Early environmental experiences exert a profound influence on brain development, with lasting effects on emotion, cognition, and behavior throughout the lifespan. However, knowledge of how environmental experiences become embedded biologically to shape neurocognitive development in humans remains remarkably limited. In this issue of Biological Psychiatry, Dunn et al. (1) test several accounts of how adversity influences peripheral DNA methylation patterns from birth through middle childhood. This work is innovative in empirically evaluating different conceptual models of adversity effects with longitudinal data on multiple forms of adversity. The findings raise critical questions regarding the mechanisms that underlie experience-driven differences in neurodevelopment. We contextualize their approach within a conceptual framework that considers underlying neuro-plasticity mechanisms and the nature of adversity experiences to inform future research on experience-related development.
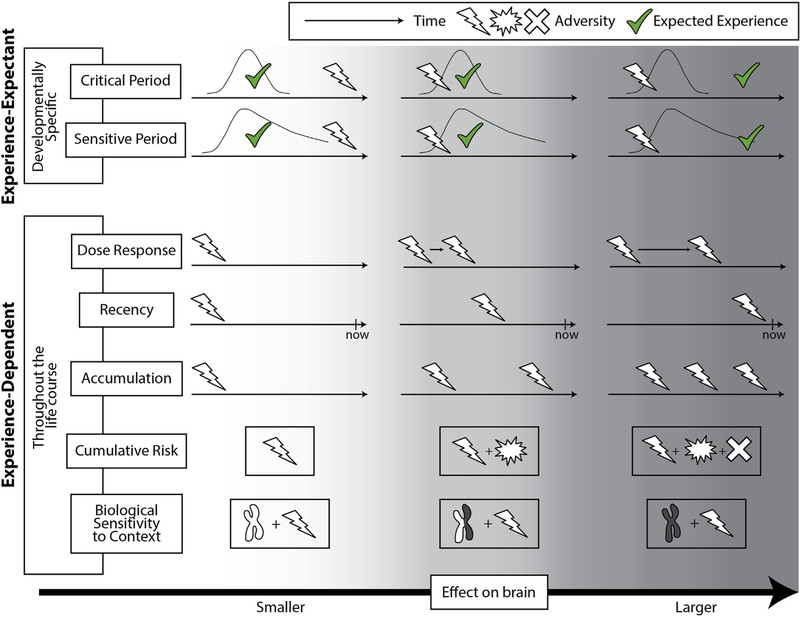
Numerous conceptual models have been proposed to describe how adverse experiences influence neurodevelopment (Figure 1). However, few studies have directly compared candidate models to determine empirically which model best accounts for the observed effects of experience. Dunn et al. (1) do so by comparing a recency model (in which recent experiences are most influential), an accumulation model (in which experiential effects increase as the number of occurrences increases), and a sensitive period model (in which experiences in specific developmental periods are most influential) to determine which account best explains observed associations between multiple forms of adversity and DNA methylation patterns.
Figure 1.
Conceptual models of environmental influence on neurodevelopment. Numerous models explain how adverse environmental experiences influence brain development. These models differ in their underlying neurobiological mechanisms, involving either experience-expectant or experience-dependent processes. Experience-expectant mechanisms are specific to development. They reflect neural preparation by specific brain substrates to encode particular types of expected experience (green checkmark) during specific windows of heightened neuroplasticity (developmental changes in neuroplasticity levels shown as curves). Critical and sensitive period models rely on experience-expectant neuroplasticity, either solely within one window (critical periods) or with some residual malleability thereafter (sensitive periods). Although most adversities do not represent expected experiences that constitute critical/sensitive period substrates, they may still influence these processes, depending on their timing. (Left panels) Adverse experiences that happen after a critical/sensitive period is completed have relatively little impact on that neural substrate. (Middle panels) Adverse experience occurring before or during critical/sensitive periods may alter their progression (e.g., accelerate or truncate the period). (Right panels) Adversities that involve the deprivation or delay of expected experience directly impact critical/sensitive period encoding of that experience. In contrast, experience-dependent mechanisms reflect neural learning in response to individual experiences. Experience-dependent mechanisms are available throughout life, though the degree of neuroplasticity associated with these mechanisms can vary across development. Dose-response, recency, and accumulation models prioritize different dimensions of experience in terms of quantity and timing. Dose-response models apply to contiguous, graded exposures like environmental toxins and predict that adversity effects scale continuously with the degree or duration of exposure. The recency model tested by Dunn et al. (1) posits that adverse events closest to the present time have the greatest effect on neurodevelopment. The accumulation model they tested posits that the effect of an adversity varies with the number of occurrences over a given window. Alternatively, the cumulative risk model predicts that the effect of experience varies as a function of the number of unique adversities experienced (regardless of timing), rather than the number of occurrences of a single adversity. Biological sensitivity to context models expect the effects of adversity to differ as a function of individual traits like physiology and (epi)genetics (e.g., genetic polymorphisms). No single conceptual model likely accounts for the entire range of complex effects of adversity on neurodevelopment.
Additional models of how adversity influences neuro-development have been proposed. These include the cumulative risk model (in which experiential effects increase with the number of distinct adversities) (2) and the dose-response model (in which changes in the degree of exposure modulate development) (3). Other models focus on how experiential effects vary as a function of individual-level traits, including genotype, temperament, or physiological reactivity (i.e., biological sensitivity to context) (4) or the type of experience (in which effects differ according to the nature of adversity experienced) (5). It is unlikely that any single model will adequately account for the widespread associations of adversity exposure with neurodevelopment.
Each of these models makes assumptions not only about the most relevant features of environmental experience but also about the underlying neurobiological mechanisms. These models differentially rely on experience-expectant versus experience-dependent neuroplasticity mechanisms (6). Experience-expectant mechanisms reflect neural preparation to biologically encode particular environmental stimuli during specific developmental windows. These mechanisms encompass triggers such as maturing inhibitory circuitry, rewiring processes such as extensive pruning of overabundant synapses, and brakes such as perineuronal net formation to actively dampen further neuroplasticity (7). Sensitive periods are governed by these experience-expectant mechanisms, with some residual malleability thereafter.
In contrast, experience-dependent mechanisms facilitate learning at all points in development (6). They co-occur with experience-expectant processes but have no ontogenetic constraints and include changes induced by experience (without previous preparation) such as synaptogenesis, synaptic strength modulation, and synaptic pruning. Experience-dependent mechanisms underlie neurodevelopmental effects of adversity in accumulation, recency, cumulative risk, duration, and adversity type models.
Dunn et al. (1) conclude that a sensitive period model best fits most DNA methylation sites associated with adversity, with experiences occurring before 3 years of age having disproportionate influences on methylation. These findings suggest that the timing of exposure may be the most relevant consideration for DNA methylation. This raises questions about the neurobiological mechanisms that might explain such a pattern. We consider this finding within the context of neurobiological features of sensitive periods.
Sensitive periods have multiple characteristics that distinguish them from experience-dependent processes (7). First, they encompass periods of heightened neuroplasticity that involve substantial, rapid changes to neural circuitry. Second, sensitive periods enable tuning the brain’s responsiveness to specific types of environmental inputs, after which additional tuning is relatively diminished and requires extensive exposure. Third, they occur for specific brain circuits only during specific windows of development, although their timing is itself malleable (e.g., in the context of adversity) (7). Fourth, sensitive periods are constrained by the development of molecular and structural regulators that protect the experience-modified circuitry and produce enduring effects on brain function. Probing sensitive period processes in human neurodevelopment requires attention to each of these elements.
First, testing sensitive period models requires precisely timed measurements. Repeated, prospective measurements at the correct developmental moments are necessary to capture the rise and fall of sensitive period neuroplasticity. Dunn et al. (1) report several methylation patterns with specific developmental timing effects characteristic of sensitive periods. For example, abuse influenced methylation patterns only in middle childhood. A hallmark of sensitive periods is that their developmental effects persist to influence mature function (7). With measurements ending in childhood, it is too early to tell if these effects endure, but further research may shed light on the long-term stability of these methylation changes.
It is also important to consider the neural circuits targeted in a sensitive period model because sensitive periods have highly specific neural substrates. DNA methylation is an established mechanism by which experiences alter neural circuits during sensitive periods (7). Peripheral measurements of experiential effects as reported by Dunn et al. (1) are more challenging to interpret. Low correlations were observed between the peripheral methylation patterns and those established within several brain regions (1). The methylated genes’ functions were also largely nonspecific to neurodevelopmental processes. Therefore, the neural substrate of these methylation effects remains unclear. An alternative interpretation is that peripheral DNA methylation itself may undergo analogous sensitive period–like phenomena, raising the question of which biological mechanisms could explain such developmentally delimited sensitivity to experiences for DNA methylation. Given the current evidence, this account remains only an intriguing possibility. A final potential interpretation is that these patterns reflect developmental variation in experience-dependent mechanisms rather than a sensitive period.
Lastly, challenges in measuring human experience influence the feasibility of testing sensitive period models. It can be difficult to determine the precise timing of exposure (or lack thereof) to specific experiences in normative human development. Moreover, duration and timing of experiences are often confounded. Several experiences tested by Dunn et al. (1) had this limitation (e.g., neighborhood disadvantage). Whether these effects reflect sensitive period or dose models remains to be determined. Furthermore, the type of experience should constitute a plausible class of expected environmental stimuli for sensitive period models. In this context, assessing complex experiences of adversity in relation to sensitive periods requires additional considerations.
Sensitive periods are inherently specific to types of experience at specific times. Knowledge of social–emotional sensitive periods has come largely from studies of extreme psychosocial deprivation with precise timing, such as children raised without a primary caregiver early in life (i.e., institutional rearing) who are later adopted into families (8,9). Here, the absence of expected inputs during specific developmental periods has revealed sensitive periods underlying language, attachment, and hypothalamic-pituitary-adrenal axis regulation, among others (5,8,9). Dunn et al.’s study (1) is notable for measuring numerous adversity types, from physical, sexual, and emotional abuse to single-parent household and financial stress, with relatively precise measurements of developmental timing.
However, many of these experiences are challenging to align with a sensitive period model as substrates. Adversity exposures are diverse in their experiential components, with some reflecting threat (e.g., abuse), some reflecting markers of social–cognitive or material deprivation (e.g., neglect, financial hardship), and many reflecting heterogeneous experiences (e.g., neighborhood disadvantage). Evidence suggests that these different dimensions of adversity have distinct influences on neurodevelopment (5). While experiences of deprivation can readily be aligned with a sensitive period model, most exposures are less straightforward. A sensitive period for trauma would require that the brain “expects” to experience threat at particular developmental periods.
Another complication when applying sensitive period models to adversity experiences is that adversity exposure alters sensitive period processes (7,9). In humans, this has been demonstrated most clearly for pubertal timing. Exposure to trauma, but not deprivation, has consistently been shown to accelerate the onset of puberty, particularly in girls (10). Across species, caregiver deprivation appears to accelerate the maturation of neural circuits underlying emotional processing and regulation, perhaps adaptive for children without a care-giver to facilitate regulation (9). In the rodent, these effects are driven by changes in the molecular triggers and brakes regulating sensitive periods (e.g., gamma-aminobutyric acidergic maturation) (9). Therefore, exposure to adversity may serve as a modulator of sensitive period mechanisms rather than as an experiential substrate driving sensitive period neuroplasticity.
A key question for the field is to determine whether a sensitive period model can be applied to all forms of adversity. Whereas a sensitive period model applies clearly to experiences of deprivation involving the absence of an expected input, it is difficult to reconcile other forms of adversity involving unexpected, atypical, or heterogeneous environmental inputs (e.g., trauma, socioeconomic disadvantage) with the nature of sensitive period substrates. Instead, developmental differences in the effects of these adversity types may be better explained by models that rely on experience-dependent mechanisms or that consider adversity as a modulator of sensitive period progression for expected experiences. Much remains to be learned about the complex mechanisms through which adversity becomes biologically embedded and how these mechanisms vary across development.
Acknowledgments and Disclosures
This work was supported by the Autism Science Foundation and the Rett Syndrome Research Foundation (to LJG-D) and National Institutes of Health Grant Nos. R01MH103291 and R01MH106482, an Early Career Research Fellowship from the Jacobs Foundation, and the International Mental Health Research Organization Rising Star Award (to KAM).
We thank Nessa Bryce for her assistance with figure construction.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Dunn EC, Soare TW, Zhu Y, Simpkin AJ, Suderman MJ, Klengel T, et al. (2019): Sensitive periods for the effect of childhood adversity on DNA methylation: Results from a prospective, longitudinal study. Biol Psychiatry 85:838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans GW, Li D, Whipple SS (2013): Cumulative risk and child development. Psychol Bull 139:1342–1396. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert ME (1997): Towards the development of a biologically based dose-response model of lead neurotoxicity. Amer Zool 37:389–398. [Google Scholar]
- 4.Belsky J, Pluess M (2009): Beyond diathesis stress: Differential susceptibility to environmental influences. Psychol Bull 135:885–908. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin KA,Sheridan MA, Lambert HK (2014): Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 47:578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenough WT, Black JE, Wallace CS (1987): Experience and brain development. Child Dev 58:539–559. [PubMed] [Google Scholar]
- 7.Takesian AE, Hensch TK (2013): Balancing plasticity/stability across brain development. Prog Brain Res 207:3–34. [DOI] [PubMed] [Google Scholar]
- 8.Nelson CA 3rd, Zeanah CH, Fox NA (2019): How early experience shapes human development: The case of psychosocial deprivation. Neural Plast 2019:1676285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callaghan BL, Tottenham N (2016): The neuro-environmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology 41:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumner JA, Colich NL, Uddin M, Armstrong D, McLaughlin KA (2019): Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol Psychiatry 85:268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]