Abstract
BACKGROUND
Hepatitis C virus (HCV) infection is a documented risk factor for chronic kidney disease (CKD) and progression to end-stage renal disease (ESRD). However, to date there are no reports on the long-term hard endpoints (ESRD and death) of anti-HCV therapy [interferon-based therapy (IBT) or new direct-acting antivirals] in CKD patients. Direct-acting antivirals are not available in Taiwan’s single-payer national health insurance database currently released for research. Therefore, we hypothesized that a retrospective analysis of the long-term outcomes of IBT in CKD patients will serve as a proxy for direct-acting antivirals to increase our understanding of progression to ESRD following HCV infection.
AIM
To evaluate the long-term outcomes (ESRD and death) of anti-HCV therapy, especially IBT, in HCV-infected patients with stage 1-5 CKD.
METHODS
We analyzed 93894 Taiwanese adults diagnosed with CKD and without HBV infection. Of these, 4.9% were infected with HCV. Of the 4582 HCV-infected CKD patients, 482 (10.5%) received IBT (treated cohort). They were matched 1:4 with 1928 untreated HCV-infected CKD patients (untreated cohort) by propensity scores and year, which further matched 1:2 by propensity scores with 3856 CKD patients without HCV infection (uninfected cohort). All participants were followed until the occurrence of ESRD, death, or the end of 2012. The association between HCV infection, IBT use, and risks of ESRD and death was analyzed using competing risk analysis.
RESULTS
Taking the uninfected cohort as a reference, the adjusted hazard ratios for ESRD, after adjusting for competing mortality, were 0.34 (0.14-0.84, P = 0.019) and 1.28 (1.03-1.60, P = 0.029) in the treated and untreated cohorts, respectively. The treated cohort had a 29% (0.54-0.92, P = 0.011) decrease in mortality compared to the untreated cohort, in which the mortality was 31% (1.18-1.45, P < 0.001) higher than in the uninfected cohort. The reduced risks of ESRD (0.14, 0.03–0.58, P = 0.007) and death (0.57, 0.41-0.79, P = 0.001) were greatest in HCV-infected CKD patients who received at least 4 mo of IBT, which accounted for 74% of the treated cohort.
CONCLUSION
Adequate anti-HCV therapy in CKD patients improves long-term renal and patient survival.
Keywords: Hepatitis C virus, Chronic kidney disease, End-stage renal disease, Anti-hepatitis C virus therapy, Cohort study
Core tip: This large nationwide retrospective cohort study used propensity score-matched and competing risk analyses to evaluate the long-term hard endpoints of hepatitis C virus (HCV) infection and anti-HCV therapy, especially interferon-based therapy, in chronic kidney disease patients. We found that untreated HCV infection in chronic kidney disease was associated with increased risks of end-stage renal disease and mortality. On the contrary, adequate anti-HCV therapy in chronic kidney disease patients improves long-term renal and patient survival.
INTRODUCTION
Hepatitis C virus (HCV) infection and chronic kidney disease (CKD) are recognized public health concerns with global implications that affect 3%[1] and 10%[2], respectively, of people worldwide. Furthermore, the two remain economic threats due to their high morbidity and mortality[3]. HCV mainly targets the liver but can also induce at least one extrahepatic manifestation in 40% of the infected patients, including renal injury, insulin resistance (IR), accelerated atherosclerosis, and cardiovascular event risk[2]. Accumulating evidence has implicated HCV infection as an important cause and consequence of CKD[1-4]. HCV is an initiating factor for CKD in the general population[5,6], regardless of the presence of conventional risk factors for CKD[6,7], including aging, diabetes, and hypertension. It is also a progression factor of CKD to end-stage renal disease (ESRD) in the general population[8,9], CKD population of any etiology[10], and patients with diabetes[11] and glomerulonephritis[12]. The immunosuppression caused by CKD[13], especially stages 3-5[1,13-15], also makes CKD patients more vulnerable to the cytopathic effects of the HCV infection[3] and to HCV infection with high viremic potential[13,14,16], independent of a history of blood transfusions[13,17]. This further decreases renal[10,18] and patient survivals[10]. In the past decades, interferon-based therapy (IBT) was shown to be beneficial for eradicating HCV in some populations[19-24]. However, there are no large-scale studies that document the long-term outcomes (renal and patient survivals) of HCV therapy in patients with CKD stages 1-5.
Although direct-acting antivirals (DAAs), the new paradigm of HCV therapy, are effective in eradicating HCV and are well tolerated in the general population, their short-term efficacy and tolerability seem promising but not representative to all patients with advanced CKD, as shown in a recent meta-analysis[25] that was small-sized and only included 16.3% Asian individuals among the 264 patients. Moreover, their long-term outcomes and safety in CKD patients have not been elucidated. The exorbitant costs of DAAs remain the biggest barrier to their universal adoption in developed countries[26]. In Taiwan, DAAs could be reimbursed by single-payer national health insurance (NHI) for a minority of HCV-infected patients since 2018 and for all those since 2019. However, DAAs were not available for research in the Taiwan’s National Health Insurance Research Database (NHIRD), which was just released for academic research in 2016. The significant risk of drug-drug interactions[26,27] is another concern in CKD patients mostly suffering from multiple comorbidities.
Even though IBT is less well-tolerated in patients with kidney disease, it has a low dropout rate (0.18)[28], it is easy to access for studies in the NHIRD, it could be offered with much less chance of sustained virological response (SVR) but at a lesser cost[27], and it has been widely used for decades with excellent therapeutic responses in Asian countries where the favorable interleukin-28B is prevalent[20]. Given the reported higher percentage of viremia in HCV-infected CKD patients[13,14,16] and the clinical benefits that are derived from HCV eradication, we hypothesized that treating HCV infection in CKD patients might improve long-term hard endpoints. In order to fill this knowledge gap and in light of the easy access to IBT in the NHIRD, we analyzed data between 1997 and 2012 to examine the long-term impact of treating HCV infection with IBT on renal and survival outcomes among CKD patients.
MATERIALS AND METHODS
Data source
This retrospective nationwide cohort study used data from Taiwan’s NHIRD. The NHIRD has been prospectively recording comprehensive nationwide healthcare data of all beneficiaries since 1995, the year when the Taiwan NHI was implemented. The NHIRD adopts ICD-9 codes and drug codes to define diseases and drugs and was released by the National Health Research Institute for academic research after all personal information was de-identified. Thus, no informed consent was required and this study was exempt from a full ethical review by the institutional review board of the Dalin Tzu Chi Hospital (B10302011). Because of the single-payer and compulsory policy, the NHI program reached coverage of more than 99% by the end of 2012. The details of the NHIRD have been described in our previous work[5,21,29-32].
Study (CKD) population
We enrolled CKD patients who had a diagnosis of ≥ 1 inpatient or ≥ 2 outpatient CKD ICD-9 codes (250.4*, 274.1*, 283.11, 403.*1, 404.*2, 404.*3, 440.1, 442.1, 447.3, 572.4, 580-588, 642.1*, 646.2*)[5,21,32] between January 1, 1997 and December 31, 2012. We excluded CKD patients < 18 years, those who had claim-based diagnoses of HBV (ICD-9 codes 070.22, 070.23, 070.32, 070.33, V02.61) during this period, or those who developed ESRD (indicating the need for long-term renal replacement therapy) before the identification of CKD. A total of 93894 CKD adults without HBV infection were eligible for analysis (Figure 1). However, the exact stage of CKD cannot be assessed from the NHIRD.
Figure 1.
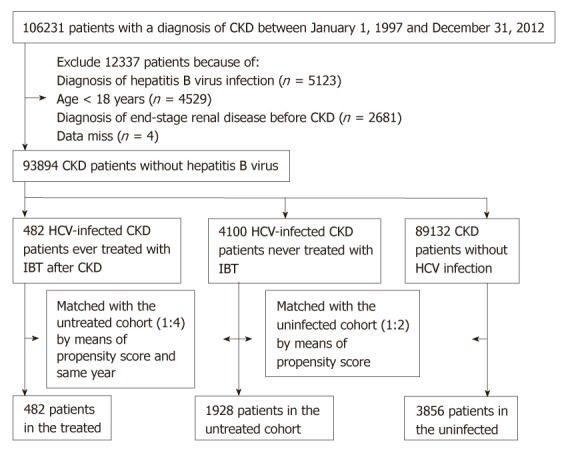
Flow diagram of the enrollment process. CKD: Chronic kidney disease; HCV: Hepatitis C virus; IBT: Interferon-based therapy.
Study cohorts
Eligible CKD enrollees were grouped into three cohorts according to a claim-based diagnosis of HCV infection (ICD-9 codes 070.41, 070.44, 070.51, 070.54, V02.62)[5,21] and the use of IBT (namely, interferon alpha, pegylated interferon alpha-2a, or pegylated interferon alpha-2b alone or in combination with ribavirin)[19,21]. There were 4582 (4.9%) CKD patients who were infected with HCV. Of the 4582 HCV-infected CKD patients, 482 (10.5%) were treated with IBT during this period (treated cohort) and the date of IBT initiation was considered the index date of the treated cohort. There were no treated HCV-infected CKD patients receiving ribavirin. Each treated patient was matched with four untreated patients who never received IBT throughout the study period by propensity score (to avoid confounding by indication bias) and during the same year of the index date (to avoid immortal time bias)[33,34]. The propensity score was estimated by the logistic regression built on age, sex, and comorbidity. The propensity score model was reliable (Hosmer–Lemeshow test P > 0.05) and provided fair discrimination between the treated and untreated cohorts (c-index > 0.6)[35] every year. Next, each untreated patient was propensity score-matched with two uninfected patients who never coded for HCV infection throughout the study period. The index dates of the untreated and uninfected cohorts were their corresponding matched dates. The propensity score model was reliable (Hosmer–Lemeshow test P = 0.999) and provided fair discrimination between the untreated and uninfected cohorts (c-index, 0.686). A total of 482 CKD patients were in the treated cohort, 1928 patients were in the untreated cohort, and 3856 patients were in the uninfected cohort for the final analysis.
Definition of hard endpoints
Follow-up started in the treated cohort after IBT was initiated, and in the untreated and uninfected cohorts after their matched dates. All patients were followed until ESRD occurrence, death, or December 31, 2012, whichever came first. Death before reaching ESRD was considered a competing risk event[29] in estimating the incidence of ESRD. In Taiwan, ESRD is a statutory major disease, and patients who develop ESRD and require long-term dialysis are issued a catastrophic illness certificate that is validated by at least two experienced nephrologists after a rigorous review of the clinical data. This grants exemption from copayment for healthcare. Thus, the diagnostic accuracy of ESRD is reliable. In the present study, all ESRD cases were identified from the Registry of Catastrophic Illness Patient Database, a part of the NHIRD.
Covariate assessment
We included the enrollee category [1 (highest status) to 4 (lowest status)] as a proxy for socioeconomic status and major comorbidity, including diabetes (ICD-9 code 250), hypertension (ICD-9 codes 401-405), coronary heart disease (ICD-9 codes 410-414), hyperlipidemia (ICD-9 codes 272-272.4), and cirrhosis (ICD-9 codes 571.2, 571.5, 571.6), which were associated with ESRD[29]. Additional confounding factors used in administrative medical databases included the number of medical visits and the Charlson comorbidity index (CCI) score[5,29]. Angiotensin-converting-enzyme inhibitor/angiotensin II receptor blocker (ACEI/ARB) was also identified because it is used as a mainstream drug against CKD progression and because of the strong correlations with ESRD and mortality[36]. Usage was defined as having used the drug for over 5% of the follow-up period.
Statistical analysis
The statistical methods of this study were reviewed by our coauthor Chung-Yi Li. The modified Kaplan-Meier method and Gray’s method[37] were used to calculate and compare the cumulative incidence of ESRD in data with competing risk. After confirming the assumption of proportional hazards (Supplementary Figure 1), we applied the modified Cox proportional hazard model to evaluate the relationship between IBT and the ESRD risk after adjusting all covariates (age per year, sex, major comorbidity, the use of ACEI/ARB, enrollee category, number of medical visits, and CCI score) and competing mortality. A sensitivity analysis was performed to evaluate the individual risk of ESRD and death between the treated and untreated cohorts as well as between the untreated and uninfected cohorts. Two stratified analyses were performed to investigate the individual impact of IBT and lack of IBT on the ESRD risk according to age, sex, comorbidity, and the use of ACEI/ARB. The impact of IBT duration, classified as < 4 mo versus ≥ 4 mo[20], on the ESRD risk was also examined. Data were managed with SAS (version 9.4; SAS Institute, Inc., Cary, NC, United States), SPSS (version 20.0; IBM Corp., New York, NY, United States), and Stata software, version 12 (StataCorp, College Station, TX, United States). A 2-sided P-value less than 0.05 was considered significant.
RESULTS
Baseline characteristics of the propensity score-matched CKD patients
Table 1 summarizes the baseline profiles of the three study cohorts. The distribution by age, sex, comorbidity, and use of ACEI/ARB, except for enrollee category, CCI score, and the number of medical visits, was balanced among the three cohorts.
Table 1.
Baseline characteristics of the three study cohorts, 1997-2012, n = 6266
| Variables |
Propensity score-matched CKD patients |
|||
| Treated (n = 482) | Untreated (n = 1928) | Uninfected (n = 3856) | P1 value | |
| Sex | 0.38 | |||
| Men | 253 (52.5) | 979 (50.8) | 2032 (52.7) | |
| Women | 229 (47.5) | 949 (49.2) | 1824 (47.3) | |
| Age in yr | 58.5 ± 10.5 | 58.5 ± 13.7 | 58.4 ± 14.1 | 0.96 |
| Interferon-based therapy duration in yr | 0.5 ± 0.8 | - | - | |
| Comorbidity | ||||
| Diabetes | 228 (47.3) | 878 (45.5) | 1727 (44.8) | 0.55 |
| Hypertension | 290 (60.2) | 1150 (59.6) | 2315 (60.0) | 0.95 |
| Coronary heart disease | 182 (37.8) | 746 (38.7) | 1519 (39.4) | 0.73 |
| Hyperlipidemia | 239 (49.6) | 961 (49.8) | 1938 (50.3) | 0.93 |
| Cirrhosis | 120 (24.9) | 446 (23.1) | 892 (23.1) | 0.68 |
| ACEI/ARB use | 14 (2.9) | 37 (1.9) | 66 (1.7) | 0.19 |
| Enrollee category | < 0.0001 | |||
| 1 | 152 (31.5) | 546 (28.3) | 1332 (34.5) | |
| 2 | 10 (2.1) | 30 (1.6) | 61 (1.6) | |
| 3 | 255 (52.9) | 968 (50.2) | 1622 (42.1) | |
| 4 | 65 (13.5) | 384 (19.9) | 841 (21.8) | |
| Number of medical visits | 38.6 ± 25.6 | 34.4 ± 26.7 | 30.0 ± 25.0 | < 0.0001 |
| Charlson comorbidity index score | 4.2 ± 2.7 | 3.5 ± 2.6 | 3.2 ± 2.7 | < 0.0001 |
Data are presented as n (%).
For comparison among three cohorts. Categorical variables given as number (percentage); continuous variable, as mean ± SD. CKD: Chronic kidney disease; ACEI/ARB: Angiotensin converting enzyme inhibitor/angiotensin receptor blocker.
Cumulative incidences of ESRD and death in the three study cohorts
During the total mean follow-up of 6.0 ± 4.4 years, 327 patients (5.2%) developed ESRD, with 5 (1.0%), 134 (7.0%), and 188 (4.9%) in the treated, untreated, and uninfected cohorts, respectively (P < 0.0001), and 1570 patients (25.1%) died, with 61 (12.7%), 648 (33.6%), and 861 (22.3%) in the treated, untreated, and uninfected cohorts, respectively (P < 0.0001) (Table 2). The 16-year cumulative incidence of ESRD was significantly higher in the untreated cohort [11.7%, 95% confidence interval (CI): 8.0%-16.1%] as compared to the treated (2.4%, 95%CI: 0.9%-5.2%) and uninfected cohorts (8.2%, 95%CI: 6.2%-10.5%), respectively (P = 0.0032). The 16-year cumulative incidence of death was significantly higher in the untreated cohort (58.0%, 95%CI: 51.5%-63.9%) as compared to the treated (41.4%, 95%CI: 8.1%-54.1%) and uninfected cohorts (37.8%, 95%CI: 34.4%-41.3%), respectively (P < 0.0001).
Table 2.
Outcomes between treated, untreated, and uninfected cohorts, n = 6266
| Treated cohort (n = 482) | Untreated cohort (n = 1928) | Uninfected cohort (n = 3856) | P value | |
| End-stage renal disease | ||||
| Events number (%) | 5 (1.0) | 134 (7.0) | 188 (4.9) | < 0.0001 |
| Competing mortality (%) | 58 (12.0) | 573 (29.7) | 775 (20.1) | < 0.0001 |
| Cumulative incidence (%) | 2.4 (95%CI: 0.9-5.2) | 11.7 (95%CI: 8.0-16.1) | 8.2 (95%CI: 6.2-10.5) | 0.0032 |
| Overall mortality | ||||
| Events number (%) | 61 (12.7) | 648 (33.6) | 861 (22.3) | < 0.0001 |
| Cumulative incidence (%) | 41.4 (95%CI: 8.1-54.1) | 58.0 (95%CI: 51.5-63.9) | 37.8 (95%CI: 34.4-41.3) | < 0.0001 |
IBT in association with ESRD risk after multivariate and competing mortality adjustment
Taking the uninfected cohort as a reference, the adjusted hazard ratios for ESRD were 1.28 (1.03-1.60, P = 0.029) and 0.34 (0.14-0.84, P = 0.019) in the untreated and treated cohorts, respectively (Table 3). In addition, diabetes (3.06, 2.37-3.95, P < 0.001), hypertension (3.51, 2.57-4.79, P < 0.001), and enrollee category 4 (1.4, 1.03-1.92, P = 0.03) were also associated with an increased risk of ESRD. Advanced aging (0.99, 0.98-0.99, P = 0.006), cirrhosis (0.61, 0.44-0.84, P = 0.003), and CCI score (0.93, 0.89-0.98, P = 0.008) were associated with a lower risk of ESRD.
Table 3.
Crude and adjusted hazard ratios for end-stage renal disease
| Variable |
Crude |
Adjusted1 |
||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| CKD patients | ||||||
| Uninfected | 1.00 | Reference | 1.00 | Reference | ||
| Treated | 0.31 | 0.13-0.77 | 0.011 | 0.34 | 0.14-0.84 | 0.019 |
| Untreated | 1.25 | 1.00-1.56 | 0.046 | 1.28 | 1.03-1.60 | 0.029 |
| Sex, men/women | 1.11 | 0.89-1.38 | 0.35 | 1.23 | 0.99-1.55 | 0.07 |
| Age, per year | 1.01 | 1.00-1.01 | 0.15 | 0.99 | 0.98-0.99 | 0.006 |
| Comorbidity, yes/no | ||||||
| Diabetes | 2.87 | 2.28-3.61 | < 0.001 | 3.06 | 2.37-3.95 | < 0.001 |
| Hypertension | 3.08 | 2.37-4.01 | < 0.001 | 3.51 | 2.57-4.79 | < 0.001 |
| Coronary heart disease | 1.28 | 1.02-1.59 | 0.031 | 0.92 | 0.72-1.16 | 0.46 |
| Hyperlipidemia | 1.29 | 1.04-1.60 | 0.023 | 0.85 | 0.68-1.07 | 0.16 |
| Cirrhosis | 0.62 | 0.46-0.83 | 0.001 | 0.61 | 0.44-0.84 | 0.003 |
| ACEI/ARB, yes/no | 1.38 | 0.65-2.92 | 0.40 | 0.96 | 0.46-2.04 | 0.92 |
| Enrollee category | ||||||
| 1 | 1.00 | Reference | 1.00 | Reference | ||
| 2 | 0.68 | 0.21-2.14) | 0.50 | 0.56 | 0.17-1.80 | 0.33 |
| 3 | 1.26 | 0.97-1.64 | 0.08 | 1.24 | 0.95-1.63 | 0.11 |
| 4 | 1.47 | 1.09-1.99 | 0.012 | 1.40 | 1.03-1.92 | 0.03 |
| Number of medical visits | 1.00 | 1.00-1.01 | 0.36 | 1.00 | 0.99-1.00 | 0.41 |
| Charlson comorbidity index score | 1.01 | 00.98-1.04 | 0.46 | 0.93 | 0.89-0.98 | 0.008 |
Adjusted for all covariates (age per year, sex, comorbidity, ACEI/ARB, enrollee category, number of medical visits, and Charlson comorbidity index score) and competing mortality. CKD: Chronic kidney disease; ACEI/ARB: Angiotensin converting enzyme inhibitor/angiotensin receptor blocker; CI: Confidence interval.
Sensitivity analysis
We compared individual ESRD and death risks between the treated versus untreated cohorts as well as between the untreated versus uninfected cohorts (Table 4). The treated cohort had 29% (0.54-0.92, P = 0.011) and 72% (0.11-0.71, P = 0.007) decreases in death and ESRD, respectively, as compared with the untreated cohort, which had 31% (1.18-1.45, P < 0.001) and 28% (1.03-1.1, P = 0.028) increases in death and ESRD, respectively, as compared with the uninfected cohort.
Table 4.
Sensitivity analysis of adjusted hazard ratios for end-stage renal disease and death between the untreated and uninfected chronic kidney disease patients as well as between the treated and untreated chronic kidney disease patients
|
Adjusted HR for ESRD1 |
Adjusted HR for death2 |
|||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Propensity score-matched CKD patients | ||||||
| Uninfected cohort (n = 3856) | 1.00 | Reference | 1.00 | Reference | ||
| Untreated HCV-infected cohort (n = 1928) | 1.28 | 1.03-1.61 | 0.028 | 1.31 | 1.18-1.45 | < 0.001 |
| Propensity score-matched CKD patients | ||||||
| Untreated HCV-infected cohort (n = 1928) | 1.00 | Reference | 1.00 | Reference | ||
| Treated HCV-infected cohort (n = 482) | 0.28 | 0.11-0.71 | 0.007 | 0.71 | 0.54-0.92 | 0.011 |
Adjusted for all covariates (age per year, sex, comorbidity, ACEI/ARB, enrollee category, number of medical visits, and Charlson comorbidity index score) and competing mortality;
Adjusted for all covariates (age per year, sex, comorbidity, ACEI/ARB, enrollee category, number of medical visits, and Charlson comorbidity index score). CKD: Chronic kidney disease; CI: Confidence interval; ACEI/ARB: Angiotensin converting enzyme inhibitor/angiotensin receptor blocker; ESRD: End-stage renal disease; HCV: Hepatitis C virus.
Impact of IBT duration on the risk of ESRD
Of those in the treated cohort, 356 (74%) participants received 4 or more mo of IBT (Table 5). These individuals had 86% (0.03-0.58, P = 0.007) and 43% (0.41-0.79, P = 0.001) decreases in ESRD and mortality, respectively.
Table 5.
The effect of the duration of interferon-based therapy for hepatitis C virus infection on the risk of end-stage renal disease and death
| IBT duration | ESRD events (%) | Adjusted HR1 (95%CI) | P value | Death events (%) | Adjusted HR2 (95%CI) | P value | |
| Propensity score-matched HCV-infected CKD patients (n = 2410) | No (n = 1928) | 134 (7.0) | 1.00 (reference) | 648 (33.6) | 1.00 (reference) | ||
| < 4 mo (n = 126) | 3 (2.4) | 0.79 (0.24-2.63) | 0.70 | 23 (18.3) | 1.18 (0.78-1.81) | 0.44 | |
| ≥ 4 mo (n = 356) | 2 (0.6) | 0.14 (0.03-0.58) | 0.007 | 38 (10.7) | 0.57 (0.41-0.79) | 0.001 |
1Adjusted for all covariates (age per year, sex, comorbidity, ACEI/ARB, enrollee category, number of medical visits, and Charlson comorbidity index score) and competing mortality; 2Adjusted for all covariates (age per year, sex, comorbidity, ACEI/ARB, enrollee category, number of medical visits, and Charlson comorbidity index score). CKD: Chronic kidney disease; CI: Confidence interval; ACEI/ARB: Angiotensin converting enzyme inhibitor/angiotensin receptor blocker; ESRD: End-stage renal disease; HCV: Hepatitis C virus.
Stratified analyses according to patient subgroups
The associations between treatment and risk reduction of ESRD were consistent across most strata, except for patients without hypertension (Supplementary Figure 2A). The associations between lack of treatment and risk increment of ESRD were consistent across all strata (Supplementary Figure 2B).
DISCUSSION
The present study is the first large national cohort to investigate the long-term effects of treating HCV infection in patients with CKD stages 1-5 of any etiology on hard endpoints after taking propensity score matching and competing risk analysis into consideration. Our most encouraging finding is a significant 66% decrease and a 28% increase in the ESRD risk in treated and untreated HCV-infected CKD patients, respectively, compared with uninfected CKD patients, during a total mean follow-up of six years. Of note, a greater risk reduction of ESRD and death occurred in those receiving ≥ 4 mo of IBT, and these risks were not attenuated when IBT was incomplete, and its duration was shorter than 4 mo. In addition to improved renal survival, the treated cohort had a significant 29% decrease in mortality compared with the untreated cohort.
Our results have three clinical implications. First, our results support the practice of routine HCV testing in all CKD patients for early detection and treatment[3,38] to ameliorate CKD progression and mortality regardless of the severity of the kidney disease. Second, our results fill the knowledge gap in the long-term impact of HCV therapy in CKD patients and serve as a reference to accelerate the study of long-term outcomes and safety of DAAs in CKD patients, given the better tolerance and efficacy of DAAs in CKD patients. Third, the renal benefits from eliminating HCV after adequate IBT suggest the pathogenic role of HCV in renal injuries.
The goal of anti-HCV treatment is to achieve SVR by viral clearance[2,26]. Anti-HCV treatment with IBT, particularly the successful attainment of SVR[24,39], has been shown to improve renal[20-22,24] and non-renal[19,20,22,39] prognosis in the general population[19-21] and patients with cirrhosis[39], diabetes[22], and glomerulonephritis[24]. Three studies that examined the association between SVR based on IBT and renal and survival benefits have been documented in patients with cirrhosis and glomerulonephritis. A hospital-based retrospective study[23] of 650 cirrhotic Japanese patients showed that failure to achieve SVR was a predictor of CKD during a mean follow-up of 6.5 yr. Recently, a multicenter prospective study[39] of 1323 cirrhotic French patients showed that the success to achieve SVR decreased the overall mortality in a median follow-up of 4.8 yr. A meta-analysis[24] involving 11 short follow-up small-scale clinical trials (one controlled and ten uncontrolled) of 225 patients with HCV-infected glomerulone-phritis indicated that IBT alleviated greater proteinuria when SVR was achieved and stabilized serum creatinine (both were used as soft renal endpoints). Regrettably, the impact on hard endpoints of ESRD and patient survival remained unknown.
The present study, despite its retrospective design, is the first large-scale analysis with a long follow-up to address the hard endpoints in HCV-treated CKD patients. Although a randomized placebo-controlled trial is the ideal design to appraise the effectiveness of an intervention, it seems impractical because of the greater expense, longer observation time, and violation of research ethics while performing randomization to placebo[20]. Despite the paucity of SVR to directly measure the therapeutic response, four Taiwanese retrospective interventional cohorts that enrolled individuals from the general population[19-21] and diabetic[22] patients used the NHIRD to address the amelioration of CKD[21], ESRD[20,22], overall mortality[20], and non-renal[19,20,22] complications conferred by IBT.
The efficacy of IBT appears convincing: In the treated cohort, IBT generally achieved an eradication rate of over 70% in Taiwan due to the prevalent and favorable genetic variation in interleukin-28B[20,22]. Another hospital-based American retrospective analysis[40] of 159 HCV-infected patients treated with IBT indicated that a history of IBT was a significant negative predictor of CKD. Similarly to the aforementioned studies, our results demonstrated renal and survival benefits in HCV-infected patients with CKD stages 1-5 who received IBT. In agreement with one American retrospective study[18] of 1603 untreated HCV-infected patients with CKD stages 3-5 during a mean follow-up of 3.8 yr, our results also found a 28% increase in the ESRD risk in untreated compared with uninfected CKD patients. We further demonstrated that treated compared with untreated CKD patients had significant 72% and 29% decreases in ESRD and mortality risks, respectively. Of note, the treated cohort receiving IBT for ≥ 4 mo experienced greater clinical benefits, but the risk of ESRD and mortality failed to decline if the duration of IBT was shorter than 4 mo, a result comparable with a prior Taiwanese study[20].
However, the impact of the degree of HCV viremia on the risk of progression to ESRD in CKD patients remains uncertain, although prior research[14,16] found a higher percentage of viremia in CKD patients with positive anti-HCV antibodies. Three studies examined the association between the degree of viremia and worsening renal outcomes in the general population[40,41] and HIV/HCV-coinfected patients[42]. A prospective cohort of 8235 HIV/HCV-coinfected patients demonstrated that patients with HCV-RNA titers over 615 IU/mL were at an increased risk for CKD during 36123 person-years of follow-up[42]. A community-based prospective cohort41 of 19984 Taiwanese individuals, with over 15 years of follow-up, revealed that HCV-RNA levels increased the risk of ESRD in a dose-dependent manner, with the highest risk in patients with HCV-RNA over 167000 IU/mL. A hospital-based retrospective analysis[40] that compared 552 HCV-infected American patients with matched controls indicated that a high baseline HCV-RNA viral load (> 700000 cps/mL) was a significant positive predictor for CKD at 74 mo.
Taken collectively, published evidence supports the clinical benefits of IBT, suggesting that eliminating the virus underlie this association. Given the higher prevalence of HCV infection[43] and the higher potential of viremia in CKD patients[13,14,16], the renal and survival benefits from HCV therapy in CKD patients seem promising. Further research is warranted to clarify how the influence of viremia and SVR correlate with clinical outcomes in CKD patients.
The exact mechanisms through which HCV therapy improves clinical outcomes have not been fully elucidated, but based on the existing clinical evidence they are most likely mediated by viral clearance[23,24,40]. HCV can trigger local and systemic oxidative stress and promote IR, endothelial dysfunction, and accelerated atherosclerosis, all of which contribute to renal injury and mortality[7]. The su-ppression of HCV replication by IBT seems to alleviate these injuries. Treating HCV infection by either IBT or DAAs in CKD patients is challenging. The former is cheaper and universal but not tolerated as well, and this might account for 10.5% of the 4582 HCV-infected CKD patients in the present study. The latter is well tolerated but very costly, which limits patient access and has a significant risk of drug-drug interactions[26,27] with concomitant polypharmacy in CKD patients that have multiple comorbidities. DAAs can be used in patients with kidney disease depending on their hepatic metabolism, but this does not guarantee the lack of adverse renal effects in cirrhotic patients, who are at a high risk of renal pharmacokinetic changes[27]. Another growing concern is the reactivation of hepatitis B[44], which is also a risk factor for CKD progression[32]. Further cost-effectiveness analyses comparing the costs of IBT with DAAs in HCV-infected CKD patients are warranted in the future.
In line with a Taiwanese study by Wu et al[22] that examined the use of IBT in a large diabetic cohort, the 16-year cumulative incidence of ESRD was greater in our untreated CKD cohort, followed by the uninfected and treated CKD cohorts. However, the 16-year cumulative incidence of ESRD was strikingly lower in the treated than in the uninfected CKD cohorts. This might be because the sample size was smaller in the treated than in the uninfected CKD cohorts. In accordance with the aforementioned study[22], the 16-year cumulative mortality was higher in our untreated CKD cohort, followed by the treated and uninfected CKD cohorts. Compared with the uninfected CKD cohort, our treated CKD cohort still had a higher cumulative mortality, which may be attributable to their higher CCI scores. Furthermore, we found that CKD patients with HCV who underwent HCV treatment had lower risk of ESRD compared to the risk in CKD patients without HCV infection. Wu et al[22] similarly observed that diabetic patients with HCV who underwent HCV treatment had a lower risk of ESRD compared to the risk in diabetic patients without HCV infection. The efficacy of anti-HCV therapy in alleviating IR, which has been convincingly demonstrated in previous research[45], may account for the abovementioned finding. IR is a prevalent feature in CKD[46] and diabetes[47]. The clinical impacts of IR include endothelial dysfunction and initiation and progression of CKD[46]. This is why IR may be a therapeutic target in the attempt to improve clinical outcomes of CKD[46] and diabetic vascular complications[48]. The mechanism through which antiviral therapy ameliorates IR was most likely mediated via viral clearance, instead of direct pharmacological effects of IBT[22]. Conjeevaram et al[45] reported that successful viral eradication was central to sustain the beneficial effects in IR. We believe that our finding should result from viral elimination in the treated patients, although this study could not directly measure SVR because of the absence of laboratory information in the NHIRD. Future research is warranted to better understand the pathological mechanism underlying this association. Our results after competing risk analysis showed that aging was associated with a lower risk of ESRD, a result consistent with prior research[8,32] showing that younger rather than older age predicted ESRD.
In addition to the universal coverage of a nationwide population to reduce selection bias, the study has several methodological strengths. First, maximum cardinality matching of treated and untreated patients was performed according to the propensity score to optimize comparability[21], which is an effective method of pseudorandomization when the effects of treatment and interventions are compared. Second, to avoid immortal time bias, we used the same year of IBT prescription time distribution[33,34]. Third, taking competing mortality into consideration to avoid overestimating the results in the untreated cohort[20] is another merit. Fourth, the well-known renoprotective ACEI/ARB was also included in the final analysis. On the whole, despite its retrospective nature, the present study provides the most persuasive data to date in addressing the long-term renal and survival benefits of treating HCV infection in CKD patients.
Some limitations of our study should be mentioned. First, the adverse reactions and actual compliance related to IBT were not assessed in the NHIRD. However, 74% of the treated CKD patients in our study used IBT for more than 4 mo. Excessive prescription of IBT is impossible under the strict regulations in Taiwan's NHI. Second, due to the lack of laboratory data in the NHIRD, the association between the HCV genotype, viral load, SVR, CKD stages (severity), and survival failed to be clarified. Third, lifestyle and family history were not available in the NHIRD either. Fourth, some patients who spontaneously cleared the virus were enrolled as untreated cohort. Nonetheless, this might lead to the underestimation of the adverse events in the untreated cohort[20]. Finally, caution is advised before applying our results to the West because of higher antiviral efficacy of IBT in Taiwan than in most Western coun-tries[22].
In conclusion, this nationwide cohort study shows that treating HCV infection with IBT in CKD patients is associated with improved long-term renal and patient survivals. Further large-scale research on the long-term clinical outcomes of DAAs in CKD patients seems to deserve attention.
ARTICLE HIGHLIGHTS
Research background
It is unknown whether adequate hepatitis C virus (HCV) treatment [interferon-based therapy (IBT) or new direct-acting antivirals (DAAs)] improves long-term renal and patient survivals in chronic kidney disease (CKD) patients with HCV infection. Yet, there is a significant value to explore this critical issue.
Research motivation
There is a significant and increasing burden of CKD, end-stage renal disease (ESRD), and HCV infection in Taiwan and worldwide. Taiwan provides an ideal setting for studying this relationship because it has a high prevalence of these three conditions. Because information on DAAs was not available in Taiwan’s single-payer national health insurance database currently released for research, we performed a retrospective analysis of IBT, in CKD patients with HCV infection to increase our understanding of their long-term outcomes following HCV infection and HCV treatment.
Research objectives
To evaluate the long-term outcomes (ESRD and death) of HCV treatment, especially IBT, in HCV-infected patients with stage 1-5 CKD.
Research methods
By analyzing the Taiwan Longitudinal Health Insurance Database 2005, the authors used propensity score-matched and competing risk analyses to evaluate the long-term effect of HCV infection with and without IBT on the risks of ESRD and death in CKD patients. All participants were followed until the occurrence of ESRD, death, or the end of 2012.
Research results
Taking the uninfected cohort as a reference, the adjusted hazard ratios for ESRD, after adjusting for competing mortality, were 0.34 (0.14-0.84, P = 0.019) and 1.28 (1.03-1.60, P = 0.029) in the treated and untreated cohorts, respectively. The treated cohort had a 29% (0.54-0.92, P = 0.011) decrease in mortality compared to the untreated cohort, in which the mortality was 31% (1.18-1.45, P < 0.001) higher than in the uninfected cohort. The reduced risks of ESRD (0.14, 0.03–0.58, P = 0.007) and death (0.57, 0.41-0.79, P = 0.001) were greatest in HCV-infected CKD patients who received at least 4 mo of IBT, which accounted for 74% of the treated cohort.
Research conclusions
To the best of our knowledge, this is the first study to investigate the long-term renal and patient outcomes in CKD patients with HCV infection and HCV treatment. Adequate HCV treatment in CKD patients improves long-term renal and patient survivals.
Research perspectives
Future prospective study is warranted to confirm our findings with new DAAs and better understand the pathological mechanism underlying this association.
Footnotes
Institutional review board statement: This study was approved by the institutional review board of the Dalin Tzu Chi Hospital (B10302011).
Informed consent statement: All patient information was de-identified in the database (LHID2005) and no informed consent was required. This study was exempt from a full ethical review by the institutional review board of the Dalin Tzu Chi Hospital (B10302011).
Conflict-of-interest statement: All authors have no conflict of interests.
Manuscript source: Unsolicited manuscript
Peer-review started: March 4, 2019
First decision: March 27, 2019
Article in press: April 19, 2019
Specialty type: Medicine, research and experimental
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ohsawa M, Shimizu Y S-Editor: Ji FF L-Editor: Filipodia E-Editor: Xing YX
Contributor Information
Yi-Chun Chen, Division of Nephrology, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi County 622, Taiwan. chenyichun0320@yahoo.com.tw; School of Medicine, Tzu Chi University, Hualien 970, Taiwan.
Chung-Yi Li, Department and Graduate Institute of Public Health, College of Medicine, National Cheng Kung University, Tainan 701, Taiwan; Department of Public Health, College of Public Health, China Medical University, Taichung 404, Taiwan.
Shiang-Jiun Tsai, Department of Medical Research, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi County 622, Taiwan.
Yen-Chun Chen, Division of Hepato-Gastroenterology, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi County 622, Taiwan.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;109:S1–99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 2.Azmi AN, Tan SS, Mohamed R. Hepatitis C and kidney disease: An overview and approach to management. World J Hepatol. 2015;7:78–92. doi: 10.4254/wjh.v7.i1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chacko EC, Surrun SK, Mubarack Sani TP, Pappachan JM. Chronic viral hepatitis and chronic kidney disease. Postgrad Med J. 2010;86:486–492. doi: 10.1136/pgmj.2009.092775. [DOI] [PubMed] [Google Scholar]
- 4.Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009;4:207–220. doi: 10.2215/CJN.03710708. [DOI] [PubMed] [Google Scholar]
- 5.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014;85:1200–1207. doi: 10.1038/ki.2013.455. [DOI] [PubMed] [Google Scholar]
- 6.Chen YC, Chiou WY, Hung SK, Su YC, Hwang SJ. Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: a 6-year nationwide cohort study across Taiwan. BMC Nephrol. 2013;14:187. doi: 10.1186/1471-2369-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon CE, Balk EM, Becker BN, Crooks PA, Jaber BL, Johnson CA, Michael MA, Pereira BJ, Uhlig K, Levin A. KDOQI US commentary on the KDIGO clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in CKD. Am J Kidney Dis. 2008;52:811–825. doi: 10.1053/j.ajkd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Tsui JI, Vittinghoff E, Shlipak MG, Bertenthal D, Inadomi J, Rodriguez RA, O'Hare AM. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271–1276. doi: 10.1001/archinte.167.12.1271. [DOI] [PubMed] [Google Scholar]
- 9.Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar-Zadeh K, Kovesdy CP. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61:1495–1502. doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JJ, Lin MY, Chang JS, Hung CC, Chang JM, Chen HC, Yu ML, Hwang SJ. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PLoS One. 2014;9:e100790. doi: 10.1371/journal.pone.0100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crook ED, Penumalee S, Gavini B, Filippova K. Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care. 2005;28:2187–2191. doi: 10.2337/diacare.28.9.2187. [DOI] [PubMed] [Google Scholar]
- 12.Noureddine LA, Usman SA, Yu Z, Moorthi RN, Moe SM. Hepatitis C increases the risk of progression of chronic kidney disease in patients with glomerulonephritis. Am J Nephrol. 2010;32:311–316. doi: 10.1159/000319456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Cavoli G, Ferrantelli A, Bono L, Tortorici C, Giammarresi C, Zagarrigo C, Schillaci O, Tralongo A, Soresi M, Rotolo U. Incidence of hepatitis C virus infection in patients with chronic kidney disease on conservative therapy. Int J Infect Dis. 2011;15:e514–e516. doi: 10.1016/j.ijid.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Valdecasas J, Bernal C, Garcia F, Cerezo S, Umana WO, von Albertini B, Kimmel PL. Epidemiology of hepatitis C virus infection in patients with renal disease. J Am Soc Nephrol. 1994;5:186–192. doi: 10.1681/ASN.V52186. [DOI] [PubMed] [Google Scholar]
- 15.Sit D, Kadiroglu AK, Kayabasi H, Yilmaz ME, Goral V. Seroprevalence of hepatitis B and C viruses in patients with chronic kidney disease in the predialysis stage at a university hospital in Turkey. Intervirology. 2007;50:133–137. doi: 10.1159/000098239. [DOI] [PubMed] [Google Scholar]
- 16.Lemos LB, Perez RM, Lemos MM, Draibe SA, Silva IS, Silva AE, Ferraz ML. Hepatitis C among predialysis patients: prevalence and characteristics in a large cohort of patients. Nephron Clin Pract. 2008;108:c135–c140. doi: 10.1159/000114452. [DOI] [PubMed] [Google Scholar]
- 17.Fabrizi F, Marcelli D, Bacchini G, Guarnori I, Erba G, Locatelli F. Antibodies to hepatitis C virus (HCV) in chronic renal failure (CRF) patients on conservative therapy: prevalence, risk factors and relationship to liver disease. Nephrol Dial Transplant. 1994;9:780–784. [PubMed] [Google Scholar]
- 18.Tartof SY, Hsu JW, Wei R, Rubenstein KB, Hu H, Arduino JM, Horberg M, Derose SF, Qian L, Rodriguez CV. Kidney Function Decline in Patients with CKD and Untreated Hepatitis C Infection. Clin J Am Soc Nephrol. 2018;13:1471–1478. doi: 10.2215/CJN.01530218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu CS, Huang CJ, Kao JH, Lin HH, Chao YC, Fan YC, Tsai PS. Interferon-based therapy decreases risks of hepatocellular carcinoma and complications of cirrhosis in chronic hepatitis C patients. PLoS One. 2013;8:e70458. doi: 10.1371/journal.pone.0070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu YC, Ho HJ, Huang YT, Wang HH, Wu MS, Lin JT, Wu CY. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut. 2015;64:495–503. doi: 10.1136/gutjnl-2014-308163. [DOI] [PubMed] [Google Scholar]
- 21.Chen YC, Hwang SJ, Li CY, Wu CP, Lin LC. A Taiwanese Nationwide Cohort Study Shows Interferon-Based Therapy for Chronic Hepatitis C Reduces the Risk of Chronic Kidney Disease. Medicine (Baltimore) 2015;94:e1334. doi: 10.1097/MD.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, Wu MS, Liu YY, Wu CY. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 23.Arase Y, Suzuki F, Kawamura Y, Suzuki Y, Kobayashi M, Matsumoto N, Akuta N, Sezaki H, Hosaka T, Ogawa K, Imai N, Seko Y, Saito S, Ikeda K, Kobayashi M, Kumada H. Development rate of chronic kidney disease in hepatitis C virus patients with advanced fibrosis after interferon therapy. Hepatol Res. 2011;41:946–954. doi: 10.1111/j.1872-034X.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 24.Feng B, Eknoyan G, Guo ZS, Jadoul M, Rao HY, Zhang W, Wei L. Effect of interferon-alpha-based antiviral therapy on hepatitis C virus-associated glomerulonephritis: a meta-analysis. Nephrol Dial Transplant. 2012;27:640–646. doi: 10.1093/ndt/gfr236. [DOI] [PubMed] [Google Scholar]
- 25.Li T, Qu Y, Guo Y, Wang Y, Wang L. Efficacy and safety of direct-acting antivirals-based antiviral therapies for hepatitis C virus patients with stage 4-5 chronic kidney disease: a meta-analysis. Liver Int. 2017;37:974–981. doi: 10.1111/liv.13336. [DOI] [PubMed] [Google Scholar]
- 26.Fabrizi F, Martin P, Messa P. New treatment for hepatitis C in chronic kidney disease, dialysis, and transplant. Kidney Int. 2016;89:988–994. doi: 10.1016/j.kint.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Isnard Bagnis C, Cacoub P. Hepatitis C Therapy in Renal Patients: Who, How, When? Infect Dis Ther. 2016;5:313–327. doi: 10.1007/s40121-016-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carvalho-Filho RJ, Feldner AC, Silva AE, Ferraz ML. Management of hepatitis C in patients with chronic kidney disease. World J Gastroenterol. 2015;21:408–422. doi: 10.3748/wjg.v21.i2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YC, Su YC, Li CY, Wu CP, Lee MS. A nationwide cohort study suggests chronic hepatitis B virus infection increases the risk of end-stage renal disease among patients in Taiwan. Kidney Int. 2015;87:1030–1038. doi: 10.1038/ki.2014.363. [DOI] [PubMed] [Google Scholar]
- 30.Tung CH, Lai NS, Li CY, Tsai SJ, Chen YC, Chen YC. Risk of rheumatoid arthritis in patients with hepatitis C virus infection receiving interferon-based therapy: a retrospective cohort study using the Taiwanese national claims database. BMJ Open. 2018;8:e021747. doi: 10.1136/bmjopen-2018-021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang JH, Tsai SJ, Liu TC, Chen YC, Lai JT. Association of Tinnitus and Other Cochlear Disorders With a History of Migraines. JAMA Otolaryngol Head Neck Surg. 2018;144:712–717. doi: 10.1001/jamaoto.2018.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YC, Li CY, Tsai SJ, Chen YC. Nationwide cohort study suggests that nucleos(t)ide analogue therapy decreases dialysis risk in Taiwanese chronic kidney disease patients acquiring hepatitis B virus infection. World J Gastroenterol. 2018;24:917–928. doi: 10.3748/wjg.v24.i8.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162:1016–1023. doi: 10.1093/aje/kwi307. [DOI] [PubMed] [Google Scholar]
- 34.Ramos R, García-Gil M, Comas-Cufí M, Quesada M, Marrugat J, Elosua R, Sala J, Grau M, Martí R, Ponjoan A, Alves-Cabratosa L, Blanch J, Bolíbar B. Statins for Prevention of Cardiovascular Events in a Low-Risk Population With Low Ankle Brachial Index. J Am Coll Cardiol. 2016;67:630–640. doi: 10.1016/j.jacc.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 35.Gershon A, Croxford R, To T, Stanbrook MB, Upshur R, Sanchez-Romeu P, Stukel T. Comparison of inhaled long-acting β-agonist and anticholinergic effectiveness in older patients with chronic obstructive pulmonary disease: a cohort study. Ann Intern Med. 2011;154:583–592. doi: 10.7326/0003-4819-154-9-201105030-00003. [DOI] [PubMed] [Google Scholar]
- 36.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 37.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 38.Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, Marcellin P, Guyader D, Fontaine H, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Leroy V, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Dharancy S, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Benhamou Y, Pilette C, Silvain C, Christidis C, Capron D, Bernard-Chabert B, Zucman D, Di Martino V, Thibaut V, Salmon D, Ziol M, Sutton A, Pol S, Roudot-Thoraval F ANRS CO12 CirVir Group. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology. 2017;152:142–156.e2. doi: 10.1053/j.gastro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Satapathy SK, Lingisetty CS, Williams S. Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection. Hepatol Int. 2012;6:369–378. doi: 10.1007/s12072-011-9284-9. [DOI] [PubMed] [Google Scholar]
- 41.Lai TS, Lee MH, Yang HI, You SL, Lu SN, Wang LY, Yuan Y, L'Italien G, Chien KL, Chen CJ REVEAL-HCV Study Group. Hepatitis C viral load, genotype, and increased risk of developing end-stage renal disease: REVEAL-HCV study. Hepatology. 2017;66:784–793. doi: 10.1002/hep.29192. [DOI] [PubMed] [Google Scholar]
- 42.Peters L, Grint D, Lundgren JD, Rockstroh JK, Soriano V, Reiss P, Grzeszczuk A, Sambatakou H, Mocroft A, Kirk O EuroSIDA in EuroCoord. Hepatitis C virus viremia increases the incidence of chronic kidney disease in HIV-infected patients. AIDS. 2012;26:1917–1926. doi: 10.1097/QAD.0b013e3283574e71. [DOI] [PubMed] [Google Scholar]
- 43.Ladino M, Pedraza F, Roth D. Opportunities for treatment of the hepatitis C virus-infected patient with chronic kidney disease. World J Hepatol. 2017;9:833–839. doi: 10.4254/wjh.v9.i19.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, Brinker A. Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System. Ann Intern Med. 2017;166:792–798. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 45.Conjeevaram HS, Wahed AS, Afdhal N, Howell CD, Everhart JE, Hoofnagle JH Virahep-C Study Group. Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology. 2011;140:469–477. doi: 10.1053/j.gastro.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Teta D. Insulin resistance as a therapeutic target for chronic kidney disease. J Ren Nutr. 2015;25:226–229. doi: 10.1053/j.jrn.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 47.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32 Suppl 2:S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang YS, Lee MH, Song HK, Hyun YY, Cha JJ, Ko GJ, Kim SH, Lee JE, Han JY, Cha DR. Aliskiren improves insulin resistance and ameliorates diabetic vascular complications in db/db mice. Nephrol Dial Transplant. 2011;26:1194–1204. doi: 10.1093/ndt/gfq579. [DOI] [PubMed] [Google Scholar]


