Abstract
Renal angioplasty and stenting (PTRAs) resolves renal artery stenosis, but inconsistently improves renal function, possibly due to persistent parenchymal damage. We developed a bioengineered fusion of a drug delivery vector (elastin-like polypeptide, ELP) with vascular endothelial growth factor (VEGF), and showed its therapeutic efficacy. We tested the hypothesis that combined ELP-VEGF therapy with PTRAs improves renal recovery more efficiently than PTRAs alone, by protecting the stenotic renal parenchyma. Unilateral renovascular disease (RVD) was induced by renal artery stenosis in 14 pigs. Six weeks later, stenotic kidney blood flow (RBF) and glomerular filtration rate (GFR) were quantified in vivo using multidetector CT. Blood and urine were collected during in vivo studies. All pigs underwent PTRAs and then were randomized into single intrarenal ELP-VEGF administration or placebo (n = 7 each) groups. Pigs were observed for four additional weeks, in vivo CT studies were repeated, and then pigs were euthanized for ex vivo studies to quantify renal microvascular (MV) density, angiogenic factor expression, and morphometric analysis. Renal hemodynamics were similarly blunted in all RVD pigs. PTRAs resolved stenosis but modestly improved RBF and GFR. However, combined PTRAs+ ELP-VEGF improved RBF, GFR, regional perfusion, plasma creatinine, asymmetric dimethlyarginine (ADMA), and albuminuria compared with PTRAs alone, accompanied by improved angiogenic signaling, MV density, and renal fibrosis. Greater improvement of renal function via coadjuvant ELP-VEGF therapy may be driven by enhanced MV proliferation and repair, which ameliorates MV rarefaction and fibrogenic activity that PTRAs alone cannot offset. Thus, our study supports a novel strategy to boost renal recovery in RVD after PTRAs.
Keywords: angioplasty, microcirculation, renal artery stenosis, renal hemodynamics, VEGF
INTRODUCTION
Current therapeutic options for chronic renovascular disease (RVD) include medical, interventional (renal angioplasty and stenting, PTRAs), and combination therapies. Although PTRAs to treat renal artery stenosis and RVD is under scrutiny, in part, because of its limited success and absence of significant advantages over medical therapy (22, 38), reduced blood flow to the kidneys imposes a risk that could promote de novo renal injury and exacerbate preexisting renal damage (57). It is possible that the limited improvement in RVD partly results from neglecting treatment of the stenotic renal parenchyma, which demands new or more comprehensive therapeutic strategies to shift the current paradigm.
Structural and functional rarefaction of the renal microvasculature, which is essential for serving the renal metabolic demands and maintaining whole body homeostasis, has been observed in several renal pathologies and constitutes a universal feature in acute and chronic renal disease regardless of the etiology (31, 32). Our laboratory (8, 9, 17, 19) and others (1, 31, 63) have demonstrated that progressive microvascular (MV) rarefaction in the kidney plays a central role in the development of renal dysfunction and renal injury progression. Renal MV damage may precede and predict renal functional decline in hypertension, diabetes, and obesity (1, 36, 47), all major causes of chronic renal disease, further supporting a cause-effect relationship between MV rarefaction and renal injury.
Vascular endothelial growth factor (VEGF) is a proangiogenic cytokine with key roles in neovascularization, repair, and preservation of the MV networks everywhere in the body to allow the vasculature to adapt to local metabolic conditions and provide adequate blood flow (29). We showed that VEGF biology is disrupted in the stenotic kidney in RVD (9), which displays a progressively decreased VEGF bioavailability and altered renal expression of upstream and downstream mediators of the VEGF pathway (37, 63). The importance of VEGF for kidney health is also highlighted by studies showing that loss of VEGF signaling associates with hypertension, podocyte loss (34), and renal injury (27), as observed in experimental RVD (8, 9, 14, 16) and in chronic kidney disease (CKD) (15, 31, 32).
We (8, 9, 37) and others (2, 39, 45) have shown that VEGF therapy protects the renal microcirculation and ameliorates renal injury in models of progressive renal failure, although this approach may be hindered by VEGF’s short plasma half-life, rapid degradation in vivo, and potential for effects in other organs besides the kidney (14, 29). Our laboratory recently developed a novel drug-delivery system derived from human elastin called elastin-like polypeptides (ELPs) (4, 5, 33) in an attempt to refine VEGF therapy. ELPs have a long plasma half-life, low immunogenicity, natural renal accumulation, and can be adapted to deliver virtually any therapeutic protein or peptide (3–5, 14, 16). We built an ELP-VEGF construct that extends the circulating time of VEGF, protects it from degradation, and increases renal accumulation and therapeutic efficacy (14, 16).
Progressive renal injury distal to the vascular obstruction may determine chances of recovery following therapeutic intervention in RVD (25a). Therefore, we aim to develop a new strategy using a novel renal targeted VEGF construct to increase the efficacy of PTRAs. We hypothesize that coadjuvant ELP-VEGF therapy during PTRAs will enhance renal recovery compared with PTRAs alone. A combined strategy could boost renal recovery in RVD, shifting current paradigms and addressing a significant burden for a patient population with increased cardiovascular morbidity and mortality (21) and limited treatments.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee at the University of Mississippi Medical Center approved all studies. Twenty-six juvenile domestic pigs (Sus scrofa domestica) were used for the study, which lasted 10 wk. In 19 pigs, unilateral RVD was induced by renal artery stenosis and high cholesterol feeding (Teklad 15% lard, 2% cholesterol swine diet) to accelerate renal injury and mimic early atherosclerotic RVD (10, 11). Renal artery stenosis was induced by engaging a 7-mm percutaneous transluminal coronary angioplasty (PTCA) balloon containing metal wire coils into the proximal-middle section of the main renal artery and inflating the balloon to 14 atm for expansion of the coil. The balloon was then deflated and removed, leaving the metal coil in place. The coil leads to progressive narrowing of the arterial lumen, as extensively demonstrated (10, 12, 37, 40, 46). Blood pressure was continuously measured by telemetry (8–10, 14).
Six weeks after RVD induction, all RVD and normal pigs were anesthetized with intramuscular telazol (5 mg/kg) and xylazine (2 mg/kg), intubated, and mechanically ventilated on room air. Anesthesia was maintained with a ketamine (0.2 mg·kg−1·min−1) and xylazine (0.03 mg·kg−1·min−1) mixture in normal saline administered via an ear cannula. With fluoroscopic guidance, a 9F arterial catheter was advanced into the renal artery. The degree of renal artery stenosis was quantified by renal angiography, as shown previously (8–10, 14). The catheter was positioned in the aorta superior to the renal arteries for in vivo multidetector computerized tomography (MDCT) studies. Saline was continuously infused through the catheter at a rate of 0.5 ml/min, and the catheter was later used to advance the balloon for PTRAs, as described in the next paragraph. MDCT studies were performed to quantify renal blood flow (RBF), glomerular filtration rate (GFR), and regional perfusion, as described previously (8–10, 14).
Immediately after 6-wk MDCT studies, 14 RVD animals underwent PTRAs using balloon dilation and placement of a tantalum stent through the lumen of the previously positioned catheter, which is now advanced to the main renal artery, as described previously (8, 13). During PTRAs, pigs were treated with a single intrarenal injection ELP-VEGF (100 μg/kg) (14), RVD+PTRAs+ELP-VEGF; n = 7) or placebo (RVD+PTRAs+saline; n = 7) through the balloon catheter while inflated in the renal artery. Animals were monitored during ELP-VEGF/placebo administration to determine any impact on heart rate or blood pressure. The other five RVD animals and an additional group of pigs served as normal controls (Normal; n = 7).
All pigs were observed for four additional weeks, and then MDCT in vivo studies were repeated as described earlier. No additional treatment/placebo was administered, and the in vivo MDCT studies assessed impacts of the interventions. Urine and systemic venous blood were collected during each in vivo study to measure plasma creatinine (ab65340; Abcam, Cambridge, UK) (15), plasma asymmetric dimethylarginine (ADMA; MBS734878, MyBioSource, San Diego, CA), and urine albuminuria (K551-100; BioVision, Milpitas, CA) following vendors’ instructions. Plasma cholesterol was determined using a Vet AXCEL chemistry analyzer.
After 10-wk in vivo studies, all pigs were allowed 3 days to recover and then euthanized with a lethal intravenous injection of pentobarbital sodium (100 mg/kg iv). Because of the widespread actions of VEGF throughout the body and its ability to induce vascular proliferation, all organs were carefully examined visually both during in vivo CT scanning and at necropsy to determine whether ELP-VEGF therapy induced development of tumors or aberrant vascularization, which were not observed anywhere.
Kidneys were removed and immersed in heparinized saline. After inspection, a lobe of kidney tissue was used for micro-CT scanning and reconstruction. Another lobe was removed, snap-frozen in liquid nitrogen, and stored at −80°C. The expression of factors involved in angiogenesis, MV remodeling, and recruitment of cell progentiors was quantified in kidney tissues, as described previously (14, 16). Another portion of the kidney was preserved in 10% formalin to assess morphology in trichrome-stained renal cross sections (8, 9, 14, 16).
Generation and purification of ELP-VEGF constructs.
The coding sequence for human VEGF-A121 was fused in frame with the ELP coding sequence, and the chimeric protein was recombinantly expressed, purified, and characterized, as recently described (14, 16, 33). Renal specificity and lack of renal and liver toxicity of ELP-VEGF therapy were recently shown, demonstrating the safety and renal targeting of the construct (3, 16). The dose for in vivo studies was the same as recently shown to be efficacious after intrarenal administration (14).
In vivo MDCT analysis was used to quantify single-kidney RBF (ml/min), GFR (ml/min), and cortical and medullary perfusion (ml·min−1·ml tissue−1), using previously validated methods (14, 16, 23, 42). Manually traced regions were selected in CT images in the aorta, renal cortex, medulla, and papilla to generate time-density curves. Time-density curves were extended with gamma-variate curve fits, and the areas under each curve segment were calculated (14, 16, 23, 42).
Ex vivo micro-CT scanning and reconstruction to quantify renal MV density were performed ex vivo, as extensively described (14, 18, 63) after the 10-wk time point. The stenotic kidney was perfused with a silicon-polymer contrast agent (Microfil, Flow Tech, Carver, MA) via the renal artery at physiological pressure. Kidney samples were scanned at 0.3° increments using a micro-CT scanner (SkyScan 1076; Bruker, Billerica, MA) and reconstructed at 9-μm resolution for image analysis. Using the Analyze software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN), the spatial density of microvessels <500 μm diameter was calculated.
Western blot analysis.
Standard protocols were followed to determine renal expression of hepatocyte growth factor (HGF, sc-71244; Santa Cruz Biotechnology, Santa Cruz, CA), matrix-metalloproteinases-2 and -9 (MMP-2; ab110186, Abcam; MMP-9; ab38898, Abcam), tissue transglutaminase (tTG, ABIN2782979; Antibodies Online, Atlanta, GA), Oct-4 (ab18976; Abcam), VEGF (ab53465; Abcam), Flk-1 (sc-505; Santa Cruz Biotechnology), angiopoietin-1 (Ang-1, ab8451; Abcam), stromal-derived factor-1 (SDF-1, AVARP07031-P050; Aviva Systems Biology, San Diego, CA), CXCR-4 (ARP30799-P050; Aviva Systems Biology), endothelial nitric oxide synthase (eNOS, ab5589; Abcam), tissue inhibitor of matrix metalloproteinases-1 (TIMP-1, orb11483; Biorbyt, San Francisco, CA), hypoxia-inducible factor-1α (HIF-1α; LS-B1503-1; Lifespan Biosciences, Seattle, WA), thrombospondin-1 (THBS; ab1823; Abcam), and osteopontin (OPN; ab8448; Abcam, UK). Our standard Western blot analysis protocol includes detection of two to four different proteins on each membrane, separated by membrane stripping and blocking in between antibodies. After the last target protein, the membrane is stripped and blocked before incubation with antibody against β-actin, which serves as a loading control for that membrane.
Histology and renal morphology.
Mid-hilar 5-μm cross sections of stenotic kidneys were stained with trichrome and used to perform renal morphometric analysis to quantify tubule-interstitial fibrosis, glomerulosclerosis, and media-to-lumen ratio using Fiji from ImageJ (National Institutes of Health (NIH), Bethesda, MD), as shown previously (10, 11, 14, 16).
Immunostaining.
Mid-hilar, 5-μm cross sections of stenotic kidneys were immunostained to quantify renal expression of factors involved in recruitment and homing of progenitor cells SDF-1 (sc-28876; Santa Cruz Biotechnology, CA), CD34 (OABI0002; Aviva Systems Biology), and resident renal stem cell marker Oct-4 (ab18976; Abcam). Immunostaining was performed using an HRP/DAB (ABC) detection IHC kit (ab64261; Abcam, UK), following the vendor’s instructions. Quantification of immunoreactivity was performed using Fiji from ImageJ (NIH), as shown previously (16).
Statistical analysis.
Results are expressed as means ± SE, as indicated. Statistical comparisons within groups were performed using paired Student’s t-test, and among groups using one-way ANOVA, with Tukey’s correction for multiple comparisons. Statistical significance was accepted for P ≤ 0.05.
RESULTS
General characteristics.
As shown in Table 1, body weight and renal hemodynamics were similar among all groups before treatment, whereas degree of stenosis and total plasma cholesterol levels were similarly elevated in all RVD pigs compared with normal controls. Furthermore, RVD pigs had similar hypertension before treatment compared with normal controls (Table 1). Heart rate and blood pressure remained unchanged during administration of ELP-VEGF or placebo.
Table 1.
General characteristics of Normal, RVD+PTRAs, and RVD+PTRAs+ELP-VEGF pigs before (6 wk) and 4 wk after (10 wk) treatment with ELP-VEGF and/or PTRAs
| Normal | RVD+PTRAs |
RVD+PTRAs+ELP-VEGF |
|||
|---|---|---|---|---|---|
| 6 Weeks | 10 Weeks | 6 Weeks | 10 Weeks | ||
| Body weight, kg | 45.1 ± 0.92 | 46.3 ± 3.4 | 53.0 ± 1.8*†† | 48.8 ± 2.4 | 60.0 ± 5.7*† |
| Urine albuminuria, mg/l | 17.8 ± 3.6 | 53.7 ± 14.3* | 42.9 ± 11.5† | 31.9 ± 7.0* | 25.0 ± 6.0†† |
| Plasma cholesterol, mg/dl | 74.9 ± 5.6 | 748.0 ± 64.3* | 883.0 ± 47.1* | 759.0 ± 54.5* | 886.0 ± 55.3*† |
| MAP, mmHg | 110.0 ± 2.0 | 132.9 ± 18.4* | 132.0 ± 11.8* | 127.7 ± 9.5* | 113.7 ± 3.8††‡‡ |
| RBF, ml/min | 439.1 ± 28.5 | 256.8 ± 32.9* | 292.4 ± 15.7* | 253.4 ± 26.1* | 351.7 ± 42.5*† |
| GFR, ml/min | 65.4 ± 2.8 | 35.1 ± 1.6* | 43.2 ± 3.9* | 39.1 ± 3.5* | 51.3 ± 3.8*†‡‡ |
| Cortical perfusion, ml·min−1·g tissue−1 | 3.9 ± 0.2 | 3.5 ± 0.4 | 3.4 ± 0.2 | 3.2 ± 0.2 | 3.8 ± 0.3† |
| Medullary perfusion, ml·min−1·g tissue−1 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.3 | 1.1 ± 0.3 | 2.3 ± 0.5† |
Data are expressed as means ± SE; n = 7 per group. Parameters were measured at 6 and 10 wk in normal animals and were not different; thus, data from normal pigs included in the table are from 10 wk only. Parameters in RVD pigs were measured at 6 (before treatment) and at 10 wk (4 wk after treatment) with PTRAs and PTRAs+ELP-VEGF and are all in table. MAP, mean arterial pressure; RBF, renal blood flow; GFR, glomerular filtration rate.
P < 0.05 versus Normal.
P < 0.05 versus 6 wk.
P between 0.05 and 0.1 versus 6 wk.
P between 0.05 and 0.1 versus RVD+PTRAs.
Also shown in Table 1, 4 wk after PTRAs, RVD pigs maintained a similar plasma cholesterol compared with normal controls, regardless of intrarenal ELP-VEGF/placebo administration. PTRAs were similarly effective at resolving renal artery stenosis in all animals [average residual stenosis was less than 14%, which was not hemodynamically significant (25a)]. Albuminuria trended toward improvement, whereas blood pressure and renal hemodynamics were improved after coadjuvant PTRAs+ELP-VEGF compared with PTRAs alone (Table 1).
Coadjuvant PTRAs+ELP-VEGF improved renal hemodynamics and function more efficiently than PTRAs.
MDCT-derived stenotic kidney RBF and GFR were similarly decreased in all pigs with RVD after 6 wk of observation, accompanied by increased plasma creatinine and ADMA (Table 1 and Fig. 1). PTRAs modestly improved RBF and GFR and had no effect on regional perfusion, whereas coadjuvant ELP-VEGF administration caused significantly greater recovery of stenotic kidney hemodynamics (RBF, GFR, and cortical and medullary perfusion) compared with pretreatment hemodynamics (Table 1 and Fig. 1). Improvements in renal hemodynamics were accompanied by a reduction in plasma ADMA and improved protein expression of eNOS, correlating with significantly decreased plasma creatinine compared with pretreatment values (Fig. 2).
Fig. 1.
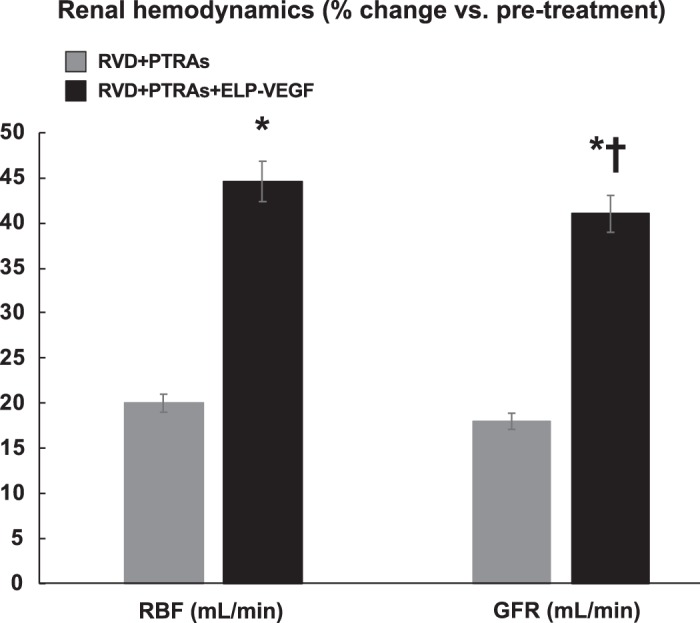
Coadjuvant intra-renal administration of elastin-like polypeptide-vascular endothelial growth factor (ELP-VEGF) during renal angioplasty and stenting (PTRAs) improved renal hemodynamics compared with pretreatment function. RBF, renal blood flow; GFR, glomerular filtration rate. *P < 0.05 versus 6 wk. †P < 0.05 versus renovascular disease (RVD)+PTRAs.
Fig. 2.
Circulating asymmetric dimethylarginine (ADMA) and representative bands (two per animal from a n = 7 per group) showing renal protein expression and quantification of endothelial nitric oxide synthase (eNOS) from normal, renal angioplasty and stenting (PTRAs) and PTRAs+ elastin-like polypeptide-vascular endothelial growth factor (ELP-VEGF)-treated pigs. Intrarenal ELP-VEGF at PTRAs resulted in a significant attenuation in systemic plasma ADMA concentration (A), a potent inhibitor of eNOS. B: ADMA attenuation was accompanied by increased expression of eNOS in the PTRAs+ELP-VEGF-treated kidney. C: these improvements in mediators of endothelial function were accompanied by a significantly greater decrease in plasma creatinine concentration in PTRAs+ELP-VEGF-treated pigs compared with pretreatment values. ADMA, asymmetric dimethylarginine. *P < 0.05 versus 6 wk. #P = 0.08 versus renovascular disease (RVD) +PTRAs. †P < 0.05 versus RVD+PTRAs.
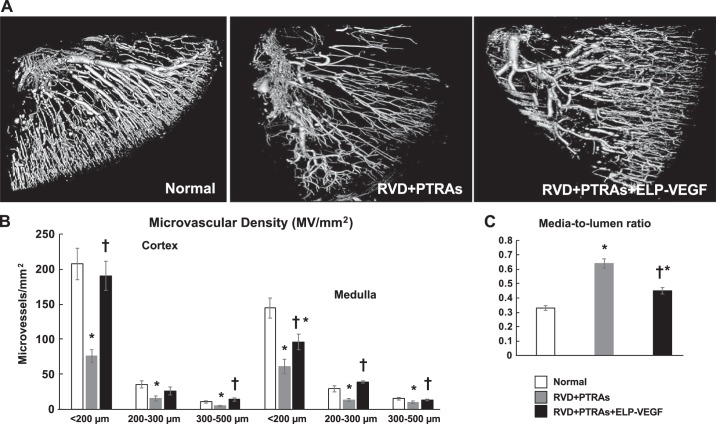
Coadjuvant PTRAs+ELP-VEGF improved renal MV density and remodeling.
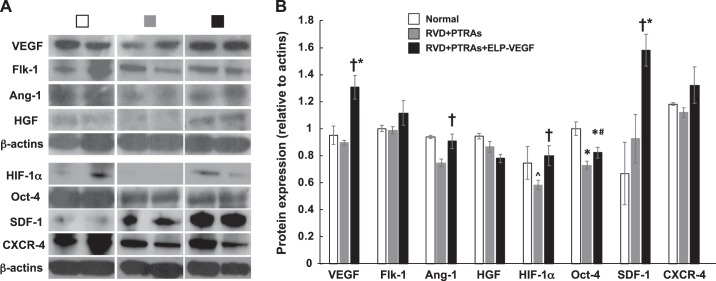
The stenotic kidney of PTRAs-treated pigs showed significant cortical and medullary MV rarefaction and remodeling compared with normal controls 4 wk after the intervention. However, administration of ELP-VEGF at PTRAs improved cortical and medullary MV density (diameters <500 µm) and remodeling, as assessed ex vivo following the 10-wk timepoint (Fig. 3). While VEGF and Flk-1 remained unchanged after PTRAs, improved stenotic kidney expression of VEGF and its Flk-1 receptor after coadjuvant PTRAs+ELP-VEGF mirrored the improvements in renal MV density (Fig. 4) compared with PTRAs alone and were accompanied by increased renal expression of Ang-1, HIF-1α, resident progenitor cell marker Oct-4 (60, 62), and mobilization factor SDF-1, suggesting improved angiogenic signaling in the ELP-VEGF-treated kidney (Fig. 4).
Fig. 3.
Representative micro-CT reconstruction of the renal microvascular (MV) architecture (one per animal from a n = 7 per group) and quantification of MV density and intrarenal MV media-to-lumen ratio from normal, renal angioplasty and stenting (PTRAs) and PTRAs+ elastin-like polypeptide-vascular endothelial growth factor (ELP-VEGF)-treated pigs. Intrarenal ELP-VEGF at PTRAs improved cortical and medullary vascular density in the stenotic kidney. Effect of PTRAs+ELP-VEGF on MV architecture (A) and quantification in renovascular disease (RVD)+PTRAs versus RVD+PTRAs+ELP-VEGF-treated animals (B). C: effect of PTRAs with and without ELP-VEGF treatment on media-to-lumen ratio, a measurement of MV remodeling. †P < 0.05 versus RVD+PTRAs. *P < 0.05 versus Normal.
Fig. 4.
A: representative bands (two per animal from a n = 7 per group) showing renal protein expression and quantification of VEGF, vascular endothelial growth factor; Flk-1, liver fetal kinase-1; Ang-1, angiopoietin-1; HGF, hepatocyte growth factor; HIF-1α, hypoxia-inducible factor-1α; SDF-1, stromal-derived factor-1; CXCR-4, chemokine receptor type 4 from normal, PTRAs and PTRAs+ELP-VEGF-treated kidneys. B: PTRAs followed by ELP-VEGF therapy improved renal expression of angiogenic factors and endothelial cell progenitor recruitment markers. †P < 0.05 versus RVD+PTRAs. *P < 0.05 versus Normal. #P = 0.06 versus RVD+PTRA. ^P = 0.06 versus Normal.
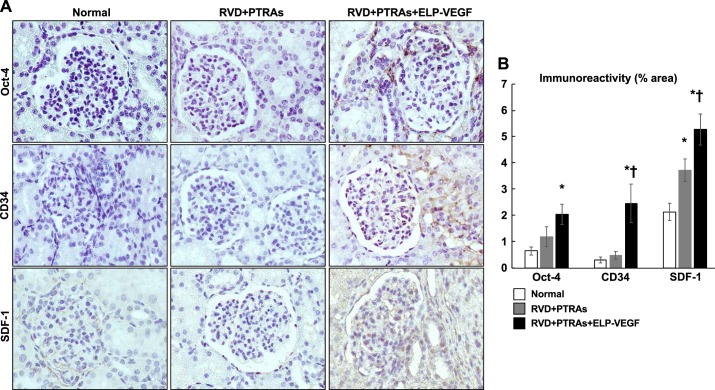
Coadjuvant PTRAs+ELP-VEGF increased renal expression of progenitor cell recruitment and activation markers.
We observed a marked increase in Oct-4 (16, 17, 19, 62), CD34, and SDF-1, specifically in the tubulointerstitial regions of the stenotic kidneys treated with PTRAs+ELP-VEGF (Fig. 5). This data underscore a distinct effect of ELP-VEGF therapy and suggest stimulation of circulating and resident cell progenitors into the kidney that was not observed after PTRAs alone.
Fig. 5.
A: representative renal cross sections (1 per animal from n = 7 per group) showing renal immunoreactivity and quantification of progenitor cell markers Oct-4, CD34, and stromal derived factor-1 (SDF-1) from normal, renal angioplasty, and stenting (PTRAs) and PTRAs+ elastin-like polypeptide-vascular endothelial growth factor (ELP-VEGF)-treated kidneys. B: coadjuvant intrarenal ELP-VEGF aministration during PTRAs improves stenotic kidney tubulointerstitial expression of progenitor cell recruitment molecules and markers of progenitor cells. Representative images captured at ×40 magnification. *P < 0.05 versus Normal. †P < 0.05 versus RVD+PTRAs.
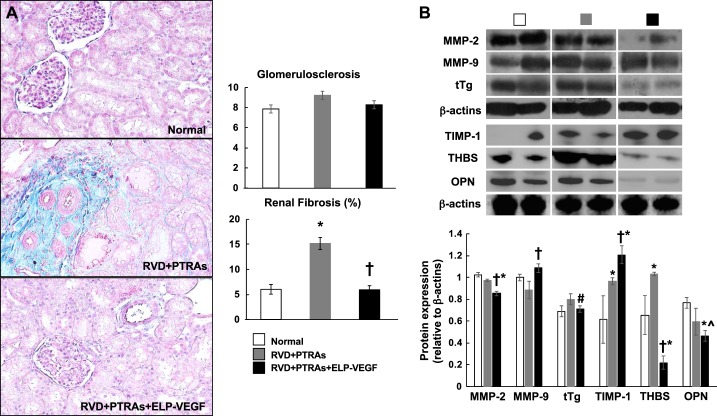
Coadjuvant PTRAs+ELP-VEGF attenuated renal fibrosis.
Tubule-interstitial fibrosis was significantly attenuated in PTRAs+ELP-VEGF-treated pigs compared with PTRAs-only-treated pigs (Fig. 6). This was accompanied by improved expression of MMP-9 and decreased expression of tTg, OPN, and THBS, suggesting increased extracellular matrix (ECM) turnover. Glomerulosclerosis was slightly, but not significantly, attenuated in PTRAs+ELP-VEGF-treated pigs (Fig. 6).
Fig. 6.
Representative renal cross sections (one per animal from a n = 7 per group) stained with trichrome, morphometric analysis, and representative bands showing renal protein expression (two per animal from a n = 7 per group) and quantification of matrix-metallopeptidase-2 and -9 (MMP-2 and MMP-9), tissue transglutaminase (tTg), tissue metallopeptidase inhibitor-1 (TIMP-1), thrombospondin (THBS), and osteopontin (OPN) from normal, renal angioplasty and stenting (PTRAs) and PTRAs+ elastin-like polypeptide-vascular endothelial growth factor (ELP-VEGF)-treated kidneys.A: coadjuvant intrarenal ELP-VEGF administration during PTRAs attenuated renal fibrosis and glomerulosclerosis. B: changes in renal morphology were accompanied by a shift in protein expression of factors involved in extracellular matrix turnover. †P < 0.05 versus RVD+PTRAs. *P < 0.05 versus Normal. #P = 0.06 versus RVD+PTRAs. ^P = 0.07 versus RVD+PTRAs.
Renal hemodynamics and blood pressure in untreated RVD pigs.
Pigs with RVD that did not undergo PTRAs or receive ELP-VEGF (n = 5) were studied 6 and 10 wk after induction of RVD, as we have previously shown (14, 16), and served as untreated controls. Stenotic kidney RBF (244.5 ± 52.2 ml/min) and GFR (38.0 ± 6.1 ml/min) were similarly reduced at the 6 wk MDCT studies, matching our observations in the RVD+PTRAs and RVD+PTRAs+ELP-VEGF groups, and remained low 4 wk later, at the 10-wk MDCT study (RBF: 250.4 ± 44.4 ml/min; GFR: 32.3 ± 5.3 ml/min). The sustained deterioration in renal function also correlated with increased mean arterial pressure, which rose from 145.2 ± 14.7 at 6 wk to 157.2 ± 12.8 at 10 wk.
DISCUSSION
This study supports the feasibility of intrarenal ELP-VEGF therapy to refine PTRAs to improve renal recovery in RVD. Our results also highlight the importance of the VEGF pathway, extending our previous contributions on the role that MV protection and integrity play in renal healing in RVD (8, 9, 14, 16). Our findings may expand the potential renal applications of VEGF-driven therapeutic angiogenesis and MV repair (14, 16) as a coadjuvant strategy to catheter-based interventions.
Randomized clinical trials show that PTRAs generally do not provide additional benefit over best medical therapy alone (22, 38), although recent evidence indicates that small subgroups of patients may benefit from catheter-based interventions (20, 53). Reasons for the disparate success of PTRAs have not been elucidated, adding uncertainty about the most suitable treatment strategy for RVD. A small randomized controlled trial demonstrated that a subgroup of patients with potentially reversible renal dysfunction experienced a proportionately greater improvement in GFR than parenchymal volume after PTRAs, supporting the “hibernating parenchyma” hypothesis (20), which posits that return of blood flow may result in recovery of renal function because irreversible injury has not yet occurred (20). Furthermore, the notion of a hibernating parenchyma (20) raises the possibility that the severity of intrarenal damage may determine the outcomes after PTRAs. Given the progressive nature of RVD, only patients whose renal artery stenosis has become severe will experience a decline in renal function, indicating that the kidneys’ compensatory mechanisms may become insufficient (25a). These patients with compromised renal function often meet the selection criteria for clinical trials, but likely already have irreversible renal damage beyond the stenosis, greatly lessening the efficacy of PTRAs. Hence, identifying patients who will likely benefit from renal revascularization remains a challenge.
We have shown that MV dysfunction, damage, and loss (8) in the stenotic kidney accompanies progressive functional deterioration and correlates with a progressive loss of renal VEGF bioavailability, highlighting the importance of VEGF for the kidney (2, 18, 37). This is further supported by studies showing that VEGF therapy improves renal function, MV proliferation, and repair in the experimental acute or chronic renal diseases (8, 9, 37, 45). However, some limitations, like VEGF’s short half-life and potential to induce vascular proliferation in other organs besides the kidney, may hinder translation of VEGF therapy into clinical settings. To address these limitations, we developed a biopolymer-delivered VEGF construct, ELP-VEGF, that targets the kidney and induces superior renoprotection compared with unconjugated VEGF (14, 16). In previous studies, we performed biodistribution studies using fluorescently labeled ELP-VEGF and showed that it preferentially accumulates in the kidney and is only detected at very low levels in other major organs 4 h after injection, minimizing the possibility that it induces aberrant vascular proliferation (or other effects) in other organ systems (14, 16). These studies were extended by assessment of the expression of VEGF and downstream mediators in major organs and the effects on liver function 4 wk after “systemic” administration in the RVD model, showing that there were no effects in any organ but the kidney (16). We also performed pharmacokinetic studies (using rodent and swine models), showing that ELP-VEGF has a significantly longer plasma half-life (14) compared with unconjugated VEGF (26, 33), supporting the extent of ELP-VEGF addressing potential limitations of VEGF therapy and enhancing the efficacy and potential clinical applicability for renal disease.
Because MV disease parallels the progression of renal damage in RVD and solely returning blood flow to the kidney may not be sufficient to ameliorate renal dysfunction, simultaneously targeting the renal microvessels may increase the efficacy of PTRAs. In the current study, PTRAs were similarly successful at resolving the vascular obstruction in all animals. However, the little structural and functional improvements observed after PTRAs suggest that some degree of preexisting renal damage cannot be ameliorated. Coadjuvant PTRAs+ELP-VEGF resulted in a greater recovery of RBF, GFR, and regional perfusion than PTRAs alone. Functional recovery was accompanied by significantly decreased media-to-lumen ratio and expanded cortical and medullary MV density. Whereas previous studies using intrarenal administration of unconjugated VEGF only increased the density of small-diameter (<200 µm) microvessels (9, 37), ELP-VEGF also expanded MV density of larger microvessels [200–500 µm (14)], suggesting more efficient MV sprouting from preexisting vessels and improved repair and remodeling of the resident vasculature. Further evidence of enhanced renoprotection is the significant decrease in plasma creatinine and ADMA in PTRAs+ELP-VEGF-treated pigs. ADMA is a pro-atherogenic inhibitor of endothelial nitric oxide synthase (eNOS) that increases in early stages of renal disease (41), facilitates endothelial dysfunction (30, 52), and may also contribute to the progression of CKD when elevated (30). Mihout et al. (49) showed that ADMA plays an important role in CKD progression in several ways, with high levels contributing to the development of hypertension, oxidative stress, increased ECM synthesis, and rarefaction of the peritubular capillaries. These contributions of increased circulating ADMA toward the progression of renal injury are in line with our observations in the RVD model, in which a trend of increasing plasma ADMA accompanies decreased MV density, increased fibrosis, and reduced renal hemodynamics. Therefore, the reduction of ADMA may have helped to improve MV endothelial function and nitric oxide bioavailablity in the stenotic kidney after PTRAs+ELP-VEGF, which could have helped in the recovery of renal MV density and hemodynamics.
Coadjuvant ELP-VEGF therapy improved the renal expression of VEGF and Flk-1 and restored renal expression of Ang-1, a downstream mediator of VEGF that plays a role in vessel maturation (28), suggesting restored angiogenic activity. The ELP-VEGF construct has a higher circulating time (14) compared with unconjugated (free) VEGF, but it is unlikely that any of the delivered VEGF remains in the kidney 4 wk after its administration. Thus, the upregulated expression of VEGF in the ELP-VEGF-treated kidney likely reflects endogenous VEGF. This notion is supported by our previous study showing a discrepancy between VEGF mRNA and protein expression and availability in the stenotic kidney that was improved after VEGF therapy (37). Therefore, it is possible that altered posttranscriptional mechanisms in the stenotic kidney that may disrupt aspects of its endogenous production (37), combined with a possible progressive loss of renal sources VEGF as fibrosis develops [e.g., podocytes and tubular cells (58)], may compromise renal bioavailability of VEGF, and that such processes may have been halted or slowed down by ELP-VEGF therapy.
Interestingly, HGF, a growth factor with the capacity to mediate VEGF stimulation (48) via HIF-1α (48, 56), was unchanged in the stenotic kidneys of PTRAs+ELP-VEGF pigs. This may suggest that the increased expression of VEGF and Ang-1 was not stimulated by HGF, but rather via another reparative mechanism. Indeed, renal expression of HIF-1α, a powerful stimulus for VEGF (48, 56), was significantly increased after ELP-VEGF therapy, likely contributing to restored VEGF signaling. Whereas increased HIF-1α expression following coadjuvant treatment indicates a likely mechanism for increased VEGF stimulation, it may also be considered paradoxical, since the most powerful stimulus for HIF-1α is hypoxia (56). Speculatively, improvements in MV density may have resulted in a more favorable milieu and, consequently, less degradation of HIF-1α (63), although future studies into other potential factors that may be upregulated by ELP-VEGF therapy and influence renal HIF-1α expression are needed. Nonetheless, HIF-1α can directly stimulate SDF-1, a progenitor cell recruitment chemokine (7) that displayed increased expression in PTRAs+ELP-VEGF-treated stenotic kidneys. Moreover, PTRAs+ELP-VEGF increased renal expression of CD34 [a marker of undifferentiated circulating progenitor cells (60)], and Oct-4 [a marker of undifferentiated resident progenitor cells (19, 60, 62)], which could indicate increased activation, recruitment, and mobilization of endothelial progenitor cells in the stenotic kidney. Endothelial cell progenitors can limit vascular injury by reconstituting the luminal barrier and restoring secretion of paracrine factors involved in vascular proliferation and tissue healing (59). Since enhanced expression of these factors was observed even 4 wk after ELP-VEGF injection, it is possible that restoration of endogenous mechanisms of MV repair, proliferation, and tissue healing persisted long term to promote renal functional recovery. However, it should be noted that one of the markers we use as a marker for undifferentiated cells, CD34, is also expressed on endothelial cells, and its increase in expression following ELP-VEGF treatment could, in fact, be indicative of increased vascularization (disclosed by micro-CT quantifications) rather than progenitor cell activation and recruitment. It is compelling, however, that we have consistently observed increases in stenotic kidney expression of other markers that cannot simply reflect increased vascularity (16), including SDF-1 and Oct-4. Future studies using other experimental approaches will be needed to fully elucidate this potential underlying mechanism.
Finally, we observed substantial attenuation of renal fibrosis in PTRAs+ELP-VEGF-treated kidneys. Decreased renal fibrosis accompanied augmented expression of MMP-9 [an enzyme involved in ECM turnover (55)] and TIMP-1, which may suggest enhanced enzymatic activity in the treated kidneys and breakdown of ECM to allow for more expansion of the renal microvasculature. MMP-9 can also contribute to angiogenesis by modulating mobilization of VEGF (50). On the other hand, expression of MMP-2 was not increased in PTRAs+ELP-VEGF-treated pigs, but decreased compared with PTRAs-treated pigs and normal controls. In vitro studies have reported that MMP-2 may act as a proinflammatory mediator in glomerular cells (44), so its decreased expression could reflect a beneficial effect of coadjuvant PTRAs+ELP-VEGF. Enhanced renal expression of MMP-9/TIMP-1 after PTRAs+ELP-VEGF was accompanied by decreased expression of tTg, an enzyme which may contribute to renal interstitial fibrosis (54) and MV remodeling (18). Furthermore, stenotic kidney expression of THBS, a glycoprotein involved in the proliferation of fibroblasts, smooth muscle cells, and mesangial cells (6, 35), increased in pigs treated with only PTRAs compared with normal controls, but was significantly decreased after coadjuvant PTRAs+ELP-VEGF. Thrombospondin-1 has also been implicated in the development of renal disease through stimulation of the profibrotic transforming growth factor-β (24, 25), which we showed to be elevated in RVD but significantly blunted after intrarenal ELP-VEGF therapy (14). Moreover, OPN, which is also profibrotic and involved in several renal pathologies (51, 61), was also reduced in PTRAs+ELP-VEGF-treated kidneys compared with normal and PTRAs-treated ones, suggesting a significant downregulation of important mediators of fibrosis following coadjuvant therapy. Coupled with the observed attenuated fibrosis and expanded microvasculature, these data suggest an antifibrotic, proangiogenic renal environment after PTRAs+ELP-VEGF that was not observed after PTRAs alone. These improvements, paired with restored VEGF signaling and a favorable environment for recruitment and/or activation of endothelial cell progenitors, may have resulted in greater MV protection that helped to improve renal hemodynamics.
In summary, our study shows that coadjuvant targeted administration of ELP-VEGF may enhance the efficacy of PTRAs in RVD, supporting the potential of a novel strategy via therapeutic angiogenesis using drug-delivery technologies. Our results support the notion that there is a point in RVD at which renal injury becomes irreversible and, therefore, nonresponsive to PTRAs alone. Future studies to define the stand-alone and coadjuvant potential of therapeutic angiogenesis for RVD with varying degrees of renal injury, length of disease, and additional time points may solidify the translational potential of this strategy toward clinical application. Future studies will also aim to determine whether beneficial effects of combined PTRAs+ELP-VEGF persist for longer than 4 wk posttreatment. These studies could have significant ramifications for the application of this new treatment to other forms of renal disease presenting with MV abnormalities.
GRANTS
This work was directly supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant R41DK-109737, and partially supported by grants R01HL-095638, P01HL-51971, P20GM-104357, and R01HL-121527 from the NIH and Grants EIA18490005, IPA34170267, and PRE34380274 from the American Heart Association.
DISCLOSURES
G. L. Bidwell is owner of Leflore Technologies LLC, a private company working to commercialize ELP-based technologies in several disease areas. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.R.C. conceived and designed research; E.G., J.E.E., M.L.W., F.M., and A.R.C. performed experiments; E.G., G.L.B., and A.R.C. analyzed data; E.G. and A.R.C. interpreted results of experiments; E.G. and A.R.C. prepared figures; E.G. drafted manuscript; E.G., J.E.E., M.L.W., F.M., G.L.B., and A.R.C. edited and revised manuscript; E.G., J.E.E., M.L.W., F.M., G.L.B., and A.R.C. approved final version of manuscript.
REFERENCES
- 1.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 3.Bidwell GL III, Mahdi F, Shao Q, Logue OC, Waller JP, Reese C, Chade AR. A kidney-selective biopolymer for targeted drug delivery. Am J Physiol Renal Physiol 312: F54–F64, 2017. doi: 10.1152/ajprenal.00143.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidwell GL III, Perkins E, Hughes J, Khan M, James JR, Raucher D. Thermally targeted delivery of a c-Myc inhibitory polypeptide inhibits tumor progression and extends survival in a rat glioma model. PLoS One 8: e55104, 2013. doi: 10.1371/journal.pone.0055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bidwell GL III, Perkins E, Raucher D. A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth. Cancer Lett 319: 136–143, 2012. doi: 10.1016/j.canlet.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 130: 503–506, 1995. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceradini DJ, Gurtner GC. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc Med 15: 57–63, 2005. doi: 10.1016/j.tcm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Chade AR, Kelsen S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ Cardiovasc Interv 3: 376–383, 2010. doi: 10.1161/CIRCINTERVENTIONS.110.951277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chade AR, Kelsen S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. Am J Physiol Renal Physiol 302: F1342–F1350, 2012. doi: 10.1152/ajprenal.00674.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106: 1165–1171, 2002. doi: 10.1161/01.CIR.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 11.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 12.Chade AR, Stewart NJ, Peavy PR. Disparate effects of single endothelin-A and -B receptor blocker therapy on the progression of renal injury in advanced renovascular disease. Kidney Int 85: 833–844, 2014. doi: 10.1038/ki.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chade AR, Tullos N, Stewart NJ, Surles B. Endothelin-a receptor antagonism after renal angioplasty enhances renal recovery in renovascular disease. J Am Soc Nephrol 26: 1071–1080, 2015. doi: 10.1681/ASN.2014040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chade AR, Tullos NA, Harvey TW, Mahdi F, Bidwell GL III. Renal therapeutic angiogenesis using a bioengineered polymer-stabilized vascular endothelial growth factor construct. J Am Soc Nephrol 27: 1741–1752, 2016. doi: 10.1681/ASN.2015040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chade AR, Williams ML, Engel J, Guise E, Harvey TW. A translational model of chronic kidney disease in swine. Am J Physiol Renal Physiol 315: F364–F373, 2018. doi: 10.1152/ajprenal.00063.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chade AR, Williams ML, Guise E, Vincent LJ, Harvey TW, Kuna M, Mahdi F, Bidwell GL III. Systemic biopolymer-delivered vascular endothelial growth factor promotes therapeutic angiogenesis in experimental renovascular disease. Kidney Int 93: 842–854, 2018. doi: 10.1016/j.kint.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119: 547–557, 2009. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB J 20: 1706–1708, 2006. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 19.Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells 28: 1039–1047, 2010. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrysochou C, Green D, Ritchie J, Buckley DL, Kalra PA. Kidney volume to GFR ratio predicts functional improvement after revascularization in atheromatous renal artery stenosis. PLoS One 12: e0177178, 2017. doi: 10.1371/journal.pone.0177178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colyer WR Jr, Cooper CJ. Cardiovascular morbidity and mortality and renal artery stenosis. Prog Cardiovasc Dis 52: 238–242, 2009. doi: 10.1016/j.pcad.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB Sr, Dworkin LD; CORAL Investigators . Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 243: 405–412, 2007. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 24.Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-β in experimental diabetic nephropathy in vivo. Diabetes 56: 2982–2989, 2007. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 25.Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-β in fibrotic renal disease in the rat in vivo. Kidney Int 65: 459–468, 2004. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 25a.de Leeuw PW. Postma CT, Spiering W, Kroon AA. Atherosclerotic renal artery stenosis: should we intervene earlier? Curr Hypertens Rep 20: 35, 2018. doi: 10.1007/s11906-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi JH, Annex BH, McCluskey ER, Zioncheck TF. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther 72: 20–32, 2002. doi: 10.1067/mcp.2002.126179. [DOI] [PubMed] [Google Scholar]
- 27.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett 328: 18–26, 2013. doi: 10.1016/j.canlet.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 30.Fliser D, Kronenberg F, Kielstein JT, Morath C, Bode-Böger SM, Haller H, Ritz E. Asymmetric dimethylarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol 16: 2456–2461, 2005. doi: 10.1681/ASN.2005020179. [DOI] [PubMed] [Google Scholar]
- 31.Futrakul N, Butthep P, Futrakul P. Altered vascular homeostasis in chronic kidney disease. Clin Hemorheol Microcirc 38: 201–207, 2008. [PubMed] [Google Scholar]
- 32.Futrakul N, Butthep P, Laohareungpanya N, Chaisuriya P, Ratanabanangkoon K. A defective angiogenesis in chronic kidney disease. Ren Fail 30: 215–217, 2008. doi: 10.1080/08860220701813335. [DOI] [PubMed] [Google Scholar]
- 33.George EM, Liu H, Robinson GG, Mahdi F, Perkins E, Bidwell GL III. Growth factor purification and delivery systems (PADS) for therapeutic angiogenesis. Vasc Cell 7: 1, 2015. doi: 10.1186/s13221-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey TW, Engel JE, Chade AR. Vascular endothelial growth factor and podocyte protection in chronic hypoxia: effects of endothelin-A receptor antagonism. Am J Nephrol 43: 74–84, 2016. doi: 10.1159/000444719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hugo C, Pichler R, Meek R, Gordon K, Kyriakides T, Floege J, Bornstein P, Couser WG, Johnson RJ. Thrombospondin 1 is expressed by proliferating mesangial cells and is up-regulated by PDGF and βFGF in vivo. Kidney Int 48: 1846–1856, 1995. doi: 10.1038/ki.1995.483. [DOI] [PubMed] [Google Scholar]
- 36.Iliescu R, Chade AR. Progressive renal vascular proliferation and injury in obese Zucker rats. Microcirculation 17: 250–258, 2010. doi: 10.1111/j.1549-8719.2010.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant 25: 1079–1087, 2010. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ASTRAL Investigators; Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 39.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Kelsen S, Hall JE, Chade AR. Endothelin-A receptor blockade slows the progression of renal injury in experimental renovascular disease. Am J Physiol Renal Physiol 301: F218–F225, 2011. doi: 10.1152/ajprenal.00089.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kielstein JT, Böger RH, Bode-Böger SM, Frölich JC, Haller H, Ritz E, Fliser D. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol 13: 170–176, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 281: F630–F638, 2001. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 44.Lelongt B, Legallicier B, Piedagnel R, Ronco PM. Do matrix metalloproteinases MMP-2 and MMP-9 (gelatinases) play a role in renal development, physiology and glomerular diseases? Curr Opin Nephrol Hypertens 10: 7–12, 2001. doi: 10.1097/00041552-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295: F1648–F1657, 2008. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Maric-Bilkan C, Flynn ER, Chade AR. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am J Physiol Renal Physiol 302: F308–F315, 2012. doi: 10.1152/ajprenal.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsumura A, Kubota T, Taiyoh H, Fujiwara H, Okamoto K, Ichikawa D, Shiozaki A, Komatsu S, Nakanishi M, Kuriu Y, Murayama Y, Ikoma H, Ochiai T, Kokuba Y, Nakamura T, Matsumoto K, Otsuji E. HGF regulates VEGF expression via the c-Met receptor downstream pathways, PI3K/Akt, MAPK and STAT3, in CT26 murine cells. Int J Oncol 42: 535–542, 2013. doi: 10.3892/ijo.2012.1728. [DOI] [PubMed] [Google Scholar]
- 49.Mihout F, Shweke N, Bigé N, Jouanneau C, Dussaule JC, Ronco P, Chatziantoniou C, Boffa JJ. Asymmetric dimethylarginine (ADMA) induces chronic kidney disease through a mechanism involving collagen and TGF-β1 synthesis. J Pathol 223: 37–45, 2011. doi: 10.1002/path.2769. [DOI] [PubMed] [Google Scholar]
- 50.Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta 1705: 69–89, 2004. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int 63: 543–553, 2003. doi: 10.1046/j.1523-1755.2003.00767.x. [DOI] [PubMed] [Google Scholar]
- 52.Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol 16: 2449–2455, 2005. doi: 10.1681/ASN.2005010076. [DOI] [PubMed] [Google Scholar]
- 53.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis 63: 186–197, 2014. doi: 10.1053/j.ajkd.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Shweke N, Boulos N, Jouanneau C, Vandermeersch S, Melino G, Dussaule JC, Chatziantoniou C, Ronco P, Boffa JJ. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-β activation and cell infiltration. Am J Pathol 173: 631–642, 2008. doi: 10.2353/ajpath.2008.080025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stetler-Stevenson WG. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol 148: 1345–1350, 1996. [PMC free article] [PubMed] [Google Scholar]
- 56.Sulpice E, Ding S, Muscatelli-Groux B, Bergé M, Han ZC, Plouet J, Tobelem G, Merkulova-Rainon T. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell 101: 525–539, 2009. doi: 10.1042/BC20080221. [DOI] [PubMed] [Google Scholar]
- 57.Textor SC, Lerman LO. Reality and renovascular disease: when does renal artery stenosis warrant revascularization? Am J Kidney Dis 63: 175–177, 2014. doi: 10.1053/j.ajkd.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol 10: 2125–2134, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95: 343–353, 2004. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 60.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med 2: a006692, 2012. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoo KH, Thornhill BA, Forbes MS, Coleman CM, Marcinko ES, Liaw L, Chevalier RL. Osteopontin regulates renal apoptosis and interstitial fibrosis in neonatal chronic unilateral ureteral obstruction. Kidney Int 70: 1735–1741, 2006. doi: 10.1038/sj.ki.5000357. [DOI] [PubMed] [Google Scholar]
- 62.Zeineddine D, Hammoud AA, Mortada M, Boeuf H. The Oct4 protein: more than a magic stemness marker. Am J Stem Cells 3: 74–82, 2014. [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol 24: 1854–1859, 2004. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]