Abstract
Network functioning during cognitive tasks is of major interest in Alzheimer's disease (AD). Cognitive functioning in AD includes variable performance in short-term memory (STM). In most studies, the verbal STM functioning in AD patients has been interpreted within the phonological loop subsystem of Baddeley's working memory model. An alternative account considers that domain-general attentional processes explain the involvement of frontoparietal networks in verbal STM beside the functioning of modality-specific subsystems. In this study, we assessed the functional integrity of the dorsal attention network (involved in task-related attention) and the ventral attention network (involved in stimulus-driven attention) by varying attentional control demands in a STM task. Thirty-five AD patients and twenty controls in the seventies performed an fMRI STM task. Variation in load (five versus two items) allowed the dorsal (DAN) and ventral attention networks (VAN) to be studied. ANOVA revealed that performance decreased with increased load in both groups. AD patients performed slightly worse than controls, but accuracy remained above 70% in all patients. Statistical analysis of fMRI brain images revealed DAN activation for high load in both groups. There was no between-group difference or common activation for low compared to high load conditions. Psychophysiological interaction showed a negative relationship between the DAN and the VAN for high versus low load conditions in patients. In conclusion, the DAN remained activated and connected to the VAN in mild AD patients who succeeded in performing an fMRI verbal STM task. DAN was necessary for the task, but not sufficient to reach normal performance. Slightly lower performance in early AD patients compared to controls might be related to maintained bottom-up attention to distractors, to decrease in executive functions, to impaired phonological processing or to reduced capacity in serial order processing.
Keywords: Alzheimer, Attention, Short-term memory, Functional MRI, Brain networks
Highlights
-
•
Patients with early AD succeeded in performing an fMRI short-term memory task.
-
•
Dorsal attention network activation did not differ between patients and controls.
-
•
Dorsal and ventral attention networks remained connected in high load task in AD.
-
•
DAN was necessary for the task, but not sufficient to reach normal performance.
1. Introduction
Memory deficits are frequently the primary cognitive symptoms in Alzheimer's disease (AD). Attention is also impaired in the early stages (Berardi et al., 2005; Finke et al., 2013; Hao et al., 2005; Parasuraman and Nestor, 1993; Park et al., 2012; Perry and Hodges, 1999; Redel et al., 2012; Rizzo et al., 2000), but patients remain able to recruit attention in selected activities (Amieva et al., 2016; Clare et al., 2010; Germain et al., 2019). Attention-related brain networks studied with resting state functional MRI (rsfMRI) were reported to be altered as the AD severity progressed (Zhang et al., 2015). There are two main attention networks. The dorsal attention network (DAN), centered on the intraparietal sulcus (IPS) and connected to the superior and middle frontal gyri, supports top-down voluntary orientation of attention (Asplund et al., 2010; Corbetta and Shulman, 2002). The ventral attention network (VAN), centered on the temporoparietal junction (TPJ) and connected to the orbitofrontal cortex, is involved in bottom-up attraction of attention by salient events (Corbetta et al., 2008; Todd et al., 2005). Neuroimaging studies have shown that the DAN and the VAN have antagonistic effects in short-term memory (STM) tasks (Corbetta et al., 2008; Majerus et al., 2012; Todd et al., 2005; Todd and Marois, 2004). When healthy subjects require attentional control to process increasing STM load, DAN activity is enhanced, whereas the VAN shows progressive deactivation (Majerus et al., 2012). Very few studies have examined the effects of AD on the cerebral activation underlying attention with task-related fMRI. Decreased activity was observed in bilateral parietal and frontal lobes during two visual selective attention tasks (Hao et al., 2005). A recent meta-analysis of task-fMRI cognitive studies in AD showed that hypoactivity compared to control participants mainly affected the default mode (DMN), VAN (such as BA 47), and visual networks (Li et al., 2015). However, increased activity was found in other parts of the DMN (such as the precuneus), VAN (such as BA 44 and BA 40), frontoparietal and somatomotor networks, possibly reflecting compensation processes.
The DAN and the VAN in AD have been more systematically explored with resting-fMRI (Brier et al., 2012). Disease-related decrease of DAN resting functional connectivity was commonly reported (Brier et al., 2012), mainly when mean mini-mental state exam score was lower than 14 (Li et al., 2012; Zhang et al., 2015). Effects on the VAN are less consistent. While studies observed decreased VAN functional connectivity (Qian et al., 2015), relative preservation (notably involving BA 44) was also reported (Li et al., 2012; Zhang et al., 2015). Moreover, the inter-network connectivity between the VAN and the DAN was recently shown to be decreased, while preserved connectivity between the DMN and the DAN was interpreted as compensatory (Li et al., 2013).
We studied the effects of early AD on DAN and VAN functioning using an fMRI paradigm that contrasted both networks in the same short-term memory task (Majerus et al., 2012). In this paradigm, increase of short-term memory load is associated with increased activity of the DAN and decreased activity of the VAN. At the behavioral level, we expected lower response accuracy and slower response times for higher load conditions, and this most strongly in AD patients compared to controls. At the neural level, we based our hypotheses on our previous study in healthy older compared to young volunteers (Kurth et al., 2016) and we anticipated a similar trend between early AD and older controls. Accordingly, we expected preserved DAN activation and lesser VAN reactivity in our mild AD patients compared to controls. We expected maintained interaction between networks in our mild AD patients, as opposed to what may occur in more advanced stages of the disease.
2. Material and methods
2.1. Participants
Probable Alzheimer patients were in a mild stage of the disease (McKhann et al., 2011), with a Mini Mental State score > 20 (Folstein et al., 1975). The diagnosis was clinical, without biomarkers of abnormal protein deposits in the brain. Controls (n = 21), without a history of neuropsychiatric disorders, were recruited from seniors' organizations and among the experimenters' acquaintances. Thirteen of fifty potential participants with AD were excluded because they were unable to perform the task in the scanner. Neuroimaging data from two more patients and one control were discarded due to excessive movement artifacts. Excluded and included patients had similar ages (excluded: 76.5 ± 5.2 years; included: 73.1 ± 8.0 years; t(46) = −1.44, p = .16). The Dementia Rating Scale (Mattis, 1976) score was lower in the excluded group (excluded: 113.08 ± 10.45; included: 122.34 ± 9.6; t(46) = 2.89, p < .05). The remaining groups of 20 healthy controls and 35 patients (Supplementary table 1) were matched for age and education (age, t(53) = 0.16, p = .31; education, t(53) = 0.84, p = .16). On the Dementia Rating Scale, each control participant performed above the cut-off score of 130, and AD patients scored lower than controls (t(53) = 6.99, p < .001). This was true for all sub-scores, apart from construction. There was also a decrease in performance for digit-symbol coding test (Wechsler, 1997) and total recall at Rey auditory verbal learning test (Rey, 1964) in patients (t(53) = 5.1, p < .0001 and t(53) = 7.8, p < .0001 respectively). The study, approved by the Ethics Committee of the University of Liège, was performed in accordance with the Declaration of Helsinki, with participants providing informed written consent. We followed the protocol of our previous study on healthy older participants (Kurth et al., 2016), and no other neuropsychological data was obtained.
2.2. Task description
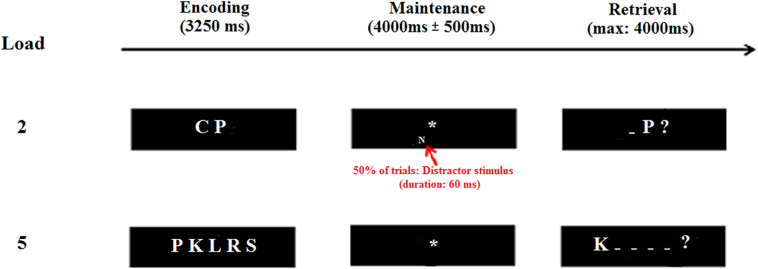
The short-term memory task, which had previously been tested in elderly participants (Kurth et al., 2016), is described in Fig. 1. In the retrieval phase, an array of lines (the number of lines corresponded to the number of consonants presented previously) was presented to the participant. A consonant was displayed in one of the positions of the lines and the participant had to decide, by pressing a yes/no button whether this consonant was presented previously and had occurred in the indicated position. An additional baseline condition consisted of the presentation of a sequence containing two or five identical vowels ordered horizontally, followed by a delay (a fixation star with a variable duration) and a response display showing the same letter in one of the two or five positions. The probe letter was presented in either upper- or lowercase, and participants had to decide whether the case was the same as in the target list. In half of the trials and for each load, a distractor stimulus was presented during the short-term memory maintenance phase. The distractor stimulus was also a letter but presented very briefly and in small and grey font to be just noticeable (Kurth et al., 2016) in order to induce stimulus-driven activity in the VAN; bottom-up attentional capture during controlled task processing usually requires that the distractor stimulus is salient and task-related, but task-irrelevant (Todd et al., 2005). This manipulation allowed us to search for possible bottom-up interference with the ongoing task (Majerus et al., 2012; Todd et al., 2005). The four STM conditions (load two with and without distractor, load five with and without distractor) and the baseline condition were presented in a single session using an event-related design. There were twenty-six trials for each STM condition and twenty trials for the baseline condition. The trials were presented in pseudorandom order, with the restriction that two trials with the same load condition could not be separated by more than five trials with a different condition. Participants were instructed to respond as accurately and quickly as possible, and response accuracy and response times were recorded.
Fig. 1.
Short term memory task. The encoding phase consisted of a sequence of two or five consonants (fixed duration: 3250 ms) followed by the appearance of a star indicating the maintenance phase (variable duration: random Gaussian distribution centered on a mean duration of 4000 ± 500 ms). In the retrieval phase (4000 ms), the participant viewed an array of lines. A consonant was displayed in the position of one of the lines and the participant had to press a yes/no button to indicate whether this consonant had been presented previously and had occurred in the indicated position. In half of the trials and for each load, a distractor stimulus was presented briefly.
2.3. Imaging procedure
Due to a change of scanner during the study, imaging data were acquired on two different machines. Imaging data for nine out of twenty healthy elderly participants and for twelve out of thirty-five AD patients were collected on a 3-Tesla head-only Siemens Allegra scanner (Siemens, Allegra, Erlangen, Germany) with the standard transmit-receive quadrature head coil. T2*-weighted functional images were acquired using a gradient echo planar imaging sequence, with TR = 2040 ms, TE = 30 ms, flip angle = 90°, FoV = 192 × 192 mm2, matrix size = 64 × 64, voxel size = 3 × 3 × 3 mm3. Thirty-four 3-mm thick transverse slices were acquired, with an interslice gap of 25%, covering the whole brain. The first three volumes were discarded to allow for magnetization equilibrium. Gradient-recalled sequences were applied directly after the functional sequences to acquire two complex images with different echo times (TE = 4.92 and 7.38 ms respectively) and generate field maps for echo planar imaging distortion correction. A structural high-resolution T1-weighted image was acquired with the T1-weighted 3D MPRAGE sequence, with TE = 4.35 ms, TR = 1960 ms, TI = 1100 ms, field of view = 230 × 173 mm2, resolution = 256 × 192 × 176, voxel size = 0.9 × 0.9 × 0.9 mm3.
Imaging data for the remaining eleven control participants and twenty-three AD patients were collected on a whole-body 3-Tesla Siemens Prisma scanner operated with a 20-channel receiver head coil (Magnetom Prisma, Siemens Medical Solutions, Erlangen, Germany). Multislice T2*-weighted functional images were acquired with a gradient-EPI sequence using axial slice orientation, covering the whole brain (36 slices, FoV = 216 × 216 mm2, voxel size 3 × 3 x 3 mm3, 25% interslice gap, matrix size 72 × 72 × 36, TR = 2260 ms, TE = 30 ms, flip angle = 90°). The three initial volumes were discarded. A gradient-recalled sequence was applied to acquire two complex images with different echo times (TE = 10.00 and 12.46 ms respectively) and generate field maps for echo planar imaging distortion correction. The other acquisition parameters were TR = 2260 ms, FoV = 192 × 192 mm2, 64 × 64 matrix, 40 transverse slices (3 mm thickness, 25% interslice gap), flip angle = 90°, bandwidth = 260 Hz/pixel. A high-resolution T1-weighted image was acquired (T1-weighted 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence, TR = 1900 ms, TE = 2.19 ms, inversion time (TI) = 900 ms, FoV = 256 × 240 mm2, matrix size = 256 × 240 × 224, voxel size = 1 × 1 × 1 mm3).
With both machines, head movement was minimized using foam pads. Stimuli were displayed on a screen positioned at the rear of the scanner, which participants could see comfortably in a mirror mounted on the standard head coil.
2.4. Functional MRI analysis
2.4.1. Preprocessing
Imaging data were preprocessed and analyzed using the SPM12 toolbox (Wellcome Department of Imaging Neuroscience) implemented in MATLAB 7.12. (Mathworks Inc., Sherborn, MA), as presented in Supplementary data.
2.4.2. Functional analyses
Individual brain responses were estimated at each voxel using a general linear model with epoch- and event-related regressors, as previously described (Kurth et al., 2016). The model assessed sustained activity over all the STM trials as a function of load (five vs. two items), and the epoch regressors ranged from each trial's onset time until the participant's response. It included event-related regressors assessing transient activity associated with distractor stimuli presentation as a function of load. This model explored the overall effects of STM load on the DAN and the VAN, independently of the STM phase. The design matrix included the four conditions described above (load two and five, with or without distractor). The realignment parameters were included as multiple regressors and the design matrix also included nuisance parameters from the artifact reduction software. We essentially searched for brain areas that were more activated in the high load (five items) than in the low load (two items) condition and vice versa. To compare the two groups, the contrast images were entered in second-level analyses, corresponding to two sample t-test random effect models. The scanner type was introduced as a covariate. As a rule, statistical inferences were performed at the voxel level at p < .05 FWE-corrected for multiple comparisons across the entire brain volume using random field theory (Worsley et al., 1996b). Moreover, to directly test hypotheses about DAN and VAN involvement, region of interest analyses were conducted by selecting a 10-mm radius sphere around the averaged published coordinates for locations of interest (Supplementary table 2) and by conducting statistical analysis directly on these regions with additional small volume corrections (Worsley et al., 1996a). To further test the reliability of the statistical significance for group differences in brain activations, we conducted a second–level Bayesian analysis. This analysis provides a probability estimate for the likelihood of a difference in activation size (expressed as a percentage value between 0 and 100) and is not susceptible to multiple comparison problems (Neumann and Lohmann, 2003).
Correlation was searched for between STM and attention performance and activation in attention networks using Pearson statistical test.
2.4.3. Psychophysiological interactions (PPI)
Differential connectivity patterns as a function of high versus low load STM conditions in each group (which represent the main experimental manipulation in this study) were explored. Intraparietal sulcus (IPS) regions were chosen as regions of interest since they belong to the DAN and could be informative about inverse relationships with VAN regions. Individual cerebral activity for the two IPS regions was extracted using a spherical 10-mm radius. A general linear model was used to perform psycho-physiological interaction analyses. At the first analysis level (fixed effect), three regressors were created: (1) psychological condition (high versus low load condition), (2) activity in the seed area, and (3) interaction of interest between the former (psychological) and the latter (physiological) regressor. The contrast images obtained allowed us to determine, for each participant, how the (positive or negative) correlation between the IPS and other brain areas was modulated by the psychological conditions. The contrast images were used at the second level (random-effect analysis) for between-group comparisons. The scanner type was introduced as a covariate. All consistent psycho-physiological interaction results are presented at a cluster level (built from a voxel level p < .001 uncorrected threshold) with a threshold of p < .05 FWE-corrected for multiple comparisons (Worsley et al., 1996b). Methodological considerations are provided as Supplementary data.
3. Results
3.1. Behavioral data
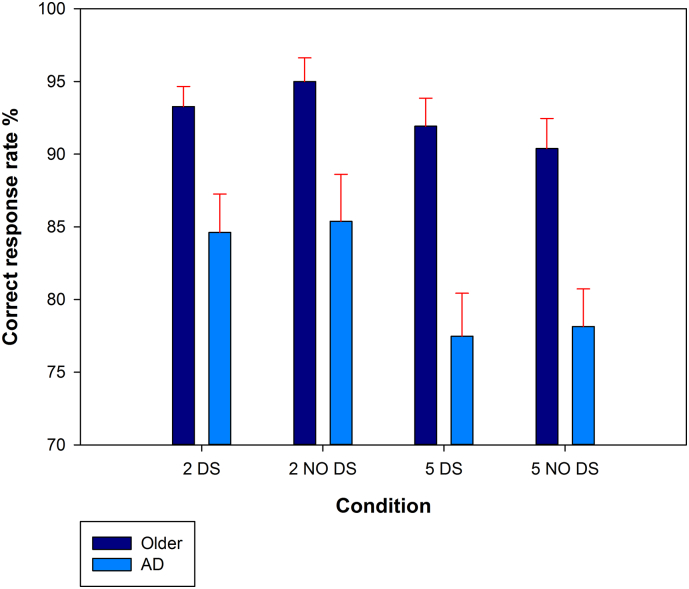
Response accuracy was assessed via a 2 (STM: two, five) by 2 (Distractor: present or absent) by 2 (Group: AD, control) ANOVA, with load and distractor stimulus as repeated-measure factors. The results revealed a main effect of group (F(1,53) = 10.51, p < .05, η2p = 0.17), with controls producing more correct responses than patients (Fig. 2). However, patients had >75% mean correct responses. There was a main effect of load (F(1,53) = 9.04, p < .05, η2p = 0.15), with better performance in the low than the high load condition, but no load by group interaction (F(1,53) = 1.55, p = .22, η2p = 0.03). No significant effect of distractor (F(1,53) = 0.24, p = 0.63, η2p = 0.004) or distractor by group interaction (F(1,53) = 0.14, p = .71, η2p = 0.001) was observed. There was no load by distractor interaction (F(1,53) = 1.03, p = .31, η2p = 0.02) or triple interaction (F(1,53) = 0.90, p = .35, η2p = 0.02).
Fig. 2.
Accuracy scores. Correct responses in short-term memory task, for two and five items, with or without (NO) distractor stimulus (DS).
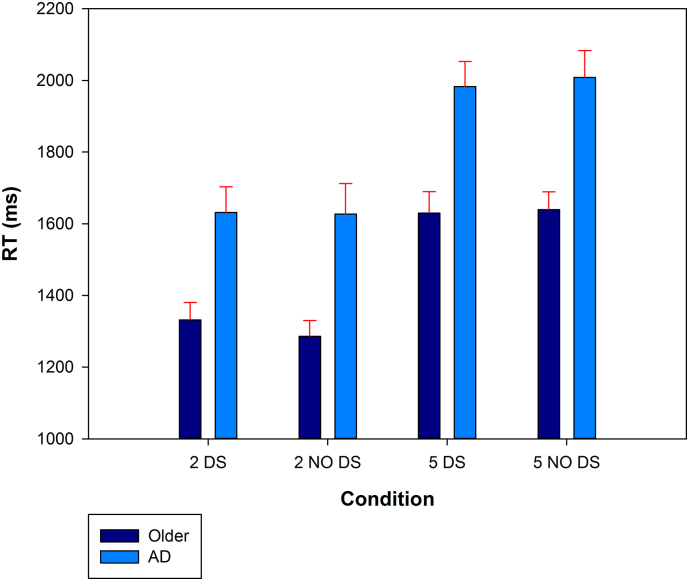
Response times for correct responses were entered in a 2 (STM load) by 2 (Distractor) by 2 (Group) ANOVA and revealed a main effect of group (F(1,53) = 11.42, p < .05, η2p = 0.18), with controls being significantly faster than AD patients; and a main effect of load (F(1,53) = 139.05, p < .001, η2p = 0.72), with low load items being processed faster than high load ones (Fig. 3). There was no load by group interaction (F(1,53) = 0.47, p = .5, η2p = 0.01). There was no main effect of distractor (F(1,53) = 0.05, p = .8, η2p = 0.001), and no interaction with the group (F(1,53) = 0.74, p = .4, η2p = 0.01). Finally, there was no load by distractor interaction (F(1,53) = 2.56, p = .12, η2p = 0.05) or triple interaction (F(1,53) = 0.22, p = .64, η2p = 0.001). Previous studies have shown that healthy controls are sensitive to distractors (Kurth et al., 2016; Majerus et al., 2012). We analyzed the distractor by load interaction separately for each group. Such an interaction was observed for reaction times (but not response accuracy) only in the healthy controls, (F(1,19) = 4.91, p < .05, η2p = 0.21); it was not significant in the AD group (F(1,34) = 1.65, p = .43, η2p = 0.02).
Fig. 3.
Reaction times. Reaction time in milliseconds (ms) in short-term memory task, for two and five items, with or without (NO) distractor stimulus (DS).
3.2. Imaging data
3.2.1. Effects of STM load
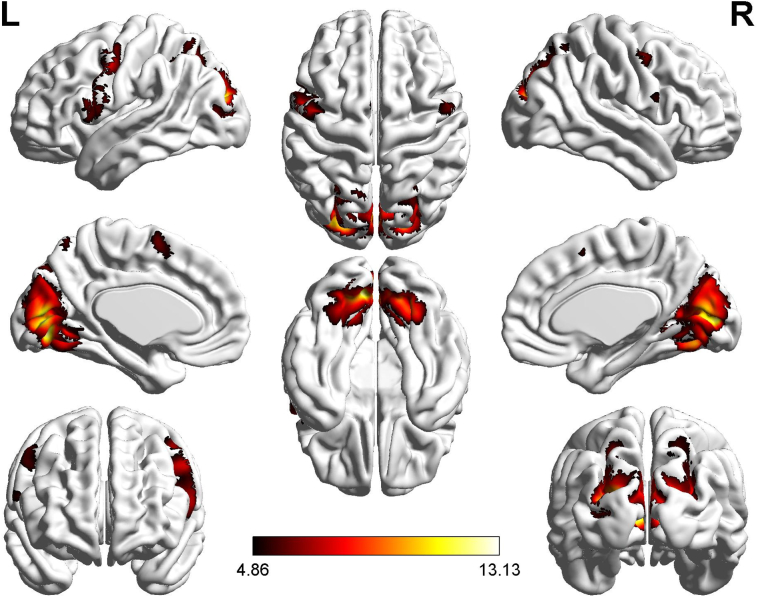
The first comparison (high > low load) explored DAN activation with increasing load. No between-group difference was observed. This was confirmed by a second-level Bayesian analysis testing group differences in regions of the DAN. For all the selected regions of interest (right and left IPS, right and left superior frontal gyrus, right middle frontal gyrus), log evidence fell between −3 and 3 and posterior probability was between 51.3% and 83.8%. To determine the load effect (five > two) common to both groups, a (null) conjunction analysis was performed. This showed common positive effects of STM load in a large set of regions extending from the occipital lobe to the superior parietal lobule, including the bilateral IPS. Other regions typically activated during a STM task (supplementary motor area, precentral frontal areas) were also activated (Table 1 and Fig. 4). A region of interest analysis confirmed activation in DAN regions for the high versus low load condition.
Table 1.
Results of the conjunction analysis for high versus low short-term memory load contrast (five versus two letters) common to the control and patient groups.
| Contrast |
Side | Cluster voxels | Z-value | MNI stereotaxic coordinates |
||
|---|---|---|---|---|---|---|
| Conjunction AD and control 5 > 2 | X | Y | Z | |||
| Precentral gyrus | L | 2181 | 6.59 | −54 | −2 | 42 |
| 6.43 | −54 | 8 | 16 | |||
| 5.45 | −44 | 0 | 30 | |||
| R | 460 | 5.32 | 54 | 0 | 48 | |
| 4.98 | 52 | 4 | 22 | |||
| 4.80 | 62 | 8 | 18 | |||
| SMA | L | 400 | 5.71 | −4 | 6 | 58 |
| 4.87 | −4 | 2 | 76 | |||
| Lingual gyrus | L | 14,970 | Inf | −8 | −80 | 4 |
| R | 14,970 | Inf | 10 | −86 | 6 | |
| Thalamus | R | 42 | 4.94 | 24 | −26 | 2 |
| Putamen | L | 108 | 4.75 | −20 | −8 | 10 |
| 4.61 | −18 | 2 | 10 | |||
| Hippocampus | L | 23 | 4.73 | −28 | −24 | −2 |
| Globus pallidus | R | 40 | 4.67 | 22 | −8 | 4 |
| Insular gyrus | L | 32 | 4.58 | −30 | 14 | 8 |
| 4.40 | −32 | 26 | 4 | |||
| MFG | R | 362 | 4.84 | 48 | 0 | 40 |
| 4.58 | 48 | 2 | 26 | |||
| SFG | R | 85 | 3.62 | 34 | −4 | 54 |
| L | 289 | 4.71 | −30 | −2 | 44 | |
| IPS | R | 476 | 5.24 | 24 | −60 | 54 |
| 4.92 | 24 | −64 | 40 | |||
| IPS | L | 511 | 5.61 | −22 | −64 | 48 |
| 5.18 | −28 | −48 | 44 | |||
MNI: Montreal Neurological Institute. AD: Alzheimer's disease. SMA: supplementary motor area. L: left. R: right. MFG: middle frontal gyrus. SFG: superior frontal gyrus. IPS: intraparietal sulcus. Expected activations in the dorsal attention network are highlighted in bold type. (p < .05 FWE-corrected).
Fig. 4.
Conjunction analysis (older controls and Alzheimer patients). Conjunction for five > two items short-term memory task. Z-values reflecting significant activation are represented according to a color scale, superimposed on a structural MRI image.
The reverse comparison (low > high load) explored the deactivation of VAN regions with increasing load. There was no between-group difference, and this was confirmed by a second-level Bayesian analysis testing group differences in regions of the VAN. For all tested regions of interest (right and left TPJ, right and left orbitofrontal cortex), the log evidence was between −3 and 3 and posterior probability was between 53.2% and 64.0%. A conjunction analysis was also performed but elicited no significant activation. To better understand the non-significant conjunction analysis, results were examined for each group separately. In the controls, the expected inverse effect of STM load was observed. The angular gyrus was increasingly deactivated and region of interest analysis confirmed deactivation of the left TPJ with increasing load (Table 2). In the AD group, the contrast did not involve significant activation in any region.
Table 2.
Whole brain (p < .05 FWE-corrected) and results from small volume corrections (in bold) for low vs. high short-term memory load contrast.
| Contrast |
Side | Cluster voxels | Z-value | MNI stereotaxic coordinates |
||
|---|---|---|---|---|---|---|
| Controls 2 > 5 | X | Y | Z | |||
| Angular gyrus | L | 226 | 4.59 | −46 | −74 | 38 |
| −52 | −68 | 32 | ||||
| TPJ | L | 8 | 3.24 | −52 | −60 | 28 |
MNI: Montreal Neurological Institute. TPJ: temporoparietal junction. L: left. Activation was observed in the ventral attention network. Neither significant activation in the Alzheimer's group nor a significant between-group difference was observed.
When individual values of DAN activation were plotted, there was no over-activation in AD participants. The values were all in the same range in controls and patients.
None of the correlations between scores at fMRI STM task or attention tests outside the scanner and activation in attention networks was significant in our AD patients.
3.2.2. Effects of distractor stimulus
For the low load condition, the presence of a distractor did not lead to group differences in brain activity. The conjunction analysis did not provide significant results either. A separate analysis of distractor effect in each group showed that controls activated a large set of VAN regions (bilateral TPJ and orbitofrontal cortex) (Supplementary table 3) while AD patients did not show significant activation. When a distractor was present in the high load condition, there was no group difference and the conjunction analysis revealed common activation in TPJ regions and the precentral cortex (Supplementary table 4).
We then compared the two load conditions. When exploring the effects of distractor in the low > high load condition, there was no between-group difference, a result confirmed by a second-level Bayesian analysis testing group differences in VAN regions of interest for this contrast. For all tested regions of interest (right and left TPJ, right and left orbitofrontal cortex), the log evidence fell between −3 and 3 and posterior probability was between 55.5% and 79.7%. A conjunction analysis was also performed but elicited no significant activation, nor was there significant activation for this contrast in either group analyzed separately.
The reverse comparison exploring the effect of distractor in the high > low load conditions did not elicit any between-group difference. This result was confirmed by a Bayesian analysis testing differences in VAN regions of interest. For all the regions of interest (right and left TPJ, right and left orbitofrontal cortex) tested, the log evidence was between −3 and 3 and posterior probability was between 55.5% and 79.7%. A conjunction analysis elicited no significant activation. When analyzing each group's results separately, no significant activation was seen in the control group. In the AD, increased activity was observed in the angular gyrus for the high > low condition and region of interest analyses showed right TPJ activation. The superior frontal gyrus and cerebellum were also activated in the AD group for this contrast (Supplementary table 5).
3.2.3. Psychophysiological interactions
We performed a psychophysiological interaction analysis to test whether the functional connectivity previously observed in older adults (Kurth et al., 2016) between DAN regions (left and right IPS) and VAN regions in high > low load condition was still present in AD. There was no between-group difference, and the conjunction analysis did not provide significant results. In the healthy older adults, seed regions (left and right IPS) were negatively correlated with left inferior parietal regions (classically involved in STM tasks) in high > low load condition (Table 3). In the AD group, there was a negative correlation between a right IPS seed region and a right TPJ region belonging to the VAN in high > low load condition, suggesting an interaction between the DAN and the VAN in AD.
Table 3.
Psychophysiological interactions in the high versus low load condition.
| Group | Relation | Seed | Region | Cluster voxels | Z-value | MNI stereotaxic coordinates |
||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Control | Negative | lIPS | lInf parietal | 177 | 4.09 | −40 | −44 | 50 |
| 3.59 | −46 | −38 | 38 | |||||
| Control | Negative | rIPS | lInf parietal | 182 | 3.62 | −46 | −40 | 44 |
| 3.61 | −48 | −42 | 52 | |||||
| AD | Negative | rIPS | rInf frontal | 77 | 4.61 | 44 | 36 | 8 |
| lMid occ | 257 | 3.94 | −28 | −72 | 38 | |||
| lSup occ | 257 | 3.61 | −22 | −72 | 26 | |||
| lTPJ | 5 | 3.36 | −46 | −58 | 20 | |||
AD: Alzheimer's disease. lIPS: left intraparietal sulcus. rIPS: right intraparietal sulcus. Inf: inferior. Mid: middle. Sup: superior. Occ: occipital. lTPJ: left temporoparietal junction. Regions displaying negative interactions with the left or right intraparietal sulcus seeds are presented. The result of region of interest analysis (small volume correction) is presented in bold. No significant regional positive interaction with the seed regions was observed.
4. Discussion
Despite extensive research, the nature of the neural networks supporting our ability to temporarily maintain verbal information remains a matter of controversy. From neuroimaging studies in healthy young participants, we know that verbal STM tasks yield a consistent pattern of fronto-parietal activation, including the bilateral dorsal prefrontal, middle and superior frontal, premotor and supplementary motor areas, as well as parietal, superior temporal and cerebellar regions (Linden et al., 2003; Majerus et al., 2006; Paulesu et al., 1993; Salmon et al., 1996; Ungerleider et al., 1998)
The present study explored the effects of early AD on the DAN and the VAN and their interactions during a STM task. Behaviorally, across-participants decreased performance and increased reaction times with increasing STM load were observed as expected (Kurth et al., 2016; Majerus et al., 2012). AD participants performed slightly worse and were slower than the controls in the fMRI environment, but the accuracy remained above 70% of correct responses in all patients, suggesting that their top-down and bottom-up attentional control abilities were sufficient to reach this performance in our study. Regarding neuroimaging, increased activation was observed in the DAN (such as in BA7 and BA6) and other STM-related regions in a conjunction analysis of “high > low load condition” in controls and AD participants, in keeping with previous studies with young and healthy older volunteers (Kurth et al., 2016; Majerus et al., 2012; Todd et al., 2005). There was no group difference, suggesting that DAN activation is relatively preserved in early AD. There was no hyper-activation either in AD participants, showing that the results were not driven by a subgroup of high performers in patients.
The reverse comparison (low > high load) elicited no group difference but no common activation either. The expected VAN deactivation (BA37) with high load was observed in controls. In AD, deactivation was not important enough in any VAN region to reach significance. A tentative explanation is that VAN remained sensitive to distractor stimuli in the high load condition, suggesting, at the neural level, that AD participants could not prevent VAN to direct bottom-up attention to distractors.
Psychophysiological analyses also showed no between-group difference in functional connectivity measures. When each group was analyzed separately, AD patients showed a “classical” negative interaction between the IPS and a TPJ region (Fox et al., 2006; Majerus et al., 2012), suggesting that the DAN and the VAN still (inversely) interact.
Previous researches on neuroimaging of STM in AD used quite variable designs that differed according to tasks, number of participants and their disease stage, and threshold applied to statistical analyses. There are reports of increased or decreased brain activation, frequently located in executive, episodic memory or modality-specific networks and sometimes in attentional networks. More specifically, hyperactivation in regions involved in STM was first suggested in an early study of verbal STM using positron emission tomography (PET) in a sample of seven AD participants compared to controls (Becker et al., 1996). Another PET study in AD used a delayed match to sample paradigm where delays varied from 1 to 16 s (Grady et al., 2001). The main result was an increase of activity in the right prefrontal cortex and in the left amygdala that was correlated with better performance in the patients. Such a correlation is consistent with a compensatory process when activity is preserved or increased in AD participants. Subsequent studies used fMRI. In a delay match to sample task, there was higher activation in the parietal and frontal lobes in MCI participants compared to healthy controls during the maintenance phase (Bokde et al., 2010). However, it was hippocampal activity that correlated to response times during the recall phase in this MCI group. Decreased activity in AD was reported during a 1-back STM task in the left frontal pole, the left ventrolateral prefrontal cortex and the right premotor cortex, while increased activation was shown in the precuneus compared to the healthy controls (Lim et al., 2008). Other studies reported correlations between STM performance and brain regions in AD. Verbal span in STM correlated to premotor glucose metabolism while visual span correlated with parieto-occipital metabolism (Collette et al., 1997). Spatial span forward performance was related to bilateral precentral sulcus and parieto-occipital thinning in AD (Foxe et al., 2016). Accordingly, working memory performance correlated with glucose metabolism in left-sided temporoparietal regions (Kobylecki et al., 2018). On the other hand, visual STM performance correlated with cortical thinning along the long axis of the MTL and associated cortical nodes of anterior and posterior MTL networks (Das et al., 2016) and decreased hippocampal volume was significantly associated with deficits in short term object-location binding in familial AD (Liang et al., 2016). In summary, there is a complex distribution of regional activity related to STM task in AD, and some regions pertaining to attentional networks may show high activation. As expected, many different regions (underlying different cognitive processes) are correlated with STM performance, including regions pertaining to the DAN.
In our mild AD patients who were still able to perform the STM task with high accuracy (even though below controls' level), preserved activation in DAN regions when task demand increased seems to temper reports of decreased frontoparietal activation in previous task fMRI studies (Hao et al., 2005) and of decreased DAN functional connectivity in resting fMRI studies (Li et al., 2012; Qian et al., 2015; Zhang et al., 2015). Our results suggests that mild AD patients could maintain DAN activity within the normal range. AD's effects on the VAN have been less studied in the task fMRI literature. Most resting fMRI studies have reported decreased functional connectivity in the VAN (Qian et al., 2015; Redel et al., 2012), although it was also shown to be relatively preserved (Li et al., 2012; Zhang et al., 2015). We found that controls and mild AD patients recruited the VAN and we observed no significant between-group differences, but AD participants maintained VAN activation for distractors in high load. This was consistent with a lesser VAN deactivation in high load previously observed in older healthy participants (Kurth et al., 2016). Psychophysiological analyses suggested that the dorsal and ventral attention networks could still functionally interact in AD, in keeping with a previous resting fMRI study where connectivity was similar in AD and control participants (Zhang et al., 2015). Disrupted VAN and DAN connectivity but increased DAN and DMN connectivity was reported in a recent resting fMRI study, but the patients were at a more advanced stage of AD (Li et al., 2013). In our healthy controls, the IPS interacted negatively with an anterior parietal region in which activation may reflect distractor stimulus processing, since it has been shown to be sensitive to bottom-up attention driven by stimulus salience (Geng and Mangun, 2009).
The discrepancies between our results and the literature could be explained by the use of heterogeneous tasks (Li et al., 2015) and patients' differing disease stages. Indeed, while our patients were in the mild stages of AD (Mini Mental State exam >20), the mean Mini Mental State exam score for patients in previous studies was 18 (Hao et al., 2005) or ranged from 18 to 27 (Li et al., 2015). The Mini Mental State exam score was even lower (12 to 13) in some resting fMRI studies (Li et al., 2012; Zhang et al., 2015). Accordingly, the breakdown in attention networks may not be as great in our mild AD patients as in people at more advanced disease stages.
Although attentional networks were recruited in our AD patients, possibly allowing to maintain performance above 70% accuracy in all participants, decreased STM performance compared to controls was nevertheless observed and might have several neural correlates and underlying cognitive causes. Our first explanation is that VAN activity (directed to bottom-up stimuli) is maintained in the high load condition. Alternately, we specifically examined encoding and retrieval of words in STM. In a previous study (Peters et al., 2009). AD patients showed reduced activation in frontal regions more anterior than in the current study (BA10 and BA44) suggesting that executive control processes (and more specifically integration and coordination processes) most probably underlaid reduced STM performance in AD patients (Baddeley et al., 1991; Belleville et al., 1996). Decreased activation around the left supramarginal gyrus (B40, much more anterior than activation in the current study) suggested that altered phonological processes could further contribute to verbal STM difficulties (Collette et al., 1999; Stopford et al., 2012). At the same time, AD patients showed increased activation in medial temporal lobe, suggesting that they might recruit alternative recognition mechanisms when performing the STM task (Peters et al., 2009). A recent study reported an impaired ability to bind item information to serial position within working memory in AD patients compared to controls (De Belder et al., 2017). The authors emphasized that spatial attention is crucially involved during item localization and retrieval in working memory.
We followed a previous fMRI protocol designed for healthy elderly volunteers, and a main limitation in this study is the lack of an extensive neuropsychological evaluation of working memory and attention in our participants.
In conclusion, we could not find significantly different activation of two key attention networks between mild AD patients and controls performing a STM task. DAN activation was maintained to perform the task, but was not sufficient to reach normal performance. In each group, DAN and VAN regions interacted during task performance. These results contrast with a common pessimistic view of profound network alteration in the literature and argue that attentional resources remain available in patients with early-stage Alzheimer's disease.
Acknowledgements/conflicts/funding sources
The authors have no conflict of interest. Funding sources were the Concerted Research Action 12/17-0 (University of Liège), InterUniversity Attraction Pole 7/11 (Belgium), and F.R.S.-FNRS (Belgium). SM and CP are Senior Research Associates at F.R.S.-FNRS, CB is Research Associate at F.R.S.-FNRS, FC is Research Director at F.R.S.-FNRS.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101892.
Appendix A. Supplementary data
Supplementary material
References
- Amieva H., Robert P.H., Grandoulier A.S., Meillon C., De Rotrou J., Andrieu S. Group and individual cognitive therapies in Alzheimer's disease: the ETNA3 randomized trial. Int. Psychogeriatr. 2016;28:707–717. doi: 10.1017/S1041610215001830. [DOI] [PubMed] [Google Scholar]
- Asplund C.L., Todd J.J., Snyder A.P., Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat. Neurosci. 2010;13:507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A.D., Bressi S., Della Sala S., Logie R., Spinnler H. The decline of working memory in Alzheimer's disease. a longitudinal study. Brain. 1991;114(Pt 6):2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- Becker J.T., Mintun M.A., Aleva K., Wiseman M.B., Nichols T., Dekosky S.T. Alterations in functional neuroanatomical connectivity in Alzheimer's disease. Positron emission tomography of auditory verbal short-term memory. Ann. N. Y. Acad. Sci. 1996;777:239–242. doi: 10.1111/j.1749-6632.1996.tb34425.x. [DOI] [PubMed] [Google Scholar]
- Belleville S., Peretz I., Malenfant D. Examination of the working memory components in normal aging and in dementia of the Alzheimer type. Neuropsychologia. 1996;34:195–207. doi: 10.1016/0028-3932(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Berardi A.M., Parasuraman R., Haxby J.V. Sustained attention in mild Alzheimer's disease. Dev. Neuropsychol. 2005;28:507–537. doi: 10.1207/s15326942dn2801_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde A.L., Karmann M., Born C., Teipel S.J., Omerovic M., Ewers M. Altered brain activation during a verbal working memory task in subjects with amnestic mild cognitive impairment. J. Alzheimers Dis. 2010;21:103–118. doi: 10.3233/JAD-2010-091054. [DOI] [PubMed] [Google Scholar]
- Brier M.R., Thomas J.B., Snyder A.Z., Benzinger T.L., Zhang D., Raichle M.E. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J. Neurosci. 2012;32:8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare L., Linden D.E., Woods R.T., Whitaker R., Evans S.J., Parkinson C.H. Goal-oriented cognitive rehabilitation for people with early-stage Alzheimer disease: a single-blind randomized controlled trial of clinical efficacy. Am. J. Geriatr. Psychiatry. 2010;18:928–939. doi: 10.1097/JGP.0b013e3181d5792a. [DOI] [PubMed] [Google Scholar]
- Collette F., Salmon E., Van der Linden M., Degueldre C., Franck G. Functional anatomy of verbal and visuospatial span tasks in Alzheimer's disease. Hum. Brain Mapp. 1997;5:110–118. doi: 10.1002/(sici)1097-0193(1997)5:2<110::aid-hbm4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Collette F., Van der Linden M., Bechet S., Salmon E. Phonological loop and central executive functioning in Alzheimer's disease. Neuropsychologia. 1999;37:905–918. doi: 10.1016/s0028-3932(98)00148-1. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.R., Mancuso L., Olson I.R., Arnold S.E., Wolk D.A. Short-term memory depends on dissociable medial temporal lobe regions in amnestic mild cognitive impairment. Cereb. Cortex. 2016;26:2006–2017. doi: 10.1093/cercor/bhv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Belder M., Santens P., Sieben A. W. F. impaired processing of serial order determines working memory impairments in Alzheimer's disease. J. Alzheimers Dis. 2017;59:1171–1186. doi: 10.3233/JAD-170193. [DOI] [PubMed] [Google Scholar]
- Finke K., Myers N., Bublak P., Sorg C. A biased competition account of attention and memory in Alzheimer's disease. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013;368 doi: 10.1098/rstb.2013.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe D., Leyton C.E., Hodges J.R., Burrell J.R., Irish M., Piguet O. The neural correlates of auditory and visuospatial span in logopenic progressive aphasia and Alzheimer's disease. Cortex. 2016;83:39–50. doi: 10.1016/j.cortex.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Geng J.J., Mangun G.R. Anterior intraparietal sulcus is sensitive to bottom-up attention driven by stimulus salience. J. Cogn. Neurosci. 2009;21:1584–1601. doi: 10.1162/jocn.2009.21103. [DOI] [PubMed] [Google Scholar]
- Germain S., Wojtasik V., Lekeu F., Quittre A., Olivier C., Godichard V. Efficacy of cognitive rehabilitation in Alzheimer disease: a 1-year follow-up study. J. Geriatr. Psychiatry Neurol. 2019;32:16–23. doi: 10.1177/0891988718813724. [DOI] [PubMed] [Google Scholar]
- Grady C.L., Furey M.L., Pietrini P., Horwitz B., Rapoport S.I. Altered brain functional connectivity and impaired short-term memory in Alzheimer's disease. Brain. 2001;124:739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- Hao J., Li K., Li K., Zhang D., Wang W., Yang Y. Visual attention deficits in Alzheimer's disease: an fMRI study. Neurosci. Lett. 2005;385:18–23. doi: 10.1016/j.neulet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Kobylecki C., Haense C., Harris J.M., Stopford C.L., Segobin S.H., Jones M. Functional neuroanatomical associations of working memory in early-onset Alzheimer's disease. Int. J. Geriatr. Psychiatr. 2018;33:176–184. doi: 10.1002/gps.4703. [DOI] [PubMed] [Google Scholar]
- Kurth S., Majerus S., Bastin C., Collette F., Jaspar M., Bahri M.A. Effects of aging on task- and stimulus-related cerebral attention networks. Neurobiol. Aging. 2016;44:85–95. doi: 10.1016/j.neurobiolaging.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Li R., Wu X., Fleisher A.S., Reiman E.M., Chen K., Yao L. Attention-related networks in Alzheimer's disease: a resting functional MRI study. Hum. Brain Mapp. 2012;33:1076–1088. doi: 10.1002/hbm.21269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Wu X., Chen K., Fleisher A.S., Reiman E.M., Yao L. Alterations of directional connectivity among resting-state networks in Alzheimer disease. AJNR Am. J. Neuroradiol. 2013;34:340–345. doi: 10.3174/ajnr.A3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.J., Hou X.H., Liu H.H., Yue C.L., He Y., Zuo X.N. Toward systems neuroscience in mild cognitive impairment and Alzheimer's disease: a meta-analysis of 75 fMRI studies. Hum. Brain Mapp. 2015;36:1217–1232. doi: 10.1002/hbm.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Pertzov Y., Nicholas J.M., Henley S.M.D., Crutch S., Woodward F. Visual short-term memory binding deficit in familial Alzheimer's disease. Cortex. 2016;78:150–164. doi: 10.1016/j.cortex.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.K., Juh R., Pae C.U., Lee B.T., Yoo S.S., Ryu S.H. Altered verbal working memory process in patients with Alzheimer's disease: an fMRI investigation. Neuropsychobiology. 2008;57:181–187. doi: 10.1159/000147471. [DOI] [PubMed] [Google Scholar]
- Linden D.E., Bittner R.A., Muckli L., Waltz J.A., Kriegeskorte N., Goebel R. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. Neuroimage. 2003;20:1518–1530. doi: 10.1016/j.neuroimage.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Majerus S., Poncelet M., Van der Linden M., Albouy G., Salmon E., Sterpenich V. The left intraparietal sulcus and verbal short-term memory: focus of attention or serial order? Neuroimage. 2006;32:880–891. doi: 10.1016/j.neuroimage.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Majerus S., Attout L., D'Argembeau A., Degueldre C., Fias W., Maquet P. Attention supports verbal short-term memory via competition between dorsal and ventral attention networks. Cereb. Cortex. 2012;22:1086–1097. doi: 10.1093/cercor/bhr174. [DOI] [PubMed] [Google Scholar]
- Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellak L., Karasu T.B., editors. Geriatric Psychiatry: Grune & Stratton. 1976. pp. 77–121. [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J., Lohmann G. Bayesian second-level analysis of functional magnetic resonance images. Neuroimage. 2003;20:1346–1355. doi: 10.1016/S1053-8119(03)00443-9. [DOI] [PubMed] [Google Scholar]
- Parasuraman R., Nestor P. Attention and driving. Assessment in elderly individuals with dementia. Clin. Geriatr. Med. 1993;9:377–387. [PubMed] [Google Scholar]
- Park M., Hood M.M., Shah R.C., Fogg L.F., Wyatt J.K. Sleepiness, parkinsonian features and sustained attention in mild Alzheimer's disease. Age Ageing. 2012;41:765–770. doi: 10.1093/ageing/afs084. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Frith C.D., Frackowiak R.S. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Perry R.J., Hodges J.R. Attention and executive deficits in Alzheimer's disease. A critical review. Brain. 1999;122(Pt 3):383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Peters F., Collette F., Degueldre C., Sterpenich V., Majerus S., Salmon E. The neural correlates of verbal short-term memory in Alzheimer's disease: an fMRI study. Brain. 2009;132:1833–1846. doi: 10.1093/brain/awp075. [DOI] [PubMed] [Google Scholar]
- Qian S., Zhang Z., Li B., Sun G. Functional-structural degeneration in dorsal and ventral attention systems for Alzheimer's disease, amnestic mild cognitive impairment. Brain Imag. Behav. 2015;9:790–800. doi: 10.1007/s11682-014-9336-6. [DOI] [PubMed] [Google Scholar]
- Redel P., Bublak P., Sorg C., Kurz A., Forstl H., Muller H.J. Deficits of spatial and task-related attentional selection in mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2012;33(195):e27–e42. doi: 10.1016/j.neurobiolaging.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Rey A. Presses Universitaires de France; Paris: 1964. L'examen Clinique en Psychologie. [Google Scholar]
- Rizzo M., Anderson S.W., Dawson J., Myers R., Ball K. Visual attention impairments in Alzheimer's disease. Neurology. 2000;54:1954–1959. doi: 10.1212/wnl.54.10.1954. [DOI] [PubMed] [Google Scholar]
- Salmon E., Van der Linden M., Collette F., Delfiore G., Maquet P., Degueldre C. Regional brain activity during working memory tasks. Brain. 1996;119(Pt 5):1617–1625. doi: 10.1093/brain/119.5.1617. [DOI] [PubMed] [Google Scholar]
- Stopford C.L., Thompson J.C., Neary D., Richardson A.M., Snowden J.S. Working memory, attention, and executive function in Alzheimer's disease and frontotemporal dementia. Cortex. 2012;48:429–446. doi: 10.1016/j.cortex.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Todd J.J., Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Todd J.J., Fougnie D., Marois R. Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychol. Sci. 2005;16:965–972. doi: 10.1111/j.1467-9280.2005.01645.x. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G., Courtney S.M., Haxby J.V. A neural system for human visual working memory. Proc. Natl. Acad. Sci. U. S. A. 1998;95:883–890. doi: 10.1073/pnas.95.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1997. WAIS-3: Wechsler Adult Intelligence Scale: Administration and Scoring Manual: Psychological Corporation. [Google Scholar]
- Worsley K.J., Marrett S., Neelin P., Evans A.C. Searching scale space for activation in PET images. Hum. Brain Mapp. 1996;4:74–90. doi: 10.1002/(SICI)1097-0193(1996)4:1<74::AID-HBM5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Marrett S., Neelin P., Vandal A.C., Friston K.J., Evans A.C. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zheng H., Liang K., Wang H., Kong S., Hu J. Functional degeneration in dorsal and ventral attention systems in amnestic mild cognitive impairment and Alzheimer's disease: an fMRI study. Neurosci. Lett. 2015;585:160–165. doi: 10.1016/j.neulet.2014.11.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material