Abstract
d-Glucosamine (GlcNH2) and several of its derivatives are known to possess immunosuppressive activities in various immune cell lines. The novel GlcNH2-containing oligosaccharide Galα1-6GlcNH2 (designated melibiosamine; MelNH2) is expected to be immunosuppressive also. In Jurkat cells (immortalized human T lymphocytes), interleukin 2 (IL-2) production (an index of the T-cell immune response) can be induced by stimulation with a mitogen, such as concanavalin A. Here, we compared the effects of GlcNH2 and MelNH2 on concanavalin A-induced IL-2 production (CIIP) in Jurkat cells and found that GlcNH2 and MelNH2 at millimolar levels both significantly suppressed CIIP without affecting cell viability. When we examined the effects of GlcNH2 and MelNH2 on the activation of the three transcription factors required for CIIP—NFAT (nuclear factor of activated T-cells), NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells), and AP-1 (activator protein 1)—we found that GlcNH2 and MelNH2 both suppressed CIIP by inhibiting the activation of NFAT and NFκB, but, unlike GlcNH2, MelNH2 also promoted the activation of AP-1. These results suggest that MelNH2 may be a potentially useful lead compound for development as an immunosuppressive or anti-inflammatory drug.
Keywords: Glucosamine, Melibiosamine, Interleukin 2, Immunosuppressive drug, Jurkat cell
Abbreviations: AP-1, activator protein 1; CIIP, ConA-induced IL-2 production; ConA, concanavalin A; CsA, cyclosporine A; GlcNH2, glucosamine; IL-2, interleukin-2; IM, ionomycin; MelNH2, melibiosamine; NFAT, nuclear factor of activated T-cells; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PIIP, PMA/IM-induced IL-2 production; PMA, phorbol 12-myristate 13-acetate
Highlights
-
•
Immunosuppressive effects of MelNH2 (Galα1-6GlcNH2) were examined in Jurkat cells.
-
•
Concanavalin A induces IL-2 production in Jurkat cells.
-
•
MelNH2 at millimolar levels dose-dependently suppressed ConA-induced IL-2 production.
-
•
MelNH2 inhibited the activation of NFAT and NFκB, which control IL-2 expression.
1. Introduction
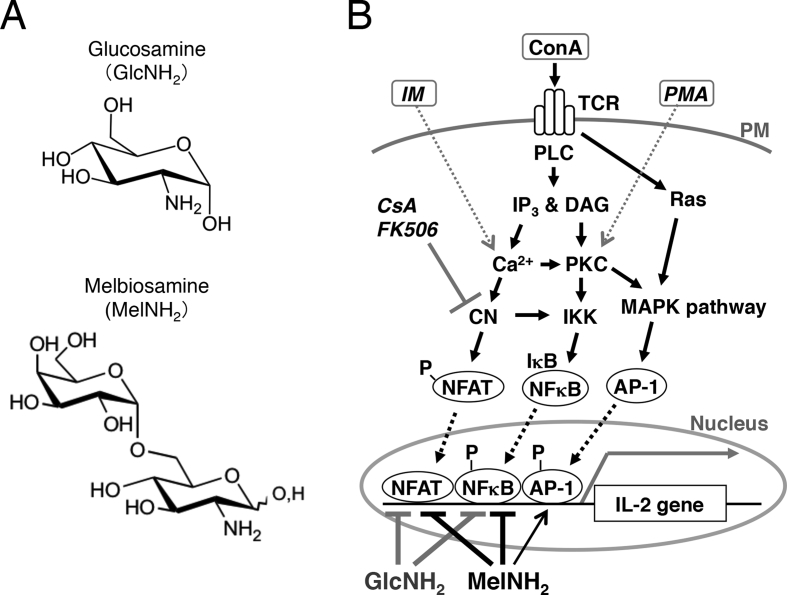
Interleukin 2 (IL-2) is an immunoregulatory cytokine produced by activated T lymphocytes. IL-2 production is considered an index of immune response in vivo. In Jurkat cells, an immortalized human T lymphocyte cell line, IL-2 production can be induced by stimulation with a mitogen such as concanavalin A (ConA) [[1], [2], [3]]. ConA binds to T-cell receptor, which triggers signaling pathways that lead to the activation of three transcription factors—nuclear factor of activated T cells (NFAT), nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), and activator protein 1 (AP-1, a complex of c-Fos and c-Jun)—which induce the expression of IL-2 mRNA (Fig. 1) [[4], [5], [6], [7]].
Fig. 1.
(A) Chemical structures of d-glucosamine and melibiosamine. (B) Hypothetical scheme for the signaling cascades leading to the expression of interleukin (IL)-2 mRNA in Jurkat cells and the actions of GlcNH2 and MelNH2. The mitogen concanavalin A (ConA) stimulates IL-2 production via T-cell receptor (TCR) and subsequent activation of the calcineurin/nuclear factor of activated T-cells (CN/NFAT), IκB kinase/nuclear factor kappa-light-chain-enhancer of activated B cells (IKK/NFκB), and mitogen-activated protein kinase/activator protein 1 (MAPK/AP-1) pathways. The immunosuppressive drugs cyclosporine A (CsA) and tacrolimus (FK506) inhibit CN via immunophilins. It was assumed that phorbol 12-myristate 13-acetate (PMA) and ionomycin (IM) together mimic the actions of ConA with regard to the induction of IL-2 expression in vitro. MelNH2 and GlcNH2 suppress the activation of NFATc1 and NFκB by ConA, whereas MelNH2, but not GlcNH2, promotes the activation of AP-1. PM, plasma membrane; PLC, phospholipase C; IP3, inositol 1,4,5-trisphosphate; DAG, diacylglycerol; PKC, protein kinase C. ‘P-’ indicates phosphate group, and the gray arrow inside the nucleus indicates IL-2 transcription.
In T lymphocytes, the immunosuppressive drugs cyclosporine A (CsA) and tacrolimus (FK506) suppress IL-2 production by inhibiting calcineurin (a Ca2+/calmodulin–dependent protein serine/threonine phosphatase) via immunophilins, which suppress the activation of NFAT (Fig. 1) [[8], [9], [10]]. CsA and tacrolimus prevent organ graft rejection and disease progression in patients with rheumatoid arthritis [11]. However, because the clinical use of these and other immunosuppressants (e.g. glucocorticoids) have serious toxicities that affect the renal, nervous, and gastrointestinal systems [[12], [13], [14], [15], [16]], novel immunosuppressants with fewer toxicities are needed.
D-Glucosamine (GlcNH2) (Fig. 1A), a naturally occurring derivative of glucose, possesses various biological and pharmacological activities, including anti-tumor and anti-inflammatory activities [[17], [18], [19], [20], [21], [22], [23], [24], [25]]. Recently, a novel GlcNH2-containing oligosaccharide, Galα1-6GlcNH2 (designated melibiosamine; MelNH2) (Fig. 1A), was found to exhibit anti-proliferative effects in human leukemia and breast cancer cells in vitro [26,27]. However, the potential biological and pharmacological activities of MelNH2 are yet to be fully elucidated.
Here, to assess the potential pharmacological activity and toxicity of MelNH2 on the immune system, we compared the effects of GlcNH2 and MelNH2 on ConA-induced IL-2 production (CIIP) in Jurkat cells. We found that MelNH2 suppressed CIIP via a mechanism that is, at least in part, different from that through which GlcNH2 suppresses CIIP, suggesting that MelNH2 may be a useful lead compound for the development of novel immunosuppressive or anti-inflammatory drugs.
2. Materials and methods
2.1. Cell line and reagents
Jurkat cells were maintained at 37 °C (5% CO2) in tissue-culture dishes containing RPMI 1640 growth medium supplemented with 10% (v/v) fetal bovine serum, 25 μg/mL penicillin, and 50 μg/mL streptomycin. MelNH2 was synthesized as described previously [26], and GlcNH2 was purchased from Sigma (St. Louis, MO); these compounds were each dissolved in phosphate-buffered saline (pH 7.4) as 0.1 M solutions, sterilized through a 0.45-μm filter, and stored at −20 °C until use. Phorbol 12-myristate 13-acetate (PMA) and ionomycin (IM) were obtained from Sigma, and CsA was purchased from Calbiochem (San Diego, CA), and these compounds were stored as 1, 0.5, and 0.5 mM stock solutions, respectively, in dimethyl sulfoxide (DMSO).
2.2. Assay of cell number and viability
Jurkat cells were pre-incubated at 37 °C (5% CO2) for 30 min in 0.5 mL RPMI 1640 (1 × 106 cells/mL) in a 24-well plate in the presence of 5–20 mM MelNH2 or GlcNH2. Then, ConA was added to each well at a final concentration of 50 μg/mL, and the cells were further incubated at 37 °C (5% CO2) for 12 h. After the addition of 0.5 mL trypan blue solution to each well, living and dead cells were counted under a microscope by using a hemocytometer.
2.3. Assay of IL-2 production by Jurkat cells
IL-2 production was assessed as described previously with some minor changes [2,28,29]. Jurkat cells were pre-incubated at 37 °C (5% CO2) for 30 min in 1 mL RPMI 1640 (1 × 106 cells/mL) in a 12-well plate in the presence of the indicated concentrations of MelNH2 or GlcNH2 or 1 μM CsA. After the addition of ConA (final concentration, 50 μg/mL) or the combination of PMA (1 μM) and IM (0.5 μM), the cells were further incubated at 37 °C (5% CO2) for 12 h. Aliquots of the culture media were collected, and the concentration of IL-2 was determined by using a Quantikine Human IL-2 ELISA Kit (R&D Systems, Minneapolis, MN). Briefly, 50-μL aliquots (in duplicate) of the culture media or IL-2 standard from the kit were added to the wells of 96-well plates pre-coated with anti-human IL-2 antibody. After incubation (at 37 °C) with biotinylated antibodies against human IL-2 and then with streptavidin–horseradish peroxidase conjugate, color was developed and the level of IL-2 was quantified by measuring absorbance at 450 nm (reference at 570 nm).
2.4. Semi-quantitative reverse transcription–polymerase chain reaction assay
Jurkat cells were pre-incubated at 37 °C (5% CO2) for 30 min in 1 mL RPMI 1640 (1 × 106 cells/mL) in a 12-well plate in the presence or absence of 15 mM GlcNH2 or MelNH2 or 5 μM CsA. After the addition of ConA (50 μg/mL), the cells were further incubated at 37 °C (5% CO2) for 3 h. Cells were collected by centrifugation (400×g, 5 min), and total RNA was prepared from the cells by using an RNeasy Mini Kit (Qiagen, Hilden, Germany). One microgram of total RNA was reverse transcribed into complementary DNA by using a ReverTra Ace qPCR Kit (Toyobo Co. Ltd., Osaka, Japan), and polymerase chain reaction amplification for IL-2 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) complementary DNA was performed as previously described [2,29]. The following primer pairs were used: IL-2, forward-5′-CAACTCCTGTCTTGCATTGCACTAA-3′, reverse-5′-AATGTGAGCATCCTGGTGAGTTTG-3′; GAPDH, forward-5′-GCACCGTCAAGGCTGAGAAC-3′, reverse-5′-ATGGTGGTGAAGACGCCAGT-3′.
2.5. Preparation of nuclear extracts and transcription factor assay
Jurkat cells were pre-incubated at 37 °C (5% CO2) for 30 min in a 10-cm dish in the presence or absence of 5–20 mM GlcNH2 or MelNH2. After the addition of PMA (1 μM) and IM (0.5 μM), or 0.2% DMSO (vehicle), the cells were further incubated at 37 °C (5% CO2) for 3 h. After incubation, the cells were collected by centrifugation (200×g, 2 min), lysed with a lysis buffer (in the kit below), and nuclear extracts were prepared by using a Nuclear Extract kit (Active Motif, Carlsbad, CA). After measurement of protein concentrations with a RC-DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA), the nuclear extracts were aliquoted and stored at −80 °C until use.
We measured the activation (the contents in nuclear extracts) of NFAT cytoplasmic 1 (NFATc1), NFκB, and phospho-c-Jun by using enzyme-linked immunosorbent assay-based TransAM NFATc1, TransAM NFκB, and TransAM AP-1 c-Jun kits (Active Motif Corp.), respectively. Briefly, nuclear extracts from control and GlcNH2- or MelNH2-treated cells were incubated in a 96-well plate containing immobilized oligonucleotides (consensus sequences) specific for the particular transcription factor, and bound transcription factor was quantified by enzyme-linked immunosorbent assay.
3. Results and discussion
3.1. Effects of MelNH2 on IL-2 production and cell viability in Jurkat cells
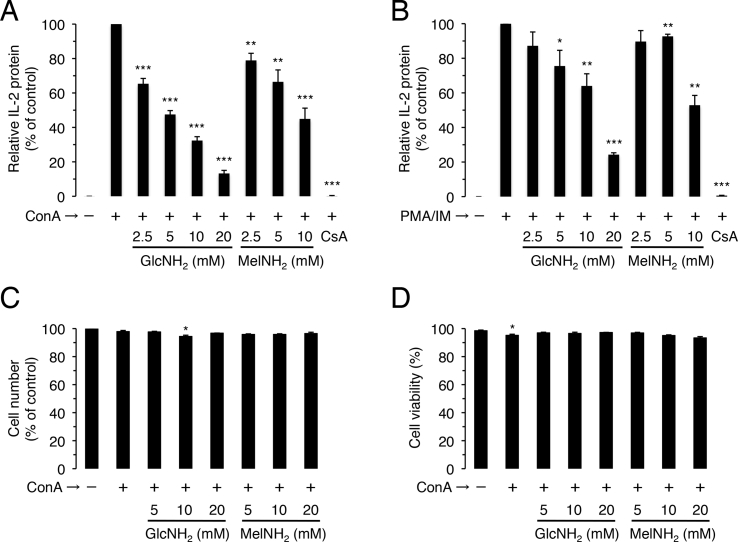
To assess the potential immunological activity and toxicity of MelNH2, we compared the effects of GlcNH2 and MelNH2 on CIIP and cell viability in Jurkat cells. As expected from previous reports, the immunosuppressive drug CsA at 1 μM strongly suppressed CIIP [28,29], and GlcNH2 at 2.5–20 mM suppressed CIIP in a dose-dependent manner (Fig. 2A) [21,23]. Similarly, MelNH2 at 2.5–10 mM suppressed CIIP in a dose-dependent manner (Fig. 2A). In addition, neither GlcNH2 nor MelNH2 at 20 mM or less had any effect on cell number and viability (Fig. 2C and D), suggesting that these compounds are not toxic to Jurkat cells.
Fig. 2.
Effects of GlcNH2 and MelNH2 on IL-2 production and cell number (viability) in Jurkat cells. Cells were pre-incubated for 30 min in the absence or presence of the indicated concentrations of GlcNH2 or MelNH2 (A–D), or 1 μM CsA (A, B). After the addition of ConA (A, C, D) or the combination of PMA (1 μM) and IM (0.5 μM) (B), cells were further incubated for 12 h and assayed for IL-2 protein production (A, B), living cell number (C), and cell viability (D). *P < 0.05, **P < 0.01, ***P < 0.001; unpaired t-test (2-tailed), as compared with control.
When administered together to Jurkat cells, PMA and IM mimic the effects of ConA by activating protein kinase C and raising cellular calcium levels, respectively (Fig. 1B) [2,3]. As expected [28,29], CsA at 1 μM strongly suppressed PMA/IM-induced IL-2 production (PIIP), and GlcNH2 at 2.5–20 mM suppressed PIIP in a dose-dependent manner. In addition, MelNH2 at 5–10 mM suppressed PIIP (Fig. 2B).
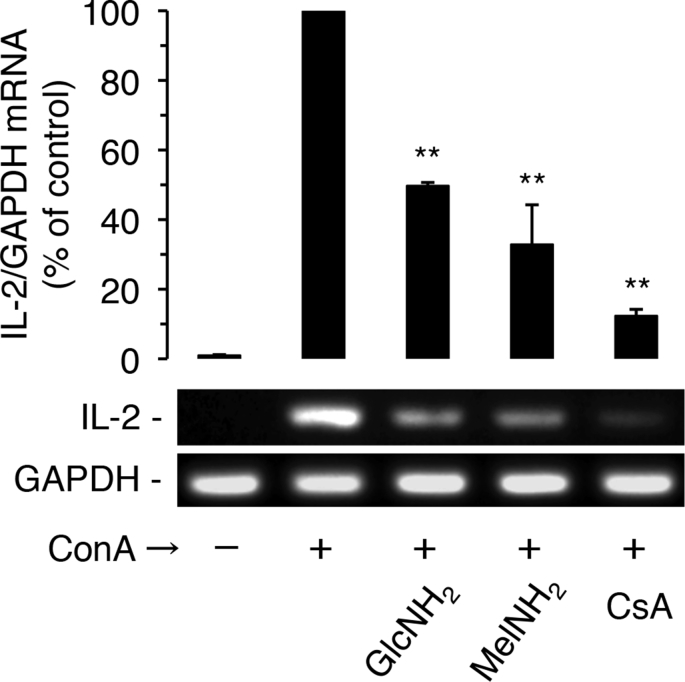
Next, we examined the effects of GlcNH2 and MelNH2 on ConA-induced IL-2 mRNA expression in Jurkat cells (Fig. 3). As expected, CsA at 1 μM strongly suppressed ConA-induced IL-2 mRNA expression; GlcNH2 and MelNH2 at 15 mM also significantly (P < 0.01) suppressed ConA-induced IL-2 mRNA expression.
Fig. 3.
Effects of GlcNH2 and MelNH2 on ConA-induced IL-2 mRNA expression in Jurkat cells. Cells were pre-incubated for 30 min in the absence or presence of 15 mM GlcNH2 or MelNH2, or 1 μM CsA. After the addition of ConA, cells were further incubated for 3 h and assayed for IL-2 mRNA expression. **P < 0.01; unpaired t-test (2-tailed), as compared with control.
Together, these results suggest that GlcNH2 and MelNH2 both suppress CIIP and PIIP by disrupting the signaling cascade downstream from where PMA and IM exert their actions but upstream of the three transcription factors that regulate IL-2 mRNA expression (Fig. 1B).
3.2. Effects of MelNH2 on the activation of NFAT, NFκB, and AP-1 in Jurkat cells
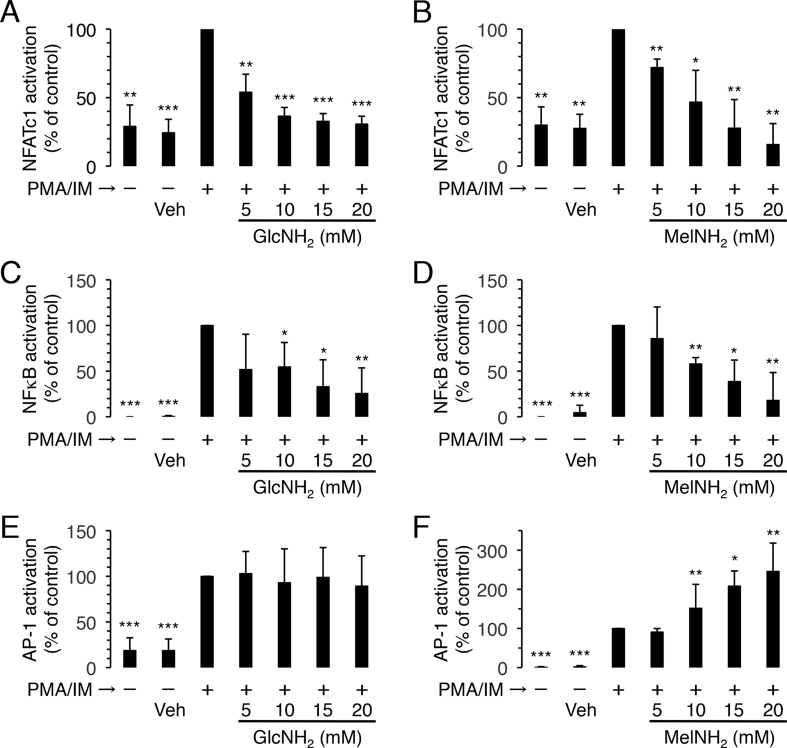
To elucidate the mechanisms underlying the immunosuppressive activities of GlcNH2 and MelNH2, we examined the effects of these compounds on the cytosol-to-nucleus translocation (i.e. the activation) of the three transcription factors that regulate IL-2 expression—NFATc1 (NFAT cytoplasmic 1), NFκB, and AP-1 (Fig. 1B). GlcNH2 and MelNH2 at 5–20 mM both dose-dependently attenuated the activation of NFATc1 and NFκB by PMA/IM (Fig. 4A–D). However, unlike GlcNH2, MelNH2 at 5–20 dose-dependently promoted the activation of AP-1 by PMA/IM (Fig. 4E and F).
Fig. 4.
Effects of GlcNH2 and MelNH2 on PMA/IM-induced activation of NFATc1, NFκB, and AP-1 in Jurkat cells. Cells were pre-incubated for 30 min in the presence of the indicated concentrations of GlcNH2 (A, C, E) and MelNH2 (B, D, F). After the addition of DMSO (0.2%) (vehicle: veh) or the combination of PMA (1 μM) and IM (0.5 μM), cells were further incubated for 3 h and assayed for the activation of NFATc1 (A, B), NFκB (C, D), and AP-1 (E, F). Data are the mean and standard deviation (bars) of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001; unpaired t-test (2-tailed), as compared with control.
These results suggest that MelNH2 and GlcNH2 both suppress PIIP (and thus CIIP) by inhibiting the activation of NFATc1 and NFκB.
3.3. Comparison of the actions of GlcNH2 and MelNH2
We previously reported that GlcNH2 at 5 mM significantly (P < 0.01) suppressed the growth of human K562 leukemia cells and human HUC-F2 (non-transformed) cells, whereas MelNH2 at 5 mM significantly (P < 0.01) suppressed the cell growth of K562 cells but not of HUC-F2 cells, as assessed at day 3 after exposure [26]. This suggests that GlcNH2 is toxic to both tumor cells and non-transformed cells, whereas MelNH2 is toxic to tumor cells but not to non-transformed cells. In the present study, we found that GlcNH2 and MelNH2 at up to 20 mM had no effect on cell number and viability in Jurkat cells, as assessed at hour 12 after exposure (Fig. 2C and D), suggesting that the two saccharides are not toxic to mammalian cells, at least within that period of exposure.
The present observation that GlcNH2 suppressed CIIP (Fig. 2, Fig. 3) by, at least in part, inhibiting the activation of NFATc1 and NFκB (Fig. 4A–D) is consistent with a previous observation that GlcNH2 reduces IL-2 production in mitogen-activated Jurkat cells by inhibiting the NFAT pathway [30]. Interestingly, MelNH2, but not GlcNH2, promoted the activation of AP-1 by PMA/IM (Fig. 1, Fig. 4E and F). Although the biological significance of this promotion of AP-1 activation by MelNH2 is currently unknown, this result suggests that GlcNH2 and MelNH2 suppress IL-2 production through different mechanisms; that is, MelNH2 has two target molecules, one of which is also the target molecule of GlcNH2 and leads to the inhibition of NFAT and NFκB activation, whereas the other stimulates AP-1 activation. In the near future, we intend to elucidate the target molecule(s) of MelNH2 in Jurkat cells, which the present results indicate reside downstream from where PMA and IM exert their actions but upstream of the three transcription factors that regulate IL-2 mRNA expression.
4. Conclusions
The present results suggest that, like GlcNH2, MelNH2 may be a promising lead compound for development as a novel immunosuppressive drug with an improved adverse effect profile compared with those of currently available immunosuppressive drugs.
Conflicts of interest
The authors declare no competing interests.
Acknowledgements
This work was supported in part by the Japan Society for the Promotion of Science [KAKENHI: grant nos. 26640106 and 15K07964].
Footnotes
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100658
Contributor Information
Osamu Hosomi, Email: ohosomi@juntendo.ac.jp.
Yuzuru Kubohara, Email: ykuboha@juntendo.ac.jp.
Transparency document
References
- 1.Abraham R.T., Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S., Akaishi E., Hosaka H., Okamura S., Kubohara Y. Zinc ions suppress mitogen-activated interleukin-2 production in Jurkat cells. Biochem. Biophys. Res. Commun. 2005;335:162–167. doi: 10.1016/j.bbrc.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Aherne S.A., O'Brien N.M. Modulation of cytokine production by plant sterols in stimulated human Jurkat T cells. Mol. Nutr. Food Res. 2008;52:664–673. doi: 10.1002/mnfr.200700385. [DOI] [PubMed] [Google Scholar]
- 4.Kvanta A., Kontny E., Jondal M., Okret S., Fredholm B.B. Mitogen stimulation of T-cells increases c-Fos and c-Jun protein levels, AP-1 binding and AP-1 transcriptional activity. Cell. Signal. 1992;4:275–286. doi: 10.1016/0898-6568(92)90067-i. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree G.R. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 6.Hayden M.S., Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K., Ishizuka S., Yokoyama C., Hatae T. Attenuation of interferon-γ mRNA expression in activated Jurkat T cells by exogenous zinc via down-regulation of the calcium-independent PKC–AP-1 signaling pathway. Life Sci. 2008;83:6–11. doi: 10.1016/j.lfs.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Liu J., Farmer J.D., Lane W.S., Friedman J., Weissman I., Schreiber S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 9.Griffith J.P., Kim J.L., Kim E.E., Sintchak M.D., Thomson J.A., Fitzgibbon M.J., Fleming M.A., Caron P.R., Hsiao K., Navia M.A. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell. 1995;82:507–522. doi: 10.1016/0092-8674(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 10.Kissinger C.R., Parge H.E., Knighton D.R., Lewis C.T., Pelletier L.A., Tempczyk C.T., Kalish V.J., Tucker K.D., Showalter R.E., Moomow E.W., Gastinel L.N., Habuka N., Chen X., Maldonado F., Barker J.E., Bacquet R., Villafranca J.E. Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature. 1995;378:641–644. doi: 10.1038/378641a0. [DOI] [PubMed] [Google Scholar]
- 11.Kitahara K., Kawai S. Cyclosporine and tacrolimus for the treatment of rheumatoid arthritis. Curr. Opin. Rheumatol. 2007;19:238–245. doi: 10.1097/BOR.0b013e328099af80. [DOI] [PubMed] [Google Scholar]
- 12.Dempster D.W. Bone histomorphometry in glucocorticoid-induced osteoporosis. J. Bone Miner. Res. 1989;4:137–141. doi: 10.1002/jbmr.5650040202. [DOI] [PubMed] [Google Scholar]
- 13.Fung J.J., Alessiani M., Abu-Elmagd K., Todo S., Shapiro R., Tzakis A., Vanthiel D., Armitage J., Jain A., Mccauley J., Selby R., Starzl T.E. Adverse effects associated with the use of FK506. Transplant. Proc. 1991;23:3105–3108. [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K. FK506: mechanism of immunosuppression and adverse-effects. J. Toxicol. Sci. 1995;20:477–479. [PubMed] [Google Scholar]
- 15.Allison A.C. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology. 2000;47:63–83. doi: 10.1016/s0162-3109(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 16.Penfornis A., Kury-Paulin S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006;32:539–546. doi: 10.1016/s1262-3636(06)72809-9. [DOI] [PubMed] [Google Scholar]
- 17.Quastel J.H., Cantero A. Inhibition of tumour growth by D-glucosamine. Nature. 1953;171:252–254. doi: 10.1038/171252a0. [DOI] [PubMed] [Google Scholar]
- 18.Bekasi J.G., Winzler R.J. Inhibitory effect of D-glucosamine on the growth of Walker 256 carcinosarcoma on protein, RNA, and DNA synthesis. Cancer Res. 1970;30:2905–2912. [PubMed] [Google Scholar]
- 19.Friedman S.J., Skehan P. Membrane-active drugs potentiate the killing of tumor cells by D-glucosamine. Proc. Natl. Acad. Sci. U.S.A. 1980;77:1172–1176. doi: 10.1073/pnas.77.2.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee D.Y., Choi I.S., Han J.H., Han S.Y. Chitosan and D-glucosamine induce expression of Th1 cytokine genes in porcine spleen cells. Immunology. 2001;64:645–648. doi: 10.1292/jvms.64.645. [DOI] [PubMed] [Google Scholar]
- 21.Ma L., Rudert W.A., Harnaha J., Wright M., Machen J., Lakomy R., Qian S., Lu L., Robbins P.D., Trucco M., Giannoukakis N. Immunosuppressive effects of glucosamine. J. Biol. Chem. 2002;277:39343–39349. doi: 10.1074/jbc.M204924200. [DOI] [PubMed] [Google Scholar]
- 22.Oh H.J., Lee J.S., Song D.K., Shin D.H., Jang B.C., Suh S.I., Park J.W., Suh M.H., Baek W.K. D-glucosamine inhibits proliferation of human cancer cells through inhibition of p70S6K. Biochem. Biophys. Res. Commun. 2007;360:840–845. doi: 10.1016/j.bbrc.2007.06.137. [DOI] [PubMed] [Google Scholar]
- 23.Zhu A., Huang J.B., Clark A., Romero R., Petty H.R. 2,5-Deoxyfructosazine, a D-glucosamine derivative, inhibits T-cell interleukin-2 production better than D-glucosamine. Carbohydr. Res. 2007;342:2745–2749. doi: 10.1016/j.carres.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ming W.C., Ming H.L., Shing H.H., Shin H.F., Chao Y.H., Lin J.B.Y., Jiann T.C., Chang D.M., Huey K.S. Glucosamine modulates T cell differentiation through down-regulating N-linked glycosylation of CD25. J. Biol. Chem. 2015;290:29329–29344. doi: 10.1074/jbc.M115.674671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Someya A., Ikegami T., Sakamoto K., Nagaoka I. Glucosamine downregulates the IL-1β-induced expression of proinflammatory cytokine genes in human synovial MH7A cells by O-GlcNAc modification-dependent and -independent mechanisms. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosomi O., Misawa Y., Takeya A., Matahira Y., Sugahara K., Kubohara Y., Yamaura F., Kudo S. Novel oligosaccharide has suppressive activity against human leukemia cell proliferation. Glycoconj. J. 2009;26:189–198. doi: 10.1007/s10719-008-9175-z. [DOI] [PubMed] [Google Scholar]
- 27.Nakazato R., Iizumi K., Sasaki H., Shigenaga A., Ogata S., Aoki D., Misawa Y., Hosomi O., Kubohara Y. Effects of a novel oligosaccharide, MelNH2, on human breast cancer cells. J. Health Sports Sci. Juntendo. 2018;9:1–10. ([non English]) [Google Scholar]
- 28.Takahashi K., Murakami M., Hosaka K., Kikuchi H., Oshima Y., Kubohara Y. Regulation of IL-2 production in Jurkat cells by Dictyostelium-derived factors. Life Sci. 2009;85:438–443. doi: 10.1016/j.lfs.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K., Murakami M., Kikuchi H., Oshima Y., Kubohara Y. Derivatives of Dictyostelium differentiation-inducing factors promote mitogen-activated IL-2 production via AP-1 in Jurkat cells. Life Sci. 2011;88:480–485. doi: 10.1016/j.lfs.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Huang J.B., Clark A.J., Petty H.R. The hexosamine biosynthesis pathway regulates IL-2 production by Jurkat T cells. Cell. Immunol. 2007;245:1–6. doi: 10.1016/j.cellimm.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.