Abstract
Enteroendocrine L cells and glucagon-like peptide 2 (GLP-2) secretion are activated in the intestinal adaptation process following bowel resection in patients with short bowel syndrome. We hypothesized that enteral activation of Takeda G protein-coupled receptor 5 (TGR5), expressed in enteroendocrine L cells, could augment endogenous GLP-2 secretion and the intestinal adaptation response. Our aim was to assess the efficacy of different TGR5 agonists to stimulate GLP-2 secretion and intestinal adaptation in a piglet short-bowel model. In study 1, parenterally fed neonatal pigs (n = 6/group) were gavaged with vehicle, olive extract (OE; 10 or 50 mg/kg), or ursolic acid (UA; 10 mg/kg), and plasma GLP-2 was measured for 6 h. In study 2, neonatal pigs (n = 6–8/group) were subjected to transection or 80% mid-small intestine resection and, after 2 days, assigned to treatments for 10 days as follows: 1) transection + vehicle (sham), 2) resection + vehicle (SBS), 3) resection + 30 mg UA (SBS + UA), and 4) resection + 180 mg/kg OE (SBS + OE). We measured plasma GLP-2, intestinal histology, cell proliferation, and gene expression, as well as whole body citrulline-arginine kinetics and bile acid profiles. In study 1, GLP-2 secretion was increased by UA and tended to be increased by OE. In study 2, SBS alone, but not additional treatment with either TGR5 agonist, resulted in increased mucosal thickness and crypt cell proliferation in remnant jejunum and ileum sections. SBS increased biliary and ileal concentration of bile acids and expression of inflammatory and farnesoid X receptor target genes, but these measures were suppressed by UA treatment. In conclusion, UA is an effective oral GLP-2 secretagogue in parenterally fed pigs but is not capable of augmenting GLP-2 secretion or the intestinal adaptation response after massive small bowel resection.
NEW & NOTEWORTHY Therapeutic activation of endogenous glucagon-like peptide 2 (GLP-2) secretion is a promising strategy to improve intestinal adaptation in patients with short bowel syndrome. This study in neonatal pigs showed that oral supplementation with a selective Takeda G protein-coupled receptor 5 (TGR5) agonist is an effective approach to increase GLP-2 secretion. The results warrant further study to establish a more potent oral TGR5 agonist that can effectively improve intestinal adaptation in pediatric patients with SBS.
Keywords: bile acids, GLP-2, short bowel syndrome, TGR5, ursolic acid
INTRODUCTION
Short bowel syndrome (SBS) is a clinical condition that mainly results from massive resection of the intestine due to clinical etiologies such as necrotizing enterocolitis, intestinal atresia, or intestinal volvulus (45, 52). The prevalence of neonatal SBS is ∼24.5 per 100,000 live births, while the mortality rate ranges from 15% to 25% (47). Because SBS is also known to be associated with premature birth, the incidence of SBS may rise with the increase in survival of premature infants (53). Because malabsorption and malnutrition are common in patients with SBS, parenteral nutrition (PN) support is often necessary. However, long-term PN, especially in infants, is associated with acute and chronic complications, including PN-associated liver disease. Moreover, PN has been shown to cause intestinal atrophy and diminished intestinal function (16, 25). Therefore, strategies to promote intestinal adaptation and restore digestive and absorptive function are vital for clinical support of patients with SBS.
Glucagon-like peptide 2 (GLP-2), an intestinal trophic enteroendocrine peptide, is synthesized and secreted from L cells located in the distal ileum (DI) and colon in response to enteral feeding (7, 8, 15). We and others have shown in neonatal pigs that circulating GLP-2 levels are depressed during total PN (TPN) and increased with enteral feeding and that exogenous GLP-2 promotes growth of the small intestine in the presence and absence of enteral nutrition (6, 9, 10, 48). Similarly, in neonatal pigs, circulating GLP-2 levels are depressed after resection of the ileum, but not proximal intestine, and the trophic adaptive response to enteral nutrition and exogenous GLP-2 is greater with an intact distal intestine and in more mature pigs (3, 23, 32, 33, 50, 52, 53, 56). In recent years, GLP-2 has been approved for therapeutic use to augment intestinal adaptation and eliminate the need for PN support in adults with SBS, and approval of GLP-2 is pending for pediatric patients with SBS (12, 29, 30). Despite recent progress, additional and more accessible therapies are warranted to improve the clinical course of patients with SBS, especially infants with increased nutrient demands for growth.
Takeda G protein-coupled receptor 5 (TGR5), also referred to as G protein-coupled bile acid receptor 1, is expressed in intestinal L cells, and its activation triggers release of GLP-1 and GLP-2 and improves glucose homeostasis (31, 39, 51). TGR5 is activated by bile acids and various natural compounds and, thus, has become a key drug target to increase GLP-1 in treatment of diabetes (1, 18, 41). The bile acids deoxycholic acid and lithocholic acid are potent physiological ligands of TGR5 (46). Ursolic acid (UA) and betulinic acid, natural pentacyclic triterpenoid carboxylic acids present in a variety of plants, are potent TGR5 agonists with in vivo activity (18, 20, 34, 43, 44). Extracts from these plants may be useful dietary supplements to augment GLP-1/GLP-2 secretion by virtue of their ability to activate intestinal TGR5. Thus, because GLP-1 and GLP-2 are normally co-secreted from L cells, we hypothesize that enteral treatment with TGR5 agonists may improve the adaptive responses of the intestine by inducing GLP-2 release.
We describe results from two studies designed to test the efficacy of different TGR5 agonists to stimulate GLP-2 secretion and augment intestinal adaptation in a piglet short bowel model. We tested a purified TGR5 agonist, UA, and an olive plant extract (OE) that contained triterpenoids, including oleanolic acid. We first tested the efficacy of the TGR5 agonists UA and OE to stimulate GLP-2 secretion in parenterally fed piglets. We further tested the capacity of these TGR5 agonists to increase GLP-2 secretion and promote intestinal adaptation in a neonatal pig model of massive mid-small bowel resection (4, 5, 36).
METHODS
The following study protocols were approved by the Animal Care and Use Committee of Baylor College of Medicine and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Study 1: TGR5 Agonist Study
Animals and surgical procedure.
Neonatal (6-day-old), cross-bred suckling pigs were obtained from a commercial farm and transported to the animal facility at the Children’s Nutrition Research Center. Upon arrival, the pigs were placed in individual cages in a room kept at 28–30°C and then underwent a surgical procedure for implantation of a silicone catheter in the jugular vein for PN infusion and blood sampling. Preoperatively, the pigs received one dose of a slow-release formulation of buprenorphine (0.12 mg/kg sc) to ensure analgesia for 72 h and one dose of the antibiotic enrofloxacin (10 mg/kg iv), which was continued at the same dose postoperatively every 12 h until the end of the study. Postoperatively, the pigs were housed in individual cages and maintained at 28–30°C with a 12:12-h light-dark cycle. After surgery, the pigs received TPN via jugular infusion at 50% of full intake (5 ml·kg−1·h−1) on day 1; thereafter, TPN intake was increased to reach 90% of full intake by day 4.
Study design and sample collection.
After 2 days of recovery from surgery, the pigs (n = 24) were randomly assigned to four treatments: 1) control formula + DMSO (control), 2) control formula + 10 mg/kg UA (UA-10), 3) control formula + 10 mg/kg OE (OE-10), and 4) control formula + 50 mg/kg OE (OE-50). UA was 98% pure, derived from sage plant (Salvia officinalis) extract (Sigma-Aldrich, St. Louis, MO). OE was derived from olive fruit and contained ≥10% triterpenes and ≥2% oleanolic acid. Both compounds were provided by Lucta SA. The compounds were diluted in DMSO (1.5 ml/kg) and given via oral gavage and flushed with sow’s milk formula (20 ml/kg). The pigs were given each treatment dose on postoperative day 3 at 1200 and 2000 and a final dose on day 4 at 0700–0800, and blood samples were obtained at 0, 15, 30, 60, 90, 120, 180, 240, 300, and 360 min. After the last blood sample, the pigs were euthanized with an intravenous injection of commercial euthanasia solution (phenytoin-pentobarbital; Beuthanasia, Merck).
Study 2: SBS TGR5 Agonist Study
Animals and surgical procedure.
The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Neonatal (6-day-old), cross-bred pigs were obtained from a commercial farm and transported to the animal facility at the Children’s Nutrition Research Center. The pigs were immediately placed in individual cages in a heated (28–30°C) room until the day of surgery. The surgical procedure involved manipulation of the bowel length and implantation of silicone catheters for intragastric formula feeding, jugular PN infusion, and carotid artery blood sampling, as previously described (36). Briefly, in the SBS groups, the pigs were subjected to an 80% mid-jejunoileal resection, with a remnant 20% representing the proximal jejunum (PJ) and DI, similar to that described previously in neonatal pigs (4, 5). In the resected SBS pigs, an ~80% length of total small intestine (calculated as 280 cm/body wt0.60) was measured from the DI end and then resected. The pigs in the sham group were subjected to the same intraoperative procedures, but, instead of small bowel resection, they underwent a mid-jejunal transection and reanastomosis, leaving them with an intact small intestine. Preoperatively, the pigs received one dose of a slow-release formulation of buprenorphine (0.12 mg/kg sc) to ensure analgesia for 72 h and one dose of the antibiotic enrofloxacin (10 mg/kg iv), which was continued at the same dose postoperatively every 12 h until the end of the study. Postoperatively, the pigs were housed in individual cages and maintained at 28–30°C with a 12:12-h light-dark cycle. All the pigs received TPN via jugular infusion at 60% of full intake (6 ml·kg−1·h−1) on the day of surgery and 80% on postoperative day 2 and, thereafter, 100% until the end of the study.
Study protocol and sample collection.
On postoperative day 2, the pigs (n = 24) were randomly assigned to four treatments: 1) sham transection + vehicle (DMSO; sham), 2) SBS + DMSO (SBS), 3) SBS + 30 mg/kg UA (SBS + UA), and 4) SBS + 180 mg/kg OE (SBS + OE). UA and OE were diluted in DMSO (1.5 ml/kg) and infused intragastrically; catheters were then flushed with cow’s milk replacer formula (20 ml/kg) to provide minimal enteral nutrition. The treatments were given every 8 h between postoperative days 2 and 10.
On day 9, before the end of the study, the pigs were primed-continuously infused for 4 h with l-[guanido-15N2]arginine HCl (22.5 µmol·kg−1·h−1), l-[5-13C, 4,4,5,5-2H4]citrulline (4.2 µmol·kg−1· h−1), l-[ring-2H5]phenylalanine (10 µmol·kg−1·h−1), and l-[3,3-2H2]tyrosine (2.5 µmol·kg−1·h−1) (Cambridge Isotope Laboratories). Blood samples were collected from carotid catheters at 0, 3, 3.5, and 4 h during the infusion.
At the end of the study, 4 h before tissue collection, each pig was injected with 5-bromodeoxyuridine (BrdU, 50 mg/kg iv bolus; Sigma Aldrich) for measurement of crypt cell proliferation index. The pigs were then euthanized with an intravenous injection of commercial euthanasia solution (Beuthanasia), and tissue weight and length were measured. PJ, DI, and liver samples were frozen for quantitative PCR analysis and fixed in formalin for histology measurements.
Histology and immunohistochemistry.
Intestinal tissues were fixed in 10% formalin and then dehydrated in 70% ethanol. Thereafter, intestinal samples were embedded in paraffin and stained with hematoxylin and eosin. Villus height and crypt depth were measured using an Axiophot microscope (Carl Zeiss) and Image version 1.60 software (National Institutes of Health). Crypt cell proliferation was measured by BrdU crypt cell labeling, as described previously (6).
Plasma GLP-2 and amino acid analysis.
In studies 1 and 2, all blood samples were collected in potassium-EDTA tubes, immediately placed in ice, and centrifuged at 1,500 g within 15 min, and plasma was stored at −80°C until assay. Plasma GLP-2 was measured using a human GLP-2 radioimmunoassay with a detection limit of 5 pmol/l that can recognize both human and porcine GLP-2 peptide, as previously described (11, 21, 22, 58). Plasma amino acids were measured using reverse-phase HPLC of their phenyl isothiocyanate derivatives (PicoTag, Waters, Milford, MA), as previously described (49).
Tissue gene expression by real-time PCR.
Total RNA was isolated from frozen ileum tissue with RNeasy Midi kits (Qiagen), and DNase was treated using an Ambion kit (catalog no. AM1906). Reverse transcription reactions were performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems) to acquire cDNA with DNase-treated RNA. Real-time quantitative PCR was performed using commercially available kits (SuperScript III Platinum Two-Step qPCR kit with SYBR green, Invitrogen) and real-time PCR machines (model CFX96, Bio-Rad). Primers were designed using Primer BLAST software (National Center for Biotechnology Information) (Table 1). Amplification efficiency was controlled by the use of an internal control (β-actin). Relative quantification of target mRNA expression was calculated and normalized to β-actin expression. All reactions were performed under the following thermal cycling conditions: 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 60 s. The comparative threshold (2CT) method was used to compare gene expression levels between treatments, which were analyzed to determine fold induction of mRNA expression.
Table 1.
Primer sequences for RT-PCR analysis
| Genes | Primer Sequence | Product Size, bp |
|---|---|---|
| ACTB | ||
| Forward | GGACCTGACCGACTACCTCA | 111 |
| Reverse | GCGACGTAGCAGAGCTTCTC | |
| TGR5 | ||
| Forward | CCATGCACCCCTGTTGCT | 66 |
| Reverse | GGTGCTGTTGGGTGTCATCTT | |
| FXR | ||
| Forward | TTTGTGTCGTTTGCGGAGAG | 128 |
| Reverse | GTTGCCCCCATTTTTACACTTG | |
| FGF19 | ||
| Forward | AAGATGCAAGGGCAGACTCA | 101 |
| Reverse | AGATGGTGTTTCTTGGACCAGT | |
| SHP | ||
| Forward | GCCTACCTGAAAGGGACCAT | 126 |
| Reverse | CAACGGGTGTCAAGCCTTTA | |
| CYP7A1 | ||
| Forward | GAAAGAGAGACCACATCTCGG | 123 |
| Reverse | GAATGGTGTTGGCTTGCGAT | |
| BSEP | ||
| Forward | TTTCATTCAGCGCCTGACCA | 105 |
| Reverse | ACTCCAATGAGAGGGCTGAC | |
| IL-1β | ||
| Forward | AGGCAGATGGTGTCTGTCATC | 125 |
| Reverse | AGGATGATGGGCTCTTCTTCAAA | |
| TNF-α | ||
| Forward | GGCCCAAGGACTCAGATCAT | 179 |
| Reverse | TGAGGTACAGCCCATCTGTC | |
| IL-6 | ||
| Forward | TCTGGGTTCAATCAGCAGACC | 128 |
| Reverse | CTAATCTGCACAGCCTCGAC | |
| EGF | ||
| Forward | TCTGAACCCGGACGGATTTG | 202 |
| Reverse | GACATCGCTCGCGAACGTAG | |
| IGF-1 | ||
| Forward | CTCTTCGCATCTCTTCTACTTGGC | 150 |
| Reverse | CCTGTGGGCTTGTTGAAATAAAA | |
| GLP-2R | ||
| Forward | CCCTGCTGTTTCTGGTTTCC | 195 |
| Reverse | GGCAGGGAACAGAAACGTTT |
ACTB, β-actin; TGR5, Takeda G protein-coupled receptor 5; SHP, small heterodimer partner; CYP7A1, cytochrome P-450 7A1; BSEP, bile salt export pump; GLP-2R, glucagon-like peptide 2 receptor.
Plasma tracer enrichment analysis and calculations.
Amino acid isotopic enrichments were determined following derivatization to their corresponding dansyl derivatives with the use of LC-MS/MS (TSQ Vantage, Thermo Fisher Scientific), as previously described (35). Amino acid flux was calculated by the isotope dilution of the infused tracers as follows:
where FluxM is the rate of appearance of the unlabeled metabolite M (μmol·kg−1·h−1), iIVM is the intravenous infusion rate of the tracer (μmol·kg−1·h−1), is the enrichment of the infused intravenous tracer, and EiIV is the plasma enrichment of metabolite M at isotopic plateau enrichment (mole percent excess).
Bile acid profile analysis.
Bile acid profiles were analyzed as described previously (17). Bile acids were extracted from tissue samples with 1:1 H2O-acetonitrile (ACN) containing chenodeoxycholic acid (CDCA) as internal standard. Intestinal mucosa from the ileum and colon was homogenized in the absence of solvent on a TissueLyzer II (Qiagen, Hilden, Germany), and 50 mg of homogenate were extracted with 500 µl of the solvent. After homogenization, the mixture was centrifuged (15,000 g, 10 min, 4°C), and the supernatant was diluted 1:2 in 1:1 H2O-ACN for ultraperformance (UPLC)-MS analysis. A similar methodology was used to extract bile acids from the liver, but the volume of extraction solvent was increased to 850 µl. Liver supernatant was diluted 1:10 in 1:1 H2O-ACN before UPLC-MS analysis. Bile acids were analyzed via UPLC-MS chromatography in an AQUITY UPLC I-Class system (Waters) connected to a Xevo-G2 quadrupole time-of-flight MS detector. Separation was run on an ACQUITY UPLC ethylene-bridged hybrid (BEH) C18 1.7-mm column (2.1 × 100 mm; Waters) using H2O and ACN as mobile phases, both containing 0.1% formic acid. Mass detection was performed in full-scan negative mode (100–1,200 Da). Bile acid concentration was determined based on standard curves with QuanLynx software (Waters).
Statistical Analysis
Values are means ± SE. In study 1, GLP-2 data were analyzed using the linear mixed effects models (MIXED) procedure in SPSS (version 8.2, SAS Institute, Cary, NC). In study 2, one-way ANOVA and Tukey’s post hoc test were performed to determine the statistical difference among sham, SBS, SBS + UA, and SBS + OE groups using GraphPad Prism software (version 5.0). P < 0.05 was considered statistically different.
RESULTS
Study 1
Plasma GLP-2.
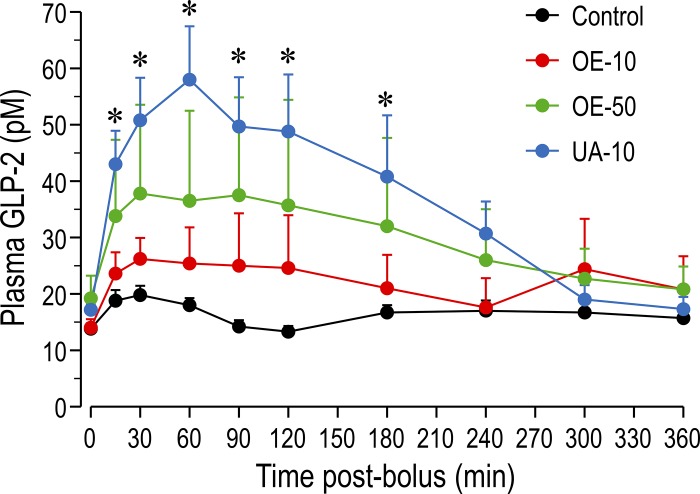
Plasma GLP-2 concentration was increased (P < 0.05) by the TGR5 agonists UA and OE (Fig. 1). Plasma GLP-2 was higher in the UA-10 (P < 0.05) than control group and tended to be higher in the OE-50 group (P < 0.10) at 15 min, 30 min, 1 h, 1.5 h, 2 h, and 3 h after treatment. Area-under-the-curve values showed a similar pattern: values were higher in the UA-10 group (222 ± 35) and tended to be higher in the OE-50 (163 ± 55) and OE-10 (135 ± 40) groups than in the control group (98 ± 7).
Fig. 1.
Plasma glucagon-like peptide (GLP)-2 concentrations at 0–360 min after treatment. Pigs receiving total parenteral nutrition were treated with ursolic acid (UA) at 10 mg/kg (UA-10), olive plant extract (OE) at 10 or 50 mg/kg (OE-10 or OE-50), or vehicle alone (control). Values are means ± SE; n = 5–6/group. *P < 0.05 vs. control.
Study 2
Body weight and organ weight.
All four groups grew at a similar rate (P > 0.05), although weight gain was slightly higher (14%) in the sham than SBS group (Table 2). As expected, tissue weight was lower (P < 0.05) and jejunum, ileum, and small intestine were shorter in all SBS groups than in the sham group. Jejunum, ileum, and small intestine weights per centimeter were greater (P < 0.05) in the SBS than sham pigs. Contrary to our expectation, the ileum was heavier (P < 0.05) in SBS than SBS + UA pigs. Liver and spleen weights were not different (P > 0.05) among the groups.
Table 2.
Body weight, organ weight, and intestine length
| Group |
||||
|---|---|---|---|---|
| Sham | SBS | SBS + UA | SBS + OE | |
| Body weight gain, g·kg−1·day−1 | 56.8 ± 4.4 | 49.8 ± 4.2 | 49.0 ± 3.1 | 50.9 ± 1.4 |
| Organ weight, g/kg body wt | ||||
| Liver | 42.1 ± 1.8 | 43.5 ± 2.1 | 45.5 ± 2.9 | 46.6 ± 1.0 |
| Jejunum | 14.0 ± 1.2a | 4.9 ± 0.4b | 4.5 ± 0.3b | 4.4 ± 0.2b |
| Ileum | 15.1 ± 1.3a | 6.9 ± 0.5b | 5.4 ± 0.3c | 5.6 ± 0.4b,c |
| Colon | 10.2 ± 1.1 | 10.1 ± 0.8 | 9.2 ± 1.1 | 11.0 ± 2.7 |
| Small intestine | 29.2 ± 2.5a | 11.8 ± 0.9b | 9.9 ± 0.6b | 10.0 ± 0.5 |
| Organ weight, g/cm | ||||
| Jejunum | 0.20 ± 0.01a | 0.33 ± 0.02b | 0.36 ± 0.02b | 0.29 ± 0.02a,b |
| Ileum | 0.22 ± 0.01a | 0.49 ± 0.05b | 0.42 ± 0.02b | 0.38 ± 0.03b |
| Colon | 0.32 ± 0.01 | 0.29 ± 0.01 | 0.29 ± 0.05 | 0.33 ± 0.03 |
| Small intestine | 0.21 ± 0.01a | 0.41 ± 0.03b | 0.39 ± 0.02b | 0.34 ± 0.02a,b |
| Organ length, cm/kg | ||||
| Jejunum | 70.9 ± 7.8a | 15.0 ± 1.2b | 12.7 ± 0.9b | 15.5 ± 1.0b |
| Ileum | 71.6 ± 7.5a | 14.5 ± 0.7b | 12.9 ± 0.7b | 14.7 ± 0.7b |
| Colon | 31.9 ± 3.5 | 34.7 ± 3.1 | 27.5 ± 2.7 | 31.8 ± 5.6 |
| Small intestine | 142.5 ± 15.3a | 29.4 ± 1.4b | 25.6 ± 1.6b | 30.2 ± 1.7b |
Values are means ± SE; n = 6–8/group. Groups are defined as follows: pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE).
Values without a common superscript are significantly different (P < 0.05).
Intestinal morphology and cell proliferation.
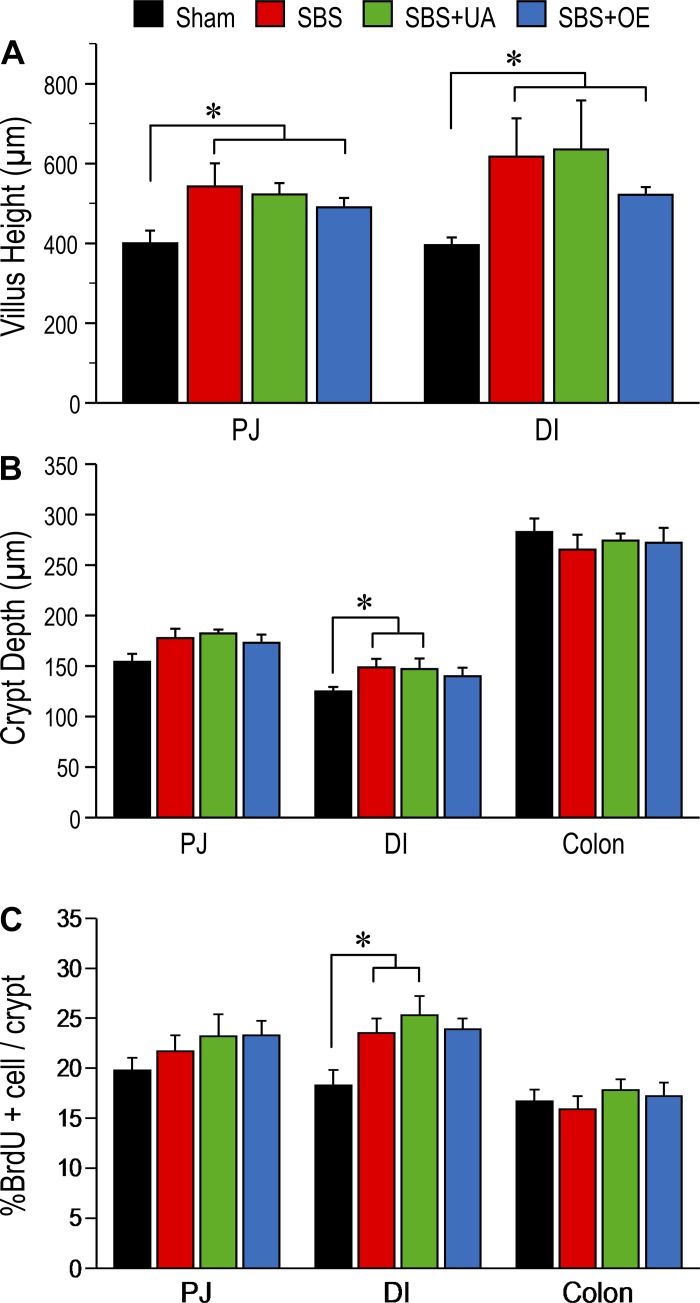
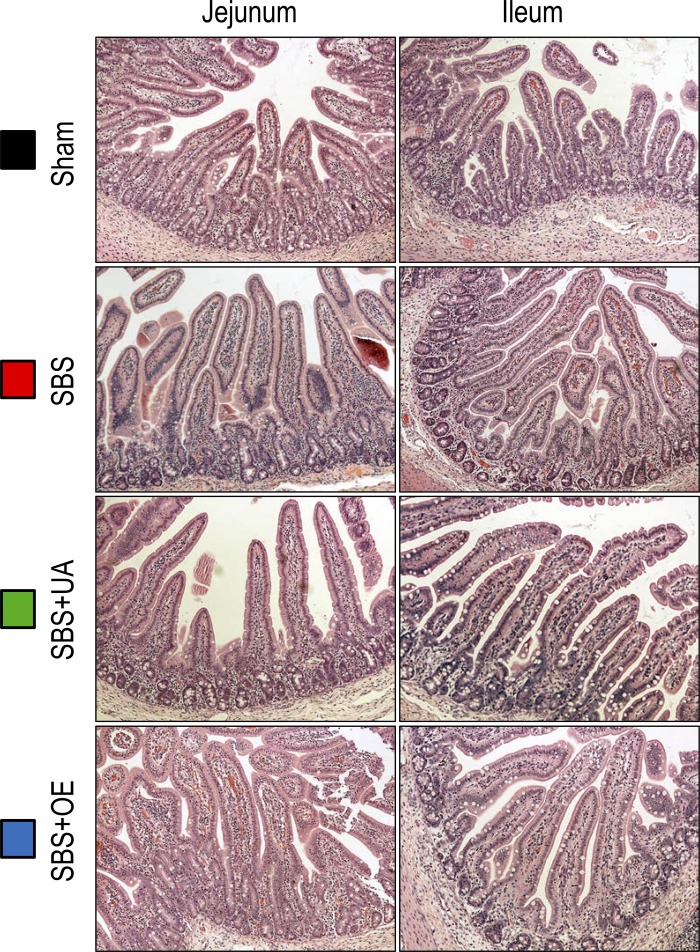
Villus height in the PJ and DI was greater (P < 0.05) in SBS than sham pigs, whereas UA and OE had no effect on villus height (Fig. 2A). Crypt depth in the DI was greater (P < 0.05) in SBS, but not SBS + UA or SBS + OE, than sham pigs (Fig. 2B). Additionally, the percentage of BrdU-positive cells in the crypt of the DI was increased (P < 0.05) in SBS compared with sham pigs (Fig. 2C). Representative histological images of sections from jejunum and ileum remnants are shown in Fig. 3.
Fig. 2.
Intestinal mucosal morphometry in pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE). A: villus height in proximal jejunum (PJ) and distal ileum (DI). B: crypt depth in PJ, DI, and colon. C: percentage of 5-bromodeoxyuridine (BrdU)-positive cells in the crypt of the DI. Values are means ± SE; n = 6–8/group. *P < 0.05, sham vs. SBS groups.
Fig. 3.
Representative histological images from remnant jejunum and ileum in pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE). Images were captured at ×200 magnification.
Secretion of GLP-2 and FGF19 and gene expression of GLP-2 receptor and other growth factors.
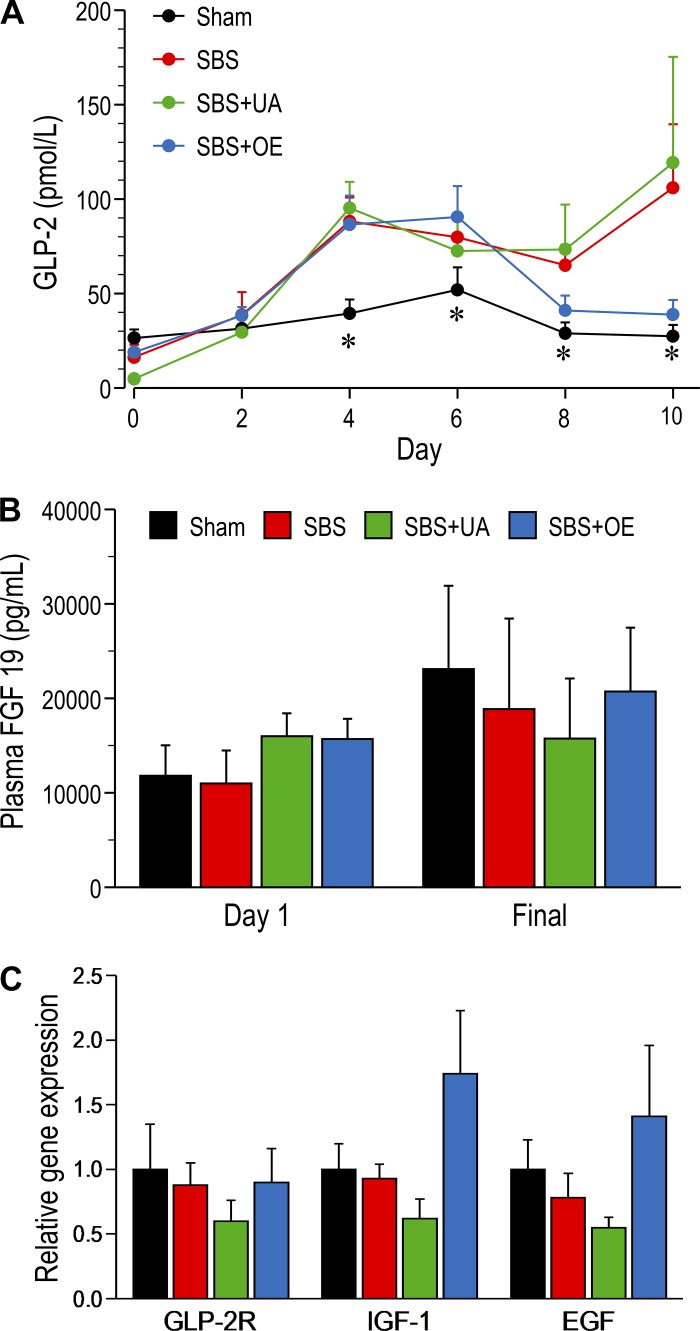
On days 0 and 2, plasma GLP-2 concentrations were similar in each group. However, on days 4, 6, 8, and 10, plasma GLP-2 concentration was higher (P < 0.05) in SBS and SBS + UA than sham pigs (Fig. 4A); in SBS + OE pigs, plasma GLP-2 concentration was higher only on days 4 and 6. Plasma GLP-2 concentration was not different between SBS, SBS + UA, and SBS + OE pigs on days 0–6 but tended to be lower in SBS + OE pigs on days 8 and 10. Plasma FGF19 concentrations were not different among the groups (Fig. 4B). Resection and TGR5 agonist treatment did not affect mRNA expression of GLP-2 receptor (GLP-2R), IGF-1, and EGF (Fig. 4C).
Fig. 4.
Plasma concentrations of glucagon-like peptide 2 (GLP-2; A) and FGF19 (B) and mRNA expression of GLP-2 receptor (GLP-2R), IGF-1, and EGF (C) in pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE). Values are means ± SE; n = 6–8/group. *P < 0.05, sham vs. SBS and SBS + UA.
Amino acid profile and tracer kinetics.
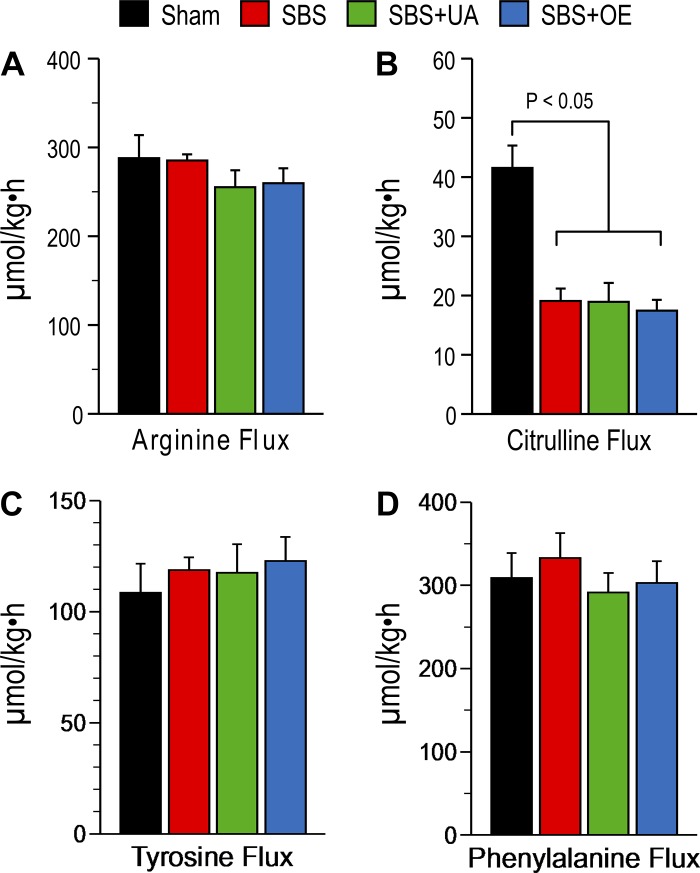
Citrulline flux was decreased (P < 0.05) in SBS, SBS + UA, and SBS + OE compared with sham pigs, but citrulline flux did not differ between the three SBS groups (Fig. 5). Arginine, tyrosine, and phenylalanine flux did not differ between any of the four groups. Citrulline and arginine concentrations were lower (P < 0.05) but glutamine concentration was higher (P < 0.05) in the resection (SBS, SBS + UA, and SBS + OE) groups than in the sham group (Table 3). Glutamine concentration was higher (P < 0.05) in SBS + UA than SBS pigs.
Fig. 5.
Flux of arginine, citrulline, tyrosine, and phenylalanine in pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE). Values are means ± SE; n = 6–8/group.
Table 3.
Plasma amino acid concentrations after 10 days of treatment
| Group |
||||
|---|---|---|---|---|
| Amino Acid | Sham | SBS | SBS + UA | SBS + OE |
| Cit | 88.4 ± 9.7a | 44.2 ± 3.2b | 38.8 ± 3.9b | 44.4 ± 4.5b |
| Arg | 58.0 ± 7.9a | 39.6 ± 3.2b | 38.5 ± 3.8b | 38.1 ± 3.1b |
| Gln | 619.0 ± 38.4a | 871.6 ± 58.9b | 1,085.8 ± 45.3a | 945.5 ± 52.3a,b |
| Orn | 41.3 ± 4.9 | 24.1 ± 1.4 | 28.9 ± 3.0 | 21.6 ± 2.1 |
| Val | 368.0 ± 18.7 | 380.2 ± 22.8 | 415.3 ± 13.6 | 411.5 ± 26.7 |
| Tyr | 31.8 ± 5.9 | 34.6 ± 6.6 | 69.7 ± 15.1 | 64.8 ± 9.0 |
| Trp | 42.2 ± 1.8 | 37.2 ± 2.5 | 42.3 ± 3.3 | 35.7 ± 2.6 |
| Thr | 1,031.3 ± 119.6 | 782.9 ± 75.4 | 912.6 ± 83.3 | 682.2 ± 45.9 |
| Ser | 408.8 ± 36.6 | 419.0 ± 36.4 | 513.9 ± 34.5 | 401.9 ± 31.6 |
| Pro | 483.8 ± 18.8 | 599.7 ± 45.8 | 640.0 ± 38.3 | 556.8 ± 25.4 |
| Phe | 185.7 ± 10.8 | 192.8 ± 30.9 | 251.5 ± 78.8 | 219.2 ± 22.1 |
| Met | 215.8 ± 19.9 | 316.8 ± 84.5 | 526.6 ± 127.7 | 346.5 ± 38.2 |
| Lys | 345.2 ± 33.8 | 333.8 ± 18.3 | 374.9 ± 32.5 | 328.6 ± 22.4 |
| Leu | 206.9 ± 16.2 | 217.1 ± 21.0 | 225.2 ± 24.0 | 213.1 ± 19.5 |
| Ile | 118.7 ± 20.1 | 123.0 ± 25.0 | 146.7 ± 22.1 | 130.8 ± 20.9 |
| His | 59.7 ± 6.1 | 69.0 ± 3.9 | 86.4 ± 8.4 | 75.1 ± 5.0 |
| Gly | 1,509.9 ± 128.4 | 1,442.8 ± 137.7 | 1,564.4 ± 78.8 | 1,148.1 ± 94.5 |
| Glu | 130.4 ± 26.0 | 142.5 ± 30.3 | 117.9 ± 21.2 | 125.2 ± 19.9 |
| Asp | 35.8 ± 9.4 | 40.1 ± 7.9 | 33.9 ± 8.3 | 37.8 ± 7.3 |
| Asn | 50.3 ± 3.7 | 58.9 ± 4.7 | 77.7 ± 6.9 | 55.0 ± 4.3 |
| Ala | 476.7 ± 45.4 | 499.5 ± 71.7 | 499.1 ± 48.6 | 473.3 ± 41.8 |
Values (means ± SE) are expressed in µmol/l; n = 6–8/group. Groups are defined as follows: pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE).
Values without a common superscript are significantly different (P < 0.05).
Bile acid profile.
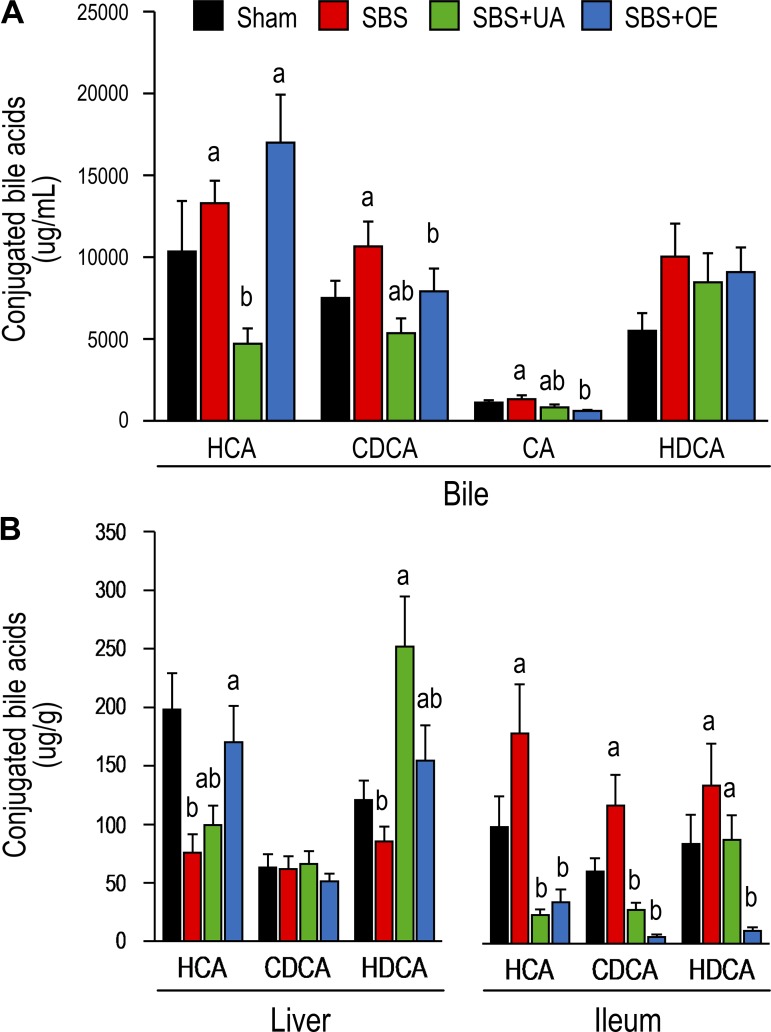
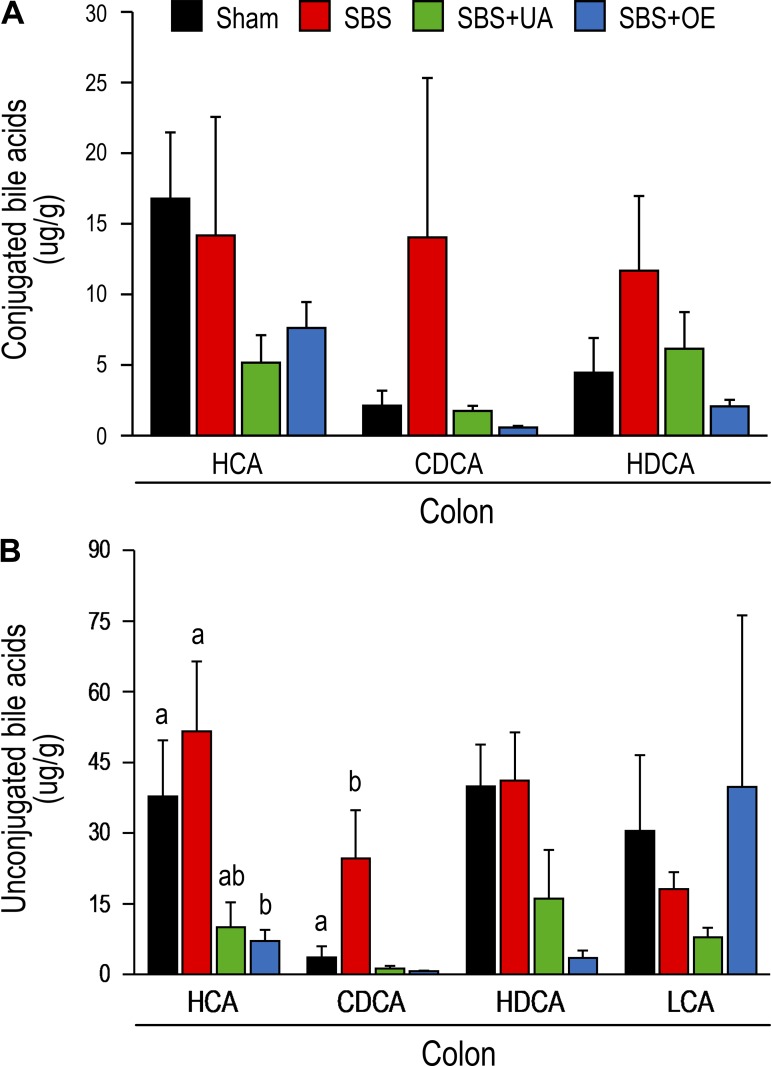
Conjugated hyocholic acid (HCA) and cholic acid concentrations were lower (P < 0.05) in bile from SBS + UA than SBS pigs, and conjugated CDCA concentration was lower (P < 0.05) in bile from SBS + OE than SBS pigs (Fig. 6A). In the liver, resection decreased (P < 0.05) conjugated HCA concentration, while treatment with UA and OE led to increased (P < 0.05) concentrations of conjugated hyodeoxycholic acid (HDCA) and HCA, respectively (Fig. 6B). In the ileum, concentrations of conjugated HCA and CDCA were decreased (P < 0.05) in the SBS + UA compared with the SBS group, and concentrations of conjugated HCA, CDCA, and HDCA were decreased (P < 0.05) in the SBS + OE compared with the SBS group. In the colon, HCA concentration was lower (P < 0.05) in the SBS + OE than SBS group (Fig. 7).
Fig. 6.
Bile acid [hyocholic acid (HCA), chenodeoxycholic acid (CDCA), cholic acid (CA), and hyodeoxycholic acid (HDCA)] profile of bile (A) and liver and ileum (B) in pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE). Values are means ± SE; n = 6–8/group. a,bValues with different superscripts are significantly different (P < 0.05).
Fig. 7.
Bile acid [hyocholic acid (HCA), chenodeoxycholic acid (CDCA), hyodeoxycholic acid (HDCA), and lithocholic acid (LCA)] profile of colon in pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE). Values are means ± SE; n = 6–8/group. a,bValues with different superscripts are significantly different (P < 0.05).
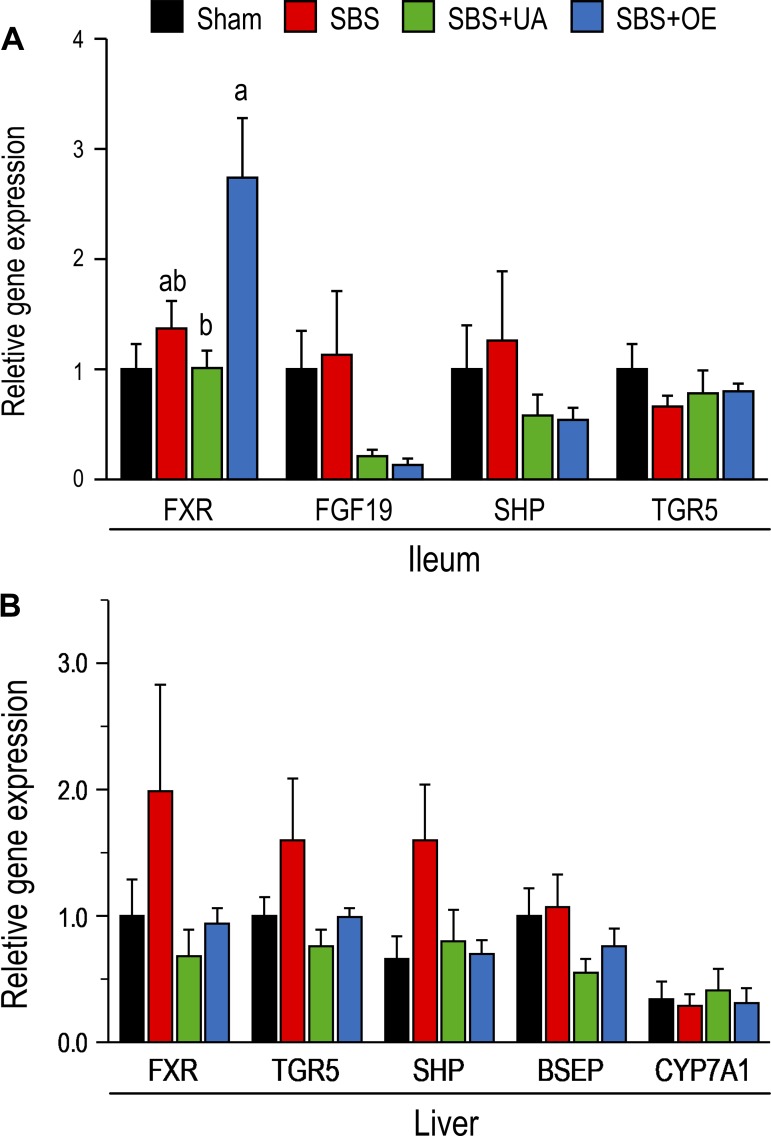
Expression of genes related to bile acid metabolism.
In the ileum, mRNA expression of farnesoid X receptor (FXR) was not affected by resection but was higher (P < 0.05) in SBS + OE than SBS + UA pigs (Fig. 8A). The mRNA expression of FGF19, small heterodimer partner (SHP), and TGR5 did not differ among the groups. In the liver, mRNA expression of SHP tended (P = 0.08) to be augmented by resection but was not altered following treatment with UA and OE (Fig. 8B). The mRNA expression of FXR, TGR5, bile salt export pump, and cytochrome P-450 7A1 did not differ among the groups.
Fig. 8.
mRNA expression of genes related to bile acid metabolism [farnesoid X receptor (FXR), FGF19, small heterodimer partner (SHP), Takeda G protein-coupled receptor 5 (TGR5), bile salt export pump (BSEP), and cytochrome P-450 7A1 (CYP7A1)] in ileum (A) and liver (B) of pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE). Values are means ± SE; n = 6–8/group. a,bValues with different superscripts are significantly different (P < 0.05).
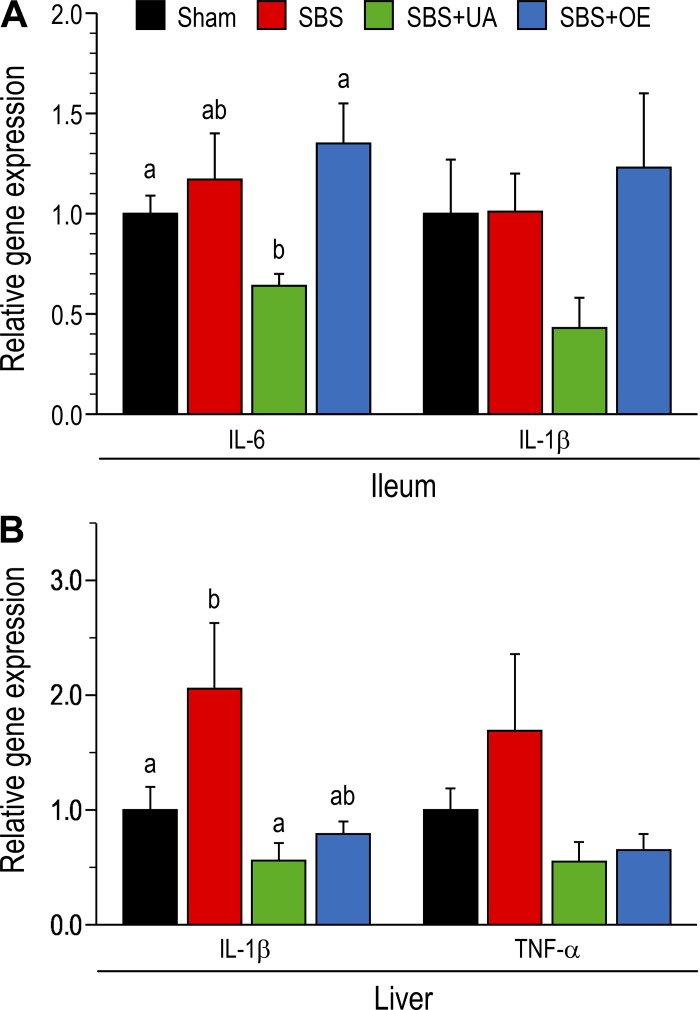
Expression of inflammatory genes in the ileum and liver.
In the ileum, resection did not affect (P > 0.05) IL-6 gene expression, whereas mRNA expression of IL-6 was lower (P < 0.05) in UA- than OE-treated pigs (Fig. 9A). In ileum tissue, mRNA expression of IL-1β was not different (P > 0.05) between groups. In the liver, resection tended (P = 0.08) to increase mRNA expression of IL-1β, whereas mRNA expression of IL-1β was lower (P < 0.05) in SBS + UA than SBS pigs. The mRNA expression of TNF-α was not different (P > 0.05) among groups (Fig. 9B). ELISA of IL-1β and TNF-α protein concentrations in liver tissue showed no difference between treatment groups (data not shown).
Fig. 9.
mRNA expression of inflammatory genes in ileum (A) and liver (B) of pigs subjected to transection surgery and treated with DMSO (sham) and pigs subjected to resection surgery and treated with vehicle [short bowel syndrome (SBS)], ursolic acid (SBS + UA), or olive plant extract (SBS + OE). Values are means ± SE; n = 6–8/group. a,bValues with different superscripts are significantly different (P < 0.05).
DISCUSSION
There is a need to develop treatments that improve intestinal adaptation following small bowel resection in patients, especially infants, with SBS to increase intestinal absorption of enteral nutrients and diminish the need for PN and the incidence of PN-associated liver disease. Past and recent studies in piglet models of SBS suggest that a key element of early intestinal adaptation following massive small bowel resection is stimulation of GLP-2 secretion and associated epithelial cell proliferation, but GLP-2 levels are reduced after 6–8 wk (23, 38, 54, 56). TGR5 is a G protein-coupled receptor expressed in enteroendocrine L cells that, when activated, stimulates the co-secretion of GLP-1 and GLP-2 (15, 26, 31). We previously showed that enteral bile acid treatment increased secretion of both GLP-1 and GLP-2, presumably by activation of TGR5 (28). The aim of the current study was to test whether oral TGR5 agonists can be used to increase GLP-2 secretion and augment the adaptive responses of the remnant intestine in a SBS pig model.
In study 1, we showed that oral gavage with UA induced a robust GLP-2 secretion in parenterally fed neonatal pigs. The response to OE treatment was not significant, but the highest dose (50 mg/kg) tended to increase GLP-2 concentrations; the responses to OE appeared to be dose-dependent and may require higher doses. The results indicate that the dose of UA tested here is capable of stimulating GLP-2 secretion to physiological plasma concentrations comparable to those in enterally fed neonatal pigs (11, 27, 28). The stimulation of GLP-2 secretion is in line with a previous study demonstrating that the TGR5 agonist betulinic acid induced bicarbonate secretion via GLP-2 release and that dipeptidyl dipeptidase IV inhibition potentiated the effect (26). Studies in diabetic rats also showed that a higher dose of UA (30 mg/kg) increases plasma GLP-1 concentration (34). Betulinic acid and UA are members of a family of pentacyclic triterpenoid compounds (e.g., oleanolic acid) that are found in various plants, including the olive (Olea europaea) (18, 20). Cell culture studies in TGR5 reporter assays show higher potencies for UA (EC50 = 1.43 μM), betulinic acid (EC50 = 1.04 μM), and oleanolic acid (EC50 = 2.25 μM) than the secondary bile acid lithocholic acid (EC50 = 5.60 μM) (18). Together, our results show that UA is an effective oral GLP-2 secretagogue in parenterally fed pigs.
Next, we examined the effects of TGR5 agonists on intestinal adaptation in a piglet short bowel model. Consistent with previous studies in neonatal pigs (23, 56), we found that plasma GLP-2 concentration, weight, length, villus height, and crypt cell proliferation in remnant intestine were increased by mid-jejunal bowel resection, whereas supplementation with UA and OE did not further improve gut growth. The failure of UA and OE to augment intestinal growth could be due to the fact that plasma GLP-2 concentrations were not increased by either TGR5 agonist. We postulate that massive jejunal resection induces a robust activation of endogenous intestinal adaptive mechanisms, including GLP-2 secretion, and may require much higher doses or more potent TGR5 agonists to induce additional trophic effects. It is also possible that the L cells, both in number and secretory capacity, are not capable of greater GLP-2 secretion during the adaptive phase and perhaps co-treatment with dipeptidyl peptidase IV inhibitors could have enhanced GLP-2 biological activity, as reported in mice (37).
We also quantified whole body citrulline kinetics in response to SBS, since citrulline is synthesized mainly in the intestine and has been suggested as a useful plasma marker of intestinal function and adaptation in patients with SBS (13, 14, 42). We also measured arginine kinetics, since citrulline is the precursor for arginine, which is a conditionally essential amino acid in neonates and becomes essential in rodents with massive small intestinal resection. Studies in parenterally fed pigs showed that GLP-2 treatment increases whole body arginine synthesis in direct correlation with intestinal mass (55). As expected, the whole body flux and plasma concentration of citrulline were markedly decreased in SBS compared with control pigs but were not increased by UA or OE treatment. Interestingly, the plasma concentration, but not whole body flux, of arginine was lower in SBS groups than in control pigs. Moreover, plasma glutamine was increased by SBS, and this response was further enhanced by UA, perhaps because the efficacy of glutamine for intestinal oxidative metabolism and citrulline production is restricted by loss of intestinal mass with SBS.
In addition to regulating the secretion of GLPs, TGR5 also modulates bile acid pool and bile acid composition, and bile acids could indirectly influence intestinal adaptation via direct activation of epithelial cells or FXR. Studies in pigs show that SBS disrupts bile acid homeostasis, resulting in reduced conversion of primary to secondary bile acids that have been linked to microbial dysbiosis (40). In pigs, primary bile acids are CDCA and HCA, whereas HDCA is a major secondary bile acid synthesized through intestinal bacterial biotransformation. In this study we found that SBS alone vs. control had limited effects on bile acid profiles, whereas UA and OE had differential effects on tissue bile acid but generally suppressed bile acid content in ileum and colon tissue. Changes in tissue gene expression related to bile acid homeostasis suggested a trend for increased FXR signaling with SBS and suppression of FXR signaling with UA and OE.
SBS has been shown to induce a proinflammatory state in the gut and liver (2, 40). Furthermore, TGR5 agonists, including UA, have been shown to be anti-inflammatory (41). Our results showed that UA seemed to suppress the gene expression of proinflammatory cytokines in the remnant intestine and liver, consistent with the anti-inflammatory effects of TGR5 agonists, but these effects were not evident in tissue cytokine protein levels.
In summary, our results show that the TGR5 agonist UA stimulates GLP-2 secretion in parenterally fed neonatal pigs under conditions of diminished basal GLP-2 secretion. In contrast, neither UA nor OE affected GLP-2 secretion or the intestinal adaptive response in neonatal pigs following massive small bowel resection. We suggest that the robust intestinal adaptation response following SBS maximized the trophic cellular mechanisms, such that they were not responsive to the doses of TGR5 agonist used in our pigs. Our results suggest that 1) these TGR5 agonists, in particular UA, may alter bile acid homeostasis and reduce the flow or uptake of bile acid in the terminal intestine and 2) higher doses or increased potency of TGR5 agonist may be necessary to augment intestinal adaptation in conditions of SBS.
GRANTS
This work was supported in part by the US Department of Agriculture, Agricultural Research Service, under Cooperative Agreement 3092-51000-060-01 and grants from Lucta SA and the National Institutes of Health (Grants DK-094616 to D. G. Burrin and P30 DK-56338 to the Texas Medical Center Digestive Diseases Center). S. Lin was supported by training fellowships from the Chinese Scholarship Council and the National Natural Science Foundation of China.
DISCLOSURES
D. G. Burrin received grant support from Lucta SA to partially fund this study. J. J. Pastor is an employee of Lucta SA. I. R. Ipharraguerre was employed by Lucta SA at the time this study was conducted.
AUTHOR CONTRIBUTIONS
J.J.P., I.R.I., and D.G.B. conceived and designed research; S.L., B.S., J.R., J.J.P., J.C.M., I.R.I., J.J.H., S.M.C., P.E.L., O.O.O., Z.F., and D.G.B. performed experiments; S.L., B.S., J.R., J.J.P., J.C.M., I.R.I., B.H., J.J.H., S.M.C., Z.F., and D.G.B. analyzed data; S.L., B.S., J.R., J.J.P., J.C.M., I.R.I., B.H., O.O.O., Z.F., and D.G.B. interpreted results of experiments; S.L. prepared figures; S.L. drafted manuscript; S.L., B.S., J.R., J.J.P., J.C.M., I.R.I., B.H., O.O.O., Z.F., and D.G.B. edited and revised manuscript; S.L., B.S., J.R., J.J.P., J.C.M., I.R.I., B.H., J.J.H., S.M.C., P.E.L., O.O.O., Z.F., and D.G.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Liwei Cui, Greg Guthrie, Lee Call, Rebecca Welch, and Tiffany Molina for assistance with this study.
REFERENCES
- 1.Agarwal S, Patil A, Aware U, Deshmukh P, Darji B, Sasane S, Sairam KV, Priyadarsiny P, Giri P, Patel H, Giri S, Jain M, Desai RC. Discovery of a potent and orally efficacious TGR5 receptor agonist. ACS Med Chem Lett 7: 51–55, 2016. doi: 10.1021/acsmedchemlett.5b00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aprahamian CJ, Chen M, Yang Y, Lorenz RG, Harmon CM. Two-hit rat model of short bowel syndrome and sepsis: independent of total parenteral nutrition, short bowel syndrome is proinflammatory and injurious to the liver. J Pediatr Surg 42: 992–997, 2007. doi: 10.1016/j.jpedsurg.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 3.Aunsholt L, Thymann T, Qvist N, Sigalet D, Husby S, Sangild PT. Prematurity reduces functional adaptation to intestinal resection in piglets. JPEN J Parenter Enteral Nutr 39: 668–676, 2015. doi: 10.1177/0148607114528714. [DOI] [PubMed] [Google Scholar]
- 4.Barnes JL, Hartmann B, Holst JJ, Tappenden KA. Intestinal adaptation is stimulated by partial enteral nutrition supplemented with the prebiotic short-chain fructooligosaccharide in a neonatal intestinal failure piglet model. JPEN J Parenter Enteral Nutr 36: 524–537, 2012. doi: 10.1177/0148607112444131. [DOI] [PubMed] [Google Scholar]
- 5.Bartholome AL, Albin DM, Baker DH, Holst JJ, Tappenden KA. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J Parenter Enteral Nutr 28: 210–222, 2004. doi: 10.1177/0148607104028004210. [DOI] [PubMed] [Google Scholar]
- 6.Burrin DG, Stoll B, Jiang R, Petersen Y, Elnif J, Buddington RK, Schmidt M, Holst JJ, Hartmann B, Sangild PT. GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am J Physiol Gastrointest Liver Physiol 279: G1249–G1256, 2000. doi: 10.1152/ajpgi.2000.279.6.G1249. [DOI] [PubMed] [Google Scholar]
- 7.Burrin D, Stoll B, Moore D. Digestive Physiology of the Pig Symposium: intestinal bile acid sensing is linked to key endocrine and metabolic signaling pathways. J Anim Sci 91: 1991–2000, 2013. doi: 10.2527/jas.2013-6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrin DG, Stoll B, Guan X. Glucagon-like peptide 2 function in domestic animals. Domest Anim Endocrinol 24: 103–122, 2003. doi: 10.1016/S0739-7240(02)00210-2. [DOI] [PubMed] [Google Scholar]
- 9.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Hadsell D. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am J Physiol Endocrinol Metab 292: E281–E291, 2007. doi: 10.1152/ajpendo.00129.2006. [DOI] [PubMed] [Google Scholar]
- 10.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Holst JJ. Glucagon-like peptide 2 dose-dependently activates intestinal cell survival and proliferation in neonatal piglets. Endocrinology 146: 22–32, 2005. doi: 10.1210/en.2004-1119. [DOI] [PubMed] [Google Scholar]
- 11.Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley GH Jr, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal piglets: how much is enough? Am J Clin Nutr 71: 1603–1610, 2000. doi: 10.1093/ajcn/71.6.1603. [DOI] [PubMed] [Google Scholar]
- 12.Carter BA, Cohran VC, Cole CR, Corkins MR, Dimmitt RA, Duggan C, Hill S, Horslen S, Lim JD, Mercer DF, Merritt RJ, Nichol PF, Sigurdsson L, Teitelbaum DH, Thompson J, Vanderpool C, Vaughan JF, Li B, Youssef NN, Venick RS, Kocoshis SA. Outcomes from a 12-week, open-label, multicenter clinical trial of teduglutide in pediatric short bowel syndrome. J Pediatr 181: 102–111.e5, 2017. doi: 10.1016/j.jpeds.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 119: 1496–1505, 2000. doi: 10.1053/gast.2000.20227. [DOI] [PubMed] [Google Scholar]
- 14.Crenn P, Vahedi K, Lavergne-Slove A, Cynober L, Matuchansky C, Messing B. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 124: 1210–1219, 2003. doi: 10.1016/S0016-5085(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 15.Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol 76: 561–583, 2014. doi: 10.1146/annurev-physiol-021113-170317. [DOI] [PubMed] [Google Scholar]
- 16.Dudley MA, Wykes LJ, Dudley AW Jr, Burrin DG, Nichols BL, Rosenberger J, Jahoor F, Heird WC, Reeds PJ. Parenteral nutrition selectively decreases protein synthesis in the small intestine. Am J Physiol Gastrointest Liver Physiol 274: G131–G137, 1998. doi: 10.1152/ajpgi.1998.274.1.G131. [DOI] [PubMed] [Google Scholar]
- 17.Gavaldà-Navarro A, Pastor JJ, Mereu A, Villarroya F, Ipharraguerre IR. Developmental regulation of the intestinal FGF19 system in domestic pigs. Am J Physiol Gastrointest Liver Physiol 314: G647–G654, 2018. doi: 10.1152/ajpgi.00312.2017. [DOI] [PubMed] [Google Scholar]
- 18.Genet C, Strehle A, Schmidt C, Boudjelal G, Lobstein A, Schoonjans K, Souchet M, Auwerx J, Saladin R, Wagner A. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J Med Chem 53: 178–190, 2010. doi: 10.1021/jm900872z. [DOI] [PubMed] [Google Scholar]
- 20.Guinda A, Rada M, Delgado T, Gutiérrez-Adánez P, Castellano JM. Pentacyclic triterpenoids from olive fruit and leaf. J Agric Food Chem 58: 9685–9691, 2010. doi: 10.1021/jf102039t. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann B, Harr MB, Jeppesen PB, Wojdemann M, Deacon CF, Mortensen PB, Holst JJ. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab 85: 2884–2888, 2000. doi: 10.1210/jc.85.8.2884. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann B, Johnsen AH, Orskov C, Adelhorst K, Thim L, Holst JJ. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides 21: 73–80, 2000. doi: 10.1016/S0196-9781(99)00176-X. [DOI] [PubMed] [Google Scholar]
- 23.Hua Z, Turner JM, Sigalet DL, Wizzard PR, Nation PN, Mager DR, Ball RO, Pencharz PB, Wales PW. Role of glucagon-like peptide-2 deficiency in neonatal short-bowel syndrome using neonatal piglets. Pediatr Res 73: 742–749, 2013. doi: 10.1038/pr.2013.44. [DOI] [PubMed] [Google Scholar]
- 25.Hughes CA, Dowling RH. Speed of onset of adaptive mucosal hypoplasia and hypofunction in the intestine of parenterally fed rats. Clin Sci (Lond) 59: 317–327, 1980. doi: 10.1042/cs0590317. [DOI] [PubMed] [Google Scholar]
- 26.Inoue T, Wang J-H, Higashiyama M, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD, Akiba Y. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol 303: G810–G816, 2012. doi: 10.1152/ajpgi.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ipharraguerre IR, Tedó G, Menoyo D, de Diego Cabero N, Holst JJ, Nofrarías M, Mereu A, Burrin DG. Bile acids induce glucagon-like peptide 2 secretion with limited effects on intestinal adaptation in early weaned pigs. J Nutr 143: 1899–1905, 2013. doi: 10.3945/jn.113.177865. [DOI] [PubMed] [Google Scholar]
- 28.Jain AK, Stoll B, Burrin DG, Holst JJ, Moore DD. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am J Physiol Gastrointest Liver Physiol 302: G218–G224, 2012. doi: 10.1152/ajpgi.00280.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 60: 902–914, 2011. doi: 10.1136/gut.2010.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeppesen PB, Gabe SM, Seidner DL, Lee HM, Olivier C. Factors associated with response to teduglutide in patients with short-bowel syndrome and intestinal failure. Gastroenterology 154: 874–885, 2018. doi: 10.1053/j.gastro.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329: 386–390, 2005. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 32.Lim DW, Diané A, Muto M, Vine DF, Nation PN, Wizzard PR, Sigalet DL, Bigam DL, Pencharz PB, Turner JM, Wales PW. Differential effects on intestinal adaptation following exogenous glucagon-like peptide 2 therapy with and without enteral nutrition in neonatal short bowel syndrome. JPEN J Parenter Enteral Nutr 41: 156–170, 2017. doi: 10.1177/0148607116665812. [DOI] [PubMed] [Google Scholar]
- 33.Lim DW, Turner JM, Wales PW. Emerging piglet models of neonatal short bowel syndrome. JPEN J Parenter Enteral Nutr 39: 636–643, 2015. doi: 10.1177/0148607114554621. [DOI] [PubMed] [Google Scholar]
- 34.Lo SH, Li Y, Cheng KC, Niu CS, Cheng JT, Niu HS. Ursolic acid activates the TGR5 receptor to enhance GLP-1 secretion in type 1-like diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 390: 1097–1104, 2017. doi: 10.1007/s00210-017-1409-9. [DOI] [PubMed] [Google Scholar]
- 35.Marini JC, Agarwal U, Didelija IC, Azamian M, Stoll B, Nagamani SC. Plasma glutamine is a minor precursor for the synthesis of citrulline: a multispecies study. J Nutr 147: 549–555, 2017. doi: 10.3945/jn.116.243592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin CR, Stoll B, Cluette-Brown J, Akinkuotu AC, Olutoye OO, Gura KM, Singh P, Zaman MM, Perillo MC, Puder M, Freedman SD, Burrin D. Use of a novel docosahexaenoic acid formulation vs. control in a neonatal porcine model of short bowel syndrome leads to greater intestinal absorption and higher systemic levels of DHA. Nutr Res 39: 51–60, 2017. doi: 10.1016/j.nutres.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okawada M, Holst JJ, Teitelbaum DH. Administration of a dipeptidyl peptidase IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome. Surgery 150: 217–223, 2011. doi: 10.1016/j.surg.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paris MC, Fuller PJ, Carstensen B, Nagy E, Taylor RG, Sourial M, Holst JJ, Hartmann B, Binesm JE. Plasma GLP-2 levels and intestinal markers in the juvenile pig during intestinal adaptation: effects of different diet regimens. Dig Dis Sci 49: 1688–1695, 2004. doi: 10.1023/B:DDAS.0000043388.52260.2f. [DOI] [PubMed] [Google Scholar]
- 39.Parker HE, Wallis K, le Roux CW, Wong KY, Reimann F, Gribble FM. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol 165: 414–423, 2012. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira-Fantini PM, Lapthorne S, Joyce SA, Dellios NL, Wilson G, Fouhy F, Thomas SL, Scurr M, Hill C, Gahan CG, Cotter PD, Fuller PJ, Hardikar W, Bines JE. Altered FXR signalling is associated with bile acid dysmetabolism in short bowel syndrome-associated liver disease. J Hepatol 61: 1115–1125, 2014. doi: 10.1016/j.jhep.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 41.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol 54: 1263–1272, 2011. doi: 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhoads JM, Plunkett E, Galanko J, Lichtman S, Taylor L, Maynor A, Weiner T, Freeman K, Guarisco JL, Wu GY. Serum citrulline levels correlate with enteral tolerance and bowel length in infants with short bowel syndrome. J Pediatr 146: 542–547, 2005. doi: 10.1016/j.jpeds.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Sakanaka T, Inoue T, Yorifuji N, Iguchi M, Fujiwara K, Narabayashi K, Kakimoto K, Nouda S, Okada T, Kuramoto T, Ishida K, Abe Y, Takeuchi T, Umegaki E, Akiba Y, Kaunitz JD, Higuchi K. The effects of a TGR5 agonist and a dipeptidyl peptidase IV inhibitor on dextran sulfate sodium-induced colitis in mice. J Gastroenterol Hepatol 30, Suppl 1: 60–65, 2015. doi: 10.1111/jgh.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez-Quesada C, López-Biedma A, Warleta F, Campos M, Beltrán G, Gaforio JJ. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J Agric Food Chem 61: 12173–12182, 2013. doi: 10.1021/jf403154e. [DOI] [PubMed] [Google Scholar]
- 45.Sangild PT, Ney DM, Sigalet DL, Vegge A, Burrin D. Animal models of gastrointestinal and liver diseases. Animal models of infant short bowel syndrome: translational relevance and challenges. Am J Physiol Gastrointest Liver Physiol 307: G1147–G1168, 2014. doi: 10.1152/ajpgi.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun 362: 793–798, 2007. doi: 10.1016/j.bbrc.2007.06.130. [DOI] [PubMed] [Google Scholar]
- 47.Schalamon J, Mayr JM, Höllwarth ME. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol 17: 931–942, 2003. doi: 10.1016/S1521-6918(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 48.Sigalet DL, de Heuvel E, Wallace L, Bulloch E, Turner J, Wales PW, Nation P, Wizzard PR, Hartmann B, Assad M, Holst JJ. Effects of chronic glucagon-like peptide-2 therapy during weaning in neonatal pigs. Regul Pept 188: 70–80, 2014. doi: 10.1016/j.regpep.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Stoll B, Chang X, Fan MZ, Reeds PJ, Burrin DG. Enteral nutrient intake level determines intestinal protein synthesis and accretion rates in neonatal pigs. Am J Physiol Gastrointest Liver Physiol 279: G288–G294, 2000. doi: 10.1152/ajpgi.2000.279.2.G288. [DOI] [PubMed] [Google Scholar]
- 50.Suri M, Turner JM, Sigalet DL, Wizzard PR, Nation PN, Ball RO, Pencharz PB, Brubaker PL, Wales PW. Exogenous glucagon-like peptide-2 improves outcomes of intestinal adaptation in a distal-intestinal resection neonatal piglet model of short bowel syndrome. Pediatr Res 76: 370–377, 2014. doi: 10.1038/pr.2014.97. [DOI] [PubMed] [Google Scholar]
- 51.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thymann T, Stoll B, Mecklenburg L, Burrin DG, Vegge A, Qvist N, Eriksen T, Jeppesen PB, Sangild PT. Acute effects of the glucagon-like peptide 2 analogue, teduglutide, on intestinal adaptation in short bowel syndrome. J Pediatr Gastroenterol Nutr 58: 694–702, 2014. doi: 10.1097/MPG.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 53.Turner JM, Wales PW, Nation PN, Wizzard P, Pendlebury C, Sergi C, Ball RO, Pencharz PB. Novel neonatal piglet models of surgical short bowel syndrome with intestinal failure. J Pediatr Gastroenterol Nutr 52: 9–16, 2011. doi: 10.1097/MPG.0b013e3181f18ca0. [DOI] [PubMed] [Google Scholar]
- 54.Uko V, Radhakrishnan K, Alkhouri N. Short bowel syndrome in children: current and potential therapies. Paediatr Drugs 14: 179–188, 2012. doi: 10.2165/11594880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 55.Urschel KL, Evans AR, Wilkinson CW, Pencharz PB, Ball RO. Parenterally fed neonatal piglets have a low rate of endogenous arginine synthesis from circulating proline. J Nutr 137: 601–606, 2007. doi: 10.1093/jn/137.3.601. [DOI] [PubMed] [Google Scholar]
- 56.Vegge A, Thymann T, Lund P, Stoll B, Bering SB, Hartmann B, Jelsing J, Qvist N, Burrin DG, Jeppesen PB, Holst JJ, Sangild PT. Glucagon-like peptide-2 induces rapid digestive adaptation following intestinal resection in preterm neonates. Am J Physiol Gastrointest Liver Physiol 305: G277–G285, 2013. doi: 10.1152/ajpgi.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wøjdemann M, Wettergren A, Hartmann B, Holst JJ. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scand J Gastroenterol 33: 828–832, 1998. doi: 10.1080/00365529850171486. [DOI] [PubMed] [Google Scholar]