/progenitor cell (MSPC) mineralization and has a broad impact on the transcriptome. A: 6 independently expanded MSPC preparations from individual wild-type (WT) and Nmp4−/− mice were established as described in materials and methods. Cultures were stained with alizarin red when mineralization was first observed. The cells derived from the Nmp4−/− mice consistently exhibited mineral days to weeks before this was observed in the WT cultures. The cell preparations 1957NKO and 1957RWT were derived from male littermates. The remaining lines were derived from male or female mice selected from random litters. B: volcano plots of RNA sequencing (RNA-seq) data from MSPCs maintained in nondifferentiating culture medium for 3 days and osteogenic differentiating culture medium for 7 days. The x-axis represents the logarithmic transformation to the base 2 of the mean fold change of mRNA expression in Nmp4−/− cells versus control cells and the y-axis represents the negative logarithm to the base 10 of the false discovery rate (FDR) value. Changes in gene expression were considered significant if the fold change of knockout (KO)/WT greater than or equal to +2 (red circles) and FDR ≤0.05 or KO/WT less than or equal to −2 (green circles) and FDR ≤0.05. The black circles represent genes that did not meet either criterion. The dotted line demarcates FDR = 0.05. C: Venn diagrams showing gene overlap between chromatin immunoprecipitation sequencing (ChIP-seq) and RNA-seq data. The former was derived from MC3T3-E1 cells (16). Genes that supported Nmp4 occupancy were required to exhibit peaks (height ≥10) within 5 to +2 kb from a transcription start site (TSS) and/or located within the range defined by the TSS and the transcription end site.
To address the mechanism for the hyperanabolic phenotype elicited by loss of Nmp4, we performed transcriptome analysis on osteogenic MSPCs as a guide for hypothesis testing. Given that there were some variations in time to mineralization between the individual Nmp4+/+ and Nmp4−/− MSPC preparations, we elected to carry out RNA-seq on the MSPCs 1584LWT versus 1515RRNmp4−/− under two distinct culture conditions. These cells exhibited a striking difference in the time to mineralization onset. We then carried out the critical phenotypic anchoring experiments with the other MSPC preparations as well as the WT and Nmp4−/− mice to show that our findings are broadly applicable.
To perform RNA-seq analysis, RNA was harvested from cells at day 3 postseeding that were maintained in nondifferentiation medium and at day 7 in culture in which the cells had been transferred to osteogenic medium 48 h postseeding. All data obtained from these studies including the differences observed in mRNA expression between the WT and Nmp4−/− MPSCs at different time points following exposure to osteogenic medium are provided in Supplemental Table S2. A volcano plot shows that Nmp4-deleted cells cultured in nondifferentiating medium for 3 days displayed a significant greater than or equal to twofold change in the expression of 5,032 genes compared with WT. Of these, there was an increase in the expression of 3,468 genes and a decrease in expression of 1,564 genes (Fig. 1B). Following this criterion, the expression profiles of 8,438 genes were not significantly affected by Nmp4 status (Fig. 1B).
Loss of Nmp4 had a similar impact on the transcriptome of MSPCs maintained in the osteogenic differentiating medium and harvested at day 7, which coincided with the initiation of mineralization. At the 7-day time point, the expression profiles of 5,313 genes were significantly altered by greater than or equal to twofold, with 3,925 genes presenting an elevation in expression compared with WT cells and 1,388 genes showing a decrease (Fig. 1B). Nmp4 status did not impact the expression of 8,151 genes in cells maintained in the osteogenic medium (Fig. 1B).
We recently reported a genome-wide ChIP-seq analysis of Nmp4 binding in MC3T3-E1 preosteoblasts that identified over 15,000 binding sites for this transcription factor (16). This cell line is an established in vitro model for early osteoblastogenesis that is similar to our primary MSPCs. To identify genes that are direct targets of Nmp4, we determined the overlap of the gene lists derived from the present MSPC RNA-seq data sets and those lists derived from our previous analysis of Nmp4 genome-wide ChIP-Seq analysis (16). The gene list used from the ChIP-seq data set contained genes that had one or more peaks associated with the TSS within −5 to +2 kb from a TSS and/or within the range defined by the TSS and the transcription end site and not within the promoter range of the same gene (Supplemental Table S1) (16). Additionally, we limited the compilation to 4,786 and 4,787 genes expressed by our MSPCs for days 3 and 7 in culture respectively. The Venn diagrams revealed that ~28% of the genes occupied by Nmp4 exhibited a significant increase in expression upon loss of this transcription factor after 3 and 7 days in culture, indicating gene repression by Nmp4. By contrast, ~9% showed a decrease in expression upon loss of Nmp4 suggesting that Nmp4 functions to directly activate these gene targets (Fig. 1C). Expression of ~63% of the genes that supported Nmp4 with significant occupancy were not strongly impacted by loss of Nmp4, suggesting that Nmp4 status alone is not sufficient to alter the expression of these genes (Fig. 1C). We conclude that in this osteogenic context Nmp4 has an extensive influence on the MSPC and osteogenic transcriptomes consistent with its widespread occupancy in their genomic landscapes.
Loss of Nmp4 alters pathways that exhibit the dual functions of driving osteogenesis and glycolysis.
To identify cellular pathways sensitive to Nmp4 status, we performed, IPA (ingenuity pathway analysis)-based network analyses on the 5032 genes that exhibited a significant change in expression between the Nmp4−/− and WT cells at day 3 (nondifferentiating medium) and on the 5,313 genes that exhibited a change in expression at day 7 (osteogenic medium) in culture. Supplemental Tables S3 and S4 list the 252 significant canonical pathways derived from transcriptome analysis of day 3 and the 201 significant canonical pathways derived from analysis of day 7 cells, respectively. The large number of affected pathways is consistent with the substantial number of genes whose expression is influenced by Nmp4 status.
Many of the canonical pathways listed in Supplemental Tables S3 and S4 were also identified in previous studies characterizing MSPC transcriptomic changes during osteogenic differentiation (11, 72, 80), thus supporting our experimental approach. For example, transforming growth factor-β signaling, insulin-like growth factor-1 (IGF-1), Wnt/β-catenin signaling, and bone morphogenic protein signaling all appear to support human adipose-derived stem cells and bone marrow stromal cell osteogenesis. Additionally, many pathways related to the triggering of cell cycle, growth, differentiation, and migration, such as axonal guidance signaling, platelet-derived growth factor signaling (signaling), integrin signaling, and actin cytoskeleton signaling, have previously been distinguished in these MSPC preparations (11, 72, 80) and were identified here.
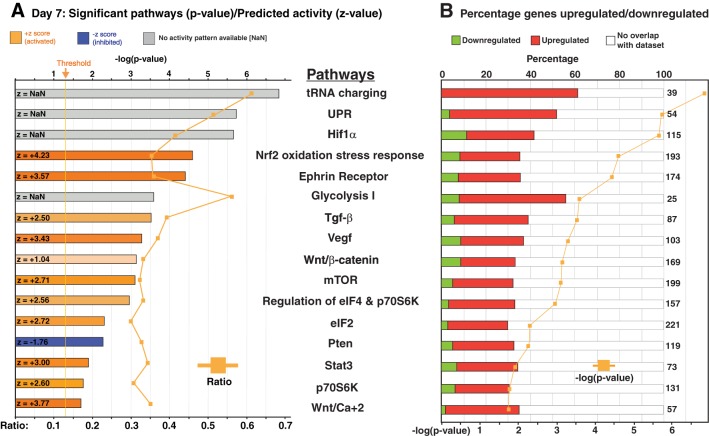
In our hypothesis-generating screen of the IPA outcomes, we identified several pathways predicted to be sensitive to Nmp4 status and drive both osteogenesis and metabolic reprogramming necessary for fueling the development of the professional secretory osteoblast (Supplemental Tables S3 and S4) (56, 79, 81, 96). Several pathways were common to cells harvested on either days 3 or 7, and we present some of these data in graphical form for day 7 (Fig. 2, A and B). The bar graphs in Fig. 2A are color coded to reflect the z-score calculated by the IPA algorithm, which predicts the direction of change for the pathway upon loss of Nmp4. An absolute z-score of 2 or more is considered significant. The activation state of the pathway is predicted to be increased if the z-score is ≥ 2, and these bars are color coded with an orange hue. Conversely, bar graphs with a blue hue indicate a z-score less than or equal to −2 representing canonical pathways with a decreased activity. Those pathways represented with a gray bar (z = NaN) indicate that the z-score algorithm cannot predict whether the pathway activity is increased or decreased in the Nmp4−/− cells.
Fig. 2.
Ingenuity Pathway Analysis (IPA) of the RNA sequencing (RNA-seq) data identified over 200 pathways significantly altered in the nuclear matrix protein 4 knockout (Nmp4−/−) cells maintained in differentiation culture medium for 7 days (see Supplemental Table S4). Here we show select canonical pathways that are sensitive to Nmp4 status and relevant to the metabolic reprogramming, protein synthesis, and secretion of the bone cells. A: the bar graphs are color coded to reflect the z-score calculated by the IPA algorithm, which predicts the direction of change for the pathway upon loss of Nmp4. An absolute z-score of 2 or more is considered significant. The activation state of the pathway is predicted to be increased if the z-score is ≥2, and these bars are color coded with an orange hue. Conversely, bar graphs with a blue hue indicate a z-score less than or equal to −2 representing canonical pathways with a decreased activity. Those pathways represented with a gray bar (z = NaN) indicate that the z-score algorithm cannot predict whether the pathway activity is increased or decreased in the Nmp4−/− cells. The orange line gives the ratio of the number of genes listed in the Nmp4 data set over the total number of genes in the IPA-annotated pathway. B: the bar graphs are color coded to reflect the percentage of genes in a particular pathway whose expressions are significantly upregulated with the loss of Nmp4 (red) and those genes whose expressions are attenuated in the null cells (green). The total numbers of genes comprising the canonical pathways are also indicated. The orange line gives the –log10(P value), and significance was defined by P ≤ 0.05 [or 1.30 = −log10(P value)]. UPR, unfolded protein response; mTOR, mammalian target of rapamycin; Pten, phosphate and tensin homolog deleted on chromosome 10; eIF, eukaryotic initiation factor; Hif1α, hypoxia-inducible factor-1α; TGF-β, transforming growth factor-β.
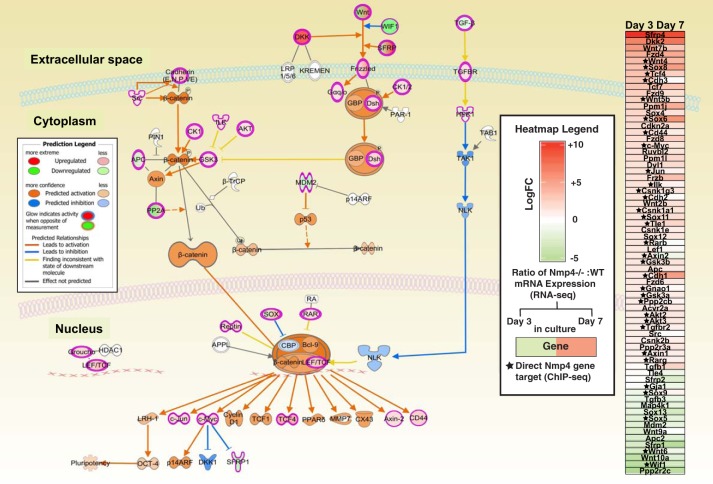
The bar graphs in Fig. 2B are color coded to reflect the percentage of genes in a particular pathway whose expressions are upregulated (red) or downregulated (green). For example, the Wnt/Ca+2 signaling pathway z-scores were +3.00Day3/+3.77Day7 (Fig. 2A; Supplemental Tables S3 and S4) indicating that loss of Nmp4 enhances the activity of this pathway. Additionally a high percentage of the genes in this pathway exhibited a significant increase in expression in the Nmp4−/− MSPCs (Fig. 2B). This is significant to the Nmp4−/− osteoblast phenotype since Wnt signaling is a major driver of bone anabolism and advances osteogenesis in part through its stimulation of glycolysis (27). The IPA's Molecule Activity Predictor algorithm allowed simulating the effects of disabling Nmp4 on the Wnt signaling pathway, which predicted elevated β-catenin activity, a key driver of osteogenesis (49) and the attenuated activity of Nemo-like kinase, a suppressor of β-catenin transcriptional activity and osteogenesis (9, 44, 74) (Fig. 3). The accompanying Wnt pathway heatmap (Fig. 3) suggests this predicted increase in Wnt signaling activity is based, in part, on the diminished expression of numerous Wnt inhibitors including Wif1, Sfrp1, Sfrp2, and Apc2 (7, 101).
Fig. 3.
The Ingenuity Pathway Analysis Molecule Activity Predictor algorithm indicated that loss of nuclear matrix protein 4 (Nmp4) elevates Wnt/β-catenin activity, a major driver of bone anabolism and suppressor of adipogenesis. Molecules in pink-red are found in the data set and are upregulated. Molecules that are green are found in the data set and are downregulated. Molecules that are gray are found in the data set but did not pass any of the filter parameters originally established for the analysis. White molecules are not in the data set but part of the pathway. Orange molecules and arrows predict activation whereas blue molecules and arrows predict inhibition. On the right side of this pathway is a heatmap of genes comprising the Wnt/β-catenin pathway derived from the RNA-seq data of wild-type (WT) and Nmp4−/− MPSCs at day 3 (uncommitted) and day 7 (early osteogenesis) in culture. Red boxes indicate increased expression in the Nmp4−/− cells compared with the WT, with greater color saturation indicating higher expression, and green color indicates reduced expression. The star indicates Nmp4 binds proximal to the transcription start site or within the intron of the gene as determined by chromatin immunoprecipitation sequencing (ChIP-seq) analysis [Childress et al. (16)]. APC, adenomatous polyposis coli protein; BCL9, B-cell lymphoma 9; CBP, histone acetyl transferase; CKI, casein kinase I; Dkk, Dickkopf; Dsh, disheveled; GSK3β, glycogen synthase kinase 3β; GBP, GSK3 binding protein; Hpk1 a.k.a. Map4k1, mitogen-activated protein kinase kinase kinase kinase 1; NLK, NEMO-like kinase; RAR, retinoic acid receptor; Tak1 a.k.a. Nr2c2, nuclear receptor subfamily 2, group C, member 2; TCF, T-cell activation factor; TGF-β, transforming growth factor-β.
Of interest, loss of Nmp4 significantly enhanced the expression of Dkk2 mRNA (see heatmap in Fig. 3). Depending on the cellular context Dkk2 can stimulate or inhibit Wnt signaling (55, 64). For example, Dkk2 is essential for osteoblast terminal differentiation and mineralization may be a novel mediator of the PTH-induced anabolic response in bone (57, 111). The activities of the Igf1 (z = +4.13Day3) and the Nrf2 signaling pathways (z = +4.33Day3; +4.23Day7) were predicted to be upregulated in Nmp4−/− cells, and although loss of Nmp4 was projected to alter the hypoxia-inducible factor-1α (Hif-1α) signaling pathway, the direction of activity could not be ascertained (z = NaNDay3; z = NaNDay7) (Fig. 2; Supplemental Tables S3 and S4). Nevertheless all pathways regulate osteogenesis as well as govern cellular metabolic reprogramming (28, 39, 82, 85). Furthermore, the phosphate and tensin homolog deleted on chromosome 10 (PTEN) network was significantly sensitive to Nmp4 status and assigned z-scores −2.50Day3 and −1.76Day7 (Fig. 2; Supplemental Tables S3 and S4) suggesting that the activity of this pathway is attenuated with loss of Nmp4. Indeed, depletion of PTEN signaling was reported to enhance osteoprogenitor expansion and glycolytic conversion (35, 110).
Of interest, loss of Nmp4 did not significantly alter the expression of Runx2 and Sp7 (Osterix), master regulators of osteogenesis, but elevated expression of the transcription factors Tcf4, Atf4, and Ddit3 (Chop, Gadd153), which all function downstream of Runx2 and Sp7 (Fig. 4). Additionally Nmp4−/− cells exhibited decreased mRNA expression of transcription factors that drive adipogenesis or chondrogenesis suggesting that loss of Nmp4 facilitates MSPC differentiation toward osteogenesis and that this predisposition is reinforced by shifts in transcriptional networks regulating the activities of the aforementioned osteogenic/metabolic pathways (Figs. 2 and 4, and Supplemental Tables S3 and S4).
Fig. 4.
Loss of nuclear matrix protein 4 (Nmp4) biases the mesenchymal stem/progenitor cells (MSPC) transcriptome toward the osteogenic lineage. Heatmap of RNA sequencing (RNA-seq) data from wild-type (WT) and Nmp4−/− MPSCs at day 3 (uncommitted) and day 7 (early osteogenesis) in culture. Red boxes indicate increased expression in the Nmp4−/−cells compared with the WT, with greater color saturation indicating higher expression, and green color indicates reduced expression. The star indicates Nmp4 binds proximal to the transcription start site or within the intron of the gene as determined by chromatin immunoprecipitation sequencing (ChIP-seq) analysis (16). Also shown, Ingenuity Pathway Analysis (IPA) canonical pathways and z-scores that support osteogenesis. Orange ovals indicate pathways that are predicted to be activated, whereas blue ovals predict that the pathways are inhibited. Pten, phosphate and tensin homolog deleted on chromosome 10; TGF-β, transforming growth factor-β; IGF-1, insulin-like growth factor-1; BMP, bone morphogenic protein.
Phenotype anchoring of our transcriptional data confirmed Nmp4−/− MSPCs exhibited an enhanced capacity for glycolytic conversion.
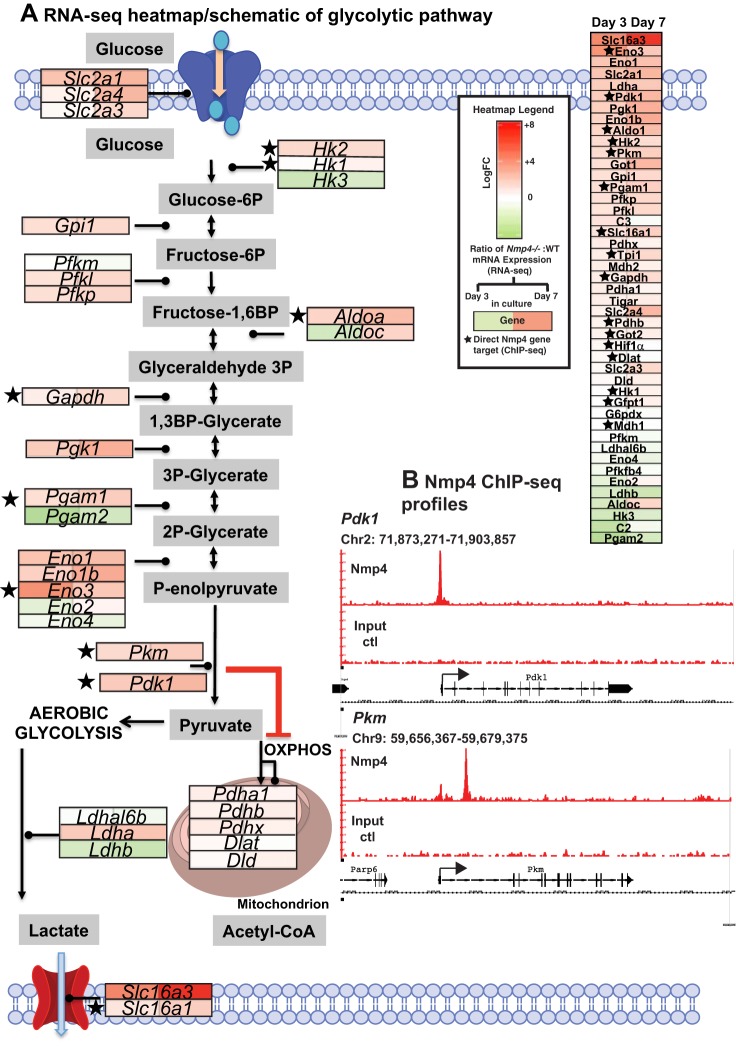
The glycolytic pathway is predicted to be altered in the Nmp4−/− cells at both days 3 and 7 in culture (Fig. 2; Supplemental Tables S3 and S4). A heatmap of several genes that comprise the glycolytic pathway showed that loss of Nmp4 greatly elevated the expression of the glucose transporter Slc2a1 (a.k.a Glut1) and increased the transcript levels of both Slc2a3 and Slc2a4 (Glut3 and Glut4; Fig. 5A). The lactate transporter Slc16a3 (a.k.a Mct4) was highly expressed in the Nmp4−/− MPSCs at both days 3 and 7 in culture (Fig. 5A). A primary function of Slc16a3 is the secretion of lactate and protons from highly glycolytic cells (22) and a recent study determined that increased levels of Slc16a3 is necessary for sustaining high glycolysis in macrophages (102). Several genes mediating the conversion of glucose to pyruvate displayed significantly elevated expression in Nmp4−/− cells (Fig. 5A). Genes responsible for regulating the switch between aerobic glycolysis and oxidative phosphorylation including Hk2, Pkm, Pdk1, and Ldha showed significantly higher mRNA levels in the Nmp4−/− cells. Additionally, our ChIP-seq analysis in MC3T3-E1 cells showed that Nmp4 binds to both Pdk1 and Pkm genes (Fig. 5B) indicating that this transacting protein directly targets key genes that regulate the glycolytic switch.
Fig. 5.
Loss of nuclear matrix protein 4 (Nmp4) perturbs the mesenchymal stem/progenitor cells (MSPC) glycolytic pathway. A: Nmp4−/− osteoprogenitors/osteoblasts exhibit significant elevated expression of several genes that drive glycolysis. Schematic of glycolysis/oxidative phosphorylation (OXPHOS) pathways with overlay of heatmap derived from RNA-sequencing (RNA-seq) data generated from WT and Nmp4−/− MPSCs harvested at day 3 (uncommitted cells) and day 7 (early osteogenesis). Red boxes indicate increased expression in the Nmp4−/− cells compared with the WT cells, with greater color saturation indicating higher expression, and green color indicates reduced expression. The star indicates Nmp4 binds proximal to the transcription start site or within the intron of the gene as determined by chromatin immunoprecipitation sequencing (ChIP-seq) analysis (16). B: ChIP-seq reveals Nmp4 binding profiles at specific gene loci in mouse MC3T3-E1 cells [Childress et al. (16); Gene Expression Omnibus accession no. GSE112693 for complete ChIP-Seq data set). The Burrows-Wheeler algorithm was used to align sequences (50-nt reads, single end) to the mouse genome (mm10). Alignments were extended in silico at their 3′-ends to a length of 150 bp, which is the average genomic fragment length in the size-selected library, and assigned to 32-nt bins along the genome. The Model-based Analysis of ChIP-Seq algorithm (v1.4.2) with a cutoff of P = 1e-7 was used to determine Nmp4 (Znf384) peak locations. The genomic loci including the chromosome number and nucleotide interval are indicated. The y-axis indicates the read scales. Arrows indicate the transcriptional start sites and direction of transcription; vertical boxes within the gene indicate exons. The Nmp4 ChIP-seq gene profiles include Pdk1 Pkm. The input DNA profiles were devoid of peaks.
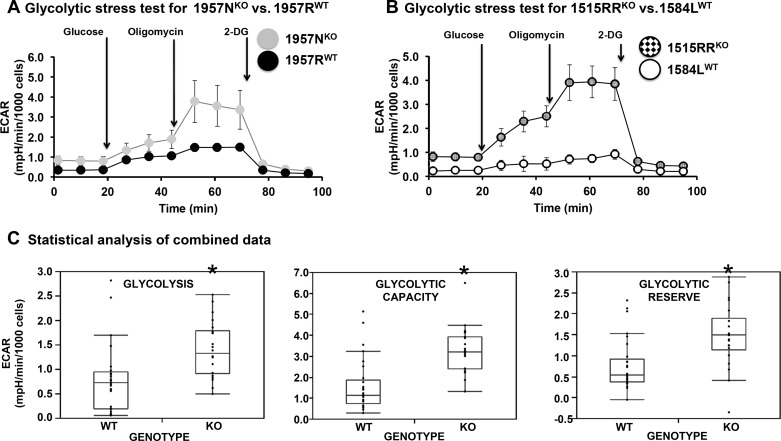
We linked our transcriptome/ChIP-seq analyses to functional data via the glycolytic stress tests (Fig. 6). WT versus Nmp4−/− cells derived from the male littermates (1957WT/1957KO) and the WT versus Nmp4−/− cells derived from the males obtained from random litters (1584LWT/1515RRKO) were cultured in nondifferentiating medium using the Seahorse analyzer. Cells were seeded directly into an analyzer well plate and grown for 24 h in culture. Subsequently, cells were incubated in medium devoid of glucose or pyruvate, and the analyzer measured the ECAR before and after a saturating amount of glucose was injected. These experiments quantified glycolytic activity (glycolysis), which was significantly elevated in Nmp4−/− cells (Fig. 6, A–C). The ECAR value was then obtained after injection of oligomycin, which inhibited oxidative phosphorylation driving the cell to use glycolysis to its maximum capacity (glycolytic capacity). Again the Nmp4−/− cells exhibited a significantly elevated level for this parameter (Fig. 6, A–C). The final injection of 2-deoxy-d-glucose, a glucose analog that inhibited glycolysis through competitive binding to glucose hexokinase, decreased ECAR confirming that the lowered medium pH was the result of increased glycolysis (Fig. 6, A and B). The glycolytic reserve, defined as the difference between glycolytic capacity and glycolysis rate, was elevated with the loss of Nmp4 (Fig. 6, A–C). We conclude that loss of Nmp4 results in the metabolic reprogramming of the MSPCs enhancing their capacity for glycolysis.
Fig. 6.
Loss of nuclear matrix protein 4 (Nmp4) enhances glycolytic capacity. The line graphs show a comparison of wild-type (WT) versus Nmp4−/− mesenchymal stem/progenitor cells (MSPC) extracellular acidification rate (ECAR) profiles that have undergone the glycolytic stress test. A: The MSPCs 1957RWT and 1957NKO were derived from male littermates. B: the MSPCs 1584LWT and 1515RRKO were derived from a random pair of male WT and Nmp4−/− mice. These graphs are representative of 4 individual tissue culture experiments (biological replicates). C: these graphs represent data from 5 separate experiments with cells from 5 different platings. In each experiment, 10 technical replicates with each cell preparation have been performed. The data are means ± SD. *P < 0.05, statistical significance. Glycolysis is the increase in ECAR measured after the glucose injection. This is the rate of glycolysis under basal conditions. Glycolytic capacity is the increase in ECAR after oligomycin injection. Glycolytic reserve is determined after 2-deoxy-d-glucose (2-DG) injection, which inhibits glycolysis. The difference between glycolytic capacity and glycolysis rates defines glycolytic reserve.
Nmp4−/− MSPCs exhibited an increased mitochondrial respiratory capacity.
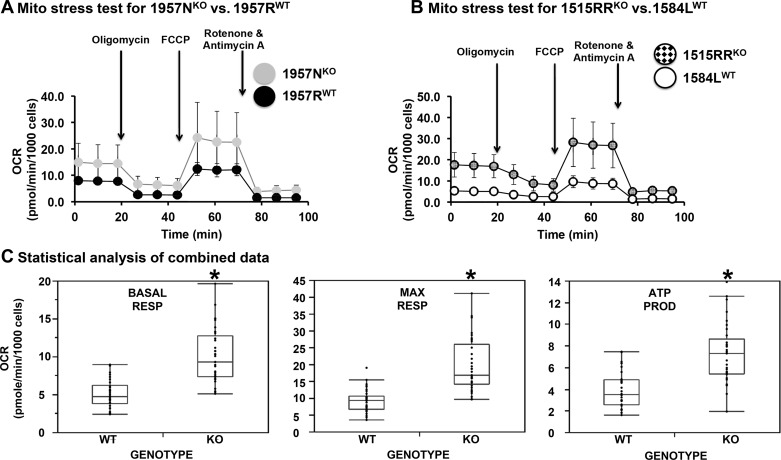
Next, the mitochondrial respiratory capacity was compared in the WT and Nmp4−/− cells. For the mitochondrial stress test, the Seahorse analyzer was used to measure basal respiration reported as oxygen consumption rate and then the cells were sequentially exposed to various compounds to assess mitochondrial electron transport chain function (Fig. 7, A–C). Our results showed that loss of Nmp4 elevated basal respiration, maximal respiration, and ATP production in MSPCs (Fig. 7, A–C). Spare respiratory capacity and nonmitochondrial respiration were also significantly elevated (data not shown). We conclude that metabolic reprogramming occurs in MSPCs as a consequence of Nmp4 loss, enhancing the capacity of these cells for oxidative phosphorylation.
Fig. 7.
Loss of nuclear matrix protein 4 (Nmp4) enhances mitochondrial respiratory capacity. The line graphs show a comparison of wild-type (WT) versus Nmp4−/− mesenchymal stem/progenitor cell (MSPC) oxygen consumption rate (OCR) profiles that have undergone the mitochondrial stress test. A: the MSPCs 1957RWT and 1957NKO were derived from male littermates. B: the MSPCs 1584LWT and 1515RRKO were derived from a random pair of male WT and Nmp4−/− mice. These graphs are representative of 5 individual tissue culture experiments (biological replicates). C: these graphs represent data from 5 separate experiments with cells from 5 different platings. In each experiment, 10 technical replicates with each cell preparation have been performed. The data are means ± SD. Statistical significance was set at P < 0.05. Basal respiration (BASAL RESP) was first measured, and then, the cells were sequentially exposed to various inhibitors of the mitochondrial electron transport chain. ATP production (ATP PROD) was based on the comparison between the basal OCR and the oligomycin-induced drop in OCR. The subsequent injection of carbonyl cyanide-4(trifluoromethoxy)phenylhydrazone (FCCP) uncoupled the electron transport chain increasing OCR and permitting the calculation of the maximal respiration rate (MAX RESP). Nonmitochondrial respiration was determined from the final injection of rotenone, a complex I inhibitor, and antimycin A, a complex III inhibitor. This parameter was significantly higher in the Nmp4−/− cells (data not shown). Spare respiratory capacity was also significantly elevated (data not shown).
Nmp4−/− osteoprogenitors exhibit enhanced protein production and secretion.
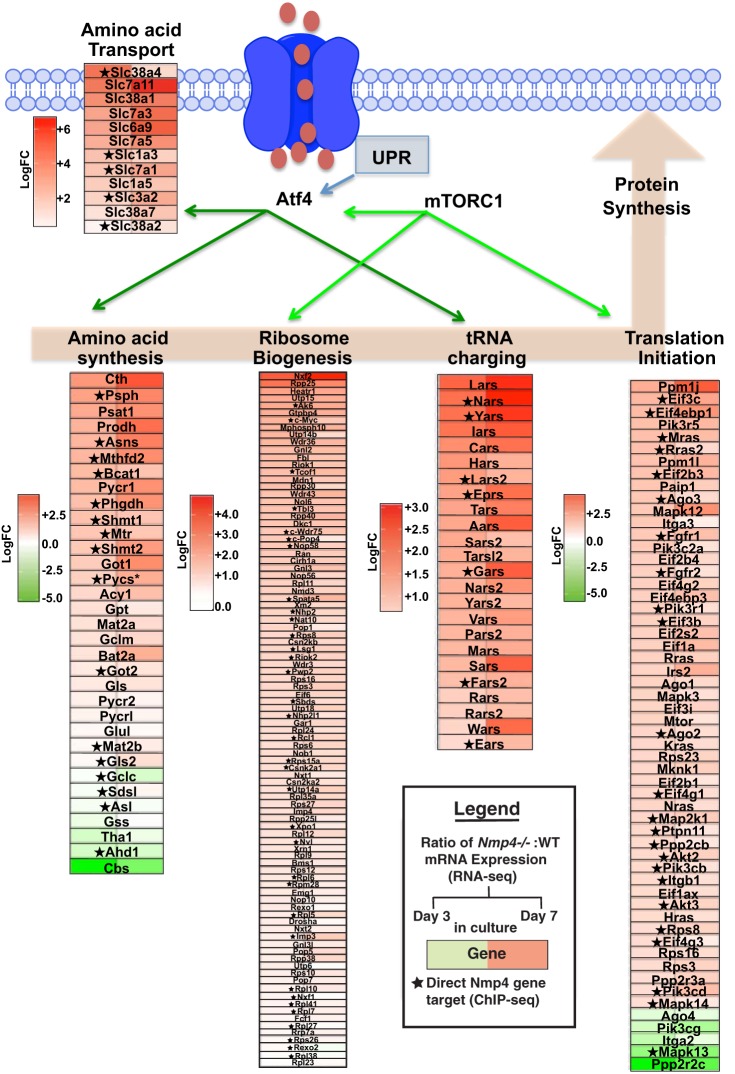
IPA analysis predicted that loss of Nmp4 elevates the activity of several cellular pathways driving protein production and delivery. Specifically, the activities of the eukaryotic initiation factor (eIF)-2 (z = +3.28Day3; +2.72Day7), mammalian target of rapamycin (mTOR; z = +2.949Day3; +2.71Day7), and the eIF4 and p70SK6 (z = +3.16Day3; +2.56Day7) signaling pathways were predicted to be upregulated in Nmp4−/− cells (Fig. 2; Supplemental Tables S3 and S4). This suggests that loss of Nmp4 stimulates anabolic processes including protein synthesis, translation initiation, and the regulation of energy production in mitochondria (71, 105). Loss of Nmp4 was projected to alter the tRNA signaling pathway, but the direction of change could not be predicted (z = NaNDay7; Fig. 2; Supplemental Table S4). Nevertheless, several genes of this pathway were significantly upregulated (Figs. 2B and 8). Indeed, the expression of numerous genes comprising the pathways of amino acid transport, amino acid biosynthesis, ribosome biogenesis, and translation initiation was significantly elevated (Fig. 8). Elevated protein production in Nmp4−/− MSPCs is also supported by our earlier report that enhanced ribosome biogenesis was sustained during induction of the unfolded protein response (UPR), which serves to expand the processing capacity of the ER for nascent secretory proteins (25, 114). This Nmp4-directed transcriptome program may allow a large protein client load to be processed through the ER without halting global osteoblast translation or inducing apoptosis (114). Indeed, the RNA-seq analysis confirmed that Nmp4−/− MSPCs exhibited elevated expression of several genes of the UPR pathway (Fig. 9) and IPA/Molecule Activity Predictor analysis predicted that protein-folding activity is elevated and UPR-induced apoptosis is attenuated with loss of Nmp4 (Fig. 9).
Fig. 8.
Genes involved in various aspects of protein synthesis are shown in the heatmaps positioned along this cellular process. These heatmaps were derived from RNA sequencing (RNA-seq) data generated from wild-type (WT) and nuclear matrix protein 4 knockout (Nmp4−/−) mesenchymal stem/progenitor cells (MSPCs) harvested at day 3 (uncommitted cells) and day 7 (early osteogenesis). Red boxes indicate increased expression in the Nmp4−/− cells compared with the WT cells, with greater color saturation indicating higher expression, and green color indicates reduced expression. The star indicates Nmp4 binds proximal to the transcription start site or within the intron of the gene as determined by chromatin immunoprecipitation sequencing (ChIP-seq) analysis (16).
Fig. 9.
The Ingenuity Pathway Analysis Molecule Activity Predictor algorithm indicated that loss of nuclear matrix protein 4 (Nmp4) elevates protein folding and attenuates endoplasmic reticulum (ER) stress-induced apoptosis. Molecules in pink-red are found in the data set and are upregulated. Molecules that are green are found in the data set and are downregulated. Molecules that are gray are found in the data set but did not pass any of the filter parameters originally established for the analysis. White molecules are not in the data set but part of the pathway. On the left side of this pathway is a heatmap of genes comprising the unfolded protein response pathway (UPR) derived from the RNA-seq data of WT and Nmp4−/− mesenchymal stem/progenitor cells (MSPCs) at day 3 (uncommitted) and day 7 (early osteogenesis) in culture. Red boxes indicate increased expression in the Nmp4−/− cells compared with the WT, with greater color saturation indicating higher expression, and green color indicates reduced expression. The star indicates Nmp4 binds proximal to the transcription start site or within the intron of the gene as determined by chromatin immunoprecipitation sequencing (ChIP-seq) analysis. AMFR, autocrine motility factor receptor; EDEM, endoplasmic reticulum-degradation-enhancing-α-mannidose-like protein; ERAD, endoplasmic reticulum-associated protein degradation; MBTPS, membrane bound transcription factor peptidase; PDI, protein disulfide isomerase; SCAP, SREBF chaperone; VCP, valosin-containing protein.
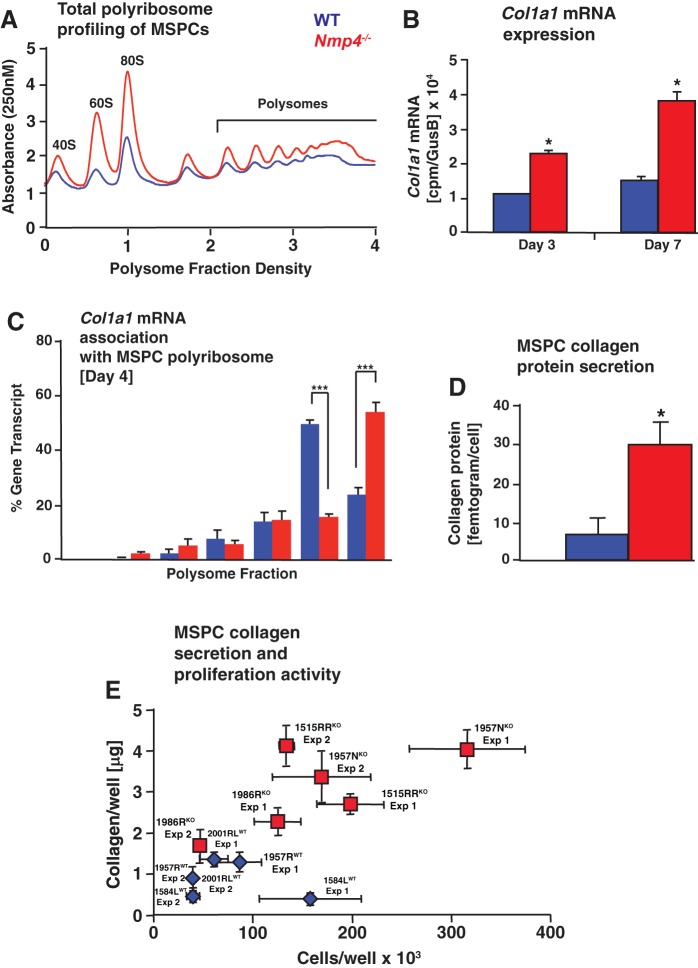
We validated the transcriptome data experimentally by measuring bone matrix production and delivery in WT and Nmp4−/− osteoprogenitors by comparing the levels of Col1a1-mRNA associated with polyribosomes and the levels of collagen protein secretion. WT versus Nmp4−/− cells derived from the littermates (1957WT/1957KO) and the WT versus Nmp4−/− cells derived from mice obtained from random litters (1584LWT/1515RRKO) were cultured in nondifferentiating medium for 4 days. We observed elevated levels of 40S and 60S ribosomal subunits and 80S monosomes and increased polysomes in Nmp4−/− MSPCs compared with WT (Fig. 10A). The RNA-seq data revealed that total Col1a1 mRNA expression was elevated in the Nmp4−/− cells (Fig. 10B). To address whether Col1a1 mRNA translation accompanied the enhanced global translation, quantitative RT-PCR analysis was performed to quantify the amount the of Col1a1 mRNA present in the polysome fractions prepared from the WT and Nmp4−/− cells (Fig. 10C). Col1a1 mRNA was present in heavy polysomes in both WT and Nmp4−/− MSPCs, suggesting efficient translation. However, there was a reproducible increase in Col1a1 mRNA in the largest fraction 7 in Nmp4−/− cells, suggesting more robust Col1a1 translation in the Nmp4-depleted cells (Fig. 10C). Thus the combination of more Col1a1 mRNA available for translation, along with increased amounts of ribosomes and more efficient Col1a1 mRNA translation, would culminate in elevated synthesis of Col1A1 protein in the Nmp4−/− cells.
Fig. 10.
Loss of nuclear matrix protein 4 (Nmp4) enhances collagen expression and secretion. Data were derived from mesenchymal stem/progenitor cell (MSPC) preparations 1584LWT, 1515RRKO, 1957RWT, and 1957NKO. A: polysome profiles of lysates prepared from wild-type (WT) and Nmp4−/− MSPCs at 4 days in culture. Representative profiles from 3 biological replicates. B: Col1a1 mRNA expression as determine by RNA sequencing (RNA-seq) in MSPCs maintained in nondifferentiation medium for 3 days in culture and 7 days in culture (5 days in osteogenic medium). C: following polysome profiling, fractions 1–7 were collected, and the percentage of Col1a1 mRNA present in each sucrose gradient fraction were quantified by quantitative RT-PCR and presented as a histogram. Data are representative of 2 biological replicates and 3 technical replicates each. Statistical analyses were performed using one-way ANOVA tests; ***P < 0.0001. D: secretion of collagen protein was measured in the acid-soluble cell-matrix layer of 1584LWT and 1515RRKO by using the Sircol assay as described in materials and methods. Loss of Nmp4 significantly enhanced the amount of collagen protein secreted/cell. * P < 0.0001. Data represent 3 biological replicates and 5–6 technical replicates each. E: secretion of collagen protein was measured in the acid-soluble cell-matrix layer by using the Sircol assay at day 4 postseeding from all MSPC preparations 1584LWT, 1957RWT, 2001RLWT, 1515RRKO, 1957NKO, and 1986RKO and presented as collagen/well (µg) versus cell number/well. All six preparations were tested independently at least twice (experiments 1 and 2) and experiments comprised 4–6 wells/preparation. All Nmp4−/− (knockout) preparations produced more collagen during the first four days of culture, regardless of cell number. Data represent average ± SD; n = 4–6 wells/group.
Collagen deposition is coupled to osteogenic proliferation (78), and Nmp4−/− MSPCs frequently exhibit a modest but significant increase in proliferative activity compared with WT (16). To evaluate changes in collagen production induced by Nmp4 deletion, independent of the confounding effects of proliferation differences, we first measured collagen production in the 1515RRKO and 1584LWT preparations that normally do not exhibit a difference in proliferation. 1515RRKO cells produced approximately three- to fourfold more collagen/cell than the 1584LWT (Fig. 10D). Next we evaluated the amount of collagen recovered/well as a function of the number of cells/well for all six MSPC preparations (Fig. 10E). All three Nmp4−/− preparations produced more collagen compared with WT cells regardless of cell number during this proliferative period in culture (Fig. 10E). Moreover, preliminary experiments with shRNA knockdown of Nmp4 in MC3T3-E1 cells yielded a similar collagen/well versus cells/well profile (data not shown). This is consistent with our previous in vivo data showing that the Nmp4−/− mice harbor more bone marrow osteoprogenitors than WT, which in turn produce more bone when stimulated (16, 41, 95). We conclude that loss of Nmp4 converts osteoprogenitors/osteoblasts into super-secretors of bone matrix while moderately enhancing their proliferative activity.
Nmp4−/− osteoblasts produce a bone matrix with improved material properties.
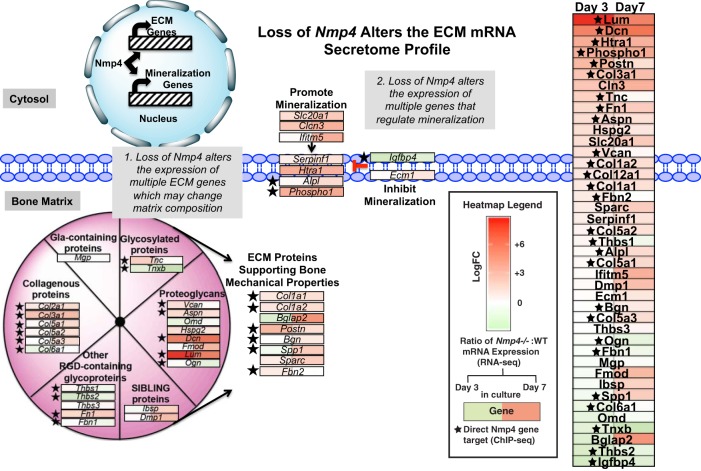
Several genes representing multiple protein classes comprising the bone matrix (6, 14, 17, 51, 69) were identified as upregulated in our RNA-seq data set suggesting enhanced matrix material properties in the null animal. The mRNA expression of this collection of genes is represented by a heatmap that displays changes between the Nmp4−/− and WT MSPCs/osteogenic cells (Fig. 11). Loss of Nmp4 significantly increased or decreased the expressions and relative ratio of several extracellular matrix (ECM) genes including those that support bone mechanical properties, e.g., Col1a1, Col1a2, Bglap2 (osteocalcin), and Spp1 (osteopontin) (Fig. 11). Also, the expression of key genes that control mineralization was altered in the Nmp4−/− cells consistent with the phenotype observed in culture. For example, the genes phosphoethanolamine/phosphocholine phosphatase (Phospho1) and alkaline phosphatase, tissue-nonspecific isozyme (Alpl), encoding phosphatases responsible for initiating mineralization (5, 43, 67, 112, 113), were highly induced in the Nmp4−/− cells as was the gene Slc20a1 a sodium-phosphate symporter also involved in the initiation of skeletal mineralization (112) (Fig. 11). Finally, the expression of several small leucine-rich proteoglycans (SLRPs) such as lumican (Lum) and decorin (Dec) was highly elevated in the Nmp4−/− cells (see Fig. 13). SLRPs play significant structural roles within the ECM and regulate collagen fibril growth, organization, and ECM assembly (12, 47, 76).
Fig. 11.
Nuclear matrix protein 4 knockout (Nmp4−/−) osteoprogenitors/osteoblasts exhibit significant elevated expression of several genes that encode proteins of the bone matrix. The schematic shows family of proteins that comprise the bone matrix. Also the expressions of key genes that control mineralization were altered in the Nmp4−/− cells consistent with the observed phenotype observed in culture. The manually annotated heatmap was derived from RNA sequencing (RNA-seq) data generated from wild-type (WT) and Nmp4−/− mesenchymal stem/progenitor cell (MSPCs) harvested at day 3 (uncommitted cells) and day 7 (early osteogenesis). Red boxes indicate increased expression in the Nmp4−/− cells compared with the WT cells, with greater color saturation indicating higher expression, and green color indicates reduced expression. The star indicates Nmp4 binds proximal to the transcription start site or within the intron of the gene as determined by chromatin immunoprecipitation sequencing (ChIP-seq) analysis (16). ECM, extracellular matrix.
Fig. 13.
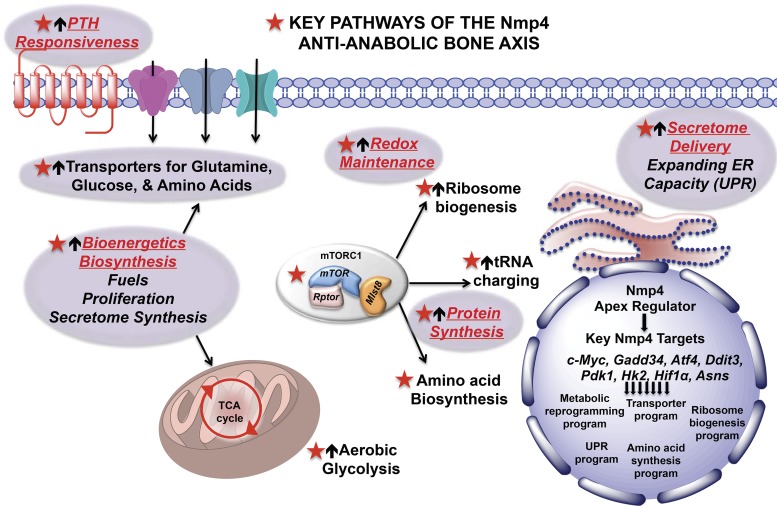
Hypothesis: nuclear matrix protein 4 (Nmp4) is an apex regulator of bone cell anabolic output. This transcription factor directly and indirectly regulates gene programs that control key stages of matrix production and delivery. It may accomplish this by regulating both the expression of master transcriptional regulators of these pathways in addition to broadly engaging several of their downstream target genes. mTOR, mammalian target of rapamycin; UPR, unfolded protein response; ER, endoplasmic reticulum; PTH, parathryoid hormone.
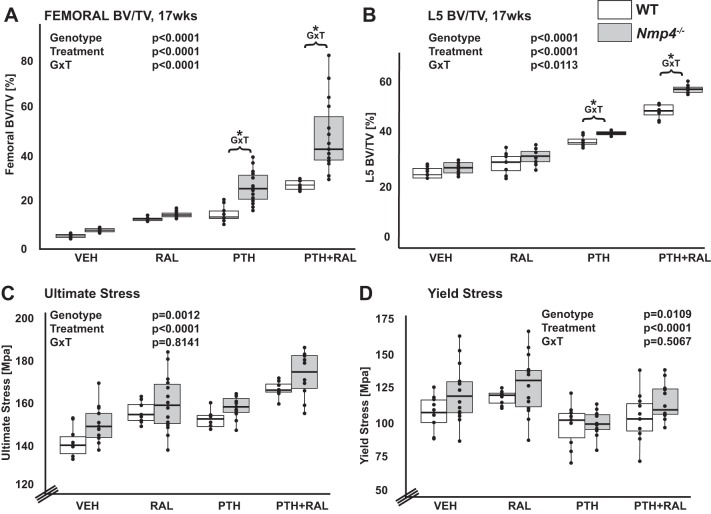
To test the biological ramifications of the transcriptional changes associated with the bone matrix genes, we evaluated skeletal tissue obtained from healthy virgin mice that had been treated with the osteoporosis therapeutics RAL, PTH, PTH + RAL, and vehicle control for 7 wk as described in materials and methods. Ovariectomized mice were not used in this experiment because ovariectomy does not change the enhanced response to anabolic drugs in the Nmp4−/− animals (16). Furthermore, all the MSPCs used in this study were derived from healthy, virgin mice. Briefly, µCT analysis showed that the PTH + RAL therapy produced more bone compared with both the PTH and RAL monotherapies at the distal femur and L5 vertebra (Fig. 12, A and B and Tables 1 and 2). There was a synergistic (greater than additive) interaction between PTH and RAL in both the WT and Nmp4−/− mice for BV/TV of the distal femur and the L5 vertebra (Table 2). However, loss of Nmp4 significantly improved the femoral bone gain and the L5 bone gain in the PTH and PTH + RAL treatments (Fig. 12, A and B, and Table 1). Nmp4 status had no impact on bone response to RAL monotherapy (Fig. 12, A and B, and Table 1). Finally, there was no significant difference between the genotypes under the VEH control treatment with respect to femoral and L5 BV/TV (Fig. 12, A and B, and Table 1). However loss of Nmp4 did significantly impact some aspects of femoral cortical geometry, such as cortical thickness, marrow area, as well as other related parameters (Table 3). Altogether, these results are similar to the data we reported in older ovariectomized mice (95).
Fig. 12.
Loss of nuclear matrix protein 4 (Nmp4) enhances therapeutically induced bone formation and femoral material properties. A and B: femoral (A) and L5 vetebral (B) bone volume per total volume (BV/TV) for all the experimental cohorts (age 17 wk) comparing WT and Nmp4−/− mice. We compared the therapies raloxifene (RAL), parathyroid hormone (PTH), and PTH + RAL to each other and to vehicle (VEH). Statistical analyses were performed using two-way ANOVA tests setting genotype and treatment as the independent variables. Statistical significance was set at P ≤ 0.05. There were a strong genotype effect and loss of Nmp4 enhanced femoral and L5 vertebral BV/TV over the cohorts. There was a strong treatment effect and PTH + RAL was the most efficacious osteoanabolic therapy for both femoral and L5 vertebral BV/TV. The analysis revealed a genotype × treatment interaction (G × T) denoted by an asterisk in the dot plot showing improved response in the PTH monotherapy and PTH + RAL combination therapy with loss of Nmp4. C and D: results of 3 point-bending analysis for ultimate stress (C) yield stress (D). There were strong genotype and treatment effects for both ultimate stress and yield stress. Data represent average ± SD; n = 8–15 mice/group.
Table 1.
Femoral and L5 trabecular architecture from WT and Nmp4−/− mice treated with vehicle, raloxifene, parathyroid hormone, and parathyroid hormone + raloxifene
| Femur BV/TV, % | Femur Tb N, mm−1 | Femur Tb Th, mm | Femur Tb Sp, mm | |
|---|---|---|---|---|
| Group | ||||
| WT VEH | 4.57 ± 0.83 | 0.970 ± 0.163 | 0.047 ± 0.003 | 0.282 ± 0.013 |
| Nmp4−/− VEH | 6.53 ± 0.93 | 1.346 ± 0.0.163 | 0.048 ± 0.002 | 0.250 ± 0.008 |
| WT RAL | 11.13 ± 1.03 | 1.885 ± 0.146 | 0.060 ± 0.001 | 0.245 ± 0.010 |
| Nmp4−/− RAL | 13.38 ± 1.37 | 2.203 ± 0.161 | 0.061 ± 0.002 | 0.227 ± 0.011 |
| WT PTH | 13.60 ± 3.39 | 2.265 ± 0.382 | 0.059 ± 0.005 | 0.230 ± 0.020 |
| Nmp4−/− PTH | 25.30 ± 6.86 | 3.304 ± 0.592 | 0.076 ± 0.008 | 0.190 ± 0.016 |
| WT PTH + RAL | 26.37 ± 2.04 | 3.591 ± 0.355 | 0.077 ± 0.005 | 0.194 ± 0.012 |
| Nmp4−/− PTH + RAL | 47.82 ± 15.70 | 5.125 ± 1.316 | 0.092 ± 0.008 | 0.139 ± 0.028 |
| Two-way ANOVA genotype | G: P < 0.0001 | G: P < 0.0001 | G: P < 0.0001 | G: P < 0.0001 |
| Nmp4-/−: 23.26a | Nmp4-/−: 2.99a | Nmp4-/−: 0.069a | WT: 0.238a | |
| WT: 13.92b | WT: 2.18b | WT: 0.061b | Nmp4-/−: 0.201b | |
| Two-way ANOVA treatment | T: P < 0.0001 | T: P < 0.0001 | T: P < 0.0001 | T: P < 0.0001 |
| P + R: 37.10a | P + R: 4.36a | P + R: 0.084a | V: 0.266a | |
| P: 19.45b | P: 2.78b | P: 0.067b | R: 0.236b | |
| R: 12.25c | R: 2.04c | R: 0.062c | P: 0.210c | |
| V: 5.55d | V: 1.16d | V: 0.047d | P + R: 0.167d | |
| Two-way ANOVA G × T | G × T: P < 0.0001 | G × T: P = 0.0015 | G × T: P < 0.0001 | G × T: P = 0.0022 |
| Nmp4−/− P + R: 47.82a | Nmp4−/− P + R: 5.13a | Nmp4−/− P + R: 0.092a | WT V: 0.282a | |
| WT P + R: 26.37b | WT P + R: 3.59b | WT P + R: 0.077b | Nmp4−/− V: 0.250b | |
| Nmp4−/− P: 25.30b | Nmp4−/− P: 3.30b | Nmp4−/− P: 0.076b | WT R: 0.245b,c | |
| WT P: 13.60c,d | WT P: 2.27c | Nmp4−/− R: 0.061c | WT P: 0.230b,c | |
| Nmp4−/− R: 13.38c | Nmp4−/− R: 2.20c | WT R: 0.060c | Nmp4−/− R: 0.227c | |
| WT R: 11.13c,d | WT R: 1.88c,d | WT P: 0.059c | WT P + R: 0.194d | |
| Nmp4−/− R: 6.53c,d | Nmp4−/− V: 1.35d,e | Nmp4−/− V: 0.048d | Nmp4−/− P: 0.190d | |
| WT V: 4.57d | WT V: 0.97e | WT V: 0.047d | Nmp4−/− P + R: 0.139e | |
| L5 BV/TV, % | L5 Tb N, mm−1 | L5 Tb Th, mm | L5 Tb Sp, mm | |
| Group | ||||
| WT VEH | 24.65 ± 2.05 | 4.37 ± 0.27 | 0.056 ± 0.002 | 0.210 ± 0.017 |
| Nmp4−/− VEH | 26.56 ± 2.11 | 4.37 ± 0.22 | 0.061 ± 0.003 | 0.204 ± 0.014 |
| WT RAL | 28.62 ± 4.10 | 5.11 ± 0.71 | 0.056 ± 0.003 | 0.201 ± 0.027 |
| Nmp4−/− RAL | 31.20 ± 2.83 | 5.24 ± 0.39 | 0.059 ± 0.003 | 0.185 ± 0.008 |
| WT PTH | 36.26 ± 2.87 | 6.60 ± 0.30 | 0.057 ± 0.003 | 0.161 ± 0.036 |
| Nmp4−/− PTH | 40.62 ± 2.23 | 6.44 ± 0.18 | 0.062 ± 0.003 | 0.153 ± 0.016 |
| WT PTH + RAL | 48.28 ± 2.72 | 8.12 ± 0.24 | 0.061 ± 0.005 | 0.129 ± 0.003 |
| Nmp4−/− PTH + RAL | 55.76 ± 3.02 | 8.07 ± 0.47 | 0.071 ± 0.004 | 0.120 ± 0.013 |
| Two-way ANOVA genotype | G: P < 0.0001 | G: P = 0.8545 | G: P < 0.0001 | G: P = 0.0324 |
| Nmp4-/−: 38.54a | Nmp4−/− = WT | Nmp4-/−: 0.063a | WT: 0.175a | |
| WT: 34.22b | WT: 0.057b | Nmp4−/−: 0.166b | ||
| Two-way ANOVA treament | T: P < 0.0001 | T: P < 0.0001 | T: P < 0.0001 | T: P < 0.0001 |
| P + R: 52.02a | P + R: 8.10a | P + R: 0.066a | V: A 0.207a | |
| P: 38.44b | P: 6.52b | P: 0.059b | R: A 0.193a | |
| R: 29.91c | R: 5.17c | R: 0.059b | P: 0.157b | |
| V: 25.61d | V: 4.37d | V: 0.0578b | P + R: 0.124c | |
| Two-way ANOVA G × T | G × T: P = 0.0133 | G × T: P = 0.6648 | G × T: P = 0.0154 | G × T: P = 0.8336 |
| Nmp4−/− P + R: 55.76a | Nmp4−/− P + R: 0.071a | |||
| WT P + R: 48.28b | Nmp4−/− P: 0.062b | |||
| Nmp4−/− P: 40.63c | Nmp4−/− V: 0.061b,c | |||
| WT P: 36.26d | WT P + R: 0.061b,c | |||
| Nmp4−/− R: 31.20e | Nmp4−/− R: 0.059b,c | |||
| WT R: EF 28.62 | WT P: 0.057c | |||
| Nmp4−/− V: 26.56f | WT V: 0.056c | |||
| WT V: 24.65f | WT R: 0.056c |
Data represent average ± SD, n = 8–15 mice/group. Nmp4, nuclear matrix protein 4; WT, wild type; V/VEH, vehicle; R/RAL, raloxifene; P/PTH, parathyroid hormone; P/PTH + R/RAL, parathyroid hormone + raloxifene; BV/TV, bone volume per total volume; Tb N, trabecular number; Tb Th, trabecular thickness; Tb Sp, trabecular spacing. Statistical analyses were performed using two-way ANOVA tests setting genotype (G) and treatment (T) as the independent variables. Statistical significance was set at P ≤ 0.05. The statistical results list the cohorts by genotype, treatment, and genotype × treatment. See text for explanation of results.
a b c d e fCohorts not connected by the same letter are statistically different; the average value of the specific parameter precedes the letter.
Table 2.
PTH and RAL synergy
| Therapy | P Value PTH Treatment | P Value RAL Treatment | P Value PTH × RAL Interaction |
|---|---|---|---|
| Femur BV/TV | |||
| PTH + RAL (WT) | <0.0001 | <0.0001 | <0.0001 |
| PTH + RAL (Nmp4−/−) | <0.0001 | <0.0001 | 0.001 |
| L5 BV/TV | |||
| PTH + RAL (WT) | <0.0001 | <0.0001 | 0.0008 |
| PTH + RAL (Nmp4−/−) | <0.0001 | <0.0001 | <0.0001 |
| Cortical area | |||
| PTH + RAL (WT) | <0.0001 | 0.0591 | 0.8056 |
| PTH + RAL (Nmp4−/−) | <0.0001 | 0.0166 | 0.5938 |
Identification of synergy between parathyroid hormone (PTH) and the anticatabolic selective estrogen receptor modulators raloxifene (RAL) using a series of two-way ANOVA tests comparing the efficacy of the PTH monotherapy, RAL monotherapy, and the combination of the 2 drugs. Nmp4, nuclear matrix protein 4; WT, wild type; BV/TV, bone volume per total volume. Statistical significance was set at P ≤ 0.05.
Table 3.
Femoral cortical architecture from WT and Nmp4−/− mice treated with vehicle, raloxifene, parathyroid hormone, and parathyroid hormone + raloxifene
| Marrow Area, mm2 | Cortical Area, mm2 | Cortical Thickness, mm | Periosteal BS, mm | Endocortical BS, mm | |
|---|---|---|---|---|---|
| Group | |||||
| WT VEH | 0.940 ± 0.049 | 0.829 ± 0.052 | 0.204 ± 0.008 | 5.393 ± 0.139 | 4.140 ± 0.114 |
| Nmp4−/− VEH | 0.913 ± 0.049 | 0.838 ± 0.039 | 0.209 ± 0.009 | 5.353 ± 0.090 | 4.078 ± 0.106 |
| WT RAL | 0.860 ± 0.022 | 0.867 ± 0.037 | 0.216 ± 0.006 | 5.344 ± 0.090 | 4.019 ± 0.095 |
| Nmp4−/− RAL | 0.879 ± 0.039 | 0.865 ± 0.047 | 0.218 ± 0.010 | 5.332 ± 0.111 | 4.004 ± 0.103 |
| WT PTH | 0.969 ± 0.048 | 0.949 ± 0.078 | 0.221 ± 0.005 | 5.617 ± 0.196 | 4.213 ± 0.108 |
| Nmp4−/− PTH | 0.931 ± 0.064 | 0.951 ± 0.062 | 0.226 ± 0.007 | 5.564 ± 0.087 | 4.142 ± 0.116 |
| WT PTH + RAL | 0.892 ± 0.043 | 0.995 ± 0.070 | 0.239 ± 0.007 | 5.552 ± 0.152 | 4.084 ± 0.106 |
| Nmp4−/− PTH + RAL | 0.852 ± 0.029 | 1.00 ± 0.067 | 0.249 ± 0.013 | 5.478 ± 0.081 | 3.978 ± 0.038 |
| Two-way ANOVA genoptype | G: P = 0.0295 | G: P = 0.3420 | G: P = 0.008 | G: P = 0.0780 | G: P = 0.0034 |
| WT: 0.915a | Nmp4−/−: 0.225a | WT: 4.11a | |||
| Nmp4−/−: 0.894b | WT: 0.220b | Nmp4−/−: 4.05b | |||
| Two-way ANOVA treatment | T: P < 0.0001 | T: P < 0.0001 | T: P < 0.0001 | T: P < 0.0001 | T: P < 0.0001 |
| P: 0.950a | P + R: 0.990a | P + R: 0.244a | P: 5.59a | P: 4.18a | |
| V: 0.927a | P: 0.954a | P: 0.223b | P + R: 5.52a | V: 4.11a | |
| P + R: 0.872b | R: 0.866b | R: 0.217b | V: 5.37b | P + R: 4.03b | |
| R: 0.869b | V: 0.833b | V: 0.206 c | R: 5.34b | R: 4.01b | |
| Two-way ANOVA G × T | G × T: P = 0.1459 | G × T: P = 0.88 | G × T: P = 0.4695 | G × T: P = 0.8513 | G × T: P = 0.4973 |
| Iap, mm4 | Iml, mm4 | Imax, mm4 | Imin, mm4 | TMD, g/cm3 HA | |
| Group | |||||
| WT VEH | 0.228 ± 0.029 | 0.144 ± 0.015 | 0.237 ± 0.029 | 0.135 ± 0.015 | 1.29 ± 0.03 |
| Nmp4−/− VEH | 0.219 ± 0.008 | 0.143 ± 0.012 | 0.224 ± 0.013 | 0.137 ± 0.012 | 0.89 ± 0.01 |
| WT RAL | 0.231 ± 0.016 | 0.144 ± 0.012 | 0.237 ± 0.016 | 0.138 ± 0.010 | 1.27 ± 0.01 |
| Nmp4−/− RAL | 0.228 ± 0.021 | 0.142 ± 0.012 | 0.235 ± 0.022 | 0.138 ± 0.014 | 0.89 ± 0.01 |
| WT PTH | 0.285 ± 0.048 | 0.179 ± 0.022 | 0.297 ± 0.053 | 0.167 ± 0.019 | 1.28 ± 0.03 |
| Nmp4−/− PTH | 0.279 ± 0.025 | 0.173 ± 0.007 | 0.281 ± 0.022 | 0.169 ± 0.010 | 0.89 ± 0.01 |
| WT PTH + RAL | 0.291 ± 0.044 | 0.173 ± 0.016 | 0.302 ± 0.043 | 0.162 ± 0.016 | 1.28 ± 0.02 |
| Nmp4−/− PTH + RAL | 0.270 ± 0.030 | 0.174 ± 0.019 | 0.276 ± 0.031 | 0.167 ± 0.017 | 0.88 ± 0.01 |
| Two-way ANOVA genotype | G: P = 0.1257 | G: P = 0.5224 | G: P = 0.0325 | G: P = 0.4557 | G: P < 0.0001 |
| WT 0.268a | WT 1.28a | ||||
| Nmp4−/−: 0.254b | Nmp4−/−: 0.886b | ||||
| Two-way ANOVA treatment | T: P < 0.0001 | T: P < 0.0001 | T: P < 0.0001 | T: P < 0.0001 | T: P = 0.2325 |
| P: 0.282a | P A 0.176a | P + R 0.289a | P: 0.168a | ||
| P + R: 0.281a | P + R: 0.173a | P: 0.289a | P + R: 0.165a | ||
| R: 0.230b | V: 0.143b | R: 0.236b | R: 0.138b | ||
| V: 0.223b | R: 0.143b | V: 0.230b | V: 0.136b | ||
| Two-way ANOVA G × T | G × T: P = 0.7276 | G × T: P = 0.9050 | G × T: P = 0.6236 | G × T: P = 0.9269 | G × T: P = 0.3597 |
Data represent average ± SD; n = 7–15 mice/group. Nmp4, nuclear matrix protein 4; WT, wild type; V/VEH, vehicle; R/RAL, raloxifene; P/PTH, parathyroid hormone; P/PTH + R/RAL, and parathyroid hormone + raloxifene; HA hydroxyapatite; Iap moment of inertia about the femoral anterior–posterior length axis; Imax maximum moment of inertia; Imin minimum moment of inertia; Iml moment of inertia about the femoral medial-lateral axis; TMD tissue mineral density. Statistical analyses were performed using two-way ANOVA tests setting genotype (G) and treatment (T) as the independent variables. Statistical significance was set at P ≤ 0.05. The statistical results list the cohorts by genotype, treatment, and genotype × treatment. See text for explanation of results.
a b cCohorts not connected by the same letter are statistically different; the average value of the specific parameter precedes the letter.
A key component of these functional investigations required the measurement of material and structural mechanical properties of the bone. Therefore, the left femurs from each animal were monotonically tested to failure. The Nmp4−/− bones exhibited a significantly higher ultimate stress, which is the stress necessary to fracture the bone at the material level, normalized for the bone geometry (Fig. 12C and Table 4). Yield stress, the stress applied to the bone after which there is permanent damage, normalized for geometry, was also significantly higher in the Nmp4−/− femurs (Fig. 12D and Table 4). Additionally, the higher value for the elastic modulus, a measure of the material’s stiffness, in Nmp4−/− bones approached significance (genotype P < 0.06, Table 4). Interestingly, numerous material properties were sensitive to the osteoporosis therapies. PTH + RAL led to significantly higher ultimate stress over RAL and PTH monotherapies in both genotypes (treatment P < 0.0001; Fig. 12C and Table 4). PTH treatment led to a modest but significantly lower yield stress than vehicle control and RAL cohorts, which were equivalent. The lower yield stress in the PTH cohorts is likely due to the increased amount of new and less mineralized bone. This would make the tissue less stiff, which is consistent with the modulus trending lowest in the PTH-treated mice (Table 4).
Table 4.
Estimated femoral material properties from WT and Nmp4−/− mice treated with vehicle, raloxifene, parathyroid hormone, and parathyroid hormone + raloxifene
| Ultimate Stress | Yield Stress | Strain to Yield, µε | Total Strain, µε | Modulus, GPa | Resilience, MPa | Toughness, MPa | |
|---|---|---|---|---|---|---|---|
| Group | |||||||
| WT VEH | 141.12 ± 6.97 | 105.73 ± 12.89 | 16,012 ± 1124 | 99,029 ± 30,556 | 7.87 ± 0.30 | 0.95 ± 0.18 | 9.19 ± 1.93 |
| Nmp4−/− VEH | 150.08 ± 8.71 | 121.23 ± 21.47 | 16,982 ± 2858 | 82,378 ± 26,470 | 8.55 ± 0.54 | 1.08 ± 0.31 | 8.86 ± 2.59 |
| WT RAL | 155.49 ± 5.00 | 117.92 ± 5.13 | 16,552 ± 371 | 83,863 ± 19,659 | 8.06 ± 0.75 | 1.06 ± 0.12 | 9.15 ± 1.86 |
| Nmp4−/− RAL | 159.48 ± 13.63 | 127.00 ± 22.37 | 16,999 ± 2556 | 75,610 ± 19,939 | 8.24 ± 1.13 | 1.20 ± 0.35 | 8.95 ± 2.10 |
| WT PTH | 152.07 ± 4.20 | 97.09 ± 15.56 | 16,881 ± 3008 | 90,210 ± 29,366 | 7.57 ± 1.23 | 0.85 ± 0.28 | 9.46 ± 2.63 |
| Nmp4−/− PTH | 157.94 ± 5.37 | 97.77 ± 9.56 | 15,021 ± 1932 | 91,519 ± 26,401 | 7.93 ± 0.30 | 0.82 ± 0.23 | 10.31 ± 2.28 |
| WT PTH + RAL | 166.39 ± 3.78 | 102.76 ± 18.16 | 15,452 ± 34324 | 69,998 ± 19,460 | 8.44 ± 0.38 | 0.89 ± 0.32 | 8.16 ± 1.90 |
| Nmp4−/− PTH + RAL | 173.39 ± 10.84 | 114.52 ± 13.66 | 15,321 ± 708 | 63,635 ± 16,321 | 8.64 ± 1.06 | 0.99 ± 0.20 | 8.09 ± 1.91 |
| Two-way ANOVA genotype | G: P = 0.0012 | G: P = 0.0109 | G: P = 0.7849 | G: P = 0.1310 | G: P = 0.0559 | G: P = 0.1304 | G: P = 0.8913 |
| Nmp4−/−: 160.22a | Nmp4−/−: 115.13a | ||||||
| WT: 153.77b | WT: 105.87b | ||||||
| Two-way ANOVA treatment | T: P < 0.0001 | T: P < 0.0001 | T: P = 0.2740 | T: P = 0.0016 | T: P = 0.0318 | T: P = 0.0031 | T: P = 0.0553 |
| P + R: 169.89a | R: 122.46a | P: A 90864a | P + R: 8.54a | R: 1.13a | |||
| R: 157.48b | V: 113.48a,b | V: A 90704a | V: 8.21a,b | V: 1.01a,b | |||
| P: 155.00b | P + R: 108.64b,c | R: 79737a,b | R: 8.15a,b | P + R: 0.94a,b | |||
| V: 145.60c | P: 97.43c | P + R: 66816b | P: 7.55b | P: 0.84c | |||
| Two-way ANOVA G × T | G × T: P = 0.8141 | G × T: P = 0.5067 | G × T: P = 0.2220 | G × T: P = 0.6425 | G × T: P = 0.7598 | G × T: P = 0.6545 | G × T: P = 0.7901 |
Data represent average ± SD; n = 7–14 mice/group. Nmp4, nuclear matrix protein 4; WT, wild type; V/VEH, vehicle; R/RAL, raloxifene; P/PTH, parathyroid hormone; P/PTH + R/RAL, and parathyroid hormone + raloxifene; BV/TV, bone volume per total volume. Statistical analyses were performed using two-way ANOVA tests setting genotype (G) and treatment (T) as the independent variables. Statistical significance was set at P ≤ 0.05. The statistical results list the cohorts by genotype, treatment, and genotype × treatment.
a b cCohorts not connected by the same letter are statistically different; the average value of the specific parameter precedes the letter.
Finally, total strain, elastic modulus, and resilience were all differentially responsive to the various therapies (treatment P < 0.05; Table 4).
Loss of Nmp4 also altered the structural properties of the femur. Yield force was significantly increased in the null bone (genotype P = 0.004; Table 5), and the increase in ultimate force neared significance (genotype P = 0.07). Total displacement, the total amount of deformation the bone undergoes before failure, was significantly lowered in the Nmp4−/− femurs (genotype P = 0.04; Table 5), and the decrease in post yield displacement, the amount of deformation that occurs after the yield point, approached significance (P = 0.06; Table 5). Finally, work-to-yield, the energy that goes in to deforming the sample, was significantly higher in the Nmp4−/− bone (genotype P = 0.03; Table 5). These results show that the Nmp4−/− osteoblast produces more matrix than WT cells and that the composition of the secretome results in improved bone material and structural properties.
Table 5.
Estimated femoral structural mechanical properties from WT and Nmp4−/− mice treated with vehicle, raloxifene, parathyroid hormone, and parathyroid hormone + raloxifene
| Yield Force, N | Ultimate Force, N | Displacement to yield, µm | Postyield Displacement, µm | Total Displacement, µm | |
|---|---|---|---|---|---|
| Group | |||||
| WT VEH | 10.08 ± 0.99 | 13.52 ± 1.16 | 162.51 ± 12.49 | 843.05 ± 320.03 | 1,005.56 ± 312.72 |
| Nmp4−/− VEH | 11.65 ± 2.44 | 14.27 ± 0.92 | 172.64 ± 26.99 | 619.27 ± 224.97 | 792.43 ± 217.81 |
| WT RAL | 11.19 ± 0.20 | 14.67 ± 0.41 | 168.22 ± 12.96 | 689.72 ± 208.95 | 861.14 ± 199.79 |
| Nmp4−/− RAL | 15.22 ± 1.20 | 15.22 ± 1.20 | 173.53 ± 15.73 | 594.32 ± 202.51 | 769.68 ± 193.22 |
| WT PTH | 11.04 ± 2.06 | 16.94 ± 1.67 | 163.51 ± 28.73 | 711.57 ± 297.33 | 875.08 ± 288.88 |
| Nmp4−/− PTH | 11.24 ± 1.79 | 17.06 ± 1.38 | 146.49 ± 18.81 | 742 ± 255.98 | 894.10 ± 252.89 |
| WT PTH + RAL | 11.50 ± 1.18 | 18.38 ± 2.31 | 152.56 ± 34.07 | 540.96 ± 205.28 | 693.53 ± 202.12 |
| Nmp4−/− PTH + RAL | 13.66 ± 2.51 | 19.43 ± 2.18 | 152.09 ± 8.08 | 458.30 ± 166.36 | 578.77 ± 109.62 |
| Two-way ANOVA genotype | G: P = 0.0037 | G: P = 0.0680 | G: P = 0.9119 | G: P = 0.0610 | G: P = 0.0379 |
| Nmp4−/−: 12.22a | WT: 858.83a | ||||
| WT: 10.95b | Nmp4-/−: 758.74b | ||||
| Two-way ANOVA treatment | T: P = 0.0243 | T: P < 0.0001 | T: P = 0.0137 | T: P = 0.0028 | T: P = 0.0005 |
| P + R: 12.58a | P + R: 18.91a | R: 170.87a | V: 731.16a | V: 898.99a | |
| R: 11.76a,b | P: 17.00b | V: 167.57a,b | P: 726.78a | P: 884.59a | |
| P: 11.14a,b | R: 14.95c | P: 155.00a,b | R: 642.02a,b | R: 815.4a | |
| V: 10.87b | V: 13.90c | P + R: 152.33b | P + R: 499.64b | P + R: 636.15b | |
| Two-way ANOVA G × T | G × T: P = 0.4051 | G × T: P = 0.7791 | G × T: P = 0.1830 | G × T: P = 0.3475 | G × T: P = 0.3910 |
| Stiffness, N/mm | Work to Yield, mJ | Post Yield Work, mJ | Total Work, mJ | ||
| Group | |||||
| WT VEH | 95.73 ± 6.19 | 0.92 ± 0.16 | 8.01 ± 2.16 | 8.93 ± 2.05 | |
| Nmp4−/− VEH | 104.39 ± 10.17 | 1.05 ± 0.31 | 7.53 ± 2.63 | 8.64 ± 2.63 | |
| WT RAL | 106.07 ± 12.65 | 1.05 ± 0.08 | 8.10 ± 1.91 | 9.15 ± 1.90 | |
| Nmp4−/− RAL | 105.42 ± 16.10 | 1.19 ± 0.34 | 7.63 ± 2.14 | 8.91 ± 2.17 | |
| WT PTH | 120.02 ± 22.68 | 0.94 ± 0.32 | 9.44 ± 2.92 | 10.38 ± 2.76 | |
| Nmp4−/− PTH | 121.91 ± 16.51 | 0.89 ± 0.23 | 10.24 ± 2.81 | 11.18 ± 2.74 | |
| WT PTH + RAL | 130.49 ± 10.66 | 0.92 ± 0.29 | 7.98 ± 1.97 | 8.97 ± 1.85 | |
| Nmp4−/− PTH + RAL | 132.83 ± 17.91 | 1.25 ± 0.45 | 7.69 ± 2.61 | 8.94 ± 2.45 | |
| Two-way ANOVA genotype | G: P = 0.3316 | G: P = 0.0315 | G: P = 0.8291 | G: P = 0.8989 | |
| Nmp4-/−: 1.10a | |||||
| WT: 0.96b | |||||
| Two-way ANOVA treatment | T: P < 0.0001 | T: P = 0.0985 | T: P = 0.0103 | T: P = 0.0159 | |
| P + R: 131.66a | P: 9.84a | P: 10.78a | |||
| P: 120.97a | R: 7.87b | R: 9.a,b | |||
| R: 105.74b | P + R: 7.83b | P + R: 8.95b | |||
| V: 100.06b | V: 7.77b | V: 8.79b | |||
| Two-way ANOVA G × T | G × T: P = 0.7575 | G × T: P = 0.2168 | G × T: P = 0.7762 | G × T: P = 0.8461 |
Data represent average ± SD; n = 7–14 mice/group. Nmp4, nuclear matrix protein 4; WT, wild type; V/VEH, vehicle; R/RAL, raloxifene; P/PTH, parathyroid hormone; P/PTH + R/RAL, and parathyroid hormone + raloxifene; BV/TV, bone volume per total volume. Statistical analyses were performed using two-way ANOVA tests setting genotype (G) and treatment (T) as the independent variables. Statistical significance was set at P ≤ 0.05. The statistical results list the cohorts by genotype, treatment, and genotype × treatment.
a b cCohorts not connected by the same letter are statistically different; the average value of the specific parameter precedes the letter.
DISCUSSION
We investigated the mechanisms underlying the hyperanabolic phenotype of Nmp4−/− MSPCs during osteogenesis. Transcriptomic data predicted that Nmp4−/− osteogenic cells have 1) a significantly increased capacity for metabolic conversion to glycolysis, 2) increased Col1a1 mRNA translation, 3) elevated collagen secretion, and 4) elaborate a matrix that improves bone material and structural mechanical properties. In each case, we were able to anchor the predicted phenotype with experimental results. Moreover, the derived mechanistic insights on Nmp4 control of bone cell phenotype were remarkably consistent between the multiple model systems used in this investigation including MSPCs, MC3T3-E1 osteoblast-like cells, and the in vivo bone studies. Nevertheless, additional model systems are required to explore Nmp4 control of bone phenotype, e.g., conditional knockout mice and CRISPR cell lines, and we are currently developing these reagents.
The Nmp4−/− MSPCs have a significantly increased capacity for metabolic conversion to glycolysis, which is a key driver of osteoblast anabolism. Our ChIP-seq and RNA-seq data show that Nmp4 directly targets and regulates key genes that direct the cell toward aerobic glycolysis including Pdk1 and Pkm. Glucose is a key nutrient for osteoblasts, and aerobic glycolysis is the dominant mode of glucose utilization in these cells (49, 53). Thus, while it is a less efficient source of energy, glycolysis can generate both anabolic growth intermediates and ATP very rapidly owing to the much higher speed of glycolysis reactions (97). In vivo, PTH-induced bone anabolism is driven in part by the hormone-mobilizing osteoblast autocrine IGF-1 signaling. This activates mTORC2, which suppresses glucose entry into the TCA cycle and shunts it into the aerobic glycolysis pathway (28). Concomitantly, PTH downregulates Sost/sclerostin expression in osteocytes, unleashing the anabolic WNT signaling pathway (98), driving osteogenesis in part by further stimulating glycolysis via the rapid increase in GLUT1 and HK2 protein expression and escalation in LDHA and PDK1 activities thus increasing glucose consumption and driving lactate over acetyl-CoA production from pyruvate (27).
Our transcriptome data identify the HIF-1α and PTEN canonical pathways as significantly sensitive to the status of Nmp4, which is consistent with the glycolytic phenotype of the null cells. Like the IGF-1 and WNT pathways, the HIF-1α and PTEN link osteogenesis and metabolic reprogramming. Stabilization of the transcription factor HIF-1α in Sp7-positive cells in postnatal mice significantly stimulated trabecular bone formation via an increase in the number of osteoblasts and also promoted bone glycolysis via the mRNA upregulation of key glycolytic enzymes including Pdk1, Ldha, and Hk2 (85). Mice expressing the stable-oxygen form of HIF-1α in osteoprogenitors exhibited an expanded pool of these cells and elevated trabecular BV/TV, very reminiscent of the Nmp4−/− skeletal response to PTH (16, 41, 95). Our pathway analysis predicted that PTEN signaling is attenuated in the Nmp4−/− cells. Interestingly, PTEN signaling antagonizes the phosphatidylinositol 3-kinase-Akt-mTORC2-p70s6k pathway and thus decreases the glycolytic rate and favors oxidative phosphorylation (77). Specifically, PTEN decreases the levels of two key enzymes involved in the Warburg effect, PKM2 and PFKFB3 (33). Therefore, conditional loss of Pten in osteoprogenitors led to increased numbers of osteoblasts and expanded bone matrix (35), whereas conditionally disabling Pten in mature osteoblasts enhanced mTOR activity and increased bone mineral density (60). Again, this Pten-deficient bone phenotype is similar to the Nmp4−/− skeleton under PTH challenge.
The Nmp4−/− MSPCs also exhibited an enhanced capacity for oxidative phosphorylation. This is consistent with a recent study showing that nondifferentiated MC3T3-E1 osteoblast-like cells exhibited both spare glycolytic and oxidative capacities (34). Additionally, this study reported that differentiated MC3T3-E1 cells met ATP demand primarily by aerobic glycolysis, whereas nondifferentiated cells generated ATP through oxidative phosphorylation (34). Further studies with our MSPCs are required to elucidate the impact of Nmp4 on metabolic reprogramming during differentiation.
The present study revealed that Nmp4−/− MSPCs exhibited increased Col1a1 mRNA translation attendant with elevated collagen secretion revealing part of the mechanism by which these cells are converted into super-secretors. Collagen comprises over 90% of the bone protein matrix and our results suggest that a large percent of Col1a1 mRNA transcripts are present in the heavy polysomes in Nmp4−/− MSPCs implying a high translation of Col1a1 transcript. This is consistent with our observed increase in collagen protein secretion in these cells. Osteoprogenitor loss of Nmp4 not only redirects metabolic programming toward cellular anabolism, but also elevates gene expression for multiple pathways involved in protein synthesis and delivery, a key step in bone formation (27, 48). Our transcriptomic analysis showed a striking increase in the mRNA expression of several genes that promote protein anabolism during osteoblast differentiation including numerous amino acid transporters, the amino acid synthase Asns and several other genes involved in amino acid synthesis, many tRNA-charging enzymes, and multiple genes driving protein translation initiation as part of the eIF4 and eIF2 pathways. The present data are consistent with our previous study showing that Nmp4−/− MSPCs exhibited significantly elevated ribosome biogenesis, the primary determinant of translational capacity and a key driver of cell growth (114).
Another key finding of the present work is that Nmp4−/− osteoblasts elaborate a matrix that improves bone material and structural mechanical properties. The expression of matrix genes that contribute to material and structural mechanical properties, e.g., collagen, osteocalcin, and SLRPs, was elevated in Nmp4−/− cells, and mechanical analysis of femurs from mice treated with osteoporosis therapies confirmed enhanced improvement in several of these key properties in the Nmp4−/− bone. Enhanced and accelerated mineralization in vitro often does not correlate with positive effects on the skeleton. For example, osteoblasts deficient in the expression of Naca (66), Sox8 (93), or Foxc1 (42) showed an ex vivo accelerated mineralization phenotype; however, animals harboring deficits in any one of these genes exhibited significant defects in bone development, formation, or mineralization (42, 66, 93). Sodium fluoride (NaF) is an osteoanabolic that increases bone mass, but the newly formed bone lacks normal structure and strength (10, 86, 99). Mechanical load is a bone anabolic signal, and the magnitude of the applied strain determines whether the response is adaptive, forming primarily lamellar bone, or injury, producing woven bone (65). Although woven bone forms faster than lamellar bone, it has inferior mechanical and material properties (8, 19). These findings are in stark contrast to our present model in which the precocious and enhanced mineralization of the Nmp4−/− osteoblast directly translates into an improved skeletal response to osteoanabolics (15, 16, 41, 95).
The molecular mechanisms underlying the improved Nmp4−/− bone quality remain to be determined, but the enhanced expression of osteocalcin by pharmacologically induced Nmp4−/− osteogenic cells (15, 16, 95) may improve the quality of the produced bone. Earlier we proposed that noncollagenous proteins act as “glue” at the collagen-mineral interface to resist the separation of the mineralized fibrils and therefore enhance bone toughness (69, 75, 84, 100). Anabolic therapies that induce the formation of osteocalcin/osteopontin-enriched bone may further enhance energy absorption capacity of bone tissue. Therefore, loss of Nmp4 may modestly alter the ratio of collagen to noncollagenous protein matrix composition, which would be enough to improve bone quality. Additionally, the present data suggest that Nmp4−/− matrix is enriched in SLRPs, which govern ECM assembly via regulation of linear/lateral fibril growth by binding to the collagen fibril surface (12, 47, 76). Thus the collagen maturation may be accelerated in Nmp4−/− bone.
The present data demonstrate that Nmp4 acts cell autonomously as a barrier to bone matrix production and mineralization. As an apex regulator of bone cell anabolic output Nmp4 directly and indirectly regulates gene programs that control key stages of matrix production and secretion and the metabolic reprogramming necessary to fuel it (Fig. 13). The Nmp4 transcriptional modus operandi is reminiscent of another apex regulator c-Myc, which like Nmp4 controls the expression of large sets of genes involved in ribosome biogenesis, metabolism, and protein synthesis, representing a cell-type-independent genetic program involved in biomass accumulation (38, 45). Furthermore, whereas c-Myc acts a general amplifier of gene expression (50, 73, 108), Nmp4 appears to act as a general attenuator and suppressor of biomass accrual (16, 114). Both the MSPC RNA-seq and Nmp4 MC3T3-E1 ChIP-seq pathway analyses (16) are consistent with this mechanistic profile. Like c-Myc, Nmp4 may directly govern the expression of master transcriptional regulators of these key networks in addition to broadly engaging some of their downstream target genes. Whether there is a functional relationship between c-Myc and Nmp4 remains to be determined. Nmp4 control of protein translation and movement through the bone cell ER is particularly intriguing since this is a potential promising area for drug target discovery. Several therapeutic strategies and multiple drugs are currently being developed to enhance the adaptive capability of the ER in the service of secretion for numerous diseases including diabetes, cancer, Alzheimer’s disease, and osteoporosis (24, 36, 40, 54, 87, 91, 104). Nmp4 control of metabolic reprogramming may also present therapeutic targets for regulating bone anabolism (48, 49, 106).
There is a critical medical need for understanding the intrinsic barriers to pharmacologically inducing bone formation in the osteoporotic skeleton (21). Investigating these pathways may help to alleviate the current limits to osteoanabolic therapy.
GRANTS
This work was supported, in whole or in part, by Department of Defense Grant PR120563, NIH Grant AR073739 and Indiana University School of Medicine Clinical and Translational Science Institute Biomedical Research Grant (to J. P. Bidwell), and NIH Grants AR-067221 (to J. M. Wallace), AR-062002 (to M. R. Allen), GM-049164 (to R. C. Wek), AR-053237 (to A. G. Robling), and NS-098772 (to N. Brustovetsky).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense, Indiana Clinical and Translational Science Institute, or NIH.
DISCLOSURES
K. R. Stayrook is an employee of Eli Lilly and Company. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
Y.S., E.W., P.J.C., R.C.W., A.L.M., Y.L., A.G.R., N.B., J.H., K.R.S., M.R.A., J.M.W., and J.P.B. conceived and designed research; Y.S., E.W., P.J.C., M.A., J.M., A.K., N.B., J.H., K.J., M.R.A., J.M.W., and J.P.B. performed experiments; Y.S., E.W., P.J.C., M.A., J.M., D.B.B., R.C.W., A.L.M., Y.L., A.G.R., N.B., J.H., K.J., D.V., K.R.S., M.R.A., and J.P.B. analyzed data; Y.S., E.W., P.J.C., M.A., J.M., D.B.B., R.C.W., A.L.M., Y.L., A.G.R., N.B., J.H., K.J., D.V., M.R.A., J.M.W., and J.P.B. interpreted results of experiments; Y.S., E.W., J.M., A.L.M., J.H., J.M.W., and J.P.B. prepared figures; Y.S., R.C.W., J.M.W., and J.P.B. drafted manuscript; Y.S., E.W., P.J.C., M.A., J.M., A.K., D.B.B., R.C.W., A.L.M., Y.L., A.G.R., N.B., J.H., D.V., K.R.S., M.R.A., J.M.W., and J.P.B. edited and revised manuscript; Y.S., E.W., P.J.C., R.C.W., A.L.M., Y.L., A.G.R., N.B., J.H., D.V., K.R.S., J.M.W., and J.P.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Selene Hernandez-Buquer, Jeanette N. McClintick, and Chris Wellbrook for technical assistance.
REFERENCES
- 1.Alford AI, Terkhorn SP, Reddy AB, Hankenson KD. Thrombospondin-2 regulates matrix mineralization in MC3T3-E1 pre-osteoblasts. Bone 46: 464–471, 2010. doi: 10.1016/j.bone.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird TD, Palam LR, Fusakio ME, Willy JA, Davis CM, McClintick JN, Anthony TG, Wek RC. Selective mRNA translation during eIF2 phosphorylation induces expression of IBTKα. Mol Biol Cell 25: 1686–1697, 2014. doi: 10.1091/mbc.e14-02-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandeira L, Lewiecki EM, Bilezikian JP. Romosozumab for the treatment of osteoporosis. Expert Opin Biol Ther 17: 255–263, 2017. doi: 10.1080/14712598.2017.1280455. [DOI] [PubMed] [Google Scholar]
- 4.Bart ZR, Hammond MA, Wallace JM. Multi-scale analysis of bone chemistry, morphology and mechanics in the oim model of osteogenesis imperfecta. Connect Tissue Res 55, Suppl 1: 4–8, 2014. doi: 10.3109/03008207.2014.923860. [DOI] [PubMed] [Google Scholar]
- 5.Bellows CG, Aubin JE, Heersche JN. Initiation and progression of mineralization of bone nodules formed in vitro: the role of alkaline phosphatase and organic phosphate. Bone Miner 14: 27–40, 1991. doi: 10.1016/0169-6009(91)90100-E. [DOI] [PubMed] [Google Scholar]
- 6.Boskey AL. Bone composition: relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep 2: 447, 2013. doi: 10.1038/bonekey.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudin E, Fijalkowski I, Piters E, Van Hul W. The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin Arthritis Rheum 43: 220–240, 2013. doi: 10.1016/j.semarthrit.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr Course Lect 45: 371–386, 1996. [PubMed] [Google Scholar]
- 9.Canalis E, Kranz L, Zanotti S. Nemo-like kinase regulates postnatal skeletal homeostasis. J Cell Physiol 229: 1736–1743, 2014. doi: 10.1002/jcp.24625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter DR, Beaupré GS. Effects of fluoride treatment on bone strength. J Bone Miner Res 5, Suppl 1: S177–S184, 1990. doi: 10.1002/jbmr.5650051372. [DOI] [PubMed] [Google Scholar]
- 11.Charoenpanich A, Wall ME, Tucker CJ, Andrews DM, Lalush DS, Loboa EG. Microarray analysis of human adipose-derived stem cells in three-dimensional collagen culture: osteogenesis inhibits bone morphogenic protein and Wnt signaling pathways, and cyclic tensile strain causes upregulation of proinflammatory cytokine regulators and angiogenic factors. Tissue Eng Part A 17: 2615–2627, 2011. doi: 10.1089/ten.tea.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen S, Birk DE. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J 280: 2120–2137, 2013. doi: 10.1111/febs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevalier Y, Quek E, Borah B, Gross G, Stewart J, Lang T, Zysset P. Biomechanical effects of teriparatide in women with osteoporosis treated previously with alendronate and risedronate: results from quantitative computed tomography-based finite element analysis of the vertebral body. Bone 46: 41–48, 2010. doi: 10.1016/j.bone.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Chiellini C, Cochet O, Negroni L, Samson M, Poggi M, Ailhaud G, Alessi MC, Dani C, Amri EZ. Characterization of human mesenchymal stem cell secretome at early steps of adipocyte and osteoblast differentiation. BMC Mol Biol 9: 26, 2008. doi: 10.1186/1471-2199-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Childress P, Philip BK, Robling AG, Bruzzaniti A, Kacena MA, Bivi N, Plotkin LI, Heller A, Bidwell JP. Nmp4/CIZ suppresses the response of bone to anabolic parathyroid hormone by regulating both osteoblasts and osteoclasts. Calcif Tissue Int 89: 74–89, 2011. doi: 10.1007/s00223-011-9496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Childress P, Stayrook KR, Alvarez MB, Wang Z, Shao Y, Hernandez-Buquer S, Mack JK, Grese ZR, He Y, Horan D, Pavalko FM, Warden SJ, Robling AG, Yang FC, Allen MR, Krishnan V, Liu Y, Bidwell JP. Genome-wide mapping and interrogation of the nmp4 antianabolic bone axis. Mol Endocrinol 29: 1269–1285, 2015. doi: 10.1210/me.2014-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi YA, Lim J, Kim KM, Acharya B, Cho JY, Bae YC, Shin HI, Kim SY, Park EK. Secretome analysis of human BMSCs and identification of SMOC1 as an important ECM protein in osteoblast differentiation. J Proteome Res 9: 2946–2956, 2010. doi: 10.1021/pr901110q. [DOI] [PubMed] [Google Scholar]
- 18.Cipriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res 27: 2419–2428, 2012. [Erratum in J Bone Miner Res 28: 431, 2013]. doi: 10.1002/jbmr.1800. [DOI] [PubMed] [Google Scholar]
- 19.Currey JD. Mechanical properties of vertebrate hard tissues. Proc Inst Mech Eng H 212: 399–411, 1998. doi: 10.1243/0954411981534178. [DOI] [PubMed] [Google Scholar]
- 20.Dalton LE, Healey E, Irving J, Marciniak SJ. Phosphoproteins in stress-induced disease. Prog Mol Biol Transl Sci 106: 189–221, 2012. doi: 10.1016/B978-0-12-396456-4.00003-1. [DOI] [PubMed] [Google Scholar]
- 21.Dede AD, Makras P, Anastasilakis AD. Investigational anabolic agents for the treatment of osteoporosis: an update on recent developments. Expert Opin Investig Drugs 26: 1137–1144, 2017. doi: 10.1080/13543784.2017.1371136. [DOI] [PubMed] [Google Scholar]
- 22.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Bröer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J 350: 219–227, 2000. doi: 10.1042/bj3500219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doultsinos D, Avril T, Lhomond S, Dejeans N, Guédat P, Chevet E. Control of the unfolded protein response in health and disease. SLAS Discov 22: 787–800, 2017. doi: 10.1177/2472555217701685. [DOI] [PubMed] [Google Scholar]
- 25.Dufey E, Sepúlveda D, Rojas-Rivera D, Hetz C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am J Physiol Cell Physiol 307: C582–C594, 2014. doi: 10.1152/ajpcell.00258.2014. [DOI] [PubMed] [Google Scholar]
- 26.Eriksen EF, Brown JP. Commentary: Concurrent administration of PTH and antiresorptives: additive effects or DXA cosmetics. Bone 86: 139–142, 2016. doi: 10.1016/j.bone.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab 17: 745–755, 2013. doi: 10.1016/j.cmet.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esen E, Lee SY, Wice BM, Long F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J Bone Miner Res 30: 1959–1968, 2015. doi: 10.1002/jbmr.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox J, Miller MA, Newman MK, Turner CH, Recker RR, Smith SY. Treatment of skeletally mature ovariectomized rhesus monkeys with PTH(1-84) for 16 months increases bone formation and density and improves trabecular architecture and biomechanical properties at the lumbar spine. J Bone Miner Res 22: 260–273, 2007. doi: 10.1359/jbmr.061101. [DOI] [PubMed] [Google Scholar]
- 30.Gallacher SJ, Dixon T. Impact of treatments for postmenopausal osteoporosis (bisphosphonates, parathyroid hormone, strontium ranelate, and denosumab) on bone quality: a systematic review. Calcif Tissue Int 87: 469–484, 2010. doi: 10.1007/s00223-010-9420-x. [DOI] [PubMed] [Google Scholar]
- 31.Gallego Romero I, Pai AA, Tung J, Gilad Y. RNA-seq: impact of RNA degradation on transcript quantification. BMC Biol 12: 42, 2014. doi: 10.1186/1741-7007-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamsjaeger S, Buchinger B, Zoehrer R, Phipps R, Klaushofer K, Paschalis EP. Effects of one year daily teriparatide treatment on trabecular bone material properties in postmenopausal osteoporotic women previously treated with alendronate or risedronate. Bone 49: 1160–1165, 2011. doi: 10.1016/j.bone.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Cao I, Song MS, Hobbs RM, Laurent G, Giorgi C, de Boer VC, Anastasiou D, Ito K, Sasaki AT, Rameh L, Carracedo A, Vander Heiden MG, Cantley LC, Pinton P, Haigis MC, Pandolfi PP. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 149: 49–62, 2012. doi: 10.1016/j.cell.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guntur AR, Gerencser AA, Le PT, DeMambro VE, Bornstein SA, Mookerjee SA, Maridas DE, Clemmons DE, Brand MD, Rosen CJ. Osteoblast-like MC3T3-E1 cells prefer glycolysis for ATP production but adipocyte-like 3T3-L1 cells prefer oxidative phosphorylation. J Bone Miner Res 33: 1052–1065, 2018. doi: 10.1002/jbmr.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guntur AR, Reinhold MI, Cuellar J Jr, Naski MC. Conditional ablation of Pten in osteoprogenitors stimulates FGF signaling. Development 138: 1433–1444, 2011. doi: 10.1242/dev.058016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamamura K, Tanjung N, Yokota H. Suppression of osteoclastogenesis through phosphorylation of eukaryotic translation initiation factor 2 alpha. J Bone Miner Metab 31: 618–628, 2013. doi: 10.1007/s00774-013-0450-0. [DOI] [PubMed] [Google Scholar]
- 37.Han HS, Ju F, Geng S. In vivo and in vitro effects of PTH1-34 on osteogenic and adipogenic differentiation of human bone marrow-derived mesenchymal stem cells through regulating microRNA-155. J Cell Biochem, 119: 3220–3235, 2018. doi: 10.1002/jcb.26478. [DOI] [PubMed] [Google Scholar]
- 38.Hann SR. MYC cofactors: molecular switches controlling diverse biological outcomes. Cold Spring Harb Perspect Med 4: a014399, 2014. doi: 10.1101/cshperspect.a014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawkins KE, Joy S, Delhove JM, Kotiadis VN, Fernandez E, Fitzpatrick LM, Whiteford JR, King PJ, Bolanos JP, Duchen MR, Waddington SN, McKay TR. NRF2 orchestrates the metabolic shift during induced pluripotent stem cell reprogramming. Cell Reports 14: 1883–1891, 2016. doi: 10.1016/j.celrep.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He L, Lee J, Jang JH, Sakchaisri K, Hwang J, Cha-Molstad HJ, Kim KA, Ryoo IJ, Lee HG, Kim SO, Soung NK, Lee KS, Kwon YT, Erikson RL, Ahn JS, Kim BY. Osteoporosis regulation by salubrinal through eIF2α mediated differentiation of osteoclast and osteoblast. Cell Signal 25: 552–560, 2013. doi: 10.1016/j.cellsig.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Y, Childress P, Hood M Jr, Alvarez M, Kacena MA, Hanlon M, McKee B, Bidwell JP, Yang FC. Nmp4/CIZ suppresses the parathyroid hormone anabolic window by restricting mesenchymal stem cell and osteoprogenitor frequency. Stem Cells Dev 22: 492–500, 2013. doi: 10.1089/scd.2012.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopkins A, Mirzayans F, Berry F. Foxc1 expression in early osteogenic differentiation is regulated by BMP4-SMAD activity. J Cell Biochem 117: 1707–1717, 2016. doi: 10.1002/jcb.25464. [DOI] [PubMed] [Google Scholar]
- 43.Huesa C, Yadav MC, Finnilä MA, Goodyear SR, Robins SP, Tanner KE, Aspden RM, Millán JL, Farquharson C. PHOSPHO1 is essential for mechanically competent mineralization and the avoidance of spontaneous fractures. Bone 48: 1066–1074, 2011. doi: 10.1016/j.bone.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol 23: 1379–1389, 2003. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji H, Wu G, Zhan X, Nolan A, Koh C, De Marzo A, Doan HM, Fan J, Cheadle C, Fallahi M, Cleveland JL, Dang CV, Zeller KI. Cell-type independent MYC target genes reveal a primordial signature involved in biomass accumulation. PLoS One 6: e26057, 2011. doi: 10.1371/journal.pone.0026057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jilka RL. Inhibiting the inhibitor: a new route to bone anabolism. J Bone Miner Res 24: 575–577, 2009. doi: 10.1359/jbmr.090228. [DOI] [PubMed] [Google Scholar]
- 47.Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol 29: 248–253, 2010. doi: 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Karner CM, Esen E, Okunade AL, Patterson BW, Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Invest 125: 551–562, 2015. doi: 10.1172/JCI78470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karner CM, Long F. Wnt signaling and cellular metabolism in osteoblasts. Cell Mol Life Sci 74: 1649–1657, 2017. doi: 10.1007/s00018-016-2425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kress TR, Sabò A, Amati B. MYC: connecting selective transcriptional control to global RNA production. Nat Rev Cancer 15: 593–607, 2015. doi: 10.1038/nrc3984. [DOI] [PubMed] [Google Scholar]
- 51.Kristensen LP, Chen L, Nielsen MO, Qanie DW, Kratchmarova I, Kassem M, Andersen JS. Temporal profiling and pulsed SILAC labeling identify novel secreted proteins during ex vivo osteoblast differentiation of human stromal stem cells. Mol Cell Proteomics 11: 989–1007, 2012. doi: 10.1074/mcp.M111.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leder BZ. Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr Osteoporos Rep 15: 110–119, 2017. doi: 10.1007/s11914-017-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee WC, Guntur AR, Long F, Rosen CJ. Energy metabolism of the osteoblast: implications for osteoporosis. Endocr Rev 38: 255–266, 2017. doi: 10.1210/er.2017-00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, Yang S, Li X, Liu D, Wang Z, Guo J, Tan N, Gao Z, Zhao X, Zhang J, Gou F, Yokota H, Zhang P. Role of endoplasmic reticulum stress in disuse osteoporosis. Bone 97: 2–14, 2017. doi: 10.1016/j.bone.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Li L, Mao J, Sun L, Liu W, Wu D. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J Biol Chem 277: 5977–5981, 2002. doi: 10.1074/jbc.M111131200. [DOI] [PubMed] [Google Scholar]
- 56.Li Q, Gao Z, Chen Y, Guan MX. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell 8: 439–445, 2017. doi: 10.1007/s13238-017-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, Harris SE, Rowe DW, Ke HZ, Wu D. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet 37: 945–952, 2005. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 58.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 59.Lindsay R, Krege JH, Marin F, Jin L, Stepan JJ. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int 27: 2395–2410, 2016. doi: 10.1007/s00198-016-3534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X, Bruxvoort KJ, Zylstra CR, Liu J, Cichowski R, Faugere MC, Bouxsein ML, Wan C, Williams BO, Clemens TL. Lifelong accumulation of bone in mice lacking Pten in osteoblasts. Proc Natl Acad Sci USA 104: 2259–2264, 2007. doi: 10.1073/pnas.0604153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lovato C, Lewiecki EM. Emerging anabolic agents in the treatment of osteoporosis. Expert Opin Emerg Drugs 22: 247–257, 2017. doi: 10.1080/14728214.2017.1362389. [DOI] [PubMed] [Google Scholar]
- 62.Luppen CA, Leclerc N, Noh T, Barski A, Khokhar A, Boskey AL, Smith E, Frenkel B. Brief bone morphogenetic protein 2 treatment of glucocorticoid-inhibited MC3T3-E1 osteoblasts rescues commitment-associated cell cycle and mineralization without alteration of Runx2. J Biol Chem 278: 44995–45003, 2003. doi: 10.1074/jbc.M306730200. [DOI] [PubMed] [Google Scholar]
- 63.Ma YL, Bryant HU, Zeng Q, Schmidt A, Hoover J, Cole HW, Yao W, Jee WS, Sato M. New bone formation with teriparatide [human parathyroid hormone-(1-34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology 144: 2008–2015, 2003. doi: 10.1210/en.2002-221061. [DOI] [PubMed] [Google Scholar]
- 64.Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene 302: 179–183, 2003. doi: 10.1016/S0378-1119(02)01106-X. [DOI] [PubMed] [Google Scholar]
- 65.McBride SH, Silva MJ. Adaptive and injury response of bone to mechanical loading. Bonekey Osteovision 1: 192, 2012. doi: 10.1038/bonekey.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meury T, Akhouayri O, Jafarov T, Mandic V, St-Arnaud R. Nuclear alpha NAC influences bone matrix mineralization and osteoblast maturation in vivo. Mol Cell Biol 30: 43–53, 2010. doi: 10.1128/MCB.00378-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Millán JL. The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int 93: 299–306, 2013. doi: 10.1007/s00223-012-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore DS. Introduction to the Practice of Statistics. New York, NY: W.H. Freeman, 2003. [Google Scholar]
- 69.Morgan S, Poundarik AA, Vashishth D. Do non-collagenous proteins affect skeletal mechanical properties? Calcif Tissue Int 97: 281–291, 2015. doi: 10.1007/s00223-015-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morinobu M, Nakamoto T, Hino K, Tsuji K, Shen ZJ, Nakashima K, Nifuji A, Yamamoto H, Hirai H, Noda M. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med 201: 961–970, 2005. doi: 10.1084/jem.20041097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J, Topisirovic I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 14: 473–480, 2015. doi: 10.4161/15384101.2014.991572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112: 295–307, 2008. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 73.Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, Zhao K, Levens D. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 151: 68–79, 2012. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nifuji A, Ideno H, Ohyama Y, Takanabe R, Araki R, Abe M, Noda M, Shibuya H. Nemo-like kinase (NLK) expression in osteoblastic cells and suppression of osteoblastic differentiation. Exp Cell Res 316: 1127–1136, 2010. doi: 10.1016/j.yexcr.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 75.Nikel O, Laurencin D, McCallum SA, Gundberg CM, Vashishth D. NMR investigation of the role of osteocalcin and osteopontin at the organic-inorganic interface in bone. Langmuir 29: 13873–13882, 2013. doi: 10.1021/la403203w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J Biol Chem 287: 33926–33933, 2012. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ortega-Molina A, Serrano M. PTEN in cancer, metabolism, and aging. Trends Endocrinol Metab 24: 184–189, 2013. doi: 10.1016/j.tem.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143: 420–430, 1990. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 79.Palomäki S, Pietilä M, Laitinen S, Pesälä J, Sormunen R, Lehenkari P, Koivunen P. HIF-1α is upregulated in human mesenchymal stem cells. Stem Cells 31: 1902–1909, 2013. doi: 10.1002/stem.1435. [DOI] [PubMed] [Google Scholar]
- 80.Pandey AC, Semon JA, Kaushal D, O’Sullivan RP, Glowacki J, Gimble JM, Bunnell BA. MicroRNA profiling reveals age-dependent differential expression of nuclear factor κB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res Ther 2: 49, 2011. doi: 10.1186/scrt90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pattappa G, Heywood HK, de Bruijn JD, Lee DA. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J Cell Physiol 226: 2562–2570, 2011. doi: 10.1002/jcp.22605. [DOI] [PubMed] [Google Scholar]
- 82.Pellegrini GG, Cregor M, McAndrews K, Morales CC, McCabe LD, McCabe GP, Peacock M, Burr D, Weaver C, Bellido T. Nrf2 regulates mass accrual and the antioxidant endogenous response in bone differently depending on the sex and age. PLoS One 12: e0171161, 2017. doi: 10.1371/journal.pone.0171161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ponnapakkam T, Katikaneni R, Sakon J, Stratford R, Gensure RC. Treating osteoporosis by targeting parathyroid hormone to bone. Drug Discov Today 19: 204–208, 2014. doi: 10.1016/j.drudis.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM, Vashishth D. Dilatational band formation in bone. Proc Natl Acad Sci USA 109: 19178–19183, 2012. doi: 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, Long F. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc Natl Acad Sci USA 111: 8673–8678, 2014. doi: 10.1073/pnas.1324290111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riggs BL, Hodgson SF, O’Fallon WM, Chao EY, Wahner HW, Muhs JM, Cedel SL, Melton LJ 3rd. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 322: 802–809, 1990. doi: 10.1056/NEJM199003223221203. [DOI] [PubMed] [Google Scholar]
- 87.Rivas A, Vidal RL, Hetz C. Targeting the unfolded protein response for disease intervention. Expert Opin Ther Targets 19: 1203–1218, 2015. doi: 10.1517/14728222.2015.1053869. [DOI] [PubMed] [Google Scholar]
- 88.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11: R25, 2010. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robling AG, Childress P, Yu J, Cotte J, Heller A, Philip BK, Bidwell JP. Nmp4/CIZ suppresses parathyroid hormone-induced increases in trabecular bone. J Cell Physiol 219: 734–743, 2009. doi: 10.1002/jcp.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rozpedek W, Markiewicz L, Diehl JA, Pytel D, Majsterek I. Unfolded protein response and PERK kinase as a new therapeutic target in the pathogenesis of Alzheimer’s disease. Curr Med Chem 22: 3169–3184, 2015. doi: 10.2174/0929867322666150818104254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sànchez-Riera L, Wilson N. Fragility fractures & their impact on older people. Best Pract Res Clin Rheumatol 31: 169–191, 2017. doi: 10.1016/j.berh.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Schmidt K, Schinke T, Haberland M, Priemel M, Schilling AF, Mueldner C, Rueger JM, Sock E, Wegner M, Amling M. The high mobility group transcription factor Sox8 is a negative regulator of osteoblast differentiation. J Cell Biol 168: 899–910, 2005. doi: 10.1083/jcb.200408013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shah AD, Shoback D, Lewiecki EM. Sclerostin inhibition: a novel therapeutic approach in the treatment of osteoporosis. Int J Womens Health 7: 565–580, 2015. doi: 10.2147/IJWH.S73244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shao Y, Hernandez-Buquer S, Childress P, Stayrook KR, Alvarez MB, Davis H, Plotkin LI, He Y, Condon KW, Burr DB, Warden SJ, Robling AG, Yang FC, Wek RC, Allen MR, Bidwell JP. Improving combination osteoporosis therapy in a preclinical model of heightened osteoanabolism. Endocrinology 158: 2722–2740, 2017. doi: 10.1210/en.2017-00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shum LC, White NS, Mills BN, de Mesy Bentley KL, Eliseev RA. Energy metabolism in mesenchymal stem cells during osteogenic differentiation. Stem Cells Dev 25: 114–122, 2016. doi: 10.1089/scd.2015.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shyh-Chang N, Ng HH. The metabolic programming of stem cells. Genes Dev 31: 336–346, 2017. doi: 10.1101/gad.293167.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol 22: 41–50, 2015. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Søgaard CH, Mosekilde L, Richards A, Mosekilde L. Marked decrease in trabecular bone quality after five years of sodium fluoride therapy–assessed by biomechanical testing of iliac crest bone biopsies in osteoporotic patients. Bone 15: 393–399, 1994. doi: 10.1016/8756-3282(94)90815-X. [DOI] [PubMed] [Google Scholar]
- 100.Sroga GE, Karim L, Colón W, Vashishth D. Biochemical characterization of major bone-matrix proteins using nanoscale-size bone samples and proteomics methodology. Mol Cell Proteomics 10: M110.006718, 2011. doi: 10.1074/mcp.M110.006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol 5: a007898, 2013. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan Z, Xie N, Banerjee S, Cui H, Fu M, Thannickal VJ, Liu G. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J Biol Chem 290: 46–55, 2015. doi: 10.1074/jbc.M114.603589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teske BF, Baird TD, Wek RC. Methods for analyzing eIF2 kinases and translational control in the unfolded protein response. Methods Enzymol 490: 333–356, 2011. doi: 10.1016/B978-0-12-385114-7.00019-2. [DOI] [PubMed] [Google Scholar]
- 104.Valenzuela V, Martínez G, Duran-Aniotz C, Hetz C. Gene therapy to target ER stress in brain diseases. Brain Res 1648, Pt B: 561–570, 2016. doi: 10.1016/j.brainres.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 105.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21: 362–369, 2006. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 106.Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, Takarada T, Iezaki T, Pessin JE, Hinoi E, Karsenty G. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell 161: 1576–1591, 2015. [Erratum in Cell 162: 1169, 2015]. doi: 10.1016/j.cell.2015.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, 2009. [Google Scholar]
- 108.Wolf E, Lin CY, Eilers M, Levens DL. Taming of the beast: shaping Myc-dependent amplification. Trends Cell Biol 25: 241–248, 2015. doi: 10.1016/j.tcb.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu X, Estwick SA, Chen S, Yu M, Ming W, Nebesio TD, Li Y, Yuan J, Kapur R, Ingram D, Yoder MC, Yang FC. Neurofibromin plays a critical role in modulating osteoblast differentiation of mesenchymal stem/progenitor cells. Hum Mol Genet 15: 2837–2845, 2006. doi: 10.1093/hmg/ddl208. [DOI] [PubMed] [Google Scholar]
- 110.Xi G, Wai C, DeMambro V, Rosen CJ, Clemmons DR. IGFBP-2 directly stimulates osteoblast differentiation. J Bone Miner Res 29: 2427–2438, 2014. doi: 10.1002/jbmr.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiao L, Fei Y, Hurley MM. FGF2 crosstalk with Wnt signaling in mediating the anabolic action of PTH on bone formation. Bone Rep 9: 136–144, 2018. doi: 10.1016/j.bonr.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yadav MC, Bottini M, Cory E, Bhattacharya K, Kuss P, Narisawa S, Sah RL, Beck L, Fadeel B, Farquharson C, Millán JL. Skeletal Mineralization Deficits and Impaired Biogenesis and Function of Chondrocyte-Derived Matrix Vesicles in Phospho1(−/−) and Phospho1/Pi t1 Double-Knockout Mice. J Bone Miner Res 31: 1275–1286, 2016. doi: 10.1002/jbmr.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yadav MC, Simão AM, Narisawa S, Huesa C, McKee MD, Farquharson C, Millán JL. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res 26: 286–297, 2011. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Young SK, Shao Y, Bidwell JP, Wek RC. Nuclear matrix protein 4 is a novel regulator of ribosome biogenesis and controls the unfolded protein response via repression of Gadd34 expression. J Biol Chem 291: 13780–13788, 2016. doi: 10.1074/jbc.M116.729830. [DOI] [PMC free article] [PubMed] [Google Scholar]