Abstract
MicroRNAs (miRNAs) are small, noncoding single-stranded RNA oligonucleotides that modulate physiological and pathological processes by modulating target gene expression. Many miRNAs display tissue-specific expression patterns, the dysregulation of which has been associated with various disease states, including kidney disease. Mounting evidence implicates miRNAs in various biological processes, such as cell proliferation and differentiation and cancer. Because miRNAs are relatively stable in tissue and biological fluids, particularly when carried by extracellular vesicles, changes in their levels may reflect the development of human disease. Urinary miRNAs originate from primary kidney and urinary tract cells, cells infiltrating the renal tissue and shed in the urine, or the systemic circulation. Although their validity as biomarkers for kidney disease has not been fully established, studies have been applying analysis of miRNAs in the urine in an attempt to detect and monitor acute and chronic renal diseases. Because appreciation of the significance of miRNAs in the renal field is on the rise, an understanding of miRNA pathways that regulate renal physiology and pathophysiology is becoming critically important. This review aims to summarize new data obtained in this field of research. It is hoped that new developments in the use of miRNAs as biomarkers and/or therapy will help manage and contain kidney disease in affected subjects.
Keywords: biomarkers, kidney, microRNAs, urine
INTRODUCTION
MicroRNAs (miRNAs) are a class of small (~20–22 nt long), noncoding single-stranded RNA oligonucleotides. These oligonucleotides modulate homeostatic and pathological processes by regulating and modulating gene expression via a posttranscriptional mechanism (32). A number of miRNAs are evolutionarily conserved and seem to target almost two-thirds of the genes in mammals (39). Many miRNAs display differential expression in different organs, the dysregulation of which has been associated with various diseases, including kidney disease. Under both homeostatic and disease conditions, the urine carries from the body solute waste that can be used as a biomarker of disease. Urinary miRNAs may be derived from the kidney and urinary tract cells and could be passively filtered through the glomerulus or secreted by renal tubules (48). Although the validity of urinary miRNAs as a biomarker for kidney disease is unknown, studies have been increasingly applying analysis of miRNAs in the urine in an attempt to detect and differentiate renal diseases (Table 1). This review aims to summarize the data obtained in this field of research.
Table 1.
Urinary miRNAs dysregulated in kidney disease
| Disease | miRNA | Expression | Reference |
|---|---|---|---|
| Ischemia-reperfusion injury | |||
| Cardiac surgery | miRNA-21 | Up | 6, 15 |
| Kidney transplantation | miRNA-24 | Up | 42 |
| Intensive care unit | miRNA-494 | Up | 38 |
| Diabetic nephropathy | miRNA-15 | Down | 76 |
| Hypertension | |||
| Salt sensitivity | hsa-miRNA-4516 | Up | 21 |
| Renovascular hypertension | miRNA-26A | Down | 95 |
| Renovascular hypertension | miRNA-21,- miRNA-93, and miRNA-200b | Up | 36 |
| Polycystic kidney disease | miRNA-1 and miRNA-133b | Down | 8 |
| Glomerulonephritis | |||
| IgA nephropathy | miRNA-146a and miRNA-155 | Up | 41, 83 |
| Focal segmental glomerulosclerosis | miRNA-10a and miRNA-30d | Up | 84 |
| Lupus nephritis | miRNA-146a | Up | 61, 93 |
| Kidney transplantation | |||
| Acute T cell-mediated rejection | miRNA-210 | Down | 44 |
| Chronic rejection | miRNA-142-3p, miRNA-204, and miRNA-211 | Up and down | 67, 73 |
| Ureteral obstruction | let-7a and miRNA-29b | Down | 57 |
| Cancer | |||
| Bladder cancer | miRNA-126 and miRNA-152 | Up and down | 4, 16, 27 |
| Renal cell carcinoma | miRNA-15 | Up | 55, 81 |
miRNA, microRNA.
BIOGENESIS AND TRANSPORT OF miRNA
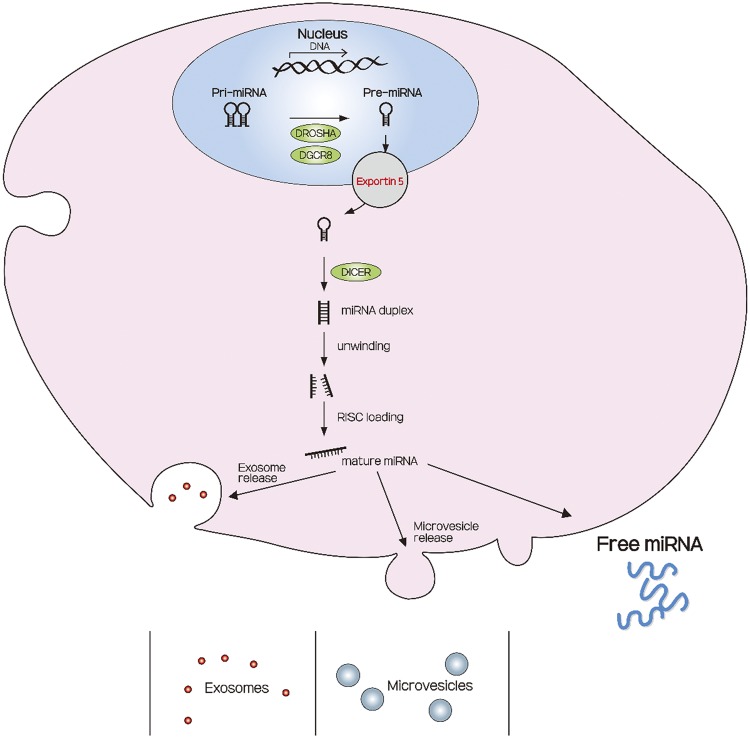
The biogenesis of miRNAs is a multistep process (Fig. 1). First, miRNAs are transcribed in the nucleus as stem-loop primary miRNA, a central building block of many RNA secondary structures, in which two regions of the same strand base pair to form a double helix that ends in an unpaired loop. These structures are then cleaved into shorter precursor miRNA by Drosha (an RNase type III enzyme) and its essential cofactor DiGeorge syndrome critical region gene 8. The precursor miRNAs are transported out of the nucleus via exportin 5 and, once in the cytosol, are cleaved into their mature double-stranded miRNAs by Dicer, another RNase type III enzyme. After cleavage, the miRNA duplex is unwound and the functional strands are assembled into the RNA-induced silencing complex and bind through their “seed sequence” to fully or partially complementary sites within the 3′-untranslated region of target mRNAs.
Fig. 1.
A schematic diagram showing microRNA (miRNA) biogenesis, release, and modes of transport. miRNA transcripts initially originate as primary miRNAs (Pri-miRNAs), which are processed into Pre-miRNAs by the Drosha enzyme and further cleaved to result in double-strand RNA duplexes by the action of a second enzyme, Dicer. The miRNA duplexes are then unwound by Dicer, and the mature miRNA guide strand is loaded into the RNA-induced silencing complex (RISC). miRNA can be exported as free miRNA, forming protein or lipid/lipoprotein complexes, or packed in extracellular vesicles (exosomes or microvesicles). [Adapted from Barreiro and Holthofer (7).]
Subsequently, miRNAs are often carried in body fluid by extracellular vesicles, membrane-enclosed particles that can be released by almost any cell. Extracellular vesicles are classified as exosomes, microparticles, and apoptotic bodies according to their origin, size, and content (7, 18). Apoptotic bodies contain parts of a dying cell, whereas exosomes and microvesicles are released during the life cycle of a cell. During their formation, exosomes and microvesicles incorporate cytosolic proteins, mRNA, and miRNA from the cell of origin and, thus, carry some of the miRNA that had been produced within the cells in body fluids. Such miRNAs are present in various body fluids, including plasma, saliva, breast milk, pleural effusion, tears, and urine (86). Extracellular vesicles seem to protect and increase the stability of miRNAs, whereas the stability of free miRNAs remains controversial. Observations from a single study indicate that miRNAs are stable in both tissue and body fluids even when stored under harsh conditions, such as −80°C (71); yet another study reported that miRNAs are unstable when stored at 4°C (74). On the other hand, miRNAs can be rendered relatively stable by several measures, since they are protected from nuclease degradation by packing within exosomes (1, 79) or by binding to plasma components, such as high-density lipoprotein-cholesterol, or proteins, such as argonaute-2 or nucleophosmin (5, 82). Therefore, research studies often assess levels of miRNAs carried by extracellular vesicles (36, 58).
TECHNIQUES FOR THE DETECTION OF miRNA LEVELS
The past few years have seen a marked rise in identified miRNAs, given that the number of miRNAs registered to the miRBase database has been rapidly expanding (25). To elucidate gene regulation under physiological and pathological conditions, various methods have been introduced for expression profiling of miRNAs. Because of the recent advent of increasingly sensitive and specific techniques, it may now be feasible to monitor miRNAs in clinical scenarios. Initially, important information regarding various forms of a miRNA was extracted from RNA blot analyses (52). Microarray technologies have been particularly useful for miRNA profiling, as they are able to screen large numbers of miRNAs simultaneously (77). More recently, quantitative real-time PCR has become the hallmark method for miRNA profiling, given its premier sensitivity and ability to detect down to 1-nt differences among miRNAs (9). Recently, newer high-throughput technologies, such as next-generation RNA sequencing, have been used to profile miRNA expression (50, 51) and have provided promising results in both tissues and biofluids (49).
miRNAs AS DIAGNOSTIC BIOMARKERS IN HUMAN DISEASES
The primary function of miRNAs seems to be regulation of gene expression and translation. Target recognition by miRNAs leads to mRNA translational repression and/or deadenylation and decay, although miRNAs have also been reported to have positive effects on transcription (62). Mammalian miRNAs may have hundreds of unique targets (20).
Increasing evidence implicates miRNA involvement in various biological processes (Fig. 2), such as cell proliferation, differentiation, and apoptosis, as well as tumorigenesis (2). Accordingly, many studies have explored the involvement of miRNAs and their correlation with various diseases, especially cancer (24). Some miRNAs were found to be markedly dysregulated during carcinogenesis, and circulating miRNAs have been regarded as biomarkers for gastric cancer, colorectal cancer, and acute myeloblastic leukemia (29, 45, 85). In addition, a number of miRNAs have been shown to play a critical role in the pathogenesis of cardiovascular diseases and may serve as diagnostic biomarkers in this field. For example, serum miRNA-499 and miRNA-210 levels are upregulated in unstable angina and non-ST-segment elevation myocardial infarction and might be a promising biomarker to support the diagnosis of acute coronary syndrome in patients in the emergency department (70). Recently, miRNAs have been demonstrated to play a vital role in kidney development, maintenance of renal function, and progression of kidney disease (Fig. 2) (46, 78). In this review, we focus on the role of miRNAs expressed in different forms of kidney diseases.
Fig. 2.
Potential uses of urinary microRNAs (miRNAs) in kidney disease. miRNAs may facilitate diagnosis and monitoring of renal conditions. Their most useful application may be to predict the clinical outcome of patients with acute or chronic kidney disease and to help decide treatment.
Acute Kidney Injury
Overall, altered expression of >50 individual miRNAs has been described in acute kidney injury (AKI) (19). A number of miRNAs have been associated with both protective and pathogenic roles in the development of AKI. For example, miRNA-21, which has the most commonly reported association with AKI, protects against injury by inhibiting apoptosis and inflammation. miRNA-21 has been shown to inhibit apoptosis by downregulating programmed cell death protein-4 and phosphatase and tensin homology (88). Inflammation may also be reduced as a result of upregulation of miRNA-21 targeting of peroxisome proliferator-activated receptor-α and decrease of NF-κB-induced inflammation (94). On the other hand, miRNA-21 is induced by transforming growth factor (TGF)-β and TNF-α and promotes fibrosis via suppression of peroxisome proliferator-activated receptor-α (11, 91). Therefore, miRNA-21 has the potential not only to protect against injury by inhibiting apoptosis and inflammation but also to amplify injury responses and promote fibrosis, which represent the healing phase of renal injury. While some of these studies assessed its expression in the kidney tissue, rather than urine, Saikumar et al. (65) reported an upregulation of miRNA-21 in kidney tissue and a subsequent increase of urinary miRNA-21 levels, suggesting its utility as a urinary biomarker for detection of AKI. Alas, the extent to which changes in tissue miRNA activity or expression consistently reflect their urinary levels needs to be determined.
Notably, urine miRNA-21 levels parallel the severity of AKI (15). The involvement of miRNA-21 has also been found in the pathophysiology of ischemia-reperfusion injury (IRI), one of the primary etiologies of AKI (40). miRNA-21 played a protective role in the response to stress and inflammation in renal IRI by downregulation of necrosis in renal tubular epithelial cells (22), whereas inhibition of miRNA-21 was protective against fibrogenesis and inflammation in a murine model of Alport syndrome, indicating a double-edged sword effect of miRNA-21 (23). Studies are needed to determine whether inhibition of miRNA-21 confers overall beneficial or detrimental effects in AKI.
miRNA-24 has also been shown to play an important role in IRI by stimulating apoptosis in endothelial and tubular epithelial cells (42). Expression of miRNA-24 was significantly elevated in kidneys of mice after IRI and in patients after kidney transplantation (42). However, no data about urinary miRNA-24 in patients with IRI are available. In a recent study in human and rat urine, Zou et al. (96) detected elevated levels of urinary miRNA-30c-5p and miRNA-192-5p in rats and patients with AKI 2 h post-IRI, showing the potential importance of these miRNAs as diagnostic markers for early IRI.
In addition to miRNA-24, miRNA-494, a cell death-associated miRNA, is upregulated early in AKI and promotes apoptosis and inflammation by downregulating activating transcription factor-3 and increasing IL-6 and monocyte chemoattractant protein-1 (38). Urinary miRNA-494 levels were 60-fold higher in patients with AKI compared with healthy controls, and their elevation preceded the increase of serum creatinine (38). These observations suggest that miRNA-494 could be used as an early biomarker portending AKI development.
Diabetic Nephropathy
Diabetic kidney disease is the most frequent underlying etiology for chronic kidney disease (CKD) and might eventuate in end-stage renal disease. Early detection of diabetic nephropathy is critical for improving clinical management, and new biomarkers are needed to identify its early stage, since microalbuminuria does not accurately predict diabetic kidney disease (75). Urinary miRNA levels differ among patients with CKD from various etiologies, with lower urinary level of miRNA-15 in patients with diabetic glomerulosclerosis (76). Subsequently, Argyropoulos et al. (3) identified a panel of 27 differentially regulated urinary miRNAs in patients with type 1 diabetes at different stages of diabetic nephropathy, which varied with its progression. A recent meta-analysis proposed that Homo sapiens (hsa)-miRNA-126 and hsa-miRNA-770 family miRNAs might have important diagnostic implications for diabetic nephropathy (59). However, data describing urinary exosomal miRNA expression in diabetic patients are too few to allow firm conclusions.
Hypertension
Several lines of evidence suggest that miRNAs regulate blood pressure. In salt-sensitive hypertension, Gildea et al. (21) identified 45 urinary exosomal miRNAs associated with salt sensitivity or inverse salt sensitivity. Of these, only hsa-miRNA-4516 could differentiate between these two phenotypical extremes from the salt-resistant group, suggesting it as a biomarker for deviant Na+ regulatory pathways in hypertension.
Renovascular hypertension may also be associated with dysregulation of miRNAs. miRNA-26a is a posttranscriptional regulator that inhibits cell differentiation and apoptosis and is linked to preservation of the glomerular filtration barrier. Zhu et al. (95) showed that renal vein levels of miRNA-26a are lower in the poststenotic kidney of both pigs and humans with renovascular hypertension and are restored after mesenchymal stem cell therapy. Hence, levels of miRNA-26a might be useful to monitor therapeutic success in renovascular disease (95). Kwon et al. (36) found lower levels of miRNA-21, miRNA-93, and miRNA-200b in urinary extracellular vesicles of patients with essential hypertension than in those with renovascular disease (36). Future studies are needed to determine the feasibility of using the levels of these miRNAs to discriminate different forms of renal damage in hypertension.
Polycystic Kidney Disease
Autosomal dominant polycystic kidney disease (ADPKD), a common genetic cause of CKD, is characterized by multiple cysts in the kidneys as well as cysts in other organs. miRNAs have been implicated in ADPKD cytogenesis by virtue of their effects on numerous target genes involved in cell proliferation as well as direct regulation of expression of PKD genes. The miRNA-17-92 cluster was found to be upregulated in mouse models of PKD (60). Moreover, transgenic overexpression of miRNA-17-92 in kidneys produced cysts in normal mice, while inactivation of miRNA-17-92 in mouse models of PKD slowed cyst growth and improved renal function. Based on results of the miRNA profile in the urine of patients with ADPKD, Ben-Dov et al. (8) reported that miRNA-1 and miRNA-133b were less abundant in ADPKD than in other patients with CKD. Whether the different miRNAs implicated in mouse compared with human models represent species differences or different etiologies of kidney damage remains to be determined, but these results clearly offer the rationale for further investigation in this field.
Glomerulonephritis
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis (14). Aberrant glycosylation of IgA1 is probably involved in the pathogenesis of IgAN, and abnormal expression of miRNA-148b in peripheral blood mononuclear cells might explain the aberrant glycosylation of IgA1 by reducing expression of the enzyme core 1, β1,3-galactosyltransferase 1 (C1GALT1) (69, 72). Urinary levels of miRNA-146a and miRNA-155 are elevated in patients with IgAN and correlate with proteinuria (83) but inversely correlate with urinary expression of cytokines, such as IL-1β and TNF-α, implicating miRNA-146a and miRNA-155 in the pathophysiology of IgAN. Urinary miRNA-10a and miRNA-30d levels in focal segmental glomerulosclerosis (FSGS) are also elevated in human and animal models and may represent a novel urine-based biomarker of kidney injury (84). In another study to identify urinary miRNAs as biomarkers for FSGS disease activity, urinary miRNA-196a, miRNA-30a-5p, and miRNA-490 discriminated between active FSGS and FSGS remission (92). Furthermore, urinary miRNA-30a-5p was helpful in predicting the response to steroid treatment (92).
Dysregulation of miRNA also has a key role in lupus nephritis, an autoimmune disorder with a complex pathophysiology. Perez-Hernandez et al. (61) recently found increased levels of miRNA-146a in urinary exosomes in human patients and in urinary sediment of a mouse model of lupus nephritis (31). Additionally, the association of miRNA-221 and miRNA-222 expression in urinary sediment with lupus nephritis disease activity (26) suggests that miRNA-221 and miRNA-222 could be biomarkers.
Kidney Transplantation
After kidney transplantation, it is important to monitor the status of transplanted kidneys, which are vulnerable to injury by several pathological processes. Currently, investigations of urinary miRNAs are underway to evaluate their prognostic value during the initial stage of graft damage. A single study has addressed the association between urinary miRNA and acute T cell-mediated rejection. Lorenzen et al. (44) reported that miRNA-210 differed between patients with acute rejection and a control group of transplant patients and that urinary miRNA-210 at the time of rejection predicted a decline in glomerular filtration rate 1 yr after transplantation. Scian et al. (67) found that miR-142-3p, miR-204, and miR-211 were differently expressed in kidney biopsy samples and urine of patients with chronic rejection, characterized by interstitial fibrosis and tubular atrophy, compared with patients with normal histology and functioning allografts. Therefore, urinary miRNAs might represent an attractive, noninvasive tool for the early detection of graft damage after transplantation.
Ureteral Obstruction
Obstructive nephropathies are frequently encountered developmental anomalies in the pediatric population and may involve renal fibrosis and inflammation (34). miRNA-21, upregulated in response to TGF-β, promotes kidney fibrosis in animal models of unilateral ureteral obstruction, and its inhibition attenuated renal fibrosis, suggesting therapeutic targets for antifibrotic therapy (91). In addition, Morizane et al. (54) showed that miRNA-34c attenuates epithelial-mesenchymal transition and renal fibrosis in mice with ureteral obstruction. In human urine and mouse kidney tissue, Papadopoulos et al. (57) reported that let-7a and miRNA-29b are involved in the development of fibrosis in ureteropelvic junction obstruction. However, because most of the miRNA data in ureteral obstruction have been obtained in animal models, more studies using human samples, including urine, are needed to confirm their clinical relevance.
Cancer
A number of studies have suggested that miRNAs regulate genes involved in cancer development, progression, and metastasis. The ratio of miRNA-126 to miRNA-152 is significantly increased in urine of patients with urothelial bladder cancer, enabling its detection at specificity of 82% and sensitivity of 72%, with an area under the curve of 0.768 (27). Another study (89) revealed that urinary miRNA-96 and miRNA-183 discriminate between patients with and those without urothelial carcinoma: expression levels of those two miRNAs in urine samples were significantly higher in patients with urothelial carcinoma than in healthy controls and, furthermore, fell after surgery. In renal cell carcinoma, von Brandenstein et al. (81) reported that the urinary level of miRNA-15, a tumor suppressor that promotes apoptosis and inhibits cell proliferation, could differentiate between malignant renal cell carcinoma and benign tumor. It would be important to determine if the levels of these miRNAs correlate with disease stage or metastasis.
Circulating miRNAs as Biomarkers of Kidney Disease
Although the function of circulating miRNAs is not fully understood, the discovery of circulating exosomes that contain miRNAs expanded the boundaries of understanding their physiological or pathological roles. Therefore, circulating miRNAs are regarded as attractive biomarker candidates (30) and may reflect kidney disease as well. Lorenzen et al. (43) found 13 deregulated miRNAs expressed in the serum of patients with AKI. Of three quantified miRNAs (miRNA-16 and miRNA-320 were downregulated, whereas miRNA-210 was upregulated), miRNA-210 was a strong predictor of survival in critically ill patients with AKI. In patients with CKD, miRNA-16, miRNA-21, miRNA-155, miRNA-210, and miRNA-638 correlate with renal function (56). However, in this study, circulating levels of some miRNAs were reduced in patients with severe CKD compared with individuals with mild or no renal impairment, demonstrating the heterogeneity of circulating miRNAs as biomarkers in individuals with severe renal damage. The extent to which changes in renal function or permselectivity affect circulating or urinary levels of miRNAs remains to be determined.
THERAPEUTIC APPROACHES INVOLVING miRNAs IN KIDNEY DISEASE
miRNAs represent potentially novel therapeutic targets. In general, there are two approaches to developing miRNA-based therapeutics: miRNA antagomirs and miRNA mimetics. The most widely adopted strategy to block miRNA function is the use of oligonucleotides (anti-miRNAs) designed against the mature miRNA sequence, which can be produced chemically (35). Anti-miRNAs are synthetically produced to contain a cholesterol group, which promotes their entrance into cells in whole animals to suppress translation of mRNA. Recently, exosome and viral vectors carrying or encoding shRNAs were also introduced as delivery methods into the cells to block mature miRNAs (10, 33, 87). In addition to anti-miRNAs, miRNA inhibition can be achieved by transgenic introduction of tandem miRNA-binding site repeats (sponge technology). miRNA sponges are competitive inhibitors of miRNA that are devised by insertion of multiple binding sites into the targets of analogous miRNAs (17). Alternatively, miRNA function can be blunted using “erasers” possessing oligonucleotides that are complementary to the miRNA sequence (13).
Conversely, when miRNAs are downregulated in renal diseases, miRNA mimetic drugs may restore their functions (12, 64). This approach generates artificial double-stranded synthetic oligonucleotides. Such RNA fragments can bind specifically to their target gene and produce posttranscriptional repression or, more specifically, translational inhibition. In addition, viral vectors and exosomes can deliver miRNA and mediate cross talk between cells and organs. Zhang et al. (90) reported that overexpression of miRNA-23a/27a in atrophic muscles, achieved using an adeno-associated virus approach, led to the release of muscle exosomes rich in miRNA-23a/27a, which, in turn, attenuated renal fibrosis. These strategies have been successful in animal models (63). However, many obstacles, such as delivery methods and safety concerns, must be overcome before miRNA-based therapies for renal disease can be translated into clinical practice.
miRNAs have rapidly progressed to the point of being tested as therapeutic targets, predominantly in oncology (28). The miRNA-based drug most studied outside the cancer field is miravirsen, which inhibits the hepatitis C virus by antagonizing miRNA-122 and is already in phase II clinical trial (37, 80). Similarly, a phase II clinical trial is in progress in patients with Alport syndrome to test an anti-miRNA-21 agent on the kidney (ClinicalTrials.gov identifier NCT02855268) based on its protective effect against TGF-β-induced fibrogenesis and inflammation observed in α3-chain collagen type IV knockout mice (23, 64a).
URINARY miRNAs AS MONITORS OF DRUG THERAPY
Recently, several studies have associated circulating miRNAs with well-known biomarkers for treatment therapy decisions to investigate the predictors of drug efficacy, especially for cancer treatment. For example, resistance to imatinib, a small molecule-targeted therapy, impedes treatment of chronic myeloid leukemia, and miRNA-451 expression levels in the plasma of patients followed sequentially are associated with response to therapy (66). Single-nucleotide polymorphisms in a miRNA target site may also predict the treatment response. The LCS6 polymorphism in the let-7-binding site predicts a poor response in patients with refractory metastatic colorectal cancer treated with anti-EGF receptor (68). Indeed, urinary miRNAs may help monitor the effects of drug therapy (53). After kidney transplantation, regular monitoring of urinary miRNA-155-5p can help discriminate between patients with and without acute rejection. Urinary miRNA-155-5p increased in patients before and during acute rejection and correlated with glomerular filtration rate. Clearly, more studies are needed to investigate the role of urinary miRNA as a monitoring tool in drug therapy.
LIMITATIONS OF URINARY miRNAs AS BIOMARKERS OF RENAL DISEASE
Measurements of urinary miRNA levels are attractive, as they may provide a noninvasive “liquid biopsy” of processes taking place in the kidney tissue. However, several open questions mandate caution when miRNAs are used as biomarkers. Whether changes in tissue miRNA activity or expression are faithfully and consistently reflected in urinary miRNA levels and the fraction of urinary miRNAs originating in kidney cells need to be determined. Most miRNAs target multiple proteins, which, in turn, might be suppressors of other proteins, decreasing their specificity for distinctive physiological or pathological processes. Consequently, changes in their levels are often difficult to interpret, and their use as therapeutic targets (inhibition or overexpression) is fraught with uncertain consequences. Furthermore, this heterogeneous functionality may account for the paucity of consistent data asserting involvement of unique miRNAs in various forms of kidney disease. Possibly, rather than single miRNAs, patterns of miRNA clusters might detect changes in human disease reproducibly. Different methodologies used to isolate, store, and quantify miRNAs also hinder the reproducibility of the findings. Some studies have examined miRNAs encapsulated in extracellular vesicles, and others have examined free miRNAs, which likely undergo some degree of degradation. Identification of the cellular source of miRNAs would also be helpful in linking their release to specific disease processes. Clearly, careful standardization and validation are warranted before the field of miRNAs is translated to clinical decision-making. Further clinical research might move the field forward in this direction.
SUMMARY
The importance of miRNAs in the kidney field is increasingly recognized, as they may disclose pathways active in kidney physiology and disease. Many studies on miRNAs have focused on the identification of reliable biomarkers for diagnosis and prognosis of a range of renal disorders (Fig. 2). Moreover, while the use of miRNAs as therapeutic agents is appealing, this field is still facing some challenges, such as safety concerns and the need for reliable organ- and cell-specific delivery systems. Therefore, the lowest-hanging fruit might still reside in the development of a novel class of diagnostic tools for early detection and monitoring of treatment success in various diseases. It is hoped that next-generation biomarker diagnosis and/or nucleic acid delivery therapy developed in future miRNA research will be applied to manage and contain kidney disease in affected patients.
GRANTS
This work was partly supported by National Institutes of Health Grants DK-100081, DK-104273, DK-120292, HL-123160, and DK-102325.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.O.S. and L.O.L. conceived and designed research; I.O.S. and L.O.L. drafted manuscript; I.O.S. edited and revised manuscript; I.O.S. and L.O.L. approved final version of manuscript.
REFERENCES
- 1.Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int 82: 1024–1032, 2012. doi: 10.1038/ki.2012.256. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 3.Argyropoulos C, Wang K, McClarty S, Huang D, Bernardo J, Ellis D, Orchard T, Galas D, Johnson J. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLoS One 8: e54662, 2013. [Erratum in PLoS One 8: 2013.] doi: 10.1371/journal.pone.0054662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong DA, Green BB, Seigne JD, Schned AR, Marsit CJ. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol Cancer 14: 194, 2015. doi: 10.1186/s12943-015-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108: 5003–5008, 2011. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvin P, Samimagham HR, Montazerghaem H, Khayatian M, Mahboobi H, Ghadiri Soufi F. Early detection of cardiac surgery-associated acute kidney injury by microRNA-21. Bratisl Lek Listy 118: 626–631, 2017. doi: 10.4149/BLL_2017_120. [DOI] [PubMed] [Google Scholar]
- 7.Barreiro K, Holthofer H. Urinary extracellular vesicles. A promising shortcut to novel biomarker discoveries. Cell Tissue Res 369: 217–227, 2017. doi: 10.1007/s00441-017-2621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Dov IZ, Tan YC, Morozov P, Wilson PD, Rennert H, Blumenfeld JD, Tuschl T. Urine microRNA as potential biomarkers of autosomal dominant polycystic kidney disease progression: description of miRNA profiles at baseline. PLoS One 9: e86856, 2014. doi: 10.1371/journal.pone.0086856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biscontin A, Casara S, Cagnin S, Tombolan L, Rosolen A, Lanfranchi G, De Pittà C. New miRNA labeling method for bead-based quantification. BMC Mol Biol 11: 44, 2010. doi: 10.1186/1471-2199-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudreau RL, Monteys AM, Davidson BL. Minimizing variables among hairpin-based RNAi vectors reveals the potency of shRNAs. RNA 14: 1834–1844, 2008. doi: 10.1261/rna.1062908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 4: 121ra18, 2012. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen HY, Zhong X, Huang XR, Meng XM, You Y, Chung AC, Lan HY. MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol Ther 22: 842–853, 2014. doi: 10.1038/mt.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caroli A, Cardillo MT, Galea R, Biasucci LM. Potential therapeutic role of microRNAs in ischemic heart disease. J Cardiol 61: 315–320, 2013. doi: 10.1016/j.jjcc.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Donadio JV, Grande JP. IgA nephropathy. N Engl J Med 347: 738–748, 2002. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 15.Du J, Cao X, Zou L, Chen Y, Guo J, Chen Z, Hu S, Zheng Z. MicroRNA-21 and risk of severe acute kidney injury and poor outcomes after adult cardiac surgery. PLoS One 8: e63390, 2013. doi: 10.1371/journal.pone.0063390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du L, Jiang X, Duan W, Wang R, Wang L, Zheng G, Yan K, Wang L, Li J, Zhang X, Pan H, Yang Y, Wang C. Cell-free microRNA expression signatures in urine serve as novel noninvasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Oncotarget 8: 40832–40842, 2017. doi: 10.18632/oncotarget.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4: 721–726, 2007. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdbrügger U, Le TH. Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol 27: 12–26, 2016. doi: 10.1681/ASN.2015010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan PC, Chen CC, Chen YC, Chang YS, Chu PH. MicroRNAs in acute kidney injury. Hum Genomics 10: 29, 2016. doi: 10.1186/s40246-016-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gildea JJ, Carlson JM, Schoeffel CD, Carey RM, Felder RA. Urinary exosome miRNome analysis and its applications to salt sensitivity of blood pressure. Clin Biochem 46: 1131–1134, 2013. [Erratum in Clin Biochem 46: 501, 2014.] doi: 10.1016/j.clinbiochem.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci USA 107: 14339–14344, 2010. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, Xia TH, Liu X, Borza DB, Grafals M, Shankland SJ, Himmelfarb J, Portilla D, Liu S, Chau BN, Duffield JS. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest 125: 141–156, 2015. doi: 10.1172/JCI75852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goradel NH, Mohammadi N, Haghi-Aminjan H, Farhood B, Negahdari B, Sahebkar A. Regulation of tumor angiogenesis by microRNAs: state of the art. J Cell Physiol 234: 1099–1110, 2019. doi: 10.1002/jcp.27051. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34: D140–D144, 2006. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan J, Wang G, Tam LS, Kwan BC, Li EK, Chow KM, Li PK, Szeto CC. Urinary sediment ICAM-1 level in lupus nephritis. Lupus 21: 1190–1195, 2012. doi: 10.1177/0961203312451334. [DOI] [PubMed] [Google Scholar]
- 27.Hanke M, Hoefig K, Merz H, Feller AC, Kausch I, Jocham D, Warnecke JM, Sczakiel G. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol 28: 655–661, 2010. doi: 10.1016/j.urolonc.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20: 460–469, 2014. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Hornick NI, Huan J, Doron B, Goloviznina NA, Lapidus J, Chang BH, Kurre P. Serum exosome microRNA as a minimally-invasive early biomarker of AML. Sci Rep 5: 11295, 2015. doi: 10.1038/srep11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hüttenhofer A, Mayer G. Circulating miRNAs as biomarkers of kidney disease. Clin Kidney J 10: 27–29, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichii O, Otsuka S, Sasaki N, Namiki Y, Hashimoto Y, Kon Y. Altered expression of microRNA miR-146a correlates with the development of chronic renal inflammation. Kidney Int 81: 280–292, 2012. doi: 10.1038/ki.2011.345. [DOI] [PubMed] [Google Scholar]
- 32.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16: 421–433, 2015. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 33.Jurj A, Pop L, Petrushev B, Pasca S, Dima D, Frinc I, Deak D, Desmirean M, Trifa A, Fetica B, Gafencu G, Selicean S, Moisoiu V, Micu WT, Berce C, Sacu A, Moldovan A, Colita A, Bumbea H, Tanase A, Dascalescu A, Zdrenghea M, Stiufiuc R, Leopold N, Tetean R, Burzo E, Tomuleasa C, Berindan-Neagoe I. Exosome-carried microRNA-based signature as a cellular trigger for the evolution of chronic lymphocytic leukemia into Richter syndrome. Crit Rev Clin Lab Sci 55: 501–515, 2018. doi: 10.1080/10408363.2018.1499707. [DOI] [PubMed] [Google Scholar]
- 34.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 35.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438: 685–689, 2005. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 36.Kwon SH, Tang H, Saad A, Woollard JR, Lerman A, Textor SC, Lerman LO. Differential expression of microRNAs in urinary extracellular vesicles obtained from hypertensive patients. Am J Kidney Dis 68: 331–332, 2016. doi: 10.1053/j.ajkd.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327: 198–201, 2010. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lan YF, Chen HH, Lai PF, Cheng CF, Huang YT, Lee YC, Chen TW, Lin H. MicroRNA-494 reduces ATF3 expression and promotes AKI. J Am Soc Nephrol 23: 2012–2023, 2012. doi: 10.1681/ASN.2012050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 40.Li YF, Jing Y, Hao J, Frankfort NC, Zhou X, Shen B, Liu X, Wang L, Li R. MicroRNA-21 in the pathogenesis of acute kidney injury. Protein Cell 4: 813–819, 2013. doi: 10.1007/s13238-013-3085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang S, Cai GY, Duan ZY, Liu SW, Wu J, Lv Y, Hou K, Li ZX, Zhang XG, Chen XM. Urinary sediment miRNAs reflect tubulointerstitial damage and therapeutic response in IgA nephropathy. BMC Nephrol 18: 63, 2017. doi: 10.1186/s12882-017-0482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorenzen JM, Kaucsar T, Schauerte C, Schmitt R, Rong S, Hübner A, Scherf K, Fiedler J, Martino F, Kumarswamy R, Kölling M, Sörensen I, Hinz H, Heineke J, van Rooij E, Haller H, Thum T. MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. J Am Soc Nephrol 25: 2717–2729, 2014. doi: 10.1681/ASN.2013121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorenzen JM, Kielstein JT, Hafer C, Gupta SK, Kümpers P, Faulhaber-Walter R, Haller H, Fliser D, Thum T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 6: 1540–1546, 2011. doi: 10.2215/CJN.00430111. [DOI] [PubMed] [Google Scholar]
- 44.Lorenzen JM, Volkmann I, Fiedler J, Schmidt M, Scheffner I, Haller H, Gwinner W, Thum T. Urinary miR-210 as a mediator of acute T-cell mediated rejection in renal allograft recipients. Am J Transplant 11: 2221–2227, 2011. doi: 10.1111/j.1600-6143.2011.03679.x. [DOI] [PubMed] [Google Scholar]
- 45.Lv ZC, Fan YS, Chen HB, Zhao DW. Investigation of microRNA-155 as a serum diagnostic and prognostic biomarker for colorectal cancer. Tumour Biol 36: 1619–1625, 2015. doi: 10.1007/s13277-014-2760-9. [DOI] [PubMed] [Google Scholar]
- 46.Macconi D, Tomasoni S, Romagnani P, Trionfini P, Sangalli F, Mazzinghi B, Rizzo P, Lazzeri E, Abbate M, Remuzzi G, Benigni A. MicroRNA-324-3p promotes renal fibrosis and is a target of ACE inhibition. J Am Soc Nephrol 23: 1496–1505, 2012. doi: 10.1681/ASN.2011121144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melkonyan HS, Feaver WJ, Meyer E, Scheinker V, Shekhtman EM, Xin Z, Umansky SR. Transrenal nucleic acids: from proof of principle to clinical tests. Ann NY Acad Sci 1137: 73–81, 2008. doi: 10.1196/annals.1448.015. [DOI] [PubMed] [Google Scholar]
- 49.Meng F, Hackenberg M, Li Z, Yan J, Chen T. Discovery of novel microRNAs in rat kidney using next generation sequencing and microarray validation. PLoS One 7: e34394, 2012. doi: 10.1371/journal.pone.0034394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng Y, Eirin A, Zhu XY, Tang H, Chanana P, Lerman A, Van Wijnen AJ, Lerman LO. The metabolic syndrome alters the miRNA signature of porcine adipose tissue-derived mesenchymal stem cells. Cytometry A 93: 93–103, 2018. doi: 10.1002/cyto.a.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng Y, Eirin A, Zhu XY, Tang H, Hickson LJ, Lerman A, van Wijnen AJ, Lerman LO. Micro-RNAS regulate metabolic syndrome-induced senescence in porcine adipose tissue-derived mesenchymal stem cells through the p16/MAPK pathway. Cell Transplant 27: 1495–1503, 2018. doi: 10.1177/0963689718795692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michael MZ. Cloning microRNAs from mammalian tissues. Methods Mol Biol 342: 189–207, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Millán O, Budde K, Sommerer C, Aliart I, Rissling O, Bardaji B, Matz M, Zeier M, Silva I, Guirado L, Brunet M. Urinary miR-155-5p and CXCL10 as prognostic and predictive biomarkers of rejection, graft outcome and treatment response in kidney transplantation. Br J Clin Pharmacol 83: 2636–2650, 2017. doi: 10.1111/bcp.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morizane R, Fujii S, Monkawa T, Hiratsuka K, Yamaguchi S, Homma K, Itoh H. miR-34c attenuates epithelial-mesenchymal transition and kidney fibrosis with ureteral obstruction. Sci Rep 4: 4578, 2014. doi: 10.1038/srep04578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mytsyk Y, Dosenko V, Borys Y, Kucher A, Gazdikova K, Busselberg D, Caprnda M, Kruzliak P, Farooqi AA, Lubov M. MicroRNA-15a expression measured in urine samples as a potential biomarker of renal cell carcinoma. Int Urol Nephrol 50: 851–859, 2018. doi: 10.1007/s11255-018-1841-x. [DOI] [PubMed] [Google Scholar]
- 56.Neal CS, Michael MZ, Pimlott LK, Yong TY, Li JY, Gleadle JM. Circulating microRNA expression is reduced in chronic kidney disease. Nephrol Dial Transplant 26: 3794–3802, 2011. doi: 10.1093/ndt/gfr485. [DOI] [PubMed] [Google Scholar]
- 57.Papadopoulos T, Casemayou A, Neau E, Breuil B, Caubet C, Calise D, Thornhill BA, Bachvarova M, Belliere J, Chevalier RL, Moulos P, Bachvarov D, Buffin-Meyer B, Decramer S, Auriol FC, Bascands JL, Schanstra JP, Klein J. Systems biology combining human- and animal-data miRNA and mRNA data identifies new targets in ureteropelvic junction obstruction. BMC Syst Biol 11: 31, 2017. doi: 10.1186/s12918-017-0411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park MY, Herrmann SM, Saad A, Widmer RJ, Tang H, Zhu XY, Lerman A, Textor SC, Lerman LO. Circulating and renal vein levels of microRNAs in patients with renal artery stenosis. Nephrol Dial Transplant 30: 480–490, 2015. doi: 10.1093/ndt/gfu341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S, Moon S, Lee K, Park IB, Lee DH, Nam S. Urinary and blood microRNA-126 and -770 are potential noninvasive biomarker candidates for diabetic nephropathy: a meta-analysis. Cell Physiol Biochem 46: 1331–1340, 2018. doi: 10.1159/000489148. [DOI] [PubMed] [Google Scholar]
- 60.Patel V, Williams D, Hajarnis S, Hunter R, Pontoglio M, Somlo S, Igarashi P. miR-17~92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA 110: 10765–10770, 2013. doi: 10.1073/pnas.1301693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perez-Hernandez J, Forner MJ, Pinto C, Chaves FJ, Cortes R, Redon J. Increased urinary exosomal microRNAs in patients with systemic lupus erythematosus. PLoS One 10: e0138618, 2015. doi: 10.1371/journal.pone.0138618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci USA 105: 1608–1613, 2008. [Erratum in Proc Natl Acad Sci USA 115: E3325, 2018.] doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol 23: 458–469, 2012. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 22: 1462–1474, 2011. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64a.Regulus Therapeutics A Phase 2, Randomized, Double-Blind, Placebo Controlled Study to Evaluate the Safety, Phamarcodynamics, Phamarcokinetics, Dose Selection, and Preliminary Efficacy of Weekly Rg 012 Injections in Patients with Alport Syndrome (Online). https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-002181-32/GB [08/10/2018].
- 65.Saikumar J, Hoffmann D, Kim TM, Gonzalez VR, Zhang Q, Goering PL, Brown RP, Bijol V, Park PJ, Waikar SS, Vaidya VS. Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicol Sci 129: 256–267, 2012. doi: 10.1093/toxsci/kfs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scholl V, Hassan R, Zalcberg IR. miRNA-451: a putative predictor marker of imatinib therapy response in chronic myeloid leukemia. Leuk Res 36: 119–121, 2012. doi: 10.1016/j.leukres.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 67.Scian MJ, Maluf DG, David KG, Archer KJ, Suh JL, Wolen AR, Mba MU, Massey HD, King AL, Gehr T, Cotterell A, Posner M, Mas V. MicroRNA profiles in allograft tissues and paired urines associate with chronic allograft dysfunction with IF/TA. Am J Transplant 11: 2110–2122, 2011. doi: 10.1111/j.1600-6143.2011.03666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sebio A, Paré L, Páez D, Salazar J, González A, Sala N, del Río E, Martín-Richard M, Tobeña M, Barnadas A, Baiget M. The LCS6 polymorphism in the binding site of let-7 microRNA to the KRAS 3′-untranslated region: its role in the efficacy of anti-EGFR-based therapy in metastatic colorectal cancer patients. Pharmacogenet Genomics 23: 142–147, 2013. doi: 10.1097/FPC.0b013e32835d9b0b. [DOI] [PubMed] [Google Scholar]
- 69.Serino G, Sallustio F, Cox SN, Pesce F, Schena FP. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol 23: 814–824, 2012. doi: 10.1681/ASN.2011060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shalaby SM, El-Shal AS, Shoukry A, Khedr MH, Abdelraheim N. Serum miRNA-499 and miRNA-210: a potential role in early diagnosis of acute coronary syndrome. IUBMB Life 68: 673–682, 2016. doi: 10.1002/iub.1529. [DOI] [PubMed] [Google Scholar]
- 71.Sharkey JW, Antoine DJ, Park BK. Validation of the isolation and quantification of kidney enriched miRNAs for use as biomarkers. Biomarkers 17: 231–239, 2012. doi: 10.3109/1354750X.2012.657246. [DOI] [PubMed] [Google Scholar]
- 72.Smith AC, Molyneux K, Feehally J, Barratt J. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol 17: 3520–3528, 2006. doi: 10.1681/ASN.2006060658. [DOI] [PubMed] [Google Scholar]
- 73.Soltaninejad E, Nicknam MH, Nafar M, Sharbafi MH, Keshavarz Shahbaz S, Barabadi M, Yekaninejad MS, Bahrami T, Ahmadpoor P, Amirzargar A. Altered expression of microRNAs following chronic allograft dysfunction with interstitial fibrosis and tubular atrophy. Iran J Allergy Asthma Immunol 14: 615–623, 2015. [PubMed] [Google Scholar]
- 74.Sourvinou IS, Markou A, Lianidou ES. Quantification of circulating miRNAs in plasma: effect of preanalytical and analytical parameters on their isolation and stability. J Mol Diagn 15: 827–834, 2013. doi: 10.1016/j.jmoldx.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Stevens LA, Greene T, Levey AS. Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol 1: 874–884, 2006. doi: 10.2215/CJN.00600206. [DOI] [PubMed] [Google Scholar]
- 76.Szeto CC, Ching-Ha KB, Ka-Bik L, Mac-Moune LF, Cheung-Lung CP, Gang W, Kai-Ming C, Kam-Tao LP. Micro-RNA expression in the urinary sediment of patients with chronic kidney diseases. Dis Markers 33: 137–144, 2012. doi: 10.1155/2012/842764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomson JM, Parker J, Perou CM, Hammond SM. A custom microarray platform for analysis of microRNA gene expression. Nat Methods 1: 47–53, 2004. doi: 10.1038/nmeth704. [DOI] [PubMed] [Google Scholar]
- 78.Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 11: 23–33, 2015. doi: 10.1038/nrneph.2014.202. [DOI] [PubMed] [Google Scholar]
- 79.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 80.van der Ree MH, van der Meer AJ, de Bruijne J, Maan R, van Vliet A, Welzel TM, Zeuzem S, Lawitz EJ, Rodriguez-Torres M, Kupcova V, Wiercinska-Drapalo A, Hodges MR, Janssen HL, Reesink HW. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antiviral Res 111: 53–59, 2014. doi: 10.1016/j.antiviral.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 81.von Brandenstein M, Pandarakalam JJ, Kroon L, Loeser H, Herden J, Braun G, Wendland K, Dienes HP, Engelmann U, Fries JW. MicroRNA 15a, inversely correlated to PKCα, is a potential marker to differentiate between benign and malignant renal tumors in biopsy and urine samples. Am J Pathol 180: 1787–1797, 2012. [Erratum in Am J Pathol 181: 1889, 2012.] doi: 10.1016/j.ajpath.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 82.Wang F, Chen C, Wang D. Circulating microRNAs in cardiovascular diseases: from biomarkers to therapeutic targets. Front Med 8: 404–418, 2014. doi: 10.1007/s11684-014-0379-2. [DOI] [PubMed] [Google Scholar]
- 83.Wang G, Kwan BC, Lai FM, Chow KM, Li PK, Szeto CC. Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Dis Markers 30: 171–179, 2011. doi: 10.1155/2011/304852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang N, Zhou Y, Jiang L, Li D, Yang J, Zhang CY, Zen K. Urinary microRNA-10a and microRNA-30d serve as novel, sensitive and specific biomarkers for kidney injury. PLoS One 7: e51140, 2012. doi: 10.1371/journal.pone.0051140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang YW, Shi DB, Chen X, Gao C, Gao P. Clinicopathological significance of microRNA-214 in gastric cancer and its effect on cell biological behaviour. PLoS One 9: e91307, 2014. doi: 10.1371/journal.pone.0091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem 56: 1733–1741, 2010. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie J, Burt DR, Gao G. Adeno-associated virus-mediated microRNA delivery and therapeutics. Semin Liver Dis 35: 81–88, 2015. doi: 10.1055/s-0034-1397352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu X, Kriegel AJ, Jiao X, Liu H, Bai X, Olson J, Liang M, Ding X. miR-21 in ischemia/reperfusion injury: a double-edged sword? Physiol Genomics 46: 789–797, 2014. doi: 10.1152/physiolgenomics.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamada Y, Enokida H, Kojima S, Kawakami K, Chiyomaru T, Tatarano S, Yoshino H, Kawahara K, Nishiyama K, Seki N, Nakagawa M. MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: correlation with stage and grade, and comparison with urinary cytology. Cancer Sci 102: 522–529, 2011. doi: 10.1111/j.1349-7006.2010.01816.x. [DOI] [PubMed] [Google Scholar]
- 90.Zhang A, Li M, Wang B, Klein JD, Price SR, Wang XH. miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J Cachexia Sarcopenia Muscle 9: 755–770, 2018. doi: 10.1002/jcsm.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zarjou A, Yang S, Abraham E, Agarwal A, Liu G. Identification of a microRNA signature in renal fibrosis: role of miR-21. Am J Physiol Renal Physiol 301: F793–F801, 2011. doi: 10.1152/ajprenal.00273.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang W, Zhang C, Chen H, Li L, Tu Y, Liu C, Shi S, Zen K, Liu Z. Evaluation of microRNAs miR-196a, miR-30a-5P, and miR-490 as biomarkers of disease activity among patients with FSGS. Clin J Am Soc Nephrol 9: 1545–1552, 2014. doi: 10.2215/CJN.11561113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng CZ, Shu YB, Luo YL, Luo J. The role of miR-146a in modulating TRAF6-induced inflammation during lupus nephritis. Eur Rev Med Pharmacol Sci 21: 1041–1048, 2017. [PubMed] [Google Scholar]
- 94.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, Li JY, Chien S. MicroRNA-21 targets peroxisome proliferators-activated receptor-α in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci USA 108: 10355–10360, 2011. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu XY, Ebrahimi B, Eirin A, Woollard JR, Tang H, Jordan KL, Ofori M, Saad A, Herrmann SM, Dietz AB, Textor SC, Lerman A, Lerman LO. Renal vein levels of microRNA-26a are lower in the poststenotic kidney. J Am Soc Nephrol 26: 1378–1388, 2015. doi: 10.1681/ASN.2014030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zou YF, Wen D, Zhao Q, Shen PY, Shi H, Zhao Q, Chen YX, Zhang W. Urinary microRNA-30c-5p and microRNA-192-5p as potential biomarkers of ischemia-reperfusion-induced kidney injury. Exp Biol Med (Maywood) 242: 657–667, 2017. doi: 10.1177/1535370216685005. [DOI] [PMC free article] [PubMed] [Google Scholar]