Abstract
BACKGROUND
The safety and feasibility of the simultaneous resection of primary colorectal cancer (CRC) and synchronous colorectal liver metastases (SCRLM) have been demonstrated in some studies. Combined resection is expected to be the optimal strategy for patients with CRC and SCRLM. However, traditional laparotomy is traumatic, and the treatment outcome of minimally invasive surgery (MIS) is still obscure.
AIM
To compare the treatment outcomes of MIS and open surgery (OS) for the simultaneous resection of CRC and SCRLM.
METHODS
A systematic search through December 22, 2018 was conducted in electronic databases (PubMed, EMBASE, Web of Science, and Cochrane Library). All studies comparing the clinical outcomes of MIS and OS for patients with CRC and SCRLM were included by eligibility criteria. The meta-analysis was performed using Review Manager Software. The quality of the pooled study was assessed using the Newcastle-Ottawa scale. The publication bias was evaluated by a funnel plot and the Begg’s and Egger’s tests. Fixed- and random-effects models were applied according to heterogeneity.
RESULTS
Ten retrospective cohort studies involving 502 patients (216 patients in the MIS group and 286 patients in the OS group) were included in this study. MIS was associated with less intraoperative blood loss [weighted mean difference (WMD) = -130.09, 95% confidence interval (CI): -210.95 to -49.23, P = 0.002] and blood transfusion [odds ratio (OR) = 0.53, 95%CI: 0.29 to 0.95, P = 0.03], faster recovery of intestinal function (WMD = -0.88 d, 95%CI: -1.58 to -0.19, P = 0.01) and diet (WMD = -1.54 d, 95%CI: -2.30 to -0.78, P < 0.0001), shorter length of postoperative hospital stay (WMD = -4.06 d, 95%CI: -5.95 to -2.18, P < 0.0001), and lower rates of surgical complications (OR = 0.60, 95%CI: 0.37 to 0.99, P = 0.04). However, the operation time, rates and severity of overall complications, and rates of general complications showed no significant differences between the MIS and OS groups. Moreover, the overall survival and disease-free survival after MIS were equivalent to those after OS.
CONCLUSION
Considering the studies included in this meta-analysis, MIS is a safe and effective alternative technique for the simultaneous resection of CRC and SCRLM. Compared with OS, MIS has less intraoperative blood loss and blood transfusion and quicker postoperative recovery. Furthermore, the two groups show equivalent long-term outcomes.
Keywords: Minimally invasive surgical procedures, Laparotomy, Colorectal neoplasms, Synchronous liver metastases, Meta-analysis
Core tip: For patients with colorectal cancer (CRC) and synchronous colorectal liver metastases (SCRLM), there is no consensus regarding whether minimally invasive surgery (MIS) or open surgery (OS) is more beneficial. We conducted this meta-analysis to compare the treatment outcomes of MIS and OS for the simultaneous resection of CRC and SCRLM. We assessed the two methods in terms of short- and long-term outcomes. Compared with OS, MIS has less intraoperative blood loss and blood transfusion and quicker postoperative recovery. Furthermore, the two groups show equivalent long-term outcomes.
INTRODUCTION
Colorectal cancer (CRC) remains one of the leading causes of cancer-related deaths worldwide. The incidence and mortality of CRC rank third (10.2%) and second (9.2%), respectively, among all cancers worldwide[1]. Moreover, according to the latest global cancer report issued by the International Agency for Research on Cancer for 2018 alone, more than 1.8 million patients were newly diagnosed with CRC, and 881000 patients died of CRC[1]. The liver is the most common organ for metastasis from colorectal neoplasms. Approximately 40% of patients with CRC eventually develop liver metastases, and 15%-20% of CRC patients have synchronous colorectal liver metastases (SCRLM) at the time of initial diagnosis; the metastases are limited to the liver in 70%-80% of these patients, but only a subset of these metastases are resectable[2-4]. Complete radical resection of primary and metastatic lesions is a potential curative treatment strategy for patients with resectable CRC and SCRLM, which is directly related to the prognosis of these patients[5,6].
The resection methods for CRC and SCRLM include simultaneous resection and staged resection. Staged resection of the initial tumor and SCRLM was first developed and performed, removing the primary CRC tumor, followed by adjuvant chemotherapy and liver metastasis tumor resection[7]. Recently, an increasing number of studies have suggested that the simultaneous resection of primary CRC and SCRLM is safe and acceptable and may become an optimal treatment strategy for patients with resectable CRC and SCRLM[8,9]. Simultaneous resection of CRC and SCRLM was only performed by laparotomy before minimally invasive surgery (MIS) such as laparoscopy or robotics was applied in the treatment of CRC[10,11]. MIS displayed superior short-term and similar long-term treatment outcomes to conventional open surgery (OS) in some studies[12,13]. However, most of the studies were case series or reports, and the sample sizes were small[14,15]. The advantages of MIS over OS remained unclear, particularly concerning the long-term outcomes. Therefore, this meta-analysis was conducted to compare the short- and long-term outcomes of MIS and OS for the simultaneous resection of primary CRC and SCRLM based on the current available literature. The present study followed the principle of PICOS.
MATERIALS AND METHODS
Search strategy
A systematic search of the Web of Science, Cochrane Library, Embase, and PubMed was performed to identify relevant studies comparing MIS and OS for simultaneous resection of CRC and SCRLM published up to December 22, 2018. The search strategy was performed using the following terms: “colon”, “rectal”, “rectum”, or “colorectal”; “liver metasta*”, “hepatic metasta*”; “robot*”, “Da Vinci”, “laparoscop*”, or “minimally invasive”; and “simultaneous”, “coinstantaneous”, “synchronous”, or “combined”. The language of the articles was not limited to any language. The references of relevant reviews and meta-analysis were also manually searched for potentially relevant studies. All relevant studies that met the inclusion criterion were reviewed for data extraction. This meta-analysis was prepared in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) statement[16], and the work was reported in line with the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) guidelines[17].
Study selection
Two authors (Ye SP and Qiu H) independently scanned the titles and abstracts from the studies identified in the electronic search. Relevant papers were further identified through perusing full texts. Disagreements were resolved by discussion and consensus between authors. Studies were included in this meta-analysis according to the following inclusion criteria: (1) They compared the treatment outcomes of MIS with OS for simultaneous resection CRC and SCRLM, and MIS was only limited to laparoscopic or robotic-assisted procedure; (2) The papers were accepted or published, and the full texts were available; and (3) The articles reported on at least three treatment outcomes mentioned below. The exclusion criteria included were as follows: (1) Patients with metachronous colorectal liver metastasis; (2) Hand-assisted laparoscopy; (3) Hepatic diseases, including benign liver lesions or primary liver malignancies through preoperative diagnosis; and (4) Noncomparative studies, duplicate studies, articles presented at meetings, review articles, conference abstracts, guidelines, case reports or series, or letters.
Data extraction and quality assessment
Two researchers (Ye SP and Qiu H) reviewed each article by a structured list and extracted data into a database independently. Disagreements of opinions were settled through discussion between authors. Data with the following items were extracted: (1) Characteristics, including first author, country, publication year, sample size, gender and age of patients, body mass index, study type, primary CRC tumor location and size, size and number of liver metastases; (2) Short-term outcomes, including operation time, intraoperative blood loss, time to intestinal function recovery, time to diet, number of blood transfusions during the operation, length of postoperative hospital stay, type (surgical or general complications) and severity (Clavien-Dindo grade ≥ 3) of postoperative complications (surgical complications including anastomotic leakage, bile leakage, ileus, abdominal abscess, wound infection, and chylous ascites; and general complications including urinary tract infection, pulmonary complications, and pleural effusion); and (3) Long-term outcomes, including overall survival and disease-free survival (DFS). Disagreements in data extraction were resolved by discussion. The time to intestinal function recovery was referred to the time to the first flatus after operation. The type and severity of complications were assessed by the sum number of the relevant complications reported in each study. The Newcastle-Ottawa scale (NOS) was used to evaluate the methodological quality of the studies[18]. The scale has three parts: Patient selection, comparability, and outcome. Two reviewers (Ai JH and Liao SJ) appraised the quality of studies independently. Disagreements were solved by consensus. The scale changes from 0 to 9 stars, and studies with a score ≥ six stars could be deemed as high quality.
Statistical analysis
The software Review Manager, version 5.3, was adopted to analyze the data. The odds ratio (OR) with 95% confidence interval (CI) was utilized to assess the dichotomous variables. The weighted mean difference (WMD) and 95%CI were analyzed for continuous variables. If continuous variables were presented as medians and ranges in some studies, the mean and standard deviation (SD) of these variables were estimated, as reported by Hozo et al[19]. The hazard ratio (HR) was used to assess the difference in the DFS and overall survival between the groups. If HR was not reported, then HR was estimated from the data of DFS and overall survival extracted from the survival curve in the included papers, as described by Tierney et al[20]. The I2 statistic was utilized to evaluate the heterogeneity among the studies[21]. The random-effects model was adopted if I2 was > 50%; otherwise, the fixed-effects model was utilized. Potential publication bias was determined by a funnel plot and assessed by the Begg’s test and Egger’s test (STATA software version 12.0 was adopted). A P-value < 0.05 was considered statistically significant.
RESULTS
Search results and study characteristics
In total, 1198 potential articles were initially retrieved from the electronic databases, and 684 potentially relevant articles were obtained for further assessment after eliminating duplicates. Next, 654 articles were excluded by screening the title and abstract. Thirty articles were retrieved for full-text evaluation. Finally, ten comparative cohort studies were included for this meta-analysis[22-31]. A flow chart of the search strategies that contains reasons for the exclusion of studies is shown in Figure 1. The study by Chen et al[28] was also included although it contained six patients with liver tumors not metastatic from the CRC diagnosed based on the postoperative pathological report because this did not influence the intraoperative procedure, and the study excluded these patients for further analysis of long-term outcomes. The characteristics of each study and study quality evaluated using the NOS are presented in Table 1. The preoperative characteristics of all patients from the two groups are shown in Table 2.
Figure 1.
Flow chart of the study search strategies. CRC: Colorectal cancer; SCRLM: Synchronous colorectal liver metastases.
Table 1.
Characteristics of the included studies
| Author-year | Country | Study type |
Gender (M/F) |
BMI (kg/m2)2 |
Age (yr)2 |
Quality score3 | |||
| MIS | OS | MIS | OS | MIS | OS | ||||
| Chen-2011 | China | Retrospective | 18/5 | 14/4 | NR | NR | 55 ± 10 | 53 ± 9 | 8 |
| Chen-2019 | China | Retrospective | 10/6 | 9/13 | 23.8 ± 3.7 | 23.3 ± 4.1 | 66.0 ± 10.4 | 64.8 ± 13.0 | 7 |
| Gorgun-2017 | America | Retrospective | 6/8 | 16/13 | 25.1 ± 0.8 | 27.5 ± 1.2 | 56.3 ± 3.3 | 57.7 ± 2.5 | 7 |
| Hu-2012 | China | Matched-pair | 10/3 | 9/4 | 21.5 ± 7.8 | 22.2 ± 8.4 | 54.0 ± 10.0 | 53.0 ± 11.0 | 8 |
| Ivanecz-2017 | Slovenia | Matched-pair | 6/4 | 6/4 | 26.9 (23.6-32.1) | 24.0 (23.1-25.5) | 62.2 ± 7.9 | 65.4 ± 8.1 | 9 |
| Lin-2015 | China | Matched-pair | 5/2 | 21/15 | 21.9 ± 2.2 | 21.2 ± 1.6 | 59.6 ± 3.4 | 57.4 ± 10.4 | 7 |
| Ma-2018 | China | Matched-pair | 9/3 | 8/4 | 23.0 (19.3-26.7) | 21.6 (75.5-24.9) | 3/91 | 1/111 | 8 |
| Ratti-2016 | Italy | Matched-pair | 14/11 | 27/23 | NR | NR | 60 (37-80) | 62 (35-81) | 8 |
| Takasu-2014 | Japan | Matched-pair | 3/4 | 3/4 | NR | NR | 74.0 ± 12.0 | 62.0 ± 4.0 | 7 |
| Tranchart-2016 | France | Matched-pair | 42/47 | 40/49 | 24.0 ± 3.6 | 24.7 ± 2.5 | 66.6 ± 10.8 | 65.0 ± 9.4 | 7 |
Dichotomous variables, >60/≤60 years;
The data are expressed as the mean ± SD or medians (range);
According to the Newcastle-Ottawa scale classification. BMI: Body mass index; F: Female; M: Male; MIS: Minimally invasive surgery; OS: Open surgery; NR: Not reported.
Table 2.
Short- and long-term outcomes of the included studies
| Author-year | Size of CRC (cm) | TD of CRC W+M/P+O | ID of CRC T1-T2/T3-T4 | CRC (C/R) | ASAs 1-2/3-4 | CEA level | Number of SCRLM | SCRLM (U/B) | Size of SCRLM (cm) |
| Chen2011-MIS | 2.5 ± 0.9 | 16/7 | NR | 3/4 | NR | 38.9 ± 3.5 | NR | NR | 5.5 ± 1.2 |
| Chen2011-OS | 2.3 ± 1.0 | 14/4 | NR | 3/4 | NR | 38.7 ± 3.3 | NR | NR | 5.6 ± 1.4 |
| Chen2019-MIS | 4.0 ± 2.0 | NR | 2/14 | NR | 1.9 (1-3) | 929 ± 1936 | NR | NR | 5.5 ± 4.2 |
| Chen2019-OS | 5.0 ± 3.0 | NR | 2/20 | NR | 2.0 (1-3) | 247 ± 728 | NR | NR | 4.7 ± 3.7 |
| Gorgun2017-MIS | 3.7 ± 0.7 | NR | 2/12 | 6/8 | 0/14 | 36.9 ± 30.4 | 1.6 ± 0.3 | 12/2 | 2.4 ± 0.7 |
| Gorgun2017-OS | 3.7 ± 0.5 | NR | 7/22 | 14/15 | 6/23 | 14.3 ± 4.9 | 2.1 ± 0.2 | 19/10 | 2.7 ± 0.2 |
| Hu2012-MIS | NR | NR | NR | 7/6 | NR | NR | NR | NR | 3.2 ± 1.0 |
| Hu2012-OS | NR | NR | NR | 7/6 | NR | NR | NR | NR | 3.5 ± 0.9 |
| Ivanecz2017-MIS | NR | 10/0 | 1/9 | 4/6 | 8/2 | 7.7 ± 7.7 | 1 (1-2) | 9/1 | 2.0 ± 1.2 |
| Ivanecz2017-OS | NR | 9/1 | 1/9 | 6/4 | 7/3 | 15.2 ± 12.5 | 1 (1-2) | 9/1 | 2.9 ± 1.5 |
| Lin2015-MIS | 5.3 ± 1.1 | 4/3 | 0/7 | 3/4 | 6/1 | 7/21 | 1.9 ± 0.9 | 7/0 | 3.3 ± 1.8 |
| Lin2015-OS | 5.7 ± 1.9 | 19/17 | 2/34 | 19/17 | 31/5 | 27/91 | 2.1 ± 1.0 | 26/10 | 4.2 ± 2.2 |
| Ma2018-MIS | NR | 10/2 | 1/11 | 7/5 | 12/0 | 7/52 | 1.0 (1-4) | 10/2 | 2.8 (0.5-10.0) |
| Ma2018-OS | NR | 9/3 | 0/12 | 7/5 | 12/0 | 11/12 | 2.5 (1-7) | 7/5 | 2.5 (1.2-6.5) |
| Ratti2016-MIS | NR | 21/5 | NR | 13/12 | 20/5 | 35.6 (2-299) | 2 (1-6) | 13/12 | 2.9 (0.5-11) |
| Ratti2016-OS | NR | 40/10 | NR | 27/23 | 44/6 | 41.6 (3.1-612) | 2 (1-7) | 27/23 | 3.4 (0.9-12) |
| Takasu2014-MIS | 4 ± 2 | 7/0 | 2/5 | 3/4 | NR | NR | 1.4 ± 0.8 | 5/2 | 1.9 ± 0.9 |
| Takasu2014-OS | 5 ± 0 | 7/0 | 1/6 | 3/4 | NR | NR | 1.5 ± 1.1 | 3/4 | 2.9 ± 1.1 |
| Tranchart2016-MIS | NR | 72/17 | 12/77 | 48/41 | 64/25 | 61 ± 75 | 1.4 ± 0.6 | 81/8 | 2.9 ± 1.9 |
| Tranchart2016-OS | NR | 63/26 | 10/79 | 51/38 | 56/33 | 31 ± 76 | 1.5 ± 0.7 | 78/11 | 2.8 ± 2.0 |
Dichotomous variables, >5/≤5 ng/mL;
Dichotomous variables, ≥5/<5 ng/mL. B: Bilobar; C: Colon; CRC: Colorectal cancer; ID: Invasion depth; M: Moderate; MIS: Minimally invasive surgery; O: Other; OS: Open surgery; P: Poor; R: Rectal; SCRLM: Synchronous colorectal liver metastases; TD: Tumor differentiation; U: Unilobar; W: Well; NR: Not reported.
Intraoperative outcomes
All studies reported the operative time and intraoperative blood loss. The operative time showed no significant difference between the two groups (WMD = 34.05 min, 95%CI: 0.65 to 67.46, P = 0.05, I2 = 84% for heterogeneity, P < 0.00001; Figure 2A). However, the intraoperative blood loss was less in the MIS group than in the OS group (WMD = -130.09, 95%CI: -210.95 to -49.23, P = 0.002, I2 = 91% for heterogeneity, P < 0.00001; Figure 2B). Seven studies reported the number of intraoperative blood transfusions in 407 patients[24-27,29-31]. The number of intraoperative blood transfusions was significantly less in the MIS group than in the OS group (OR = 0.53, 95%CI: 0.29 to 0.95, P = 0.03, I2 = 0% for heterogeneity, P = 0.81; Figure 2C).
Figure 2.
Forest plots of the meta-analysis for intraoperative outcomes. A: The operative time was compared between the minimally invasive surgery (MIS) and open surgery (OS) groups; B: Intraoperative estimated blood loss was compared between the MIS and OS groups; C: Number of intraoperative blood transfusions was compared between the MIS and OS groups; D: Time to bowel functional recovery was compared between the MIS and OS groups; E: Time to start diet was compared between the MIS and OS groups; F: Length of postoperative hospital stay was compared between the MIS and OS groups. MIS: Minimally invasive surgery; OS: Open surgery; CI: Confidence interval; SD: Standard deviation.
Postoperative outcomes
In this meta-analysis, postoperative outcomes included the time to bowel functional recovery, time to start diet, length of postoperative hospital stay, and postoperative complications. Five studies reported the time to bowel functional recovery in 222 patients[23,24,26,30,31]. The pooled mean time to bowel functional recovery was shorter in the MIS group than in the OS group (WMD = -0.88 d, 95%CI: -1.58 to -0.19, P = 0.01, I2 = 94% for heterogeneity, P < 0.00001; Figure 2D). Four studies provided the time to recovery of diet in 162 patients[24,26,27,31]. The time to recovery of diet for MIS was significantly shorter than that for OS (WMD = -1.54 d, 95%CI: -2.30 to -0.78, P < 0.0001, I2 = 88% for heterogeneity, P < 0.0001; Figure 2E). All studies reported the length of hospital stay, but only six studies clearly recorded the length of postoperative hospital stay, including 87 patients in the MIS group and 136 patients in the OS group[22-24,27,29,30]. According to the pooled data from the six studies, the patients in the MIS group were discharged earlier than those in the OS group after operation (WMD = -4.06 d, 95%CI: -5.95 to -2.18, P < 0.0001, I2 = 69% for heterogeneity, P = 0.006; Figure 2F).
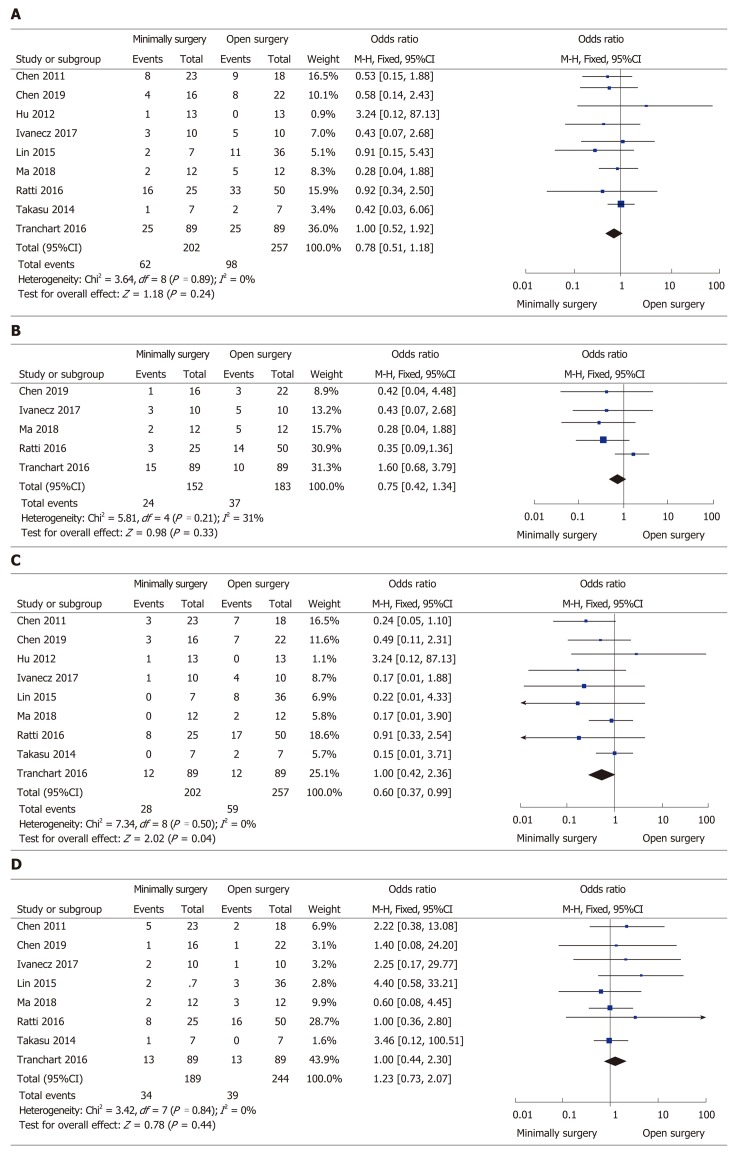
All but one study[26] clearly reported the rate of overall postoperative complications, ranging from 7.1% to 34.8% in the MIS group and from 0% to 50% in the OS group, without a significant difference between the two groups (OR = 0.78, 95%CI: 0.51 to 1.18, P = 0.24, I2 = 0% for heterogeneity, P = 0.89; Figure 3A). The severity (Clavien–Dindo grade ≥ 3) of complications was reported in five studies[24,25,27,28,31], and the number of severe complications was not significantly different in the two groups (OR = 0.75, 95%CI: 0.42 to 1.34, P = 0.33, I2 = 31% for heterogeneity, P = 0.21; Figure 3B). Different types (surgical or general complications) of postoperative complications were compared for further analysis. The surgical complications were reported in nine studies[22-25,27-31], and the pooled data identified that the rate of surgical complications in the MIS group was significantly reduced compared with the OS group (OR = 0.60, 95%CI: 0.37 to 0.99, P = 0.04; I2 = 0% for heterogeneity, P = 0.50; Figure 3C). The general complications between the groups retrieved from eight papers[22-25,27,28,30,31] showed no significant difference (OR = 1.23, 95%CI: 0.73 to 2.07, P = 0.44, I2 = 0% for heterogeneity, P = 0.84; Figure 3D).
Figure 3.
Forest plots of the meta-analysis for complications. A: Overall complications were compared between the minimally invasive surgery (MIS) and open surgery (OS) groups; B: Severe (Clavien–Dindo grade ≥ 3) complications were compared between the MIS and OS groups; C: Surgical complications were compared between the MIS and OS groups; D: General complications were compared between the MIS and OS groups. MIS: Minimally invasive surgery; OS: Open surgery; CI: Confidence interval.
Long-term outcomes
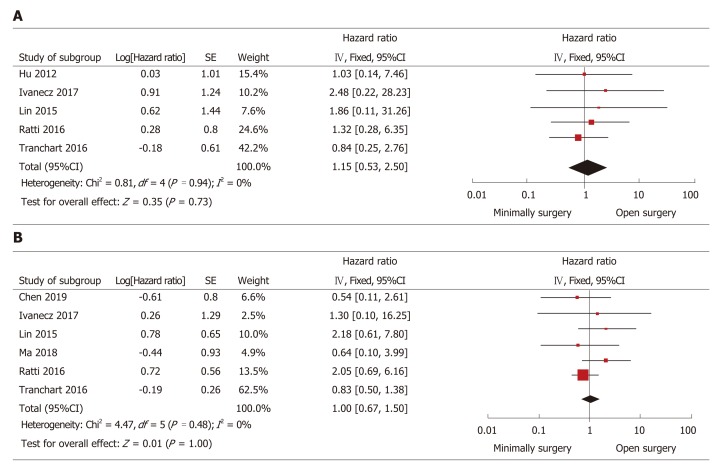
The HR was used to assess the differences in the long-term outcomes (DFS and overall survival) between the groups. The HR was estimated from the survival data that were extracted from the survival curve reported in the studies. All studies provided the Kaplan-Meier curves of overall survival, whereas the 95%CI could not be calculated in half of the studies. Another five studies[23-25,29,31] showed that HR was not significantly different between the groups (HR = 1.15, 95%CI: 0.53 to 2.50, P = 0.73, I2 = 0% for heterogeneity, P = 0.94; Figure 4A). Seven studies reported DFS curves, but the 95%CI could not be calculated in one study because it only provided the median follow-up time without a range[23-25,27,28,31]. Thus, synthetic analysis of the DFS data in the other six studies was conducted. The HR of DFS was not significantly different (HR = 1.00, 95%CI: 0.67 to 1.50, P = 1.00, I2 = 0% for heterogeneity, P = 0.48; Figure 4B).
Figure 4.
Forest plots of the meta-analysis for long-term outcomes. A: The overall survival was compared between the minimally invasive surgery (MIS) and open surgery (OS) groups; B: Disease-free survival was compared between the MIS and OS groups. MIS: Minimally invasive surgery; OS: Open surgery; CI: Confidence interval; SE: Standard error.
Publication bias
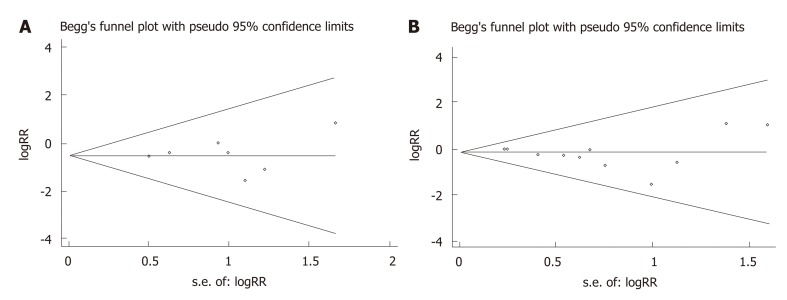
As shown in Figure 5, the possible publication bias of the number of intraoperative blood transfusions and overall complications between the groups was assessed by the funnel plot, Egger’s test, and Begg’s test. The funnel plot of the number of intraoperative blood transfusion was symmetrically distributed, and none of the studies included was outside the 95%CI (Figure 5A). No significant publication bias was detected from statistical tests based on the number of intraoperative blood transfusions (Begg’s test P = 1.000; Egger’s test P = 0.897). The funnel plot of the overall complications showed a symmetric distribution of all studies, and all were inside the 95%CI (Figure 5B). The pooled analysis suggested that there was no significant publication bias in the overall complications (Begg’s test P = 0.283; Egger’s test P = 0.127).
Figure 5.
Funnel plots of the number of intraoperative blood transfusions and postoperative complications in patients between the minimally invasive surgery and open surgery groups. A: Intraoperative blood transfusions; B: Postoperative complications. RR: Relative risk.
DISCUSSION
In past decades, the resection timing of primary CRC and SCRLM has been controversial. Some scholars hold the opinion that staged resection can reduce the rate of operative complications and make the occult micrometastases become perceptible[32,33]. However, some authors believe that simultaneous resection can reduce the burden of tumors, as well as reduce the economic and psychological burden of patients, leading to patients undergoing one operative procedure instead of two[34]. Recently, with the advances in the strategy of perioperative management and overall critical care, the operation strategy for resectable patients has been questioned. An increasing number of authors support that the optimal operation timing is gradually changing from staged resection to simultaneous resection[35,36].
Traditionally, simultaneous resection of primary CRC and SCRLM is usually performed by laparotomy. Conventional laparotomy always requires a long abdominal incision for adequate exposure of the operative field, causing severe pain and incision complications[29]. Laparotomy is also associated with serious physical and psychological operative trauma to the patients. With the improvements in surgical technique and apparatus, MIS showed great advantages in some surgeries such as proctocolectomy or hepatectomy[11,13]. Furthermore, reports regarding the safety and efficacy of combined resection of CRC and SCRLM have been increased. However, most were case series or reports limited by a small sample size[14,15]. Our meta-analysis of ten comparative studies with 502 patients provided a large body of information and identified the advantages of MIS over OS in simultaneous resection of CRC and SCRLM.
The pooled results revealed that the operative time was similar between the groups. The operation time depended on the characteristics of primary tumors and liver metastases, severe degree of abdominal adhesion and obesity, and experience of the surgical teams[37,38]. These might also be the sources of heterogeneity (P < 0.00001, I2 = 84%). The intraoperative blood loss was critical to evaluate the quality of surgery. The most significant finding in this meta-analysis was the reduction of blood loss in MIS vs OS (P = 0.002). Moreover, the number of intraoperative blood transfusions was also less in the MIS group than in the OS group (P = 0.03). Less blood loss indicated a lower blood transfusion rate. Studies have reported that perioperative blood transfusion might lead to poor survival, and transfused patients had more post-operative complications[39-41]. High heterogeneity (P < 0.00001, I2 = 91%) among groups was observed in this regard that was likely to be related to the methods used to estimate the blood loss and diverse experience of the surgical teams.
Postoperative recovery was also analyzed in the present meta-analysis. Intere-stingly, the time to both intestinal function recovery and start of the diet in patients accepting MIS was shorter than that in patients undergoing OS (P = 0.01, P < 0.0001). The cause was that the MIS technique shortened the intestinal exposure time and reduced intestinal irritation. Furthermore, another target to assess the postoperative recovery was the length of postoperative hospital stay. As expected, the length of postoperative hospital stay was shorter in the MIS group than in the OS group (P = 0.01). Different surgical teams had different postoperative management concepts, which might interpret the high heterogeneity in the above indexes of postoperative recovery.
Another issue of concern for MIS is the postoperative complications; all studies reported postoperative complications, but the number of complications reported in one article[26] was too obscure for further analysis. No significant difference was found in the rate (P = 0.24) or severity (Clavien–Dindo grade ≥ 3, P = 0.33) of overall postoperative complications. Our meta-analysis also analyzed the type of post-operative complications. The general complications were not different between the groups, but the surgical complications were fewer in the MIS group than in the OS group. Thus, our results suggested that MIS is a safe and effective technique.
A key factor in evaluating the quality of surgery is the long-term oncological outcomes. Nine studies provided overall survival curves, and seven studies reported DFS curves. However, the 95%CI of HR could not be calculated in some studies according to the data extracted from the studies. Finally, further analysis for overall survival and DFS could only be conducted in six and five studies, respectively. The pooled data showed that the HR of DFS and overall survival was similar in the two groups.
Several limitations should be considered in this meta-analysis such as the heterogeneity. First, the surgeries were performed by different surgical teams with different experience and skills, as well as different perioperative management. Second, although these studies were focused on CRC and SCRLM, the involved types of surgeries were different because of different tumor locations. Third, these were all retrospective studies with a small size, without RCTs available. Finally, the number of studies included in our meta-analysis was not large enough because studies focused on MIS and OS for the simultaneous resection of CRC and SCRLM were limited in the electronic databases. Based on the above limitations, caution must be taken in analyzing the results pooled from the ten studies.
In conclusion, MIS was proven to be a safe and effective technique in the treatment of patients with CRC and SCRLM. Compared with OS, MIS has less blood loss and blood transfusion, a quicker postoperative recovery, and equivalent long-term outcomes. Further randomized controlled trials are needed to further confirm these advantages.
ARTICLE HIGHLIGHTS
Research background
Colorectal cancer (CRC) is a common cause of cancer-related deaths, especially in cases with liver metastases. Simultaneous surgical resection of primary colorectal tumors and synchronous colorectal liver metastases (SCRLM) is an effective strategy to improve the overall survival rate. Application of minimally invasive surgery (MIS) is increasing, but the true benefits of MIS remain unclear.
Research motivation
We hope to offer guidance on the clinical care of MIS in CRC and SCRLM.
Research objectives
To help identify which optimal surgery method is suitable for patients with CRC and SCRLM.
Research methods
A systematic search was conducted in PubMed, EMBASE, Web of Science, and Cochrane Library databases for studies concerning minimally invasive and open surgery (OS) for the simultaneous resection of CRC and SCRLM. We followed the PRISMA agreement. The meta-analysis was performed using Review Manager Software, and the quality was assessed using the Newcastle-Ottawa scale.
Research results
Two hundred and sixteen patients in the mini-invasive group and 286 patients in the open group were included in this study. Intraoperative blood loss (P = 0.002) and blood transfusion (P = 0.03) were less, the recovery of intestinal function (P = 0.01) and diet (P < 0.0001) was faster, the length of postoperative hospital stay (P < 0.0001) was shorter, and the number of surgical complications was lower (P = 0.04) in the MIS group. However, the operation time, rates and severity of overall morbidity, as well as the rates of general morbidity showed no significant difference between the minimally invasive and OS groups. Moreover, the overall survival and disease-free survival after MIS were similar to those after OS.
Research conclusions
The current meta-analysis showed that MIS is an optimal strategy for the simultaneous resection of CRC and SCRLM.
Research perspectives
The results of the current meta-analysis may help researchers to develop guidelines about surgical methods in CRC and SCRLM more perfectly.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors deny any conflict of interest.
Data sharing statement: No additional data are available.
PRISMA 2009 Checklist statement: This meta-analysis was prepared and revised in accordance with the PRISMA 2009 Checklist.
Peer-review started: March 1, 2019
First decision: April 11, 2019
Article in press: May 8, 2019
P-Reviewer: De Bree E, Ozturk E, Sturesson C S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Zhang YL
Contributor Information
Shan-Ping Ye, Department of General Surgery, First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
Hua Qiu, Department of General Surgery, First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
Shi-Jun Liao, Department of General Surgery, First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
Jun-Hua Ai, Department of General Surgery, First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
Jun Shi, Department of General Surgery, First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China. shijunyfy@sina.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Veen T, Søreide K. Can molecular biomarkers replace a clinical risk score for resectable colorectal liver metastasis? World J Gastrointest Oncol. 2017;9:98–104. doi: 10.4251/wjgo.v9.i3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–474. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 5.Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN Americas Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Selection for hepatic resection of colorectal liver metastases: Expert consensus statement. HPB (Oxford) 2013;15:91–103. doi: 10.1111/j.1477-2574.2012.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayo SC, Pulitano C, Marques H, Lamelas J, Wolfgang CL, de Saussure W, Choti MA, Gindrat I, Aldrighetti L, Barrosso E, Mentha G, Pawlik TM. Surgical management of patients with synchronous colorectal liver metastasis: A multicenter international analysis. J Am Coll Surg. 2013;216:707–16; discussion 716-8. doi: 10.1016/j.jamcollsurg.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, Barbas AS, Abdalla EK, Choti MA, Vauthey JN, Ludwig KA, Mantyh CR, Morse MA, Clary BM. Simultaneous resections of colorectal cancer and synchronous liver metastases: A multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]
- 8.Slesser AA, Simillis C, Goldin R, Brown G, Mudan S, Tekkis PP. A meta-analysis comparing simultaneous versus delayed resections in patients with synchronous colorectal liver metastases. Surg Oncol. 2013;22:36–47. doi: 10.1016/j.suronc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Feng Q, Wei Y, Zhu D, Ye L, Lin Q, Li W, Qin X, Lyu M, Xu J. Timing of hepatectomy for resectable synchronous colorectal liver metastases: For whom simultaneous resection is more suitable--a meta-analysis. PLoS One. 2014;9:e104348. doi: 10.1371/journal.pone.0104348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR, Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, Hausken J, Tønnessen TI, Abildgaard A, Barkhatov L, Yaqub S, Røsok BI, Bjørnbeth BA, Andersen MH, Flatmark K, Aas E, Edwin B. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg. 2018;267:199–207. doi: 10.1097/SLA.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 12.Wei M, He Y, Wang J, Chen N, Zhou Z, Wang Z. Laparoscopic versus open hepatectomy with or without synchronous colectomy for colorectal liver metastasis: A meta-analysis. PLoS One. 2014;9:e87461. doi: 10.1371/journal.pone.0087461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues TFDC, Silveira B, Tavares FP, Madeira GM, Xavier IP, Ribeiro JHC, Pereira RMOS, Siqueira SL. Open, laparoscopic, and robotic-assisted hepatectomy in resection of liver tumors: a non-systematic review. Arq Bras Cir Dig. 2017;30:155–160. doi: 10.1590/0102-6720201700020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takorov I, Belev N, Lukanova T, Atanasov B, Dzharov G, Djurkov V, Odisseeva E, Vladov N. Laparoscopic combined colorectal and liver resections for primary colorectal cancer with synchronous liver metastases. Ann Hepatobiliary Pancreat Surg. 2016;20:167–172. doi: 10.14701/ahbps.2016.20.4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatwell C, Bretagnol F, Farges O, Belghiti J, Panis Y. Laparoscopic resection of colorectal cancer facilitates simultaneous surgery of synchronous liver metastases. Colorectal Dis. 2013;15:e21–e28. doi: 10.1111/codi.12068. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, Henry DA, Boers M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62:1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takasu C, Shimada M, Sato H, Miyatani T, Imura S, Morine Y, Ikemoto T, Kanamoto M, Kurita N, Eto S, Utsunomiya T. Benefits of simultaneous laparoscopic resection of primary colorectal cancer and liver metastases. Asian J Endosc Surg. 2014;7:31–37. doi: 10.1111/ases.12066. [DOI] [PubMed] [Google Scholar]
- 23.Lin Q, Ye Q, Zhu D, Wei Y, Ren L, Zheng P, Xu P, Ye L, Lv M, Fan J, Xu J. Comparison of minimally invasive and open colorectal resections for patients undergoing simultaneous R0 resection for liver metastases: A propensity score analysis. Int J Colorectal Dis. 2015;30:385–395. doi: 10.1007/s00384-014-2089-2. [DOI] [PubMed] [Google Scholar]
- 24.Ratti F, Catena M, Di Palo S, Staudacher C, Aldrighetti L. Impact of totally laparoscopic combined management of colorectal cancer with synchronous hepatic metastases on severity of complications: A propensity-score-based analysis. Surg Endosc. 2016;30:4934–4945. doi: 10.1007/s00464-016-4835-8. [DOI] [PubMed] [Google Scholar]
- 25.Tranchart H, Fuks D, Vigano L, Ferretti S, Paye F, Wakabayashi G, Ferrero A, Gayet B, Dagher I. Laparoscopic simultaneous resection of colorectal primary tumor and liver metastases: A propensity score matching analysis. Surg Endosc. 2016;30:1853–1862. doi: 10.1007/s00464-015-4467-4. [DOI] [PubMed] [Google Scholar]
- 26.Gorgun E, Yazici P, Onder A, Benlice C, Yigitbas H, Kahramangil B, Tasci Y, Aksoy E, Aucejo F, Quintini C, Miller C, Berber E. Laparoscopic versus open 1-stage resection of synchronous liver metastases and primary colorectal cancer. Gland Surg. 2017;6:324–329. doi: 10.21037/gs.2017.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma K, Wang XY, Chen JH. [Laparoscopic versus open surgery for simultaneous resection of synchronous colorectal liver metastases] Zhonghua Wai Ke Za Zhi. 2018;56:516–521. doi: 10.3760/cma.j.issn.0529-5815.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Chen YW, Huang MT, Chang TC. Long term outcomes of simultaneous laparoscopic versus open resection for colorectal cancer with synchronous liver metastases. Asian J Surg. 2019;42:217–223. doi: 10.1016/j.asjsur.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Hu MG, Ou-yang CG, Zhao GD, Xu DB, Liu R. Outcomes of open versus laparoscopic procedure for synchronous radical resection of liver metastatic colorectal cancer: A comparative study. Surg Laparosc Endosc Percutan Tech. 2012;22:364–369. doi: 10.1097/SLE.0b013e31825af6b2. [DOI] [PubMed] [Google Scholar]
- 30.Chen KY, Xiang GA, Wang HN, Xiao FL. Simultaneous laparoscopic excision for rectal carcinoma and synchronous hepatic metastasis. Chin Med J (Engl) 2011;124:2990–2992. [PubMed] [Google Scholar]
- 31.Ivanecz A, Krebs B, Stozer A, Jagric T, Plahuta I, Potrc S. Simultaneous Pure Laparoscopic Resection of Primary Colorectal Cancer and Synchronous Liver Metastases: A Single Institution Experience with Propensity Score Matching Analysis. Radiol Oncol. 2017;52:42–53. doi: 10.1515/raon-2017-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallance AE, van der Meulen J, Kuryba A, Charman SC, Botterill ID, Prasad KR, Hill J, Jayne DG, Walker K. The timing of liver resection in patients with colorectal cancer and synchronous liver metastases: A population-based study of current practice and survival. Colorectal Dis. 2018;20:486–495. doi: 10.1111/codi.14019. [DOI] [PubMed] [Google Scholar]
- 33.Kaibori M, Iwamoto S, Ishizaki M, Matsui K, Saito T, Yoshioka K, Hamada Y, Kwon AH. Timing of resection for synchronous liver metastases from colorectal cancer. Dig Dis Sci. 2010;55:3262–3270. doi: 10.1007/s10620-009-1124-6. [DOI] [PubMed] [Google Scholar]
- 34.Fukami Y, Kaneoka Y, Maeda A, Takayama Y, Onoe S, Isogai M. Simultaneous resection for colorectal cancer and synchronous liver metastases. Surg Today. 2016;46:176–182. doi: 10.1007/s00595-015-1188-1. [DOI] [PubMed] [Google Scholar]
- 35.Lupinacci RM, Andraus W, De Paiva Haddad LB, Carneiro D' Albuquerque LA, Herman P. Simultaneous laparoscopic resection of primary colorectal cancer and associated liver metastases: A systematic review. Tech Coloproctol. 2014;18:129–135. doi: 10.1007/s10151-013-1072-1. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Li Q, Wang C, Zhu H, Shi Y, Zhao G. Simultaneous vs. staged resection for synchronous colorectal liver metastases: A meta-analysis. Int J Colorectal Dis. 2011;26:191–199. doi: 10.1007/s00384-010-1018-2. [DOI] [PubMed] [Google Scholar]
- 37.Pai A, Alsabhan F, Park JJ, Melich G, Sulo S, Marecik SJ. The Impact of Obesity on the Perioperative, Clinicopathologic, and Oncologic Outcomes of Robot Assisted Total Mesorectal Excision for Rectal Cancer. Pol Przegl Chir. 2017;89:23–28. doi: 10.5604/01.3001.0010.3906. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Xiao Y, Wu L, Li B, Li H. Laparoscopic liver resection as a safe and efficacious alternative to open resection for colorectal liver metastasis: A meta-analysis. BMC Surg. 2013;13:44. doi: 10.1186/1471-2482-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel SV, Brennan KE, Nanji S, Karim S, Merchant S, Booth CM. Peri-operative blood transfusion for resected colon cancer: Practice patterns and outcomes in a population-based study. Cancer Epidemiol. 2017;51:35–40. doi: 10.1016/j.canep.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Jagoditsch M, Pozgainer P, Klingler A, Tschmelitsch J. Impact of blood transfusions on recurrence and survival after rectal cancer surgery. Dis Colon Rectum. 2006;49:1116–1130. doi: 10.1007/s10350-006-0573-7. [DOI] [PubMed] [Google Scholar]
- 41.Mazzeffi M, Tanaka K, Galvagno S. Red Blood Cell Transfusion and Surgical Site Infection After Colon Resection Surgery: A Cohort Study. Anesth Analg. 2017;125:1316–1321. doi: 10.1213/ANE.0000000000002099. [DOI] [PubMed] [Google Scholar]