Abstract
BACKGROUND
Inflammatory cytokines play a vital role in the occurrence of osteoarticular injury and inflammation. Whether inflammation-associated factors interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF) are involved in the pathogenesis of keen articular cartilage injury remains poorly understood.
AIM
To measure the levels of inflammatory factors [IL-1β, IL-6, TNF-α and VEGF] in patients with knee articular cartilage injury.
METHODS
Fifty-five patients with knee articular cartilage injury were selected as patient groups, who were divided into three grades [mild (n = 20), moderate (n = 19) and severe (n = 16)] according to disease severity and X-ray examinations. Meanwhile, 30 healthy individuals who underwent physical examination were selected as the control group. The levels of IL-1β, IL-6, TNF-α and VEGF were measured by ELISA and immunohistochemical staining.
RESULTS
Compared with the control group, patient groups displayed significantly higher levels of IL-1β, IL-6, TNF-α and VEGF, and the extent of increase was directly proportional to the severity of injury (P < 0.05). In addition, the number of cells with positive staining of IL-1β, IL-6, TNF-α and VEGF in the synovial membrane were significantly increased, along with increased disease severity (P < 0.05). After treatment, the scores of visual analogue scale and the Western Ontario and McMaster University of Orthopaedic Index in patient groups were 2.26 ± 1.13 and 15.56 ± 7.12 points, respectively, which were significantly lower than those before treatment (6.98 ± 1.32 and 49.48 ± 8.96). Correlation analysis suggested that IL-1β and TNF-α were positively correlated with VEGF.
CONCLUSION
IL-1β, IL-6, TNF-α and VEGF levels are increased in patients with knee articular cartilage injury, and are associated with the disease severity, indicating they might play an important role in the occurrence and development of knee articular cartilage injury. Furthermore, therapeutically targeting them might be a novel approach for the treatment of keen articular cartilage injury.
Keywords: Knee articular cartilage injury, Interleukin-1β, Interleukin-6, Tumor necrosis factor-α, Vascular endothelial growth factor
Core tip: Increased levels of interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α and vascular endothelial growth factor are found in patients with knee articular cartilage injury, and are associated with the disease severity, suggesting that they might be involved in the pathogenesis of knee articular cartilage injury. Furthermore, therapeutically targeting them might be beneficial for the treatment of knee articular cartilage injury.
INTRODUCTION
Knee articular cartilage injury is a type of joint disease with a high incidence, and is characterized by degenerative changes, such as disintegration and reduction of articular cartilage matrix. This leads to the formation of osteophytes, accompanied by aseptic inflammation of the synovial membrane[1-3]. It has been proven that in-flammatory cytokines play a vital role in the occurrence of osteoarticular injury and inflammation[4-6]. Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) can trigger the production of matrix metalloproteinases, causing degradation of articular cartilage matrix, and eventually leading to osteochondral injury[7]. IL-6 is a multifunctional cytokine that induces the proliferation and differentiation of immune cells, and plays a central role in the regulation of the complicated immune network in the body. This can lead to long-lasting chronic inflammation by inducing and activating various immune cells in vivo[8-10]. Vascular endothelial growth factor (VEGF) is a platelet-derived growth factor, which is mainly expressed in articular osteoblasts and synovial fibroblasts[11]. Elevated VEGF promotes neovascularization, and is considered to be one of the strongest angiogenic factors in the body[12]. Considering the role of inflammatory cytokines in the pathogenesis of oestoarticular injury, whether IL-1β, IL-6, TNF-α and VEGF are involved in the pathogenesis of keen articular cartilage injury remains poorly understood. The purpose of this study is to explore the expression profiles of IL-1β, IL-6, TNF-α and VEGF in patients with knee articular cartilage injury, with an attempt to evaluate the clinical significance of the occurrence and development of the disease.
MATERIALS AND METHODS
Patients
A total of 55 patients consisting of 26 males and 29 females with knee osteoarthritis caused by knee articular cartilage injury who were treated in our hospital from January 2017 to July 2017 were selected. All patients were diagnosed according to the diagnostic criteria of knee osteoarthritis formulated by the American College of Rheumatology[13]. Patients who had been treated with hormone therapy within 3 mo, or patients with severe organ diseases or surgical contraindications, were excluded. Patients with knee articular cartilage injury were further divided into three grades [mild (n = 20), moderate (n = 19) and severe (n = 16)] according to clinical symptoms, disease severity and X-ray examinations. Another 30 healthy individuals who underwent physical examinations during the same period were selected as the control group. No significant differences were observed in these two groups regarding age, sex and other aspects (P > 0.05). This study was approved by the ethics committee of our hospital, and informed consents were received from all participants prior to this study.
Measurement of IL-1β, IL-6, TNF-α and VEGF levels by ELISA
The fasting venous blood was drawn in the morning and centrifuged at × 2000 g for 20 min to obtain serum, which was used to measure the levels of IL-1β; IL-6; TNF-α and VEGF by ELISA according to the manufacturer’s instructions.
Immunohistochemical staining
Synovial specimens were collected during the replacement surgery and were fixed, dehydrated and immersed in wax. After embedding in paraffin, synovial specimens were sliced and processed by heating antigen retrieval. After blocking the endogenous peroxidase activity with freshly made 0.3% H202 in methanol for 20 min, primary antibodies against IL-1β, IL-6, TNF-α or VEGF (Cell Signaling Technology) were added and incubated for 1 h, followed by washing and addition of a biotinylated secondary antibody (Cell Signaling Technology) for a 10 min incubation. Then, DAB substrate was added for development, followed by counter-staining and differentiation, and subsequently mounted with mounting medium (Simpo-Mount). Mounted slides were observed and recorded under a light-field microscope. The number of positive cells with IL-1β, IL-6, TNF-α and VEGF in synovial tissues was counted.
Evaluation indexes
Changes in clinical indicators [visual analog scale (VAS) and Western Ontario and McMaster University of Orthopaedic Index (WOMAC)] before and after treatment were observed, and the overall scores before and after treatment (i.e. the fourth week) were taken as the main evaluating indicators. VAS is an evaluating indicator that can accurately express the subjective pain of the patients. WOMAC scores are on physical function, stiffness and the degree of pain. It was divided into five grades: 0 points (no), 1 (mild), 2 (moderate), 3 (severe) and 4 (extremely severe).
Statistical analysis
The data were processed by Statistical Product and Service Solutions (SPSS) 19.0 software [International Business Machines Corporation] and displayed as mean ± SD. Student t-tests were performed to compare the differences between the two groups, and one-way ANOVA was conducted to compare the difference among multiple groups. The correlation between two variables was analyzed by Pearson correlation analysis. P < 0.05 indicated a statistical difference.
RESULTS
Elevated levels of IL-1β, IL-6, TNF-α and VEGF in patients
The levels of IL-1β, IL-6, TNF-α and VEGF in the serum of patients group were 74.91 ± 3.48, 40.35 ± 27.18, 67.12 ± 3.15 and 224.42 ± 31.06 ng/L, respectively, which were significantly higher than those of the control group (13.41 ± 1.50, 12.25 ± 2.58, 6.91 ± 4.20 and 135.48 ± 20.41 ng/L) (P < 0.05) (Figure 1).
Figure 1.
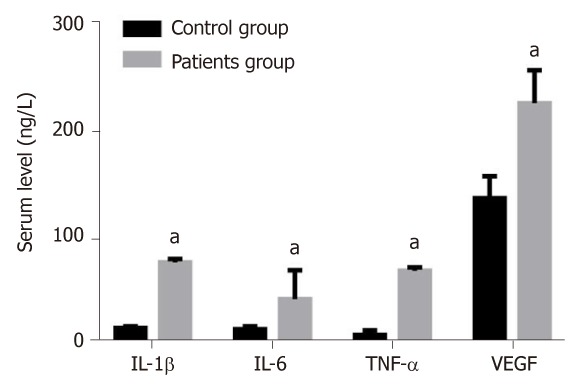
Levels of interleukin-1β, interleukin-6, tumor necrosis factor-α and vascular endothelial growth factor in patient and control groups. aP < 0.05, vs control group. IL-1β: Interleukin-1β; TNF-α: Tumor necrosis factor-α; VEGF: Vascular endothelial growth factor.
Levels of IL-1β, IL-6, TNF-α and VEGF in patients with different degrees of knee articular cartilage injury
The levels of IL-1β, IL-6, TNF-α and VEGF showed an increased trend from patients with mild, moderate or severe knee articular cartilage injury, with statistically significant differences (P < 0.05) (Figure 2).
Figure 2.
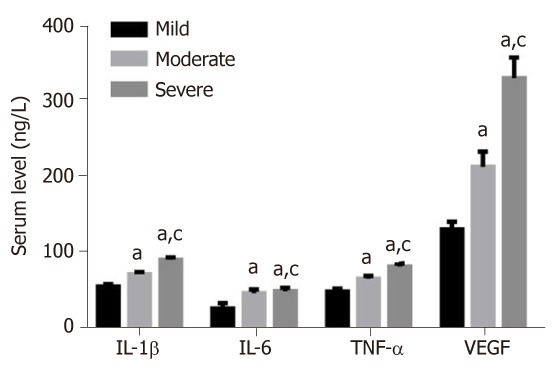
Serum levels of interleukin-1β, interleukin-6 and tumor necrosis factor-α in patients with different degrees of knee articular cartilage injury. aP < 0.05, vs mild; cP < 0.05, vs moderate group. IL-1β: Interleukin-1β; TNF-α: Tumor necrosis factor-α; VEGF: Vascular endothelial growth factor.
Immunohistochemical staining analysis of the levels of IL-1β, IL-6, TNF-α and VEGF in synovial membrane
As seen in Figure 3, the number of positive cells of IL-1β, IL-6, TNF-α and VEGF in synovial membrane in patients with severe knee articular cartilage injury were significantly higher than those in patients with moderate knee articular cartilage injury. These were also higher than those in the group of patients with mild knee articular cartilage injury (P < 0.05).
Figure 3.

Immunohistochemical staining analysis of the number of positive cells of interleukin-1β, interleukin-6, tumor necrosis factor-α and vascular endothelial growth factor in synovial membrane (%). aP < 0.05, vs mild; cP < 0.05, vs moderate group. IL-1β: Interleukin-1β; TNF-α: Tumor necrosis factor-α; VEGF: Vascular endothelial growth factor.
Changes of clinical indices in patients before and after treatment
After the treatment, the scores of VAS and WOMAC in patients were 2.26 ± 1.13 and 15.56 ± 7.12 points, respectively, which were significantly lower than those before treatment (6.98 ± 1.32 vs 49.48 ± 8.96 points) (P < 0.05) (Figure 4).
Figure 4.
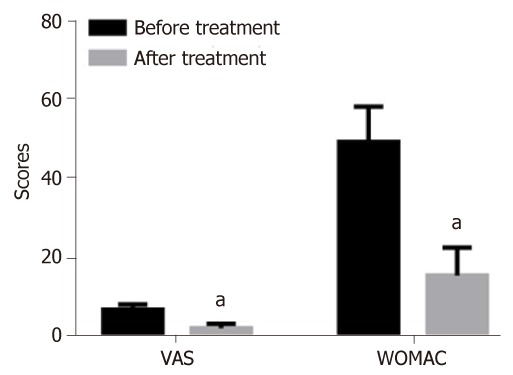
Comparisons of changes in clinical indices before and after treatment. aP < 0.05, vs before treatment. VAS: Visual analogue scale; WOMAC: Western Ontario and McMaster University of Orthopaedic Index.
Correlation analysis of inflammatory factors with VEGF
Pearson correlation analysis results showed that serum inflammatory factors (IL-1β and TNF-α) in patients were positively correlated with VEGF (r = 0.6763, r = 0.4856, P < 0.01) (Figure 5). However, there was no significant correlation between IL-6 and VEGF.
Figure 5.
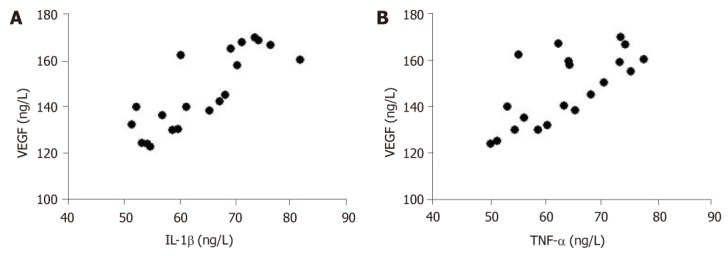
Correlation analysis of interleukin-1β (A) or tumor necrosis factor-α (B) with vascular endothelial growth factor. IL-1β: Interleukin-1β; TNF-α: Tumor necrosis factor-α; VEGF: Vascular endothelial growth factor.
DISCUSSION
In recent years, many studies have found that osteoarthritis patients have immune abnormalities, and abnormal secretions of cytokines might damage cartilage function and metabolism[14,15]. In addition, aggravated gradual decomposition of cartilage matrix can bring irreversible injury to the joint structure of patients, which is manifested as the corresponding clinical symptoms, thus accelerating the progress of articular cartilage injury[16,17]. IL-1β, IL-6 and TNF-α are secreted from macrophages and immune cells, which mainly come from the lining cells of the synovial membrane. These participate in the occurrence and development of osteoarthritis[18,19]. VEGF is an important factor in promoting neovascularization, and abnormal cell proliferation and differentiation may lead to the persistence of synovial inflammation[20]. The mechanism of VEGF in promoting the progress of osteoarthritis is believed to promote the proliferation of vascular endothelial cells and the growth of tumor lymphatic vessels, as well as upregulate the expression of anti-apoptotic proteins[21]. This thereby accelerates the formation of neovascularization in synovial pannus, and the subsequent division of endothelium cells, which is an important factor leading to increased vascular permeability[22].
Consistent with the role of inflammatory cytokines in the pathogenesis of osteoarthritis, we showed in this study that levels of IL-1β, IL-6 and TNF-α in the serum of patients with knee articular cartilage injury group were significantly higher than those in the control group (P < 0.05). In addition, immunohistochemical staining showed that the numbers of cells with positive staining of IL-1β, TNF-α and VEGF were statistically increased with disease progression. This suggests that an imbalance of cellular and humoral immunity in vivo might be involved in the pathophysiological process of the damage and progress of synovial membrane in knee osteoarthritis[23]. Patients with osteoarthritis are characterized by articular cartilage degeneration and changes of cartilage matrix composition, and the decrease of anti-angiogenic factors indirectly enhances the functions of pro-angiogenic factors, such as VEGF, in the pathogenesis of osteoarthritis. It was reported that different levels of VEGF are detected in patients with osteoarthritis[24]. In accordance with this, our study showed that, along with osteoarthritis progression, the serum level of VEGF in patients was significantly increased, further supporting the role of VEGF in the pathogenesis and development of osteoarthritis.
VAS is an evaluating indicator that can accurately reflect the subjective pain of patients. Through assessment of physical function, stiffness and the degree of pain, WOMAC scores are currently widely used in the treatment of osteoarthritis to evaluate the therapeutic effect of drugs. In this study, we showed that VAS and WOMAC in patients with knee articular cartilage injury after treatment were significantly lower than those before treatment, indicating that the clinical symptoms were greatly improved. Furthermore, Pearson correlation analysis showed that serum inflammatory factors (IL-1β and TNF-α) in patients was positively correlated with VEGF, indicating that the imbalance of the immune state in vivo, and the increase of factors related to neovascularization, might contribute to the development of osteoarthritis[25].
In conclusion, IL-1β, IL-6, TNF-α and VEGF levels are significantly increased in patients with knee articular cartilage injury, and are associated with the severity of disease. This suggests they might play an important role in the occurrence and development of knee articular cartilage injury. Thus, targeting them might be a novel approach for the treatment of knee articular cartilage injury.
ARTICLE HIGHLIGHTS
Research background
Inflammatory cytokines play a vital role in the occurrence of osteoarticular injury and inflammation. Whether inflammation-associated factors interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF) are involved in the pathogenesis of keen articular cartilage injury remains poorly understood.
Research motivation
The main topic is the inflammatory cytokines in knee articular cartilage injury.
Research objectives
To measure the levels of inflammatory factors [IL-1β, IL-6, TNF-α and VEGF] in patients with knee articular cartilage injury.
Research methods
Fifty-five patients with knee articular cartilage injury were selected as the patients groups, and were divided into three grades [mild (n = 20), moderate (n = 19) and severe (n = 16)] according to disease severity and X-ray examinations. Meanwhile, 30 healthy individuals who underwent physical examination were selected as the control group. The levels of IL-1β, IL-6, TNF-α and VEGF were measured by ELISA and immunohistochemical staining.
Research results
Compared with the control group, the patients group displayed significantly higher levels of IL-1β, IL-6, TNF-α and VEGF, and the extent of increase was directly proportional to the severity of injury (P < 0.05). In addition, the number of cells with positive staining of IL-1β, IL-6, TNF-α and VEGF in the synovial membrane was significantly increased, along with an increase in disease severity (P < 0.05). After treatment, the scores of visual analogue scale (VAS) and the Western Ontario and McMaster University of Orthopaedic Index in the patient group were 2.26 ± 1.13 and 15.56 ± 7.12 points, respectively, which were significantly lower than those before treatment (6.98 ± 1.32 and 49.48 ± 8.96). The correlation analysis suggested that IL-1β and TNF-α were positively correlated with VEGF.
Research conclusions
IL-1β, IL-6, TNF-α and VEGF levels are increased in patients with knee articular cartilage injury, and are associated with disease severity. This indicates that they might play an important role in the occurrence and development of knee articular cartilage injury. In addition, therapeutically targeting them might be a novel approach for the treatment of keen articular cartilage injury.
Research perspectives
Abnormal levels of IL-1β, IL-6, TNF-α and VEGF were found in patients with knee articular cartilage injury, indicating that they might be novel therapeutic targets for the treatment of knee articular cartilage injury.
Footnotes
Institutional review board statement: This study has been approved by the ethnic committee of Affiliated Zhongshan Hospital of Dalian University.
Conflict-of-interest statement: All authors have no conflict-of-interest to state.
Manuscript source: Unsolicited manuscript
Peer-review started: February 24, 2019
First decision: March 10, 2019
Article in press: April 9, 2019
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, Wang Y S-Editor: Ji FF L-Editor: Filipodia E-Editor: Xing YX
Contributor Information
Zhen-Wei Wang, Department of Sports Medicine, Affiliated Zhongshan Hospital of Dalian University, Dalian 16000, Liaoning Province, China.
Le Chen, Department of Sports Medicine, Affiliated Zhongshan Hospital of Dalian University, Dalian 16000, Liaoning Province, China.
Xiao-Rui Hao, Department of Sports Medicine, Affiliated Zhongshan Hospital of Dalian University, Dalian 16000, Liaoning Province, China.
Zhen-An Qu, Department of Sports Medicine, Affiliated Zhongshan Hospital of Dalian University, Dalian 16000, Liaoning Province, China.
Shi-Bo Huang, Department of Sports Medicine, Affiliated Zhongshan Hospital of Dalian University, Dalian 16000, Liaoning Province, China.
Xiao-Jun Ma, Department of Sports Medicine, Affiliated Zhongshan Hospital of Dalian University, Dalian 16000, Liaoning Province, China.
Jian-Chuan Wang, Department of Sports Medicine, Affiliated Zhongshan Hospital of Dalian University, Dalian 16000, Liaoning Province, China.
Wei-Ming Wang, Department of Sports Medicine, Affiliated Zhongshan Hospital of Dalian University, Dalian 16000, Liaoning Province, China. wangwm_01@126.com.
References
- 1.Ma J, Niu DS, Wan NJ, Qin Y, Guo CJ. Elevated chemerin levels in synovial fluid and synovial membrane from patients with knee osteoarthritis. Int J Clin Exp Pathol. 2015;8:13393–13398. [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelnaby R, El Deeb S, Khachab A, Bläsius K, Tingart M, Rath B. Plasma level of Osteopontin does not respond to total replacement Surgery in patients with severe Primary knee/Hip Osteoarthritis. J Orthop. 2017;14:354–357. doi: 10.1016/j.jor.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokogawa N, Toribatake Y, Murakami H, Hayashi H, Yoneyama T, Watanabe T, Tsuchiya H. Differences in Gait Characteristics of Patients with Lumbar Spinal Canal Stenosis (L4 Radiculopathy) and Those with Osteoarthritis of the Hip. PLoS One. 2015;10:e0124745. doi: 10.1371/journal.pone.0124745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 5.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70:185–193. doi: 10.1016/j.cyto.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan PW, Liu DY, Chu XD, Hao YQ, Zhu C, Qu Q. Effects of preventive administration of juanbi capsules on TNF-alpha, IL-1 and IL-6 contents of joint fluid in the rabbit with knee osteoarthritis. J Tradit Chin Med. 2010;30:254–258. doi: 10.1016/s0254-6272(10)60052-0. [DOI] [PubMed] [Google Scholar]
- 8.Funck-Brentano T, Cohen-Solal M. Subchondral bone and osteoarthritis. Curr Opin Rheumatol. 2015;27:420–426. doi: 10.1097/BOR.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8 Suppl 2:S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagao M, Hamilton JL, Kc R, Berendsen AD, Duan X, Cheong CW, Li X, Im HJ, Olsen BR. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Sci Rep. 2017;7:13027. doi: 10.1038/s41598-017-13417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M, Yang S, Zhang D, Shui P, Song S, Yao J, Dai Y, Sun Q. Fructopyrano-(1→4)-glucopyranose inhibits the proliferation of liver cancer cells and angiogenesis in a VEGF/VEGFR dependent manner. Int J Clin Exp Med. 2014;7:3859–3869. [PMC free article] [PubMed] [Google Scholar]
- 13.Cojocaru IM, Ştefănescu V, Traşcă D, Şerban-Pereţeanu A, Chicoş B, Cojocaru M. Multiple Intracerebral Hemorrhages in an Old Patient with Rheumatoid Arthritis. Rom J Intern Med. 2015;53:365–373. doi: 10.1515/rjim-2015-0048. [DOI] [PubMed] [Google Scholar]
- 14.Steinhaus ME, Christ AB, Cross MB. Total Knee Arthroplasty for Knee Osteoarthritis: Support for a Foregone Conclusion? HSS J. 2017;13:207–210. doi: 10.1007/s11420-017-9558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong R, Gao J, Si Y, Zhao D. Combination of circulating miR-19b-3p, miR-122-5p and miR-486-5p expressions correlates with risk and disease severity of knee osteoarthritis. Am J Transl Res. 2017;9:2852–2864. [PMC free article] [PubMed] [Google Scholar]
- 16.Loures FB, Carrara RJ, Góes RFA, Albuquerque RSPE, Barretto JM, Kinder A, Gameiro VS, Marchiori E. Anthropometric study of the knee in patients with osteoarthritis: intraoperative measurement versus magnetic resonance imaging. Radiol Bras. 2017;50:170–175. doi: 10.1590/0100-3984.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willett M, Duda J, Gautrey C, Fenton S, Greig C, Rushton A. Effectiveness of behavioural change techniques in physiotherapy interventions to promote physical activity adherence in patients with hip and knee osteoarthritis: a systematic review protocol. BMJ Open. 2017;7:e015833. doi: 10.1136/bmjopen-2017-015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim SH, Hong BY, Oh JH, Lee JI. Relationship between knee alignment and the electromyographic activity of quadriceps muscles in patients with knee osteoarthritis. J Phys Ther Sci. 2015;27:1261–1265. doi: 10.1589/jpts.27.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bar-Or D, Rael LT, Thomas GW, Brody EN. Inflammatory Pathways in Knee Osteoarthritis: Potential Targets for Treatment. Curr Rheumatol Rev. 2015;11:50–58. doi: 10.2174/1573397111666150522094131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai J, Li G, Shen M, Sui D, Lin S. Primary central nervous system histiocytic sarcoma mimicking glioma. Neurol India. 2014;62:684–685. doi: 10.4103/0028-3886.149409. [DOI] [PubMed] [Google Scholar]
- 21.Minchenko OH, Garmash IA, Kovalevska OV, Tsymbal DO, Minchenko DO. Expression of phosphoribosyl pyrophosphate synthetase genes in U87 glioma cells with ERN1 knockdown: effect of hypoxia and endoplasmic reticulum stress. Ukr Biochem J. 2014;86:74–83. [PubMed] [Google Scholar]
- 22.Ozawa A, Kadowaki E, Haga Y, Sekiguchi H, Hemmi N, Kaneko T, Maki T, Sakabe K, Hara S, Yamamoto M, Arishima K, Sakaue M. Acetylcholine esterase is a regulator of GFAP expression and a target of dichlorvos in astrocytic differentiation of rat glioma C6 cells. Brain Res. 2013;1537:37–45. doi: 10.1016/j.brainres.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Wang XP, Deng XL, Li LY. MicroRNA-584 functions as a tumor suppressor and targets PTTG1IP in glioma. Int J Clin Exp Pathol. 2014;7:8573–8582. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SS, Joo YS, Kim WU, Min DJ, Min JK, Park SH, Cho CS, Kim HY. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001;19:321–324. [PubMed] [Google Scholar]
- 25.Sun XP, Dong X, Lin L, Jiang X, Wei Z, Zhai B, Sun B, Zhang Q, Wang X, Jiang H, Krissansen GW, Qiao H, Sun X. Up-regulation of survivin by AKT and hypoxia-inducible factor 1α contributes to cisplatin resistance in gastric cancer. FEBS J. 2014;281:115–128. doi: 10.1111/febs.12577. [DOI] [PubMed] [Google Scholar]


