Abstract
Diabetes is a worldwide health problem. Roux-en-Y gastric bypass (RYGB) leads to rapid resolution of type 2 diabetes (T2D). Decreased hepatic insulin resistance is key, but underlying mechanisms are poorly understood. We hypothesized that changes in intestinal function and subsequent changes in portal venous milieu drive some of these postoperative benefits. We therefore aimed to evaluate postoperative changes in portal milieu. Two rat strains, healthy [Sprague-Dawley (SD)] and obese diabetic [Zucker diabetic fatty (ZDF)] rats, underwent RYGB or control surgery. After 4 wk, portal and systemic blood was sampled before and during an intestinal glucose bolus to investigate changes in intestinal glucose absorption (Gabsorp) and utilization (Gutil), and intestinal secretion of incretins and glucagon-like peptide-2 (GLP-2). Hepatic activity of dipeptidyl peptidase-4 (DPP4), which degrades incretins, was also measured. RYGB decreased Gabsorp in both rat strains. Gutil increased in SD rats and decreased in ZDF rats. In both strains, there was increased expression of intestinal hexokinase and gluconeogenesis enzymes. Systemic incretin and GLP-2 levels also increased after RYGB. This occurred without an increase in secretion. Hepatic DPP4 activity and expression were unchanged. RYGB perturbs multiple intestinal pathways, leading to decreased intestinal glucose absorption and increased incretin levels in both healthy and diabetic animals. In diabetic rats, intestinal glucose balance shifts toward glucose release. The portal vein as the gut-liver axis may integrate these intestinal changes to contribute to rapid changes in hepatic glucose and hormone handling. This fresh insight into the surgical physiology of RYGB raises the hope of less invasive alternatives.
NEW & NOTEWORTHY Portal milieu after gastric bypass surgery is an underinvestigated area. Roux-en-Y gastric bypass perturbs multiple intestinal pathways, reducing intestinal glucose absorption and increasing incretin levels. In diabetic rats, the intestine becomes a net releaser of glucose, increasing portal glucose levels. The portal vein as the gut-liver axis may integrate these intestinal changes to contribute to changes in hepatic glucose handling. This fresh insight raises the hope of less invasive alternatives.
Keywords: bariatric surgery, glucose metabolism, intestinal metabolism, metabolic surgery
INTRODUCTION
The epidemic of type 2 diabetes (T2D) affects almost 400 million people worldwide (11) and is increasing in parallel with the obesity epidemic. Although medical therapy is the mainstay of treatment, Roux-en-Y gastric bypass (RYGB) remains the fastest and most effective treatment available for obesity-induced T2D (18, 32). However, RYGB cannot feasibly be offered on a global scale, and our incomplete mechanistic understanding of T2D resolution hinders the development of less invasive alternatives. Proposed mechanisms include foregut exclusion (28), decreased intestinal glucose absorption (35), increased intestinal glucose utilization (Gutil) (31), increased intestinal gluconeogenesis (37) and increased glucagon-like peptide (GLP)-1 levels (33), as well as more recent data on changes in bile salts and microbiome (3, 17, 29, 36). Roux limb hypertrophy has also been described in animal models (9, 35).
Each of these previous studies has focused on a single mechanism, none of which alone can fully explain the early metabolic benefits of RYGB. We believe that several of these antidiabetic mechanisms are enhanced after RYGB, leading to early improved hepatic insulin sensitivity and glucose handling (5). We hypothesize that the change in foregut intestinal anatomy perturbs the intestine at several levels: morphology, glucose absorption and utilization, and metabolism. As the portal vein drains the intestine, portal milieu will be altered. Previous studies looking at intestinal glucose metabolism have shown inconsistent results, with both increased utilization (30) and increased glucose release (37) being reported. This is an important area for further research. We have previously shown that changes in portal nutrient sensing can alter hepatic glucose handling through a neurally mediated pathway (24). We further hypothesize changes in portal milieu after RYGB drive the beneficial hepatic effects.
In these studies, we describe the multiple antidiabetic mechanisms of RYGB and elucidate the net effect on the portal vein. Using two rat models, we sample both the portal and systemic circulations to specifically characterize the contribution of the small intestine to portal nutrient and hormone content. We also measure intestinal weight, to ascertain whether hypertrophy could account for responses to RYGB, and expression of enzymes involved in glycolysis and gluconeogenesis. We propose these intestinal changes through portal milieu drive hepatic changes.
METHODS
Surgical Procedures
Animal studies were performed in accordance with protocols prospectively approved by the Harvard Medical Area Standing Committee on Animals. Male Sprague-Dawley (SD, 220–240 g; Harlan) and male Zucker diabetic fatty (ZDF, 9 wk; Charles River) rats were used. The SD rat is a healthy rodent model, and animals were ordered by weight according to their growth curve to match ZDF age. The ZDF rat is an obese diabetic model that has a mutation in the leptin receptor gene (fa gene) leading to polyphagia, obesity, glucose intolerance, and fasting hyperglycemia (34). We selected 9-wk-old ZDF rats with random glucose >250 mg/dl to reduce the variability in the diabetic phenotype.
Rats were acclimatized for 7 days under a 12:12-h light-dark cycle (lights on 7:00 AM) with ad libitum access to standard rat chow (Purina 5053) for SD rats and high-fat diet (Purina 5008) for ZDF rats. After an overnight fast, rats were anesthetized using isoflurane (1–3% in oxygen).
RYGB
RYGB was performed as previously described by our group (4, 35). In summary, the stomach was divided using a linear stapler to create the gastric pouch and gastric remnant. A 16-cm biliopancreatic (BP) limb and 10-cm Roux limb were constructed [Supplemental Fig. S1A (All Supplemental data can be found at https://doi.org/10.6084/m9.figshare.7764506.v1)], and hand-sewn gastrojejunal (GJ) and jejuno-jejunal (JJ) anastomosis were performed (PDS 6/0 suture). Animals were maintained on liquid diet for 5 days postoperatively and then switched to a solid diet with ad libitum access to rat chow (Purina 5053 for SD rats, Purina 5008 for ZDF rats).
Control Surgery
In the control group (Supplemental Fig. S1B), the small intestine was divided (16 cm from the ligament of Treitz) and then anastomosed (PDS 6/0 interrupted sutures). For the control group in this study, two possible options were considered. Although the option of a laparotomy alone would have been technically easier, it would only have controlled for the effects of anesthesia but not the effects of intestinal anastomosis. We therefore elected to perform intestinal transection and anastomosis, which would control for the latter. In our acute studies (25), baseline portosystemic gradient was −6.4 ± 1.5 mg/dl, corresponding to intestinal Gutil of 32 mg/h. This contrasts with the transection-anastomosis SD rat control group Gutil of 61 mg/h (P < 0.05). This suggests that, in control animals, healing of the intestinal anastomosis may contribute to increased Gutil. In the RYGB groups, additional factors are contributing to increased Gutil and intestinal glucose balance.
For oral intake, animals had ad libitum access to water. From postoperative day 1 to 5, a liquid diet (Liquid Nutrition chocolate-flavored; CVS Pharmacy, Woonsocket, RI) was provided, and animals remained on the metallic tray. On postoperative day 6, a solid diet was resumed with rat chow. Animals were weighed daily. Solid food intake was measured daily from postoperative day 6 onward, and, for this purpose, animals were caged separately postoperatively.
Portal and Systemic Sampling Experiments
Following a 4-wk period of recovery, animals underwent portal and systemic sampling experiments. After an overnight fast, rats were anaesthetized using isoflurane (1–3% in oxygen). Experiments were consistently started at 9:00 AM to avoid the confounding factor of diurnal variation and conducted under anesthesia to allow for accurate catheter placement and organ harvest at the end of the experiment
Portal and systemic venous blood was sampled before and during an intestinal glucose bolus to measure glucose and hormone levels for each group (Supplemental Fig. S2, A and B). Portosystemic gradients were used to calculate intestinal glucose fluxes and hormone secretion (see Calculation of Intestinal Glucose Fluxes and Calculation of Hormone Secretion). In brief, for systemic sampling, a Silastic catheter was advanced through the right jugular vein in the right atrium to sample mixed systemic venous blood. For portal sampling, a Silastic catheter was inserted through the superior mesenteric vein (SMV) in the portal vein. Portal and systemic blood was sampled before (0 min) and then 10, 30, and 60 min after the start of the glucose bolus (200 μl from each catheter at each time point), and glucose level was measured (LifeScan OneTouch glucometer).
In control rats (Supplemental Fig. S2C), the intestinal catheter was placed in the duodenum (just distal to the pylorus), and they received a whole intestinal glucose bolus (2 g/kg), with the pylorus ligated to prevent backflow. In RYGB animals (Supplemental Fig. S2D), the intestinal catheter was placed in the Roux limb (just distal to the GJ anastomosis) and secured with a silk suture, and a glucose bolus (2 g/kg) was delivered in the Roux limb.
Tissue Harvest and Storage
At the end of each experiment, the intestine was harvested. The entire small intestine was weighed (total intestinal weight), four 10-cm intestinal segments were weighed, and the following mucosal samples were taken: BP limb (5–15 cm distal to the ligament of Treitz), Roux limb (entire segment of intestine), common limb (0–10 cm distal to JJ anastomosis), and terminal ileum (0–10 cm proximal to the cecum). Corresponding segments of intestine were harvested from control animals. Samples were rapidly frozen in liquid N2 and stored at −80°C.
Enzyme Assays
Hepatic dipeptidyl peptidase-4 (DPP4) activity was measured using a fluorescent assay kit (MAK088; Sigma-Aldrich, St. Louis, MO). One DPP4 unit is defined as the amount of enzyme that hydrolyzes the nonfluorescent DPP4 substrate to yield 1.0 mmol of the fluorescent product 7-amino-4-methyl coumarin per minute at 37°C. Fluorescence was quantified using λex = 360 nm and λem = 460 nm (Spectramax M5; Molecular Devices, Sunnyvale, CA).
Real-Time PCR for mRNA Expression
Expression of mRNA for intestinal and liver proteins involved in glucose metabolism was determined relative to β-actin (internal reference). RNA was extracted from tissue samples using the mirVana mRNA Isolation Kit (Ambion, Grand Island, NY) and quantified (Spectramax M5; Molecular Devices). RNA (2 μg) was reverse transcribed [Superscript III and oligo(dT); Invitrogen-Life Technologies] to generate cDNA. Real-time PCR was then performed (ABI 7900HT; Applied Biosystems) on a 384-well plate using SYBR Green (Life Technologies). Primers used were phosphenolpyruvate carboxykinase (PEPCK): forward CTCACCTCTGGCCAAGATTGGTA, reverse GTTGCAGGCCCAGTTGTTGA; glucose-6-phosphatase (G-6-Pase): forward AACGTCTGTCTGTCCCGGATCTAC, reverse ACCTCTGGAGGCTGGCATTG; and DPP4: forward TCCCAACTCCAGAGGACAAC, reverse CAGGGCTTTGGAGATCTGAG.
Calculation of Intestinal Glucose Fluxes
Intestinal glucose fluxes were calculated as previously described (25), using the portosystemic glucose gradient (GPS; Supplemental Fig. S3, A and B):
where GP is portal glucose level and GS is systemic glucose level at a given time point.
At baseline (0 min), before the intestinal glucose infusion was started, GPS was negative (i.e., GP < GS), reflecting the net use of glucose by the intestine in the fasting state. Gutil (mg/h) was calculated:
where GPS0 is the portosystemic gradient at 0 min, and PBF is portal blood flow (ml/min).
After intestinal glucose infusion was started, GPS became positive, indicating glucose absorption by the intestine in the portal bloodstream. The area under the curve of GPS (2), corrected for baseline gradient, was used to estimate intestinal glucose absorption (Gabsorp; mg/h) as follows:
where GPS(AUC) is area under the curve, or “portosystemic area,” of GPS from 0 to 60 min (mg·dl−1·min−1).
Calculation of Hormone Secretion
Hormone levels in portal and systemic blood were determined at 0, 10, 30, and 60 min. The portosystemic hormone gradient was calculated:
where GLP-1P and GLP-1S are portal and systemic GLP-1 levels (pg/ml), respectively.
GLP-1PS was positive at baseline, as expected, and increased following glucose infusion, indicating GLP-1 secretion by the intestine in the portal blood (Supplemental Fig. S3C). The area under the curve of the GLP-1 portosystemic gradient (GLP-1PS; Supplemental Fig. S3D) was used to calculate intestinal GLP-1 secretion (GLP-1secrete; ng/h) as follows:
where GLP-1PS(AUC) is area under the curve, or portosystemic area, of GLP-1PS from 0 to 60 min (pg/ml min)
Similar calculations were used for GLP-2 and gastric inhibitory polypeptide (GIP).
Portal Flow Measurement
Portal blood flow was measured, for use in intestinal glucose flux calculations, using transabdominal ultrasound Doppler (Vevo 2100; VisualSonics, Toronto, Canada). The portal vein, which runs from the splenic vein-SMV confluence to its bifurcation into left and right branches, was identified. The mean diameter was calculated from two measurements (at the confluence and the bifurcation). The mean flow velocity was measured in the sagittal section view. Portal blood flow was calculated as the product of vein area and mean velocity.
Portal vein diameter was 2.2 ± 0.09 mm and portal flow was 9.6 ± 0.7 ml/min in SD rats. Portal vein diameter was 1.9 ± 0.04 mm and portal flow was 8.5 ± 0.5 ml/min in ZDF rats. There was no significant difference between RYGB and control rats in either strain.
Statistical Analysis
Data analysis was performed using Excel. The two-tailed unpaired t-test was used for planned comparison of two groups. Data are reported as means ± SE.
RESULTS
RYGB Causes Weight Loss and Reduced Food Intake
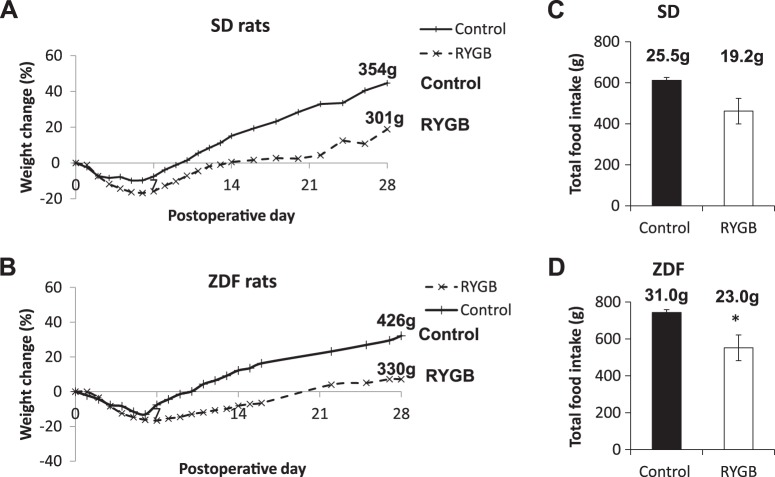
RYGB led to a greater degree of weight loss compared with controls in both SD and ZDF rats. The weight difference was significant from postoperative day 6 onward in both rat strains (SD day 28 weight change +18.9% vs. +44.6, RYGB vs. control, P < 0.001; ZDF day 28 weight change −4.6 vs. +22.9%, P < 0.01; Fig. 1 and Supplemental Fig. S4). There was a trend to reduced solid food intake (postoperative day 5 to 28) following RYGB in SD rats (total intake 462 ± 62 vs. 612 ± 14 g, RYGB vs. control, P = 0.06) and a significant reduction in food intake following RYGB in ZDF rats (total intake 552 ± 70 vs. 743 ± 16 g, RYGB vs. control, P < 0.01). None of the animals developed diarrhea or other suggestion of intestinal malabsorption.
Fig. 1.
Postoperative weight change and food intake. Weight changes for Sprague-Dawley (SD, A) and Zucker diabetic fatty (ZDF, B) rats are plotted as %initial weights before surgery. The value at the end of each line is the mean weight at 28 days for that group. Total food intake from postoperative day 6 to 28 (23 days) is shown for SD (C) and ZDF (D) rats. The value above each bar is the calculated average daily food intake for that experimental group. RYGB, Roux-en-Y gastric bypass. *P < 0.05 vs. control.
Intestinal Hypertrophy After RYGB
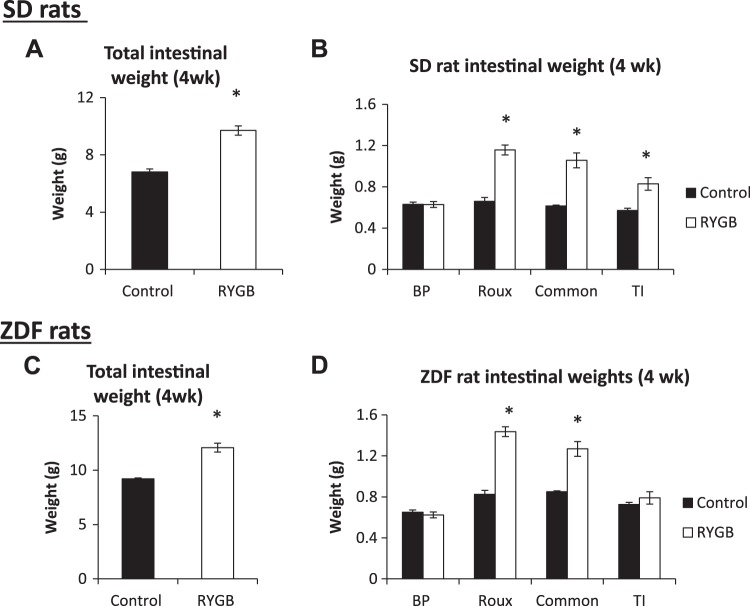
Total intestinal weight increased following RYGB in SD (9.7 ± 0.3 vs. 6.8 ± 0.2 g, RYGB vs. control, respectively; P < 0.001) and ZDF (12.1 ± 0.4 vs. 9.2 ± 0.1 g; P < 0.05) rats. In both strains, the weight increase was particularly marked in the Roux limb, but significant increases also occurred in the common limb and terminal ileum (Fig. 2). The combined effects of decrease in total weight (Fig. 1) and increase in intestinal weight following RYGB led to a substantial increase in intestinal weight as a percent of total body weight in both species. For SD rats, this ratio nearly doubled (control 1.9% vs. RYGB 3.6%, P < 0.001), and there was also a significant increase in ZDF rats (2.2 vs. 3.7%, P < 0.05).
Fig. 2.
Intestinal weight. Total intestinal (A and C) and segmental intestinal (B and D) weights for Sprague-Dawley (SD, A and B) and Zucker diabetic fatty (ZDF, C and D) rats. BP, biliopancreatic; RYGB, Roux-en-Y gastric bypass; TI, terminal ileum. *P < 0.05 vs. control.
Strain-Specific Effects on Intestinal Glucose Balance
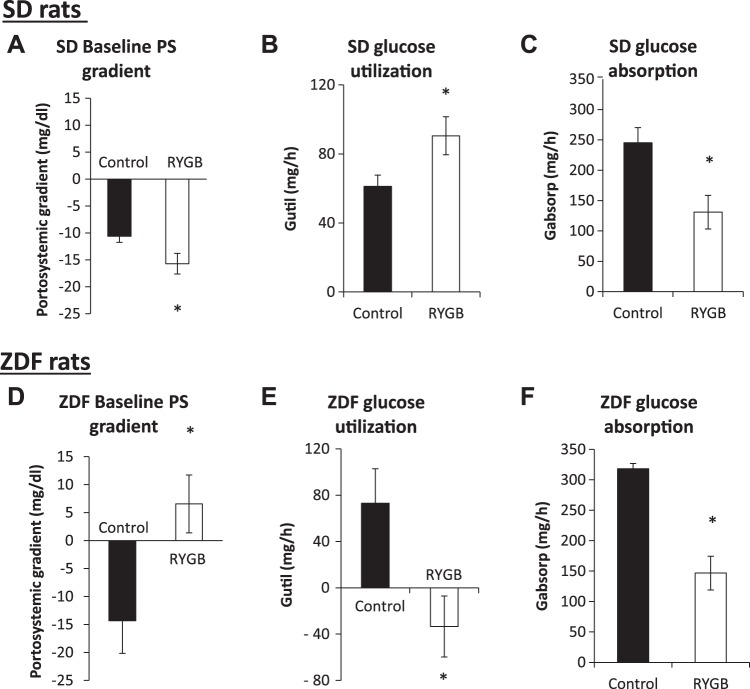
Gutil is derived from the portosystemic gradient for glucose in fasted rats (see methods). In both strains in the control group, the negative gradient (Fig. 3, A and D) corresponds to net Gutil (Fig. 3, B and E), indicative of greater intestinal utilization over production. In SD rats, the lower portosystemic gradient after RYGB (−15.7 ± 1.9 vs. −10.6 ± 1.1 mg/dl in controls; Fig. 3A) corresponds to an increase in Gutil (91 ± 11 vs. 61 ± 7 mg/h, P < 0.05; Fig. 3B). In contrast, the baseline portosystemic gradient became positive in ZDF rats following RYGB (+6.6 ± 5.2 vs. −14.3 ± 5.8 mg/dl; Fig. 3B), which reflects a negative Gutil (−33 ± 26 vs. 73 ± 30 mg/h, P < 0.05; Fig. 3E) or the emergence of net intestinal glucose release.
Fig. 3.
Intestinal glucose utilization and absorption. The baseline portosystemic (PS) gradient (before glucose infusion) was more negative in Sprague-Dawley (SD) rats following Roux-en-Y gastric bypass (RYGB) than in control SD rats (A). In contrast, the baseline portosystemic gradient in Zucker diabetic fatty (ZDF) rats, which was also negative in the control group, was positive after RYGB (D). Therefore, net intestinal glucose utilization increased in SD rats (B) but decreased in ZDF rats (E). Intestinal glucose absorption (after glucose infusion) decreased in both SD (C) and ZDF (F) rats after RYGB vs. the respective values in the control groups. *P < 0.05 vs. control.
Intestinal Glucose Absorption Reduced
As Gutil is derived from the portosystemic gradient before glucose infusion, Gabsorp is derived from the area under the curve of the portosystemic gradient over 1 h of a glucose infusion. RYGB reduced Gabsorp compared with control surgery in both SD (131 ± 28 vs. 246 ± 25 mg/h, P < 0.01; Fig. 3C) and ZDF (146 ± 20 vs. 318 ± 9 mg/h, P < 0.01; Fig. 3F) rats. Between the rat strains, control Gabsorp was higher in ZDF rats than in SD rats (P = 0.02; see discussion). Notably, the postoperative Gabsorp after RYGB was similar in both strains (P = 0.71), with a greater decrease seen after RYGB in ZDF rats.
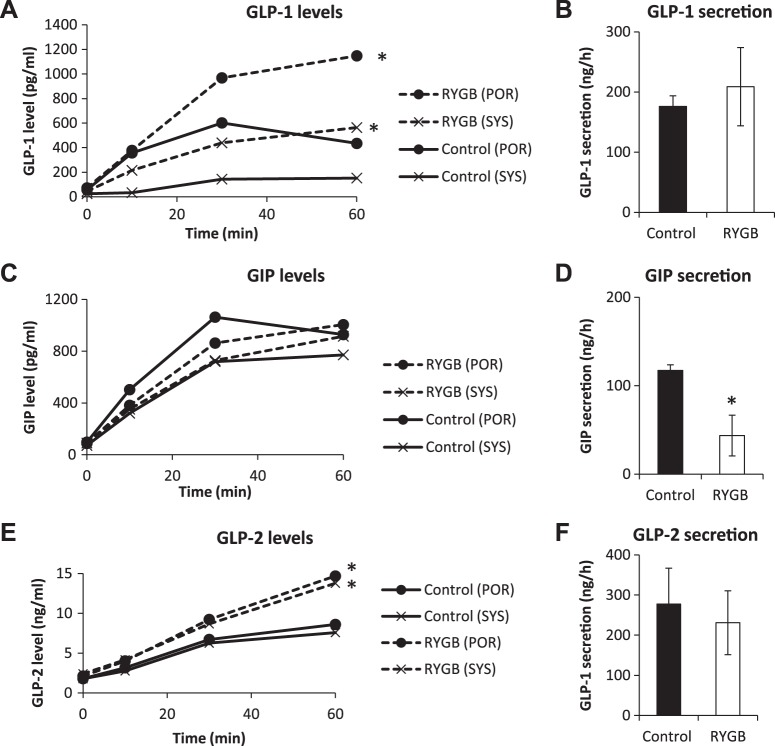
Incretin Hormone Levels and Secretion
Our strategy of measuring both portal and systemic blood levels was applied to probing the secretion of GLP-1, GIP, and GLP-2.
GLP-1.
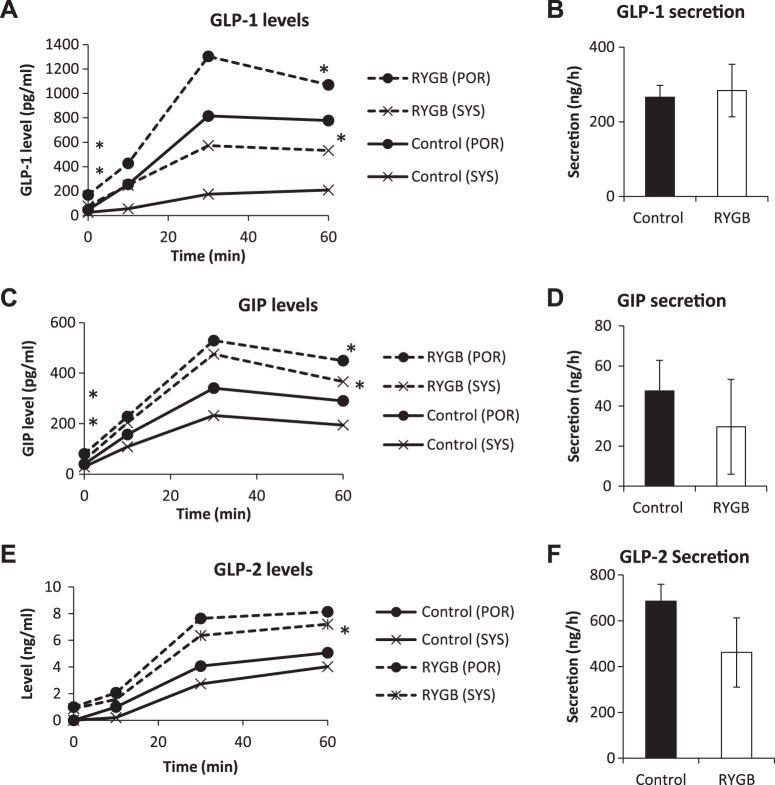
In SD rats after RYGB, systemic and portal GLP-1 levels were higher at 0 (systemic 77 ± 15 vs. 25 ± 5 pg/ml, P < 0.01; portal 169 ± 41 vs. 46 ± 15 pg/ml, P < 0.01) and at 60 (systemic 532 ± 115 vs. 209 ± 58 pg/ml, P < 0.05; portal 1,070 ± 107 vs. 778 ± 126 pg/ml, P < 0.05; Fig. 4A) min after the intestinal glucose bolus compared with controls. However, because the areas under the curve of the portosystemic gradient (portosystemic areas) were nearly equivalent (data not shown), it appeared that GLP-1 secretion did not differ between the groups (283 ± 99 vs. 266 ± 45 ng/h, RYGB vs. control, P = 0.81; Fig. 4B).
Fig. 4.
Hormone secretion in Sprague-Dawley (SD) rats. Portal and systemic levels of glucagon-like peptide (GLP)-1 (A), gastric inhibitory polypeptide (GIP, C), and GLP-2 (E) were measured over the course of intestinal glucose infusion in SD rats. Secretion rates of GLP-1 (B), GIP (D), and GLP-2 (F) were calculated from the portosystemic area as described in methods. RYGB, Roux-en-Y gastric bypass. *P < 0.05 vs. control.
In ZDF rats, baseline systemic and portal GLP-1 levels remained unchanged by RYGB. However, systemic and portal levels were higher than controls at 60 min (systemic 600 ± 119 vs. 153 ± 50 pg/ml, P < 0.05; portal 1,051 ± 256 vs. 435 ± 141 pg/ml; P < 0.05; Fig. 5A). As with SD rats, GLP-1 secretion did not change substantially (209 ± 65 vs. 176 ± 18 ng/h, RYGB vs. control, P = 0.64; Fig. 5B).
Fig. 5.
Hormone secretion in Zucker diabetic fatty (ZDF) rats. Portal and systemic levels of glucagon-like peptide (GLP)-1 (A), gastric inhibitory polypeptide (GIP, C), and GLP-2 (E) were measured over the course of intestinal glucose infusion in ZDF rats. Secretion rates of GLP-1 (B), GIP (D), and GLP-2 (F) were calculated from the portosystemic area as described in methods. RYGB, Roux-en-Y gastric bypass. *P < 0.05 vs. control.
GIP.
In SD rats after RYGB, both systemic and portal GIP levels were higher at baseline (systemic 56 ± 6 vs. 29 ± 6 pg/ml, P < 0.01; portal 81 ± 16 vs. 40 ± 6 pg/ml, P < 0.05) and at 60 min (systemic 366 ± 40 vs. 195 ± 28 pg/ml, P < 0.05; portal 450 ± 39 vs. 290 ± 43 pg/ml, P < 0.05; Fig. 4C). However, the portosystemic areas for GIP were similar in control and RYGB groups, which led to values of GIP secretion that were not different between the groups (30 ± 24 vs. 48 ± 15 ng/h, RYGB vs. control, P = 0.37; Fig. 4D).
In ZDF rats, RYGB did not alter systemic or portal GIP levels either at baseline or at 60 min. However, the reduced portosystemic area after RYGB translates to a significant reduction in GIP secretion (44 ± 23 vs. 117 ± 6 ng/h, RYGB vs. control, P < 0.05; Fig. 5, C and D), suggesting decreased GIP degradation. This contrasts with SD rats, where GIP levels were elevated after RYGB. This may be because baseline GIP levels are higher in ZDF rats than SD rats (see Species comparison).
GLP-2.
In SD rats after RYGB, there were no differences in baseline systemic and portal GLP-2 levels. At 60 min, systemic and portal levels were higher (systemic 7.2 ± 1.3 vs. 4.0 ± 0.6 ng/ml, P < 0.05; portal 8.1 ± 1.6 vs. 5.1 ± 0.7 ng/ml, P = 0.08; Fig. 4E). There was no significant change in GLP-2 secretion (462 ± 151 vs. 686 ± 74 ng/h, P = 0.26; Fig. 4F).
In ZDF rats after RYGB, there were no differences in baseline systemic and portal GLP-2 levels. At 60 min, systemic and portal levels were higher (systemic 13.8 ± 2.0 vs. 7.6 ± 1.6 ng/ml, P < 0.05; portal 16.3 ± 1.1 vs. 8.6 ± 2.0 ng/ml, P < 0.05; Fig. 5E). Again, as with SD rats, GLP-2 secretion was not different after RYGB (231 ± 80 vs. 277 ± 89 ng/h, P = 0.71; Fig. 5F).
Species comparison.
We then compared baseline hormone levels between control SD rats and control ZDF rats to determine whether the diabetic phenotype had an effect. There was no difference in baseline GLP-1 level (25 ± 5 vs. 25 ± 7 pg/ml, SD vs. ZDF, P = 0.97). Baseline GIP levels were significantly elevated in ZDF rats (29 ± 6 vs. 71 ± 10 pg/ml, SD vs. ZDF, P < 0.01), as were baseline GLP-2 levels (0 vs. 1.8 ± 0.4 ng/ml, SD vs. ZDF, P < 0.01). As described above, there were also species-specific changes in baseline hormone levels after RYGB: in SD rats, baseline systemic GLP-1 increased (3-fold), GIP increased (2-fold), and GLP-2 was unchanged; in ZDF rats, baseline systemic incretin and GLP-2 levels were unchanged.
Intestinal and Hepatic Gene Expression
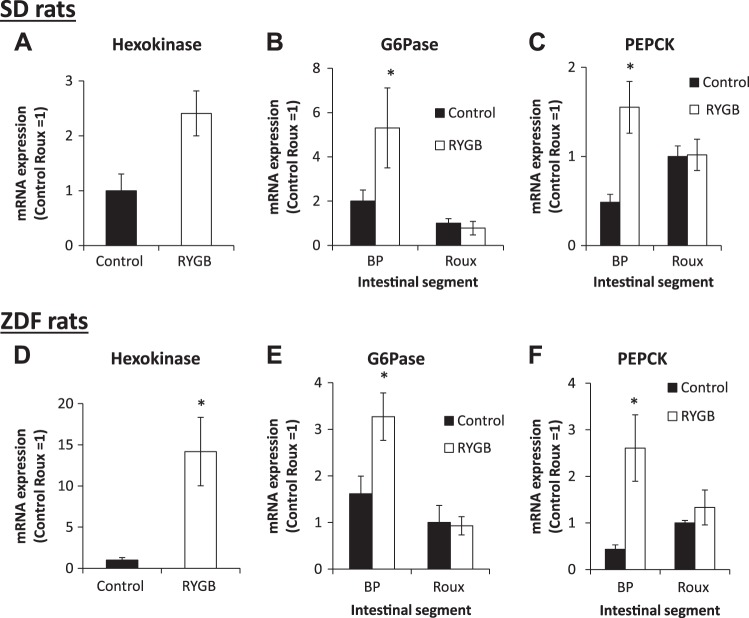
Intestinal enzymes.
After RYGB in SD rats, hexokinase expression increased 2.5-fold in the Roux limb (Fig. 6A). Expression of the enzymes of gluconeogenesis, G-6-Pase (Fig. 6B) and PEPCK (Fig. 6C), increased in the BP limb two- and threefold, respectively, but remained unchanged in the Roux limb.
Fig. 6.
Intestinal gene expression in Sprague-Dawley (SD) and Zucker diabetic fatty (ZDF) rats 4 wk postoperative. After Roux-en-Y gastric bypass (RYGB) in SD rats, hexokinase expression increased in the Roux limb (A), and glucose-6-phosphatase (G-6-Pase, B) and phosphenolpyruvate carboxykinase (PEPCK, C) expression increased in the biliopancreatic (BP) limb but not the Roux limb. A similar pattern of changes was seen in ZDF rats (D–F). The mRNA expression was normalized, for the purposes of comparison, by setting Roux limb expression to 1. *P < 0.05 vs. control.
In ZDF rats, there was a 14-fold increase in Roux limb hexokinase expression after RYGB (Fig. 6D). Expression of G-6-Pase (Fig. 6E) and PEPCK (Fig. 6F) increased in the BP limb two- and sixfold, respectively, and remained unchanged in the Roux limb.
Hepatic DPP4.
There was no difference in either DPP4 activity or expression between control and RYGB groups in either SD or ZDF rats (Fig. 7).
Fig. 7.
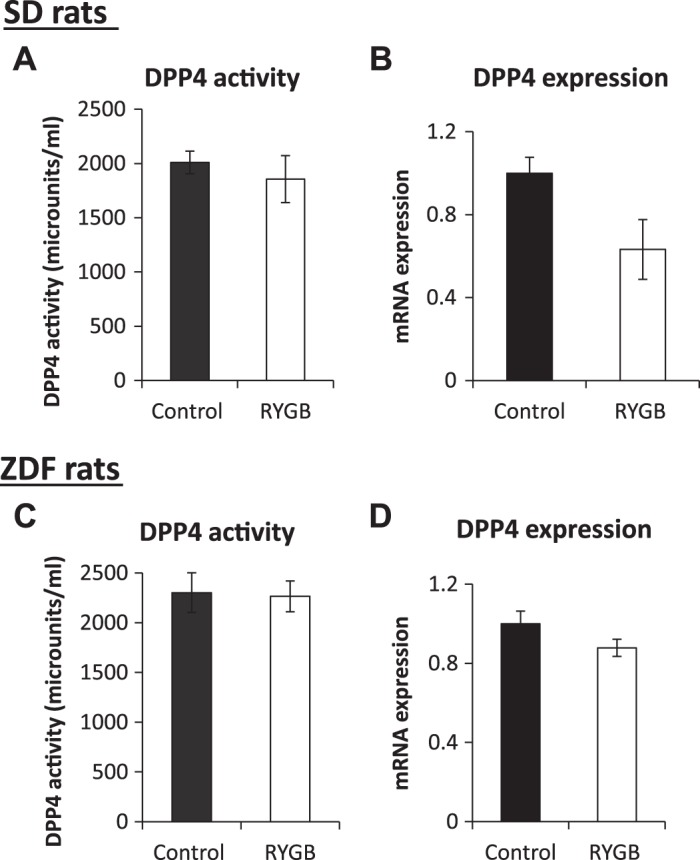
Hepatic dipeptidyl peptidase-4 (DPP4) activity and expression. Hepatic DPP4 activity and mRNA expression in Sprague-Dawley (SD, A and B) and Zucker diabetic fatty (ZDF, C and D) rats. RYGB, Roux-en-Y gastric bypass.
DISCUSSION
The intriguing weight-independent antidiabetic effect of RYGB is well known, and yet the mechanisms underlying this metabolic benefit remain elusive. Although several mechanisms have been proposed, the interaction among these mechanisms is also unclear. It is likely that the major anatomical changes after surgery lead to multiple physiological changes that, in combination, lead to changes in glucose homeostasis. With clinical data showing rapid changes in hepatic glucose handling and a role for increased incretin levels (15), we investigated not only the systemic circulation but also changes in portal milieu.
We hypothesized that RYGB leads to several changes in intestinal function and through changes in portal milieu lead to changes in hepatic metabolism. Our results highlight multiple pathways through which RYGB exerts its metabolic effects: 1) intestinal hypertrophy occurred in both strains, with an almost twofold increase in the ratio of intestinal weight to body weight in SD and ZDF rats; 2) RYGB reduced intestinal glucose absorption rate. Of note, glucose absorption was markedly increased in control diabetic rats, and RYGB reduced glucose absorption to the same lower level in both normal SD and diabetic ZDF models; 3) intestinal Gutil increased in SD rats, with increased hexokinase expression and increased intestinal mass likely contributing to this. In contrast, in ZDF rats, there was net intestinal glucose release, despite similar intestinal hypertrophy and intestinal expression changes. This has therapeutic relevance (see Effects on Intestinal Glucose Balance); 4) levels of both incretins increased after RYGB in SD rats, and GLP-1 levels increased in ZDF rats. This occurred without increased secretion of these hormones, suggesting decreased degradation, and without a change in hepatic DPP4 activity; and 5) GLP-2 levels also increased in both SD and ZDF rats following RYGB and may be driving the intestinal hypertrophy.
Intestinal Glucose Absorption Decreased
RYGB reduced intestinal glucose absorption by 47% in SD and 54% in ZDF rats, consistent with previous work in which acute exclusion of the same length of intestine led to a 59% decrease (23) and decreased absorptive capacity in ex vivo studies (35). This further supports the role of the foregut following RYGB (28).
The animals did not develop diarrhea and thus exhibited no obvious signs of malabsorption of glucose or other nutrients. Therefore, the rate of glucose absorption may be reduced, and total glucose absorption remained unchanged. The concept of glycemic index in diabetes has shown certain foods have a better glucose response than others (13). Of note, we observed elevated glucose absorption in control ZDF versus control SD rats, which correlates with the clinical observation in T2D patients (8) and likely contributes to the diabetic phenotype. Reducing the rate of glucose absorption in essence lowers the glycemic index of food, without necessarily reducing total glucose absorption. However, we did not measure glucose content in the feces to verify this.
A role for bile in the Roux limb in promoting glucose absorption was demonstrated in a study using minipigs: RYGB led to the absence of bile in the Roux limb and an associated reduction in glucose absorption that was restored when bile was added back to that limb (3). In contrast, a clinical study showed a more rapid appearance of ingested glucose in the systemic circulation after RYGB compared with before (12). Patients consumed a mixed meal, and, after RYGB with the absence of the pylorus, a more rapid transit of glucose in the small intestine may explain this observation. The difference in our study is the effects of the pylorus were circumvented because glucose was infused in the Roux limb after RYGB (pylorus absent) or the duodenum in control animals (distal to the pylorus).
Effects on Intestinal Glucose Balance
In SD rats, RYGB shifted intestinal glucose balance toward Gutil. In contrast, in ZDF rats, balance shifted to intestinal glucose release despite similar intestinal hypertrophy and changes in intestinal mRNA expression.
Several factors may explain this difference. First, the portal vein drains the entire intestine (as well as other tissues that sense nutrient intake, notably the pancreas), and therefore utilization and absorption entail the entire intestine while hexokinase expression was only measured in the Roux limb. The increase in hexokinase may be adaptive because of the Roux limb being transposed more proximally in the nutrient stream following RYGB, and the changes in the Roux limb may not be representative of the entire intestine. Second, mRNA expression may not represent protein expression and function. Therefore, although intestinal expression of hexokinase, PEPCK, and G-6-Pase increased, the relative sizes of these changes at the posttranslational level are unknown. Gutil may, in fact, increase in both rat strains after RYGB, with others showing glucose is directed toward aerobic glycolysis and metabolic pathways that support tissue growth (30), and intestinal gluconeogenesis only increasing in diabetic rats with the resultant difference in intestinal glucose balance. Third, other biochemical pathways are likely involved and may account for the disease-specific difference, and this requires further investigation. Our experiments were important to determine the effect of the diabetic phenotype on intestinal glucose balance after RYGB.
The direction of change in post-RYGB intestinal glucose balance remains a topic of debate in the literature. In the Long Evans diet-induced obesity rat model, Roux and common limbs intestinal glucose uptake of labeled glucose from the systemic circulation increased after RYGB (30), which would shift intestinal glucose balance toward utilization, consistent with our SD rat data. In another study in a diabetic mouse model of enterogastric anastomosis, intestinal gluconeogenesis increased (37), which would shift intestinal glucose balance toward net intestinal glucose release, consistent with our ZDF rat data. The difference between the two models in our study, and between the two previous studies, may reflect the animal model used. Diabetes has been shown to increase intestinal gluconeogenesis in rats (19). Furthermore, endogenous glucose production rate is higher in the mouse (19, 37), and the intestinal gluconeogenesis rate may be correspondingly higher.
There is a paucity of human data on portal milieu after RYGB, possibly because of technical challenges of portal sampling. Analogous to the different rat strains, patients undergoing RYGB form a heterogeneous group, which includes diabetic and nondiabetic patients, and with body mass index ranging from 18 to >60 (7, 14), so differences in response to RYGB are likely to exist. In one study, post-RYGB fasting portal glucose level was only 0.2 mmol/l (4 mg/dl) higher than the systemic level in obese nondiabetic patients (10), whereas no difference was noted in the eight diabetic patients. Although the authors concluded there was no evidence to support the hypothesis of intestinal gluconeogenesis after RYGB, a subsequent comment on this study questions this conclusion (20). Portal glucose level depends on both intestinal gluconeogenesis and intestinal glucose uptake. Intestinal gluconeogenesis was not directly measured in the study by Hayes et al. (10), but it may actually be the factor responsible for preventing the drop in portal glucose level in the fasted state. In contrast, in another clinical study using positron emission tomography-computed tomography imaging, Roux limb glucose disposal increased (6). Furthermore, this was associated with better glucose tolerance, suggesting that intestinal glucose disposal has benefits both in the fasting and postprandial states. Thus, a similar discrepancy in the impact of RYGB on intestinal gluconeogenesis appears in both animal and clinical studies.
Hormone
Clinical studies have identified increased incretin levels in the early weight-independent and diet-independent effects of RYGB (15, 16), and reduced incretin levels after RYGB may play a role in weight regain and diabetes relapse (31). Although the “hindgut hypothesis,” with accelerated transit to the GLP-1-secreting cells of the terminal ileum (26), and Roux limb hypertrophy (9) have been proposed to explain increased GLP-1 levels, these hypotheses have not been verified by measuring incretin secretion after RYGB, and our data question its importance.
Previous work from our laboratory showed that acute foregut exclusion, i.e., accelerated nutrient delivery to the distal intestine, actually reduced GLP-1 secretion (23). This study confirms increased GLP-1 levels without increased secretion, suggesting reduced degradation, and without a change in hepatic DPP4 expression and activity. Reduced degradation may be occurring at other sites, e.g., plasma. Consistent with this notion, decreased plasma DPP4 activity in humans following RYGB has been reported (1). A further explanation for increased DPP4 levels is an increased stability of the post-RYGB DPP4. Of note, baseline GLP-1 levels after RYGB were increased in SD rats but not in ZDF rats, whereas glucose-stimulated GLP-1 levels were increased in both species. This may also be the result of differences in stability of GLP-1 and requires further investigation.
GLP-2 levels (at 60 min) also increased, again without an increase in secretion, and could be driving intestinal hypertrophy, with total intestinal weight increasing by 43% in SD rats and 31% in ZDF rats, with most marked increases in the Roux limb (1.75-fold in both strains), consistent with other studies (9, 35). This kind of hypertrophy and elevated GLP-2 level are analogous to that seen after massive small bowel resection in a rat model (27).
Of note, comparing diabetic and healthy control animals, baseline GIP and GLP-2 levels were higher in diabetic rats, despite a lower secretion, again suggesting markedly decreased degradation. The increased GLP-2 levels correspond to the significantly higher intestinal weight in ZDF rats (6.8 ± 0.2 vs. 9.2 ± 0.1 g, SD vs. ZDF, P < 0.001) and the higher ratio of intestinal weight to total body weight (1.9 ± 0.1 vs. 2.2 ± 0.1%, SD vs. ZDF, P = 0.07). The increased GIP level may be a compensatory mechanism to drive the incretin effect in the setting of increased intestinal glucose absorption (shown in our data) and insulin resistance. Our previous work has shown that stimulation of the portal glucose sensor sodium–glucose transporter 3 increases GIP secretion (24).
Portal Theory: Portal Vein as Site for Interplay of Multiple Mechanisms
Because the portal vein drains the intestine into the liver, changes in intestinal function will affect portal milieu, which through a portal sensor impacts hepatic metabolism. In this conceptualization, the portal vein forms the anatomical and physiological gut-liver axis, linking the anatomical changes of RYGB and its hepatic effects. In the ZDF rat, the shift to intestinal glucose release in the portal vein after RYGB will stimulate the portal glucose sensor, which we have previously shown to have a heightened glucose-lowering effect in ZDF rats (24). This novel hypothesis requires further validation and study of other pathways that could be contributing to increased intestinal glucose release in ZDF rats.
Changes in portal hormone content may also be driving downstream hepatic effects. Portal GLP-1 is detected by a portal GLP-1 sensor to improve glucose tolerance (38). Portal GLP-1 infusion triggers firing in afferent fibers of the hepatic branch of the vagus nerve (22) and in efferent fibers to the pancreas to cause insulin secretion (21).
In summary, RYGB has several metabolic effects, with the intestine forming the hub of a surgical physiological network, and this may underlie its superiority to even intensive medical therapy. The reduction in glucose absorption will have an antidiabetic effect per se. In the diabetic state, net intestinal glucose release leads and stimulation of the portal glucose sensor may lead to downstream hepatic effects. For the first time, we show that increased GLP-1 and GLP-2 levels occur because of decreased degradation rather than increased secretion. This critical insight into the surgical physiology of RYGB can help in developing a less invasive alternative to RYGB that can be made more widely available.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1 R01-DK-084064 (A. Tavakkoli).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.P., D.B.R., and A.T. conceived and designed research; A.P. performed experiments; A.P. analyzed data; A.P., D.B.R., and A.T. interpreted results of experiments; A.P., D.B.R., and A.T. prepared figures; A.P., D.B.R., and A.T. drafted manuscript; A.P., D.B.R., and A.T. edited and revised manuscript; A.P., D.B.R., and A.T. approved final version of manuscript.
REFERENCES
- 1.Alam ML, Van der Schueren BJ, Ahren B, Wang GC, Swerdlow NJ, Arias S, Bose M, Gorroochurn P, Teixeira J, McGinty J, Laferrère B. Gastric bypass surgery, but not caloric restriction, decreases dipeptidyl peptidase-4 activity in obese patients with type 2 diabetes. Diabetes Obes Metab 13: 378–381, 2011. doi: 10.1111/j.1463-1326.2011.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach Knudsen KE, Jørgensen H, Canibe N. Quantification of the absorption of nutrients derived from carbohydrate assimilation: model experiment with catheterised pigs fed on wheat- or oat-based rolls. Br J Nutr 84: 449–458, 2000. [PubMed] [Google Scholar]
- 3.Baud G, Daoudi M, Hubert T, Raverdy V, Pigeyre M, Hervieux E, Devienne M, Ghunaim M, Bonner C, Quenon A, Pigny P, Klein A, Kerr-Conte J, Gmyr V, Caiazzo R, Pattou F. Bile diversion in Roux-en-Y gastric bypass modulates sodium-dependent glucose intestinal uptake. Cell Metab 23: 547–553, 2016. doi: 10.1016/j.cmet.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Bhutta HY, Rajpal N, White W, Freudenberg JM, Liu Y, Way J, Rajpal D, Cooper DC, Young A, Tavakkoli A, Chen L. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One 10: e0122273, 2015. doi: 10.1371/journal.pone.0122273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bojsen-Møller KN, Dirksen C, Jørgensen NB, Jacobsen SH, Serup AK, Albers PH, Hansen DL, Worm D, Naver L, Kristiansen VB, Wojtaszewski JF, Kiens B, Holst JJ, Richter EA, Madsbad S. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 63: 1725–1737, 2014. doi: 10.2337/db13-1307. [DOI] [PubMed] [Google Scholar]
- 6.Cavin JB, Couvelard A, Lebtahi R, Ducroc R, Arapis K, Voitellier E, Cluzeaud F, Gillard L, Hourseau M, Mikail N, Ribeiro-Parenti L, Kapel N, Marmuse JP, Bado A, Le Gall M. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs. sleeve gastrectomy. Gastroenterology 150: 454–64.e9, 2016. doi: 10.1053/j.gastro.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Choban PS, Flancbaum L. The effect of Roux limb lengths on outcome after Roux-en-Y gastric bypass: a prospective, randomized clinical trial. Obes Surg 12: 540–545, 2002. doi: 10.1381/096089202762252316. [DOI] [PubMed] [Google Scholar]
- 8.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 82 : G241–G248, 2002. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 9.Hansen CF, Bueter M, Theis N, Lutz T, Paulsen S, Dalbøge LS, Vrang N, Jelsing J. Hypertrophy dependent doubling of L-cells in Roux-en-Y gastric bypass operated rats. PLoS One 8: e65696, 2013. doi: 10.1371/journal.pone.0065696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes MT, Foo J, Besic V, Tychinskaya Y, Stubbs RS. Is intestinal gluconeogenesis a key factor in the early changes in glucose homeostasis following gastric bypass? Obes Surg 21: 759–762, 2011. doi: 10.1007/s11695-011-0380-7. [DOI] [PubMed] [Google Scholar]
- 11.International Diabetes Federation Diabetes Atlas (Online). https://www.idf.org. [Accessed March 1, 2017].
- 12.Jacobsen SH, Bojsen-Møller KN, Dirksen C, Jørgensen NB, Clausen TR, Wulff BS, Kristiansen VB, Worm D, Hansen DL, Holst JJ, van Hall G, Madsbad S. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia 56: 2250–2254, 2013. doi: 10.1007/s00125-013-3003-0. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins DJ, Wolever TM, Jenkins AL, Thorne MJ, Lee R, Kalmusky J, Reichert R, Wong GS. The glycaemic index of foods tested in diabetic patients: a new basis for carbohydrate exchange favouring the use of legumes. Diabetologia 24: 257–264, 1983. [DOI] [PubMed] [Google Scholar]
- 14.Jiang F, Zhu H, Zheng X, Tu J, Zhang W, Xie X. Duodenal-jejunal bypass for the treatment of type 2 diabetes in Chinese patients with an average body mass index <24 kg/m2. Surg Obes Relat Dis 10: 641–646, 2014. doi: 10.1016/j.soard.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30: 1709–1716, 2007. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93: 2479–2485, 2008. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 5: 178ra41, 2013. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 386: 964–973, 2015. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 19.Mithieux G, Bady I, Gautier A, Croset M, Rajas F, Zitoun C. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am J Physiol Endocrinol Metab 286: E370–E375, 2004. doi: 10.1152/ajpendo.00299.2003. [DOI] [PubMed] [Google Scholar]
- 20.Mithieux G. Comment about intestinal gluconeogenesis after gastric bypass in human in relation with the paper by Hayes et al., Obes. Surg. 2011. Obes Surg 22: 1920–1922, 2012. doi: 10.1007/s11695-012-0755-4. [DOI] [PubMed] [Google Scholar]
- 21.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol Endocrinol Metab 271: E808–E813, 1996. doi: 10.1152/ajpendo.1996.271.5.E808. [DOI] [PubMed] [Google Scholar]
- 22.Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A. The hepatic vagal nerve is receptive to incretin hormone glucagon-like peptide-1, but not to glucose-dependent insulinotropic polypeptide, in the portal vein. J Auton Nerv Syst 61: 149–154, 1996. doi: 10.1016/S0165-1838(96)00071-9. [DOI] [PubMed] [Google Scholar]
- 23.Pal A, Rhoads DB, Tavakkoli A. Foregut exclusion disrupts intestinal glucose sensing and alters portal nutrient and hormonal milieu. Diabetes 64: 1941–1950, 2015. doi: 10.2337/db14-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal A, Rhoads DB, Tavakkoli A. Effect of portal glucose sensing on systemic glucose levels in SD and ZDF rats. PLoS One 11: e0165592, 2016. doi: 10.1371/journal.pone.0165592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal A, Rhoads DB, Tavakkoli A. Customization of biliopancreatic limb length to modulate and sustain antidiabetic effect of gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol 314: G287–G299, 2018. doi: 10.1152/ajpgi.00276.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patriti A, Aisa MC, Annetti C, Sidoni A, Galli F, Ferri I, Gullà N, Donini A. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery 142: 74–85, 2007. doi: 10.1016/j.surg.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Perez A, Duxbury M, Rocha FG, Ramsanahie AP, Farivar RS, Varnholt H, Ito H, Wong H, Rounds J, Zinner MJ, Whang EE, Ashley SW. Glucagon-like peptide 2 is an endogenous mediator of postresection intestinal adaptation. JPEN J Parenter Enteral Nutr 29: 97–101, 2005. doi: 10.1177/014860710502900297. [DOI] [PubMed] [Google Scholar]
- 28.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 244: 741–749, 2006. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509: 183–188, 2014. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341: 406–410, 2013. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santo MA, Riccioppo D, Pajecki D, Kawamoto F, de Cleva R, Antonangelo L, Marçal L, Cecconello I. Weight regain after gastric bypass: influence of gut hormones. Obes Surg 26: 919–925, 2016. doi: 10.1007/s11695-015-1908-z. [DOI] [PubMed] [Google Scholar]
- 32.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes–5-year outcomes. N Engl J Med 76: 641–651, 2017. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151: 1588–1597, 2010. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiota M, Printz RL. Diabetes in Zucker diabetic fatty rat. Methods Mol Biol 933: 103–123, 2012. doi: 10.1007/978-1-62703-068-7_8. [DOI] [PubMed] [Google Scholar]
- 35.Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol 297: G950–G957, 2009. doi: 10.1152/ajpgi.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol 28: 727–740, 2014. doi: 10.1016/j.bpg.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, Foretz M, Leclerc J, Duchampt A, Zitoun C, Thorens B, Magnan C, Mithieux G, Andreelli F.. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 8: 201–211, 2008. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D’Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148: 4965–4973, 2007. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]