Abstract
Ketosis is a metabolic adaptation to fasting, nonalcoholic fatty liver disease (NAFLD), and prolonged exercise. β-OH butyrate acts as a transcriptional regulator and at G protein-coupled receptors to modulate cellular signaling pathways in a hormone-like manner. While physiological ketosis is often adaptive, chronic hyperketonemia may contribute to the metabolic dysfunction of NAFLD. To understand how β-OH butyrate signaling affects hepatic metabolism, we compared the hepatic fasting response in control and 3-hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2) knockdown mice that are unable to elevate β-OH butyrate production. To establish that rescue of ketone metabolic/endocrine signaling would restore the normal hepatic fasting response, we gave intraperitoneal injections of β-OH butyrate (5.7 mmol/kg) to HMGCS2 knockdown and control mice every 2 h for the final 9 h of a 16-h fast. In hypoketonemic, HMGCS2 knockdown mice, fasting more robustly increased mRNA expression of uncoupling protein 2 (UCP2), a protein critical for supporting fatty acid oxidation and ketogenesis. In turn, exogenous β-OH butyrate administration to HMGCS2 knockdown mice decreased fasting UCP2 mRNA expression to that observed in control mice. Also supporting feedback at the transcriptional level, β-OH butyrate lowered the fasting-induced expression of HMGCS2 mRNA in control mice. β-OH butyrate also regulates the glycemic response to fasting. The fast-induced fall in serum glucose was absent in HMGCS2 knockdown mice but was restored by β-OH butyrate administration. These data propose that endogenous β-OH butyrate signaling transcriptionally regulates hepatic fatty acid oxidation and ketogenesis, while modulating glucose tolerance.
NEW & NOTEWORTHY Ketogenesis regulates whole body glucose metabolism and β-OH butyrate produced by the liver feeds back to inhibit hepatic β-oxidation and ketogenesis during fasting.
Keywords: β-OH butyrate; β-oxidation; fasting, gluconeogenesis; ketogenesis
INTRODUCTION
Hepatocytes acutely produce ketones to spare glucose utilization during a fast, when insulin signaling is low and hepatic lipid oxidation is dominant (4). Yet, with the rise in nonalcoholic fatty liver disease (NAFLD) and the adoption of ketogenic diets, ketosis is a prolonged metabolic state for many individuals. In fact, both lean and obese NAFLD patients with simple steatosis have elevated circulating β-OH butyrate concentrations (3, 25). On the other hand, individuals with inborn errors of metabolism that impair ketogenesis are hypoketotic and display profound metabolic abnormalities (6, 20, 35). Given the broad incidence of states with altered ketogenic flux, it is important to fully understand the metabolic consequences of ketone signaling. β-OH butyrate, the predominant ketone, is canonically thought to provide energy to the central nervous system during a sustained fast. However, in the last decade it has been established that many metabolites, including β-OH butyrate, also act as hormone-like signaling molecules and allosteric modulators that alter fundamental cellular processes (26, 41).
Normally, acetoacetate and β-OH butyrate exist in a 1:1 ratio and total circulating ketone concentrations rarely exceed 0.3 mM (4). However, under conditions that favor ketogenesis, β-OH butyrate concentrations rise dramatically to be nearly fourfold higher than acetoacetate concentrations (4, 42). This elasticity in hepatic β-OH butyrate production and serum β-OH butyrate concentrations allows for β-OH butyrate to induce metabolic state specific signaling. In fact, β-OH butyrate is the ligand for two G protein-coupled receptors, GPR109a and GPR41 (16, 43). The EC50 for β-OH butyrate agonism of GPR109a, 0.7 mM, is well above fed state concentrations and well within the concentrations observed during an extended fast (up to 6 mM) (11, 32). At concentrations above 1 mM, β-OH butyrate regulates gene expression by globally modifying transcription as an endogenous histone deacetylase (HDAC) inhibitor, sequestering carbohydrate responsive element binding protein in the cytosol of hepatocytes, and may act directly at the butyrate response element in promoter regions of target genes (8, 29, 41).
Cotter et al. (7) performed the first study using dsDNA antisense oligonucleotides (ASO) to knockdown expression of hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2), an enzyme essential for ketogenesis. Through these studies they confirmed the essential role of basal hepatic ketone production in gluconeogenesis, de novo lipogenesis, and mitochondrial metabolism. They additionally aimed to understand the role of blocking ketogenesis in the ketogenic obese state. However, interpretation of these studies was limited, because HMGCS2 knockdown limited ad libitum calorie consumption and weight gain on a high-fat diet (7).
To specifically understand the role of ketones in the metabolic adaptation to a ketogenic state, we tested the effect of HMGCS2 knockdown in fasting. Furthermore, we administered β-OH butyrate to restore ketone signaling in fasted HMGCS2 knockdown mice. Given that β-OH butyrate regulates gene expression and is a key metabolite during prolonged food deprivation, we hypothesized that inhibiting β-OH butyrate production would increase hepatic glucose output to meet the metabolic demand during a fast. We further hypothesized that decreasing β-OH butyrate production would increase hepatic β-oxidative flux, in an attempt to provide more of the ketogenic substrate, acetyl-CoA. In turn, we hypothesized that exogenous β-OH butyrate administration would restore the normal fasting response.
MATERIALS AND METHODS
Animals.
All studies were conducted using 12- to 16-wk-old male WT C57BL/6J mice purchased from Jackson Laboratories (Bar Harbor, ME). Mice were kept on a 14-h light/10-h dark schedule and housed three to four mice per cage until 1 wk before study initiation, at which point animals were individually housed. Ad libitum access to NIH-31 chow (Harlan Laboratories, Indianapolis, IN) and water was available. All studies were approved by the University of Arizona Institutional Animal Care and Use Committee.
3-Hydroxy-3-methylglutaryl-CoA Synthase II knockdown.
Twelve-week-old WT C57BL/6J mice received twice-weekly intraperitoneal injections of 25 mg/kg of murine Hmgcs2-targeted ASO (IONIS 191229; 5′-CTGTTTGTCACTGCTGGATG-3′) or scramble control ASO (IONIS 141923; 5′-CCTTCCCTGAAGGTTCCTCC-3′) for 4 wk before experimentation. The control ASO does not have complementarity to known genes and was employed to demonstrate the specificity of target reduction. These ASOs incorporate several chemical modifications to improve potency, duration of action, and tolerability. The internucleotide phosphates are modified with a phosphorothioate substitution, in which one of the nonbridging oxygen atoms is substituted with sulfur. Additionally, the compound incorporates five 2′-O-(2-methoxyethyl) modified ribonucleosides at the 3′- and 5′-ends with ten 2′-O-deoxyribonucleosides in between to support RNaseH-1-mediated target mRNA degradation. These modifications improve the binding affinity for target mRNA as well as stability against exonuclease-mediated degradation. Treatment with this HMGCS2-targeted ASO reduces hepatic HMGCS2 mRNA by 90% in adult mice (7, 12).
Injection studies.
Mice were singly housed 1 wk before experimentation. Sixteen hours before being euthanized (6 PM), all mice were switched to Sani-Chip bedding (cat. no. 7090 Sani-Chips; Harlan Laboratories;) and food was removed from mice in the fasted group. Mice in the fed group retained ad libitum access to food. All mice had ad libitum access to water. Intraperitoneal injections of 5.7 mmol/kg dl-sodium β-OH butyrate (CAS no. 150-83-4; Sigma-Aldrich, St. Louis, MO) in 0.01 M PBS or PBS alone were given at 0.1 ml/10 g body wt at 9, 7, 5, 3, and 1 h before mice were euthanized. Euthanization began at 10 AM, 5 h after lights on, and was completed within 1 h. Throughout this paper, mice referred to as fed had ad libitum access to food up until the moment they were euthanized at 10 AM, and mice referred to as fasted had no access to food from 6 PM to 10 AM and were 16-h fasted at the time of euthanization.
Glucose tolerance test.
Intraperitoneal glucose (2.5 g/kg; 0.1 ml/10 g body wt) was given to 4-h fasted individually housed mice. All glucose tolerance tests began at 1 PM, and glucose was measured in whole blood, collected from the tail vein, by glucometer (manufacturer no. D2ASCCONKIT; Bayer, Leverkusen, Germany) at 0, 15, 30, 60, 90, and 120 min after glucose injection. At 15 min after glucose injection, a larger bleed (~50 μl blood) was taken from the tail vein to measure glucose stimulated serum insulin. Blood was immediately stored on ice and within 2 h of collection, blood was allowed to clot at room temperature for 30 min, and serum was collected after centrifugation at 3,000 g for 30 min at 4°C. Serum was stored at −80°C.
Insulin tolerance test.
Intraperitoneal insulin (0.75 U/kg; 0.1 ml/10 g body wt) was given to 4-h fasted individually housed mice. All insulin tolerance tests began at 1 PM, and glucose was measured in whole blood, collected from the tail vein, by a glucometer (manufacturer no. D2ASCCONKIT; Bayer) at 0, 30, 60, 90, and 120 min after insulin injection.
Tissue collection.
Mice were euthanized by decapitation following bell jar delivery of isoflurane anesthesia. Trunk blood was collected and stored on ice, while livers were snap frozen on dry ice. Within 2 h of collection, blood was allowed to clot at room temperature for 30 min and serum was collected after centrifugation at 3,000 g for 30 min at 4°C. All tissues and serum were stored at −80°C. Before analysis, frozen livers were powdered using a liquid nitrogen cooled mortar and pestle to obtain homogenous liver samples.
Serum assays.
Serum triglycerides (cat. no. T7531; Ponte Scientific, Canton, MI), glucose (cat. no. G7519, Pointe Scientific), nonesterified fatty acids (HR Series NEFA-HR; Wako Diagnostics, Richmond, VA), and β-OH butyrate (cat. no. 700190; Cayman Chemicals, Pittsburgh, PA) were analyzed by colorimetric assay. Serum insulin was analyzed by ELISA (cat. no. 80-INSMSU-E01,E10; Alpco, Salem, NH).
Real-time quantitative RT-PCR.
Whole liver mRNA was isolated from powered liver samples with TRI Reagent (Life Technologies, Grand Island, NY), and phenol contamination was eliminated by using water-saturated butanol and ether as previously described (19). cDNA was synthesized by reverse transcription with Verso cDNA synthesis kit (Thermo Scientific, Waltham, MA), and quantitative PCR performed using SYBR 2X mastermix (Bio-Rad Laboratories, Hercules, CA) and the Bio-Rad iQ5 iCycler (Bio-Rad Laboratories). Expression of β-actin (ACTβ), peroxisome-proliferator activated receptor-α (PPAR-α), HMGCS2, phosphoenolpyruvate carboxykinase (PEPCK), uncoupling protein 2 (UCP2), and carnitine palmitoyltransferase 1 (CPT1) mRNA were measured using the primer pairs previously published (11). LinReg PCR analysis software was used to determine the efficiency of amplification from raw output data (36). ACTβ served as the reference gene for calculating fold change in gene expression using the efficiency −ΔΔCt method (22).
Western blot analysis.
Powdered liver was lysed in RIPA lysis buffer (sc-364162; Santa Cruz, Dallas, TX) containing a protease inhibitor cocktail (P50700-1; Research Products International, Mt. Prospect, IL). Extracted proteins were quantified by Pierce BCA Protein Assay Kit (no. 23225; Thermo Scientific, Rockford, IL), and 24 µg protein was separated using 4–12% gradient bis-Tris gels (Life Technologies, Carlsbad, CA). Proteins were transferred to nitrocellulose membranes using a Bio-Rad Trans-Blot Turbo (Bio-Rad). Membranes were blocked for 1 h at room temperature in TBS with 0.1% Tween 20 (TBST) and 5% nonfat dry milk (NFDM). Primary antibodies including rabbit polyclonal anti-CPT1A (15184–1-AP; 0.33 µg/ml; Proteintech, Rosemont, IL) and mouse monoclonal anti-β-tubulin (T8328; 0.5 µg/ml; Sigma Aldrich) were diluted in TBST with 1% NFDM and incubated on a rocking platform overnight at 4°C. Membranes were washed four times for 5 min each in TBST, and IRDye 680RD or 800CW-conjugated secondary antibodies (LI-COR, Lincoln, NE) were diluted 1:5,000 in TBST with 1% NFDM and incubated with membranes for 1 h at room temperature. Membranes were washed as before and imaged with a LI-COR Odyssey CLx. Image Studio software version 3.1 (LI-COR) was used for densitometry analyses.
Liver metabolic analysis.
Total liver lipids were extracted from powdered liver samples. Briefly, 10–20 mg of sample were sonicated in 100 µl PBS. One millilter of 100% ethanol was added to each sample and vortexed for 10 min. Following 5 min of centrifugation at 16,000 g at 4°C, the supernatant was transferred to a fresh tube for analysis of liver nonesterified fatty acids (NEFAs) (HR Series NEFA-HR; Wako Diagnostics, Richmond, VA) and triglycerides (cat. no. T7531; Ponte Scientific, Canton, MI). Triglyceride values obtained by this extraction method were compared against those obtained by the Folch method to confirm a complete extraction (10). Values determined by the two methods were highly correlated (R2 = 0.9848). Liver glycogen content was quantified by a colorimetric assay as previously described (24). Liver PEPCK activity was quantified as previously described (11).
Liver acetoacetate concentrations were measured using a modified method combining those of Walker (46) and Salway (38). Briefly, 10–20 mg of powdered liver tissue were sonicated in 100 µl PBS. One-hundred microliters of 20% trichloroacetate in ddiH2O were added to each sample, which was subsequently incubated on ice for 30 min. We the centrifuged the samples for 15 min at 12,000 g at 4°C and transferred the supernatant to a fresh tube for use in the assay. A 1-M acetoacetate standard was generated according to the Ljunggren method by the mixture of 1.3 ml 100% ethyl acetoacetate, 5.1 ml of 2 N NaOH, and 3.6 ml distilled water that was incubated at 40°C for 1 h and neutralized to pH 7 (23). We prepared fresh diazo reagent required for the assay as previously described by Walker (46). To begin the assay, 40 µl of each sample or standard were put into duplicate tubes. One set of tubes was boiled at 100°C for 5 min to degrade acetoacetate and determine the background signal generated by the sample. The second set of tubes remained on ice during this 5 min. We then added 45 µl of 1 M sodium acetate-acetic acid buffer (pH 5.2) followed by 270 µl of the freshly prepared diazo reagent (pH 5.0) to each tube and shook the tubes vigorously by hand for 15 s. We incubated the tubes for 30 min at room temperature in the dark to allow color development. 90 µl of 2 N NaOH was added to stop the reaction, and the tubes were again shaken vigorously for 15 s. Samples and standards with and without heat treatment were plated at 300 µl/well in a 96-well plate, and absorption was measured at 480 nm on a Spectramax M2 (Molecular Devices, Sunnyvale, CA). For analysis, we subtracted the absorbance of the heat-treated sample the absorbance of the normal sample to determine signal above background. The concentrations were then calculated based on the standard curve. Liver acetoacetate is expressed as nanomoles per grams of tissue. Liver β-OH butyrate was assayed from the same tissue extract as prepared for the acetoacetate assay (cat. no. 700190; Cayman Chemicals).
Statistical analysis.
All statistical analyses were completed in SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC). First, we used a mixed model ANOVA to assess the effect of ASO treatment (scramble control or HMGCS2) in mice that were fed or fasted. Post hoc comparisons were made between ASO treatments within the hypoketonemic fed or hyperketonemic fasted state. Next, we analyzed the response to exogenous β-OH butyrate or saline administration in hyperketonemic control mice or hypoketonemic HMGCS2 ASO-treated mice. Post hoc comparisons were focused on injection (β-OH butyrate or saline) within scramble control or HMGCS2 ASO-treated mice. For measures of glucose and insulin tolerance, we used a repeated measures ANOVA model including time, ASO, and their interaction. A Bonferroni correction was used to correct for multiple comparisons. Glucose-stimulated insulin secretion (glucose tolerance test area under the curve, and insulin tolerance test area under the curve were analyzed by ANOVA with ASO as the sole main effect. Throughout all analyses the independent variables were set as classification variables. Figures were created in GraphPad PRISM Version 6.0 for Windows (GraphPad Software, San Diego, CA; https://www.graphpad.com/) and are displayed as mean ± SE. A α of P ≤ 0.05 was classified as a significant response. The number of individual observations (n) are displayed in the figures.
RESULTS
Hepatic metabolism differs in mice unable to upregulate ketogenesis.
ASO treatment did not affect body weight at the time of euthanization (control scramble ASO, 26.96 ± 0.7042 g; and HMGCS2 ASO, 26.95 ± 0.5645 g; P > 0.05). To validate that treatment with the HMGCS2-targeted ASO effectively knocked down HMGCS2 mRNA expression and rendered mice deficient in ketogenic potential, we assessed hepatic HMGCS2 mRNA, hepatic acetoacetate and β-OH butyrate concentrations, and serum β-OH butyrate concentrations in the fed and fasted states. HMGCS2-targeted ASO treatment decreased HMGCS2 mRNA expression by 95% and reduced serum β-OH butyrate concentrations independent of nutritional state (Fig. 1, A and B; P < 0.05). Additionally, fasted liver β-OH butyrate concentrations were lower in HMGCS2 knockdown mice, while fed and fasted liver acetoacetate were unaffected by HMGCS2 knockdown (Fig. 1, C and D).
Fig. 1.
Ketogenic response to fasting in control and 3-hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2) knockdown (KD) mice. A–D: hepatic HMGCS2 mRNA expression (A), serum β-OH butyrate (µM; B), hepatic acetoacetate (nmol/g liver tissue; C), and β-OH butyrate (nmol/g liver tissue; D). Direct comparisons were made between antisense oligonucleotides (ASO)-injected groups within nutritional state. NS, nonsignificant. P > 0.05. Number inside bar denotes n per group.
To further assess the hepatic response to fasting we measured changes in liver and serum metabolites, hepatic mRNA and protein expression, and enzyme activity. We chose to examine enzymes involved in β-oxidation, gluconeogenesis, and ketogenesis, three predominant pathways involved in the hepatic response to fasting and the ketogenic state which we were investigating. The potential for hepatic glucose output was assessed by measuring the fast-induced decrease in hepatic glycogen content and serum glucose concentrations and the increase in hepatic PEPCK mRNA expression and enzymatic activity. To assess fasting lipid metabolism, serum and liver NEFA and triglyceride (TAG) concentrations; hepatic PPAR-α, CPT1, and UCP2 mRNA expression; and hepatic CPT1 protein expression were measured.
HMGCS2 knockdown decreased fed state liver glycogen yet increased fasted state liver glycogen concentrations (Fig. 2A; P < 0.05). Fed state serum glucose was decreased by HMGCS2 knockdown, while fasted serum glucose was increased by HMGCS2 knockdown (Fig. 2B). HMGCS2 knockdown did not affect basal serum insulin concentrations or the glucose:insulin ratio in fed or fasted mice (Fig. 2C; P > 0.05). The lower fed state serum glucose levels in HMGCS2 ASO-treated mice suggests that mice with impaired ketogenesis may better clear glucose. Accordingly, we performed a glucose tolerance test, which revealed that HMGCS2 ASO treatment improved glucose tolerance resulting in a decreased area under the curve (Fig. 2, E and F; P < 0.005). Impressively, HMGCS2 knockdown improved glucose tolerance despite reducing glucose stimulated serum insulin concentrations (Fig. 2G; P = 0.02). Control and HMGCS2 knockdown mice were equally insulin sensitive, as assessed by an insulin tolerance test (Fig. 2H).
Fig. 2.
Glucose homeostasis in response to fasting in control and 3-hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2) knockdown (KD) mice. A–D: hepatic glycogen (mg/g liver tissue; A), serum glucose (mg/dl; B), insulin (ng/ml; C), and glucose:insulin ratio (D). Direct comparisons were made between antisense oligonucleotides (ASO)-injected groups within nutritional state. NS, nonsignificant; P > 0.05. E: glucose tolerance test in 4-h fasted mice (control and HMGCS2 KD; n = 23). *P < 0.05, significant difference between control and KD mice in a repeated measures ANOVA analysis. F–H: glucose tolerance test area under the curve (AUC; F), glucose-stimulated serum insulin (G), and insulin tolerance test AUC (H). Bars were analyzed by a two-sided unpaired t-test. Number inside bar denotes n per group.
Hepatic ketone synthesis depends on oxidation of fatty acids. Fed and fasted serum NEFA concentrations were comparable between HMGCS2 and scramble control ASO-treated mice (Fig. 3A; P > 0.05). HMGCS2 knockdown decreased fasted serum TAG concentrations and tended to decrease fed serum TAG concentrations (Fig. 3B; P = 0.08). NEFAs mobilized from adipose tissue during fasting accumulate in the liver and either are metabolized through β-oxidation or are resynthesized into TAGs. Fasted hepatic NEFA and TAG concentrations were unaffected by ASO treatment, while HMGCS2 knockdown tended to decrease hepatic NEFA (P = 0.09) and TAG (P = 0.06) concentrations in the fed state (Fig. 3, C and D). Unsaturated fatty acids bind to and activate PPAR-α, which feeds forward to increase expression of hepatic PPAR-α mRNA (1, 17, 34). Since fasting induced a similar degree of lipid accumulation in control and HMGCS2 ASO-treated mice, we expected the similar level of fasted PPAR-α mRNA expression seen in both groups of mice (Fig. 4A; P > 0.05). However, HMGCS2 knockdown lowered fed state PPAR-α mRNA (Fig. 4A; P < 0.05), perhaps due to the trend for diminished fed hepatic lipids.
Fig. 3.
Effect of fasting and β-OH butyrate (βHB) on lipid homeostasis in control and 3-hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2) knockdown (KD) mice. A–D: fed and fasted saline injected control and HMGCS2 KD mice. E–H: fasted saline or βHB injected control and HMGCS2 KD mice. A and E: serum nonesterified fatty acids (NEFAs; µM). B and F: serum triacylglycerol (TAG; mg/dl). C and G: hepatic NEFAs (µmol/g liver tissue). D and H: hepatic TAG (mg/g liver tissue). A–D: direct comparisons were made between antisense oligonucleotides (ASO)-injected groups within nutritional state. E–H; direct comparisons were made between injection (PBS or βHB) within ASO-treated group. NS, nonsignificant. P > 0.05. Number inside bar denotes n per group.
Fig. 4.
Hepatic gene expression in response to fasting in control and 3-hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2) knockdown (KD) mice. A–F: hepatic peroxisome-proliferator activated receptor-α (PPAR-α) mRNA expression (A), uncoupling protein 2 (UCP2) mRNA expression (B), phosphoenolpyruvate carboxykinase (PEPCK) mRNA expression (C), PEPCK enzymatic activity (D), carnitine palmitoyltransferase 1 (CPT1) mRNA (E), and CPT1 protein expression (F). Direct comparisons were made between ASO-injected groups within nutritional state. NS, nonsignificant. P > 0.05. Number inside bar denotes n per group.
PPAR-α upregulates genes critical for gluconeogenesis, β-oxidation, and ketogenesis (13, 34). In response to a fast, the mitochondrial NAD+-to-NADH ratio decreases (47) and flux through β-oxidation becomes limited by mitochondrial NAD+ availability. To sustain high β-oxidative capacity, the mitochondrial uncoupling protein UCP2 is upregulated in the fasted liver. This PPAR-α target gene allows unrestricted NAD+ regeneration and is required for maximal fasting induced lipid oxidation and ketone synthesis (33, 40). HMGCS2 knockdown tended to increase fed state liver UCP2 mRNA expression (P = 0.09) and robustly increased fasted UCP2 mRNA expression relative to control mice (Fig. 4B; P < 0.05).
Hepatic PEPCK mRNA expression, an early enzyme in gluconeogenesis, was depressed by HMGCS2 knockdown in the fed state (P < 0.05), however there was no difference in fasted PEPCK mRNA expression or enzymatic activity of PEPCK in either nutritional state (Fig. 4, C and D; P > 0.05). Long-chain fatty acid entry into the mitochondria and flux through β-oxidation is largely controlled by the activity of CPT1 (9). Expression of hepatic CPT1 mRNA was unaffected by HMGCS2 knockdown in the fed state yet tended to be elevated in the fasted state in HMGCS2 knockdown mice (Fig. 4E; P = 0.09). Indeed, HMGCS2 knockdown has previously been shown to elevate hepatic CPT1 mRNA expression (7). Surprisingly, HMGCS2 knockdown decreased fed and fasted CPT1 protein expression (Fig. 4F; P < 0.05).
β-OH butyrate normalizes the fasting response in HMGCS2 knockdown mice.
Mice that are unable to upregulate ketone synthesis in response to a fast more robustly upregulate hepatic UCP2 mRNA expression and tend to express more hepatic CPT1 mRNA yet lower CPT1 protein than fasting control mice. Moreover, mice that were unable to produce ketones did not respond to a fast with a drop in serum glucose concentrations. To confirm that these derangements in the fasting response were due to a lack of β-OH butyrate signaling, we reintroduced β-OH butyrate to HMGCS2 knockdown and control mice over the final 9 h of food deprivation.
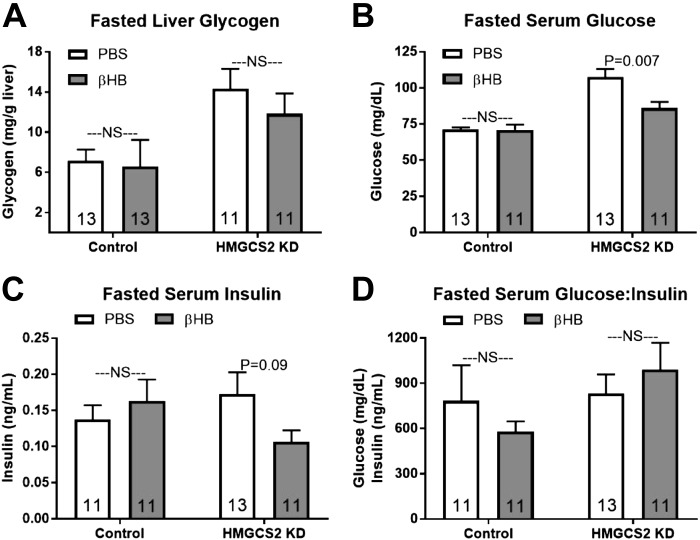
Fasted liver glycogen concentrations were unaffected by β-OH butyrate injections independent of ASO treatment (Fig. 5A; P > 0.05). β-OH butyrate administration did not alter fasting serum glucose levels in control mice, but in HMGCS2 knockdown mice β-OH butyrate injections lowered serum glucose concentrations, resulting in serum glucose concentrations comparable to fasting controls (Fig. 5B). β-OH butyrate injections did not alter fasted serum insulin in control mice but tended to decrease fasted serum insulin in HMGCS2 knockdown mice (Fig. 5C; P = 0.09). The fasted basal glucose:insulin ratio was unaffected by β-OH butyrate in either ASO-treated group (Fig. 5D; P > 0.05).
Fig. 5.
Effect of β-OH butyrate (βHB) on fasting glucose homeostasis in control and 3-hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2) knockdown (KD) mice. A–D: hepatic glycogen (mg/g liver tissue; A), serum glucose (mg/dl; B), insulin (ng/m; C), and glucose:insulin ratio (D). Direct comparisons were made between injection (PBS or βHB) within antisense oligonucleotides (ASO)-treated group. NS, nonsignificant; P > 0.05. Number inside bar denotes n per group.
β-OH butyrate injections had no effect on fasted serum NEFA concentrations independent of HMGCS2 expression but decreased fasted serum TAG concentrations in control mice (Fig. 3, E and F). Fasted hepatic NEFA and TAG concentrations were unaltered by β-OH butyrate in either control or HMGCS2 knockdown mice (Fig. 3, G and H).
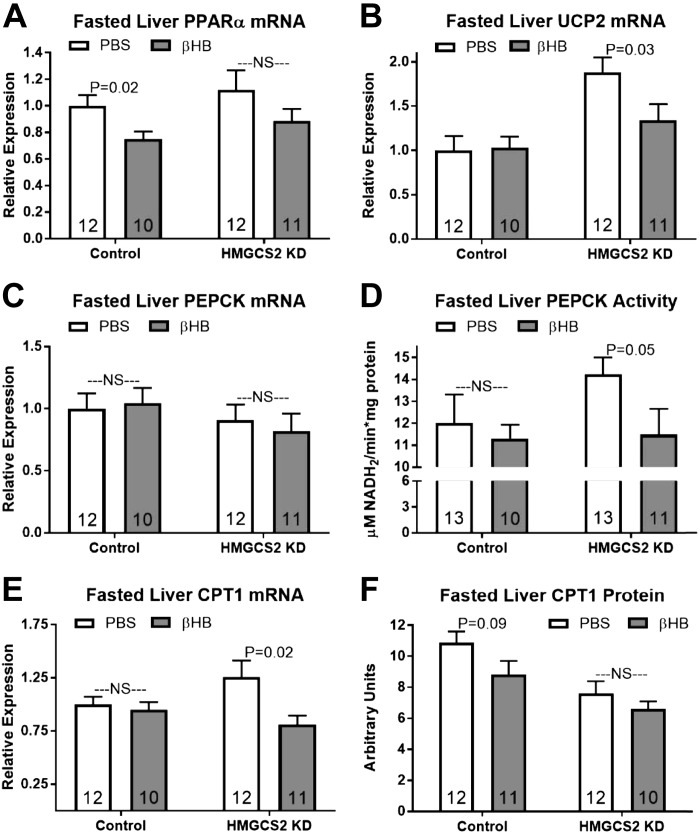
β-OH butyrate injections decreased fasted hepatic PPAR-α mRNA expression by 25% in control mice and 21% in HMGCS2 knockdown mice, although this only reached significance in control mice (Fig. 6A; P < 0.05). Fasted hepatic UCP2 mRNA was decreased by β-OH butyrate administration in HMGCS2 knockdown mice, restoring expression to that seen in control mice (Fig. 6B; P < 0.05).
Fig. 6.
Effect of β-OH butyrate (βHB) on fasting hepatic gene expression in control and 3-hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2) knockdown (KD) mice. A–F: hepatic peroxisome-proliferator activated receptor-α (PPAR-α) mRNA expression (A), uncoupling protein 2 (UCP2) mRNA expression (B), phosphoenolpyruvate carboxykinase (PEPCK) mRNA expression (C), PEPCK enzymatic activity (D), carnitine palmitoyltransferase 1 (CPT1) mRNA (E), and CPT1 protein expression (F). Direct comparisons were made between injection (PBS or βHB) within antisense oligonucleotides (ASO)-treated group. NS, nonsignificant. P > 0.05. Number inside bar denotes n per group.
β-OH butyrate injections did not affect fasted PEPCK mRNA expression in mice from either ASO treatment (Fig. 6C; P > 0.05). Although mRNA expression was unaffected, β-OH butyrate injection decreased hepatic PEPCK enzymatic activity in HMGCS2 knockdown mice (Fig. 6D; P = 0.05). β-OH butyrate injections also decreased fasted hepatic CPT1 expression in knockdown mice, returning transcript levels to those seen in fasted controls (Fig. 6E; P < 0.05). Fasted hepatic CPT1 protein expression tended to be decreased by β-OH butyrate injections in control but not HMGCS2 knockdown mice (Fig. 6F; P = 0.09).
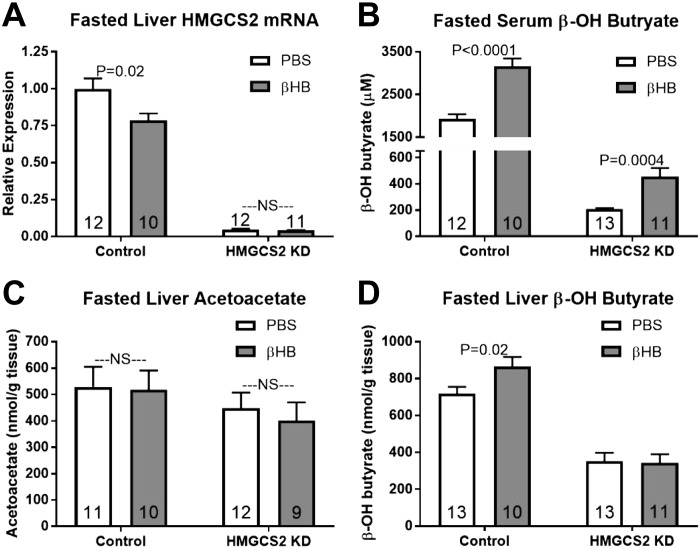
Fasted HMGCS2 mRNA expression in knockdown mice of all injection groups remained over 20-fold lower than fasted controls. In control mice, β-OH butyrate treatment lowered fasting HMGCS2 mRNA expression (Fig. 7A; P < 0.05). β-OH butyrate injections increased serum β-OH butyrate in control and HMGCS2 knockdown mice relative to saline injection (Fig. 7B; P < 0.05). β-OH butyrate injection did not affect liver acetoacetate concentrations in either ASO-treated group but increased liver β-OH butyrate concentrations in control mice (Fig. 7, C and D; P < 0.05).
Fig. 7.
Effect of β-OH butyrate (βHB) on fasting ketogenesis in control and 3-hydroxy-3-methylglutaryl-CoA synthase II (HMGCS2) knockdown (KD) mice. A–D: hepatic HMGCS2 mRNA expression (A), serum βHB (µM; B), hepatic acetoacetate (nmol/g liver tissue; C), and βHB (nmol/g liver tissue; D). Direct comparisons were made between injection (PBS or βHB) within antisense oligonucleotides (ASO)-treated group. NS, nonsignificant. P < 0.05. Number inside bar denotes n per group.
DISCUSSION
The switch from fed state hepatic glucose import and fatty acid synthesis to fasted state glucose and ketone export and fatty acid oxidation is critical to providing peripheral tissues with fuel during periods of food deprivation. Ketones are classically thought to serve as an alternative energy source during a fast, sparing glucose utilization for red blood cells. However, in the past 10 years, the metabocrine actions of β-OH butyrate have been recognized. In fact, β-OH butyrate, a ligand for two G protein-coupled receptors, alters second messenger pathways and signals intracellularly to affect pathway flux and gene expression (16, 41, 43). Almost half a century ago, ketones were first proposed to exert fine regulatory control over metabolism in the fasted state (39). Today, research highlighting β-OH butyrate as a regulator of metabolic rate, energy expenditure, autophagy, lipid and glucose metabolism, and feeding behavior has revitalized interest in the importance of this signaling metabolite in the adaptation to food deprivation (37). Indeed, our research establishes that β-OH butyrate regulates the normal hepatic fasting response.
Hepatic glucose output is central to the metabolic adaptations of fasting. Fasting serum glucose was elevated after eliminating hepatic production of ketones (Fig. 2B), while reintroducing ketone signaling through exogenous β-OH butyrate administration depressed serum glucose concentrations in HMGCS2 knockdown mice (Fig. 5B). Fasting hepatic glycogen concentrations were higher in HMGCS2 knockdown mice (Fig. 2A). Thus any increase in fasting hepatic glucose output with HMGCS2 knockdown was due to increased gluconeogenesis, not glycogenolysis. Hepatic glucose production is regulated both at the transcriptional and posttranslational level (21, 50). β-OH butyrate infusion in 24-h fasted miniature pigs decreases hepatic glucose production by 52% and reduces blood glucose concentrations (28). These changes are evident within 20 min after initiating β-OH butyrate infusion, proposing that β-OH butyrate mediated suppression of hepatic glucose production is not transcriptionally regulated. We show that β-OH butyrate suppresses fasting PEPCK activity in HMGCS2 knockdown mice without affecting PEPCK mRNA expression (Fig. 6, C and D). Posttranslational acetylation of PEPCK reduces activity, diminishing hepatic glucose production, and further targets PEPCK protein for degradation (21, 48). β-OH butyrate is known to inhibit HDAC activity (8). Through this mechanism, β-OH butyrate may increase PEPCK acetylation, resulting in decreased PEPCK activity and diminished hepatic glucose output. Accordingly, HMGCS2 knockdown using this same ASO enhances hepatic gluconeogenic flux from pyruvate and lactate (7).
HMGCS2 knockdown did not alter peripheral insulin-stimulated glucose clearance during an insulin tolerance test (Fig. 2H). In line with these results, β-OH butyrate infusion during a euglycemic insulin clamp in healthy humans had no effect on whole body insulin stimulated glucose clearance (2). Although acute (<4 h) β-OH butyrate exposure fails to affect insulin action (14, 44, 49), chronic (>16 h) β-OH butyrate exposure dose dependently inhibits insulin stimulated glucose uptake in oxidative skeletal muscle (44). Chronically, elevated ketones promote peripheral insulin resistance and alter β-cell metabolism, disrupting glucose homeostasis (14, 51). Therefore, in disease states that can result in chronic ketosis, such as NAFLD, persistently elevated β-OH butyrate concentrations may exacerbate insulin resistance.
We observed that mice that were unable to produce ketones had improved glucose tolerance, despite a decreased glucose-stimulated insulin response and no effect of HMGCS2 knockdown on insulin sensitivity assessed by insulin tolerance test (Fig. 2, E–H). In turn, we hypothesize that HMGCS2 knockdown mice responded to the intraperitoneal glucose challenge with a more severe inhibition of endogenous glucose production, which would explain the improved glucose tolerance despite no effect on insulin sensitivity. This hypothesis agrees with the data of Cotter et al. (7), who showed that hepatic glucose production was lower in HMGCS2 knockdown mice than in control mice. Admittedly, there is also a possibility that the stress of blood collection, to allow for measurement of oral glucose stimulated insulin secretion, differentially affected adrenergic stress response and subsequently glucose tolerance in HMGCS2 knockdown and control mice.
β-Oxidative and ketogenic flux are interconnected (25), and CPT1 and HMGCS2 control flux through these pathways (27, 45). Accordingly, HMGCS2 mRNA expression was highly correlated with serum β-OH butyrate concentrations in all mice (excluding β-OH butyrate injected groups; R2 = 0.94). Regulation of enzyme expression by pathway substrates or products is common. Carnitine increases transcription and activity of hepatic CPT1, while fatty acids increase CPT1 and UCP2 mRNA expression by increasing PPAR-α-driven transcription (5, 15). Mitochondrial uncoupling, induced by hepatic UPC2, is essential for regenerating the NAD+ necessary for β-oxidation and ketogenesis during fasting (33, 40). We show that β-OH butyrate injection decreased fasting CPT1 and UCP2 mRNA expression in HMGCS2 knockdown mice and tended to decrease fasting CPT1 protein expression in control mice (Fig. 6, B, E, and F). In addition to being upregulated by long-chain fatty acids (18), we show that HMGCS2 mRNA expression is downregulated by the terminal ketogenic product β-OH butyrate (Fig. 7A). Control of CPT1, UCP2, and HMGCS2 expression by both fatty acids and β-OH butyrate allows for transcriptional fine tuning of potential β-oxidative and ketogenic activity by both the substrate and product to meet the metabolic needs of the individual. Much like the negative feedback of β-OH butyrate on its own synthesis through inhibition of adipose tissue lipolysis (43), we show here that β-OH butyrate signaling feeds back to inhibit its own production and protect against excess ketosis by limiting expression of enzymes directly involved in its synthesis.
CPT1 mRNA and protein are differentially regulated by acute β-OH butyrate administration and chronic limitation of ketogenic flux. Acutely, β-OH butyrate downregulates CPT1 mRNA in HMGCS2 knockdown mice, while tending to downregulate CPT1 protein in control mice (Fig. 6, E and F). Surprisingly, HMGCS2 knockdown, which dramatically reduces ketogenic flux, decreases hepatic CPT1 protein content in both the fed and fasted state (Fig. 4F). The acetyl-CoA produced in β-oxidation is utilized in both the TCA cycle and ketone synthesis. We hypothesize that by limiting acetyl-CoA dissipation through ketogenesis, HMGCS2 knockdown increases hepatic acetyl-CoA concentrations, which feed back to limit CPT1 protein expression. Supportive of increased hepatic acetyl-CoA, Cotter et al. (7) show that HMGCS2 knockdown increases blood acetyl-carnitine concentrations. A build-up of acetyl-CoA is known to limit flux through β-oxidation (30). The trend for increased CPT1 mRNA expression with HMGCS2 knockdown suggests that β-OH butyrate feeds back to inhibit CPT1 gene transcription independent of acetyl-CoA-mediated downregulation of CPT1 protein expression.
During fasting, liver β-OH butyrate concentrations reach ~700 nmol/g tissue (Fig. 1D). With an average hepatic density of 1.05 ± 0.01 g/ml, we estimate the concentration to be ~735 µM, within the concentration range needed for β-OH butyrate to affect gene transcription through HDAC inhibition or ChREBP regulation (29, 31, 41). Therefore, the transcriptional regulation of fasting gene expression by β-OH butyrate may be mitigated directly by hepatic β-OH butyrate.
Conboy et al. (6) recently detailed a number of human mutations in the HMGCS2 gene that result in profound whole body metabolic abnormalities. HMGCS2 deficiency presents in the first year of life with hyperlipidemia and hypertriglyceridemia. Individuals with these mutations are often hypoglycemic (35), paralleling what we observed in fed state HMGCS2 knockdown mice (Fig. 2B). Lee et al. (20) describes another model of limited ketogenesis. Loss of hepatic CPT2 expression similarly results in hyperlipidemia and hypoglycemia in high-fat diet-fed mice (20). The rise in discovered inborn errors in ketone metabolism calls for pertinent research aimed at understanding the interplay among ketone, lipid, and glucose metabolism.
GRANTS
This work is supported by the Animal Health and Production and Animal Products: Improved Nutritional Performance, Growth, and Lactation of Animals Program Grant 2015-70007-24236 from the United States Department of Agriculture National Institute of Food and Agriculture (to B. J. Renquist), Arizona Biomedical Research Commission Early Stage Investigator Award ADHS14-082986 (to B. J. Renquist), Arizona Biomedical Research Commission Investigator Grant ADHS17-000007403 (to B. J. Renquist), and National Heart, Lung, and Blood Institute T32 Training Grant Project 5T32-HL-007249-42 (to C. E. Geisler).
DISCLAIMERS
The funding sources for this work had no role in study design, in the collection, analysis or interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.J.R. conceived and designed research; C.E.G., S.G., R.L.B., and B.J.R. performed experiments; C.E.G., R.L.B., and B.J.R. analyzed data; C.E.G., S.G., R.L.B., and B.J.R. interpreted results of experiments; C.E.G. and B.J.R. prepared figures; C.E.G. drafted manuscript; C.E.G., S.G., R.L.B., and B.J.R. edited and revised manuscript; C.E.G., S.G., R.L.B., and B.J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mark Graham and Ionis Pharmaceuticals, Carlsbad, CA for kindly providing both the HMGCS2 and control antisense oligonucleotides.
REFERENCES
- 1.Banner CD, Göttlicher M, Widmark E, Sjövall J, Rafter JJ, Gustafsson JA. A systematic analytical chemistry/cell assay approach to isolate activators of orphan nuclear receptors from biological extracts: characterization of peroxisome proliferator-activated receptor activators in plasma. J Lipid Res 34: 1583–1591, 1993. [PubMed] [Google Scholar]
- 2.Bratusch-Marrain PR, DeFronzo RA. Failure of hyperketonemia to alter basal and insulin-mediated glucose metabolism in man. Horm Metab Res 18: 185–189, 1986. doi: 10.1055/s-2007-1012266. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 48: 634–642, 2005. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 4.Cahill GF Jr, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA Jr, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest 45: 1751–1769, 1966. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatelain F, Kohl C, Esser V, McGarry JD, Girard J, Pegorier JP. Cyclic AMP and fatty acids increase carnitine palmitoyltransferase I gene transcription in cultured fetal rat hepatocytes. Eur J Biochem 235: 789–798, 1996. doi: 10.1111/j.1432-1033.1996.00789.x. [DOI] [PubMed] [Google Scholar]
- 6.Conboy E, Vairo F, Schultz M, Agre K, Ridsdale R, Deyle D, Oglesbee D, Gavrilov D, Klee EW, Lanpher B. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase deficiency: unique presenting laboratory values and a review of biochemical and clinical features. JIMD Rep 40: 63–69, 2017. doi: 10.1007/8904_2017_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter DG, Ercal B, Huang X, Leid JM, d’Avignon DA, Graham MJ, Dietzen DJ, Brunt EM, Patti GJ, Crawford PA. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest 124: 5175–5190, 2014. doi: 10.1172/JCI76388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 133, Suppl 7: 2485S–2493S, 2003. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 9.Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res 41: 197–239, 2002. doi: 10.1016/S0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]
- 10.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 11.Geisler CE, Hepler C, Higgins MR, Renquist BJ. Hepatic adaptations to maintain metabolic homeostasis in response to fasting and refeeding in mice. Nutr Metab (Lond) 13: 62, 2016. doi: 10.1186/s12986-016-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hepler C, Foy CE, Higgins MR, Renquist BJ. The hypophagic response to heat stress is not mediated by GPR109A or peripheral β-OH butyrate. Am J Physiol Regul Integr Comp Physiol 310: R992–R998, 2016. doi: 10.1152/ajpregu.00513.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Im SS, Kim MY, Kwon SK, Kim TH, Bae JS, Kim H, Kim KS, Oh GT, Ahn YH. Peroxisome proliferator-activated receptor α is responsible for the up-regulation of hepatic glucose-6-phosphatase gene expression in fasting and db/db mice. J Biol Chem 286: 1157–1164, 2011. doi: 10.1074/jbc.M110.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanikarla-Marie P, Jain SK. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic Biol Med 95: 268–277, 2016. doi: 10.1016/j.freeradbiomed.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlic H, Lohninger S, Koeck T, Lohninger A. Dietary L-carnitine stimulates carnitine acyltransferases in the liver of aged rats. J Histochem Cytochem 50: 205–212, 2002. doi: 10.1177/002215540205000208. [DOI] [PubMed] [Google Scholar]
- 16.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108: 8030–8035, 2011. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA 94: 4318–4323, 1997. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostiuk MA, Keller BO, Berthiaume LG. Palmitoylation of ketogenic enzyme HMGCS2 enhances its interaction with PPARα and transcription at the Hmgcs2 PPRE. FASEB J 24: 1914–1924, 2010. doi: 10.1096/fj.09-149765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs S, Fischaleck M, Blum H. A simple and loss-free method to remove TRIzol contaminations from minute RNA samples. Anal Biochem 387: 136–138, 2009. doi: 10.1016/j.ab.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Choi J, Selen Alpergin ES, Zhao L, Hartung T, Scafidi S, Riddle RC, Wolfgang MJ. Loss of hepatic mitochondrial long-chain fatty acid oxidation confers resistance to diet-induced obesity and glucose intolerance. Cell Reports 20: 655–667, 2017. doi: 10.1016/j.celrep.2017.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin YY, Lu JY, Zhang J, Walter W, Dang W, Wan J, Tao SC, Qian J, Zhao Y, Boeke JD, Berger SL, Zhu H. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell 136: 1073–1084, 2009. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Ljunggren G. The preparation of acetoacetic solutions (in German). Biochem Z 145: 422–425, 1924. [Google Scholar]
- 24.Lo S, Russell JC, Taylor AW. Determination of glycogen in small tissue samples. J Appl Physiol 28: 234–236, 1970. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- 25.Männistö VT, Simonen M, Hyysalo J, Soininen P, Kangas AJ, Kaminska D, Matte AK, Venesmaa S, Käkelä P, Kärjä V, Arola J, Gylling H, Cederberg H, Kuusisto J, Laakso M, Yki-Järvinen H, Ala-Korpela M, Pihlajamäki J. Ketone body production is differentially altered in steatosis and non-alcoholic steatohepatitis in obese humans. Liver Int 35: 1853–1861, 2015. doi: 10.1111/liv.12769. [DOI] [PubMed] [Google Scholar]
- 26.Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E. The nuclear receptor LXR is a glucose sensor. Nature 445: 219–223, 2007. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 27.Monsénégo J, Mansouri A, Akkaoui M, Lenoir V, Esnous C, Fauveau V, Tavernier V, Girard J, Prip-Buus C. Enhancing liver mitochondrial fatty acid oxidation capacity in obese mice improves insulin sensitivity independently of hepatic steatosis. J Hepatol 56: 632–639, 2012. doi: 10.1016/j.jhep.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Müller MJ, Paschen U, Seitz HJ. Effect of ketone bodies on glucose production and utilization in the miniature pig. J Clin Invest 74: 249–261, 1984. doi: 10.1172/JCI111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa T, Ge Q, Pawlosky R, Wynn RM, Veech RL, Uyeda K. Metabolite regulation of nucleo-cytosolic trafficking of carbohydrate response element-binding protein (ChREBP): role of ketone bodies. J Biol Chem 288: 28358–28367, 2013. doi: 10.1074/jbc.M113.498550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oram JF, Bennetch SL, Neely JR. Regulation of fatty acid utilization in isolated perfused rat hearts. J Biol Chem 248: 5299–5309, 1973. [PubMed] [Google Scholar]
- 31.Overmoyer BA, McLaren CE, Brittenham GM. Uniformity of liver density and nonheme (storage) iron distribution. Arch Pathol Lab Med 111: 549–554, 1987. [PubMed] [Google Scholar]
- 32.Pan JW, Rothman TL, Behar KL, Stein DT, Hetherington HP. Human brain β-hydroxybutyrate and lactate increase in fasting-induced ketosis. J Cereb Blood Flow Metab 20: 1502–1507, 2000. doi: 10.1097/00004647-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, Ricquier D, Miroux B, Thompson CB. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J 22: 9–18, 2008. doi: 10.1096/fj.07-8945com. [DOI] [PubMed] [Google Scholar]
- 34.Pineda Torra I, Jamshidi Y, Flavell DM, Fruchart JC, Staels B. Characterization of the human PPARα promoter: identification of a functional nuclear receptor response element. Mol Endocrinol 16: 1013–1028, 2002. doi: 10.1210/mend.16.5.0833. [DOI] [PubMed] [Google Scholar]
- 35.Pitt JJ, Peters H, Boneh A, Yaplito-Lee J, Wieser S, Hinderhofer K, Johnson D, Zschocke J. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase deficiency: urinary organic acid profiles and expanded spectrum of mutations. J Inherit Metab Dis 38: 459–466, 2015. doi: 10.1007/s10545-014-9801-9. [DOI] [PubMed] [Google Scholar]
- 36.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339: 62–66, 2003. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 37.Rojas-Morales P, Tapia E, Pedraza-Chaverri J. β-Hydroxybutyrate: A signaling metabolite in starvation response? Cell Signal 28: 917–923, 2016. doi: 10.1016/j.cellsig.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Salway JG. The simultaneous determination of acetoacetate and glucose in capillary blood. Clin Chim Acta 25: 109–116, 1969. doi: 10.1016/0009-8981(69)90235-6. [DOI] [PubMed] [Google Scholar]
- 39.Senior B, Loridan L. Direct regulatory effect of ketones on lipolysis and on glucose concentrations in man. Nature 219: 83–84, 1968. doi: 10.1038/219083a0. [DOI] [PubMed] [Google Scholar]
- 40.Sheets AR, Fülöp P, Derdák Z, Kassai A, Sabo E, Mark NM, Paragh G, Wands JR, Baffy G. Uncoupling protein-2 modulates the lipid metabolic response to fasting in mice. Am J Physiol Gastrointest Liver Physiol 294: G1017–G1024, 2008. doi: 10.1152/ajpgi.00016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339: 211–214, 2013. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens JM, Sulway MJ, Watkins PJ. Relationship of blood acetoacetate and 3-hydroxybutyrate in diabetes. Diabetes 20: 485–489, 1971. doi: 10.2337/diab.20.7.485. [DOI] [PubMed] [Google Scholar]
- 43.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. (D)-β-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280: 26649–26652, 2005. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 44.Tardif A, Julien N, Pelletier A, Thibault G, Srivastava AK, Chiasson JL, Coderre L. Chronic exposure to β-hydroxybutyrate impairs insulin action in primary cultures of adult cardiomyocytes. Am J Physiol Endocrinol Metab 281: E1205–E1212, 2001. doi: 10.1152/ajpendo.2001.281.6.E1205. [DOI] [PubMed] [Google Scholar]
- 45.Vilà-Brau A, De Sousa-Coelho AL, Mayordomo C, Haro D, Marrero PF. Human HMGCS2 regulates mitochondrial fatty acid oxidation and FGF21 expression in HepG2 cell line. J Biol Chem 286: 20423–20430, 2011. doi: 10.1074/jbc.M111.235044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker PG. A colorimetric method for the estimation of acetoacetate. Biochem J 58: 699–704, 1954. doi: 10.1042/bj0580699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103: 514–527, 1967. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong Y, Lei QY, Zhao S, Guan KL. Regulation of glycolysis and gluconeogenesis by acetylation of PKM and PEPCK. Cold Spring Harb Symp Quant Biol 76: 285–289, 2011. doi: 10.1101/sqb.2011.76.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada T, Zhang SJ, Westerblad H, Katz A. β-hydroxybutyrate inhibits insulin-mediated glucose transport in mouse oxidative muscle. Am J Physiol Endocrinol Metab 299: E364–E373, 2010. doi: 10.1152/ajpendo.00142.2010. [DOI] [PubMed] [Google Scholar]
- 50.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413: 131–138, 2001. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 51.Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab 80: 1584–1590, 1995. doi: 10.1210/jcem.80.5.7745004. [DOI] [PubMed] [Google Scholar]