Abstract
AIM
To investigate the changes in intraocular pressure (IOP) before and after intraocular surgery measured with Goldmann applanation tonometry (GAT) and pascal dynamic contour tonometry (PDCT), and assessed their agreement.
METHODS
Patients who underwent trans pars plana vitrectomy (TPPV) with or without cataract extraction (CE) were included. The IOP was measured in both eyes with GAT and PDCT pre- and postoperatively, where the non-operated eyes functioned as control.
RESULTS
Preoperatively, mean IOP measurements were 16.3±6.0 mm Hg for GAT and 12.0±2.8 mm Hg for PDCT for the operated eyes. Postoperatively, the mean IOP dropped to 14.3±5.6 mm Hg for GAT (P=0.011) and rose up to 12.7±2.6 mm Hg for PDCT (P=0.257). Bland-Altman analysis showed a poor agreement between GAT and PDCT with a mean difference of 2.9 mm Hg preoperatively and 95% limits of agreement ranging from -3.2 to 9.0 mm Hg. Postoperatively, the mean difference was 1.2 mm Hg with 95% limits of agreement ranging from -8.3 to 10.7 mm Hg. There were no significant differences between the TPPV and TPPV+CE group, except when measured with PDCT postoperatively (P=0.012).
CONCLUSION
The IOP is reduced after surgery when measured with GAT and remained stable when measured with PDCT. However, the agreement between GAT and PDCT is poor. Although PDCT may be a more accurate predictor of the true IOP, it seems less suitable for daily use in the clinical practice.
Keywords: glaucoma, intraocular pressure, intraocular surgery, trans pars plana vitrectomy
INTRODUCTION
Several studies reported a change in intraocular pressure (IOP) after intraocular surgery. Most of them focused on cataract extraction (CE), the most performed type of surgery in the world[1]–[5]. Trans pars plana vitrectomy (TPPV) is another type of intraocular surgery that is performed regularly in the ophthalmic practice. The indications and number of TPPVs performed are increasing. Although less is known about the change in IOP after TPPV than after CE, studies have shown that vitrectomy causes an elevation of 5%-35% of the IOP postoperatively[6]–[8]. In patients who underwent CE the IOP decreased significantly, ranging from 14.2% to 21.1% at one month follow-up[2]–[5]. Also, after CE the number of IOP-lowering medications was decreased in glaucoma patients[4],[9]. Patients with a high preoperative IOP or patient who develop a deep anterior chamber postoperatively tend to have a greater reduction in postoperative IOP, respectively 3.7±2.5 mm Hg and 2.3±1.0 mm Hg[10]–[14]. After TPPV the IOP and risk of glaucoma may increase, but studies are inconsistent[15]–[17]. The reason for the effect of TPPV on IOP still remains unclear. Chang theorized that oxidative stress would affect the cells of the trabecular meshwork, causing a rise in IOP[18]. However, TPPV having an effect on the biomechanical properties of the cornea could also be a possible explanation.
The gold standard for measuring the IOP still is Goldmann applanation tonometry (GAT)[19]. GAT measures the force that is needed to applanate the central cornea. Therefore it is affected by the biomechanical properties of the cornea, like the central corneal thickness and rigidity[20]. Pascal dynamic contour tonometry (PDCT) is another method of measuring the IOP[21]. In PDCT the measuring tip is made to match the contour of the cornea, thus being less affected by the biomechanical properties of the cornea[6],[20]–[21].
The objective of this study is to assess whether there are different changes in IOP after different types of intraocular surgery (TPPV with or without CE).
SUBJECTS AND METHODS
Ethical Approval
The Medical Ethics Committee of the Erasmus University approved the study and the study adhered to the tenets of the Declaration of Helsinki. For this study retrospective data of regular visits of patients was used; therefore, neither a written informed consent was required nor did the patients receive a stipend.
Participants
All patients undergoing TPPV surgery with or without CE by phacoemulsification with intraocular lens (IOL) implant between March and July at the Erasmus Medical Center Rotterdam, the Netherlands, were considered eligible. Eyes with an ocular trauma were excluded from the study. Other exclusion criteria were: patients who were less mobile, patients with cognitive impairment, patients with a corneal transplant, and patients with surgical complications. Intraocular surgery consisted of TPPV or a combination of TPPV and CE. The IOP was measured preoperatively, and 1mo postoperatively.
Surgical Procedure
In all patients, a 25-gauge TPPV with or without laser coagulation was performed. Depending on the indication for TPPV, the tamponades air, SF6-gas or oil were used. In the TPPV+CE group, CE was performed in addition to the TPPV. CE consisted of phacoemulsification with IOL implantation through a 2.4-mm corneoscleral incision. The corneal incisions were self-sealing and left unsutured. Postoperatively, all patients were treated with prednisolone 1.0% eye drops four times daily for one week, and thereafter the eyedrops were tapered with 1 drop a week, in the operated eye. All surgical procedures were completed without complications by the same surgeon (Kılıç E). To minimize device-dependent IOP changes the same surgical device was used[22]–[23].
Ophthalmic Examination
Patients underwent a comprehensive ophthalmic examination before and after surgery. IOP was measured in both eyes, where the non-operated eye served as a control. A drop of topical oxybuprocaine 0.4% mixed with fluorescence sodium 0.25% was introduced into both eyes. IOP was measured with GAT (Haag-Streit, Köniz, Switzerland) and PDCT (SMT Swiss Microtechnology, Zurich, Switzerland). Both devices had been calibrated conform manufacturers standards. For a GAT measurement there is no quantitative quality measurement, but PDCT registers the quality of the measurement. This measurement (Q) is a number between 1 to 5, where Q1 is good, Q2 and Q3 are acceptable, and Q4 and Q5 are poor measurements. If the PDCT quality measurement was Q4 or Q5 the measurement was discarded. IOP was first measured with GAT to prevent bias from the semiautomatic recording of PDCT. After two to three minutes IOP was measured with PDCT. Measurements with both GAT and PDCT were taken twice. A third measurement was taken if the difference in the first two measurements was more than 2 mm Hg or if the quality of the PDCT measurement exceeded Q3. If 2 measurements were taken, the measurement with the best Q was used or the average if the Q of both measurements were equal. The median was used if 3 measurements were taken.
The method of IOP measurement was exactly the same before and after surgery. The measurement was taken one hour before start of the intraocular surgery, before the start of the pre-operative preparations. One month postoperatively, IOP was also measured with GAT and PDCT according to a similar procedure. To minimize the possible effect of diurnal variation in IOP the measurements were performed within a time frame of one hour[24]. All IOP measurements were performed by the same investigator (Kovacic H), except of the postoperative GAT measurements (ophthalmic residents). The one-month postoperative appointments were tracked with the electronic patient database of the hospital. General data of the patient, such as age, gender, previous surgery, pre-operative refraction, and axial length were recorded.
Statistical Analysis
Analysis focused on the differences between GAT and PDCT. First, we assessed the effect of the quality of the PDCT measurement on IOP. Next, the agreement between GAT and PDCT was evaluated statistically. Scatterplots were made for the preoperative and postoperative averages of the GAT and PDCT. The Bland-Altman method, using Medcalc 13.3.0 statistical software (Ostend, Belgium), was applied to plot the difference in IOP between the two measurements versus the mean of the two measurements[25].
The paired samples t-test was used to assess the differences between pre- and postoperative IOP measurements taken with GAT and those taken with PDCT. The Shapiro-Wilk test was used to test the continuous data for normality. Differences in IOP between the TPPV and TPPV+CE group were evaluated with the independent samples t-test or the Mann-Whitney U test for continuous variables and the Chi-square test for categorical variables. We also checked whether there were any postoperative differences in IOP related to the type of tamponade used during TPPV. Linear regression models were used to examine the associations between IOP and the type of operation adjusted for age, gender and variables showing a significant univariate difference between the TPPV and TPPV+CE group.
Statistical analyses were performed using SPSS v22.0 for Windows (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered statistically significant.
RESULTS
Forty-six eyes of 46 patients were included for analysis and 46 contralateral eyes for comparison. The mean±standard deviation age was 64.1±13.7y, spherical equivalent was -1.4±4.2 D, axial length 23.9±1.7 mm, and 26 (56.5%) were female. A total of 13 (28.3%) patients were on glaucoma medication, 2 (4.3%) had a history of glaucoma surgery. Figure 1 shows a flowchart of the missing IOP measurements for GAT and PDCT.
Figure 1. Flow chart of included eyes.
Concerning the quality of measurement values of the PDCT, no significant differences were observed in the IOP between Q1, Q2 and Q3 preoperative (P=0.941) as well as postoperative (P=0.608).
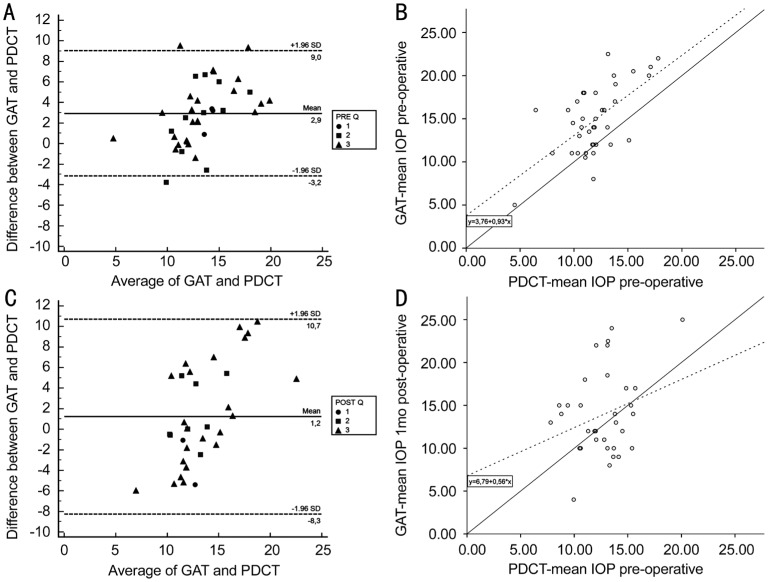
Figure 2A and 2C display the Bland-Altman analysis pre- and postoperative, respectively. The mean difference between GAT and PDCT preoperatively was 2.9 mm Hg with 95% limits of agreement ranging from -3.2 mm Hg to 9.0 mm Hg. Postoperatively, the mean difference between the two methods was 1.2 mm Hg with 95% limits of agreement ranging from -8.3 mm Hg to 10.7 mm Hg. Figure 2B and 2D present the preoperative linear regression analysis with IOP(GAT) = 3.8+0.9 IOP(PDCT), and the postoperative linear regression analysis with IOP(GAT) = 6.8+0.6 IOP(PDCT).
Figure 2. Bland-Altman plot of the difference in preoperative (A) and postoperative (C) intraocular pressure (IOP) between Goldmann applanation tonometry (GAT) and Pascal dynamic contour tonometry (PDCT) plotted against the average IOP, with corresponding scatterplot of the preoperative (B) and postoperative (D) average of GAT versus the average of the PDCT.
In the Bland-Altman plot the solid line represents the mean difference and the dashed lines represent the 95% limits of agreement. The quality of the PDCT measurements are shown as circles (Q1), squares (Q2), and triangles (Q3). In the scatterplot the solid line represents the line y=x. The dashed line represents the regression line.
Table 1 show the difference in pre- and postoperative IOP for the operated and non-operated eyes, respectively, measured with GAT and PDCT. The mean IOP decreased significantly when measured with GAT (P=0.011), but remained stable when measured with PDCT. As expected, for the non-operated (control) eyes there was neither a significant difference in IOP measured with GAT (P=0.673) nor with PDCT (P=0.137).
Table 1. Preoperative IOP of the operated eyes and non-operated eyes measured with GAT and PDCT.
| Parameters | Preoperative | Postoperative | P |
| Operated eyes | |||
| IOP GAT | 16.3±6.0 | 14.3±5.6 | 0.011 |
| IOP PDCT | 12.0±2.8 | 12.7±2.6 | 0.257 |
| Non-operated eyes | |||
| IOP GAT | 15.9±7.1 | 15.6±8.8 | 0.673 |
| IOP PDCT | 12.3±2.9 | 13.0±2.5 | 0.137 |
GAT: Goldmann applanation tonometry; PDCT: Pascal dynamic contour tonometry; IOP: Intraocular pressure.
mean±SD, mm Hg
The indications for TPPV were epiretinal membrane (n=12 eyes), retinal detachment (n=13 eyes), macular hole (n=6 eyes), vitreous hemorrhage (n=5 eyes), vitreomacular traction syndrome (n=5 eyes), floaters (n=3 eyes), and diagnostic vitreous biopsy (n=2 eyes). The types of tamponade used were air (n=23 eyes), SF6-gas (n=12 eyes) and oil (n=11 eyes). There were no significant differences in type of tamponade used between patients that underwent TPPV and those that underwent TPPV+CE. Furthermore, differences in IOP between air, gas, and oil tamponades were not significant when measured with GAT (P=0.327) and with PDCT (P=0.813).
In the univariate analysis no significant differences in the baseline demographics of the patients between TPPV and TPPV+CE were observed, except for previous surgery (P<0.001). Table 2 shows the IOP between the TPPV and TPPV+CE group. Preoperatively, no significant differences in IOP were found when measured with GAT (P=0.402) and PDCT (P=0.063) between the TPPV and TPPV+CE group. Postoperatively, there were also no significant differences in IOP measured with GAT (P=0.320); however, with PDCT the IOP was significantly lower in the TPPV+CE group compared to the TPPV group (P=0.012). Multivariate analysis (adjusted for age, gender and previous surgery) did not change results. Concerning the change in IOP (ΔIOP=postoperative IOP—preoperative IOP), in the TPPV+CE group the ΔIOP was significantly lower when measured with GAT (P=0.044), while the ΔIOP was significantly higher in the TPPV group when measured with PDCT (P=0.001). No differences in outcomes were observed after exclusion of the TPPV's for retinal re-detachment.
Table 2. Differences in IOP between the TPPV and TPPV+CE group measured with GAT or PDCT.
| Parameters | Preoperative |
Postoperative |
ΔIOPa |
|||
| Mean±SD | P | Mean±SD | P | Mean±SD | P | |
| GAT | ||||||
| TPPV | 15.4±6.2 | 0.402 (0.938)b | 15.3±6.8 | 0.320 (0.655)b | -0.4±4.5 | 0.044 |
| TPPV+CE | 16.9±5.8 | 13.5±4.3 | -3.4±5.0 | |||
| PDCT | ||||||
| TPPV | 10.9±2.4 | 0.063 (0.193)b | 13.8±2.2 | 0.012 (0.038)b | 3.0±3.4 | 0.001 |
| TPPV+CE | 12.6±2.7 | 11.7±2.3 | -1.0±2.4 | |||
aPostoperative IOP—preoperative IOP; bAdjusted for age, gender and previous surgery; IOP: Intraocular pressure; GAT: Goldmann applanation tonometry; PDCT: Pascal dynamic contour tonometry; TPPV: Trans pars plana vitrectomy; CE: Cataract extraction.
DISCUSSION
In the present study, we found a reduction in IOP after surgery when measured with GAT, while the IOP remained unchanged when measured with PDCT.
When measured with GAT a significant decrease in postoperative IOP was observed, which is consistent with previous studies[2]–[3]. Although corneal hysteresis was not actually measured, this decrease might at least partly be explained by a measurement error due to changes in the biomechanical properties of the cornea. Changes in corneal biomechanical properties were shown before by de Freitas Valbon et al[26]. They found a significantly lower corneal resistance 30d after surgery, probably causing an underestimation of measured IOP. However, other biomechanical properties did not differ 30d postoperatively. Also, another study showed that differences between PDCT and GAT are associated with biomechanical properties such as central corneal thickness and corneal hysteresis[27]. However, the pathophysiological mechanism of the reduction in IOP after CE still remains unclear. There are many different theories explaining the possible mechanism. One of them is that CE leads to deepening of the anterior chamber, facilitating aqueous outflow[14],[28]–[29]. Another theory is that the surgical technique used may play an important role[30]. Furthermore, it has been suggested that interleukin-1 (IL-1) plays an important role in the increase of the postoperative aqueous outflow facility. One study found that IL-1 is being released together with tumor necrosis factors by cultured cells of the trabecular meshwork. This in turn may lead to an increased production of matrix metalloproteinases, which enhances tissue remodeling and reduces the resistance of the extracellular matrix, thus increasing the facility of outflow[31].
We found no significant difference in the pre- versus postoperative IOP when measured with PDCT. It could be that GAT is underestimating the IOP due to surgery-induced changes in biomechanical properties of the cornea. PDCT would not have this effect since the measuring tip of the PDCT is concave and contour-matched, thus being less affected by the change in the biomechanical properties of the cornea.
In non-operated eyes, which functioned as control, no significant change occurred postoperatively when measured with either GAT or PDCT. The mean IOP measured one month postoperative was consistent with the mean IOP at baseline, as to be expected.
The mean IOP in operated as well as non-operated eyes was higher when measured with GAT compared to PDCT when measured preoperatively. It could be that GAT is systematically measuring a higher IOP. One previous study comparing GAT and PDCT in phakic and in pseudophakic eyes also found this effect in phakic eyes (18.0 and 17.0 mm Hg, respectively)[20]. Another recent study comparing GAT and PDCT in eyes after vitrectomy also found a 3.1 mm Hg higher IOP measured with GAT in gas-filled eyes[32]. In contrast, several other studies reported PDCT measurements to be higher than GAT with differences ranging from 0.6 to 5.1 mm Hg[6],[33]–[34]. The present study showed a poor agreement between the two methods. This finding is in line with several other studies and implies that measurements with GAT and PDCT should not be used interchangeably[33],[35].
According to our knowledge, this is the first study comparing GAT with PDCT before and after TPPV and TPPV+CE surgery. The IOP only differed between these groups when it was measured postoperatively with PDCT, which could be a true IOP decrease not detected by GAT due to measurement artifacts caused by possible changes in the biomechanical properties of the cornea after surgery. Two studies compared the IOP measured with GAT in patients who underwent TPPV and TPPV+CE. Both did not find a significant difference between TPPV and TPPV+CE measured with GAT, which is in agreement with the current GAT results[36]–[37]. In our study, the change in mean IOP differed between the TPPV and TPPV+CE group when it was measured with GAT as well as PDCT. This might suggest that there is a difference in the change of biomechanical properties of the cornea after TPPV and TPPV+CE.
Although the different TPPV indications and tamponades may result in heterogeneity, it is not likely that this influenced our results. For example, it is well known that in eyes with retinal detachment IOP tends to be lower. However, we found no significant differences in preoperative IOP between TPPV for the indication of retinal detachment versus other indications when measured with GAT (P=0.352) as well as PDCT (P=0.857). Therefore, it is not likely that this has influenced the presented results. Only one study investigated the change in IOP after TPPV by comparing GAT with PDCT. However, they focused on the difference between GAT and PDCT when different types of tamponades were used, and found that the change in IOP was independent of used tamponade[6]. Similarly, we could not find any significant difference in IOP between the different tamponade-types measured with either GAT or PDCT. Another possible limitation of this study is that preoperative GAT and PDCT measurements were taken by the same trained researcher, whereas postoperative GAT measurements were taken by ophthalmic residents. This could have led to interobserver variability, which has already been proven to be a problem with GAT in previous studies[33],[35],[38]–[40]. Nevertheless, the variability of the postoperative measurements was similar to the preoperative measurements, which diminishes the effect of interobserver variability.
In conclusion, the present study shows a reduced IOP after surgery when measured with GAT and stable IOPs when measured with PDCT. This difference might be influenced by changes in the corneal biomechanical properties after the surgery. However, the difference in pre- and postoperative IOP might also be due to GAT systematically measuring higher IOP's. Evaluation of corneal biomechanical properties such as corneal hysteresis and corneal resistance factor combined with pre- and postoperative measurements of GAT and PDCT is needed to further explore these findings.
Acknowledgments
Foundation: Supported by Stichting Nederlands Oogheelkundig Onderzoek (SNOO).
Conflicts of Interest: Kovacic H, None; Wolfs RCW, None; Kılıç E, None; Ramdas WD, None.
REFERENCES
- 1.He M, Wang W, Huang WY. Variations and trends in health burden of visual impairment due to cataract: a global analysis. Invest Ophthalmol Vis Sci. 2017;58(10):4299–4306. doi: 10.1167/iovs.17-21459. [DOI] [PubMed] [Google Scholar]
- 2.Saccà S, Marletta A, Pascotto A, Barabino S, Rolando M, Giannetti R, Calabria G. Daily tonometric curves after cataract surgery. Br J Ophthalmol. 2001;85(1):24–29. doi: 10.1136/bjo.85.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leelachaikul Y, Euswas A. Long-term intraocular pressure change after clear corneal phacoemulsification in Thai glaucoma patients. Chotmaihet Thangphaet. 2005;88(Suppl 9):S21–S25. [PubMed] [Google Scholar]
- 4.Armstrong JJ, Wasiuta T, Kiatos E, Malvankar-Mehta M, Hutnik CML. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: A systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26(6):511–522. doi: 10.1097/IJG.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 5.Coh P, Moghimi S, Chen RI, Hsu CH, Masís Solano M, Porco T, Lin SC. Lens position parameters as predictors of intraocular pressure reduction after cataract surgery in glaucomatous versus nonglaucomatous eyes. Invest Ophthalmol Vis Sci. 2016;57(6):2593–2599. doi: 10.1167/iovs.16-19384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaldson MJ, Bhatnagar P, Dhrami-Gavazi E, Santos RA, Barile GR, Del Priore LV, Iranmanesh R, Schiff WM, Chang S. Pascal dynamic contour tonometry versus goldmann applanation tonometry in gas and air-filled eyes after vitrectomy surgery. Retina. 2009;29(4):481–486. doi: 10.1097/IAE.0b013e31818c5dc9. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg RS, Peyman GA, Huamonte FU. Elevation of intraocular pressure after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 1976;200(2):157–161. doi: 10.1007/BF00414365. [DOI] [PubMed] [Google Scholar]
- 8.Han DP, Lewis H, Lambrou FH, Jr, Mieler WF, Hartz A. Mechanisms of intraocular pressure elevation after pars plana vitrectomy. Ophthalmology. 1989;96(9):1357–1362. doi: 10.1016/s0161-6420(89)32715-1. [DOI] [PubMed] [Google Scholar]
- 9.Shingleton BJ, Gamell LS, O'Donoghue MW, Baylus SL, King R. Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25(7):885–890. doi: 10.1016/s0886-3350(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 10.Shrivastava A, Singh K. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol. 2010;21(2):118–122. doi: 10.1097/ICU.0b013e3283360ac3. [DOI] [PubMed] [Google Scholar]
- 11.Guan H, Mick A, Porco T, Dolan BJ. Preoperative factors associated with IOP reduction after cataract surgery. Optom Vis Sci. 2013;90(2):179–184. doi: 10.1097/OPX.0b013e31827ce224. [DOI] [PubMed] [Google Scholar]
- 12.Yang HS, Lee J, Choi S. Ocular biometric parameters associated with intraocular pressure reduction after cataract surgery in normal eyes. Am J Ophthalmol. 2013;156(1):89–94.e1. doi: 10.1016/j.ajo.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Liu CJ, Cheng CY, Ko YC, Lau LI. Determinants of long-term intraocular pressure after phacoemulsification in primary angle-closure glaucoma. J Glaucoma. 2011;20(9):566–570. doi: 10.1097/IJG.0b013e3181efe1e9. [DOI] [PubMed] [Google Scholar]
- 14.Shin HC, Subrayan V, Tajunisah I. Changes in anterior chamber depth and intraocular pressure after phacoemulsification in eyes with occludable angles. J Cataract Refract Surg. 2010;36(8):1289–1295. doi: 10.1016/j.jcrs.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Mi CW, Thompson JT. Long-term follow-up of intraocular pressure after vitrectomy in eyes without preexisting glaucoma. Retina. 2015;35(12):2543–2551. doi: 10.1097/IAE.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 16.Fang Y, Long QQ, Wang XQ, Jiang R, Sun XH. Intraocular pressure 1y after vitrectomy in eyes without a history of glaucoma or ocular hypertension. Clin Ophthalmol. 2017;11:2091–2097. doi: 10.2147/OPTH.S144985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miele A, Govetto A, Fumagalli C, Donati S, Biagini I, Azzolini C, Rizzo S, Virgili G. Ocular hypertension and glaucoma following vitrectomy: a systematic review. Retina. 2018;38(5):883–890. doi: 10.1097/IAE.0000000000001651. [DOI] [PubMed] [Google Scholar]
- 18.Chang S. LXII Edward Jackson lecture: open angle glaucoma after vitrectomy. Am J Ophthalmol. 2006;141(6):1033–1043. doi: 10.1016/j.ajo.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Goldmann H, Schmidt T. Applanation tonometry. Ophthalmologica. 1957;134(4):221–242. doi: 10.1159/000303213. [DOI] [PubMed] [Google Scholar]
- 20.Heinrich MA, Eppig T, Langenbucher A, Walter S, Behrens-Baumann W, Viestenz A. Comparison of Goldmann applanation and dynamic contour tonometry before and after cataract surgery. J Cataract Refract Surg. 2012;38(4):683–689. doi: 10.1016/j.jcrs.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Kanngiesser HE, Kniestedt C, Robert YC. Dynamic contour tonometry: presentation of a new tonometer. J Glaucoma. 2005;14(5):344–350. doi: 10.1097/01.ijg.0000176936.16015.4e. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Park SH, Choi KS. Fluctuation of infusion pressure during microincision vitrectomy using the constellation vision system. Retina. 2015;35(12):2529–2536. doi: 10.1097/IAE.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 23.Falabella P, Stefanini FR, Lue JC, Pfister M, Reyes-Mckinley J, Koss MJ, Teixeira A, Schor P, Humayun MS. Intraocular pressure changes during vitrectomy using constellation vision system's intraocular pressure control feature. Retina. 2016;36(7):1275–1280. doi: 10.1097/IAE.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 24.Lee YW, Kim JM, Shim SH, Kim DY, Bae JH, Park KH. The influence of a vitrectomy on the diurnal intraocular pressure. J Ophthalmol. 2015;2015:427808. doi: 10.1155/2015/427808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 26.de Freitas Valbon B, Ventura MP, da Silva RS, Canedo AL, Velarde GC, Ambrósio R., Jr Central corneal thickness and biomechanical changes after clear corneal phacoemulsification. J Refract Surg. 2012;28(3):215–219. doi: 10.3928/1081597X-20111103-02. [DOI] [PubMed] [Google Scholar]
- 27.Mangouritsas G, Mourtzoukos S, Mantzounis A, Alexopoulos L. Comparison of Goldmann and Pascal tonometry in relation to corneal hysteresis and central corneal thickness in nonglaucomatous eyes. Clin Ophthalmol. 2011;5:1071–1077. doi: 10.2147/OPTH.S23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang GF, Gonzalez E, Lee R, Chen YC, He MG, Lin SC. Association of biometric factors with anterior chamber angle widening and intraocular pressure reduction after uneventful phacoemulsification for cataract. J Cataract Refract Surg. 2012;38(1):108–116. doi: 10.1016/j.jcrs.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slabaugh MA, Bojikian KD, Moore DB, Chen PP. The effect of phacoemulsification on intraocular pressure in medically controlled open-angle glaucoma patients. Am J Ophthalmol. 2014;157(1):26–31. doi: 10.1016/j.ajo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Meyer MA, Savitt ML, Kopitas E. The effect of phacoemulsification on aqueous outflow facility. Ophthalmology. 1997;104(8):1221–1227. doi: 10.1016/s0161-6420(97)30154-7. [DOI] [PubMed] [Google Scholar]
- 31.Wang N, Chintala SK, Fini ME, Schuman JS. Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification. Invest Ophthalmol Vis Sci. 2003;44(5):1977–1981. doi: 10.1167/iovs.02-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamas N, Fuest M, Koutsonas A, Roessler G, Mazinani BE, Walter P, Plange N. Goldmann applanation tonometry versus dynamic contour tonometry after vitrectomy. J Glaucoma. 2016;25(8):663–668. doi: 10.1097/IJG.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 33.Jordão ML, Costa VP, Rodrigues Mde L, Paula JS. Comparison of dynamic contour tonometry and Goldmann applanation tonometry in relation to central corneal thickness in primary congenital glaucoma. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):117–121. doi: 10.1007/s00417-012-2027-3. [DOI] [PubMed] [Google Scholar]
- 34.Duba I, Wirthlin AC. Dynamic contour tonometry for post-LASIK intraocular pressure measurements. Klin Monbl Augenheilkd. 2004;221(5):347–350. doi: 10.1055/s-2004-812895. [DOI] [PubMed] [Google Scholar]
- 35.Anderson MF, Agius-Fernandez A, Kaye SB. Comparison of the utility of Pascal dynamic contour tonometry with Goldmann applanation tonometry in routine clinical practice. J Glaucoma. 2013;22(5):422–426. doi: 10.1097/IJG.0b013e31824cb10c. [DOI] [PubMed] [Google Scholar]
- 36.Yang HK, Woo SJ, Park KH, Park KH. Intraocular pressure changes after vitrectomy with and without combined phacoemulsification and intraocular lens implantation. Korean J Ophthalmol. 2010;24(6):341–346. doi: 10.3341/kjo.2010.24.6.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arikan Yorgun M, Toklu Y, Mutlu M, Ozen U. Clinical outcomes of 25-gauge vitrectomy surgery for vitreoretinal diseases: comparison of vitrectomy alone and phaco-vitrectomy. Int J Ophthalmol. 2016;9(8):1163–1169. doi: 10.18240/ijo.2016.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang AS, Alencar LM, Weinreb RN, Tafreshi A, Deokule S, Vizzeri G, Medeiros FA. Repeatability and reproducibility of Goldmann applanation, dynamic contour, and ocular response analyzer tonometry. J Glaucoma. 2013;22(2):127–132. doi: 10.1097/IJG.0b013e3182254ba3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albis-Donado O, Bhartiya S, Gil-Reyes M, Casale-Vargas G, Arreguin-Rebollar N, Kahook MY. Citius, altius, fortius: agreement between perkins and dynamic contour tonometry (Pascal) and the impact of altitude. J Curr Glaucoma Pract. 2018;12(1):40–44. doi: 10.5005/jp-journals-10028-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotecha A, White E, Schlottmann PG, Garway-Heath DF. Intraocular pressure measurement precision with the Goldmann applanation, dynamic contour, and ocular response analyzer tonometers. Ophthalmology. 2010;117(4):730–737. doi: 10.1016/j.ophtha.2009.09.020. [DOI] [PubMed] [Google Scholar]