Abstract
Sex is an important biological variable that impacts diverse physiological and pathological processes, including the progression of diabetic nephropathy. Diabetic nephropathy is one of the most common complications of diabetes mellitus and is the leading cause of end-stage renal disease. The endothelial nitric oxide synthase-deficient (eNOS−/−) db/db mouse is an appropriate and valuable model to study mechanisms in the development of diabetic nephropathy because of the similarities of the features of diabetic kidney disease in this model to those in humans. The aim of the present study was to determine whether there was a sex difference in renal injury in eNOS−/− db/db mice. Both male and female eNOS−/− db/db mice showed hyperglycemia, obesity, and renal hypertrophy. However, there was no significant difference in those variables between male and female mice. Furthermore, both male and female diabetic mice showed progressive albuminuria and significantly greater levels of serum creatinine and blood urea nitrogen compared with the same sex of wild-type mice (nondiabetic controls). Although all three variables in female eNOS−/− db/db mice had a tendency to be greater than those in male eNOS−/− db/db mice, those sex differences were not statistically significant. Moreover, both male and female eNOS−/− db/db mice showed significant mesangial expansion, higher glomerular injury scores, profound renal fibrosis, and substantial accumulation of fibronectin and collagen type IV proteins. However, sex differences in those structural changes were not observed. Similarly, survival rates of male and female eNOS−/− db/db mice were comparable. Taken together, the results from the present study suggest no sex difference in renal structural and functional damage in eNOS−/− db/db mice.
Keywords: diabetic nephropathy, endothelial nitric oxide synthase-deficient db/db mouse, mouse model of diabetic nephropathy, renal injury, sex difference
INTRODUCTION
Sex is an important biological variable that impacts diverse physiological and pathological processes. Recent evidence has demonstrated that sex steroids and sex chromosomes can have a marked impact on the biology of such diverse tissues as neurons and renal cells (37). It is also evident that there are sex differences in the incidence, age of onset, manifestation, severity, and development as well as responses to treatment in various diseases, including heart disease, hypertension, obesity, and acute or chronic renal ischemia (1, 12, 22, 45). In kidneys, sex differences have been reported in structure and/or function as well as in the occurrence and development of several kidney diseases, such as diabetic nephropathy (DN) (8, 17, 37, 43). Therefore, sex is an important aspect of rigor and reproducibility of research, an issue emphasized by the National Institutes of Health (NIH) recently.
DN is one of the most common complications of diabetes mellitus and is the leading cause of end-stage renal disease (10, 11). Early features of DN include renal/glomerular hypertrophy with thickening of the glomerular basement membrane and expansion of the glomerular mesangium, which eventually develop into glomerulosclerosis, tubular atrophy, renal fibrosis, and renal insufficiency (21, 26, 39, 48). However, there are no known therapies currently available that can treat the progressive lesion of renal histology and loss of renal function in DN. Therefore, understanding the mechanisms underlying renal injury and how to efficiently intervene in the pathological processes in the setting of diabetes is critically important for the many millions of patients with DN. To this end, tremendous efforts have been made to develop reliable animal models of DN that can mimic human disease. Over the past 20 yr, various murine models of DN have been established and used by many investigators in the field. Although some of these mouse models have shown promise, none has completely satisfied the criteria for a progressive mouse model of DN as proposed by the Animal Models of Diabetic Complications Consortium (2, 3). Recently, the mouse model with double knockout of endothelial nitric oxide synthase (eNOS) and leptin receptor (eNOS−/−db/db) was established by Zhao et al. (47) and identified as the most robust model of type 2 DN that has been described to date. eNOS−/− db/db mice have many advantages for studying DN over the other models of diabetes because they develop significant albuminuria, decreased glomerular filtration rate, mesangial expansion, glomerular basement membrane thickening, arteriolar hyalinosis, glomerulosclerosis, and tubulointerstitial injury, all of which are features of DN in humans (3, 47). Therefore, these double-knockout mice are an appropriate and valuable model to study DN. However, it is not known whether there are sex differences in renal injury and renal dysfunction in eNOS−/− db/db mice. The aim of the present study was to compare the severity of DN between male and female eNOS−/− db/db mice so that we can provide a rationale of sex selection for investigators who use this mouse model.
MATERIALS AND METHODS
Animals.
A total of 12 wild-type (WT) C57BLKS/J mice (7 male and 5 female mice) and 27 eNOS−/− db/db mice (13 male and 14 female mice) were used in the present study. The number of mice used for different analysis presented in this study was based on experimental design and quality of samples collected. All procedures were approved by the University of North Texas Health Science Center (UNTHSC) Institutional Animal Care and Use Committee. eNOS−/− db/db mice were generated from homozygous eNOS−/− and heterozygous Leprdb (eNOS−/− db) mice on the C57BLKS/J background. The eNOS−/− db breeding pairs were purchased from The Jackson Laboratory (JAX no. 8340, Bar Harbor, ME) and were bred at UNTHSC. eNOS−/− db/db mice were identified by genotyping at an age of 3–4 wk in combination with their phenotypes at an age of 8 wk. Genotyping was performed by PCR using the High-Resolution Melting protocol provided by the vendor. The phenotypes that were used to verify the genotype of eNOS−/− db/db mice included the level of fasting blood glucose, body weight, size, and shape. Age-matched WT C57BLKS/J mice were also purchased from The Jackson Laboratory (JAX no. 000662). These WT mice were shipped to our institution at 4 wk of age and were maintained under the same conditions as eNOS−/− db/db mice. All mice were maintained at the UNTHSC animal facility under local and NIH guidelines. These mice were housed in a specific pathogen-free facility with a temperature-controlled room and regulated with a 12:12-h light-dark cycle with free access to water and food (standard chow diet, LabDiet, St. Louis, MO) containing 25.1% fiber, 0.29% Na+, 19.3% protein, 13.5% fat, and 15.8% calories from fat.
Measurement of blood glucose.
Fasting blood glucose was evaluated with a LifeScan One Touch glucometer (Johnson & Johnson, Milpitas, CA) by tailed blood sampling from conscious mice at 2:00 PM after fasting for 4 h initiated at 10:00 AM.
Measurements of blood urea nitrogen and serum creatinine.
Blood samples were collected from the heart at 11:00 AM on the second day of week 24 when mice were euthanized using isofluorane or intraperitoneal injection of ketamine with xylazine (100 mg/kg + 10 mg/kg). Blood was set aside for 45 min at room temperature for clotting and then centrifuged to separate the serum. Serum samples were stored at −80°C in a freezer until assays were conducted. Blood urea nitrogen (BUN) was measured using the QuantiChrom Urea Assay kit (DIUR-100, BioAssay System, Hayward, CA) following the protocol provided by the manufacturer. Serum creatinine levels were measured in the O’Brien Kidney Center at the University of Texas Southwestern Medical Center by the P/ACE MDQ Capillary Electrophoresis System (Beckman Coulter, Fullerton, CA).
Measurements of urine output and urinary albumin excretion.
Twenty-four-hour urine samples were collected through metabolic cages (catalog no. 370 0M022, Braintree Scientific, Braintree, MA). Urinary albumin and creatinine excretion was determined using Albuwell M kits (Exocell, Philadelphia, PA). Albumin excretion rate was expressed as the ratio of urinary albumin concentration to urinary creatinine concentration (ACR; in µg/mg).
Renal tissue preparation.
Mice were anesthetized using isofluorane or intraperitoneal injection of ketamine with xylazine (100 mg/kg + 10 mg/kg). After mice had been perfused with physiological saline solution through the left ventricle to wash out blood, their left kidneys were removed and immediately snap frozen for extracting renal cortical proteins. Mice were then perfused with 4% paraformaldehyde, and the right kidneys were excised and decapsulated. The right kidneys were cut in half through a midsagittal plane and fixed with 4% paraformaldehyde. The fixed kidneys were dehydrated through a graded series of ethanol, infiltrated and embedded in paraffin, sectioned (4–5 µm), and mounted on glass slides for histological and immunohistochemistry examinations.
Isolation of the renal cortex and extraction of cortical proteins.
The renal cortex was separated from the other regions of the kidney with a sharp blade and then minced with two sharp blades. The minced cortical tissue was then sonicated in a lysis buffer followed by centrifugation at 20,817 g for 15 min at 4°C. Supernatants were collected for Western blot analysis.
Western blot analysis.
Whole cell lysates were fractionated by 10% SDS-PAGE, transferred to PVDF membranes, and probed with primary antibodies against fibronectin (FN; rabbit polyclonal, catalog no. ab2413, lot no. GR232441-3, Abcam), collagen type IV (Col IV; rabbit polyclonal, catalog no. ab135802, lot no. GR95279-9, Abcam), and α-tubulin (catalog no. sc-5286, Santa Cruz Biotechnology). Bound antibodies were visualized with Super Signal West Femto or Pico Luminol/Enhancer Solution (Thermo Scientific, Rockford, IL). The specific protein bands were visualized and captured using the AlphaEase FC Imaging System (Alpha Innotech, San Leandro, CA). The integrated density value of each band was measured by drawing a rectangle outlining the band using AlphaEase FC software with auto background subtraction. Abundance of the targeted proteins was evaluated by normalizing the integrated density value of their bands to that of the α-tubulin band on the same blot. All of the primary antibodies were validated by the manufacturers.
Histological analysis and assessment of glomerular injury.
Periodic acid-Schiff (PAS) staining was performed to evaluate glomerular histology and glomerulosclerosis, which was indicated by the glomerular score. Paraffin-embedded kidney sections (5 μm) were stained with PAS (Sigma-Aldrich, St. Louis, MO). Images were captured using an Olympus DP70 digital camera with DP manager software (version 2.2.1). The glomerular scores in PAS-stained sections were graded by a blind observer using a scale of 0–4: 0 was assigned to normal glomeruli, 1 was assigned to glomeruli with mesangial expansion, 2 was assigned to glomeruli in which sclerosis encompassed <50% of the glomerulus, 3 was assigned to glomeruli with lesions encompassing 50–75% of the glomerulus, and 4 was assigned to glomeruli with lesions encompassing >75% of the glomerulus or fully collapsed glomeruli. For each mouse, the glomerular score was evaluated in >50 glomeruli from 5 sections (>10 glomeruli/section), and those individual scores were averaged to obtain a mean value for that mouse.
Masson’s trichrome staining.
Kidney paraffin sections (4–5 µm thick) were stained with Masson’s trichrome to evaluate the severity of renal fibrosis. The staining reagents were purchased from Sigma, and staining was performed according to the protocol provided by the manufacturer. However, in one kidney sample from a male and female eNOS−/− db/db mouse, we used made-in-house reagents to stain the sections by following the protocols previously described (4). Images were captured using an Olympus DP70 digital camera with DP manager software (version 2.2.1). Fibrotic areas (stained blue by Masson’s trichrome) were quantified as a total number of pixels by a blind observer using the neural networks machine learning algorithm in the R environment. The neural network model used to count the fibrotic area was built using the “neuralnet” package in the R environment. The model has an accuracy rate of 96% on randomly selected samples.
Immunohistochemical staining.
After deparaffinization of kidney sections, antigen retrieval was achieved by heating the sections in 10 mM citrate buffer in a microwave for 10 min. Sections were blocked by 5% goat serum for 30 min at room temperature and then incubated with anti-Col IV antibody (rabbit polyclonal, catalog no. ab6586, lot no. GR193836-7, Abcam) at 1:100 or anti-FN antibody (rabbit polyclonal, catalog no. ab2413, lot no. GR232441-3, Abcam) at 1:100 at 4°C overnight. Sections were incubated with anti-rabbit poly horseradish peroxidase immunohistochemistry reagent (catalog no. IHC-2291, General Bioscience) at room temperature for 1 h followed by an incubation with peroxidase substrate solution for 2–3 min, stained in hematoxylin solution for 90 s, dehydrated in an incubator at 60°C for 30 min, and coverslipped with resinous mounting medium. Sections were examined using an Olympus microscope (BX41) and an Olympus DP70 digital camera with DP manager software (version 2.2.1). Images were uniformly adjusted for brightness and contrast. Semiquantification of the glomerular staining was conducted by a blinded observer using ImageJ (version 1.50b, NIH) following the instructions previously described (31).
Statistical analysis.
Data were reported as means ± SE. One-way ANOVA plus Student-Newman-Keuls post hoc analysis and a Student’s unpaired t-test were used to analyze differences among multiple groups and between two groups, respectively, as indicated in Figs. 1–7. The log rank test was used to analyze the survival distributions of male and female mice (Fig. 7). P values of <0.05 were considered statistically significant. Statistical analyses were performed using SigmaStat (Jandel Scientific, San Rafael, CA).
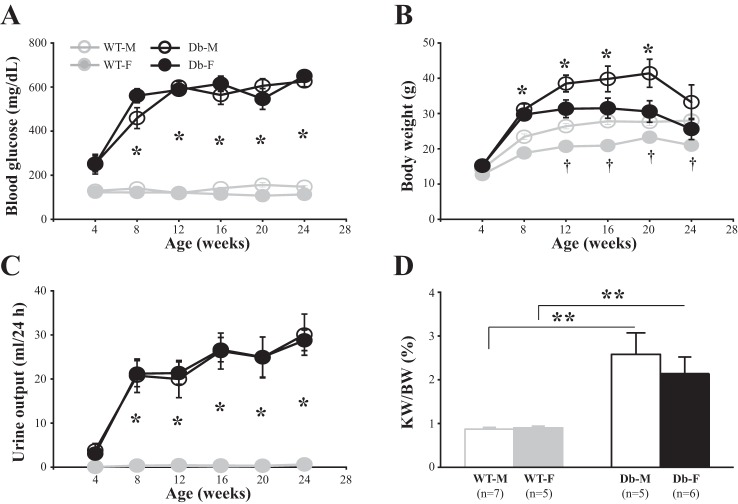
Fig. 1.
Blood glucose level (A), body weight (B), urine output (C), and ratio of kidney weight to body weight (D) in male wild-type (WT-M), female wild-type (WT-F), male endothelial nitric oxide synthase-deficient (eNOS−/−) db/db (Db-M), and female eNOS−/− db/db (Db-F) mice. In A–C, *P < 0.05 (one-way ANOVA), Db-M vs. WT-M and Db-F vs. WT-F mice; †P < 0.05, WT-M vs. WT-F mice. In A, 5–7 mice were used in all age groups of WT-M and WT-F mice. In B and C, 8–10 mice were used in all age groups of WT-M and WT-F mice. However, for eNOS−/− db/db mice in A–C, 9–13 mice were used in the age groups of 4–20 wk and 5 mice in the group of 24 wk for both Db-M and Db-F mice. In D, ratios of kidney weight (KW) to body weight (BW) were evaluated at 24 wk. **P < 0.01 (Student’s t-test), comparison between the groups indicated. n, number of mice in each group.
Fig. 7.
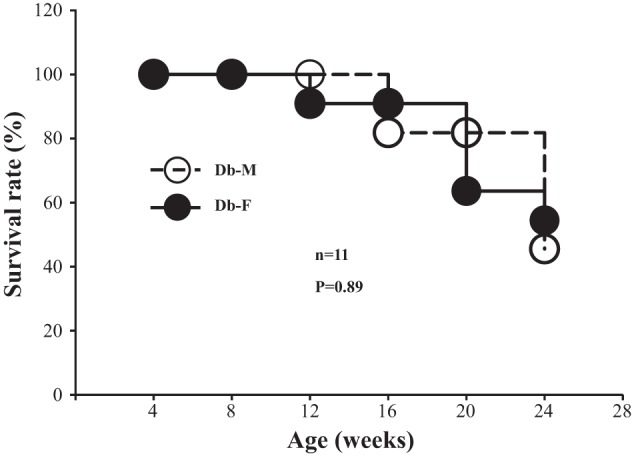
Survival rate of male and female wild-type (WT) and endothelial nitric oxide synthase-deficient (eNOS−/−) db/db mice. The log rank test was conducted to compare survival rates of male and female mice. n, number of mice entering the study (at the age of 4 wk) in each group.
RESULTS
Blood glucose level, body weight, urine output, and renal hypertrophy in male and female eNOS−/− db/db mice.
The blood glucose level was measured after 4 h of fasting (from 10:00 AM to 2:00 PM). In WT (nondiabetic) male and female mice, fasting blood glucose levels were maintained at normal range throughout the entire period of the study (24 wk), and no sex differences were observed. However, the level of fasting blood glucose in both male and female eNOS−/− db/db mice was significantly elevated as early as 8 wk of age. The hyperglycemia in both male and female diabetic mice was sustained throughout the study. There were no differences in blood glucose levels between male and female diabetic mice (Fig. 1A). Of note, the One Touch system used in this study had a cutoff reading of blood glucose level at 650 mg/dl. In a few mice, there was an overshoot of blood glucose level (>650 mg/dl) at some time points. For those, we used the value of 650 mg/dl for data analysis. This limitation of methodology might have masked authentic development of hyperglycemia in both male and female diabetic mice.
Body weights of male WT mice were significantly greater than those of female WT mice from 12 wk on. Both male and female eNOS−/− db/db mice showed obesity as early as 8 wk of age. Although the body weights of male diabetic mice had a tendency to being greater than those of female diabetic mice, the differences did not reach statistical significance (Fig. 1B). Furthermore, both male and female eNOS−/− db/db mice showed significant polyuria. Twenty-four-hour urine outputs of both sex diabetic mice were significantly increased from 8 wk on, and this high urine flow was sustained throughout the 24 wk of study. No sex differences in diabetes-induced polyuria were observed (Fig. 1C).
Renal hypertrophy is one feature of early DN. Both male and female eNOS−/− db/db mice showed marked renal hypertrophy, as indicated by a significant increase in the ratio of kidney weight to body weight. However, there were no sex differences in renal hypertrophy in diabetic mice (Fig. 1D).
Comparison of renal dysfunction between male and female eNOS−/− db/db mice.
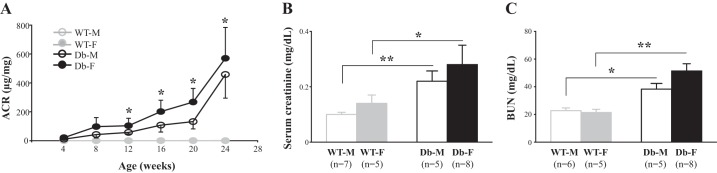
To examine if there were sex differences in impairment to renal function in this DN mouse model, we assessed albumin excretion rates, serum creatinine levels, and BUN. Both male and female diabetic mice showed progressive albuminuria starting from 12 wk of age (Fig. 2A). Similarly, levels of serum creatinine and BUN at 24 wk were significantly elevated in both sexes of eNOS−/− db/db mice compared with the same sex of WT mice (Fig. 2, B and C). All three variables in female eNOS−/− db/db mice had a tendency to be greater than those in male eNOS−/− db/db mice. However, the differences did not reach statistically significant levels (Fig. 2, A–C).
Fig. 2.
Albumin excretion rate (A), serum creatinine (B), and blood urea nitrogen (BUN; C) in male wild-type (WT-M), female wild-type (WT-F), male endothelial nitric oxide synthase-deficient (eNOS−/−) db/db (Db-M), and female eNOS−/− db/db (Db-F) mice. In A, the albumin excretion rate is indicated by the albumin-to-creatinine ratio (ACR). *P < 0.05 (Student’s t-test) for both Db-M vs. WT-M and Db-F vs. WT-F mice. Four mice were used in all age groups of WT-M and WT-F mice. For eNOS−/− db/db mice, nine male and six female mice were included in the age groups of 4–20 wk and four mice were included in the group of 24 wk for both Db-M and Db-F mice. In B and C, blood samples were collected from mice at 24 wk. *P < 0.05 and **P < 0.05 (Student’s t-test). n, number of mice used in each group.
Comparison of histological impairment between male and female eNOS−/− db/db mice.
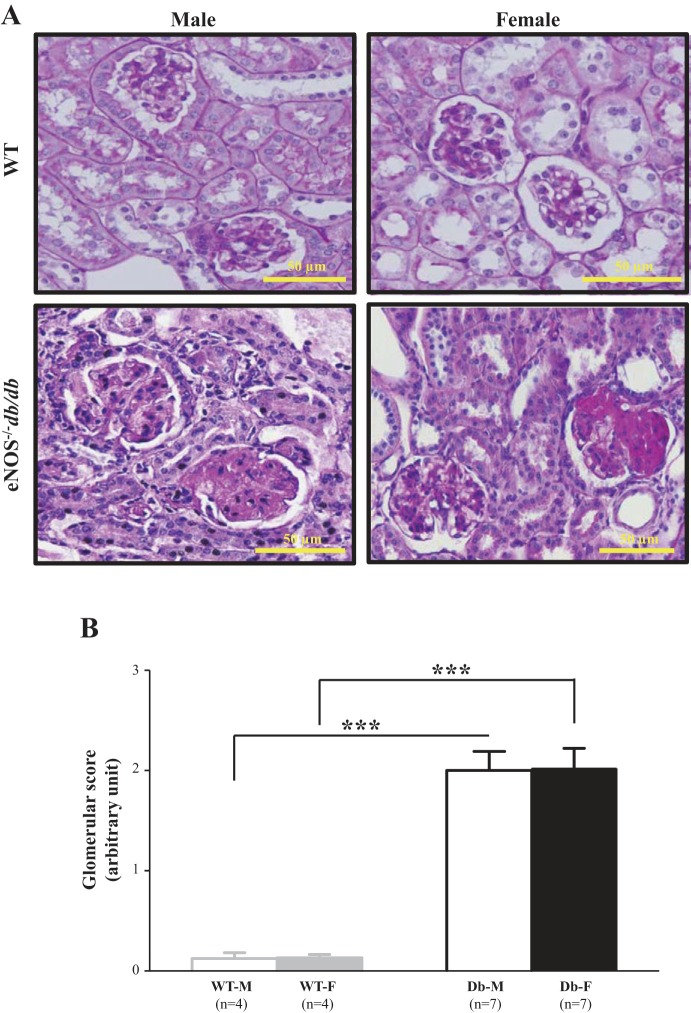
In agreement with previously published studies (24, 46, 47), profound renal injuries were observed in eNOS−/− db/db mice. These included substantial glomerular mesangial expansion and glomerulosclerosis as well as glomerular and interstitial fibrosis. The glomerular injuries in diabetic mice were evaluated using glomerular scores. As shown in Fig. 3, glomerular scores of both male and female eNOS−/− db/db mice were significantly greater than those of the same sex of WT mice. However, there were no differences in glomerular injuries between male and female diabetic mice.
Fig. 3.
Glomerular histopathology in male and female endothelial nitric oxide synthase-deficient (eNOS−/−) db/db mice. A: representative images of periodic acid-Schiff (PAS) staining from male and female wild-type (WT) and eNOS−/− db/db mice at 24 wk. B: summarized glomerular injury scores from four male and female WT mice (WT-M and WT-F, respectively) and seven male and female eNOS−/− db/db mice (Db-M and Db-F, respectively). About 15–20 glomeruli from 1 kidney section were counted, and 5 sections were taken from 1 mouse. ***P < 0.01 (Student’s t-test), comparison between the groups as indicated.
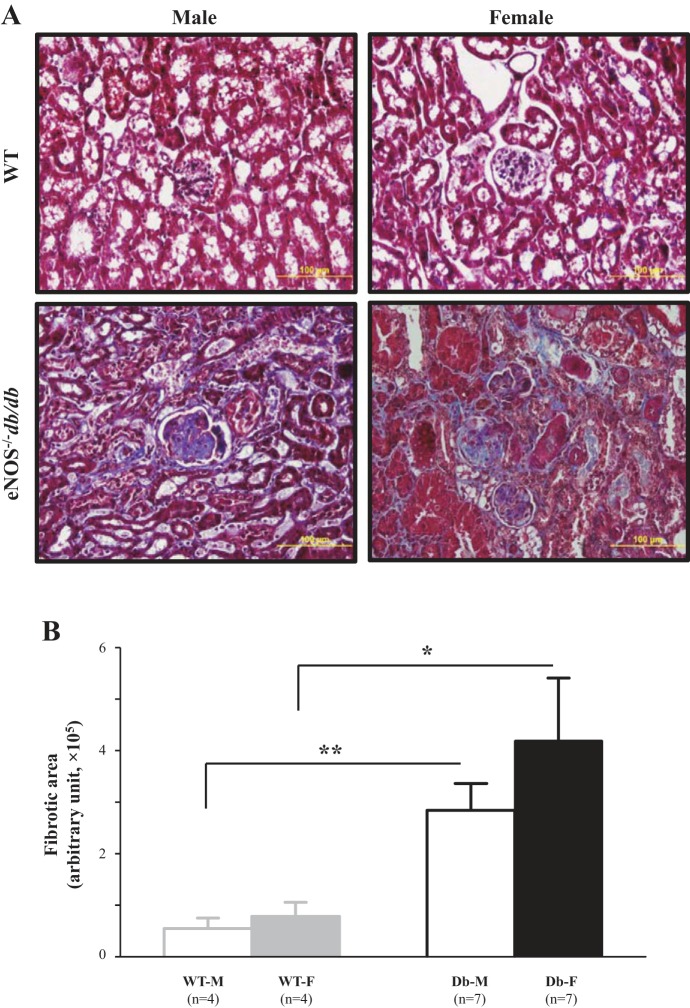
Renal fibrosis, including glomerular and interstitial fibrosis, is one characteristic of DN. Histological evidence of renal fibrosis was evaluated by staining collagen with aniline blue using the Masson’s trichrome staining protocol. The significant increases in aniline blue-positive areas in kidney sections from eNOS−/− db/db mice versus WT mice indicated marked renal fibrosis in diabetic mice (Fig. 4). However, there were no differences in staining between male and female WT mice and in fibrotic areas between male and female eNOS−/− db/db mice (Fig. 4).
Fig. 4.
Renal fibrosis in male and female endothelial nitric oxide synthase-deficient (eNOS−/−) db/db mice. A: representative Masson’s trichrome staining of kidney sections at 24 wk. B: semiquantitative scores of renal fibrosis from four male and female wild-type (WT) mice (WT-M and WT-F, respectively) and seven male and female eNOS−/− db/db mice (Db-M and Db-F, respectively). About 15–20 glomeruli from 1 kidney section were counted, and 5 sections were taken from 1 mouse. *P < 0.05 and **P < 0.01 (Student’s t-test), comparison between the groups as indicated.
Accumulation of extracellular matrix proteins in male and female eNOS−/− db/db mice.
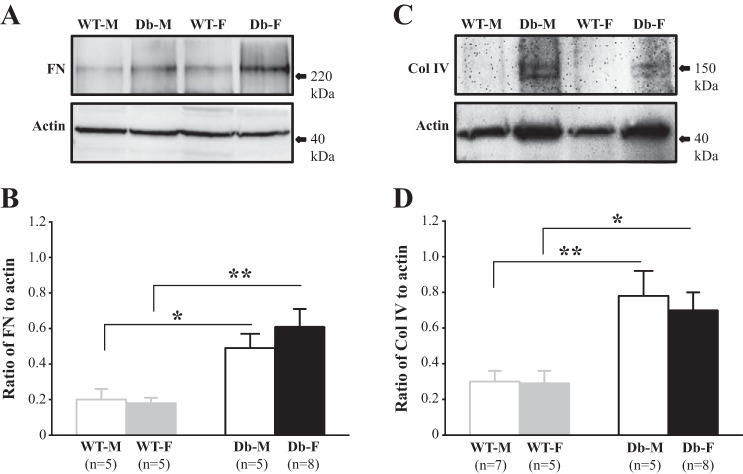
Overproduction of extracellular matrix (ECM) proteins and deposition of these proteins in the mesangium are important contributors to mesangial expansion in diabetic kidney disease (7, 13, 14, 36). FN and Col IV are major components of increased glomerular ECM in DN (9, 13, 14, 27, 44). We conducted Western blot analysis using renal cortical protein extracts from both male and female WT and eNOS−/− db/db mice at 24 wk. As shown in Fig. 5, the abundance of both FN and Col IV proteins was significantly increased in both sexes of diabetic mice. However, no statistically significant differences in levels of ECM proteins were observed between male and female diabetic mice (Fig. 5, B and D).
Fig. 5.
Abundance of renal cortical fibronectin (FN) and collagen IV (Col IV) proteins in male and female wild-type (WT) and endothelial nitric oxide synthase-deficient (eNOS−/−) db/db mice. A and C: representative Western blots of renal cortical extracts showing the protein abundance of FN (A) and Col IV (C) in the cortex of kidney from WT male (WT-M) and female (WT-F) mice and eNOS−/− db/db male (Db-M) and female (Db-F) mice at 24 wk. β-Actin was used as the loading control. B and D: summary densitometric data from experiments presented in A and C, respectively. *P < 0.05 and **P < 0.01 (Student’s t-test), comparison between the groups as indicated. n, number of mice in each group.
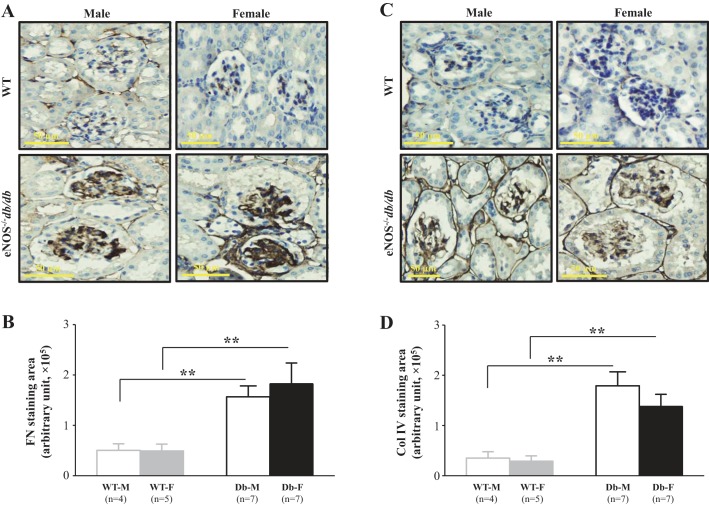
Immunohistochemistry was then used to examine expression levels of glomerular FN and Col IV proteins in both male and female WT and eNOS−/− db/db mice. Glomerular staining areas and intensities for both FN and Col IV were markedly increased in eNOS−/− db/db mice compared with WT mice (Fig. 6, A and C), suggesting an accumulation of ECM proteins in the glomeruli. Semiquantitation of the positively stained areas in the glomeruli showed significant increases in the expression levels of both FN and Col IV in diabetic mice. However, there were no statistically significant differences in the abundance of ECM proteins between male and female eNOS−/− db/db mice (Fig. 6, B and D).
Fig. 6.
Glomerular expression of fibronectin (FN) and collagen IV (Col IV) in male and female wild-type (WT) and endothelial nitric oxide synthase-deficient (eNOS−/−) db/db mice at an age of 24 wk. A and C: representative light microscopic images of immunohistochemistry of FN (A) and Col IV (C). Original magnification was ×200. B and D: semiquantitation of glomerular stain of FN (B) and Col IV (D) from four male and five female WT mice (WT-M and WT-F, respectively) and seven male and female eNOS−/− db/db mice (Db-M and Db-F, respectively). About 15–20 glomeruli from 1 kidney section were counted, and 5 sections were taken from 1 mouse. **P < 0.01 (Student’s t-test), comparison between the groups as indicated.
Survival rates of male and female eNOS−/− db/db mice.
Eleven male and female eNOS−/− db/db mice entered the present study (at 4 wk of age). During the 24-wk period of study, we started losing female diabetic mice at ~12 wk (1 of 11 mice) and male diabetic mice at ~16 wk (2 of 11 mice). At the end of 24 wk (the duration of the present study), there were 5 male mice and 6 female mice. There were no sex differences in the survival rate (Fig. 7).
DISCUSSION
The present study showed that both male and female eNOS−/− db/db mice developed overt DN, as indicated by the substantial decline of renal function, profound mesangial expansion/glomerulosclerosis, renal fibrosis, and significant accumulation of ECM proteins. Those functional and structural impairments are consistent with previously published studies using the same mouse model (24, 46, 47). However, the major finding in the present study was the lack of sex differences in the severity of renal injury in this genetic mouse model of DN. Although most variables evaluated in the present study, such as albumin excretion rates, serum creatinine levels, BUN levels, renal fibrosis, and abundance of glomerular FN protein, showed a tendency to be greater in female eNOS−/− db/db mice than those in male eNOS−/− db/db mice, those differences were not significant statistically. Therefore, our results suggest that sex may not be a factor contributing to the development and progression of DN in eNOS−/− db/db mice.
Sex differences in the development of diabetes mellitus and its renal complications have been reported in some mouse models of DN. For instance, Gurley et al. (16) reported that, in different strains of mice with streptozotocin-induced DN, male mice demonstrated more robust hyperglycemia and renal injury than female mice. Studies of Ins2Akita mice also showed more severe DN in male mice than in female mice (18). In the db/db mouse model, although a head-to-head study has not been performed between male and female mice, db/db male mice generally have two times as much albuminuria compared with female mice (25). Sexual dimorphism on the progression of DN has also been observed in humans with diabetes mellitus. Several studies have suggested that men with both type 1 and 2 diabetes mellitus have significantly higher rates of decline in glomerular filtration rate and increased risk of developing microalbuminuria than women (8, 28–30, 32, 35). Those observations are further supported by the evidence that estrogens can protect the kidney from renal injury (40, 41). However, we did not find significant differences in renal injury between male and female eNOS−/− db/db mice. We speculate that no sex difference in DN in our model may be attributed to loss of the eNOS pathway. Previously published studies have demonstrated that estrogens can enhance nitric oxide signaling by activation of eNOS (15, 19, 20, 23). It is also evident that the eNOS pathway is renoprotective, and activation of the pathway is a downstream mechanism for renoprotection by estradiol (5, 6, 34, 38, 42). In the mouse model presented in the present study (eNOS−/− db/db), eNOS deficiency resulted in loss of a renoprotective mechanism of estrogens in female mice. Therefore, the advantage of renoprotective sex hormones in female mice did not exist any more in eNOS−/− db/db mice. This could also explain why some indicators of renal injury had a tendency to be worse in female eNOS−/− db/db mice than male mice.
The biological variable of sex has been recently proposed by NIH as an important aspect of rigor and reproducibility of research, since one’s sex impacts the incidence, age of onset, manifestation, severity, and rate of disease progression as well as responses to treatment in diverse diseases, including cardiovascular, renal, and metabolic diseases (1, 17, 33, 45). Sex selection should be considered in the animal studies of those diseases. The eNOS−/− db/db mouse is a recently developed mouse model and has proven to be quite a representative model of advanced DN (24, 46, 47). As previously described in published studies (24, 46, 47) and presented in this study, eNOS−/− db/db mice develop most features of DN in humans. Therefore, the eNOS−/− db/db mouse is an adequate model to study diabetic kidney disease. The lack of sex differences in the development of DN affords more flexibility in mouse selection when using this mouse model.
We noticed that albumin excretion rates in our eNOS−/− db/db mice were substantially lower compared with studies from the research group that originally developed this model (24, 46, 47). The reason for the discrepancy is not clear but may be attributed to variability in the albumin and creatinine assays. Indeed, the ACR in different ages (from 4 to ~24 wk) of eNOS−/− db/db mice in their studies (24, 46) were significantly greater than the ACR in the same age group of eNOS−/− db/db mice in the present study. Nevertheless, in the present study, albumin excretion rates were ~1,000-fold greater in both male and female eNOS−/− db/db mice at 24 wk compared with the same age/sex of WT mice and compared with the same sex of eNOS−/− db/db mice at 4 wk (Fig. 2A), which meets the criterion of DN proposed by the Animal Models of Diabetic Complications Consortium (2, 3).
Several limitations exist in the present study. First, we did not measure arterial blood pressure of eNOS−/− db/db mice. Whether there were sex differences in this important physiological variable is not known. As is frequently seen in patients with diabetes mellitus, blood pressure is moderately elevated in eNOS−/− db/db mice (46). However, one study demonstrated that the DN in eNOS−/− db/db mice was not the result of hypertension (47). Thus, a lack of blood pressure data does not affect the conclusion of this study. Second, the present study did not provide information on lipid metabolism in male and female eNOS−/− db/db mice. Abnormalities in lipid panels, such as high levels of blood cholesterols and triglycerides, are associated with type 2 diabetes and its complications. Whether there are sex differences in lipid metabolism and how much they influence the progression of DN in this mouse model warrant further investigation. Third, the estrous cycle of female eNOS−/− db/db mice in the present study was not known. It is not clear whether there were differences in female hormones among the female diabetic mice because of differences in the cycle periods and whether the variability of the estrous cycle influenced the results in female eNOS−/− db/db mice. Indeed, we did observe a large SD in some measures of renal function and histology in female eNOS−/− db/db mice. Finally, this study was limited to sex difference in renal complications of eNOS−/− db/db mice. The results from the present study should not be extended to other complications that may be associated with this mouse model.
In summary, we showed no sex differences in renal structural and functional damage in eNOS−/− db/db mice. Because this mouse model provides many advantages for studying DN over other models of diabetes (3, 47), eNOS−/− db/db mice are an appropriate and valuable model for studying the mechanisms of DN progression. However, since its initial description in 2006 (47), the use of this mouse model has been limited, which may potentially be a result of time-consuming breeding strategies to introduce multiple mutations in these mice. Our findings suggest that both sexes of the double-knockout mice produced from the breeders can be used to study DN without concerns of sex influences on the reproducibility of experiments. Therefore, the present study provides another advantage of using the eNOS−/− db/db model over other DN models for which male mice are preferably selected because of more severe renal injury in male mice than in female mice (16, 18, 25).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1-RO1-DK-115424-01 (to R. Ma), American Heart Association Southwest Affiliate Grant-In-Aid 16GRNT27780043 (to R. Ma), and an award from the Harry S. Moss Heart Trust (to R. Ma).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.M., W.L., P.Y.S., B.H.D., L.H., and S.C. performed experiments; Y.M., W.L., L.H., L.A.W., Z.Z., and R.M. analyzed data; Y.M., W.L., and R.M. interpreted results of experiments; Y.M., W.L., P.Y.S., B.H.D., L.H., S.C., P.W., L.A.W., M.-G.R., and Z.Z. edited and revised manuscript; Y.M., W.L., P.Y.S., B.H.D., L.H., S.C., P.W., L.A.W., M.-G.R., Z.Z., and R.M. approved final version of manuscript; P.Y.S. and R.M. prepared figures; R.M. conceived and designed research; R.M. drafted manuscript.
ACKNOWLEDGMENTS
We thank the O’Brien Kidney Center at the University of Texas Southwestern Medical Center for the serum creatinine assay in mice.
REFERENCES
- 1.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol 37: 746–756, 2017. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breyer MD, Böttinger E, Brosius FC III, Coffman TM, Harris RC, Heilig CW, Sharma K; AMDCC . Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 3.Brosius FC III, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T; Animal Models of Diabetic Complications Consortium . Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson FL, Hladik C. Histotechnology, a Self-Instructional Text. Chicago: American Society for Clinical Pathology, 2009. [Google Scholar]
- 5.Chander V, Chopra K. Renal protective effect of molsidomine and l-arginine in ischemia-reperfusion induced injury in rats. J Surg Res 128: 132–139, 2005. doi: 10.1016/j.jss.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Chatauret N, Coudroy R, Delpech PO, Vandebrouck C, Hosni S, Scepi M, Hauet T. Mechanistic analysis of nonoxygenated hypothermic machine perfusion’s protection on warm ischemic kidney uncovers greater eNOS phosphorylation and vasodilation. Am J Transplant 14: 2500–2514, 2014. doi: 10.1111/ajt.12904. [DOI] [PubMed] [Google Scholar]
- 7.Chavers BM, Bilous RW, Ellis EN, Steffes MW, Mauer SM. Glomerular lesions and urinary albumin excretion in type I diabetes without overt proteinuria. N Engl J Med 320: 966–970, 1989. doi: 10.1056/NEJM198904133201503. [DOI] [PubMed] [Google Scholar]
- 8.Clotet S, Riera M, Pascual J, Soler MJ. RAS and sex differences in diabetic nephropathy. Am J Physiol Renal Physiol 310: F945–F957, 2016. doi: 10.1152/ajprenal.00292.2015. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MP, Lautenslager GT, Shearman CW. Increased urinary type IV collagen marks the development of glomerular pathology in diabetic d/db mice. Metabolism 50: 1435–1440, 2001. doi: 10.1053/meta.2001.28074. [DOI] [PubMed] [Google Scholar]
- 10.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J, St Peter W, Guo H, Hu Y, Kats A, Li S, Li S, Maloney J, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis Suppl 63: A7, 2014. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007. doi: 10.1681/ASN.2007020220. [DOI] [PubMed] [Google Scholar]
- 12.Gillis EE, Sullivan JC. Sex differences in hepertension: recent advances. Hypertension 68: 1322–1327, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol 284: F144–F154, 2003. doi: 10.1152/ajprenal.00158.2002. [DOI] [PubMed] [Google Scholar]
- 14.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 15.Guetta V, Quyyumi AA, Prasad A, Panza JA, Waclawiw M, Cannon RO III. The role of nitric oxide in coronary vascular effects of estrogen in postmenopausal women. Circulation 96: 2795–2801, 1997. doi: 10.1161/01.CIR.96.9.2795. [DOI] [PubMed] [Google Scholar]
- 16.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- 17.Harris AN, Lee HW, Osis G, Fang L, Webster KL, Verlander JW, Weiner ID. Differences in renal ammonia metabolism in male and female kidney. Am J Physiol Renal Physiol 315: F211–F222, 2018. doi: 10.1152/ajprenal.00084.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haseyama T, Fujita T, Hirasawa F, Tsukada M, Wakui H, Komatsuda A, Ohtani H, Miura AB, Imai H, Koizumi A. Complications of IgA nephropathy in a non-insulin-dependent diabetes model, the Akita mouse. Tohoku J Exp Med 198: 233–244, 2002. doi: 10.1620/tjem.198.233. [DOI] [PubMed] [Google Scholar]
- 19.Hodgin JB, Knowles JW, Kim HS, Smithies O, Maeda N. Interactions between endothelial nitric oxide synthase and sex hormones in vascular protection in mice. J Clin Invest 109: 541–548, 2002. doi: 10.1172/JCI0214066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohmann N, Xia N, Steinkamp-Fenske K, Förstermann U, Li H. Estrogen receptor signaling and the PI3K/Akt pathway are involved in betulinic acid-induced eNOS activation. Molecules 21: 973, 2016. doi: 10.3390/molecules21080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 233: 4–11, 2008. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 22.Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res 67: 594–603, 2005. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Kim KH, Bender JR. Membrane-initiated actions of estrogen on the endothelium. Mol Cell Endocrinol 308: 3–8, 2009. doi: 10.1016/j.mce.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Li Y, Overstreet JM, Chung S, Niu A, Fan X, Wang S, Wang Y, Zhang MZ, Harris RC. Inhibition of epidermal growth factor receptor activation is associated with improved diabetic nephropathy and insulin resistance in type 2 diabetes. Diabetes 67: 1847–1857, 2018. doi: 10.2337/db17-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Like AA, Lavine RL, Poffenbarger PL, Chick WL. Studies in the diabetic mutant mouse. VI. Evolution of glomerular lesions and associated proteinuria. Am J Pathol 66: 193–224, 1972. [PMC free article] [PubMed] [Google Scholar]
- 26.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol 14: 1358–1373, 2003. doi: 10.1097/01.ASN.0000065640.77499.D7. [DOI] [PubMed] [Google Scholar]
- 27.Matsubara T, Araki M, Abe H, Ueda O, Jishage K, Mima A, Goto C, Tominaga T, Kinosaki M, Kishi S, Nagai K, Iehara N, Fukushima N, Kita T, Arai H, Doi T. Bone morphogenetic protein 4 and Smad1 mediate extracellular matrix production in the development of diabetic nephropathy. Diabetes 64: 2978–2990, 2015. doi: 10.2337/db14-0893. [DOI] [PubMed] [Google Scholar]
- 28.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 39: 1116–1124, 1990. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 29.Parving HH, Gall MA, Skott P, Jorgensen HE, Lokkegaard H, Jorgensen F, Nielsen B, Larsen S. Prevalence and cause of albuminuria in no-insulin-dependent diabetic patients. Kidney Int 41: 758–762, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, Holl RW. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 30: 2523–2528, 2007. doi: 10.2337/dc07-0282. [DOI] [PubMed] [Google Scholar]
- 31.Rangan GK, Tesch GH. Quantification of renal pathology by image analysis. Nephrology (Carlton) 12: 553–558, 2007. doi: 10.1111/j.1440-1797.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 32.Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med 158: 998–1004, 1998. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- 33.Reckelhoff JF, Samson WK. Sex and gender differences in cardiovascular, renal and metabolic diseases. Am J Physiol Regul Integr Comp Physiol 309: R1057–R1059, 2015. doi: 10.1152/ajpregu.00417.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhoden EL, Rhoden CR, Lucas ML, Pereira-Lima L, Zettler C, Belló-Klein A. The role of nitric oxide pathway in the renal ischemia-reperfusion injury in rats. Transpl Immunol 10: 277–284, 2002. doi: 10.1016/S0966-3274(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 35.Savage S, Nagel NJ, Estacio RO, Lukken N, Schrier RW. Clinical factors associated with urinary albumin excretion in type II diabetes. Am J Kidney Dis 25: 836–844, 1995. doi: 10.1016/0272-6386(95)90565-0. [DOI] [PubMed] [Google Scholar]
- 36.Schlöndorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20: 1179–1187, 2009. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 37.Shah K, McCormack CE, Bradbury NA. Do you know the sex of your cells? Am J Physiol Cell Physiol 306: C3–C18, 2014. doi: 10.1152/ajpcell.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata Y, Takaoka M, Maekawa D, Kuwahara C, Matsumura Y. Involvement of nitric oxide in the suppressive effect of 17beta-estradiol on endothelin-1 overproduction in ischemic acute renal failure. J Cardiovasc Pharmacol 44, Suppl 1: S459–S461, 2004. doi: 10.1097/01.fjc.0000166315.38258.e1. [DOI] [PubMed] [Google Scholar]
- 39.Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int 71: 846–854, 2007. doi: 10.1038/sj.ki.5002180. [DOI] [PubMed] [Google Scholar]
- 40.Singh AP, Singh N, Singh Bedi PM. Estrogen attenuates renal IRI through PPAR-γ agonism in rats. J Surg Res 203: 324–330, 2016. doi: 10.1016/j.jss.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 41.Singh AP, Singh N, Bedi PMS. Estradiol mitigates ischemia reperfusion-induced acute renal failure through NMDA receptor antagonism in rats. Mol Cell Biochem 434: 33–40, 2017. doi: 10.1007/s11010-017-3034-9. [DOI] [PubMed] [Google Scholar]
- 42.Singh AP, Singh N, Pathak D, Bedi PMS. Estrdiol attenuates ischemia reperfusion-induced acute kidney injury through PPAR-γ stimulated eNOS activation in rats. Mol Cell Biochem 453: 1–9, 2019. doi: 10.1007/s11010-018-3427-4. [DOI] [PubMed] [Google Scholar]
- 43.Škrtić M, Lytvyn Y, Bjornstad P, Reich HN, Scholey JW, Yip P, Sochett EB, Perkins B, Cherney DZI. Influence of sex on hyperfiltration in patients with uncomplicated type 1 diabetes. Am J Physiol Renal Physiol 312: F599–F606, 2017. doi: 10.1152/ajprenal.00357.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Striker LJ, Peten EP, Elliot SJ, Doi T, Striker GE. Mesangial cell turnover: effect of heparin and peptide growth factors. Lab Invest 64: 446–456, 1991. [PubMed] [Google Scholar]
- 45.Sullivan JC, Gillis EE. Sex and gender differences in hypertensive kidney injury. Am J Physiol Renal Physiol 313: F1009–F1017, 2017. doi: 10.1152/ajprenal.00206.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang MZ, Wang S, Yang S, Yang H, Fan X, Takahashi T, Harris RC. Role of blood pressure and the renin-angiotensin system in development of diabetic nephropathy (DN) in eNOS−/− db/db mice. Am J Physiol Renal Physiol 302: F433–F438, 2012. doi: 10.1152/ajprenal.00292.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol 17: 2664–2669, 2006. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziyadeh FN, Sharma K. Role of transforming growth factor-β in diabetic glomerulosclerosis and renal hypertrophy. Kidney Int Suppl 51: S34–S36, 1995. [PubMed] [Google Scholar]